Abstract
γδ T cells (γδT) belong to a distinct T cell lineage that performs immune functions different from αβ T cells (αβT). Previous studies have established that Erk1/2 MAPKs are critical for positive selection of αβT cells. Additional evidence also suggests that elevated Erk1/2 activity promotes γδT cell generation. RasGRP1, a guanine nucleotide releasing factor for Ras, plays an important role in positive selection of αβT cells by activating the Ras-Erk1/2 pathway. In this report, we demonstrate that RasGRP1 is critical for TCR-induced Erk1/2 activation in γδT cells but exerts different roles for γδT cell generation and activation. Deficiency of RasGRP1 does not obviously affect γδT cell numbers in the thymus but leads to increased γδT cells, particularly CD4−CD8+ γδT cells, in the peripheral lymphoid organs. The virtually unhindered γδT cell development in the RasGRP1−/− thymus proved to be cell intrinsic, while the increase in CD8+ γδT cells is caused by non-cell-intrinsic mechanisms. Our data provides genetic evidence that decreased Erk1/2 activation in the absence of RasGRP1 is compatible for γδT cell generation. Although RasGRP1 is dispensable for γδT cell generation, RasGRP1-deficient γδT cells are defective in proliferation following TCR stimulation. Additionally, RasGRP1-deficient γδT cells are impaired to produce IL-17 but not IFNγ. Together, these observations have revealed that RasGRP1 plays differential roles for γδ and αβ T cell development but is critical for γδT cell proliferation and production of IL-17.
Introduction
Two lineages of T cells marked by the expression of two distinct antigen receptors, αβ and γδ T cell receptors (TCRs), are generated during intrathymic development. T cell development in the thymus can be divided into CD4−CD8− (double negative, DN), CD4+CD8+ (double positive, DP), and CD4+CD8− or CD4−CD8+ (single positive, SP) stages. DN thymocytes contain the most immature T cells and can be further divided from DN1 to DN4 based on CD25 and CD44 or cKit expression (1). Functional TCRs must be generated through somatic V(D)J recombination in the TCR loci for generation of either αβ T cells (αβT) or γδ T cells (γδT) (2). V(D)J recombination in the TCR loci is tightly regulated in a developmental-stage-specific manner. At the DN 2 and 3 stages, TCRγ, δ, and β loci rearrange. Formation of functional γδTCR directs progenitor cells to the γδ lineage (3). TCRβ associates with the pre-TCRα chain to form the pre-TCR, which drives DN thymocyte maturation to the DP stage and full commitment to the αβT cell lineage (4). DN2 thymocytes are mostly committed to the T cell lineage. γδT lineage commitment mainly occurs at the DN2 stage but can also happen at the DN3 stage (5). At the DP stage, the TCRα gene rearranges and in-frame rearranged α gene produces a functional chain to associate with the TCRβ chain to drive DP thymocytes to mature to the SP stage (6). In normal thymus and peripheral lymphoid organs, γδT is the minor lineage while αβT is the dominant lineage. Most γδT cells reside in the DN population, and γδT cells expressing CD4 or CD8 co-receptor are rare.
It has been well documented that expression of a functional γδTCR or αβTCR in developing thymocytes is essential for the generation of their respective T cell lineages. Defects in formation of a functional γδTCR or αβTCR can cause a complete absence of γδ or αβ T cell lineage, respectively (3, 7, 8). Our knowledge of TCR signal transduction has primarily come from studies on the αβTCR, as γδT cells are rare. It is well known that TCR stimulation leads to the activation of PLCγ1 via orchestrated actions of proximal protein kinases such as Lck, Zap70, and Itk, and adaptor molecules such as SLP-76 and LAT (9-12). Activated PLCγ1 produces two critical second messengers, diacylglycerol (DAG) and inositol-1,4,5-trisphosphate (IP3), that are crucial for relaying proximal signaling to the activation of distal signaling cascades (13). IP3 binds to its receptor in ER, leading to depletion of calcium from ER and subsequent calcium influx through the CRAC channel, which leads to the activation of the calcineurin-NFAT pathway (14). DAG associates with multiple effector molecules, including the RasGRP1, PKCθ, and PKDs, to induce the activation of downstream signaling cascades such as the RasGRP1-Ras-Erk1/2 and PKCθ-CARM1/Bcl10-IKK-NFκB pathways (13, 15, 16).
Evidence suggests that αβTCR and γδTCR signaling share at least some common features. Deficiency of some proximal signaling molecules such as Lck, SLP76, and LAT impacts both αβT cell and γδT cell generation (17). Additionally, expression of an αβTCR at early DN stages in transgenic mice can drive thymocytes to adopt the γδ fate even though they express the αβTCR, suggesting that the timing of γδTCR versus αβTCR signaling rather than the quality of the signaling plays an important role in γδ versus αβ lineage fate decision (18). While similarities between αβ and γδ TCRs exist, differences between them have also been reported. Murine αβTCR but not γδTCR contains the CD3δ chain (19, 20). The threshold of γδTCR signaling appears lower than αβTCR. Under the same stimulating condition, γδTCR induces stronger Erk1/2 activation than αβTCR. Furthermore, strong TCR signaling and Erk1/2 activation has been reported to promote γδ differentiation (19, 21-24). However, substantial genetic evidence demonstrating the requirement of Erk1/2 for γδT cell lineage development is lacking.
RasGRP1, a guanine nucleotide exchange factor for Ras, is critical for the activation of the Ras-Erk1/2 pathway in αβT cells (25, 26). RasGRP1 promotes positive selection of conventional αβT cells, particularly those expressing TCR with low affinity to self-peptide-MHC complex (27). We have recently found that RasGRP1 also promotes the development of the invariant NKT cells (28). However, positive selection of thymocytes with relative high affinity to self-peptide-MHC complex, such as regulatory T cells, is less dependent on RasGRP1 (29). While the importance of RasGRP1 in αβT cells is becoming clear, whether RasGRP1 functions as the upstream activator for Erk1/2 during TCRγδ signaling and its importance for γδT development and activation are not well understood. In this report, we demonstrate that RasGRP1 is important for TCR-induced Erk1/2 activation in γδT cells but is dispensable for γδT generation. Deficiency of RasGRP1 does not obviously affect γδT cell numbers in the thymus but leads to increased γδT cells, particularly CD4−CD8+ γδT cells, in the peripheral lymphoid organs. Although RasGRP1 is not required for γδT cell ontogeny, it is critical for TCR-induced γδT cell proliferation and production of IL-17.
Materials and methods
Mice
The C57BL6/J mice were purchased from the Jackson Laboratory. The RasGRP1−/− mice were previously described (25) and were backcrossed onto B6 background for 9 generations. All mice used were between 6 and 8 weeks of age according to a protocol approved by the Duke University Institute Animal Care and Use Committee. Thymocytes, splenocytes, and lymph node cells were prepared following standard procedures.
Antibodies and flow cytometry
Cells were stained with antibodies in PBS containing 2% FCS. PE-Cy7-conjugated anti-CD4 (RM4-5), APC-Cy7-conjugated anti-CD8α (53-6.7), APC-conjugated anti-CD8β.2 (53-5.8), FITC- or APC-conjugated anti-TCRβ (H57-597), FITC- or PE-conjugated anti-TCRγδ (GL3), PE-conjugated anti-IL-17A (TC11-18H10), APC-conjugated anti-IFNγ (XMG1.2), PE/Cy7-conjugated anti-ICOS (C398.4A), PE-conjugated anti-CD5 (53-7.3), APC-conjugated anti-CD25 (PC61), FITC-conjugated anti-CD122 (TM-β1), Biotin-conjugated anti-NKG2D (C7), APC-conjugated anti-CD44 (IM7), Biotin-conjugated anti-OX40 (OX-86), Biotin-conjugated anti-CD27 (LG.3A10), APC-conjugated anti-CD90.1 (HIS51), and PE- or APC-conjugated anti-CD90.2 (30-H12) were purchased from Biolegend or BD Biosciences. Biotin-conjugated antibodies were stained with PE-, PE-Cy5-, or APC-conjugated streptavidin. For intracellular staining, cells were permeabilized using the Foxp3 staining kit (eBioscience) after cell surface staining, followed by APC-conjugated anti-TCRβ or unconjugated anti-Ki-67 (B56, BD Biosciences), anti-IL-17A, or anti-IFNγ. An Alexa Fluor® 488-conjugated goat anti-mouse IgG (H+L) (Invitrogen) was used to detect anti-Ki67 antibodies. Anti-TCRVγ1.1 (clone 2.11) and - TCRVδ6.3 (clone 9D3) antibodies were previously reported (30). Cell death was identified using a 7AAD or Live/Dead® fixable dead cell stain kit (Invitrogen). Pacific Blue- or APC-conjugated Annexin V (BD Biosciences) was used to detect apoptotic cells. Data of stained samples were collected using a Canto II flow-cytometer (BD Biosciences) and analyzed using FlowJo software (TreeStar, Inc.).
Bone marrow reconstitution
WT C57BL/6J mice were lethally irradiated (1100 rad) four hours before adoptive transfer. Bone marrow cells from age- and sex-matched Thy1.1+ B6 and Thy1.2+ RasGRP1−/− were mixed at a 1:1 ratio. Ten to twenty million mixed cells were then intravenously injected into each recipient mouse. The resulting chimeric mice were analyzed 7 to 8 weeks later.
Western blot
Total γδT cells were isolated from WT and RasGRP1−/− thymocytes, splenocytes, and LN cells using MACS purification and then FACS sorting using a MoFlo Cell Sorter (Beckman Coulter), with post-sort purity >98%. Thymic and splenic γδT cells were also similarly purified from these mice. Four hundred thousand sorted γδT cells were rested in 0.5ml PBS at 37°C for 30 minutes. Cells were then either left untreated or stimulated with an anti-CD3ε antibody (500A2, 5μg/ml, BD Biosciences) for 5 minutes. The 500A2 antibody is capable of inducing TCR signaling without cross-linking by a secondary antibody. Cells were lysed in 1% NP-40 lysis buffer (1% NP-40, 150mM NaCl, 50mM Tris, pH 7.4) with protease and phosphatase inhibitors. Proteins in lysates were subjected to Western blot analysis with anti-phospho-Erk1/2, total Erk1/2 antibodies (Cell Signaling Technology), and an anti-RasGRP1 antibody (Santa Cruz Biotechnology). For loading control, the blots were stripped and reprobed with anti-β-actin (Sigma).
γδ T cell proliferation and cytokine production
Sorted WT and RasGRP1−/− γδT were labeled with 10 μM CFSE at room temperature for 9 minutes as previously described (31). Cells were seeded at 5 ×105 cells/well in a 48-well plate precoated with PBS or with 10 μg/ml soluble anti-CD3ε (2C11). After incubation at 37°C for 72 hours, cells were stained for death with Live/Dead® fixable staining before being analyzed by flow cytometry.
For cytokine production, 2 × 106 freshly isolated thymocytes, splenocytes, and LN cells were seeded in each well in a 48 well-plate. The cells were left unstimulated or stimulated with PMA (25 ng/ml) and ionomycin (500 ng/ml) in the presence of a Golgi Plug for 5h. Cells were then surface stained for TCRγδ, TCRβ, CD4, CD8, CD27, and CD44, followed by intracellular staining for IFNγ and IL-17A.
Real-time PCR
Total RNAs were extracted from sorted cells and cDNAs were obtained using the Superscript III First-Strand Synthesis System (Invitrogen). Real-time PCR was prepared using the RealMasterMix (Eppendorf) and performed on the Mastercycler®Ep realplex2 system (Eppendorf). Primers for RasGRPs and actin were published previously (25) or are listed as follows: Egr1: forward, 5′-gtccttttctgacatcgctctga-3′ and reverse 5′-cgagtcgtttggctgggata-3′; Egr2: forward, 5′-ttgaccagatgaacggagtg-3′ and reverse 5′-cagagatgggagcgaagcta-3′; Id3: forward, 5′-cacttaccctgaactcaacgcc-3′ and reverse 5′-cccattctcggaaaagccag-3′. Relative mRNA expression was normalized with β-actin and was presented as an arbitrary unit (a.u.) of fold change using the 2−ΔΔCT method.
Statistics
For statistical analysis, two-tail Student t-tests were performed: *, p<0.05. **, p<0.01, ***, p<0.001.
Results
RasGRP1 is dispensable for intrathymic γδT cell development
We first examined mRNA levels of RasGRP1 and other RasGRP family members in γδT cells since it has been unclear whether these molecules are expressed in these cells. RasGRP1, 2, and 4 mRNAs could be detected in both γδT cells and αβT cells sorted from the thymus and spleens (Figures 1A and 1B). αβT cells appeared to express higher levels of RasGRP1 and RasGRP2 than γδT cells. The differences were particularly obvious in the peripheral lymphoid organs. In contrast, expression of RasGRP4 was similar between αβT and γδT cells. RasGRP3 was undetectable in either γδT cells or αβT cells.
Figure 1. RasGRP1 is dispensable for γδ T cell development in the thymus.
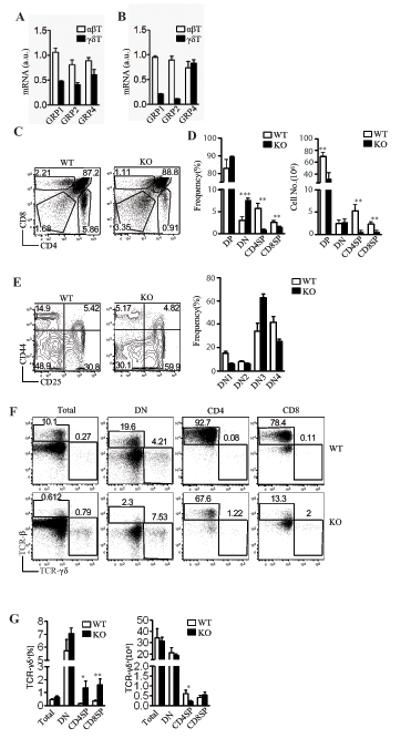
(A, B) Expression of RasGRPs in αβT and γδT cells. mRNA levels of RasGRPs from sorted αβT and γδT cells from thymus (A) and spleen (B) were determined by real-time qPCR. (C) CD4 and CD8 staining of total thymocytes from WT and RasGRP1−/− mice. (D) Decrease of DP and SP thymocytes in RasGRP1−/− mice. Data shown are mean ± SEM. (E) Inefficient DN3 to DN4 transition in the absence of RasGRP1−/−. Representative FACS plots of CD25 and CD44 expression in gated CD4−CD8− DN thymocytes are shown. (F) Representative FACS plots of TCRγδ and TCRβ staining of the indicated thymocytes. Total thymocytes as well as gated DN and SP cells are shown. (G) Percentages (left panel) and absolute number (right panel) of γδT cells within total, CD4SP, CD8SP, and DN thymocytes in WT and RasGRP1−/− mice. Mice used in the study were 6-8 weeks of age. Data shown are representative of at least three experiments. *P<0.05, **P<0.01, ***P<0.001 determined by the Student t-test.
To determine the role of RasGRP1 in γδT cells, we analyzed RasGRP1−/− mice. As previously reported (21, 24), CD4+CD8− and CD4−CD8+ single-positive (SP) thymocytes were markedly decreased in RasGRP1−/− (KO) mice as compared to WT mice (Figures 1C and 1D). In addition, we also found that the CD4+CD8+ double positive (DP) thymocyte number as well as total thymic cellularity of RasGRP1−/− mice was decreased about 50% (Figure 1D). Further analysis of DN thymocytes revealed an increase in CD25+CD44− DN3 cells and a decrease in CD25−CD44− DN4 cells in RasGRP1−/− mice (Figure 1E). This data suggested RasGRP1 deficiency resulted in a partial blockage of DN3 to DN4 transition, a process called β-selection due to its requirement of the pre-TCR signal. Thus, besides promoting positive selection, RasGRP1 may also be involved in pre-TCR signaling and plays a role for β-selection.
In contrast to the striking decrease in αβT cells and DP thymocytes, the overall percentage of γδT cells in the RasGRP1−/− thymus was increased about twofold as compared to WT controls (Figures 1F and 1G). Although rare, the relative ratio of γδT cells in the CD4 or CD8 SP population was also increased. The total thymic as well as DN and CD8+ γδT cell subsets in RasGRP1−/− mice were similar to WT mice. However, the CD4+ γδT cell number was slightly decreased in RasGRP1−/− mice. Thus, although RasGRP1 is critical for DP to SP maturation and is important for efficient DN3 to DN4 transition, it is virtually dispensable for intrathymic γδT cell development.
Enrichment of γδT in the peripheral lymphoid organs in RasGRP1−/− mice
As previously reported (25, 27), CD4+ and CD8+ T cell percentages and numbers in the spleen and lymph nodes (LNs) were decreased in RasGRP1−/− mice as compared to WT mice (Figure 2A). Different from the decrease in αβT cells, the percentages and absolute numbers of the overall γδT cells did not decrease and in fact even increased in the spleen (Figure 2B, 2D) and LNs (Figure 2C, 2E) of RasGRP1−/− mice. The percentages of splenic total and DN γδT cells were slightly increased in the spleen but more substantially increased in the LNs in RasGRP1−/− mice. The total and DN γδT cell numbers in the WT and RasGRP1−/− spleens were similar but were increased two- to fourfold in RasGRP1−/− LNs. The reason for the selective increase of γδT cells in the LNs but not in the spleen is unclear but could be caused by altered expression of chemokine receptors or homing molecules such as CD62L in the absence of RasGRP1. The total CD4SP γδT cells were slightly decreased in both RasGRP1−/− spleens and LNs. However, CD8SP γδT cells were drastically increased both in percentages and absolute numbers in both RasGRP1−/− spleens and LNs. While CD8αSP γδT cells accounted for around 0.5% of CD8SP T cells in WT mice, up to 40% LN and 20% splenic CD8SP cells from RasGRP1−/− mice were γδT cells. About 90% of CD8SP γδT cells in WT and RasGRP1−/− thymus and LN were CD8α+β+. In the spleen, CD8α+β+ cells accounted for 65% and 85% of WT and RasGRP1−/− CD8SP γδT cells, respectively (Figure 2F). As to be demonstrated in figure 7, the CD8+ γδT cells were capable of producing high levels of IFNγ. At present, it is unclear whether RasGRP1 is involved in regulating CD4/CD8 expression.
Figure 2. Enrichment of γδT cells in the peripheral lymphoid organs in RasGRP1−/− mice.
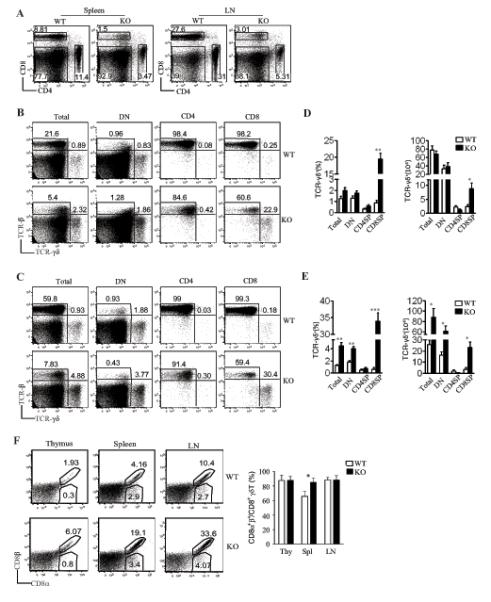
(A) Decreased CD4 and CD8 T cells in RasGRP1−/− spleen and LNs. Representative dot plots of CD4 and CD8 staining of live-gated cells are shown. (B, C) Representative dot plot for assessment of γδT and αβT cells in the spleens (B) and LNs (C) in RasGRP1−/− and WT mice. (D, E) Mean ± SEM presentation of percentages and absolute numbers of γδT cells in the spleens (D) and LNs (E). (F) Expression of CD8α and CD8β heterodimer in γδT cells. Representative dot plots of CD8α and CD8β expression on gated TCRβ−TCRγδ+ cells from WT and RasGRP1−/− thymus (Thy), spleen (spl), and LNs (LN). Bar graph shows mean ± SEM (n=3) of percentages of CD8α+β+ cells within the CD8α+ γδT cells. Data shown are representative of at least three experiments. *P<0.05, **P<0.01, ***P<0.001 determined by the Student t-test.
Figure 7. Defective IL-17 production by RasGRP1−/− γδT cells.
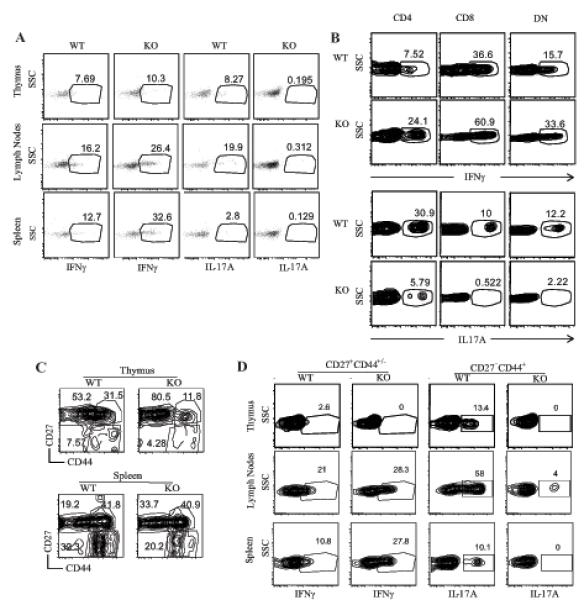
(A) WT and RasGRP1−/− thymocytes, splenocytes, and LN cells were stimulated with PMA and ionomycin in the presence of Golgi plug for 5 hours. Stimulated cells were cell surface stained for TCRβ and TCRγδ, followed by intracellular cytokine staining for IFNγ and IL17A. Dot plots show IL-17A and IFNγ expression in gated total TCRβ−TCRγδ+ cells. (B) IFNγ and IL-17A expression in CD4+, CD8α+, and DN γδT cells. LN cells were similarly treated and stained as in (A) with the addition of CD4 and CD8α staining. Contour-plots show IFNγ and IL-17A staining in gated WT and RasGRP1−/− CD4+, CD8α+, and DN γδT cells. (C) γδT cell subsets based on ex vivo CD27 and CD44 staining. Contour plots show CD27 and CD44 expression in gated TCRβ−TCRγδ+CD4− CD8− thymocytes and splenocytes from WT and RasGRP1KO mice. (C) Differential effects of RasGRP1 deficiency on IL-17A production by CD27−CD44+ γδT cells and IFNγ expression by CD27+CD44+/- γδT cells. Data shown represent three experiments.
Cell-intrinsic and -extrinsic mechanisms control γδ T cell development in RasGRP1−/− mice
Since the RasGRP1−/− mice we studied were germ-line knockout and the overall T cell lymphopenia could significantly affect γδT cell development, we investigated whether γδT cell development in RasGRP1−/− mice was caused by cell-intrinsic mechanisms. We generated and analyzed mixed bone marrow (BM) chimeric mice reconstituted with Thy1.1+ WT and Thy1.2+ RasGRP1−/− BM cells at a 1:1 ratio. Chimeric mice were analyzed 8 weeks after transfer. The ratio of Thy1.1+ WT to Thy1.2+ RasGRP1−/− CD4−CD8− DN thymocytes were about 2:1 (Figure 3A), suggesting that RasGRP1 may be involved in early T cell development. Within the DN population, Thy1.2+ RasGRP1−/− CD25+CD44− DN3 and CD25−CD44− DN4 cells were substantially increased and decreased, respectively, as compared to Thy1.1+ WT controls (Figure 3B), indicating that RasGRP1 is intrinsically important for efficient β-selection. These observations, together with those demonstrating critical roles of RasGRP1 for DP to SP maturation (25), indicate RasGRP1 plays crucial roles at multiple stages for αβT cell development in a cell-autonomous manner. Within TCRαβ+ T cells, most were Thy1.1+ WT and Thy1.2+ RasGRP1−/− cells were virtually undetectable (Figure 3C). However, the ratio of γδT cells originated from RasGRP1−/− to WT BM was similar to the ratio of total DN cells between these two origins. As mentioned earlier, CD8+ γδT cells were drastically increased in RasGRP1−/− mice, but such an increase was not observed in the chimeric mice (Figure 3D). Thus, the overall RasGRP1-independent generation of γδT cells in RasGRP1−/− mice was cell intrinsic. However, the expansion of CD8+ γδT cells in these mice was not cell intrinsic.
Figure 3. Contribution of cell-intrinsic and -extrinsic mechanisms for γδ T cell development in RasGRP1-deficient mice.
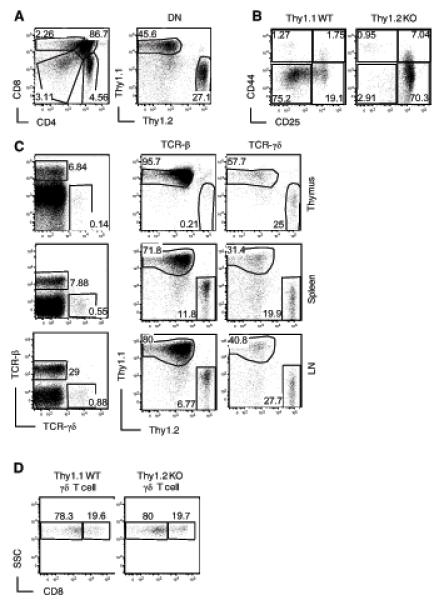
(A) Left panel, CD4 and CD8α staining on total live thymocytes from lethally irradiated recipient mice 8 weeks after reconstituted with WT (Thy1.1) and RasGRP1−/− (Thy1.2) BM. Right panels, Thy1.1 and Thy1.2 expression on gated CD4−CD8− DN thymocytes. (B) Impaired β-selection in the absence of RasGRP1. Dot plots show CD44 and CD25 staining on gated Thy1.1+ WT and Thy1.2+ RasGRP1−/− CD4−CD8− DN thymocytes from the recipient mice. (C) Left panel, TCRβ and TCRγδ staining of live-gated thymocytes from recipient mice. Middle and right panels, Thy1.1 and Thy1.2 staining on gated TCRβ+ and TCRγδ+ thymocytes, respectively. (D) CD8α staining of Thy1.1+ WT and Thy1.2+ RasGRP1−/− γδT cells in the chimeric mice. Data are representative of three experiments.
Differential effects of RasGRP1 deficiency on αβT and γδT cell homeostasis
The TCR-mediated Ras-Erk1/2 pathway plays a crucial role in T cell survival and proliferation. One possibility that may cause increased γδT cells in RasGRP1−/− mice would be that they have proliferative and/or survival advantage over αβT cells. Previous studies have demonstrated that RasGRP1 prevents conventional CD4+ T cells and CD8+ T cells as well as iNKT cells from apoptosis (28, 29). By staining freshly isolated cells with Annexin-V and 7-AAD, we also observed increased apoptosis of RasGRP1−/− αβT cells. However, no increase of apoptosis was observed in RasGRP1−/− γδT cells as compared with WT controls. In fact, slight decreases of apoptosis were observed in γδT cells from RasGRP1−/− spleen and LNs (Figure 4A).
Figure 4. Assessment of T cell survival and homeostatic proliferation.
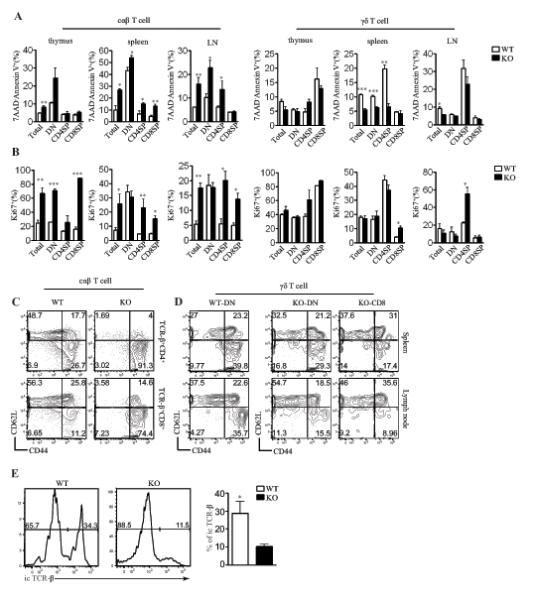
(A) Increased apoptosis of αβT but not γδT cells in the absence of RasGRP1. Freshly isolated thymocytes, splenocytes, and LN cells from WT and RasGRP1−/− mice were stained with anti-CD4, anti-CD8, anti-TCRβ, anti-TCRγδ, 7AAD, and annexin V. Bar figures show mean ± SEM of apoptotic cells within the indicated populations. (B) Increased Ki67+ αβT but not γδT cells in the absence of RasGRP1. Cells were similarly stained and analyzed as described in (A), except that Ki67 were determined by intracellular staining. (C, D) CD44 and CD62L expression on αβT (C) and γδT cells (D) from WT and RasGRP1−/− mice. Data from WT CD8+ γδT cells was not presented since they were rare. (E) Decreased intracellular TCRβ+ cells within the γδT cells in the absence of RasGRP1. Histograms show intracellular staining of TCRβ in gated γδT cells from WT and RasGRP1−/− thymus. Data shown represent three experiments. *P<0.05, **P<0.01, ***P<0.001 determined by the Student t-test.
In RasGRP1−/− mice, the total peripheral αβT cell numbers are decreased. Under such a lymphopenic condition, T cells may undergo homeostatic proliferation. Expression of nuclear Ki67, a protein that is upregulated in actively cycling cells, was obviously increased in freshly isolated RasGRP1−/− αβT cells as compared to WT controls (Figure 4B). Consistent with increased Ki67 expression in RasGRP1−/− αβT cells, most of these cells were CD44highCD62L−, which was consistent with homeostatic proliferation of these cells (Figure 4C). In contrast, Ki67 expression in RasGRP1−/− γδT cells was similar to WT control (Figure 4B). Furthermore, most RasGRP1−/− γδT cells displayed a naïve CD44lowCD62L+ phenotype (Figure 3D). Of note, Ki67 positive CD4SP RasGRP1−/− γδT cells were increased as compared to WT controls (Figure 4B), likely caused by homeostatic proliferation due to the decrease of this population of γδT cells in RasGRP1−/− mice.
Together, these observations suggest that RasGRP1−/− αβT cells undergo enhanced homeostatic proliferation in vivo and that there is no obvious increase in homeostatic proliferation of most γδT cells in RasGRP1−/− mice except the CD4SP subset. Our data also suggest that γδT cells may sense different homeostatic cues compared with αβT cells since the overall αβT cell lymphopenia resulted in increased homeostatic proliferation of αβT but not γδT cells in RasGRP1−/− mice. The enrichment of γδT cells in the absence of RasGRP1 is likely an active process involved in γδT cell generation since the percentage of intracellular TCRβ positive γδT cells was significantly decreased in RasGRP1−/− mice (Figure 4E).
Requirement of RasGRP1 for TCR-induced Erk1/2 activation in γδT cells
As mentioned earlier in Figure 1A, in addition to RasGRP1, RasGRP2 and 4 are also expressed in γδT cells. We examined whether RasGRP1 deficiency may affect RasGRP2 and RasGRP4 expression. As shown in Figure 5A, RasGRP2 mRNA level decreased about 50% while RasGRP4 mRNA level increased about twentyfold in RasGRP1KO γδT cells compared to WT controls. Increased Erk1/2 activity has been found to promote γδT cell development (21, 23). It is further known that RasGRP1 plays an important role in TCR-induced Erk1/2 activation in total thymocytes and αβT cells (25, 27). The minimal requirement of RasGRP1 for γδT cell development and the drastic increase in RasGRP4 expression in RasGRP1KO γδT cells raise the possibility that RasGRP1 may be dispensable for TCR-induced Erk1/2 activation, or elevated RasGRP4 expression may compensate the loss of RasGRP1 for TCR-induced Erk1/2 activation in γδT cells. To determine the role of RasGRP1 for TCR-induced Erk1/2 activation, we sorted γδT cells from RasGRP1−/− and WT mice. Sorted cells were left unstimulated or stimulated with a soluble anti-CD3 antibody, and Erk1/2 phosphorylation was examined by Western blot analysis. As shown in Figure 5B, Erk1/2 phosphorylation was decreased in RasGRP1−/− γδT cells. However, induction of Erk1/2 phosphorylation could still be detected in RasGRP1−/− γδT cells. Thus, RasGRP1 is required for optimal TCR-induced Erk1/2 activation in γδT cells, and other Ras activation mechanisms are not able to fully compensate for the loss of RasGRP1 for TCR-induced Erk1/2 activation.
Figure 5. Requirement of RasGRP1 for TCR-induced Erk1/2 activation in γδT cells.
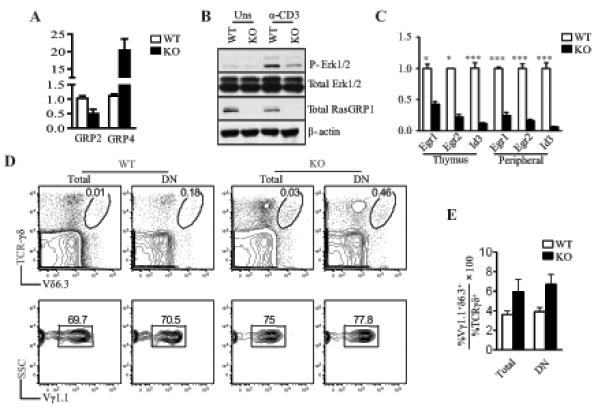
(A) Altered RasGRP2 and RasGRP4 expression in RasGRP1KO γδT cells. mRNA levels of RasGRP2 and RasGRP4 in sorted WT and RasGRP1KO γδT cells were determined by qRT-PCR. (B) Decreased Erk1/2 activation in RasGRP1KO γδT cells. Sorted γδT cells from WT and RasGRP1−/− mice were rested in PBS for 30 minutes and then left unstimulated or stimulated with an anti-CD3ε antibody for 5 minutes. Erk1/2 phosphorylation was determined by immunoblotting analysis. (C) Decreased Egr1/2 and Id3 expression in RasGRP1−/− γδT cells. RNA isolated from WT and RasGRP1−/− γδT cells were reversely transcribed, and mRNA levels of indicated molecules were determined by real-time qPCR. Data shown are representative of two experiments. (D) Slight increase in Vγ1+Vδ6.3+ γδT cells in RasGRP1−/− mice. Upper panels show TCRγδ and TCRVδ6.3 staining of total thymocytes from WT and RasGRP1−/− mice. Lower panels show TCRVγ1.1 staining of gated TCRγδ+TCRVδ6.3+ cells. (E) Percentages of TCRVγ1.1+Vδ6.3+ cells within the γδT cells. Data shown are representative of two (A-C) and three (D, E) experiments.
Activation of the Ras-Erk1/2 pathway leads to induction of Egr1-3, which promotes Id3 transcription. Both Id3 and Egr1/2 are involved in γδT cell development. Id3-deficient mice contain increased γδT cells that are dominantly Vγ1+Vδ6.3+ innate γδT cells, which display an activating phenotype (32-36). Egr1/2 and Id3 expression was substantially decreased in RasGRP1−/− γδT cells (Figure 5C). The decreased expression of Id3 cells may contribute to the approximately twofold increase in Vγ1+Vδ6.3+ γδT cells in RasGRP1−/− mice (Figures 5D, 5E).
Requirement of RasGRP1 for TCR-induced γδT cell proliferation
RasGRP1 activates the Ras-Erk1/2 pathway in peripheral T cells and is critical for peripheral αβT cell activation (25-27). The dispensable role of RasGRP1 for γδT cell development and homeostasis raises the question whether this protein participates in γδT cell activation. Due to downregulation of γδTCR following TCR activation, we sorted γδT cells and labeled them with CFSE. CFSE-labeled γδT cells were left unstimulated or stimulated with plate-bound anti-CD3 for 72 hours. While WT γδT cells proliferated vigorously, RasGRP1−/− γδT cells were defective in proliferation (Figure 6A). No obvious difference in cell death was observed between WT and RasGRP1KO γδT cells during in vitro stimulation (Figure 6B), suggesting that defective γδT cell proliferation in the absence of RasGRP1 was not caused by increased cell death. In WT mice, anti-CD3ε-induced Erk1/2 phosphorylation in splenic γδT cells was stronger than in thymic γδT cells. Both thymic and splenic RasGRP1KO γδT cells displayed decreased Erk1/2 phosphorylation as compared to WT control (Figure 6C). Given the role of the Ras-Erk1/2 pathway for αβT cell activation, impaired Erk1/2 activation in RasGRP1KO γδT cells may lead to their proliferative defect. RasGRP1KO γδT cells expressed slightly increased CD25 and CD122 compared to WT control. They expressed similar levels of costimulatory molecules ICOS, CD5, OX40, and NKG2D as compared to WT controls (Figure 6D), suggesting that RasGRP1 is not required for the expression of these molecules. However, whether RasGPR1 is involved in costimulatory signaling in γδT cells is unclear at present. Together, these observations indicate that RasGRP1 is crucial for TCR-induced γδT cell proliferation.
Figure 6. Requirement of RasGRP1 for TCR-induced γδ T cell proliferation.
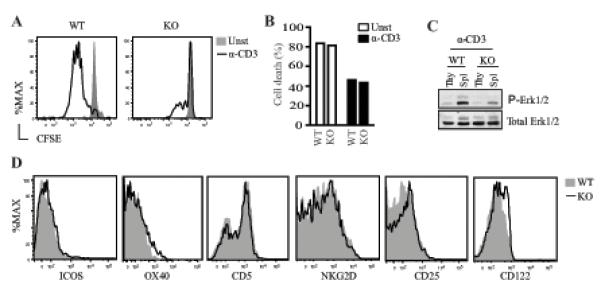
(A, B) CFSE dilution assay to assess γδT cell proliferation and death. CFSE-labeled purified WT and RasGRP1−/− γδT and αβT cells were left unstimulated or stimulated with a plate-bound anti-CD3ε antibody for 72 hours. Cultured cells were stained with Live/Dead before analyzed by flow cytometry. Histograms show CFSE intensity on gated live γδT cells (A). Bar figure shows death rate of cultured γδT cells (B). (C) Impaired Erk1/2 activation in both thymic and splenic γδT cells in the absence of RasGRP1. (D) Expression of costimulatory molecules and cytokine receptors by RasGRP1KO γδT cells. Data shown are representative of two (A-C) and three (D) experiments.
Defective IL-17 production by γδT cells in the absence of RasGRP1
γδT cells are an important innate source of IL-17 and IFNγ (37-39). To examine whether RasGRP1 deficiency affects cytokine production by γδT cells, we stimulated freshly isolated thymocytes, splenocytes, and LN cells with PMA and ionomycin for 5 hours in the presence of a Golgi plug, and we then analyzed cytokine production by intracellular staining. IL-17A production by RasGRP1−/− γδT cells from the thymus, spleen, and LN was substantially decreased (Figure 7A). Different from IL-17, IFNγ production was increased in RasGRP1−/− γδT cells as compared to WT γδT cells. Further comparison of cytokine expression in CD4+, CD8+, and DN γδT cells revealed that CD8+ γδT cells produced higher levels of IFNγ but lower levels of IL-17A than CD4+ and DN γδT cells in WT mice. In the absence of RasGRP1, IFNγ expression was increased but IL-17A expression was substantially decreased in these γδT cell populations (Figure 7B).
γδT cells can be divided into functional subsets based on CD27 and CD44 expression, with the CD27−CD44+ mainly producing IL-17 and the CD27+CD44+/- producing IFNγ (40). In RasGRP1KO thymus and spleen, the percentages of CD27−CD44+ γδT cells were decreased about 50% and 30% respectively as compared to WT controls, suggesting that RasGRP1 is important for the generation/maintenance of this population of γδT cells (Figure 7C). Moreover, IL-17 production by RasGRP1KO CD27−CD44+ γδT cells was substantially decreased compared to WT control. In contrast to CD27−CD44+ γδT cells, there were about 50%-70% increases in CD27+CD44− γδT cells in RasGRP1KO thymus and spleen. Moreover, IFNγ production by splenic and LN CD27+CD44+/- γδT cells from RasGRP1KO mice appeared to increase (Figure 7D). Together, these observations revealed that RasGRP1 is not only important for the generation/maintenance of the CD27−CD44+ γδT cell subset but also critical for this subset of cells to produce IL-17.
Discussion
An important signaling event following both αβTCR and γδTCR stimulation is the activation of Erk1/2. In αβT cells, RasGRP1 is a critical upstream activator for Erk1/2 through the Ras-Raf-Mek1/2 signaling cascade following TCR engagement (27). Genetic evidence has revealed that both RasGRP1 and Erk1/2 are critical for maturation of T cells adopting the αβT cell lineage, including conventional αβT cells (25, 27, 41), iNKT cells (28), and Treg (29). In this report, we have demonstrated that RasGRP1 is important for Erk1/2 in γδT cells following TCR stimulation. Thus, although additional RasGRPs and other guanine-nucleotide-releasing factors such as Sos are expressed in γδT cells, they cannot fully compensate for the loss of RasGRP1 for Erk1/2 activation. Extending previous studies demonstrating that RasGRP1 is critical for positive selection of αβT cells (25, 27), we now find that RasGRP1 is also important for efficient β-selection as evidenced by the accumulation of DN3 cells and decreased DN4 thymocytes, suggesting that RasGRP1 participates in pre-TCR signaling.
In RasGRP1−/− mice, total as well as CD4−CD8− DN and CD8 SP γδT cell numbers in the thymus are comparable to WT control. The virtually normal development of RasGRP1−/− γδT cells in mixed chimeric mice further supports the minimal role of RasGRP1 for γδT cell development. CD4−CD8− DN γδT cells, and CD8 SP γδT cells in particular, expand in RasGRP1−/− peripheral lymphoid organs. However, such expansion of RasGRP1−/− γδT cells is not observed in mixed BM chimeric mice, suggesting that cell-extrinsic factors lead to the expansion of γδT cells in the peripheral lymphoid organs in RasGRP1−/− mice. These extrinsic factors could be cytokines produced by RasGRP1−/− conventional αβT cells under the lymphopenic environment or by other cell types. Furthermore, CD4+Foxp3+ Treg numbers in RasGRP1−/− mice were decreased (29), which could also contribute to peripheral γδT cell expansion.
It has been reported that TCR signal strength influences αβT and γδT cell lineage commitment with strong TCR signal and enhanced Erk1/2 activity favoring the γδT lineage (21-23). It is intriguing that γδT cell development is virtually intact in RasGRP1−/− mice with an obvious decrease in Erk1/2 activation. One potential explanation is that the relative strengths of Erk1/2 signaling dictate γδT versus αβT differentiation. The commitment of the progenitor to the αβT lineage or cells that have committed to the αβT fate could be more sensitive to the decreased Erk1/2 activity than those that are committing to or have committed to the γδT lineage. It is important to note that our data does not rule out the role of Erk1/2 for γδT cell development since a low level of Erk1/2 phosphorylation can still be induced in RasGRP1−/− γδT cells. Furthermore, TCR stimulation induces stronger Erk1/2 activation in peripheral γδT cells than thymic γδT cells, suggesting that differential regulation of Erk1/2 activation between thymic and peripheral γδT cells. The low level of TCR-induced Erk1/2 activity in thymic γδT cells may suggest the possibility that developing γδT cells can be less sensitive to the decrease of Erk1/2 activation in the absence of RasGRP1 than peripheral γδT cells. Additionally, we have recently demonstrated that in addition to Erk1/2, RasGRP1 also functions as an upstream activator for PI3K/Akt and mTOR in thymocytes. In RasGRP1−/− thymocytes, not only Erk1/2 but also PI3K/Akt and mTOR activation is impaired following TCR engagement (42, 43). RasGRP1 could promote αβT cell development not only via Erk1/2 but also through other downstream signaling pathways.
The Ras-Erk1/2 pathway plays an important role in transcriptional activation of Egr1/2, which in turn promotes Id3 expression (44). In RasGRP1-deficient γδT cells, Egr1/2 and Id3 expression is decreased, which is consistent with the importance of RasGRP1 for Erk1/2 activation in γδT cells. Proper expression of Id3 ensures normal γδT cell development. Loss of Id3 leads to preferential generation and expansion of “innate” γδT cells. However, elevated Id3 level also leads to increases in γδT cells (32, 34-36, 45). These data suggest a narrow window of Id3 expression for proper development of innate γδT cells. Although γδT cells are enriched in Id3−/− mice and are relatively enriched in RasGRP1−/− mice, differences between these two strains are obvious. In Id3−/− mice, the Vγ1+Vδ6.3+ innate γδT cells are predominantly increased (32, 34, 36). In RasGRP1−/− mice, there is less than a twofold increase in such innate γδT cells. The differences between these two strains of mice can be explained by the influence of RasGRP1 deficiency on multiple downstream effector molecules and the incomplete loss of Id3 expression.
Most γδ T cells in adult WT mice are CD4−CD8− DN. Although CD4SP or CD8SP γδ T cells can be easily detected in fetal thymus, they are rare in postnatal thymus, spleen, and LN (48, 49). In RasGRP1−/− mice, CD8SP γδT cells are substantially increased but CD4SP γδT cells are decreased. In both WT and RasGRP1−/− thymus, spleen, and LN, most of the CD8SP γδT cells express the CD8α and β heterodimer. Although γδT cells are not MHC-I restricted and extracellular factors play a critical role in the expansion of CD8SP γδT cells in RasGRP1−/− mice, expression of the CD8αβ heterodimer could be involved in the expansion of these CD8SP γδT cells in RasGRP1−/− mice since the CD8αβ heterodimer is able to associate with MHC-I. Of note, IL-7 has been reported to be able to expand CD8α+β+ γδT cells from WT fetal thymus (50). It would be interesting to determine whether IL-7 level is elevated and is responsible for the expansion of CD8SP γδT cells in RasGRP1−/− mice. Enrichment of CD8SP γδT cells has also been observed in several other genetically manipulated mice such as Id3−/− mice (34). Those Id3−/− CD8SP γδT cells express the CD8αα homodimer and are thus different from the RasGRP1−/− CD8α+β+ γδT cells. It is possible that combined action of altered intrinsic properties, such as abnormal expression of Id3 and other molecules, and extrinsic factors causes the expansion of CD8SP γδT cells in RasGRP1−/− mice.
RasGRP1 is differentially required for αβ versus γδ T cell development. However, it is critical for both αβ and γδ T cell activation. RasGRP1 may exert its role in γδT cell activation through multiple mechanisms. For example, it can directly promote mature γδT cell activation by activating the Ras-Erk1/2 pathway, mTOR, and PI3K-Akt. Alternatively but not mutually exclusively, RasGRP1 may be required for licensing or arming γδT cells during their development to ensure competence for activation after they mature, a phenomenon that occurs during NK cell development (46, 47). Further studies utilizing conditional RasGRP1-deficient mice will help to distinguish such possibilities.
IL-17-producing γδT cells play important roles during microbial infection and the pathogenesis of autoimmune and inflammatory diseases (51-53). Generation of IL-17-producing γδT cells appears to be developmentally programmed during intrathymic development that is at least partially dependent on RORγt and the Notch-Hes pathway but is independent on Stat3 (54). RasGRP1-deficient mice contain decreased CD27−CD44+ subset but increased CD27+CD44− subset in both thymus and peripheral lymphoid organs, suggesting that RasGRP1 is involved in differentiation of γδT cells into the different effector subsets. Although it is most likely that RasGRP1 mediates γδTCR signaling to regulate γδ effector T cell differentiation, we cannot rule out that RasGRP1 may be involved in other receptor signaling to control γδT differentiation. Within the CD27−CD44+ RasGRP1KO γδT cells, the ratio of IL-17A-producing cells is also decreased, suggesting that RasGRP1 participates in IL-17A production by these cells. At present, it is unclear how RasGRP1 promotes IL-17 production by γδT cells. In αβT cells, mTOR complex 1 signaling is critical for Th17 differentiation (55, 56). Interestingly, RasRP1 is critical for mTOR activation in T cells by activating Ras-Erk1/2 signaling (42, 43). Future studies should determine whether RasGRP1promotes IL-17-producing γδT cells through inducing mTOR activation and whether RasGRP1 can be a potential target for γδT-cell-mediated diseases.
In summary, we identified several important functions of RasGRP1 in T cells. We demonstrated that RaGRP1 is involved in αβT cell maturation from the DN to DP stage, is important for TCR-induced Erk1/2 activation in both thymic and splenic γδT cells, is dispensable for overall γδT cell development but important for the generation/maintenance of IL-17-producing γδT cells, and, finally, is critical for TCR-induced γδT cell proliferation.
Acknowledgments
We thank Drs. Yuan Zhuang, Michael Kulis, and Tommy O’Brien for critically reviewing the manuscript and Nancy Martin and Mike Cook in the Duke Cancer Center Flow Cytometry Core Facility for sorting cells.
This study is supported by funding from the National Institutes of Health (R01AI076357, R01AI079088, and R21AI079873), and the American Cancer Society (RSG-08-186-01-LIB) to X-P.Z, and the Chinese National Science Foundation (31071237).
Abbreviations
- TCR
T cell receptor
- mTOR
mammalian target of rapamycin
- SP
single positive
- DN
double negative
- DP
double positive
References
- 1.Godfrey DI, Kennedy J, Suda T, Zlotnik A. A developmental pathway involving four phenotypically and functionally distinct subsets of CD3-CD4-CD8- triple-negative adult mouse thymocytes defined by CD44 and CD25 expression. J Immunol. 1993;150:4244–4252. [PubMed] [Google Scholar]
- 2.Shinkai Y, Koyasu S, Nakayama K, Murphy KM, Loh DY, Reinherz EL, Alt FW. Restoration of T cell development in RAG-2-deficient mice by functional TCR transgenes. Science. 1993;259:822–825. doi: 10.1126/science.8430336. [DOI] [PubMed] [Google Scholar]
- 3.Itohara S, Mombaerts P, Lafaille J, Iacomini J, Nelson A, Clarke AR, Hooper ML, Farr A, Tonegawa S. T cell receptor δ gene mutant mice: independent generation of αβ T cells and programmed rearrangements of γδ TCR genes. Cell. 1993;72:337–348. doi: 10.1016/0092-8674(93)90112-4. [DOI] [PubMed] [Google Scholar]
- 4.von Boehmer H, Aifantis I, Feinberg J, Lechner O, Saint-Ruf C, Walter U, Buer J, Azogui O. Pleiotropic changes controlled by the pre-T-cell receptor. Curr Opin Immunol. 1999;11:135–142. doi: 10.1016/s0952-7915(99)80024-7. [DOI] [PubMed] [Google Scholar]
- 5.Ciofani M, Knowles GC, Wiest DL, von Boehmer H, Zuniga-Pflucker JC. Stage-specific and differential notch dependency at the αβ and γδ T lineage bifurcation. Immunity. 2006;25:105–116. doi: 10.1016/j.immuni.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Krangel MS, Carabana J, Abbarategui I, Schlimgen R, Hawwari A. Enforcing order within a complex locus: current perspectives on the control of V(D)J recombination at the murine T-cell receptor α/δ locus. Immunol Rev. 2004;200:224–232. doi: 10.1111/j.0105-2896.2004.00155.x. [DOI] [PubMed] [Google Scholar]
- 7.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes α and β block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 8.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor α β-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 9.Finco TS, Kadlecek T, Zhang W, Samelson LE, Weiss A. LAT is required for TCR-mediated activation of PLCγ1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Sommers CL, Burshtyn DN, Stebbins CC, DeJarnette JB, Trible RP, Grinberg A, Tsay HC, Jacobs HM, Kessler CM, Long EO, Love PE, Samelson LE. Essential role of LAT in T cell development. Immunity. 1999;10:323–332. doi: 10.1016/s1074-7613(00)80032-1. [DOI] [PubMed] [Google Scholar]
- 11.Clements JL, Yang B, Ross-Barta SE, Eliason SL, Hrstka RF, Williamson RA, Koretzky GA. Requirement for the leukocyte-specific adapter protein SLP-76 for normal T cell development. Science. 1998;281:416–419. doi: 10.1126/science.281.5375.416. [DOI] [PubMed] [Google Scholar]
- 12.Schaeffer EM, Debnath J, Yap G, McVicar D, Liao XC, Littman DR, Sher A, Varmus HE, Lenardo MJ, Schwartzberg PL. Requirement for Tec kinases Rlk and Itk in T cell receptor signaling and immunity. Science. 1999;284:638–641. doi: 10.1126/science.284.5414.638. [DOI] [PubMed] [Google Scholar]
- 13.Zhong XP, Guo R, Zhou H, Liu C, Wan CK. Diacylglycerol kinases in immune cell function and self-tolerance. Immunol Rev. 2008;224:249–264. doi: 10.1111/j.1600-065X.2008.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu Rev Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL, Lin X. CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IκB kinase β to the immunological synapse through CARMA1. Mol Cell Biol. 2004;24:164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isakov N, Altman A. Protein kinase CΩ in T cell activation. Annu Rev Immunol. 2002;20:761–794. doi: 10.1146/annurev.immunol.20.100301.064807. [DOI] [PubMed] [Google Scholar]
- 17.Kreslavsky T, von Boehmer H. γδTCR ligands and lineage commitment. Semin Immunol. 2010;22:214–221. doi: 10.1016/j.smim.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Terrence K, Pavlovich CP, Matechak EO, Fowlkes BJ. Premature expression of T cell receptor (TCR)αβ suppresses TCRγδ gene rearrangement but permits development of γδ lineage T cells. J Exp Med. 2000;192:537–548. doi: 10.1084/jem.192.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Siegers GM, Swamy M, Fernandez-Malave E, Minguet S, Rathmann S, Guardo AC, Perez-Flores V, Regueiro JR, Alarcon B, Fisch P, Schamel WW. Different composition of the human and the mouse γδ T cell receptor explains different phenotypes of CD3γ and CD3δ immunodeficiencies. J Exp Med. 2007;204:2537–2544. doi: 10.1084/jem.20070782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayes SM, Love PE. Distinct structure and signaling potential of the γδ TCR complex. Immunity. 2002;16:827–838. doi: 10.1016/s1074-7613(02)00320-5. [DOI] [PubMed] [Google Scholar]
- 21.Haks MC, Lefebvre JM, Lauritsen JPH, Carleton M, Rhodes M, Miyazaki T, Kappes DJ, Wiest DL. Attenuation of γδ TCR Signaling Efficiently Diverts Thymocytes to the αβ Lineage. Immunity. 2005;22:595–606. doi: 10.1016/j.immuni.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Hayes SM, Laird RM, Love PE. Beyond αβ/γδ lineage commitment: TCR signal strength regulates γδ T cell maturation and effector fate. Semin Immunol. 2010;22:247–251. doi: 10.1016/j.smim.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreslavsky T, Garbe AI, Krueger A, von Boehmer H. T cell receptor-instructed αβ versus γδ lineage commitment revealed by single-cell analysis. J Exp Med. 2008;205:1173–1186. doi: 10.1084/jem.20072425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hayes SM, Li LQ, Love PE. TCR signal strength influences αβ/γδ lineage fate. Immunity. 2005;22:583–593. doi: 10.1016/j.immuni.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Dower NA, Stang SL, Bottorff DA, Ebinu JO, Dickie P, Ostergaard HL, Stone JC. RasGRP is essential for mouse thymocyte differentiation and TCR signaling. Nat Immunol. 2000;1:317–321. doi: 10.1038/79766. [DOI] [PubMed] [Google Scholar]
- 26.Ebinu JO, Stang SL, Teixeira C, Bottorff DA, Hooton J, Blumberg PM, Barry M, Bleakley RC, Ostergaard HL, Stone JC. RasGRP links T-cell receptor signaling to Ras. Blood. 2000;95:3199–3203. [PubMed] [Google Scholar]
- 27.Priatel JJ, Teh SJ, Dower NA, Stone JC, Teh HS. RasGRP1 transduces low-grade TCR signals which are critical for T cell development, homeostasis, and differentiation. Immunity. 2002;17:617–627. doi: 10.1016/s1074-7613(02)00451-x. [DOI] [PubMed] [Google Scholar]
- 28.Shen S, Chen Y, Gorentla BK, Lu J, Stone JC, Zhong XP. Critical Roles of RasGRP1 for Invariant NKT Cell Development. J Immunol. 2011;187:4467–4473. doi: 10.4049/jimmunol.1003798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Priatel JJ, Chow MT, Teh HS. Preferential development of CD4 and CD8 T regulatory cells in RasGRP1-deficient mice. J Immunol. 2008;180:5973–5982. doi: 10.4049/jimmunol.180.9.5973. [DOI] [PubMed] [Google Scholar]
- 30.Pereira P, Hermitte V, Lembezat MP, Boucontet L, Azuara V, Grigoriadou K. Developmentally regulated and lineage-specific rearrangement of T cell receptor Vα/δ gene segments. Eur J Immunol. 2000;30:1988–1997. doi: 10.1002/1521-4141(200007)30:7<1988::AID-IMMU1988>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 31.Zhong XP, Hainey EA, Olenchock BA, Jordan MS, Maltzman JS, Nichols KE, Shen H, Koretzky GA. Enhanced T cell responses due to diacylglycerol kinase ζ deficiency. Nat Immunol. 2003;4:882–890. doi: 10.1038/ni958. [DOI] [PubMed] [Google Scholar]
- 32.Ueda-Hayakawa I, Mahlios J, Zhuang Y. Id3 restricts the developmental potential of γ δ lineage during thymopoiesis. J Immunol. 2009;182:5306–5316. doi: 10.4049/jimmunol.0804249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong GW, Zuniga-Pflucker JC. γδ and αβ T cell lineage choice: resolution by a stronger sense of being. Semin Immunol. 2010;22:228–236. doi: 10.1016/j.smim.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Verykokakis M, Boos MD, Bendelac A, Adams EJ, Pereira P, Kee BL. Inhibitor of DNA binding 3 limits development of murine slam-associated adaptor protein-dependent “innate” γδ T cells. PLoS One. 2010;5:e9303. doi: 10.1371/journal.pone.0009303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verykokakis M, Boos MD, Bendelac A, Kee BL. SAP protein-dependent natural killer T-like cells regulate the development of CD8+ T cells with innate lymphocyte characteristics. Immunity. 2010;33:203–215. doi: 10.1016/j.immuni.2010.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lauritsen JP, Wong GW, Lee SY, Lefebvre JM, Ciofani M, Rhodes M, Kappes DJ, Zuniga-Pflucker JC, Wiest DL. Marked induction of the helix-loop-helix protein Id3 promotes the γδ T cell fate and renders their functional maturation Notch independent. Immunity. 2009;31:565–575. doi: 10.1016/j.immuni.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hayday AC. γδ T cells and the lymphoid stress-surveillance response. Immunity. 2009;31:184–196. doi: 10.1016/j.immuni.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 38.Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, Craft J, Yin Z. γδ T cells provide an early source of interferon γ in tumor immunity. J Exp Med. 2003;198:433–442. doi: 10.1084/jem.20030584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shibata K, Yamada H, Hara H, Kishihara K, Yoshikai Y. Resident Vγ1+ T cells control early infiltration of neutrophils after Escherichia coli infection via IL-17 production. J Immunol. 2007;178:4466. doi: 10.4049/jimmunol.178.7.4466. [DOI] [PubMed] [Google Scholar]
- 40.Ribot JC. CD27 is a thymic determinant of the balance between interferon-γ -and interleukin 17-producing T cell subsets. Nat Immunol. 2009;10:427–436. doi: 10.1038/ni.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer AM, Katayama CD, Pages G, Pouyssegur J, Hedrick SM. The role of erk1 and erk2 in multiple stages of T cell development. Immunity. 2005;23:431–443. doi: 10.1016/j.immuni.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong XP, Shin J, Gorentla BK, O’Brien T, Srivatsan S, Xu L, Chen Y, Xie D, Pan H. Receptor signaling in immune cell development and function. Immunol Res. 2011;49:109–123. doi: 10.1007/s12026-010-8175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bain G, Cravatt CB, Loomans C, Alberola-Ila J, Hedrick SM, Murre C. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2:165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 45.Matsuda S, Miwa Y, Hirata Y, Minowa A, Tanaka J, Nishida E, Koyasu S. Negative feedback loop in T-cell activation through MAPK-catalyzed threonine phosphorylation of LAT. EMBO J. 2004;23:2577–2585. doi: 10.1038/sj.emboj.7600268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jonsson AH, Yokoyama WM. Natural killer cell tolerance licensing and other mechanisms. Adv Immunol. 2009;101:27–79. doi: 10.1016/S0065-2776(08)01002-X. [DOI] [PubMed] [Google Scholar]
- 47.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher AG, Ceredig R. γδ T cells expressing CD8 or CD4low appear early in murine foetal thymus development. Int Immunol. 1991;3:1323–1328. doi: 10.1093/intimm/3.12.1323. [DOI] [PubMed] [Google Scholar]
- 49.Itohara S, Nakanishi N, Kanagawa O, Kubo R, Tonegawa S. Monoclonal antibodies specific to native murine T-cell receptor γδ: analysis of γδ T cells during thymic ontogeny and in peripheral lymphoid organs. Proc Natl Acad Sci U S A. 1989;86:5094–5098. doi: 10.1073/pnas.86.13.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leclercq G, De Smedt M, Plum J. Presence of CD8αCD8β positive TCR γδ thymocytes in the fetal murine thymus and their in vitro expansion with interleukin-7. Eur J Immunol. 1992;22:2189–2193. doi: 10.1002/eji.1830220902. [DOI] [PubMed] [Google Scholar]
- 51.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing γδ T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 52.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EDC, Mills KHG. Interleukin-1 and IL-23 induce innate IL-17 production from γδ T Cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shibata K, Yamada H, Sato T, Dejima T, Nakamura M, Ikawa T, Hara H, Yamasaki S, Kageyama R, Iwakura Y, Kawamoto H, Toh H, Yoshikai Y. Notch-Hes1 pathway is required for the development of IL-17-producing γδ T cells. Blood. 2011;118:586–593. doi: 10.1182/blood-2011-02-334995. [DOI] [PubMed] [Google Scholar]
- 55.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, Magnuson MA, Boothby M. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–753. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Delgoffe GM, Pollizzi KN, Waickman AT, Heikamp E, Meyers DJ, Horton MR, Xiao B, Worley PF, Powell JD. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat Immunol. 2011;12:295–303. doi: 10.1038/ni.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


