Abstract
Background
Immigrants to developed countries have low rates of aeroallergen sensitization and asthma, but less is known about both food allergy and the role of parental immigration status.
Objective
To evaluate the relationship between personal and parental nativity on the risk of food sensitization.
Methods
3550 subjects <21 years old from the National Health and Examination Survey 2005-2006 were included. Odds ratios were generated using logistic regression which adjusted for race/ethnicity, gender, age, and household income, and accounted for the complex survey design. Nativity was classified as US-born or foreign-born and the age of immigration was estimated. Head of household nativity was used as a proxy for parental nativity. Food sensitization was defined as at least one specific-IgE ≥ 0.35 kU/L to milk, egg or peanut. Aeroallergen specific sensitizations, and the presence of asthma, allergic rhinitis or eczema were also assessed.
Results
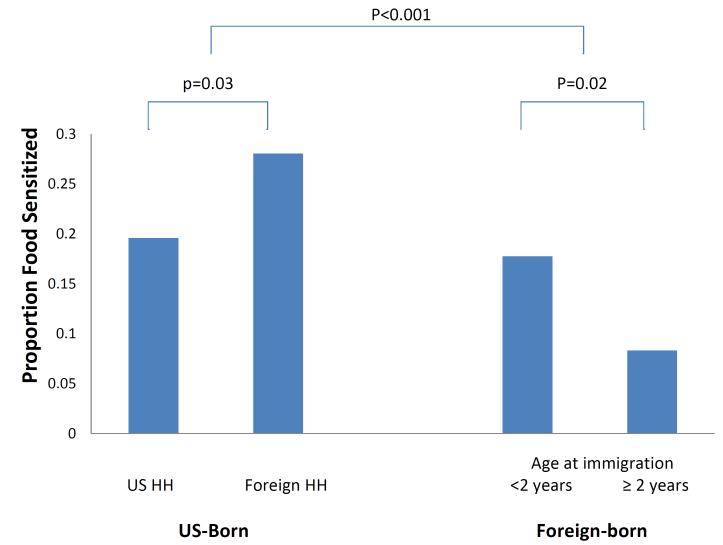
Compared to those born outside the US, US-born children and adolescents had higher odds of sensitization to any food (OR 2.05, 95% CI 1.49-2.83, p<0.001). Among the foreign-born, those who arrived before 2 years of age had higher odds of food sensitization than those who arrived later (OR 2.68, 95% CI 1.19-6.08, p=0.02). Within the US-born group, in contrast, children of immigrants were at the highest risk (OR 1.53, 95% CI 1.05-2.24, p=0.02).
Conclusion
While foreign-born children and adolescents are at lower risk of food sensitization compared to those born in the US, among those born in the US, the children of immigrants are at the highest risk.
Keywords: Food allergy, food sensitization, immigration, nativity, child, hay fever, asthma, eczema, aeroallergen
Introduction
Food allergy is a problem of complex etiology that appears to be rapidly increasing in prevalence(1). Large discrepancies in the rates of food allergy are found by geography(2) and ethnicity(1, 3, 4). Previous studies of asthma have generally shown that immigrants to developed countries have lower rates of asthma and allergic sensitization than native born populations of the same(5-15) and different ethnicities(16, 17). Despite the fact that immigrants to developed countries tend to have lower rates of most sensitizations than native born populations, ethnic minorities in developed countries tend to have high rates of allergic sensitization(4, 14, 18), and some data indicate that children of immigrants are at particular risk for various atopic diseases(14, 19, 20). However, the relationship between immigration and food allergy has only rarely been studied(5, 20, 21), even though the pathophysiologic mechanisms of food allergy may be distinct from other allergic disorders.
In our clinical practice, we have noted a disproportionate rate of food allergy among first-generation Americans, and thus hypothesized that personal and parental nativity may affect the risk of food sensitization differently. In order to examine the effects of immigration to the US on the risk of food sensitization, we examined a large, nationally representative sample of children and adolescents living in the US. As a country of immigrants representing many generations, the US offers a unique opportunity to examine multi-generational effects of immigration on the risk of allergic disease. In this study, we sought specifically to investigate whether native and foreign-born residents of the US have different risks of food sensitization, whether the risk of food sensitization differs by immigration status of a child’s parent, and whether the timing of immigration affects the risk of food and aeroallergen sensitization.
Methods
Survey Design
We examined the National Health and Nutrition Examination Survey (NHANES) 2005-2006. NHANES is a publicly available cross-sectional national survey conducted by the Centers for Disease Control (CDC) with a target population of the civilian non-institutionalized US population. The survey has a complex, multistage sampling design with oversampling of low-income persons, adolescents, African-Americans and Mexican Americans. The first stage is a questionnaire administered in the subject’s home, with follow-up stages for physical examination and laboratory studies in a mobile van. The survey is approved by the Institutional Review Board of the National Center for Health Statistics, CDC, and informed consent was given by all participants. For this study, subjects between 1 and 21 years old with complete data for milk, egg, and peanut IgE, family income, age, country of birth, gender, and head of household country of birth were included.
Measurement and definition of exposures and outcomes
The main outcome measure was food sensitization, defined by a specific IgE of at least 0.35 kU/L to milk, egg or peanut. Allergen specific IgEs were analyzed using the Pharmacia Diagnostics Immunocap 1000 system (Kalamazoo, MI). Because no information on clinical reactivity was available, sensitivity analyses were done using food-specific IgE cut-offs that have previously been identified as being indicative of probable food allergy(4, 22, 23). Accordingly, probable food allergy was defined as at least one of the following: If less than 2 years of age, IgE to milk ≥ 5 kU/L, egg ≥ 2 kU/L, or peanut ≥ 14 kU/L, and if at least 2 years of age, IgE to milk ≥ 15 kU/L, egg ≥ 7 kU/L, or peanut ≥ 14 kU/L (4). We also assessed the relationship between immigration and sensitization to aeroallergens, and to history of health professional’s diagnosis of asthma, hay fever, and eczema. Because of the difficulty in diagnosing asthma in young children, sensitivity analyses were conducted of asthma outcomes including only those aged 6 and above. In addition to the foods mentioned above, specific IgE was measured in all subjects to: D. farinae, D. pteronyssinus, cat, dog, cockroach, and Alternaria. Seasonal sensitizations, which were only assessed in subjects at least 6 years of age, were defined as a specific IgE of at least 0.35 kU/L for at least one of: ragweed, rye grass, Bermuda grass, oak, birch, and thistle.
The main exposures were the nativity of the subject and the subject’s head of household, which were both defined as US-born or foreign-born. The nativity of head of household was used as a proxy for the parent’s nativity. Data from the 2010 census support this use, showing that more than 98% of the time a child is a relative of the head of household with whom he/she lives; 89% of the time the head of household is a parent(24). Analyses were first done comparing US-born to foreign-born children and adolescents. The US-born group was then split into those living with a US-born householder (“US-born/non-immigrant household”) and those living with a foreign-born householder (“US-born/immigrant household”). In order to examine whether the age of immigration affected the odds of food sensitization, we used a variable for length of stay in the US which categorized subjects as having lived in the US for < 1 year, 1-<5 years, 5-<10 years, 10-<15 years, 15-<20 years, or 20-<30 years. We subtracted the midpoint of these ranges from the subject’s age to estimate the age at immigration and then dichotomized as < 2 years or ≥ 2 years. This inflection point was chosen to be consistent with previous reports(25), and because visual inspection of the data identified this age as a natural point for dichotomization.
Potential confounders included age, gender, poverty income ratio and race/ethnicity. Poverty income ratio is a continuous measure of the ratio of the household’s income to the national poverty line. Race/ethnicity was by self report and was defined as Hispanic, non-Hispanic Caucasian, African-American and “other”, except for analyses of age of immigration, for which ethnicity was dichotomized as Hispanic vs. Non-Hispanic to avoid empty strata. Other potential confounders/mediators evaluated separately included serum vitamin D level, history of eczema, BMI z score (calculated as described previously(26)), household size, head of household education level and marital status and cockroach in the home, defined by the question “In the past 12 months, have you seen any cockroaches in your home?”.
Statistical Methods
Logistic regression was used to estimate odds ratios for each sensitization according to category of personal and householder nativity, adjusting for age, gender, poverty income ratio and race/ethnicity. For comparisons among foreign-born children and adolescents, ethnicity was categorized as Hispanic/Non-Hispanic as indicated in the results in order to minimize strata with no data. Descriptive statistics were generated for the different groups; the distributions of age and poverty income ratio were compared by simple linear regression. Chi-squared statistics were generated to compare the distribution of gender and race/ethnicity between the groups. Graphs of the proportion of subjects sensitized to a given food by age and place of birth were generated using locally weighted regression (LOWESS) smoothing with a bandwidth of 0.8; these analyses did not use the survey weights. All other analyses accounted for the complex survey design by using the weights and sampling units provided with the dataset and were done using STATA 11.0/SE (Statacorp, College Station, TX).
Results
Population Characteristics
3550 children and adolescents with complete case information were included in this analysis. 2495 were born in the United States and lived with an US-born head of household, 714 were born in the US and lived with a non-US-born head of household, and 341 were born outside the US. Among the foreign-born, 97 were estimated to have arrived in the US before the age of 2 years, and 244 at age 2 or above. Compared to US-born children and adolescents, foreign-born children and adolescents were older, lived in poorer households, and were more likely to be Hispanic or of other race/ethnicity. Other demographic differences among US-born and foreign-born groups are shown in Table 1.
Table 1.
Demographic Features of US-born children and adolescents.
| US-born | Foreign-born | Foreign- vs. US-born | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Living in non- immigrant households |
Living in immigrant households |
P value | Arrived <2 years of age |
Arrived ≥ 2 years of age |
P value | P value | |
| Number | 2495 | 714 | 97 | 244 | |||
|
| |||||||
| Mean age (years) | 11.4 | 9.2 | <0.001 | 10.1 | 14.7 | <0.001 | <0.001 |
|
| |||||||
| Mean poverty income ratio | 2.7 | 2.1 | 0.002 | 2.5 | 1.7 | 0.09 | 0.001 |
|
| |||||||
| Ethnicity | |||||||
| Hispanic | 8% | 62% | 39% | 61% | |||
| Non-Hispanic Caucasian | 70% | 22% | <0.001 | 26% | 16% | 0.37 | <0.001 |
| African-American | 16% | 6% | 10% | 8% | |||
| Other | 6% | 10% | 25% | 14% | |||
| Male Gender | 53% | 47% | 0.07 | 53% | 51% | 0.87 | 0.92 |
|
| |||||||
| Any Food Sensitization | 20% | 28% | <0.001 | 18% | 8% | 0.02 | <0.001 |
|
| |||||||
| Milk sensitization | 10% | 19% | 0.001 | 10% | 3% | 0.04 | 0.007 |
|
| |||||||
| Egg Sensitization | 6% | 9% | 0.12 | 2% | 0.1% | 0.009 | 0.001 |
|
| |||||||
| Peanut Sensitization | 10% | 10% | 0.99 | 10% | 6% | 0.32 | 0.15 |
All analyses account for survey design of the study as discussed in the methods section. Poverty income ratio is defined as ratio of household income to the poverty line. P values represent unadjusted comparisons.
Twenty percent of subjects were sensitized to at least one food; 11% to milk, 6% to egg, and 10% to peanut. See Table 1 and supplementary figures 1 and 2 in the online repository for more details.
Comparisons of US-born vs. Foreign-born children and adolescents
After adjusting for race/ethnicity, age, gender and household income, US-born children and adolescents were significantly more likely to be sensitized to any food (OR 2.05, 95% CI 1.49-2.83, p<0.001), milk (OR 2.10, 95% CI 1.13-3.92, p=0.02), and egg (OR 6.52, 95% CI 1.56-27.20, p=0.01) than foreign-born children and adolescents. There was no evidence of heterogeneity by race/ethnicity for the relationship between nativity and food sensitization (OR: 1.84 for Hispanics, 1.24 for Caucasians, 1.49 for African Americans and 1.50 for “other”, p>0.3 for all interaction terms).
The trend was the same when higher levels of specific IgE were used to identify subjects with probable food allergy (OR: 2.15, 95% CI 0.71-6.49, p=0.16) although these analyses were underpowered given the small numbers of subjects meeting these cut-offs; only three foreign-born subjects had specific IgE putting them in the probable food allergy category.
Compared to foreign-born children and adolescents, US-born children and adolescents were also statistically significantly more likely to be sensitized to cat (OR 2.11, 95% CI 1.68-2.65), dog (OR 2.96, 95% CI 1.48-5.91), and Alternaria (OR 4.80, 95% CI 1.28-17.94), and were less likely to be sensitized to cockroach (OR 0.52, 95% CI 0.38-0.70). In order to assess whether the lower odds of cockroach sensitization among US-born children was due to exposure to cockroach, a question that asked whether cockroaches were seen in the home in the past year was included. US-born subjects were less likely to report seeing cockroaches in their homes (OR 0.56, 95% CI 0.37-0.83), but adjusting for this did not substantially change the relationship between cockroach sensitization and nativity (OR 0.54, 95% CI 0.40-0.74).
US-born children and adolescents were also more likely to report a diagnosis of asthma (OR 2.19, 95% CI 1.02-4.68,). When analyses were restricted to only those aged 6 and above, the relationship between nativity and asthma was of similar magnitude but was no longer statistically significant (OR 2.12, 95% CI 0.98-4.60). There were not statistically significant differences in the rates of reported diagnosis of hay fever (OR 2.96, 95% CI 0.39-22.39) or eczema (OR 1.97, 95% CI 0.65-5.95). (Table 2) Seasonal allergen sensitization, which was only assessed on those aged 6 years and older, was more common among US born children and adolescents (OR 1.76, 95% CI 1.16-2.66, p=0.01).
Table 2.
Comparison of US-born to foreign-born children and adolescents
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Foods | |||
| Sensitization to any food | 2.05 | 1.49-2.83 | <0.001 |
| Milk | 2.10 | 1.13-3.92 | 0.02 |
| Egg | 6.52 | 1.56-27.20 | 0.01 |
| Peanut | 1.52 | 0.88-2.62 | 0.13 |
| Other sensitizations | |||
| Dustmite | 1.20 | 0.73-1.99 | 0.44 |
| Cockroach | 0.52 | 0.38-0.70 | <0.001 |
| Cat | 2.11 | 1.68-2.65 | <0.001 |
| Dog | 2.96 | 1.48-5.91 | 0.004 |
| Alternaria | 4.80 | 1.28-17.94 | 0.02 |
| Asthma † | 2.19 | 1.02-4.68 | 0.045 |
| Hay fever† | 2.96 | 0.39-22.39 | 0.27 |
| Eczema‡ | 1.97 | 0.65-5.95 | 0.21 |
All analyses are adjusted for age, gender, poverty income ratio and ethnicity. Food sensitization is defined as at least one specific IgE to milk, egg, or peanut ≥ 0.35 kU/L. N=3550 for all analyses except for
3546 and
3540. Bold are statistically significant
Comparisons within US-born groups of children and adolescents
US-born children were then divided into two groups based on householder nativity. Compared to those not living in immigrant households, those living in immigrant households were significantly more likely to be sensitized to any food (OR 1.53, 95% CI 1.05-2.24, p=0.02), to milk (OR 1.74, 95% CI 1.06-2.86, p=0.04), and to egg and peanut, although not statistically significantly. The trend was the also the same with probable food allergy (OR 1.92, 95% CI 0.71-5.23, p=0.18). (Table 3)
Table 3.
Comparison of US-born children and adolescents living in immigrant households vs. US-born children and adolescents living in non- immigrant Households
| Odds Ratio | 95% CI | P value | |
|---|---|---|---|
| Foods | |||
| Sensitization to any food | 1.53 | 1.05-2.24 | 0.03 |
| Milk | 1.74 | 1.06-2.86 | 0.03 |
| Egg | 1.42 | 0.69-2.91 | 0.31 |
| Peanut | 1.21 | 0.74-1.98 | 0.42 |
| Other sensitizations | |||
| Dust mite | 1.16 | 0.78-1.72 | 0.45 |
| Cockroach | 1.69 | 1.02-2.77 | 0.04 |
| Cat | 1.17 | 0.75-1.84 | 0.47 |
| Dog | 1.08 | 0.79-1.68 | 0.70 |
| Alternaria | 1.01 | 0.66-1.56 | 0.95 |
| Asthma† | 0.73 | 0.44-1.22 | 0.21 |
| Hay fever† | 0.23 | 0.05-1.02 | 0.053 |
| Eczema‡ | 0.91 | 0.64-1.31 | 0.60 |
All analyses are adjusted for age, gender, poverty income ratio and ethnicity. Food sensitization is defined as at least one specific IgE to milk, egg, or peanut ≥ 0.35 kU/L. N=3209 for all analyses except for
3205 and
3200. Bold are statistically significant.
Unlike the US-born versus foreign-born comparison, where trends were consistent across foods, aeroallergens and atopic diseases, there did not appear to be any relationship between living in immigrant versus US-born households and the rate of aeroallergen sensitizations, except for cockroach (OR 1.69, 95% CI 1.02-2.77). Adjusting for the sighting of cockroach in the home did not materially alter this relationship (adjusted OR: 1.66, 95% CI 0.99-2.76). Rates of asthma, hay fever and eczema were actually lower in those living in immigrant households, although the differences were not statistically significant (OR 0.73, 95% CI 0.44-1.22 for asthma, OR 0.23, 95% CI 0.05-1.02 for hay fever, and OR 0.91, 95% CI 0.64-1.31 for eczema). Similar results were found when analyses were restricted to only those older than 6 (OR for asthma 0.81, 95% CI 0.49-1.33).
Comparisons within groups of foreign-born children and adolescents
Compared to children and adolescents who immigrated at an estimated age of at least 2 years old, those who immigrated at earlier ages had higher odds of any food sensitization (OR 2.69, 95% CI 1.19-6.08, p=0.02), although the differences in individual food sensitizations were not statistically significantly: milk sensitization (OR 3.37, 95% CI 0.81-13.94, p=0.09), egg sensitization (OR 10.05, 95% CI 0.99-102.19, p=0.051), and peanut sensitization (OR 2.51, 95% CI 0.89-7.12, p=0.08). The relationship between sensitization to aeroallergens and age of immigration was more mixed and not statistically significant; the odds ratio comparing those who immigrated earlier to those who immigrated later was 0.47 for dust mite (95% CI 0.21-1.04, p=0.06), 1.07 for cockroach (95% CI 0.50-2.28, p=0.85), 2.88 for cat (95% CI 0.69-12.01, p=0.14), 2.27 for dog (95% CI 0.55-9.37, p=0.24) and 4.97 for Alternaria (95% CI 0.79-31.23, p=0.08). There was no difference in the rate of asthma (OR 1.03, 95% CI 0.21-5.01, p=0.97), or eczema (OR 3.98, 95% CI 0.40-39.85, p=0.22). If only those over aged 6 were included, there remained no association with asthma (OR 1.52 95% CI 0.33-7.10, p=0.57).
Potential Mediators of These Relationships
The differences in food sensitization between US-born and foreign-born children and adolescents, between US-born children and adolescents living and not-living in immigrant households, and between those arriving at younger than 2 years of age and those arriving later, were independent of vitamin D status, history of eczema, BMI z score, household size, educational status of the head of household, and marital status of the head of household when each of these potential confounder/mediators were included separately in the multivariate model. (Supplemental Table 1) The relationship between food sensitization and head of household immigration history did not differ by gender of the household head (p=0.18 for the interaction).
Ethnic differences in odds of sensitization to food
Among US-born children and adolescents, all other ethnic groups had higher rates of food sensitization than Caucasians (OR 1.59, 95% CI 1.12-2.67 for Hispanic; OR 2.43, 95% CI 1.12-2.67 for African-American; and OR 1.63, 95% CI 1.06-2.52 for “other” race/ethnicity children and adolescents vs. Caucasian children and adolescents). When the presence of a foreign-born head of household was adjusted for, the difference between Hispanic and Caucasian children and adolescents was no longer significant (OR 1.29, 95% CI 0.90-1.85), but there continued to be significantly higher rates of food sensitization for African-American (OR 2.44, 95% CI 1.63-3.64) and “other” (OR 1.52, 95% CI 1.01-2.29) children and adolescents compared to Caucasian children and adolescents, suggesting that ethnic differences in the rates of food sensitization are independent of migration history.
Discussion
In this large, nationally representative US sample, we found that both personal and parental nativity were risk factors for food sensitization. As has previously been reported for respiratory allergy, foreign birth was associated with a lower risk of food sensitization. However, foreign born children who immigrate during infancy lose some of the protective effects of foreign birth, suggesting that early life exposure to a developed country confers increased risk of sensitization. While we might therefore expect that children of immigrants born in the US would have a risk of food sensitization similar to other US-born children, we found that US-born children of immigrants are actually at greater risk of food sensitization. This finding corroborates our clinical observations and suggests that foreign-born parentage may interact with birth in a developed country to increase the risk of food sensitization.
It is not surprising that foreign-born immigrants to the US have low rates of food sensitization. Previous studies report that rates of aeroallergen sensitization, with a few exceptions, are lower among immigrants to the developed world than native-born residents(5-17). Our findings extend this to food sensitization, and additionally find age of immigration as an important risk factor for food sensitization. These findings are consonant with the “hygiene hypothesis”, which posits that infectious exposures during critical developmental periods modulate the immune response in a way that is protective against allergy(27). Recently, as evidence for this theory has grown, attention has shifted from individual exposures to the prenatal and cross-generational effects of infectious exposures(28-30). Based on data showing that maternal and fetal responses are highly correlated(31), some have speculated that the effects of changes in the environment on allergic risk accrete slowly over generations, arguing that “a cumulative increase in the level of immunological responsiveness occurs through cross-generational effects on immunity”(32). Our findings that the US-born children of immigrants are at the highest risk of sensitization contradict this theory, and raise the possibility that instead of cross-generational transmission of low allergy risk, there are in fact parental factors specific to immigrants which promote allergy.
Genetic factors are one possible explanation. There is considerable evidence that the human immune system evolved in response to infectious pressures related to intestinal infections(33), and it is theorized that the same genetic polymorphisms both protect against intestinal parasite infections and predispose to allergy(34-39). Thus, those with the highest genetic fitness in an environment of high pathogen density may be most at risk for allergy under the pathogen poor conditions of the US. With subsequent generations, admixture into the majority European descended US population would dilute this genetic propensity.
Another possibility is that the abrupt change in environment that occurs with immigration increases sensitization. For example, others have suggested that novel allergen exposures are responsible for the high rates of allergy in children of immigrants(21), although the fact that immigrants themselves had such low rates of allergic sensitization argues against this. Another possibility is that the specific infectious history of the immigrant mother may contribute to sensitization in her child. Chronic intestinal infections, especially by helminthes, cause a huge burden of disease worldwide, infecting an estimated 1/6 to 1/3 of the world’s population at any given time(40, 41). In contrast to infectious agents found on European farms, they cause a modified Th2 response(41) rather than Th1 deviation(41, 42).Perhaps, upon immigration to the US, the legacy of these previous infections involves an intrauterine environment which can confer Th2 deviation on subsequent children, increasing their risk of sensitization.
Other environmental conditions distinctive to immigrants may also predispose to food sensitization. Although the specific causal exposures may not be clear, urban living, maternal history of low socioeconomic status and current poverty, all more common among immigrants, have been associated with allergy promoting immune deviation(43, 44). The fact that cockroach sensitization was more common among children and adolescents living in immigrant households, even after accounting for family income, suggests that there may be residual confounding by socio-economic status within our data, perhaps explaining some of the relationship between food sensitization and residence in an immigrant household. It’s worth noting that if an unmeasured socio-economic factor is responsible for these findings, it stands in contrast to other conditions, such as adverse birth outcomes and asthma, where protective cultural practices are thought to explain the better outcomes of children of immigrants even in the face of worse socio-economic status(45, 46), and where increased acculturation has been associated with poorer outcomes by some(25, 46-48). Exclusive breast feeding(45, 46), a practice more common among immigrants, has been associated with increased risk of food sensitization(49); perhaps it plays a role here.
Although US-born children and adolescents living in immigrant households were more likely to be sensitized to foods than US-born children and adolescents not living in immigrant households, they were generally no more likely to be sensitized to aeroallergens (except cockroach) or to report a history of diagnosis of asthma, hay fever or eczema. It is biologically plausible that food sensitization, and allergy, may have distinct risk factors than sensitization to aeroallergens, and food allergy distinct risk factors than asthma, hay fever and eczema, even though these diseases are often correlated. Sensitization to the major food allergens is thought to develop within the first few years of life, and thus factors that work during this time may be more important for food than for aeroallergen sensitizations, which tend to develop later in childhood. This trend may also be evident in the fact that peanut sensitization was much less related to personal or parental nativity than the other foods. Peanut sensitization tends to increase with age, and is frequently related to cross-reactivity with pollens (50). Other “atopic” diseases such as asthma and eczema also have complex allergic and non-allergic risk factors, which may be distinct from those for food sensitization.
Cockroach sensitization followed distinct patterns from the remainder of specific sensitizations; both foreign born subjects and those living in immigrant households were more likely to be sensitized to cockroach than US born non-immigrants. This may be related to household exposures; both of these groups were more likely to report seeing a cockroach in the past year than those living in non-immigrant households, although adjusting for household cockroach did not remove the association with immigration, suggesting that the relationship between nativity category and cockroach sensitization is not a result of differences in cockroach exposure. Although this is the best cockroach exposure data available in NHANES, it is limited in that the question only refers to the year prior; there may be residual confounding not adjusted for in these analyses. . However, another factor to explain this pattern is that cockroach and intestinal helminthes are cross-reactive(51), and the cockroach sensitization found among immigrants may be related to a history of intestinal infections.
One potential limitation to the approach taken here is that head of household nativity status is an imprecise measure of parental nativity. That being said, census data indicate that about 90% of children in similar surveys are children of the named head of household, and it is a reasonable assumption that the nativity of the head of the household would track closely with the biological parent’s. Moreover, given that misclassification of the parent’s nativity is likely unrelated to food sensitization status, we would expect that any misclassification would result in an underestimate of the effect of parental nativity in our analysis. Similarly, for our analyses of age at immigration, we were only able to approximate age of immigration. Like any misclassification of immigration status of the parent, we would expect that misclassification of this variable would result in a conservative bias to our effect estimates, and that the real relationship is even stronger than we were able to find here. Another limitation is that we only had information about food sensitization, but not food reactivity. Many of the sensitized subjects may not be clinically reactive to the foods. When we looked at the higher levels of food specific IgE very predictive of food allergy, the same trends were seen, although the analyses were underpowered. Further studies will need to be done to determine whether clinical food allergy follows the same patterns we saw here.
In summary, in this large population representative of the non-institutionalized US population, we found that the risk of food sensitization immediately increases upon immigration to the US during early childhood, and that those living in immigrant households but born in the US are at even greater risk for food sensitization than US-born children and adolescents who do not live in immigrant households. Identifying the reasons for these findings may provide insight into the mechanisms of food allergy and its rapid increase in prevalence.
Supplementary Material
Clinical Implications.
Although foreign-born children and adolescents are at lower risk of food sensitization than those born in the US, among the US-born, children of immigrants are at the highest risk.
Figure 1.
Proportion of subjects sensitized to at least one food (milk, egg or peanut) by group. HH: head of household. P values calculated from multiple logistic regression adjusting for age, gender, race/ethnicity and income.
Acknowledgments
Sources of support: The study described was made possible by Grant Number 1KL2RR025006-01 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at http://www.ncrr.nih.gov/. Information on Re-engineering the Clinical Research Enterprise can be obtained from http://nihroadmap.nih.gov/clinicalresearch/overview-translational.asp.
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- CDC
Centers for Disease Control
- HH
Head of household
- BMI
Body Mass Index
- OR
Odds ratio
- CI
Confidence Interval
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Corinne A. Keet, Division of Allergy and Immunology, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD.
Robert A. Wood, Division of Allergy and Clinical Immunology, Department of Medicine, Johns Hopkins University School of Medicine, Baltimore, MD.
Elizabeth C. Matsui, Division of Allergy and Immunology, Department of Pediatrics, Johns Hopkins University School of Medicine, Baltimore, MD.
References
- 1.Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594–602. doi: 10.1016/j.jaci.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 2.Lack G. Epidemiologic risks for food allergy. J Allergy Clin Immunol. 2008;121(6):1331–6. doi: 10.1016/j.jaci.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 3.Sicherer SH, Munoz-Furlong A, Sampson HA. Prevalence of seafood allergy in the United States determined by a random telephone survey. J Allergy Clin Immunol. 2004;114(1):159–65. doi: 10.1016/j.jaci.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 4.Liu AH, Jaramillo R, Sicherer SH, Wood RA, Bock SA, Burks AW, et al. National prevalence and risk factors for food allergy and relationship to asthma: results from the National Health and Nutrition Examination Survey 2005-2006. J Allergy Clin Immunol. 2010;126(4):798–806. e13. doi: 10.1016/j.jaci.2010.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eldeirawi K, McConnell R, Freels S, Persky VW. Associations of place of birth with asthma and wheezing in Mexican American children. J Allergy Clin Immunol. 2005;116(1):42–8. doi: 10.1016/j.jaci.2005.03.041. [DOI] [PubMed] [Google Scholar]
- 6.Brugge D, Lee AC, Woodin M, Rioux C. Native and foreign born as predictors of pediatric asthma in an Asian immigrant population: a cross sectional survey. Environ Health. 2007;6:13. doi: 10.1186/1476-069X-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brugge D, Woodin M, Schuch TJ, Salas FL, Bennett A, Osgood ND. Community-level data suggest that asthma prevalence varies between U.S. and foreign-born black subpopulations. J Asthma. 2008;45(9):785–9. doi: 10.1080/02770900802179957. [DOI] [PubMed] [Google Scholar]
- 8.Subramanian SV, Jun HJ, Kawachi I, Wright RJ. Contribution of race/ethnicity and country of origin to variations in lifetime reported asthma: evidence for a nativity advantage. Am J Public Health. 2009;99(4):690–7. doi: 10.2105/AJPH.2007.128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dumanovsky T, Matte TD. Variation in adult asthma prevalence in Hispanic subpopulations in New York City. J Asthma. 2007;44(4):297–303. doi: 10.1080/02770900701344140. [DOI] [PubMed] [Google Scholar]
- 10.Holguin F, Mannino DM, Anto J, Mott J, Ford ES, Teague WG, et al. Country of birth as a risk factor for asthma among Mexican Americans. Am J Respir Crit Care Med. 2005;171(2):103–8. doi: 10.1164/rccm.200402-143OC. [DOI] [PubMed] [Google Scholar]
- 11.Svendsen ER, Gonzales M, Ross M, Neas LM. Variability in childhood allergy and asthma across ethnicity, language, and residency duration in El Paso, Texas: a cross-sectional study. Environ Health. 2009;8:55. doi: 10.1186/1476-069X-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migliore E, Pearce N, Bugiani M, Galletti G, Biggeri A, Bisanti L, et al. Prevalence of respiratory symptoms in migrant children to Italy: the results of SIDRIA-2 study. Allergy. 2007;62(3):293–300. doi: 10.1111/j.1398-9995.2007.01238.x. [DOI] [PubMed] [Google Scholar]
- 13.Netuveli G, Hurwitz B, Sheikh A. Ethnic variations in incidence of asthma episodes in England & Wales: national study of 502,482 patients in primary care. Respir Res. 2005;6:120. doi: 10.1186/1465-9921-6-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rottem M, Szyper-Kravitz M, Shoenfeld Y. Atopy and asthma in migrants. Int Arch Allergy Immunol. 2005;136(2):198–204. doi: 10.1159/000083894. [DOI] [PubMed] [Google Scholar]
- 15.Ventura MT, Munno G, Giannoccaro F, Accettura F, Chironna M, Lama R, et al. Allergy, asthma and markers of infections among Albanian migrants to Southern Italy. Allergy. 2004;59(6):632–6. doi: 10.1111/j.1398-9995.2004.00448.x. [DOI] [PubMed] [Google Scholar]
- 16.Gruber C, Meinlschmidt G, Bergmann R, Wahn U, Stark K. Is early BCG vaccination associated with less atopic disease? An epidemiological study in German preschool children with different ethnic backgrounds. Pediatr Allergy Immunol. 2002;13(3):177–81. doi: 10.1034/j.1399-3038.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 17.Pereg D, Tirosh A, Lishner M, Goldberg A, Shochat T, Confino-Cohen R. Prevalence of asthma in a large group of Israeli adolescents: influence of country of birth and age at migration. Allergy. 2008;63(8):1040–5. doi: 10.1111/j.1398-9995.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- 18.Barnes KC, Grant AV, Hansel NN, Gao P, Dunston GM. African Americans with asthma: genetic insights. Proc Am Thorac Soc. 2007;4(1):58–68. doi: 10.1513/pats.200607-146JG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hjern A, Haglund B, Bremberg S, Ringback-Weitoft G. Social adversity, migration and hospital admissions for childhood asthma in Sweden. Acta Paediatr. 1999;88(10):1107–12. doi: 10.1080/08035259950168171. [DOI] [PubMed] [Google Scholar]
- 20.Cataldo F, Accomando S, Fragapane ML, Montaperto D. Are food intolerances and allergies increasing in immigrant children coming from developing countries ? Pediatr Allergy Immunol. 2006;17(5):364–9. doi: 10.1111/j.1399-3038.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 21.Asseyr AF, Businco L. Atopic sensitization in children of Somali immigrants in Italy. J Investig Allergol Clin Immunol. 1994;4(4):192–6. [PubMed] [Google Scholar]
- 22.Perry TT, Matsui EC, Kay Conover-Walker M, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcome. J Allergy Clin Immunol. 2004;114(1):144–9. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107(5):891–6. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 24.U.S. Census Bureau [cited 2011 June 30];America’s Families and Living Arrangements. 2010 Available from: http://www.census.gov/population/www/socdemo/hh-fam/cps2010.html.
- 25.Eldeirawi K, McConnell R, Furner S, Freels S, Stayner L, Hernandez E, et al. Associations of doctor-diagnosed asthma with immigration status, age at immigration, and length of residence in the United States in a sample of Mexican American School Children in Chicago. J Asthma. 2009;46(8):796–802. [PubMed] [Google Scholar]
- 26.Keet CA, McCormack MC, Peng RD, Matsui EC. Age- and atopy-dependent effects of vitamin D on wheeze and asthma. J Allergy Clin Immunol. 2011;128(2):414–6. e5. doi: 10.1016/j.jaci.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thornton CA, Macfarlane TV, Holt PG. The hygiene hypothesis revisited: role of materno-fetal interactions. Curr Allergy Asthma Rep. 2010;10(6):444–52. doi: 10.1007/s11882-010-0148-5. [DOI] [PubMed] [Google Scholar]
- 28.Ege MJ, Bieli C, Frei R, van Strien RT, Riedler J, Ublagger E, et al. Prenatal farm exposure is related to the expression of receptors of the innate immunity and to atopic sensitization in school-age children. J Allergy Clin Immunol. 2006;117(4):817–23. doi: 10.1016/j.jaci.2005.12.1307. [DOI] [PubMed] [Google Scholar]
- 29.Brand S, Teich R, Dicke T, Harb H, Yildirim AO, Tost J, et al. Epigenetic regulation in murine offspring as a novel mechanism for transmaternal asthma protection induced by microbes. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.04.035. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 30.Ege MJ, Mayer M, Normand AC, Genuneit J, Cookson WO, Braun-Fahrlander C, et al. Exposure to environmental microorganisms and childhood asthma. N Engl J Med. 2011;364(8):701–9. doi: 10.1056/NEJMoa1007302. [DOI] [PubMed] [Google Scholar]
- 31.Djuardi Y, Wibowo H, Supali T, Ariawan I, Bredius RG, Yazdanbakhsh M, et al. Determinants of the relationship between cytokine production in pregnant women and their infants. PLoS One. 2009;4(11):e7711. doi: 10.1371/journal.pone.0007711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friberg IM, Bradley JE, Jackson JA. Macroparasites, innate immunity and immunoregulation: developing natural models. Trends Parasitol. 2010;26(11):540–9. doi: 10.1016/j.pt.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 33.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Bresolin N, Clerici M, et al. The landscape of human genes involved in the immune response to parasitic worms. BMC Evol Biol. 2010;10:264. doi: 10.1186/1471-2148-10-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fumagalli M, Pozzoli U, Cagliani R, Comi GP, Riva S, Clerici M, et al. Parasites represent a major selective force for interleukin genes and shape the genetic predisposition to autoimmune conditions. J Exp Med. 2009;206(6):1395–408. doi: 10.1084/jem.20082779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maizels RM. Parasite immunomodulation and polymorphisms of the immune system. J Biol. 2008;8(7):62. doi: 10.1186/jbiol166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moller M, Gravenor MB, Roberts SE, Sun D, Gao P, Hopkin JM. Genetic haplotypes of Th-2 immune signalling link allergy to enhanced protection to parasitic worms. Hum Mol Genet. 2007;16(15):1828–36. doi: 10.1093/hmg/ddm131. [DOI] [PubMed] [Google Scholar]
- 37.Peisong G, Yamasaki A, Mao XQ, Enomoto T, Feng Z, Gloria-Bottini F, et al. An asthma-associated genetic variant of STAT6 predicts low burden of ascaris worm infestation. Genes Immun. 2004;5(1):58–62. doi: 10.1038/sj.gene.6364030. [DOI] [PubMed] [Google Scholar]
- 38.Hopkin J. Immune and genetic aspects of asthma, allergy and parasitic worm infections: evolutionary links. Parasite Immunol. 2009;31(5):267–73. doi: 10.1111/j.1365-3024.2009.01104.x. [DOI] [PubMed] [Google Scholar]
- 39.Kouriba B, Chevillard C, Bream JH, Argiro L, Dessein H, Arnaud V, et al. Analysis of the 5q31-q33 locus shows an association between IL13-1055C/T IL-13-591A/G polymorphisms and Schistosoma haematobium infections. J Immunol. 2005;174(10):6274–81. doi: 10.4049/jimmunol.174.10.6274. [DOI] [PubMed] [Google Scholar]
- 40. [cited 2011 July 7];WHO Fact Sheet on Intestinal Worms. 2011 Available from: http://www.who.int/intestinal_worms/en/
- 41.Hewitson JP, Grainger JR, Maizels RM. Helminth immunoregulation: the role of parasite secreted proteins in modulating host immunity. Mol Biochem Parasitol. 2009;167(1):1–11. doi: 10.1016/j.molbiopara.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Figueiredo CA, Barreto ML, Rodrigues LC, Cooper PJ, Silva NB, Amorim LD, et al. Chronic intestinal helminth infections are associated with immune hyporesponsiveness and induction of a regulatory network. Infect Immun. 2010;78(7):3160–7. doi: 10.1128/IAI.01228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J, Visness CM, Sampson HA. Food allergen sensitization in inner-city children with asthma. J Allergy Clin Immunol. 2005;115(5):1076–80. doi: 10.1016/j.jaci.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Sternthal MJ, Coull BA, Chiu YH Mathilda, Cohen S, Wright RJ. Associations among maternal childhood socioeconomic status, cord blood IgE levels, and repeated wheeze in urban children. J Allergy Clin Immunol. 2011;128(2):337–345. e1. doi: 10.1016/j.jaci.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Acevedo-Garcia D, Soobader MJ, Berkman LF. Low birthweight among US Hispanic/Latino subgroups: the effect of maternal foreign-born status and education. Soc Sci Med. 2007;65(12):2503–16. doi: 10.1016/j.socscimed.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 46.Mendoza FS. Health disparities and children in immigrant families: a research agenda. Pediatrics. 2009;124(Suppl 3):S187–95. doi: 10.1542/peds.2009-1100F. [DOI] [PubMed] [Google Scholar]
- 47.Mosnaim GS, Sadowski LS, Durazo-Arvizu RA, Sharp LK, Curtis LM, Shalowitz MU, et al. Parental language and asthma among urban Hispanic children. J Allergy Clin Immunol. 2007;120(5):1160–5. doi: 10.1016/j.jaci.2007.08.040. [DOI] [PubMed] [Google Scholar]
- 48.Eldeirawi KM, Persky VW. Associations of acculturation and country of birth with asthma and wheezing in Mexican American youths. J Asthma. 2006;43(4):279–86. doi: 10.1080/0277090060022869. [DOI] [PubMed] [Google Scholar]
- 49.Hong X, Wang G, Liu X, Kumar R, Tsai HJ, Arguelles L, et al. Gene polymorphisms, breast-feeding, and development of food sensitization in early childhood. J Allergy Clin Immunol. 2011;128(2):374–81. e2. doi: 10.1016/j.jaci.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niggemann B, Schmitz R, Schlaud M. The high prevalence of peanut sensitization in childhood is due to cross-reactivity to pollen. Allergy. 2011;66(7):980–1. doi: 10.1111/j.1398-9995.2011.02561.x. [DOI] [PubMed] [Google Scholar]
- 51.Santos AB, Rocha GM, Oliver C, Ferriani VP, Lima RC, Palma MS, Sales VS, Aalberse RC, Chapman MD, Arruda LK. Cross-reactive IgE antibody responses to tropomyosins from Ascaris lumbricoides and cockroach. J Allergy Clin Immunol. 2008;121(4):1040–6. e1. doi: 10.1016/j.jaci.2007.12.1147. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.