Abstract
Receptor editing is the process that replaces the heavy chain or light chain variable region genes in a B-cell immunoglobulin receptor that is already productively rearranged. It is a major mechanism in the bone marrow for maintaining B-cell tolerance to autoantigens. We propose that a pathological autoimmune process can use receptor editing to induce the de novo creation and activation of B cells with autoreactive receptors in the peripheral immune system.
Keywords: receptor editing, autoimmunity, B cell, autoreactivity
Receptor editing is the replacement of a productively rearranged immunoglobulin light chain or heavy chain variable region gene with another one, either on the same chromosome, on the homologous chromosome, or through an isotype switch (i.e., κ light chain to λ light chain) [1]. Evidence from both transgenic and wild type mice have demonstrated that this process is an important mechanism in the maintenance of tolerance at the earlier stages of B-cell ontogeny in the bone marrow. If a developing B cell expresses a heavy chain/light chain combination that recognizes an autoantigen with sufficient affinity, it can be signaled to continue to express the Ig gene recombination machinery, including the rag1 and rag2 genes. It thereby undergoes further gene rearrangements that replace either the light chain or the heavy chain variable regions, so that a new B-cell receptor is produced that is not autoreactive.
Thus, the role of receptor editing in the bone marrow is well established in the suppression of autoimmunity. More controversial is the possibility of receptor editing in the peripheral lymphoid system, also termed receptor revision [2]. Although several laboratories have demonstrated the expression of the rag genes in the spleen and lymph nodes, particularly after an antigenic challenge, much of this phenomenon has been explained by the peripheralization of immature B cells [3]. In vitro experiments in mice have demonstrated that B cells can apparently be induced to upregulate rag genes by stimulation with LPS and IL-4 [4,5], although it cannot be ruled out that these observations are explained by selective survival and proliferation of immature B cells.
In the setting of autoimmune disease, particularly lupus, the characterization of Ig gene usage by autoantibody producing B cells has shown increased receptor editing [6,7]. In general, however, it cannot be ascertained where and when in B-cell ontogeny this process might have occurred. It has been assumed that the increased editing is a result of a frustrated effort by the immune system to suppress autoimmunity, presumably in the bone marrow. However, some data are consistent with the maintenance of tolerance by peripheral receptor editing [8], and in one case the timing of somatic hypermutation in the Ig gene sequence suggested that peripheral receptor editing actually resulted in the creation of an autoantibody [9].
In this regard, work from Youinou and colleagues has provided striking evidence for the increased expression of the rag genes in vivo in the setting of human autoimmunity or through in vitro stimulation by anti-IgM and other signals, including IL-6 [10–13]. Whether this is truly a reinduction of the recombination machinery or a selective survival of rag expressing cells cannot be determined with certainty. However, the parallel between this work and the results in the mouse system (admittedly with a different cytokine) is provocative.
We have more recently approached this issue in a highly defined system in which we can differentiate more clearly processes that occur at various stages of B-cell ontogeny. The transfer of CD4 T cells from normal mice (bm12) into normal mice of another strain (C57BL/6) that differs only in the MHC class II locus induces a chronic graft-versus-host (cGVH) syndrome that produces autoantibodies and immunopathology that parallel spontaneous lupus [14]. The model depends on cognate interaction of the donor CD4 T cells with the recipient B cells [15]. We have modified this system such that we can separately transfer the stimulating CD4 T cells and the responding B cells to an immunodeficient (rag1 knockout) mouse, and produce the same response of anti-DNA and anti-chromatin autoantibodies. Thus, we can preselect the transferred B cells in various ways, and determine which B cells are capable of losing tolerance in this system. We thereby have shown, perhaps surprisingly, that the B cells that respond best after transfer are mature peripheral B cells, i.e., those that have lost the CD93 marker [16].
Some of our earlier work with the cGVH model had already revealed anomalies related to receptor editing. In one set of experiments, we utilized the hen egg lysozyme (HEL)-anti-HEL double transgenic system developed by Goodnow [17,18]. These mice (on a C57BL/6 background) have an IgM/IgD, κ, conventional transgene that results in a high affinity anti-HEL antibody. In the presence of a transgene that produces a soluble form of HEL, the double transgenic mice achieve tolerance largely by downregulating their surface Ig receptor. They also show evidence of increased light chain receptor editing in the bone marrow [19]. The transfer of MHC Class II incompatible CD4 T cells form bm12 mice into the HEL/anti-HEL double transgenic recipients breaks this ‘self’ tolerance, and results in the production of high levels of anti-HEL serum antibodies [17]. This is accompanied by a decreased level of receptor editing in the bone marrow, as shown by lower numbers of non-HEL binding B cells, decreased levels of endogenous κ chain rearrangements, and decreased rag2 expression [20].
In another system, we have utilized recipient mice on a normal C57BL/6 background that also expressed a site-directed immunoglobulin heavy chain transgene that came from an anti-DNA monoclonal antibody. This transgene, named 56R, had been modified by the insertion of an additional arginine residue, which resulted in even stronger binding with double-stranded DNA. In fact, very few light chains are able to pair with 56R to produce an immunoglobulin that isn’t an anti-DNA autoantibody [21]. BALB/c mice with the 56R transgene undergo extensive light chain editing in order to express the 56R transgene with a light chain that does not result in autoreactivity. In the C57BL/6 background, however, some 56R-expressing anti-DNA B cells do escape tolerance, in a T-independent process that is not yet understood [22].
Not unexpectedly, the transfer of MHC class II incompatible CD4 T cells from bm12 mice into C57BL/6.56R recipients resulted in increased levels of anti-DNA antibodies as part of the cGVH reaction [23]. Surprisingly, however, the serum antibody detected was produced not only by the chromosome containing the heavy-chain transgene (as marked by allotype), but also from the endogenous heavy chain chromosome. PCR typing of the heavy chain variable regions from spontaneous hybridomas from these cGVH mice showed a pattern of gene usage that was even more remarkable. Every one of the 56 IgG producing hybridomas failed to show evidence of the 56R transgene, although 18/22 IgM hybridomas were transgene positive. Thirty-two of the IgG hybridomas were IgG2a anti-DNA (and thus could be tested serologically for allotype), and of these 30 used the endogenous Igh genes (IgG2ab, or more properly designated as IgG2c). Two used the transgenic chromosome (IgG2aa), and yet failed to reveal the transgene by PCR. Sequencing showed that they had rearranged a different VH gene, presumably by the process of receptor invasion at the site of a cryptic heptamer [2]. Somehow the loss of tolerance induced by the abnormal T-cell help of the allogeneic T cells in the cGVH bypassed the use of a pre-existing anti-DNA heavy chain gene and selected other heavy chain variable region genes from the repertoire.
How can the remarkable switch away from an anti-DNA site directed transgenic heavy chain gene be understood in the context of loss of B-cell tolerance to DNA forced by abnormal allogeneic T-cell help? As the transgene bearing cells are prevalent in C57BL/6.56R mice, the edited B cells clearly are undergoing selective activation in this model. We have shown in several mouse lupus models that the b allotype in heavy chains is indeed favored for anti-nuclear autoantibodies; however, this effect is modest, and it would not explain why even the a allotype anti-DNA heavy chain hybridomas no longer expressed the transgene [24,25]. It seems likely that the B cells that expressed heavy-chain edited anti-DNA antibodies must have been subjected to selection by the allogeneic T cells at a stage in B-cell ontogeny not readily available to the 56R+ B cells. We propose that this process occurs in the peripheral lymphoid system. In the initial polyclonal activation of the peripheral B cells by the alloreactive T cells, receptor editing would occur and produce some combinations of new heavy and new (or old) light chains which are DNA reactive. In the ubiquitous presence of antigen and the ongoing help from alloreactive T cells, these anti-DNA B cells would become fully activated and secrete autoantibodies. In contrast, in the absence of peripheral editing, a B cell would either not express an anti-DNA Ig or would be in a state of anergy that would not be susceptible to activation by allogeneic help.
This switching of Ig chain usage in the periphery could occur by secondary receptor revision involving further gene rearrangements. This would imply either the persistent expression of the genetic recombination machinery, including the rag1 and rag2 genes, or its reinduction under the stimulus of the cGVH. This possibility is supported by Youinou’s results with human cells [10–12]. It is also possible that some B cells may migrate to the periphery already harboring two productively rearranged Ig chains, one of which would result in an autospecificity and would not normally be expressed on the cell surface [26,27]. This would allow the dual expressing cell to escape tolerization, and the autoreactive Ig would be activated during the cGVH.
We are now testing the potential for receptor editing to occur in mature B cells under the stimulus of alloreactive T cell help. If this occurs, it would imply the de novo creation of anti-DNA B cells in the periphery, when most of the tolerance checkpoints have already been bypassed. An important final checkpoint would be the requirement for ongoing T cell help. In the cGVH, the promiscuity of the alloreactive T cells would provide the mechanism to bridge this final checkpoint. We would postulate that such a process might also occur in spontaneous lupus, and that either abnormal T cell help or abnormal B-cell independence of such help would be key factors in this loss of tolerance that leads to anti-DNA autoantibody production (Figure).
Figure 1. BCR specificity dictates selective outcome at multiple checkpoints.
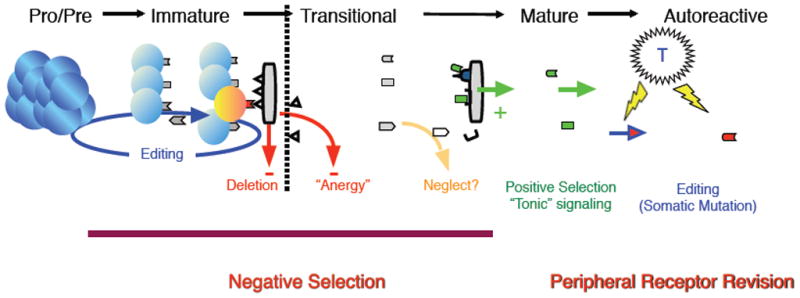
B cells that interact avidly with soluble autoantigens are purged from the active repertoire at several stages of ontogeny by negative selection. We propose an additional process whereby mature B cells can acquire a new autoreactivity by receptor revision or somatic mutation [28]. The creation and survival of such autoreactive cells would depend on abnormal immunoregulation, such as ongoing T-cell help. (Figure modified from M.P. Cancro [29]).
Take-home messages.
Autoreactive B cells in the bone marrow are frequently rescued by a secondary rearrangement of their heavy or light chain genes, which displaces the autoantigen specificity of their immunoglobulin receptor. This process is called receptor editing.
Abnormalities in receptor editing have been described in human lupus and in mouse lupus models, and in other autoimmune diseases.
In systemic autoimmunity, receptor editing in the periphery may actually create autoimmune B-cell receptors, and thereby bypass the checkpoints for B-cell tolerance.
Acknowledgments
Dr. Eisenberg’s work is supported by the Alliance for Lupus Research, the Lupus Research Institute, the Arthritis Foundation, the American Autoimmune Related Disease Association, the Lupus Foundation of South Jersey and the NIH (R01-AR-34156; R01-AI063626).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Luning Prak ET, Monestier M, Eisenberg RA. B cell receptor editing in tolerance and autoimmunity. Ann N Y Acad Sci. 2011;1217:96–121. doi: 10.1111/j.1749-6632.2010.05877.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nemazee D, Weigert M. Revising B cell receptors. J Exp Med. 2000;191:1813–1817. doi: 10.1084/jem.191.11.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu W, Nagaoka H, Jankovic M, Misulovin Z, Suh H, Rolink A, Melchers F, Meffre E, Nussenzweig MC. Continued RAG expression in late stages of B cell development and no apparent re-induction after immunization. Nature. 1999;400:682–687. doi: 10.1038/23287. [DOI] [PubMed] [Google Scholar]
- 4.Hikida M, Mori M, Takai T, Tomochika K, Hamatani K, Ohmori H. Reexpression of RAG-1 and RAG-2 genes in activated mature mouse B cells. Science. 1996;274:2092–2094. doi: 10.1126/science.274.5295.2092. [DOI] [PubMed] [Google Scholar]
- 5.Hikida M, Ohmori H. Rearrangement of lambda light chain genes in mature B cells in vitro and in vivo. Function of reexpressed recombination-activating gene (RAG) products. J Exp Med. 1998;187:795–799. doi: 10.1084/jem.187.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dörner T, Foster SJ, Farner NL, Lipsky PE. Immunoglobulin kappa chain receptor editing in systemic lupus erythematosus. J Clin Invest. 1998;102:688–694. doi: 10.1172/JCI3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klonowski KD, Monestier M. Ig heavy-chain gene revision: leaping towards autoimmunity. Trends Immunol. 2001;22:400–405. doi: 10.1016/s1471-4906(01)01953-6. [DOI] [PubMed] [Google Scholar]
- 8.Wang YH, Diamond B. B cell receptor revision diminishes the autoreactive B cell response after antigen activation in mice. J Clin Invest. 2008;118:2896–2907. doi: 10.1172/JCI35618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brard F, Shannon M, Prak EL, Litwin S, Weigert M. Somatic mutation and light chain rearrangement generate autoimmunity in anti-single-stranded DNA transgenic MRL/lpr mice. J Exp Med. 1999;190:691–704. doi: 10.1084/jem.190.5.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillion S, Rochas C, Devauchelle V, Youinou P, Jamin C. Central and peripheral RAG protein re-expression: underestimate mechanisms of tolerance? Scand J Immunol. 2006;64:185–189. doi: 10.1111/j.1365-3083.2006.01801.x. [DOI] [PubMed] [Google Scholar]
- 11.Hillion S, Youinou P, Jamin C. Peripheral expression of RAG in human B lymphocytes in normal and pathological conditions is dependent on interleukin-6. Autoimmun Rev. 2007;6:415–420. doi: 10.1016/j.autrev.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 12.Rochas C, Hillion S, Youinou P, Jamin C, Devauchelle-Pensec V. RAG-mediated secondary rearrangements of B-cell antigen receptors in rheumatoid synovial tissue. Autoimmun Rev. 2007;7:155–159. doi: 10.1016/j.autrev.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Hillion S, Rochas C, Youinou P, Jamin C. Signaling pathways regulating RAG expression in B lymphocytes. Autoimmun Rev. 2009;8:599–604. doi: 10.1016/j.autrev.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Eisenberg RA. The Chronic Graft-versus-Host Model of Systemic Autoimmunity. In: Nemazee D, editor. B Cell Biology in Autoimmunity. Basel: Karger; 2002. pp. 228–43. [DOI] [PubMed] [Google Scholar]
- 15.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Autoantibodies in chronic graft versus host result from cognate T-B interactions. Journal of Experimental Medicine. 1990;171:503–517. doi: 10.1084/jem.171.2.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhury A, Cohen PL, Eisenberg RA. Mature B cells preferentially lose tolerance in the chronic graft-versus-host disease model of systemic lupus erythematosus. J Immunol. 2007;179:5564–5570. doi: 10.4049/jimmunol.179.8.5564. [DOI] [PubMed] [Google Scholar]
- 17.Feuerstein N, Chen F, Madaio M, Maldonado M, Eisenberg RA. Induction of autoimmunity in a transgenic model of B cell receptor peripheral tolerance: changes in coreceptors and B cell receptor-induced tyrosine-phosphoproteins. J Immunol. 1999;163:5287–5297. [PubMed] [Google Scholar]
- 18.Goodnow CC. Transgenic mice and analysis of B-cell tolerance. Annu Rev Immunol. 1992;10:489–518. doi: 10.1146/annurev.iy.10.040192.002421. [DOI] [PubMed] [Google Scholar]
- 19.Tze LE, Baness EA, Hippen KL, Behrens TW. Ig light chain receptor editing in anergic B cells. J Immunol. 2000;165:6796–6802. doi: 10.4049/jimmunol.165.12.6796. [DOI] [PubMed] [Google Scholar]
- 20.Feuerstein N, DeSimone DC, Eisenberg RA, Finkel TH. Chronic graft-versus-host reaction is associated with a decrease in Ig light chain receptor editing in bone marrow self-reactive B cells. Eur J Immunol. 2004;34:1361–1370. doi: 10.1002/eji.200324743. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Jiang Y, Prak EL, Radic M, Weigert M. Editors and editing of anti-DNA receptors. Immunity. 2001;15:947–957. doi: 10.1016/s1074-7613(01)00251-5. [DOI] [PubMed] [Google Scholar]
- 22.Tsao PY, Jiao J, Ji MQ, Cohen PL, Eisenberg RA. T Cell-Independent Spontaneous Loss of Tolerance by Anti-Double-Stranded DNA B Cells in C57BL/6 Mice. J Immunol. 2008;181:7770–7777. doi: 10.4049/jimmunol.181.11.7770. [DOI] [PubMed] [Google Scholar]
- 23.Sekiguchi DR, Eisenberg RA, Weigert M. Secondary heavy chain rearrangement: a mechanism for generating anti-double-stranded DNA B cells. J Exp Med. 2003;197:27–39. doi: 10.1084/jem.20020737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris SC, Cheek RL, Cohen PL, Eisenberg RA. Allotype-specific immunoregulation of autoantibody production by host B cells in chronic graft-versus host disease. Journal of Immunology. 1990;144:916–922. [PubMed] [Google Scholar]
- 25.Halpern MD, Fisher CL, Cohen PL, Eisenberg RA. Influence of the Ig H chain locus on autoantibody production in autoimmune mice. Journal of Immunology. 1992;149:3735–3740. [PubMed] [Google Scholar]
- 26.Liu S, Velez MG, Humann J, Rowland S, Conrad FJ, Halverson R, Torres RM, Pelanda R. Receptor editing can lead to allelic inclusion and development of B cells that retain antibodies reacting with high avidity autoantigens. J Immunol. 2005;175:5067–5076. doi: 10.4049/jimmunol.175.8.5067. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Li H, Weigert M. Autoreactive B cells in the marginal zone that express dual receptors. J Exp Med. 2002;195:181–188. doi: 10.1084/jem.20011453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W, Smith D, Aviszus K, Detanico T, Heiser RA, Wysocki LJ. Somatic hypermutation as a generator of antinuclear antibodies in a murine model of systemic autoimmunity. J Exp Med. 2010;207:2225–2237. doi: 10.1084/jem.20092712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cancro MP. B cells and aging: gauging the interplay of generative, selective, and homeostatic events. Immunol Rev. 2005;205:48–59. doi: 10.1111/j.0105-2896.2005.00272.x. [DOI] [PubMed] [Google Scholar]


