Abstract
EEG studies suggested that the N170 ERP and Gamma‐band responses to faces reflect early and later stages of a multiple‐level face‐perception mechanism, respectively. However, these conclusions should be considered cautiously because EEG‐recorded Gamma may be contaminated by noncephalic activity such as microsaccades. Moreover, EEG studies of Gamma cannot easily reveal its intracranial sources. Here we recorded MEG rather than EEG, assessed the sources of the M170 and Gamma oscillations using beamformer, and explored the sensitivity of these neural manifestations to global, featural and configural information in faces. The M170 was larger in response to faces and face components than in response to watches. Scrambling the configuration of the inner components of the face even if presented without the face contour reduced and delayed the M170. The amplitude of MEG Gamma oscillations (30–70 Hz) was higher than baseline during an epoch between 230–570 ms from stimulus onset and was particularly sensitive to the configuration of the stimuli, regardless of their category. However, in the lower part of this frequency range (30–40 Hz) only physiognomic stimuli elevated the MEG above baseline. Both the M170 and Gamma were generated in a posterior‐ventral network including the fusiform, inferior‐occipital and lingual gyri, all in the right hemisphere. The generation of Gamma involved additional sources in the visual system, bilaterally. We suggest that the evoked M170 manifests a face‐perception mechanism based on the global characteristics of face, whereas the induced Gamma oscillations are associated with the integration of visual input into a pre‐existent coherent perceptual representation. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: face processing, M170, gamma oscillations
INTRODUCTION
Human faces are one of the most important sources of social information and, despite some interindividual variation, they are perceived by humans with unmatched expertise. Therefore, it is not surprising that face perception has been extensively investigated in attempt to unveil the perceptual mechanisms accounting for this expertise as well as its neural underpinnings. Performance studies pointed to a variety of perceptual processes, such as global, featural and configural analysis [Maurer et al., 2002], probably occurring in cascade. The neural mechanisms involved in face perception have been investigated using a variety of techniques. Functional Magnetic Resonance Imagery (fMRI) revealed a distributed neural system containing at least three distinct brain areas that are activated during face processing [Haxby et al., 2000; Ishai et al., 2005; Kanwisher, 2010; Kanwisher and Yovel, 2006; Pitcher et al., 2011]. Electrical event‐related potentials (ERP) revealed that the brain responds to faces as a distinct category already at about 170 ms from stimulus onset [Bentin et al., 1996], and perhaps even sooner [e.g., Latinus and Taylor, 2006]. EEG oscillations, particularly in the Gamma band suggested a multilevel hierarchical model for face processing [Zion‐Golumbic and Bentin, 2007]. Finally, MEG studies combined a fine time resolution with a fairly good spatial resolution to shed light on the timing at which different neural mechanisms are activated during face processing [for a review see Watanabe et al., 2005].
Although the first MEG study using face images as stimuli was conducted as early as 1991 [Lu et al., 1991], investigations of face processing using MEG have proliferated only since the late 1990's. At that time, several studies described a face‐selective component in the posterior area of human brain, the M170, which, like the N170 ERP, is significantly larger in response to faces than the nonface categories [Halgren et al., 2000; Liu et al., 2000; Sams et al., 1997; Sato et al., 1999; Streit et al., 1999; Swithenby et al., 1998; Watanabe et al., 1999]. The special link between the MEG and face processing has been established by Xu et al. (2005). In that study the M170 amplitude was larger for faces than for houses, cars, or shoes in all participants. Moreover, reflecting the special expertise for faces, it was not larger in response to cars in car experts than in car naives, and it was not larger in response to cars than in response to shoes in either group. Source localization studies implicated lateral temporal regions, particularly the fusiform gyrus in the generation of this component [Deffke et al., 2007; Henson et al., 2007; Itier et al., 2006; Japee et al., 2009; Schweinberger et al., 2007; Taylor et al., 2008, 2011; Watanabe et al., 2003]. Other studies aimed at elaborating the functional significance of the perceptual mechanisms manifested by the M170 and their role in normal face processing as well as their defective activity when face processing is impaired [e.g., Dobel et al., 2008; Duchaine and Nakayama, 2006; Harris et al., 2005; Taylor et al., 2008]. For instance, it has been shown that, like the N170, the peak of the M170 is delayed for inverted than upright faces [Itier et al., 2006; Liu et al., 2000; Watanabe et al., 2003] but, unlike the N170, the M170 amplitude is considerably larger for famous or personally familiar faces than for unfamiliar faces [Harris and Aguirre, 2008; Kloth et al., 2006]. Moreover, the M170 amplitude was larger when episodically studied unfamiliar faces were successfully recognized at test, than when they were not [Liu et al., 2002; Tanskanen et al., 2005; Xu et al., 2005]. Hence, the perceptual mechanism manifested by M170 modulations seems to be associated with the initial stages in face recognition probably reflecting conscious detection of faces and possibly their individuation.
Surprisingly, relative to the wealth of studies that investigated the M170 component only a few studies investigated MEG oscillations during face processing. Moreover, the absolute majority of those studies tapped face‐memory processes focusing particularly on the theta and alpha bands [Guderian and Duzel, 2005; Jokisch and Jensen, 2007; Nieuwenhuis et al., 2008; Tuladhar et al., 2007] or in processing emotions expressed by faces [Chen et al., 2010]. Yet, a very recent report, showed that MEG‐recorded Gamma oscillations in the 50–100 Hz range was larger in response to up‐right than inverted faces [Dobel et al., 2011], suggesting that this neural manifestation is sensitive to the configuration of the face. This new finding corroborates several previous MEG Gamma studies outside the face‐processing domain which showed that coherent visual objects could induce strong and sustained Gamma oscillations [Gruber et al., 2008; Hoogenboom et al., 2006; Kaiser et al., 2004].
Studying MEG oscillations (particularly in the Gamma band) during the encoding of faces and face components might reveal aspects of face perception which are not evident in the modulation of the M170 potential. Illustrating this possibility, several EEG studies demonstrated dissociations as well as associations between the mechanisms reflected in the N170 component and in EEG oscillations. For example, Bentin and coworkers found that the EEG oscillations in the Gamma band (>20 Hz) were of higher amplitude when the face was normally configured than when its inner features were scrambled [Zion‐Golumbic and Bentin, 2007], higher in response to upright than inverted faces [Anaki et al., 2007], and higher for famous than unfamiliar faces [Anaki et al., 2007; Zion‐Golumbic et al., 2008]. This contrasted with the demonstrated N170 sensitivity to face features as well as their global shape and insensitivity to the configuration of the inner face components or to the face identity. Based on these results, Bentin and coworkers suggested that the N170 is primarily associated with the detection and categorization of faces at an early processing stage, whereas Gamma fluctuations reflect the activation of an integrated face representation at a later stage. Another study reported by this group showed that face memory modulates EEG oscillations in different frequency ranges between 4 and 16 Hz whereas Gamma seems to be specifically associated with the reactivation of perceptual semantic representations during the formation of new episodic traces [Zion‐Golumbic et al., 2010].
Whereas these EEG studies were insightful, additional evidence is required to better understand the roles of the face‐perception mechanism manifested by the Gamma oscillations and compare it with the mechanism reflected by the evoked response (N170/M170). First, in some of these studies the neural activity affecting the EEG oscillations (particularly in the Gamma band) was contaminated with other electrical sources, particularly microsaccades, which are augmented by using of a nose reference [Keren et al., 2010a; Yuval‐Greenberg et al., 2008]. Second, these studies did not attempt to unveil the intracranial sources of the face‐related Gamma oscillations. Third, the EEG is predominantly sensitive to neural activity originating in radially oriented sources in human brain; to more comprehensively understand how faces are processed in the human brain, it is necessary to also examine the activity from tangentially oriented neural sources.
MEG can help solving the above three problems. First, the magnetic signal originating from the human brain is reference‐free and considerably less affected by muscular artifacts from microsaccades and other eye movements [Keren et al., 2010b]. Second, magnetic signals recorded on the scalp are less spatially smeared or attenuated by the cerebrospinal fluid, skull and scalp as is the scalp EEG activity. Therefore, coupled with modern high‐density detector grids covering the whole head, MEG provides reliable solutions to the distribution of intracranial sources of neural activity. Third, MEG is predominantly sensitive to tangentially oriented neural sources rather to the radial ones. Taking advantage of these benefits, in the current study we adopted the experimental design used in Zion‐Glumbic and Bentin (2007) and assessed the relative sensitivity of the MEG‐recorded Gamma oscillations as well as the M170 to global, featural and configural processing of faces and neural sources. Based on these data we sought to shed additional light on the neural systems accounting for the face processing in humans.
METHODS
Participants
Twenty‐four students (14 men and 10 women, 29.13 years old on the average) from Bar‐Ilan University and Hebrew University of Jerusalem were paid to participate in the experiment. All had normal or corrected‐to‐normal vision and no history of neurological disorders. They signed an informed consent to participate, and all procedures were in compliance with the Code of Ethics of the World Medical Association (Declaration of Helsinki), as well as approved by the Research Ethics Board of the two participating universities. Six participants (4 men) were excused due to excessive movement artifacts (over 1 cm), and four additional participants (1 man) were removed only from the source localization since head movements extended over 0.5 cm. Hence, the MEG analysis was based on 18 participants and the source analysis on 14 participants.
Stimuli and Apparatus
The stimuli were different transformations of 80 photographs of different faces, 80 photographs of different watches, and 120 photographs of different flowers. The faces were edited to form four stimulus conditions with 80 different stimuli in each condition: normally‐configured full‐faces, scrambled full‐faces (the spatial location of the inner components was scrambled within a normal face‐contour), normally‐configured inner components (presented without the face‐contour), and scrambled inner components (presented without the face‐contour). The watches were edited to form two conditions with 80 different stimuli in each condition: normally‐configured watches and scrambled watches (see Fig. 1 for examples).
Figure 1.
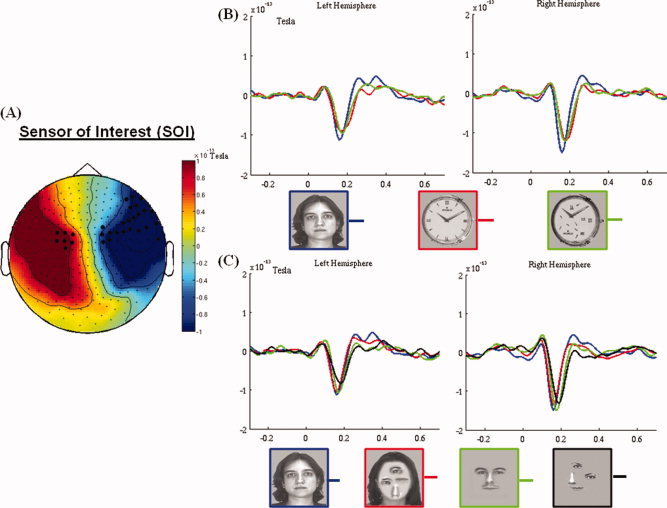
(A) The spatial distribution of M170. The black dots show the sensors where the M170 amplitude is significantly larger (P < 0.05) for normally configured faces than for normally configured watches in the time window of 140–180 ms. (B) M170 elicited by faces, watches and scrambled watches show face sensitivity. (C) The modulation of the M170 by scrambling the inner components with and without the face contour. Note the sensitivity of the M170 to the global face configuration. The M170 components in B and C are averaged over the face‐sensitive sensors in each hemisphere. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
The normally‐configured and scrambled stimuli sets for each category were equated for luminance, and contrast, and had a similar distribution of energy across spatial frequencies. The stimuli were presented at the center of the screen and seen from a distance of ∼ 50 cm, occupied 11.5° × 16° in the visual field.
Task and Procedure
The task was an oddball target monitoring in which stimuli from different experimental categories were presented one after another and participants were requested to press a button when a rarely presented flower appeared on the screen. This procedure ensured that all stimulus categories of interest in this study were equally task‐relevant (indeed, all irrelevant distracters). The 600 stimuli were fully randomized and presented in six blocks of 100 stimuli each, with a short (1–3 minutes) break between blocks for refreshment. Each stimulus was presented for 700 ms with interstimulus intervals varying between 500 and 1,250 ms.
MEG Recording
MEG recordings were conducted with a whole‐head, 248‐channel magnetometer array (Magnes 3600 WH, 4D Neuroimaging) in a magnetically shielded room. Reference coils, which were located ∼ 30 cm above the head and oriented by the x, y and z axis, were used to remove environmental noise. Participants laid supine with their head in the MEG helmet, inside a dimly lit room. Before data acquisition, three localization coils were placed at the two preauricular points and nasion to localize the participant's head relative to the MEG sensors. The system allowed head localization to the precision of 1 mm. Head localization was completed before and after the experiment, to confirm that participants remained still throughout the session (smaller than 5 mm of motion). The MEG was digitized at a rate of 1,017 Hz and low‐pass filtered online at 400 Hz. An additional channel recorded the 50 Hz signal from the power outlet and the average power‐line response to a power cycle was subtracted from every MEG sensor. This allowed cleaning the 50 Hz line‐power noise and its harmonics without using a notch‐filter. Stimuli were back‐projected on a screen placed in front of the subjects, by a video projector situated outside the room. E‐prime (Psychology Software Tools Inc.) was used for experimental control. Participants pressed a button on a response‐pad each time a flower was presented (20% of the trials).
MEG Analysis for M170 and Gamma Activity
Data analysis was performed in MATLAB (Mathworks, Andover, MA) using the Fieldtrip toolbox [Oostenveld et al., 2011]. MEG data were band‐pass filtered offline between 0.8 and 80 Hz, and segmented from 500 ms prestimulus presentation to 1,000 ms post‐stimulus. This long time window was used to avoid the edge‐effect in the wavelet analysis. Epochs contaminated by muscle activity, large drift, and jump artifacts in the SQUIDs were first removed, then eye‐movements and blinks were corrected by using an ICA procedure [Jung et al., 2000]. The segmented data of all conditions were first down‐sampled to 300 Hz to speed up the data decomposition. Then they were decomposed into a set of independent components, from which components reflecting eye‐movement induced artifacts were determined by visual inspection of the 2D component scalp maps and components time courses. These components were nullified and the predown‐sampled data reconstructed. Two malfunctioning MEG sensors (A74 and A204) were discarded from all analyses.
M170 Analysis
The averaged MEG waveforms were smoothed by applying a low‐pass filter of 17 Hz (24 dB) and baseline‐corrected using an epoch between –300 and –100 ms before stimulus onset. Due to the nature of the magnetic field generated by electric currents in the brain, the magnetic fields in the two hemisphere were reversed in polarity, that is, a magnetic minimum was measured over the right whereas a magnetic maximum was measured over the left hemisphere [usually the magnetic minimum is denoted by a negative polarity; Harris and Aguirre, 2008 see Fig. 1A]. For purposes of comparison between the two hemispheres and comparing with previous N170 studies, amplitudes for the sensors in the left hemisphere were multiplied by −1 to correct for this polarity difference.
The analysis of the M170 began by selecting sensors that showed face‐sensitivity. To locate such sensors we compared the M170 elicited by normally configured faces and normally configured watches at each of the remaining 246 sensors. To control for Type‐I error rate due to the multiple comparisons over sensors we ran a spatial cluster‐based permutation test over all the sensors. That is, for each participant we randomly reassigned values to conditions and reran the relevant t tests. This procedure was repeated 1,000 times, yielding a data‐driven distribution of t values. The significance levels reported are relative to this distribution, which has been corrected by a cluster‐based test statistic [Maris and Oostenveld, 2007]. A cluster is defined by at least 2 adjacent sensors reaching the predetermined significant level (P < 0.05). The dependent variable for this analysis was the mean amplitude recorded at each sensor between a time‐window of 140–180 ms.1 Sensors showing a significantly higher amplitude for faces than for watches were considered face‐sensitive and selected for further analysis. This procedure yielded two clusters (see the dark points in Fig. 1A). Finally, a representative sensor was constructed for each cluster by averaging all the sensors in a cluster, and the M170 was analyzed based on the two representative sensors.
The statistical evaluation of the experimental manipulations compared the amplitudes of the M170 averaged across the face‐sensitive clusters in the left and in the right hemisphere in each experimental condition. This amplitude was automatically determined as the most negative peak between 130 and 230 ms followed by visual scrutiny to ensure the negative values representing real peaks. The larger epoch used to calculate these amplitudes was necessary because the range of the M170 latencies across the six experimental conditions was larger than for the normally configured faces and watches. A Stimulus‐type (full faces, inner components, watches) × Configuration (normally configured, scrambled) × Hemisphere (right, left) ANOVA with repeated measures was conducted for M170 peak amplitudes and for its latencies. For factors with more than two levels, the degrees of freedom were corrected when necessary using the Greenhouse‐Geisser epsilon. Significant main effects and interactions were followed by post hoc contrasts or by subsequent 1‐way ANOVAs for each level of the interacting factors.
Analysis of Gamma Oscillations
Time‐frequency representations (TFRs) of the spectral power were computed using a Gaussian Morlet wavelet method with an adaptive time window of eight cycles for each frequency (ΔT = 8/f). This procedure was applied to frequencies ranging from 20 to 80 Hz in steps of 1 Hz, and the results were baseline corrected by subtracting the mean power value of the time between −300 and −100 ms prior the stimulus onset from the post‐stimulus power at each frequency. The induced Gamma oscillations were calculated by applying the wavelet analysis to individual trials and averaging the time‐frequency plots.
For the statistical analysis of the induced TFRs, we defined a “window of interest” (WOI), which was limited by the stimulus exposure time (700 ms) but excluding the activity evoked by the onset and the offset of the stimuli on the screen. Based on these criteria the predetermined WOI was 230–570 ms (Fig. 3b).
Figure 3.
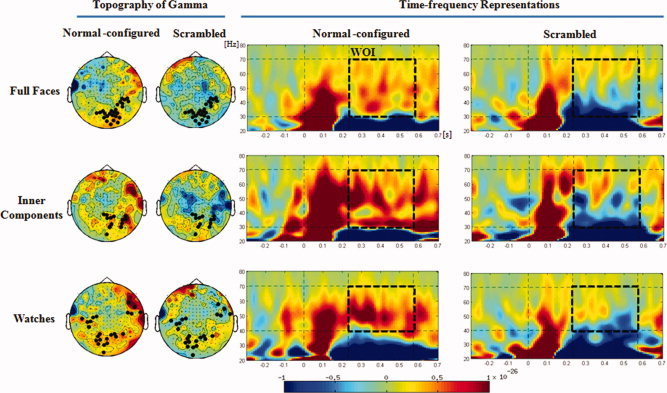
Time‐frequency representations of induced MEG oscillations elicited by each of the six types of stimuli, and their topography. The black dots in the topography show the sensors where Gamma oscillations are significantly higher in the normally configured condition than in the scrambled condition. Note that whereas physiognomic stimuli elicited Gamma higher than the baseline in the entire frequency range, in the lower range (30–40 Hz) the Gamma elicited by watches was lower than the baseline. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
To locate sensors sensitive to the configuration of the stimulus we compared the average Gamma amplitude elicited by normally configured and scrambled stimuli within the WOI separately for each category using cluster‐based permutation tests at each sensor. As aforementioned, this procedure was repeated 1,000 times, yielding a data‐driven distribution of t values. The significance levels reported are relative to this distribution, which has been corrected by cluster‐based test statistic [Maris and Oostenveld, 2007]. The sensitivity of Gamma oscillations to the stimulus type was assessed comparing the mean Gamma amplitude recorded at each sensor during the WOI in response to normally configured stimuli (see additional details in the Results section).
Source Space Analysis
Source estimation for both the M170 and the Gamma oscillations was based on Synthetic Aperture Magnetometry (SAM) beamformer approach [Robinson and Vrba, 1999; http:/kurage.nimh.nih.gov/meglab/Meg/SAM2]. SAM is a nonlinear minimum‐variance beamformer algorithm. It uses the signal covariance calculated from the magnetic signals recorded at MEG sensors to construct optimum spatial filters at each voxel in the brain, by minimizing the correlations with all other analyzed voxels. Here the SAM beamformer estimated the source power for each voxel on a local sphere head model in 5‐mm steps, which resulted in a 3D spatial distribution of the power of the neuronal sources. Since we did not have the structural MRIs of our participants, a template MRI [Colin27 average, Holmes et al., 1998] was adjusted to each individual head shape using SPM8 (http://www.fil.ion.ucl.ac.uk/spm). The individual SAM 3D distributions were overlaid on the adjusted MRIs, normalized to Talairach space for group analysis which was conducted by using Analysis of Functional NeuroImages (AFNI; see http://afni.nimh.nih.gov/afni). The choice of the Colin template instead of MNI brain was due to practical considerations: AFNI statistics is based on Talairach coordinates, which is natural to the Colin template but is hard with the MNI template. Dual‐state images were calculated by dividing integrated power of a defined WOI for each condition by integrated power over a time window of defined baseline (pseudo‐F ratios; details please see the results for M170 and Gamma), yielding estimates of relative increases (or decreases) for each condition.
To be specific, for each participant we first calculated a global covariance matrix adopting the segmented data from −100 to 600 ms relative to the onset of stimuli across the six types of stimuli. The segmented data was band‐pass filtered (1–17 Hz for M170, 30–70 Hz, and 40–70 Hz for Gamma elicited by physiognomic stimuli and watches, respectively – see results). The beamformer weights were calculated based on the above covariance matrix calculated using data from all trials. Then for M170 an event‐related SAM (SAMerf) was performed for each stimulus type, which generated source images of M170 based on the average waveform at each voxel location for each participant. To reveal the face‐sensitive sources and to allow comparison with previous findings, the source images of M170 from normally configured full faces, scrambled full faces, normally configured inner components, and scrambled inner components were compared to normally configured watches separately for each stimulus type by running paired t‐tests (using the 3dtest module in AFNI). The sources of Gamma oscillations were assessed by applying induced SAM, which compared the activity within the WOI with a prestimulus onset baseline. This analysis was followed by t‐tests over all participants aimed at examining which voxel (if any) was activated significantly above zero. All the resulting images were further corrected for multiple comparisons based on Monte Carlo simulation of random noise distribution (using 3dClustSim module of AFNI, Forman et al., 1995).
RESULTS
M170 Effects
A conspicuous M170 was measured at lateral temporal sensors bilaterally in response to all six stimulus categories (Fig. 1). Yet, the number of sensors showing significantly larger M170 amplitude in response to faces than watches was larger over the right than the left hemisphere; specifically, whereas at medial sites face‐sensitivity was found bilaterally in a similar number of sensors, at lateral sites face sensitivity was found only for right hemisphere sensors (Fig. 1A)
The three‐way ANOVA of the peak amplitude showed that, on the average, the M170 was more robust at right hemisphere than at left hemisphere sensors [F(1,17) = 9.8, P < 0.01]. There was no main effect of Stimulus‐type [F(2,34) = 2.5; P = 0.095], but a significant Stimulus‐type x Hemisphere interaction [F(2,34) = 3.92; P < 0.05] indicated that the M170 amplitude was modulated differently by Stimulus‐type in each hemisphere. The interaction was further investigated by separate analyses of the Stimulus‐type effect at each hemisphere by one‐way ANOVAs. This analysis showed that the M170 amplitude was significantly modulated by Stimulus‐type in the right hemisphere [F(2,34) = 5.2; P < 0.025], but not in the left hemisphere [F(2,34) < 1]. Post hoc contrasts limited to the right hemisphere showed that full faces and inner components elicited similar M170 potentials (P = 0.417), both significantly higher than that elicited by watches (P < 0.05, for both comparisons). The main effect of Configuration was significant [F(1,17) = 14.1, P < 0.01], but it was also modulated by Stimulus‐type [Configuration x Stimulus‐type interaction, F(2,34) = 3.4, P < 0.05]. Post hoc comparisons showed that the amplitude was larger for normally configured inner components than for scrambled inner components [P < 0.01], and slightly larger for normally configured than scrambled faces [P = 0.057]. In contrast, Configuration had no effect on the M170 elicited by watches [P = 0.83]. Finally, one‐way ANOVAs followed by post hoc contrasts showed that for normally configured stimuli there was no difference between the M170 elicited by full faces and inner components [P = 0.72], and both were significantly larger than the M170 elicited by watches [P < 0.05, for both comparisons]. In contrast, for scrambled stimuli, the M170 amplitude was considerably larger for faces than for inner components as well as for watches [P < 0.05, for all comparisons], and there was no difference between the latter two stimulus types [P = 0.99].
A similar analysis conducted on the latency of M170 yielded a significant main effect of Stimulus‐type [F(2,34) = 24.6, P < 0.001], which was modulated by Configuration [Stimulus‐type × Configuration interaction, F(2,34) = 7.6, P < 0.01]. Post hoc contrasts showed that the M170 elicited by normally configured inner components peaked earlier (177 ms) than that elicited by scrambled inner components [185 ms, P < 0.05]. In contrast, there were no Configuration effects for full faces and watches [P = 0.23 and P = 0.066, respectively]. The main effect of Hemisphere was significant [F(1,17) = 9.5, P = 0.007], indicating that the peak of M170 was earlier in the left hemisphere (170 ms) than in the right hemisphere (176 ms). There were no additional significant effects.
Sources of M170
The active windows used for estimating the sources of the M170 by SAMerf were 100 ms long, located during the time course of the MEG so that they included the peak of the M170 component for all participants. Based on these guidelines the widows for full faces and watches encompassed the epoch of 100–200 ms from stimulus onset, whereas for the inner components it was between 130 and 230 ms. A control window of −150 to −50 ms was used as baseline.
To assess face‐sensitive sources of the M170, we looked for voxels at which the activity elicited by faces during the predetermined time window exceeded the activity elicited by watches. The distribution of these regions for each stimulus type condition is presented in Figure 2, and the brain structures included in those regions are listed in Table 1.
Figure 2.
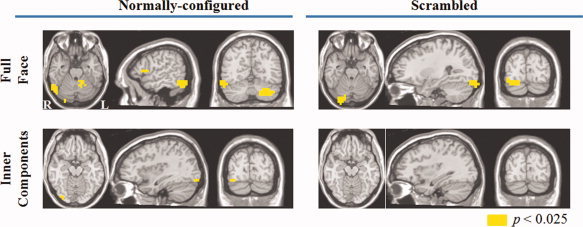
Neural sources of the M170 elicited by normally configured faces, normally configured inner components, scrambled faces and scrambled inner components. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table I.
Source solutions for M170
| Stimulus | Talairach coordinates | Neural substrates | ||
|---|---|---|---|---|
| x | y | z | ||
| Normally configured full face | 53 | −57 | −18 | Right fusiform gyrus |
| 38 | −84 | −10 | Right inferior occipital gyrus | |
| 58 | −57 | −10 | Right inferior temporal gyrus | |
| 23 | −67 | −6 | Right lingual gyrus | |
| Scrambled full face | 28 | −92 | −18 | Right fusiform gyrus |
| 28 | −89 | −14 | Right inferior occipital gyrus | |
| 53 | −62 | 27 | Right middle temporal gyrus | |
| 46 | −60 | 32 | Right angular gyrus | |
| 28 | −74 | −10 | Right lingual gyrus | |
| Normally configured inner components | 32 | −82 | −12 | Right fusiform gyrus |
| 33 | −92 | −13 | Right inferior occipital gyrus | |
| 36 | −78 | −8 | Right middle occipital gyrus | |
| 63 | −47 | 7 | Right middle temporal gyrus | |
| Scrambled inner components | N/A | N/A | ||
Normally configured faces and inner components, as well as scrambled faces elicited significantly larger activity than watches in several posterior lateral and ventro‐temporal regions, but only in the right hemisphere. In contrast, there was no region in the brain in which the activity elicited by scrambled inner components during the M170 time window differed from that elicited by watches (Table 1).
Specifically, the analysis showed that the activity in the right fusiform gyrus and right inferior occipital gyrus was sensitive to faces regardless of their configuration as well as to inner components, but only if the latter were normally configured. The right inferior temporal gyrus was sensitive only to normally configured faces.
Gamma Oscillations
Conspicuous and sustained Gamma oscillations were observed during the WOI for all the three categories of normally configured stimuli particularly at sensors covering the posterior scalp (Fig. 3). For physiognomic stimuli, sensors showing Gamma oscillations were more abundant in the right hemisphere than in the left, whereas for watches the distribution of active sensors was more bilateral and included also a few more anterior clusters (Fig. 3a). This activity ranged between 30 and 70 Hz for faces and inner components and between 40 and 70 Hz for watches. Indeed, between 30 and 40 Hz, the MEG activity in the Gamma‐band elicited by watches was deactivated relative to the baseline. The Gamma elicited by scrambled stimuli was considerably weaker and limited to the higher parts of this frequency range.
In the 30–70 Hz range, the permutation tests revealed that the difference between normally configured and scrambled physiognomic stimuli was significant (P < 0.05 for both faces and inner components). Limiting the analysis to the range of 40–70 Hz, there was no difference among the three types of normal‐configured stimuli [all P values >0.2] and for all three categories the amplitude of Gamma was higher for normally configured than for scrambled stimuli (P = 0.068 for faces, P = 0.05 for inner components and P = 0.065 for watches).
Sources of the Oscillation Between 30 and 70 Hz
We used induced SAM to assess brain regions where the amplitude of Gamma oscillations was significantly larger compared to baseline. This analysis was restricted to MEG elicited by normally configured stimuli and focused on the 30–70 Hz range for faces and inner components and 40–70 Hz range for watches. The time window used for this analysis was 230–570 ms and the activity during this epoch was compared to an equally long baseline lasting from −440 ms to −100 ms.
As illustrated in Figure 4 and detailed in Table 2, the induced SAM solutions revealed several brain structures where the amplitude of Gamma oscillations were significantly stronger than baseline (P < 0.005) and, therefore, seemed to be sources of the MEG‐recorded Gamma. For faces, almost all the sources of Gamma were in the right hemisphere. Some of these sources contributed, in fact, to the Gamma oscillations elicited by all three categories (right fusiform gyrus, right cuneus, right lingual gyrus, right middle occipital gyrus, and left middle occipital gyrus). Other sources, particularly the left cuneus, contributed to Gamma elicited by inner components and watches, but not that elicited by faces (Table 2).
Figure 4.
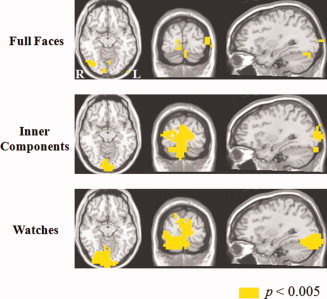
Neural sources of Gamma oscillations elicited by normally configured faces, inner components and watches. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Table II.
Source solutions for gamma oscillation
| Stimulus | Talairach coordinates | Neural substrates | ||
|---|---|---|---|---|
| x | y | z | ||
| Normally configured full face | 33 | −72 | −14 | Right fusiform gyrus |
| 14 | −92 | 8 | Right cuneus | |
| 8 | −92 | −8 | Right lingual gyrus | |
| 43 | −68 | −8 | Right middle occipital gyrus | |
| 37 | −72 | −3 | Right inferior occipital gyrus | |
| −42 | −87 | 2 | Left middle occipital gyrus | |
| Normally configured inner components | 21 | −91 | −18 | Right fusiform gyrus |
| 3 | −102 | −3 | Right cuneus | |
| 5 | −97 | −14 | Right lingual gyrus | |
| 30 | −97 | 9 | Right middle occipital gyrus | |
| −3 | −102 | −3 | Left cuneus | |
| −9 | −90 | 14 | Left middle occipital gyrus | |
| Normally configured watch | 40 | −61 | −13 | Right fusiform gyrus |
| 2 | −102 | −3 | Right cuneus | |
| 10 | −88 | −12 | Right lingual gyrus | |
| 12 | −100 | 18 | Right middle occipital gyrus | |
| 29 | −95 | −8 | Right inferior occipital gyrus | |
| −2 | −102 | −3 | Left cuneus | |
| −20 | −100 | 7 | Left middle occipital gyrus | |
DISCUSSION
In the current study we used MEG to investigate the neural mechanisms underlying face perception. We determined the sites at which the M170 sensitivity to faces was evident, and explored modulations of this component as well as the modulations of MEG amplitude in the Gamma range (30–70 Hz) to unveil the perceptual mechanisms associated to these neural manifestations of face processing. Specifically, we focused on their sensitivity to a face's gestalt and to individual face components by manipulating the spatial organization of the inner components within the face contour and in the absence of the face contour. The results indicated that the presence or absence of the contour and the spatial organization of the face components affected the M170 and the MEG Gamma oscillations differently, suggesting the involvement of different neural mechanisms as the processing of the face proceeds. Peaking at around 160 ms from stimulus onset, the M170 was modulated by the configuration of the physiognomic stimuli as well as by the existence or absence of the face contour. The M170 was not sensitive to the spatial organization of watches. In contrast, Gamma was sensitive primarily to the stimulus configuration starting at about 230 ms with less sensitivity to the stimulus type. Taking advantage of the good spatial resolution of MEG, we distinguished intracranial sources of the neural activity associated with face processing at the categorical level and neural activity associated with processing a visual stimulus' configuration regardless of its category.
The M170 recorded at a cluster of sensors with probable sources in the right ventro‐temporal cortex showed the typical higher amplitude in response to faces than to nonface stimuli (watches). We did not find face sensitivity at any earlier latency, which stands in contrast to several previous studies in which MEG distinguished between faces and nonface stimulus categories as early as 100 ms [the M100; e.g., Halgren et al., 2000 using a magnetometer, and Itier et al., 2006; Liu et al., 2002; Susac et al., 2009, 2010; Taylor et al., 2011 using a gradiometer]. On the other hand, the current data join other previous publications in which, like here, the first MEG component that distinguished faces from other stimuli was the M170 [Watanabe et al., 1999, using a magnetometer; for a review see Watanabe et al., 2005].2
Given the fairly similar distribution of the M170 and the N170, it is tempting to assume that these MEG and EEG components are manifestations of the same intracranial sources, reflecting similar perceptual mechanisms, which probably differ at the different latencies. In fact there are studies suggesting that the M170 and the N170 might have the same sources [e.g., Deffke et al., 2007, who used a gradiometer to record the MEG]. However, the present results provide only moderate support to the hypothesis that these two neural manifestations are associated with the same perceptual mechanism. Whereas the peaks of both N170 and M170 are delayed by manipulations altering the canonical gestalt of the face (by inversion, scrambling or isolating inner components), the modulation of their amplitudes by the experimental variables used in this study differed. The N170 was larger when inner components are presented outside of the face contour and was not affected by the configuration of the face [Daniel and Bentin, 2010; Zion‐Golumbic and Bentin, 2007]. In contrast, the amplitude of the M170 was reduced when the spatial configuration of the inner components was scrambled and was not affected by removing the face contour. Further, unlike the consistent face‐inversion effects on the N170, the amplitude of the M170 is not affected by face inversion [Harris and Nakayama, 2007; Itier et al., 2006; Taylor et al., 201, but see Kloth et al., 2006, all using gradiometers]. Hence, our ad hoc conclusion is that both these components are associated with basic level categorization of faces, based on a rapid detection of global features [e.g., Bentin et al., 2006; Hole, 1994; Maurer et al., 2002; Young et al., 1987]. Yet, the N170 seems to be slightly more sensitive than the M170 to individual facial components, whereas the M170 seems to be slightly more sensitive than the N170 to their configuration. It is possible that this difference is due to the fact that although the N170 and the M170 are generated in roughly the same brain regions, they reflect the activity of different dipoles. The EEG primarily reflects activation originated from the radial‐orientated dipoles while the MEG predominantly tracks the activation coming from the tangential‐oriented dipoles. However, whereas this direct comparison between M170 and N170 is insightful its outcome should be treated with caution because the conductivity of head model compartments for EEG and MEG is different.
In contrast to the M170, the MEG activity in the Gamma band was affected mostly (but not exclusively) by the configuration of stimuli. In a relatively lower Gamma range (30–40 Hz), the present results replicated the pattern of EEG Gamma modulations described by Zion‐Golumbic and Bentin (2007), roughly in the same frequency range (25–45 Hz). That is, the amplitude of MEG activity in the Gamma range elicited by physiognomic stimuli was higher than that elicited by watches, there was no difference between normally configured full faces and normally configured face components while scrambling the configuration of the physiognomic stimuli reduced the Gamma amplitude significantly. Importantly, unlike the EEG Gamma oscillations, which were probably contaminated by microsaccades [Yuval‐Greenberg et al., 2008], the currently recorded MEG activity was probably less influenced by eye movements, if at all [Dobel et al., 2011; Keren et al., 2010b]. The difference between the time‐frequency patterns of the EEG‐ and MEG‐recorded Gamma support this hypothesis: Whereas in the EEG study the induced Gamma oscillations lasted about 100 ms and their frequency range was wide (20–80 Hz), the MEG Gamma was limited to a relatively narrower range (30–70 Hz) and were sustained for at least 340 ms. These patterns suggest that in the Zion‐Golumbic and Bentin (2007) study the real EEG activity in the Gamma band was partly veiled by a strong spike (putatively reflecting the microsaccades), whereas the MEG oscillations in the current study were not.
In the 40–70 Hz range the MEG Gamma was sensitive to the stimulus configuration but not to the stimulus category. That is, the Gamma amplitude elicited by normally configured faces and face components was not different than that elicited by watches; however, all coherent, meaningful stimuli elicited higher Gamma amplitudes than scrambled stimuli. Considering the tentative pattern of EEG‐Gamma modulation by stimulus category and configuration together with the current MEG‐Gamma modulations, we suggest that the neural oscillations in the Gamma range manifest the integration of coherent visual input into a previously existent perceptual representation [Anaki et al., 2007; Bertrand and Tallon‐Baudry, 2000; Tallon‐Baudry and Bertrand, 1999; Zion‐Golumbic et al., 2008].
The current MEG Gamma results support a functional connection between Gamma and N250r [see Gruber and Muller, 2005]. There is ample evidence that the amplitude N250r is higher in response to repeated than nonrepeated stimuli over inferior occipitotemporal regions [Schweinberger, 2011; Schweinberger et al., 2004, 2007]. Previous studies have demonstrated that both induced EEG Gamma and N250r are sensitive to repetition starting at around 230 ms in the posterior region [e.g., Gruber et al., 2004; Gruber and Muller, 2002, 2005; Schweinberger, 2011; Schweinberger et al., 2004, 2007]. Moreover, both are more prominent in response to familiar compared to unknown faces; and higher in response to upright compared to inverted full faces and nonface stimuli [Anaki et al., 2007; Zion‐Golumbic and Bentin, 2007 for EEG Gamma; Schweinberger et al., 2004, 2007 for N250r]. The current MEG Gamma results corroborate the above EEG findings by showing a strong activation for normally configured—familiar stimuli starting at 230 ms with a predominantly posterior distribution. Moreover, similar to the N250r, the 30–40 Hz Gamma exhibited a face‐selective morphology in a similar time range. Yet, we notice that in the present study this observation is post hoc and, therefore, the functional relation between Gamma and N250r should be addressed in future studies.
Consistent with previous fMRI and MEG studies [Deffke et al., 2007; Halgren et al., 2000; Haxby et al., 2000; Ishai et al., 2005; Itier et al., 2006; Japee et al., 2009; Pitcher et al., 2011; Taylor et al., 2011], the sources of the M170 were located in the fusiform, inferior occipital and lingual gyri but only in the right hemisphere. Although this finding is in line with the well established right lateralization of face processing and, at medial sites face‐sensitivity seemed to be more bilateral, the absence of clear face‐sensitive activity in the left hemisphere is intriguing, given that such activity has been reported before (e.g., Dobel et al., 2008; Harris and Nakayama, 2007). It is possible that this pattern reflects shortcomings of the beamformer algorithm. Particularly, the currently algorithm does not provide a satisfactory solution to highly correlated activities between voxels. This weakness might have veiled the correlated activities between left and right hemisphere, or make the weaker neural activity in the left hemisphere less consistent leading to a Type‐II error.
Interestingly, focusing on faces, we observe that almost all the sources of M170 were also sources of Gamma, while there were a few sources of Gamma that were not associated with the generation of the M170. These additional sources of Gamma were in the right middle occipital gyrus, the right cuneus and the left middle occipital gyrus. The additional sources as well as the different modulations of the evoked M170 and the induced Gamma oscillations by the factors manipulated in this study, suggest that these two neural manifestations of processing physiognomic stimuli reflect different perceptual mechanisms and different stages in a multiple‐levels face processing, albeit Gamma oscillations might not be specific to faces.
The functional dissociation of the M170 and the Gamma oscillations was also evident in two recent intracranial EEG recording studies [Engell and McCarthy, 2010, 2011; see also Vidal, 2010]. Recording electrocorticogram from subdural electrodes in the ventral occipito‐temporal cortex of epileptic patients, Engell and McCarthy found that whereas Gamma oscillations were modulated by selective attention, the intracranial face‐selective N200 (which might be considered analogous to the M170) was not. Furthermore, they found that whereas both line‐drawn faces and face photographs evoked conspicuous N200 components, which were larger than those elicited by Greebles, there was no difference between the Gamma oscillations elicited by line‐drawn faces and Greebles, both significantly smaller than the Gamma oscillations elicited by face photographs. Based on these results, the authors suggested that the face‐N200 tracked an obligatory response to the perception of a face, possibly related to structural encoding, whereas the Gamma oscillations reflected more elaborative processing of faces instead of responding to face features. Using MEG and a different set of experimental manipulations we replicated the dissociation between the face‐sensitive evoked potentials (be it M170, N170, or N200) and the induced face‐sensitive Gamma oscillations beyond the limited recording sites imposed by the clinical recording setup. On the one hand, the M170 sensitivity to the face contour, which was not seen in Gamma and the earlier onset of the M170 relative to the induced Gamma, suggest that the M170 is associated with a face perception mechanism based on the global characteristics of faces. On the other hand, the higher sensitivity of Gamma to spatially scrambling of the stimuli relative to that of the M170, as well as the relatively similar Gamma amplitudes for physiognomic stimuli and watches, suggest that these MEG oscillations are associated with the integration of visual input (regardless of stimulus type) into a pre‐existent coherent perceptual representation [cf., Gruber et al., 2008]. Together, the M170 and the induced Gamma reflect two functionally dissociated mechanisms in the cascade processing of faces, which might partake in a distributed neural network in charge of face processing [Haxby et al., 2000; Ishai et al., 2005; Liu et al., 2010; Pitcher et al., 2011].
Supporting information
Additional Supporting Information may be found in the online version of this article.
Suppporting Information
Footnotes
This time epoch was selected since the mean latency of M170 for normally configured faces and normally configured watches was 168 ms.
The analysis of the M100 is described and discussed in the Supporting Information.
REFERENCES
- Anaki D, Zion‐Golumbic E, Bentin S ( 2007): Electrophysiological neural mechanisms for detection, configural analysis and recognition of faces. Neuroimage 37: 1407–1416. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G ( 1996): Electrophysiological studies of face perception in humans. J Cogn Neurosci 8: 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Golland Y, Flevaris A, Robertson LC, Moscovitch M ( 2006): Processing the trees and the forest during initial stages of face perception: Electrophysiological evidence. J Cogn Neurosci 18: 1406–1421. [DOI] [PubMed] [Google Scholar]
- Bertrand O, Tallon‐Baudry C ( 2000): Oscillatory gamma activity in humans: A possible role for object representation. Int J Psychophysiol 38: 211–223. [DOI] [PubMed] [Google Scholar]
- Chen YH, Edgar JC, Holroyd T, Dammers J, Thonnessen H, Roberts TPL, Mathiak K ( 2010): Neuromagnetic oscillations to emotional faces and prosody. Eur J Neurosci 31: 1818–1827. [DOI] [PubMed] [Google Scholar]
- Daniel S, Bentin S ( 2012): Age‐related changes in processing faces from detection to identification: ERP evidence. Neurobiol. Aging, 33, e1–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deffke I, Sander T, Heidenreich J, Sommer W, Curio G, Trahms L, Lueschow A ( 2007): MEG/EEG sources of the 170‐ms response to faces are co‐localized in the fusiform gyrus. Neuroimage 35: 1495–1501. [DOI] [PubMed] [Google Scholar]
- Dobel C, Putsche C, Zwitserlood P, Junghofer M ( 2008): Early left‐hemispheric dysfunction of face processing in congenital prosopagnosia: An MEG study. PLoS One 3: e2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobel C, Junghöfer M, Gruber T ( 2011): The role of gamma‐band activity in the representation of faces: Reduced activity in the fusiform face area in congenital prosopagnosia. PLoS ONE 6:e19550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchaine BC, Nakayama K ( 2006): Developmental prosopagnosia: A window to content‐specific face processing. Curr Opin Neurobiol 16: 166–173. [DOI] [PubMed] [Google Scholar]
- Engell AD, McCarthy G ( 2010): Selective attention modulates face‐specific induced gamma oscillations recorded from ventral occipitotemporal cortex. J Neurosci 30: 8780–8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engell AD, McCarthy G ( 2011): The relationship of gamma oscillations and face‐specific ERPs recorded subdurally from occipitotemporal cortex. Cereb Cortex 21: 1213–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Gruber T, Muller MM ( 2005): Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb Cortex 15: 109. [DOI] [PubMed] [Google Scholar]
- Gruber T, Maess B, Trujillo‐Barreto NJ, Muller MM ( 2008): Sources of synchronized induced Gamma‐Band responses during a simple object recognition task: A replication study in human MEG. Brain Res 1196: 74–84. [DOI] [PubMed] [Google Scholar]
- Guderian S, Duzel E ( 2005): Induced theta oscillations mediate large‐scale synchrony with mediotemporal areas during recollection in humans. Hippocampus 15: 901–912. [DOI] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmaki V, Hari R ( 2000): Cognitive response profile of the human fusiform face area as determined by MEG. Cereb Cortex 10: 69–81. [DOI] [PubMed] [Google Scholar]
- Harris AM, Duchaine BC, Nakayama K ( 2005): Normal and abnormal face selectivity of the M170 response in developmental prosopagnosics. Neuropsychologia 43: 2125–2136. [DOI] [PubMed] [Google Scholar]
- Harris A, Nakayama K ( 2007): Rapid face‐selective adaptation of an early extrastriate component in MEG. Cereb Cortex 17: 63–70. [DOI] [PubMed] [Google Scholar]
- Harris AM, Aguirre GK ( 2008): The effects of parts, wholes, and familiarity on face‐selective responses in MEG. J Vis 8: 4 1–12. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI ( 2000): The distributed human neural system for face perception. Trends Cogn Sci 4: 223–233. [DOI] [PubMed] [Google Scholar]
- Henson RN, Mattout J, Singh KD, Barnes GR, Hillebrand A, Friston K ( 2007): Population‐level inferences for distributed MEG source localization under multiple constraints: Application to face‐evoked fields. Neuroimage 38: 422–438. [DOI] [PubMed] [Google Scholar]
- Herrmann MJ, Ehlis AC, Ellgring H, Fallgatter AJ ( 2005): Early stages (P100) of face perception in humans as measured with event‐related potentials (ERPs). J Neural Transm 112: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Hole GJ ( 1994): Configurations! factors in the perception of unfamiliar faces. Perception 23: 65–74. [DOI] [PubMed] [Google Scholar]
- Holmes CJ, Hoge R, Colin L, Woods R, Toga AW, Evans AC ( 1998): Enhancement of MR images using registration for signal averaging, J Comput Assist Tomogr 22: 324–333. [DOI] [PubMed] [Google Scholar]
- Hoogenboom N, Schoffelen JM, Oostenveld R, Parkes LM, Fries P ( 2006): Localizing human visual gamma‐band activity in frequency, time and space. Neuroimage 29: 764–773. [DOI] [PubMed] [Google Scholar]
- Ishai A, Schmidt CF, Boesiger P ( 2005): Face perception is mediated by a distributed cortical network. Brain Res Bull 67: 87–93. [DOI] [PubMed] [Google Scholar]
- Itier RJ, Herdman AT, George N, Cheyne D, Taylor MJ ( 2006): Inversion and contrast‐reversal effects on face processing assessed by MEG. Brain Res 1115: 108–120. [DOI] [PubMed] [Google Scholar]
- Japee S, Crocker L, Carver F, Pessoa L, Ungerleider LG ( 2009): Individual differences in valence modulation of face‐selective M170 response. Emotion 9: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jokisch D, Jensen O ( 2007): Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J Neurosci 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ ( 2000). Removal of eye activity artifacts from visual event‐related potentials in normal and clinical subjects. Clin Neurophysiol 111: 1745–1758. [DOI] [PubMed] [Google Scholar]
- Kanwisher N ( 2010): Functional specificity in the human brain: A window into the functional architecture of the mind. Proc Natl Acad Sci USA 107: 11163–11170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G ( 2006): The fusiform face area: A cortical region specialized for the perception of faces. Philos Trans R Soc Lond B Biol Sci 361: 2109–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser J, Buhler M, Lutzenberger W ( 2004): Magnetoencephalographic gamma‐band responses to illusory triangles in humans. Neuroimage 23: 551–560. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval‐Greenberg S, Deouell LY ( 2010a): Saccadic spike potentials in gamma‐band EEG: Characterization, detection and suppression. Neuroimage 49: 2248–2263. [DOI] [PubMed] [Google Scholar]
- Keren AS, Yuval‐Greenberg S, Gao Z, Bentin S, Deouell LY ( 2010b) Saccadic spike potentials in gamma‐band EEG and MEG: Characterization, detection and suppression. Presented at meeting of the Organization for Human Brain Mapping, Barcelona. June 6–10, 2010.
- Kloth N, Dobel C, Schweinberger SR, Zwitserlood P, Bolte J, Junghofer M ( 2006): Effects of personal familiarity on early neuromagnetic correlates of face perception. Eur J Neurosci 24: 3317–3321. [DOI] [PubMed] [Google Scholar]
- Latinus M, Taylor MJ ( 2006): Face processing stages: Impact of difficulty and the separation of effects. Brain Res 1123: 179–187. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N ( 2002): Stages of processing in face perception: An MEG study. Nat Neurosci 5: 910–916. [DOI] [PubMed] [Google Scholar]
- Liu J, Harris A, Kanwisher N ( 2010): Perception of face parts and face configurations: An FMRI study. J Cogn Neurosci 22: 203–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Higuchi M, Marantz A, Kanwisher N ( 2000): The selectivity of the occipitotemporal M170 for faces. Neuroreport 11: 337–341. [DOI] [PubMed] [Google Scholar]
- Lu ST, Hamalainen MS, Hari R, Ilmoniemi RJ, Lounasmaa OV, Sams M, Vilkman V ( 1991): Seeing faces activates three separate areas outside the occipital visual cortex in man. Neuroscience 43: 287–290. [DOI] [PubMed] [Google Scholar]
- Maris E, Oostenveld R ( 2007): Nonparametric statistical testing of EEG‐ and MEG‐data. J Neurosci Methods 164: 177–190. [DOI] [PubMed] [Google Scholar]
- Maurer D, Grand RL, Mondloch CJ ( 2002): The many faces of configural processing. Trends Cogn Sci 6: 255–260. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis ILC, Takashima A, Oostenveld R, Fernandez G, Jensen O ( 2008): Visual areas become less engaged in associative recall following memory stabilization. Neuroimage 40: 1319–1327. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM ( 2011): FieldTrip: Open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011: 156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher D, Walsh V, Duchaine B ( 2011): The role of the occipital face area in the cortical face perception network. Exp Brain Res 209: 481–493. [DOI] [PubMed] [Google Scholar]
- Robinson SE, Vrba J ( 1999). Recent advances in biomagnetism In: Yoshimoto T, Kotani M, Kuriki S, Karibe S, Nakasato N, editors. Functional Neuroimaging by Synthetic Aperture Magnetometry (SAM). Sendai, Japan: Tohoku University Press; pp 302–305. [Google Scholar]
- Sams M, Hietanen JK, Hari R, Ilmoniemi RJ, Lounasmaa OV ( 1997): Face‐specific responses from the human inferior occipito‐temporal cortex. Neuroscience 77: 49–55. [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura K, Nakamura A, Sugiura M, Ito K, Fukuda H, Kawashima R ( 1999): Different time course between scene processing and face processing: A MEG study. Neuroreport 10: 3633–3637. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR ( 2011). Neurophysiological correlates of face recognition In: Calder AJ, Rhodes G, Johnson MH, Haxby JV, editors. The Handbook of Face Perception. Oxford: Oxford University Press; pp 345‐366. [Google Scholar]
- Schweinberger SR, Huddy V, Burton AM ( 2004): N250r: A face‐selective brain response to stimulus repetitions. Neuroreport 15: 1501–1505. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Kaufmann JM, Moratti S, Keil A, Burton AM ( 2007): Brain responses to repetitions of human and animal faces, inverted faces, and objects: An MEG study. Brain Res 1184: 226–233. [DOI] [PubMed] [Google Scholar]
- Streit M, Ioannides AA, Liu L, Wolwer W, Dammers J, Gross J, Gaebel W, Muller‐Gartner HW ( 1999): Neurophysiological correlates of the recognition of facial expressions of emotion as revealed by magnetoencephalography. Brain Res Cogn Brain Res 7: 481–491. [DOI] [PubMed] [Google Scholar]
- Susac A, Ilmoniemi RJ, Pihko E, Nurminen J, Supek S ( 2009): Early dissociation of face and object processing: A magnetoencephalographic study. Hum Brain Mapp 30: 917–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susac A, Ilmoniemi RJ, Pihko E, Ranken D, Supek S ( 2010): Early cortical responses are sensitive to changes in face stimuli. Brain Res 1346: 155–164. [DOI] [PubMed] [Google Scholar]
- Swithenby SJ, Bailey AJ, Brautigam S, Josephs OE, Jousmaki V, Tesche CD ( 1998): Neural processing of human faces: A magnetoencephalographic study. Exp Brain Res 118: 501–510. [DOI] [PubMed] [Google Scholar]
- Tallon‐Baudry C, Bertrand O ( 1999): Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci 3: 151–162. [DOI] [PubMed] [Google Scholar]
- Tanskanen T, Nasanen R, Montez T, Paallysaho J, Hari R ( 2005): Face recognition and cortical responses show similar sensitivity to noise spatial frequency. Cereb Cortex 15: 526–534. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Bayless SJ, Mills T, Pang EW ( 2011): Recognising upright and inverted faces: MEG source localisation. Brain Res 1381: 167–174. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Mills T, Smith ML, Pang EW ( 2008): Face processing in adolescents with and without epilepsy. Int J Psychophysiol 68: 94–103. [DOI] [PubMed] [Google Scholar]
- Tuladhar AM, ter Huurne N, Schoffelen JM, Maris E, Oostenveld R, Jensen O ( 2007): Parieto‐occipital sources account for the increase in alpha activity with working memory load. Hum Brain Mapp 28: 785–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JRVJR, Ossandón T, Jerbi K, Dalal SS, Minotti L, Ryvlin P, Kahane P, Lachaux JP ( 2010): Category‐specific visual responses: An intracranial study comparing gamma, beta, alpha, and ERP response selectivity. Front Hum Neurosci 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S, Kakigi R, Koyama S, Kirino E ( 1999): Human face perception traced by magneto‐ and electro‐encephalography. Cogn Brain Res 8: 125–142. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Kakigi R, Puce A ( 2003): The spatiotemporal dynamics of the face inversion effect: A magneto‐ and electro‐encephalographic study. Neuroscience 116: 879–895. [DOI] [PubMed] [Google Scholar]
- Watanabe S, Miki K, Kakigi R ( 2005): Mechanisms of face perception in humans: A magneto‐ and electro‐encephalographic study. Neuropathology 25: 8–20. [DOI] [PubMed] [Google Scholar]
- Xu Y, Liu J, Kanwisher N ( 2005): The M170 is selective for faces, not for expertise. Neuropsychologia 43: 588–597. [DOI] [PubMed] [Google Scholar]
- Young AW, Hellawell D, Hay DC ( 1987): Configurational information in face perception. Perception 16: 747–759. [DOI] [PubMed] [Google Scholar]
- Yuval‐Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY ( 2008): Transient induced gamma‐band response in EEG as a manifestation of miniature saccades. Neuron 58: 429–441. [DOI] [PubMed] [Google Scholar]
- Zion‐Golumbic E, Bentin S ( 2007): Dissociated neural mechanisms for face detection and configural encoding: Evidence from N170 and induced gamma‐band oscillation effects. Cereb Cortex 17: 1741–1749. [DOI] [PubMed] [Google Scholar]
- Zion‐Golumbic E, Golan T, Anaki D, Bentin S ( 2008): Human face preference in gamma‐frequency EEG activity. Neuroimage 39: 1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion‐Golumbic E, Kutas M, Bentin S ( 2010): Neural dynamics associated with semantic and episodic memory for faces: Evidence from multiple frequency bands. J Cogn Neurosci 22: 263–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Suppporting Information


