Abstract
Background
Established psychosocial risk factors increase the risk for experimentation among Mexican-origin youth. Now we comprehensively investigate the added contribution of select polymorphisms in candidate genetic pathways associated with sensation seeking, risk taking, and smoking phenotypes to predict experimentation.
Methods
Participants, (N=1,118 Mexican origin youth) recruited from a large population-based cohort study in Houston, Texas, provided prospective data on cigarette experimentation over three years. Psychosocial data were elicited twice—baseline and final follow-up. Participants were genotyped for 672 functional and tagging variants in the dopamine, serotonin and opioid pathways.
Results
After adjusting for gender and age, with a Bayesian False Discovery Probability set at 0.8 and prior probability of 0.05, six gene variants were significantly associated with risk of experimentation. After controlling for established risk factors, multivariable analyses revealed that participants with six or more risk alleles were 2.25 (95%CI: 1.62–3.13) times more likely to have experimented since baseline compared to participants with five or fewer. Among committed never smokers (N=872), three genes (OPRM1, SNAP25, HTR1B) were associated with experimentation as were all psychosocial factors. Among susceptible youth (N=246) older age at baseline, living with a smoker, and three different genes (HTR2A, DRD2, SLC6A3) predicted experimentation.
Conclusions
Our findings, which have implications for development of culturally-specific interventions, need to be validated in other ethnic groups.
Impact
These results suggest that variations in select genes interact with a cognitive predisposition toward smoking. In susceptible adolescents, the impact of the genetic variants appears to be larger compared to committed never smokers.
Introduction
Smoking remains the leading modifiable risk factor for lung cancer, increasing risk for the disease between 10- to 20-fold, depending on the duration and intensity (1). Moreover, compared to smoking initiation in late adolescence or early adulthood, early smoking initiation is associated with increased DNA damage in the lung (2) as well as higher levels of nicotine dependence and more years smoked (3), all factors that increase the risk of developing lung cancer (2,4). The social, economic, and individual benefits to be gained by preventing youth from experimenting with cigarettes and becoming adult smokers are therefore profound.
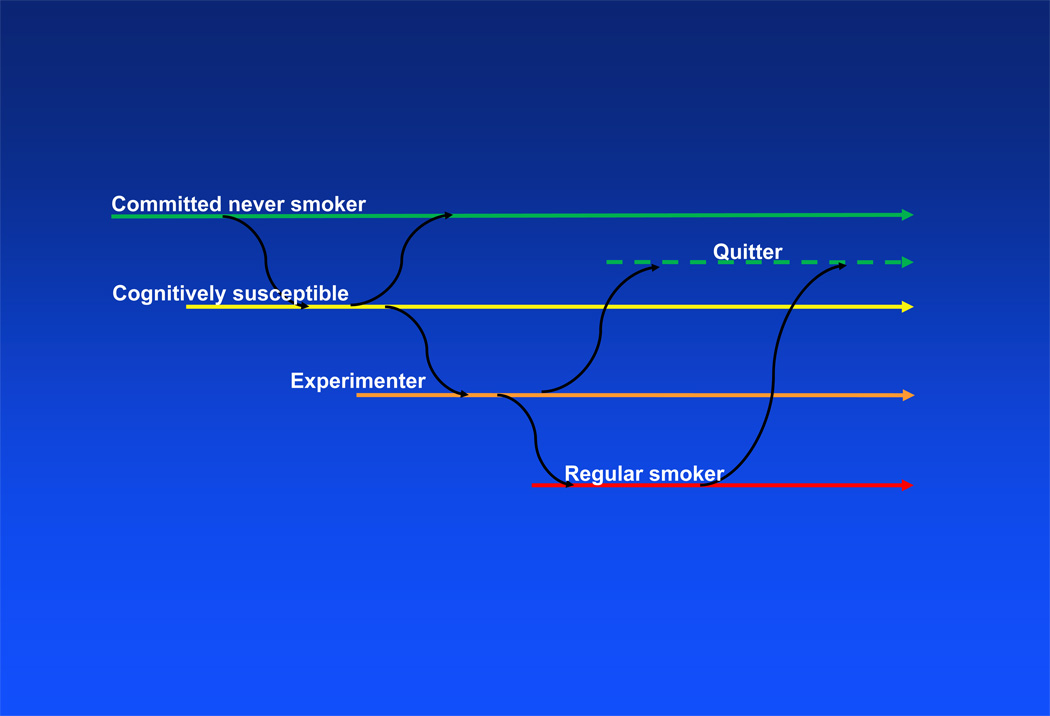
Despite the large body of literature available on the prevalence of smoking behavior among US adolescents, research examining the transition from never smoking to experimentation (defined as having tried cigarettes, but not smoking on a monthly basis) with cigarettes is limited, especially among Hispanic adolescents. We (5), and others (6,7), have reported that cognitive susceptibility to smoking, a construct that combines peer social influence with behavioral intentions, is possibly the strongest single predictor of cigarette experimentation. We found that 45% of youth who self-reported as cognitively susceptible to smoking subsequently experimented with cigarettes, compared to 15% of their peers who self-reported as being committed never smokers (5). Understanding factors associated with this transition is crucial to smoking prevention efforts because up to 64% of experimenters transition to daily smoking within one year of starting (8), most make the transition to regular smoking by age 18 (9), and almost 90% of adults who currently smoke initially experimented with smoking when they were less than 18 years of age (10). Figure 1 presents transitions on the smoking continuum from committed never smoker, to susceptible, to experimenting, and finally regular smoking.
Figure 1.
Movement between cognitive susceptibility, experimentation and regular smoking over time
Hispanics are the largest and most rapidly increasing ethnic group in the United States, and those of Mexican origin comprise the majority of this subgroup (11). Nationally, Mexican origin youth report the highest rates of cognitive susceptibility to smoking – 29% compared to 21% in non-Hispanic whites and 23% in non-Hispanic blacks (12) and in Texas Hispanic adolescents exhibit the highest rates of experimentation with cigarettes among all ethnic groups – 55% compared to 48% in non-Hispanic whites and 46% in non-Hispanic blacks report ever experimenting with cigarettes (13), underscoring their increased risk for adult smoking.
In order to enhance prevention efforts in this growing population, we evaluated the trajectory from never smoking to experimenting with cigarettes in a longitudinal cohort of Mexican origin adolescents between 11 and 13 years of age at enrollment who were followed for five years. Among this sample of over a thousand youth, we have shown (5) that being a boy and older at enrollment, holding positive beliefs about the social benefits of smoking, living with a smoker, and having had at least one school detention all significantly predicted becoming an experimenter over the course of the four-year study. We also reported that baseline cognitive susceptibility to smoking status modified the relationship between experimentation at follow-up and the other established risk factors examined (5), suggesting that risk factors associated with experimentation vary by susceptibility status.
Contemporaneously, family, twin, candidate and genome wide association studies provide compelling evidence of a role for genetic variants governing smoking initiation, nicotine dependence and cessation (14,15) with estimated heritability rates ranging from 40–60% for initiation (16,17) and around 50% for persistence (18). We now expand our analysis to assess the role of 58 genes and 672 SNPs covering genetic variants in candidate pathways that influence sensation seeking behavior, risk taking behavior, and smoking phenotypes, in the context of established demographic and psychosocial risk factors, among these same youth during the transition from never-smoking to experimentation with cigarettes. We include SNPs associated with sensation seeking and risk taking behavior because both behaviors are associated with smoking initiation (19–21). Because our goal is to examine potential gene-environment interactions associated with the transition from never smoking to experimenting with cigarettes, in the current study we focus on youth who had not experimented with cigarettes at baseline when they enrolled in the cohort.
Participants and Methods
Participant recruitment
Between May 2005 and December 2006, youth were recruited from a population-based cohort of Mexican-American households in the Houston metropolitan area. A detailed description of the cohort recruitment methodology has been published (22). A total of 3,000 households with potential age-eligible adolescents (11 to 13 years) were identified from the cohort database. Of the first 1,425 potential participants’ parents or legal guardians contacted to assess interest in the study, just over 90% agreed to enroll their child (N=1,328). The institutional review board at M. D. Anderson Cancer Center approved all aspects of this study.
Data collection
Data on smoking phenotype were collected via personal interview on five sequential encounters. Baseline and final interviews were completed in the home -- the second home survey was completed 30 months (SD=4.8 months) after baseline. The second, third and fourth interviews, were completed roughly 6, 12, and 18 months respectively following baseline, over the telephone. At baseline, after consenting into the study, each participant completed a 5-minute personal interview during which basic demographic (gender, age, nativity status (US or Mexico)) acculturation data (23), and parental educational attainment (available for 93.5% of the study participants) were collected. To assure confidentiality from parents overhearing the child’s responses, the remainder of the survey was completed on a personal digital assistant (PDA). All participants provided buccal samples at baseline. A detailed description of baseline data collection procedures has been published (24).
Measures
Our primary outcome variable was new experimentation with cigarettes, assessed using two questions derived from the Youth Risk Behavior Surveillance Survey (25), “Have you ever smoked a whole cigarette?” and “Have you ever tried a cigarette, even a puff?” Participants who answered “yes” to either question were defined as experimenters. All others were considered never experimenters. Participants who responded “no” at baseline, but “yes” to either question at any follow-up were designated as ‘new experimenters.’
We further examined the influence of several established psychosocial risk factors: risk taking tendencies (21), outcome expectations related to smoking (26), household social influence (27–29), and cognitive susceptibility to smoking (5,7). The measures used to assess these constructs are detailed in Table 1.
Table 1.
Description of the psychosocial measures used to assess each construct
| Construct | Description |
|---|---|
| Linguistic acculturation | Assessed at baseline using 4 items from the Language Use subscale of the Brief Acculturation Scale for Hispanics (23), which ascertains language used when reading, speaking at home, with friends, and thinking. This scale had good internal reliability (alpha=0.76). |
| Risk taking | Assessed using two items “I look for dangerous things to do, just for excitement” and “If I got a chance to skydive from an airplane, I’d do it.” Responses were made on a four-point scale ranging from “just like me” to “not at all like me” and were averaged. For descriptive purposes the variable was categorized into “high” and “low” based on the median split, in the multivariable analysis it was entered as continuous. Higher scores indicate greater risk taking tendencies. |
| Outcome expectations | Assessed at baseline using a 7-item scale developed by Dalton et al (26). Examples include “I think smoking would help me to feel more comfortable at parties” and “I think smoking would make me look more mature.” Responses are made on a 4-point scale ranging from “strongly disagree” to “strongly agree.” We have found the scale to have good internal consistency (Cronbach’s alpha = 0.86). For descriptive purposes the variable was categorized into “none” and “some” however, in the multivariable analysis it was entered as continuous. Higher scores indicate more positive perceived consequences ascribed to smoking behavior. |
| Household social influence | For each potential household member (i.e. father, mother, sister(s), brother(s), and other(s)) the participant was asked “Does your father smoke?” and “Do you live with your father?” To ensure the variable reflected social influence from all household members with whom the participant currently lived, we summed the number of smoking household members residing with participant. In the multivariable analyses this variable was coded as “none” and “at least one,” with “none” serving as the reference category. |
| Cognitive susceptibility | Combines behavioral intentions and peer influence and was assessed among never-smokers only (7). To be coded as “non-susceptible” participants responded “no” to “Do you think you will try a cigarette soon?”; and “definitely not” to “If one of your best friends were to offer you a cigarette would you smoke it?” & “Do you think you will be smoking cigarettes 1 year from now?” All other participants were coded as susceptible. |
DNA collection
Saliva samples were obtained in Oragene vials (DNA Genotek, Ottawa, Ontario, Canada). DNA extraction was performed using a “Purifier” solution with alcohol precipitation per the manufacturer’s protocol. The median yield of DNA from 2 mL of saliva captured in 2 mL of Oragene•DNA was 110 µg.
SNP selection
Candidate genes were identified from published reviews (e.g. 30) and pub-med searches using the following key words: sensation seeking, risk taking, gambling, smoking onset and initiation. This list was cross-referenced with the Gene Ontology Database (31) and Kegg Pathway to confirm pathway information. Tagging SNPs were selected from the International HapMap Project (Release 21 with NCBI build 36; 32). The following selection criteria were used: located in the respective gene or within 10 kb upstream or downstream of the gene ends to cover the regulatory regions; minor allele frequency (MAF) >5% in various ethnic groups; and not already represented by a current tag SNP at an LD of r2>0.80. We also included SNPs in coding (synonymous SNPs, nonsynonymous SNPs) and regulatory regions (promoter, splicing site, 5-UTR, and 3-UTR). In addition, functional SNPs and SNPs previously reported to be associated with smoking phenotypes were included. The gene, position, allelic change and chromosome are summarized in Supplemental Table 1.
Genotyping
A total of 1274 samples were sent for genotyping. We designed an Illumina GoldenGate assay for candidate SNPs (Illumina, San Diego, California). Ninety-three percent of the SNPs had Illumina SNP scores of >0.6. DNA samples (250 ng) were genotyped following the standard three-day Illumina protocol. Data from the array were autocalled by the BeadArray Reader (Illumina Inc). Cluster definitions for each SNP were determined using Illumina BeadStudio Genotyping Module v 2.3.41. Genotype calls were made when a genotype yielded a quality score (Gencall value) of 95% or higher. Among these markers, 1.2% of calls were missing (8 out of 672). Seventy blind duplicate pairs were included, and the concordance of SNP genotype calls was greater than 99%.
Statistical analyses
Chi-square tests were used to examine associations between experimenter status and both categorical demographic and smoking-related covariates. Student’s t-tests and one-way analysis of variance were used to examine mean differences on the continuous covariates. Linkage disequilibrium (LD) between SNPs was assessed by calculating pairwise Lewontin’s D′ and r2 using Haploview version 3.32.
Two multivariable models were developed (one with and one without genotype data). Psychosocial risk factors with a significant bivariate association (p<0.05) with new experimenting were entered into unconditional logistic regression analyses. We used Bayesian False Discovery Probability tests (BFDP; 33) to evaluate the chance of false positive associations for the variants studied. We set four levels of prior probability (0.01, 0.03, 0.05, and 0.07), prior odds ratio (OR) at 1.5, and the selected level of noteworthiness for BFDP at 0.8, the recommended threshold by Wakefield (33).
Because a priori we do not know the mode of inheritance, we tested each significant SNP using dominant, recessive, and additive models and selected the most parsimonious. SNPs retained in the multivariable model were summed to create a genetic risk score. SNPs that exhibited a protective effect were reverse coded prior to creating the genetic risk score. Thus, higher scores on the genetic risk score reflected increased risk.
Next principle components analysis was performed to test for the possible underlying ethnic stratification, with the use of EIGENSTRAT software (34). We first applied the principal components analysis to the genotype data to infer continuous axes of genetic variation, which are defined as eigenvectors. We then employed the top axes of variation as the covariates in the unconditional logistic regressions to develop the multivariable models.
Finally, having determined that cognitive susceptibility modifies the influence of the psychosocial variables on experimentation (5) and because including the genotype data did not significantly attenuate the odds ratios derived from the first model, we applied a standard methodology (35,36) to determine if cognitive susceptibility to smoking moderated (modified) the influence of the genotype data on experimentation. The interaction term (cognitive susceptibility by genetic risk score) and the main effects were simultaneously entered into unconditional logistic regression models. The interaction reflects the interaction between putative risk alleles and being cognitively susceptible to smoking.
Results
DNA from 1,274 youth enrolled was available for analysis. The 120 youth who reported experimenting at baseline were excluded as were an additional 36 for whom complete follow-up information was missing. The final sample size was 1,118; 584 (52.2%) were girls.
Table 2 summarizes demographic characteristics and psychosocial risk factors. Of the 211 (18.9%) participants who began experimenting over the course of the study, 62.6% were boys compared with 44.3% for never experimenters (p<0.001). Experimenters were significantly more likely to be 13 at baseline (42.7% vs. 21.3%; p<0.001) and live with at least one smoker (55.4% vs. 35.3%; p<0.001). A higher proportion of experimenters held positive outcome expectations about smoking (56.9% vs. 34.6%; p<0.001), reported risk taking tendencies (70.1% vs. 50.5%; p<0.001), and being cognitively susceptible to smoking (43.6% vs. 17.0%) than never experimenters.
Table 2.
Distribution of demographic characteristics and smoking-related covariates by new experimenter status (N=1,118).
| Experimenter Status | ||||
|---|---|---|---|---|
| New Experimenter |
Never Experimenter |
|||
| N (%) | N (%) | N (%) | p-value | |
| Overall | 1,118 (100.0) | 211 (18.9) | 907 (81.1) | |
| Demographic Characteristics | ||||
| Gender | ||||
| Females | 584 (52.2) | 79 (37.4) | 505 (55.7) | |
| Males | 534 (47.8) | 132 (62.6) | 402 (44.3) | < 0.001 |
| Age (years) | ||||
| 11 or 12 | 835 (74.7) | 121 (57.3) | 714 (78.7) | |
| 13 | 283 (25.3) | 90 (42.7) | 193 (21.3) | < 0.001 |
| Mean (SD) | 11.89 (0.84) | 12.18 (0.87) | 11.75 (0.80) | < 0.001 |
| Country of birth | ||||
| Mexico | 297 (26.6) | 48 (22.7) | 249 (27.5) | |
| US | 821 (73.4) | 163 (77.3) | 658 (72.5) | 0.16 |
| Linguistic acculturation | ||||
| Low | 256 (22.9) | 41 (19.4) | 215 (23.8) | |
| High | 861 (77.1) | 170 (80.6) | 691 (76.2) | 0.18 |
| Parental education (N=1,051) | ||||
| < HS | 619 (65.6) | 64 (32.3) | 301 (35.3) | |
| ≥ HS grad | 324 (34.4) | 134 (67.7) | 552 (64.7) | 0.43 |
| Smoking-related Covariates | ||||
| No. of household members who smoke | ||||
| None | 681 (60.9) | 94 (44.6) | 587 (64.7) | |
| One | 341 (30.5) | 84 (39.8) | 257 (28.4) | |
| ≥ Two | 96 (8.6) | 33 (15.6) | 63 (6.9) | < 0.001 |
| Mean (SD) | 0.55 (0.76) | 0.75 (0.84) | 0.43 (0.66) | < 0.001 |
| Risk taking | ||||
| Averse | 512 (45.8) | 63 (29 9) | 449 (49.5) | |
| Some | 606 (54.2) | 148 (70.1) | 458 (50.5) | < 0.001 |
| Mean (SD) | 1.68 (0.81) | 1.95 (0.90) | 1.58 (0.76) | < 0.001 |
| Outcome expectations | ||||
| None | 684 (61.2) | 91 (43.1) | 593 (65.4) | |
| Some | 434 (38.8) | 120 (56.9) | 314 (34.6) | < 0.001 |
| Mean (SD) | 1.26 (0.40) | 1.35 (0.42) | 1.19 (0.35) | < 0.001 |
| Susceptible at baseline | ||||
| No | 872 (78.0) | 119 (56.4) | 753 (83.0) | |
| Yes | 246 (22.0) | 92 (43.6) | 154 (17.0) | < 0.001 |
Genotyping was completed on a total of 672 SNPs; eight SNPs had missing genotype data for all participants, 12 SNPs failed the Hardy Weinberg Equilibrium Test (p<0.00001) for the entire data set, and 86 SNPs failed the frequency test (MAF<0.05). Thus 574 SNPs were included in the analysis. There were 38 SNPs with p<0.05 based on the best model fit (additive, dominant, or recessive model). After controlling for false discovery, we identified 12 SNPs with a statistically significant BFDP at 0.8 and prior probability of 0.05. Finally using unconditional logistic regression analysis with backward selection and adjusting for age and sex, 6 of the 12 SNPs were selected at p≤0.05. Table 3 presents the distribution of the six SNPs retained for the multivariable analyses – three in the dopamine pathway (rs12422191 on DRD2, rs10052016 on SLC6A3, and rs8119844 on SNAP25), two in the serotonin pathway (rs6297 on HTR1B and rs9567732 on HTR2A), and an opioid receptor variant (rs9322451 on OPMR1).
Table 3.
Distribution of genes by new experimenter status (N=1,118)
| Experimenter Status | |||||||
|---|---|---|---|---|---|---|---|
| Genes | M* | New Experimenter |
Never Experimenter |
HapMap minor allele |
MATCh minor allele |
||
| N (%) | N (%) | N (%) | p-value | % | % | ||
| 1,118 (100.0) | 211 (18.9) | 907 (81.1) | |||||
| OPRM1 (rs9322451) | D | ||||||
| 0 | 822 (73.5) | 174 (21.2) | 648 (78.8) | ||||
| 1 | 296 (26.5) | 37 (12.5) | 259 (87.5) | 0.001 | 16.7 | 14.4 | |
| HTR1B (rs6297) | D | ||||||
| 0 | 990 (88.6) | 117 (17.9) | 813 (82.1) | ||||
| 1 | 128 (11.4) | 34 (26.6) | 94 (73.4) | 0.018 | 8.3 | 5.9 | |
| SNAP25 (rs8119844) | R | ||||||
| 0 | 1,089 (97.4) | 200 (18.4) | 889 (81.6) | ||||
| 1 | 29 (2.6) | 11 (37.9) | 18 (62.1) | 0.008 | 15.0 | 16.1 | |
| SLC6A3 (rs10052016) | D | ||||||
| 0 | 620 (55.5) | 133 (21.5) | 487 (78.5) | ||||
| 1 | 498 (44.5) | 78 (15.7) | 420 (84.3) | 0.014 | 77.0 | 74.8 | |
| HTR2A (rs9567732) | D | ||||||
| 0 | 530 (47.7) | 117 (22.1) | 413 (77.9) | ||||
| 1 | 588 (52.6) | 94 (16.0) | 494 (84.0) | 0.009 | -- | -- | |
| DRD2 (rs12422191) | D | ||||||
| 0 | 980 (87.7) | 175 (17.9) | 805 (82.1) | ||||
| 1 | 138 (12.3) | 36 (26.1) | 102 (73.9) | 0.021 | 5.0 | 6.6 | |
NB: M = Model; D = Dominant, R = Recessive
To perform the principle components analysis, we used N=1,118 participants and 536 SNPs, which were shown to be un-associated with the experimentation outcome at a significance level 0.05 based on the best model fit (additive, dominant, or recessive model). We did not observe significant ethnic stratification in our data from the principle components analysis. Because only the top two eigenvalues (derived from the top two principle components) were significantly larger than the subsequent eigenvalues, we used these two largest principle components in our analyses (Tables 4a and 4b; 37,38). We also considered controlling for the top three and top ten largest principal components, but found no significant differences in the resulting multivariable models.
Table 4.
| a: Logistic Regression for New Experimentation (N=1118) | ||||||
|---|---|---|---|---|---|---|
| Psychosocial factors only |
Psychosocial and genetic factors |
|||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Social and psychological factors | ||||||
| Cognitively susceptible | 2.34 | 1.62 – 3.37 | < 0.001 | 2.55 | 1.74 – 3.73 | < 0.001 |
| Age 13 at baseline | 2.13 | 1.51 – 2.99 | < 0.001 | 2.27 | 1.59 – 3.23 | < 0.001 |
| Male | 1.87 | 1.35 – 2.59 | < 0.001 | 1.96 | 1.40 – 2.75 | < 0.001 |
| Outcome expectations | 1.69 | 1.11 – 2.57 | 0.014 | 1.65 | 1.07 – 2.53 | 0.023 |
| Household member smokes | 1.51 | 1.22 – 1.86 | < 0.001 | 1.53 | 1.23 – 1.90 | < 0.001 |
| Risk taking tendencies | 1.35 | 1.12 – 1.64 | 0.002 | 1.30 | 1.07 – 1.59 | 0.009 |
| Gene (SNP) | ||||||
| SNAP25 (rs8119844) | – | – | – | 0.26 | 0.11 – 0.61 | 0.002 |
| OPRM1 (rs9322451) | – | – | – | 0.53 | 0.35 – 0.81 | 0.003 |
| HTR1B (rs6297) | – | – | – | 1.89 | 1.17 – 3.03 | 0.009 |
| SLC6A3 (rs10052016) | – | – | – | 0.64 | 0.45 – 0.90 | 0.009 |
| HTR2A (rs9567732) | – | – | – | 0.70 | 0.50 – 0.98 | 0.038 |
| DRD2 (rs12422191) | – | – | – | 2.10 | 1.31 – 3.36 | 0.002 |
| AIC Criteria | 958.41 | 930.10 | ||||
| b: Logistic Regression for New Experimentation Stratified by Cognitive Susceptibility | ||||||
|---|---|---|---|---|---|---|
| Committed Never Smoker (N = 872) |
Susceptible (N = 246) |
|||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | |
| Social and psychological factors | ||||||
| Age 13 at baseline | 2.04 | 1.31 – 3.20 | 0.002 | 3.00 | 1.61 – 5.62 | 0.001 |
| Male | 2.08 | 1.37 – 3.17 | 0.001 | 1.64 | 0.89 – 3.02 | 0.116 |
| Outcome expectations | 2.87 | 1.63 – 5.06 | < 0.001 | 0.93 | 0.48 – 1.80 | 0.825 |
| Household member smokes | 1.52 | 1.15 – 2.01 | 0.003 | 1.63 | 1.12 – 2.38 | 0.011 |
| Risk taking tendencies | 1.46 | 1.14 – 1.88 | 0.003 | 1.17 | 0.83 – 1.64 | 0.379 |
| Gene (SNP) | ||||||
| SNAP25 (rs8119844) | 0.21 | 0.08 – 0.53 | 0.001 | 0.42 | 0.04 – 4.10 | 0.454 |
| OPRM1 (rs9322451) | 0.54 | 0.32 – 0.92 | 0.025 | 0.54 | 0.26 – 1.11 | 0.095 |
| HTR1B (rs6297) | 2.10 | 1.20 – 3.66 | 0.009 | 1.44 | 0.58 – 3.58 | 0.433 |
| SLC6A3 (rs10052016) | 0.85 | 0.56 – 1.28 | 0.429 | 0.37 | 0.20 – 0.68 | 0.002 |
| HTR2A (rs9567732) | 0.80 | 0.53 – 1.21 | 0.287 | 0.46 | 0.25 – 0.86 | 0.015 |
| DRD2 (rs12422191) | 1.49 | 0.81 – 2.74 | 0.199 | 4.88 | 1.89 – 12.56 | 0.001 |
| AIC Criteria | 640.95 | 291.66 | ||||
New experimentation includes participants who reported that they had not experimented at baseline and reported that they had experimented on one or more of the four subsequent contacts.
Including the genes in the model (Table 4a) did not significantly attenuate the impact of the psychosocial factors as seen by overlapping confidence intervals. Being cognitively susceptible to smoking was the strongest predictor of new experimentation (OR=2.55; 95% CI: 1.74–3.73), followed by being 13 at baseline (OR=2.27; 95% CI: 1.59–3.23), male (OR=1.96; 95% CI: 1.40–2.75), holding positive outcome expectations about smoking (OR=1.65; 95% CI: 1.07–2.53), living with somebody who smokes (OR=1.53; 95% CI: 1.23–1.90) and exhibiting risk taking tendencies (OR=1.30; 95% CI: 1.07–1.59). All six SNPs were significantly associated with new experimentation. Four SNPs, within genes rs9322451 on OPMR1 (OR=0.53; 95% CI: 0.35–0.81), rs10052016 on SLC6A3 (OR=0.64; 95% CI: 0.45–0.90), rs9567732 on HTR2A (OR=0.70; 95% CI: 0.50–0.98) and rs8119844 on SNAP25 (OR=0.26; 95% CI: 0.11–0.61) were protective, while two others, specifically rs6297 on HTR1B (OR=1.89; 95% CI: 1.17–3.03) and rs12422191 on DRD2 (OR=2.10; 95% CI: 1.31–3.36) were associated with increased risk. Participants who carried six risk alleles were just over two times (OR=2.25; 95% CI: 1.62–3.13) more likely to have experimented since baseline, compared to participants with five or fewer risk alleles (data not shown).
The interaction (cognitive susceptibility by genetic risk score) was significant (OR=1.59; 95% CI: 1.06–2.39; data not shown), therefore, we stratified by cognitive susceptibility status (Table 4b). We found that among committed never smokers, all psychosocial variables maintained statistical significance as did three SNPs: rs9322451 on OPRM1 (OR=0.55; 95% CI: 0.33–0.94), rs6297 on HTR1B (OR=2.10; 95% CI: 1.20–3.66) and rs8119844 on the SNAP25 gene (OR=0.21; 95% CI: 0.08–0.53). However, among susceptible youth, only two of the variables maintained significance (being 13 years at baseline and living with a smoker), as did three different SNPs, specifically, rs10052016 on SLC6A3 (OR=0.37; 95% CI: 0.20–0.68), rs9567732 on HTR2A (OR=0.46; 95% CI: 0.25–0.86), and rs12422191 on DRD2 (OR=4.88; 95% CI: 2.89–12.56).
In each model adding the genetic information to the psychosocial factors increased the area under the Receiver Operating Characteristic (ROC) curve (Table 5), this increase was greatest in the model based on susceptible youth only. Among susceptible youth, adding the genetic information resulted in a 7% AUC increase from 69% (95% CI: 62–76) to 76% (95% CI: 70–82), compared to a 4% AUC increase from 70% (95% CI: 65–75) to 74% (95% CI: 69–79) among the committed never smokers (or non-susceptible youth).
Table 5.
Area Under the Curve (AUC) and 95% CI for each model’s ROC Curve
| Psychosocial factors only |
Psychosocial and genetic factors |
|||
|---|---|---|---|---|
| AUC | 95% CI | AUC | 95% CI | |
| All | 0.74 | 0.70 – 0.78 | 0.77 | 0.73 – 0.81 |
| Committed Never Smoker | 0.70 | 0.65 – 0.75 | 0.74 | 0.69 – 0.79 |
| Susceptible | 0.69 | 0.62 – 0.76 | 0.76 | 0.70 – 0.82 |
Discussion
In this longitudinal study, we evaluated the added predictive value of select variants of candidate smoking-related phenotype genes in the dopamine, serotonin and opioid pathways on smoking experimentation as well as the impact of gene-environment interaction. Our results confirm the relationship between experimenting with cigarettes and established psychosocial and demographic risk factors and identify six SNPs significantly associated with new experimentation: three in the dopamine pathway, two in the serotonin pathway and an opioid receptor. Adding these SNPs to the multivariable models significantly improved the risk prediction of each model examined. Participants who carried six risk alleles were two times more likely to have experimented since baseline, compared to participants with five or fewer risk alleles. Our overall experimentation rate of 26.3%, and 44.3% among those who were 13 at baseline, appears comparable to rates reported elsewhere. In Brazil, 30% of 7th to 10th grade (39) and up to 50% of 15 to 16 year-old European students (40) report experimenting
Overall cognitive susceptibility to smoking, a psychosocial risk factor that assesses both peer influence and behavioral intentions (7) and an early phase in the transition from never to ever smoking (5) was the strongest psychosocial risk factor (over 2-fold) for experimentation. Risk factors associated with being susceptible to smoking include having friends who smoke, living with a smoker, having lower subjective social status and more detentions at school, and reporting more temptations to try smoking (41). We, and others, have shown that smoking initiation is strongly influenced by gender and age (5,42,43). Higher perceived outcome expectations, which assess the perceived social benefits from smoking, are associated with both intentions to smoke (44) and experimenting with cigarettes (5,45). Our data confirmed a roughly 70% increased risk of experimenting associated with positive outcome expectations. Among committed never smokers only, the risk increased to almost 3-fold and holding positive outcome expectations exerted the strongest independent influence on experimenting among all psychosocial risk factors examined. Having a parent who smokes is a strong, consistent predictor of smoking initiation among youth (27–29). Consistent with previous work, risk taking tendencies, which tend to peak in adolescences (46), predicted experimentation (20,47,48).
Next we focus on the results from the stratified analyses. Among committed never smokers three of the SNPs (OPRM1, SNAP25, HTR1B) were associated with experimentation as were all the psychosocial factors. However, among susceptible youth, it appears that the psychosocial factors are less relevant because only increasing age and living with a smoker had an impact. In addition, three different SNPs (HTR2A, DRD2, SLC6A3) were associated with experimentation in this subgroup. Adding the genetic data improved the predictiveness of both models as assessed by the area under the curve; this improvement was more marked among susceptible participants. It must be noted that these genetic variants are not predicting a response to nicotine, rather sensation seeking, risk taking or curiosity, as this analysis only included participants who had not tried cigarettes at baseline. In summary, psychosocial risk factors appear to be more important predictors of experimenting among the committed never smokers (non-susceptible youth) relative to the susceptible youth, based on previous work (5) and the current analysis. The genetic risk factors appear to be more important predictors of experimenting among the susceptible youth than the committed never smokers, as fewer psychosocial risk factors maintained significance among the susceptible youth compared to committed never smokers.
The dopaminergic system is important in mediating the pleasurable feelings of reward and novelty seeking behavior. Individuals with a high level of such behaviors are likely to be more susceptible to cues for potential reward, such as initiating smoking. We found that a SNP on DRD2 (rs12422191) was significantly associated with new experimentation; in the analysis stratified on susceptibility status, DRD2 maintained significance among participants identified as susceptible to smoking only, among whom this variant exhibited the strongest association with smoking behavior (OR=4.88) among all genes and psychosocial factors examined.
There are conflicting reports about the association between DRD2 and smoking behavior among both adolescents and adults (49–52). Among adults, we found rs1800497 on DRD2 (which is in perfect LD with rs12422191 for both CEU and Mexican populations) was associated with continuation of smoking. Carriers of the rare allele are reported to exhibit an earlier age at onset of smoking (51) and in an adolescent Caucasian population, to be associated with progression to smoking (53). Neuroimaging findings support a functional role of the gene in regulation of DRD2 availability in the frontal cortex (54), and Noble (55) has shown that carriers of at least one variant allele have 40% fewer receptors relative to wildtype carriers.
The dopamine transporter gene (SLC6A3) regulates dopamine reuptake into presynaptic terminals and has also been studied extensively in relation to smoking behavior and cessation (56). We found the rare allele of SLC6A3 (rs10052016) to be protective against experimentation in susceptible participants. The 9 repeat allele of SLC6A3 VNTR sequence in the 3’ UTR has previously shown to have a protective in several diverse populations, including young Israeli women (57) and adolescents (58).
The opioid receptors and their endogenous ligands have long been implicated in addiction, sensation seeking behaviors, and smoking initiation (59). The OPMR1 variant had a protective effect among the full sample and among committed never smokers, but was not significantly associated with new experimentation among susceptible youth.
Gene variants in serotonin receptors are logical candidates for studying inter-individual variation in smoking initiation, since the neurotransmitter plays a role in modulating social behavior. The 5-hydroxytryptamine (serotonin) receptor HTR1B variant, rs6297, was associated with an increased risk of new experimentation among the full sample (OR=1.89) and just over a two-fold increased risk of new experimentation among the committed never smokers. This synonomous exonic SNP has been associated with aggressive behavior in children (60), and impulsivity, although not consistently (61).
The SNAP complex mediates presynaptic vesicle trafficking and participates in the release of dopamine and other neurotransmitters (62). The SNAP25 (rs8119844) variant exhibited the strongest association with new experimentation of any of the genes and psychosocial factors we examined in the overall analysis, and among committed never smokers. Committed never smokers who carry the minor AA allele are almost five times more likely to have tried cigarettes than their peers. To the best of our knowledge, the relationship between SNAP25 and experimenting with cigarettes has not been reported in the literature before. However, SNAP25 is associated with negative affect (62) and with ADHD (63), both of which are associated with an increased risk for smoking among adolescents (64,65).
The current study has both strengths and limitations. The prospective design allowed us to examine experimentation at follow-up among participants who had not experimented at baseline and to test for moderation effects. Further, participants were from a population-based cohort and included roughly equal numbers of girls and boys. All psychosocial constructs were assessed using validated measures, and data were collected using PDAs to ensure participant privacy and quality of the data. The participants represent a large ethnically homogenous and predominantly low-income sample of Mexican origin youth, an understudied population. A final strength is the high retention rate: 87% of the youth provided data on all five contacts.
Conversely, the main limitation of this study is the lack of an independent replication sample; thus we must consider our findings preliminary. However given the large number of SNPs tested, we did adjust the significance level for each SNP included in this analysis using a BFDP approach (30). Although the percent of SNPs failing the H-W test is higher than expected, we did not observe a systematic pattern with regards the SNPs that failed suggesting it did not impact our results. A third limitation is that participants were all of Mexican origin, and results may not generalize to other ethnicities. In addition, we did not have biochemical validation of the participants’ smoking status (e.g., salivary cotinine). However, we informed participants during the consent process that they might be selected to provide a saliva sample to check their smoking status; this "bogus pipeline" procedure has been shown to increase the validity of self-reported smoking status (66).
Conclusion
This is one of the first studies to examine the role of both genetic and non-genetic factors associated with the transition from never smoking to cigarette experimentation among Mexican origin youth. The results confirm and extend previous findings (e.g. the strong association between SNAP25 and experimenting) and suggest that the impact of both psychosocial and genetic factors differs depending upon the susceptibility status of the adolescents. Our results need to be confirmed with an independent sample of Mexican origin youth (in particular with regards to SNAP25 as in general with recessive genes the number of risk SNPs is smaller compared to dominant) and among youth of other ethnic backgrounds, as they have implications for development of culturally-specific interventions. In conclusion, with regards the genes we identified, which are related to the very beginning of the smoking trajectory, when youth are contemplating whether to try cigarettes (i.e. risk of experimentation), there appear to be differences in genotype between youth who think they will try cigarettes compared to their peers who think they will not try cigarettes in the future.
Supplementary Material
Acknowledgements
We thank the cohort staff for conducting all field interviews and maintaining the high participation rates. We thank the participants for providing the data and their parents for permitting their children to join the study. Without their support this research would not be possible. Finally, we thank Dr. Amy R. Spelman, who created Figure 1. Dr. Margaret R. Spitz, the PI, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis
Funding: This research is supported by the National Cancer Institute grants [CA105203 to MRS, CA126988 to AVW]; funds collected pursuant to the Comprehensive Tobacco Settlement of 1998 and appropriated by the 76th legislature to The University of Texas M. D. Anderson Cancer Center; by the Caroline W. Law Fund for Cancer Prevention, and by the Dan Duncan Family Institute. The funders did not contribute to the design and conduct of the study, the data collection, analysis, and interpretation of the data, the preparation, review, or approval of the manuscript.
References
- 1.Coyle YM. Lifestyle, genes, and cancer. Methods Mol Biol. 2009;472:25–56. doi: 10.1007/978-1-60327-492-0_2. [DOI] [PubMed] [Google Scholar]
- 2.Wiencke JK, Thurston SW, Kelsey KT, Varkonyi A, Wain JC, Mark EJ, et al. Early age at smoking initiation and tobacco carcinogen DNA damage in the lung. J Natl Cancer Inst. 1999;91:614–619. doi: 10.1093/jnci/91.7.614. [DOI] [PubMed] [Google Scholar]
- 3.Wilkinson AV, Schabath MB, Prokhorov AV, Spitz MR. Factors associated with smoking initiation. Cancer Causes Control. 2007;18:635–644. doi: 10.1007/s10552-007-9008-6. [DOI] [PubMed] [Google Scholar]
- 4.Etzel CJ, Bach PB. Estimating individual risk for lung cancer. Semin Respir Crit Care Med. 2011;32:3–9. doi: 10.1055/s-0031-1272864. [DOI] [PubMed] [Google Scholar]
- 5.Spelman AR, Spitz MR, Kelder SH, Prokhorov AV, Bondy ML, Frankowski RF, et al. Cognitive susceptibility to smoking: Two paths to experimenting among Mexican origin youth. Cancer Epidemiol Biomarkers Prev. 2009;18:3459–3467. doi: 10.1158/1055-9965.EPI-09-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson C. Cognitive susceptibility to smoking and initiation of smoking during childhood: A longitudinal study. Prev Med. 1998;27:129–134. doi: 10.1006/pmed.1997.0255. [DOI] [PubMed] [Google Scholar]
- 7.Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15:355–361. doi: 10.1037//0278-6133.15.5.355. [DOI] [PubMed] [Google Scholar]
- 8.McNeill AD, Jarvis MJ, Stapleton JA, Russell MA, Eiser JR, Gammage P, et al. Prospective study of factors predicting uptake of smoking in adolescents. J Epidemiol Community Health. 1989;43:72–78. doi: 10.1136/jech.43.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giovino GA, Henningfield JE, Tomar SL, Escobedo LG, Slade J. Epidemiology of tobacco use and dependence. Epidemiol Rev. 1995;17:48–65. doi: 10.1093/oxfordjournals.epirev.a036185. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin EA, Lee L, Evans N, Pierce JP. Smoking initiation rates in adults and minors: United States, 1944-1988. Am J Epidemiol. 1994;140:535–543. doi: 10.1093/oxfordjournals.aje.a117280. [DOI] [PubMed] [Google Scholar]
- 11.Tienda M, Mitchell F, editors. Multiple origins, uncertain destines: Hispanics and the American Future. Washington DC: The National Academies Press; 2006. [PubMed] [Google Scholar]
- 12.Gfroerer J, Caraballo R. Racial/Ethnic Differences among youths in cigarette smoking and Susceptibility to start smoking -- United States, 2002—2004. MMWR. 2006;55:1275–1277. [PubMed] [Google Scholar]
- 13.Youth risk behavior surveillance system (YRBSS) results. [Accessed September 22, 2011]; [ http://apps.nccd.cdc.gov/YouthOnline/App/Default.aspx].
- 14.Maes HH, Neale MC, Kendler KS, Martin NG, Heath AC, Eaves LJ. Genetic and cultural transmission of smoking initiation: An Extended Twin Kinship Model. Behav Genet. 2006;36:795–808. doi: 10.1007/s10519-006-9085-4. [DOI] [PubMed] [Google Scholar]
- 15.Munafò MR, Johnstone EC. Genes and cigarette smoking. Addiction. 2008;103:893–904. doi: 10.1111/j.1360-0443.2007.02071.x. [DOI] [PubMed] [Google Scholar]
- 16.Heath AC, Madden PA, Slutske WS, Martin NG. Personality and the inheritance of smoking behavior: A genetic perspective. Behav Genet. 1995;25:103–117. doi: 10.1007/BF02196921. [DOI] [PubMed] [Google Scholar]
- 17.McGue M, Elkins I, Iacono WG. Genetic and environmental influences on adolescent substance use and abuse. Am J Med Genet. 2000;96:671–677. doi: 10.1002/1096-8628(20001009)96:5<671::aid-ajmg14>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 18.Madden PA, Pedersen NL, Kaprio J, Koskenvuo MJ, Martin NG. The epidemiology and genetics of smoking initiation and persistence: cross-cultural comparisons of twin study results. Twin Res. 2004;7:82–97. doi: 10.1375/13690520460741471. [DOI] [PubMed] [Google Scholar]
- 19.Martin CA, Kelly TH, Rayens MK, Brogli BR, Brenzel A, Smith WJ, et al. Sensation seeking, puberty, and nicotine, alcohol, and marijuana use in adolescence. J Am Acad Child Adolesc Psychiatry. 2002;41:1495–1502. doi: 10.1097/00004583-200212000-00022. [DOI] [PubMed] [Google Scholar]
- 20.Burt RD, Dinh KT, Peterson AV, Sarason IG. Predicting adolescent smoking: A prospective study of personality variables. Prev Med. 2000;30:115–125. doi: 10.1006/pmed.1999.0605. [DOI] [PubMed] [Google Scholar]
- 21.Wilkinson AV, Shete S, Bondy ML, Prokhorov AV, Spitz MR, Sargent JD. Exposure to smoking imagery in the movies and experimenting with cigarettes among Mexican origin youth. Cancer Epidemiol Biomarkers Prev. 2009;18:3435–3442. doi: 10.1158/1055-9965.EPI-09-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson AV, Spitz MR, Strom SS, Prokhorov AV, Barcenas CH, Cao Y, et al. Effects of nativity, age at migration, and acculturation on smoking among adult Houston residents of Mexican descent. Am J Public Health. 2005;95:1043–1049. doi: 10.2105/AJPH.2004.055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Norris AE, Ford K, Bova CA. Psychometrics of a brief acculturation scale for Hispanics in a probability sample of urban Hispanic adolescents and young adults. Hisp J Behav Sci. 1996;18:29–38. [Google Scholar]
- 24.Wilkinson AV, Waters AJ, Vasudevan V, Bondy ML, Prokhorov AV, Spitz MR. Correlates of smoking susceptibility among Mexican origin youth. BMC Public Health. 2008;8:337. doi: 10.1186/1471-2458-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Youth risk behavior surveillance system (YRBSS) questionnaires. [Accessed July 11, 2011]; [ http://www.cdc.gov/healthyyouth/yrbs/questionnaire_rationale.htm].
- 26.Dalton MA, Sargent JD, Beach ML, Bernhardt AM, Stevens M. Positive and negative outcome expectations of smoking: Implications for prevention. Prev Med. 1999;29:460–465. doi: 10.1006/pmed.1999.0582. [DOI] [PubMed] [Google Scholar]
- 27.Bricker JB, Peterson AV, Anderson MR, Leroux BG, Bharat RK, Sarason IG. Close friends’, parents’, and older siblings’ smoking: Reevaluating their influence on children’s smoking. Nicotine Tob Res. 2006;8:217–226. doi: 10.1080/14622200600576339. [DOI] [PubMed] [Google Scholar]
- 28.Fleming CB, Hyoshin K, Harachi TW, Catalano RF. Family processes for children in early elementary school as predictors of smoking initiation. J Adolesc Health. 2002;30:184–189. doi: 10.1016/s1054-139x(01)00327-5. [DOI] [PubMed] [Google Scholar]
- 29.Wilkinson AV, Shete S, Prokhorov AV. The moderating role of parental smoking on their children’s attitudes toward smoking among a predominantly minority sample: a cross-sectional analysis. Subst Abuse Treat Prev Policy. 2008;3:18. doi: 10.1186/1747-597X-3-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreek MJ, Nielsen DA, LaForge KS. Genes associated with addiction: alcoholism, opiate, and cocaine addiction. Neuromolecular Med. 2004;5:85–108. doi: 10.1385/NMM:5:1:085. [DOI] [PubMed] [Google Scholar]
- 31. [Accessed September 29, 2011]; http://pid.nci.nih.gov/.
- 32. [Accessed September 29, 2011]; http://www.hapmap.org.
- 33.Wakefield J. A Bayesian measure of the probability of false discovery in genetic epidemiology studies. Am J Hum Genet. 2007;81:208–227. doi: 10.1086/519024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nat Genet. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- 35.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 36.Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. Am J Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- 37.Nassir R, Kosoy R, Tian C, White PA, Butler LM, Silva G, et al. An ancestry informative marker set for determining continental origin: validation and extension using human genome diversity panels. BMC Genet. 2009;10:39. doi: 10.1186/1471-2156-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian C, Kosoy R, Lee A, Ransom M, Belmont JW, Gregersen PK, et al. Analysis of East Asia genetic substructure using genome-wide SNP arrays. PLoS One. 2008;3:e3862. doi: 10.1371/journal.pone.0003862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Silva MA, Rivera IR, Carvalho AC, Guerra AH, Jr, Moreira TC. The prevalence of variables associated with smoking in children and adolescents. J Pediatr. 2006;82:365–370. doi: 10.2223/JPED.1525. [DOI] [PubMed] [Google Scholar]
- 40.Hibell B, Andersson B, Bjarnasson T, Ahlstrom S, Balakireva O, Kokkevi A, et al., editors. ESPAD Report: Alcohol and Other Drug Use among Students in 35 European countries (2003) Stockholm: The Swedish Council for Information on Alcohol and Other Drugs (CAN), The Pompidou Group at the Council of Europe; 2004. [Google Scholar]
- 41.Wilkinson AV, Waters AJ, Vasudevan V, Prokhorov AV, Bondy ML, Spitz MR. Correlates of smoking susceptibility among Mexican origin youth. BMC Public Health. 2008;8:337. doi: 10.1186/1471-2458-8-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elder JP, Campbell NR, Litrownik AJ, Ayala GX, Slymen DJ, Parra-Medina D, et al. Predictors of cigarette and alcohol susceptibility and use among Hispanic migrant adolescents. Prev Med. 2000;31:115–123. doi: 10.1006/pmed.2000.0693. [DOI] [PubMed] [Google Scholar]
- 43.Mucha L, Stephenson J, Morandi N, Dirani R. Meta-analysis of disease risk associated with smoking, by gender and intensity of smoking. Gender Med. 2006;3:279–291. doi: 10.1016/s1550-8579(06)80216-0. [DOI] [PubMed] [Google Scholar]
- 44.Abroms L, Simons-Morton B, Haynie DL, Chen R. Psychosocial predictors of smoking trajectories during middle and high school. Addiction. 2005;100:852–861. doi: 10.1111/j.1360-0443.2005.01090.x. [DOI] [PubMed] [Google Scholar]
- 45.Brady SS, Song AV, Halpern-Felsher BL. Adolescents report both positive and negative consequences of experimentation with cigarette use. Prev Med. 2008;46:585–590. doi: 10.1016/j.ypmed.2008.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hair EC, Park MJ, Ling TJ, Moore KA. Risky behaviors in late adolescence: co-occurrence, predictors, and consequences. J Adolesc Health. 2009;45:253–261. doi: 10.1016/j.jadohealth.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Prokhorov AV, de Moor CA, Hudmon KS, Hu S, Kelder SH, Gritz ER. Predicting initiation of smoking in adolescents: evidence for integrating the stages of change and susceptibility to smoking constructs. Addict Behav. 2002;27:697–712. doi: 10.1016/s0306-4603(01)00203-9. [DOI] [PubMed] [Google Scholar]
- 48.Lejuez CW, Aklin WM, Bornovalova MA, Moolchan ET. Differences in risk-taking propensity across inner city adolescent ever- and never-smokers. Nicotine Tob Res. 2005;7:71–79. doi: 10.1080/14622200412331328484. [DOI] [PubMed] [Google Scholar]
- 49.Laucht M, Becker K, Frank J, Schmidt MH, Esser G, Treulein J, et al. Genetic variation in dopamine pathways differentially associated with smoking progression in adolescence. J Am Acad Child Adolesc Psychiatry. 2008;47:673–681. doi: 10.1097/CHI.0b013e31816bff77. [DOI] [PubMed] [Google Scholar]
- 50.Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2002;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- 51.Comings DE, Ferry L, Bradshaw-Robinson S, Burchette R, Chiu C, Muhleman D. The dopamine D2 receptor (DRD2) gene: a genetic risk factor in smoking. Pharmacogenetics. 1996;6:73–79. doi: 10.1097/00008571-199602000-00006. [DOI] [PubMed] [Google Scholar]
- 52.Zuo Y, Gilbert DG, Rabinovich NE, Riise H, Needham R, Huggenvik JI. DRD2-related TaqIA polymorphism modulates motivation to smoke. Nicotine Tob Res. 2009;11:1321–1329. doi: 10.1093/ntr/ntp141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Audrain-McGovern J, Lerman C, Wileyto EP, Shields PG. Interacting effects of genetic predisposition and depression on adolescent smoking progression. Am J Psychiatry. 2004;161:1224–1230. doi: 10.1176/appi.ajp.161.7.1224. [DOI] [PubMed] [Google Scholar]
- 54.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noble EP. D2 dopamine receptor gene in psychiatric and neurologic disorders and its phenotypes. Am J Med Genet B Neuropsychiatr Genet. 2003;116B:103–125. doi: 10.1002/ajmg.b.10005. [DOI] [PubMed] [Google Scholar]
- 56.Stapleton JA, Sutherland G, O'Gara C. Association between dopamine transporter genotypes and smoking cessation: a meta-analysis. Addict Biol. 2007;12:221–226. doi: 10.1111/j.1369-1600.2007.00058.x. [DOI] [PubMed] [Google Scholar]
- 57.Segman RH, Kanyas K, Karni O, Lerer E, Goltser-Dubner T, Pavlov V, et al. Why do young women smoke? IV. Role of genetic variation in the dopamine transporter and lifetime traumatic experience. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:533–540. doi: 10.1002/ajmg.b.30507. [DOI] [PubMed] [Google Scholar]
- 58.Timberlake DS, Haberstick BC, Lessem JM, Smolen A, Ehringer M, Hewitt JK, et al. An association between the DAT1 polymorphism and smoking behavior in young adults from the National Longitudinal Study of Adolescent Health. Health Psychol. 2006;25:190–197. doi: 10.1037/0278-6133.25.2.190. [DOI] [PubMed] [Google Scholar]
- 59.Zhang L, Kendler KS, Chen X. The mu-opioid receptor gene and smoking initiation and nicotine dependence. Behav Brain Funct. 2006;4(2):28. doi: 10.1186/1744-9081-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Davidge KM, Atkinson L, Douglas L, Lee V, Shapiro S, Kennedy JL, et al. Association of the serotonin transporter and 5-HTlDb receptor genes with extreme, persistent and pervasive aggressive behaviour in children. Psychiatry Genet. 2004;14:143–146. doi: 10.1097/00041444-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 61.Conner TS, Jensen KP, Tennen H, Furneaux HM, Kranzler HR, Covault J. Functional polymorphisms in the serotonin 1B receptor gene (HTR1B) predict self-reported anger and hostility among young men. Am J Med Genet B Neuropsychiatr Genet. 2010;53B:67–78. doi: 10.1002/ajmg.b.30955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sheese BE, Voelker P, Posner MI, Rothbart MK. Genetic variation influences on the early development of reactive emotions and their regulation by attention. Cogn Neuropsychiatry. 2009;14:332–355. doi: 10.1080/13546800902844064. [DOI] [PubMed] [Google Scholar]
- 63.Coghill D, Banaschewski T. The genetics of attention-deficit/hyperactivity disorder. Expert Rev Neurother. 2009;9:1547–1565. doi: 10.1586/ern.09.78. [DOI] [PubMed] [Google Scholar]
- 64.Audrain-McGovern J, Rodriguez D, Kassel JD. Adolescent smoking and depression: evidence for self-medication and peer smoking mediation. Addiction. 2009;104:1743–1756. doi: 10.1111/j.1360-0443.2009.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modesto-Lowe V, Danforth JS, Neering C, Easton C. Can we prevent smoking in children with ADHD: a review of the literature. Conn Med. 2010;74:229–236. [PubMed] [Google Scholar]
- 66.Murray DM, O'Connell CM, Schmid LA, Perry CL. The validity of smoking self-reports by adolescents: A reexamination of the bogus pipeline procedure. Addict Behav. 1987;12:7–15. doi: 10.1016/0306-4603(87)90003-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.