Abstract
Background
Although serum level of alpha-fetoprotein (AFP) has long been used to complement imaging tests in the screening and diagnosis of hepatocellular carcinoma (HCC), whether it can be used as a predictive marker of long-term risk for developing HCC in patients with hepatitis B virus (HBV) has not been extensively evaluated and thus remains controversial.
Methods
We retrospectively conducted a clinic-based longitudinal cohort study including 617 Korean American patients with HBV who had been followed for up to 22 years (median follow-up time, 6.2 years) to evaluate the association between baseline serum AFP level and the long-term risk of HCC.
Results
The median baseline AFP value of these patients was 3.8 ng/ml. Compared to patients with lower-than-median AFP value, those with higher-than-median baseline serum AFP had a significantly increased risk of developing HCC with an hazard ratio (HR) of 2.73 (95% confidence interval [CI] 1.25–5.99), independent of other major HCC risk factors. In addition, we calculated the cumulative incidence of HCC during different years of follow-up time by baseline serum AFP, and found that the cumulative incidence of HCC was significantly higher in HBV patients with high baseline serum AFP compared to those with low baseline serum AFP in each of the five follow-up time periods examined.
Conclusions
Our results indicated that AFP was a strong independent prospective predictor of long-term HCC risk in high-risk HBV patients. More targeted prevention and early detection of HCC may be considered for these patients.
Keywords: Alpha-fetoprotein (AFP), hepatitis B virus (HBV), hepatocellular carcinoma (HCC)
INTRODUCTION
Hepatocellular carcinoma (HCC) is the fifth most common solid malignancy and the third leading cause of cancer mortality worldwide, with approximately 625,000 new cases and 600,000 deaths in each year (1–3). Hepatitis B virus (HBV) is the most significant hepatocarcinogen which is responsible for up to 80% of HCC worldwide. Currently there are over 400 million patients with chronic HBV infection globally which corresponds to over 5% of the world’s population; and it is estimated that about 20% of these infected individuals may eventually develop HCC (4, 5). The hepatocarcinogenesis from HBV infection to HCC development has been increasingly recognized as a multifactorial and multistep process. Observational studies have identified potential demographic factors, viral status, clinical variables, and genetic components in HBV patients that are associated with the risk of HCC (6–8). Although acclaimed instrumental, many of these factors remain controversial in their roles for predicting the long-term risk of developing HCC (6). Thus, validation of these significant risk factors for HCC would allow further stratification of HBV patients and selection of those with the highest risk of HCC to receive more intensive, targeted, and personalized prevention strategies.
Alpha-fetoprotein (AFP) was first identified in 1956 as a serum glycoprotein generated by the yolk sac and the liver during fetal life (9). Generally, normal people have a low AFP level, which may be elevated under certain diseases. Serum AFP has long been used as a diagnostic marker for HCC, but with controversies (10). Although still widely used in the current clinics, the use of AFP in HCC diagnosis has been challenged in recent years due to the associated false positive and false negative findings (11). In addition, the role of AFP as a prospective predictive marker or a surveillance indicator of HCC among HBV patients has not been extensively evaluated and thus remains controversial (12–15). For example, in a prospective study including 463 patients with liver cirrhosis, AFP was not significantly associated with HCC development within a mean follow-up time of 3.2 years (14). In comparison, Oka et al. reported a significant positive correlation between high baseline AFP value and increased incidence of HCC in a cohort of 260 Japanese patients within a 5-year follow-up period (12). Consistently, Colombo et al. reported that the risk of developing HCC in patients with persistently high levels of AFP was more than 10 times higher than those patients with persistently low AFP levels (15). The lack of consistency in findings from these studies are likely accounted for by various factors, such as study design, population size and characteristics, patient enrollment criteria, HCC etiology, and follow-up time. Nonetheless, given the broad application of AFP measurement in the current clinics on HCC screening, surveillance, diagnosis, and treatment, additional studies on the predictive role of baseline AFP in long-term risk of developing HCC are warranted to better assist clinicians to make informed decisions on HCC prevention and management.
To further assess the relationship between baseline AFP and future HCC risk, we retrospectively conducted a clinic-based longitudinal cohort study based on a unique population of Korean American HBV patients in the Greater Philadelphia area who have been followed for up to 22 years. In this study, we evaluated the differences in HCC incidence between HBV patients with high and low level of baseline AFP values. To our knowledge, this is one of the largest clinic-based cohort studies with a long-time follow-up.
MATERIALS AND METHODS
Study population
The subjects in this study were identified from an existing clinic-based patient cohort. The patients were consecutively enrolled from those who visited the Liver Disease Prevention Center at Thomas Jefferson University Hospital for treatment of chronic HBV or HCV infection and liver diseases, such as cirrhosis, fibrosis, and/or HCC. There were no restrictions on age, gender, ethnicity, and disease stage in patient enrollment. Enrollment was initiated from 1988 and is still ongoing. As of October 2010, the cohort included more than 2,600 patients, of which 90% were of Korean ancestry. The cohort included patients with different etiologies and conditions, including non-cancer patients with mono-infection of HBV, hepatitis C virus (HCV), or without viral infection; and also included cancer patients with HBV-related HCC, HCV-related HCC, or HCC without viral infection. For each patient, viral infection status was clinically determined before enrollment. All demographic, clinical, and follow-up data for study subjects were obtained from medical chart review and consultation with the treating physicians. For the purpose of this study, we included all those patients who (a) were non-cancer patients who had only HBV infection at their first clinical visits, (b) had recorded AFP measurement within 12 months after their first clinical visit, (c) had been followed for a minimum of 12 months, and (d) was not diagnosed with HCC within 12 months of their first clinical visit. Because the majority (>90%) of the patients who visited the Liver Disease Prevention Center were of Korean ancestry, we further restricted the study to Korean patients to eliminate the confounding effect from population stratification. This study has been reviewed and approved by the Institutional Review Board (IRB) at the Thomas Jefferson University.
Data collection
Demographic and clinical data were abstracted from patient medical records to create a patient-level longitudinal database. Demographic variables collected included age, gender, ethnicity, smoking status, drinking status, self-reported family history of HBV infection, family history of cirrhosis, and family history of cancer. Clinical variables were collected for the first clinical visit with a recorded AFP value, including the presence of liver cirrhosis, serum AFP, alkaline phosphatase (ALP), Alanine Aminotransferase (ALT), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), HBV DNA load, count of white blood cell (WBC) and platelets, prothrombin time, total protein, albumin, total bilirubin, direct bilirubin, ferritin, blood urine nitrogen (BUN), triglyceride, low-density lipid protein (LDL), and creatinine, etc. Missing data in these variables were noticed due to the retrospective nature of the chart review. The rate of missing data for these variables in the initial patients’ visits ranged from 0.91% (AST) to 39.9% (LDL). Liver cirrhosis and HCC status were determined by the combined use of clinical diagnosis and imaging studies (ultrasound, computed tomography, or magnetic resonance imaging) (16). Treatment data including antiviral nucleoside and nucleotide analogs and interferons were also obtained. More than 95% of these patients received nucleoside/nucleotide analog-based antiviral treatments. Less than 5% of patients received interferon-based or combined treatments. For those patients who developed HCC during follow-up, treatments usually included transarterial embolization/chemoembolization (TACE) radiofrequency ablation (RFA), cryoablation, chemotherapy, and/or surgery.
Statistical Analysis
Statistical analyses were performed using the SAS software version 9.2 (SAS Institute, Cary, NC). Continuous variables were expressed as means with standard deviation (SD), and were compared using the student’s t test or ANOVA as appropriate. Categorical variables were compared using the chi-square test or Fisher’s exact test, where appropriate. The primary outcome of this study was the diagnosis of HCC. Time to the HCC diagnosis was defined as the time from the date of first visit to the date of HCC diagnosis, or the date of last follow-up if the patient was still alive without HCC diagnosis at the time of this analysis. Patients free of HCC at their death or at the last follow-up date were censored for the analysis. Incidence rates of HCC were calculated by dividing the number of incident HCC cases by the total number of person-years of follow-up. Cumulative incidence of HCC by follow-up year was derived using the Nelson-Aalen method (17). Kaplan-Meier analysis was used to compare the cumulative risk for developing HCC in patients with different levels of serum AFP. The log rank test was used to determine the statistical significance of associations. The baseline AFP value was treated as continuous predictor variable and grouped based on the median and tertile distribution in all patients included in the analyses. Cox proportional hazards model was performed to determine the association between the independent variables and the risk of developing HCC. The risk of HCC was estimated using hazard ratio (HR) and 95% confidence interval (CI) after adjustment for age, gender, smoking, drinking, cirrhosis, family history of HBV, family history of cirrhosis, and family history of cancer, where appropriate. All statistical tests in this study were two-sided, and P<0.05 was considered statistically significant.
RESULTS
Population Characteristics
From our database of patients enrolled from 1988 to 2010, we identified a total of 617 cancer-free Korean HBV patients who met the criteria described in the study population in Materials and Method. Patients who developed HCC within 12 months of their first clinical visits were excluded from the analyses. The basic demographic characteristics of the 617 patients are listed in Table 1. The median (SD) age of all patients was 42.2 (11.1) years. The majority of patients were males (67.6%), never smokers (66.8%), and never drinkers (60.9%). Approximately 36.8% of the patients had cirrhosis at the time of enrollment. There were an equal number of patients with and without a family history of HBV infection (49.4% vs. 50.6%, respectively). The majority of patients did not have a family history of cirrhosis (81.2%) or family history of cancer (66.0%). The median level of baseline AFP (first measured within 12 months of first clinic visit) of these patients was 3.8 ng/ml (Table 1).
Table 1.
Baseline characteristics of the HBV patients included in this study
| Variables | Number (%) of patients (N=617) |
|---|---|
| Age (Mean ± SD *) | 42.2 ± 11.1 |
| Gender | |
| Male | 417 (67.6) |
| Female | 200 (32.4) |
| Smoking status | |
| Never | 412 (66.8) |
| Ever | 205 (33.2) |
| Drinking status | |
| Never | 376 (60.9) |
| Ever | 241 (39.1) |
| Cirrhosis | |
| No | 390 (63.2) |
| Yes | 227 (36.8) |
| Family history of HBV infection | |
| No | 305 (49.4) |
| Yes | 312 (50.6) |
| Family history of cirrhosis | |
| No | 501 (81.2) |
| Yes | 116 (18.8) |
| Family history of cancer | |
| No | 407 (66.0) |
| Yes | 210 (34.6) |
| AFP | |
| ≤ median | 300 (48.6) |
| > median | 317 (51.4) |
SD, standard deviation
Incidence rate and risk factors associated with the development of HCC
There were a total of 3,785 person-years of follow-up in the 617 study subjects, with the average (SD) follow-up time period of 6.2 (4.7) years (range: 1.0–22.2 years). During follow-up, a total of 61 HBV patients developed HCC after 12 months. We analyzed the association of major demographic variables and baseline serum AFP value with the risk of developing HCC using multivariate Cox proportional hazard model (Table 2). We found that male patients, ever smokers, and ever drinkers exhibited an increased but non-significant risk of HCC development with an HR of 1.90 (95% CI 0.84–4.30), 1.65 (0.81–3.34), and 1.10 (0.56–2.16), respectively. Compared to patients ≤39 years old, patients who were 40–49 years old, 50–59 years old, and over 60 years old showed an increased risk for developing HCC, with an HR (95%CI) of 1.45 (0.71–2.96), 6.28 (3.12–12.63), and 2.11 (0.85–11.40), respectively. As expected, patients with cirrhosis had a statistically significantly increased risk of developing HCC (HR=8.02, 95% CI 3.38–19.05). Furthermore, patients who reported a family history of cirrhosis also had a significantly increased risk of HCC with an HR (95% CI) of 3.20 (1.08–9.43). However, no significant associations were observed between HCC risk and self-reported family history of HBV infection (HR 0.94, 95% CI 0.52–1.69) and family history of cancer (HR 1.13, 95% CI 0.65–1.99). There were 300 patients with a baseline AFP level that was lower than the median value. In this group, 15 (5.0%) patients developed HCC in an average of 7.9 years. In comparison, 46 of 317 (14.5%) patients with an AFP level higher than the median value developed HCC in an average of 4.6 years (chi square test < 0.0001, data not shown). Multivariate Cox proportional hazard analysis indicated that compared to patients with a lower-than-median serum AFP, those with a higher-than-median serum AFP value had a 2.73-fold (95% CI 1.25–5.99) increase in the risk for developing HCC (Table 2). The incidence rates of HCC per 100,000 person-years by baseline serum AFP level increased from 801 per 100,000 person-years for the low AFP group to 2405 per 100,000 person-years for the high AFP group (Table 2). Moreover, a significant difference in AFP levels was identified between HBV patients who developed and who did not develop HCC during follow up (P value for t test, 0.008, data not shown).
Table 2.
The associations of demographic variables and serum AFP value with the risk of developing HCC in HBV patients.
| Variables | Number (%) of patients (N=617) | Person-years of follow-up | Number (%) of HCC cases (N=61) | Incidence rate per 100,000 person-years | HR (95% CI) * | P value |
|---|---|---|---|---|---|---|
| Age (Year) | ||||||
| ≤39 | 233(40.88) | 1651 | 8 | 485 | Reference | |
| 40–49 | 194(34.04) | 1179 | 17 | 1442 | 1.45 (0.71–2.96) | 0.30 |
| 50–59 | 108(18.95) | 531 | 26 | 4896 | 6.28 (3.12–12.63) | <0.0001 |
| ≥60 | 35(6.14) | 148 | 3 | 2027 | 2.11 (0.85–11.40) | 0.09 |
| Sex | ||||||
| Female | 200(32.41) | 1160 | 9 | 776 | Reference | |
| Male | 417(67.59) | 2625 | 52 | 1981 | 1.90 (0.84–4.30) | 0.12 |
| Smoking status | ||||||
| Never | 412(66.77) | 2544 | 29 | 1140 | Reference | |
| Ever | 205(33.23) | 1241 | 32 | 2579 | 1.65 (0.81–3.34) | 0.17 |
| Drinking status | ||||||
| No | 376(60.94) | 2369 | 30 | 1266 | Reference | |
| Yes | 241(39.06) | 1416 | 31 | 2189 | 1.10 (0.56–2.16) | 0.78 |
| Cirrhosis | ||||||
| No | 390(63.21) | 2315 | 7 | 302 | Reference | |
| Yes | 227(36.79) | 1470 | 54 | 3673 | 8.02 (3.38–19.05) | <0.0001 |
| Family history of HBV | ||||||
| No | 305(49.31) | 1894 | 31 | 1637 | Reference | |
| Yes | 312(50.57) | 1891 | 30 | 1586 | 0.94 (0.52–1.69) | 0.83 |
| Family history of cirrhosis | ||||||
| No | 501(81.20) | 3106 | 45 | 1449 | Reference | |
| Yes | 116(18.80) | 679 | 16 | 2356 | 3.20 (1.08–9.43) | 0.04 |
| Family history of cancer | ||||||
| No | 407(65.96) | 2547 | 37 | 1453 | Reference | |
| Yes | 210(34.04) | 1238 | 24 | 1939 | 1.13 (0.65–1.99) | 0.67 |
| AFP | ||||||
| ≤median | 300(48.62) | 1872 | 15 | 801 | Reference | |
| >median | 317(51.38) | 1913 | 46 | 2405 | 2.73 (1.25–5.99) | 0.01 |
Adjusted for age, sex, smoking status, drinking status, cirrhosis, family history of HBV, family history of cirrhosis, family history of cancer, and AFP, where appropriate.
Cumulative incidence of HCC by serum AFP level during complete follow-up
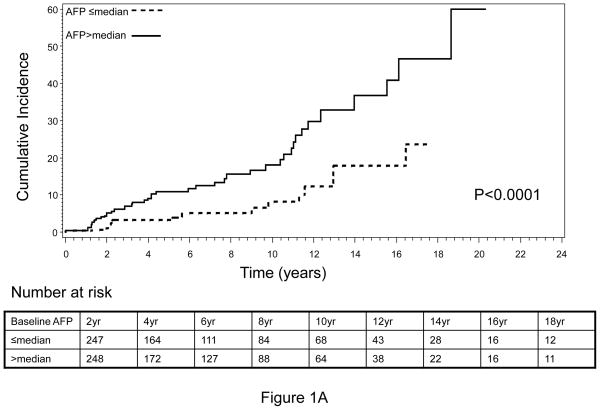
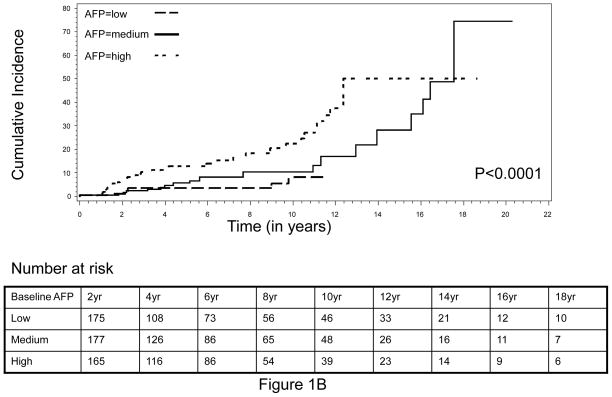
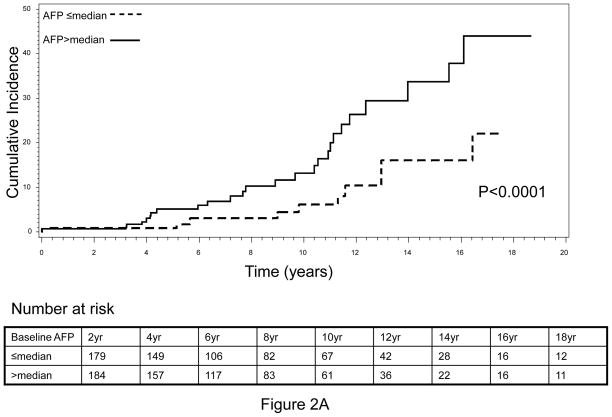
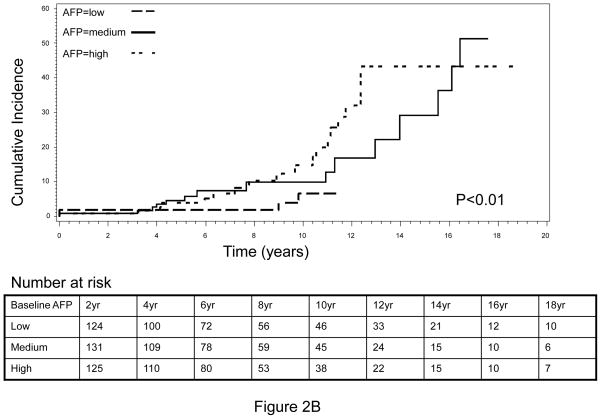
The cumulative incidence of HCC during the complete follow-up period (1988–2010) is shown in Figure 1. An increasing trend of cumulative HCC incidence over the follow-up period was noted, with significantly higher cumulative incidence observed in HBV patients with higher-than-median baseline serum AFP level compared to those HBV patients with lower-than-median AFP levels (log-rank P<0.0001, Figure 1A). Very similar results were obtained when the tertile distribution of baseline serum AFP value was used to separate patients, indicating that the association between elevated baseline AFP and increased risk of HCC was dose-dependent (Figure 1B). Because the population in this analysis included HBV patients whose AFP level was measured within 12 months of the first clinical visit and who had a minimum of 12 months follow-up period, there was a possibility that some patients with high AFP value actually already had cancer but were not diagnosed at the time of AFP measurement. The inclusion of these patients might confound our results, because the elevated AFP value in these patients was more like a marker of cancer detection than a predictive marker of long-term cancer risk. To address this potential cofounder, we calculated the average time from first clinic visit to AFP measurement as well as the average time from first clinic visit to HCC diagnosis. We found that for 419 of the 617, the AFP measurement was conducted at the same date of their first clinic visit. The average time of AFP measurement from the first clinic visit was 1.3 months whereas the average time of HCC diagnosis from the first clinic visit was 5.1 years. Moreover, we further restricted the patient cohort to include only those patients who had the baseline serum AFP measurement done within 6 months of first clinical visit as well as had been followed up for a minimum of 36 months and excluded those patients who developed HCC within 12 months. This approach yielded a total of 363 non-cancer HBV patients, among which 37 patients developed HCC after 36 months of follow-up (data not shown). We then evaluated the cumulative incidence of HCC within this patient cohort. Very similar results to that of the previous cohort were attained (Figure 2). That is, the cumulative incidence of HCC was again significantly higher in those patients with a higher-than-median AFP value, compared to those with a lower-than-median AFP value (Figure 2A). Furthermore, we found that this effect was also in a significant dose-response manner when the tertile, instead of median, distribution of baseline AFP was used to group subjects (Figure 2B).
Fig. 1.
Cumulative incidence of HCC by (A) median and (B) tertile distribution of baseline AFP level, conducted within patients with baseline AFP measured within 12 months of first clinical visit and a minimum of 12 months’ follow-up.
Fig. 2.
Cumulative incidence of HCC by (A) median and (B) tertile distribution of baseline AFP level, conducted within patients with baseline AFP measured within 6 months of first clinical visit and a minimum of 36 months’ of follow-up.
Cumulative incidence of HCC by serum AFP level at different years of follow-up
Finally, we conducted analyses to calculate the cumulative incidence of HCC during different years of follow-up time by serum AFP level using the Nelson-Aalen method (Table 3). We found that the cumulative incidence of HCC was significantly higher in patients with higher baseline serum AFP than those with lower AFP value, in each of the follow-up time periods examined. In patients with lower-than-median baseline AFP value, the cumulative incidence of HCC was 2.4, 3.9, 5.2, 10.0, and 17.9 at the end of 3, 6, 9, 12, and more than 18 years of follow-up, respectively. In comparison, HCC incidence was 6.6, 10.9, 15.5, 27.8, and 40.8, respectively, in patients with higher-than-median baseline AFP values (Table 3, upper panel). Very similar results were observed when the analysis was conducted within those 363 patients whose baseline AFP was measured within 6 months of first clinical visit and had been followed for more than 36 months (Table 3, lower panel).
Table 3.
Cumulative incidence of HCC by baseline serum AFP level and different years of follow-up in patients.
| Years of follow-up | Baseline serum AFP(≤ median) Cumulative incidence | Baseline serum AFP(≥median) Cumulative incidence |
|---|---|---|
|
In patients with more than 12 month’s follow-up*
| ||
| 3 | 2.4 | 6.6 |
| 6 | 3.9 | 10.9 |
| 9 | 5.2 | 15.5 |
| 12 | 10.0 | 27.8 |
| ≥18 | 17.9 | 40.8 |
|
| ||
|
In patients with more than 36 month’s follow-up**
| ||
| 6 | 1.7 | 5.1 |
| 9 | 3.0 | 10.3 |
| 12 | 8.0 | 24.1 |
| ≥18 | 16.1 | 37.8 |
Within patients who had an AFP value within 12 months of first clinical visit and who had been follow-up for at least 12 months.
Within patients who had an AFP value within 6 months of first clinical visit and who had been follow-up for at least 36 months
DISCUSSION
Despite the wide application of serum AFP level in complementing other imaging-based approaches in HCC diagnosis, there is uncertainty about whether baseline level of AFP can predict the long-term risk of developing HCC in HBV patients (10, 12, 14). Through a retrospective analysis of a clinic-based well-characterized and homogeneous HBV patient cohort that have been followed for up to 22 years, we found that baseline serum AFP level was a strong indicator of the development of HCC, independent of other demographic risk factors including age, gender, smoking, drinking, cirrhosis, family history of HBV, family history of cirrhosis, and family history of cancer. With a median follow-up time of 6.2 years, the HBV patients who had high baseline serum AFP value exhibited a 2.73-fold increase in HCC risk compared to patients with low AFP value. In addition, the cumulative incidence of HCC at the end of 18 years follow-up was significantly higher in patients with high baseline AFP (40.8%) than those with low baseline AFP (17.9%).
Compared to prospectively designed population-based or clinic-based studies, a potential caveat for retrospective analysis of clinic-based cohort was patient selection bias (18). However, when we compared the major demographic characteristics between the 617 HBV patients included in this study to the rest of HBV patients in our cohort that were not included in this study due to lack of measurement of baseline AFP value and/or short follow-up time, we did not identify any significant differences (P value ranged from 0.21 to 0.89, data not shown), indicating the chance was low for bias in patient selection in our study. In addition, the risk association of other demographic variables calculated by multivariate analyses was consistent with the findings from previous reports in other Asian populations. For example, compared to patients less than 40 years old, patients who were between 40 to 49 and 50–59 had a 1.45-fold and 6.28-fold increase in HCC risk, respectively, consistent with the previous reports that older patients were more likely to develop HCC in HBV patients (17, 19). However, the risk was 2.11 (95% CI 0.85–11.40) in patients with an age of more than 60 years in our study, which was probably due to the small number (N=3) of HCC patients in this age group that led to an unstable estimate. Moreover, we found that HBV patients in our population who had liver cirrhosis had a more than 8-fold increase in the risk of developing HCC, which was also consistent with many previous reports on the significant positive correlation between cirrhosis and HCC in previous studies. For example, Chen et al. reported that cirrhosis was associated with a 21.8-fold increased HCC risk in a population-based HBV patient cohort (19). The difference in the magnitude of increase in HCC risk between our study (8.02) and the study from Chen et al. (21.8) could be due to the differences in patient ethnicity, sample size, and clinic and population-based studies. Further, consistently we found in our study that the family history of liver cirrhosis was also an independent predictor of HCC development (Table 2). Other previously reported significant risk factors such as male gender, ever smokers, and ever drinkers were also found in our study to be associated with increased HCC risk. However, these associations did not reach statistical significance. These discrepancies could be due to the nature of clinic-based study in which most patients have higher disease severity than those patients in the general HBV population. The severity of HBV infection and relevant induced condition could overshadow the effects of other demographic risk factors on HCC development. In consistency with this notion, the lack of significant association between several major demographic and clinical risk factors and the risk of developing HCC was also noted in other independent clinic-based studies (13, 14, 20, 21).
The major strength of our study is the unique and highly homogenous HBV patient population that has been completely collected in a single institute. In addition, in comparison to many previous studies that did not differentiate HCC cases with different etiologies, our study was focused on HCC risk in patients infected with HBV only. All subjects were of Korean ethnicity to eliminate the confounding effects of population stratification. In addition, the majority of the patients in our study were infected with HBV at birth or childhood, making our population an ideal resource to study the long-term outcome of HBV infection at the population level. Our study also has limitations. First, due to the nature of clinic-based research, the findings from this study might not be generalized to the general population with HBV infection. Instead, the conclusion of our study might be more applicable to high-risk HBV patients who visit clinics and seek medical advice and/or treatment. Moreover, different AFP levels have been reported in different ethnic groups. For example, it has been reported that in the general male population, AFP levels were higher in Africans than in Asians (22). O’Brien et al. found that normal Asian and African American women exhibited a higher AFP level than Hispanic and White American women (23). Consistently, Lok et al. reported a higher AFP level in African Americans than Caucasians (24). Since our study is restricted to Korean HBV patients, whether the finding can be generalized to other ethnic groups remains a task of further evaluation. Second, all the data used in this study were obtained from medical chart review instead of in-person interview, although we had strict criteria for quality control of chart review as well as frequent validation with treating physicians. Third, we also had missing values for some major clinical variables such as the status of HBe Ag and anti-HBe, viral DNA load, levels of ALT and AST, etc. These variables were not included in our multivariate adjustment and thus might confound our results. However, when we used the first available measurement of these variables within 12 months of first patient clinical visit, the rate of missing data was significantly reduced yet the inclusion of these variables in the adjustment of multivariate analyses did not significantly changed our results (high vs. low AFP, HR=2.37, 95% CI 1.19–3.87, data not shown). Fourth, although we observed an increased HCC risk associated with drinking status, a commonly recognized HCC risk factor, the association did not reach statistical significance (Table 2). This might be due to the nature of our clinic-based cohort in which the severity of HBV patients has overshadowed the causal relationship of drinking. It is also possible that the current categorization of never and ever drinkers is not sufficient to accurately reflect the intensity of drinking. This issue may not be able to be addressed using our current patient population and thus, warrants further investigations, especially in a prospective setting. Finally, the cut-off of AFP used in this study was determined using the median baseline value among all patients (3.8 ng/ml). Thus, it remains to be determined whether this cut-off is applicable to different patients in other clinical settings. A cut-off value ranged from 5 to 20 ng/ml has been reported in other clinic-based studies as having good discriminative capability (12, 14, 24, 25). Using a cut-off of 20 ng/ml in our study resulted in a small number of patients whose AFP was higher than the cut-off, which in turn led to an unstable estimate. However, a cut-off of both 5 ng/ml and 10 ng/ml significantly differentiated HBV patients with different risks of developing HCC in our study (log rank P<0.001 in both analyses). Nonetheless, future larger studies are still warranted to further determine the optimum clinically applicable AFP value in clinic-based HBV patients. Taken together, these caveats limit the clinical applicability of the findings in our study. Prospective studies in independent populations are warranted to further validate our findings.
In summary, we retrospectively conducted a clinic-based longitudinal cohort study to determine the predictive role of baseline AFP value in the prediction of long-term risk of developing HCC in HBV patients. Our conclusion was that elevated serum AFP was significantly associated with increased risk of HCC in HBV patients who seek medical advice/treatment. The effect of high AFP on increased risk of HCC might last for many years after the AFP measurement. Although it still remains a topic of further debate as to whether AFP should be an integral component for HCC surveillance in the general HBV patient population, our study indicated that high levels of serum AFP were associated with higher risk of developing HCC in non-cancer HBV patients. Thus, more targeted prevention and early detection of HCC may be considered for these patients.
Acknowledgments
Financial supports: The work reported here was supported by Tobacco Grant from the Pennsylvania Department of Health HRFF 09F RFA-08-07-06, National Cancer Institute Grants CA153099 and CA159047, American Cancer Society Grant IRG0806001, and a Research Scholar Award from the V Foundation for Cancer Research.
Footnotes
Conflict of interest: None declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118(12):3030–44. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–76. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 4.Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942–56. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 5.Blum HE, Moradpour D. Viral pathogenesis of hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17 (Suppl 3):S413–20. doi: 10.1046/j.1440-1746.17.s3.37.x. [DOI] [PubMed] [Google Scholar]
- 6.Kao JH, Chen PJ, Chen DS. Recent advances in the research of hepatitis B virus-related hepatocellular carcinoma: epidemiologic and molecular biological aspects. Adv Cancer Res. 108:21–72. doi: 10.1016/B978-0-12-380888-2.00002-9. [DOI] [PubMed] [Google Scholar]
- 7.Farazi PA, DePinho RA. Hepatocellular carcinoma pathogenesis: from genes to environment. Nat Rev Cancer. 2006;6(9):674–87. doi: 10.1038/nrc1934. [DOI] [PubMed] [Google Scholar]
- 8.Aravalli RN, Steer CJ, Cressman EN. Molecular mechanisms of hepatocellular carcinoma. Hepatology. 2008;48(6):2047–63. doi: 10.1002/hep.22580. [DOI] [PubMed] [Google Scholar]
- 9.Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest. 1956;8(2):174. doi: 10.3109/00365515609049266. [DOI] [PubMed] [Google Scholar]
- 10.Sherman M. Alphafetoprotein: an obituary. J Hepatol. 2001;34(4):603–5. doi: 10.1016/s0168-8278(01)00025-3. [DOI] [PubMed] [Google Scholar]
- 11.Bruix J, Sherman M. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 12.Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19(1):61–6. [PubMed] [Google Scholar]
- 13.Chen TH, Chen CJ, Yen MF, Lu SN, Sun CA, Huang GT, et al. Ultrasound screening and risk factors for death from hepatocellular carcinoma in a high risk group in Taiwan. Int J Cancer. 2002;98(2):257–61. doi: 10.1002/ijc.10122. [DOI] [PubMed] [Google Scholar]
- 14.Velazquez RF, Rodriguez M, Navascues CA, Linares A, Perez R, Sotorrios NG, et al. Prospective analysis of risk factors for hepatocellular carcinoma in patients with liver cirrhosis. Hepatology. 2003;37(3):520–7. doi: 10.1053/jhep.2003.50093. [DOI] [PubMed] [Google Scholar]
- 15.Colombo M, de Franchis R, Del Ninno E, Sangiovanni A, De Fazio C, Tommasini M, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325(10):675–80. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 16.Digumarthy SRSD, Saini S. MRI in detection of hepatocellular carcinoma (HCC) Cancer Imaging. 2005;5(1):20–24. doi: 10.1102/1470-7330.2005.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen CJYH, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH REVEAL-HBV Study Group. Risk of Hepatocellular Carcinoma Across a Biological Gradient of Serum Hepatitis B Virus DNA Level. JAMA. 2006;295(1):65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 18.Frazee RC, Roberts JW, Symmonds RE, Snyder SK, Hendricks JC, Smith RW, et al. A prospective randomized trial comparing open versus laparoscopic appendectomy. Ann Surg. 1994;219(6):725–8. doi: 10.1097/00000658-199406000-00017. discussion 728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen CJ, Yang HI, Iloeje UH. Hepatitis B virus DNA levels and outcomes in chronic hepatitis B. Hepatology. 2009;49(5 Suppl):S72–84. doi: 10.1002/hep.22884. [DOI] [PubMed] [Google Scholar]
- 20.Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, et al. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53(10):1494–8. doi: 10.1136/gut.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuen MF, Tanaka Y, Fong DY, Fung J, Wong DK, Yuen JC, et al. Independent risk factors and predictive score for the development of hepatocellular carcinoma in chronic hepatitis B. J Hepatol. 2009;50(1):80–8. doi: 10.1016/j.jhep.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Sizaret P, Tuyns A, Martel N, Jouvenceaux A, Levin A, Ong YW, et al. Alpha-Fetoprotein levels in normal males from seven ethnic groups with different hepatocellular carcinoma risks. Ann N Y Acad Sci. 1975;259:136–55. doi: 10.1111/j.1749-6632.1975.tb25410.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien JE, Dvorin E, Drugan A, Johnson MP, Yaron Y, Evans MI. Race-ethnicity-specific variation in multiple-marker biochemical screening: alpha-fetoprotein, hCG, and estriol. Obstet Gynecol. 1997;89(3):355–8. doi: 10.1016/S0029-7844(96)00524-8. [DOI] [PubMed] [Google Scholar]
- 24.Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, et al. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 138(2):493–502. doi: 10.1053/j.gastro.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Trevisani F, D’Intino PE, Morselli-Labate AM, Mazzella G, Accogli E, Caraceni P, et al. Serum alpha-fetoprotein for diagnosis of hepatocellular carcinoma in patients with chronic liver disease: influence of HBsAg and anti-HCV status. J Hepatol. 2001;34(4):570–5. doi: 10.1016/s0168-8278(00)00053-2. [DOI] [PubMed] [Google Scholar]