Abstract
Group B streptococcus (GBS) is a major cause of neonatal sepsis and meningitis. Despite aggressive campaigns using antenatal prophylactic antibiotic therapy, infections continue. Developing an effective maternal vaccine is a public health priority. Antibody (Ab) to the capsular polysaccharide (CPS) is considered the dominant “protective” immune mediator. Here we study the fine specificity and potential host reactivity of a panel of well-characterized murine monoclonal Abs against the type III CPS by examining the binding of the Abs to intact and neuraminidase-digested GBS, purified CPS, synthetic carbohydrate structures, and cells. The results showed marked differences in the fine specificity among these mAbs to a single carbohydrate structure. Cross-reactions with synthetic GD3 and GT3 carbohydrates, representing structures found on surfaces of neural and developing cells, were demonstrated using carbohydrate array technology. The anti-CPSIII mAbs did not react with cells expressing GD3 and GT3, nor did mAbs specific for the host carbohydrates cross-react with GBS, raising questions about the physiological relevance of this cross-reaction. But in the process of these investigations, we serendipitously demonstrated cross-reactions of some anti-CPSIII mAbs with antigens, likely carbohydrates, found on human leukocytes. These studies suggest caution in the development of a maternal vaccine to prevent infection by this important human pathogen.
Keywords: Monoclonal Ab, group B streptococci, cross-reaction, fine specificity, carbohydrate array, capsular polysaccharide
INTRODUCTION
Group B streptococcus (GBS)1, or Streptococcus agalactiae, causes invasive infections of newborns, pregnant women, and adults with underlying medical conditions. Although the bacteria are sensitive to antibiotics such as penicillin, case fatality rates are estimated to be 5–20% in neonates and 15–32% in adults [1]. Neonatal GBS infection is most commonly seen as bacteremia, meningitis, and pneumonia. Prevention of GBS infections is a major public health priority [1]. Maternal colonization with GBS is generally the source of neonatal infection. Newborns acquire infections with GBS either via ascending infection following premature rupture of the membranes or during passage through the birth canal. Maternal colonization is common, ranging from 10–30% in different studies [1]. Approximately 1–2% of infants born to colonized mothers will develop neonatal GBS infection. The absence of maternal Ab to GBS capsular antigens has been identified as an important factor in the pathogenesis of GBS infections in newborns [2, 3]. Presumably, transplacental transfer of anti-GBS maternal Ab results in sufficient immunity in the neonate to protect against the development of infection. The absence of maternal Ab in the face of vaginal colonization with GBS places the infant at highest risk. Thus maternal immunization to elicit anti-GBS Ab has been proposed for prevention [1, 4–9].
Two lines of evidence have suggested the capsular polysaccharide (CPS) as a vaccine antigen: Ab-mediated protection and the definition of the CPS as a virulence factor. Lancefield originally demonstrated the protective efficacy of anti-GBS Abs using rabbit antisera [10–13] and established that protective efficacy is serotype specific. Using MAbs we, and others, have confirmed these findings, and demonstrated that the protective epitope on the type III CPS contains sialic acid [14–17]. The role of the CPS in bacterial virulence has been demonstrated. Variants lacking the capsule have markedly reduced virulence [18–20]. Transposon mutagenesis has demonstrated that sialylation of the CPS is critical for pathogenicity [18]. The capsule is anti-complementary, inhibiting the activation of the alternate pathway of complement activation [21]. Phase variants and transposon mutants lacking the capsule spontaneously activate complement [20, 22]. Sialic acid plays an important role in the ability of GBS CPS to inhibit complement deposition [22]. It has been postulated that Abs against sialic acid containing epitopes of CPS protect by blocking this complement-inhibiting activity of the polysaccharide, thus facilitating C3 deposition, perhaps by both classical and alternate pathways, and interacting with Fc receptors on PMNs [23].
In the studies reported here, we have examined the fine specificity of mAbs to the type III CPS (CPSIII) using ELISA, immunofluorescence and carbohydrate arrays, in which synthetic carbohydrates are spotted at high density on microscope slides [24, 25]. The results demonstrate that even among a group of mAbs to the same carbohydrate Ag, there is considerable heterogeneity of fine specificity. Moreover, microarray results and Ab binding to cells point to potential cross-reactions of some anti-CPSIII mabs with host glycans. The biological relevance of these results has yet to be defined.
MATERIALS AND METHODS
Ethics statement
All animal studies were performed under protocols approved by the Children’s Hospital IACUC (Animal Welfare Assurance Number A4336-01) and carried out in accordance with PHS Policy on the Humane Care and the NIH Guide for the Care and Use of Laboratory Animals. The animal facility is certified by AAALAC. Peripheral blood was obtained from a healthy adult donor who provided written informed consent under a protocol approved by both the LSU Health Sciences Center-New Orleans Institutional Review Board and the Children’s Hospital of New Orleans Institutional Review Board.
Reagents and cells
MAbs used in these studies are described in table I. Mel-1 (clone R24) was obtained from Signet Labs, (Dedham, MA) [26], anti-CD60b (clone JONES) from Biomeda (Foster City, CA) [27], MB3.6 from Chemicon (Temecula, CA) [28], and A2B5 from Chemicon [29]. All other mAbs were prepared in our laboratories. Secondary Abs conjugated to enzymes and fluorochromes were purchased from Zymed/Invitrogen (Carlsbad, CA). RIN, HeLa, and H9 cell lines were serially passaged in our laboratories [30–32]. Human peripheral blood mononuclear cells were purified on hypaque ficoll gradients. GBS were clinical isolates passaged in the laboratory on Todd-Hewitt agar and broth. In some experiments, GBS were treated with Clostrida perfringens neuraminidase (Sigma Aldrich, St. Louis MO) as described elsewhere [14]. The purified GBS type III capsular polysaccharide was the gift of the late Milan Blake (US Food and Drug Administration, Bethesda MD). Synthetic GD3 and GT3 carbohydrates were obtained from the Glycan Array Synthesis Core-D of the Consortium for Functional Glycomics (La Jolla, CA) as oligosaccharides (CFG stock numbers Te79 and Te97 respectively for GD3 and GT3), biotinylated oligosaccharides (CFG stock numbers 107, B108), or as polyacrylamide agarose (PAA)-multi-biotin-conjugated multimers of GD3 (CFG stock number PA-189). A control multi-biotin-PAA with no glycan attached was also obtained (Glycotech Corp. Gaithersberg, MD). Oligomers of the biotinylated saccharides were made using neutravidinavidin or streptavidin (Pierce, Rockford, IL), mixing the saccharide with the avidin in a 10X molar excess of sugar to avidin, and removing free disaccharide on a Zeba Desalt Column (Pierce). Avidin conjugates of the biotin-PAA structures were made by mixing equal masses of the two components.
Table I.
Antibodies used in this study.
| Antibody | Isotype | Reference | Immunogen | Epitope Specificity | Protection |
|---|---|---|---|---|---|
| 1. Anti-CPS type III antibodies | |||||
| S9 | IgM | [14] | GBS | complete CPSIII | in vivo |
| S3.1A6 | IgG1 | [35] | Tet-CPSIII | complete CPSIII 3-D epitope | in vitroa |
| S3.2A6 | IgG1 | [35] | Tet-CPSIII | complete CPSIII 3-D epitope | in vitroa |
| S3.1B1 | IgG2a | [35] | Tet-CPSIII | complete CPSIII 3-D epitope | in vitroa |
| SB3 | IgM | [17] | GBS | non-sialylated CPSIII | nob |
| SF23 | IgG3 | [17] | GBS | non-sialylated CPSIII | no |
| SS8 | IgG2a | [17] | GBS | complete CPSIII | in vivo |
| SV18 | IgM | [17] | GBS | complete CPSIII | in vivo |
| SE11 | IgM | [17] | GBS | non-sialylated CPSIII | no |
| 2. Anti-ganglioside Abs, non CPSIII Abs binding GBS, and control Abs | |||||
| B6.1 | IgM | [52] | C. albicans | Isotype control | NTc |
| L2-I5 | IgG3 | [53] | C. trachomatis | Isotype control | NT |
| S3 | IgM | [14] | GBS | GBS group carbohydrate | No |
| S7 | IgM | [14] | GBS | GBS group carbohydrate | No |
| A2B5 | IgM | [29] | chick retina cells | GT3 and 0-acetyl GT3 ganglioside | NT |
| Mel-1 (clone R24) | IgG3 | [26] | melanoma cells | GD3 ganglioside | NT |
| anti-CD60b (clone JONES) | IgM | [27] | retinal cells | 9-0-acetyl GD3 | NT |
| MB3.6 | IgG3 | [28] | melanoma cells | disialo GD3 ganglioside | NT |
These Abs opsonize live GBS for ingestion and killing by PMNs; unpublished data from H.J. Jennings, et al.
Unpublished data, D. Pritchard, M. Egan.
Not tested
Carbohydrate array
The carbohydrate array was synthesized by the Glycan Array Synthesis Core-D of the Consortium for Functional Glycomics as described elsewhere [24, 25]. The microarrays were printed by robotic pin deposition of 0.5 nl droplets of 100μM glycan onto N-hydroxysuccinimide activated glass microscope slides. Two hundred different synthetic glycans were spotted onto each array. The slides were incubated with the primary Abs at 10 μg/ml in PBS 0.5% Tween-20 (Sigma), followed by washing, and then incubation with FITC-conjugated anti-mouse Ig. The slides were washed and fluorescence intensities were measured in a ScanArray 5000 (PerkinElmer, Waltham, MA) confocal scanner. IMAGENE image analysis software (BioDiscovery, El Segundo, CA) was used for image analyses. Signal-to-background was typically >20:1, and no background subtractions were performed. Microsoft EXCEL software was used for data plotting.
Immunological analyses
ELISA was performed by coating Imulon II microtiter plates with soluble antigen at 10 μg/ml in PBS (or other concentration as indicated), or by attaching intact GBS by glutaraldehyde fixation[14]. After blocking the plates with blotto (PBS, 10% powdered skim milk, and 0.01% Tween-20), primary Abs, diluted in blotto, were incubated on the coated plates for 18 hr at 4°. The plates were washed with PBS/0.01% Tween-20 and alkaline phosphatase-conjugated secondary Abs, diluted in blotto, were added. Following a minimum 6 hr incubation at room temperature, the plates were again washed, and substrate (p-nitrophenyl phosphate, Sigma Aldrich, 0.5 mg/ml in 10% diethanolamine buffer, pH 9.8) was added. Absorbance at 405 nm was read 10–60 minutes later on a plate reader (EL-320, Bio-Tek, Winooski VT). For inhibition ELISA, the Ab and inhibitor were premixed one hour prior to addition to the coated ELISA plates. ELISA results are shown as mean and SEM. If no error bars are visible, then they are so small as to be obscured by the symbols. Flow cytometry was performed on a FACSstar or LSRII flow cytometer (BD Biosciences, San Jose, CA). Cells were incubated in the primary Ab 10 μg/ml diluted in PBS/1% bovine serum albumin/0.01% sodium azide for 1 hour, washed 3X, and then in FITC-conjugated anti-mouse IgG or IgM, as appropriate. Three to ten thousand cells were analyzed each flow cytometer run. Immunoblots were performed using H9 cell lysates, either treated or not with PNGaseF (NEB, Ipswich MA). One million H9 cells were lysed in 1% Triton-X100 in tris buffered saline in the presence of a protease inhibitor cocktail (Sigma-Aldrich). Lysate (150μg) was denatured in the presence of glycoprotein denaturing buffer (NEB) and 1% NP40 at 100° for 10 min, and digested with 500 U of PNGaseF for 1.5 hr at 37°. The lysates were subjected to SDS-PAGE on 4–15% gradient gels and then electrophoretically blotted onto PVDF membranes. Following exposure to the test antibodies, binding was detected with alkaline phosphatase-conjugated anti-mouse IgG, A, and M antibodies, exposure to chemiluminescent substrate (SuperSignal West Pico, Thermo Scientific) and analysis on Gel-Doc (Bio-Rad, Hercules CA).
Immunizations
Outbred NIH-Swiss mice were obtained from NCI (Bethesda, MD). Two experiments were performed. In the first, three groups of 5 mice were immunized with 20 μg of GD3-biotin-neutravidin, GT3-biotin-neutravidin, or neutravidin alone at three monthly intervals. The primary immunization was given sc in complete Freund’s adjuvant (Difco, Detroit, MI), boosters ip in incomplete Freund’s. Mice were bled prior to immunization, two weeks post each booster, and 6 weeks after the second booster. In the second experiment, two groups were immunized with either GD3-PAA-biotin-neutravidin or PAA-biotin-neutravidin. Dosing, time, and route of immunization and timing of bleeds were as for the first immunization.
RESULTS
Heterogeneity of mAb fine specificity for type III GBS capsular polysaccharide
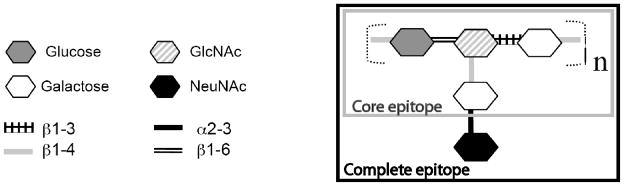
Type III GBS are among the dominant serotypes causing neonatal infection. The type III capsular polysaccharide of GBS (CPSIII) consists of a repeating subunit of glucose-(β1–6)-N-acetyl glucosamine-(β1–3)-galactose-(β1–4), with a side chain of N-acetyl-neuraminic acid-(β2–3)-galactose joined by a (β1–4) linkage from the N-acetyl glucosamine to the galactose (figure 1) [33]. It has long been known that there is a degree of heterogeneity in Abs to CPSIII, defined by reactivity with neuraminidase-treated CPSIII. We have assembled a panel of well-characterized mAbs to CPSIII, shown in Table I. The anti-GBS mAbs were developed in three different laboratories. S3, S7, S9 are IgM mAbs that derive from a single mouse immunized with live GBS [14, 34]. S3 and S7 react with all GBS isolates, bind to the group-specific carbohydrate antigen, and are neither protective in vitro nor in vivo. S9 binds to a sialic acid-dependent epitope (figure 1) on the intact or complete type III capsular polysaccharide (CPSIII). S9 is highly effective in opsonophagocytic assays and protects well in vivo. Mabs S3 1A6, 2A6, and 1B1 were derived by immunization with CPSIII conjugated to tetanus toxoid. They recognize a conformational epitope on the complete CPSIII [35]. They have opsonic activity in vitro (H.J. Jennings, et al., unpublished data), and have not been tested in vivo. The remaining mAbs [17] to CPSIII detect two different structures (figure 1): the sialic acid-containing complete epitope (mAbs SS8 SV18) and the core epitope resulting from neuraminidase digestion (mAbs SB3, SF23, SE10). In this group, Abs to the complete epitope are protective in vivo, whereas those to the non-sialylated core Ag offer protection neither in vivo nor in vitro [17].
Figure 1. Structure of CPSIII of GBS.
The structure consists of a repeating backbone chain of glucose, N-acetyl glucosamine, and galactose, with a side chain of galactose and neuraminic acid. Boxes enclose the core epitope and the sialic acid-containing complete antigen.
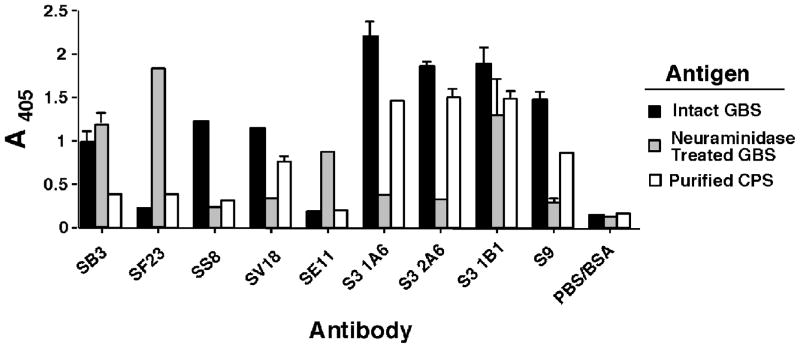
In figure 2, we examined the effect of neuraminidase treatment and extraction of purified CPSIII on the binding of a panel of anti-CPSIII mAbs. The conditions chosen for the neuraminidase digestion (time and neuraminidase concentration) were titrated to give complete digestion of the S9 epitope. Comparison of binding to intact or neuraminidase-treated type III GBS shows three distinct patterns of reactivity: some mAbs bind to both equally (SB3 and S3.1B1), some bind preferentially to intact GBS (SS8, SV18, S3.1A6, S3.2A6, and S9), whereas some bind only to neuraminidase-treated GBS (SF23 and SE11). It has previously been shown that mAb S3.1B1 does not bind to CPS of S. pneumoniae type 14, which consists of the GBS CPSIII core structure lacking sialic acid [35]. This was recently confirmed by direct binding ELISA (data not shown). Two possibilities may explain this discrepancy, either the digestion of sialic acid from the surface of GBS is not complete, or the epitope recognized by mAb S3.1B1 is stabilized by other structures on the surface of GBS. Regardless of the explanation, the data emphasize the heterogeneity among Abs, even those that fail to bind to CPS of S. pneumoniae type 14 (eg. S3.1B1, S3.1A6, and S3.2A6). Further heterogeneity in specificity among these mAbs is demonstrated by binding to the purified CPSIII (figure 2). In general, Abs that bound intact GBS also bound CPSIII, whereas those that only bound neuraminidase-treated GBS did not bind CPSIII. However, Ab SS8 binds well to intact GBS, but not to purified CPS. Both mAbs SB3 and S3.1B1 bind to intact and to neuraminidase-treated GBS, but only S3.1B1 binds to the purified CPSIII.
Figure 2. Binding of anti-CPSIII mAbs to intact GBS, neuraminidase-treated GBS, or purified CPSIII.
Elisa plates were coated with intact or neuraminidase treated GBS, or with purified capsular polysaccharide. Mabs were incubated in the wells at 3 μg/ml and detected with alkaline phosphatase-conjugated anti-mouse Ig. Data are the absorbance at 405nm, mean and SEM.
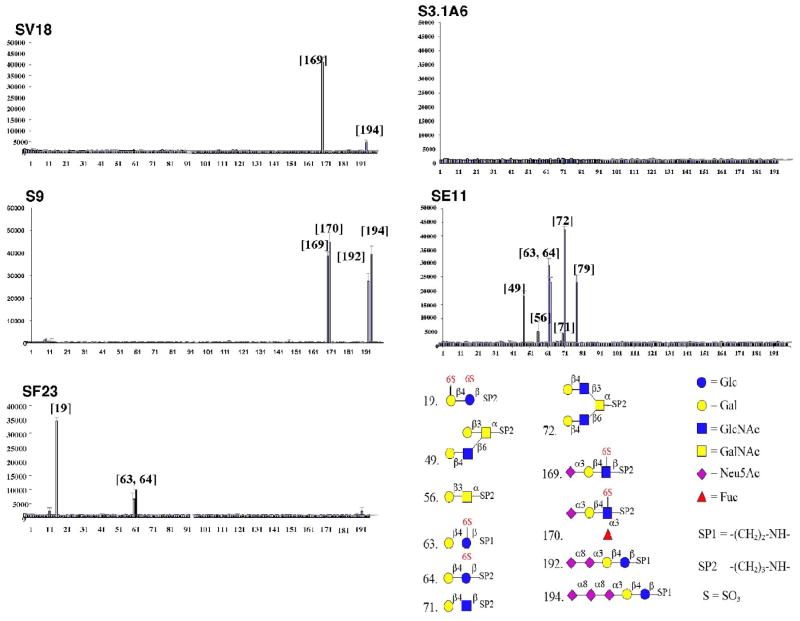
The heterogeneity of binding of these mAbs was further demonstrated using a glycan array, in which 200 synthetic glycans are spotted at high density onto microscope slides [24, 25]. Many of the glycans were chosen to represent structures found on mammalian cells. Each Ab that showed any binding demonstrated a unique pattern of reactivity (figure 3). Glucose, galactose, and/or N-acetyl-glucosamine were prominent components in most glycans bound by the mAbs, and these are key constituents of CPSIII. Those mAbs that bound only to neuraminidase-treated GBS (SF23 and SE11) only bound to glycans lacking sialic acid, and conversely, those reacting only with intact GBS (S9 and SV18) bound only glycans containing sialic acid. Four mAbs (S3.1A6, S3.2A6, S3.1B1, and SB3) did not bind to any glycans, indicating that they either bound three dimensional epitopes too complex to be constructed synthetically, or that the simple epitopes they bound were not represented on the array. One Ab shown in figure 2 was not tested on the glycan array.
Figure 3. Binding of anti-CPSIII mAbs to carbohydrate array demonstrates fine specificity differences.
MAbs at 10 μg/ml were placed onto the microscope slide containing the carbohydrate array and incubated. Following washing they were incubated with fluorochrome-conjugated anti-mouse Ig and the signal read for each spot. The results are shown with the different carbohydrates arrayed on the horizontal axis and signal intensity (mean and SEM) on the vertical. The lower right panel shows the structure of the different carbohydrates with which the anti-CPSIII mAbs react.
Cross-reaction between GD3/GT3 gangliosides and CPSIII
MAb S9, and to a lesser degree SV18, bind to array carbohydrates that contain the carbohydrate structure of gangliosides GD3 and GT3. Because the GD3 and GT3 carbohydrate structures are found on gangliosides expressed on cell surfaces [26–28, 36–38] and Abs to GD3 and GT3 gangliosides have been reported in autoimmune diseases [39], it is possible that immunization with CPSIII could induce autoreactive Abs, or alternatively immunological tolerance to such self-antigens might limit the ability of a vaccine to induce anti- CPSIII Abs. We therefore studied whether anti-CPSIII mAbs bind to the native self-antigen, or whether mAbs to the cell-surface gangliosides react with CPSIII.
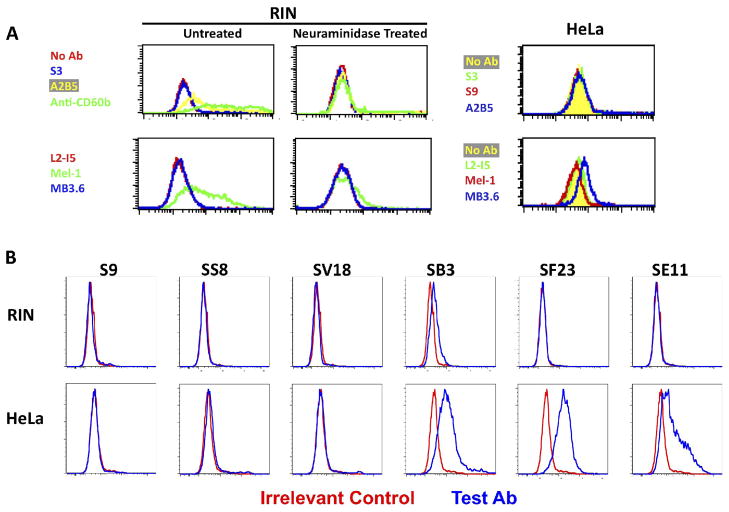
Flow cytometry was performed on cells that express GD3 and GT3 gangliosides on the cell surface using anti-GBS mAbs and anti-ganglioside mAbs (figure 4). Rat insulinoma cells, designated RIN, express gangliosides containing both GD3 and GT3, as demonstrated by binding of mAbs A2B5, Mel-1, and anti-CD60b, but not with MB3.6 (figure 4A). As expected, neuraminidase treatment of the cells destroyed the epitopes and eliminated Ab binding. No binding of S3 (anti-type B carbohydrate) was demonstrated. HeLa cells expressed disialo-GD3 ganglioside, defined by mAb MB3.6, to a low degree, but were not bound by the other anti-ganglioside mAbs. We next tested a panel of anti-GBS mabs for binding to cell surface molecules on RIN and HeLa cells (figure 4B). MAbs to the complete, sialic acid-dependent epitope (S9, SS8, and SV18) did not bind to either cell type, despite the cross-reaction with GD3 and GT3 demonstrated on the microarray. But surprisingly, mAbs to the sialic acid-independent, core polysaccharide determinants (SB3, SF23, and SE11) all bound to HeLa cells. SB3 may also bind weakly to RIN.
Figure 4. Binding of anti-CPSIII mAbs to cells expressing GD3/GT3 gangliosides on the cell surface.
Flow cytometry was used to study the binding of anti-GBS, anti-GD3, and anti-GT3 Abs to the surface of rat insulinoma cell line RIN and HeLa cells. Cell number is on the vertical axis, fluorescent intensity on the horizontal. (A) RIN cells were either treated with neuraminidase, or not, to demonstrate the sialic acid dependence of Ab binding; the HeLa cells were untreated. Binding of Abs was detected with FITC-conjugated isotype (murine IgM or IgG) specific Ab. IgM mAbs are at the top and IgG3 mAbs at the bottom in A. (B) Binding of anti-GBS mAbs to RIN (top) and HeLa (bottom) cells. Each graph compares binding of the test mAb (blue) to an irrelevant control mAb (red). FIGURE 4 SHOULD BE PUBLISHED IN COLOR.
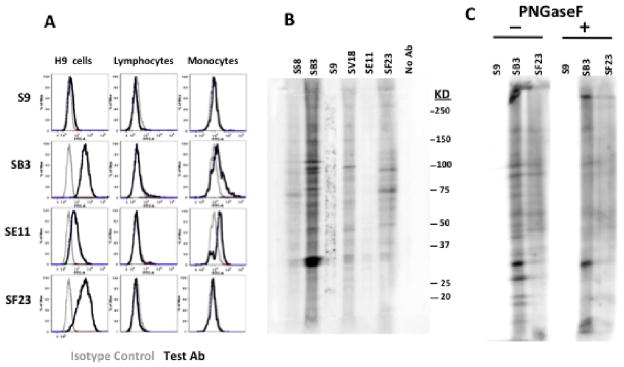
To explore this unexpected cross-reaction further, we tested the binding of mAbs SB3, SF23, and SE11 (and S9 as a negative control) to other human cells: H9 T-cell lymphoma cell line, and to freshly explanted human peripheral blood mononuclear cells (figure 5A). All three sialic acid-independent mAbs reacted with H9 cells by flow cytometry, whereas S9 did not. None bound to freshly explanted human lymphocytes, while SE11 and SB3 did bind to monocytes. The different patterns of binding of these mAbs to cell surface antigens again highlight the specificity differences among these mAbs, here measured as cross-reactions. To further examine the specificity of the cross-reaction, immunoblots were performed on H9 lysates. Figure 5B shows that the mAbs react with multiple bands, presumably with the carbohydrate determinants expressed on different cellular glycoproteins. Minor differences in the binding patterns and larger differences in the degree of binding can be observed among the antibodies. There is also considerable “background” staining in some lanes, especially in the range between the 37 and 150 KD markers. We observe this often when using anti-carbohydrate antibodies in Western blots. PNGaseF digestion of the cell lysates (figure 5C) indicates that a good part of the reactivity is due to recognition of carbohydrate determinants by the anti-GBS mAbs. Because incomplete digestion cannot be ruled out, we cannot determine whether the remaining binding demonstrates non-carbohydrate specificity or simply incomplete removal of the carbohydrate determinants. We do not believe that the observed cross-reactions are due to mycoplasma contamination of the cells and cross-reactivity with mycoplasma antigens rather than cellular antigens, for the following reasons: 1. We have examined the cells by staining with Hoechst dye and have found no evidence of mycoplasma contamination (not shown), 2. We have examined low passage cells and observed no difference in binding of the anti-GBS mAbs (not shown), 3. Cells have been treated with oxoquinolone antibiotic (Mycoplasma Removal Agent, MP Biomedicals, Solon OH) for two weeks and there was no alteration in binding of the cells with anti-GBS mabs (not shown), and 4. Reactivity was observed on freshly explanted human monocytes (figure 5B) which are not contaminated with mycoplasma.
Figure 5. Binding of anti-GBS mAbs to lymphoid cells and monocytes.
(A) Flow cytometry was performed to measure binding to cell-surface antigens on H9 lymphoma cells, and freshly explanted peripheral blood mononuclear cells. Cell number is on the vertical axis, fluorescent intensity on the horizontal. Each graph compares binding of the test mAb (black) to an isotype-matched control mAb (grey). Lymphocytes and monocytes were examined together, and the cell populations segregated on the basis of forward and side scatter characteristics. (B and C). Immunoblots were performed on H9 cell lysates. In B the lysates were not treated with any enzymes, in C cells were digested (or not) with PNGaseF to remove carbohydrate determinants from glycoproteins. Lysates were blotted onto membranes and incubated with the indicated antibodies, followed by detection with alkaline-phosphatase conjugated anti-mouse Ig and chemiluminescent detection.
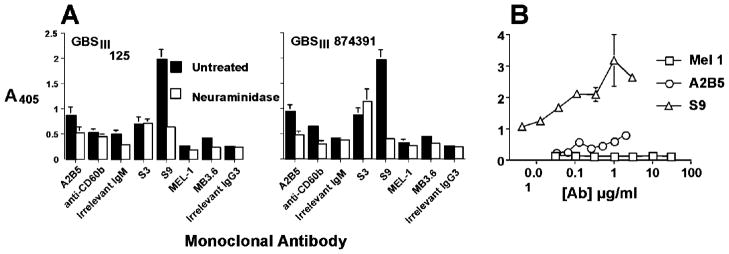
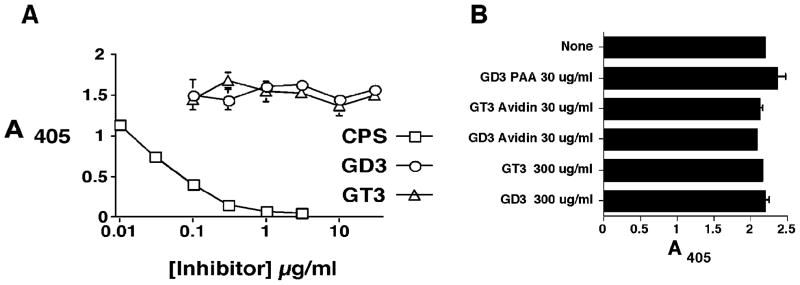
The cross-reaction was probed in the opposite direction by testing whether anti-GD3 or GT3-ganglioside mAbs bind to intact GBS (figure 6). The results show a low level of binding of mAbs A2B5, anti-CD60b, MB3.6 to two different GBS isolates. Neuraminidase treatment of GBS results in loss of binding activity. The binding of the anti-ganglioside mAbs is several orders of magnitude less than that of anti-CPSIII Ab (figure 6B). The relative avidity of the anti-CPSIII Ab for GBS capsular polysaccharide versus that for the GD3/GT3 carbohydrates was tested by competitive inhibition ELISA (figure 7). Neither monomeric nor multimeric GD3 or GT3 were able to inhibit the binding of S9 to GBS, whereas purified CPSIII was highly effective.
Figure 6. Abs specific for GD3 and GT3 bind GBS only weakly.
The binding of the indicated mAbs to intact GBS, either treated with neuraminidase or not, was measured by ELISA. In panel A, the binding of mAbs at 10 μg/ml (except S9, which was 1 μg/ml) was tested against two independent isolates of GBSIII. In panel B, mAbs were titrated against GBSIII strain 125.
Figure 7. GD3 and GT3 synthetic saccharides do not inhibit binding of S9 mAb to CPSIII.
Panel A. The binding of S9 to intact GBSIII strain 125 was inhibited with the indicated concentrations of purified CPSIII, soluble monomeric GD3, or GT3. Ab and inhibitor were premixed and incubated prior to transfer to microtiter plates coated with GBS. Binding of S9 Ab was detected with alkaline phosphatase-conjugated goat anti-mouse IgM. Binding in the absence of an inhibitor was 1.72 + 0.07. Panel B. Multimeric forms of GD3 and GT3 were tested for their ability to inhibit the binding of S9 to CPSIII. The indicated concentration of inhibitor was premixed with S9 Ab prior to plating into microtiter wells. The GD3 and GT3 were either monomeric, tetrameric (avidin-GD3 and avidin-GT3), or highly multimeric (GD3-PAA)
To determine whether immunization with GD3 or GT3 can elicit Abs that cross-react with GBS, mice were immunized with oligomeric, or highly branched polymeric forms of the carbohydrates created by complexing biotinylated monomeric or PAA-biotin polymeric forms of the carbohydrates with neutravidin. Antigen was given initially in CFA, followed by booster immunization in IFA. Although Ab to the immunogen was obtained, there was no evidence of the induction of anti-GBS Ab in these mice (data not shown).
DISCUSSION
Ab to the capsular polysaccharide has been shown to be a critical component of protective immunity to group B streptococcus, and thus is felt to be essential for an effective vaccine [1–8, 10–13, 21, 24]. To evaluate the antigenic structures identified by anti-CPS Abs, we studied a panel of anti-CPSIII mAbs whose protective efficacy has previously been examined [14, 17, 35]. Two important results emerged. First is the degree of heterogeneity of antigen binding demonstrated by Abs directed against this structure, and second is the potential immunologic cross-reaction between CPSIII and cell surface carbohydrates. Cross-reactions between capsular polysaccharides and self-antigens are known to exist, perhaps the best studied being that between N. meningitides type B and a polysialic acid determinant structure found on developing neural cells [40, 41]. Such cross-reactions suggest caution in the development of vaccines to GBS.
It has long been known that there is a degree of heterogeneity in Abs to CPSIII, defined by reactivity to neuraminidase-treated CPSIII. Some Abs react with the core structure lacking sialic acid, which is otherwise identical to the S. pneumoniae type 14 CPS (figure 1). Protective Abs require the presence of sialic acid for Ab recognition, the so-called complete antigen. The studies presented in figure 2 demonstrate that even among mAbs specific for the complete epitope, there are differences in the degree of dependence on sialic acid. This is best demonstrated by mAb S3.1B1 which fails to bind CPS of S. pneumoniae type 14, but does react with the neuraminidase digested GBS. Abs that bind to sialic-acid dependent epitopes are generally more protective [12, 14, 17, 42]. It has been debated whether sialic acid is a functional part of the epitope or is important for maintaining the overall three dimensional structure of the epitope bound by the Abs [43]. The microarray study demonstrates that there is further heterogeneity among anti-CPSIII Abs to either the core structure or to the sialic acid-dependent complete epitope. Among the mAbs dependent upon sialic acid for binding, S9 tolerates fucose and an additional sialic acid when compared to SV18. But an even greater difference is seen between these two mAbs and S3 1A6, 1B1, and 2A6, which fail to react with any of the simple carbohydrate structures. Although it is possible that the target epitopes for these mAbs are not represented on the array, it seems more likely that these mAbs identify a conformational determinant requiring an intact 3D structure, an interpretation consistent with previously reported NMR studies [35, 43]. Thus it is likely that sialic acid plays two roles in the antigenic structure of CPSIII. First it is a key component of the epitope identified by some protective mAbs, eg S9 and SV18. Second it plays a role in maintaining the 3D structures that are identified by mAbs S3 1A6, 1B1, and 2A6. Despite this wealth of heterogeneity in the fine specificity of the mAbs, the single most important determinant of protective efficacy remains whether it binds to the complete, sialic-acid containing epitope, or not [12, 14, 17, 42]. Beyond that, Ab avidity is important [14]. The heterogeneity of the Ab response to CPSIII described here, combined with the observations of others cited in the paragraphs above, are in contrast to recent claims of a single CPSIII epitope [44].
Interestingly, the mAbs elicited by immunization with CPS-protein conjugates did not bind to simple sugars represented on the microarray and have been shown to bind to conformational structures [35], whereas Abs elicited by intact GBS (S9, SF23, SV18, and SE11) tend to bind to the oligosaccharides found on the microarray. It has previously been shown that polyclonal sera elicited by CPS-tetanus toxoid immunization primarily bound conformational structures. This also was observed in rabbits [45], and in humans[8]. A recent examination of immune sera elicited by CPS-conjugate vaccines suggested that the dominant epitope was the “core” epitope [44], but the design of the study may have overlooked conformational epitopes. The CPS employed in the production of the conjugate used to elicit the mAbs described here was isolated so that full sialic acid content was preserved, and confirmed by chemical analysis. In contrast, the infection/immunization protocols using intact/live GBS, have no control over the structure of the CPS exposed to the immune system.
GD3 and GT3 are carbohydrate structures found on a variety of normal tissues and tumors, particularly on neural cells undergoing maturation [26–28, 36–38]. Because a GBS vaccine is proposed for maternal usage and transplacental transfer of Abs, the presence of cross-reactive epitopes on developing neural tissue raises concerns. We have tried to determine whether this cross-reaction, identified on synthetic sugars, has physiologic significance. We find no evidence that these mAbs bind to GD3 or GT3 gangliosides. Thus it appears that although mAbs S9 and SV18 can bind to the carbohydrate structure present in high density in the spotted microarray, they do not bind to more sparsely spread epitopes on the cell surface (figures 4 and 5), or when monomeric or oligomeric GD3 or GT3 is coated onto microtiter plates (data not shown). This is consistent with anti-CPS mAbs requiring the highly repetitive structures present on CPSIII for antigen recognition and binding. Surprisingly we found that mAbs SB3, SE11, and SF23, which bind to non-protective core epitope(s) of CPSIII, bind to surface antigens on human cell lines and primary monocytes. The target of this binding on the cell surface is not known, but is presumed to be the carbohydrate portions of cell-surface glycoproteins. For example, compounds 49 and 72 on the microarray (figure 3) that are bound by SE11 represent O-glycans of Core-2 structures found in normal tissues. Because the mAbs that cross-react are targeted to non-protective epitopes on the CPSIII, it may still be possible to design a vaccine that elicits only Abs that are directed against the protective, sialic acid-dependent epitopes. Perhaps this may be accomplished using the CPS-tetanus toxoid conjugates, which seem to target Ab responses to conformational sialic-acid dependent structures unique to the CPSIII.
An immunologic cross-reaction between a microbial polysaccharide and a self-Ag could explain why some individuals fail to respond immunologically to the microbial polysaccharide. The absence of maternal Ab to GBS capsular antigens has been identified as an important factor in the pathogenesis of GBS infections in newborns [2, 3], and a proportion of women fail to respond to immunization with CPSIII [46]. The failure of individuals to respond may partially result from self-tolerance to critical determinants of the CPSIII structure. The binding, albeit weak, of anti-GD3/GT3 ganglioside mAbs to GBS suggests that there is a degree of resemblance between the CPSIII and the gangliosides. The weak immunogenicity of the N. meningitides type B CPS, which resembles polysialic acid epitopes found on neural cells, has been overcome by using N-propionated CPS as an immunogen [47]. Alternatively, the cross-reaction between a microbial Ag and a self-Ag could result in molecular mimicry causing autoimmunity, a process that may explain the relationship between Guillain-Barre syndrome and Campylobacter jejuni infection [48, 49]. In the case of Guillain-Barre syndrome it appears that the cross-reaction is between GD1 and GM1 gangliosides and the bacteria’s lipo-oligosaccharide. In the case we describe here, the cross-reaction is to the protective Ag of the CPS.
The carbohydrate microarray that was used in these studies was originally designed to probe carbohydrate interactions on mammalian cells [24, 25], and thus glycans were designed to represent such structures. The use of such “biased” microarrays to study the fine specificity of Ab responses to a microbial polysaccharide may identify those Abs with the potential to bind human cells, and thus because of molecular mimicry result in a sub-optimal immune response. Such microarrays may have utility in screening candidate vaccine antigens, although we do not fully understand the physiologic relevance of the reactivity on carbohydrate microarrays.
In view of the difficulties in developing a maternal vaccine for GBS, and the success of antenatal screening and intrapartum antibiotic prophylaxis in decreasing the prevalence of neonatal GBS infection [9, 50], the impetus for developing such a vaccine seems to have lessened. Yet despite near universal implementation of screening programs in the United States, a number of children are born each year with the disease, particularly those whose mothers have tested negative for GBS on culture [9]. As a result, a recently published authoritative survey of neonatal GBS infection, primarily by authors at the US Centers for Disease Control and Prevention, concludes that new strategies, such as the development of vaccines against group B streptococcus, continue to hold the most promise for further prevention of early-onset group B streptococcal disease [9]. Our findings of potential immunologic cross-reactions among anti-GBS Abs and human cell surface Ags, and the heterogeneity of the Ab response to CPS antigens, will certainly complicate these efforts. But, our results also suggest that administration of CPS conjugated to tetanus toxoid is potentially a safe route of immunization. MAbs elicited by this immunogen do not react with the carbohydrate microarray representing mammalian glycans (figure 3). It is likely that the fully sialylated CPS in this immunogen results in Abs directed against conformational structures unique to the CPS, thereby avoiding the generation of Abs that cross-react with other carbohydrate structures. The conjugation of CPS to carrier protein drives a memory immune response, producing higher titers of more effective Abs. These data and those of previous studies of immunization in animals and humans [4, 8, 51] support the ongoing development of a CPS-based vaccine for the prevention of GBS newborn infections.
Whether the cross-reactions we have detected in vitro can predict the potential development of autoimmunity remains undefined. Using anti-GBS mAbs that reacted with GD3 and GT3 synthetic carbohydrates spotted onto a microarray, we were unable to observe any reaction with those antigens as expressed on cells or in lower density forms of the synthetic carbohydrate. Nor do antibodies to GD3 or GT3 react with group B streptococci. The observed cellular cross-reactions may reflect differences in glycosylation patterns in mice and humans. The mAbs we studied were raised in mice, but tested on human cells. It is possible that in humans, B-cell clones producing such cross-reactive Abs would be deleted, or otherwise silenced. Further in vivo analyses would be necessary to determine the physiological importance of these observed cross-reactions.
In conclusion, we have examined the fine specificity and cross-reactions of a panel of mAbs to the type III CPS of group B streptococcus, the major protective antigen of this bacterium. Although all mAbs all recognize a single repetitive carbohydrate epitope, there are multiple fine specificity differences among these mAbs. These differences are noted when assaying: 1) reactivity to intact vs neuraminidase-digested GBSIII in ELISA, 2) binding to purified native CPSIII in ELISA, 3) binding to synthetic glycans on microarray, 4) surface immunofluorescence of intact mammalian cells, and 5) immunoblotting mammalian cell lysates. Cross-reactions of anti-GBS mAbs with synthetic glycans mimicking mammalian structures, and with human cell lines and peripheral blood monocytes raise concerns. But additional experiments suggest that these observed cross-reactions may be of limited physiological relevance.
HIGHLIGHTS.
Group B streptococcal type III capsular polysaccharide (CPSIII) is an important Ag
We compared a panel of different mAbs to CPSIII using multiple immunoassays
MAbs showed unique patterns of fine specificity and host-reactivity
These studies raise caution in developing CPSIII-based vaccines
Acknowledgments
This work supported by US Public Health Service Grant AI51086 and by The Research Institute for Children. These studies are part of NIGMS and The Consortium for Functional Glycomics (GM62116). We thank Megan Weydert, Chad Gustafson, and Kejing Song for technical assistance.
Footnotes
Abbreviations used in this manuscript: CPSIII, group B streptococcal capsular polysaccharide, type III; GBS, group B streptococci; GD3, glycan Neu5Ac(α2–8)Neu5Ac(α2–3)Gal (β1–4)Glc; GT3, glycan Neu5Ac(α2–8)Neu5Ac (α2–8)Neu5Ac(-α2–3)Gal(β1–4)Glc; PAA, polyacrylamide
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevention CfDCa. Prevention of perinatal group B streptococcal disease: a public health perspective. Morbidity and Mortality Weekly Report. 1996;45(RR-7):1–24. [PubMed] [Google Scholar]
- 2.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. New Eng J Med. 1976;294:753–6. doi: 10.1056/NEJM197604012941404. [DOI] [PubMed] [Google Scholar]
- 3.Baker CJ, Kaspar DL. Immunologic investigation of infants with septicemia or meningitis due to group B streptococcus. J Infect Dis. 1977;136:598–604. doi: 10.1093/infdis/136.supplement.s98. [DOI] [PubMed] [Google Scholar]
- 4.Wessels MR, Paoletti LC, Kasper DL, DiFabio JL, Michon F, Holme K, et al. Immunogenicity in animals of a polysaccharide-protein conjugate vaccine against type III group B Streptococcus. J Clin Invest. 1990;86(’):1428–33. doi: 10.1172/JCI114858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madoff LC, Paoletti LC, Tai JY, Kasper DL. Maternal immunization of mice with group B streptococcal type III polysaccharide-beta C protein conjugate elicits protective antibody to multiple serotypes. J Clin Invest. 1994;94:286–92. doi: 10.1172/JCI117319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paoletti LC, Wessels MR, Rodewald AK, Shroff AA, Jennings HJ, Kasper DL. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by maternal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Inf Immun. 1994;62:3236–43. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baker CJ, Rench MA, Edwards MS, Carpenter RJ, Hays BM, Kasper DL. Immunization of pregnant women with a polysaccharide vaccine of group B streptococcus. New Eng J Med. 1988;319:1180–85. doi: 10.1056/NEJM198811033191802. [DOI] [PubMed] [Google Scholar]
- 8.Kasper DL, Paoletti LC, Wessels MR, Guttormsen H-K, Carey VJ, Jennings HJ, et al. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Invest. 1996;98:2308–14. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dyke MK, Phares CR, Lynfield R, Thomas AR, Arnold KE, Craig AS, et al. Evaluation of universal antenatal screening for group B streptococcus. N Engl J Med. 2009 Jun 18;360(25):2626–36. doi: 10.1056/NEJMoa0806820. [DOI] [PubMed] [Google Scholar]
- 10.Lancefield RC. A serologic differentiation of specific types of bovine hemolytic streptococci (group B) J Exp Med. 1934;59:441–58. doi: 10.1084/jem.59.4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lancefield RC, McCarty M, Everly WN. Multiple mouse-protective antibodies directed against group B streptococci. J Exp Med. 1975;142:165–79. doi: 10.1084/jem.142.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancefield RC, Freimer EH. Type specific polysaccharide antigens of group B streptococci. Journal of Hygiene (london) 1966;64:191–9. doi: 10.1017/s0022172400040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancefield RC. Two serological types of group B hemolytic streptococci with related, but not identical, type specific substances. J Exp Med. 1938;67:25–40. doi: 10.1084/jem.67.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pincus SH, Shigeoka AO, Moe AA, Ewing LP, Hill HR. Protective efficacy of IgM monoclonal antibodies in experimental group B streptococcal infection is a function of antibody avidity. J Immunol. 1988;140:2779–85. [PubMed] [Google Scholar]
- 15.Shigeoka AO, Pincus SH, Rote NS, Pritchard DG, Santos JI, Hill HR. Monoclonal antibody preparations for immunotherapy of experimental GBS infection. Antibiot Chemother. 1985;35:254–66. doi: 10.1159/000410379. [DOI] [PubMed] [Google Scholar]
- 16.Shigeoka AO, Pincus SH, Rote NS, Hill HR. Protective efficacy of hybridoma type-specific antibody against experimental infection with group-B Streptococcus. J Infect Dis. 1984;149:363–72. doi: 10.1093/infdis/149.3.363. [DOI] [PubMed] [Google Scholar]
- 17.Egan ML, Pritchard DG, Dillon HC, Gray BM. Protection of mice from infection with type III group B streptococcus using monoclonal antibodies. J Exp Med. 1983;158:1006–20. doi: 10.1084/jem.158.3.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wessels MR, Rubens CE, Benedi JV, Kasper DL. Definition of a bacterial virulence factor: sialylation of the group B streptococcal capsule. Proc Natl Acad Sci USA. 1989;86:8983–7. doi: 10.1073/pnas.86.22.8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pincus SH, Cole RL, Wessels MR, Corwin MD, Kamanga-Sollo E, Hayes SF, et al. Group B streptococcal opacity variants. J Bacteriol. 1992;174:3739–49. doi: 10.1128/jb.174.11.3739-3749.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pincus SH, Cole RL, Kamanga-Sollo E, Fischer SH. Interaction of group B streptococcal opacity variants with the host defense system. Infect Immun. 1993;61:3761–8. doi: 10.1128/iai.61.9.3761-3768.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edwards MS, Kasper DL, Jennings HJ, Baker CJ, Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982;128:1278–83. [PubMed] [Google Scholar]
- 22.Marques MB, Kasper DL, Pangburn MK, Wessels MR. Prevention of C3 deposition by capsular polysaccharide is a virulence mechanism of type III group B streptococci. Inf Immun. 1992;60:3986–93. doi: 10.1128/iai.60.10.3986-3993.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell JR, Baker CJ, Edwards MS. Deposition and degradation of C3 on type III group B streptococci. Inf Immun. 1991;59:1978–83. doi: 10.1128/iai.59.6.1978-1983.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paulson JC, Blixt O, Collins BE. Sweet spots in functional glycomics. Nature Chemical Biology. 2006;2:238–48. doi: 10.1038/nchembio785. [DOI] [PubMed] [Google Scholar]
- 25.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, et al. Printed covalent glycan array for ligand profiling of diverse glycan binding proteins. Proc Natl Acad Sci USA. 2004;101:17033–38. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pukel CS, Lloyd KO, Travassos LR, Dippold WG, Oettgen HF, Old LJ. GD3, a prominent ganglioside of human melanoma. Detection and characterisation by mouse monoclonal antibody. The Journal of experimental medicine. 1982 Apr 1;155(4):1133–47. doi: 10.1084/jem.155.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Constantine-Paton M, Blum AS, Mendez-Otero R, Barnstable CJ. A cell surface molecule distributed in a dorsoventral gradient in the perinatal rat retina. Nature. 1986 Jan 1;324(6096):459–62. doi: 10.1038/324459a0. [DOI] [PubMed] [Google Scholar]
- 28.Cheresh DA, Harper JR, Schulz G, Reisfeld RA. Localization of the gangliosides GD2 and GD3 in adhesion plaques and on the surface of human melanoma cells. Proc Natl Acad Sci USA. 1984 Sep 1;81(18):5767–71. doi: 10.1073/pnas.81.18.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrer RG, Quarles RH. GT3 and its O-acetylated derivative are the principal A2B5-reactive gangliosides in cultured O2A lineage cells and are down-regulated along with O-acetyl GD3 during differentiation to oligodendrocytes. Journal of Neuroscience Res. 1999;57:371–80. [PubMed] [Google Scholar]
- 30.Wang H-W, Muguira M, Liu W-D, Zhang T, Chen C, Aucoin R, et al. Identification of an INSM1-binding site in the insulin promoter: negative regulation of the insulin gene transcription. J Endocrinol. 2008 Jul 1;198(1):29–39. doi: 10.1677/JOE-08-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chesebro B, Wehrly K. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J Virol. 1988;62:3779–88. doi: 10.1128/jvi.62.10.3779-3788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pincus SH, Fang H, Wilkinson RA, Marcotte TK, Robinson JE, Olson WC. In vivo efficacy of anti-gp41, but not anti-gp120, immunotoxins in a mouse model of HIV infection. J Immunol. 2003;170:2236–41. doi: 10.4049/jimmunol.170.4.2236. [DOI] [PubMed] [Google Scholar]
- 33.Wessels MR, Pozsgay V, Kasper DL, Jennings HJ. Structure and immunochemistry of an oligosaccharide repeating unit of the capsular polysaccharide of type III group B Streptococcus. A revised structure for the type III group B streptococcal polysaccharide antigen. Journal of biological chemistry. 1987;262:8262–7. [PubMed] [Google Scholar]
- 34.Shigeoka AO, Pincus SH, Rote NS, Hill HR. Protective efficacy of hybridoma type-specific antibody against experimental infection with group-B Streptococccus. Journal of infection disease. 1984;149:363. doi: 10.1093/infdis/149.3.363. [DOI] [PubMed] [Google Scholar]
- 35.Zou W, Mackenzie R, Thérien L, Hirama T, Yang Q, Gidney MA, et al. Conformational epitope of the type III group B Streptococcus capsular polysaccharide. J Immunol. 1999 Jul 15;163(2):820–5. [PubMed] [Google Scholar]
- 36.Farrer RG, Quarles RH. Expression of sulfated gangliosides in the central nervous system. J Neurochem. 1997 Feb 1;68(2):878–81. doi: 10.1046/j.1471-4159.1997.68020878.x. [DOI] [PubMed] [Google Scholar]
- 37.Irwin LN, Michael DB, Irwin CC. Ganglioside patterns of fetal rat and mouse brain. J Neurochem. 1980 Jun 1;34(6):1527–30. doi: 10.1111/j.1471-4159.1980.tb11235.x. [DOI] [PubMed] [Google Scholar]
- 38.Schlosshauer B, Blum AS, Mendez-Otero R, Barnstable CJ, Constantine-Paton M. Developmental regulation of ganglioside antigens recognized by the JONES antibody. J Neurosci. 1988 Feb 1;8(2):580–92. doi: 10.1523/JNEUROSCI.08-02-00580.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gillard BK, Thomas JW, Nell LJ, Marcus DM. Antibodies against ganglioside GT3 in the sera of patients with type I diabetes mellitus. J Immunol. 1989 Jun 1;142(11):3826–32. [PubMed] [Google Scholar]
- 40.Finne J, Bitter-Suermann D, Goridis C, Finne U. An IgG monoclonal antibody to group B meningococci cross-reacts with developmentally regulated polysialic acid units of glycoproteins in neural and extraneural tissues. J Immunol. 1987 Jun 15;138(12):4402–7. [PubMed] [Google Scholar]
- 41.Finne J, Finne U, Deagostini-Bazin H, Goridis C. Occurrence of alpha 2–8 linked polysialosyl units in a neural cell adhesion molecule. Biochem Biophys Res Commun. 1983 Apr 29;112(2):482–7. doi: 10.1016/0006-291x(83)91490-0. [DOI] [PubMed] [Google Scholar]
- 42.Paoletti LC, Madoff LC, Kasper DL. Surface structures of group B streptococcus important in human immunity. In: Fischetti VA, Novick RP, Ferretti JJ, Prtnoy DA, Rood JI, editors. Gram-Positive Pathogens. Washington, D.C: ASM Press; 2000. pp. 137–53. [Google Scholar]
- 43.Brisson J-R, Uhrinova S, Woods RJ, van der Zwan M, Jarrell HC, Paoletti LC, et al. NMR and molecular dynamics studies of the conformational epitope of the type III group B Streptococcus type III capsular polysaccharide. Biochemistry. 1997;36:3278–92. doi: 10.1021/bi961819l. [DOI] [PubMed] [Google Scholar]
- 44.Safari D, Dekker HAT, Rijkers GT, van der Ende A, Kamerling JP, Snippe H. The immune response to group B streptococcus type III capsular polysaccharide is directed to the -Glc-GlcNAc-Gal- backbone epitope. Glycoconj J. 2011 Dec 1;28(8–9):557–62. doi: 10.1007/s10719-011-9354-1. [DOI] [PubMed] [Google Scholar]
- 45.Marques MB, Kasper DL, Shroff AA, Michon F, Jennings HJ, Wessels MR. Functional activity of antibodies to the group B polysaccharide of group Bstreptococci elicited by a polysaccharide-protein conjugate vaccine. Infection and immunity. 1994;62:1593. doi: 10.1128/iai.62.5.1593-1599.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baker CJ, Rench MA, Kasper DL. Response to type III polysaccharide in women whose infants have had invasive group B streptococcal infection. New Eng J Med. 1990;322:1857–60. doi: 10.1056/NEJM199006283222606. [DOI] [PubMed] [Google Scholar]
- 47.Pon RA, Lussier M, Yang QL, Jennings HJ. N-Propionylated group B meningococcal polysaccharide mimics a unique bactericidal capsular epitope in group B Neisseria meningitidis. The Journal of experimental medicine. 1997 Jun 2;185(11):1929–38. doi: 10.1084/jem.185.11.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaida K, Ariga T, Yu RK. Antiganglioside antibodies and their pathophysiological effects on Guillain-Barré syndrome and related disorders--a review. Glycobiology. 2009 Jul 1;19(7):676–92. doi: 10.1093/glycob/cwp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu RK, Usuki S, Ariga T. Ganglioside molecular mimicry and its pathological roles in Guillain-Barré syndrome and related diseases. Inf and Immunity. 2006 Dec 1;74(12):6517–27. doi: 10.1128/IAI.00967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrag SJ, Zywicki S, Farley MM, Reingold AL, Harrison LH, Lefkowitz LB, et al. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. New england journal of medicine. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 51.Paoletti LC, Wessels MR, Rodewalk AK, Shroff AA, Jennings HJ, Kasper DL. Neonatal mouse protection against infection with multiple group B streptococcal (GBS) serotypes by matenal immunization with a tetravalent GBS polysaccharide-tetanus toxoid conjugate vaccine. Infection and immunity. 1994;62:3236. doi: 10.1128/iai.62.8.3236-3243.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li R-K, Cutler J. Chemical definition of an epitope/adhesin on Candida Albicans. J Biol Chem. 1993;268:18293–99. [PubMed] [Google Scholar]
- 53.Nano FE, Caldwell HD. Expression of the Chlamydial Genus-Specific Lipopolysaccharide Epitope in Escherichia coli. Science. 1985;228:742–4. doi: 10.1126/science.2581315. [DOI] [PubMed] [Google Scholar]