Abstract
Background
Weight loss and changes in growth are noted in children treated with interferonα
Objectives
To prospectively determine changes in weight, height, body mass index and body composition during and after treatment of children with hepatitis C.
Methods
Children treated with PEG-IFNα2a +/− ribavirin in the PEDS-C trial underwent anthropometric measurements, DXA scan, dietary and activity assessments during and after treatment.
Results
114 (55% male) children mean age 11±3 years were randomized, and 107 received treatment for at least 24 weeks. Subjects were divided into 3 groups according to duration of treatment: 24 (N=14), 48 (N=82), or 72 (N=11) weeks. Decrements of up to 0.50 z score were observed for weight, height and BMI while on therapy among all groups (P≤0.01 compared to baseline). In the group treated for 48 weeks, 29 (33%) subjects had greater than 0.5 unit decrement in height-for-age Z score. While weight-for-age and BMI z scores returned to baseline after cessation of therapy, mean HAZ score was slower to rebound, still lower than baseline at 96 weeks post-therapy for the long treatment duration group (P=0.03) and lower than baseline in most children treated for 48 weeks. Percent body fat, fat-free mass z scores and triceps skinfold z scores decreased with therapy. Dietary energy intake and levels of physical activity did not change during treatment.
Conclusions
PEG-IFNα2a was associated with significant changes in body weight, linear growth, body mass index and body composition in children. These effects were generally reversible with cessation of therapy, although height-for-age z scores had not returned to baseline after 2 years of observation in many. Longer term growth data are needed among children treated for chronic HCV.
Introduction
Chronic hepatitis C virus (HCV) infection in childhood is usually a mild, slowly progressive disease. The extended natural history has not been clearly elucidated, but some children develop progressive liver disease with cirrhosis (1) and even cancer (2). Peginterferon (PEG-IFNα) with ribavirin (RV) until recently was the standard treatment for chronic HCV in adults, and is approved for children as young as 3 years of age. There may be significant benefit to treating HCV infection in children, while liver disease is mild, confounding factors are few, and IFNα is usually well tolerated (3).
Potential side effects of IFNα in children have been a concern in the decision to use this agent. Weight loss, slow weight gain, and changes in linear growth have been reported in children treated with IFNα for either chronic hepatitis B or HCV (4,5). Some reversibility of these effects has been documented after cessation of treatment, but few details are known about this side effect. In particular, the question of whether and how changes in weight represent changes in body composition has never been studied. In addition, the long-term effects, especially on linear growth, have not been fully elucidated.
The Pediatric Study of Hepatitis C (PEDS-C) was a randomized controlled multi-center trial of the safety and efficacy of PEG-IFNα2a (PEGASYS®, Roche Pharmaceuticals, Nutley, NJ) with and without RV (COPEGUS®, Roche Pharmaceuticals) in children and adolescents with chronic hepatitis C. The results regarding safety and efficacy of this treatment have been previously reported (6). Subjects were followed longitudinally for up to 2 years after treatment in order to ascertain changes in anthropometric status in children treated with PEG-IFNα2a, and to determine if these changes were associated with other factors, including dietary intake and physical activity during and after treatment. Additional analyses were planned to investigate changes in weight, growth, and body composition in relation to type of antiviral therapy and receipt of at least 80% of total calculated dose of PEG-IFNα2a. Informed consent was obtained from parents or guardians of all subjects, and the study was approved by Institutional Review Boards of all participating institutions.
Methods
Patients
Eligible subjects included treatment-naïve children age 5 to 18 years with chronic HCV infection documented by the presence of HCV RNA in plasma on two occasions at least 6 months apart. Details of the trial were previously published (7) and the study was registered at www.ClinicalTrials.gov, NCT00100659. Subjects were enrolled by investigators at 11 North American centers from December 2004 until May 2006. All two year off-therapy follow-up visits were completed by February 2010.
All children received PEG-IFNα2a. Although some children also received RV, PEG-IFNα2a is the agent considered responsible for weight loss commonly observed in treated patients. In a generalized estimating equations model adjusted for baseline age and sex, we found no statistical difference in weight, growth or body composition outcomes between subjects who received RV and those who did not, so the groups were combined for the current analysis. The cohort was divided into 3 groups based on duration of PEG-IFNα2a treatment (24, 48 or 72 weeks), regardless of RV treatment. Post-treatment follow-up continued for up to 96 weeks.
Virologic Testing
Qualitative HCV RNA was assessed with Roche COBAS AMPLICOR HCV Qualitative PCR, v2.0 with a lower limit of detection of 60 IU/mL. HCV viral genotyping was performed at entry using a line-probe assay (LiPA, Innogenetics; Ghent, Belgium). Sustained virologic response (SVR) was defined as undetectable HCV RNA 24 weeks after the end of treatment.
Treatment
Subjects were randomly assigned with equal allocation to receive either PEG-IFNα2a and RV or PEG-IFNα2a and placebo. PEG-IFNα2a was administered in a dose of 180 μg/1.73m2 body surface area (maximum 180 μg) subcutaneously once weekly. Ribavirin was administered in a dose of 15 mg/kg/day orally in 2 doses (maximum 1200 mg/day if ≥ 75 kg and 1000 mg if < 75 kg), using 100 mg tablets. Placebo tablets were supplied in the same dosing regimen as RV. Patients without detectable HCV RNA at 24 weeks were continued on treatment for another 24 weeks, whereas those who had detectable HCV RNA at 24 weeks were considered treatment failures. Treatment was stopped in those subjects who had been given PEG-IFNα2a with RV and were still viremic. Patients who failed treatment with PEG-IFNα2a plus placebo were offered “open-label” therapy with PEG-IFNα2a plus RV for another 48 weeks (stopping after 24 weeks if HCV RNA remained positive). This strategy led to 3 groups based on duration of PEG-IFNα2a treatment of 24, 48 or 72 weeks (Figure 1). Further details of the study design have been previously reported (7). Drug dose reductions were instituted for toxicities according to protocol.
Figure 1.
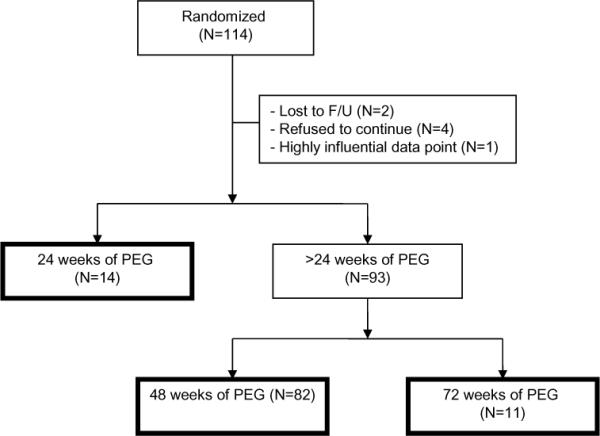
Derivation and definitions of the study population demonstrating the three therapy duration groups.
Growth, Body Composition and Nutritional Status
Body weight was measured by an electronic digital scale accurate to 0.1 kg. Standing height was measured by stadiometer to the nearest 0.1 cm. Triceps, biceps, iliac, and subscapular skinfold thicknesses were measured to the nearest 0.5 mm using Lange skin calipers. Mid-arm circumference (MAC) was measured to the nearest 0.1 cm using a flexible non-stretchable plastic tape, and mid-arm muscle area was calculated (MAC2/4π). All measurements were made by a single observer at each site, who had been trained in the standardized measurement procedures. Anthropometric measurements were performed in duplicate and reported as the mean of the two measurements. Weight-for-age, height-for-age, body mass index (BMI) and triceps skinfold z scores (8) were calculated using the 2000 US NCHS reference values (www.cdc.gov/nchs). Anthropometric data were measured at baseline and weeks 24, 48 and 72 of treatment, as well as up to 96 weeks post-treatment.
Dual-energy x-ray absorptiometry (DXA) scans were performed using either Hologic, Inc. (QDR4500A and QDR4500W models, Bedford, MA) or GE Lunar Prodigy (GE Medical System, Fairfield, CT) bone densitometers. Scans were performed on a single densitometer at each center. Data was standardized across sites and read at a central site. The software versions used for acquisition varied from version 11.2 to 12.7.3.1 (APEX 2.2) on Hologic machines, and from 8.10 to 11.20 on Lunar machines. Whole body scans were performed according to manufacturer specific procedures. Quality control for these measures included cross-calibration of all devices as well as longitudinal monitoring of each site scanner's accuracy and stability. Hologic DXA longitudinal quality control was carried out by scanning, either daily or no less than 3 times per week, the Hologic Spine phantom and by performing a weekly Table Top Radiographic Uniformity Test (Airscan). GE Lunar DXA longitudinal quality control was provided by scanning, either daily or no less than 3 times per week, the lunar calibration block and the Lunar aluminum spine phantom. Intra-manufacturer cross-calibration was performed by scanning a set of European Spine, Hologic Block, and Whole Body Phantoms at the beginning of the study period. For inter-manufacturer cross-calibration of the whole body bone mineral density, standardization equations were used (9). A “gold standard” site was chosen to which other sites were calibrated. No inter-manufacturer cross-calibration was performed for the whole body soft tissue measurements, as there was no standardization equation or converting formula available to use. All subject and cross-calibration scans were analyzed centrally by the DXA Core Laboratory (University of California, San Francisco) using Hologic software release 12.3 and Lunar software release 11.4. Data were expressed as grams of fat, grams of fat-free mass, and total body weight. Percent body fat (%BF) was defined as (total grams of fat/body weight × 100). Percent body fat z score (%BFZ) and fat-free mass z score (FFMZ) were calculated using the Pediatric Body Composition Reference Charts of the Children's Nutrition Research Center at USDA/Baylor College of Medicine (http://www.bcm.edu/bodycomplab/Flashapps/AllDXArefsChartpage.html) (10).
Dietary intake assessment was obtained using 3-day food records (11). Each 3-day record consisted of a 2-day patient/parent-completed food diary and a 24-hour diet recall with the assistance of a study-designated site dietitian during an interview with the subject and parent. Detailed instructions were provided to each subject at the baseline visit. Each 3-day record included at least one weekday and one weekend day. The total intake of calories from each 3-day period was determined using the Food Processor SQL© Nutrition Analysis Software (ESHA Research, Salem, OR). Dietary energy intake (kcal/day) was calculated as the average intake over the 3 days for each visit. Energy intake was expressed as a percentage of the subject's resting energy expenditure (REE), as calculated by the Schofield equations (12).
Physical activity was assessed by parent responses to questions designed to determine changes in the amount and strenuousness of activity during each month prior to the protocol defined study visit (13). In one question, parents were asked to compare the child's level of activity to other children of the same age and sex on a 5-point scale. In a second question, parents estimated the percent of time the child spent in activities of varying strenuousness during weekdays and weekend days. Week estimates of each activity level were calculated based on 5 × weekday measure + 2 × weekend measure.
The study was conducted under an Investigational New Drug application and was approved by the institutional review boards of the participating sites. All parents/guardians provided written informed consent and children over 12 years provided written assent prior to enrollment. The Data Coordinating Center was the Maryland Medical Research Institute in Baltimore, MD.
Statistical Analysis
Baseline characteristics were described using mean ± SD for continuous data and N (%) for categorical data. Physical activity data that were continuously distributed were highly skewed and were described by median and inter-quartile range (IQR). Outcomes of interest included change from baseline for weight-for-age z score (WAZ), height-for-age z score (HAZ), BMI z score (BMIZ), triceps skinfold z score (TSFZ), % body fat z score (%BFZ), and fat-free mass z score (FFMZ), all of which were approximately normally distributed. Subjects were treated with PEG-IFNα2a for either 24, 48, or 72 weeks and then followed thereafter for up to 96 weeks post-therapy. The analysis of changes in weight, height, and body composition over time was performed with a repeated-measures, generalized linear model assuming a normal distribution with an identity link function. The within-subjects correlation was modeled as autoregressive (AR1). Changes in outcome from baseline were assessed with an interaction term of weeks on study drug (24, 48, or 72) by weeks off study drug (0, 24, 48, or 96), adjusted for baseline age and sex. The effect of several independent variables, including duration of PEG-IFNα2a treatment, sustained viral response (SVR), energy intake during and after treatment, change in physical activity during and after treatment, receipt of ribavirin, and receipt of at least 80% of total dose of PEG-IFNα2a, were explored but not retained in the final model, since none were statistically significant modifiers. All tests were two-sided with P<0.05 used to indicate statistical significance. Results and plots were generated using SAS/STAT and SAS/GRAPH software, Version 9.2 of the SAS System for Windows, SAS Institute Inc., Cary, NC, USA.
Results
Patients
114 children were eligible for treatment and randomized to receive PEG-IFNα2a with either RV (N=55) or placebo (N=59) (Figure 1). Six subjects were either lost to follow-up or withdrew prior to completing at least 24 weeks of therapy, and one subject in the 72-week treatment group was omitted after a sensitivity analysis showed this subject to have highly influential anthropometry z scores. This resulted in a final study cohort of 107 subjects. Twelve subjects in the RV arm had no virologic response (VR) at week 24, at which point treatment was discontinued, and two from the placebo arm were lost to follow-up at 24 weeks, resulting in 14 children who had only 24 weeks of PEG-IFNα2a. Children who had VR at week 24 (N=66) received 24 more weeks of treatment, as did those who crossed over from placebo to RV (N=27). Among these 93 children, 82 (16 from the crossover group) were treated for a total of 48 weeks, while the remaining 11 children in the crossover group had VR at week 48 and received a total of 72 weeks of PEG-IFNα2a. Follow-up visits occurred at 24, 48, and 96 weeks after discontinuation of therapy. Due to attrition, loss of contact or retreatment, not all subjects were available at all follow-up visits.
Subjects at baseline were on average 11 years of age (range 5 to 17), 45% female, mostly Caucasian (82%), and of normal weight, height, BMI, percent body fat, and fat-free mass. A majority of subjects had no more than mild disease (82% no more than mild histologic activity, 100% with no more than mild steatosis, and 96% with no more than portal-periportal fibrosis). Additional baseline characteristics are shown in Table 1.
Table 1.
Baseline characteristics for 107 subjects.
| N (%) or Mean±SD | |
|---|---|
| Patient Characteristics | |
| Age (Years) | 11.1±3.5 |
| 5–11 | 59 (55%) |
| 12–17 | 48 (45%) |
| Female gender | 48 (45%) |
| Non-Caucasian race (n=97) | 17 (18%) |
| Weight for age Z score (WAZ) (n=106) | 0.56±1.17 |
| Height for age Z score (HAZ) (n=106) | 0.17±1.05 |
| Body Mass Index Z score (BMI Z )(n=106) | 0.60±1.11 |
| BMI Z >2.00 | 10 ( 9%) |
| Percent body fat Z score (n=77) | 0.00±1.15 |
| Fat-free mass Z score (n=80) | 0.01±0.92 |
| Mode of acquisition (n=90) | |
| Maternal-infant | 80 (89%) |
| Transfusion | 8 ( 9%) |
| Sexual contact, intravenous drug use | 2 ( 2%) |
| Genotype (n=106) | |
| 1 | 85 (80%) |
| 2 | 7 ( 7%) |
| 3 | 13 (12%) |
| 6 | 1 ( 1%) |
| Histology Results (n=104) | |
| Histology Activity Index | |
| None | 0 ( 0%) |
| Minimal (1 – 3) | 33 (32%) |
| Mild (4 – 6) | 52 (50%) |
| Moderate (7 – 9) | 17 (16%) |
| Marked (10 – 12) | 2 ( 2%) |
| Steatosis | |
| None | 59 (57%) |
| Minimal (≤ 5% of tissue) | 36 (35%) |
| Mild (6 – 33%) | 9 ( 9%) |
| Fibrosis Score | |
| None | 13 (13%) |
| Portal-Periportal fibrosis (Ishak 1–2) | 86 (83%) |
| Bridging Fibrosis (Ishak 3–4) | 4 ( 4%) |
| Cirrhosis (Ishak 5–6) | 1 ( 1%) |
Virologic Responses
Primary efficacy and safety of treatment in this study has been reported (6). In the 107 subjects included in this study cohort, the SVR rate was 56% among 52 children who received PEG-IFNα2a and RV, and 22% among 55 who received PEG-IFNα2a with placebo (P=0.0003).
Weight, Stature and BMI
Table 2 shows the change from baseline for WAZ, HAZ, and BMIZ for the 3 treatment duration groups (24, 48, and 72 weeks of PEG-IFNα2a), after adjusting for baseline age and sex. Significant decreases were seen in age-adjusted weight, height and BMI z scores while on therapy, with longer treatment associated with greater decreases. The most marked decreases for WAZ and HAZ were seen in the 11 children who received therapy for 72 weeks (mean ± SE decrease −0.50±0.13 and −0.50±0.07, respectively; P<0.0001 vs. baseline for each), while for BMIZ, the greatest decrease occurred for subjects receiving 48 weeks of therapy (−0.40±0.04, P<0.0001). Thereafter, z scores rebounded toward baseline levels, especially among the largest treatment group (48 weeks of therapy) (Figure 2.) This group at 96 weeks post-treatment was not statistically different from baseline for any of the three z scores. In the small group of children treated for 72 weeks, HAZ score was still significantly different from baseline at the end of follow up (Supplementary Figure 1.)
Table 2.
Change in WAZ, HAZ, and BMIZ from baseline, adjusted for baseline age and sex (n=106).1
| Weeks on | Weeks off | Change in WAZ |
Change in HAZ |
Change in BMIZ |
||||
|---|---|---|---|---|---|---|---|---|
| PEG IFNα2a | PEG IFNα2a | N | Mean±SE | P Δ=0 | Mean±SE | P Δ=0 | Mean±SE | P Δ=0 |
| 24 | 0 | 106 | −0.31±0.03 | <0.0001 | −0.14±0.02 | <0.0001 | −0.33±0.04 | <0.0001 |
| 24 | 11 | −0.15±0.07 | 0.03 | −0.11±0.05 | 0.05 | −0.16±0.07 | 0.03 | |
| 48 | 9 | −0.05±0.10 | 0.63 | −0.03±0.12 | 0.81 | −0.10±0.09 | 0.28 | |
| 96 | 7 | −0.08±0.18 | 0.65 | 0.14±0.18 | 0.43 | −0.21±0.15 | 0.15 | |
| 48 | 0 | 88 | −0.43±0.04 | <0.0001 | −0.28±0.02 | <0.0001 | −0.40±0.04 | <0.0001 |
| 24 | 73 | −0.08±0.04 | 0.06 | −0.22±0.03 | <0.0001 | −0.01±0.05 | 0.77 | |
| 48 | 63 | −0.01±0.05 | 0.88 | −0.16±0.04 | <0.0001 | 0.00±0.05 | 0.94 | |
| 96 | 53 | 0.02±0.07 | 0.81 | −0.08±0.06 | 0.18 | −0.03±0.07 | 0.66 | |
| 72 | 0 | 11 | −0.50±0.13 | <0.0001 | −0.50±0.07 | <0.0001 | −0.35±0.14 | 0.01 |
| 24 | 11 | −0.04±0.12 | 0.76 | −0.42±0.09 | <0.0001 | 0.14±0.14 | 0.35 | |
| 48 | 11 | 0.03±0.12 | 0.77 | −0.39±0.11 | 0.0004 | 0.18±0.12 | 0.14 | |
| 96 | 7 | 0.01±0.14 | 0.92 | −0.41±0.19 | 0.03 | 0.13±0.09 | 0.16 | |
Abbreviations: WAZ = weight-for-age z-score; HAZ = height-for-age z-score; BMIZ = body mass index z-score.
One subject is missing WAZ, HAZ, and BMIZ at baseline.
Figure 2.
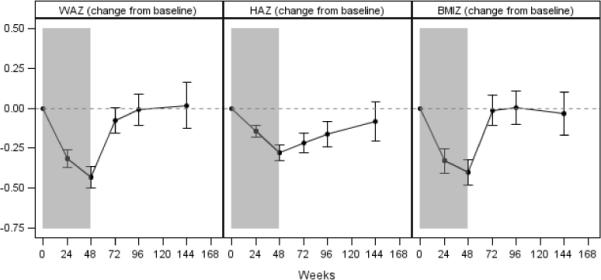
Change from baseline in weight-for-age z score (WAZ), height-for-age z score (HAZ), and body mass index z score (BMIZ) among subjects treated with peginterferonα2a for 48weeks. Error bars indicate 95% confidence interval. The shaded regions indicate time on peginterferonα2a. Results are from a generalized linear model, adjusted for baseline age and sex.
Further analysis was performed in the largest group, subjects treated for 48 weeks. In this group, 29 subjects (33%) had greater than a 0.5 unit decrement in HAZ score at one or more time points during the study. In particular, 10 (34%) subjects crossed this threshold during treatment but rebounded to within 0.5 unit decrease in HAZ by the end of trial, 7 (24%) continued to have more than a 0.5 unit decrement in HAZ score by the end of the trial, and 12 (41%) exceeded a 0.5 unit decrease only after therapy was discontinued. Among subjects <12 years of age at baseline, 19 (41%) experienced a drop in HAZ score of 0.5 or larger compared with 10 (24%) among subjects 12 years or older (P=0.11 by Fisher exact test). The multivariate model described in Methods was re-run for the 48-week treatment group by including WAZ score changes, specifically to examine the effect of weight loss on HAZ score. The data suggest that subjects who experienced more than a 0.50 decrement in WAZ had an additional mean decrement of 0.15 unit in HAZ score at all follow-up periods. Those with <0.50 WAZ decrement rebounded at 48 and 96 weeks post-treatment to near baseline levels, while those with >0.50 WAZ decrement remained below baseline levels (data not shown).
Body Composition
Serial measures of body composition as measured by DXA are presented in Table 3 for the whole cohort and Figure 3 for the group treated for 48 weeks. Significant reductions in percent body fat z score (%BFZ) were noted during treatment. After 24 or 48 weeks of PEG-IFNα2a, %BFZ was lower than baseline by 0.12±0.05 units and 0.14±0.06 units, respectively (P=0.02 for both comparisons). After discontinuation of therapy, %BFZ returned to baseline levels in all subjects, except for a mean increase of 0.33±0.08 units (P<0.0001) at 48 weeks post-therapy among subjects in the 24-week therapy group.
Table 3.
Change in percent fat Z-score and fat-free mass Z-score from baseline, adjusted for baseline age and sex.
| Weeks on | Weeks off | Change in %BFZ |
Change in FFMZ |
||||
|---|---|---|---|---|---|---|---|
| PEG IFNα2a | PEG IFNα2a | N | Mean±SE | P Δ=0 | N | Mean±SE | P Δ=0 |
| 24 | 0 | 68 | −0.12±0.05 | 0.02 | 70 | −0.33±0.05 | <0.0001 |
| 24 | 6 | 0.02±0.05 | 0.60 | 6 | −0.26±0.08 | 0.001 | |
| 48 | 5 | 0.33±0.08 | <0.0001 | 5 | −0.46±0.20 | 0.02 | |
| 96 | 4 | 0.31±0.34 | 0.37 | 3 | −0.28±0.08 | 0.0003 | |
| 48 | 0 | 56 | −0.14±0.06 | 0.02 | 56 | −0.37±0.05 | <0.0001 |
| 24 | 44 | −0.02±0.06 | 0.79 | 45 | 0.02±0.06 | 0.75 | |
| 48 | 40 | −0.01±0.07 | 0.91 | 40 | 0.06±0.07 | 0.36 | |
| 96 | 28 | 0.04±0.12 | 0.71 | 30 | −0.02±0.09 | 0.81 | |
| 72 | 0 | 4 | −0.24±0.22 | 0.29 | 3 | 0.11±0.21 | 0.60 |
| 24 | 4 | −0.14±0.40 | 0.74 | 3 | 0.33±0.30 | 0.27 | |
| 48 | 4 | −0.11±0.45 | 0.80 | 3 | 0.47±0.27 | 0.08 | |
| 96 | 1 | 0.37±0.26 | 0.16 | 1 | 0.61±0.20 | 0.002 | |
Abbreviations: %BFZ = percent fat z-score; FFMZ = fat-free mass z-score.
Figure 3.
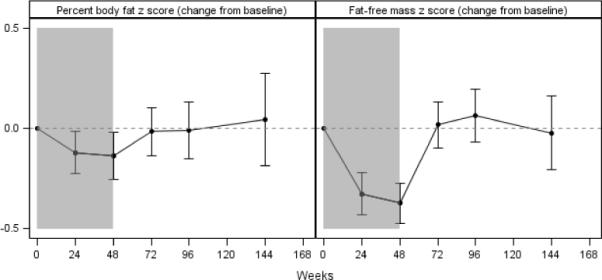
Change from baseline in percent body fat z score (%BFZ) and fat-free mass z score (FFMZ) among subjects treated with peginterferonα2a for 48 weeks. Error bars indicate 95% confidence interval. The shaded regions indicate time on peginterferonα2a. Results are from a generalized linear model, adjusted for baseline age and sex.
Concurrent with these changes in body fat, significant decrements in fat-free mass z score (FFMZ) were observed during treatment in all groups (Supplemental Figures 2 and 3). After 24 or 48 weeks of PEG-IFNα2a, FFMZ was lower than baseline by 0.33±0.05 units and 0.37±0.05 units, respectively (P<0.0001 for both comparisons). Variable trends in FFMZ were observed after the discontinuation of treatment. In the group treated for 24 weeks, FFMZ was still lower than baseline at 24 weeks post-therapy (−0.26±0.08, P=0.001), 48 weeks post-therapy (−0.46±0.20, P=0.02), and 72 weeks post-therapy (−0.28±0.08, P=0.0003). In the larger group treated for 48 weeks, FFMZ returned to baseline levels after treatment was stopped. In the smaller group treated for 72 weeks, FFMZ rebounded to levels surpassing baseline levels.
Measures of peripheral body composition mirrored these DXA trends (Supplemental Table 1). Triceps skinfold z scores (TSFZ) were significantly lower than baseline in subjects treated for either 24 or 48 weeks (−0.17±.07, P=0.01 and −0.27±.09, P=0.002, respectively)(Supplemental Figure 4). Mid-arm muscle circumference (MAC) and midarm muscle area (MAMA) were not different from baseline during antiviral therapy, suggesting no accretion of peripheral lean mass. After therapy ceased, expected increments in MAC and MAMA were observed.
Dietary Intake
Mean (± SD) dietary energy intake was 139 (±43)% of estimated resting energy expenditure (REE) at baseline, 148 (± 54)% of resting REE while on antiviral therapy, and 133 (± 51)% when subjects were off treatment (P=0.06, data not shown).
Physical Activity
Assessment of physical activity at baseline, during and after treatment is shown in Table 4. No effect of treatment on either comparison of treated children to peers or distribution of level of activity as assessed by parent questionnaires was observed.
Table 4.
Physical activity (107 subjects).
| Baseline | On Therapy | Off Therapy | P | |
|---|---|---|---|---|
| Physical Activity | ||||
| Subjects (n) | 106 | 107 | 92 | -- |
| In comparison to other children the same age and sex, the subject is … | 0.56 | |||
| A lot less active | 0 ( 0%) | 0 ( 0%) | 1 ( 1%) | |
| A little less active | 9 ( 9%) | 26 (13%) | 9 ( 9%) | |
| About average | 62 (59%) | 105 (54%) | 56 (54%) | |
| A little more active | 23 (22%) | 48 (25%) | 28 (27%) | |
| A lot more active | 11 (10%) | 14 ( 7%) | 9 ( 9%) | |
| Median score (IQR)1 | 3 (3 – 4) | 3 (3 – 4) | 3 (3 – 4) | 0.59 |
| Percent of week spent … 2 | ||||
| Sleeping | 39 (36 – 43) | 39 (35 – 42) | 39 (35 – 42) | 0.63 |
| Sedentary or seated activities | 52 (42 – 70) | 56 (42 – 70) | 51 (36 – 67) | 0.20 |
| Light or casual activities | 16 (11 – 21) | 15 (8 – 22) | 15 (10 – 21) | 0.76 |
| Moderate or stop/start activities | 8 (4 – 12) | 7 (3 – 11) | 8 (4 – 13) | 0.17 |
| Intense or sustained activities | 2 (0 – 7) | 2 (0 – 5) | 4 (0 – 8) | 0.09 |
Response categories scored: 1=a lot less active, 2=a little less active, 3=about average, 4=a little more active, and 5=a lot more active.
Physical activity measures were collected on a single weekday and a single weekend day. Week estimates based on (5 × weekday measure) + (2 × weekend day measure).
Discussion
In this large cohort of children with chronic HCV infection treated with antiviral therapy, we observed significant changes in weight, height and body composition, some of which persisted after discontinuation of treatment. The nature and timing of these effects were not explained by reductions in dietary intake or energy expenditure.
There has long been a concern about effects of prolonged treatment with IFNα or its pegylated counterpart on the nutritional status of children. The mechanism of weight loss, slow weight gain, or decreased growth velocity associated with this cytokine has not been elucidated. Some patients report nausea, anorexia, early satiety or diarrhea while being treated with these agents, but these symptoms are often short-lived and are not universal.
A number of studies have evaluated this potential safety concern in children with chronic viral hepatitis. In 1996, weight loss was noted in children treated for 6 months for chronic hepatitis B or chronic hepatitis C. Among 11 children with HBV or HCV infection, treatment with recombinant IFN-alpha 2a and 2b for at least 24 weeks was associated with a mean weight-for-age z score decrement of 0.81 unit after 3 months of therapy (P<0.001) and a decrement of 0.45 unit after 6 months of treatment (P<0.05). Mean weight-for-height z scores were reduced from +0.18 to −0.74 after 3 months of therapy (P<0.01); BMI was not reported (5). In that study, children returned to baseline WAZ values by 1 year after treatment and height was not affected by anti-viral therapy Mean energy intake was recorded in only 2 of the 11 subjects. It was reported to be normal at baseline but reduced by 13 and 26%, respectively, after three months of anti-viral therapy (5).
Temporary effects on weight and height were noted in children with chronic hepatitis B treated for only 24 weeks with IFNα with or without lamivudine (14). As treatment durations increased for chronic hepatitis C in children, especially those with genotype 1 infection, additional data regarding this effect has accumulated. In 56 children treated with IFNα2b and ribavirin for 48 weeks, changes in both weight and height percentiles were reported (15). Although compensatory weight gain was noted after treatment was discontinued, the linear growth catch-up was only partial after the 24 weeks follow-up period, and longer follow-up of that cohort is underway. These effects were seen with the use of pegylated interferons as well. In a multinational study of 65 HCV-infected children treated with PEG-IFNα2a and ribavirin for 24 (N=18) or 48 (N=47) weeks, weight and height z scores were not different from baseline at the end of treatment (16). However, in another European study, this time with PEG-IFNα2b and ribavirin, weight loss was recorded in 19% of children, and noted to be regained after 24 weeks follow-up (17). In that cohort, linear growth velocity decreased during treatment in the majority (70%) of subjects and increased during off-treatment follow-up. Decrease in mean height percentile at the end of 24 weeks of follow-up was greater in those children who had received longer duration of therapy. None of these studies included analysis of body composition, in order to determine the body compartment(s) that were compromised during IFNα treatment, and none included assessment of energy intake and physical activity during and after therapy.
Possible etiologies of growth delay in patients with chronic hepatitis include increased energy requirements, decreased energy intake, or gastrointestinal malabsorption, but the medical literature contains few systematic studies of the nutritional status of patients undergoing antiviral therapy (18). Among adults with HCV treated with pegylated interferon and ribavirin, 14 of 15 patients lost weight during treatment, with a mean weight loss of 3.1 kg at 24 weeks(19). This weight loss was associated with lower dietary energy intake, on average 9.7% lower than baseline at week 1 and persisting through week 12. Data from the current study do not demonstrate a similar change in dietary intake in this group of children, since in our sample there was actually a trend to an increase in energy intake while on treatment.
Our data demonstrate that weight and height z scores declined in tandem, as opposed to sequential changes as might be expected if nutritional factors such as decreased intake or increased energy losses were responsible for the slower linear growth velocity. These findings implicate an alternative mechanism for these constitutional effects, such as direct effects of the interferon on somatic growth. In addition, whole body DXA data suggested that both body fat and fat-free mass components were affected by this growth arresting effect. Arm anthropometric data also suggested a reduction in peripheral fat mass that returned to baseline after therapy was completed. It is important to note that baseline liver histology and response to therapy did not affect these growth outcomes, indicating that neither underlying liver disease nor uncontrolled viral replication contributed to the observed changes in weight, growth, or body composition.
Many children with chronic HCV infection are good candidates for treatment. Successful therapy will substantially decrease long-term risk for serious complications such as cirrhosis and hepatocellular carcinoma. Less measurable, but no less significant, benefits include mitigation of concern about later perinatal transmission and real or perceived social stigmatization, and fewer risks for liver disease cofactors such as obesity and alcohol consumption in infected children and adolescents. Interferon-based therapies are the mainstay of HCV treatment in both children and adults. However, growth disruption is a uniquely pediatric concern. We have demonstrated significant effects on weight, BMI and body composition in a large cohort of children treated with PEG-IFNα2a, including measurable effects on linear growth and body composition that persisted after the treatment was discontinued in some groups. There was no difference in HAZ scores effects in pre-adolescent compared to adolescent children. It is critical that longer-term growth and metabolic data are gathered to determine the ultimate impact of this therapy, so that informed decisions can be made about optimal timing of treatment in this vulnerable population.
Supplementary Material
Acknowledgment
The authors would like to acknowledge the contribution of Dr. Roman Shypailo, Department of Pediatrics, Children's Nutrition Research Center, Baylor College of Medicine, Houston TX, for assistance with calculating body composition z scores.
Funding Supported by a cooperative agreement between the National Institute of Diabetes and Digestive and Kidney Diseases and the Food and Drug Administration (contract no. 1UO1DK067767-01.CRC) and in part by National Institutes of Health/National Center for Research Resources Colorado CTSI grant no. UL1 RR025780 and the following study sites: M01-RR-00069, Children's Hospital Colorado, Aurora, CO; M01-RR-02172, Children's Hospital Boston, Boston, MA; M01-RR-01271, University of California, San Francisco, CA; 5-M01-RR-020359-01, Children's National Medical Center, Washington, DC; M01-RR-00645, Columbia University Medical Center, New York, NY; M01-RR-00082, University of Florida, Gainesville, FL; M01-RR-00037, University of Washington, Seattle, WA; 5-M01-RR-000240, Children's Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA; U01-DK-067767-02, Johns Hopkins Medical Center, Baltimore, MD; M01-RR-08084, University of Cincinnati, Cincinnati, OH; and M01-RR-00750, Indiana University, Indianapolis, IN. CD was supported in part by K24 HD058795. The contents of this report are the authors' sole responsibility and do not necessarily represent the official views of the National Institutes of Health. Additional support was provided by Hoffmann-La Roche for study medications, the data coordinating center, and central laboratory costs.
List of Abbreviations
- PEDS-C
Pediatric Study of Hepatitis C
- IFNα
interferon-alfa
- BMI
body mass index
- WAZ
weight for age z score
- HAZ
height for age z score
- BMIZ
body mass index z score
- PEG-IFNα2a
peginterferon alfa-2a
- RV
ribavirin
- SVR
sustained virologic response
- CDC
Centers for Disease Control
- DXA
dual-energy x-ray absorptiometry
- HCV
hepatitis C virus
- %BF
% body fat
- %LBM
% lean body mass
- MAMA
mid-arm muscle area
- MAC
mid-arm circumference
- TS
triceps skinfold
- TSFZ
triceps skinfold z score
- REE
resting energy expenditure
References
- 1.Bortolotti F, Verucchi G, Cammà C, et al. Long-Term Course of Chronic Hepatitis C in Children: From Viral Clearance to End-Stage Liver Disease. Gastroenterology. 2008;134(7):1900–1907. doi: 10.1053/j.gastro.2008.02.082. [DOI] [PubMed] [Google Scholar]
- 2.González-Peralta R, Langham MR, Jr, Andres JM, et al. Hepatocellular carcinoma in 2 young adolescents with chronic hepatitis C. Journal Of Pediatric Gastroenterology And Nutrition. 2009;48:630–635. doi: 10.1097/MPG.0b013e318170af04. [DOI] [PubMed] [Google Scholar]
- 3.Rodrigue JR, Balistreri W, Haber B, et al. Peginterferon with or without ribavirin has minimal effect on quality of life, behavioral/emotional, and cognitive outcomes in children. Hepatology. 2011;53(5):1468–1475. doi: 10.1002/hep.24248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Comanor L, Minor J, Conjeevaram HS, et al. Impact of chronic hepatitis B and interferon-alfa therapy on growth of children. J Vir Hepat. 2001;8:139–147. doi: 10.1046/j.1365-2893.2001.00262.x. [DOI] [PubMed] [Google Scholar]
- 5.Gottrand F, Michaud L, Guimber D, et al. Influence of recombinant interferon alpha on nutritional status and growth pattern in children with chronic viral hepatitis. European Journal of Pediatrics. 1996;155(12):1031–1034. doi: 10.1007/BF02532525. [DOI] [PubMed] [Google Scholar]
- 6.Schwarz KB, Gonzalez-Peralta RP, Murray KF, et al. The combination of ribavirin and peginterferon is superior to peginterferon and placebo for children and adolescents with chronic hepatitis C. Gastroenterology. 2011 Feb;140(2):450–458. e451. doi: 10.1053/j.gastro.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray KF, Rodrigue JR, Gonzalez-Peralta RP, et al. for the PEDS-C Clinical Research Network. Design of the PEDS-C trial: pegylated interferon +/− ribavirin for children with chronic hepatitis C viral infection. Clinical Trials. 2007;4(6):661–673. doi: 10.1177/1740774507085445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 2010;91(3):635–642. doi: 10.3945/ajcn.2009.28385. [DOI] [PubMed] [Google Scholar]
- 9.Hui SL, Gao S, Zhou XH, et al. Universal standardization of bone density measurements: a method with optimal properties for calibration among several instruments. Journal of Bone Mineral Research. 1997;12(9):1463–1470. doi: 10.1359/jbmr.1997.12.9.1463. [DOI] [PubMed] [Google Scholar]
- 10.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-ray absorptiometry body composition reference values from NHANES. PLoS ONE. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burrows TL, Martin RJ, Collins CE. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. Journal of the American Dietetic Association. 2010;110(10):1501–1510. doi: 10.1016/j.jada.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Schofield W. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39C(suppl 1):5–41. [PubMed] [Google Scholar]
- 13.Sallis JF, Haskell WL, Wood PD, et al. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. doi: 10.1093/oxfordjournals.aje.a113987. [DOI] [PubMed] [Google Scholar]
- 14.Kuloglu Z, Kansu A, Demirceken F, et al. The influence of interferon-alpha and combination interferon-alpha and lamivudine therapy on height and weight in children with chronic hepatitis B infection. Journal of Pediatric Endocrinology & Metabolism. 2007;20(5):615–620. doi: 10.1515/jpem.2007.20.5.615. [DOI] [PubMed] [Google Scholar]
- 15.González-Peralta R, Kelly DA, Haber B, et al. Interferon alfa-2b in combination with ribavirin for the treatment of chronic hepatitis C in children: Efficacy, safety, and pharmacokinetics. Hepatology. 2005;42:1010–1018. doi: 10.1002/hep.20884. [DOI] [PubMed] [Google Scholar]
- 16.Sokal EM, Bourgois A, Stéphenne X, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection in children and adolescents. J Hepatol. 2010;52(6):827–831. doi: 10.1016/j.jhep.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 17.Wirth S, Ribes-Koninckx C, Calzado MA, et al. High sustained virologic response rates in children with chronic hepatitis C receiving peginterferon alfa-2b plus ribavirin. J Hepatol. 2010;52(4):501–507. doi: 10.1016/j.jhep.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 18.Piche T, Schneider SM, Tran A, Benzaken S, Rampal P, Hebuterne X. Resting energy expenditure in chronic hepatitis C. J Hepatol. 2000;33(4):623–627. doi: 10.1034/j.1600-0641.2000.033004623.x. [DOI] [PubMed] [Google Scholar]
- 19.Hamer C. The impact of combination therapy with peginterferon alpha-2a and ribavirin on the energy intake and body weight of adult hepatitis C patients. J Hum Nutr Diet. 2008;21(5):486–493. doi: 10.1111/j.1365-277X.2008.00882.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


