Abstract
OBJECTIVE
Fetal neutrophilia is present in two-thirds of cases with the fetal inflammatory response syndrome (FIRS). The mechanisms responsible for this finding have not been elucidated. Granulocyte Colony-Stimulating Factor (G-CSF) is the primary physiologic regulator of neutrophil production and plays a key role in the rapid generation and release of neutrophils in stressful conditions (i.e., infection). The objective of this study was to determine: 1) whether FIRS was associated with changes in fetal plasma G-CSF concentrations; and 2) if fetal plasma G-CSF concentrations correlated with fetal neutrophil counts, chorioamnionitis, neonatal morbidity/mortality and cordocentesis-to-delivery interval.
STUDY DESIGN
Percutaneous umbilical cord blood sampling was performed in a population of patients with preterm labor (n=107). A fetal plasma interleukin-6 (IL-6) concentration >11 pg/mL was used to define FIRS. Cord blood G-CSF was measured by a sensitive and specific immunoassay. An absolute neutrophil count was determined and corrected for gestational age. Receiver operating characteristic (ROC) curve, survival analysis and Cox proportional hazard model were employed.
RESULTS
1) G-CSF was detected in all fetal blood samples; 2) fetuses with FIRS had a higher median fetal plasma G-CSF concentration than those without FIRS (p<0.001); 3) a fetal plasma G-CSF concentration ≥134 pg/mL (derived from an ROC curve) was associated with a shorter cordocentesis-to-delivery interval, a higher frequency of chorioamnionitis (clinical and histological), intra-amniotic infection, and composite neonatal morbidity/mortality than a fetal plasma concentration below this cut-off; and 4) a fetal plasma G-CSF concentration ≥134 pg/mL was associated with a shorter cordocentesis-to-delivery interval (hazard ratio 3.2; 95% confidence interval 1.8-5.8) after adjusting for confounders.
CONCLUSIONS
1) G-CSF concentrations are higher in the peripheral blood of fetuses with FIRS than in fetuses without FIRS; and 2) a subset of fetuses with FIRS with elevated fetal plasma G-CSF concentrations are associated with neutrophilia, a shorter procedure-to-delivery interval, chorioamnionitis and increased perinatal morbidity and mortality.
Keywords: G-CSF, FIRS, interleukin-6, pregnancy, preterm labor, fetal plasma, cordocentesis
INTRODUCTION
The fetal inflammatory response syndrome (FIRS), originally described in pregnancies complicated by spontaneous preterm labor and preterm prelabor rupture of membranes (PROM), is operationally defined as a fetal plasma interleukin-6 (IL-6) concentration of >11 pg/mL [1,2]. Soluble tumor necrosis factor receptor- 1 and -2, IL- 1β, IL-8, and CRP are also associated with FIRS [3-5]. Funisitis and chorionic vasculitis are considered the histological counterpart of FIRS [6,7]. A solid body of evidence supports the view that intra-amniotic infection/inflammation are considered to play a major role in the patophysiology of FIRS [8-12]. Fetuses with FIRS had a higher rate of neonatal morbidity including respiratory distress syndrome (RDS) [1,13], suspected or proven neonatal sepsis [1,13], pneumonia [1], bronchopulmonary dysplasia [14-16], necrotizing enterocolitis [1], intraventricular hemorrhage [1], periventricular leucomalacia as well as cerebral palsy [17-26] and a shorter cordocentesis-to-delivery interval in patients presenting with preterm PROM than those without FIRS [27]. Therefore, intra-amniotic infection/inflammation, a condition with an elevation of pro-inflammatory cytokines [28-41], caspase-1 (a component of inflammasome) [42], anti-inflammatory cytokines [43], chemokines [44-50], proteases/anti-proteases [51] , angiogenic factors [52], matrix-metalloproteinase [53-61], coagulation factors [62-65], adipocytokines [66-70], anti-microbial peptides [71,72], and prostaglandins [73,74] in amniotic fluid, is associated with neonatal morbidity/mortality among very preterm neonates [75-85].
Granulocyte colony-stimulating factor (G-CSF) is a cytokine produced mainly by monocytes and macrophages [86,87]. The production of the G-CSF is highly regulated and not constitutive [86]. In healthy adults, the circulating concentrations of G-CSF are usually below the limits of detection, and when detectable, are generally <100 pg/mL. During infection, however, G-CSF concentrations increase dramatically and may exceed 2000 pg/mL [88]. The biological activities of G-CSF include stimulation of neutrophil progenitors resulting in clonal expansion, increasing the bone marrow neutrophil storage pool [89] as well as the neutrophil count in peripheral blood [90], and improvement of mature neutrophil functions (e.g. phagocytosis and oxidative burst) [91]. Collectively, G-CSF is considered to act as a physiologic regulator and an emergency signal to increase neutrophil production/function under stressful conditions or infection [86].
Although septic preterm neonates tend to develop neutropenia [92], two-thirds of fetuses with FIRS have fetal neutrophilia [93]. The mechanisms responsible for fetal neutrophilia in FIRS have not been elucidated. The objective of this study was to determine: 1) whether FIRS was associated with changes in fetal plasma G-CSF concentrations; and 2) if fetal plasma G-CSF concentrations correlated with fetal neutrophil counts, clinical or histologic chorioamnionitis, neonatal morbidity/mortality and cordocentesis-to-delivery interval.
PATIENTS AND METHODS
Patients and eligibility
This retrospective cross-sectional study included singleton pregnancies with spontaneous preterm labor and intact membranes who were admitted to Hutzel Women’s Hospital between March 1992 and June 1995. Patients were offered amniocentesis for the diagnosis of microbial invasion of the amniotic cavity and the assessment of fetal lung maturity. Patients who consented to amniocentesis were asked to participate in a research protocol that included cordocentesis to assess the fetal status. Exclusion criteria were multiple gestations, clinical signs of chorioamnionitis, vaginal bleeding, fetal distress, and unavailability of the fetal plasma samples for this study.
Clinical definition
Spontaneous preterm labor was diagnosed in the presence of regular uterine contractions (at least 3 in 30 minutes) and documented cervical change in patients with a gestational age between 20 and 36 6/7 weeks [1]. FIRS was defined as a fetal plasma IL-6 concentration greater than 11 pg/mL [1]. Intra-amniotic infection was defined as a positive microbiological culture of amniotic fluid. Clinical chorioamnionitis was diagnosed in the presence of a temperature elevation to 37.8°C or higher and two or more of the following criteria: uterine tenderness, malodorous vaginal discharge, fetal tachycardia >160 beats/min, and maternal leukocytosis >15,000 cells/mm3 [94]. Composite neonatal morbidity/mortality was defined as the presence of any of the following conditions: respiratory distress syndrome (RDS) suspected or proved neonatal sepsis, pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage, necrotizing enterocolitis and perinatal death. The definitions of these neonatal complications have previously been described in detail [1]. The corrected neutrophil count was calculated from the ratio between the observed fetal neutrophil count and the expected mean fetal neutrophil count [95] according to gestational age at cordocentesis.
All patients provided written informed consent prior to the collection of samples. The collection and utilization of samples for research was approved by the Human Investigation Committee of Wayne State University, (Detroit, MI) and the Institutional Review Board of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD/NIH/DHHS). Many of these samples have been used in previous studies.
Clinical procedures and assays
All patients had a detailed ultrasonographic examination before amniocentesis and cordocentesis were performed. Electronic fetal monitoring was performed before and after the procedure to evaluate fetal well-being. Amniocentesis and cordocentesis procedures were performed with the freehand technique under ultrasound guidance. A 22-gauge needle was used, and a path was chosen for needle insertion that allowed the amniocentesis and cordocentesis procedures to be carried out with a single percutaneous needle insertion in approximately 95% of patients. Amniotic fluid studies included Gram stain, microbial cultures for aerobic and anaerobic bacteria as well as genital mycoplasmas, and the lecithin/sphyngomyelin ratio. The results of these tests were used for subsequent clinical management decisions. Fetal cord blood was collected into ethylenediaminetetra-acetic acid (EDTA) tubes. Kleihauer-Betke stains were performed on fetal blood, and all specimens were found to be free of maternal blood. Fetal blood was analyzed and complete white blood cell, platelet, and differential cell counts were performed. Results were made available for clinical management.
Plasma G-CSF and IL-6 concentrations were determined with commercially available enzyme-linked immunoassays obtained from R&D Systems (Minneapolis, MN). Briefly, the immunoassay utilized the quantitative sandwich technique and analyte concentrations were determined by interpolation from the standard curves. The inter- and intra-assay coefficients of variation for G-CSF were 4.8 % and 2.9 %, respectively, and those for IL-6 were 8.3% and 3.3% respectively. The sensitivities of the assays for G-CSF and IL-6 were 0.99 pg/mL and 0.06 pg/mL, respectively. The results of the analytes reported herein were not available for clinical decision-making. The presence or absence of acute inflammatory lesions in the extra-placental membranes (histologic chorioamnionitis) was assessed as previously described [7].
Statistical analysis
The Kolmogorov-Smirnov or Shapiro-Wilk test was used to determine if the data was normally distributed. A two-tailed Mann-Whitney U test was used to compare continuous nonparametric variables. Comparisons between proportions were performed using Chi-square or Fisher’s exact tests. Correlation between two continuous variables was determined using Spearman’s rank correlation test. Receiver operating characteristic (ROC) curve analysis was employed to determine a fetal plasma G-CSF concentration that identified patients who subsequently delivered within 7 days after cordocentesis. Kaplan-Meier survival analysis with a log rank test and Cox proportional hazard model were applied to examine the interval from cordocentesis-to-delivery according to fetal plasma G-CSF concentrations, while adjusting for confounding factors. A p-value <0.05 was considered statistically significant. Analysis was performed with SPSS, version 12 (SPSS Inc., Chicago, IL, USA).
RESULTS
Demographic and clinical characteristics
This study included 107 women who presented with spontaneous preterm labor and intact membranes, 23% (25/107) of which had a diagnosis of FIRS. Seventy-six patients (71%) delivered at less than 37 weeks of gestation. There were 2 perinatal deaths, and in both cases, the infants were delivered before 24 weeks of gestation. Intra-amniotic infection was diagnosed in 11.2% (12/107) of cases. The organisms were Ureaplasma urealyticum (n=7), Fusobacterium species (n=3), Pseudomonas aeruginosa (n=1) and Corynebacterium jeikeium (n=1). As expected, patients with FIRS had a significantly higher rate of clinical chorioamnionitis, microbiological proven intra-amniotic infection, histologic chorioamnionitis, composite neonatal morbidity/mortality and a shorter cordocentesis-to-delivery interval than those without FIRS (all p<0.05; see Table I).
Table I.
Clinical characteristics of the study population
| No FIRS n = 82 |
FIRS n=25 |
p | |
|---|---|---|---|
| Age (years) | 22.0 (20.0-27.0) | 23 (19.0-25.5) | 0.6 |
| GA at admission (weeks) | 32.0 (29.1-33.0) | 29.0 (25.1-31.3) | 0.003 |
| Interval to delivery (days) | 27.0 (9.9-46.0) | 1.6 (0.5-13.9) | <0.001 |
| Preterm delivery | 53 (64.6%) | 23 (92%) | 0.01 |
| GA at delivery (weeks) | 35.8 (33.6-38.0) | 29.4 (25.1-35.0) | <0.001 |
| Birthweight (grams) | 2,608 (2,097-3,042)μ | 1,230 (740-2,622) | <0.001 |
| Clinical chorioamnionitis | 2 (2.4%) | 5 (20%) | 0.007 |
| Positive amniotic fluid culture | 3 (3.7%) | 9 (36.0%) | <0.001 |
| Delivery within 24 hours | 7 (8.5%) | 8 (32%) | 0.003 |
| Delivery within 48 hours | 8 (9.8%) | 15 (60%) | <0.001 |
| Delivery within 7 days | 16 (19.5%) | 18 (72%) | <0.001 |
| Histologic chorioamnionitis | 8 (22.9%) γ | 12 (63.2%)π | 0.003 |
| Composite neonatal morbidity | 27 (32.9%) | 19 (76%) | <0.001 |
| Fetal interleukin-6 (pg/ml) | 5.2 (3.2-6.7) | 69.1 (18.4-169.4) | <0.001 |
| Fetal neutrophil count (x109/L) | 1.5 (0.7-2.4)α | 2.5 (1.4-3.9)β | 0.028 |
| Corrected fetal neutrophil count | 1.8 (1.3-2.5)α | 3.4 (1.1-8.9)β | 0.028 |
| Fetal G-CSF (pg/ml) | 55.7 (30.1-86.1) | 714.4 (104.3-1918.7) | <0.001 |
FIRS: Fetal Inflammatory Response Syndrome
Values are expressed as median (interquartile range) or number (percent)
GA: gestational age; G-CSF: granulocyte colony stimulating factor
μ: n=81; γ: n=35; π: n=19; α: n=75; β: n=21
Neutrophil count and fetal plasma G-CSF concentration in fetuses with FIRS
Immunoreactive G-CSF was detected in all samples of fetal blood. Fetuses with FIRS had a median absolute neutrophil count, corrected neutrophil count and plasma G-CSF concentration higher than those without FIRS (all p<0.05; see Table I and Figure 1). Among patients with FIRS, the fetal plasma G-CSF concentration was correlated with fetal plasma IL-6 concentration (Spearman’s rho = 0.5; p=0.02; Figure 2a) and the corrected fetal neutrophil count (Spearman’s rho = 0.6; p=0.007; Figure 2b). The fetal plasma G-CSF concentration had an inverse correlation with gestational age at cordocentesis (Spearman’s rho = −0.5; p=0.02) and birthweight (Spearman’s rho = −0.7; p<0.001). In contrast, among patients who subsequently delivered at term (n=31), there was a significant relationship between fetal plasma G-CSF concentration and gestational age at cordocentesis (Spearman’s rho = 0.6; p=0.001, especially between 27 and 35 weeks, Figure 2c) as well as fetal neutrophil count (Spearman’s rho = 0.8; p<0.001, Figure 2d). The median fetal plasma G-CSF concentration of women who presented with spontaneous preterm labor between 21 and 35 weeks and subsequently delivered at term was 45.3 pg/mL and ranged between 8.0 and 134.0 pg/mL.
Figure 1. Comparison of the fetal plasma granulocyte-colony stimulating factor (G-CSF) concentration between fetuses with FIRS and those without FIRS.
Fetuses with FIRS had a median fetal plasma concentration of G-CSF higher than those without FIRS [FIRS: median 714.4 pg/mL, interquartile range (IQR) 104-1918 pg/mL vs. without FIRS: median 55.7 pg/mL, IQR 30.1-86.1 pg/mL; p<0.001]. The y-axis is depicted in log scale.
Figure 2. Relationships between fetal plasma G-CSF concentration and other variables.
Among patients with FIRS, fetal plasma G-CSF concentrations correlated with fetal plasma IL-6 concentration (Spearman’s rho = 0.5; p=0.02; Figure 2a) and corrected fetal neutrophil count (Spearman’s rho = 0.6; p=0.007; Figure 2b). In contrast, among patients who subsequently delivered at ≥37 weeks, there was a significant relationship between fetal plasma G-CSF concentration and gestational age at cordocentesis (Spearman’s rho = 0.6; p=0.001; Figure 2c) as well as absolute fetal neutrophil count (Spearman’s rho = 0.8; p<0.001; Figure 2d).
Elevated fetal plasma G-CSF concentration is associated with a shorter cordocentesis-to-delivery interval and a higher frequency of chorioamnionitis
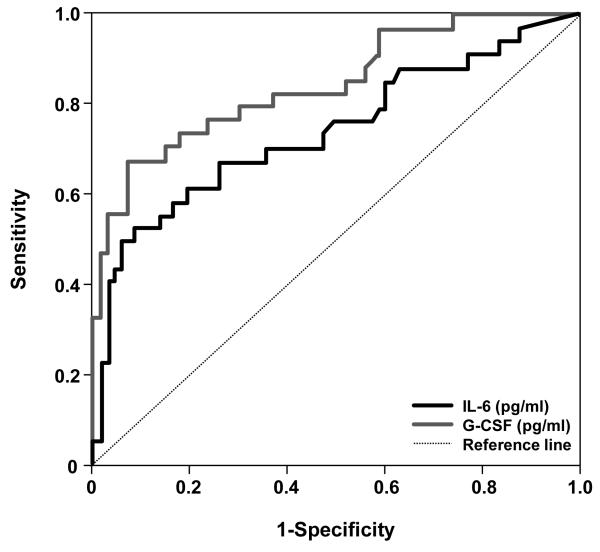
The prevalence of patients who delivered within 7 days after cordocentesis was 32% (34/107). ROC curve analysis indicated that a fetal plasma G-CSF concentration ≥134 pg/mL had an area under the curve of 0.85 (95% CI 0.76-0.92; p<0.001), a sensitivity of 68% (23/34), a specificity of 92% (67/73), a positive predictive value of 79% (23/29), and a negative predictive value of 86% (67/78) for the identification of patients who subsequently delivered within 7 days. The corresponding indices for fetal plasma IL-6 concentration >11 pg/mL were 0.72 (0.60-0.83), 52.9%, 90.4%, 72% and 80.5%, respectively (see Figure 3).
Figure 3. Receiver operating characteristic (ROC) curve analysis for the identification of patients who subsequently delivered within 7 days after cordocentesis.
A fetal plasma G-CSF concentration ≥134 pg/mL (grey line) and a fetal plasma IL-6 concentration >11 pg/mL (black line) had an area under the curve of 0.85 (95% CI 0.76-0.92; p<0.001) and 0.72 (95% CI 0.60-0.83; p<0.001), respectively, for the identification of patients who subsequently delivered within 7 days.
Women with a fetal plasma G-CSF concentration ≥134 pg/mL had a higher frequency of clinical chorioamnionitis (17.2% vs. 2.6%; p=0.02), intra-amniotic infection (37.9% vs. 1.3%; p<0.001), histologic chorioamnionitis (63.6% vs. 18.8%; p=0.001) and composite neonatal morbidity/mortality (69% vs. 33.3%; p<0.001; see Table II) than those with a fetal plasma G-SCF concentration below this cutoff.
Table II.
Clinical characteristics of the study population according to fetal plasma concentrations of G-CSF ≥134 or < 134 pg/ml
| G-CSF <134 pg/ml (n=78) |
G-CSF ≥ 134 pg/ml (n=29) |
p | |
|---|---|---|---|
| Age (years) | 22.0 (19.8-27) | 23.0 (19-25.5) | 0.7 |
| GA at cordocentesis (weeks) | 31.8 (28.9-33.0) | 30.5 (25.4-32.3) | <0.001 |
| Cervical dilatation (cm) | 1.5 (0.9-2.5) | 2.5 (1.0-4.0)γ | 0.009 |
| Interval to delivery (days) | 29.9 (13.6-48.7) | 1.6 (0.5-4.9) | <0.001 |
| Preterm delivery | 48 (61.5%) | 28 (96.6%) | <0.001 |
| GA at delivery (weeks) | 36.1 (34.0-38.0) | 30.5 (25.5-34.7) | <0.001 |
| Birthweight (grams) | 2,640 (2,200-3,104)μ | 1680 (747-2,342) | <0.001 |
| Clinical chorioamnionitis | 2 (2.6%) | 5 (17.2%) | 0.02 |
| Positive amniotic fluid culture | 1 (1.3%) | 11 (37.9%) | <0.001 |
| Exposure to antenatal steroids prior to cordocentesis |
19 (24.4%) | 13 (44.8%) | 0.04 |
| Exposure to tocolysis prior to cordocentesis |
68 (87.2%) | 27 (93.1%) | 0.5 |
| Spontaneous delivery | 66 (84.6%) | 24 (82.8%) | 0.8 |
| Histologic chorioamnionitis | 6 (18.8%) α | 14 (63.6%) β | 0.001 |
| Composite neonatal morbidity | 26 (33.3%) | 20 (69.0%) | 0.001 |
Values are expressed as median (interquartile range) or number (percent)
G-CSF: granulocyte colony stimulating factor; GA: gestational age;
Tocolysis: magnesium sulfate, indocin, or terbutaline
γ: n=28;μ: n=77; α: n=32; β: n=22
Fetal plasma G-CSF concentration: an independent predictor of procedure-to-delivery interval
To assess the relationship between fetal plasma G-CSF concentration and the duration of the cordocentesis-to-delivery interval, Kaplan-Meier survival analysis was employed. Spontaneous labor and delivery was considered the event of interest, and the cordocentesis-to-delivery interval of patients who were delivered for fetal or maternal indications was treated as censored observations (a censoring time equal to the cordocentesis-to-delivery interval).
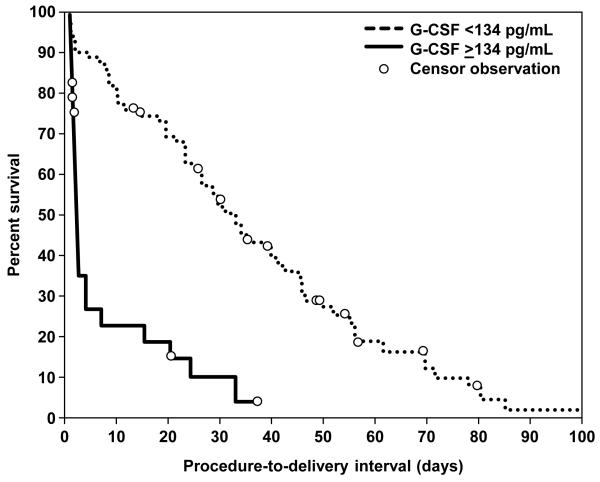
The median cordocentesis-to-delivery interval in patients with a fetal plasma G-CSF concentration ≥134 pg/mL was significantly shorter than that of those with a fetal plasma G-CSF concentration below this cutoff value [G-CSF >134 pg/mL; median procedure-to-delivery interval 1.75 days, interquartile (IQR) 1-6.4 days, censored 5 out of 29 events vs. G-CSF <134 pg/mL; median procedure-to-delivery interval 33 days, IQR range 18-55 days, censored 12 out of 78 events; p<0.0001; see Figure 4). Cox proportional hazard modeling was used to examine the relationship between the duration of the cordocentesis-to-delivery interval and fetal plasma G-CSF concentrations while adjusting for cervical dilatation at admission, the status of amniotic fluid culture, gestational age at cordocentesis, the presence of clinical chorioamnionitis, and the exposure to antenatal steroids or tocolysis prior to cordocentesis. Only fetal plasma G-CSF concentration ≥134 pg/mL and cervical dilatation were independent predictors of cordocentesis-to-delivery interval with a hazard ratio of 3.2 (95% CI 1.8-5.8) and 1.6 (95% CI 1.3-1.9), respectively (Table III).
Figure 4. Survival curves for cordocentesis-to-delivery interval according to fetal plasma concentrations of G-CSF.
Spontaneous labor and delivery was entered in the analysis as the event of interest. Patients who delivered by fetal or maternal indications were censored. Women with fetal plasma concentrations of G-CSF >134 pg/mL had a significantly shorter procedure-to-delivery interval than those with a fetal plasma G-CSF concentration below this cutoff value: [G-CSF >134 pg/mL; median procedure-to-delivery interval 1.75 days, interquartile (IQR) 1-6.4 days, censored 5 out of 29 events vs. G-CSF <134 pg/mL; median procedure-to-delivery interval 33 days, IQR range 18-55 days, censored 12 out of 78 events; log rank test p<0.0001].
Table III.
Hazard ratio for the procedure-to-delivery interval (days)*
| Independent variables | Hazard ratio | 95% Confidence Interval |
|---|---|---|
| Fetal plasma G-CSF ≥134 pg/ml | 3.2 | 1.8-5.8 |
| Cervical dilation (cm) | 1.6 | 1.3-1.9 |
| Gestational age at procedure (weeks) | 1.1 | 0.9-1.15 |
| Clinical chorioamnionitis | 2.6 | 0.8-8.7 |
| Exposure to antenatal steroids prior to cordocentesis | 0.9 | 0.6-1.6 |
| Exposure to tocolysis prior to cordocentesis | 1.2 | 0.5-3.0 |
| Positive amniotic fluid culture | 1.2 | 0.5-2.9 |
n=106 (one patient had no information on cervical dilatation)
A subset of FIRS has a high fetal plasma G-CSF concentration
Only 72% (18/25) of fetuses with FIRS had a high fetal plasma G-CSF concentration, while 62% (18/29) of fetuses with a high fetal plasma G-CSF concentration had a diagnosis of FIRS. Patients who had both fetal plasma G-CSF concentrations >134 pg/mL and IL-6 concentrations >11 pg/mL had a significantly shorter procedure-to-delivery interval (median 1.1 day, IQR 0.4-1.8 days) than those who had either isolated fetal plasma IL-6 concentrations >11 pg/mL (median 26 days, IQR 2-46 days; p=0.01) or fetal plasma G-CSF concentrations >134 pg/mL alone (median 3.5 days, IQR 0.8-20.2 days; p=0.03) (Table IV). There was no significant difference in the cordocentesis-to-delivery interval between those who had fetal plasma IL-6 concentrations >11 pg/mL and those who had high fetal plasma G-SCF concentrations alone (p=0.2). The frequency of intra-amniotic infection was the highest in a subset of patients with an elevation of both cytokines in fetal plasma [high for both cytokines 50% (9/18) vs. high for G-CSF alone 18.2% (2/11), high for IL-6 alone 0% (0/7) and low for both cytokines 1.4% (1/71); Chi-square for trend: p<0.001, see Table IV).
Table IV.
Procedure-to-delivery interval (days) and composite neonatal morbidity/mortality according to fetal plasma G-CSF and IL-6 concentrations
| Number (%) | GA at delivery (weeks) |
Procedure-to-delivery interval (days) |
Positive amniotic fluid culture |
|
|---|---|---|---|---|
| IL-6 ≤ 11 pg/ml G-CSF < 134 pg/ml |
71 (66.4%) | 36.1 (34.0-38.0) | 30 (14-49) | 1 (1.4%) |
| IL-6 > 11 pg/ml G-CSF < 134 pg/ml |
7 (6.5%) | 35.1 (34.8-37.9) | 26 (2.0-46.0) | 0 |
| IL-6 ≤ 11 pg/ml G-CSF > 134 pg/ml |
11 (10.3%) | 33.6 (31.3-35.9) | 3.5 (0.8- 20.2) | 2 (18.2%) |
| IL-6 > 11 pg/ml G-CSF ≥ 134 pg/ml |
18 (16.8%) | 28.4 (24.4-31.1) | 1.1 (0.4-1.8) | 9 (50%) |
Values are expressed as median (interquartile range) or number (percent)
GA: gestational age; G-CSF: granulocyte colony stimulating factor; IL: interleukin
DISCUSSION
Principal findings of the study
1) Fetuses with FIRS had a higher median plasma G-CSF concentration than those without FIRS; 2) patients with spontaneous preterm labor with intact membranes and a fetal plasma G-CSF concentration >134 pg/mL had a shorter cordocentesis-to-delivery interval, a higher frequency of chorioamnionitis (clinical and histological) and microbiologically proven intra-amniotic infection and composite neonatal morbidity/mortality than those with a fetal plasma G-CSF concentration below this cutoff; 3) fetal plasma G-CSF concentration was an independent predictor of cordocentesis-to-delivery interval after adjustment for other confounders; 4) a subset of patients with FIRS who had elevated plasma G-CSF concentrations had a significantly shorter procedure-to-delivery interval and higher frequency of intra-amniotic infection than those who had either isolated high fetal plasma IL-6 or high G-CSF concentration alone; 5) among patients with FIRS, the fetal plasma G-CSF concentration correlated with fetal plasma IL-6 concentration and with corrected fetal neutrophil count; and 6) in the group of women with an episode of spontaneous preterm labor who delivered at term, there was a significant relationship between gestational age and fetal G-CSF concentration as well as between fetal plasma G-CSF concentration and absolute fetal plasma neutrophil count.
Physiologic roles of G-CSF
Monocytes and macrophages are the primary sources of G-CSF [86,96,97], although this hematopoietic growth factor can be produced by endothelial cells [98], fibroblasts [99] and mesothelial cells [100]. Monocytes and macrophages can be stimulated to produce G-CSF upon incubation with lipopolysaccharides (LPS), IL-1, IL-3, IL-4, interferon gamma and granulocyte-macrophage colony-stimulating factor [101]. G-CSF exerts its biological activities through binding to a specific G-CSF receptor which has been located on both hematopoietic cells (such as myeloid progenitors, mature granulocytes, platelets, monocytes, lymphocytes) [102-105] and non-hematopoietic cells (such as vascular endothelial cells [106], cardiac myocytes [107], intestinal villi [108], lung [109], kidney [110], skeletal muscle [110], neural stem cells [111] and syncytiotrophoblast [112,113]). G-CSF plays a key role in the rapid generation and release of neutrophils from the storage pool in stressful conditions such as infection [101,113,114]. Moreover, G-CSF not only attracts neutrophils [115], but also improves neutrophil function by increasing phagocytosis, superoxide release, and delays neutrophil apoptosis [116].
Conflicting results have been reported on the change of G-CSF concentrations in the umbilical cord blood of preterm neonates as a function of gestational age. Either an increase [90,117-119], a decrease [120,121] or no change [122,123] in the circulating G-CSF concentration with gestational age has been reported. In this study, we demonstrate for the first time that fetal plasma G-CSF concentrations increased as a function of gestational age (Spearman Rho = 0.6) in patients presenting with preterm labor who delivered at term. Moreover, plasma G-CSF concentration strongly correlated with the absolute fetal neutrophil count (Spearman Rho = 0.8) suggesting that G-CSF is a physiologic regulator of neutrophil count in peripheral blood of preterm fetuses.
The roles of G-CSF in growth and maturation beyond hematopoiesis remain unknown. Recent animal experiments suggested that G-CSF is an essential neurotrophic factor which plays a role in the proliferation, differentiation and functional integration of neural cells in the hippocampus, an area important for memory formation and development of motor skills [124,125]. Moreover, the recombinant form of G-CSF may have a beneficial role in necrotizing enterocolitis [126], ischemic heart disease and hypoxic-ischemic brain injury [111,127-129] by inhibiting apoptosis, promoting the local neural stem cells [130] and recruiting bone marrow-derived stem cells to the sites of injury [131].
Fetuses with FIRS have a higher plasma G-CSF concentration than those without FIRS
Accumulating evidence suggests that fetuses with FIRS have evidence of multi-organ involvement as identified by biochemical and biophysical changes observed in the adrenal gland [132], heart [133], lung [14], brain [18,19,24,134], skin [135] and hematopoietic systems [93,136]. The latter includes an elevation of neutrophil count [93] as well as phenotypic evidence of monocyte-neutrophil activation in patients who delivered within 72 hours of cordocentesis [136]. Moreover, umbilical cord blood from patients with acute funisitis, the histological counterpart of FIRS [7], had phenotypic [higher median mean channel brightness of CD14, CD64, and CD66b on granulocytes and of CD64 on monocytes] and metabolic changes (increased basal intracellular reactive oxygen species and oxidative burst in monocytes) on leukocytes consistent with activation of the fetal innate immune response[137]. Indeed, microarray analysis of leukocyte RNA revealed differential expression of 541 unique genes. Moreover, ontological and pathway analyses yielded significant enrichment of biological processes including antigen processing and presentation, immune response, and processes critical to cellular metabolism[138]. The increased plasma G-CSF concentration in fetuses with FIRS and the relationship between the fetal plasma G-CSF concentrations and the corrected neutrophil count observed in the current study could, at least in part, explain the quantitative and qualitative changes in neutrophils of fetuses with FIRS.
Is an increased fetal plasma G-CSF concentration beneficial or detrimental to the fetus?
Experimental G-CSF knock-out mice are viable, fertile, and apparently healthy, but they have a chronic neutropenia and markedly impaired ability to control bacterial infection [139]. These observations suggest that the function of G-CSF is beneficial to maintain the normal quantitative physiologic balance of neutrophil production and is an emergency granulopoietic signal against infections. Several studies in newborn animals with experimental sepsis [140] and in human neonates with sepsis indicate that the administration of recombinant G-CSF increases the neutrophil count [141], improves neutrophil function [142] and might reduce sepsis-related mortality [143]. However, a concern is the potential detrimental effects of recombinant G-CSF treatment in aggravating acute lung injury in neonates with RDS [144,145].
The most recent Cochrane review concluded that there is insufficient evidence to support the introduction of recombinant G-CSF either as a treatment of established infection to reduce mortality or as a prophylaxis to prevent systemic infection in high-risk neonates [146]. However, this subject should be further investigated since there is evidence that pharmacologic G-CSF treatment may reduce mortality in a subset of neonates with severe neutropenia [146,147].
Elevated fetal plasma G-CSF concentration is associated with a shorter cordocentesis-to-delivery interval, chorioamnionitis and neonatal morbidity/mortality
Immunoreactive G-CSF has been detected in amniotic fluid, neonatal urine and tracheobronchial secretion as well as umbilical cord, neonatal and maternal blood. Patients who delivered preterm, especially those with detectable endotoxin in the amniotic fluid and those with clinical or histologic chorioamnionitis, had a median amniotic fluid concentration of G-CSF higher than those who delivered at term or those without clinical/histologic chorioamnionitis [148-151]. Women in labor at term had a higher mean amniotic fluid concentration of G-CSF than those without labor [148]. Similarly, neonates born from mothers with clinical chorioamnionitis and those with signs of infection had a higher mean G-CSF concentration in serum, urine and tracheobronchial secretion than those who were born from mothers without clinical chorioamnionitis or signs of infection [118,149,150]. Consistent with these findings, the current study demonstrated that elevated fetal plasma G-CSF concentration was associated with clinical/histological chorioamnionitis, intra-amniotic infection and composite neonatal morbidity/mortality.
Elevated maternal serum G-CSF concentration has been reported in patients with clinical chorioamnionitis [149]. In asymptomatic women at 24 and 28 weeks of gestation, an elevation of this cytokine is associated with subsequent early spontaneous preterm delivery (<32 weeks), but not with late preterm birth [152]. The authors also noted that spontaneous preterm delivery often occurs within 4 weeks after the blood sampling in such cases [152]. However, a recent study in asymptomatic women between 6 and 18 weeks of gestation reported an association between elevated maternal serum G-CSF concentrations and spontaneous preterm birth with an odds ratio of 1.52 (for each one standard deviation increase) [153]. Consistent with these observations, in the current study, fetal plasma G-CSF concentration is an independent predictor of cordocentesis-to-delivery interval after adjustment for other potential confounders. Although dexamethasone has been shown to increase the production of G-CSF in mononuclear cells of term and preterm infants [154], a fetal plasma G-CSF concentration >134 pg/mL is associated with a shorter cordocentesis-to-delivery interval after adjustment for the exposure of antenatal steroids.
The increased fetal plasma G-CSF concentration observed herein is unlikely to result mainly from transplacental passage from the mother’s circulation since the G-CSF concentration in different fetal compartments (amniotic fluid, umbilical cord blood or neonatal serum: mean 5,520 pg/mL, 8,281 pg/mL and 4,364 pg/mL, respectively) is much higher than that observed in maternal blood (mean 186 pg/mL) during clinical chorioamnionitis [149]. Despite the relative low transplacental passage of G-CSF, a randomized clinical trial demonstrated that the administration of recombinant G-CSF to women during preterm labor could increase neutrophil proliferative pool in the neonatal bone marrow after preterm delivery [155].
FIRS may have multiple etiologies
The original definition of FIRS was described in fetuses with preterm labor and preterm PROM, and was often associated with microbial invasion of the amniotic cavity [1]. To date, FIRS has been largely observed in pregnancy complications involving infection. However, similar to systemic inflammatory response syndrome in adults [156], the fetus might also be able to mount an inflammatory response to non-microbial-related insults (eg: fetal anemia due to Rh alloimmunization) [157]. In this study, only a subset of fetuses with FIRS had an elevation of plasma G-CSF concentrations. This subgroup of patients had a shorter procedure-to-delivery interval, and had a higher rate of a positive microbial culture in amniotic fluid than those with FIRS without G-CSF elevation. It is possible that FIRS accompanied by an elevation of G-CSF concentration is a consequence of bacterial or fungal-related insults [139,158,159]. Consistent with this view, plasma G-CSF concentrations have been proposed to be an early marker of proven bacterial or fungal infection in neonates undergoing an evaluation for sepsis [122]. The possibility that a subset of fetuses with FIRS who presents with systemic inflammatory response (elevation of IL-6) is a consequence of non-bacterial or non-fungal infection (e.g., fetal anemia ue to Rh alloimmunization [157], viral infection or other non-infectious etiologies) should be considered.
Strength and limitations of the study
This study is the first description of plasma G-CSF concentration ue to Rh alloimmunization [157] in preterm fetuses with FIRS and those who subsequently delivered at term. We consider patients who presented with preterm labor without intra-amniotic infection and subsequently delivered at term be the closest to normal fetuses that could have cordocentesis performed, since this procedure could not have been performed in pregnant women without a clinical indication. Previous studies of the changes in plasma G-CSF concentrations as a function of gestational age of “normal” preterm neonates may be inaccurate for the following reasons: 1) preterm neonates are not “normal” by definition (they have to be delivered for maternal or fetal indications); 2) the difficulty in the accurate assessment of infection in premature infants; and 3) the high rate of subclinical intrauterine infection in patients with early preterm delivery.
In conclusion, our observations suggest that G-CSF is detectable in the plasma of preterm fetuses. The fetal plasma G-CSF concentration is higher in fetuses with FIRS than in those without FIRS. The elevation of G-CSF correlates with the corrected fetal neutrophil counts suggesting that G-CSF is responsible for fetal neutrophilia in FIRS. A subset of fetuses with FIRS, who had a fetal plasma G-CSF concentration ≥134 pg/mL had a higher frequency of chorioamnionitis, composite neonatal morbidity/mortality, and a shorter cordocentesis-to-delivery interval than those with a fetal plasma G-CSF concentration below this cut-off.
Acknowledgment
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, NIH, DHHS.
Reference List
- 1.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol. 1998;179:194–202. doi: 10.1016/s0002-9378(98)70272-8. [DOI] [PubMed] [Google Scholar]
- 2.Gotsch F, Romero R, Kusanovic JP, Mazaki-Tovi S, Pineles BL, Erez O, et al. The fetal inflammatory response syndrome. Clin Obstet Gynecol. 2007;50:652–83. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 3.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med. 2003;14:85–90. doi: 10.1080/jmf.14.2.85.90. [DOI] [PubMed] [Google Scholar]
- 4.Mestan K, Yu Y, Thorsen P, Skogstrand K, Matoba N, Liu X, et al. Cord blood biomarkers of the fetal inflammatory response. J Matern Fetal Neonatal Med. 2009;22:379–87. doi: 10.1080/14767050802609759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH, et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol. 2000;183:1070–7. doi: 10.1067/mob.2000.108885. [DOI] [PubMed] [Google Scholar]
- 6.Kim CJ, Yoon BH, Romero R, Moon JB, Kim M, Park SS, et al. Umbilical arteritis and phlebitis mark different stages of the fetal inflammatory response. Am J Obstet Gynecol. 2001;185:496–500. doi: 10.1067/mob.2001.116689. [DOI] [PubMed] [Google Scholar]
- 7.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH, et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2002;11:18–25. doi: 10.1080/jmf.11.1.18.25. [DOI] [PubMed] [Google Scholar]
- 8.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 9.Park JS, Romero R, Yoon BH, Moon JB, Oh SY, Han SY, et al. The relationship between amniotic fluid matrix metalloproteinase-8 and funisitis. Am J Obstet Gynecol. 2001;185:1156–61. doi: 10.1067/mob.2001.117679. [DOI] [PubMed] [Google Scholar]
- 10.Moon JB, Kim JC, Yoon BH, Romero R, Kim G, Oh SY, et al. Amniotic fluid matrix metalloproteinase-8 and the development of cerebral palsy. J Perinat Med. 2002;30:301–6. doi: 10.1515/JPM.2002.044. [DOI] [PubMed] [Google Scholar]
- 11.Lee SE, Romero R, Jung H, Park CW, Park JS, Yoon BH. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294–6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Park CW, Lee SM, Park JS, Jun JK, Romero R, Yoon BH. The antenatal identification of funisitis with a rapid MMP-8 bedside test. J Perinat Med. 2008 doi: 10.1515/JPM.2008.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000;183:1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI, et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol. 1999;181:773–9. doi: 10.1016/s0002-9378(99)70299-1. [DOI] [PubMed] [Google Scholar]
- 15.Kramer BW. Antenatal inflammation and lung injury: prenatal origin of neonatal disease. J Perinatol. 2008;28(Suppl 1):S21–S27. doi: 10.1038/jp.2008.46. [DOI] [PubMed] [Google Scholar]
- 16.Kramer BW, Kallapur S, Newnham J, Jobe AH. Prenatal inflammation and lung development. Semin Fetal Neonatal Med. 2009;14:2–7. doi: 10.1016/j.siny.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leviton A. Preterm birth and cerebral palsy: is tumor necrosis factor the missing link? Dev Med Child Neurol. 1993;35:553–8. doi: 10.1111/j.1469-8749.1993.tb11688.x. [DOI] [PubMed] [Google Scholar]
- 18.Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 19.Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 20.Dammann O, Leviton A. Infection remote from the brain, neonatal white matter damage, and cerebral palsy in the preterm infant. Semin Pediatr Neurol. 1998;5:190–201. doi: 10.1016/s1071-9091(98)80034-x. [DOI] [PubMed] [Google Scholar]
- 21.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birth weight infants. Developmental Epidemiology Network Investigators. Pediatr Res. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Dammann O, Leviton A. Role of the fetus in perinatal infection and neonatal brain damage. Curr Opin Pediatr. 2000;12:99–104. doi: 10.1097/00008480-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 23.Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Ment Retard Dev Disabil Res Rev. 2002;8:46–50. doi: 10.1002/mrdd.10005. [DOI] [PubMed] [Google Scholar]
- 24.Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. BJOG. 2003;110(Suppl 20):124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]
- 25.Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- 26.Berger A, Witt A, Haiden N, Kaider A, Klebermasz K, Fuiko R, et al. Intrauterine infection with Ureaplasma species is associated with adverse neuromotor outcome at 1 and 2 years adjusted age in preterm infants. J Perinat Med. 2009;37:72–8. doi: 10.1515/JPM.2009.016. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS, et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol. 1998;179:186–93. doi: 10.1016/s0002-9378(98)70271-6. [DOI] [PubMed] [Google Scholar]
- 28.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest. 1990;85:1392–400. doi: 10.1172/JCI114583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Santhanam U, Avila C, Romero R, Viguet H, Ida N, Sakurai S, et al. Cytokines in normal and abnormal parturition: elevated amniotic fluid interleukin-6 levels in women with premature rupture of membranes associated with intrauterine infection. Cytokine. 1991;3:155–63. doi: 10.1016/1043-4666(91)90037-e. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC, et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol. 1989;160:1117–23. doi: 10.1016/0002-9378(89)90172-5. [DOI] [PubMed] [Google Scholar]
- 31.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH, et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2000;182:135–41. doi: 10.1016/s0002-9378(00)70502-3. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp. 1992;167:205–20. doi: 10.1002/9780470514269.ch13. [DOI] [PubMed] [Google Scholar]
- 33.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC, et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol. 1989;161:336–41. doi: 10.1016/0002-9378(89)90515-2. [DOI] [PubMed] [Google Scholar]
- 34.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins. 1989;37:13–22. doi: 10.1016/0090-6980(89)90028-2. [DOI] [PubMed] [Google Scholar]
- 35.Baumann P, Romero R, Berry S, Gomez R, McFarlin B, Araneda H, et al. Evidence of participation of the soluble tumor necrosis factor receptor I in the host response to intrauterine infection in preterm labor. Am J Reprod Immunol. 1993;30:184–93. doi: 10.1111/j.1600-0897.1993.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 36.Romero R, Avila C, Brekus CA, Morotti R. The role of systemic and intrauterine infection in preterm parturition. Ann N Y Acad Sci. 1991;622:355–75. doi: 10.1111/j.1749-6632.1991.tb37880.x. [DOI] [PubMed] [Google Scholar]
- 37.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol. 1991;165:969–71. doi: 10.1016/0002-9378(91)90450-6. [DOI] [PubMed] [Google Scholar]
- 38.Romero R, Sepulveda W, Mazor M, Brandt F, Cotton DB, Dinarello CA, et al. The natural interleukin-1 receptor antagonist in term and preterm parturition. Am J Obstet Gynecol. 1992;167:863–72. doi: 10.1016/s0002-9378(12)80003-2. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Gomez R, Galasso M, Mazor M, Berry SM, Quintero RA, et al. The natural interleukin-1 receptor antagonist in the fetal, maternal, and amniotic fluid compartments: the effect of gestational age, fetal gender, and intrauterine infection. Am J Obstet Gynecol. 1994;171:912–21. doi: 10.1016/s0002-9378(94)70058-3. [DOI] [PubMed] [Google Scholar]
- 40.Romero R, Erez O, Espinoza J. Intrauterine infection, preterm labor, and cytokines. J Soc Gynecol Investig. 2005;12:463–5. doi: 10.1016/j.jsgi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 42.Gotsch F, Romero R, Chaiworapongsa T, Erez O, Vaisbuch E, Espinoza J, et al. Evidence of the involvement of caspase-1 under physiologic and pathologic cellular stress during human pregnancy: a link between the inflammasome and parturition. J Matern Fetal Neonatal Med. 2008;21:605–16. doi: 10.1080/14767050802212109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gotsch F, Romero R, Kusanovic JP, Erez O, Espinoza J, Kim CJ, et al. The anti-inflammatory limb of the immune response in preterm labor, intra-amniotic infection/inflammation, and spontaneous parturition at term: a role for interleukin-10. J Matern Fetal Neonatal Med. 2008;21:529–47. doi: 10.1080/14767050802127349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C, et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol. 1998;78:5–10. doi: 10.1016/s0301-2115(97)00236-4. [DOI] [PubMed] [Google Scholar]
- 45.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H, et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol. 1999;181:989–94. doi: 10.1016/s0002-9378(99)70337-6. [DOI] [PubMed] [Google Scholar]
- 46.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R, et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–73. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 47.Mittal P, Romero R, Kusanovic JP, Edwin SS, Gotsch F, Mazaki-Tovi S, et al. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am J Reprod Immunol. 2008;60:246–57. doi: 10.1111/j.1600-0897.2008.00620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hamill N, Romero R, Gotsch F, Kusanovic JP, Edwin S, Erez O, et al. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–27. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nhan-Chang CL, Romero R, Kusanovic JP, Gotsch F, Edwin SS, Erez O, et al. A role for CXCL13 (BCA-1) in pregnancy and intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2008;21:763–75. doi: 10.1080/14767050802244946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E, et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med. 2005;18:405–16. doi: 10.1080/14767050500361703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Helmig BR, Romero R, Espinoza J, Chaiworapongsa T, Bujold E, Gomez R, et al. Neutrophil elastase and secretory leukocyte protease inhibitor in prelabor rupture of membranes, parturition and intra-amniotic infection. J Matern Fetal Neonatal Med. 2002;12:237–46. doi: 10.1080/jmf.12.4.237.246. [DOI] [PubMed] [Google Scholar]
- 52.Pacora P, Romero R, Chaiworapongsa T, Kusanovic JP, Erez O, Vaisbuch E, et al. Amniotic fluid angiopoietin-2 in term and preterm parturition, and intra-amniotic infection/inflammation. J Perinat Med. 2009;37:503–11. doi: 10.1515/JPM.2009.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P, et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol. 1998;179:1248–53. doi: 10.1016/s0002-9378(98)70141-3. [DOI] [PubMed] [Google Scholar]
- 54.Maymon E, Romero R, Pacora P, Gervasi MT, Edwin SS, Gomez R, et al. Matrilysin (matrix metalloproteinase 7) in parturition, premature rupture of membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;182:1545–53. doi: 10.1067/mob.2000.107652. [DOI] [PubMed] [Google Scholar]
- 55.Maymon E, Romero R, Pacora P, Gomez R, Athayde N, Edwin S, et al. Human neutrophil collagenase (matrix metalloproteinase 8) in parturition, premature rupture of the membranes, and intrauterine infection. Am J Obstet Gynecol. 2000;183:94–9. doi: 10.1067/mob.2000.105344. [DOI] [PubMed] [Google Scholar]
- 56.Maymon E, Romero R, Chaiworapongsa T, Kim JC, Berman S, Gomez R, et al. Value of amniotic fluid neutrophil collagenase concentrations in preterm premature rupture of membranes. Am J Obstet Gynecol. 2001;185:1143–8. doi: 10.1067/mob.2001.118166. [DOI] [PubMed] [Google Scholar]
- 57.Maymon E, Romero R, Pacora P, Gomez R, Mazor M, Edwin S, et al. A role for the 72 kDa gelatinase (MMP-2) and its inhibitor (TIMP-2) in human parturition, premature rupture of membranes and intraamniotic infection. J Perinat Med. 2001;29:308–16. doi: 10.1515/JPM.2001.044. [DOI] [PubMed] [Google Scholar]
- 58.Fortunato SJ, Menon R. Distinct molecular events suggest different pathways for preterm labor and premature rupture of membranes. Am J Obstet Gynecol. 2001;184:1399–405. doi: 10.1067/mob.2001.115122. [DOI] [PubMed] [Google Scholar]
- 59.Fortunato SJ, Menon R, Lombardi SJ. MMP/TIMP imbalance in amniotic fluid during PROM: an indirect support for endogenous pathway to membrane rupture. J Perinat Med. 1999;27:362–8. doi: 10.1515/JPM.1999.049. [DOI] [PubMed] [Google Scholar]
- 60.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S, et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol. 2002;187:1125–30. doi: 10.1067/mob.2002.127312. [DOI] [PubMed] [Google Scholar]
- 61.Maymon E, Romero R, Pacora P, Gervasi MT, Bianco K, Ghezzi F, et al. Evidence for the participation of interstitial collagenase (matrix metalloproteinase 1) in preterm premature rupture of membranes. Am J Obstet Gynecol. 2000;183:914–20. doi: 10.1067/mob.2000.108879. [DOI] [PubMed] [Google Scholar]
- 62.Erez O, Romer R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med. 2009;22:971–82. doi: 10.3109/14767050902994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Matern Fetal Neonatal Med. 2010;23:23–33. doi: 10.3109/14767050902994770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Erez O, Romer R, Vaisbuch E, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, et al. Changes in amniotic fluid concentration of thrombin-antithrombin III complexes in patients with preterm labor: evidence of an increased thrombin generation. J Matern Fetal Neonatal Med. 2009;22:971–82. doi: 10.3109/14767050902994762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Erez O, Romero R, Vaisbuch E, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Matern Fetal Neonatal Med. 2010;23:23–33. doi: 10.3109/14767050902994770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mazaki-Tovi S, Romero R, Kusanovic JP, Erez O, Gotsch F, Mittal P, et al. Visfatin/Pre-B cell colony-enhancing factor in amniotic fluid in normal pregnancy, spontaneous labor at term, preterm labor and prelabor rupture of membranes: an association with subclinical intrauterine infection in preterm parturition. J Perinat Med. 2008;36:485–96. doi: 10.1515/JPM.2008.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazaki-Tovi S, Romero R, Vaisbuch E, Erez O, Mittal P, Chaiworapongsa T, et al. Dysregulation of maternal serum adiponectin in preterm labor. J Matern Fetal Neonatal Med. 2009;22:887–904. doi: 10.1080/14767050902994655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mazaki-Tovi S, Romero R, Vaisbuch E, Kusanovic JP, Erez O, Mittal P, et al. Adiponectin in amniotic fluid in normal pregnancy, spontaneous labor at term, and preterm labor: a novel association with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:120–30. doi: 10.3109/14767050903026481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vaisbuch E, Mazaki-Tovi S, Kusanovic JP, Erez O, Than NG, Kim SK, et al. Retinol binding protein 4: an adipokine associated with intra-amniotic infection/inflammation. J Matern Fetal Neonatal Med. 2010;23:111–9. doi: 10.3109/14767050902994739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kusanovic JP, Romero R, Mazaki-Tovi S, Chaiworapongsa T, Mittal P, Gotsch F, et al. Resistin in amniotic fluid and its association with intra-amniotic infection and inflammation. J Matern Fetal Neonatal Med. 2008;21:902–16. doi: 10.1080/14767050802320357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21. doi: 10.1080/jmf.13.1.2.21. [DOI] [PubMed] [Google Scholar]
- 72.Erez O, Romero R, Tarca AL, Chaiworapongsa T, Kim YM, Than NG, et al. Differential expression pattern of genes encoding for anti-microbial peptides in the fetal membranes of patients with spontaneous preterm labor and intact membranes and those with preterm prelabor rupture of the membranes. J Matern Fetal Neonatal Med. 2009;22:1103–15. doi: 10.3109/14767050902994796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Romero R, Emamian M, Wan M, Quintero R, Hobbins JC, Mitchell MD. Prostaglandin concentrations in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1461–7. doi: 10.1016/s0002-9378(87)80245-4. [DOI] [PubMed] [Google Scholar]
- 74.Romero R, Quintero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, et al. Arachidonate lipoxygenase metabolites in amniotic fluid of women with intra-amniotic infection and preterm labor. Am J Obstet Gynecol. 1987;157:1454–60. doi: 10.1016/s0002-9378(87)80243-0. [DOI] [PubMed] [Google Scholar]
- 75.Landmann E, Misselwitz B, Steiss JO, Gortner L. Mortality and morbidity of neonates born at <26 weeks of gestation (1998-2003). A population-based study. J Perinat Med. 2008;36:168–74. doi: 10.1515/JPM.2008.016. [DOI] [PubMed] [Google Scholar]
- 76.Goepfert AR, Andrews WW, Carlo W, Ramsey PS, Cliver SP, Goldenberg RL, et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–81. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 77.Elovitz MA, Brown AG, Breen K, Anton L, Maubert M, Burd I. Intrauterine inflammation, insufficient to induce parturition, still evokes fetal and neonatal brain injury. Int J Dev Neurosci. 2011 doi: 10.1016/j.ijdevneu.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kemp MW, Saito M, Nitsos I, Jobe AH, Kallapur SG, Newnham JP. Exposure to in utero lipopolysaccharide induces inflammation in the fetal ovine skin. Reprod Sci. 2011;18:88–98. doi: 10.1177/1933719110380470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sood BG, Madan A, Saha S, Schendel D, Thorsen P, Skogstrand K, et al. Perinatal systemic inflammatory response syndrome and retinopathy of prematurity. Pediatr Res. 2010;67:394–400. doi: 10.1203/PDR.0b013e3181d01a36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Malaeb S, Dammann O. Fetal inflammatory response and brain injury in the preterm newborn. J Child Neurol. 2009;24:1119–26. doi: 10.1177/0883073809338066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Paananen R, Husa AK, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154:39–43. doi: 10.1016/j.jpeds.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 82.Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, et al. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48:946–54. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- 83.Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, et al. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol. 2009;24:1179–89. doi: 10.1177/0883073809338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nelson KB. Causative factors in cerebral palsy. Clin Obstet Gynecol. 2008;51:749–62. doi: 10.1097/GRF.0b013e318187087c. [DOI] [PubMed] [Google Scholar]
- 85.Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, et al. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199:651–7. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Calhoun DA, Christensen RD. Human developmental biology of granulocyte colony-stimulating factor. Clin Perinatol. 2000;27:559–76. vi. doi: 10.1016/s0095-5108(05)70039-7. [DOI] [PubMed] [Google Scholar]
- 87.Groopman JE, Molina JM, Scadden DT. Hematopoietic growth factors. Biology and clinical applications. N Engl J Med. 1989;321:1449–59. doi: 10.1056/NEJM198911233212106. [DOI] [PubMed] [Google Scholar]
- 88.Watari K, Asano S, Shirafuji N, Kodo H, Ozawa K, Takaku F, et al. Serum granulocyte colony-stimulating factor levels in healthy volunteers and patients with various disorders as estimated by enzyme immunoassay. Blood. 1989;73:117–22. [PubMed] [Google Scholar]
- 89.Goldman S, Ellis R, Dhar V, Cairo MS. Rationale and potential use of cytokines in the prevention and treatment of neonatal sepsis. Clin Perinatol. 1998;25:699–710. [PubMed] [Google Scholar]
- 90.Rondini G, Chirico G. Hematopoietic growth factor levels in term and preterm infants. Curr Opin Hematol. 1999;6:192–7. doi: 10.1097/00062752-199905000-00011. [DOI] [PubMed] [Google Scholar]
- 91.Ahmad M, Fleit HB, Golightly MG, La Gamma EF. In vivo effect of recombinant human granulocyte colony-stimulating factor on phagocytic function and oxidative burst activity in septic neutropenic neonates. Biol Neonate. 2004;86:48–54. doi: 10.1159/000077585. [DOI] [PubMed] [Google Scholar]
- 92.Mohan P, Brocklehurst P. Granulocyte transfusions for neonates with confirmed or suspected sepsis and neutropaenia. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003956. CD003956. [DOI] [PubMed] [Google Scholar]
- 93.Gomez R, Berry SM, Yoon BH, Mazor M, Athayde N, Ghezzi F, Romero R. The hematologic profile of the fetus with systemic inflammatory response syndrome. Am J Obstet Gynecol. 1998 [Google Scholar]
- 94.Gibbs RS, Blanco JD, St Clair PJ, Castaneda YS. Quantitative bacteriology of amniotic fluid from women with clinical intraamniotic infection at term. J Infect Dis. 1982;145:1–8. doi: 10.1093/infdis/145.1.1. [DOI] [PubMed] [Google Scholar]
- 95.Davies NP, Buggins AG, Snijders RJ, Jenkins E, Layton DM, Nicolaides KH. Blood leucocyte count in the human fetus. Arch Dis Child. 1992;67:399–403. doi: 10.1136/adc.67.4_spec_no.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ernst TJ, Ritchie AR, Demetri GD, Griffin JD. Regulation of granulocyte- and monocyte-colony stimulating factor mRNA levels in human blood monocytes is mediated primarily at a post-transcriptional level. J Biol Chem. 1989;264:5700–3. [PubMed] [Google Scholar]
- 97.Schibler KR, Liechty KW, White WL, Christensen RD. Production of granulocyte colony-stimulating factor in vitro by monocytes from preterm and term neonates. Blood. 1993;82:2478–84. [PubMed] [Google Scholar]
- 98.Zsebo KM, Yuschenkoff VN, Schiffer S, Chang D, McCall E, Dinarello CA, et al. Vascular endothelial cells and granulopoiesis: interleukin-1 stimulates release of G-CSF and GM-CSF. Blood. 1988;71:99–103. [PubMed] [Google Scholar]
- 99.Koeffler HP, Gasson J, Ranyard J, Souza L, Shepard M, Munker R. Recombinant human TNF alpha stimulates production of granulocyte colony-stimulating factor. Blood. 1987;70:55–9. [PubMed] [Google Scholar]
- 100.Demetri GD, Zenzie BW, Rheinwald JG, Griffin JD. Expression of colony-stimulating factor genes by normal human mesothelial cells and human malignant mesothelioma cells lines in vitro. Blood. 1989;74:940–6. [PubMed] [Google Scholar]
- 101.Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–808. [PubMed] [Google Scholar]
- 102.Nicola NA, Peterson L. Identification of distinct receptors for two hemopoietic growth factors (granulocyte colony-stimulating factor and multipotential colony-stimulating factor) by chemical cross-linking. J Biol Chem. 1986;261:12384–9. [PubMed] [Google Scholar]
- 103.Nicola NA, Vadas MA, Lopez AF. Down-modulation of receptors for granulocyte colony-stimulating factor on human neutrophils by granulocyte-activating agents. J Cell Physiol. 1986;128:501–9. doi: 10.1002/jcp.1041280320. [DOI] [PubMed] [Google Scholar]
- 104.Elliott MJ, Vadas MA, Eglinton JM, Park LS, To LB, Cleland LG, et al. Recombinant human interleukin-3 and granulocyte-macrophage colony-stimulating factor show common biological effects and binding characteristics on human monocytes. Blood. 1989;74:2349–59. [PubMed] [Google Scholar]
- 105.Lopez AF, Eglinton JM, Gillis D, Park LS, Clark S, Vadas MA. Reciprocal inhibition of binding between interleukin 3 and granulocyte-macrophage colony-stimulating factor to human eosinophils. Proc Natl Acad Sci U S A. 1989;86:7022–6. doi: 10.1073/pnas.86.18.7022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bussolino F, Wang JM, Defilippi P, Turrini F, Sanavio F, Edgell CJ, et al. Granulocyte- and granulocyte-macrophage-colony stimulating factors induce human endothelial cells to migrate and proliferate. Nature. 1989;337:471–3. doi: 10.1038/337471a0. [DOI] [PubMed] [Google Scholar]
- 107.Liongue C, Wright C, Russell AP, Ward AC. Granulocyte colony-stimulating factor receptor: stimulating granulopoiesis and much more. Int J Biochem Cell Biol. 2009;41:2372–5. doi: 10.1016/j.biocel.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 108.Calhoun DA, Lunoe M, Du Y, Christensen RD. Granulocyte colony-stimulating factor is present in human milk and its receptor is present in human fetal intestine. Pediatrics. 2000;105:e7. doi: 10.1542/peds.105.1.e7. [DOI] [PubMed] [Google Scholar]
- 109.Avalos BR, Gasson JC, Hedvat C, Quan SG, Baldwin GC, Weisbart RH, et al. Human granulocyte colony-stimulating factor: biologic activities and receptor characterization on hematopoietic cells and small cell lung cancer cell lines. Blood. 1990;75:851–7. [PubMed] [Google Scholar]
- 110.Calhoun DA, Donnelly WH, Jr., Du Y, Dame JB, Li Y, Christensen RD. Distribution of granulocyte colony-stimulating factor (G-CSF) and G-CSF-receptor mRNA and protein in the human fetus. Pediatr Res. 1999;46:333–8. doi: 10.1203/00006450-199909000-00015. [DOI] [PubMed] [Google Scholar]
- 111.Solaroglu I, Jadhav V, Zhang JH. Neuroprotective effect of granulocyte-colony stimulating factor. Front Biosci. 2007;12:712–24. doi: 10.2741/2095. [DOI] [PubMed] [Google Scholar]
- 112.McCracken S, Layton JE, Shorter SC, Starkey PM, Barlow DH, Mardon HJ. Expression of granulocyte-colony stimulating factor and its receptor is regulated during the development of the human placenta. J Endocrinol. 1996;149:249–58. doi: 10.1677/joe.0.1490249. [DOI] [PubMed] [Google Scholar]
- 113.Uzumaki H, Okabe T, Sasaki N, Hagiwara K, Takaku F, Tobita M, et al. Identification and characterization of receptors for granulocyte colony-stimulating factor on human placenta and trophoblastic cells. Proc Natl Acad Sci U S A. 1989;86:9323–6. doi: 10.1073/pnas.86.23.9323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nicola NA. Granulocyte colony stimulating factor. Methods Enzymol. 1985;116:600–19. doi: 10.1016/s0076-6879(85)16046-5. [DOI] [PubMed] [Google Scholar]
- 115.Wang JM, Chen ZG, Colella S, Bonilla MA, Welte K, Bordignon C, et al. Chemotactic activity of recombinant human granulocyte colony-stimulating factor. Blood. 1988;72:1456–60. [PubMed] [Google Scholar]
- 116.Molloy EJ, O’Neill AJ, Grantham JJ, Sheridan-Pereira M, Fitzpatrick JM, Webb DW, et al. Granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor have differential effects on neonatal and adult neutrophil survival and function. Pediatr Res. 2005;57:806–12. doi: 10.1203/01.PDR.0000156500.13600.B5. [DOI] [PubMed] [Google Scholar]
- 117.Cairo MS, Gillan ER, Buzby JS, van d V, Suen Y. Circulating steel factor (SLF) and G-CSF levels in preterm and term newborn and adult peripheral blood. Am J Pediatr Hematol Oncol. 1993;15:311–5. [PubMed] [Google Scholar]
- 118.Gessler P, Kirchmann N, Kientsch-Engel R, Haas N, Lasch P, Kachel W. Serum concentrations of granulocyte colony-stimulating factor in healthy term and preterm neonates and in those with various diseases including bacterial infections. Blood. 1993;82:3177–82. [PubMed] [Google Scholar]
- 119.Weimann E, Rutkowski S, Reisbach G. G-CSF, GM-CSF and IL-6 levels in cord blood: diminished increase of G-CSF and IL-6 in preterms with perinatal infection compared to term neonates. J Perinat Med. 1998;26:211–8. doi: 10.1515/jpme.1998.26.3.211. [DOI] [PubMed] [Google Scholar]
- 120.Bailie KE, Irvine AE, Bridges JM, McClure BG. Granulocyte and granulocyte-macrophage colony-stimulating factors in cord and maternal serum at delivery. Pediatr Res. 1994;35:164–8. doi: 10.1203/00006450-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 121.Wilimas JA, Wall JE, Fairclough DL, Dancy R, Griffin C, Karanth S, et al. A longitudinal study of granulocyte colony-stimulating factor levels and neutrophil counts in newborn infants. J Pediatr Hematol Oncol. 1995;17:176–9. doi: 10.1097/00043426-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 122.Kennon C, Overturf G, Bessman S, Sierra E, Smith KJ, Brann B. Granulocyte colony-stimulating factor as a marker for bacterial infection in neonates. J Pediatr. 1996;128:765–9. doi: 10.1016/s0022-3476(96)70327-x. [DOI] [PubMed] [Google Scholar]
- 123.Shimada M, Minato M, Takada M, Takahashi S, Harada K. Plasma concentration of granulocyte-colony-stimulating factor in neonates. Acta Paediatr. 1996;85:351–5. doi: 10.1111/j.1651-2227.1996.tb14031.x. [DOI] [PubMed] [Google Scholar]
- 124.Diederich K, Sevimli S, Dorr H, Kosters E, Hoppen M, Lewejohann L, et al. The role of granulocyte-colony stimulating factor (G-CSF) in the healthy brain: a characterization of G-CSF-deficient mice. J Neurosci. 2009;29:11572–81. doi: 10.1523/JNEUROSCI.0453-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Diederich K, Schabitz WR, Kuhnert K, Hellstrom N, Sachser N, Schneider A, et al. Synergetic effects of granulocyte-colony stimulating factor and cognitive training on spatial learning and survival of newborn hippocampal neurons. PLoS One. 2009;4:e5303. doi: 10.1371/journal.pone.0005303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Canpolat FE, Yurdakok M, Ozsoy S, Haziroglu R, Korkmaz A. Protective effects of recombinant human granulocyte colony stimulating factor in a rat model of necrotizing enterocolitis. Pediatr Surg Int. 2006;22:719–23. doi: 10.1007/s00383-006-1728-2. [DOI] [PubMed] [Google Scholar]
- 127.Kim BR, Shim JW, Sung DK, Kim SS, Jeon GW, Kim MJ, et al. Granulocyte stimulating factor attenuates hypoxic-ischemic brain injury by inhibiting apoptosis in neonatal rats. Yonsei Med J. 2008;49:836–42. doi: 10.3349/ymj.2008.49.5.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yata K, Matchett GA, Tsubokawa T, Tang J, Kanamaru K, Zhang JH. Granulocyte-colony stimulating factor inhibits apoptotic neuron loss after neonatal hypoxia-ischemia in rats. Brain Res. 2007;1145:227–38. doi: 10.1016/j.brainres.2007.01.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lu CZ, Xiao BG. Neuroprotection of G-CSF in cerebral ischemia. Front Biosci. 2007;12:2869–75. doi: 10.2741/2278. [DOI] [PubMed] [Google Scholar]
- 130.Schneider A, Kruger C, Steigleder T, Weber D, Pitzer C, Laage R, et al. The hematopoietic factor G-CSF is a neuronal ligand that counteracts programmed cell death and drives neurogenesis. J Clin Invest. 2005;115:2083–98. doi: 10.1172/JCI23559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, et al. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–10. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 132.Yoon BH, Romero R, Jun JK, Maymon E, Gomez R, Mazor M, et al. An increase in fetal plasma cortisol but not dehydroepiandrosterone sulfate is followed by the onset of preterm labor in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol. 1998;179:1107–14. doi: 10.1016/s0002-9378(98)70114-0. [DOI] [PubMed] [Google Scholar]
- 133.Romero R, Espinoza J, Goncalves LF, Gomez R, Medina L, Silva M, et al. Fetal cardiac dysfunction in preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2004;16:146–57. doi: 10.1080/14767050400009279. [DOI] [PubMed] [Google Scholar]
- 134.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST, et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol. 1997;177:797–802. doi: 10.1016/s0002-9378(97)70271-0. [DOI] [PubMed] [Google Scholar]
- 135.Kim YM, Romero R, Chaiworapongsa T, Espinoza J, Mor G, Kim CJ. Dermatitis as a component of the fetal inflammatory response syndrome is associated with activation of Toll-like receptors in epidermal keratinocytes. Histopathology. 2006;49:506–14. doi: 10.1111/j.1365-2559.2006.02542.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB, et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol. 1995;173:1315–20. doi: 10.1016/0002-9378(95)91378-5. [DOI] [PubMed] [Google Scholar]
- 137.Kim SK, Romero R, Chaiworapongsa T, Kusanovic JP, Mazaki-Tovi S, Mittal P, et al. Evidence of changes in the immunophenotype and metabolic characteristics (intracellular reactive oxygen radicals) of fetal, but not maternal, monocytes and granulocytes in the fetal inflammatory response syndrome. J Perinat Med. 2009;37:543–52. doi: 10.1515/JPM.2009.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Madsen-Bouterse SA, Romero R, Tarca AL, Kusanovic JP, Espinoza J, Kim CJ, et al. The transcriptome of the fetal inflammatory response syndrome. Am J Reprod Immunol. 2010;63:73–92. doi: 10.1111/j.1600-0897.2009.00791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lieschke GJ, Grail D, Hodgson G, Metcalf D, Stanley E, Cheers C, et al. Mice lacking granulocyte colony-stimulating factor have chronic neutropenia, granulocyte and macrophage progenitor cell deficiency, and impaired neutrophil mobilization. Blood. 1994;84:1737–46. [PubMed] [Google Scholar]
- 140.Cairo MS, Mauss D, Kommareddy S, Norris K, van d V, Modanlou H. Prophylactic or simultaneous administration of recombinant human granulocyte colony stimulating factor in the treatment of group B streptococcal sepsis in neonatal rats. Pediatr Res. 1990;27:612–6. doi: 10.1203/00006450-199006000-00016. [DOI] [PubMed] [Google Scholar]
- 141.Gillan ER, Christensen RD, Suen Y, Ellis R, van d V, Cairo MS. A randomized, placebo-controlled trial of recombinant human granulocyte colony-stimulating factor administration in newborn infants with presumed sepsis: significant induction of peripheral and bone marrow neutrophilia. Blood. 1994;84:1427–33. [PubMed] [Google Scholar]
- 142.Drossou-Agakidou V, Kanakoudi-Tsakalidou F, Sarafidis K, Taparkou A, Tzimouli V, Tsandali H, et al. Administration of recombinant human granulocyte-colony stimulating factor to septic neonates induces neutrophilia and enhances the neutrophil respiratory burst and beta2 integrin expression. Results of a randomized controlled trial. Eur J Pediatr. 1998;157:583–8. doi: 10.1007/s004310050884. [DOI] [PubMed] [Google Scholar]
- 143.Kocherlakota P, La Gamma EF. Preliminary report: rhG-CSF may reduce the incidence of neonatal sepsis in prolonged preeclampsia-associated neutropenia. Pediatrics. 1998;102:1107–11. doi: 10.1542/peds.102.5.1107. [DOI] [PubMed] [Google Scholar]
- 144.Papoff P. Infection, neutrophils, and hematopoietic growth factors in the pathogenesis of neonatal chronic lung disease. Clin Perinatol. 2000;27:717–31. viii. doi: 10.1016/s0095-5108(05)70047-6. [DOI] [PubMed] [Google Scholar]
- 145.Suratt BT, Eisner MD, Calfee CS, Allard JB, Whittaker LA, Engelken DT, et al. Plasma granulocyte colony-stimulating factor levels correlate with clinical outcomes in patients with acute lung injury. Crit Care Med. 2009;37:1322–8. doi: 10.1097/CCM.0b013e31819c14fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Carr R, Modi N, Dore C. G-CSF and GM-CSF for treating or preventing neonatal infections. Cochrane Database Syst Rev. 2003 doi: 10.1002/14651858.CD003066. CD003066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kuhn P, Messer J, Paupe A, Espagne S, Kacet N, Mouchnino G, et al. A multicenter, randomized, placebo-controlled trial of prophylactic recombinant granulocyte-colony stimulating factor in preterm neonates with neutropenia. J Pediatr. 2009;155:324–30. doi: 10.1016/j.jpeds.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 148.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine. 1993;5:81–8. doi: 10.1016/1043-4666(93)90027-3. [DOI] [PubMed] [Google Scholar]
- 149.Calhoun DA, Chegini N, Polliotti BM, Gersting JA, Miller RK, Christensen RD. Granulocyte colony-stimulating factor in preterm and term pregnancy, parturition, and intra-amniotic infection. Obstet Gynecol. 2001;97:229–34. doi: 10.1016/s0029-7844(00)01120-0. [DOI] [PubMed] [Google Scholar]
- 150.Stallmach T, Hebisch G, Joller-Jemelka HI, Orban P, Schwaller J, Engelmann M. Cytokine production and visualized effects in the feto-maternal unit. Quantitative and topographic data on cytokines during intrauterine disease. Lab Invest. 1995;73:384–92. [PubMed] [Google Scholar]
- 151.Raynor BD, Clark P, Duff P. Granulocyte colony-stimulating factor in amniotic fluid. Infect Dis Obstet Gynecol. 1995;3:140–4. doi: 10.1155/S1064744995000482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD, et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 2000;182:625–30. doi: 10.1067/mob.2000.104210. [DOI] [PubMed] [Google Scholar]
- 153.Whitcomb BW, Schisterman EF, Luo X, Chegini N. Maternal serum granulocyte colony-stimulating factor levels and spontaneous preterm birth. J Womens Health (Larchmt ) 2009;18:73–8. doi: 10.1089/jwh.2008.0883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Witek-Janusek L, Mathews HL. Differential effects of glucocorticoids on colony stimulating factors produced by neonatal mononuclear cells. Pediatr Res. 1999;45:224–9. doi: 10.1203/00006450-199902000-00011. [DOI] [PubMed] [Google Scholar]
- 155.Calhoun DA, Christensen RD. A randomized pilot trial of administration of granulocyte colony-stimulating factor to women before preterm delivery. Am J Obstet Gynecol. 1998;179:766–71. doi: 10.1016/s0002-9378(98)70080-8. [DOI] [PubMed] [Google Scholar]
- 156.Nystrom PO. The systemic inflammatory response syndrome: definitions and aetiology. J Antimicrob Chemother. 1998;41(Suppl A):1–7. doi: 10.1093/jac/41.suppl_1.1. [DOI] [PubMed] [Google Scholar]
- 157.Vaisbuch E, Romero R, Gomez R, Kusanovic JP, Mazaki-Tovi S, Chaiworapongsa T, et al. An elevated fetal interleukin-6 concentration can be observed in fetuses with anemia due to Rh alloimmunization: implications for the understanding of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med. 2011;24:391–6. doi: 10.3109/14767058.2010.507294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liechty KW, Schibler KR, Ohls RK, Perkins SL, Christensen RD. The failure of newborn mice infected with Escherichia coli to accelerate neutrophil production correlates with their failure to increase transcripts for granulocyte colony-stimulating factor and interleukin-6. Biol Neonate. 1993;64:331–40. doi: 10.1159/000244007. [DOI] [PubMed] [Google Scholar]
- 159.Basu S, Hodgson G, Zhang HH, Katz M, Quilici C, Dunn AR. “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood. 2000;95:3725–33. [PubMed] [Google Scholar]