Abstract
Background
The ventral tegmental area (VTA) is a pivotal relay site within the reinforcement circuit that has been shown to play a role in ethanol-motivated behaviors. The primary dopamine projections within this system originate in the VTA and innervate several areas including the nucleus accumbens (NAc) and prefrontal cortex (PFC), and the PFC has afferent glutamate projections to the VTA and the NAc. The following studies utilized two different operant paradigms, one focusing on reinforcer-seeking and one on reinforcer drinking, (both with an ethanol and a sucrose reinforcer solution) to elucidate regulation of these behaviors by the posterior VTA, and the specific roles of dopamine and glutamate in this region.
Methods
The present experiments assessed the effects of microinjections of the glutamate (AMPA/kainate) antagonist CNQX and the dopamine D1-like antagonist SCH23390 in the posterior VTA, as well as transient chemical inactivation of this region using tetrodotoxin (TTX). In four separate experiments, (two Dopamine, two Glutamate, both with TTX) male Long Evans rats were trained to complete a single response requirement that resulted in access to 10% ethanol or 2% sucrose for a 20-min drinking period.
Results
Prior to microinjections, ethanol-reinforced subjects were consuming ~0.45–0.65 g/kg ethanol and making ~50 responses during intermittent non-reinforced aCSF sessions (Sucrose Groups had similar baseline response levels). Overall, TTX inactivation of the VTA consistently decreased reinforcer-seeking but not intake in all experiments. CNQX also dose-dependently decreased ethanol-seeking, with no significant effect on sucrose-seeking or reinforcer intake. SCH23390 had no significant effects on reinforcer-seeking, and very moderately decreased intake of both ethanol and sucrose.
Conclusions
Inactivation of the posterior VTA implicated this region in reinforcer-seeking as opposed to reinforcer intake. Overall, the present findings provide support for the importance of posterior VTA glutamate activity specifically in ethanol-seeking behavior in animals consuming pharmacologically relevant amounts of ethanol.
Keywords: Ethanol, Sucrose, Appetitive, Consummatory
INTRODUCTION
Dopamine and glutamate within the mesocorticolimbic system play a role in ethanol-reinforced behaviors (for reviews Koob & Nestler, 1997; Tupala & Tiihonen, 2004; VTA specific Morikawa & Morrisett, 2010). The primary dopamine projections originate in the ventral tegmental area (VTA) and innervate areas including the nucleus accumbens (NAc), prefrontal cortex (PFC) and amygdala. GABA projections feed back from the NAc to the VTA, while the PFC has afferent glutamate projections to the VTA and NAc (Berger et al., 1976; Groenewegen et al., 1990; Oades & Halliday, 1987; Sesack et al., 1989). Ethanol increases dopamine activity in the VTA (Brodie et al., 1999) and rats will self-administer ethanol directly into this region (Rodd et al., 2004). With regard to anterior versus posterior differentiation of the VTA, one group has demonstrated that the posterior and not the anterior VTA in selected alcohol-preferring and nonselected rats is involved in ethanol-reinforcement (Rodd et al., 2004; 2005a) and recently cocaine-reinforcement (Rodd et al., 2005b).
The VTA is a pivotal site of feedforward and feedback information within this circuit. It has been proposed that the PFC/NAc glutamate projection underlies “unmanageable motivation to seek drugs” (Kalivas et al., 2005), and glutamate activity in the VTA is necessary for interaction between the PFC and NAc. Specifically, PFC-stimulated increases of extracellular dopamine in the NAc can be blocked with a glutamate antagonist when administered into the VTA but not into NAc (Karreman et al., 1996; You et al., 1998). Similarly, PFC-induced dopamine in the limbic striatum was blocked by either tetrodotoxin (TTX) inactivation or by glutamate antagonist administration in the VTA (Karreman & Moghaddam, 1996; Taber et al., 1995). Moreover, intra-VTA glutamate antagonists blocked cocaine reinstatement (Sun et al., 2005) as well as the development and expression of morphine conditioned place preferences (Harris et al., 2004) and decreasing evoked glutamate release in the VTA also blocked reinstatement of heroin-seeking (Bossert et al., 2004). CNQX is an AMPA/kainate antagonist, and burst firing of dopamine neurons in the VTA evoked by stimulation of glutamatergic inputs is blocked by administration of AMPA receptor antagonists (Blythe et al., 2007; Di Loreto et al., 1992; Georges & Aston-Jones, 2002). While enhancement of glutamatergic release in the VTA increases reinstatement of cocaine-seeking, this effect is blocked by administration of CNQX into the VTA (Schmidt et al., 2009). Within the VTA, dendritically released dopamine activates D1 receptors on the terminals of glutamatergic inputs (Sesack & Pickel, 1992; Smith et al., 1996) which in turn modulate the activity of dopaminergic VTA outputs (Albin et al., 1992; Kalivas, 1993; Overton & Clark, 1992), and dendritically released dopamine in the VTA affects the rewarding properties of cocaine (Ranaldi & Wise, 2001). The present experiments will examine the effects of the AMPA/kainate antagonist, CNQX, and the dopamine D1 antagonist, SCH23390, administered in the posterior VTA on ethanol-reinforced responding.
Common operant models used to assess pharmacological manipulation of reinforced responding use schedules of reinforcement (i.e., fixed/progressive ratios) that require animals to work for each small dose of a given drug of abuse. In the case of ethanol, each “sip” of ethanol must be earned, and therefore these approaches focus on a combined seeking/drinking response and the maintenance of ethanol drinking rather than the initiation of ethanol drinking. The following studies utilized two different operant paradigms, one focusing on reinforcer-seeking and one on reinforcer consumption, (both with an ethanol and a sucrose reinforcer) to attempt to elucidate specific neurochemical regulation of these behaviors. The model dissects these behaviors into their distinct components, and the present studies examined the impact of the posterior VTA and the effects of neurochemical manipulations within this region on ethanol seeking and intake.
MATERIALS and METHODS
Subjects
Subjects were male Long Evans rats (Harlan Sprague-Dawley, Indianapolis, IN). Body weights were 175–210 g at the start of the experiments, and ad libitum access to food and water was maintained except as noted below. The rats were housed individually (lights on 6:00AM to 6:00PM), and animal care procedures were in accordance with NIH’s Guide for the Care and Use of Laboratory Animals and approved by the Institutional Animal Care and Use Committee.
Apparatus
Daily sessions were conducted in modular operant chambers (Med-Associates; East Fairfield, VT) (30cm × 30cm × 24.5 cm) equipped with a houselight, retractable lever, and retractable graduated cylinder tube with rubber stopper and a stainless steel sipper-spout with double ball bearings to prevent leakage. All chambers were housed in sound attenuated enclosures with exhaust fans that masked external noise. Electrical inputs/outputs of each chamber were controlled by an IBM compatible PC (Med-Associates software).
Ethanol solutions were prepared volume/volume (v/v) in water from 95% (v/v) ethanol. For sucrose/ethanol solutions, the sucrose solution was prepared weight/volume and used as a solute. The non-NMDA glutamate (AMPA/kainate) antagonist was CNQX (6-Cyano-7-nitroquinoxaline-2,3-dione) and the dopamine D1-like antagonist was SCH23390 [R(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride)] (Sigma Aldrich, Inc., St. Louis, MO). The drugs were dissolved in artificial cerebrospinal fluid (aCSF; Harvard Apparatus, Holliston, MA) immediately prior to microinjection. Tetrodotoxin (TTX; Fugu sp., EMD Chemicals Inc., San Diego, CA) was prepared in phosphate buffered saline. Microinjections were administered through sterile stainless steel injectors (16mm; 33 gauge) connected to 25.0 μl syringes (Hamilton, Reno, NV) using KD Scientific Infusion Pumps (Model 101).
Surgery and Microinjection Procedures
Surgeries were conducted over a one-week period (see below for exact timing within each experiment) approximately 24 hours after an operant session, and sessions were resumed 24 hours after surgery. Rats were anesthetized with sodium pentobarbital (60 mg/kg i.p.) and placed in the stereotaxic apparatus (myNeurolab, St. Louis, MO, Benchmark Digital Stereotaxic) with the incisor bar set at 3.3 mm below the interaural line. Bilateral stainless steel guide cannulae (15mm; 26 gauge) were implanted that terminated 1 mm dorsal to the VTA (target coordinates with a 10 degree angle: AP −5.3, ML ±2.1, V −7.0; Paxinos & Watson, 1998). Removable wire obturators (33 gauge) were inserted the full length of the guide cannulae to limit obstruction by tissue and to maintain patency..
SCH23390 was tested at doses of 0.5, 1.0 and 2.0 μg/0.5μl [dose range based on See et al. (2001) that showed that 2.0 μg in the BLA decreased cocaine-seeking and on Sharf et al. (2002) that showed that 2–4 μg in the VTA decreased food-reinforced responding]. CNQX was tested at doses of 0.25, 0.5, and 1.0 μg/0.5 μl. [dose range based on Harris et al. (2004), Karreman & Moghaddam (1996) and Taber et al. (1995) that used comparable doses in the VTA to block morphine-induced conditioned place preference and alter DA release]. The sodium channel blocker tetrodotoxin (TTX) was tested at a single dose (3ng/0.5 μl). When microinjected into a variety of locations, the effects of TTX were undetectable in <6 hours (Lorenzini et al., 1995), produced dissociable changes in behavioral function in adjacent brain areas (i.e., BLA versus central nucleus of the amygdala: McLaughlin & See, 2003), and regionally confined, in that 10ng at a volume of 1 μl (more than twice that used in the present experiments) had a radial spread of under 1mm (Zhuravin et al., 1994). Rats received a total of seven microinjections (three aCSF and four drug), consistent with the use of 6–10 microinjections at the same volume in several brain regions (Andrzejewski et al., 2005; Bachtell et al., 2005; Sun & Rebec, 2005) including the posterior VTA (Nowak et al., 2000) that caused no visible signs of tissue damage.
For injections, rats were placed in a small holding tub (27 × 17 × 12 cm) and drug solutions were injected bilaterally (0.5 μl/site) over one-minute using a stainless steel injector that extended 1 mm past the end of the guide cannula. Injectors were left in place for 30 additional seconds to allow for drug diffusion. After injection procedures, rats were moved back to the animal carrier for 10 minutes prior to the start of the operant session.
Experimental Sessions
Training
Four separate experiments were conducted (two Seeking and two Intake Experiments, each with either the dopamine or glutamate antagonist and all four with a single TTX treatment) each with an ethanol- and a sucrose-reinforced group. Daily sessions were conducted 5 days/week, and for all sessions the houselight indicated the start (on with concurrent lever extension) and end (off with concurrent sipper or lever retraction depending on session type; see below). Subjects were initially trained to lever-press on a fixed-ratio (FR) one schedule that resulted in 15 sec of sipper-tube access with 10% sucrose in 30-min sessions. Subjects were water-restricted for the initial 2–5 sessions only, after which food and water were available ad lib in the home cage. Over a three-week period, the training/sucrose-fading (modified from Samson, 1986) procedures involved: increasing the FR from 1–4, decreasing the sucrose concentration to 2% (2S), and in the ethanol groups introducing ethanol and increasing the concentration from 2 to 10% (10E) while fading the sucrose out completely (final solutions: Sucrose Group: 2S; Ethanol Group: 10E). The procedural separation between seeking (lever-pressing) and consumption was then instituted. Following a single response requirement (RR), access to the sipper tube was provided for 20 min. Over 2–4 weeks, the RR was increased from 4–10 (Intake Experiments) or 4–20 (Seeking Experiments), and then 15–19 sessions at the final RR were conducted prior to surgery (i.e., 3–4 weeks depending on day of surgery). Following surgery, two more weeks of reinforced sessions were conducted with no other treatment [RR after surgery was 1, then 4, 10 (stayed at 10 for Intake Exp.) 15 and 20 (for Seeking Exp.)]. This was followed by a week of sham injections (injector the same length as the guide cannulae, injection pump run but no fluid injected) prior to drug testing to acclimate subjects to the brief restraint. Immediately following Friday’s session before the first week of drug testing, a 16mm obturator (same length as the injector) was inserted into the cannulae and then removed in order to control for any disturbance of the tissue prior to the first drug injection.
Drug-Testing Schedule for Seeking Experiments
Monday-Wednesday and Friday sessions were “normal”, reinforced sessions with the RR20 schedule. On Thursdays, drug treatment preceded a non-reinforced extinction session. For these sessions, the lever extended into the chamber for twenty minutes, after which the lever retracted and the sipper-tube was not extended into the chamber. The sipper-tube was still in place (outside the chamber/reach of the rat) with the appropriate solution to control for scent cues that would normally be present on a reinforced session. The series of treatment weeks was nine weeks long, and seven of those weeks were microinjection weeks. The first and final injections were aCSF, and the remaining five were three doses of the specific ligand (SCH23390 or CNQX), one TTX, and one aCSF which were administered in a counterbalanced, randomized order to all subjects. The other two weeks of the nine week series were sessions intended to “uncouple” the injection/extinction association, since microinjections could become a predictor of the impending extinction condition. A sham injection preceded a reinforced session the week after the first drug administration (i.e., week 3 of 9 extinction weeks) and an extinction session with no preceding microinjection was conducted in week seven.
Drug-Testing Schedule for Intake Experiments
Monday-Wednesday and Friday sessions were “normal”, reinforced sessions with the RR10 schedule. On Thursdays, a reinforced session used a RR1, and was preceded by drug treatment. The RR1 schedule was used to control for the timing of drug activity between subjects in the two experimental paradigms (i.e., microinjection, ten-minute wait time, nearly immediate performance of behavior of interest: seeking or drinking), and to help ensure that the subjects would gain access to the reinforcer to be able to assess intake on that day. As in the Seeking Experiments, seven weeks of microinjection treatment were conducted: an initial aCSF, five weeks of drug/TTX/aCSF (counterbalanced, randomized order), and a final aCSF treatment.
Histology
Within 24 hours of the final operant session, rats were deeply anesthetized (120 mg/kg sodium pentobarbital, i.p.) and transcardially perfused with phosphate buffered saline followed by 10% formalin. Brains were removed, stored in 10% formalin for 7–14 days, then cut (30 μm sections) on a cryostat (Ultrapro Lite, Vibratome Company, St. Louis, MO), mounted and stained with cresyl violet to determine injection locations using light microscopy.
Statistical Analyses
Total session intakes were determined from the change in fluid volume in the sipper-tube. Ethanol intakes (g/kg) were calculated from intake volume and daily body weight measures. Latency to lever-press was measured to the nearest tenth of a second (from houselight on to first response). Assessment of ethanol-reinforced behavior (lever-presses and g/kg intake) following the first versus the last aCSF microinjection in all four experiments (i.e., the aCSF treatments that “bookended” all drug manipulations including an additional aCSF microinjection) was conducted using two-way repeated measures analysis of variance (RMANOVA) with Treatment (first vs. last microinjection) and Experiment (DA vs. GLU) as the main variables. This was conducted to establish that behavior did not shift overall from the beginning to the end of the experiments, as well as to determine if baseline behavior levels were comparable across experiments. In addition, ethanol intake on all non-test days (the four days each week with no microinjection) was assessed to confirm the consistency of behavior over the course of the experiments using two-way RMANOVA with Day and Experiment (DA vs. GLU) as the main variables. Day 1 for all four experiments was the Monday of the sham injection week (i.e., days 1–7 preceded any tissue penetration for all experiments) and the final day was the Friday after the last microinjection (i.e., Seeking Experiments had a total of 40 non-test days while Intake Experiments had a total of 32 non-test days). Then for each experiment, seeking data (total lever-presses during an extinction session) were analyzed using one-way RMANOVA with Treatment (aCSF, TTX or one of three drug doses) as the main variable. Intake data [volume (mls) for sucrose groups and dose (g/kg) for ethanol groups] were also analyzed using one-way RMANOVA with Treatment (aCSF or one of three drug doses) as the main variable. For the Intake Experiments, however, the effects of TTX were analyzed separately since some animals in this treatment condition failed to complete the response requirement and did not gain access to the reinforcer solution. Therefore, the aCSF and TTX conditions were compared using paired t-tests, and the data were analyzed both with zero entered if the subject failed to respond, and with subjects excluded from the analyses if they failed to respond. This approach was used to make sure that all subjects were represented, but also to assess the intake response in a way that was not confounded by possible drug effects on the seeking response. Finally, latency to first lever-press in the sucrose- and ethanol-reinforced groups in all four experiments was assessed to address possible motor-impairing effect of TTX. Since there were some subjects that failed to complete the RR following this treatment, the Seeking and Intake Experiments were collapsed (i.e., groups were combined that had the same training and RR) across drug to increase the final group sizes for analyses (Table 2 shows all group sizes by total and number of animals responding). Latencies were then analyzed by paired t-test. Post hoc comparisons were performed following ANOVAs using Student-Newman-Keuls Method when appropriate (p<0.05).
Table 2.
Mean (±SEM) latency (sec) to lever-press in both ethanol- and sucrose-reinforced groups following either aCSF or TTX microinjection.
Data are collapsed over the Seeking and Intake experiments (i.e., experiments with the same training protocol and response requirements) and represent only animals that responded following both microinjections (indicated in parentheses after the group name; number of subjects responding as a function of total number in that group). Open circles denote marginal effect of TTX relative to aCSF, within the ethanol groups only (p<.08).
| Seeking Experiments | Intake Experiments | ||||
|---|---|---|---|---|---|
| aCSF | TTX | aCSF | TTX | ||
|
|
|
||||
| EtOH (9/17) | 122.1 (54.8) | 303.9 (124.2)○ | EtOH (9/14) | 46.0 (15.1) | 166.7 (71.3)○ |
| SUC (15/16) | 51.9 (12.8) | 190.7 (83.2) | SUC (13/17) | 111.6 (63.7) | 101.7 (44.6) |
RESULTS
Only subjects with correct bilateral cannulae placement that completed the entire experiment with no blocked cannulae or failure to respond during baseline/aCSF treatment days were included in the data analyses (see Figures 1 and 2). Final group totals were: DA Seeking: Ethanol = 9 Sucrose = 10; DA Intake: Ethanol = 8; Sucrose = 10; GLU Seeking: Ethanol = 8; Sucrose = 6; GLU Intake: Ethanol = 6; Sucrose = 7. Table 1 shows ethanol-reinforced lever-presses and intake (g/kg) for the first versus last aCSF microinjections in all four experiments. There were no significant differences in lever-pressing between experiments [F(1,15)=0.69, p=.42] or from the first to last microinjection [F(1,15)=0.20, p=.66]. Similarly, there were no significant differences in intake between experiments [F(1,11)=3.61, p=.08] or from the first to last microinjection [F(1,11)=2.35, p=.15]. In the Intake Experiments, ethanol intake (g/kg) on non-test days did not differ significantly by experiment [F(1,358)=4.19, p=.06] (DA = 0.70 ± .04; GLU = 0.56 ± .05), but there was a significant effect of Day [F(31,358)=2.09, p<.01] and Day × Experiment interaction [F(31,358)=2.08, p<.01]. Post hoc testing indicated that the difference in mean intake on Day 5 (0.78 ± .08) vs. Day 23 (0.54 ± .07) approached significance (p=.056), with no other differences between days and no meaningful pattern to the interaction effect. In the Seeking Experiments, ethanol intake (g/kg) on non-test days did not differ significantly by experiment [F(1,540)=4.24, p=.06] (DA = 0.88 ± .06; GLU = 0.71 ± .06), but there was a significant effect of Day [F(39,540)=2.19, p<.01] with no Day × Experiment interaction [F(39,540)=0.98, p=.50]. Post hoc testing revealed the Day effect to be due to the tendency to decrease intake on Fridays following microinjection/extinction relative to the initial days of testing. Specifically, significant differences (p<.05) were found between Day 1 vs. Days 12, 24, 32 and 36 as well as between Days 2 and 3 vs. Days 12 and 36. (Note that Day 16 was a sham injection/no extinction Friday and Day 28 was a no injection/extinction Friday, and neither differed from these initial days.) Finally, mean body weight (±SEM) on Day 1 vs. 32 in the ethanol groups was 430g (±8) vs. 488g (±10), so the absence of an overall decrease in g/kg ethanol intake from the start to end of the experiments suggests an increase in volume (mls) in order to compensate for the increase in body weight. On these non-test days, there were a few failures to complete the response requirement with no discernable pattern over the experiment progression or for day of the week [Seeking Experiments: 43/680 possible at RR 20, slightly more common on Friday after extinction (M=12, T=8, W=5, F=18), 6 in first aCSF week and 3 in final aCSF week; Intake Experiments: 14/448 possible at RR10, distributed over days, 1 in first aCSF week and 1 in final aCSF week]. Overall, then, there is no evidence of a general decrement in behavior over the course of the experiments or the repeated microinjections.
Figure 1.
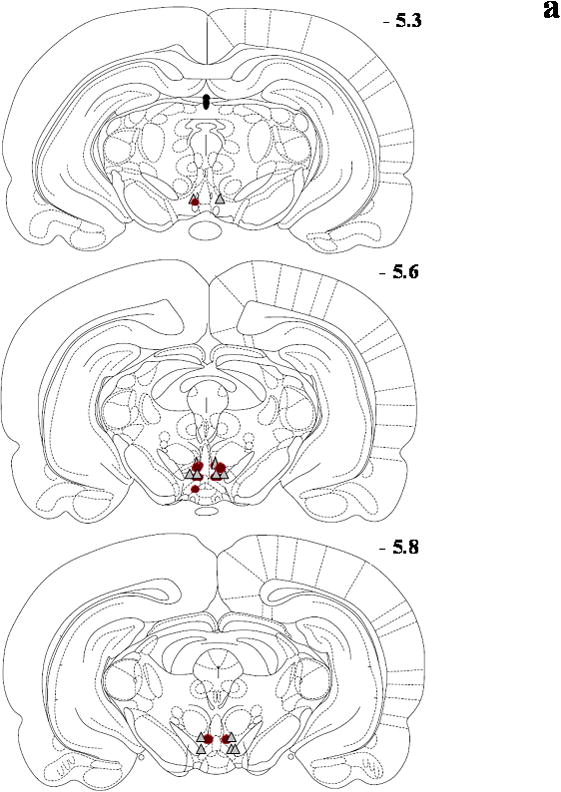
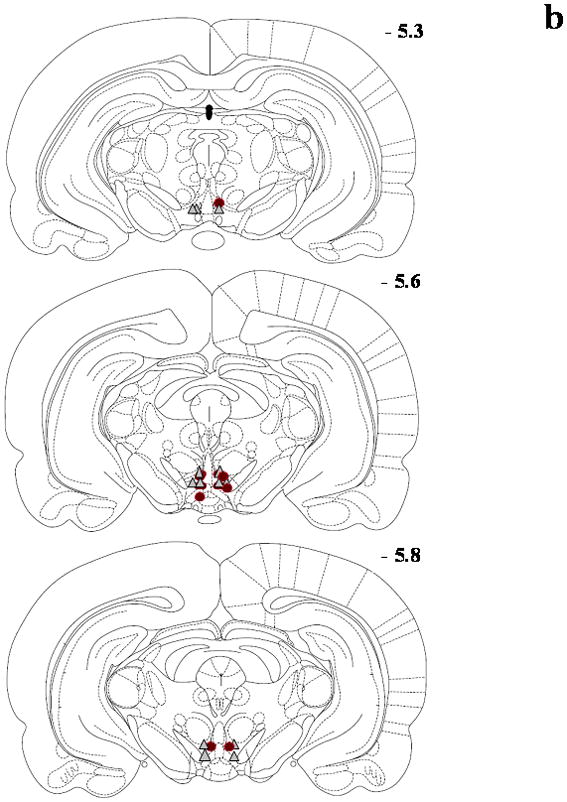
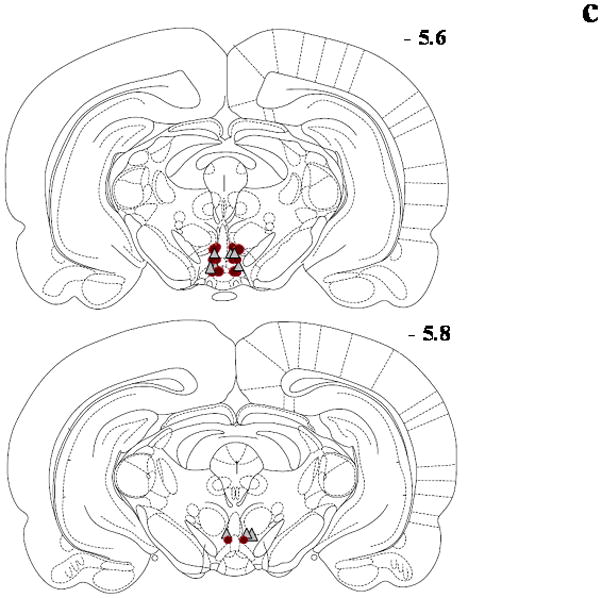
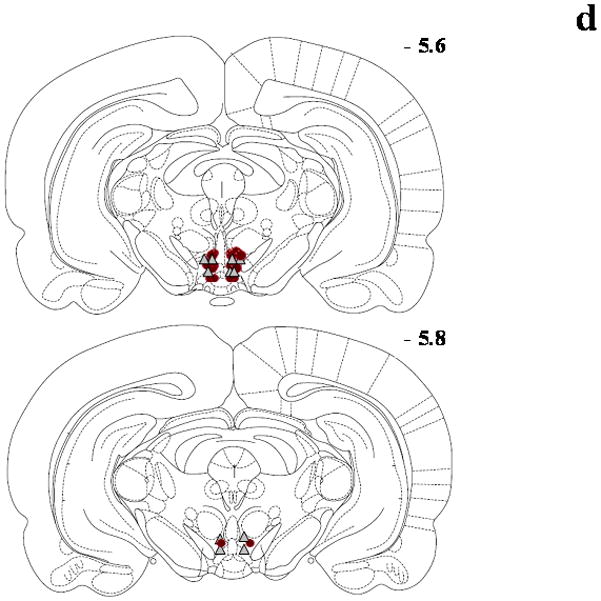
Schematic diagrams adapted from the rat brain atlas (Paxinos and Watson, 1998) representing the bilateral cannula placement (symbol = microinjection site; circle = DA and triangle = GLU) in the posterior VTA for the Seeking Experiments/Ethanol groups (panel a; n=17), Seeking Experiments/Sucrose groups (panel b; n=16), Intake Experiments/Ethanol groups (panel c; n=14) and Intake Experiments//Sucrose groups (panel d; n=17). Each section represents the approximate position in the anteroposterior plane relative to bregma, and overlapping placements are not shown for clarity. Subjects with cannula placement outside of the posterior VTA were not included in the data analyses and are not shown here.
Figure 2.
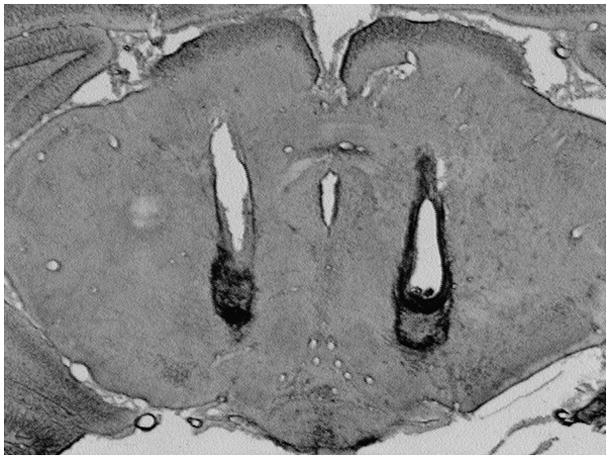
Photograph of a sample brain section showing typical bilateral cannulae placement with microinjection evidence into the posterior VTA (subject from DA Intake Experiment, sucrose group).
Table 1.
Mean (±SEM) ethanol-reinforced responding (seeking and intake) on the first versus the last aCSF microinjection sessions for all four experiments.
| Lever-Presses | ||
|---|---|---|
| 1st aCSF | last aCSF | |
|
|
||
| DA Exp | 35.2 (9.4) | 39.2 (15.4) |
| GLU Exp | 56.6 (15.1) | 39.0 (15.7) |
|
| ||
| Intake (g/kg) | ||
| 1st aCSF | last aCSF | |
|
| ||
| DA Exp | 0.56 (.04) | 0.56 (.05) |
| GLU Exp | 0.46 (.10) | 0.29 (.05) |
Table 2 shows lever-press latencies by experiment type and group (sucrose vs. ethanol) following aCSF and TTX, as well as the number of subjects responding as a function of total number of subjects. In the Seeking Experiments there was no significant effect of TTX in either the sucrose [t(14)= −1.64, p=.12] or the ethanol groups [t(8)= −2.02, p=.08] and similarly in the Intake Experiments there was no significant effect of TTX in the sucrose [t(12)=0.13, p=.90] or the ethanol groups [t(8)= −2.05, p=.08]. Following TTX microinjections, a higher percentage of animals in the ethanol-reinforced groups failed to make a lever-press response (Seeking Experiment: 47%; Intake Experiment: 36%) as compared to the sucrose-reinforced groups (Seeking Experiment: 6%; Intake Experiment: 24%).
Seeking Experiments
Figure 3 shows lever-presses over the drug manipulations from both Seeking Experiments.
Figure 3.

Mean (±SEM) reinforcer-seeking (number of lever-presses) in both the Dopamine (left half) and Glutamate (right half) experiments of subjects in both the sucrose- and ethanol-reinforced groups. Open bars represent bilateral microinjection of aCSF into the posterior VTA (the aCSF administration that was included in the counterbalanced drug administrations). Increasing shading denotes increasing dose of the antagonists (Dopamine Exp.: SCH23390 at 0.5, 1.0 and 2.0 μg/0.5 μL; Glutamate Exp.: CNQX at 0.25, 0.5 and 1.0 μg/0.5 μL), and the hatched bars denote TTX treatment. Asterisks indicate significant difference from aCSF treatment.
Dopamine and TTX Experiment
In the ethanol group, there was a significant effect of microinjection treatments on lever-pressing overall [F(4,32)=2.96, p<.05], with post hoc testing indicating that TTX decreased responding relative to aCSF and also relative to the low dose of SCH23390. However, no dose of the dopamine antagonist had any significant effect on ethanol-seeking relative to aCSF. In the sucrose group, there was also a significant effect of treatments [F(4,36)=2.71, p<.05], with post hoc testing again indicating that TTX decreased responding relative to aCSF and also relative to all doses of SCH23390. However, no dose of the dopamine antagonist had any significant effect on sucrose-seeking relative to aCSF.
Glutamate and TTX Experiment
In the ethanol group, there was a significant effect of treatments on lever-pressing [F(4,28)=4.04, p<.01], with post hoc testing indicating that TTX decreased responding relative to aCSF and relative to the low dose of CNQX. In addition, the high dose of the glutamate antagonist (1.0 μg/0.5 μl) decreased ethanol-seeking relative to aCSF. In the sucrose group, there was also a significant effect of treatments [F(4,20)=2.91, p<.05]. Post hoc testing again indicated that TTX decreased responding relative to aCSF. However, no dose of the glutamate antagonist had any significant effect on sucrose-seeking relative to aCSF.
Intake Experiments
Dopamine and TTX Experiment
There were no reliable effects of the dopamine antagonist, SCH23390, on ethanol intake (g/kg) [F(3,21)=0.90, p=.46] (See Figure 4). There was a slight, dose-dependent decrease in ethanol drinking, but it was neither statistically nor pharmacologically significant (~0.12 g/kg total decrease at the high dose). Similarly, while there was a dose-dependent trend to decrease intake in the sucrose group, there was no significant effect of SCH23390 on sucrose consumption (mls) [F(3,27)=2.73, p=.07] (See Figure 5).
Figure 4.
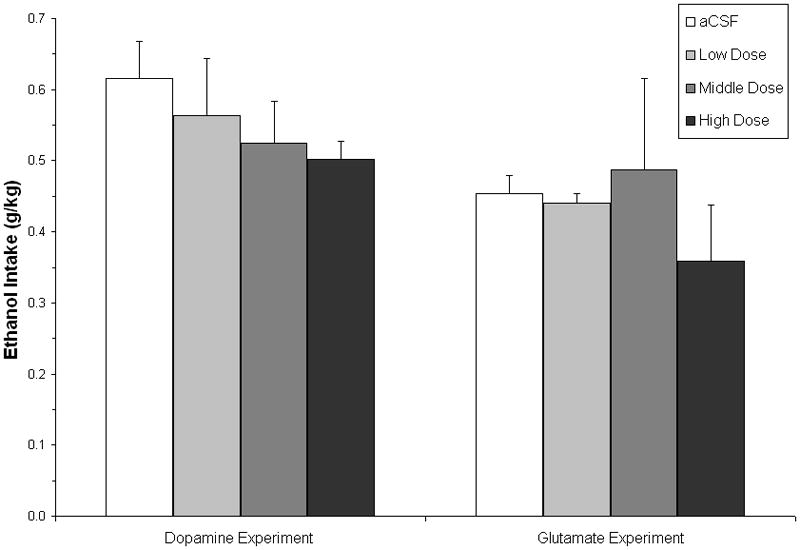
In the ethanol-reinforced groups, mean (±SEM) 10% ethanol intake (g/kg) in both the Dopamine (left) and Glutamate (right) experiments. Open bars represent bilateral microinjection of aCSF into the posterior VTA (the aCSF administration that was included in the counterbalanced drug administrations) and increasing shading denotes increasing dose of the antagonists (Dopamine Exp.: SCH23390 at 0.5, 1.0 and 2.0 μg/0.5 μL; Glutamate Exp.: CNQX at 0.25, 0.5 and 1.0 μg/0.5 μL).
Figure 5.

In the sucrose-reinforced groups, mean (±SEM) 2% sucrose intake (mls) in both the Dopamine (left) and Glutamate (right) experiments. Open bars represent bilateral microinjection of aCSF into the posterior VTA (the aCSF administration that was included in the counterbalanced drug administrations) and increasing shading denotes increasing dose of the antagonists (Dopamine Exp.: SCH23390 at 0.5, 1.0 and 2.0 μg/0.5 μL; Glutamate Exp.: CNQX at 0.25, 0.5 and 1.0 μg/0.5 μL).
Three subjects in both the ethanol- and sucrose-reinforced groups failed to complete the response requirement following TTX in this experiment, which is consistent with the finding from both Seeking Experiments that TTX decreased reinforcer seeking. However, whether these subjects were removed from the analyses or whether a zero was assigned for total intake, TTX had no significant effect on intake compared to aCSF. For the ethanol dose (g/kg) analysis - only subjects that gained access to the sipper tube: [t(4)=0.65, p=.55] [TTX 0.57 g/kg (±0.03)]; all subjects: [t(7)=1.89, p=.10] [TTX 0.36 g/kg (±0.04)]. For the sucrose volume (mls) analysis -only subjects that gained access to the sipper tube: [t(6)= −0.3, p=.78] [TTX 5.9 mls (±0.7)]; all subjects: [t(9)=1.60, p=.14] [TTX 4.2 mls (±1.1)].
Glutamate and TTX Experiment
There were no reliable effects of the glutamate antagonist, CNQX, on ethanol intake (g/kg) [F(3,14)=0.89, p=.47] (See Figure 4). In the sucrose group, there was a main effect of treatment on consumption (mls) [F(3,18)=3.56, p<.05] reflecting higher intake following the high dose relative to the low and middle doses of CNQX, however there were no significant effects of CNQX relative to aCSF (See Figure 5).
Two subjects in the ethanol- and one in the sucrose-reinforced group failed to complete the response requirement following TTX in this experiment, which is again consistent with the finding from both Seeking Experiments that TTX decreased reinforcer seeking. And similar to the other Intake Experiment, whether these subjects were removed from the analyses or whether a zero was assigned for total intake, TTX had no significant effect on intake compared to aCSF. For the ethanol dose (g/kg) analysis - only subjects that gained access to the sipper tube: [t(3)=0.24, p=.83] [TTX 0.42 g/kg (±0.05)]; all subjects: [t(5)=1.52, p=.19] [TTX 0.28 g/kg (±0.10)]. For the sucrose (mls) analysis - only subjects that gained access to the sipper tube: [t(5)= −0.38, p=.72] [TTX 8.8 mls (±2.0)]; all subjects: [t(6)=0.13, p=.90] [TTX 7.6 mls (±2.2)].
DISCUSSION
These experiments examined the effects of an AMPA/kainate antagonist and a dopamine D1 antagonist administered into the posterior VTA in two procedurally distinct phases of reinforcer-directed behavior (seeking and intake) using two reinforcer solutions (ethanol and sucrose). One important aspect of the procedural approach was the assessment of both the temporary inactivation of the VTA together with neurotransmitter-specific manipulations in that region in the same animals. Overall, the findings consistently showed in two separate experiments that TTX-inactivation of the posterior VTA decreased reinforcer-seeking by more than 75% of baseline in subjects trained to respond for both ethanol and sucrose reinforcement. In two parallel experiments, however, the same TTX treatment had no effect on either ethanol or sucrose drinking.
TTX has been previously tested in other behavioral paradigms in different brain regions at doses as high as 5–10ng/side, with no significant effects on locomotor activity (Fuchs & See, 2002; Lorenzini et al., 1995; McLaughlin & See, 2003). There are several pieces of evidence in the present study that the attenuation of the seeking response was not due solely to motor impairment. Latency to the first lever-press response in all four experiments was not significantly increased by TTX, and there was no effect of TTX on the drinking response in the Intake Experiments. While latencies did tend to increase following TTX administration, this nonsignificant effect was greater in the ethanol groups and more ethanol-reinforced subjects failed to make a lever-press response as compared to sucrose-reinforced subjects. Since there is no ethanol on board during the seeking portion of the experimental sessions, the reinforcer-specific nature of the TTX effects suggests that the response attenuation is motivational in nature rather than a general performance deficit. However, specific tests of locomotor behavior are necessary to completely rule out any general sedating/motor impairing effects of TTX.
While the neurochemical/neuroanatomical systems that regulate ethanol-seeking and drinking are likely to overlap to some degree, we have previously found evidence that they are also distinct (Czachowski, 2005; Czachowski et al., 2001; Czachowski et al., 2002). The two behavioral paradigms are designed to procedurally assess these behaviors, with the seeking procedure maximizing an appetitive response requirement and the inatke procedure dropping the response requirement to one on days of pharmacological manipulation. The response requirement remains in effect, rather than providing “free” access to the reinforcer, because there is evidence that free versus earned food and ethanol reinforcement are differentially regulated (Brown et al., 1982; Clifton et al., 1991; Salamone et al., 1991; 1994). Even so, if an animal fails to complete the single response to gain access to the solutions, then an assessment of intake is either not possible, or confounded by influence of the drug manipulation of the seeking response (i.e,. in the case where zeros are used to represent intake in the absence of access to the sipper tube). In the present experiment, only TTX resulted in some subjects failing to complete the seeking requirement, and whether or not subjects were included in the analyses that did or did not complete the seeking response, there was no effect of TTX on consumption of ethanol or sucrose. However, when subjects were included with zero for intake, an effect did begin to emerge (as evidenced by p values approaching 0.05). This suggests that in paradigms where the seeking/drinking behaviors are combined (i.e., fixed-ratio responding or progressive ratio responding where each “sip” is earned), that some effects on intake may reflect an effect on the seeking response as opposed to an effect purely on intake behavior. The approach used in the present studies strongly suggests that TTX inactivation of the posterior VTA decreased only the seeking behavior of both the ethanol and sucrose reinforcers, and does not affect consumption.
Ethanol-seeking was blocked not only by TTX-inactivation of the VTA, but also by intra-VTA administration of the glutamate antagonist, CNQX. The decrease in the seeking response was dose-dependent and consistent with findings that a similar dose (0.3 μg/0.5 μl) also administered in the caudal region of the VTA blocked cocaine-induced reinstatement but not food-induced reinstatement (Schmidt et al., 2009). The attenuation of ethanol-seeking by CNQX in this study did not generalize to seeking for a highly palatable calorie source (2% sucrose). This suggests that the effects of the glutamate manipulations may be specific to the psychoactive, reinforcing properties of ethanol, and that this portion of the mesocorticolimbic circuitry is involved in regulating something above and beyond food or calorie-related behavior. It should be noted that it is unlikely that the decrease in ethanol-seeking was produced by an interaction between ethanol and CNQX, since no ethanol was on board during extinction sessions.
The very moderate attenuation of ethanol and sucrose and intake by SCH23390, a dopamine D1-like antagonist, on the other hand, suggests that dopamine function within the posterior VTA may play only a tangential role in general consummatory behaviors. This is consistent with findings that SCH23390 in this region decreased the reinforcing effects of both cocaine (Ranaldi & Wise, 2001) and food (Sharf et al., 2005). The doses of SCH23390 necessary to produce significant effects on cocaine and food responding were in the 2–4 μg/0.5 μl range, and even at the 4ug dose the attenuating effect was overcome by increasing the amount of food presented after each response (Sharf et al., 2005). In the present study, the effects of SCH23390 (0.5–2 μg/0.5 μl) were not significant and only beginning to become apparent at the highest dose tested. Since rats in the seeking/self-administration paradigm can consume ethanol for 20 uninterrupted minutes, this may be similar to increasing the amount of reinforcer as in Sharf et al. (2005), resulting in overcoming the effects of the D1 antagonist. In addition, previous studies have shown that chronic ethanol drinking in alcohol-preferring P rats (8 weeks of access to 15% ethanol) increases the sensitivity of the posterior VTA to the effects of ethanol (Rodd et al., 2005c), and that activation of dopamine neurons was related to this effect (Rodd et al., 2005a). One possible explanation for the somewhat surprising lack of effect of the D1 antagonist in the present study could be that the long term ethanol drinking in these animals (12–14 weeks by the time of drug testing) sensitized the dopamine receptors in the VTA to ethanol, thereby requiring a higher dose of the antagonist to see an effect.
There are no dopamine afferents to the VTA and no dopamine D1 receptors on dopaminergic neurons in the VTA, therefore blockade of these D1 receptors affects only dendritically released dopamine within the VTA (Harrison et al., 1990; Lu et al., 1997; Ranaldi et al., 2001; Yung et al.,1995). Dopamine D1 receptors in the VTA are localized on GABAergic and glutamatergic afferents and activation of these receptors facilitates release of GABA and glutamate (Sesack & Pickel, 1992; Smith et al., 1996; Steffensen et al., 1998). Therefore, while SCH23390 could act at both of these sites on the GABA and glutamate cells, administration of CNQX would alter only glutamate activity in the VTA. Also, when the VTA was chemically inactivated by TTX, the behavioral effects resembled those resulting from CNQX, with the exception that the complete “removal” of posterior VTA afferent activity by TTX attenuated behaviors that were not reinforcer specific. Overall, the findings implicate the importance of glutamate activity, possibly from the primary projection from the PFC, in specifically regulating ethanol-seeking. However, the anatomical specificity of this region can only be definitively confirmed by conducting similar manipulations of dopamine and glutamate receptors in adjacent brain regions, and of particular interest would the anterior VTA, a region adjacent to the posterior VTA but showing no involvement in ethanol reinforcement (Rodd et al., 2004; 2005a).
The amount of ethanol consumed by the rats in this study on both non-test days and on aCSF treatment days (0.56–0.88 and 0.45–0.65 g/kg, respectively) was in a range considered to be “discriminable” and pharmacologically relevant (Hodge et al., 2001), especially given that it was consumed in under twenty minutes. Based upon blood alcohol levels measured in this paradigm in the same strain of rats, the present intake levels would yield blood ethanol concentrations in the 40–60mg% range (Czachowski et al., 2002). We propose that the present findings provide support for the importance of posterior VTA glutamate activity specifically in ethanol-seeking behavior in animals consuming single daily bouts of pharmacologically relevant amounts of ethanol. Further investigation of higher doses of the D1-like antagonist, as well as direct manipulation of PFC and NAc function are necessary to further identify the key players and their specific roles in this complex circuit.
Acknowledgments
This work was supported by grants from the National Institute on Alcohol Abuse and Alcoholism R01AA016101 (CLC). Special thanks to Dr. Kimberly Leite-Morris for her technical expertise and assistance with neuroanatomical confirmation and histology.
References
- Albin RL, Makowiec RL, Hollingsworth ZR, Dure LS, IV, Penney JB, Young AB. Excitatory amino acid binding sites in the basal ganglia of the rat: a quantitative autoradiographic study. Neuroscience. 1992;46:35–48. doi: 10.1016/0306-4522(92)90006-n. [DOI] [PubMed] [Google Scholar]
- Andrzejewski ME, Spencer RC, Kelley AE. Instrumental learning, but not performance, requires dopamine D1-receptor activation in the amygdala. Neuroscience. 2005;135:335–345. doi: 10.1016/j.neuroscience.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachtell RK, Whisler K, Karanian D, Self DW. Effects of intranucleus accumbens shell administration of dopamine agonists and antagonists on cocaine-taking and cocaine-seeking behaviors in the rat. Psychopharm. 2005;183:41–53. doi: 10.1007/s00213-005-0133-1. [DOI] [PubMed] [Google Scholar]
- Berger B, Thierry AM, Tassin JP, Moyne MA. Dopaminergic innervation of the rat prefrontal cortex: a fluorescence histochemical study. Brain Res. 1976;106:133–145. doi: 10.1016/0006-8993(76)90078-0. [DOI] [PubMed] [Google Scholar]
- Blythe SN, Wokosin D, Atherton JF, Bevan MD. Cellular mechanisms underlying burst firing in substantia nigra dopamine neurons. J Neurosci. 2009;29:15531–15541. doi: 10.1523/JNEUROSCI.2961-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert JM, Liu SY, Lu L, Shaham Y. A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J Neurosci. 2004;24:10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodie MS, Pesold C, Appel SB. Ethanol directly excites dopaminergic ventral tegmental area reward neurons. Alcohol Clin Exp Res. 1999;23:1848–1852. [PubMed] [Google Scholar]
- Brown ZW, Gill K, Abitbol M, Amit Z. Lack of effect of dopamine receptor blockade on voluntary ethanol consumption in rats. Behav Neural Biol. 1982;36:291–294. doi: 10.1016/s0163-1047(82)90915-3. [DOI] [PubMed] [Google Scholar]
- Clifton PG, Rusk IN, Cooper SJ. Effects of dopamine D1and dopamine D2 antagonists on the free feeding and drinking patterns of rats. Behav Neurosci. 1991;105:272–281. doi: 10.1037//0735-7044.105.2.272. [DOI] [PubMed] [Google Scholar]
- Czachowski CL. Manipulations of serotonin function in the nucleus accumbens core produce differential effects on ethanol- and sucrose-seeking and intake. Alcohol Clin Exp Res. 2005;29:1146–1155. doi: 10.1097/01.alc.0000171944.50381.86. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Chappell AM, Samson HH. The effects of raclopride in the nucleus accumbens on ethanol-seeking and consumption. Alcohol Clin Exp Res. 2001;25:1431–1440. doi: 10.1097/00000374-200110000-00005. [DOI] [PubMed] [Google Scholar]
- Czachowski CL, Santini LA, Legg BH, Samson HH. Separate measures of ethanol seeking and drinking in the rat: effects of remoxipride. Alcohol. 2002;28:39–46. doi: 10.1016/s0741-8329(02)00236-7. [DOI] [PubMed] [Google Scholar]
- Di Loreto S, Florio T, Scarnati E. Evidence that non-NMDA receptors are involved in the excitatory pathway from the pedunculopontine region to nigrostriatal dopaminergic neurons. Exp Brain Res. 1992;89:79–86. doi: 10.1007/BF00229003. [DOI] [PubMed] [Google Scholar]
- Fuchs RA, See RE. Basolateral amygdala inactivation abolishes conditioned stimulus- and heroin-induced reinstatement of extinguished heroin-seeking behavior in rats. Psychopharm. 2002;160:425–433. doi: 10.1007/s00213-001-0997-7. [DOI] [PubMed] [Google Scholar]
- Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: A novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewegen HJ, Berendse HW, Wolters JG, Lohman AHM. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. In: Uylings HBM, VanEden CG, DeBruin JPC, Corner MA, Feenstra MGP, editors. Progress in Brain Research. Vol. 85. Elsevier Science Publishers; Amsterdam: 1990. pp. 95–118. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Byrne R, Aston-Jones G. Glutamate-associated plasticity in the ventral tegmental area is necessary for conditioning environmental stimuli with morphine. Neurosci. 2004;129:841–847. doi: 10.1016/j.neuroscience.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Harrison MB, Wiley RG, Wooten GF. Selective localization of striatal D1 receptors to striatonigral neurons. Brain Res. 1990;528:317–322. doi: 10.1016/0006-8993(90)91674-6. [DOI] [PubMed] [Google Scholar]
- Hodge CW, Cox AA, Bratt AM, Camarini R, Iller K, Kelley SP, Mehmert KK, Nannini MA, Olive MF. The discriminative stimulus properties of self-administered ethanol are mediated by GABA(A) and NMDA receptors in rats. Psychopharmacol. 2001;154(1):13–22. doi: 10.1007/s002130000619. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. Neurotransmitter regulation of dopamine neurons in the ventral tegmental area. Brain Res Rev. 1993;18:75–113. doi: 10.1016/0165-0173(93)90008-n. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron. 2005;45:647–650. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Karreman M, Moghaddam B. The prefrontal cortex regulates basal release of dopamine in the limbic striatum: an effect mediated by ventral tegmental area. J Neurochem. 1996;66:589–598. doi: 10.1046/j.1471-4159.1996.66020589.x. [DOI] [PubMed] [Google Scholar]
- Karreman M, Westerink BH, Moghaddam B. Excitatory amino acid receptors in the ventral tegmental area regulate dopamine release in the ventral striatum. J Neurochem. 1996;67:601–607. doi: 10.1046/j.1471-4159.1996.67020601.x. [DOI] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsych Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Tassoni G. Time-dependent deficits of rat’s memory consolidation induced by tetrodotoxin injections into the caudate-putamen, nucleus accumbens, and globus pallidus. Neurobio Learn Mem. 1995;63:87–93. doi: 10.1006/nlme.1995.1008. [DOI] [PubMed] [Google Scholar]
- Lu X-Y, Churchill L, Kalivas PW. Expression of D1 receptor mRNA in projections from the forebrain to the ventral tegmental area. Synapse. 1997;25:205–214. doi: 10.1002/(SICI)1098-2396(199702)25:2<205::AID-SYN11>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, See RE. Selective inactivation of the dorsomedial prefrontal cortex and the basolateral amygdala attenuates conditioned-cued reinstatement of extinguished cocaine-seeking behavior in rats. Psychopharm. 2003;168:57–65. doi: 10.1007/s00213-002-1196-x. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: Interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol. 2010;91:235–288. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McBride WJ, Lumeng L, Li TK, Murphy JM. Involvement of dopamine D2 autoreceptors in the ventral tegmental area on alcohol and saccharin intake of the alcohol-preferring P rat. Alcohol Clin Exp Res. 2000;24:476–483. [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D. Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse. 1992;10:131–140. doi: 10.1002/syn.890100208. [DOI] [PubMed] [Google Scholar]
- Paxinos W, Watson C. The rat brain in stereotaxic coordinates. 4. Academic Press; San Diego: 1998. [Google Scholar]
- Ranaldi R, Wise RA. Blockade of D1 dopamine receptors in the ventral tegmental area decreases cocaine reward: Possible role for dendritically released dopamine. J Neurosci. 2001;21(15):5841–5846. doi: 10.1523/JNEUROSCI.21-15-05841.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li TK, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring and Wistar rats. Alcohol Clin Exp Res. 2004;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior ventral tegmental area of Wistar rats: Evidence for involvement of serotonin-3 receptors and dopamine neurons. J Pharm Exper Ther. 2005b;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, McQueen VK, Davids MR, Hsu CC, Murphy JM, Li TK, Lumeng L, McBride WJ. Chronic ethanol drinking by alcohol-preferring rats increases the sensitivity of the posterior ventral tegmental area to the reinforcing effects of ethanol. Alcohol Clin Exp Res. 2005c;29(3):358–366. doi: 10.1097/01.alc.0000156127.30983.9d. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Zhang Y, Murphy JM, Goldstein A, Zaffaroni A, Li TK, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol and acetaldehyde within the ventral tegmental area of alcohol-preferring (P) rats: involvement of dopamine and serotonin. Neuropsychopharmacol. 2005a;30(2):330–338. doi: 10.1038/sj.npp.1300561. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, McCullough LD, Carriero DL, Berkowitz RJ. Nucleus accumbens dopamine release increases during instrumental lever pressing for food but not free food consumption. Pharmacol Biochem Behav. 1994;49:25–31. doi: 10.1016/0091-3057(94)90452-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens dopamine depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacol. 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Samson HH. Initiation of ethanol reinforcement using a sucrose-substitution procedure in food- and water-sated rats. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci. 2009;30:1358–1369. doi: 10.1111/j.1460-9568.2009.06904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See RE, Kruzich PJ, Grimm JW. Dopamine, but not glutamate, receptor blockade in the basolateral amygdala attenuates conditioned reward in a rat model of relapse to cocaine-seeking behavior. Psychopharm. 2001;154:301–310. doi: 10.1007/s002130000636. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlableled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Sharf R, Lee DY, Ranaldi R. Microinjections of SCH 23390 in the ventral tegmental area reduce operant responding under a progressive ratio schedule of food reinforcement in rats. Brain Res. 2005;1033:179–185. doi: 10.1016/j.brainres.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Parent A. Synaptic innervation of midbrain dopaminergic neurons by glutamate-enriched terminals in the squirrel monkey. J Comp Neurol. 1996;364:231–253. doi: 10.1002/(SICI)1096-9861(19960108)364:2<231::AID-CNE4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun W, Akins CK, Mattingly AE, Rebec GV. Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacol. 2005;30:2073–2081. doi: 10.1038/sj.npp.1300744. [DOI] [PubMed] [Google Scholar]
- Sun WL, Rebec GV. The role of prefrontal cortex D1-like and D2-like receptors in cocaine-seeking behavior in rats. Psychopharm. 2005;177:315–323. doi: 10.1007/s00213-004-1956-x. [DOI] [PubMed] [Google Scholar]
- Taber MT, Das S, Fibiger HC. Cortical regulation of subcortical dopamine release: Mediation via the ventral tegmental area. J Neurochem. 1995;65:1407–1410. doi: 10.1046/j.1471-4159.1995.65031407.x. [DOI] [PubMed] [Google Scholar]
- Tupala E, Tiihonen J. Dopamine and alcoholism: neurobiological basis of ethanol abuse. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1221–1247. doi: 10.1016/j.pnpbp.2004.06.022. [DOI] [PubMed] [Google Scholar]
- You Z-B, Tzschentke TM, Brodin E, Wise RA. Electrical stimulation of the prefrontal cortex increases cholecystokinin, glutamate, and dopamine release in the nucleus accumbens: an In Vivo microdialysis study in freely moving rats. J Neurosci. 1998;18:6492–6500. doi: 10.1523/JNEUROSCI.18-16-06492.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung KK, Bolam JP, Smith AD, Hersch SM, Ciliax BJ, Levey AI. Immunocytochemical localization of D1 and D2 dopamine receptors in the basal ganglia of the rat: light and electron microscopy. Neuroscience. 1995;65:709–730. doi: 10.1016/0306-4522(94)00536-e. [DOI] [PubMed] [Google Scholar]
- Zhuravin IA, Brozek G, Bures J. Differential contribution of motor cortex and caudate nucleus to instrumental tongue forelimb synchronization in rats: a functional ablation study. Neurosci. 1994;58:193–200. doi: 10.1016/0306-4522(94)90166-x. [DOI] [PubMed] [Google Scholar]


