Abstract
Background
Surgical brain injury (SBI) is damage to functional brain tissue resulting from neurosurgical manipulations such as sharp dissection, electrocautery, retraction, and direct applied pressure. Brain edema is the major contributor to morbidity with inflammation, necrosis, oxidative stress and apoptosis likely playing smaller roles. Effective therapies for SBI may improve neurological outcomes and postoperative morbidities associated with brain surgery. Previous studies show an adrenergic correlation to blood-brain barrier control. The alpha-2 receptor agonist dexmedetomidine (DEX) has been shown to improve neurological outcomes in stroke models. We hypothesized that DEX may reduce brain edema and improve neurological outcomes in a rat model of SBI.
Methods
Male Sprague-Dawley rats (n=63) weighing 280–350g were randomly assigned to one of four intraperitoneal (IP) treatment groups: sham IP, vehicle IP, DEX 10 mg/kg, and DEX 30 mg/kg. Treatments were given 30 minutes before SBI. These treatment groups were repeated to observe the physiologic impact of DEX on mean arterial blood pressure (MAP), heart rate (HR), and blood glucose on SBI naïve animals. Rats were also assigned to four postinjury IV treatment groups: sham IV, vehicle IV, DEX 10/5, and DEX 30/15 (DEX group doses were 10 and 30 mg/kg/hr, with 5 and 15 mg/kg initial loading doses respectively). Initial loading doses began 20 minutes after SBI, followed by 2 hours of infusion. SBI animals were subjected to neurological testing 24 hours after brain injury by a blinded observer, promptly killed, and brain water content measured via the dry/wet weight method.
Results
All treatment groups showed a significant difference in ipsilateral frontal brain water content and neurological scores when compared with sham animals. However, there was no difference between DEX-treated and vehicle animals. Physiologic monitoring showed treatment with low or high doses of DEX significantly decreased MAP and HR, and briefly increased blood glucose compared with naïve or vehicle-treated animals.
Conclusions
DEX administration did not reduce brain edema or improve neurological function after SBI in this study. The statistical difference in brain water content and neurological scores when comparing sham treatment to vehicle and DEX treatments shows consistent reproduction of this model. Significant changes in MAP, HR, and blood glucose after DEX as compared to vehicle and sham treatments suggest appropriate delivery of drug.
Introduction
The National Neurosurgical Procedural Statistics 2006 Survey from the American Association of Neurological Surgeons (AANS) reports 592,443 cranial surgeries were performed in that year alone.1 Though this total included a variety of procedures, all of them share a common pathology. Surgical brain injury (SBI) is damage to normal brain tissue caused by surgical manipulation. Sharp dissection, electrocauterization, retraction, or direct application of any foreign materials are examples of interventions that cause this damage. A simplified rodent model has been developed for the purpose of studying SBI and therapies that could improve postoperative outcomes.2 Brain edema is the major contributor to SBI in this model, followed by smaller contributions from inflammation, necrosis, oxidative stress, and apoptosis.2–6
It has been hypothesized that the central noradrenergic system has correlations to blood-brain barrier (BBB) control, via projections from the locus ceruleus which follow the brain microvasculature.7–9 The nonselective alpha adrenergic receptor antagonist phenoxybenzamine decreased brain water content in a cold-induced model of vasogenic edema in rats.10 Electrical stimulation of the locus ceruleus has been shown to increase BBB permeability by increasing central norepinephrine levels.11 A study using a porcine model of sepsis showed the alpha-1 adrenergic receptor agonist methoxamine induces brain microvessel endothelial cell swelling.12 The alpha-2 receptor agonist clonidine significantly decreased postburn edema on rat skin when administered for two hours after injury.13
Dexmedetomidine (DEX) is a powerful alpha-2 receptor agonist that has been shown to be neuroprotective in animal models of stroke.14–19 We hypothesized that DEX would decrease brain edema and improve neurological outcomes after SBI in rats.
Methods
All procedures were approved by the Loma Linda University Institutional Animal Care and Use Committee (Loma Linda, CA) and conform to the United States Public Health Service Policy on Humane Care and Use of Laboratory Animals and the Guide for the Care and Use of Laboratory Animals (1996). A total of 63 adult male Sprague-Dawley rats weighing 280–350g were used.
Surgical Brain Injury
SBI was performed as previously described.2 Briefly, animals were either anesthetized via induction with 4% isoflurane then maintained on 2.5% isoflurane (preinjury groups), or given ketamine 100 mg/kg and xylazine 10 mg/kg via intraperitoneal (IP) injection (postinjury groups). IP anesthesia was used for better continuous sedation during surgery because animals were frequently moved from supine to prone during catheter placement, and the long-lasting sedation allowed for better control of animals attached to infusion pumps postoperatively. A midline scalp incision was made, and a 5 mm by 5 mm cranial window, beginning at the bregma, was removed using a Micro Drill (Fine Science Tools, Foster City, CA, USA). The dura mater was then incised and reflected to expose the right frontal lobe. Puncture through the dura represented initial brain injury time. The right frontal lobe was sharply dissected down to the base of the brain (assessed stereotactically by the blade contacting bone) at 2 mm lateral and 1 mm anterior to the bregma, and then removed. Electrocautery (Veterinary Electrosurgical Unit, Macan, Chicago, IL, USA) was applied briefly (~2 seconds total) to the remaining medial and posterior surfaces of exposed brain. Hemostasis was achieved by direct pressure with absorbent tamponade applicators before replacing the bone over the cranial window. The scalp was closed and the animal placed into a heated recovery cage for postoperative monitoring. All animals received 0.9% Saline 1 mL subcutaneously either before or immediately after surgery for hydration. Sham surgeries proceeded similarly except without incision of dura or brain injury (craniotomy only). Buprenorphine 0.03 mg/kg was administered subcutaneously at the start of surgery to every animal anesthetized with isoflurane, and given as needed postoperatively for pain both to isoflurane and ketamine/xylazine-anesthetized animals.
Pretreatment
In this arm of the study, treatments were given as one-time IP doses 30 minutes before SBI, based on previous studies with stroke models.14,15 Animals were randomly assigned to one of four groups: sham IP (n=6), vehicle IP (n=8), DEX 10 mg/kg (n=8), DEX 30 mg/kg (n=8). Saline 0.9% was the vehicle used, and all treatment concentrations were calculated such that volumes administered across groups would be similar.
Physiologic Monitoring
To determine the physiologic effects of treatment, SBI naïve animals underwent arterial blood pressure, heart rate (HR), and blood glucose monitoring (n=4 per preinjury IP treatment group). These animals underwent 4% isoflurane induction followed by 2.5% isoflurane to maintain anesthesia. Body temperature throughout the procedure was monitored via rectal probe and kept at 37±1 degrees Celsius via a heat lamp. A right groin cut-down procedure was used to gain access to the femoral artery which was cannulated for continuous hemodynamic monitoring (Digi-Med Blood Pressure Analyzer™ 400, Micro-Med, Inc., Louisville, KY, USA) and periodic sampling of blood glucose levels via an Aviva Accu-Check® blood glucose meter system with strips (Roche Diagnostics, Indianapolis, IN, USA). Physiologic variables were measured before IP dosing, and intermittently for one hour after. Animals from this study arm were monitored daily for 7 days before being incorporated into the SBI groups.
Posttreatment
Rats were randomly assigned to four postinjury infusion groups:13 sham IV (n=7), vehicle IV (n=8), DEX 10/5 (n=8), DEX 30/15 (n=8). The two DEX group doses were 10 and 30 mg/kg/hr, with 5 and 15 mg/kg initial loading doses respectively. Initial loading doses were given 20 minutes after SBI, followed by 2 hours of drug infusion (Standard Infusion Only Pump 22 Syringe Pump, Harvard Apparatus, Holliston, MA, USA).
Exclusion Criteria
Animals that died before outcome measurements were excluded. Otherwise, all collected data were incorporated into the results.
Neurological Testing
A blinded observer tested all surviving animals 24 hours after brain injury. The observer used a modified 21-point sensorimotor test that measures spontaneous activity, body proprioception, response to vibrissae touch, limb symmetry, lateral turning ability, forepaw outstretching, and climbing ability, allowing a maximum of 3 points for each category.20 The bilateral tactile stimulation test (BTS) was used to measure the extent of sensorimotor deficit.21 This test has two parts: limb affinity (LA), which tests for forelimb preference by placing adhesive labels on the forelimbs and observing which side the animal removes first, and magnitude of asymmetry (MOA), which measures the magnitude of stimulation needed for a response by placing labels of changing sizes on the forelimbs and observing which are removed first. Animals received four rounds of LA testing with a fifth round given if 25% or 75% affinity for the unaffected (ipsilateral) limb are shown. Only rats showing a more than 70% affinity for the ipsilateral limb undergo MOA. Brain injury will dampen sensorimotor function to the contralateral limb, causing the animal to respond more readily to ipsilateral input until a large enough stimulus to the affected area gains precedence.
Brain Water Content
Animals were killed, immediately after neurological testing, at 24 hours for brain harvesting. Brains were divided into 6 regions: ipsilateral and contralateral frontal and parietal lobes, cerebellum, and brainstem. These sections were weighed immediately (wet weight), then placed in a 100 degree Celsius oven for 48 hours before being weighed again (dry weight). Brain water content was calculated as a percentage via the following formula: .1
Statistical Analysis
One-way analysis of variance was used to test for significance among the four groups, and the Tukey method was used for pairwise multiple comparisons. A p-value of less than 0.05 was deemed statistically significant. Unless otherwise noted, results are listed as mean ± standard deviation. Tukey corrected 95% confidence intervals showing the difference between means are listed as difference in means ± margin of error. A Pearson analysis was also conducted to analyze for correlation of brain water content with neurological outcomes. All statistical analyses were conducted using SigmaStat 3.5 (Aspire Software International, Ashburn, VA, USA).
Results
Mortality
Of the 63 animals used, 11 died (17%) after SBI from uncontrollable or occult intracerebral hemorrhage before data collection times and therefore were excluded from the brain water and neurological results. Mortality per group: sham IP=0% (0/6), vehicle IP=25% (2/8), DEX 10 =0% (0/8), DEX 30=25% (2/8), sham IV=0% (0/7), vehicle IV=50% (4/8), DEX 10/5=14% (1/8), DEX 30/15=25% (2/8). Two animals from the DEX 10 mg/kg dosing group (n=6) died during physiologic monitoring due to cardiopulmonary arrest secondary to anesthesia overdose. The initial gas delivery system was giving a higher concentration than expected. The data collected were incorporated into the final results along with an additional two full animal datasets taken after the system was corrected. This did not change the statistical significance of the results.
Pretreatment and posttreatment with DEX does not reduce brain water content
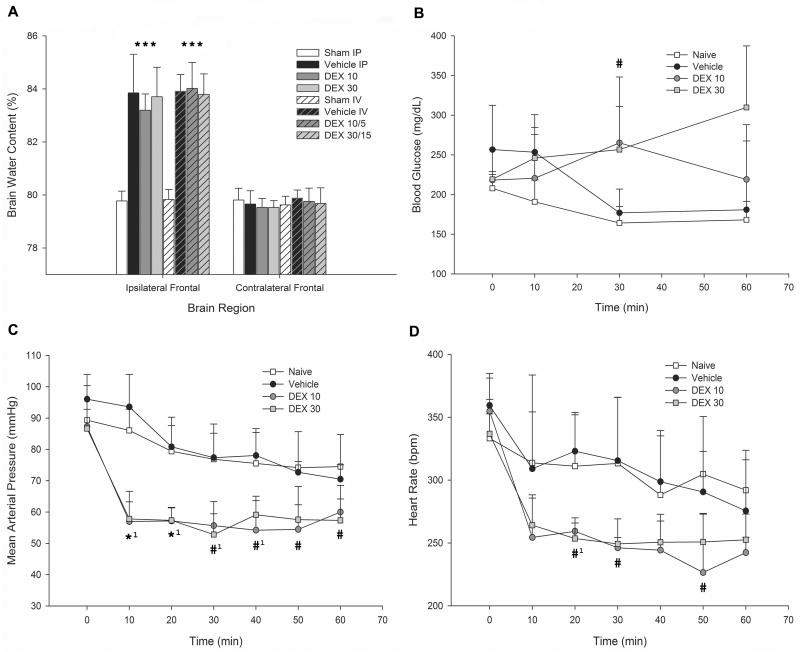
Ipsilateral frontal lobe water content in the sham IP group (79.8% ± 0.4%) was significantly lower (p<0.001) when compared with vehicle IP (83.9% ± 1.5%; difference in means 4.1% ± 1.4%), DEX 10 (83.2% ± 0.6%; difference in means 3.5% ± 0.6%), and DEX 30 (83.7% ± 1.1%; difference in means 3.9% ± 1.1%) groups (Figure 1A). The same was true when comparing ipsilateral frontal lobes of the sham IV group (79.8% ± 0.4%) with vehicle IV (83.9% ± 0.6%; difference in means 4.1% ± 0.8%), DEX 10/15 (84.0% ± 1.0%; difference in means 4.2% ± 0.9%), and DEX 30/15 (83.8% ± 0.8%; difference in means 4.0% ± 0.8%) groups (p<0.001). However, there was no statistical difference between the frontal brain water content of vehicle IP and DEX 10 (p=0.594; difference in means 0.7% ± 1.4%) and DEX 30 (p=0.994; difference in means 0.1% ± 1.7%) groups, or the vehicle IV and DEX 10/5 (p=0.996; difference in means 0.1% ±1.1%) and DEX 30/15 (p=0.994; difference in means 0.1% ± 1.0%) groups. All other brain regions remained similar in brain water content across groups.
Figure 1.
A) Postoperative brain water content. Sham intraperitoneal (IP) (n=6), vehicle IP (n=6), dexmedetomidine (DEX) 10 (n=8), DEX 30 (n=6), sham IV (n=7), vehicle IV (n=4), DEX 10/5 (n=8), DEX 30/15 (n=6). *P<0.001 when compared with respective sham group. B–D) Physiologic monitoring results (n=4 per study group) for B) blood glucose, C) mean arterial blood pressure (MAP), and D) heart rate (HR). *1P<0.001, #1P<0.01, and #P<0.05 after one-way ANOVA testing of the treatment groups in the labeled time period. Tukey’s pairwise multiple comparison procedure (results not displayed) showed at least a P<0.05 when comparing MAP of DEX 10 and DEX 30 with naïve and vehicle at 10–50 min, and with naïve at 60 min. P<0.05 comparing HR of DEX 10 with naïve and vehicle at 20 and 30 min, and with DEX 10 compared with naïve and vehicle at 50 min. P<0.05 comparing blood glucose of DEX 10 with naïve and vehicle at 30 min, and DEX 30 with naïve at 30 min. Values shown as mean + SD.
Pretreatment and posttreatment with DEX does not improve neurological scores
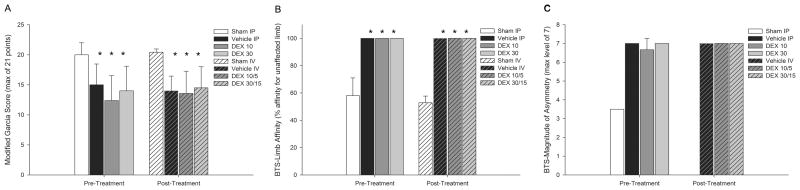
There was a significant difference in the modified 21-point sensorimotor scores between the sham IP group (20 ± 2) and the DEX 10 (12 ± 4; p=0.004; difference in means 8 ± 3), and DEX 30 (14 ± 4; p=0.039; difference in means 6 ± 4) groups, as well as between the sham IV group (20 ± 1) and the vehicle IV (14 ± 2; p=0.009; difference in means 6 ± 3), DEX 10/5 (14 ± 4; p=0.001; difference in means 6 ± 3), and DEX 30/15 (15 ± 4; p=0.007; difference in means 5 ± 3) groups (Figure 2A). Vehicle and DEX groups showed 100% affinity for the unaffected limb compared with sham IP (58.0% ± 13.0%; p<0.001) and sham IV (52.9% ± 4.9%; p<0.001) groups (which had equal affinity for both limbs) in the BTS LA tests for both pre- and posttreatment arms (Figure 2B). Only one animal from the sham IP, and no animals from the sham IV, group qualified for BTS MOA testing (as would be expected), and therefore statistical comparisons with shams were not possible (Figure 2C). Though every vehicle and DEX-treated animal qualified for BTS MOA testing, there was no difference in asymmetry with nearly all animals scoring at the highest level. There was no significant difference between vehicle and DEX groups for any of the neurological tests.
Figure 2.
Neurological test results. A) Modified 21-point Garcia sensorimotor test (maximum score = 21 points); sham intraperitoneal (IP) (n=6), vehicle IP (n=6), dexmedetomidine (DEX) 10 (n=8), DEX 30 (n=6), sham IV (n=7), vehicle IV (n=4), DEX 10/5 (n=7), and DEX 30 (n=6). B) Bilateral tactile stimulation (BTS) limb affinity (LA) test; sham IP (n=5), vehicle IP (n=5), DEX 10 (n=6), DEX 30 (n=4), sham IV (n=7), vehicle IV (n=4), DEX 10/5 (n=7), and DEX 30 (n=6). C) BTS magnitude of asymmetry (MOA) test; sham IP (n=1), vehicle IP (n=5), DEX 10 (n=6), DEX 30 (n=4), vehicle IV (n=4), DEX 10/5 (n=7), and DEX 30 (n=6). *P<0.05 when compared with respective sham group. Note: only one animal from the sham IP group qualified for BTS-MOA testing and no animals qualified from the sham IV group, therefore no statistical comparison is possible. Values shown as mean + SD.
Pearson’s analysis showed a negative correlation coefficient (r = −0.589, p<0.001) between brain water content, and modified 21-point sensorimotor scores, and a positive correlation coefficient (r = 0.884, p<0.001) between brain water content and BTS-LA scores. This is interpreted to mean that worsening edema correlates with poorer neurological scores.
DEX significantly decreases mean arterial blood pressure (MAP) and HR, while increasing blood glucose
Physiologic monitoring showed a statistically significant decrease in MAP and HR in DEX-treated groups when compared to vehicle and sham groups (Figure 1C and D). MAP decreased within 10 minutes of DEX administration and remained significantly lower throughout the procedure compared to naïve and vehicle groups (p<0.001). HR also decreased significantly by the 20-minute mark and remained low (p=0.003).
Blood glucose levels showed a period of significant elevation after 30 minutes in DEX groups (Figure 1B). Though the higher dose DEX 30 group showed continuing elevation at 60 minutes, there was no statistical difference between groups by the end of the procedure (p=0.051).
Discussion
In this study we used a rat model of SBI to test the ability of DEX to reduce brain edema and improve neurological outcomes after intracranial surgery. Pre- and posttreatment with DEX did not improve either of these outcomes when measured 24 hours after injury as compared with control groups (Figures 1 and 2).
Brain swelling has long been an indicator of poor outcome in stroke patients, with increases of just 1% in brain water content causing a 4% increase in volume leading to morbidity through mass effect.22 In SBI, mass effect is unlikely the cause of morbidity because the resection space would negate the effect of soft tissue volume expansion to a large extent. However, this study correlates increased brain water around the injury site with poorer neurologic outcomes, and previous studies using this same model of surgical resection have shown neurologic improvements with decreased brain water in the injured tissue.2,4,23 Differences in water content between samples may occur due to variations in the size of resection, which may explain the 2–3% range in the 95% confidence intervals between means of some groups while neurologic scores remain similar. Therefore, we hypothesized that a reduction of brain water content to within 1% of the sham operated level would most likely show important changes in outcomes. Lack of brain water reduction in this study may have due to several factors. Previous studies showed that noradrenergic blockade could decrease brain edema via control of the BBB; however, sympatholysis from alpha-2 receptor stimulation alone may not be enough to reduce edema.10 Large-scale, gross disruption of tissues by sharp dissection exposes interstitial tissues to a considerable amount of blood and surgical irrigation, likely making the contribution of a leaking BBB minute in comparison. Mechanisms that allow for water drainage (e.g., upregulation of aquaporins) may be more important in the first hours after injury; therefore timing and duration of treatment may also be a factor.24 Future studies may test a later time point with a longer infusion period (such as would be appropriate in a neurological critical care setting) to see if DEX has any ability to decrease BBB permeability and improve brain edema since there does seem to be a statistical correlation between brain water content and neurological outcomes in SBI. Because there was no difference in brain water content, no further steps were taken to investigate BBB permeability in this study as the clinical result was lacking.
Several previous studies have shown significant neurological improvement in DEX treated stroke animals.14–19 However, the results of this study describe no difference when compared to control groups. This may have been due to the larger area of injury creating a greater deficit in SBI compared to other models, with sensorimotor scores for the vehicle and treatment groups comparable to a permanent middle cerebral artery occlusion.20 Also, the treatment effect may have been too small for detection in this study. The goal of this study was to improve postoperative morbidities that may be associated with intracranial surgery, and only large effects would likely have measurable or relevant changes. Therefore this study was powered to detect what we deemed as clinically significant results, which may have caused a type II error.
Hypotension and increased blood glucose have been associated with worsening neurological outcomes in stroke models.25 However, previous studies describing the effect of DEX in stroke reported neuroprotection regardless of significant hypotension and hyperglycemia.14,16–19 The effects of arterial blood pressure and blood glucose have not been studied in SBI. Therefore, it is possible that the physiologic changes in this study could have masked a positive neurological outcome by concurrently causing injury, or that DEX actually does not affect these outcomes at all. Future studies may attempt to control for hyperglycemia and hypotension in order to further describe any neuroprotective effects of DEX in this model. The agreement of the data presented here with previous studies monitoring physiologic changes while using DEX suggest that the drug was delivered appropriately.14,16–19,26–27
The overall mortality in this study was higher than previous SBI studies, especially in the posttreatment arm.2–6 Although there are no studies to correlate this, it is possible that the IV infusions may have caused an intravascular change that predisposed SBI animals to hemorrhage at the injury site even after initial hemostasis. We are unable to determine if the DEX groups showed a significant decrease in mortality in this treatment arm due to the small number of animals used.
Several reports have shown that alpha-2 receptor agonists like DEX and clonidine may be used to increase patient comfort during “awake” craniotomies, decrease postoperative shivering after neurosurgery, and ameliorate hemodynamic and neuroendocrinal responses to skull-pin head-holder application.27–30 However, we were unable to demonstrate a significant effect from DEX administration before or after SBI in decreasing postoperative brain edema or improving short-term neurological deficits, which may be relevant when considering anesthetic technique in intracranial surgery.
Conclusion
The data suggest that administration of DEX shortly before or just after brain injury has no effect on brain water content or short-term neurological outcomes in SBI. DEX decreased MAP and HR, while increasing blood glucose, showing that the drug was delivered effectively. These physiologic changes did not affect outcomes. Significant differences between sham and treatment groups in brain water content and neurological scores suggest that the model was reproduced consistently.
Acknowledgments
Funding: NIH Grant NS43338
We would like to thank Suzanne Marcantonio for her technical expertise.
Footnotes
Reprints will not be available from the authors.
DISCLOSURES:
Name: Michael Benggon, MD
Contribution: This author helped design the study, conduct the study, analyze the data, and write the manuscript
Attestation: Michael Benggon has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Han Chen, MD
Contribution: This author helped design the study, conduct the study, and analyze the data
Attestation: Han Chen has seen the original study data, reviewed the analysis of the data, and approved the final manuscript
Name: Richard Applegate, MD
Contribution: This author helped write the manuscript
Attestation: Richard Applegate approved the final manuscript
Name: Robert Martin, MD
Contribution: This author helped write the manuscript
Attestation: Robert Martin approved the final manuscript
Name: John H. Zhang, MD, PhD
Contribution: This author helped design the study and write the manuscript
Attestation: John H. Zhang has seen the original study data, approved the final manuscript, and is the author responsible for archiving the study files
This manuscript was handled by: Gregory J. Crosby, MD
The authors declare no conflicts of interest.
Contributor Information
Michael Benggon, Department of Anesthesiology, Loma Linda University School of Medicine, Loma Linda, California.
Han Chen, Department of Physiology and Pharmacology, Loma Linda University School of Medicine, Loma Linda, California.
Richard Applegate, Department of Anesthesiology, Loma Linda University School of Medicine, Loma Linda, California.
Robert Martin, Department of Anesthesiology, Loma Linda University School of Medicine, Loma Linda, California.
John H. Zhang, Department of Physiology and Pharmacology, Department of Anesthesiology, and Department of Neurosurgery, Loma Linda University School of Medicine, Loma Linda, California.
References
- 1.American Association of Neurological Surgeons. AANS. Rolling Meadows; IL: 2008. [Accessed 23 May 2011]. National Neurosurgical Procedural Statistics 2006 Survey. ( https://www.myaans.org/desktopmodules/directory/ProcedStat2006.pdf) [Google Scholar]
- 2.Jadhav V, Matchett G, Hsu FPK, Zhang JH. Inhibition of Src tyrosine kinase and effect on outcomes in a new in vivo model of surgically induced brain injury. J Neurosurg. 2007;106:680–86. doi: 10.3171/jns.2007.106.4.680. [DOI] [PubMed] [Google Scholar]
- 3.Matchett G, Hahn J, Obenaus A, Zhang JH. Surgically induced brain injury in rats: the effect of erythropoietin. J Neurosci Meth. 2006;158:234–41. doi: 10.1016/j.jneumeth.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi M, Jadhav V, Obenaus A, Colohan A, Zhang JH. Matrix metalloproteinase inhibition attenuates brain edema in an in vivo model of surgically-induced brain injury. Neurosurgery. 2007;61:1067–76. doi: 10.1227/01.neu.0000303203.07866.18. [DOI] [PubMed] [Google Scholar]
- 5.Hyong A, Jadhav V, Lee S, Tong W, Rowe J, Zhang JH, Tang J. Rosiglitazone, a PPAR gamma agonist, attenuates inflammation after surgical brain injury in rodents. Brain Res. 2008;1215:218–24. doi: 10.1016/j.brainres.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lo W, Bravo T, Jadhav V, Titova E, Zhang JH, Tang J. NADPH oxidase inhibition improves neurological outcomes in surgically-induced brain injury. Neurosci Lett. 2007;414:228–32. doi: 10.1016/j.neulet.2006.12.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raichle ME, Hartman BK, Eichling JO, Sharpe LG. Central noradrenergic regulation of cerebral blood flow and vascular permeability. P Natl Acad Sci USA. 1975;72:3726–30. doi: 10.1073/pnas.72.9.3726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalaria RN, Stockmeier CA, Harik SI. Brain microvessels are innervated by locus ceruleus noradrenergic neurons. Neurosci Lett. 1989;97:203–8. doi: 10.1016/0304-3940(89)90164-x. [DOI] [PubMed] [Google Scholar]
- 9.Harik S. Blood-brain barrier sodium/potassium pump: modulation by central noradrenergic innervation. P Natl Acad Sci USA. 1986;83:4067–70. doi: 10.1073/pnas.83.11.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borges N, Sarmento A, Azevedo I. Dynamics of experimental vasogenic brain oedema in the rat: changes induced by adrenergic drugs. J Auton Pharmacol. 1999;19:209–17. doi: 10.1046/j.1365-2680.1999.00137.x. [DOI] [PubMed] [Google Scholar]
- 11.Sarmento A, Borges N, Lima D. Influence of electrical stimulation of locus ceruleus on the rat blood-brain barrier permeability to sodium fluorescein. Acta Neurochir. 1994;127:215–19. doi: 10.1007/BF01808769. [DOI] [PubMed] [Google Scholar]
- 12.Moss RF, Parmar NK, Tighe D, Davies DC. Adrenergic agents modify cerebral edema and microvessel ultrastructure in porcine sepsis. Crit Care Med. 2004;32:1916–21. doi: 10.1097/01.ccm.0000139917.26914.dd. [DOI] [PubMed] [Google Scholar]
- 13.Cassuto J, Tarnow P, Yregard L, Lindblom L, Rantfors J. Adrenoceptor subtypes in the control of burn-induced plasma extravasation. Burns. 2005;31:123–29. doi: 10.1016/j.burns.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman WE, Kochs E, Werner C, Thomas C, Albrecht RF. Dexmedetomidine improves neurologic outcome from incomplete ischemia in the rat. Reversal by the alpha-2 adrenergic antagonist atipamezole. Anesthesiology. 1991;75:328–32. doi: 10.1097/00000542-199108000-00022. [DOI] [PubMed] [Google Scholar]
- 15.Engelhard K, Werner C, Eberspacher E, Bachl M, Blobner M, Hildt E, Hutzler P, Kochs E. The effect of the α2-agonist dexmedetomidine and the N-methyl-D-aspartate antagonist S(+)-ketamine on the expression of apoptosis-regulating proteins after incomplete cerebral ischemia and reperfusion in rats. Anesth Analg. 2003;96:524–31. doi: 10.1097/00000539-200302000-00041. [DOI] [PubMed] [Google Scholar]
- 16.Kuhmonen J, Pokorny J, Miettinen R, Haapalinna A, Jolkkonen J, Riekkinen P, Sivenius J. Neuroprotective effects of dexmedetomidine in the gerbil hippocampus after transient global ischemia. Anesthesiology. 1997;87:371–7. doi: 10.1097/00000542-199708000-00025. [DOI] [PubMed] [Google Scholar]
- 17.Kuhmonen J, Haapalinna A, Sivenius J. Effects of dexmedetomidine after transient and permanent occlusion of the middle cerebral artery in the rat. J Neural Transm. 2001;108:261–71. doi: 10.1007/s007020170071. [DOI] [PubMed] [Google Scholar]
- 18.Goyagi T, Nishikawa T, Tobe Y, Masaki Y. The combined neuroprotective effects of lidocaine and dexmedetomidine after transient forebrain ischemia in rats. Acta Anaesthesiol Scand. 2009;53:1176–83. doi: 10.1111/j.1399-6576.2009.01976.x. [DOI] [PubMed] [Google Scholar]
- 19.Sato K, Kimura T, Nishikawa T, Tobe Y, Masaki Y. Neuroprotective effects of a combination of dexmedetomidine and hypothermia after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2010;54:377–82. doi: 10.1111/j.1399-6576.2009.02139.x. [DOI] [PubMed] [Google Scholar]
- 20.Garcia JH, Wagner S, Liu KF, Hu XJ. Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation Stroke. 1995;26:627–34. doi: 10.1161/01.str.26.4.627. [DOI] [PubMed] [Google Scholar]
- 21.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–87. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 22.Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the european cooperative acute stroke study (ECASS) I. Stroke. 1999;30:2631–36. doi: 10.1161/01.str.30.12.2631. [DOI] [PubMed] [Google Scholar]
- 23.Jadhav V, Ostrowski RP, Tong W, Matus B, Jesunathadas R, Zhang JH. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–42. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papadopoulos MC, Manley GT, Krishna S, Verkman AS. Aquaporin-4 facilitates reabsorption of excess fluid in vasogenic brain edema. FASEB J. 2004;18:1291–3. doi: 10.1096/fj.04-1723fje. Epub 2004 Jun 18. [DOI] [PubMed] [Google Scholar]
- 25.Li PA, Shamloo M, Smith ML, Katsura K, Siesjo BK. The influence of plasma glucose concentrations on ischemic brain damage is a threshold function. Neurosci Lett. 1994;177:63–5. doi: 10.1016/0304-3940(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 26.Dyck JB, Maze M, Haack C, Vuorilehto L, Shafer SL. The pharmacokinetics and hemodynamic effects of intravenous and intramuscular dexmedetomidine hydrochloride in adult human volunteers. Anesthesiology. 1993;78:813–20. doi: 10.1097/00000542-199305000-00002. [DOI] [PubMed] [Google Scholar]
- 27.Uyar AS. Dexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull-pin head-holder application during craniotomy. J Neurosurg Anesthesiol. 2008;20:174–79. doi: 10.1097/ANA.0b013e318177e5eb. [DOI] [PubMed] [Google Scholar]
- 28.Bekker AY, Kaufman B, Samir H, Doyle W. The use of dexmedetomidine infusion for awake craniotomy. Anesth Analg. 2001;92:1251–3. doi: 10.1097/00000539-200105000-00031. [DOI] [PubMed] [Google Scholar]
- 29.Rozet I, Muangman S, Vavilala MS, Lee LA, Souter MJ, Domino KJ, Slimp JC, Goodkin R, Lam AM. Clinical experience with dexmedetomidine for implantation of deep brain stimulators in parkinson's disease. Anesth Analg. 2006;103(5):1224–28. doi: 10.1213/01.ane.0000239331.53085.94. [DOI] [PubMed] [Google Scholar]
- 30.Stapelfeldt C, Lobo EP, Brown R, Talke PO. Intraoperative clonidine administration to neurosurgical patients. Anesth Analg. 2005;100:226–32. doi: 10.1213/01.ANE.0000142122.57201.6B. [DOI] [PubMed] [Google Scholar]