Abstract
Objectives
To test the hypothesis that the degree vascular burden and/or age of onset may influence the degree to which cognition can improve during the course of treatment in late life depression.
Design
Measurement of cognition both prior to and following 12-weeks of treatment with Sertraline.
Setting
University Medical Centers (Washington University and Duke University)
Participants
166 individuals with late life depression.
Intervention
Sertraline treatment.
Measurements
The cognitive tasks were grouped into five domains (language, processing speed, working memory, episodic memory, and executive function). We measured vascular risk using the Framingham Stroke Risk Profile measure. We measured T2 based white matter hyperintensities using the Fazekas criteria.
Results
Both episodic memory and executive function demonstrated significant improvement among adults with late life depression during treatment with sertraline. Importantly, older age, higher vascular risk scores, and lower baseline Mini-Mental state exam scores predicted less change in working memory. Further, older age, later age of onset, and higher vascular risk scores predicted less change in executive function.
Conclusions
These results have important clinical implications, in that they suggest that a regular assessment of vascular risk in older adults with depression is necessary as a component of treatment planning and in predicting prognosis, both for the course of the depression itself and for the cognitive impairments that often accompany depression in later life.
Keywords: Cognition, Vascular Depression, Treatment, white matter
Numerous studies document the presence of a range of cognitive impairments in late life depression (e.g., 1, 2), including reductions in working memory, executive function, episodic memory, and processing speed (1, 2). One hypothesis is that the presence of such cognitive impairments in late life depression reflects frontal striatal and/or hippocampal dysfunction that may result – at least in part - from vascular disease (3–6), and that this may be particularly true for depression with a later age of onset. Given this hypothesis, it is not surprising that cognitive impairment persists following depression treatment in older adults (for a review, see 7). As such, the goal of the current paper is to examine the influence of vascular risk and age of onset on cognitive improvement following depression treatment in older adults.
A growing body of research now documents that more severe cognitive impairment among older adults with depression predicts poorer outcome (8, 9) and a poorer response to treatment (10–13). Further, older adults with greater evidence of white matter impairment (13, 14) or reduced hippocampal and cortical volumes (15, 16) also show a poorer response to treatment. All of these results are consistent with the hypotheses that cognitive impairments in late life depression result from vascular or other neural changes that contribute to the onset or recurrence of depression in late life, and are not transient or state related manifestations of the presence of depression. If so, it is not surprising that such cognitive deficits persist even among individuals who respond to depression treatment.
However, there is also evidence that cognitive impairments are present in depression with an initial onset in young adulthood or middle age. These deficits are often present in many of the same cognitive domains impaired in late life depression (17), though the robustness of such cognitive impairments in early or midlife adult depression has been mixed across studies (e.g., 18). Further, there is evidence that at least some of these cognitive impairments can improve during treatment in younger depressed adults. Specifically, Douglas and Porter recently reviewed this literature and concluded that measures of episodic memory, verbal fluency and processing speed varied as a function of clinical state in depression, with deficits in executive function and attention showing more stable and trait - like characteristics in younger adults with depression (7).
Such findings in younger adults raise the possibility that at least some aspects of cognitive dysfunction in late life depression may not necessarily result from accruing vascular changes, but may reflect state or even trait aspects of depression. If so, one might expect more robust evidence for improvement in cognitive function across the course of treatment in late-life depression. Several studies have found some evidence for such improvement, with the magnitude of improvement in cognitive function correlated with the magnitude of improvement in depression in some studies (19–25). However, a number of other studies have either found no improvement in cognition as a function of treatment in late-life depression, or that the level of cognitive function in antidepressant responders was still below non-depressed individuals (e.g., 1, 16, 23, 26–30).
One important factor that these studies have not taken into account is the role that vascular burden and the presence of white matter hyperinstensities may play in moderating cognitive change. Some studies have found that older adults with depression are either less likely to respond to antidepressant treatment, or slower to respond (e.g., 31). This could reflect the influence of vascular changes and white matter alterations in older adults with depression. If so, then the degree vascular burden, white matter hyperintensities, and/or age of onset may influence the degree to which cognition can improve during treatment in late-life depression. The goal of this study was to test these hypotheses by examining the degree to which vascular risk, white matter hyperintensities, and age of onset predict the degree of cognitive improvement during 12-weeks of sertraline treatment in a large sample of older adults with DSM-IV Major Depression.
Methods
Participants
Participants were recruited as part of an NIMH funded study through advertising and physician referral to Washington University (WU) and Duke University. Patients were recruited into the study if they met DSM-IV criteria for major depression (MDD) by Structured Clinical Interview for Axis I DSM-IV Disorders (SCID-IV) (32) administered by a research psychiatrist, and were age ≥ 60 years of age. Exclusion criteria included: 1) severe or unstable medical disorders; 2) known primary neurological disorders; 3) history of other Axis I disorders prior to the depression diagnosed by SCID; 4) current suicidal risk; 5) a current MDD episode that had failed to respond to adequate trials of two prior antidepressants for at least 6 weeks at therapeutic doses; 6) use of psychotropic prescription or nonprescription drugs or herbals (e.g. hypericum) within three weeks or 5 half lives; 7) inpatient status; or 8) Clinical Dementia Rating (CDR) > 0 or Mini-Mental State Examination score <21 (27). Of 362 phone screens at WU and 374 at Duke there were 181 clinic screens at WU and 135 at Duke (see (13) for details). The 316 clinic screens resulted in 217 participants (120 at WU and 97 at Duke) being enrolled in a 12-week treatment trial with sertraline. Of these participants, 190 completed treatment (109 at WU and 81 at Duke). Written informed consent approved by the relevant Institutional Review Board was obtained for all subjects. This trial is registered at clinical trials.gov Treatment Outcome of Vascular Depression NCT00045773.
Sertraline Treatment
Sertraline was selected as the SSRI in this study because it is among the more selective 5- HT re-uptake inhibitors, has an excellent profile for safety and effectiveness in the treatment of major depression in the context of comorbid illness (33) has linear kinetics and minimal age effect on clearance (34). The primary results of depression response to treatment have been previously reported (13). Briefly, the treatment consisted of an initial dose of sertraline at 25 mg for one day to rule out drug sensitivity, then 50 mg daily, with subsequent dose changes at 2, 4 and 6 weeks (to 100, 150 and 200 mg per day respectively). At any point patients who had side effects could be titrated to a lower dose. Medication adherence was assessed on each visit by self-report. At the end of treatment, the mean final dose was 114, with 64 on <100 mg, 60 on 100–125 mg, 46 on 150–175 mg, and 34 on 200 mg.
Measures
We assessed depression severity using the Montgomery-Asberg Depression Rating Scale (MADRS)(35). The MADRS was administered by a research psychiatrist at the start of the trial and during each week of the trial. We assessed overall cardiovascular risk using the Framingham Stroke Risk Profile (36). The Framingham Stroke Risk Profile generates a composite score using the following vascular risk factors to predict 10-year probability of stroke in both men and women: age, systolic blood pressure, the use of antihypertensive therapy, diabetes mellitus, cigarette smoking, cardiovascular disease (coronary heart disease, cardiac failure, or intermittent claudication), atrial fibrillation, and left ventricular hypertrophy by electrocardiogram. This score has been positively associated with white matter hyperintensities (37) and negatively associated with total brain volume (38). We also assessed baseline global cognitive function using the Clinical Dementia Rating (CDR)(39) and Mini-Mental State exam (40). We assessed age at onset from the SCID-IV and all available medical and psychiatric records.
Neuropsychological Function
Participants were administered a large battery of neuropsychological tests that covered cognitive domains relevant to understanding late-life depression at both baseline (prior to the start of medications) and at the end of the 12-weeks of treatment. The neuropsychological testing was performed by a trained examiner who was supervised by a Ph.D. level psychologist (D.B. and K. W. B.). We grouped the cognitive tasks into rationally motivated domains described below based on the prior literature regarding the cognitive processes tapped by each of the tasks. The domains were executive function, processing speed, episodic memory, working memory and language processing. To combine the tasks within each cognitive domain, we created Z-scores for the primary dependent measure of interest using the scores from both baseline and follow-up across all participants and then summed the Z-scores (the results would not have been different if only the baseline was used to create Z-scores). For the majority of variables, better performance is indicated by a higher score. We reverse scored any items (e.g., reaction time on Trails B) for which good performance was indicated by a lower value. Cronbach’s coefficient alpha (a measure of internal consistency) was computed for each domain from the baseline data. For further details see Sheline et al., (43), which describes the baseline analyses of neuropsychological function in this sample.
Executive Function
This domain included verbal fluency (total phonological and semantic), Trails B (reverse scored time to completion), the color-word interference condition of the Stroop (number completed), the Initiation-Perseveration subscales of the Mattis, and categories completed from the Wisconsin Card Sorting Test. The coefficient alpha for this domain was .73.
Processing Speed
This domain included Symbol-digit modality (number completed), the color naming condition of the Stroop task (number completed), and Trails A (reverse scored time to completion),. The coefficient alpha for this domain was .80.
Episodic Memory
This domain included word list learning (total correct), logical memory (total correct immediate), constructional praxis (Rey-Osterrieth Complex Figure Test memory performance), and the Benton Visual Retention Test (total correct). The coefficient alpha for this domain was .76.
Language Processing
This domain included the Shipley Vocabulary Test (number correct), the Boston Naming Test (number correct), and the Word reading condition of the Stroop (number completed). The coefficient alpha for this domain was .67.
Short-Term/Working Memory
This domain included digit span forward (number of trials correctly completed), digit span backwards (number of trials correctly completed), and ascending digits (number of trials correctly completed). The coefficient alpha for this domain was .68.
MR Imaging
T1 and T2 MRI images were collected using a Siemens Sonata 1.5T scanner at Washington University School of Medicine and a GE 1.5T scanner at Duke University. See Text, Supplemental Digital Content 1 for details on pulse sequences and processing.
T2-Weighted Hyperintensities
Hyperintensities were assessed blinded to treatment data using the modified Fazekas criteria, which is a widely used measure of white matter burden that allows comparison to a large number of previous studies. All ratings were conducted at Washington University School of Medicine by RCM and YIS. The modified Fazekas criteria (35) describe MRI hyperintensities in three regions (periventricular, deep white matter, and subcortical gray matter regions) using ascending degree of severity. The dependent variable was a total score summing severity scores in all three regions. See Text, Supplemental Digital Content 1 for details on scoring.
Results
Of the 217 individuals enrolled in the trial, 211 had usable neuropsychological data at baseline. Of the 190 individuals who completed the trial, 166 (105/109 at WU, 61/81 at Duke) were able to provide cognitive data at the 12-week follow up. We began by comparing the demographic, clinical and cognitive characteristics of the participants with usable neuropsychological data at baseline (211) who did (166) and did not have data at study completion (45). As shown in Table 1, these two groups did not differ in age, education, gender, race, baseline MADRS, baseline Mini-Mental State, vascular risk, or on any of the 5 cognitive domains. The individuals who did not have neuropsychological data at study completion had a slightly but significantly later age of onset than those individuals who did not.
Table 1.
Demographic, Clinical and Cognitive Characteristics of Participants
| Completers | Non-Completers | Comparison of Completers & Non- Completers |
|||
|---|---|---|---|---|---|
| Variable | Mean | SD | Mean | SD | |
| Demographic Characteristics | |||||
| Age (in years) | 68.1 | 7.1 | 69.4 | 7.9 | t(209) = 1.03, p=.31 |
| Gender (% Female) | 56 | 53 | X2(1) = 0.10, p=.75 | ||
| Race (% Caucasian) | 91 | 96 | X2(1) = 1.10, p=.58 | ||
| Education (in years) | 14.2 | 3.1 | 14.4 | 2.8 | t(209) = 0.56 p=.58 |
| Clinical Characteristics | |||||
| Age of Onset (in years) | 51.2 | 18.0 | 57.3 | 15.5 | t(209) = 2.06, p=.04 |
| Baseline MADRS | 26.2 | 4.3 | 25.6 | 4.8 | t(209) = −.93, p=.354 |
| Vascular Risk Score | 11.6 | 4.5 | 13.5 | 5.6 | t(209) = 1.8, p=.07 |
| Mini-Mental State Exam | 27.7 | 2.0 | 27.7 | 2.1 | t(209) = −.32 p=.75 |
| Baseline Cognitive Function | |||||
| Language (Z-score) | −.02 | 0.8 | −.02 | 0.9 | t(209) = 0.05, p=.97 |
| Processing Speed (Z-score) | −.01 | 0.8 | 0.03 | 0.8 | t(209) = 0.27, p=.79 |
| Working Memory (Z-score) | −.01 | 0.8 | −.04 | 0.9 | t(209) = −.21, p=.83 |
| Episodic Memory (Z-score) | −.11 | 0.8 | −.13 | 0.7 | t(209) = −.18, p=.86 |
| Executive Function (Z-score) | −.06 | 0.7 | 0.02 | 0.7 | t(209) = 0.65, p=.51 |
Did Cognition Improve Across the Course of Treatment?
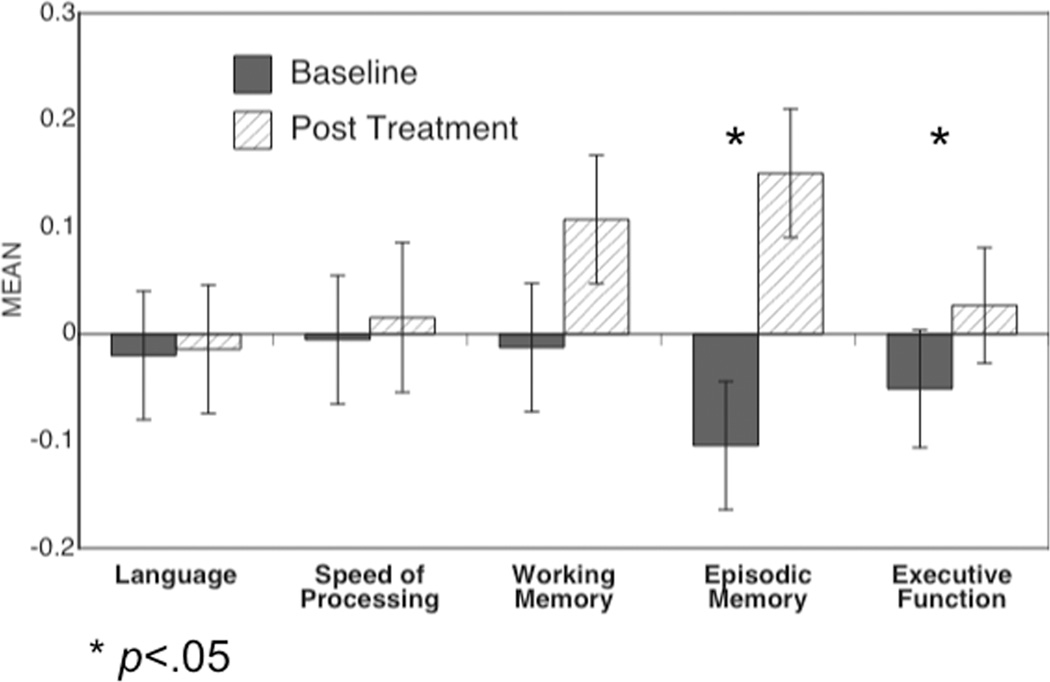
To examine whether performance in any of the five cognitive domains improved across treatment, we used a repeated measures ANOVA with time point (baseline, follow-up) and cognitive domain (Language, Processing Speed, Working Memory, Episodic Memory, Executive Function) as within subject factors, and the domain Z-score as the dependent measure. This analysis revealed a main effect of time (F(1,165) = 19.3, p<.0001) and a time by cognitive domain interaction (F(4,660) = 11.1, p<.001). As shown in Figure 1, follow-up contrasts for each cognitive domain indicated that only episodic memory and executive function improved over the course of treatment.
Figure 1.
Graph of performance in each of the five cognitive domains at baseline and at post treatment. Significance of change in each cognitive domain was assessed with posthoc contrast (F tests with 1,165 dfs).
What factors predict improved cognition?
We computed residualized change scores for each of the five cognitive domains, using baseline performance to predict post treatment performance. We then used Pearson’s Product Moment Correlations to compute the correlations between age, age of onset, vascular risk score, baseline Mini-Mental State Exam scores, and baseline MADRS depression scores with the 5 cognitive domain residualized change scores. The Fazekas scores were not normally distributed, and thus we used Spearman Rank Order correlations to examine the relationship to cognitive change for this variable. As shown in Table 2, older age predicted less improvement in processing speed, working memory, and executive function. Later age of onset predicted less improvement in executive function. Higher vascular risk predicted less change in working memory and executive function. More severe white matter hyperintensities predicted less change in processing speed. Of note, the results were essentially identical when partial correlations were used, examining the relationship between posttreatment performance and the predictors, covaring for baseline cognitive performance.
Table 2.
Correlations of Predictor Variables with Residualized Change Scores (Baseline to Post Treatment) in Each Cognitive Domain
| Variable | Language Function |
Processing Speed |
Working Memory |
Episodic Memory |
Executive Function |
|---|---|---|---|---|---|
| Age | −.06 | −.18* | −.25*** | −.14 | −.17* |
| Age of Onset | −.03 | −.04 | −.06 | −.03 | −.24*** |
| Vascular Risk Score | .07 | −.07 | −.24*** | −.11 | −.17* |
| White Matter Hyperintensities (Fazekas Scores) | .07 | −.16* | −.12 | −.14 | .05 |
| Baseline MADRS | −.03 | −.11 | −.10 | −.16* | −.12 |
| Mini-Mental State | −.06 | −.10 | .15# | .07 | .13 |
| Change in MADRS from Baseline to Post Treatment | −.19* | −.01 | −.14 | −.08 | −.03 |
N = 166
p = .05
p < .05
p < .01
p<.005
We also examined whether the magnitude of improvement in depression predicted the magnitude of improvement in cognition. To do so, we created a residualized change score for depression using baseline MADRS scores to predict post treatment MADRS scores, and correlated this with the residualized change scores for cognition. As shown in Table 2, a greater reduction in MADRS scores predicted a greater improvement in language function, but did not predict improvement in the other cognitive domains.
Cognitive Improvement as a Function of Remitter Status
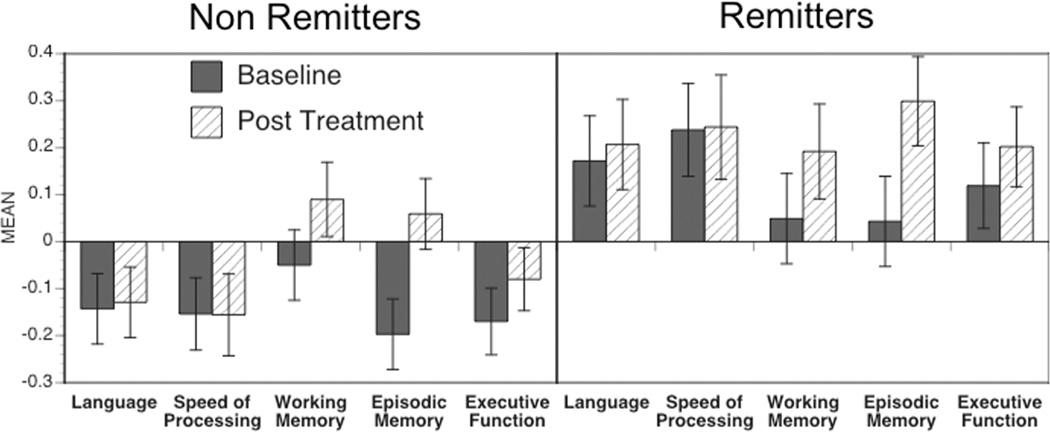
Some prior research suggests that cognitive improvement over the course of treatment may vary as a function of whether an individual was considered to have remitted to treatment in terms of depression status (22, 30). Thus, we examined cognitive change as a function of remitter status, with a remitter defined as someone who achieved a final MADRS score of 7 or less. Of the 166 individuals with neuropsychological data at both baseline and follow-up, 63 were remitters and 103 were not. We computed a repeated measures ANOVA with time (baseline, post treatment) and cognitive domain as within subject factors, and remitter status as a between subject factor. This ANOVA again revealed a time by cognitive domain interaction, F(4,656) = 9.92, p<.001, and revealed a main effect of remitter status, F(1,164) = 8.37, p=.004. However, there was no significant two-way interaction between remitter status and time, F(1,164) = 1.24, p=.27) or three-way interaction between remitter status, time and cognitive domain, F(4,656) = 1.56, p=.184). As shown in Figure 2, the main effect of remitter status reflected the fact that the non-remitters had overall worse cognitive performance than the remitters at both baseline and post treatment.
Figure 2.
Graph of performance in each of the five cognitive domains at baseline and at post treatment, plotted separately for those individuals whose depression remitted by the end of treatment (MADRS score of 7 or less) and for those individuals who depression did not remit by the end of treatment.
Discussion
The goal of the current study was to examine the degree to which cognitive function improved during the course of antidepressant treatment among depressed older individuals, and to determine whether factors such as the degree of vascular burden, white matter hyperintensities, and/or age of onset influenced the degree to which cognition improved during treatment in late life depression. We found that both episodic memory and executive function improved from baseline to post treatment, and that this improvement occurred for individuals whose depression remitted and for those whose depression did not remit. However, working memory improved only among depressed individuals whose depression remitted. Of note, we cannot definitely attribute these changes to the treatment, as we did not have a placebo control group. However, importantly, we found that a number of factors moderated the degree of improvement in cognition (whether it was specifically due to treatment or responsivity to practice) particularly among those individuals whose depression did not fully remit. Specifically, older age, higher vascular risk scores, and lower baseline Mini-Mental state exam scores predicted less improvement in working memory. Further, older age, later age of onset, and higher vascular risk scores predicted less improvement in executive function. In addition, more severe white matter hyperintensities predicted less improvement in processing speed.
The fact that episodic memory improved is consistent with prior work suggesting that impairments in episodic memory may be associated with state components of depression and may be more likely to improve with the remission of depression than some other cognitive functions (7). However, we also saw improvements in executive function, a domain in which improvements have not been as consistently demonstrated in prior studies (7). This is a cognitive domain which Douglas and Porter argued may reflect more trait like aspects of depression and which has been associated with white matter abnormalities in late life depression (3). Interestingly, the predictors of change in episodic memory and executive function were very different. The degree of change in executive function, but not episodic memory, was predicted by older age, older age of onset and higher vascular risk scores. In contrast, baseline depression severity predicted change in episodic memory, but not executive function. Similar to executive function, change in working memory was also predicted by older age and higher vascular risk scores. Thus, although both episodic memory and executive function improved over the course of treatment, the predictors of the magnitude of change in executive function are consistent with a critical role for vascular burden in constraining executive function (as well as working memory) and the degree to which it can improve in response to depression treatment.
In our prior work with this sample, we found that more severe white matter hyperintensities were associated with worse cognitive function in all domains at baseline (13). Interestingly, in the current analyses we also found that more severe white matter hyperintensities predicted less improvement in processing speed. This relationship with processing speed is consistent with the hypothesis that vascular changes leading to white matter alternations may contribute to at least some of the cognitive impairments found in late life depression (3–6). It is somewhat surprising that vascular burden and not white matter hyperintensities predicted change in some of the other cognitive domains, such as executive function and working memory. However, it may be that our measure of white matter hyperintensities, which was restricted to periventricular, deep white matter, and subcortical gray matter regions, did not capture changes in white matter in other brain regions which may also be related to vascular changes.
We also found that individuals whose depression remitted during treatment showed overall better cognitive function. This result is consistent with our prior work (in this same sample) showing that baseline cognitive function predicted response to treatment (13), and with other work showing that impaired cognitive function in late life depression is associated with a poorer response to treatment (e.g., 8, 9–13).
There were several limitations to the current study. First, we did not recruit a control sample of older adults without depression, as the purpose of the study was to examine treatment response in depressed older adults. Thus, we could not directly address the question of whether cognitive function in our depressed individuals was worse than controls at baseline, or whether the degree of cognitive improvement would have resulted in a level of cognitive performance that no longer differed from non-depressed individuals. However, the large body of research showing that late life depression is associated with impaired cognition relative to matched controls makes it likely that we would have also found that our sample was impaired relative to an appropriate control group. Second, we did not have a placebo control group, so we cannot definitely determine that the cognitive change we did observe reflected a response to treatment rather than practice effects or placebo effects. However, this does not minimize the importance or utility of identifying predictors of cognitive change, regardless of the source of change. In other words, the ability to benefit from practice may be key to various cognitive enhancement approaches, and thus information about the factors that may identify who or who would show responsivity to either antidepressant therapy or practice may be useful in individualized treatment planning.
In summary, we found that depressed older adults showed significant improvement in episodic memory and executive function across the course of a 12-week treatment with sertraline. Further, we found that factors such as age, age of onset and vascular risk scores predicted the amount of change in cognitive domains such as executive function and working memory, a result consistent with the hypothesis that vascular burden may play a critical role in constraining the degree of cognitive improvement that can be obtained in some domains among older adults treated for depression. These results have important clinical implications, in that they suggest that a regular assessment of vascular risk in older adults with depression is necessary as a component of treatment planning and in predicting prognosis, both for the course of the depression itself and for the cognitive impairments that often accompany depression in later life.
Supplementary Material
Acknowledgments
Disclosures and Acknowledgments: The authors would like to thank Dan Blazer MD, PhD for serving as an advisor to the study, Caroline Hellegers MA for her assistance with study coordination at Duke and Tony Durbin M.S. and Brigitte Mittler for their assistance with study coordination at Washington University. Drs. Sheline, Doraiswamy, Taylor, Steffens, and Krishnan have received grants and/or speaking/consulting fees from antidepressant manufacturers but do not own stock in these companies. Dr. Krishnan is also a coinventor on a patent that is licensed to Cypress Biosciences and owns stock in CeneRx. Dr. Doraiswamy also owns stock in EnergyInside.
Grant Support: This work was supported by a Collaborative R01 for Clinical Studies of Mental Disorders Grant Number MH60697 (YIS) and MH62158 (PMD). Dr. Sheline also receives support from NIMH K24 65421. Additionally this work was supported by a grant (RR00036) to the WUSM General Clinical Research Center and by a grant from Pfizer, Inc to pay for drug costs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61(6):587–595. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- 2.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive function in late life depression: relationships to depression severity, cerebrovascular risk factors and processing speed. Biol Psychiatry. 2006;60(1):58–65. doi: 10.1016/j.biopsych.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 3.Sheline YI, Price JL, Vaishnavi SN, Mintun MA, Barch DM, Epstein AA, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165(4):524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexopoulos GS. Vascular disease, depression, and dementia. J Am Geriatr Soc. 2003;51(8):1178–1180. doi: 10.1046/j.1532-5415.2003.51373.x. [DOI] [PubMed] [Google Scholar]
- 5.Alexopoulos GS. The vascular depression hypothesis: 10 years later. Biol Psychiatry. 2006;60(12):1304–1305. doi: 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment: findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63(3):273–279. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 7.Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust N Z J Psychiatry. 2009;43(12):1105–1117. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin RC, Gallagley A, Gourlay M, Jackson A, Burns A. Prognosis of late life depression: a three-year cohort study of outcome and potential predictors. Int J Geriatr Psychiatry. 2006;21(1):57–63. doi: 10.1002/gps.1424. [DOI] [PubMed] [Google Scholar]
- 9.Potter GG, Kittinger JD, Wagner HR, Steffens DC, Krishnan KR. Prefrontal neuropsychological predictors of treatment remission in late-life depression. Neuropsychopharmacology. 2004;29(12):2266–2271. doi: 10.1038/sj.npp.1300551. [DOI] [PubMed] [Google Scholar]
- 10.Alexopoulos GS, Kiosses DN, Heo M, Murphy CF, Shanmugham B, Gunning-Dixon F. Executive dysfunction and the course of geriatric depression. Biol Psychiatry. 2005;58(3):204–210. doi: 10.1016/j.biopsych.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Sneed JR, Keilp JG, Brickman AM, Roose SP. The specificity of neuropsychological impairment in predicting antidepressant non-response in the very old depressed. Int J Geriatr Psychiatry. 2008;23(3):319–323. doi: 10.1002/gps.1889. [DOI] [PubMed] [Google Scholar]
- 12.Story TJ, Potter GG, Attix DK, Welsh-Bohmer KA, Steffens DC. Neurocognitive correlates of response to treatment in late-life depression. Am J Geriatr Psychiatry. 2008;16(9):752–759. doi: 10.1097/JGP.0b013e31817e739a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheline YI, Pieper CF, Barch DM, Welsh-Boehmer K, McKinstry RC, MacFall JR, et al. Support for the vascular depression hypothesis in late-life depression: results of a 2-site, prospective, antidepressant treatment trial. Arch Gen Psychiatry. 2010;67(3):277–285. doi: 10.1001/archgenpsychiatry.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alexopoulos GS, Kiosses DN, Choi SJ, Murphy CF, Lim KO. Frontal white matter microstructure and treatment response of late-life depression: a preliminary study. Am J Psychiatry. 2002;159(11):1929–1932. doi: 10.1176/appi.ajp.159.11.1929. [DOI] [PubMed] [Google Scholar]
- 15.Simpson SW, Baldwin RC, Burns A, Jackson A. Regional cerebral volume measurements in late-life depression: relationship to clinical correlates, neuropsychological impairment and response to treatment. Int J Geriatr Psychiatry. 2001;16(5):469–476. doi: 10.1002/gps.364. [DOI] [PubMed] [Google Scholar]
- 16.O'Brien JT, Lloyd A, McKeith I, Gholkar A, Ferrier N. A longitudinal study of hippocampal volume, cortisol levels, and cognition in older depressed subjects. Am J Psychiatry. 2004;161(11):2081–2090. doi: 10.1176/appi.ajp.161.11.2081. [DOI] [PubMed] [Google Scholar]
- 17.Castaneda AE, Tuulio-Henriksson A, Marttunen M, Suvisaari J, Lonnqvist J. A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord. 2008;106(1–2):1–27. doi: 10.1016/j.jad.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 18.Grant MM, Thase ME, Sweeney JA. Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry. 2001;50:35–43. doi: 10.1016/s0006-3223(00)01072-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Savaskan E, Muller SE, Bohringer A, Schulz A, Schachinger H. Antidepressive therapy with escitalopram improves mood, cognitive symptoms, and identity memory for angry faces in elderly depressed patients. Int J Neuropsychopharmacol. 2008;11(3):381–388. doi: 10.1017/S1461145707007997. [DOI] [PubMed] [Google Scholar]
- 20.Gallassi R, Di Sarro R, Morreale A, Amore M. Memory impairment in patients with late-onset major depression: the effect of antidepressant therapy. J Affect Disord. 2006;91(2–3):243–250. doi: 10.1016/j.jad.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Doraiswamy PM, Krishnan KR, Oxman T, Jenkyn LR, Coffey DJ, Burt T, et al. Does antidepressant therapy improve cognition in elderly depressed patients? J Gerontol A Biol Sci Med Sci. 2003;58(12):M1137–M1144. doi: 10.1093/gerona/58.12.m1137. [DOI] [PubMed] [Google Scholar]
- 22.Devanand DP, Pelton GH, Marston K, Camacho Y, Roose SP, Stern Y, et al. Sertraline treatment of elderly patients with depression and cognitive impairment. Int J Geriatr Psychiatry. 2003;18(2):123–130. doi: 10.1002/gps.802. [DOI] [PubMed] [Google Scholar]
- 23.Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, et al. Changes in cognitive functioning following treatment of late-life depression. Am J Psychiatry. 2000;157(12):1949–1954. doi: 10.1176/appi.ajp.157.12.1949. [DOI] [PubMed] [Google Scholar]
- 24.Beats BC, Sahakian BJ, Levy R. Cognitive performance in tests sensitive to frontal lobe dysfunction in the elderly depressed. Psychol Med. 1996;26(3):591–603. doi: 10.1017/s0033291700035662. [DOI] [PubMed] [Google Scholar]
- 25.Abas MA, Sahakian BJ, Levy R. Neuropsychological deficits and CT scan changes in elderly depressives. Psychol Med. 1990;20(3):507–520. doi: 10.1017/s0033291700017025. [DOI] [PubMed] [Google Scholar]
- 26.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant BH, Zmuda MD, et al. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized, double-blind clinical trial with nortriptyline and paroxetine. J Psychiatr Res. 2003;37(2):99–108. doi: 10.1016/s0022-3956(02)00085-7. [DOI] [PubMed] [Google Scholar]
- 27.Nebes RD, Butters MA, Mulsant BH, Pollock BG, Zmuda MD, Houck PR, et al. Decreased working memory and processing speed mediate cognitive impairment in geriatric depression. Psychol Med. 2000;30(3):679–691. doi: 10.1017/s0033291799001968. [DOI] [PubMed] [Google Scholar]
- 28.Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, et al. Persistence of neuropsychologic deficits in the remitted state of late-life depression. Am J Geriatr Psychiatry. 2006;14(5):419–427. doi: 10.1097/01.JGP.0000203130.45421.69. [DOI] [PubMed] [Google Scholar]
- 29.Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, et al. Executive dysfunction in medicated, remitted state of major depression. J Affect Disord. 2008;111(1):46–51. doi: 10.1016/j.jad.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 30.Culang ME, Sneed JR, Keilp JG, Rutherford BR, Pelton GH, Devanand DP, et al. Change in cognitive functioning following acute antidepressant treatment in late-life depression. Am J Geriatr Psychiatry. 2009;17(10):881–888. doi: 10.1097/jgp.0b013e3181b4bf4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zanardi R, Cusin C, Rossini D, De Ronchi D, Serretti A. Comparison of response to fluvoxamine in nondemented elderly compared to younger patients affected by major depression. J Clin Psychopharmacol. 2003;23(6):535–539. doi: 10.1097/01.jcp.0000095344.32154.d3. [DOI] [PubMed] [Google Scholar]
- 32.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for the DSM-IV-TR Axis I disorders. Washington, D.C.: American Psychiatric Press; 2001. [Google Scholar]
- 33.Cipriani A, La Ferla T, Furukawa TA, Signoretti A, Nakagawa A, Churchill R, et al. Sertraline versus other antidepressive agents for depression. Cochrane Database Syst Rev. 2009;(2):CD006117. doi: 10.1002/14651858.CD006117.pub2. [DOI] [PubMed] [Google Scholar]
- 34.DeVane CL, Pollock BG. Pharmacokinetic considerations of antidepressant use in the elderly. J Clin Psychiatry. 1999;60(Suppl 20):38–44. [PubMed] [Google Scholar]
- 35.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to changes. British Journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 36.Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: A risk profle from the Framingham Study. Stroke. 1991;22:312–318. doi: 10.1161/01.str.22.3.312. [DOI] [PubMed] [Google Scholar]
- 37.Jeerakathil T, Wolf PA, Beiser A, Massaro J, Seshadri S, D'Agostino RB, et al. Stroke risk profile predicts white matter hyperintensity volume: the Framingham Study. Stroke. 2004;35(8):1857–1861. doi: 10.1161/01.STR.0000135226.53499.85. [DOI] [PubMed] [Google Scholar]
- 38.Seshadri S, Wolf PA, Beiser A, Elias MF, Au R, Kase CS, et al. Stroke risk profile, brain volume, and cognitive function: the Framingham Offspring Study. Neurology. 2004;63(9):1591–1599. doi: 10.1212/01.wnl.0000142968.22691.70. [DOI] [PubMed] [Google Scholar]
- 39.Morris JC. The Clinical Demential Rating (CDR): Current version and scoring rules. Neurology. 1993;43:2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 40.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.