Abstract
Background & Aims
Endoscopic therapy (ET) is frequently used to treat patients with painful chronic pancreatitis (CP), but little is known about outcomes of patients for whom ET was not successful that then underwent surgery, or outcomes after ET compared with only medical treatment. We evaluated utilization and long-term effectiveness of ET in a well-defined cohort of patients with CP.
Methods
We analyzed data from 146 patients with CP who participated in the North American Pancreatitis Study 2 study at the University of Pittsburgh Medical Center (UPMC) from 2000 to 2006; 71 (49%) received ET at UPMC. Success of ET and surgery were defined by cessation of narcotic therapy and resolution of episodes of acute pancreatitis. Disease progression was followed from its onset until January 1, 2011 (mean 8.2±4.7 yrs).
Results
Patients who underwent ET had more symptoms (pain, recurrent pancreatitis) and had more complex pancreatic morphology (based on imaging) than patients that received medical therapy. ET had a high rate of technical success (60/71 cases, 85%); its rates of clinical success were 51% for 28/55 patients for which follow-up data was available (mean time of 4.8±3.0 yrs) and 50% for 12/24 patients who underwent surgery after receiving ET. Patients that responded to ET were significantly older, had a shorter duration of disease before ET, had less constant pain, and required fewer daily narcotics than patients who did not respond to ET. Among the 36 symptomatic patients who received medical therapy and were followed for a mean time period of 5.7±4.1 yrs, 31% improved and 53% had no change in symptoms; of these, 21% underwent surgery.
Conclusions
ET is clinically successful for 50% of patients with symptomatic CP. When ET is not successful, surgery has successful outcomes in 50% of patients. Symptoms resolve in 31% of symptomatic patients who receive only medical therapy.
Keywords: NAPS2 study, pancreas, inflammation, treatment comparison, endoscopic retrograde cholangiopancreatography (ERCP), endoscopy
Introduction
Chronic pancreatitis (CP) is a progressive inflammatory disease of the pancreas characterized by destruction of pancreatic parenchyma and subsequent fibrosis, leading to exocrine and/or endocrine insufficiency.1 Abdominal pain is a prominent symptom in many CP patients and is often debilitating. An important mechanism for pain in CP is intraductal hypertension as a result of pancreatic duct (PD) obstruction from stones or strictures2,–4. Other suggested mechanisms of pain include ongoing inflammation, peripheral or central neuropathic processes, and pancreatic or peripancreatic complications (e.g. pseudocysts, biliary stricture, compression of surrounding structures).3–5
Endoscopic and surgical treatments in CP patients are pursued to relieve pain and address local complications.6–32 Although effectiveness of endoscopic therapy (ET) has been evaluated in several studies, most are limited by small numbers of patients, incomplete follow-up, and lack of medically managed control arm.6–20 Moreover, most studies have used pain as an indication for and outcome of ET and do not present a comprehensive evaluation of ET for all indications (e.g. recurrent acute pancreatitis [RAP], PD leak, biliary stricture, etc.). There is also a paucity of data describing the utilization of ET within a cohort of CP patients. There are few studies comparing outcomes of surgery with ET, and existing data on surgical treatment focuses mainly on patients who have not undergone prior ET.21–32 Few data are available on the outcomes of pancreatic surgery after failed ET, which is more representative of the current clinical practice.
We evaluated the utilization, effectiveness, and long-term clinical outcomes of ET in a well phenotyped cohort of CP patients and compared it with patients who were managed medically.
Methods
Patient Cohort
The North American Pancreatitis Study 2 (NAPS2) is a multicenter study in which 540 CP and 460 RAP patients were prospectively enrolled between 2000 and 2006 from nineteen secondary and tertiary referral centers with interest in pancreatic diseases in the United States between 2000 and 2006.33 The inclusion criteria for CP was presence of definitive changes on imaging studies (primarily computerized tomography [CT] scan or endoscopic retrograde cholangiopancreatography [ERCP]) or histology.
The present study included CP patients (n=150) in the NAPS2 cohort enrolled from the University of Pittsburgh Medical Center (UPMC). Of these, 4 were excluded due to missing or insufficient information relevant to this study (n=2), diagnosis of IPMN (n=1) or a change in diagnosis to (RAP (n=1) thereby resulting in a final study population of 146 patients. All patients provided informed consent for review of their records; the study was approved by the University of Pittsburgh Institutional Review Board.
Data Collection
We reviewed study data collected for all patients at the time of NAPS2 enrollment as well as medical records both prior to enrollment and during the follow-up period (i.e. until 1/1/2011). Data in this study is therefore presented relating to the patients’ presentation at the time of initial evaluation at UPMC and relevant to the timing and follow-up after ET and/or surgery. Data was recorded regarding chronic (not associated with episodes of AP) pain characteristics (intermittent, constant), use and type of pain medication (non-narcotic, narcotic) and frequency of pain (intermittent, daily), presence of RAP, jaundice/cholestasis, diabetes, and steatorrhea. Baseline pancreatic and biliary findings, including pancreatic morphology, pancreatic and/or biliary ductal dilation, strictures, stones, leak, pseudocysts, and divisum on abdominal imaging studies (CT scan, magnetic resonance imaging [MRI], ultrasound [USG] and ERCP) were recorded for all patients. ERCP reports were reviewed for the number and indications of procedures, diagnostic findings, therapeutic interventions, complications, and duration of therapy. Surgical reports were reviewed for procedure type, operative findings, and technical success. Endoscopic or surgical treatment prior to evaluation at UPMC was also recorded.
Patient Classification
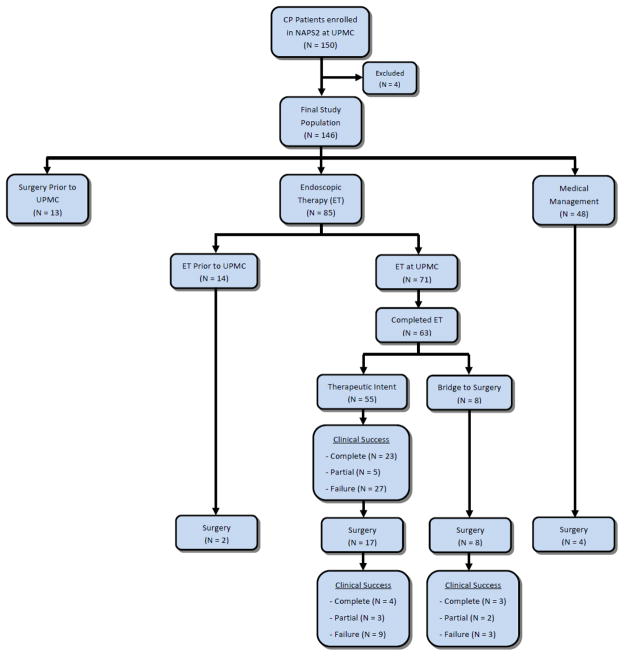
We classified patients according to pancreatic morphology to evaluate technical and clinical success in homogenous groups6. Categories were based on the presence of dominant PD morphology: 1) strictures in the PD in head/body, 2) stones in the PD in head/body, 3) both strictures and stones, 4) complex pathology (strictures, stones) but dilation in the PD body/tail. Patients who did not have these findings were categorized as follows: 5) isolated biliary stricture, 6) isolated pancreatic divisum, 7) isolated papillary stenosis, or 8) isolated leak/pseudocyst. For each patient we also noted the treatment modality - endoscopic, surgical, or medical (Figure 1). Indications for ET included symptoms related to CP, failed medical therapy, and changes on imaging amenable to endoscopic treatment (i.e., stones, strictures, PD dilation, etc).
Figure 1.
Schematic representation of patient stratification and data analysis
Bridge Patients
A subset of patients for whom the gastroenterologist and gastrointestinal surgeon believed surgery was the optimal approach were classified as “bridge to surgery” patients. Their pancreatic morphology typically demonstrated an inflammatory mass or extensive calcifications in the pancreatic head. ET was performed as a temporary measure to delay the surgery until resolution of inflammatory changes, assess response to ductal decompression as a predictor of response to surgery or in patients who refused surgery in favor of ET. Clinical success of ET was not assessed in these patients due to the short duration of treatment and short follow-up between ET and surgery.
Technical Outcomes and Complications of ET
All ERCPs at UPMC were performed by one of four expert therapeutic endoscopists. The technical success of ET (Table 1) was determined for each morphologic finding requiring therapy and was divided into three categories: complete, partial, failure. If more than one morphologic feature was present (e.g. - strictures and stones), complete success required that all features be resolved. ET was considered complete if no further ERCP sessions were performed after the initial treatment series, and no stents were left in situ. Otherwise, ET was considered to be ongoing at the time of last follow-up. “Temporary stent” refers to stents without internal fixation, placed to reduce the risk of post-ERCP pancreatitis. Migration was confirmed on abdominal x-ray at 1 week; otherwise, stents were endoscopically removed.
Table 1.
Criteria used for technical and clinical success and failure of endoscopic therapy based on anatomic findings and presenting symptoms*
| Complete Success | Failure | |
|---|---|---|
|
| ||
| Technical Outcome | ||
|
|
||
| PD stricture | Successful dilation, with the stricture waist disappearing, and/or successful stent placement across stricture | Dilation is without appreciable effect, and/or a stent could not be placed across stricture |
|
|
||
| PD stone | Complete removal of intraductal stones with no stones remaining | Stone burden cannot be appreciably reduced |
|
|
||
| PD leak | Resolution of the leak on pancreatogram with no extravasation of contrast or recurrent fluid collection | Ongoing leak refractory to ET and/or requiring surgical treatment |
|
|
||
| CBD stricture | Successful dilation, with the stricture waist disappearing, and/or successful stent placement across stricture | Dilation is without appreciable effect, and/or stent could not be placed across stricture |
|
|
||
| Divisum | Successful stent placement in the dorsal duct and/or minor papillotomy | Inability to place a stent in the dorsal duct or perform a minor papillotomy |
|
|
||
| Papillary stenosis | Successful dilation, stent placement across the papilla, and/or pancreatic sphincterotomy | Inability to place a stent across the papilla or perform a pancreatic sphincterotomy |
|
| ||
| Clinical Outcome | ||
|
|
||
| Pain | Reduction in frequency of pain (not daily) and discontinuation of narcotic medications | No reduction in the frequency of pain and/or ongoing use of daily narcotics |
|
|
||
| RAP | No further episodes of acute pancreatitis following ET | No change in the frequency of episodes of RAP |
| Jaundice/cholestasis | Resolution of jaundice or cholestasis without need for PTC or surgery | No change in jaundice/cholestasis and/or need for PTC or surgery |
Partial success was an intermediate category between complete success and failure
Post-ERCP pancreatitis was defined as new or worsened abdominal pain with amylase and/or lipase elevation ≥3 times the upper limit of normal more than 24 hours post procedure. Cholangitis was defined as a temperature ≥38 degrees Celsius for 24–48 hours post-procedure felt to be of biliary origin. Bleeding was defined as clinical evidence of gastrointestinal blood loss after ET irrespective of transfusion requirement or further procedures to achieve hemostasis. Hospitalization for 24 hour observation without any other complications following ERCP was also recorded as a complication.
Clinical Outcomes of ET and Surgery
Follow-up data were collected through 1/1/2011. The presence of symptoms (pain, RAP, jaundice/cholestasis) was recorded for every clinical contact during follow-up. Clinical success of ET and surgery (Table 1) were divided into three categories: complete, partial, and failure. If more than one symptom was present (e.g. chronic pain and RAP), complete success required fulfilling criteria for all symptoms. The need for surgery at any time during follow-up was considered clinical failure of ET.
Medical Management
Patients who underwent no ET or surgery following their diagnosis of CP were considered to be managed medically. Medical management included analgesic medications (non-narcotic, narcotic), pancreatic enzyme supplementation, counseling for abstinence from alcohol and tobacco, and dietary recommendations. Follow-up symptoms were classified as: 1) remained asymptomatic (never developed symptoms), 2) improved (discontinued or remained off narcotics with improved pain frequency), and 3) ongoing or worsened (continued or increased pain and narcotic usage).
Statistical Methods
Descriptive statistics are presented as proportions for categorical data and as mean ± standard deviation or median (interquartile range) as applicable. Bivariate comparisons were performed using chi-squared or Fischer exact test for categorical data and student-t or Mann-Whitney U test for continuous data depending on the data distribution. A p-value of <0.05 was considered significant.
Results
Patient Cohort
Overall, age at initial diagnosis of CP was 44±18 years, 52% were male and 89% were Caucasian. The etiology was alcohol in 60 (40%), idiopathic in 47 (32%), genetic in 17 (12%), hypertriglyceridemia in 8 (5%), divisum in 8 (5%), SOD in 3 (2%), autoimmune in 2 (1%), and biliary in 1 (1%) patient. Symptoms at the time of initial evaluation at UPMC included abdominal pain (93 patients, 64%), RAP (71 patients, 48%), jaundice/cholestasis (17 patients, 12%), diabetes (13 patients, 9%), and steatorrhea (35 patients, 24%). Abdominal pain was characterized as constant in 50/93 (54%) patients. Analgesic medications were used by 80/93 (86%) patients - non-narcotic in 7/80 (9%), intermittent narcotic in 30/80 (38%), daily narcotic in 43/80 (54).
Treatment Groups
The distribution of patients into groups and follow-up based on treatment type is shown in Figure 1. Of the 146 CP patients, 13 (9%) had undergone pancreatic surgery prior to evaluation at UPMC, 48 (33%) were managed medically, and 85 (58%) underwent ET (14/85 [16%] at outside facilities, 17/85 [20%] both at outside facilities and at UPMC, and 54/85 [64%] only at UPMC). Compared to patients who were managed medically, patients who underwent ET were significantly younger, more likely to have any pain, constant pain, required more narcotics, and were more likely to have jaundice/cholestasis (Table 2).
Table 2.
Initial clinical data and anatomic findings in chronic pancreatitis patients treated medically or with endoscopic therapy.
| Endoscopic Therapy (n = 85) | Medical Management (n = 48) | P value | |
|---|---|---|---|
| Demographics | |||
|
|
|||
| Age at diagnosis# | 41 (31–53) | 53 (32–66) | 0.04 |
|
|
|||
| Male gender – n (%) | 43 (51) | 26 (54) | 0.69 |
|
|
|||
| White race – n (%) | 73 (86) | 44 (92) | 0.33 |
|
|
|||
| Smoker – n (%) | 59 (69) | 28 (58) | 0.20 |
|
| |||
| Etiology – n (%) | |||
|
|
|||
| Alcohol | 35 (41) | 22 (46) | 0.60 |
|
|
|||
| Idiopathic | 22 (26) | 19 (40) | 0.16 |
|
|
|||
| Genetic | 10 (12) | 4 (8) | 0.54 |
|
|
|||
| Triglyceride | 5 (6) | 2 (4) | 0.67 |
|
|
|||
| Divisum | 8 (9) | 0 (0) | 0.028 |
|
|
|||
| SOD | 3 (4) | 0 (0) | 0.19 |
|
|
|||
| Autoimmune | 2 (2) | 0 (0) | 0.28 |
|
| |||
| Biliary | 0 (0) | 1 (2) | 0.18 |
|
| |||
| Symptoms* - n (%) | |||
|
|
|||
| Pain present | 62 (72) | 22 (46) | 0.002 |
|
|
|||
| Constant pain | 36 (42) | 8 (17) | 0.002 |
|
|
|||
| Narcotic usage | 50 (59) | 15 (31) | 0.002 |
|
|
|||
| RAP | 45 (53) | 21 (44) | 0.31 |
|
|
|||
| Jaundice/cholestasis | 16 (19) | 1 (2) | 0.005 |
|
|
|||
| Diabetes | 7 (8) | 5 (10) | 0.67 |
|
|
|||
| Steatorrhea | 18 (21) | 16 (33) | 0.12 |
|
| |||
| Imaging/ERCP Findings* - n (%) | |||
|
|
|||
| PD dilation | 68 (80) | 26 (54) | 0.002 |
|
|
|||
| PD stone | 35 (41) | 16 (33) | 0.37 |
|
|
|||
| PD stricture | 39 (46) | 4 (8) | <0.001 |
|
|
|||
| Pseudocyst | 20 (24) | 9 (19) | 0.52 |
|
|
|||
| PD leak | 8 (9) | 0 (0) | 0.028 |
|
|
|||
| Divisum | 16 (19) | 1 (2) | 0.005 |
|
|
|||
| CBD stricture | 20 (24) | 2 (4) | 0.004 |
|
|
|||
| Papillary stenosis | 13 (15) | -- | -- |
Data collected at the time of initial evaluation at UPMC
median (interquartile range)
Imaging findings and Morphological Groups
Imaging findings on cross-sectional studies and/or ERCP of the ET and medically managed groups are presented in Table 2. Overall, frequent findings included PD dilation, PD stones, and PD strictures which were present in 71%, 38%, and 32% of patients, respectively. Less common findings included papillary stenosis (11%), pseudocysts (22%), PD leak (6%), and CBD dilation (17%). Compared with patients managed medically, patients who underwent ET were more likely to have PD dilation, PD strictures, pancreas divisum, PD leak, and CBD stricture.
Patients who underwent ET were categorized based on morphologic findings into the following groups: PD strictures 18 (25%), PD stones 15 (21%), PD stones plus strictures 14 (20%), complex morphology 5 (7%), isolated pancreas divisum 4 (6%), isolated papillary stenosis 7 (10%), isolated PD leak/pseudocyst 4 (6%), and isolated biliary stricture 4 (6%).
Endoscopic Therapy
Of the 85 patients who underwent ET, 14 had ET performed only at outside institutions. Due to lack of procedure details in these patients, subsequent analysis is presented only for patients who underwent ET at UPMC (Table 3). In these 71 patients, ERCP was performed with therapeutic intent in 63 (89%) and as a bridge to surgery in 8 (11%) patients. A total of 247 ERCP sessions were performed (median 2, range 1–17). In 13 (18%) patients with pancreatic divisum, treatment was performed through the minor papilla. Overall, complete or partial technical success was achieved in 60/71 (85%) patients.
Table 3.
Details of endoscopic therapy in chronic pancreatitis patients
| Patients with ET at UPMC (n = 71) | |
|---|---|
|
| |
| Number of ERCPs* | 2 (1, 4)
|
| Duration of therapy (months)* | 4 (<1, 29)
|
| Bridge to surgery – n (%) | 8 (11) |
|
| |
| Pancreatic Therapy |
|
| Pancreatic sphincterotomy – n (%) | 43 (61)
|
| Dilation performed – n (%) | 24 (34)
|
| Number of dilations* | 2 (1, 2.75)
|
| Dilation size (mm)* | 4 (4, 6)
|
| Stone extraction – n (%) | 26 (37)
|
| ESWL performed – n (%) | 6 (8)
|
| PD stent placement – n (%) | 53 (75)
|
| Temporary stent – n (%) | 28 (39)
|
| Number of stents* | 1 (1, 1.25)
|
| Therapeutic Stent – n (%) | 44 (62)
|
| Number of stents* | 2 (1, 3)
|
| Number of sittings* | 2 (1,3)
|
| Stent length (cm)* | 7 (7, 9)
|
| Stent size (Fr)* | 7 (7, 7) |
|
| |
| Biliary Therapy |
|
| Biliary sphincterotomy – n (%) | 31 (44)
|
| Dilation – n (%) | 7 (10)
|
| Stent placement – n (%) | 19 (27)
|
| Number of stents* | 2 (1, 4)
|
| Number of sittings* | 2 (1, 4)
|
| Stent length (cm)* | 7 (7, 9)
|
| Stent size (Fr)* | 10 (10, 10) |
|
| |
| Transgastric Pseudocyst drainage – n (%) | 3 (4)
|
| EUS – ERCP – Both | 1 – 1 – 1
|
| Celiac Plexus Block – n (%) | 4 (6) |
median (interquartile range)
ERCP – endoscopic retrograde cholangiopancreatography; ESWL – extracorporeal shock wave lithotripsy; EUS – endoscopic ultrasound
There were several notable differences in ET among the morphologic groups. Patients with isolated PD stones underwent fewer ERCP sessions (mean 2.1) than patients with isolated strictures (mean 5.0, p=0.024), strictures plus stones (mean 4.1, p=0.066), or complex morphology (mean 4.8, p=0.001). The mean duration of therapy was also shorter for the PD stone group (9 months) compared with the stricture (39 months, p=0.023) and complex groups (44 months, p=0.049). More PD stents were placed in the stone plus stricture group (100%) compared with the isolated stone group (67%, p=0.037) and isolated stricture group (72%, p=0.032). The presence of concurrent biliary strictures and the rate of technical success were similar among all morphologic groups.
Success and Complications of ET
Of the 71 patients who underwent ET at UPMC, complete follow-up data were available for 63 (89%) patients. Four patients were lost to follow-up, three were still undergoing ET at last follow-up, and one was diagnosed with pancreatic cancer during ET. The mean duration of follow-up after completion of ET was 4.8±3.0 years (range 0.5–13 years). Complete or partial clinical success was seen in 28/55 (51%) patients who completed ET with a therapeutic intent (Table 4). Excluding patients who also underwent ET at outside institutions prior to enrollment, clinical success of ET performed only at UPMC was seen in 26/45 (58%) patients. Patients who responded to ET were more likely to be older at the start of ET (47 vs. 40 yrs, p=0.10), have less constant pain (21 vs. 52%, p=0.031), require less daily narcotics (14 vs. 56%, p=0.001), and have a shorter duration between diagnosis of CP and start of ET (median 4 vs. 40 months, p=0.017) (table 5). There was no significant difference in clinical success between the morphologic groups (data not shown). Among the 14 patients who underwent biliary stenting and had follow-up data, resolution of biliary stricture was achieved in 9/14 (64%).
Table 4.
Complications and technical and clinical success of endoscopic therapy and surgery in 71 chronic pancreatitis patients who underwent endoscopic therapy at UPMC
| N (%) | |
|---|---|
| Complications of ET (n=247 ERCPs) |
|
| Total including all hospitalizations | 29 (12)
|
| Total minus hospitalizations for observation | 12 (5)
|
| Pancreatitis | 9 (4)
|
| Infection | 3 (1) |
|
| |
| Technical Success of ET (n=70 completed ET) |
|
| Complete | 54 (77)
|
| Partial | 6 (9)
|
| Failure | 10 (14) |
|
| |
| Clinical Success of ET (n= 55 patients) * |
|
| Complete | 23/55 (42)
|
| Partial | 5 (9)
|
| Failure | 27 (49) |
|
| |
| Clinical Success of Surgery |
|
| Therapeutic ET Group ** | 17/60 (23)
|
| Complete Clinical Success | 4/16 (25%)
|
| Partial Clinical Success | 3 (19%)
|
| Clinical Failure | 9 (56%)
|
| Bridge Group | 8/8 (100%)
|
| Complete Clinical Success | 3/8 (38%)
|
| Partial Clinical Success | 2 (25%)
|
| Clinical Failure | 3 (38%) |
55/71 patients completed ET with therapeutic intent and have follow up data.
16/17 patients completed surgery and have follow up data.
Table 5.
Factors associated with long term clinical response to endoscopic therapy in chronic pancreatitis patients
| Response to ET (n = 28) | No Response to ET (n = 27) | P value | |
|---|---|---|---|
| Age at diagnosis (years)* | 47 ± 19 | 40 ± 13 | 0.10 |
|
|
|||
| Constant pain (%) | 6 (21) | 14 (52) | 0.031 |
|
|
|||
| Daily narcotics (%) | 4 (14) | 15 (56) | 0.001 |
|
|
|||
| Duration of disease (months)** | 4 (1, 12) | 40 (1, 60) | 0.017 |
mean +/− standard deviation;
median (interquartile range)
No difference in response based on etiology or morphologic groups
The overall incidence of complications related to ERCP was 12% (29/247 sessions). Of these, 17 (59%) were hospitalizations for observation alone. Specific complications included post-ERCP pancreatitis in 4% (9/247) and cholangitis in 1% (3/247). There was no ERCP-related bleeding, perforation, or death. There was no significant difference in complication rates between the morphologic groups or the bridge/therapeutic ET groups (data not shown).
Surgery
Of the 132 patients with follow-up data, 44 (33%) patients underwent surgery (Figure 1). Among patients undergoing ET at UPMC, 25/71 (35%) required surgery. The rate of surgery among patients undergoing ET with therapeutic intent (i.e. due to clinical failure of ET) was 31% (17/55). All 8 (100%) patients in the bridge to surgery group underwent surgery. Patients with isolated strictures were less likely to undergo surgery than patients with isolated stones (20 vs. 62%, p=0.025), stones plus strictures (20 vs. 54%, p=0.062), and complex morphology (20 vs. 60%, p=0.091), despite a similar clinical failure rate of ET. The mean time from completion of ET to surgery was 7 months (range 0–68 months).
Surgical procedures included Whipple (10), Frey (6), Puestow (2), Beger (1), Duval (1), total pancreatectomy with auto-islet cell transplant (1), distal pancreatectomy (1), and other (3). Technical success of surgery was observed in 24/25 (96%) patients. One patient was diagnosed with pancreatic cancer during surgery. The mean duration of follow-up after surgery was 4.3±3.4 years (range 1–13 years). Complete or partial clinical success with surgery was seen in 12/24 (50%) patients. There was no significant difference in response to surgery between the morphologic groups (data not shown). No factors were identified as significantly associated with clinical success of surgery, although there was a trend toward response for patients with short duration of disease (54 vs. 87 months, p=0.19), and in the bridge to surgery group compared with the therapeutic ET group (63% vs. 44%, p=0.40).
Medical Management
Overall, 48/146 (33%) patients were managed medically. One quarter of patients (12/48) were asymptomatic at the time of initial presentation and all remained asymptomatic during the follow up period. There were no significant morphologic differences between symptomatic and asymptomatic patients (data not shown). The mean duration from time of CP diagnosis to last follow up in medically managed patients was 5.7±4.1 years (range 0.3–19 years). Of the 36/48 (75%) patients who had symptomatic disease, 11 (31%) had significant improvement in symptoms, 19 (53%) had no change or an increase in symptoms, and 6 (17%) were lost to follow-up. There were no significant differences in morphology between patients who improved and those who remained symptomatic (data not shown). Four of nineteen (21%) persistently symptomatic patients underwent surgery during follow-up, with clinical success of surgery comparable to that of the ET group.
Endocrine and Exocrine Dysfunction
Among the 132 patients with complete follow-up data, 19 (14%) developed new diabetes and 8 (6%) developed new steatorrhea during follow-up. There was no significant difference in the development of diabetes and steatorrhea between patients based on the type of treatment received (data not shown).
Mortality
The 10 (7%) patients who died during follow up were equally distributed in the ET and medically managed groups. The cause of death included pancreatic cancer (4), other malignancy (2), infection (2), GI bleed (1), and spontaneous ulcer perforation (1). The mean duration between diagnosis of CP and death was 4.7 years (range 0.5–15 years).
Discussion
We found that patients undergoing ET were more symptomatic and had more complex morphology than patients who were managed medically. ET was safe, technically successful in most patients and achieved long-term clinical success in over half of patients. Long-term clinical success was also achieved in half of all patients who underwent surgery after failed ET or in whom ET was used as a bridge to surgery.
CP patients comprise a heterogeneous population with a variety of symptoms, morphologic features, and disease-related complications. Nearly 40% of patients who underwent ET in our study had more than one symptom (pain, RAP, jaundice), and all but one patient (biliary stricture alone) who underwent ET presented with abdominal pain as a component of their disease. Similarly, nearly 40% of patients who underwent ET had an additional morphologic finding outside the PD (biliary stricture, pseudocyst). These findings highlight the presence of clinical symptoms and morphological features that can guide selection of patients for ET and/or surgery.
Morphologic features may affect the technical and clinical success of ET in CP patients. Because previous studies have shown a higher rate of clinical success of ET in patients with PD strictures over stones,6 we took morphology into account when stratifying patients into homogenous groups before evaluating the efficacy of ET. While we noted differences in terms of the number of ERCPs and stents placed (higher in patients with strictures vs. without strictures), technical and clinical success rates of ET and surgery were similar amongst morphologic groups. Our results suggest that specific baseline morphologic characteristics may not predict long-term clinical outcomes of ET.
Rather than focusing on short term clinical efficacy which may diminish over time, we assessed the long-term clinical outcomes of ET. Clinical success in our study (51–58%) was somewhat lower than other large series (54–94%) with medium to long-term follow-up.6–17 A lower response in our study may be due to long duration of follow-up, inclusion of patients with multiple morphologic features, and differences in assessing outcome of ET (e.g. narcotic discontinuation in our study vs. hospitalizations, pain scores, etc.). As in previous studies, we found that a shorter duration of disease was associated with a higher clinical success.7–8, 12, 22–23 This suggests that a degree of irreversibility develops as CP progresses, and may indicate a role for endoscopic or surgical intervention early in disease course. In contrast to prior studies, we found that patients with less severe symptoms (lack of constant pain or use of daily narcotics) were more likely to achieve clinical success after ET. Presence of constant pain may be an indicator of more advanced disease, different pain mechanisms, or visceral hyperalgesia, thereby affecting the success of ET. Daily narcotic use may confer a degree of narcotic dependence that impedes discontinuation of narcotics post-ET independent of improvement in pain. In fact, prior CP surgical series have shown that patients on opiods preoperatively are more likely to continue them postoperatively despite improvement in pain.23 Failure of endoscopic ductal decompression suggests a pathogenesis of pain other than intraductal hypertension.
In our cohort, 44/146 (30%) overall and 27/85 (32%) patients after ET underwent surgery. Clinical success after surgery was seen in 50%. The rate and outcomes of surgery in prior studies have been highly variable. Surgery rates in CP patients were much higher in earlier studies when ET was not routinely performed (56 to 72%).23,34 Most studies either assessed effectiveness of surgery as initial treatment (i.e. without prior attempts at ET), or do not provide details regarding ET prior to surgery. The clinical success rates in these series have ranged from 53 to 95%, 21–32 although recurrence of pain over very long follow-up (>10 years) has been reported to be as high as 56–65%.34–36
Clinical success after surgery depends on whether surgery is performed in naïve patients or in those who have failed ET. A unique aspect of our study was the evaluation of surgical outcomes after failed ET. This represents a negative selection of patients, which may explain our somewhat lower surgical success rate compared to previous studies. Our approach, however, is consistent with the current guidelines, which suggest ET as an initial approach, reserving surgery for cases of failure or recurrence of symptoms.38 Interestingly, the overall clinical success rate of ET plus surgery was 66 %, similar to surgical series. Three other studies report detailed descriptions of ET and subsequent surgery, with clinical success in 63–79% of patients.6,9,15 Two of these studies have small numbers of patients (8 and 12 patients), 9,15 while the third does not report follow-up duration after surgery6.
Our study differs from previous reports in stratifying patients according to whether ET was performed with a therapeutic intent or as a bridge to surgery. The bridge group comprises patients for whom surgery was felt to be the ideal treatment. ET was performed as a temporary measure to permit resolution of inflammatory changes in the pancreatic/peripancreatic area, to accommodate patient’s request for initial attempt at ET rather than surgery, or to assess whether surgery will be beneficial (e.g. decompression of PD prior to consideration of a Puestow’s operation). Not surprisingly, surgery in these patients did show a trend toward better success than those in the therapeutic ET group (63 vs. 44%). The decision to perform ET as a bridge to surgery should be made in collaboration between gastroenterologist, therapeutic endoscopist and surgeon to individualize treatment approach.
Another unique aspect of our study was the inclusion of a medically managed arm. This reflects the natural history of CP in patients treated at a referral center. We identified no prior studies that directly compare medical and endoscopic management in a cohort of CP patients with long-term results. Although most medically managed patients (75%) were symptomatic at the time of presentation, their symptoms were milder and morphology less complex when compared with patients who underwent ET, justifying a conservative approach to treatment. Interestingly, spontaneous improvement was seen in 11 (31%) patients, somewhat lower than prior studies describing the natural history of CP (35–70%).34–37 This difference may be explained by the longer duration of follow-up (>10 years) in other natural history studies.
Limitations of our study include a retrospective analysis at a single tertiary academic center. Such retrospective studies can overestimate clinical success and underestimate complications. Our cohort likely suffers from referral bias as patients who have more symptomatic or complex disease are more likely to be referred to a tertiary center. Follow-up data were available for 89% of patients which provides a good, but not comprehensive degree of data completeness. We did not assess short-term effectiveness of ET was not performed, as effectiveness is thought to diminish over time, making long-term results a more useful assessment of overall effectiveness. Finally, the cohort presented here does not include CP patients treated at UPMC who were not enrolled in the NAPS2 study.
In conclusion, we evaluated and compared the long-term outcomes of ET with those of surgery and medical management in a large cohort of CP patients treated at a tertiary academic center. In expert hands, ET was safe and achieved long-term clinical success in over half of patients. Among patients who failed ET, surgery achieved clinical success in an additional half of patients. Overall, clinical success of ET and surgery was seen in 63%. We propose a stepwise approach for managing CP, starting with medical management. Patients who are symptomatic and have appropriate morphologic features should be considered for ET early in disease course. A subset of patients will require surgical intervention, with or without ET as a bridge to surgery, based on an individualized assessment of risk and benefit. An interdisciplinary approach is critical to providing effective, safe, and lasting palliation of symptoms.
Acknowledgments
The authors thank Michelle L Kienholz, Department of Medicine, University of Pittsburgh for critical review and editorial assistance.
Funding Source: This research was supported by National Institutes of Health (NIDDK) - DK061451 (DCW), the National Pancreas Foundation (DCW), Robert and Vicki Hall, and Andrew and Michelle Aloe.
Footnotes
This study was presented as a Poster at the Digestive Disorders Week, May 2011 at Chicago, IL, USA
Potential conflict of interest relevant to this manuscript: None
Authorship criteria and contributions:
Bridger Clarke: Study design, study supervision, data acquisition and interpretation, statistical analysis, drafting and revising the article, final approval of the version to be published.
Adam Slivka: Patient enrollment, data interpretation, revising the article, final approval of the version to be published.
Yutaka Tomizawa, MD: Data acquisition and interpretation, revision the article, final approval of the version to be published.
Michael Sanders MD, Georgios Papachristou: Data interpretation, revising the article, final approval of the version to be published.
David C. Whitcomb: Obtaining funding, patient ascertainment, data interpretation, revising the article, final approval of the version to be published.
Dhiraj Yadav: Study design, study supervision, data interpretation, statistical analysis, drafting and revising the article, final approval of the version to be published.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sarner M, Cotton P. Classification of pancreatitis. Gut. 1984;25:756–759. doi: 10.1136/gut.25.7.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jalleh RP, Aslam M, Williamson RC. Pancreatic tissue and ductal pressures in chronic pancreatitis. Br J Surg. 1991;78:1235–7. doi: 10.1002/bjs.1800781028. [DOI] [PubMed] [Google Scholar]
- 3.Di Magno EP. Toward understand (and management) of painful chronic pancreatitis. Gastroenterology. 1999;116:1152–1257. doi: 10.1016/s0016-5085(99)70031-4. [DOI] [PubMed] [Google Scholar]
- 4.Fasanella KE, Davis B, Lyon J, et al. Pain in chronic pancreatitis and pancreatic cancer. Gastroenterol Clin North Am. 2007 Jun;36(2):335–64. doi: 10.1016/j.gtc.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Di Sebastiano P, di Mola FF, Bockman DE, et al. Chronic pancreatitis: the perspective of pain generation by neuroimmune interaction. Gut. 2003;52(6):907–11. doi: 10.1136/gut.52.6.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosch T, Daniel S, Scholz M, et al. Endoscopic treatment of chronic pancreatitis: a multicenter study of 1000 patients with long-term follow-up. Endoscopy. 2002;34:765–771. doi: 10.1055/s-2002-34256. [DOI] [PubMed] [Google Scholar]
- 7.Dumonceau JM, Deviere J, Le Moine O, et al. Endoscopic pancreatic drainage in chronic pancreatitis associated with ductal stones: long-term results. Gastrointest Endosc. 1996;43:547–555. doi: 10.1016/s0016-5107(96)70189-x. [DOI] [PubMed] [Google Scholar]
- 8.Adamek HE, Jakobs R, Buttmann A, et al. Long-term follow-up of patients with chronic pancreatitis and pancreatic stones treated with extracorporeal shock-wave lithotripsy. Gut. 1999;45:402–405. doi: 10.1136/gut.45.3.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delhaye M, Matos C, Deviere J, et al. Endoscopic management of chronic pancreatitis. Gastrointest Endosc Clin N Am. 2003;13:717–42. doi: 10.1016/s1052-5157(03)00070-9. [DOI] [PubMed] [Google Scholar]
- 10.Tadenuma H, Ishihara T, Yamaguchi T, et al. Lont-term results of extracorporeal shock wave lithotripsy and endoscopic therapy for pancreatic stones. Clin Gastroenterol Hepatol. 2005;3:1128–1135. doi: 10.1016/s1542-3565(05)00530-6. [DOI] [PubMed] [Google Scholar]
- 11.Eleftheriadis N, Dinu F, Delhaye M, et al. Long-term outcome after pancreatic stenting in severe chronic pancreatitis. Endoscopy. 2005;37:223–30. doi: 10.1055/s-2005-860988. [DOI] [PubMed] [Google Scholar]
- 12.Binmoeller KF, Jue P, Seifert H, et al. Endoscopic pancreatic stent drainage in chronic pancreatitis and a dominant stricture: Long-term results. Endoscopy. 1995;27:638–644. doi: 10.1055/s-2007-1005780. [DOI] [PubMed] [Google Scholar]
- 13.Delhaye M, Arvanitakis M, Verset G, et al. Long-term clinical outcome after endoscopic pancreatic ductal drainage for patients with painful chronic pancreatitis. Clin Gastroenterol Hepatol. 2004;2(12):1096–106. doi: 10.1016/s1542-3565(04)00544-0. [DOI] [PubMed] [Google Scholar]
- 14.Dite P, Zboril V, Cikankova E, et al. Endoscopic therapy of chronic pancreatitis. Hepatogastroenterology. 1996;43:1633–1637. [PubMed] [Google Scholar]
- 15.Smits ME, Badiga SM, Rauws EA, et al. Long-term results of pancreatic stents in chronic pancreatitis. Gastrointest Endosc. 1995;42:461–7. doi: 10.1016/s0016-5107(95)70051-x. [DOI] [PubMed] [Google Scholar]
- 16.Dumonceau JM, Costamagna G, Tringali A, et al. Treatment of painful calcified chronic pancreatitis: extracorporeal shock wave lithotripsy versus endoscopic treatment: a randomized controlled trial. Gut. 2007;56:545–552. doi: 10.1136/gut.2006.096883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cremer M, DeviUre J, Delhaye M, et al. Stenting in severe chronic pancreatitis: Results of medium-term follow-up in seventy-six patients. Endoscopy. 1991;23:171–176. doi: 10.1055/s-2007-1010649. [DOI] [PubMed] [Google Scholar]
- 18.Cahen DL, Gouma DJ, Nio Y, et al. Endoscopic versus surgical drainage of the pancreatic duct in chronic pancreatitis. N Engl J Med. 2007;356:676–684. doi: 10.1056/NEJMoa060610. [DOI] [PubMed] [Google Scholar]
- 19.Delhaye M, Vandermeeren A, Baize M, Cremer M. Extracorporeal shock-wave lithotripsy of pancreatic calculi. Gastroenterology. 1992;102:610–620. doi: 10.1016/0016-5085(92)90110-k. [DOI] [PubMed] [Google Scholar]
- 20.Sherman S, Lehman GA, Hawes RH, et al. Pancreatic ductal stones: Frequency of successful endoscopic removal and improvement in symptoms. Gastrointest Endosc. 1991;37:511–517. doi: 10.1016/s0016-5107(91)70818-3. [DOI] [PubMed] [Google Scholar]
- 21.Sakorafas GH, et al. Pancreatoduodenectomy for chronic pancreatitis: longterm results in 105 patients. Arch Surg. 2000;135:517–23. doi: 10.1001/archsurg.135.5.517. [DOI] [PubMed] [Google Scholar]
- 22.Jimenez RE, et al. Outcome of pancreaticoduodenectomy with pylorus preservation or with antrectomy in the treatment of chronic pancreatitis. Ann Surg. 2000;231:293–300. doi: 10.1097/00000658-200003000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alexakis N, Connor S, Ghaneh P, et al. Influence of opioid use on surgical and long-term outcome after resection for chronic pancreatitis. Surgery. 2004;136(3):600–608. doi: 10.1016/j.surg.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 24.Greenlee HB, Prinz RA, Aranha GV. Long-term results of side-to-side pancreaticojejunostomy. World J Surg. 1990;14:70–76. doi: 10.1007/BF01670548. [DOI] [PubMed] [Google Scholar]
- 25.Bradley EL. Long-term results of pancreatojejunostomy in patients with chronic pancreatitis. Am J Surg. 1987;153(2):207–13. doi: 10.1016/0002-9610(87)90816-6. [DOI] [PubMed] [Google Scholar]
- 26.Izbicki JR, Bloechle C, Broering DC, et al. Extended drainage versus resection in surgery for chronic pancreatitis: a prospective randomized trial comparing the longitudinal pancreaticojejunostomy combined with local pancreatic head excision with the pylorus-preserving pancreatoduodenectomy. Ann Surg. 1998;228:771–9. doi: 10.1097/00000658-199812000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Beger HG, Schlosser W, Friess HM, et al. Duodenum preserving head resection in chronic pancreatitis changes the natural course of the disease: a single-center 26-year experience. Ann Sur. 1999;230(4):512–9. doi: 10.1097/00000658-199910000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Traverso LW, Kozarek RA. Pancreatoduodenectomy for chronic pancreatitis: anatomic selection criteria and subsequent long-term outcome analysis. Ann Surg. 1997;226 (4):429–438. doi: 10.1097/00000658-199710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strate T, Taherpour Z, Bloechle C, et al. Long-term follow-up of a randomized trial comparing the beger and frey procedures for patients suffering from chronic pancreatitis. Ann Surg. 2005;241(4):591–8. doi: 10.1097/01.sla.0000157268.78543.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor RH, Bagley FH, Braasch JW, Warren KW. Ductal drainage or resection for chronic pancreatitis. Am J Surg. 1981;141:28–33. doi: 10.1016/0002-9610(81)90007-6. [DOI] [PubMed] [Google Scholar]
- 31.Riediger H, Adam U, Fischer E, et al. Long-term Outcome After Resection for Chronic Pancreatitis in 224 Patients. J Gastrointest Surg. 2007;11:949–960. doi: 10.1007/s11605-007-0155-6. [DOI] [PubMed] [Google Scholar]
- 32.Frey CF, Amikura K. Local resection of the head of the pancreas combined with longitudinal pancreaticojejunostomy in the management of patients with chronic pancreatitis. Ann Surg. 1994;220:492–507. doi: 10.1007/BF02348284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Whitcomb DC, Yadav D, Adam S, Hawes RH, Brand RE, Anderson MA, et al. Multicenter approach to recurrent acute and chronic pancreatitis in the United States: the North American Pancreatitis Study 2 (NAPS2) Pancreatology. 2008;8(4–5):520–31. doi: 10.1159/000152001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ammann RW, Muellhaupt B. The natural history of pain in alcoholic chronic pancreatitis. Gastroenterology. 1999;116:1132–40. doi: 10.1016/s0016-5085(99)70016-8. [DOI] [PubMed] [Google Scholar]
- 35.Cavallini G, Frulloni L, Pederzoli P, et al. Long-term follow-up of patients with chronic pancreatitis in Italy. Scand J Gastroenterol. 1998;33(8):880–9. doi: 10.1080/00365529850171567. [DOI] [PubMed] [Google Scholar]
- 36.Lankisch PG, Lohr-Happe A, Otto J, et al. Natural course in chronic pancreatitis: pain, exocrine and endocrine pancreatic insufficiency and prognosis of the disease. Digestion. 1993;54:148–155. doi: 10.1159/000201029. [DOI] [PubMed] [Google Scholar]
- 37.Miyake H, Harada H, Kunichika K, et al. Clinical course and prognosis of chronic pancreatitis. Pancreas. 1987;2:378–385. doi: 10.1097/00006676-198707000-00003. [DOI] [PubMed] [Google Scholar]
- 38.ASGE Guidelines: the role of ERCP in diseases of the biliary tract and pancreas. Gastrointest Endosc. 2005;62:1–8. doi: 10.1016/j.gie.2005.04.015. [DOI] [PubMed] [Google Scholar]