Abstract
Purpose
To determine if time to treatment (TTT) has an effect on overall survival (OS) in patients with unresectable or medically inoperable stage III non-small cell lung cancer (NSCLC), and if patient or treatment factors are associated with TTT.
Methods and Materials
A total of 237 consecutive patients with stage III NSCLC treated at University of Michigan Hospital (UM) or the Veterans Affairs Ann Arbor Healthcare System (VA) were evaluated. Patients were treated with either palliative or definitive radiotherapy (RT), and received RT alone (n = 106) or either sequential (n = 69) or concurrent chemoradiation (n = 62). The primary endpoint was overall survival (OS).
Results
Median follow-up was 69 months and median TTT was 57 days. On univariate analysis, the risk of death did not increase significantly with longer TTT (p = 0.093). However, subset analysis showed that there was a higher risk of death with longer TTT in patients who survived ≥ 5 years (p = 0.029). Younger age (p = 0.027), male gender (p = 0.013), lower Karnofsky Performance Score (KPS) (p = 0.002), and treatment at the VA (p = 0.001) were significantly associated with longer TTT. However, on multivariable analysis only lower KPS remained significantly associated with longer TTT (p = 0.003).
Conclusion
Time to treatment is significantly associated with OS in patients with stage III NSCLC who lived longer than 5 years, though it is not a significant factor in stage III patients as a whole. Lower KPS is associated with longer TTT.
Keywords: Non-small cell lung cancer, stage III, time to treatment, treatment delay, overall survival
Introduction
Lung cancer is the leading cause of cancer-related death in the United States. In 2007, there were 211,380 new lung cancer cases and 160,390 deaths (1), of which 80% to 85% were due to non-small cell lung cancer (NSCLC). Surgical resection is the most effective method of controlling early-stage NSCLC. Most patients with T1-2 N0 tumors are curable by resection, with 5 year overall survival (OS) of 75–80% (2). Five-year OS rates for completely resected T3 N0 lesions are in the range of 30–50%. However, once mediastinal lymph nodes are involved, survival decreases to 15–20% (3). In addition, there is an inverse relationship between tumor volume and local control. Based upon a reported mean doubling time for squamous cell carcinoma of the lung, biologic models estimated a 0.31% per day decrease in the probability of local tumor control with increasing time from cancer detection (4). Therefore, some patients may be at risk of becoming incurable while waiting to initiate therapy (5). Thus, to maximize the probability of cure it is theoretically advantageous to treat patients with minimal delay.
Many clinical studies have shown that time to treatment (TTT) is highly variable (4–16). For example, estimated TTT from referral to initiation of RT for patients with stage IIIB squamous cell carcinoma of the lung in the Unites States versus Canada is 9 versus 34 days, respectively (5). Studies from Europe have reported median TTT of 15-54 days for RT for patients with lung cancer (5, 7, 10–12,14–16). However, the influence of diagnostic and therapeutic delays on clinical outcomes is poorly understood. Some studies have indicated that such delays negatively affect prognosis (5,9,15) while others have not shown such an association (7,10–14,16).
Most previous studies on TTT are derived from single-institution experiences with heterogeneous patient cohorts composed of both early and advanced stage lung cancer patients treated with older RT techniques (5,7,9,10,12,15,16). Approximately 30%-40% of NSCLC patients have unresectable stage IIIA/B disease at diagnosis, and newer chemotherapy agents, improved supportive care measures, and advances in radiation technology have resulted in improved outcomes (17,18). Yet, treatment with combined chemoradiation using contemporary 3-D CT-based planning is logistically more complicated and requires more time for treatment planning. Knowledge of the possible influence of TTT on survival and the factors associated with TTT in a large cohort of patients treated with modern chemotherapy regimens and RT techniques would be clinically valuable.
In this study we aimed to investigate: 1) the effect of TTT on overall survival (OS); and 2) the factors associated with TTT in patients with unresectable or medically inoperable stage III NSCLC treated by RT alone or combined chemoradiation.
Materials and Methods
Patient Selection
This is an Institutional Review Board approved retrospective study. Eligible subjects included all patients with stage III NSCLC treated with radiation-based therapy at University of Michigan Hospital (UM) and the Veterans Affairs Ann Arbor Healthcare System (VA) between January 1992 and July 2004. Patients treated with surgical resection or for recurrent disease were excluded. Thus, of a total n = 353 patients, 116 were excluded. All 237 patients involved in this study were restaged according to American Joint Cancer Committee (AJCC) 2002 criteria: stage IIIA (T1-3 N2 M0, T3 N1 M0) and IIIB (T1-3 N3 M0, T4 N0-3 M0). CT was used for the staging the majority of patients. Positron emission tomography (PET) was performed based on physician's preference at University of Michigan Hospital; and it was routinely not performed at the Veterans' hospital during the study period. A total of 75 patients (55 at UM and 20 at VA) underwent PET scan. Charlson scales were used to rate comorbidity (19). A consistent 3-D conformal technique was used throughout the study period. Patients treated with RT for palliative or curative intent, as well as patients who participated in a RT dose-escalation trial during the study period were included in the analysis. Sequential or concurrent chemotherapy was allowed.
General Treatment Decision
Treatment decisions were generally made by an institutional tumor board consisting of thoracic surgeons, medical oncologists, and radiation oncologists. Treatment strategies were determined by the treating physician based on tumor status, performance status and comorbidities. Most of the patients with stage III NSCLC were treated with RT with definitive or palliative intent either with or without chemotherapy depending on treatment era (more concurrent chemoradiation in recent years) and the patient's eligibility for chemotherapy.
Radiation Therapy Technique
Patients who enrolled in the dose-escalation trial received RT based on the following protocol specifications. Patients underwent planning CT and treatment while lying supine in a low-density cradle and breathing freely. At the time of simulation, fluoroscopy was performed from both the anterior and lateral projections to measure the maximum excursion of the primary tumor in the superior-inferior and anterior-posterior directions. Treatment-planning CT scans were performed with patients in the treatment position, including the entire thorax, using a maximum of 5-mm cuts through the target volume, and were performed using IV contrast, if possible. The GTV included only the primary tumor, any hilar or mediastinal lymph nodes with a short-axis diameter of at least 1 cm on CT, and any abnormal findings detected on bronchoscopy or mediastinoscopy. The primary GTV was usually drawn using a lung CT window and level, whereas abnormal lymph node volumes were drawn using a mediastinal window and level. The clinical target volume (CTV) was routinely created by expanding the GTV by 0.5 cm. Clinically uninvolved hilar, mediastinal, and supraclavicular nodal regions were not purposely included in the CTV. The planning target volume (PTV) was created by expanding the CTV by a minimum of 0.5 cm for setup error. An additional margin (typically 0.5–1.0 cm) was added when necessary to account for respiratory motion. The 100% isodose line was defined at the isocenter, and dose was prescribed to this point. Plans were optimized to attempt to cover 99% of the PTV by the 95% isodose line.
Typically, a single treatment plan was used, which consisted of a median of three non-coplanar static fields (range: 2–7). All patients received daily treatment with beams ranging in energy from 6 to 25 MV. Tissue heterogeneity was corrected using the equivalent path length algorithm. The fraction size was 2.1 Gy for on-trial patients. For patients who received radiation in the off-trial setting, the radiation technique was similar to that described above, except that fraction size ranged from 1.8 Gy to 3.0 Gy. The radiation dose for the whole group ranged from 30 Gy in 3-Gy fractions to 102.9 Gy in 2-or 2.1-Gy fractions, with a median dose of 60 Gy in 2-Gy fractions. The median BED was 72.2 Gy (range, 39-124.5 Gy). There were 169 patients treated with definitive radiotherapy and 68 with a palliative dose of radiotherapy. The median BED was 78 Gy (95% confidence interval [CI], 77-79 Gy) for patients treated with definitive intent and 50 Gy (95% CI, 47-53Gy) for patients treated with palliative intent.
Data Analysis and Statistical Considerations
Overall survival (OS) was the primary endpoint of this study. Survival was defined as the time between the pathological diagnosis date and the date of death. Time to treatment was defined as the interval between first radiological diagnosis of the lung cancer and the commencement of treatment.
Age at diagnosis, gender, Karnofsky Performance Score (KPS), percent of total body weight loss (<5 vs. ≥5%), smoking history (yes vs. no), pre-RT oxygen use (yes vs. no), Charlson's comorbidity score, treatment intent and chemotherapy use (yes vs. no) were collected from patient's hospital medical records and are the factors evaluated for association with TTT.
Data were considered right-censored for OS outcomes if no events occurred by the end of follow-up. The Kaplan-Meier method was used to estimate TTT curves for different groups, and the Log-Rank test was used to assess significance between groups. Cox's proportional hazards model was used for multivariate analysis to test the significance of TTT effects on OS and to estimate the simultaneous impact of patient and treatment factors on TTT. Fisher's Exact Test was used to compare the differences in survival rates among groups. All p values were two-sided, with p ≤ 0.05 considered significant.
Results
Patient and Treatment Characteristics
Patient and treatment characteristics are provided in Table 1. In this group of patient, 68 had squamous cell carcinoma, 77 adenocarcinoma, 34 large cell carcinoma and 58 had unknown histologic subtype of NSCLC. A total of 106 patients were treated with RT alone, 69 with sequential chemoradiation and 62 with concurrent chemoradiation; 168 with definitive RT and 69 with palliative RT. The commonly used chemotherapy agents were carboplatin, etoposide, paclitaxel, cisplatin and vinorelbine. For sequential chemoradiation, 24 patients were treated with carboplatin and paclitaxel, 19 with cisplatin and vinorelbine, 6 with cisplatin and etoposide and 20 with other regimens. For concurrent chemoradiation, 21 patients were treated with carboplatin and etoposide, 18 with cisplatin and etoposide, 17 with carboplatin and paclitaxel and 6 with other regimens.
Table 1. Patient, tumor and treatment characteristics and their effects on time to treatment (univariate analysis).
| Characteristic | No. of Patients | Time to Treatment (days) | Range (days) | P value | |
|---|---|---|---|---|---|
| Age | Median | 65 years | |||
| Range | 34 – 89 years | 0.027‡ | |||
| 34-65 years | 121 (51.1%) | 55.0 | 0 - 223 | ||
| >65 years | 116 (48.9%) | 62.0 | 9 - 377 | ||
| Gender | 0.013‡ | ||||
| Male | 170 (71.7%) | 60.0 | 0 - 377 | ||
| Female | 67 (28.3%) | 54.0 | 1 - 223 | ||
| KPS | Median | 80 | |||
| Range | 60 – 100 | 0.002‡ | |||
| < 80 | 11 (4.6%) | 60.0 | 7 - 147 | ||
| ≥ 80 | 226 (95.4%) | 57.0 | 0 - 377 | ||
| Weight loss | 0.368‡ | ||||
| ≥ 5% | Yes | 85 (35.9%) | 53.0 | 6 – 377 | |
| No | 152 (64.1%) | 60.0 | 0 - 324 | ||
| Smoking | 0.656‡ | ||||
| history | Yes | 211 (89.0%) | 56.0 | 0 - 377 | |
| No | 26 (11.0%) | 67.0 | 9 - 186 | ||
| Oxygen use | 0.171‡ | ||||
| pre-RT | 46.0 | 14 - | |||
| Yes | 19 (8.0%) | 147 | |||
| No | 218 (92.0%) | 58.0 | 0 - 377 | ||
| Comorbidity | Median | 2.0 | |||
| score | Range | 0-10 | 0.087‡ | ||
| 0 - 2.0 | 152 (64.1%) | 54.0 | 6 - 377 | ||
| > 2 | 85 (35.9) | 64.0 | 0 - 265 | ||
| Hospital | 0.001‡ | ||||
| UM | 154 (65.0%) | 55.0 | 1.0 -265 | ||
| VA | 83 (35.0%) | 67.0 | 0 - 377 | ||
| Treatment | 0.747† | ||||
| regimen | RT | 106 (44.7%) | 54.0 | 0 - 336 | |
| SCTRT | 69 (29.1%) | 56.0 | 1 - 194 | ||
| CTRT | 62 (26.2%) | 59.0 | 9 - 377 | ||
Abbreviations: KPS = Karnofsky performance score, UM = University of Michigan Hospital, VA = Veterans Affairs Ann Arbor Healthcare System, RT= Radiotherapy, SCTRT = Sequential chemoradiation, CTRT = Concurrent chemoradiation
Log-rank test, categorical covariates or continuous covariate transform to categorical covariates by the median.
Cox-regression analysis, continuous covariate.
Time to Treatment and Overall Survival
The median TTT for the entire group was 57.0 days (95% CI: 52.8–61.2 days, range 0–377 days). The number of patients with TTT ≤ 60, 61–90, and > 90 days were 128, 58, and 51, respectively. Figure 1 shows the TTT for the entire study group. There was no significant association between TTT ≤ 60, 60–90, or > 90 days and OS in this group of patients (median OS was 10.2, 14.9 and 16.4 months, respectively, p = 0.065). Patients in this study group with TTT ≤ 60 days appeared to have worse survival than those with TTT >90 days, but this effect appeared to be due to confounding factors such as KPS, body weight loss, treatment regimen, and biologically equivalent RT dose as described that stated in a previous study (20).
Figure 1.
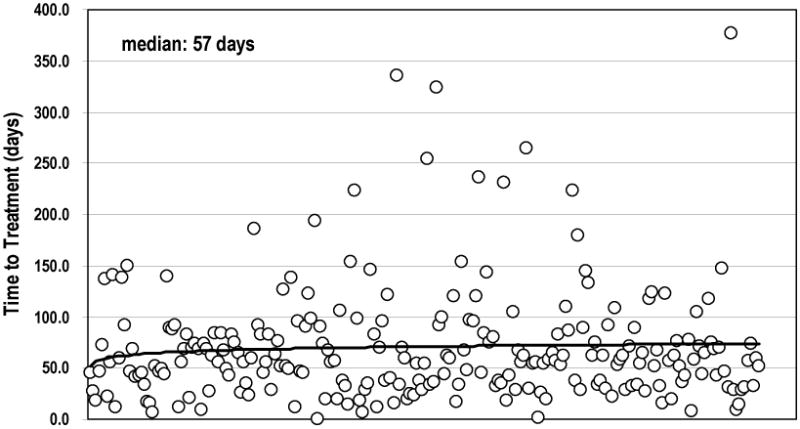
Time to treatment of all patients (X-axis present different patient).
Figure 2 shows no significant effect of TTT on OS in the entire patient cohort. Among the 10 patients with TTT > 6 months, 3-year and 5-year survival rates were 10% and 0%, respectively, while among the 226 patients with TTT < 6 months, 3-year and 5-year survival rates were 15% and 7.4%, respectively (3-year, p=0.548; 5-year, p=0.466).
Figure 2.
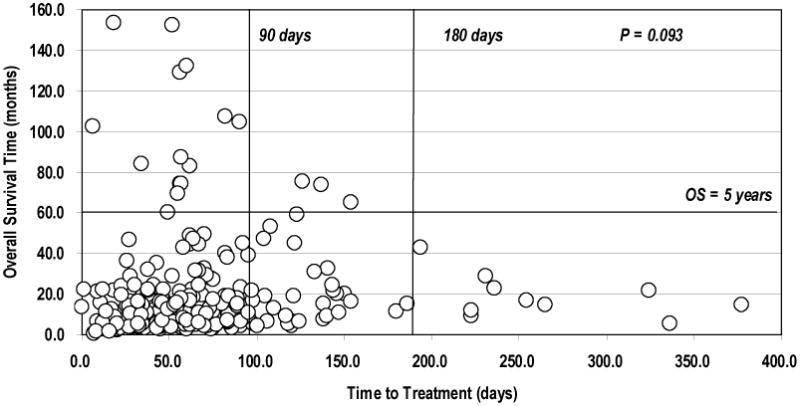
Time to treatment and overall survival time. There was no correlation between time to treatment and overall survival (Cox-regression analysis, continuous covariate, p = 0.093).
Univariate analysis demonstrated that TTT had no significant impact on OS for the entire cohort of patients (HR 0.998, 95% CI: 0.995–1.000, p = 0.093), or for patients with squamous cell carcinoma (HR 0.996, 95% CI: 0.990–1.002, p = 0.247), adenocarcinoma (HR 0.997, 95% CI: 0.992–1.003, p = 0.344), or large cell carcinoma (HR 0.995, 95% CI: 989–1.002, p = 0.133). TTT also had no significant effect on OS for patients treated with RT alone (HR 0.997, 95% CI: 0.994–1.001, p = 0.115), sequential chemoradiation (HR 0.958, 95% CI: 0.917–1.002, p = 0.060), or concurrent chemoradiation (HR 0.996, 95% CI: 957–1.036, p = 0.848); or for patients treated with definitive RT (HR 0.998, 95% CI: 0.995–1.002, p = 0.362) or palliative RT (HR 0.997, 95% CI: 993–1.002, p = 0.214).
Upon categorizing patients by duration of OS, longer TTT was a significant negative prognostic factor in patients with OS ≥ 5 years (HR 1.029, 95% CI: 1.003–1.055, p = 0.029), but not in patients with OS ≥ 2 years (HR 1.004, 95% CI: 0.996–1.012, p = 0.319) or OS ≥ 3 years (HR 1.008, 95% CI: 0.995–1.022, p = 0.207).
Factors Associated with Time to Treatment
The factors that were evaluated for association with TTT are shown in Table 1. Univariate analysis demonstrated that male gender (Figure 3A, p = 0.013) and care at the VA (Figure 3B, p = 0.001) were significantly associated with longer TTT. Older age (HR 0.985, 95% CI: 0.972–0.998, p = 0.027) and higher KPS (HR 0.964, 95% CI: 0.942– 0.986, p = 0.002) were significantly associated with shorter TTT. Weight loss, smoking history, pre-RT oxygen use and higher co-morbidity score were not significantly associated with TTT.
Figure 3.
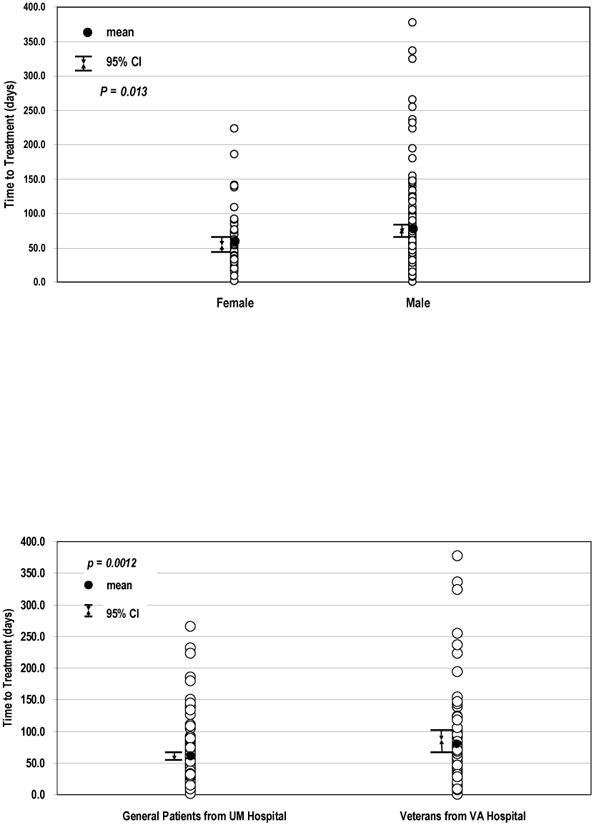
Factors associated with time to treatment. Figure 3A: Time to treatment by gender with greater median time to treatment in men than in women (Log-rank, p = 0.013). Figure 3B: Time to treatment by institution with greater median time to treatment in the VA cohort than in the UM hospital cohort (Log-rank, p = 0.001).
In terms of treatment variables, there was no significant difference in TTT between patients treated with RT alone or with sequential or concurrent chemoradiation (p = 0.747). The median TTT for patients treated with RT alone, sequential chemoradiation, and concurrent chemoradiation was 54.0 days (95% CI: 43.9–64.1 days, range 0–336 days), 56.0 days (95% CI: 40.9–71.1 days, range 1–194 days), and 59.0 days (95% CI: 54.8–63.2 days, range 9–377 days), respectively.
Multivariate Cox proportional hazards regression analysis was performed to further evaluate the independent effect of patient, tumor and treatment factors on TTT (Table 2). This analysis included factors with p ≤ 0.1 on univariate analysis. KPS remained significantly associated with TTT (HR 0.962, 95% CI: 0.937–0.987, p = 0.003). Older age, gender, and co-morbidity score were not associated with TTT (p ≥ 0.084). In terms of treatment factors, treatment at the VA was not significantly correlated with longer TTT on multivariate analysis (HR 1.318, 95% CI: 0.966–1.798, p = 0.082).
Table 2. Patient and treatment characteristics associated with time to treatment (multivariate analysis).
| Factors | Estimated HR of treatment wait (95% CI) | P value* |
|---|---|---|
| Age | 0.988 (0.974 – 1.002) | 0.084 |
| Gender | 1.110 (0.806 – 1.527) | 0.523 |
| KPS | 0.962 (0.937 – 0.987) | 0.003 |
| Comorbidity score | 0.920 (0.832 –1.017) | 0.104 |
| Treatment at VA | 1.318 (0.966 – 1.798) | 0.082 |
Abbreviations: KPS = Karnofsky performance score, VA = Veterans Affairs Ann Arbor Healthcare System.
CI = confidence interval, HR = hazard ratio (risk of treatment wait).
Multivariate Cox's proportional hazards model.
Discussion
This study demonstrated that longer TTT is not significantly correlated with OS in a large cohort of patients with stage III NSCLC treated with RT. However, longer TTT may be associated with an increased risk of death in patients who were treated within 90 days and the patients who survived beyond 5 years after treatment of stage III NSCLC. Higher KPS was associated with shorter TTT on both univariate (p = 0.002) and multivariate analyses (p = 0.003) and treatment at the VA Healthcare System was associated with longer TTT (p = 0.001) on univariate analysis.
From a biologic point of view, prolonged TTT may result in increased tumor burden, which would have a potential negative effect on prognosis. Hasegawa et al. examined volume doubling times (VDT) of lung cancers detected on mass-screening CT scans (21). The VDT varied significantly by histology: adenocarcinoma, 533 ± 381 days; squamous cell carcinoma, 129 ± 97 days; and small cell lung cancer, 97 ± 46 days. Others have reported that the doubling times for squamous cell carcinoma and adenocarcinoma are 88 days and 161 days, respectively (4,21,22). Thus, even lung cancers that are barely radiographically detectable may have originated months and even years ago. However, the growth of tumors is exponential, and even if the pre-clinical history is long, the growth rate at the time of radiographic identification will be more rapid due to the cell number effect. Hence, the duration of time to diagnosis or treatment may be an important prognostic factor.
In the present study, the median TTT, defined as the time from radiographic diagnosis to initiation of therapy, for the entire cohort was 57 days. O'Rourke et al. studied 29 lung cancer patients awaiting RT in the United Kingdom (UK) and showed that the median time between diagnostic and RT planning CT scans was 54 days (range 18–131 days) (5). In another single-center UK study, Bozcuk et al. reported a median time of 48 days from referral request to a specialist to initiation of treatment (10). A Swedish study by Koyi et al. analyzed 134 patients with stage I–IV NSCLC and demonstrated that the median time from the first visit to a specialist to diagnosis was 9 days, and from diagnosis to treatment was 79 days (7). Similarly, among 466 Swedish patients with stage I-IV NSCLC, Myrdal et al. found a median of approximately 48 days from first visit to a specialist and the start of treatment (11).
Clinical investigations on the effect of TTT on lung cancer prognosis have demonstrated mixed results (5,7–16). For instance, several studies did not show a correlation between longer treatment wait times and OS. In a series of 132 patients with stage I–IV small cell lung cancer and NSCLC with median treatment delay of 15 days from diagnosis to treatment start, Salomaa et al. concluded that longer delays did not correlate with worse prognosis (12). In this study, the majority (67%) of patients had stage IIIB-IV lung cancer, and the median time between the first visit with a specialist and diagnosis was shorter for these patients (median 13 versus 22 days for stage IIIB–IV versus I–IIIA, respectively, p = 0.001). Moreover, patients with a longer TTT had a lower risk of death. The study by Myrdal et al. also reported an association between short time to treatment and a poorer prognosis with a significant interaction between tumor stage and shorter TTT, especially among patients with stage IIIB-IV disease (11). Similarly, Bozcuk H et al. found no correlation between the time from receipt of referral to first treatment and OS (10). One possible explanation for the lack of correlation between TTT and outcome is that patients with more advanced disease often have more severe symptoms which require earlier initiation of treatment (10–12).
In this study, a large cohort of patients with stage IIIA/B NSCLC treated with modern therapy and techniques were analyzed to investigate the effect of TTT on OS. As was reported in the majority of previous studies (11–14), the results of the current study suggest that TTT is not a significant prognostic factor for the entire patient cohort. However, longer TTT may increase the risk of death in patients who become long-term survivors, suggesting that shorter TTT could eventually benefit some patients. Unfortunately, there are no reliable methods to predict which patients are going to become long-term survivors.
The growth of tumors is highly variable, but may be partly dependent on histology (4, 21,22). However, similar to prior studies, the current study did not show any effect of TTT on OS in either squamous cell carcinoma or adenocarcinoma (12). Of note, Kashiwabara et al. (23) studied 198 asymptomatic patients with stage I–IV SCLC and NSCLC detected on screening CTs and found that a 1-year delay prior to consultation or treatment was associated with a poor outcome. Overall, the evidence indicates that there is no strong effect of TTT on OS, possibly because the usual range of TTT is not long enough to allow significant tumor growth (24). Alternatively, overall survival of patients with lung cancer may be so uniformly poor that a small effect cannot be detected. However, TTT may become a more important factor for patients with more rapidly growing tumors or with longer survival times.
Few studies have investigated factors associated with longer TTT. In the present study, longer TTT was noted in males (median, 60 vs. 54 days, p = 0.013) and the VA cohort (median, 67 versus 55 days, p = 0.001), while shorter TTT was noted in older patients (p = 0.027) and patients with higher KPS (p = 0.002). On multivariable analysis, higher KPS (HR= 0.962, 95% CI 0.937- 0.987, p = 0.003) remained associated with shorter TTT. Thus, the dominant factor affecting TTT in this study was performance status.
Although the current study did not show any benefit with shorter TTT, ,it is still important to initiate treatment with RT as soon as possible after diagnosis because of the presence of symptoms, patient anxiety and many other reasons of patients with lung cancer. As such, there are many recommendations regarding optimal limits on TTT. The Swedish Lung Cancer Study Group (11) recommends that 80% of all patient diagnostic tests should be completed within 4 weeks from consultation with a specialist and that treatment should start within 2 weeks of diagnosis. Canadian recommendations (25) specify a maximum of 4 weeks between the first visit to a general practitioner and diagnosis and less than 2 weeks from diagnosis to surgery. The British Thoracic Society recommends the following maximal times from first visit to a respiratory specialist to specific treatments: 8 weeks for thoracotomy, 4 weeks for definitive RT, and 2 weeks for palliative RT (26). However, no evidence-based recommendations exist for TTT for combined modality treatments for stage III NSCLC.
There are several limitations of the current study. First, it represents the experience in two different health systems and patient populations. Due to non-uniform clinical conditions and the presence of other confounding factors, the possible effects of TTT may have been obscured. Second, the study may be underpowered to detect relatively small differences in outcomes. Third, the standard of care for treatment and staging of NSCLC evolved over the duration of this study, with more recent patients being staged with PET and more frequently treated with combined chemoradiation. Fourth, retrospective studies are always hypothesis generating and should be validated in prospective cohorts before definitive conclusions can be drawn.
In summary, this study assessed the effect of TTT on overall survival in a cohort of patients with stage IIIA/B NSCLC receiving modern treatment regimens. Overall, there was no significant effect of TTT on overall survival. However, longer TTT may increase the risk of death in long-terms survivors. Higher KPS is significantly correlated with shorter TTT and KPS is a confounding factor for OS in this group of patients, while younger age, male gender, and treatment in the VA Healthcare System may be associated with longer TTT. Future prospective studies are needed to establish optimal TTT guidelines in patients receiving chemoradiation for stage III NSCLC.
Acknowledgments
This work was partially supported by the Pardee Foundation and a Career Developmental Award from American Society of Clinical Oncology. We express our gratitude to all our patients. We also thank Brady T. West of the Center for Statistical Consultation and Research for his expertise on data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Deslauriers J, Gregoire J. Surgical therapy of early non–small cell lung cancer. Chest. 2000;117(Suppl. 1):104S–109S. doi: 10.1378/chest.117.4_suppl_1.104s. [DOI] [PubMed] [Google Scholar]
- 3.Deslauriers J. Current surgical treatment of nonsmall cell lung cancer. Eur Respir J. 2002;35(Suppl):61S–70S. doi: 10.1183/09031936.02.00271302. [DOI] [PubMed] [Google Scholar]
- 4.Mackillop WJ, Bates JH, O'Sullivan B, Withers HR. The effect of delay in treatment on local control by radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34:243–250. doi: 10.1016/0360-3016(95)02049-7. [DOI] [PubMed] [Google Scholar]
- 5.O'Rourke N, Edwards R. Lung cancer treatment waiting times and tumour growth. Clin Oncol (R Coll Radiol) 2000;12:141–144. doi: 10.1053/clon.2000.9139. [DOI] [PubMed] [Google Scholar]
- 6.Mackillop WJ, Zhou Y, Quirt CF. A comparison of delays in the treatment of cancer with radiation in Canada and the United States. Int J Radiat Oncol Biol Phys. 1995;32:531–539. doi: 10.1016/0360-3016(94)00662-5. [DOI] [PubMed] [Google Scholar]
- 7.Koyi H, Hillerdal G, Branden E. Patient's and doctors' delays in the diagnosis of chest tumors. Lung Cancer. 2002;35:53–7. doi: 10.1016/s0169-5002(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 8.Jensen AR, Mainz J, Overgaard J. Impact of delay on diagnosis and treatment of primary lung cancer. Acta Oncol. 2002;41:147–52. doi: 10.1080/028418602753669517. [DOI] [PubMed] [Google Scholar]
- 9.Robinson E, Mohilever J, Zidan J, Sapir D. Delay in diagnosis of cancer. Possible effects on the stage of disease and survival. Cancer. 1984;54:1454–1460. doi: 10.1002/1097-0142(19841001)54:7<1454::aid-cncr2820540739>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 10.Bozcuk H, Martin C. Does treatment delay affect survival in non-small cell lung cancer? A retrospective analysis from a single UK centre. Lung Cancer. 2001;34:243–52. doi: 10.1016/s0169-5002(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 11.Myrdal G, Lambe M, Hillerdal G, et al. Effect of delays on prognosis in patients with non-small cell lung cancer. Thorax. 2004;59:45–49. [PMC free article] [PubMed] [Google Scholar]
- 12.Salomaa ER, Sallinen S, Hiekkanen H, Liipo K. Delays in the diagnosis and treatment of lung cancer. Chest. 2005;128:2282–2288. doi: 10.1378/chest.128.4.2282. [DOI] [PubMed] [Google Scholar]
- 13.Falk SJ, Girling DJ, White RJ, et al. Immediate versus delayed palliative thoracic radiotherapy in patients with unresectable locally advanced non-small cell lung cancer and minimal thoracic symptoms: randomised controlled trial. BMJ. 2002;325:465. doi: 10.1136/bmj.325.7362.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aragoneses FG, Moreno N, Leon P, Fontan EG, Folque E. Influence of delays on survival in the surgical treatment of bronchogenic carcinoma. Lung Cancer. 2002;36:59–63. doi: 10.1016/s0169-5002(01)00458-5. [DOI] [PubMed] [Google Scholar]
- 15.Christensen ED, Harvald T, Jendresen M, et al. The impact of delayed diagnosis of lung cancer on the stage at the time of operation. Eur J Cardiothorac Surg. 1997;12:880–4. doi: 10.1016/s1010-7940(97)00275-3. [DOI] [PubMed] [Google Scholar]
- 16.Liberman M, Liberman D, Sampalis JS, Milder DS. Delays to surgery in non-small-cell lung cancer. Can J Surg. 2006;49:31–36. [PMC free article] [PubMed] [Google Scholar]
- 17.Furuse K, Fukuoka M, Kawakara M, et al. Phase III study of concurrent versus sequential thoracic radiotherapy in combination with mitomycin, vindesine and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol. 1999;17:2692–2699. doi: 10.1200/JCO.1999.17.9.2692. [DOI] [PubMed] [Google Scholar]
- 18.Curran WJ, Scott CB, Langer CJ, et al. Long-term benefit is observed in a phase III comparison of sequential vs. concurrent chemo-radiation for patients with unresectable stage III NSCLC: RTOG 9410[Abstract] Proc ASCO. 2003;22:621A. [Google Scholar]
- 19.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 20.Li Wang, Candace R Correa, Zhao Lujun, et al. The effect of radiation dose and chemotherapy on overall survival in 237 patients with stage III Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2008. doi: 10.1016/j.ijrobp.2008.06.1935. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasegawa M, Sone S, Takashima S, et al. Growth rate of small lung cancers detected on mass CT screening. Br J Radiol. 2000;73:1252–1259. doi: 10.1259/bjr.73.876.11205667. [DOI] [PubMed] [Google Scholar]
- 22.Geddes DM. The natural history of lung cancer: a review based on rates of tumour growth. Br J Dis Chest. 1979;73:1–17. [PubMed] [Google Scholar]
- 23.Kashiwabara K, Koshi S, Itonaga K, et al. Outcome in patients with lung cancer found on lung cancer mass screening roentgenograms, but who did not subsequently consult a doctor. Lung Cancer. 2003;40:67–72. doi: 10.1016/s0169-5002(02)00505-6. [DOI] [PubMed] [Google Scholar]
- 24.Porta M, Gallen M, Malats N, Planas J. Influence of ‘diagnostic delay’ upon cancer survival: an analysis of five tumour sites. J Epidemiol Community Health. 1991;45:225–230. doi: 10.1136/jech.45.3.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simunovic M, Gagliardi A, McCready D, et al. A snapshot of waiting times for cancer surgery provided by surgeons affiliated with regional cancer centres in Ontario. CMAJ. 2001;165:421–425. [PMC free article] [PubMed] [Google Scholar]
- 26.British Thoracic Society. Thorax; BTS recommendations to respiratory physicians for organising the care of patients with lung cancer: The Lung Cancer Working Party of the BritishThoracic Society Standards of Care Committee; 1998. pp. S1–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]


