Abstract
Introduction
The specific aims of the study were to evaluate the two-year overall survival (OS) and progression-free survival (PFS), toxicity profile, and best objective response rate in patients with locally advanced, clinically unresectable esophageal cancer receiving cetuximab, cisplatin, irinotecan, and thoracic radiotherapy (TRT) within a multi-institutional cooperative group setting.
Methods
Eligible patients (cT4 M0 or medically unresectable, biopsy proven, non-cervical esophageal cancer) were to receive four 21-day cycles of cetuximab 400 mg/m2 (days 1, cycle 1), cetuximab 250 mg/m2 (days 8, 15, cycle 1; then days 1, 8, 15 for subsequent cycles), cisplatin 30 mg/m2 (days 1, 8 all cycles), and irinotecan 65 mg/m2 (days 1, 8 all cycles). TRT was administered at 1.8 Gy in 28 daily fractions to a total dose of 50.4 Gy, to begin with day 1, cycle 3. The primary endpoint was 2-year OS, with an accrual goal of 75 patients with adenocarcinoma.
Results
The study was closed due to slow accrual, with 21 eligible patients (11 squamous, 10 adenocarcinoma) enrolled from May 2005 to September 2007. Two-year OS and PFS (95% CI) were 33.3% (14.6–57.0%) and 23.8% (8.2–47.2%), respectively. Kaplan-Meier estimates of median (95% CI) OS and PFS were 11.2 (6.4–43.6) and 6.4 (3.7–12.0) months, respectively. The overall response rate (95% CI) among 17 evaluable patients was 17.6% (3.8–43.4%), including 6% confirmed complete responders and 12% unconfirmed partial responders. Two deaths were due to protocol treatment (sudden death & GI necrosis). Ten (47.6%) and 6 (28.6%) patients had Grade 3/4 toxicity, respectively: 52.4% hematologic, 23.8% fatigue, 19.0% nausea, 19.0% dehydration, and 19.0% anorexia.
Conclusions
Concomitant cetuximab, cisplatin, irinotecan, and TRT was poorly tolerated in the first North American cooperative group trial testing this regimen for locally advanced esophageal cancer, as treatment-related mortality approached 10%. Single institution phase II cetuximab-based combined modality trials have yielded encouraging results in preliminary analyses. The SWOG GI Committee endorses enrollment to open clinical trials in order to clarify the therapeutic ratio of cetuximab-based combined modality approaches for esophageal cancer.
Keywords: Cetuximab, Cisplatin, Irinotecan, Combined modality, Chemoradiotherapy, Esophagus Cancer
INTRODUCTION
An estimated 16,640 new cases of esophageal cancer will be diagnosed in the United States in 2010, accompanied by 14,500 deaths from the disease.1 It is the fifth leading cause of cancer death in men over the age of 40 years. National Comprehensive Cancer Network guidelines recommend the concomitant administration of cisplatin, 5-fluorouracil (5-FU), and thoracic radiotherapy (TRT) as definitive therapy for patients with locally advanced esophageal cancer. Both cisplatin and 5-FU have proven to be relatively effective radiosensitizing agents in pre-clinical and clinical experience over the past two decades. RTOG 85-01 demonstrated a 27% 5-year survival compared to 0% for patients receiving the combined-modality regimen over TRT (6,400 cGy) alone.2,3 Despite the addition of systemic cytotoxic agents to TRT, local failure within the gross tumor volume remains the most common cause of treatment failure.4 A Patterns of Care analysis by the American College of Radiology for the period of 1996 - 1999 suggests that 56% of patients with esophageal cancer receive combined-modality therapy as definitive therapy.5 Infusional 5-FU delivered over several days can be cumbersome and the toxicity profile may be so profound as to preclude an adequate number of courses, despite its efficacy. Thus, there is a pressing need for effective, less toxic and novel treatment programs for patients with locally advanced esophageal cancer.6
Irinotecan, a topoisomerase I enzyme inhibitor, is a semi-synthetic, watersoluble derivative of the plant Camtotheca acuminata and inhibits topo-1, a nuclear enzyme, via binding and stabilization of the topo-1/DNA cleavable complex.7 A 22% objective response rate in advanced esophageal and gastric cancer has been reported, using irinotecan combined with a 5-FU/folinic acid backbone.8 This study and other irinotecan-based studies (408 total combined patients) with esophageal and gastric cancer, suggest response rates of 14 – 65%.7,9,10,11,12,13,14,15
In vitro and in vivo data suggest that irinotecan exhibits significant radiosensitizing properties.16,17,18,19,20 Phase I experience with single-agent irinotecan and TRT noted that 60 mg/m2 weekly for 5 to 6 weeks could be safely administered in a combined-modality setting.21 Based upon published Phase II experience of weekly irinotecan and cisplatin for advanced esophagus cancer that demonstrated a 57% overall response rate (including two clinical complete responses) along with a nearly 15 month median actuarial survival, investigators added TRT to this regimen for patients with Stage II or III lesions.12 A dose-escalation study of weekly irinotecan, fixed-dose cisplatin, and TRT, following four weeks of induction therapy (irinotecan 65 mg/m2 weekly and cisplatin 30 mg/m2 weekly), determined that the maximum tolerated dose of irinotecan was 65 mg/m2 weekly for five weeks.22 TRT was administered at 5,040 cGy in standard 180 cGy fractions. This combination of non-5-FU-based chemoradiotherapy was shown to be safe and therapeutically active against primary esophageal cancer. Moreover, the pathologic complete response rate of 32% was consistent with prior results in the literature that included infusional fluorinated pyrimidine-based chemoradiotherapy. The same group recently reported the results of a Phase II study of induction weekly irinotecan and cisplatin followed by the same regimen concurrent with TRT to 5,040 cGy, followed by surgery.23 R0 resection was obtained in 69% of the patients, and the pathologic complete response rate was 16%. Post-induction PET response was correlated with better clinical outcomes. A retrospective analysis of induction cisplatin and irinotecan followed by concurrent cisplatin, irinotecan, and TRT with a median follow-up of two years reported a 2-year OS of 42% and acceptable tolerability of this regimen.24
Cetuximab is a novel chimeric monoclonal antibody directed against the external domain of the epidermal growth factor receptor (EGFR). This agent is able to inhibit the activity of tyrosine kinase on the inner surface of the cell membrane. This results in inhibition of downstream events within the signal transduction cascade from the cell surface to the nucleus. Preclinical data suggest that cetuximab has radiosensitizing properties.25 Its utility combined with radiotherapy has been demonstrated for squamous cell carcinoma of the head and neck.26 Cetuximab has been safely used in combination with cisplatin27,28 as well as with irinotecan,29,30 and evidence suggests that cetuximab acts to enhance irinotecan’s cytotoxic properties by downregulating EGFR pathways the topoisomerase inhibitor is known to upregulate.31
Consequently, a novel form of antitumor activity may occur to support this study's hypotheses that:
As definitive therapy for primary locally advanced and clinically unresectable esophageal cancer, cetuximab, in combination with cisplatin, irinotecan, and TRT will produce a favorable response rate and survival.
In combination with cisplatin, irinotecan, and TRT, cetuximab will cause significantly less clinical toxicity than 5-FU-based chemoradiotherapy, a current standard of care for patients receiving either neoadjuvant or definitive combined-modality therapy for esophageal cancer. Cisplatin and irinotecan can be administered in full doses when given with cetuximab.
Patients with primary esophageal tumors expressing low levels of ERCC-1 and high levels of EGFR will exhibit an encouraging PFS and clinical complete response rate, following definitive treatment with cetuximab, cisplatin, irinotecan and external beam radiation.
MATERIALS AND METHODS
Eligible patients had pathologically documented squamous cell carcinoma or adenocarcinoma of the thoracic esophagus (≥ 20 cm from the incisors) or gastroesophageal (GE) junction, and all had measurable or evaluable cT4 M0 or unresectable disease. History, physical examination, EUS or EGD, chest radiography, PET scans with CT or MRI, as well as adequate renal and hepatic function, absence of prior cancer, absence of prior chemotherapy or radiotherapy, Zubrod performance status 0–2, and IRB-approved informed consent were all required. A baseline EKG and pulmonary function studies were recommended. Bronchoscopy with negative cytology was required for patients with a primary tumor < 26 cm from the incisors. Patients with clinical evidence of tracheo-esophageal fistulas were ineligible for this trial.
Induction chemotherapy
Two cycles of cetuximab 400 mg/m2 IV loading dose on day 1 followed by 250 mg/m2 (days 18, 15; and 22, 29, and 36), cisplatin 30 mg/m2 IV bolus (days 1, 8; and 22, 29), and irinotecan 65 mg/m2 (days 1, 8; and 22, 29) combination chemotherapy were administered. Cisplatin was administered after adequate hydration. Cetuximab was provided courtesy of ImClone, a wholly owned subsidiary of Eli Lilly and Company, and Bristol-Myers-Squibb. Standard pre-treatment agents were administered.
Concurrent chemotherapy
Two cycles of cetuximab 250 mg/m2 IV (days 43, 50, and 57; 64, 71, and 78), cisplatin 30 mg/m2 IV (days 43, 50; and 64, 71), and irinotecan 65 mg/m2 IV (days 43, 50; and 64, 71) were given beginning with the initiation of TRT.
Radiotherapy
3D-conformal megavoltage radiotherapy (IMRT was not allowed) was administered at 1.8 Gy in 28 daily fractions (excluding weekends and holidays) to a total dose of 50.4 Gy, to begin with concurrent chemotherapy. The gross tumor volume (GTV) included the primary tumor mass and involved lymph nodes. This GTV included the celiac nodal region in patients with tumor in the distal third of the esophagus. A clinical target volume (CTV) was derived by expanding the GTV 5 cm superiorly and inferiorly for the primary tumor and at least 2 cm around any involved lymph nodes. For patients with tumor extending at least 2 cm above the carina, the CTV included the supraclavicular nodal regions. Margins for expansion to the planning target volume (PTV) were left to the discretion of the treating radiation oncologist. Heterogeneity corrections were applied, and at least 95% of the CTV received the prescribed dose. All TRT plans were centrally reviewed by the Quality Assurance Review Center (Providence, RI) for compliance with the study parameters.
Growth factor support
The use of granulocyte-colony stimulating factor (G-CSF) was permitted only for patients who developed Grade 3 or 4 neutropenia. G-CSF was not permitted for primary prevention of neutropenia.
Dose Modifications
No dose modifications for radiotherapy were allowed. A hemogram was performed prior to systemic therapy on each infusion day. Irinotecan was to be held for WBC < 3000/ul, ANC < 1000/ul, or platelets < 100,000/ul; for febrile neutropenia or bleeding complications; for Grade 2+ mucositis or diarrhea; or for Grade 4 fatigue lasting more than three days. Cisplatin was dose reduced for a moderate increase in creatinine but was omitted for creatinine > 2.0 mg/dl, permanently discontinued for Grade 3+ peripheral neuropathy, and dose reduced by 25% for other Grade 3+ non-hematologic toxicity. Cetuximab was discontinued for Grade 4 acneiform rash, delayed and potentially dose reduced for Grade 3 rash, but no dose modifications were made for Grade 1 or 2 rash. Grade 1 or 2 infusion reaction from cetuximab was an indication for a permanent 50% dose reduction; Grade 3 or 4 infusion reactions led to permanent discontinuation of the drug. All patients were prophylactically treated with oral tetracycline and topical clindamycin.
Assessment of response
The objective response was evaluated according to the RECIST criteria. All toxicities were scored according to the CTCAE (NCI Common Terminology Criteria for Adverse Events), version 3.0.
Statistical methods
The main objective of the study was to assess the two-year OS of this novel therapeutic combination. This primary endpoint was driven by accrual of patients with adenocarcinoma, although although up to 25 patients with squamous cell tumors were eligible for enrollment. The regimen was to be considered promising if the true OS at two years was at least 50%, but not of further interest if the true survival rate was less than 35%. With a planned 75 adenocarcinoma patients, the power of a one-sided 0.05 level test to detect a 35% vs. a 50% two-year overall survival was 0.91. Additional endpoints included assessment of the toxicity profile of this regimen, best objective response to therapy, and progression-free survival.
RESULTS
The study was closed due to slow accrual, with 22 patients enrolled from May 2005 to September 2007. One patient was ineligible due to involvement of the cervical esophagus. The baseline characteristics of the 21 eligible patients are described in Table 1. The median age was 61 years (range 43 – 83 years).
Table 1.
baseline patient characteristics/demographics (n = 21)
| Age | |
|---|---|
| Median | 61.4 (43–83) |
| Sex | |
| Male | 15 (71%) |
| Female | 6 (29% |
| Race | |
| Caucasian | 15 (71%) |
| African-American | 4 (19%) |
| Asian | 2 (10%) |
| Histology | |
| Adenocarcinoma | 10 (48%) |
| Squamous cell | 11 (52%) |
Toxicities
Toxicity data for the 21 eligible patients are presented in Table 2. Eighteen patients (85.7%) exhibited a maximum Grade 3 or higher toxicity. Of the eight (38.1%) patients experiencing Grade 4 or higher toxicity, five had squamous cell and 3 had adenocarcinoma histology. Treatment-related mortality was observed in two patients (9.5%, sudden death and GI necrosis), both of whom has squamous cell histology. In both cases, the treating physician felt that protocol treatment may have been a contributing factor. The most common Grade 3 or higher toxicities were leucopenia (42.9%), neutropenia (28.6%), fatigue (23.8%), lymphopenia (19.0%), dehydration (19.0%), and gastrointesintal complaints (diarrhea (23.8%), nausea (19.0%), and anorexia (19.0%)). Febrile neuropenia was seen in fewer than 5% of patients. Eighteen patients (85.7%) were able to complete treatment as planned (2 deaths, 1 stopped due to side effects).
Table 2.
Maximum grade of adverse events by category (Maximum grade experienced by patient for each category) (n = 21)
| Grade | 3 (%) | 4 (%) | 5 (%) |
|---|---|---|---|
| Hematologic | |||
| Anemia | 14.3 | -- | -- |
| Leukopenia | 33.3 | 9.5 | -- |
| Lymphopenia | 9.5 | 9.5 | -- |
| Neutropenia | 14.3 | 14.3 | -- |
| Neutropenia, febrile | 4.8 | -- | -- |
| Nonhematologic | |||
| Acneiform rash | 4.8 | -- | -- |
| Anorexia | 19.0 | -- | -- |
| CNS ischemia | -- | 4.8 | -- |
| Creatinine | -- | 4.8 | -- |
| Dehydration | 19.0 | -- | -- |
| Diarrhea | 23.8 | -- | -- |
| Dysphagia | 14.3 | -- | -- |
| Esophagitis | 9.5 | -- | -- |
| Fatigue | 23.8 | -- | -- |
| GI necrosis | -- | -- | 4.8 |
| GI pain: abdomen | 4.8 | -- | -- |
| GI pain: esophagus | 4.8 | -- | -- |
| GI perforation: colon | 4.8 | -- | -- |
| Hyperglycemia | 4.8 | -- | -- |
| Hypoalbuminemia | 4.8 | -- | -- |
| Hypocalcemia | 4.8 | -- | -- |
| Hypokalemia | 9.5 | -- | -- |
| Hypomagnesemia | 4.8 | -- | -- |
| Hyponatremia | 4.8 | -- | -- |
| Nausea | 19.0 | -- | -- |
| Neuropathy | 4.8 | -- | -- |
| Renal failure | -- | 4.8 | -- |
| Skin lesions | 4.8 | -- | -- |
| Sudden death | -- | -- | 4.8 |
| Thrombosis/embolism | -- | 4.8 | -- |
| Typhlitis | -- | 4.8 | -- |
| Vomiting | 14.3 | -- | -- |
| Weight loss | 4.8 | -- | -- |
Response
Objective responses were assessed by the investigators at the treating institution using RECIST criteria; 17 were evaluable. The overall response rate was 17.6% (95% CI: 3.8 – 43.4%), including 1 (5.9%) complete response and 2 (11.8%) unconfirmed partial responses. All responders had squamous cell histology. Three patients (17.6%) exhibited stable disease, 4 (23.5%) had progressive disease, and 6 (35.3%) with inadequate assessment are considered nonresponders. These results are summarized in Table 3.
Table 3.
Best objective response by RECIST criteria (n = 17 measurable)
| Total | ||
|---|---|---|
| Complete response | 1 | 6% |
| Unconfirmed partial response | 2 | 12% |
| Stable/no response | 3 | 18% |
| Progressive disease | 4 | 24% |
| Symptomatic deterioration | 1 | 6% |
| Assessment inadequate | 6 | 35% |
| Total | 17 | 100% |
Survival
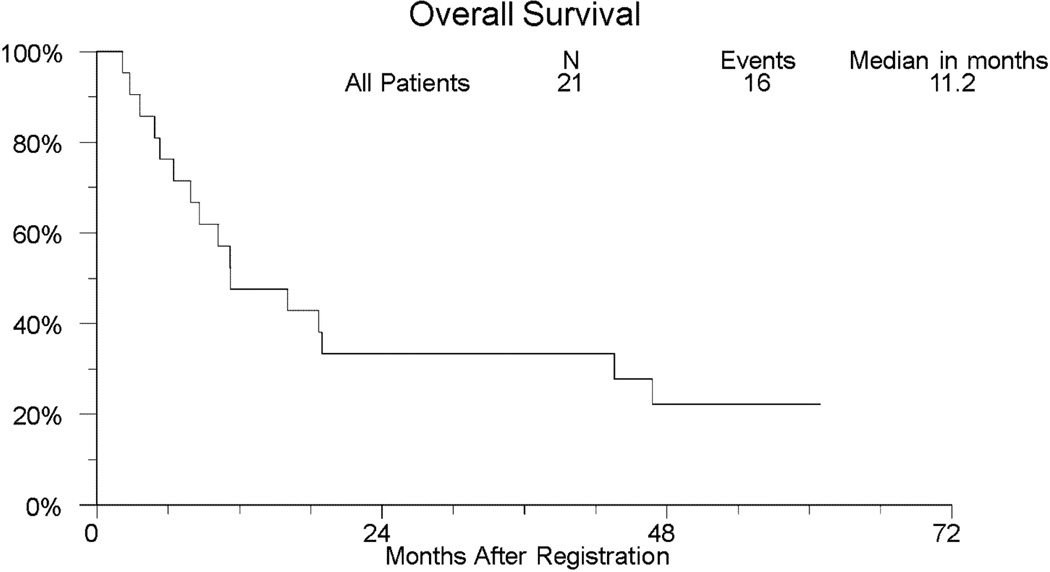
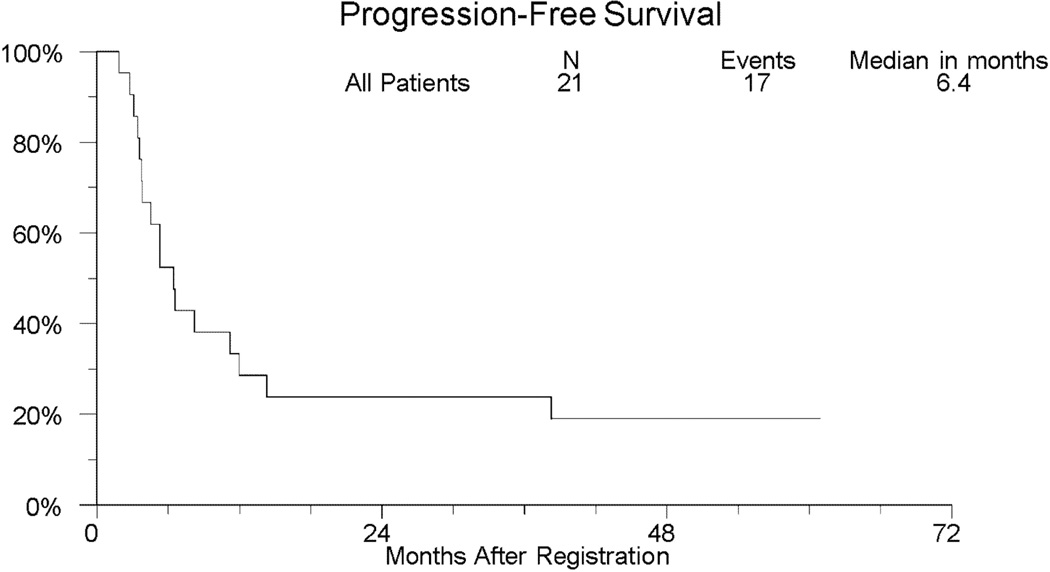
With over 3.5 years of followup among those last known alive, the median (95% CI) overall survival was 11.2 months (6.4 – 43.6 months). The median (95% CI) progression-free survival was 6.4 months (range 3.7 – 12.0 months). Two-year overall and progression-free survival is 33.3% (95% CI: 14.6% – 57.0%) and 23.8% (95% CI: 8.2% – 47.2%), respectively. Two-year OS (95% CI) within the 11 patients with squamous cell histology and the 10 patients with adenocarcinoma is 45.5% (16.0%–74.9%) and 20.0% (0.0%–44.8%), respectively. Kaplan-Meier curves for overall and progression-free survival for the entire study population are shown in Figures 1 and 2, respectively.
Fig 1.
Kaplan-Meier Curve of Overall Survival (OS) in SWOG S0414
Fig 2.
Kaplan-Meier Curve of Progression-Free Survival (PFS) in SWOG S0414
DISCUSSION
The long-term results of RTOG 85-01 suggest that one of four good performance patients with locally advanced esophageal cancer, who are able to successfully receive concomitant chemoradiotherapy will be alive at the five-year mark.3 Patients with clinically T4 primary esophageal cancers often have suboptimal performance status, in part, due to the symptoms consistent with invasion of adjacent structures,32 althought preoperative combined modality approaches have been reported with variable success.33,34
Building upon platform of RTOG 85-01, investigators have attempted to both increase the intensity of treatment by adding surgery (trimodality) often following preoperative combined modality therapy, as well as adding full-dose systemic therapy. Induction strategic approaches allows both the opportunity of administering full-dose systemic therapy as well as serving as in in-vivo assessment of response with novel systemic therapeutics prior the adminsitration of a concomitant chemoradiotherapy platform. Several investigators have successfully tested induction systemic combination chemotherapy and/or molecular targeted therapeutics, prior to concomitant chemotherapy.35
One of the combination cytotoxic chemotherapy regimens upon which the present study was build, included irinotecan and cisplatin. This doublet yielded encouraging objective responses, including some clincal complete responses, in patients with unresectable or metastatic esophageal cancer.12,13 Furthermore, a phase 1 trial of weekly irinotecan and cisplatin plus concomitant thoracic radiotherapy (TRT), from an experienced group of investigators noted an acceptable toxicity profile along with encouraging observations of symptomatic improvement and objective responses.21 A phase II trial by the MD Anderson Cancer Center group modified this regimen by first administering induction irinotecan and cisplatin for 1–2 cycles followed by chemoradiotherapy using a 5-fluorouracil (5-FU), paclitaxel, TRT backbone prior to surgery for patients with clinically resectable disease.36 More recently, the Spanish Cooperative Group for Digestive Tumor Therapy’s phase II trial which included induction irinotecan and cisplatin followed by concomitant chemoradiotherapy with the same two drugs prior to surgical resection, noted acceptable toxicity and modest activity compared to the historical published experience of cisplatin, 5-FU, and TRT in patients with clinicall y resectable disease.37 When this group and others tested this regimen in patients with clinically unresectable disease, the results were even less favorable.22,38
Over the past decade, there has been signifiant progress in characterizing mechanisms of tumor growth that result from altered regulation of various parts of the signal transduction pathway. At the turn of the century, the epidermal growth factor receptor (EGFR), a member of the ErbB family of growth factor receptor tyrosine kinases, was reported to be overexpressed in a number of epithelial tumors of the upper aerodigestive tract, including esophageal cancer, hence suggesting a target that could used to make progress in this difficult to treat solid tumor.39,40 Midway during the decade, the concomitant administration of the chimeric monocloncal antibody against the EGFR, cetuximab, and ionizing radiation, was shown to yield a superior survival outcome in patients with head and neck cancer.23,24
Based on encouraging pre-clinical and clinical data cetuximab and ionizing radiation as well as the phase I/II results of irinotecan-cisplatin and TRT, in 2004 SWOG, a federally funded cancer research group, designed a phase II clinical trial for esophageal cancer patients. SWOG-0414 is the first U.S. multi-institutional, prospective, cooperative group trial incorporating the novel combination of cetuximab, cisplatin and irinotecan in patients with locally advanced or unresectable esophageal cancer. This regimen was poorly tolerated, with 38% of patients experiencing Grade 4 or 5 toxicities and a treatment-related mortality approaching 10%. The objective response rate was disappointing, with only 1 CR and 2 unconfirmed PRs to therapy, all among patients with squamous cell histology.
No patients in this study experienced Grade 3 or higher pulmonary toxicity. This is in contrast to the recently reported results of ECOG 2205, a Phase II study examining the addition of cetuximab to concurrent oxaliplatin, 5-FU, and 45 Gy TRT delivered as neoadjuvant therapy prior to surgery for patients with resectable adenocarcinoma of the esophagus.41 Despite a promising pathologic CR rate, 4/22 patients died from ARDS postoperatively, compared to no incidence of ARDS in ECOG 1201 without cetuximab. Other reports of concurrent chemoradiation for esophageal cancer using cetuximab have not shown significant pulmonary toxicity.42,43
This trial was closed due to slow accrual. A potential confounding factor is that SWOG sites were concurrently accruing to SWOG-0356, a protocol for resectable esophageal adenocarcinoma. Patients with technically respectable cT4 disease may have preferentially been enrolled on SWOG-0356, skewing the population to the medically unresectable patients for this trial. This could also explain the fact that the majority of patients enrolled on this trial had squamous tumors and the poor accrual for adenocarcinomas. Furthermore, we may have chosen a suboptimal doublet to combined with TRT, as others have reported superior tolerability with a carboplatin-paclitaxel backbone during concomitant therapy.33,39,44 Additionally, investigators have recently shown that the actual expression of EGFR may be low to non-existent for esophageal adenocarcinomas.45
Given the unfavorable results of this trial, we would not recommend further development of this particular regimen for patients with locally advanced or medically unresectable esophageal cancer. Several other studies in esophageal and non-small cell lung cancer have shown safe and effective combination of cetuximab and radiotherapy, and we believe that alternate cetuximab-based chemoradiotherapy regimens may hold promise for esophageal cancer.39,40,46 While recent reoprts of combining chemoradiotherapy with oral tyrosine kinase inhibitors against EGFR, such as erlotinib, demonstrate feasibility, we believe that the major symptom of dysphagia in esophageal cancer patients will likely make the patient compliance unreliable in a prospective, multi-institutional effort for this patient population.47,48 The RTOG is currently conducting a Phase III trial examining the addition of cetuximab to paclitaxel, cisplatin and TRT for unresectable esophageal cancer, and we would encourage enrollment to this pivotal trial. Additionally, the SCOPE1 trial in the UK is currently open to accrual.49 This multicenter Phase II/III study randomizes patients with locally advanced esophageal cancer to induction cisplatin and capecitabine followed by the same regimen concurrent with TRT (5,000 cGy in 200 cGy fractions) versus the investigational arm which simply adds cetuximab to the induction and concurrent chemotherapy of the control arm. The results of these two randomized trials will help to answer the question of the role of cetuximab in this patient population.
Acknowledgements
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926, CA52654, CA20319, CA63850, CA45807, CA45377, CA35090, CA35178, CA04915, CA35176, CA45808 and in part by Bristol-Myers Squibb, ImClone Systems, Inc., a wholly owned subsidiary of Eli Lilly and Company, and Response Genetics. Cetuximab was provided by an alliance between ImClone, wholly owned subsidiary of Eli Lilly Company and Bristol-Myers Squibb
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented, in part, at the 51st Annual Meeting of the American Society of Radiation Oncology, Chicago, 2009 and the 2010 Gastrointestinal Cancers Symposium, Orlando, 2010.
References
- 1.Jemal A, Siegel R, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Herskovic A, Martz K, A-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med. 1992;326:1593–1598. doi: 10.1056/NEJM199206113262403. [DOI] [PubMed] [Google Scholar]
- 3.Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01) JAMA. 1999;281:1623–1627. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 4.Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer. 2011 doi: 10.1002/cncr.26586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suntharalingam M, Moughan J, Coia LR, et al. The national practice for patients receiving radiation therapy for carcinoma of the esophagus: results of the 1996–1999 Patterns of Care Study. Int J Radiat Oncol Biol Phys. 2003;56:981–987. doi: 10.1016/s0360-3016(03)00256-6. [DOI] [PubMed] [Google Scholar]
- 6.Ilson DH. Oesophageal cancer: new developments in systemic therapy. Cancer Treat Rev. 2003;29:525–532. doi: 10.1016/s0305-7372(03)00104-x. [DOI] [PubMed] [Google Scholar]
- 7.Kawato Y, Aonuma M, Hirota Y, et al. Intracellular roles of SN-38, a metabolite of the camptothecin derivative CPT-11, in the antitumor effect of CPT-11. Cancer Res. 1991;51:4187–4191. [PubMed] [Google Scholar]
- 8.Blanke CD, Haller DG, Benson AB, et al. A phase II study of irinotecan with 5-fluorouracil and leucovorin in patients with previously untreated gastric adenocarcinoma. Ann Oncol. 2001;12:1575–1580. doi: 10.1023/a:1013129315036. [DOI] [PubMed] [Google Scholar]
- 9.Lin L-S, Hecht J. A phase II trial of irinotecan in patients with advanced adenocarcinoma of the gastroesophageal (GE) junction. Proc Am Soc Clin Oncol. 2000;19:289a. abstract no. 1130. [Google Scholar]
- 10.Enzinger PC, Kulke MH, Clark JW, et al. A phase II trial of irinotecan in patients with previously untreated advanced esophageal and gastric adenocarcinoma. Dig Dis Sci. 2005;50:2218–2223. doi: 10.1007/s10620-005-3038-2. [DOI] [PubMed] [Google Scholar]
- 11.Findlay MPN, Ackland S, Gebski V, et al. Phase II study of irinotecan, leucovorin, and 5-FU (ILF) in advanced gastric cancer. Proc Am Soc Clin Oncol. 2001;20:165a. abstract no. 655. [Google Scholar]
- 12.Pozzo C, Barone C, Szanto J, et al. Irinotecan in combination with 5-fluorouracil and folinic acid or with cisplatin in patients with advanced gastric or esophageal-gastric junction adenocarcinoma: results of a randomized phase II study. Ann Oncol. 2004;15:1773–1781. doi: 10.1093/annonc/mdh473. [DOI] [PubMed] [Google Scholar]
- 13.Ilson DH, Saltz L, Enzinger P, et al. Phase II trial of weekly irinotecan plus cisplatin in advanced esophageal cancer. J Clin Oncol. 1999;17:3270–3275. doi: 10.1200/JCO.1999.17.10.3270. [DOI] [PubMed] [Google Scholar]
- 14.Ajani JA, Baker J, Pisters PW, et al. CPT-11 plus cisplatin in patients with advanced, untreated gastric or gastroesophageal junction carcinoma: results of a phase II study. Cancer. 2002;94:641–646. doi: 10.1002/cncr.10279. [DOI] [PubMed] [Google Scholar]
- 15.Gold PJ, Carter G, Livingston R. Phase II trial of irinotecan (CPT-11) and mitomycin-C (MMC) in the treatment of metastatic esophageal and gastric cancers. Proc Am Soc Clin Oncol. 2001;20:162a. abstract no. 644. [Google Scholar]
- 16.Lamond JP, Wang M, Kinsella TJ, et al. Concentration and timing dependence of lethality enhancement between topotecan, a topoisomerase I inhibitor, and ionizing radiation. Int J Radiat Oncol Biol Phys. 1996;36:361–368. doi: 10.1016/s0360-3016(96)00328-8. [DOI] [PubMed] [Google Scholar]
- 17.Roffler SR, Chan J, Yeh MY. Potentiation of radioimmunotherapy by inhibition of topoisomerase I. Cancer Res. 1994;54:1276–1285. [PubMed] [Google Scholar]
- 18.Wang DS, Ueno Y, Oyamade H, et al. Enhancement of the antitumor effect of gamma-ray irradiation in combination with camptothecin against human colorectal adenocarcinoma. Biol Pharm Bull. 1996;19:354–359. doi: 10.1248/bpb.19.354. [DOI] [PubMed] [Google Scholar]
- 19.Kirichenko AV, Rich TA, Newman RA, et al. Potentiation of murine MCA-4 carcinoma radioresponse by 9-amino-20(S)-camptothecin. Cancer Res. 1997;57:1929–1933. [PubMed] [Google Scholar]
- 20.Omura M, Torigoe S, Kubota N. SN-38, a metabolite of the camptothecin derivative CPT-11, potentiates the cytotoxic effects of radiation in human colon adenocarcinoma cells grown as spheroids. Radioth Oncol. 1997;43:197–201. doi: 10.1016/s0167-8140(97)01924-5. [DOI] [PubMed] [Google Scholar]
- 21.Blumenschein G, Ajani J, Fairweather J, et al. Phase I study of CPT-11 plus radiotherapy in patients with locally advanced upper GI carcinomas. Proc Am Soc Clin Oncol. 2000;19:322a. abstract no. 1274. [Google Scholar]
- 22.Ilson DH, Bains M, Kelsen DP, et al. Phase I trial of escalating-dose irinotecan given weekly with cisplatin and concurrent radiotherapy in locally advanced esophageal cancer. J Clin Oncol. 2003;21:2926–2932. doi: 10.1200/JCO.2003.02.147. [DOI] [PubMed] [Google Scholar]
- 23.Ilson DH, Minsky BD, Ku GY, et al. Phase 2 trial of induction and concurrent chemoradiotherapy with weekly irinotecan and cisplatin followed by surgery for esophageal cancer. Cancer. 2011 Oct 11; doi: 10.1002/cncr.26591. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 24.Watkins JM, Zauls AJ, Kearney PL, et al. Toxicity, response rates and survival outcomes of induction cisplatin and irinotecan followed by concurrent cisplatin, irinotecan and radiotherapy for locally advanced esophageal cancer. Jpn J Clin Oncol. 2011;41:334–342. doi: 10.1093/jjco/hyq208. [DOI] [PubMed] [Google Scholar]
- 25.Raben D, Helfrich B, Chan DC, et al. The effects of cetuximab alone and in combination with radiation and/or chemotherapy in lung cancer. Clin Cancer Res. 2005;11:795–805. [PubMed] [Google Scholar]
- 26.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. New Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 27.Baselga J, Trigo JM, Bourhis J, et al. Phase II multicenter study of the antiepidermal growth factor receptor monoclonal antibody cetuximab in combination with platinum-based chemotherapy in patients with platinum-refractory metastatic and/or recurrent squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5568–5577. doi: 10.1200/JCO.2005.07.119. [DOI] [PubMed] [Google Scholar]
- 28.Herbst RS, Arquette M, Shin DM, et al. Phase II multicenter study of the epidermal growth factor receptor antibody cetuximab and cisplatin for recurrent and refractory squamous cell carcinoma of the head and neck. J Clin Oncol. 2005;23:5578–5587. doi: 10.1200/JCO.2005.07.120. [DOI] [PubMed] [Google Scholar]
- 29.Lim R, Sun Y, Hsieh RK, et al. Cetuximab plus irinotecan in pretreated metastatic colorectal cancer patients: the ELSIE study. World J Gastroenterol. 2011;17:1879–1888. doi: 10.3748/wjg.v17.i14.1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Custem E, Kohne CH, Lang I, et al. Cetuximab Plus Irinotecan, Fluorouracil, and Leucovorin As First-Line Treatment for Metastatic Colorectal Cancer: Updated Analysis of Overall Survival According to Tumor KRAS and BRAF Mutation Status. J Clin Oncol. 2011;29:2011–2019. doi: 10.1200/JCO.2010.33.5091. [DOI] [PubMed] [Google Scholar]
- 31.Liu X, Guo W-J, Z X-W. Cetuximab enhances the activities of irinotecan on gastric cancer cell lines through downregulating the EGFR pathway upregulated by irinotecan. Cancer Chemother Pharmacol. 2011;68:871–878. doi: 10.1007/s00280-011-1559-2. [DOI] [PubMed] [Google Scholar]
- 32.Seto Y, Chin K, Gomi K, et al. Treatment of thoracic esophageal carcionma invading adjacent structures. Cancer Sci. 2007;98:937–942. doi: 10.1111/j.1349-7006.2007.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujita H, Sueyoshi S, Tanaka T, et al. Prospective non-randomized trial comparing esophagectomy-followed-by-chemoradiotherapy versus chemoradiotherapy-followed-by-esophagectomy for T4 esophageal cancers. J Surg Oncol. 2005;90:209–219. doi: 10.1002/jso.20259. [DOI] [PubMed] [Google Scholar]
- 34.Koike R, Nishimura Y, Nakamatsu K, et al. Concurrent chemoradiotherapy for esophageal cancer with malignant fistula. Int J Radiat Oncol Biol Phys. 2008;70:1418–1422. doi: 10.1016/j.ijrobp.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 35.Ruhstaller T, Widmer L, Schuller JC, et al. Multicenter phase II trial of preoperative induction chemotherapy followed by chemoradiation with docetaxel and cisplatin for locally advanced esophageal carcinoma (SAKK 75/02) Ann Oncol. 2009;20:1522–1528. doi: 10.1093/annonc/mdp045. [DOI] [PubMed] [Google Scholar]
- 36.Ajani JA, Walsh G, Komaki R, et al. Preoperative induction of CPT-11 and cisplatin chemotherapy followed by chemoradiotherapy in patients with locoregional carcinoma of the esophagus or gastroesophageal junction. Cancer. 2004;100:2347–2354. doi: 10.1002/cncr.20284. [DOI] [PubMed] [Google Scholar]
- 37.Rivera F, Galan M, Tabernero J, et al. Phase II trial of preoperative irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for resectable locally advanced gastric and esophagogastric junction adenocarcinoma. Int J Radiat Oncol Biol Phys. 2009;75:1430–1436. doi: 10.1016/j.ijrobp.2008.12.087. [DOI] [PubMed] [Google Scholar]
- 38.Rivera F, Galan M, Tabernero J, et al. Phase II trial of induction irinotecan-cisplatin followed by concurrent irinotecan-cisplatin and radiotherapy for unresectable, locally advanced gastric and oesophageal-gastric junction adenocarcinoma. Cancer Chemother Pharmacol. 2011;67:75–82. doi: 10.1007/s00280-010-1285-1. [DOI] [PubMed] [Google Scholar]
- 39.Morgan S, Grandis JR. ErbB receptors in the biology and pathology of the aerodigestive tract. Exper Cell Res. 2009;315:572–582. doi: 10.1016/j.yexcr.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schrump DS, Nguyen DM. Novel molecular targeted therapy for esophageal cancer. J Surg Oncol. 2005;92:257–261. doi: 10.1002/jso.20367. [DOI] [PubMed] [Google Scholar]
- 41.Kleinberg LR, Catalano PJ, Gibson MK, et al. ECOG 2205: A phase II study to measure response rate and toxicity of neo-adjuvant chemoradiotherapy (CRT) (IMRT permitted) with oxaliplatin (O) and infusional 5-fluorouracil (5-FU) plus cetuximab (C225) in patients with operable adenocarcinoma of the esophagus: high risk of post-op adult respiratory distress syndreome (ARDS) Int J Radiat Oncol Biol Phys. 2010;78:S72. abstract no. 154. [Google Scholar]
- 42.Safran H, Suntharalingam M, Dipetrillo T, et al. Cetuximab with concurrent chemoradiation for esophagogastric cancer: assessment of toxicity. Int J Radiat Oncol Biol Phys. 2008;70:391–395. doi: 10.1016/j.ijrobp.2007.07.2325. [DOI] [PubMed] [Google Scholar]
- 43.Ruhstaller T, Pless M, Dietrich D, et al. Cetuximab in combination with chemoradiotherapy before surgery in patients with resectable, locally advanced esophageal carcinoma: A prospective, multicenter phase Ib/II trial (SAKK 75/06) J Clin Oncol. 2011;29:626–631. doi: 10.1200/JCO.2010.31.9715. [DOI] [PubMed] [Google Scholar]
- 44.Ruppert BN, Watkins JM, Shirai K, et al. Cisplatin/irinotecan versus carboplatin/paclitaxel as definitive chemoradiotherapy for locoregionally advanced esophageal cancer. Am J Clin Oncol. 2010;33:346–352. doi: 10.1097/COC.0b013e3181aaca26. [DOI] [PubMed] [Google Scholar]
- 45.Harper N, Li Y, Farmer R, et al. Epidermal growth factor expression in esophageal adenocarcinoma: a clinically relevant target? J Gastrointest Surg. 2011 doi: 10.1007/s11605-011-1778-1. [DOI] [PubMed] [Google Scholar]
- 46.DeVita F, Orditura M, Martinelli E, et al. A multicenter phase II study of induction chemotherapy with FOLFOX-4 and cetuximab followed by radiation and cetuximab followed by radiation and cetuximab in locally advanced oesophageal cancer. Brit J Cancer. 2011;104:427–432. doi: 10.1038/sj.bjc.6606093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li G, Hu W, Wang J, et al. Phase II study of concurrent chemoradiation in combination with erlotinib for locally advanced esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2010;78:1407–1412. doi: 10.1016/j.ijrobp.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 48.Dobelbower MC, Russo SM, Raisch KP, et al. Epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib, and concurrent 5-fluorouracil, cisplatin and radiotherapy for patients with esophageal cancer: a phase I study. Anti-Cancer Drugs. 2006;17:95–102. doi: 10.1097/01.cad.0000185178.26862.4c. [DOI] [PubMed] [Google Scholar]
- 49.Hurt CN, Nixon LS, Griffiths GO, et al. SCOPE1: a randomised phase II/III multicentre clinical trial of definitive chemoradiation, with or without cetuximab, in carcinoma of the oesophagus. BMC Cancer. 2011;11:466–476. doi: 10.1186/1471-2407-11-466. [DOI] [PMC free article] [PubMed] [Google Scholar]