Abstract
We examined the usability of smartphones for accessing a web-based e-Diary for self-monitoring symptoms in children and adolescents with sickle cell disease (SCD). One group of participants (n=10; mean age 13.1 ± 2.4 years; 5M; 5F) responded to questions using pre-completed paper-based measures. A second group (n=21; mean age 13.4 ± 2.4 years; 10M;11F) responded based on pain and symptoms they experienced over the previous 12 hours. The e-Diary was completed with at least 80% accuracy when compared to paper-based measures. Symptoms experienced over the previous 12 hours included feeling tired (33.3%), headache (28.6%), coughing (23.8%), lack of energy/fatigue (19.0%), yellowing of the eyes (19.0%), pallor (19.0%), irritability (19.0%), stiffness in joints (19.0%), general weakness (14.3%), and pain (14.3%), rating on average as 2.0 ± 1.7 (on 0 to 10 scale). Overall, sleep was good (8.1 ± 1.4 on the 0 to 10 scale). In conclusion, children with SCD were able to use smartphones to access a web-based e-Diary for reporting pain and symptoms. Smartphones may improve self-reporting of symptoms and communication between patients and their health care providers, who may consequently be able to improve pain and symptom management in children and adolescents with SCD in a timely manner.
Keywords: e-Diary, pain, symptoms, children and adolescents, sickle cell disease
INTRODUCTION
With emerging interactive and communication technologies (e-health), new media to deliver health interventions are now available. The utilization of smartphones and the internet to communicate directly and quickly with health care providers may improve pain management for children and adolescents with sickle cell disease (SCD) who are at increased risk for under-treatment for pain and disruption in activities of daily living due to pain(1,2). Handheld wireless devices in the form of smartphones have the ability to improve the self-monitoring of pain and other symptoms at home. This technology may also minimize barriers (lack of transportation to comprehensive centers and access to knowledgeable providers, both of which limit access to hospital and clinic based interventions) and give providers an opportunity to intervene before symptoms become severe (3–5).
We developed an innovative wireless pain intervention program for children and adolescents with SCD that incorporated an electronic pain and symptom monitoring diary (e-Diary) that is easily accessible using a smartphone. To date, electronic pain diaries have been developed to monitor pain in children and adolescents with gastrointestinal symptoms (6) and recurrent and chronic pain (7–9). Higher rates of completion are the major advantage of e-Diaries over paper diaries, as reported by these investigators. Palermo and colleagues (7) demonstrated that children completed significantly more e-Diary entries when compared with paper diaries (mean 6.6 vs 3.8 daily entries, p<0.001).
Pain diaries involve repeated assessment of pain and concurrent symptoms and are increasingly administered electronically, either on small, portable computers (10), wireless devices such as personal digital assistants, also known as PDAs (7–10) or smartphones (5). Electronic (e-Diary) assessments were reported to have high compliance rates (11–14), as they are programmed with alarms and reminders that foster compliance. Compliance rates were reported to be higher with e-Diaries (83% vs 47%) when compared to paper diaries (7). Researchers and clinicians have instant access to data (12, 15–19), which promotes timely responses and facilitates communication of pain and symptoms (11,12,16).
The two-fold purpose of this study was 1) to describe and test the technical features of an e-Diary for self-monitoring of pain and symptoms using a smartphone; and 2) to test usability of the e-Diary by having participants with sickle cell disease (SCD) describe their pain and symptoms over the previous 12 hours.
METHODS
A pilot study was conducted in a small group of participants (n=10) with SCD who were asked to use a smartphone and answer questions on the e-Diary as presented on pre-completed paper based measures. A second pilot study was conducted in a small group of participants (n=21) with SCD who did not participate in the first pilot study and were asked to use a smartphone to answer questions on the e-Diary about the pain and symptoms they experienced over the previous 12 hours. The Institutional Review Board of the University of California Los Angeles (UCLA) approved the study procedures.
Sample & Setting
Participants were children and adolescents with SCD who were recruited from the Sickle Cell Disease Foundation of California (SCDFC). The SCDFC is a nonprofit community based organization involved in programs such as 1) LifeSteps/Youth Program, which targets adolescents and young adults with SCD, ages 13–25 years and provides educational workshops and social service activities; 2) Together We Can! Learning Center which offers one –on- one academic support services on Saturdays to school aged children; and 3) Camp Crescent Moon, a medical camp for children with sickle cell disease ages 8–14 years. SCDFC’s serves the sickle cell community residing in the greater Los Angeles area. In 2006 the SCDFC expanded and opened a satellite office in the Inland Empire (San Bernardino and Riverside Counties) approximately 75 miles from Los Angeles to address the lack of services available for individuals with SCD. The SCDFC offers services to an estimated 5,000 individuals throughout the state of California. The ethnic mix of the population served is approximately ninety percent (90%) African-American. The others (10%) are Hispanics and other origins.
Eligibility
Children and adolescents were eligible to participate if: 1) they were 10 to 17 years of age; 2) diagnosed with SCD; and 3) able to speak, read, write, and understand English. They were excluded if they had major cognitive or neurological impairments (as reported by parents) that may have impacted their ability to understand and use the web-based e-Diary that is accessed by a smartphone. Eligible participants received flyers and were invited by the special projects coordinator from the SCDFC to participate in the study.
Development and Testing of the Web-based e-Diary
One of the co-authors (AG) from the computer science department of the University of California Los Angeles developed a customized web-based e-Diary that included items from well-validated paper-based measures (25, 27–30) of pain and symptoms that were previously used by children and adolescents with SCD (20–22). The web-based e-Diary was designed so that it could be accessed by using the smartphone.
Different from electronic pain diaries that were previously reported in the literature (6–9), the e-Diary is a web-based application, and not a local application that resides on the smartphone. Even though a local application is faster than a web-based application, it has the disadvantage of being tied to a single wireless handheld device (e.g. smartphone), that is, it can work only on the device for which it was developed. We anticipated that technological advances will inevitably occur with wireless handheld devices, and we may want to have the flexibility to use different or more advanced wireless handheld devices. In addition, a web-based application may be accessed, not only by the handheld wireless devices, but also by desktop computers, laptops, netbooks, and other portable electronic devices with wireless connectivity. The speed of wireless connection in the web-based application, when compared to the local application residing on the device was not a problem during the testing. There was greater advantage to having more control and flexibility with the web-based application that would allow changes to occur more readily as technology progresses, thereby increasing the lifetime of the application.
The e-Diary was designed so that when data were submitted, they were immediately and directly transmitted wirelessly by a service provider to a secured server purchased specifically for the study where it was safely stored in a database. Data stored on the server were immediately downloaded to Excel spreadsheets so that they could be imported into a statistical software program for analyses. This aspect of the web-based e-Diary minimized time delay and errors typically encountered during entries of paper diaries. Since data were not stored locally in the device, privacy was maintained, and the risk for loss of confidentiality was minimized in the event that the smartphone was lost or stolen. Furthermore, the smartphone could be deactivated remotely if lost or stolen.
Measures on the e-Diary
The items on the e-Diary were taken from measures with well-established reliability and validity and were previously used by children and adolescents with sickle cell disease in our previous studies (20–24). These measures are described below.
Symptoms
A list of 27 symptoms that were derived from our previous study (21) was presented with three to five symptoms per screen at a time. Each screen had the following at the top “Check if you had any of the signs and/or symptoms over the last 12 hours.” The symptoms included: 1) general symptoms (fever, headache, tiredness/fatigue, dizziness, general weakness); 2) neurological symptoms (one side weaker than the other side, change in level of alertness, change in behavior, more irritable); 3) changes in color (yellowing of the eyes, being pale, changes in skin color); 4) respiratory symptoms (sniffling, coughing, changes in breathing); 5) gastrointestinal symptoms (nausea, vomiting, change in appetite, diarrhea, constipation); 6) genito-urinary symptoms (difficulty with passing urine, pain with passing urine, painful erection); 7) musculoskeletal symptoms (swelling of hands/feet, tenderness, stiffness in joints); and 8) pain (21).
Pain
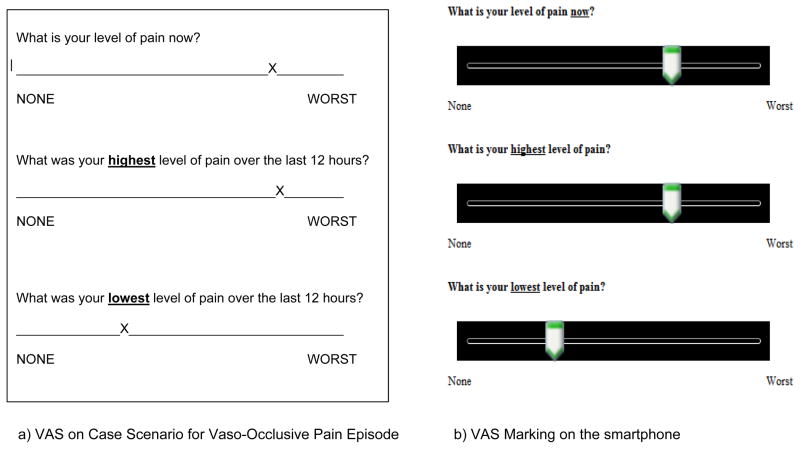
If pain was checked, the screens that followed assessed the “current,” “worst,” and “least” pain, using Visual Analog Scales (VAS). The VAS used in this study was a horizontal line anchored by the words “none” on the left side and “worst pain” on the right side (25). Unlike the traditional paper version, where the VAS is 10 cm (100 mm) in length, the VAS in the e-Diary was not measured with a ruler, but electronically quantified on a 10-unit metric. The 10-unit metric was selected because the 0 to 10 metric was commonly used by clinicians when assessing pain. Von Baeyer and colleagues (26) recommended that all pediatric pain scales have a 10-unit metric so as to decrease confusion from the many differing numerical values. The VAS lines were preceded with the following questions: 1) “What is your level of pain now?”; 2) “What was your highest level of pain in the past 12 hours?”; and 3) “What was your lowest level of pain in the past 12 hours?”
A screen with a body outline diagram modified with permission from the authors of the Adolescent Pediatric Pain Tool (APPT) was included in the subsequent screens to allow marking of areas on the body where pain was felt (25, 27–30). The body outline diagram for marking the location of pain was modified in the electronic version to include additional areas not previously demarcated in the original instrument. The list of pain quality descriptors was included in several screens where the participant was asked to “Select all words that describe your pain” (25, 27–30). The list had 67 words that were previously tested in children to describe pain quality and included sensory, affective, evaluative, and temporal words from the APPT. The words were presented in similar groupings to that in the original APPT (30), and were presented three to five words per screen.
Medications
Several screens were included to assess utilization of medications. One question was “What medications did you take for pain in the last 12 hours?” This question was linked to the medications list typically prescribed for painful episodes in patients with SCD (20, 31). For each medication selected, the following questions were asked: 1) “How many times was this medication taken in the last 12 hours?”; 2) “What time was this medication last taken?”; and 3) “How much did this medication help?”. Responses to the third question included: “don’t know”; (0) “did not help at all”; (1) “helped a little”; (2) “helped some”; (3) “helped quite a bit”; (4) “helped a lot” (23). The final question asked was: “What other medications did you take in the last 12 hours?” A corresponding text box was provided after the question.
Other Strategies for Pain
A list of non-pharmacological strategies used to relieve pain (32, 33) was included to allow participants to “Check if you did any of the following to help with your pain”: The strategies were: 1) Deep breathing; 2) Relaxation exercises; 3) Heat packs; 4) Hot bath/shower; 5) Massage; 6) Imagery; 7) Distraction [TV, Nintendo]; 8) Talking with friends/parents; or 9) None of the above. For each strategy selected, the following questions were asked: 1) “How many times was this activity done in the last 12 hours?”; 2) “What time was this activity last done?”; and 3) “How much did this activity help?”. Responses to the third question were: “don’t know”; (0) “did not help at all”; (1) “helped a little”; (2) “helped some”; (3) “helped quite a bit”; (4) “helped a lot” (Jacob, et al, 2003b). The final question was: “What other activities did you do in the last 12 hours to help with pain?” A corresponding text box was provided after the question.
Sleep
Sleep was assessed with the question, “How much sleep did you have during the night?” (Jacob, et al, 2006). This question was followed by these responses: (0) “did not sleep at all (0 hours)”; (1) “slept a little (<3 hours)”; (2) “slept some (3–5 hours)”; (3) “slept quite a bit (5–8 hours)”; and (4) “slept a lot (>8 hours)”.
Thoughts/Feelings
A checklist that included words to describe thoughts and feelings (Watson, et al, 1988) was presented in two screens. The top of the screens had the following: “Select from the following list to describe your thoughts/feelings over the past 12 hours.” The thoughts and feelings were: 1) Happy/Delighted/Joyful; 2) Content/Calm; 3) Anxious; 4) Depressed; 5) Angry/Mad/Upset; 6) Fearful/Scared/Frightened; 7) Sad/Lonely/Gloomy; 8) Miserable; 9) Worried/Bothered; 10) Nervous/Jittery; 11) Ashamed; Guilty; 12) Tired
Fluids
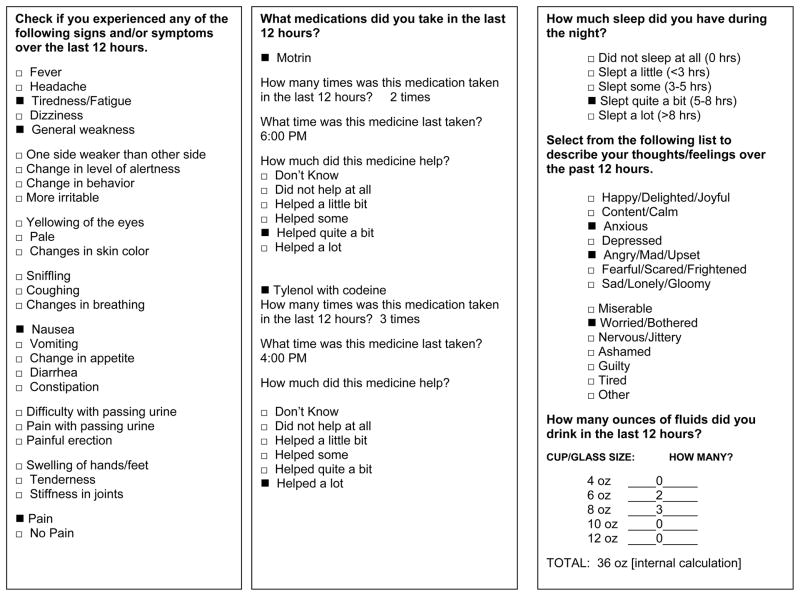
The amount of fluids taken was assessed with the question, “How much did you drink in the last 12 hours?” The responses included the size of cup or glass (4 to 12 ounce size), and how many servings of that cup or glass were taken. An internal calculator showed the total amount taken in ounces (Figure 1c).
Figure 1.
Examples of entries in pre-completed paper based measures for a) symptoms, b) medications, and c) sleep, feelings, fluids
Health Care Use
Two items were included to assess utilization of health care services. The first question was, “In the last 12 hours, did you go to: 1) Sickle Cell Clinic; 2) Medical Office; 3) Urgent Care Clinic; 4) Other Clinic; 5) ED; 6) Day Hospital; or 7) None of the above?” The second question was “In the last 12 hours, have you been: 1) hospitalized, 2) discharged from the hospital, or 3) none of the above?”.
Additional Questions
Two additional screens had questions that asked participants, 1) “Do you have questions?” and 2) “Do you have other comments?” A corresponding text box was provided after each question.
Pre-Testing Procedures
An initial pre-test occurred in a private and quiet conference room at the UCLA School of Nursing. The purpose of the initial testing was to test the technical features of the smartphone. A 16 year old female with SCD used the smartphone to enter items from a completed paper version of the study measures to the e-Diary on the smartphone. Technical changes were made to the e-Diary based upon the initial testing to improve appearance of the images on each screen, navigation (layout and tabs for ease of finding information), function (clear/submit, back, cursor movement), clarification of the wording of questions, as well as deletion/addition of some items.
A second pre-test took place in a private and quiet conference room arranged by the Sickle Cell Disease Foundation. The purpose of the second pre-test was to refine and further improve the smartphone screens in terms of touch, navigation, user interaction, readability, functionalities, visual appearance, and overall layout. In addition, the data transfer function was also tested to check that data were correctly downloaded to Excel spreadsheets so that they could be imported into a statistical software program for analyses. To accomplish this, three participants (10 to 13 years) with SCD were asked to enter answers from the pre-completed paper-based measures to the e-Diary. These measures included a predetermined selection of pain and symptoms typically experienced by individuals with SCD if they had 1) upper respiratory infection, 2) acute chest pain, and 3) vaso-occlusive pain episode. Changes were made to refine the e-Diary and simplify the procedure for downloading data to Excel spreadsheets. Table 1 provides examples of the changes that were made during the pretesting procedures.
Table 1.
Examples of Technical Changes Made During the Testing Procedures
Screen Layout
|
Fonts and Punctuations
|
Appearance
|
Function
|
Pilot Study 1
Participants (n=10) were recruited by the special projects coordinator of the SCDFC and were scheduled during a one-hour session that occurred on a Saturday morning. The ten participants were given three pre-completed paper-based measures representing symptoms and pain typically experienced by individuals with SCD. The purpose of the first pilot study was to validate that: 1) all the technical features of the smartphone were functioning properly, 2) participants were able to accurately use the e-Diary in the same manner that the paper-based measures are used, and 3) all data were correctly downloaded to Excel spreadsheets and imported into a statistical software program for analyses. Participants were given three sets of the paper-based measures that had already been filled out with typical pain and symptom information usually experienced by children with SCD with: 1) upper respiratory infection, 2) acute chest pain, and 3) vaso-occlusive pain episode. These three sets of pre-completed paper-based measures were the same ones used during the pretesting procedures described above. The ten participants provided a total of 30 responses. We hypothesized that responses to the questions would be 100% accurate when participants entered the items from the pre-completed paper-based measures to the e-Diary.
Pilot Study 2
Participants (n=21) in Study 2 were recruited by the special projects coordinator of the SCDFC and were scheduled during a one-hour session that occurred on a Saturday morning. The purpose of the second pilot study was to test that participants were able to use the e-Diary to describe their own pain and symptoms over the previous 12 hours.
Data Analysis
Data were downloaded to Excel spreadsheets and were retrieved using a statistical software program (SPSS, version 18.0, Chicago, IL). Descriptive statistics (frequency, percentages, means, standard deviations) were used to describe the pain and symptoms of participants over the previous 12 hours.
RESULTS
Pilot Study 1
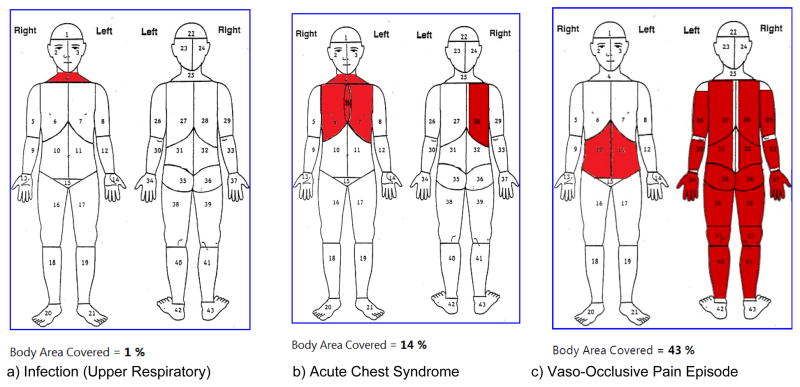
Participants (n=10) in the first pilot study were children and adolescents with sickle cell disease recruited from the programs at the Sickle Cell Disease Foundation of California who had a mean age of 13.1 ± 2.4 years (range 10 to 17 years; 5 males and 5 females). The median school grade was 7th grade (range 4 to 11th grade). They completed the e-Diary with at least 80% accuracy (Table 2) when compared with the pre-completed paper-based measures. Figure 2 presents an example of e-Diary markings on the body outline diagram for location of pain for upper respiratory infection (2a), acute chest pain (2b), and vaso-occlusive pain episode (2c). Figure 3 presents an example of the VAS pain markings for current, highest, and lowest intensity ratings for the vaso-occlusive pain episode which participants entered from the paper-based measures (Figure 3a) to the e-Diary (Figure 3b).
Table 2.
Symptoms experienced the previous 12 hours
| n (%) | |
|---|---|
| General Symptoms | |
| Headache | 6 (28.6%) |
| Tiredness/Fatigue | 4 (19.0%) |
| Dizziness | 1 (4.8%) |
| Neurological Symptoms | |
| General Weakness | 3 (14.3%) |
| Irritability | 3 (14.3%) |
| Color Changes | |
| Yellowing of the eyes | 4 (19.0%) |
| Pale | 4 (19.0%) |
| Respiratory Symptoms | |
| Sniffling | 2 (9.6%) |
| Coughing | 5(23.8%) |
| GastrointestinaI | |
| Constipation | 1 (4.8%) |
| Pain | |
| Painful erection | 1 (4.8%) |
| Stiffness in joints | 4 (19.0%) |
| Pain | 3 (14.3%) |
Figure 2.
Body Outline Diagram for Case Scenarios a) Infection, b) Acute Chest Syndrome, c) Vaso-Occlusive Episode
Figure 3.
Example of markings on Visual Analog Scales on paper-based measures (a) and on e-Diary using the smartphone (b).
Pilot Study 2
Participants (n=21) in the second pilot study were children and adolescents with sickle cell disease recruited from the programs at the Sickle Cell Disease Foundation of California who had a mean age of 13.4 ± 2.4 years (range 10 to 17 years; 10 males and 11 females). The median school grade was 7th grade (range 5 to 11th grade). The majority of the participants had HgbSS (n=10; 47.6%). The remainder had HgbSC (n=6; 28.6%), or other/unknown (n=5; 23.8%). On average some reported having no pain crisis (n=6; 28.6%) during the previous 12 months, and the others have reported at least 1 to 5 crises (7; 33.3%), or more than 5 crises (8; 38.1%) the previous 12 months. They reported having history of acute chest syndrome (n=7; 33.3%), avascular necrosis (n=7; 33.3%), asthma (n=5; 23.8%), and splenectomy (n=4; 19.0%). They were asked to rate their own symptom experiences over the previous 12 hours using the smartphone, to test their ability to use the smartphone, how easy (on a scale of 0 = not useful at all to 5 = very useful) and how much of a bother (on a scale 0=not a bother at all to 5 = a lot of bother) it would be if they were to answer the questions.
Symptoms experienced the previous 12 hours
The most frequently marked symptoms (Table 2) were 1) headache (28.6%); 2) respiratory symptoms such as coughing (23.8%) and sniffling (9.6%); 3) general symptoms such as tiredness/fatigue (19.0%), yellowing of the eyes (19.0%), paleness (19.0%), general weakness (14.3%), and irritability (19.0%). One had indicated dizziness (4.8%) and constipation (4.8%).
Pain experienced the previous 12 hours
Few participants indicated having pain (14.3%) and stiffness in joints (19.0%). One reported having a painful erection (4.8%) the previous 12 hours. The mean pain intensity ratings at the time of completion were (2.0 ± 1.7); range from 1 (mild) to 4 (moderate) on 0 to 10 (electronically quantified metric unit with 10=highest pain score). The medication indicated for pain was ibuprofen (9.5%); no one indicated acetaminophen with codeine or other combination opioids typically prescribed for moderate pain.
Perception of Sleep During the Night
Sleep scores ranged from 0 (did not sleep at all) to 10 (slept a lot; ≥ 8 hours). The mean sleep score was 8.1 ± 1.4 on the 0 to 10 scale; range was 5 to 10.
Thoughts/Feelings reported the previous 12 hours
The majority of the participants indicated feelings of being happy (71.4%) and/or content (42.9%). Some indicated feeling tired (33.3%) and a few had indicated being anxious (9.5%); angry (9.5%), sad (9.5%), nervous (9.5%), miserable (4.8%), and/or other (14.3%)
The participants took an average of 3.9 ± 1.6 minutes to complete the e-Diary questions using the smartphone. When asked how useful the e-Diary would be in helping them talk about pain and symptoms with their doctor or nurse, the mean rating was 4.1 ± 1.5 (on a scale of 0 = not useful at all to 5 = very useful), indicating that it was useful. When asked how much of a bother would it be to answer the questions on the e-Diary using the smartphone, the mean rating 0.6 ± 1.1 (on a scale of 0 = not a bother at all to 5 = a lot of bother), indicating that it would not be much of a bother.
DISCUSSION
We pre-tested the web-based e-Diary for self-monitoring of pain and symptoms and then pilot tested it in two small groups of children and adolescents with sickle cell disease. We found that the participants were able to record pain and symptoms on the e-Diary using the smartphone. The smartphone was easy to use and efficient to complete (<5 minutes). Changes and revisions were made after the pretesting which improved the responsiveness of the screens to touch, navigation, functionalities (submit, clear, cursor movement), visual appearance and layout. Data showed that the participants were able to accurately (80%) enter items from pre-completed paper-based measures to the web-based e-Diary accessed by using the smartphone. In items with less than 100% accuracy, participants may have made their markings based upon their own signs and symptoms in the previous 12 hours, rather than from the paper based measures. For example, on the screen with “Check if you experienced any of the following signs and/or symptoms over the last 12 hours,” they marked negatively based on their own symptoms, rather than the symptom (e.g. fever) that was marked on the paper based measures.
The e-Diary was also used by children and adolescents to describe their own pain and symptoms, sleep, and thoughts and feelings over the previous 12 hours. Data were downloaded immediately to an Excel spreadsheet and imported readily into a statistical software program. The simple download allows immediate analyses of data, which is a major advantage when compared to traditional paper-based measures. Therefore, data may be monitored in real time by clinicians and researchers enhancing the opportunity to detect health problems early. The direct download of data into the analysis program also minimized the errors, personnel cost, and time delay associated with having to enter data from paper based measures.
The overall pain intensity reported by participants was mild (2.0 on 0 to 10 scale), which was lower than previous reports of overall pain intensity in adult patients (4.5 on 0 to 9 scale) who completed daily diaries at home (49) when they participated in the Pain in Sickle Cell Epidemiology (PiSCES). It is interesting to note that one of the most commonly reported symptoms was feeling tired and, yet the mean sleep score was 8.1 (0=did not sleep at all to 10=slept a lot). The feeling of being tired was accompanied by yellowing of the eyes, paleness, general weakness, and irritability. It is therefore possible that some participants may have low steady state hemoglobin that may explain the feeling of being tired. Because the pain intensity ratings were mild and ibuprofen was the pain medication reported, the feeling of being tired was not likely related to narcotic administration. Participants were specifically invited by the Sickle Cell Disease Foundation of California (SCDFC), a community based organization. Therefore, the pain and symptoms experiences may be different from a normative sample, and may not be representative of children and adolescents with SCD who have more or less frequent pain episodes at home and/or who were routinely receiving follow-up services in comprehensive medical centers.
To our knowledge, the web-based e-Diary was currently the only one available for children and adolescents with SCD that includes measures of the multiple dimensions of pain (i.e. intensity, location, quality descriptors for affective, evaluative, sensory, and temporal dimensions). A unique feature of the web-based e-Diary was that it included items specific to sickle cell disease, such as symptoms that may potentially trigger pain episodes (21, 34), and pain medications that were not typically included in previously reported e-Diaries (5, 7). Previous studies of e-Diaries utilized the Children’s Symptom Inventory (CSI), with 35 symptoms. Some of these symptoms were not applicable to SCD, and items that were highly relevant to SCD, such as pain with urination, and pain in the genitals were not included in the CSI (6, 7, 35).
Previous studies in SCD have used paper pain diaries which asked participants to report similar questions to the web-based e-Diary used in our study. For example, McClish and colleagues (49) used a paper pain diary in adults in the Pain in Sickle Cell Epidemiology Study (PiSCES). The PiSCES diary was modeled after the one used in the Multicenter Study of Hydroxyurea (50). The paper pain diary (49, 50) asked patients to record daily 1) worst sickle cell pain intensity, on a scale from 0 (none) to 9 (unbearable), 2) whether or not they were in a sickle cell crisis, and 3) whether they had gone for an unscheduled physician visit, emergency department (ED) visit or were hospitalized due to sickle cell pain. Our e-Diary was different in that we used a 10-unit metric as a measure of pain intensity. The 0 to 10 metric was commonly used by clinicians when assessing pain. Von Baeyer and colleagues (26) recommended that all pediatric pain scales have a 10-unit metric so as to decrease confusion from the many differing numerical values. Our e-Diary also included other dimensions of pain (location, evaluative, affective, sensory, temporal quality), as well as other factors that could affect the overall pain ratings such as fluids, sleep, thoughts/feelings, symptoms, medications and other strategies used for pain.
The diary used in the PiSCES study (49) also asked adult participants to mark a body chart, indicating where they hurt. However, the analyses were limited to the number of participants marking a specific body area and the number of body areas marked as done in other pain studies (7, 27, 28), and did not account for change in body surface area. In our web-based e-Diary, not only the number of body location but also the surface area could be marked and was also quantifiable by percentage of body surface area (Figure 2). We believed this was important, since our previous data (20) in children and adolescents with SCD showed that although the intensity may not change significantly, the spatial distribution of the pain changed daily (up to 50% to 60%). Participants were able to highlight portions of a body area that were painful; but the segments were preserved from the original paper-based measure. Therefore the markings may not truly represent the depth and specific markings within a segmented area. However, the electronic percentage calculation of the segmented area represented a proportion from the total body outline and could be reflective of the pain areas in the body. Although pain quality descriptors were selected on separate screens of the e-Diary to describe quality of pain, participants were not be able to indicate descriptive pain terms to the different sites on the body outline.
In contrast to previous studies that utilized a 7-point cartoon type faces pain scale (7), our study used a VAS that was anchored by “none” and “worst”, with the scale starting at “none”. The VAS in the e-Diary was based on word anchors of “none” to “worst pain” which does not display numbers. The VAS allows participants a wider range of responses to rate their current, worst, and least pain, without the use of numbers that limits responses to the number 10. A disadvantage is that cartoon type faces scale can be used by younger children while the VAS would have to be used by schoolage children that were tested. However, it is very unlikely that younger children than those who participated in the study will be expected to self-monitor pain and symptoms using the smartphone. Similar to our study, McClellan and colleagues (5) used a smartphone to ask participants with SCD to record daily pain using an e-diary. However, it only had a few items to assess the morning and evening pain, followed by questions about sleep quality, functional limitations, and use of the coping skills program. The pain diary was limited to pain intensity, without assessing other dimensions (location on a body outline diagram, quality), other symptoms, or medications and other strategies.
Our study utilized a smartphone device that would allow similar features as personal digital assistants (PDAs) that were previously reported in the literature (7–9). Additional features of the smartphones include the ability to make direct phone calls, access resources on the internet, and also send/receive text messages to clinicians as well as peers. The smartphone may potentially facilitate communication in a more timely manner than the typical paper-based measures and allow immediate contact with care providers in ways that were not previously possible. Furthermore, smartphone devices are more commonly used than PDAs. The smartphone allows for the development of web-based applications that overcome the issue of developing phone specific programs. Because smartphones use finger touch, they do not require a stylus or pen, which may potentially break or be lost.
We did not encounter technical problems, such as not being able to connect to internet, loss of data, or data not transmitting to the server during either of the two pilot studies. However, it is possible that these technical problems may be encountered once the smartphones are used in naturalistic settings (home, school, hospital). The smartphone device may be lost, and power failures and damage to the unit may occur (6–9). To minimize loss of data, the e-Diary was designed so that data were immediately transmitted using wireless connection. Therefore, we anticipate that data would not be lost even if the device was lost, or broken afterwards.
We were using the smartphone as part of a wireless pain intervention program in a larger study of children and adolescents with SCD. The program allowed them to self-monitor their symptoms. The program also allowed a health care provider to track symptoms remotely over time. An algorithm was pre-programmed in such a way that an alert message was sent to the health care provider: 1) immediately for e-diary entries of symptoms requiring immediate attention such as fever, changes in breathing, painful erection, severe pain (rating of 8 or more on the on the 0 to 10 VAS); 2) if symptoms were checked in three consecutive entries (an equivalent of having unrelieved symptoms for 24 hours), for symptoms not requiring immediate attention, but which may be potentially serious; 3) if symptoms were checked in five consecutive entries (an equivalent of having the symptoms checked for 48 hours), for symptoms not requiring immediate attention, but which may potentially lead to sickle cell related complications. The healthcare provider was responding and making appropriate referrals to an emergency department or hematology clinic when needed. Furthermore, the healthcare provider offered clinical guidance (individually tailored treatments based on standardized algorithms), as well as promoted patient-care provider communication and patient self-care management in a timely manner (36–38).
The American Society of Pediatric Hematology/Oncology Sickle Cell Summit recommended optimizing access to care and pain treatments from knowledgeable health care providers, such as hematologists and pain specialists (39). The web-based e-Diary may improve pain management for children and adolescents with SCD who are at increased risk for under-treatment and disruption in activities of daily living due to pain (40–41). The handheld wireless technology, such as the smartphone, may potentially minimize barriers (e.g. lack of transportation to comprehensive centers and access to knowledgeable providers) that not only limit access to hospital and clinic based interventions, but also lead to treatment delays (3–5).
In conclusion, this study provides support for the usability of smartphones to access a web-based e-Diary for self-monitoring of pain and symptoms in children and adolescents with SCD. Usability testing is a procedure used for evaluating technology using a small sample of users (2 to 3 initially, 4 to 8 to drive a useful iterative cycle) to identify technical performance problems, determine time to complete, ease of use, and incorporate user feedback and comments during development (45).
This study also provided support for the feasibility of using the smartphones in children and adolescents with sickle cell disease, who pilot tested the e-Diary using the smartphone by describing the pain and symptoms experienced the previous 12 hours. Smartphones may be critical to improve communications between individuals with SCD and health care providers, to increase accessibility and acceptability of distance treatment programs (5, 42–44), and teach children and adolescents about self-monitoring of pain and symptoms, so that they may be addressed in a timely manner to minimize delay in seeking treatment. The majority of children and adolescents were able to access online information, making smartphones an excellent medium to deliver health promotion and preventive treatment strategies (42–44).
Electronic diaries were used in children as young as six years for measurement of symptoms and behaviors in children with recurrent and chronic pain (7–9, 46). We currently use the smartphones to access the web-based e-Diary in the Wireless Pain Intervention Program for at Risk Youths with Sickle Cell Disease. The smartphone may be particularly useful for those children and adolescents with sickle cell disease who are more severely affected by the disease and frequently use the health care system. Wireless technology may potentially be a cost-effective method for measuring health outcomes, engaging participants in self-monitoring and self-management behavioral interventions, and timely communication between patient and healthcare providers. A major benefit of wireless technology is the automatic data transfer which may potentially improve frequency and contact between the patient and health care providers, and consequently minimize delay in treatments and health care costs associated with ED visits and hospitalizations (47–48).
Acknowledgments
Funding was received from the National Institute of Health, National Heart Blood & Lung Institute American Recovery & Reinvestment Act Grant #1RC1 HL100301-01
The project described was supported by Award Number RC1HL100301 from the National Heart, Lung, And Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, And Blood Institute or the National Institutes of Health. We thank all the children and adolescents from the Sickle Cell Disease Foundation of California (SCDFC) who participated in the study. We are grateful to the SCDFC and their staff, particularly Tara Ragin, who facilitated accessing participants, distribution of recruitment flyers, scheduling of participants, and making private room arrangements during the usability testing cycles. The authors wish also to acknowledge the assistance provided by Meredith Pelty, PsyD, and the University of California Los Angeles, nursing students, Miya Villanueva, Ashley Ponce, Cecilia Dong, and Anjana Gokhale for assisting with different aspects of the research. Finally, we appreciate the expertise provided by Judith E. Beyer, PhD, RN, who provided editorial comments and thoughts related to pain measurement challenges in sickle cell disease.
References
- 1.Harper AC. Telehealth. In: Roberts MC, editor. Handbook of pediatric psychology. 3. New York: Guilford Press; 2003. pp. 735–746. [Google Scholar]
- 2.Spittaels H, De Bourdeaudhuij I, Vandelanotte C. Evaluation of a website-delivered computertailored intervention for increasing physical activity in the general population. Preventive Medicine. 2007;44(3):209–217. doi: 10.1016/j.ypmed.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Devineni T, Blanchard EB. A randomized controlled trial of an Internet-based treatment for chronic headache. Behavior Research and Therapy. 2005;43(3):277–292. doi: 10.1016/j.brat.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Taylor W, Jerome M, Fitzpatrick K. Laptop computers in health care. Physician Assist. 1993;17(3):105–8. [PubMed] [Google Scholar]
- 5.McClellan CB, Schatz JC, Puffer E, et al. Use of Handheld Wireless Technology for a Home-based Sickle Cell Pain Management Protocol. J Pediatr Psychol. 2009;34(5):564–573. doi: 10.1093/jpepsy/jsn121. [DOI] [PubMed] [Google Scholar]
- 6.Walker LS, Sorrells SC. Brief report: Assessment of children’s gastrointestinal symptoms for clinical trials. J Pediatr Psychol. 2002;27(3):303–7. doi: 10.1093/jpepsy/27.3.303. [DOI] [PubMed] [Google Scholar]
- 7.Palermo TM, Valenzuela D, Stork PP. A randomized trial of electronic versus paper pain diaries in children: impact on compliance, accuracy, and acceptability. Pain. 2004;107(3):213–219. doi: 10.1016/j.pain.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Stinson J, Petroz G, Tait G, et al. E-Ouch: Usability testing of an electronic chronic pain diary for adolescents with Arthritis. Clinical Journal of Pain. 2006;22(3):295–305. doi: 10.1097/01.ajp.0000173371.54579.31. [DOI] [PubMed] [Google Scholar]
- 9.Stinson J, Stevens BJ, Feldman B, et al. Construct validity of a multidimensional electronic pain diary for adolescents with arthritis. Pain. 136:281–292. doi: 10.1016/j.pain.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 10.Morren M, van Dulmen S, Ouwerkerk J, et al. Compliance with momentary pain measurement using electronic diaries: a systematic review. Eur J Pain. 2009;13(4):354–65. doi: 10.1016/j.ejpain.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Hufford MR, Shields AL, Shiffman S, et al. Reactivity to ecological momentary assessment: an example using undergraduate problem drinkers. Psychol Addict Behav. 2002;16(3):205–11. [PubMed] [Google Scholar]
- 12.Stone AA, Broderick JE, Schwartz JE, et al. Intensive momentary reporting of pain with an electronic diary: reactivity, compliance, and patient satisfaction. Pain. 2003;104(1–2):343–51. doi: 10.1016/s0304-3959(03)00040-x. [DOI] [PubMed] [Google Scholar]
- 13.Stone AA, Shiffman S, Schwartz JE, et al. Patient compliance with paper and electronic diaries. Control Clin Trials. 2003 Apr;24(2):182–99. doi: 10.1016/s0197-2456(02)00320-3. [DOI] [PubMed] [Google Scholar]
- 14.Salaffi F, Stancati A, Procaccini R, et al. Assessment of circadian rhythm in pain and stiffness in rheumatic diseases according the EMA (Ecologic Momentary Assessment) method: patient compliance with an electronic diary. Reumatismo. 2005;57(4):238–49. doi: 10.4081/reumatismo.2005.238. [DOI] [PubMed] [Google Scholar]
- 15.Christensen CP, Althausen PL, Mittleman MA, et al. The nonarthritic hip score: reliable and validated. Clin Orthop Relat Res. 2003;(406):75–83. doi: 10.1097/01.blo.0000043047.84315.4b. [DOI] [PubMed] [Google Scholar]
- 16.Aaron LA, Mancl L, Turner JA, et al. Reasons for missing interviews in the daily electronic assessment of pain, mood, and stress. Pain. 2004;109(3):389–98. doi: 10.1016/j.pain.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 17.Gaertner J, Elsner F, Pollmann-Dahmen K, et al. Electronic pain diary: a randomized crossover study. J Pain Symptom Manage. 2004;28(3):259–67. doi: 10.1016/j.jpainsymman.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 18.Jamison RN, Raymond SA, Slawsby EA, et al. Pain assessment In patients with low back pain: comparison of weekly recall and momentary electronic data. J Pain. 2006;7(3):192–9. doi: 10.1016/j.jpain.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 19.Sorbi MJ, Peters ML, Kruise DA, et al. Electronic momentary assessment in chronic pain I: psychological pain responses as predictors of pain intensity. Clin J Pain. 2006;22(1):55–66. doi: 10.1097/01.ajp.0000148624.46756.fa. [DOI] [PubMed] [Google Scholar]
- 20.Jacob E, Miaskowski C, Savedra M, et al. Changes in intensity, location, and quality of vaso-occlusive pain in children with sickle cell disease. Pain. 102(1–2):187–193. doi: 10.1016/s0304-3959(02)00374-3. 003a. [DOI] [PubMed] [Google Scholar]
- 21.Jacob E, Beyer JE, Miaskowski C, et al. Are there Phases to the Acute Painful Episode in Children with Sickle Cell Disease. Journal of Pain & Symptom Management. 2005;29(4):392–400. doi: 10.1016/j.jpainsymman.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 22.Jacob E, Miaskowski C, Savedra M, et al. Management of pain in children with sickle cell disease. Journal of Pediatric Hematology & Oncology. 2003b;25(4):307–311. doi: 10.1097/00043426-200304000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Jacob E, Mueller BU. Pain Experience of Children with Sickle Cell Disease Who Had Prolonged Hospitalizations for Acute Painful Episodes. Pain Medicine. 2008a;9(1):13–21. doi: 10.1111/j.1526-4637.2006.00252.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacob E, Hockenberry M, Mueller BM, et al. Analgesic Response to Morphine in Children with Sickle Cell Disease. Journal of Pain Management. 2008;2(1):179–190. [PMC free article] [PubMed] [Google Scholar]
- 25.Tesler MD, Savedra MC, Holzemer WL, et al. The word-graphic rating scale as a measure of children’s and adolescents’ pain intensity. Research in Nursing & Health. 1991;14(5):361–371. doi: 10.1002/nur.4770140507. [DOI] [PubMed] [Google Scholar]
- 26.Von Baeyer CL, Hicks CL. Support for a common metric for pediatric pain intensity scales. Pain Research and Management. 2000;5(2):157–60. [Google Scholar]
- 27.Savedra MC, Holzemer WL, Tesler MD, et al. Assessment of postoperation pain in children and adolescents using the Adolescent Pediatric Pain Tool. Nursing research. 1993;42(1):5–9. [PubMed] [Google Scholar]
- 28.Savedra MC, Tesler MD, Holzemer WL, et al. Pain location: validity and reliability of body outline markings by hospitalized children and adolescents. Research in Nursing & Health. 1989;12(5):307–314. doi: 10.1002/nur.4770120506. [DOI] [PubMed] [Google Scholar]
- 29.Van Cleve LJ, Savedra MC. Pain location: Validity and reliability of body outline markings by 4 to 7-year-old children who are hospitalized. Pediatr Nurs. 1993;19(3):217–20. [PubMed] [Google Scholar]
- 30.Wilkie DJ, Holzemer WL, Tesler MD, et al. Measuring pain quality: validity and reliability of children’s and adolescents’ pain language. Pain. 1990;41(2):151–159. doi: 10.1016/0304-3959(90)90019-A. [DOI] [PubMed] [Google Scholar]
- 31.Jacob E, Miaskowski C, Savedra M, et al. Quantification of Analgesic Use in Children With Sickle Cell Disease. Clinical Journal of Pain. 2007;23(1):8–14. doi: 10.1097/01.ajp.0000210938.58439.dd. [DOI] [PubMed] [Google Scholar]
- 32.Ballas SK. Pain Management of Sickle Cell Disease. Hematology/Oncology Clinics of North America. 2005;19(5):785–802. doi: 10.1016/j.hoc.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Beyer JE. Home Treatment of Pain for Children and Adolescents With Sickle Cell Disease. Pain Manag Nurs. 2004;5(3):126–135. doi: 10.1016/j.pmn.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Jacob E, Sockrider MM, Dinu M, et al. Respiratory Symptoms and Acute Painful Episodes in Children and Adolescents with Sickle Cell Disease. Journal of Pediatric Hematology & Oncology Nursing. 2010;27(1):33–39. doi: 10.1177/1043454209344578. [DOI] [PubMed] [Google Scholar]
- 35.Meesters C, Muris P, Ghys A, et al. The Children’s Somatization Inventory: further evidence for its reliability and validity in a pediatric and a community sample of Dutch children and adolescents. J Pediatr Psychol. 2003;28(6):413–22. doi: 10.1093/jpepsy/jsg031. [DOI] [PubMed] [Google Scholar]
- 36.Marceau LD, Link C, Jamison RN, et al. Electronic diaries as a tool to improve pain management: Is there any evidence? Pain Medicine. 2007;8:S101–S109. doi: 10.1111/j.1526-4637.2007.00374.x. [DOI] [PubMed] [Google Scholar]
- 37.Smith MY, DePue JD, Rini C. Computerized decision-support systems for chronic pain management in primary care. Pain Medicine. 2007;8:S155–S166. [Google Scholar]
- 38.Hochlehnert A, Richter A, Blaudau HB, et al. A computerized-based information-tool for chronic pain patients: Computerized information to support the process of shared decision-making. Patient Education and Counselling. 2006;61:92–98. doi: 10.1016/j.pec.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Hassell K, Pace B, Wang W, et al. Sickle cell disease summit: from clinical and research disparity to action. Am J Hematol. 2009;84(1):39–45. doi: 10.1002/ajh.21315. [DOI] [PubMed] [Google Scholar]
- 40.Harper P, Ersser S, Gobbi M. How military nurses rationalize their postoperative pain assessment decisions. J Adv Nurs. 2007;59(6):601–11. doi: 10.1111/j.1365-2648.2007.04369.x. [DOI] [PubMed] [Google Scholar]
- 41.Spittaels H, De Bourdeaudhuij I. Who participates in a computer-tailored physical activity program delivered through the Internet? A comparison of participants’ and non-participants’ characteristics. Int J Behav Nutr Phys Act. 2007;19;4:39. doi: 10.1186/1479-5868-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spittaels H, De Bourdeaudhuij I, Vandelanotte C. Evaluation of a website-delivered computer-tailored intervention for increasing physical activity in the general population. Prev Med. 2007;44(3):209–17. doi: 10.1016/j.ypmed.2006.11.010. Epub 2007 Jan 2. [DOI] [PubMed] [Google Scholar]
- 43.Spittaels H, De Bourdeaudhuij I, Brug J, et al. Effectiveness of an online computer-tailored physical activity intervention in a real-life setting. Health Educ Res. 2007;22(3):385–96. doi: 10.1093/her/cyl096. [DOI] [PubMed] [Google Scholar]
- 44.Spittaels H, De Bourdeaudhuij I. Implementation of an online tailored physical activity intervention for adults in Belgium. Health Promot Int. 2006;21(4):311–9. doi: 10.1093/heapro/dal030. [DOI] [PubMed] [Google Scholar]
- 45.Molich R. A critique of “How to Specify the Participant Group Size for Usability Studies: A Practicioner’s Guide”. Journal of Usability Studies. 2010;5(3):124–128. [Google Scholar]
- 46.Palermo TM, Valenzuela D. Use of pain diaries to assess recurrent and chronic pain in children. Suffer Child. 2003;3:1–19. [Google Scholar]
- 47.Kauf TL, Coates TD, Huazhi L, Mody-Patel N, Hartzema AG. The cost of health care for children and adults with sickle cell disease. Am J Hematol. 2009;84(6):320–2. doi: 10.1002/ajh.21408. [DOI] [PubMed] [Google Scholar]
- 48.Lanzkron S, Haywood C, Jr, Segal JB, Dover GJ. Hospitalization rates and costs of care of patients with sickle-cell anemia in the state of Maryland in the era of hydroxyurea. Am J Hematol. 2006;81(12):927–932. doi: 10.1002/ajh.20703. [DOI] [PubMed] [Google Scholar]
- 49.McClish DK, Smith WR, Dahman BA, Levenson JL, Roberts JD, Penberthy LT, Aisiku IP, Roseff SD, Bovbjerg VE. Pain Site Frequency and Location in Sickle Cell Disease: the PiSCES Project. Pain. 2009 Sep;145(1–2):246–251. doi: 10.1016/j.pain.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Charache S, Terrin ML, Moore RD, Dower GJ, McMahon RP, Barton FB, Waclawiw M, Eckert SV. Design of the multicenter study of hydroxyurea in sickle cell anemia. Investigators of the Multicenter Study of Hydroxyurea in Sickle Cell Anemia. Control Clin Trials. 1995;16:432–446. doi: 10.1016/s0197-2456(95)00098-4. [DOI] [PubMed] [Google Scholar]