Abstract
All-trans retinal is a potent photosensitizer that is released in photoreceptor outer segments by the photoactivated visual pigment following the detection of light. Photoreceptor outer segments also contain high concentrations of polyunsaturated fatty acids, and are thus particularly susceptible to oxidative damage such as that initiated by light via a photosensitizer. Upon its release, all-trans retinal is reduced within the outer segment to all-trans retinol, through a reaction requiring metabolic input in the form of NADPH. The phototoxic potential of physiologically generated all-trans retinal was examined in single living rod photoreceptors obtained from frog (Rana pipiens) retinas. Light-induced oxidation was measured with fluorescence imaging using an oxidation-sensitive indicator dye from the shift in fluorescence between the intact and oxidized forms. Light-induced oxidation was highest in metabolically compromised rod outer segments following photoactivation of the visual pigment rhodopsin, and after a time interval sufficiently long to ensure the release of all-trans retinal. Furthermore, light-induced oxidation increased with the concentration of exogenously added all-trans retinal. The results show that the all-trans retinal generated during the detection of light can mediate light-induced oxidation. Its removal through reduction to all-trans retinol protects photoreceptor outer segments against light-induced oxidative damage.
Introduction
The photoreceptor cells of the retina are the sensory neurons responsible for converting light to an electrical signal. Light transduction takes place in the outer segment, a modified cilium full of membrane disks on the surface of which incoming photons are captured and converted to a biochemical signal (1). The environment and composition of photoreceptor cells make them particularly prone to light-induced damage (2). Photoreceptor outer segments are particularly vulnerable to lipid peroxidation, as they are extraordinarily rich in polyunsaturated fatty acids, especially docosahexaenoic acid (3), and experience high O2 levels due to their proximity to the choriocapillaris. In addition, they are also continuously bombarded by light, which aids in the production of oxygen radicals. These three elements, polyunsaturated fatty acids, light, and oxygen, are major factors favoring lipid peroxidation (4). Oxidative damage to the retina can lead to blindness and has been associated with pathologies like photic retinopathy (5), retinopathy of prematurity (6), and Age-Related Macular Degeneration (7). Light-induced damage of the retina has been extensively studied for many years beginning with the work of Noell et al (8). The level of and action spectrum for classic light-induced damage correlate with the amount and absorption spectrum of rhodopsin (5, 9, 10), suggesting that the visual pigment is involved as a primary light receptor. This notion is bolstered by the resistance to light damage of the Rpe65-deficient mice (11), which cannot generate 11-cis retinal and lack rhodopsin (12).
All-trans retinal is an obligate intermediate of the detection of light by vertebrate photoreceptor cells. It is a highly reactive aldehyde, as well as a potent photosensitizer (13, 14). It can lead to light-induced lipid peroxidation and damage through the generation of reactive species, such as singlet oxygen (13–15), and can mediate the photooxidative damage of rod outer segment proteins in vitro(16). Although all-trans retinal absorbs maximally ~380 nm (17), its absorption spectrum extends well above 400 nm into the visible range, where the eye is transparent (18). Because of its toxic properties, all-trans retinal has been suspected to be a mediator of light damage for many years (19).
All-trans retinal is released in photoreceptor outer segments from the photoactivated visual pigment, as part of the process of visual pigment regeneration that follows the absorption and detection of light. Following its release, all-trans retinal is reduced to all-trans retinol in a reaction that requires NADPH as a cofactor (20). The removal of all-trans retinal can be enhanced through the transport of all-trans retinol out of the outer segment and to the adjacent retinal pigment epithelial cells by the Interphotoreceptor retinoid-binding protein (IRBP) (21, 22), though the physiological significance of such IRBP action has been called into question (23, 24). In the retinal pigment epithelium, all-trans retinol is recycled into 11-cis retinal, which is brought back to the photoreceptor outer segment to regenerate the visual pigment (25).
We have examined the photooxidative potential of physiologically generated all-trans retinal in single isolated living rod photoreceptors with fluorescence imaging. We have used a dye, BODIPY 581/591 undecanoic acid (BODIPY C11), whose fluorescence properties shift as a result of oxidation. We find that all-trans retinal released from bleached rhodopsin can mediate light-induced oxidation and its removal through reduction to all-trans retinol protects photoreceptors against such damage.
Materials and Methods
Grass frogs (Rana pipiens) were from NASCO (Fort Atkinson, WI) or Carolina Biologicals (Burlington, NC). All animal procedures were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee of the Medical University of South Carolina and with the recommendations of the Panel on Euthanasia of the American Veterinary Medical Association. Animals were sacrificed after being dark-adapted for at least 3 hours, the eyes were enucleated, and the retinas were excised under infrared light in frog Ringer’s solution (in mmol/L): 110 NaCl, 2.5 KCl, 1.6 MgCl2, 1 CaCl2, 5 HEPES, 5 glucose, pH = 7.55. Isolated intact rod photoreceptors and broken off rod outer segments were obtained as described (26), transferred to 100 μL chambers (Warner Instruments, Hamden, CT), and allowed to settle for 10 min.
For BODIPY C11 (Molecular Probes/Life Technologies, Carlsbad, CA ) measurements, isolated cells were incubated with 10 μM of the dye for 30 min and excess dye was removed by washing with Ringer’s. For retinol fluorescence measurements, no dye was added. The chambers were placed on the stage of an inverted Zeiss Axiovert 100 microscope (Carl Zeiss, Thornwood, NY) and imaged with a Zeiss 40× Plan Neofluar oil immersion objective lens (N.A. = 1.3). Fluorescence was excited with light of different wavelengths from a Xenon continuous arc light source (Sutter Instrument Company Novato, CA), and the fluorescence image was acquired with a Hamamatsu C4742 CCD camera (Hamamatsu Photonics, Hamamatsu-City, Japan). Retinol and retinal fluorescence were excited with 360 nm light and fluorescence emission was collected >420 nm. Intact BODIPY C11 fluorescence was excited with 555 nm and measured at 617 nm; for oxidized BODIPY C11, fluorescence was excited with 490 nm and measured at 528 nm. For an experiment, a dark-adapted cell was bleached by exposing it to long wavelength light (>530 nm from a 150-W halogen lamp illuminator) for 1 min. Fluorescence measurements were carried out immediately before bleaching and at different times after bleaching. Image acquisition and analysis were carried out using the Intelligent Imaging Innovations (Denver, CO) software. Fluorescence intensity was measured over defined regions of interest (ROI) in the outer segment and a background area, and corrected for background. Experiments were carried out at room temperature.
For experiments with exogenous retinal and decanal, dark-adapted isolated cells were exposed to a Ringer’s solution containing different concentrations of aldehyde (0 – 100 μM) with 1% bovine serum albumin (BSA) as carrier for 10 min. Subsequently, the solution containing the aldehyde was removed, and the chamber containing the cells was washed with Ringer’s. For light-induced oxidation measurements, the cells were then loaded with 10 μM BODIPY C11 for 10 min, and excess dye was removed by washing with Ringer’s. For all-trans retinal fluorescence measurements, the cells were not loaded with BODIPY C11.
Measurement of oxidation with BODIPY C11
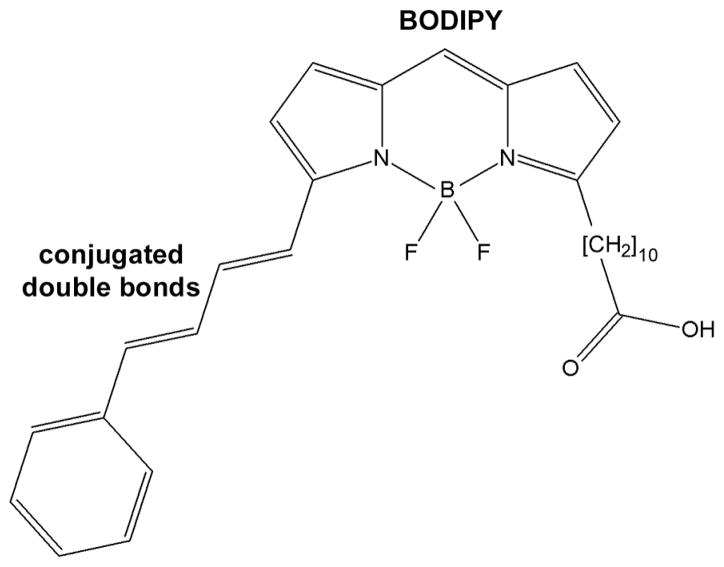
BODIPY C11 is a ratiometric indicator dye. The dye has been characterized extensively and the effects of oxidation on its fluorescence properties are well established (27–29). Its structure is shown in Scheme 1. The intact molecule has excitation/emission maxima at 581/591 nm. Oxidation destroys the conjugated double bond system, so that the fluorescence of the oxidized dye originates from the BODIPY part, which has excitation/emission maxima at 505/515 nm. To measure oxidation with BODIPY C11, fluorescence is measured in two channels, one for measuring the intact dye ( “red” signal) and one for measuring the oxidized dye ( “green” signal). The fluorescence ratio “green”/“red” is a measure of the proportion of the oxidized dye, and is independent of the dye concentration. Measuring the oxidation state of the dye with two fluorescence measurements could raise a concern in the case of dark-adapted cells: the first measurement exposes the cell to light and therefore the second measurement is not carried out with a dark-adapted cell anymore. However, the two measurements, although they are carried out in sequence, together take less than 2 sec to complete. This time is insufficient for a significant amount of all-trans retinal to be released from photoactivated rhodopsin (22). Furthermore, the level of BODIPY C11 oxidation is not affected by the exposure to 1 min of bleaching light (see Figure 3). Thus, the procedure for measuring the oxidation state of BODIPY C11 is valid in the case of dark-adapted cells as well. Light-induced oxidation was measured by exposing a cell to UV light (1 mW/cm2; 360 nm) or to visible light (30,000 lux; >400 nm).
Scheme 1.
Structure of BODIPY 581/591 undecanoic acid (BODIPY C11).
Figure 3.
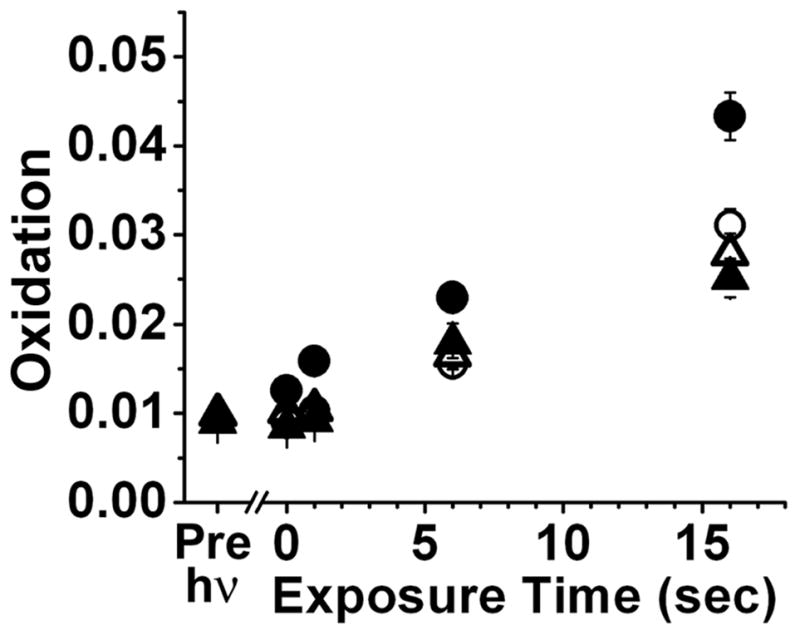
Increase of rod outer segment oxidation with increase in time of exposure to 360 nm light. Oxidation is measured as the “green”/“red” ratio of the fluorescence of the oxidized and intact forms of BODIPY C11. Rod outer segments of metabolically intact cells, exposed immediately (△, n = 15) and 60 min after rhodopsin bleaching (○, n = 20), and broken off rod outer segments, exposed immediately (▲, n = 12) and 60 min after rhodopsin bleaching (●, n = 21). The ratio reflecting the oxidation of BODIPY C11 in the cells before rhodopsin bleaching is shown as the Pre-hν point. Error bars represent standard errors.
Results and Discussion
Exposure of dark-adapted isolated vertebrate rod photoreceptors to light results in the isomerization of the rhodopsin chromophore from 11-cis to all-trans and the subsequent release of all-trans retinal. In rod outer segments that have access to the metabolic machinery of the inner segment that can supply the necessary NADPH, the released all-trans retinal is reduced to all-trans retinol, resulting in a large increase in outer segment fluorescence (30, 31). On the other hand, broken-off rod outer segments have been separated from the NADPH supply, and all-trans retinal cannot be reduced. In that case, the outer segment fluorescence increase is much smaller (31, 32). A comparison between metabolically intact rod outer segments with attached ellipsoids and metabolically compromised broken-off rod outer segments is shown in Figure 1. These two types of rod outer segments provide good experimental preparations to study light-induced oxidation mediated by the physiologically generated all-trans retinal.
Figure 1.
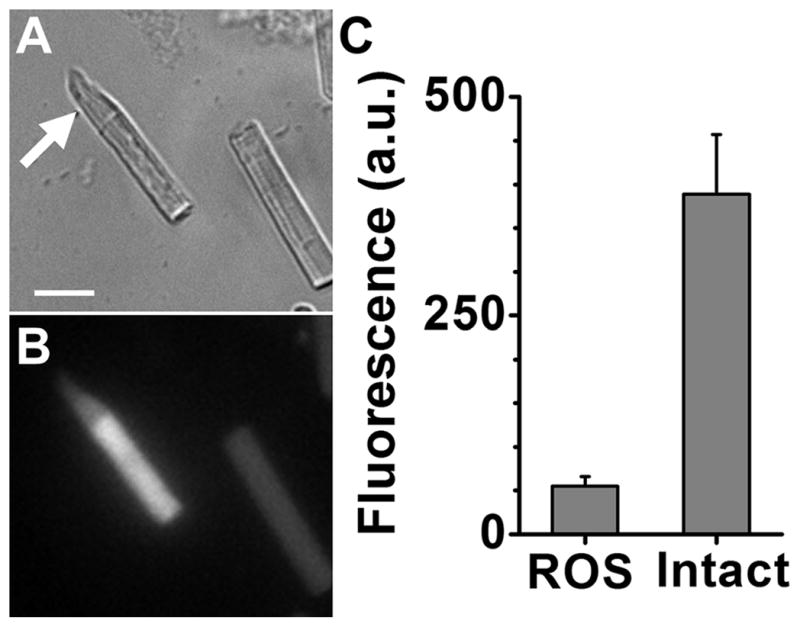
In the absence of the metabolic input provided by the inner segment of the cell, the all-trans retinal released from bleached rhodopsin is not converted to all-trans retinol. (A) Brightfield image of a frog rod outer segment with attached ellipsoid (arrow) and another broken off rod outer segment lacking an ellipsoid. Bar is 10 μm. (B) Fluorescence image (excitation: 360 nm; emission: > 420 nm) of the field in panel A, 60 min after bleaching of rhodopsin. (C) Rod outer segment fluorescence intensity 60 min after bleaching in broken off rod outer segments (ROS, n = 22) and in rod outer segments with attached ellipsoids (Intact, n = 14). Error bars represent standard errors.
Light-induced oxidation can be measured in single rod outer segments after loading them with BODIPY C11 (Figure 2). Exposure of a broken-off rod outer segment, 60 min after the bleaching of rhodopsin, to 360 nm UV light results in the oxidation of BODIPY C11 as shown by the decline in the fluorescence of the intact form (red pseudocolor, Figure 2B and 2D) and an increase in the fluorescence of the oxidized form (green pseudocolor, Figure 2C and 2E). The oxidation of BODIPY C11 was measured as the “green”/“red” fluorescence ratio. When rod outer segments were exposed to 360 nm UV light, the “green”/“red” fluorescence ratio increased, indicating increased oxidation of BODIPY C11. Oxidation increased linearly with the time of exposure to the UV light (Figure 3). Oxidation was measured in outer segments with or without access to NADPH and immediately or 60 min after bleaching of rhodopsin – comprising four conditions in total. The increase was higher for rod outer segments separated from the inner segment and exposed to the UV light 60 min after the bleaching of rhodopsin (●, Figure 3). These would be the outer segments with the highest levels of accumulated all-trans retinal: 60 min after rhodopsin bleaching, all-trans retinal has been released from photoactivated rhodopsin (22), and, due to the lack of NADPH, has not been reduced to all-trans retinol (32). Immediately after rhodopsin bleaching, the light-induced oxidation of BODIPY C11 was lower and was the same regardless of NADPH availability (△ and ▲, Figure 3). This would be consistent with there being no time for the release of sufficient amount of all-trans retinal from the photoactivated pigment. Light-induced oxidation of the dye was also lower 60 min after rhodopsin bleaching for the outer segments of metabolically intact rods (○, Figure 3), which would be consistent with the released all-trans retinal having been reduced to all-trans retinol.
Figure 2.
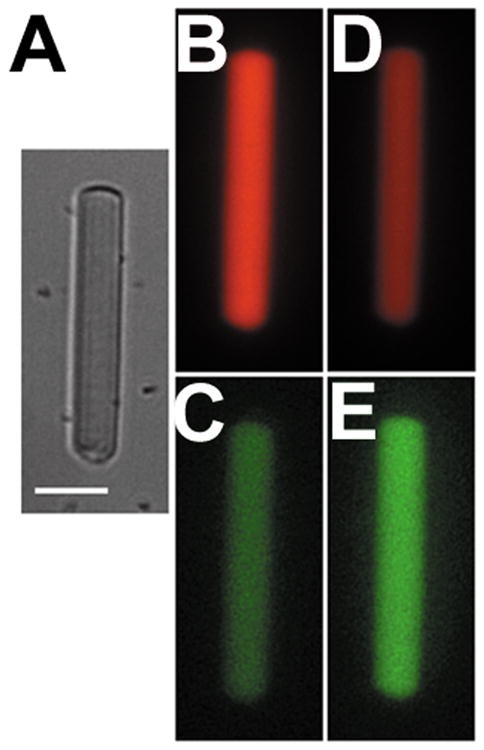
Light-induced oxidation of BODIPY C11 in a rod outer segment, 60 min after bleaching of rhodopsin. (A) Bright field image of a broken off rod outer segment loaded with BODIPY C11. Bar is 10 μm. (B) and (C), fluorescence images of the outer segment, acquired with 555/617 nm (B) and 490/528 nm (C) excitation/emission filters, respectively. (D) and (E), fluorescence images of the same cell after a total exposure to 16 s of 360 nm light, acquired with 555/617 nm (D) and 490/528 nm (E) excitation/emission filters, respectively. The intensity scaling is the same for images B and D and images C and E.
Exposure to visible light (>400 nm) also resulted in increase in oxidation in single rod outer segments (Figure 4). In this case, instead of measuring the total oxidation of BODIPY C11, as in the experiment in Figure 3, the change in dye oxidation due to light exposure was measured. As with UV light, in metabolically intact rod outer segments there was no significant difference between the light-induced oxidation immediately and 60 min after rhodopsin bleaching, consistent with reduction of the all-trans retinal released after bleaching. In broken-off rod outer segments however, allowing time for the release of all-trans retinal resulted in significantly higher light-induced oxidation.
Figure 4.
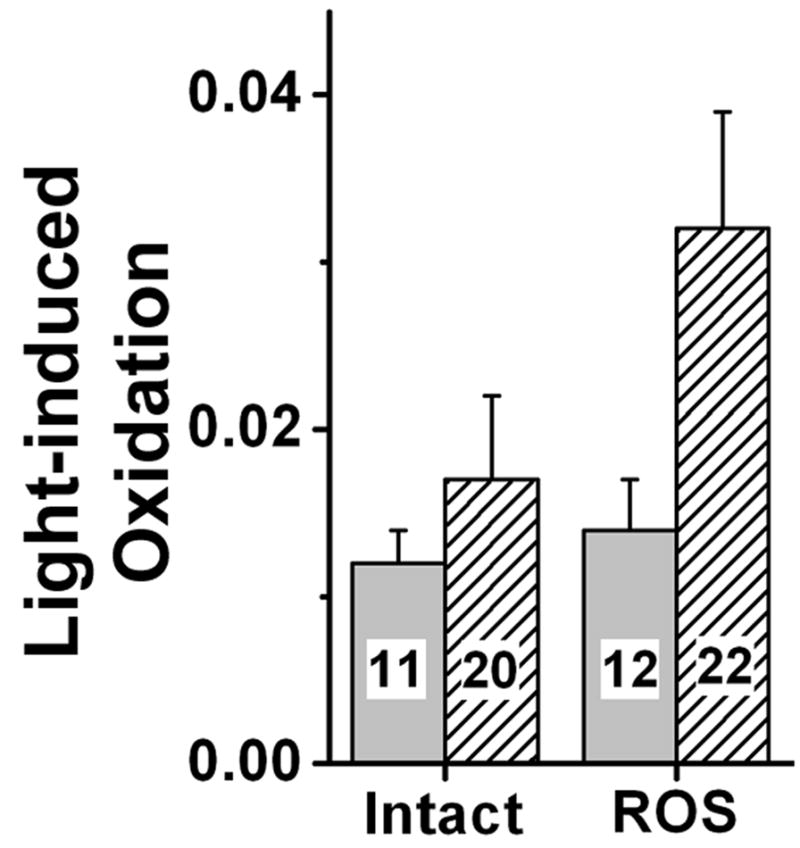
Rod outer segment oxidation induced by visible light. Metabolically intact (Intact) and broken-off (ROS) rod outer segments loaded with BODIPY C11 were exposed to 10 min of visible light (30,000 lux; λ > 400 nm) immediately (light grey) and 60 min after rhodopsin bleaching (hatched). Light-induced oxidation was measured as the change in the “green”/“red” BODIPY C11 ratio caused by the light exposure. The numbers of outer segments are shown within each bar. Error bars represent standard errors.
In experiments with UV as well as with visible light, there was significant light-induced oxidation under all conditions. Importantly, light-induced oxidation was the same in metabolically intact cells immediately and 60 min after bleaching, and in broken-off outer segments immediately after bleaching. The source of this oxidation is unlikely to be all-trans retinal, as rod outer segments under these three conditions contain small amounts of it: of the retinoid released from photoactivated rhodopsin only 10–20% is present as all-trans retinal in the rod outer segments of metabolically intact cells (22); immediately after bleaching, even in broken off rod outer segments, only a small amount of all-trans retinal has been released from photoactivated rhodopsin. One possible source of this photooxidation is BODIPY C11 itself, which, as a light-absorbing pigment, is also a photosensitizer. This is supported by the observation that photooxidation increases with the concentration of BODIPY present in the outer segment (data not shown), but does not exclude the possible contribution from other photosensitizers. Because the photosensitizing action of BODIPY C11 could interfere with measurements of the contribution of other photosensitizers, its concentration was keptapproximately the same across cells by ensuring similar levels of loading. The concentration of BODIPY C11 was independently assessed from the fluorescence of the intact dye at the beginning of an experiment.
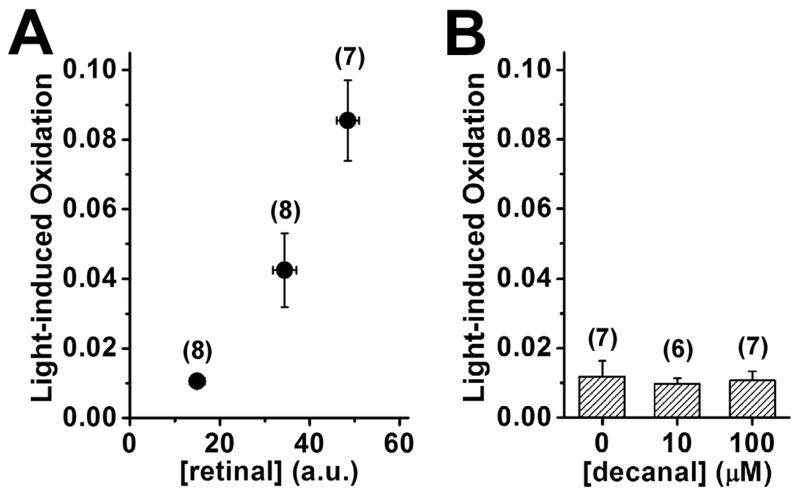
The higher photooxidation by both UV and visible light in broken-off rod outer segments 60 min after rhodopsin bleaching can be readily explained by the accumulation of all-trans retinal. If this were the case, we would expect the addition of exogenous all-trans retinal to isolated rod outer segments to result in higher light-induced oxidation. The experiment was carried out with dark-adapted broken-off rod outer segments, which, being dark-adapted, do not have significant levels of endogenous all-trans retinal and, lacking NADPH sources, cannot metabolize the retinal supplied exogenously. The relative concentration of exogenous all-trans retinal accumulating in the outer segments was independently measured from its fluorescence from cells loaded under identical conditions. Figure 5A shows that light-induced oxidation (by 10 sec of 360 nm light) increased with the concentration of exogenous all-trans retinal, in accordance with expectations.
Figure 5.
Higher concentrations of exogenously added all-trans retinal, but not of decanal, result in increased light-induced oxidation in rod outer segments. Dark-adapted broken-off rod outer segments were loaded with different concentrations of all-trans retinal or decanal and subsequently with BODIPY C11. Light-induced oxidation was measured as the change in the “green”/“red” BODIPY C11 ratio caused by exposure to 10 sec of 360 nm light. (A) Light-induced oxidation increased with the concentration of exogenous all-trans retinal loaded in the outer segment. The concentrations of all-trans retinal used for loading were 10, 33, and 100 μM. The relative concentration of the exogenous all-trans retinal that accumulated in the outer segments was measured in separate experiments from its fluorescence (n = 10 cells for each retinal concentration). The basal fluorescence in control outer segments exposed to 1% BSA with no retinal added was 2.3±0.6 (Mean±SEM, n = 7). (B) Light-induced oxidation was not affected by loading with different concentrations of decanal. For both panels, the numbers of cells used for measuring light-induced oxidation are given in parentheses. Error bars represent standard errors.
It is possible that the higher photooxidation observed in the presence of all-trans retinal is not due to its photosensitizer properties but to its being an aldehyde that depletes free radical scavenger pools. This possibility was examined by loading dark-adapted broken-off rod outer segments with different concentrations of decanal. Figure 5B shows that loading the cells with different concentrations of decanal had no effect on light-induced oxidation (by 10 sec of 360 nm light). The concentrations of decanal used (10 and 100 μM) were the same as the low and high concentrations of all-trans retinal (in the experiments of Figure 5A), and so, broadly similar concentrations of aldehyde accumulating in the outer segments would be expected. The lack of an effect on photooxidation suggests that the higher light-induced oxidation by all-trans retinal is by virtue of its photosensitizer properties and not through depletion of scavenger pools.
It is possible that the increased level of photooxidation in broken-off rod outer segments could be due to a lack of reducing power. This is unlikely however, because photooxidation immediately after bleaching was the same in both metabolically intact and metabolically compromised rod outer segments. It should be noted that this observation is somewhat surprising, because the higher level of reducing power of metabolically intact cells should be associated with a larger concentration of free radical scavengers. Scavengers would drain off the free radicals generated by light, and so, in the presence of the same concentrations of photosensitizers, photooxidation would be expected to be lower in metabolically intact cells. A possible explanation for the lack of any observable effect of scavengers would be the spatial proximity between BODIPY C11 and all-trans retinal, as both of them are fairly hydrophobic and should dwell in outer segment membranes.
The all-trans retinal present in the metabolically intact cells 60 min after rhodopsin bleaching should also support photooxidation. Its concentration however is only 10–20% of that of all-trans retinal present in broken-off rod outer segments 60 min after bleaching and its effect was probably masked by the photooxidation mediated by BODIPY C11. It should be pointed out that the concentrations of all-trans retinal in these metabolically intact isolated cells arise partly from the slow removal of all-trans retinol, which allows for a significant back reaction. In the intact eye, all-trans retinol would be robustly removed by IRBP, which would result in even lower concentrations of all-trans retinal and levels of photooxidation (22). Thus, and within the resolution of the measurements, it would appear that although all-trans retinal can mediate photooxidation in the rod outer segment, the removal processes present in the intact retina would minimize its effects on light-induced oxidation. The contribution of all-trans retinal to light-induced oxidation would be expected to be significant mainly under conditions that would hinder its clearance, such as limited NADPH supply, lack of access to the retinol dehydrogenase, or a defect in the retinol dehydrogenase activity itself.
The results provide no information on the reactive species generated by all-trans retinal and responsible for the observed light-induced oxidation. It is very likely that singlet oxygen, 1O2, is a mediator (13, 14), but other free radical intermediates may play a role as well.
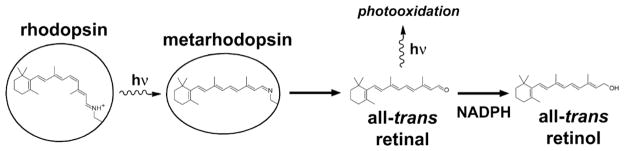
To summarize (Scheme 2), the results show that the all-trans retinal released from photoactivated rhodopsin during the course of light detection can mediate light-induced oxidation in single living rod photoreceptors. Because its absorption spectrum has a λmax ~ 380 nm and extends well into the visible range, all-trans retinal can mediate oxidation by visible light. The reduction of all-trans retinal to all-trans retinol (λmax ~ 325 nm) shifts the absorption spectrum further away from the visible range and protects photoreceptors from light-induced oxidation.
Scheme 2.
Reactions that affect the levels of all-trans retinal in a rod outer segment. Light isomerizes the 11-cis retinyl chromophore of rhodopsin to all-trans, generating a range of photointermediates, collectively referred to here as metarhodopsin. Subsequently, the photointermediates decay, and all-trans retinal is released and reduced to all-trans retinol. In the absence of sufficient levels of NADPH, all-trans retinal accumulates, promoting photooxidation of rod outer segment components.
Acknowledgments
Supported by NIH/NEI grant EY014850 and an unrestricted award to the Department of Ophthalmology at MUSC from Research to Prevent Blindness, Inc.
Footnotes
This invited paper is part of the Symposium in Print “Retinal Photodamage”
References
- 1.Ebrey T, Koutalos Y. Vertebrate photoreceptors. Prog Retin Eye Res. 2001;20:49–94. doi: 10.1016/s1350-9462(00)00014-8. [DOI] [PubMed] [Google Scholar]
- 2.Boulton M, Rozanowska M, Rozanowski B. Retinal photodamage. J Photochem Photobiol B. 2001;64:144–161. doi: 10.1016/s1011-1344(01)00227-5. [DOI] [PubMed] [Google Scholar]
- 3.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 4.Wiegand RD, Jose JG, Rapp LM, Anderson RE. Free radicals and damage to ocular tissues. In: Armstrong D, RSS, Cutler RG, Slater TF, editors. Free Radicals in Molecular Biology, Aging and Disease. Raven Press; New York: 1984. pp. 317–353. [Google Scholar]
- 5.Noell WK, Albrecht R. Irreversible effects on visible light on the retina: role of vitamin A. Science. 1971;172:76–79. doi: 10.1126/science.172.3978.76. [DOI] [PubMed] [Google Scholar]
- 6.Kretzer FL, Hittner HM, Johnson AT, Mehta RS, Godio LB. Vitamin E and retrolental fibroplasia: ultrastructural support of clinical efficacy. Ann N Y Acad Sci. 1982;393:145–166. doi: 10.1111/j.1749-6632.1982.tb31240.x. [DOI] [PubMed] [Google Scholar]
- 7.Winkler BS, Boulton ME, Gottsch JD, Sternberg P. Oxidative damage and age-related macular degeneration. Mol Vis. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- 8.Noell WK, V, Walker S, Kang BS, Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966;5:450–473. [PubMed] [Google Scholar]
- 9.Organisciak DT, Noell WK. The rod outer segment phospholipid/opsin ratio of rats maintained in darkness or cyclic light. Invest Ophthalmol Vis Sci. 1977;16:188–190. [PubMed] [Google Scholar]
- 10.Williams TP, Howell WL. Action spectrum of retinal light-damage in albino rats. Invest Ophthalmol Vis Sci. 1983;24:285–287. [PubMed] [Google Scholar]
- 11.Grimm C, Wenzel A, Hafezi F, Yu S, Redmond TM, Reme CE. Protection of Rpe65-deficient mice identifies rhodopsin as a mediator of light-induced retinal degeneration. Nat Genet. 2000;25:63–66. doi: 10.1038/75614. [DOI] [PubMed] [Google Scholar]
- 12.Redmond TM, Yu S, Lee E, Bok D, Hamasaki D, Chen N, Goletz P, Ma JX, Crouch RK, Pfeifer K. Rpe65 is necessary for production of 11-cis-vitamin A in the retinal visual cycle. Nat Genet. 1998;20:344–351. doi: 10.1038/3813. [DOI] [PubMed] [Google Scholar]
- 13.Delmelle M. Retinal sensitized photodynamic damage to liposomes. Photochem Photobiol. 1978;28:357–360. doi: 10.1111/j.1751-1097.1978.tb07718.x. [DOI] [PubMed] [Google Scholar]
- 14.Krasnovsky AA, Jr, Kagan VE. Photosensitization and quenching of singlet oxygen by pigments and lipids of photoreceptor cells of the retina. FEBS Lett. 1979;108:152–154. doi: 10.1016/0014-5793(79)81198-9. [DOI] [PubMed] [Google Scholar]
- 15.Shvedova AA, Alekseeva OM, Kuliev I, Muranov KO, Kozlov Yu P, Kagan VE. Damage of photoreceptor membrane lipids and proteins induced by photosensitized generation of singlet oxygen. Curr Eye Res. 1982;2:683–689. doi: 10.3109/02713688209019997. [DOI] [PubMed] [Google Scholar]
- 16.Sun H, Nathans J. ABCR, the ATP-binding cassette transporter responsible for Stargardt macular dystrophy, is an efficient target of all-trans-retinal-mediated photooxidative damage in vitro. Implications for retinal disease. J Biol Chem. 2001;276:11766–11774. doi: 10.1074/jbc.M010152200. [DOI] [PubMed] [Google Scholar]
- 17.Hubbard R, Brown PK, Bownds D. Methodology of vitamin A and visual pigments. Methods in Enzymology. 1971;18C:615–653. [Google Scholar]
- 18.Norren DV, Vos JJ. Spectral transmission of the human ocular media. Vision Res. 1974;14:1237–1244. doi: 10.1016/0042-6989(74)90222-3. [DOI] [PubMed] [Google Scholar]
- 19.Rozanowska M, Sarna T. Light-induced damage to the retina: role of rhodopsin chromophore revisited. Photochem Photobiol. 2005;81:1305–1330. doi: 10.1562/2004-11-13-IR-371. [DOI] [PubMed] [Google Scholar]
- 20.Futterman S, Hendrickson A, Bishop PE, Rollins MH, Vacano E. Metabolism of glucose and reduction of retinaldehyde in retinal photoreceptors. J Neurochem. 1970;17:149–156. doi: 10.1111/j.1471-4159.1970.tb02195.x. [DOI] [PubMed] [Google Scholar]
- 21.Okajima TI, Pepperberg DR, Ripps H, Wiggert B, Chader GJ. Interphotoreceptor retinoid-binding protein: role in delivery of retinol to the pigment epithelium. Exp Eye Res. 1989;49:629–644. doi: 10.1016/s0014-4835(89)80059-4. [DOI] [PubMed] [Google Scholar]
- 22.Wu Q, Blakeley LR, Cornwall MC, Crouch RK, Wiggert BN, Koutalos Y. Interphotoreceptor retinoid-binding protein is the physiologically relevant carrier that removes retinol from rod photoreceptor outer segments. Biochemistry. 2007;46:8669–8679. doi: 10.1021/bi7004619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palczewski K, Van Hooser JP, Garwin GG, Chen J, Liou GI, Saari JC. Kinetics of visual pigment regeneration in excised mouse eyes and in mice with a targeted disruption of the gene encoding interphotoreceptor retinoid-binding protein or arrestin. Biochemistry. 1999;38:12012–12019. doi: 10.1021/bi990504d. [DOI] [PubMed] [Google Scholar]
- 24.Ripps H, Peachey NS, Xu X, Nozell SE, Smith SB, Liou GI. The rhodopsin cycle is preserved in IRBP “knockout” mice despite abnormalities in retinal structure and function. Vis Neurosci. 2000;17:97–105. doi: 10.1017/s095252380017110x. [DOI] [PubMed] [Google Scholar]
- 25.Saari JC. Biochemistry of visual pigment regeneration: the Friedenwald lecture. Invest Ophthalmol Vis Sci. 2000;41:337–348. [PubMed] [Google Scholar]
- 26.Koutalos Y, Cornwall MC. Microfluorometric measurement of the formation of all-trans-retinol in the outer segments of single isolated vertebrate photoreceptors. Methods Mol Biol. 2010;652:129–147. doi: 10.1007/978-1-60327-325-1_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drummen GP, Gadella BM, Post JA, Brouwers JF. Mass spectrometric characterization of the oxidation of the fluorescent lipid peroxidation reporter molecule C11-BODIPY(581/591) Free Radic Biol Med. 2004;36:1635–1644. doi: 10.1016/j.freeradbiomed.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Pap EH, Drummen GP, Post JA, Rijken PJ, Wirtz KW. Fluorescent fatty acid to monitor reactive oxygen in single cells. Methods Enzymol. 2000;319:603–612. doi: 10.1016/s0076-6879(00)19056-1. [DOI] [PubMed] [Google Scholar]
- 29.Pap EH, Drummen GP, Winter VJ, Kooij TW, Rijken P, Wirtz KW, Op den Kamp JA, Hage WJ, Post JA. Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY(581/591) FEBS Lett. 1999;453:278–282. doi: 10.1016/s0014-5793(99)00696-1. [DOI] [PubMed] [Google Scholar]
- 30.Chen C, Tsina E, Cornwall MC, Crouch RK, Vijayaraghavan S, Koutalos Y. Reduction of all-trans retinal to all-trans retinol in the outer segments of frog and mouse rod photoreceptors. Biophys J. 2005;88:2278–2287. doi: 10.1529/biophysj.104.054254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsina E, Chen C, Koutalos Y, Ala-Laurila P, Tsacopoulos M, Wiggert B, Crouch RK, Cornwall MC. Physiological and microfluorometric studies of reduction and clearance of retinal in bleached rod photoreceptors. J Gen Physiol. 2004;124:429–443. doi: 10.1085/jgp.200409078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen C, Koutalos Y. Rapid formation of all-trans retinol after bleaching in frog and mouse rod photoreceptor outer segments. Photochem Photobiol Sci. 2010;9:1475–1479. doi: 10.1039/c0pp00124d. [DOI] [PMC free article] [PubMed] [Google Scholar]