Abstract
Background
Alcohol increases the expression of Group 1 metabotropic glutamate receptors (mGluRs), their associated scaffolding protein Homer2, and stimulates phosphatidylinositol 3-kinase (PI3K) within the nucleus accumbens (NAC). Moreover, functional studies suggest that NAC Group 1 mGluR/Homer2/PI3K signaling may be a potential target for pharmacotherapeutic intervention in alcoholism.
Methods
Immunoblotting was conducted to examine the effects of alcohol consumption under Drinking-in-the-Dark (DID) procedures on Group 1 mGluR-associated proteins in C57BL/6J (B6) mice. Follow-up behavioral studies examined the importance of Group 1 mGluR/Homer2/PI3K signaling within the NAC shell for limited access alcohol drinking. Finally, immunoblotting examined whether the NAC expression of Group 1 mGluR-associated proteins is a genetic correlate of high alcohol drinking using a selectively bred high DID (HDID-1) mouse line.
Results
Limited access alcohol drinking under DID procedures up-regulated NAC shell Homer2 levels, concomitant with increases in mGluR5 and NR2B. Intra-NAC shell blockade of mGluR5, Homer2, or PI3K signaling, as well as transgenic disruption of the Homer binding site on mGluR5 decreased alcohol consumption in B6 mice. Moreover, transgenic disruption of the Homer binding site on mGluR5 and Homer2 deletion both prevented the attenuating effect of mGluR5 and PI3K blockade upon intake. Finally, the basal NAC shell protein expression of mGluR1 and Homer2 was increased in offspring of HDID-1 animals.
Conclusions
Taken together, these data further implicate Group1 mGluR signaling through Homer2 within the NAC in excessive alcohol consumption.
Keywords: mGluR5 receptors, PI3 kinase, Homer, mGluR1 receptors, HDID-1
Introduction
Alcohol modulates amino acid neurotransmission through both ionotropic and metabotropic glutamate receptors (mGluRs) (Lovinger, 1996; Minami et al., 2003; Neiber et al., 1998; Wirkner et al., 2000), which are scaffolded at post-synaptic sites by the Homer family of proteins (c.f. Shiraishi-Yamaguchi and Furuichi, 2007). Homers interact directly with a proline-rich motif (PxxF) on Group 1 mGluRs (mGluR1/5), while mGluR/Homer complexes are coupled with PSD-95 by the Shank protein family (e.g., Tu et al., 1999) that links to the NMDA receptor/PSD-95/GKAP complex (Ehrengruber et al., 2004; Naisbitt et al., 1999). Alcohol administration, including binge alcohol drinking under Scheduled High Alcohol Consumption (SHAC) procedures, increases indices of glutamate receptor/Homer2 signaling, including phosphotidylinositol-3-kinase (PI3K) activation (Cozzoli et al., 2009; Goulding et al., 2011; Neasta et al., 2010; Obara et al., 2009; Szumlinski et al., 2008). Moreover, elevated NAC Homer2/PI3K activity is a genetic correlate of binge alcohol drinking under SHAC procedures (Cozzoli et al., 2009). At least in alcohol-preferring P rats, alcohol-induced increases in NAC glutamate receptor/Homer2 expression are restricted to the core subregion (Obara et al., 2009), while, in both C57BL/6J (B6) and DBA2/J mice, repeated, experimenter-administered, alcohol injections tend to produce parallel changes in glutamate receptor/Homer expression within both the NAC shell and core (Goulding et al., 2011). Earlier studies of drinking-induced changes in NAC glutamate receptor/Homer/PI3K expression (including those changes elicited by a history of binge alcohol drinking) failed to discriminate between NAC subregions (Cozzoli et al., 2009; Neasta et al., 2010; Szumlinski et al., 2008) and so it remains to be determined whether or not a history of binge alcohol drinking differentially influences glutamate-related protein adaptations within specific NAC subregions
Understanding how binge alcohol drinking influences glutamate receptor/Homer2/PI3K expression within the NAC has behavioral relevance as inhibition of PI3K within the NAC reduces alcohol drinking under SHAC procedures (Cozzoli et al., 2009) and in rat models of intermittent alcohol access (Neasta et al., 2011). Moreover, the “anti-binge” effects of NAC PI3K blockade are (1) not additive with those produced by NAC infusions of mGluR5 antagonists and (2) absent in mice with a point mutation on mGluR5 disrupting Homer interactions (Cozzoli et al., 2009). In order to fully understand the role of a particular neurobiological substrate in alcoholism, we argue that one has to approach the research question from different angles and using different models. Thus, to increase our understanding of the role played by mGluR/Homer/PI3K signaling in the neurobiology of binge alcohol drinking, the present study sought to replicate, and importantly to extend, our earlier behavioral data derived from studies employing SHAC binge alcohol drinking procedures (Cozzoli et al., 2009). To accomplish this, the present studies employed a procedurally-simple, and increasingly popular (e.g., Crabbe et al., 2009; Blednov and Harris, 2008; Gupta et al., 2008; Kamdar et al., 2007; Moore and Boehm, 2009; Mulligan et al., 2011) alternate murine model of binge alcohol intake termed Drinking-in-the-Dark (DID) (Rhodes et al., 2005). While alcohol intake under DID procedures is not affected by mGluR5 deletion (Blednov and Harris, 2008), it can be attenuated by systemic pretreatment with mGluR5 antagonists (Blednov and Harris, 2008; Gupta et al., 2008). Moreover, the results of a recent cDNA microarray study and meta-analysis indicate significant positive (1–1.5-fold) associations in PI3K mRNA expression within striatal structures and binge alcohol drinking under DID procedures (Mulligan et al., 2011). These data support the working hypothesis that increased mGluR5 signaling through PI3K within the NAC is a mechanism contributing to the manifestation of excessive alcohol intake.
Materials and Methods
Animals and General Husbandry
C57BL/6J mice
The majority of subjects used in this study were adult male, inbred C57BL/6J (B6) mice (8 weeks of age; 25–30 g; The Jackson Laboratory, Bar Harbor, ME). For all drinking experiments, animals were single-housed in polyethylene cages in a temperature (25°C)- and humidity (71%)-controlled colony room under a 12-hour reverse light cycle (lights off at 11:00 A.M.; lights on at 11:00 P.M.). Food and water was available ad libitum.
F/R knock-in (KI) mGluR5 and Homer2 knock-out (KO) mice
To examine the functional relevance of mGluR5-Homer interactions and of Homer2 for binge drinking behavior, we employed (1) male transgenic mice with a phenylalanine (F) to arginine (R) point mutation at amino acid position 1128 of mGluR5 (F/R KI) that disturbs the physical interaction between Homers and mGluR5 (Cozzoli et al., 2009) and (2) male Homer2 null mutant mice (e.g., Szumlinski et al., 2005). For both strains, male mice were generated from heterozygous breeder pairs (C57BL/6J × 129Xi/SvJ background) and housed under conditions described for the B6 mice above. All testing for behavior commenced when mice were 7–8 weeks of age.
HDID-1 mice
Whole brains were obtained from the 15th generation of a mouse line (generated in the laboratory of Dr. J.C. Crabbe, Oregon Health and Science University, Portland, OR) selectively bred to exhibit a high blood alcohol concentrations (BACs) under DID procedures (hereafter referred to as HDID-1 mice) versus their HS/Npt founder population as described previously (Crabbe et al., 2009). Animals were alcohol-naïve.
All experimental protocols were approved by the Institutional Animal Care and Use Committee of our respective institutions and were consistent with the guidelines provided by the National Institute of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH publication number 80-23, revised 1996).
Drinking-in-the-Dark Procedures
To elicit consistently high alcohol consumption, we employed a version of the Drinking-in-the-Dark (DID) model, in which mice consume between 3.5–5.0 g/kg alcohol in a 2-hour period (Crabbe et al., 2009; Gupta et al., 2008; Moore and Boehm, 2009; Rhodes et al., 2005). In brief, at 3 hours after lights out, the home cage water bottle was removed from the cage and replaced with a 50 ml sipper tube containing 20% alcohol in tap water (v/v). Mice were allowed to drink from the tubes for a total of 2 hours, at which point the alcohol bottles were removed from the cages and the home cage water bottles were replaced. In certain experiments, mice were also presented with 5% alcohol to determine whether or not our manipulations influenced alcohol sensitivity.
Immunoblotting
B6 mice were subjected to 30 consecutive days of alcohol or water drinking under DID procedures (see above). Twenty-four hours following the last drinking session, tissue from the NAC core and shell were processed by immunoblotting as described in detail previously (Cozzoli et al., 2009). A second immunoblotting study was conducted on tissue derived from flash-frozen brains of selectively bred HDID-1 and control mice from their heterogeneous HS/Npt stock founder line (Crabbe et al., 2009). As in our previous report (Cozzoli et al., 2009), the p(Tyr)p85α PI3K binding motif was used to index PI3K activity (Zhang et al., 2006) in the HDID-1 immunoblotting study. Unfortunately, at the time of immunoblotting for core-shell differences in the effects of drinking under DID procedures, this antibody was no longer commercially available. As activated PI3K phosphorylates Akt at Ser473 (e.g, Chua et al., 2009; Sarbassov et al., 2005), we examined the relative levels of pSer473-Akt (Cell Signaling Technology, Beverly, MA, USA; 1:250 dilution), versus total Akt (Cell Signaling Technology, Beverly, MA, USA; 1:1000 dilution) following chronic drinking under DID procedures to index PI3K activity in this study. Unfortunately, the results for Akt activation within both the NAC core and shell were highly variable (see Figure 1), precluding any confirmation of alcohol-induced changes in PI3K activity and we had insufficient core and shell tissue remaining from this study to conduct any further immunoblotting assays with statistical confidence (n=5–6/group). Thus, upon the advent of a new commercially available antibody that recognizes p-(Tyr458) on the p85 regulatory subunit of PI3K (Cell Signaling Technology; 1:1000 dilution), we revisited the question of binge alcohol-induced changes in NAC PI3K activity in tissue homogenate from the entire NAC of mice with a 30-day drinking history under DID procedures that was available for assay in our laboratory.
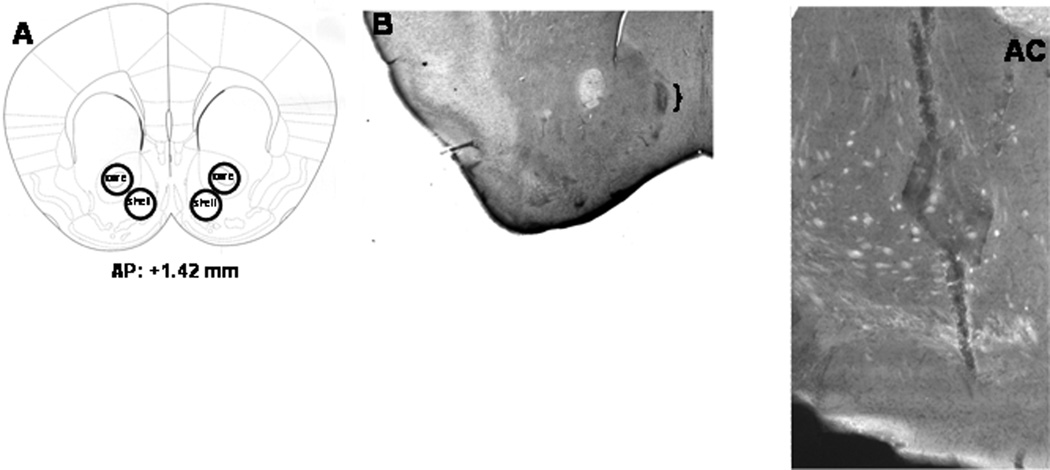
Figure 1. Verification of micropunches, specificity of transduction, and microinjector placements.
A, Shematic of a coronal section of the mouse brain, indicating representative sites of micropunches for the accumbens core and shell. Below the section is the approximate level from Bregma where the punches were acquired (modified from Paxinos and Franklin, 2004). B, Representative micrograph of the placement of the microinjector tip within the NAC shell of B6 mice. Only mice whose microinjectors were located within the boundaries of NAC shell were included in the statistical analysis of the data. C, Representative 10X micrograph of immunostaining in the left NAC shell of a B6 mouse infused with our AAV-shRNA vector. Other examples of AAV transduction, as well as verification of protein knock-down by immunoblotting procedures, is provided in Cozzoli et al. (2009).
Surgical and infusion procedures
The procedures for implanting bilateral guide cannulae and microinjecting drugs or AAVs into the NAC shell of mice were very similar to those described in detail in previous studies (Cozzoli et al., 2009; Kapasova and Szumlinski, 2008; Szumlinski et al., 2008). It should be noted that the AAV-infused animals described in the present report were studied previously for the effects of Homer2b knock-down upon binge alcohol drinking under SHAC procedures (Cozzoli et al., 2009) and testing for AAV-infused mice for binge alcohol drinking under DID procedures commenced following a 1-week wash-out period in which mice had free-access to the home cage water bottle. All details pertaining to the construction and infusion of our AAV constructs are provided in Cozzoli et al. (2009), as is immunoblotting evidence that our AAV-shRNA construct produced enduring Homer2 knock-down selectively within the NAC shell that was apparent at the end of the behavioral testing of the animals (i.e., at the end of the experiments presented in this report).
For behavioral pharmacological experiments, mice were presented with 20% alcohol (v/v) for 2 hours, every day, until stable intake was established (<10% variability across three consecutive presentations; approximately 3–4 presentations). The mGluR5 antagonist MPEP [2-methyl-6-(phenylethynyl)pyridine hydrochloride] (Sigma-Aldrich, St. Louis, MO, USA; 0, 0.1, 0.3, 1.0, and 3.0 µg/side), the mGluR1a antagonist CPCCOEt [7-(hydroxyimino)cyclopropa[b] chromen-1a-carboxylate ethyl ester] (Tocris Cookson, Ellisville, MO, USA; 0 and 3.0 µg/side), and the non-selective PI3K antagonist wortmannin (Sigma-Aldrich, St. Louis, MO, USA; 0 and 50 ng/side), were infused as described previously (Cozzoli et al., 2009). As wortmannin is well-characterized to inhibit also polo-like kinase 1 (PLK-1) (Liu et al., 2007), we conducted a follow-up experiment in which animals were infused with IC50 doses of the highly selective PI3K antagonist LY 294002 [2-(4-Morpholinyl)-8-phenyl-4H-1-benzopyran-4-one hydrochloride] (Tocris; 0 and 0.17 ng/side; Walker et al. 2000) and the highly selective PLK-1 antagonist cyclapolin 9 [7-Nitro-5-(trifluoromethyl)-2-benzo thiazolecarboxamide-3-oxide] (Tocris; 38 pg/side) or 0.1% DMSO vehicle. For all microinjection studies, mice were returned to their home cage immediately following microinjection and presented with the 20% alcohol-containing sipper tube for 2 hours. Within each experiment, mice received at least two alcohol bottle presentations between intra-NAC drug tests to examine for potential carryover effects of pretreatment and to ensure that alcohol drinking had re-stabilized post-infusion. As observed under SHAC procedures (Cozzoli et al., 2009), in none of the experiments were carryover effects observed (data not shown), indicating that a transient inactivation of mGluR5/PI3K signaling does not affect subsequent binge drinking. The order of the dosing was randomized across test days and the maximally effective antagonist dose for reducing alcohol intake was also examined for effects upon water and 5% sucrose intake during a 2-hour session. Standard cresyl violet histochemical procedures were used to verify injector localization (Figure 1B).
Statistical analysis
All data were analyzed for significance using SPSS. Immunoblotting data were statistically evaluated using Student’s t tests and ANOVAs were employed for all behavioral studies. When appropriate, post- hoc comparisons were made using Fisher’s least significant difference test. α = 0.05 for all analyses.
Results
Immunoblotting following alcohol drinking under DID procedures
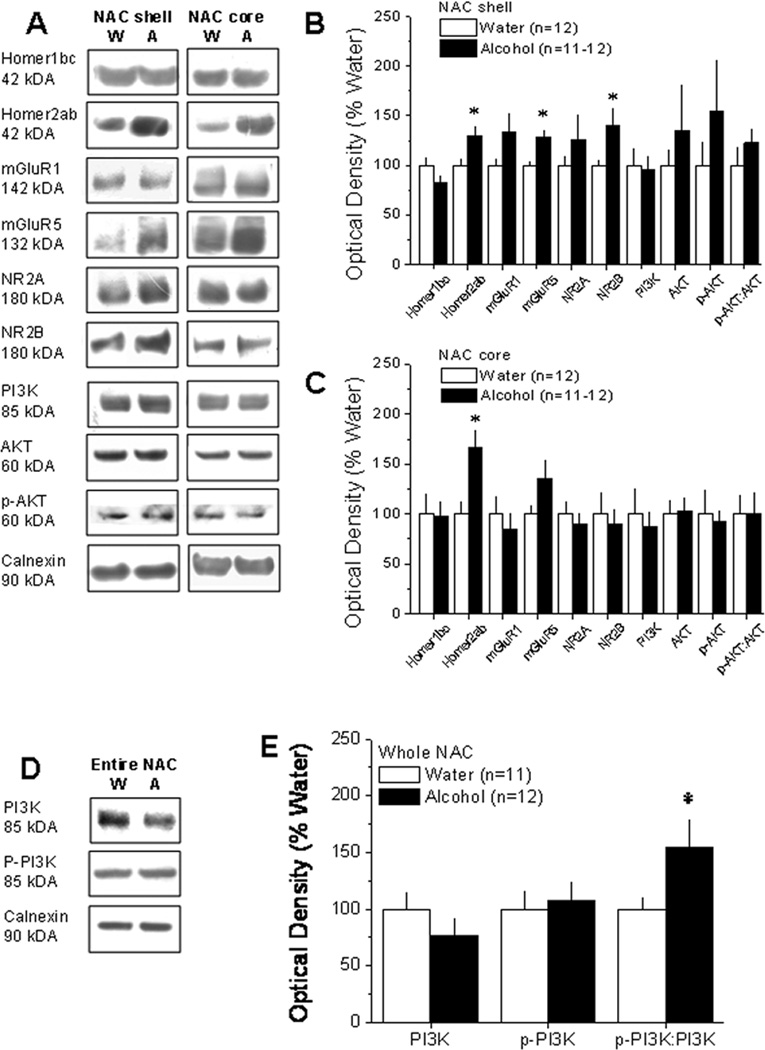
The average alcohol intake exhibited by the B6 mice under DID procedures was approximately 4.8 g/kg/2 hour. As summarized in Figure 2B, DID drinking significantly increased NAC shell levels of Homer2a/b (t21=2.515, p=.02), mGluR5 (t22=3.806, p=.001), and NR2B (t22=2.129, p=.05). In contrast, the NAC shell levels of Homer1b/c, mGluR1, NR2A, and PI3K were unaffected by alcohol intake (all p’s > 0.10). Our data for both total and p-Ser473-Akt (the index of PI3K activity employed in this experiment) was highly variable and the DID-induced elevation in the relative amount of p-Ser473-Akt failed to reach statistical significance (p=.35). As shown in Figure 2C, with the exception increased Homer2a/b (t20=3.253, p=.004), no statistically significant alcohol-induced changes in protein expression were observed in the NAC core (all p’s>0.1). While we were unable to assay for core-shell distinctions in the relative expression p-Tyr(458)-PI3K due to insufficient tissue, an analysis of the relative levels of phospho-to-total levels of p85 within entire NAC tissue revealed an up-regulation of the phospho-to-total protein ratio (t21=2.16, p=.04), without significant alterations in total levels of either total p85 or p-Tyr(458)-PI3K (p’s>.25).
Figure 2. 30 days of alcohol drinking under DID procedures up-regulated indices of glutamate signaling within the NAC.
A, Representative immunoblots for the total protein levels of Homer1b/c, Homer2a/b, mGluR1, mGluR5, NR2A, NR2B, PI3K, Akt, p-Ser473-Akt (p-AKT), and Calnexin in the NAC shell and core of mice killed at 24 hours withdrawal after 30 days of 2-hour access to 20% alcohol (A) or water (W). B, Summary of the change in protein expression within the NAC shell at 24 hours withdrawal from 30 days of alcohol drinking under DID procedures (Alcohol), expressed as a percent of the average protein expression of water-drinking controls (Water). Compared to water controls, excessive alcohol intake under DID procedures significantly increased NAC shell mGluR5, Homer2a/b, and NR2B expression. C, A parallel study conducted on tissue from the NAC core revealed a significant increase in Homer2a/b expression only. D, Representative immunoblots for the total protein levels of the p85 subunit of PI3K, p-Tyr458-p85 and their ratio in tissue from the entire NAC of mice taken at 24 hours withdrawal from a 30-day history of alcohol intake under DID procedures. E, Alcohol drinking mice exhibited greater relative expression of the phosphorylated versus total isoform of p85. Data represent the mean ± SEM of the number of animals indicated in the figure. *p<0.05 (t-tests).
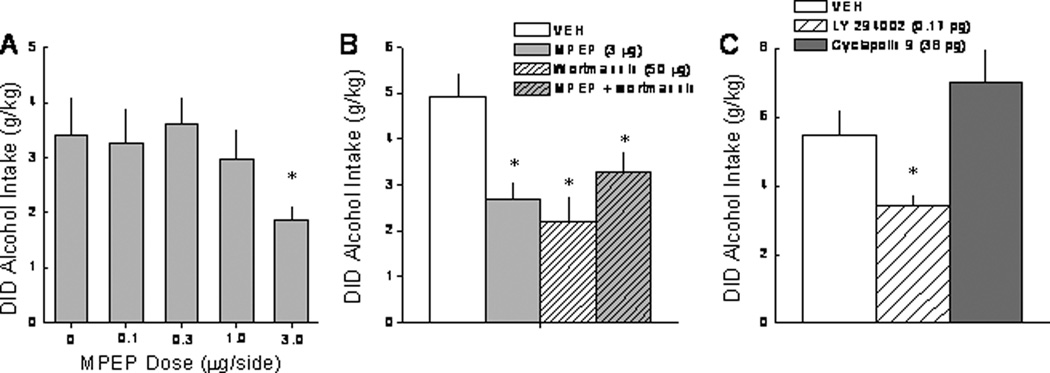
NAC blockade of mGluR5 and PI3K on alcohol drinking under DID procedures in B6 mice
An intra-NAC infusion of the mGluR5 antagonist MPEP reduced alcohol intake in the DID paradigm at the highest dose tested (Figure 3A) [F(3,46)=3.05, p=0.04]. While the 3.0 µg MPEP dose reduced alcohol intake, it did not significantly affect water or 5% sucrose intake under identical conditions (Table 1; p>0.05). As reported previously under SHAC conditions (Cozzoli et al., 2009), an intra-NAC infusion of the mGluR1 selective antagonist CPCCOEt (3 µg/side) produced a moderate, but non-significant reduction in alcohol consumption under DID procedures (VEH: 3.41 ± 0.66 g/kg; CPCCOEt: 2.18 ± 0.46 g/kg; p>0.05). An intra-NAC infusion of the non-selective PI3K inhibitor wortmannin (50 ng/side) reduced alcohol drinking by B6 mice and these effects were not additive with those of the mGluR5 antagonist MPEP (Figure 3B) [F(4,38)=7.76, p<0.0001; post-hoc tests]. Similar to MPEP, an infusion of wortmannin failed to reduce significantly water or 5% sucrose consumption in these mice (Table 1; p>0.05), although the reduction in water intake approached statistical significance [t14=1.973, p=.07]. Addressing the PI3K-specificity of the wortmannin effect, an infusion of highly PI3K-selective antagonist LY 294002 (0.17 ng/side) also reduced alcohol intake below that exhibited by animals infused with 0.1% DMSO vehicle, while infusion of the highly selective PLK-1 inhibitor cyclapolin 9 (38 pg/side) was ineffective (Figure 3C) [Drug effect: F(2,12)=5.34, p=0.02; post-hoc tests].
Figure 3. Blockade of NAC shell mGluR5 and PI3K, but not mGluR1, reduces limited access alcohol drinking in B6 mice.
Summary of the effects of an intra-NAC shell infusion of the mGluR5 antagonist MPEP (A), the non-selective PI3K antagonist wortmannin, as well as the combination of MPEP + wortmannin (B) and the selective PI3K antagonist LY 294002 (C) upon 20% alcohol intake during a 2-hour period under DID procedures. The data represent the mean ± SEM of the animals indicated in the figure. *p<0.05 vs. respective vehicle pre-treatment (i.e., 0 dose).
Table 1.
Summary of the means ± SEM of the effects of intra-NAC infusion of vehicle, MPEP, and wortmannin on the intake of water and 5% sucrose (in ml’s) in the DID model.
| Water (n=7–8) |
5% Sucrose (n=8) |
|
|---|---|---|
| Vehicle | 26.51±4.86 | 2.09±0.37 |
| 3.0 µg MPEP | 23.02±5.11 | 2.13±0.44 |
| 50 ng Wortmannin | 13.13±4.73 | 2.15±0.43 |
mGluR5-Homer binding in binge alcohol drinking under DID procedures
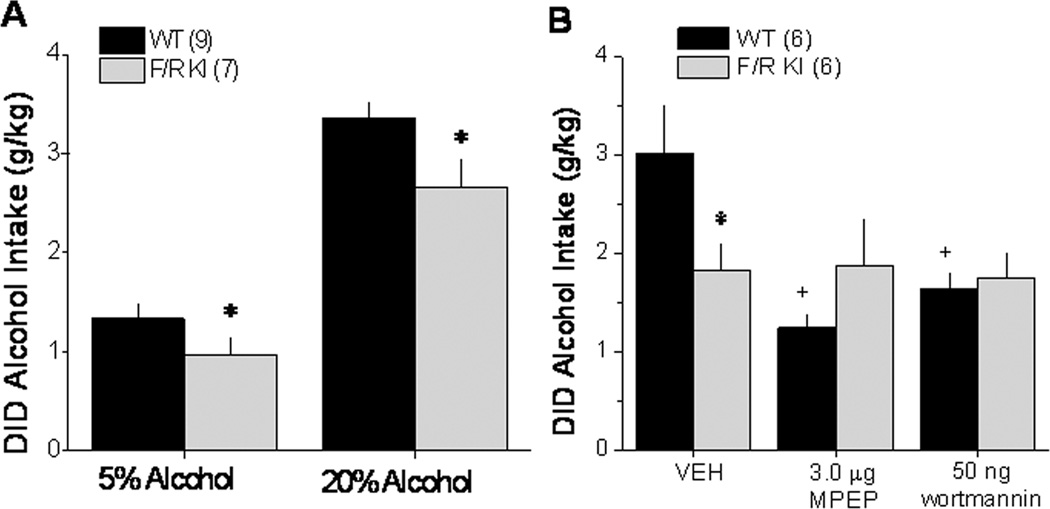
Compared to their WT littermates, male F/R KI mice exhibited blunted alcohol drinking at both high (20%) and low (5%) alcohol concentrations (Figure 4A) [Alcohol effect: F(1,14)=224.71, p<.0001; F/R KI effect: F(1,14)=5.39, p=.04; interaction: p=.22]. As illustrated in Figure 4B, the attenuating effects of our intra-NAC pharmacological manipulations upon the intake of 20% alcohol under DID procedures appeared to be selective for WT animals [Pretreatment × Genotype interaction: F(2,20)=7.389, p=0.004]. Deconstruction of our interaction along the Genotype factor confirmed in WT mice a significant reduction in drinking by both intra-NAC MPEP and wortmannin [F(2,10)=10.92, p=.003; post-hoc tests]. In contrast, neither of these manipulations altered drinking in KI animals (one-way ANOVA, p>0.05).
Figure 4. mGluR5-Homer binding is important for alcohol drinking under DID procedures and for the attenuating effects of NAC mGluR5 and PI3K blockade.
A, Summary of the average alcohol intake of wild-type (WT) and F1128R mGluR5 knock-in (F/R KI) mice with disrupted Homer-mGluR5 interactions. B, Summary of the effects of an intra-NAC shell infusion of effective doses of MPEP and wortmannin (see Figure 3) upon 20% alcohol intake under DID procedures exhibited by WT and F/R KI mice. The data represent the means ± SEM of the number of animals indicated in the figure. *p<0.05 WT vs. F/R KI, +p<0.05 vs. vehicle (0 dose).
Homer2 and binge alcohol drinking under DID procedures
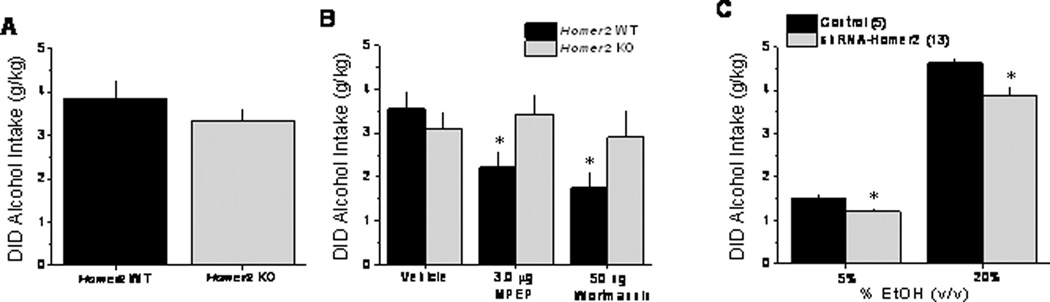
In contrast to the results of our studies using the F/R KI mice, but consistent with earlier data for mGluR5 KO animals (e.g., Bledinov and Harris, 2008), Homer2 KO mice exhibited WT-levels of 20% alcohol intake under DID procedures when tested in the absence of any manipulation (Figure 5A). Despite this, WT-KO differences were observed regarding the attenuating effects of intra-NAC MPEP and wortmannin pretreatment upon the intake of 20% alcohol (Figure 5B) [Pretreatment × Genotype: F(3,30)=3.93, p=.02]. Deconstruction of our interaction along the Genotype factor confirmed a significant reduction in drinking by intra-NAC MPEP and wortmannin in WT mice [F(3,15)=6.82, p=.004; post-hoc tests], but not in Homer2 KO animals (p>.05).
Figure 5. NAC shell Homer2 plays a role in mediating alcohol drinking under DID procedures and is necessary for the attenuating effects of NAC mGluR5 and PI3K blockade.
A, Summary of the average intake of 20% alcohol by Homer2 wild-type (Homer2 WT) and constitutive knock-out (Homer2 KO) mice. B, Summary of the effects of an intra-NAC shell infusion of effective doses of MPEP and wortmannin (see Figure 3) upon the 20% alcohol intake under DID procedures exhibited by Homer2 WT and KO mice. C, Summary of the effects of intra-NAC shell infusions of Homer2 shRNA or a scrambled control shRNA (control) upon the average alcohol intake of B6 mice over the course of 4 days of alcohol drinking under DID procedures. The data represent the mean ± SEM of the number of animals indicated in the figure. *p<0.05 vs. vehicle (Panel B) or control (Panel C).
To examine the possibility that some developmental compensation in the Homer2 KO mouse might be masking the effects of gene deletion upon limited access alcohol drinking, we investigated the impact of knocking-down Homer2b levels within the NAC shell upon alcohol consumption under DID procedures. Homer2b knock-down significantly reduced alcohol intake in the DID model and this effect was observed at both 5% and 20% alcohol, indicating a downward shift in the dose-response function by this pretreatment (Figure 5C) [Alcohol effect: F(1,16)=388.69, p<.0001; shRNA effect: F(1,16)=6.94, p=.02; interaction: p=.14]. Moreover, this attenuation was specific to alcohol consumption as we showed previously in these same animals that NAC shell Homer2b knock-down did not affect water or sucrose intake (Cozzoli et al., 2009).
Immunoblotting in selectively bred HDID-1 mice
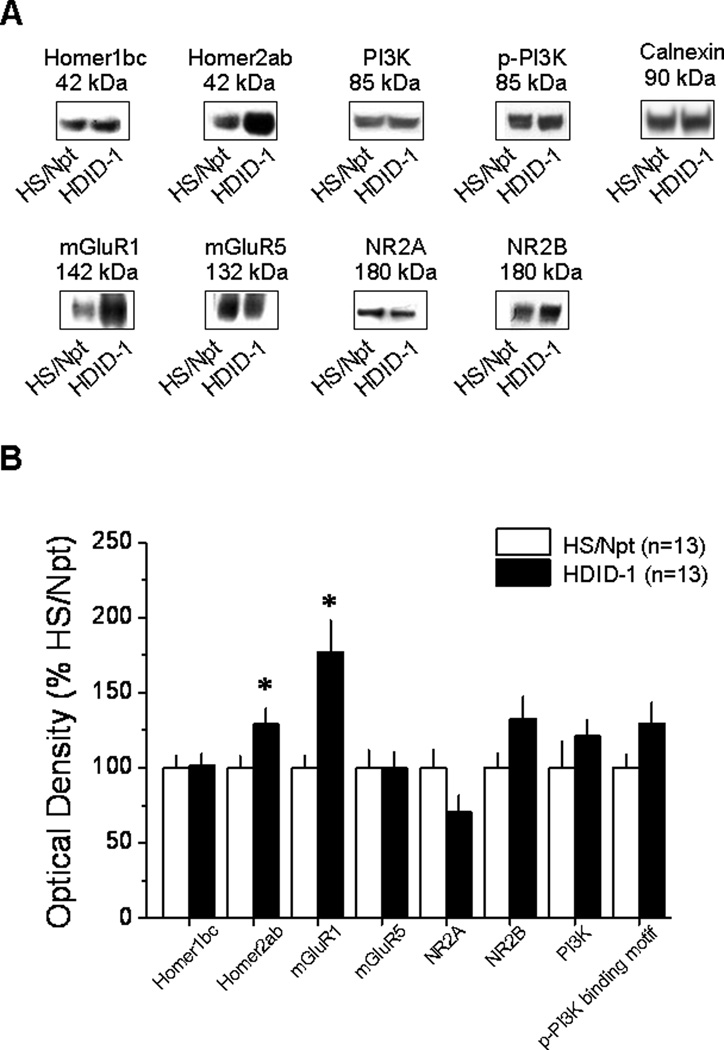
As shown in Figure 6, Homer2a/b (t26=−2.21, p=.036) and mGluR1 (t26=−3.289, p=.003) expression were increased significantly within the NAC shell in alcohol-naïve HDID-1 mice, compared to HS/Npt controls. While there appeared to be strain-dependent differences in the expression of NR2A, NR2B, total PI3K and phospho-p85α PI3K binding motif, these differences were not statistically reliable (for all proteins, p=0.09). Additionally, strain differences in protein expression seemed to occur preferentially within the NAC shell as no significant differences in protein expression were found in the NAC core (Table 2).
Figure 6. Differences in NAC shell Homer2 and mGluR1 expression in selectively bred high DID mice.
A, Representative immunoblots for the total protein levels of Homer1bc, Homer2ab, mGluR1, mGluR5, NR2A, NR2B, PI3K, p-(Tyr)p85α (p-PI3K), and Calnexin in the 15th generation (S15) selectively bred HDID-1 mice compared with the HS/Npt founder population. B, Summary of the differences in basal protein expression within the NAC shell of HDID-1 mice, expressed as a percent of average levels of proteins in HS/Npt control mice. Data represent the means ± SEM of the number of animals indicated in the figure. *p<0.05 vs. HS/Npt (t-tests).
Table 2.
Comparison of the means ± SEM of the basal protein expression of Homer1b/c, Homer2a/b, mGluR1, mGluR5, NR2A, NR2B, PI3K, and p-(Tyr)p85α PI3K binding motif [p-PI3K] within the NAC core in the 15th generation offspring of mice selectively bred for a high DID drinking phenotype (HID-1; n=14), compared with the HS/Npt founder population (HS/Npt; n=14). The data are expressed as a percent of the mean optical density values of HS/Npt animals.
| HS/Npt | HDID-1 | |
|---|---|---|
| Homer1b/c | 100±8.67 | 106.88±9.42 |
| Homer2a/b | 100±14.22 | 82.03±12.53 |
| mGluR1 | 100±16.05 | 151.12±20.72 |
| mGluR5 | 100±8.52 | 96.76±12.66 |
| NR2A | 100±17.13 | 109.43±16.17 |
| NR2B | 100±7.32 | 98.39±8.86 |
| PI3K | 100±15.51 | 106.82±20.0 |
| p-PI3K | 100±17.30 | 100.81±15.15 |
| binding motif |
Discussion
Here we show that a prolonged (30-day) history of binge alcohol intake under DID procedures up-regulates PI3K activity within the entire NAC and increases the expression of mGluR5, Homer2 and NR2B in the NAC shell subregion. Also, basal Homer2, as well as mGluR1, expression within the NAC shell is a genetic correlate of binge drinking in selectively bred HDID-1 mice. Moreover, we confirm an important role for mGluR5-Homer2-PI3K signaling within the NAC shell in maintaining high levels of alcohol intake and demonstrate conclusively that intact protein-protein interactions with Homer2 are required for the attenuating effects of mGluR5 and PI3K antagonists upon binge drinking.
NAC subregional differences in the relation between binge alcohol drinking and Homer2 expression?
The NAC core and shell are components of functionally and anatomically distinct subcircuits (Heimer et al., 1997), both of which are involved in regulating specific aspects of drug self-administration (c.f., Everitt et al., 2008). While many of the previous studies examining for alcohol effects upon Homer/glutamate receptor expression did not discriminate between NAC subregions (e.g., Cozzoli et al., 2009; Neasta et al., 2010; Szumlinski et al., 2008), a persistent increase in Group 1 mGluR/Homer/NR2 protein expression occurs selectively within the NAC core in P rats with an extensive (6-month) history of alcohol drinking (Obara et al., 2009). Herein, NAC core Homer2a/b expression was elevated (~ 66%) at 24 hrs withdrawal from a 1-month history of binge alcohol intake under DID procedures (Figure 2A,C); however, no other protein change within the NAC core reached statistical significance, although there was a trend for a rise (~40%; p=.11) in mGluR5 levels. In contrast to the P rat study by Obara et al. (2009) where protein changes were observed only in the core subregion, but consistent with data from the entire NAC tissue of alcohol-drinking mice (Cozzoli et al., 2009; Szumlinski et al., 2008), a month-long history of binge alcohol drinking under DID procedures elevated both Homer2a/b and NR2B levels within the NAC shell. Interestingly, we also observed an alcohol-induced rise in NAC shell mGluR5 levels in the present study (Figure 2A,B) that was not observed in our previous reports for alcohol-drinking B6 mice (Cozzoli et al., 2009; Szumlinski et al., 2008). In all likelihood, the more pronounced effect of binge alcohol intake upon mGluR5 expression observed within both the NAC shell and core in the present study reflects some combination of the daily pattern and the total duration of alcohol drinking, as no changes in NAC mGluR5 expression are observed in: (1) B6 mice with a relative short history of binge alcohol drinking under SHAC procedures (6 exposures; daily intakes of ~ 1.5 g/kg/30 min; Cozzoli et al., 2009); (2) B6 or DBA2/J mice subjected to repeated (8), intermittent injections of 2 g/kg alcohol (Goulding et al., 2011); or (3) B6 mice with a long history of alcohol intake under 24-hr free-access conditions (3 months; daily intakes of ~11 g/kg; Szumlinski et al., 2005).
It remains to be determined whether or not the existing discrepancies regarding the subregional localization of alcohol-induced increases in NAC glutamate receptors/Homer2a/b relates to: (1) species or strain (within mouse) differences in the relative responsiveness of the NAC shell and core to alcohol; (2) differences in the daily availability of alcohol (i.e., short vs. long-access); (3) differences in the duration of alcohol access (1 month vs. 6 months); or some combination thereof. However, in support of the latter possibilities, activity within the NAC core is theorized to be involved in the transition to habitual drug-seeking and –taking behavior (c.f., Everitt et al., 2008). While requiring further study, it is quite possible that with prolonged alcohol self-administration experience (>1 month), there is a progressive transition in excitatory signaling to more dorsal structures within the striatum, commencing with increased Homer2a/b expression. In some support of this assertion, there is a progressive and cumulative alcohol-induced increase in NR2B-dependent NMDA receptor activity and trafficking of the receptor to the plasma membrane within dorsomedial striatum of rats subjected to repeated injections of 2 g/kg alcohol (Wang et al., 2011) and increased NR2B-dependent NMDA receptor activity within dorsal striatum is observed in rats with a history of chronic alcohol drinking (5–6 g/kg/day; Wang et al., 2010). However, it still remains to be determined whether or not this latter effect reflects an alcohol history-dependent transition in glutamatergic signaling from ventral to more dorsal striatal subregions.
In our earlier SHAC study (Cozzoli et al., 2009), the rise in Homer2/glutamate receptor expression within entire NAC homogenate co-occurred with elevated indices of PI3K activity as assayed using an antibody against the p-(Tyr)p85α PI3K binding motif (Zhang et al., 2006). As this antibody was no longer commercially available at the time of this study, we employed 2 strategies to examine for binge alcohol-induced changes in PI3K under DID procedures. We first processed NAC core and shell tissue using an antibody against p-Ser473-Akt, as this site is known to be phosphorylated by PI3K (c.f., Steelman et al., 2011). While p-Ser473-Akt tended to rise in the NAC shell (~25%) of alcohol-drinking animals, the group difference was not statistically significant (Figure 2). These findings contrast with reported increases in p-Ser473-Akt, as well as p-Thr308-Akt, in the entire NAC of rats with a 2-month history of intermittent access to 20% alcohol (Neasta et al., 2011). Whether our modest alcohol effects upon NAC p-Ser473-Akt levels relates to the shorter duration of alcohol exposure, the species studied, or the fact that our mice did not undergo repeated bouts of alcohol withdrawal during the drinking phase of the experiment remains to be determined. Ser473-Akt can be phosphorylated by at least 92 different kinases, which include: PI3K, as well as PKCα, PKCβII, PKCε, mTOR and choline kinase (Chua et al., 2009). In brain, alcohol affects the expression/translocation of all of these PKC isozymes (c.f., Newton and Ron, 2007), as well as mTOR activity (Li and Ren, 2007; Neasta et al., 2010). However, the experimental parameters that influence the direction and magnitude of alcohol’s effects upon the activity of these kinases within specific brain regions are not fully characterized (c.f., Newton and Ron, 2007). Thus, the possibility remains that binge alcohol drinking may have opposing influences on PI3K and one or more of these other Akt-targeting kinases, which could mask our ability to detect group differences in Akt activation. Arguing in favor of this latter explanation, we were able to demonstrate that a month-long history of binge drinking under DID procedures increases the relative expression of p-(Tyr458)-p85 in the NAC (Figure 2D,E) – a very direct indicator of increased PI3K activation (c.f., Osaki et al., 2009). While it is unfortunate that we were unable to assay for core-shell differences using this new, direct, marker of PI3K activity, the present data are sufficient to demonstrate cross-model generalization of binge alcohol-induced increases in NAC PI3K activity during short-term withdrawal and further implicate heightened NAC PI3K activity as an important mediator of continued, excessive alcohol drinking.
NAC shell Homer2/mGluR1 expression is a genetic correlate of binge drinking under DID procedures
The comparison of basal protein expression between HDID-1 mice and HS/Npt mice revealed differences in protein expression within the NAC shell only (Figure 6 vs. Table 2). As observed for whole NAC tissue homogenate from mice selectively bred SHAC versus SLAC mice (Cozzoli et al., 2009), basal Homer2a/b expression and mGluR1 expression were significantly elevated in the NAC shell of HDID-1 mice (Figure 6). This consistency in findings across distinct selectively bred mouse lines, coupled with recent data derived from immunoblotting studies of inbred mouse strains with divergent alcohol preference/intake (Goulding et al., 2011), provide very strong evidence that elevated Group1 mGluR and Homer2a/b expression within the NAC shell is a genetic correlate of an excessive alcohol drinking phenotype. The phenotypic characterization of the HDID-1 line is still in its infancy and thus, it is not possible at the present time to know whether or not the elevated basal protein expression of HDID-1 mice might correlate with other behavioral traits. However, HDID-1 mice were reported recently to not differ from HS/Npt controls regarding bitter or sweet tastant sensitivity or regarding their total daily intake of 3–25% alcohol under 2-bottle-choice, free-access drinking procedures (Crabbe et al., 2011). While HDID-1 lines drank less water overall and tended to prefer moderate alcohol concentrations (e.g., 9% alcohol), compared to HS/Npt mice, their intake and preference for alcohol concentrations greater than 25% was significantly less than that exhibited by mice from the founder population (Crabbe et al., 2011). Thus, while Homer2b over-expression within the NAC promotes alcohol-induced increases in both dopamine and glutamate within the NAC (Goulding et al., 2011; Szumlinski et al., 2005; 2008) and facilitates aspects of alcohol reward and reinforcement under place-conditioning, operant, and continuous access drinking procedures (Goulding et al., 2001; Szumlinski et al., 2005; 2008), the available data regarding the phenotype of HDID-1 mice argue that that elevated basal NAC mGluR1/Homer2 expression is genetic correlate of high BACs attained under limited alcohol-access procedures and not a genetic correlate of bitter/sweet tastant sensitivity or alcohol intake/preference under free-access conditions.
The behavioral relevance of NAC mGluR1 expression for alcohol intake has been questioned as neither systemic treatment with, nor intra-NAC infusion of, the selective mGluR1a antagonist CPCCOEt significantly reduces measures of alcohol reward/reinforcement, despite eliciting what appears to be a dose-dependent attenuation of behavior (e.g., Figure 2; Cozzoli et al., 2009; Hodge et al., 2006; Schroeder et al., 2005; but see Lominac et al., 2005). Prolonged (3 to 6 months) alcohol self-administration experience under free-access home-cage drinking conditions elevates NAC mGluR1 levels and this effect persists for at least 2 weeks into withdrawal (Obara et al., 2009; Szumlinski et al., 2008). Given our data for SHAC/SLAC (Cozzoli et al., 2009) and HDID-1 mice (Figure 6), we recommend that the role for mGluR1 in regulating alcohol intake be re-visited using more potent and soluble mGluR1 antagonists (e.g., JNJ1625685) in future studies concerning the receptor substrates underpinning excessive alcohol consumption, as well as the genetic basis of this phenomenon.
In contrast to our earlier report of SHAC/SLAC mice (Cozzoli et al., 2009), HDID-1 and HS/Npt mice did not differ in their basal expression p-(Tyr)p85α PI3K binding motif within the NAC (Figure 6), suggesting that elevated basal NAC PI3K activity is not a consistent genetic correlate of excessive alcohol intake across binge drinking models and, when more tissue becomes available from HDID-1 mice, as well as from mice from a 2nd replicate line (HDID-2), we will apply the p-(Tyr458)-p85 antibody only now commercially available, to prove or disprove the validity of this hypothesis. Within both the NAC core and shell of mice, the basal protein expression/activational state of kinases downstream of Group1 mGluRs, including PI3K, is strain-dependent (Goulding et al., 2011). Thus, it is quite possible that our failure to detect significant differences in basal PI3K activity between HDID-1 and HS/Npt mice relates to the fact that the genetic background of both lines is comprised of 8 different inbred mouse strains with varying degrees of alcohol intake (see Crabbe et al., 2009), which presumably vary in their degree of basal kinase activity within the NAC. While the SHAC/SLAC mice in our earlier study (Cozzoli et al., 2009) were also derived from this same HS/Npt founder population, the SLAC line was selectively bred for low BAC and no comparison was made to tissue from the heterogeneous HS/Npt founder population. Thus, it is entirely possible that the SHAC-SLAC differences observed in our previous report reflected lower PI3K activity in the SLAC animals, rather than higher PI3K activity in the SHAC animals – a hypothesis that cannot be tested as these selected lines are no longer available. Nevertheless, the fact that we were able to detect significant differences in the basal NAC shell expression of Homer2 and mGluR1 between HDID-1 and the heterogeneous HS/Npt line only strengthens the assertion that NAC shell Homer2-associated signaling is a molecular correlate of genetic vulnerability to consume excessive amounts of alcohol. If relevant to humans, idiopathic increases in NAC shell Homer2a/b expression is predicted to promote the primary reinforcing properties of alcohol, in turn rendering an individual more vulnerable for developing an alcohol drinking problem and maintaining that drinking problem in the face of adverse consequences.
The functional relevance of Homer2a/b signaling for binge drinking under DID procedures
An examination of the functional relevance of NAC shell Group 1 mGluR/Homer2/PI3K signaling for maintaining high intake of 20% alcohol under DID procedures demonstrated that interruption of signaling significantly lowered the alcohol intake of mice below or around those predicted to result in BACs ≤ 80 mg% (see Rhodes et al., 2005; Crabbe et al., 2009). As reported previously in other alcohol drinking models (Cozzoli et al., 2009; Neasta et al., 2011), an intra-NAC shell infusion of both the non-selective PI3K inhibitor wortmannin and the PI3K-selective inhibitor LY 294002 attenuated alcohol consumption in the DID model (Figures 3, 4B, 5B). In addition to PI3K, wortmannin is also a potent inhibitor of PLK1, as well as a number of other kinases (e.g., Elling et al., 2008). However, intra-NAC infusion of the IC50 dose of the selective PLK1 inhibitor cyclapolin 9 failed to influence alcohol intake under DID procedures, arguing against an important role for at least this kinase in wortmannin’s anti-binge drinking effects. As reported previously (Cozzoli et al., 2009), the attenuating effect of wortmannin was not additive with that produced by mGluR5 inhibition (Figure 3C) nor was it apparent in mice with disrupted mGluR5-Homer interactions (Figure 4B), indicating an important role for mGluR5 in mediating wortmannin’s inhibitory effect upon drinking. Extending these earlier data, we show here that an intra-NAC infusion of neither MPEP nor wortmannin was effective at reducing alcohol intake in Homer2 KO mice (Figure 5B), indicating for the first time that Homer2 is critical for the mGluR5/PI3K activity mediating excessive alcohol intake.
It is interesting to note that while Homer2 deletion was without any significant effect upon alcohol drinking under DID procedures (Figure 5A), shRNA-mediated knock-down of Homer2b within the NAC shell was sufficient to reduce alcohol intake in this paradigm. Moreover, the attenuating effect of NAC Homer2b knock-down was apparent at both 5 and 20% alcohol, indicating a downward shift in the alcohol dose-response function by this manipulation or a reduction in the efficacy of alcohol to maintain intake (Figure 5C). The discrepancy in findings between our KO and shRNA studies for Homer2 is reminiscent of distinctions between the effects of constitutive mGluR5 deletion versus systemic mGluR5 antagonist pretreatment reported previously for mice drinking under DID procedures (Blednov and Harris, 2008). While the precise reason for the “normal” DID phenotype of constitutive mGluR5 or Homer2 KO mice cannot be discerned from the results of the present study, a potential explanation relates to some developmental compensation(s) that mask effects of gene deletion upon limited access alcohol drinking. At least in the case of the Homer2 KO, this “masking” might relate to the scheduling of alcohol access for a time during the mouse’s circadian cycle when fluid consumption is typically high (i.e., 3 hours after lights out) as Homer2 KO mice exhibit WT circadian patterns of food and water intake (Szumlinski et al., 2005) and these animals exhibit marked reductions in alcohol intake under continuous-access home cage drinking and under limited-access (15 minute) operant conditions (Szumlinski et al., 2005). Alternatively, as the same shRNA-infused mice were tested under both SHAC (Cozzoli et al., 2009) and DID procedures, it is possible that the prior SHAC experience of the shRNA-infused mice may have influenced subsequent alcohol intake under DID conditions. While there is no published study directly comparing baseline alcohol intake under different binge drinking procedures, the rank order of alcohol intake under DID procedures exhibited by various inbred mouse strains (from highest to lowest: C57BL/6J, BALB/cJ, BALB/cByJ, FVB/NJ, CBA/J, A/J, BTBRT+tf/J, C3H/HeJ, AKR/J, LP/J, 129S1/SvlmJ, DBA/2J; Rhodes et al., 2005) is distinct from that exhibited by inbred strains drinking under SHAC procedures (from highest to lowest: AKR/J, A/J, C57BL/6J, BALB/cJ, C3H/HeJ, LP/J, DBA/2J, CBA/J; D.A. Finn, unpublished data). Such data argue that baseline intake under one binge drinking procedure does not necessarily predict baseline intake under the alternate procedure. Nevertheless, the observation that inhibition of mGluR5/Homer2/PI3K signaling within the NAC shell attenuated alcohol consumption, coupled with the fact that the attenuating effects of intra-NAC mGluR5 and PI3K antagonists require intact Homer2 expression, as well as, intact mGluR5-Homer2 interactions, further implicate the mGluR5/Homer2/PI3K signaling cascade as a viable target for therapeutic intervention in alcoholism.
Acknowledgements
These studies were conducted as part of the Integrative Neuroscience Initiative on Alcoholism consortium of the National Institute on Alcohol Abuse and Alcoholism and were supported by NIH grants AA016650 (INIA West) to KKS and AA13519 (INIA West) and AA10760 to JCC. This work was also funded by grants from the Department of Veterans Affairs to JCC and portions of the work were also funded by the National Institute on Drug Abuse grants DA00266 and DA011742 to PFW. We would like to thank Scott Goulding, Dr. Ilona Obara, William Ramirez, Daniel Maliniak and Hoda Abou-Ziab (all UCSB) for expert technical assistance, as well as Dr. Deborah A. Finn (OHSU) for her helpful comments on this manuscript.
Footnotes
Financial Disclosures
All of the authors report no biomedical financial interests or potential conflicts of interest.
References
- Bird MK, Lawrence AJ. Group 1 metabotropic glutamate receptors: involvement in drug-seeking and drug-induced plasticity. Curr Mol Pharmacol. 2009;2:83–94. doi: 10.2174/1874467210902010083. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Harris RA. Metabotropic glutamate receptor 5 (mGluR5) regulation of ethanol sedation, dependence and consumption: relationship to acamprosate actions. Int J Neuropsychopharmacol. 2008;11:775–793. doi: 10.1017/S1461145708008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua BT, Gallego-Ortega D, Ramirez de Molina A, Ullrich A, Lacal JC, Downward J. Regulation of Akt(ser473) phosphorylation by choline kinase in breast carcinoma cells. Mol Cancer. 2009;8:131. doi: 10.1186/1476-4598-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, Obara I, Rahn A, Abou-Ziab H, Tyrrel B, Marini C, Yoneyama N, Metten P, Snelling C, Dehoff MH, Crabbe JC, Finn DA, Klugmann M, Worley PF, Szumlinski KK. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: Functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Metten P, Rhodes JS, Yu CH, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiatry. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrivieres S, Krause K, Dyer A, Frank J, Blomeyer D, Lathrop M, Mann K, Banaschewski T, Laucht M, Schumann G. Nucleotide sequence variation within the PI3K p85 alpha gene associates with alcohol risk drinking behaviour in adolescents. PLoS One. 2008;3:e1769. doi: 10.1371/journal.pone.0001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrengruber MU, Kato A, Inokuchi K, Hennou S. Homer/Vesl proteins and their roles in CNS neurons. Molecular Neurobiology. 2004;29:213–227. doi: 10.1385/MN:29:3:213. [DOI] [PubMed] [Google Scholar]
- Elling RA, Fucini RV, Romanowski MJ. Structures of the wild-type and activated catalytic domains of Brachydanio rerio Polo-like kinase 1 (Plk1): changes in the active-site conformation and interactions with ligands. Acta Crystallogr D Biol Crystallogr. 2008;64:909–918. doi: 10.1107/S0907444908019513. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass JT, Olive MF. Glutamatergic substrates of drug addiction and alcoholism. Biochem Pharmacol. 2008;75:218–265. doi: 10.1016/j.bcp.2007.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulding SP, Obara I, Lominac KD, Gould AT, Miller BW, Klugmann M, Szumlinski KK. Accumbens Homer2-mediated signaling: a factor contributing to mouse strain differences in alcohol drinking? Genes, Brain, and Behav. 2011:111–126. doi: 10.1111/j.1601-183X.2010.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Syed YM, Revis AA, Miller SA, Martinez M, Cohn KA, Demeyer MR, Patel KY, Brzezinska WJ, Rhodes JS. Acute effects of acamprosate and MPEP on ethanol Drinking-in-the-Dark in male C57BL/6J mice. Alcohol Clin Exp Res. 2008;32:1992–1998. doi: 10.1111/j.1530-0277.2008.00787.x. [DOI] [PubMed] [Google Scholar]
- Heimer L, Alheid GF, de Olmos JS, Groenewegen HJ, Haber SN, Harlan RE, Zahm DS. The accumbens: beyond the core-shell dichotomy. J Neuropsychiatry Clin Neurosci. 1997;9:354–381. doi: 10.1176/jnp.9.3.354. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Miles MF, Sharko AC, Stevenson RA, Hillmann JR, Lepoutre V, Besheer J, Schroeder JP. The mGluR5 antagonist MPEP selectively inhibits the onset and maintenance of ethanol self-administration in C57BL/6J mice. Psychopharmacology (Berl) 2006;183:429–438. doi: 10.1007/s00213-005-0217-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamdar NK, Miller SA, Syed YM, Bhayana R, Gupta T, Rhodes JS. Acute effects of naltrexone and GBR 12909 on ethanol drinking-in-the-dark in C57BL/6J mice. Psychopharmacology. 2007;192:207–217. doi: 10.1007/s00213-007-0711-5. [DOI] [PubMed] [Google Scholar]
- Kammermeier PJ. Endogenous homer proteins regulate metabotropic glutamate receptor signaling in neurons. J Neurosci. 2008;28:8560–8567. doi: 10.1523/JNEUROSCI.1830-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapasova Z, Szumlinski KK. Strain differences in alcohol-induced neurochemical plasticity: a role for accumbens glutamate in alcohol intake. Alcohol Clin Exp Res. 2008;32:617–631. doi: 10.1111/j.1530-0277.2008.00620.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Ren J. Chronic alcohol consumption alters mammalian target of rapamycin (mTOR), reduces ribosomal p70s6 kinase and p4E-BP1 levels in mouse. Exp Neurol. 2007;204:840–844. doi: 10.1016/j.expneurol.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang N, Wu J, Dai W, Rosenblum JS. Polo-like kinases inhibited by wortmannin. Labeling site and downstream effects. J Biol Chem. 2007;282:2505–2511. doi: 10.1074/jbc.M609603200. [DOI] [PubMed] [Google Scholar]
- Lominac KD, Kapasova Z, Hannun RA, Patterson C, Middaugh LD, Szumlinski KK. Behavioral and neurochemical interactions between group 1 mGluR antagonists and ethanol: potential insight into their anti-addictive properties. Drug Alcohol Depend. 2006;85:142–156. doi: 10.1016/j.drugalcdep.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Lovinger DM. Interactions between ethanol and agents that act on the NMDA-type glutamate receptor. Alcohol Clin Exp Res. 1996;20:187A–191A. doi: 10.1111/j.1530-0277.1996.tb01773.x. [DOI] [PubMed] [Google Scholar]
- Minami I, Kengaku M, Smitt PS, Shigemoto R, Hirano T. Long-term potentiation of mGluR1 activity by depolarization-induced Homer1a in mouse cerebellar Purkinje neurons. Eur J Neurosci. 2003;17:1023–1032. doi: 10.1046/j.1460-9568.2003.02499.x. [DOI] [PubMed] [Google Scholar]
- Moore EM, Boehm SL., 2nd Site-specific microinjection of baclofen into the anterior ventral tegmental area reduces binge-like ethanol intake in male C57BL/6J mice. Behav Neurosci. 2009;123:555–563. doi: 10.1037/a0015345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan MK, Rhodes JS, Crabbe JC, Mayfield RD, Adron Harris R, Ponomarev I. Molecular profiles of drinking alcohol to intoxication in C57BL/6J mice. Alcohol Clin Exp Res. 2011;35:659–670. doi: 10.1111/j.1530-0277.2010.01384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Alcohol abuse increases, dependence declines across decade: Young adult minorities emerge as high-risk subgroups. [Accessed May 18, 2011];2004 Jun 10; [News Releases NIAAA Web site] Available at: http://www.niaaa.nih.gov/NewsEvents/NewsReleases/Pages/NESARCNews.aspx#chart.
- Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neasta J, Hamida SB, Yowell QV, Carnicella S, Rob D. AKT signaling pathway in the nucleus accumbens mediates excessive alcohol drinking behaviors. Biol Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.03.019. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neiber K, Poelchen W, Sieler D, Illes P. Inhibition by ethanol of excitatory amino acid receptors in rat locus coeruleus neurons in vitro. Naunyn Schmiedebergs Arch Pharmacol. 1998;357:299–308. doi: 10.1007/pl00005171. [DOI] [PubMed] [Google Scholar]
- Newton PM, Ron D. Protein kinase C and alcohol addiction. Pharmacol Res. 2007;55:570–577. doi: 10.1016/j.phrs.2007.04.008. [DOI] [PubMed] [Google Scholar]
- Obara I, Bell RL, Goulding SP, Reyes C, Larson LA, Ary AW, Truitt WA, Szumlinski KK. Differential effects of chronic ethanol consumption and withdrawal on homer/glutamate receptor expression in subregions of the accumbens and amygdala of P rats. Alcohol Clin Exp Res. 2009;33:1924–1934. doi: 10.1111/j.1530-0277.2009.01030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M, Oshimura M, Ito H. PI3K-Akt pathway: Its functions and alterations in human cancer. Apoptosis. 2009;9:667–676. doi: 10.1023/B:APPT.0000045801.15585.dd. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The mouse brain in stereotaxic coordinates. Maryland Heights, MO: Academic Press; 2004. [Google Scholar]
- Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Rong R, Ahn JY, Huang H, Nagata E, Kalman D, Kapp JA, Tu J, Worley PF, Snyder SH, Ye K. PI3 kinase enhancer-Homer complex couples mGluRI to PI3 kinase, preventing neuronal apoptosis. Nat Neurosci. 2003;6:1153–1161. doi: 10.1038/nn1134. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Schroeder JP, Overstreet DH, Hodge CW. The mGluR5 antagonist MPEP decreases operant ethanol self-administration during maintenance and after repeated alcohol deprivations in alcohol-preferring (P) rats. Psychopharmacology (Berl) 2005;179:262–270. doi: 10.1007/s00213-005-2175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraishi-Yamaguchi Y, Furuichi T. The Homer family proteins. Genome Biol. 2007;8:206. doi: 10.1186/gb-2007-8-2-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steelman LS, Chappell WH, Abrams SL, Kempf RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F, Mazzarino MC, Donia M, Fagone P, Malaponte G, Nicoletti F, Libra M, Milella M, Tafuri A, Bonati A, Basecke J, Cocco L, Evangelisti C, Martelli AM, Montalto G, Cervello M, McCubrey JA. Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity to therapy-implications for cancer and aging. Aging (Albany NY) 2011;3:192–222. doi: 10.18632/aging.100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Ary AW, Lominac KD, Klugmann M, Kippin TE. Accumbens Homer 2 overexpression facilitates alcohol-induced neuroplasticity in C57BL/6J mice. Neuropsychopharmacology. 2008;33:1365–1378. doi: 10.1038/sj.npp.1301473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szumlinski KK, Lominac KD, Oleson EB, Walker JK, Mason A, Dehoff MH, Klugmann M, Cagle S, Welt K, During M, Worley PF, Middaugh LD, Kalivas PW. Homer2 is necessary for EtOH-induced neuroplasticity. J Neurosci. 2005;25:7054–7061. doi: 10.1523/JNEUROSCI.1529-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Town M, Naimi TS, Mokdad AH, Brewer RD. Health care access among U.S. adults who drink alcohol excessively: missed opportunities for prevention. Prev Chronic Dis. 2006;3:A53. [PMC free article] [PubMed] [Google Scholar]
- Tu JC, Xiao B, Naisbitt S, Yuan JP, Petralia RS, Brakeman P. Coupling of mGluR/Homer and PSD-95 complexes by the Shank family of postsynaptic density proteins. Neuron. 1999;23:583–592. doi: 10.1016/s0896-6273(00)80810-7. [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, Stephens L, Hawkins PT, Wymann MP, Williams RL. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 2000;6:909–919. doi: 10.1016/s1097-2765(05)00089-4. [DOI] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Ron D. Ethanol-mediated long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum. Channels (Austin) 2011;5(4) doi: 10.4161/chan.5.3.14856. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Lanfranco MF, Gibb SL, Yowell QV, Carnicella S, Ron D. Long-lasting adaptations of the NR2B-containing NMDA receptors in the dorsomedial striatum play a crucial role in alcohol consumption and relapse. J Neurosci. 2010;30:10187–1098. doi: 10.1523/JNEUROSCI.2268-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirkner K, Eberts C, Poelchen W, Allgaier C, Illes P. Mechanism of inhibition by ethanol of NMDA and AMPA receptor channel functions in cultured rat cortical neurons. Naunyn Schmiedebergs Arch Pharmacol. 2000;362:568–576. doi: 10.1007/s002100000262. [DOI] [PubMed] [Google Scholar]
- Zhang X, Mi J, Wetsel WC, Davidson C, Xiong X, Chen Q, Ellinwood EH, Lee TH. PI3 kinase is involved in cocaine behavioral sensitization and its reversal with brain area specificity. Biochem Biophys Res Commun. 2006;340:1144–1150. doi: 10.1016/j.bbrc.2005.12.114. [DOI] [PubMed] [Google Scholar]