Abstract
Increases in colonization with serotypes of Streptococcus pneumoniae not contained within the 7-valent pneumococcal conjugate vaccine (PCV) have been reported among children following introduction. Serotype 6C has emerged as prevalent in nasopharyngeal colonization and acute otitis media (AOM), though it is uncommonly recovered from children with invasive pneumococcal disease. Vaccine serotypes within PCV7 have been replaced by nonvaccine serotypes without significant changes in the overall carriage rate. We hypothesize 1) that serotypes vary in their ability to evade host defenses and establish AOM following colonization and 2) the observed reduction in pneumococcal otitis results from a reduced disease potential by some ‘replacement serotypes’. We compared the capacity of S. pneumoniae serotypes 6C and 19A to produce experimental otitis media (EOM) in a chinchilla model. The proportion of chinchillas that developed culture positive EOM and density of middle ear infection was evaluated. EOM was found in 28/82 (34%) ears challenged with 6C compared to 13/18(72.2%) with 19A [p=0.0003]. When disease due to 6C did occur, it was characterized by lowdensity infection. Our findings demonstrate that challenge with serotype 6C results in EOM less frequently than 19A. These data support the need for greater knowledge regarding differences among serotypes to produce AOM.
Keywords: Streptococcus pneumoniae, complement, virulence
1. Introduction
A newly identified pneumococcal serotype, 6C, has emerged as one of the prevalent serotypes in the nasopharynx among children and adults in the US and Brazil [1] [2] [3] [4, 5]. These isolates were previously serotyped as 6A pneumococci by quellung reaction, but their capsule biosynthetic locus differs from that of serotype 6A pneumococci with a single substitution of a galactose unit with a glucose unit [6]. Factor 6d antisera as well as monoclonal antibodies can now distinguish serotype 6A and 6C isolates [7].
Recent studies demonstrate an increase in prevalence of serotype 6C in the nasopharynx subsequent to the introduction of PCV7 [8]. In Massachusetts, approximately 3% of children < 7 yrs are colonized with serotype 6C [3]. Serotype 6C has been reported as one of the common serotypes causing pneumococcal otitis media in children with recurrent AOM or treatment failure [9]. Its importance as a cause of invasive pneumococcal disease (IPD) is less well established. In Spain and US, some researchers have observed a small increase in IPD, however it remains a relatively uncommon cause of IPD in children and adults [1, 2, 10–12]. Surveillance studies in Massachusetts identified serotype 6C in 0.9% of 557 IPD cases in children < 5 years between 2001 and 2011 [13]. Most recently Green and colleagues have reported 44 cases of IPD due to 6C between 2000 and 2009 [5]. In Brazil, Serotype 6C was recovered from 16 of 709 cases of pneumococcal meningitis over an 11 year period [2]. Serotype 19A is the most common serotype in the nasopharynx of Massachusetts children, especially sequence type (ST) ST199. ST199 has emerged as prevalent among pneumococcal isolates causing nasopharyngeal colonization, IPD and OM.[9, 14] [15–20]. Serotype 19A has been reported as the most common cause of pneumococcal otitis [9] consistent with our previous observation that colonization followed by barotraumas with serotype 19A resulted in frequent experimental otitis media in our model [21] [22].
Despite the observations that overall pneumococcal colonization prevalence has not declined in children in the US and other countries [3, 23] [24], a substantial reduction in AOM has been reported [25, 26] (Abstract # 1343, presented at IDSA Oct,2011). We hypothesize that serotypes vary in their ability to evade host defenses and establish AOM following colonization and that the observed reduction in pneumococcal otitis results from a reduction in the potential for some of the ‘replacement serotypes’ to cause AOM following colonization (compared to PCV7 serotypes). Although this reduced potential will not be seen in each individual serotype, the overall distribution will result in a lower incidence of AOM following colonization. We compared the relative virulence of 6C and 19A in production of middle ear disease in a well established chinchilla experimental otitis media model Our goals were to evaluate difference in capacity to produce disease following colonization and to determine if such differences contribute to the reduction in pneumococcal AOM despite the unchanged prevalence of colonization following the introduction of PCV7.
2. Materials and methods
2.1 Bacterial isolates and growth conditions
Serotype 6C S. pneumoniae strains were isolated from patients with either invasive pneumococcal disease or asymptomatic nasopharyngeal (NP) carriage in Massachusetts. Colony morphology and optochin sensitivity were used to initially identify bacteria as S. pneumoniae. Six 6C strains were selected for further animal studies. These six isolates included four sequence types as well as carriage and invasive isolates. Sequence types 1292 and 1379 were the most common reported among 57 disease causing strains collected from 8 children’s hospitals [5]; 1379 is also the most common among our collection of 6C isolates recovered from NP cultures in Massachusetts children (unpublished). ST 1390 and 1692 were chosen from our collection of carriage isolates. Serotypes 19A isolates, from clonal complex 199, were used for comparison. Characteristics of the eight strains used for animal studies are shown in Table 1. We have previously evaluated the 19A strains in our animal model [21, 22].
Table 1.
Serotype, source and MLST of strains used in animal model
| Strain ID | Serotype | Source | MLST |
|---|---|---|---|
| PT8114 | 6C | Carriage | 1390 |
| NP7029 | 6C | Carriage | 1390 |
| 05AR0443 | 6C | Invasive | 1379 |
| 07AR0125 | 6C | Invasive | 1292 |
| BR1064 | 6C | Carriage | 1692 |
| ND6012 | 6C | Carriage | 1692 |
| FG23 | 19A | Carriage | 199 |
| SP4564 | 19A | Invasive | 199 |
2.2 Serotyping
Pneumococcal isolates were initially serotyped at our laboratory, using the quellung reaction with Danish antisera (Statens Seruminstitute, Copenhagen, Denmark). Intial identification of serotype 6C was performed with factor 6d antiserum and additional confirmation of the serotype was provided by Dr. Moon Nahm’s laboratory by inhibition ELISA using monoclonal antibodies Hyp6AG1 and Hyp6AM3 that specifically reacted with 6C capsule [7].
2.3 Antibodies and complement reagents
Human complement used in the flow cytometry experiments was purchased from Sigma Chemical Company (St. Louis, MO). In some experiments, the alternative complement pathway was selectively activated by adding EGTA and Mg2+ to serum, both to a final concentration of 10 mM [27]. Heat inactivation of human complement (56 °C for 30 min) was used as a control in some experiments. Fluorescein isothiocyanate (FITC) - conjugated sheep anti-human C3c and anti-human C4 (Biodesign) were used in flow cytometry assays as described previously [28] [29]. Anti-goat IgG, anti-human IgG, and anti-mouse IgG conjugated to FITC (Sigma) were used as secondary antibodies.
2.4 Multilocus sequence typing
The genotypes of the pneumococcal strains used in this study were analyzed by multilocus sequence typing (MLST) [30]. MLST of the serotype 6C NP strains was performed at the Imperial College London, whereas the invasive strains were first sequenced at the Partners Center for Personalized Genomic Medicine and MLST was determined using web-based software (http://www.mlst.net/)
2.5 Evaluation of complement and antibody deposition on surface of S. pneumoniae
Flow cytometry was utilized to quantitate C3 binding to the surface of pneumococci, as described previously [31]. Briefly, 2 × 108 bacteria were washed twice in HBSS (Cambrex, Inc.) containing 2% bovine serum albumin (BSA) (Sigma) and Human complement was added to a final concentration of 5% and allowed to incubate before fixing the cells in paraformaldehyde. The C3 fragments bound to the bacterial surface was detected using anti-human C3c-FITC (Biodesign, Saco, ME) as described previously [32].
2.6 Experimental chinchilla otitis media model
All procedures and manipulations were performed using sedation analgesia with a mixture of ketamine and xylazine given intramuscularly in accordance with approved IACUC protocols at Boston University Medical Center. Isolates of S. pneumoniae grown to the mid-log phase were introduced into each nare followed by barotrauma, created by withdrawing 250 μl of air from the middle ear through the superior bullae of both ears, which promotes ascension of bacteria into the middle ear. Daily tympanometry and otomicroscopy were performed to determine the presence of fluid behind the tympanic membrane and signs of infection including bulging of memebrane and erythema. Once abnormality was identified, the middle ear cavity was accessed 48–72 hrs later as described previously [33]and quantitative cultures were obtained. The lower limit of detection of viable organisms in MEF using this dilution series was 100 colony forming units (CFU)/ml [29]. Direct and indirect ear examination was performed every 3 to 4 days until the middle ear cultures were sterile on two consecutive samples.
2.7 Statistical analysis
Fisher’s exact and the Rank sum tests were used to calculate statistical significance for the differences in the proportions of culture-positive middle ears in animals challenged with 6C or 19A strains. Geometric mean fluorescence (GMF) intensities of C3 deposition with 95% confidence intervals were analyzed. In all analyses, P values of <0.05 were considered to indicate a statistically significant difference.
3. Results
3.1 Complement binding to Serotype 6C compared with 19A and 15BC
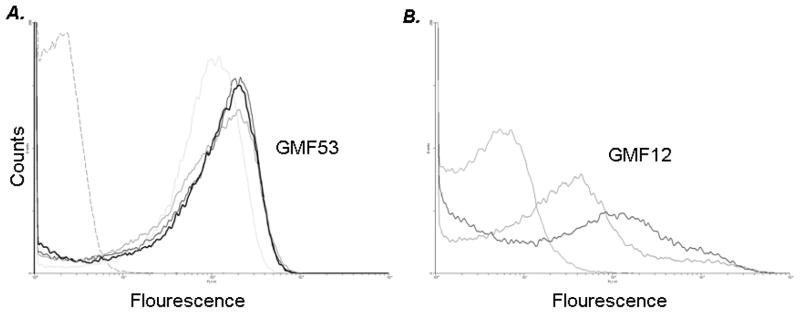
As prior studies [31] correlated C3 binding with capacity to produce experimental otitis media (EOM) in our model, we evaluated C3 binding to the four carriage and two invasive 6C isolates and two 19A strains using flow cytometry. The mean geometric C3 fluorescence cluster was 54 for the six 6C strains with a range of 47–58 and for the two 19A strains it was 12 No differences in C3 binding between ST or isolate source (IPD or carriage) were observed between isolates. All six 6C strains demonstrated higher C3 binding than either of the 19A strains. Control experiments performed with no complement showed minimal fluorescence (<10) (data not shown). We also measured binding of IgG in the human complement source to exclude the possibility that differences in C3 binding between the invasive and carriage isolates resulted from differences in antibody binding and no differences were observed (data not shown). Thus, the 6C strains bound high amounts of C3, while 19A isolates bound lower amounts of C3. C3 binding histograms to four randomly selected 6C and two 19A isolates following incubation with human complement are shown in Fig 1.
Figure 1. Mean Geometric Complement binding to 6C and 19A strains.
C3 binding to serotype 6C (n=4), 19A(n=2) isolates. A. Bacteria were incubated with 8% human complement (HC). C3 binding to the four 6C strain is illustrated in A. The isotype control (no human complement in the reaction mixture) is illustrated by broken grey line on the left of histogram. B. Bacteria were incubated with 8% human complement (HC). C3 binding to the two 19A strains is illustrated in B. The isotype control (no human complement in the reaction mixture) is illustrated by solid grey line on the left of histogram. Data shown is representative of two separate and comparable experiments. The x-axis represents fluorescence on a log10 scale and the y-axis the number of events (counts). Geometric mean fluorescence (GMF) for the 4 6C strains and 2 19A is included.
3.2 Nasopharyngeal colonization with serotype 6C compared to 19A
Colonization is a critical step in the disease process. We compared the density of NP colonization among the six 6C isolates and two 19A isolates following intranasal challenge with ~107CFU/100μl per nare and no significant difference in NP colonization was observed among or between 6C and 19A isolates, despite differences in C3 binding. NP lavage at 24 hours following challenge identified a mean concentration of 104 CFU/ml in all eight groups of animals. By day 4 the density of NP colonization increased to 105–106CFU/ml in all groups; there was no difference in animals challenged with 19A or 6C (data not shown).
3.3 Serotype 6C S. pneumoniae demonstrate reduced capacity to ascend Eustachian tube and produce middle ear disease following barotrauma compared to serotype 19A
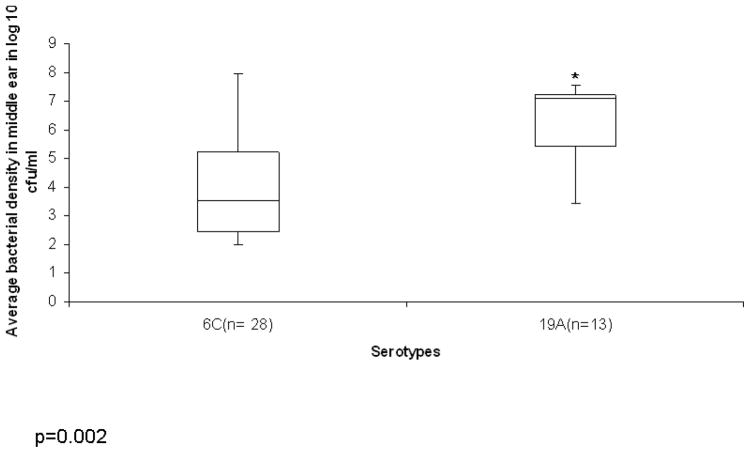
The 6C strains were of four different MLST types, the 19A strains were both MLST 199. The source, serotype, and MLST of the different strains used in the animal model and flow studies are shown in Table 1. EOM was observed in 28/82 (34%) ears challenged with 6C compared to 13/18(72.2%) challenged with 19A [p=0.0003] (Table 2). When culture positive middle ear disease due to serotype 6C was observed, it was characterized by low-density infection (~10E3) as compared to culture positive disease due to 19A (~10E6). The average middle ear bacterial density in animals with culture positive disease amongst serotype 6C and 19A were statistically different (p=0.002) and are shown in Fig 2. 3/10 (30%) animals developed bacteremia in the 19 A groups as compared to 5/41(12.1%) in the 6C groups (p= 0.23).
Table 2.
Culture positive middle ear disease following barotrauma by serotype
| Serotype | Strains (No.) a | No. ears challenged | Cx+ MEFb/No. challenged (%) Day 7–8 |
Cx+ MEF/No. challenged (%) Day 11–12 |
Cx+ MEF/No. challenged (%) Day 14–15 |
No Blood cx+/No. of animal (%) |
|---|---|---|---|---|---|---|
| 6C | 6 | 82 | 28/82 (34%) | 30/82 (36.5%) | 21/82 (25.6%) | 5/41 (12.1%) |
| 19A | 2 | 18 | 13/18* (72.2%) | 14/18 (77.7%) | 14/18 (77.7%) | 3/10** (30%) |
p=0.0003 (19A vs 6C)
p=0.23 (bacteremia 19A vs 6C)
No- Number
Cx+ - culture positive; MEF- Middle ear fluid
Figure 2. Bacterial density in animals with Cuture positive EOM.
The box plot represents average Bacterial density in log 10 cfu/ml of animals with culture positive disease for serotypes 6C and 19A. The y-axis represents average bacterial density in log 10 cfu/ml and the x-axis the serotypes. *signifies p value of 0.002. The top and bottom of the box plot represents the 25th and 75th percentile range. The line across the box shows the median geometric mean and the top and bottom bars show the maximum and minimum values.
4. Discussion
To our knowledge, these data are the first to evaluate the serotype 6C in an experimental model of AOM. Our findings demonstrate that nasopharyngeal challenge with serotype 6C followed by barotrauma results in EOM less frequently compared to serotype 19A. This difference is not due to differences in ability to colonize, as both serotypes demonstrated similar density of colonization. When disease due to 6C occurred, it was characterized by low-density infection compared to 19A. Bacteremia was less common in chinchillas challenged with 6C compared to 19A. All six 6C strains were of similar virulence in the model, despite difference in source (carriage vs. IPD) and/or ST. C3 binding to selected 6C strains is significantly higher than to 19A strains suggesting that evading C3 deposition enables pneumococci to overcome host defenses.
Our observation that both 19A and 6C serotypes colonize well supports data from Massachusetts children that suggests that 19A and 6C are amongst the most prevalent in the nasopharynx in children less than 5 years [3]. The colonization of mucosal surfaces is a requirement for the development of either mucosal or invasive pneumococcal disease [34, 35]. We also demonstrated that the differences in observed C3 binding do not impact on early colonization density. . As colonization was not followed through to clearance, we are unable to identify if any correlation between C3 binding and eventual clearance of colonization could exist. However, other researchers have reported that the rate of S. pneumoniae clearance from the nasopharynx was not affected in C3 knockout mice [36].
Recent reports have identified serotype 6C from children with recurrent AOM or children with persistent AOM despite antibiotic treatment [9]. Our data indicate that 6C strains can cause AOM, albeit less frequently than serotype 19A. These data also suggest a potential mechanism. Previous work in our lab had identified complement deposition on S. pneumoniae as a correlate of virulence for pneumococcal experimental otitis media[31]. All of the 6C strains studied were uniformly identified as high complement binders and demonstrated relatively low virulence in our animal model. Serotype 19A has been reported to exhibit a greater than the average virulence capacity for AOM compared to all pneumococcal serotypes (OR 1.6) [37], and has been implicated as most common pathogen in AOM failure after introduction of PCV7 in France[38]. The decreased binding of complement for strains 19A potentially explains its capacity to escape from innate immunity and the observed virulence. In contrast, serotype 6C strains, which all bind more complement relative to 19A strains, fail to effectively escape host defenses. Even when serotype 6C produces middle ear disease, the middle ear density is lower than serotype 19A.
Our observations are consistent with the hypothesis that the redistribution of serotypes in carriage in children results in an overall reduction in the likelihood of pneumococcal otitis media occurring following colonization. As overall pneumococcal colonization prevalence has not declined in US and other countries where vaccine was introduced, [23] [3] [24] the emergence of serotype 6C is likely related to biochemical difference between the polysaccharide in serotype 6A and 6C that results in different higher functional antibody activity against the 6A serotypes strains compared to 6C among children immunized with PCV7. Serotype 6C is not included in the next generation PCV13 and it is unknown whether antibody generated against the added serotype 6A polysaccharide will be sufficiently cross reactive with serotype 6C to provide protection against colonization and/or disease. Park reported that PCV7 induces only low concentrations of opsonic antibody against isolates of 6C compared with serotype 6A in serum specimens from PCV-7 immunized children[12]. Although, recent data suggests greater functional activity against 6C in individuals immunized with PCV 13 [39].
The chinchilla otitis media model requires initial NP colonization followed by ascension through the Eustachian tube following barotrauma for establishing middle ear infection. The successful ascension of bacterial otopathogens into the middle ear, bacterial replication, and subsequent inflammatory response require overcoming host defense mechanisms within the nasopharynx and middle ear. Specific attributes that enable pneumococci to breach host epithelial and tissue barriers during the progression from colonization to invasive infection are not fully characterized but are reported to include capsule as well as pneumococcal surface proteins [40] [41]. Inoculation of relatively low virulent serotypes like 6C directly into the bulla leads to development of EOM (data not shown), which suggests that observed difference results primarily from prevention of ascension rather than protection after reaching the middle ear.
This study is limited by the selection of strains; we compared 6C serotypes of differing ST against strains of 19A of a single ST. Although we only tested 6C strains from four sequence types, strains originated from both invasive and carriage sources. Selecting a single ST may not fully represent all 19A strains present in the community, however to date clonal differences in virulence have not been established. Our hypothesis was that currently circulating 6C strains have a lower capacity to produce EOM. Therefore we did not pursue capsular switch experiments, which would be needed to determine whether the basis which would be needed to determine whether the basis for the observed limited virulence lies in the capsule or depends on the specific genetic background of the strain. In the past we have demonstrated that virulence in our model correlates with C3 binding [31] and that both capsule and pneumococcal surface proteins contribute to the resultant C3 binding. Our data is also limited to the chinchilla model and may not be fully analogous to disease in humans even though the model employs initial nasopharyngeal colonization followed by barotrauma.
These data support the need for additional research regarding differences among serotype specific capacity to ascend the Eustachian tube and produce otitis media in order to predict the long term impact of serotype replacement in the nasopharynx on AOM. Serotypes 19A, 6C, 23A and B, 15B/C, 35B, 11A have become the most prevalent carried serotypes. The capsule has been reported as critical for pathogenesis of invasive pneumococcal disease and resistance to host defenses [42] [43] [44]. Certain capsular serotypes have been associated with an increased potential to produce invasive pneumococcal disease as compared to other serotypes [45]. Yildirim et al. demonstrated that with the exception of 19A, most of the prevalent serotypes have relatively lower invasive capacities[13]. Thereby potentially explaining the reduction in IPD despite no overall change in carriage. Insufficient data are available regarding the capacity of these common serotypes to ascend the Eustachian tube and produce AOM, however we hypothesize that the same concept as for IPD is likely correct.
Our data also suggest that measurement of C3 binding among pneumococcal strains has the potential to be a surrogate for predicting the likelihood that a specific serotype would cause frequent AOM as well as greater understanding of how pneumococci overcome host defenses to ascend the Eustachian tube.
Acknowledgments
We thank Loc Truong for expert technical assistance. These studies were supported in part by a NIH/NCRR, K award KL2RR025770 to VS, and NIAID grant R01 A1068043 to MMP. Additional grant support provided by the Shereta Seelig Trust.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Millar EV, Pimenta FC, Roundtree A, Jackson D, Carvalho Mda G, Perilla MJ, Reid R, Santosham M, Whitney CG, Beall BW, O’Brien KL. Pre- and post-conjugate vaccine epidemiology of pneumococcal serotype 6C invasive disease and carriage within Navajo and White Mountain Apache communities. Clin Infect Dis. 2010;51:1258–65. doi: 10.1086/657070. [DOI] [PubMed] [Google Scholar]
- 2.Campos LC, Carvalho Mda G, Beall BW, Cordeiro SM, Takahashi D, Reis MG, Ko AI, Reis JN. Prevalence of Streptococcus pneumoniae serotype 6C among invasive and carriage isolates in metropolitan Salvador, Brazil, from 1996 to 2007. Diagn Microbiol Infect Dis. 2009;65:112–5. doi: 10.1016/j.diagmicrobio.2009.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs MR, Bajaksouzian S, Bonomo RA, Good CE, Windau AR, Hujer AM, Massire C, Melton R, Blyn LB, Ecker DJ, Sampath R. Occurrence, distribution, and origins of Streptococcus pneumoniae Serotype 6C, a recently recognized serotype. J Clin Microbiol. 2009;47:64–72. doi: 10.1128/JCM.01524-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Green MC, Mason EO, Kaplan SL, Lamberth LB, Stovall SH, Givner LB, Bradley JS, Tan TQ, Barson WJ, Hoffman JA, Lin PL, Hulten KG. Increase in prevalence of Streptococcus pneumoniae serotype 6C at Eight Children’s Hospitals in the United States from 1993 to 2009. J Clin Microbiol. 2011;49:2097–101. doi: 10.1128/JCM.02207-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park IH, Park S, Hollingshead SK, Nahm MH. Genetic basis for the new pneumococcal serotype, 6C. Infect Immun. 2007;75:4482–9. doi: 10.1128/IAI.00510-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007;45:1225–33. doi: 10.1128/JCM.02199-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199:320–5. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey JR, Adlowitz DG, Pichichero ME. New patterns in the otopathogens causing acute otitis media six to eight years after introduction of pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2010;29:304–9. doi: 10.1097/INF.0b013e3181c1bc48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jacobs MR, Good CE, Bajaksouzian S, Windau AR. Emergence of Streptococcus pneumoniae serotypes 19A, 6C, and 22F and serogroup 15 in Cleveland, Ohio, in relation to introduction of the protein-conjugated pneumococcal vaccine. Clin Infect Dis. 2008;47:1388–95. doi: 10.1086/592972. [DOI] [PubMed] [Google Scholar]
- 11.Rolo D, Ardanuy C, Calatayud L, Pallares R, Grau I, Garcia E, Fenoll A, Martin R, Linares J. Characterization of invasive pneumococci of serogroup 6 from adults in Barcelona, Spain, in 1994 to 2008. J Clin Microbiol. 2011;49:2328–30. doi: 10.1128/JCM.02545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park IH, Moore MR, Treanor JJ, Pelton SI, Pilishvili T, Beall B, Shelly MA, Mahon BE, Nahm MH. Differential effects of pneumococcal vaccines against serotypes 6A and 6C. J Infect Dis. 2008;198:1818–22. doi: 10.1086/593339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yildirim I, Hanage WP, Lipsitch M, Shea KM, Stevenson A, Finkelstein J, Huang SS, Lee GM, Kleinman K, Pelton SI. Serotype specific invasive capacity and persistent reduction in invasive pneumococcal disease. Vaccine. 2010;29:283–8. doi: 10.1016/j.vaccine.2010.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pelton SI, Huot H, Finkelstein JA, Bishop CJ, Hsu KK, Kellenberg J, Huang SS, Goldstein R, Hanage WP. Emergence of 19A as virulent and multidrug resistant pneumococcus in Massachusetts following universal immunization of infants with pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2007;26:468–72. doi: 10.1097/INF.0b013e31803df9ca. [DOI] [PubMed] [Google Scholar]
- 15.Moore MR, Gertz RE, Jr, Woodbury RL, Barkocy-Gallagher GA, Schaffner W, Lexau C, Gershman K, Reingold A, Farley M, Harrison LH, Hadler JL, Bennett NM, Thomas AR, McGee L, Pilishvili T, Brueggemann AB, Whitney CG, Jorgensen JH, Beall B. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J Infect Dis. 2008;197:1016–27. doi: 10.1086/528996. [DOI] [PubMed] [Google Scholar]
- 16.Gertz RE, Jr, McEllistrem MC, Boxrud DJ, Li Z, Sakota V, Thompson TA, Facklam RR, Besser JM, Harrison LH, Whitney CG, Beall B. Clonal distribution of invasive pneumococcal isolates from children and selected adults in the United States prior to 7-valent conjugate vaccine introduction. J Clin Microbiol. 2003;41:4194–216. doi: 10.1128/JCM.41.9.4194-4216.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pai R, Moore MR, Pilishvili T, Gertz RE, Whitney CG, Beall B. Postvaccine genetic structure of Streptococcus pneumoniae serotype 19A from children in the United States. J Infect Dis. 2005;192:1988–95. doi: 10.1086/498043. [DOI] [PubMed] [Google Scholar]
- 18.van Gils EJ, Veenhoven RH, Hak E, Rodenburg GD, Keijzers WC, Bogaert D, Trzcinski K, Bruin JP, van Alphen L, van der Ende A, Sanders EA. Pneumococcal conjugate vaccination and nasopharyngeal acquisition of pneumococcal serotype 19A strains. JAMA. 2010;304:1099–106. doi: 10.1001/jama.2010.1290. [DOI] [PubMed] [Google Scholar]
- 19.Munoz-Almagro C, Esteva C, de Sevilla MF, Selva L, Gene A, Pallares R. Emergence of invasive pneumococcal disease caused by multidrug-resistant serotype 19A among children in Barcelona. J Infect. 2009;59:75–82. doi: 10.1016/j.jinf.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers GL, Arguedas A, Cohen R, Dagan R. Global serotype distribution among Streptococcus pneumoniae isolates causing otitis media in children: potential implications for pneumococcal conjugate vaccines. Vaccine. 2009;27:3802–10. doi: 10.1016/j.vaccine.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Thomas JC, Figueira M, Fennie KP, Laufer AS, Kong Y, Pichichero ME, Pelton SI, Pettigrew MM. Streptococcus pneumoniae clonal complex 199: genetic diversity and tissue-specific virulence. PLoS One. 2011;6:e18649. doi: 10.1371/journal.pone.0018649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laufer AS, Thomas JC, Figueira M, Gent JF, Pelton SI, Pettigrew MM. Capacity of serotype 19A and 15B/C Streptococcus pneumoniae isolates for experimental otitis media: Implications for the conjugate vaccine. Vaccine. 2010;28:2450–7. doi: 10.1016/j.vaccine.2009.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dunais B, Bruno P, Carsenti-Dellamonica H, Touboul P, Dellamonica P, Pradier C. Trends in nasopharyngeal carriage of Streptococcus pneumoniae among children attending daycare centers in southeastern France from 1999 to 2006. Pediatr Infect Dis J. 2008;27:1033–5. doi: 10.1097/INF.0b013e31817bb8cf. [DOI] [PubMed] [Google Scholar]
- 24.Vestrheim DF, Hoiby EA, Aaberge IS, Caugant DA. Impact of a pneumococcal conjugate vaccination program on carriage among children in Norway. Clin Vaccine Immunol. 2010;17:325–34. doi: 10.1128/CVI.00435-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grijalva CG, Poehling KA, Nuorti JP, Zhu Y, Martin SW, Edwards KM, Griffin MR. National impact of universal childhood immunization with pneumococcal conjugate vaccine on outpatient medical care visits in the United States. Pediatrics. 2006;118:865–73. doi: 10.1542/peds.2006-0492. [DOI] [PubMed] [Google Scholar]
- 26.Zhou F, Shefer A, Kong Y, Nuorti JP. Trends in acute otitis media-related health care utilization by privately insured young children in the United States, 1997–2004. Pediatrics. 2008;121:253–60. doi: 10.1542/peds.2007-0619. [DOI] [PubMed] [Google Scholar]
- 27.Platts-Mills TA, Ishizaka K. Activation of the alternate pathway of human complements by rabbit cells. J Immunol. 1974;113:348–58. [PubMed] [Google Scholar]
- 28.Ram S, Cullinane M, Blom AM, Gulati S, McQuillen DP, Monks BG, O’Connell C, Boden R, Elkins C, Pangburn MK, Dahlback B, Rice PA. Binding of C4b-binding protein to porin: a molecular mechanism of serum resistance of Neisseria gonorrhoeae. J Exp Med. 2001;193:281–95. doi: 10.1084/jem.193.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bouchet V, Hood DW, Li J, Brisson JR, Randle GA, Martin A, Li Z, Goldstein R, Schweda EK, Pelton SI, Richards JC, Moxon ER. Host-derived sialic acid is incorporated into Haemophilus influenzae lipopolysaccharide and is a major virulence factor in experimental otitis media. Proc Natl Acad Sci U S A. 2003;100:8898–903. doi: 10.1073/pnas.1432026100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enright MC, Spratt BG. A multilocus sequence typing scheme for Streptococcus pneumoniae: identification of clones associated with serious invasive disease. Microbiology. 1998;144:3049–60. doi: 10.1099/00221287-144-11-3049. [DOI] [PubMed] [Google Scholar]
- 31.Sabharwal V, Ram S, Figueira M, Park IH, Pelton SI. Role of complement in host defense against pneumococcal otitis media. Infect Immun. 2009;77:1121–7. doi: 10.1128/IAI.01148-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Figueira MA, Ram S, Goldstein R, Hood DW, Moxon ER, Pelton SI. Role of complement in defense of the middle ear revealed by restoring the virulence of nontypeable Haemophilus influenzae siaB mutants. Infect Immun. 2007;75:325–33. doi: 10.1128/IAI.01054-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babl FE, Pelton SI, Li Z. Experimental acute otitis media due to nontypeable Haemophilus influenzae: comparison of high and low azithromycin doses with placebo. Antimicrob Agents Chemother. 2002;46:2194–9. doi: 10.1128/AAC.46.7.2194-2199.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray BM, Converse GM, 3rd, Dillon HC., Jr Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980;142:923–33. doi: 10.1093/infdis/142.6.923. [DOI] [PubMed] [Google Scholar]
- 35.Faden H, Duffy L, Wasielewski R, Wolf J, Krystofik D, Tung Y. Relationship between nasopharyngeal colonization and the development of otitis media in children. Tonawanda/Williamsville Pediatrics. J Infect Dis. 1997;175:1440–5. doi: 10.1086/516477. [DOI] [PubMed] [Google Scholar]
- 36.van Rossum AM, Lysenko ES, Weiser JN. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect Immun. 2005;73:7718–26. doi: 10.1128/IAI.73.11.7718-7726.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shouval DS, Greenberg D, Givon-Lavi N, Porat N, Dagan R. Site-specific disease potential of individual Streptococcus pneumoniae serotypes in pediatric invasive disease, acute otitis media and acute conjunctivitis. Pediatr Infect Dis J. 2006;25:602–7. doi: 10.1097/01.inf.0000220231.79968.f6. [DOI] [PubMed] [Google Scholar]
- 38.Couloigner V, Levy C, Francois M, Bidet P, Hausdorff WP, Pascal T, Boucherat M, Bingen E, Mariani P, Pierrot S, Bille E, Carbonnelle E, Varon E, Cohen R. Pathogens Implicated in Acute Otitis Media Failures After 7-valent Pneumococcal Conjugate Vaccine Implementation in France: Distribution, Serotypes, and Resistance Levels. Pediatr Infect Dis J. 31:154–58. doi: 10.1097/INF.0b013e3182357c8d. [DOI] [PubMed] [Google Scholar]
- 39.Cooper D, Yu X, Sidhu M, Nahm MH, Fernsten P, Jansen KU. The 13-valent pneumococcal conjugate vaccine (PCV13) elicits cross-functional opsonophagocytic killing responses in humans to Streptococcus pneumoniae serotypes 6C and 7A. Vaccine. 2011;29:7207–11. doi: 10.1016/j.vaccine.2011.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bergmann S, Hammerschmidt S. Versatility of pneumococcal surface proteins. Microbiology. 2006;152:295–303. doi: 10.1099/mic.0.28610-0. [DOI] [PubMed] [Google Scholar]
- 41.Rosenow C, Ryan P, Weiser JN, Johnson S, Fontan P, Ortqvist A, Masure HR. Contribution of novel choline-binding proteins to adherence, colonization and immunogenicity of Streptococcus pneumoniae. Mol Microbiol. 1997;25:819–29. doi: 10.1111/j.1365-2958.1997.mmi494.x. [DOI] [PubMed] [Google Scholar]
- 42.Magee AD, Yother J. Requirement for capsule in colonization by Streptococcus pneumoniae. Infect Immun. 2001;69:3755–61. doi: 10.1128/IAI.69.6.3755-3761.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyams C, Camberlein E, Cohen JM, Bax K, Brown JS. The Streptococcus pneumoniae capsule inhibits complement activity and neutrophil phagocytosis by multiple mechanisms. Infect Immun. 2010;78:704–15. doi: 10.1128/IAI.00881-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melin M, Jarva H, Siira L, Meri S, Kayhty H, Vakevainen M. Streptococcus pneumoniae capsular serotype 19F is more resistant to C3 deposition and less sensitive to opsonophagocytosis than serotype 6B. Infect Immun. 2009;77:676–84. doi: 10.1128/IAI.01186-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brueggemann AB, Peto TE, Crook DW, Butler JC, Kristinsson KG, Spratt BG. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J Infect Dis. 2004;190:1203–11. doi: 10.1086/423820. [DOI] [PubMed] [Google Scholar]