Abstract
Rationale
Multiple sclerosis (MS) and its mouse model, experimental autoimmune encephalomyelitis (EAE), are inflammatory disorders of the central nervous system (CNS). The function of platelets in inflammatory and autoimmune pathologies is thus far poorly defined.
Objective
Here we addressed the role of platelets in mediating CNS inflammation in EAE.
Results
We found that platelets were present in human MS lesions as well as in the CNS of mice subjected to EAE but not in the CNS from control non-diseased mice. Platelet depletion at the effector-inflammatory phase of EAE in mice resulted in significantly ameliorated disease development and progression. EAE suppression upon platelet depletion was associated with reduced recruitment of leukocytes to the inflamed CNS, as assessed by intravital microscopy, and with a blunted inflammatory response. The platelet-specific receptor glycoprotein Ib alpha (GPIbα) promotes both platelet adhesion as well as inflammatory actions of platelets, and, targeting of GPIbα attenuated EAE in mice. Moreover, targeting another platelet adhesion receptor, glycoprotein IIb/IIIa (GPIIb/IIIa) also reduced EAE severity in mice.
Conclusions
Thus, platelets contribute to the pathogenesis of EAE by promoting CNS inflammation. Targeting platelets may therefore represent an important new therapeutic approach for MS treatment.
Keywords: Platelets, EAE, inflammation, autoimmune disease
Introduction
Multiple sclerosis (MS) is an inflammatory degenerative disease of the central nervous system (CNS).1–5 MS and its mouse counterpart, experimental autoimmune encephalomyelitis (EAE), are initiated by infiltration of the neuronal tissue by T-cells autoreactive to antigens of the myelin sheath.6–9 The subsequent breakdown of the blood brain barrier (BBB) facilitates the recruitment of further inflammatory effector cells such as mononuclear cells and macrophages,1,10 and the activation of resident inflammatory microglial cells that contribute to the development of CNS lesions.11 Inhibiting the inflammatory response is a promising therapeutic approach in EAE and MS.1,9 For instance, leukocyte integrins or their endothelial counter-receptors that contribute to inflammatory cell recruitment, represent therapeutic targets in EAE and MS.9,12,13
Besides their well established role in haemostasis and thrombosis, platelets contribute to inflammatory processes. Upon inflammatory stimulation, platelets rapidly adhere to the endothelium or to the subendothelial extracellular matrix at sites of vascular endothelial injury.14–17 Several platelet adhesion receptors, such as the glycoprotein (GP) VI, the integrin GPIIbIIIa (also designated as αIIbβ3-integrin or CD41/CD61-integrin) or the GPIb/IX/V complex mediate platelet adhesion or aggregation.18 Platelet-derived chemokines and cytokines can trigger inflammation in a paracrine fashion, whereas the direct adhesive interactions of platelets with endothelial cells and inflammatory cells promote leukocyte recruitment to the inflamed tissue. Distinct platelet adhesion receptors have been implicated in these processes, including P-selectin, junctional adhesion molecule-C or GPIb. The latter receptor consists of the GPIbα and GPIbβ chains and is a component of the GPIb/IX/V complex that acts as the von Willenbrand Factor (vWF) receptor mediating platelet adhesion to the inflamed endothelial surface and the subendothelial matrix, whereas the interaction of GPIb with the leukocyte integrin Mac-1 promotes leukocyte/platelet interactions and thereby inflammatory cell recruitment. Platelets are thus recognized as an important link between haemostasis and inflammation.14,15,19–27
A recent microarray approach analyzing differentially expressed genes in plaques from patients with multiple sclerosis revealed an upregulation of the message of the platelet-specific GPIIb in patients with chronic demyelinating disease.28 Furthermore, a recent report demonstrated increased activation of platelets in the peripheral blood from patients suffering from multiple sclerosis.29 These observations prompted us to investigate, whether platelets could play a role in the pathogenesis of EAE. The present work demonstrates for the first time that platelets constitute a substantial component of the inflammatory response in the course of EAE by promoting the inflammatory response in the CNS. Targeting platelets or their interactions with inflammatory cells may thus represent a novel therapeutic approach to ameliorate inflammatory lesions in MS.
Materials and Methods
“Reagents”, “Induction of EAE in mice”, “Intravital Microscopy after EAE induction”, “Isolation of primary mouse cells”, “Adhesion assays”, “Reverse transcriptase-PCR and real-time RT-PCR”, “RNA isolation and microarray analysis”, “Detection of platelets in human MS tissue” and “Data presentation and statitiscs” are described in detail in the Online Supplement
Results
Platelets are present in the CNS of mice after induction of EAE and in human MS lesions
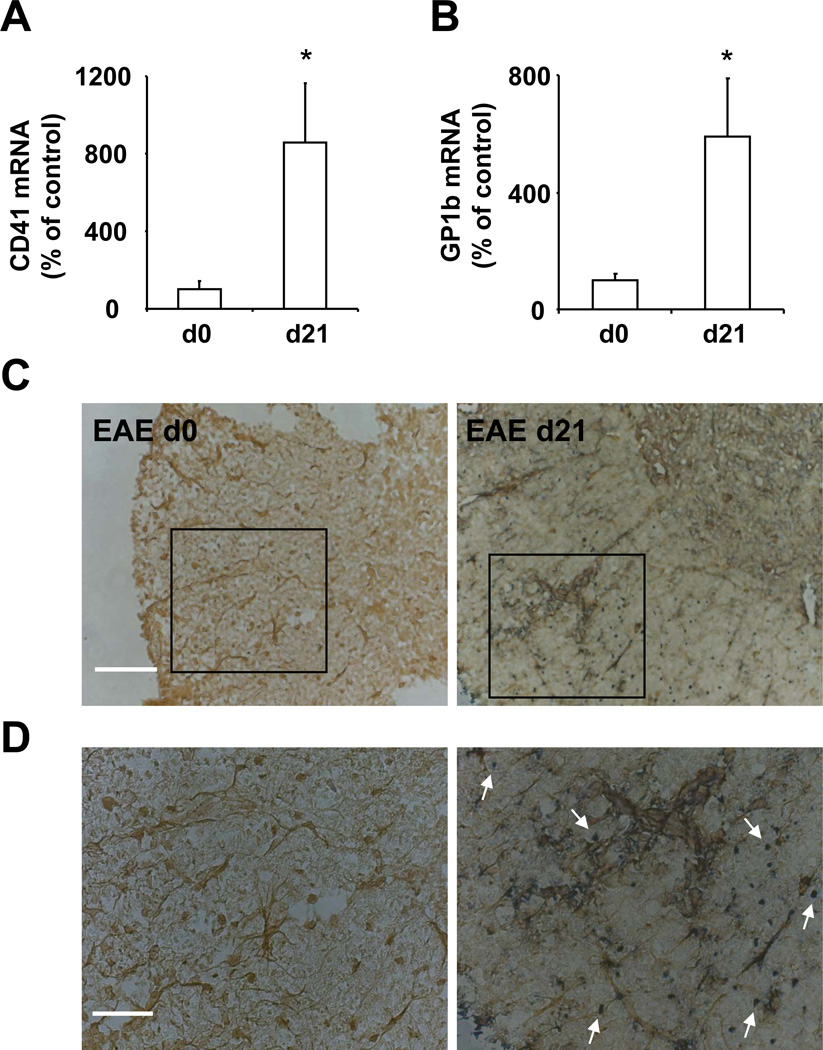
To address a potential contribution of platelets to the inflammatory lesions of EAE, we first assessed for the presence of platelets in the CNS after EAE induction. EAE was induced in mice using myelin oligodendrocyte glycoprotein (MOG). At different time points post EAE induction we analyzed the inflamed spinal cords for the presence of platelets as assayed by the detection of the platelet specific CD41 (GPIIb) and GPIb (CD42b). Before sacrificing the mice and extracting the spinal cords, mice were perfused, in order to efficiently remove circulating blood including circulating platelets, thus allowing for the assessment of only tissue-associated platelets or platelets permanently adherent on the endothelium of the inflamed CNS vessels. First, as compared to non-inflamed spinal cords, we found elevated levels of CD41 mRNA and GPIb mRNA in the inflamed spinal cords of mice on day 21 after EAE induction, representing the effector phase of the disease (Figure 1A and 1B). These results are in line with a previous report that demonstrated an upregulation of CD41 mRNA in chronic human MS lesions.28 Second, using immunohistochemistry with an antibody for the platelet-specific GPIb we could visualize platelets in inflamed spinal cords, in parts localizing in inflamed areas containing activated microglial cells, which were stained by the marker Iba-1 (Figure 1C and 1D). Furthermore, the presence of platelets in inflamed spinal cords was assessed by western blot analysis for platelet specific GPIIb. Platelets were detectable at the effector phase of the disease (i.e. after clinical disease onset), whereas no platelets were found in non-inflamed spinal cord tissues (Online Figure I). In addition to inflamed spinal cords, platelets were detectable in inflamed brain sections of mice subjected to EAE (Online Figure I).
Figure 1. Platelets are present in spinal cord during EAE.
(A, B, C, D) EAE was induced in female WT mice and spinal cords were collected after extensive systemic perfusion with saline on day 0 and day 21 post immunization. On d 0 or d 21 samples were analyzed for mRNA levels of platelet specific (A) CD41 or (B) CD42b (GPIbα). Data are mean +/− SEM (n=4–5) and are shown as % of control. The ratio of CD41 mRNA / actin mRNA or GPIb/ actin mRNA on day 0 represents the 100 % control. * indicates p<0.05. (C, D) Tissues from mice on d 0 and d 21 after EAE induction were analyzed for the platelet specific marker CD42 (GPIb) (dark blue) and the microglial marker Iba-1 (brown) by immunohistochemistry. The arrows indicate platelets. Scale Bar (C): 100 µm; Scale Bar (D): 50 µm.
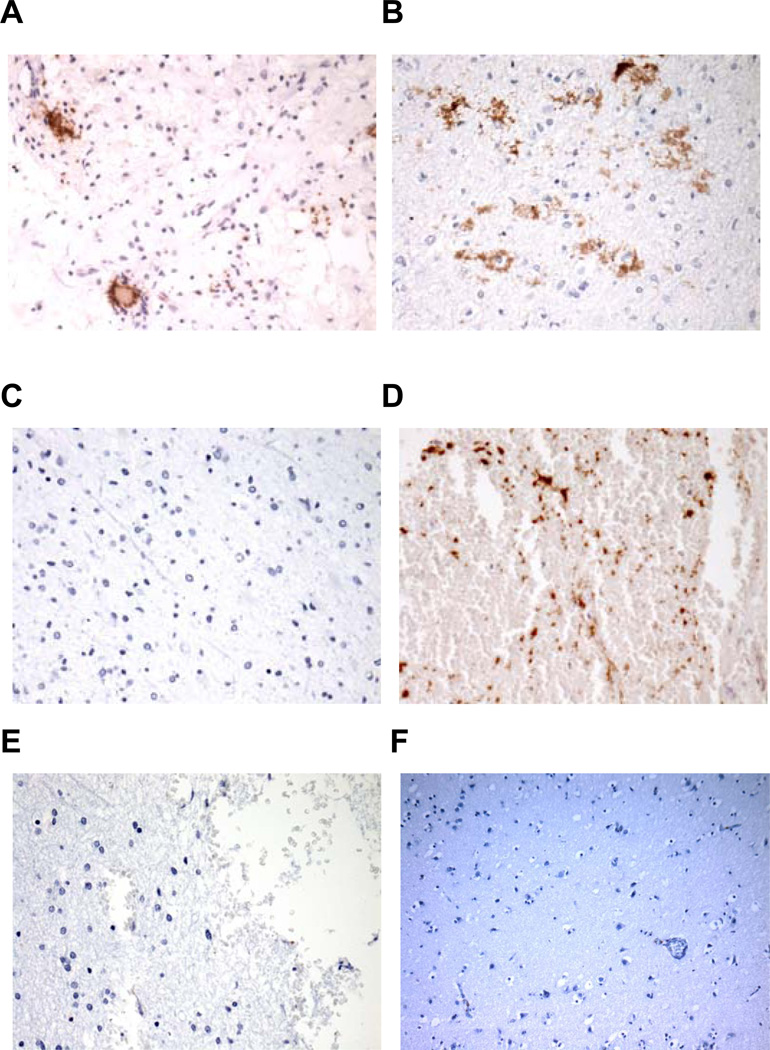
Moreover, we assessed for the presence of platelets in lesions from human MS patients. Four cases of patients with multiple sclerosis were analysed. All cases displayed active demyelination plaques including perivascular chronic inflammatory infiltrates composed of lymphocytes and plasma cells, macrophage infiltration, myelin breakdown with relative preservation of axons and gliosis. Positive platelet staining with anti-human CD42b was observed in vessels and capillaries, in areas of hemorrhage as well as in the extracellular space, which could represent potentially extravasated platelets. Normal adjacent brain areas, as well as sections from normal human brain tissue displayed minimal to no positive platelet staining. Sections stained using IgG control antibody were also negative (Figure 2). Furthermore, a chronic active plaque from a 54year-old female patient with clinically definite MS30,31 from a different center fulfilling the criteria of chronic active lesions32 also displayed abundant platelets as assessed by GPIb staining (Online Figure I).
Figure 2. Platelets are present in multiple sclerosis lesions.
Pathological deposition of platelets in brain sections from MS patients with active demyelination plaques. Paraffin-embedded brain sections from 3 MS cases (Panels A: case 1, B and C: case 2, D–E were from case 3). Panel F is a representative image from normal human brain; four cases of normal human brains were studied with similar results. Panels A–D and F were stained with mouse anti-human CD42b mAb, whereas panel E was stained with isotype control IgG1. Active demyelination plaques (panels A, B and D) are shown, whereas panel C represents normal tissue adjacent to the plaque of case 2. Representative sections (panels A–E) are shown at 400X magnification, panel F is shown at 200× magnification.
Platelets contribute to EAE pathogenesis
Since platelets were present in the inflamed CNS after EAE induction, we then examined their impact on EAE using pharmacological depletion of platelets. Efficient platelet depletion was achieved with an intraperitoneal (i.p.) injection of rabbit anti-mouse platelet serum (depletion of over 97% at 24 h post injection) (Online Figure II), as previously described.24 Platelet depletion lasted for at least 72 hours (data not shown). The platelet-depleting serum did not alter the number of circulating total leukocytes (Online Figure II), as previously described.33 Neither control nor platelet-depleting serum had any immune-stimulatory effects, as assessed by TNF-alpha and C3 levels in the serum of mice (data not shown).
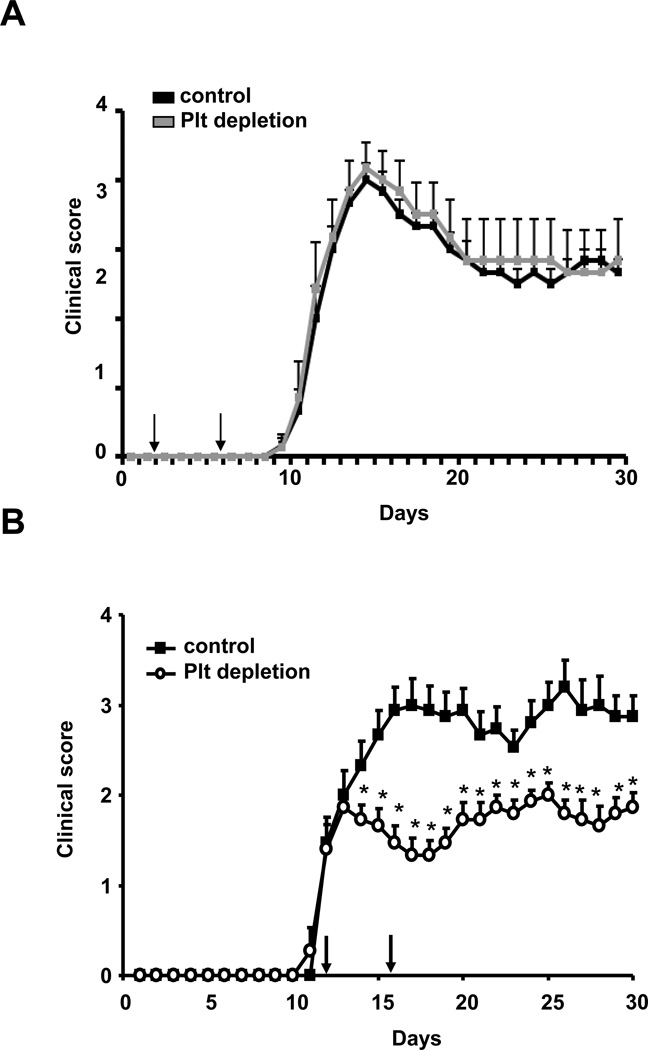
We performed platelet depletion either in the immunization phase or in the inflammatory effector phase of EAE, when clinical disease is evident. In the former case, mice received an i.p. injection of platelet-depleting serum or control serum at two different time points, on days 2 and 6, whereas in the latter case, mice received platelet-depleting serum or control serum on days 12 and 16 post immunization (day 12 represents the average begin of clinical disease in EAE, i.e. the effector phase). Whereas platelet depletion during the immunization phase of disease did not affect EAE development (Fig. 3A), depletion of platelets resulted in a significant and persistent reduction in EAE disease severity already two days after application of the depleting agent (Figure 3B). Consistent with reduced EAE disease severity upon platelet depletion in the effector phase, we found ameliorated loss of axonal integrity, as assessed by axonal staining using the neurofilament-200 (NF-200) as a marker, in mice that received the platelet depleting serum as compared to mice receiving control serum (Online Figure III). Moreover, reduced demyelination was also found upon platelet depletion as analyzed by Luxol fast blue staining of the spinal cords (Online Figure III). Taken together, our findings suggest a role of platelets in the effector phase of EAE, while platelets do not interfere with the immunization phase of EAE.
Figure 3. Platelets contribute to the pathogenesis of EAE.
(A) To test the impact of platelets on EAE pathogenesis, EAE was induced in WT mice and on day 2 and 6 post induction mice were treated with control serum (control) or with platelet-depleting serum (Plt depletion) and clinical disease score was assessed (n=10 mice per group). (B) EAE was induced in WT mice and on days 12 and 16 post induction (indicated by the arrows) mice were treated with control serum (control) or with platelet-depleting serum and the clinical disease score was assessed. (n=15 mice per group). * indicates p<0.05 as compared to the control group on the same day.
Platelets contribute to the inflammatory response during EAE pathogenesis
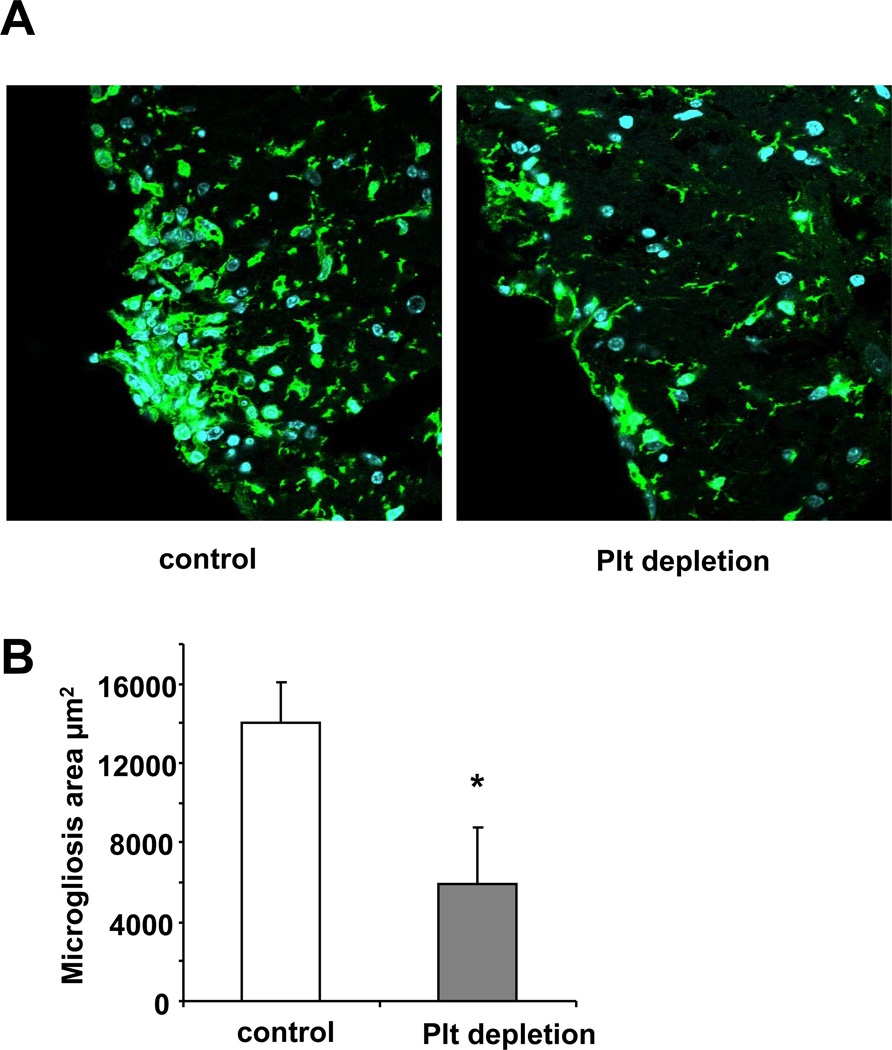
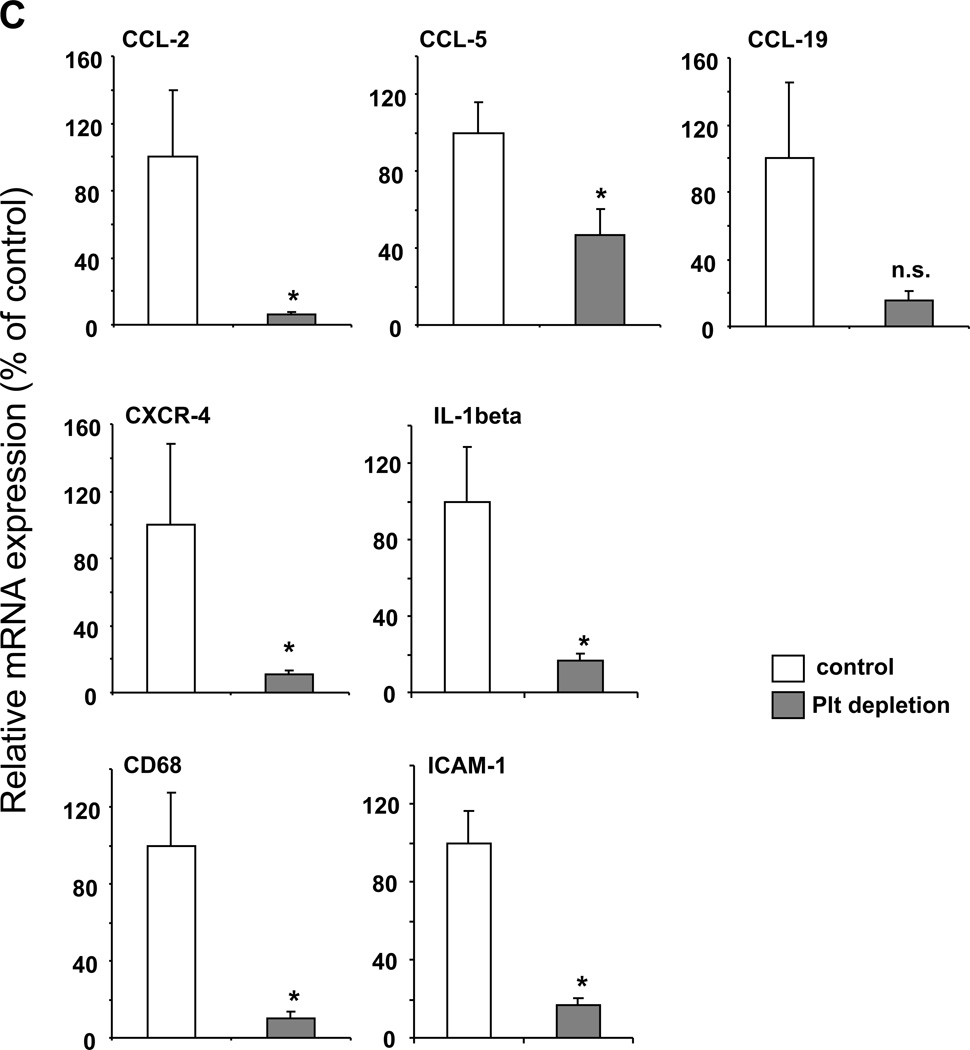
The data that platelet depletion during the effector phase of EAE reduced EAE severity indicated that platelets contribute to inflammation in the course of EAE. Indeed, inflammation in EAE was blunted upon platelet depletion on days 12 and 16 post immunization, as evidenced by studying different components of the inflammatory response of EAE. First, activation of resident microglial cells is a marker of the inflammatory response in the course of EAE and activated microglia contribute to CNS inflammation and myelin sheath lesions.11 Interestingly, we observed a significant reduction of microgliosis area in the inflamed spinal cord upon platelet depletion in mice, as compared to control mice (Figure 4A, B). Second, we performed microarray analysis of the inflamed spinal cords from EAE mice that were treated with control or platelet depleting serum. Several inflammatory and immune factors and pathways were significantly downregulated in the inflamed spinal cords upon platelet depletion. Selected proinflammatory factors such as cytokines, chemokines or adhesion molecules that were significantly downregulated in the spinal cords upon platelet depletion are summarized in Online Table I. We confirmed the downregulation of selected pro-inflammatory factors (the chemokines CCL-2, CCL-5, the chemokine receptor CXCR-4, the cytokine IL-1beta, the macrophage marker CD68 and the adhesion molecule ICAM-1) in the spinal cord upon platelet depletion by real-time PCR analysis (Figure 4C).
Figure 4. Platelet depletion reduces the inflammatory response during EAE.
To analyze the influence of platelet depletion on the inflammatory response in EAE, the disease was induced in WT mice and on days 12 and 16 post induction mice were treated with control serum (control) or with platelet-depleting serum. (A, B) On day 21, staining for microglia cells using the antibody to the marker Iba-1 was performed. The area of microgliosis was reduced in spinal cords upon platelet depletion. Representative images showing microgliosis areas are depicted. (B) The area of microgliosis was analyzed by morphometry. Six spinal cords per group and 3 non-consecutive sections per spinal cord were analyzed. Data are mean +/− SEM. * indicates p<0.05. (C) Spinal cords from control treated or platelet depleted mice were isolated on day 21 after induction of EAE. Real-time PCR analysis for the mRNA levels of the chemokines CCL-2, CCL-5 and CCL-19, the chemokine receptor CXCR-4, the cytokine IL-1β, the macrophage marker CD68 and the adhesion molecule ICAM-1 was performed. Data are mean +/− SEM (n = 5 mice per group) and are shown as percent of control. The mRNA expression of the respective molecule in spinal cords from control treated mice represents the 100 % control. * indicates p<0.05 as compared to control serum treated animals.
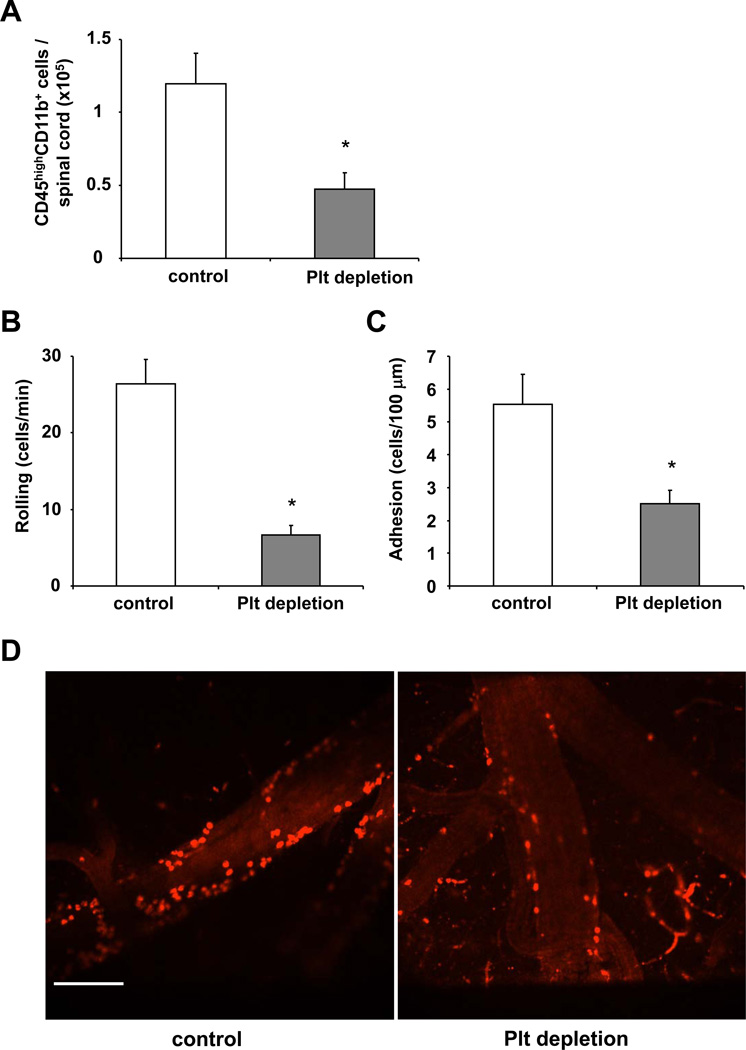
Third, platelets contributed to inflammatory cell recruitment in the course of EAE. We observed decreased accumulation of inflammatory cells to the inflamed CNS upon platelet depletion. In particular, mice treated with the platelet-depleting or the control serum on days 12 and 16 post immunization, were euthanized on day 21. Immediately prior to euthanasia, mice were perfused with saline to get rid of circulating cells, the inflamed spinal cords were extracted, leukocytes were isolated and their total number was counted. Flow cytometry analysis of the leukocyte populations isolated from inflamed spinal cords was performed for CD45 and CD11b and we assessed the numbers of CD45highCD11b+ cells representing recruited monocytes / macrophages.34–36 Upon platelet depletion, a significant decrease in the numbers of CD45highCD11b+ cells in the inflamed spinal cord after EAE induction was observed (Figure 5A). In addition, we studied the recruitment of different leukocyte subpopulations into the inflamed spinal cord of mice that received platelet-depleting or control serum by performing quantitative PCR analysis for the mRNA of CD4, CD8, CD11b. Upon platelet depletion the mRNA levels of CD4, CD8 and CD11b were reduced, as compared to mice receiving control serum, implying reduced recruitment of the respective cells to the inflamed spinal cord (Online Figure IV). Moreover, the level of granulocytes was significantly reduced after platelet depletion, as assessed by ELISA for myeloperoxidase (Online Figure IV). That platelet depletion expectedly resulted in reduced platelet accumulation in the inflamed CNS was demonstrated by the marked decrease in platelet-specific GPIb mRNA in the inflamed CNS of mice receiving the platelet-depleting serum as compared to the control serum (data not shown).
Figure 5. Role of platelets for leukocyte recruitment to the inflamed CNS in mice.
(A) EAE was induced in WT mice and on days 12 and 16 post induction mice were treated with control serum (control) or with platelet-depleting serum. Then, spinal cord tissue from WT mice treated with control serum (control) or with platelet depleting serum was collected after extensive systemic perfusion with saline on day 21 post immunization and leukocytes were isolated with percoll gradient centrifugation and analyzed by flow cytometry. The number of CD45highCD11b+ cells per spinal cord is shown. Data are mean +/− SEM (n=4–5 mice per group). * indicates p<0.05. (B, C, D) To test for the impact of platelets on leukocyte recruitment to the inflamed CNS, EAE was induced in WT mice and after 2 weeks mice, were treated without (control) or with platelet-depleting serum. Twenty-four h thereafter the number of labelled rolling (B) or firmly adherent (C) leukocytes to postcapillary venules was determined using spinning disc intravital microscopy. Data are mean +/− SEM and are shown as number of rolling leukocytes/minute or number of adherent leukocytes/100µm (n=3–4 mice per group). * indicates p<0.05 as compared to control. (D) Representative offline images of intravital microscopy experiments showing adherent leukocytes in control mice (left panel) or in mice after platelet depletion (right panel). Scale Bar: 100 µm.
To substantiate the impact of platelets on leukocyte recruitment to the inflamed CNS in vivo, we induced EAE in mice and assessed the effect of platelet depletion on leukocyte recruitment to the CNS by spinning disc intravital microscopy.15,37 Fourteen days post EAE immunization mice were treated without or with the platelet depleting reagent and 24 hours thereafter, leukocyte recruitment to the inflamed CNS was assessed by intravital microscopy. A 70% and 50% reduction in the number of rolling leukocytes and of firmly adherent leukocytes, respectively, was observed in the vessels of the inflamed CNS of thrombocytopenic mice, as compared to control mice (Figure 5B–D and Online Videos I–II). In the absence of EAE disease, there was virtually no leukocyte adhesion to the vessel wall independent of whether platelets were depleted or not (Online Table II).
Together, these findings suggest that platelets substantially contribute to EAE disease severity in mice by mediating the overall inflammatory response in the course of EAE, through promoting leukocyte recruitment to the inflamed spinal cord and the upregulation of multiple inflammatory cytokines, chemokines and adhesion molecules.
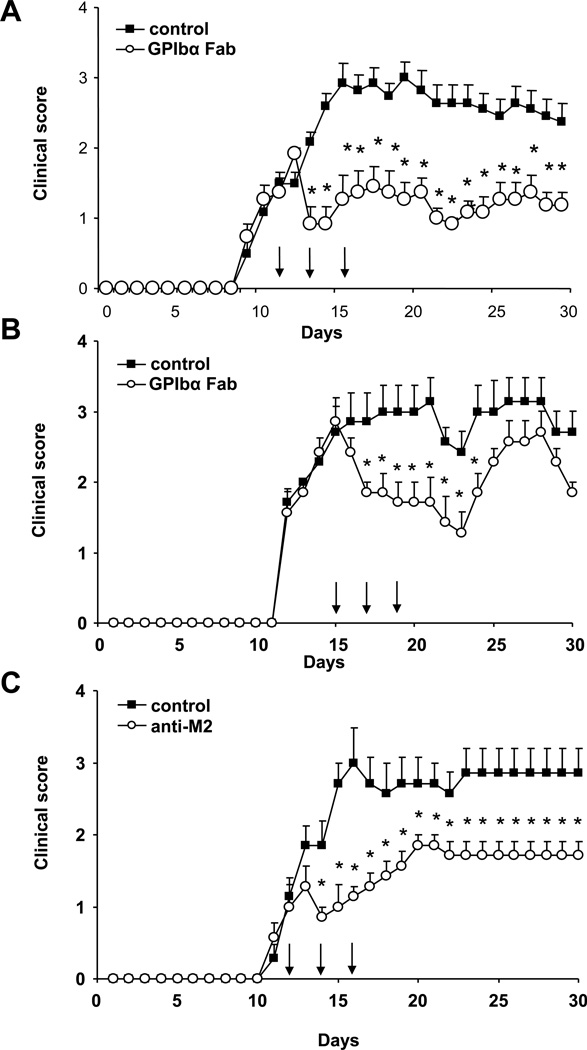
Targeting platelet GPIbα ameliorates EAE in mice
Our findings so far demonstrated that platelets are present in the inflamed CNS tissue after EAE induction and contribute to disease severity by promoting the inflammatory response including inflammatory cell recruitment to the inflamed CNS. We therefore addressed next whether targeting specific platelet receptors could represent a therapeutic approach in EAE. Platelet GPIbα is a component of the GPIb/IX/V complex that interacts with vWF mediating platelet interactions with the vessel wall and thereby platelet recruitment14, as well as contributes to inflammatory actions of platelets in parts due to its propensity to bind to leukocyte integrin Mac-1 and promote leukocyte/platelet adhesion and leukocyte recruitment.26 We therefore engaged reagents blocking GPIbα in EAE. First, we utilized a blocking Fab to GPIbα that is capable of blocking platelet-vessel wall interactions and thus platelet accumulation.38 To evaluate the therapeutic potential of GPIbα blockade in EAE, we treated mice with the blocking Fab to platelet GPIbα at the beginning of the effector phase of the disease, on days 12, 14 and 16, and found a prolonged amelioration of EAE (Figure 6A). In the human disease setting, treatment is given after diagnosis of the disease. To mimic this situation we also treated mice with the blocking Fab to platelet GPIbα on days 15, 17 and 19, i.e. after the clinical onset of disease. Strikingly, treatment of mice with the Fab to GPIbα after disease onset resulted in a transient but significant clinical recovery from EAE, as compared to Fab control treatment (Figure 6B). Second, we engaged the antibody anti-M2 that was raised against the binding site of Mac-1 for GPIbα and specifically blocks this interaction and thereby platelet-dependent inflammatory cell recruitment39–41. Anti-M2 antibody blocked the adhesion of primary bone-marrow derived macrophages42,43 to immobilized surface-adherent platelets44,45 in vitro (Online Figure V), without affecting further Mac-1-dependent adhesive interactions, e.g. to fibrinogen or ICAM-1 (Online Figure V), as previously described.39–41 Treatment with antibody anti-M2 on days 12, 14 and 16 resulted in a significant and prolonged reduction of clinical EAE symptoms as compared to control antibody (Figure 6C). When the blocking Fab to GPIbα was used in combination with the antibody anti-M2, no additive inhibitory effect was observed, indicating that both reagents interfere with the same pathway of platelet-mediated inflammation in EAE (Online Figure VI).
Figure 6. Inhibition of platelet GPIbα or its interaction with leukocyte Mac-1 ameliorates EAE in mice.
(A, B) EAE was induced in female WT mice. Mice were treated i.p. with Fab to GPIbα or control Fab (each 75µg/mouse) (A) on days 12, 14 and 16 or (B) on days 15, 17 and 19 (indicated by the arrows). Clinical disease scores are shown. Data are mean +/− SEM (n=11–12 mice per group in A; n=7 mice per group in B). * indicates p<0.05 as compared to control Fab group on the same day. (C) Similarly, mice were treated i.v. with anti-M2 antibody blocking specifically the Mac-1/GPIbα interaction or control rabbit antibody on days 12, 14 and 16 (each at 47 µg/mouse), as indicated by the arrows. Clinical scores are shown. Data are mean +/− SEM (n=7 mice per group). * indicates p<0.05 as compared to control antibody group on the same day.
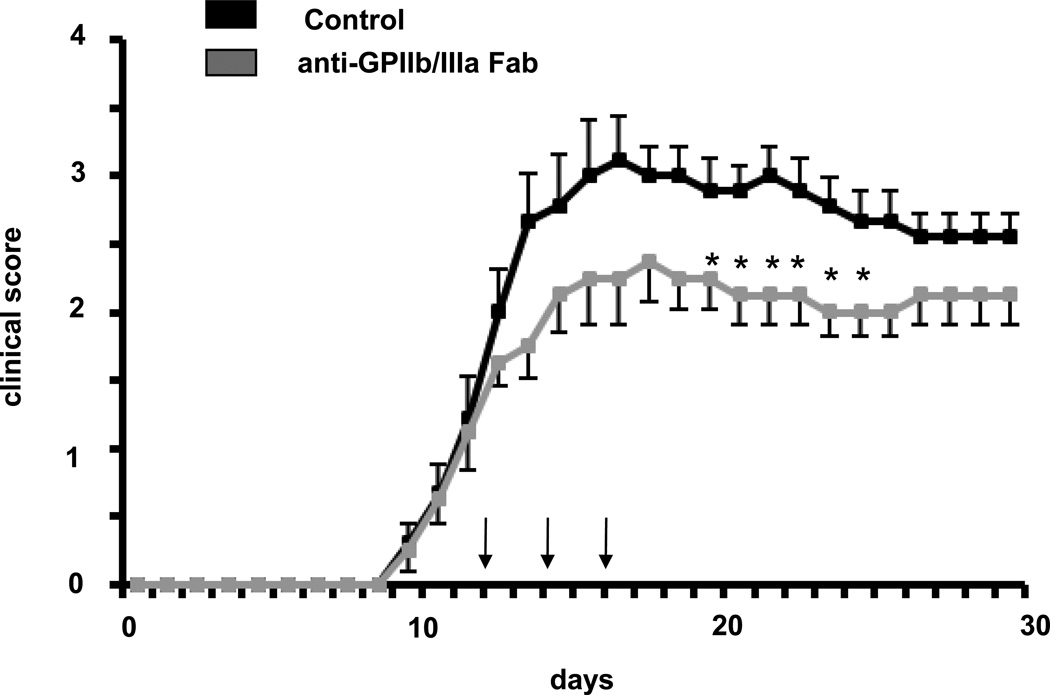
In order to assess whether the function of platelets in EAE can be solely attributed to GPIb or the GPIb–Mac-1 interaction molecularly, we engaged an inhibitor of the major platelet adhesive receptor GPIIbIIIa. Blocking Fab to GPIIbIIIa provided a significant reduction in EAE disease severity (Figure 7). Together, these data demonstrate that targeting platelet GPIbα or other platelet receptors, such as GPIIbIIIa could represent a specific therapeutic strategy for EAE treatment.
Figure 7. Inhibition of platelet GPIIbIIIa ameliorates EAE in mice.
EAE was induced and mice were treated with a blocking Fab against GPIIb/IIIa or control (each 100 µg/mouse) on day 12 i.v. and subsequently on days 14 and 16 i.p. (again 100 µg/mouse each). Data are mean +/− SEM (n=10 mice per group). * indicates p<0.05 as compared to control IgG.
Discussion
The inflammatory response is a major component of MS pathogenesis and thus an important therapeutic target. Here we demonstrated unequivocally that platelets contribute substantially to the inflammatory response and pathogenesis of EAE and provide clear evidence that targeting platelets is a novel therapeutic strategy in EAE and thereby potentially in human MS.
Previous reports have shown the message of the platelet specific receptor CD41 (αIIb–integrin, GPIIb) in chronic lesions of patients with multiple sclerosis,28 the presence of platelets in murine neuroinflammation46 as well as the relevance of platelet derived interleukin-1alpha for cerebrovascular inflammation47. A recent study has demonstrated increased levels of platelet activation in the peripheral blood of MS patients29. Consistently, we were able to demonstrate here that platelets are present in the inflamed vessels and the inflamed parenchyma of the CNS during EAE in mice; in addition, platelets were detected by immunohistology in human chronic active MS lesions.
EAE is a chronic inflammatory disease of the nervous system associated with destruction of myelin sheaths and axonal damage.48–50 The myelin sheath damage is mediated by the inflammatory response including recruited inflammatory cells, a process which is facilitated by the disruption of the BBB1. Recruited monocytes / macrophages participate in the phagocytosis of myelin and secrete proinflammatory cytokines.11 Our experiments utilizing platelet depletion in the course of EAE demonstrated unequivocally that platelets contribute substantially to the inflammatory cell recruitment and thereby to the exacerbation of EAE disease severity. Platelet depletion suppressed EAE when applied in the effector phase of the disease, whereas platelet depletion during the immunization phase did not influence EAE disease induction. These data point to a role of platelets in the inflammatory phase of EAE and in EAE disease exacerbation rather than in EAE disease induction.
Platelets may aggravate inflammation in EAE, as they are capable of enhancing monocyte adhesion to endothelial cells.51 Alternatively, platelets could locally secrete proinflammatory mediators,16 activating recruited inflammatory cells or resident microglia. As pointed out by our mRNA microarray analysis of inflamed spinal cords, the inflammatory response was blunted in the absence of platelets. Different pro-inflammatory factors including chemokines, cytokines and endothelial adhesion molecules were reduced in the spinal cords of mice that received the platelet-depleting serum. It is likely that these different pathways are interconnected with each other, which merits further investigation.
We also demonstrated that the platelet adhesion receptor GPIbα may represent a therapeutic target in EAE and MS. Platelet GPIbα mediates both platelet-vessel wall interactions and platelet accumulation, as well as platelet-dependent leukocyte recruitment. Blocking Fab to GPIbα as well as an antibody that specifically interferes with the interaction of GPIbα with leukocyte Mac-1 suppressed EAE in mice. This is in accordance with previous findings that blocking antibodies to Mac-1 ameliorated clinical severity of EAE in mice without affecting the number of sick animals52, whereas EAE development was reduced in Mac-1−/− mice53. However, the inhibitory effect on EAE mediated by blocking platelet GPIbα is not restricted to the GPIb/Mac-1-interaction. Presumably, alternative GPIb-mediated pathways, such as platelet adhesion to von Willebrand factor17;54 may participate in the function of platelets in EAE, which should be addressed in future studies. Moreover, blockade of another platelet adhesion receptor, GPIIbIIIa also reduced EAE disease severity. Thus, the contribution of platelets to EAE is probably multi-faceted. We think that strategies to interfere with platelet accumulation and platelet-leukocyte interactions, e.g. by targeting GPIbα, GPIIbIIIa, as shown here, or other platelet adhesion receptor systems, such as the interaction between P-selectin and its ligand on leukocytes, P-selectin glycoprotein ligand-1, might represent therapeutic approaches in EAE and MS. Besides the here engaged model for primary progressive EAE disease, which reflects a disease subtype of human MS, there is also a relapsing-remitting mouse model,55 representing a more common MS subtype. The effect of platelets should be studied in relapsing-remitting EAE in future investigations to further substantiate the therapeutic potential of platelet-targeting therapies in MS.
Finally, a recent study demonstrated the crosstalk between coagulation and brain inflammation. In this study a proteomic approach identified tissue factor and protein C inhibitor within chronic active lesions and thrombin inhibition was used to reduce EAE severity.56 Our present data underline this connection between coagulation, platelets and neuro-inflammation and suggest that targeting platelets and their receptors may represent a novel attractive therapeutic approach to ameliorate the inflammatory response in multiple sclerosis.
Supplementary Material
Novelty and Significance.
What is known?
mRNA of platelet-specific genes is upregulated in human chronic multiple sclerosis (MS) lesions
Platelets in peripheral blood of patients with MS are in an activated state
What new information does this article contribute?
Platelets accumulate in inflamed lesions of the CNS in the course of experimental autoimmune encephalomyelitis (EAE) in mice, a mouse model for human MS
Platelets significantly contribute to the inflammatory response in the effector phase of EAE
Platelet depletion or targeting platelet adhesion receptors, such as GPIbα ameliorates EAE disease severity
MS is a devastating disease associated with severe impairment and high mortality. MS and its mouse model EAE are characterized by the infiltration of inflammatory cells into the CNS mediating tissue damage. Emerging evidence suggests that platelets may be critical contributors of inflammatory cell recruitment. However, little was known about the functional role of platelets in EAE and MS. Here, we present evidence supporting the notion that platelets contribute to the inflammatory response and disease severity of EAE. We demonstrate that platelets are present in the inflamed spinal cord tissue in the course of EAE, as well as in human MS lesions. Platelets play a crucial role for leukocyte recruitment to the inflamed CNS. Platelet depletion ameliorated EAE in mice and blunted inflammatory response, whereas the induction of the disease was not affected. Interfering with platelet GPIbα or the Mac-1/GPIbα-interaction attenuated EAE in mice. Therefore platelets
Acknowledgements
We thank the cooperative Human Tissue Network (CHTN) of the University of Pennsylvania for providing us with normal human brain tissue. The authors would like to acknowledge D. Winkler, J. Hammer and G. Sanchez-Howard for help with mouse work, Dr. F. Bosetti for help with immunohistochemistry, and Dr. R. Hodes and Dr. D.S. Singer for critically reading the manuscript.
Sources of Funding
This research was supported by the Intramural Research Program of the NIH, National Cancer Institute (TC), and National Institute on Aging (KGB), the NIH grants HL57506, HL085816 and HL073852 (DIS), the National Multiple Sclerosis Society grant RG3411B4/1 (JWR) and the Novartis Foundation for Therapeutic Research (TC). HFL is supported by the IZKF program of the University of Tübingen (1868-0-0), the Tuebingen Platelet Investigative Consortium (TuePIC) funded by the Clinical Research Unit (KFO 274) of the German Research Foundation and the Volkswagen Foundation (Lichtenberg program).
Non-standard Abbreviations and Acronyms
- BBB
blood brain barrier
- CD
cluster of differentiation
- CNS
central nervous system
- EAE
experimental autoimmune encephalomyelitis
- GP
glycoprotein
- ICAM-1
intercellular adhesion molecule-1
- Mac-1
Macrophage-1 antigen
- MOG
myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- NF-200
neurofilament-200
- TNF-alpha
tumor necrosis factor alpha
- vWF
von Willenbrand Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
DIS and YW are co-inventors of small molecule inhibitors targeting the Mac-1/GPIbα interaction.
Reference list
- 1.Steinman L. Elaborate interactions between the immune and nervous systems. Nat Immunol. 2004;5:575–581. doi: 10.1038/ni1078. [DOI] [PubMed] [Google Scholar]
- 2.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 3.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Miller SD, Turley DM, Podojil JR. Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol. 2007;7:665–677. doi: 10.1038/nri2153. [DOI] [PubMed] [Google Scholar]
- 5.Weiner HL, Selkoe DJ. Inflammation and therapeutic vaccination in CNS diseases. Nature. 2002;420:879–884. doi: 10.1038/nature01325. [DOI] [PubMed] [Google Scholar]
- 6.Bettelli E, Pagany M, Weiner HL, Linington C, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J Exp Med. 2003;197:1073–1081. doi: 10.1084/jem.20021603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McFarland HF, Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 8.Yan SS, Wu ZY, Zhang HP, Furtado G, Chen X, Yan SF, Schmidt AM, Brown C, Stern A, LaFaille J, Chess L, Stern DM, Jiang H. Suppression of experimental autoimmune encephalomyelitis by selective blockade of encephalitogenic T-cell infiltration of the central nervous system. Nat Med. 2003;9:287–293. doi: 10.1038/nm831. [DOI] [PubMed] [Google Scholar]
- 9.Ransohoff RM. Natalizumab for multiple sclerosis. N Engl J Med. 2007;356:2622–2629. doi: 10.1056/NEJMct071462. [DOI] [PubMed] [Google Scholar]
- 10.Weber MS, Prod'homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 11.Heppner FL, Greter M, Marino D, Falsig J, Raivich G, Hovelmeyer N, Waisman A, Rulicke T, Prinz M, Priller J, Becher B, Aguzzi A. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat Med. 2005;11:146–152. doi: 10.1038/nm1177. [DOI] [PubMed] [Google Scholar]
- 12.Hu X, Wohler JE, Dugger KJ, Barnum SR. beta2-integrins in demyelinating disease: not adhering to the paradigm. J Leukoc Biol. 2010;87:397–403. doi: 10.1189/jlb.1009654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xie C, Alcaide P, Geisbrecht BV, Schneider D, Herrmann M, Preissner KT, Luscinskas FW, Chavakis T. Suppression of experimental autoimmune encephalomyelitis by extracellular adherence protein of Staphylococcus aureus. J Exp Med. 2006;203:985–994. doi: 10.1084/jem.20051681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, len-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 16.Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 18.Langer HF, Gawaz M. Platelet-vessel wall interactions in atherosclerotic disease. Thromb Haemost. 2008;99:480–486. doi: 10.1160/TH07-11-0685. [DOI] [PubMed] [Google Scholar]
- 19.Langer HF, Daub K, Braun G, Schonberger T, May AE, Schaller M, Stein GM, Stellos K, Bueltmann A, Siegel-Axel D, Wendel HP, Aebert H, Roecken M, Seizer P, Santoso S, Wesselborg S, Brossart P, Gawaz M. Platelets recruit human dendritic cells via Mac-1/JAM-C interaction and modulate dendritic cell function in vitro. Arterioscler Thromb Vasc Biol. 2007;27:1463–1470. doi: 10.1161/ATVBAHA.107.141515. [DOI] [PubMed] [Google Scholar]
- 20.Wegmann F, Petri B, Khandoga AG, Moser C, Khandoga A, Volkery S, Li H, Nasdala I, Brandau O, Fassler R, Butz S, Krombach F, Vestweber D. ESAM supports neutrophil extravasation, activation of Rho, and VEGF-induced vascular permeability. J Exp Med. 2006;203:1671–1677. doi: 10.1084/jem.20060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein E. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 22.Zarbock A, Singbartl K, Ley K. Complete reversal of acid-induced acute lung injury by blocking of platelet-neutrophil aggregation. J Clin Invest. 2006;116:3211–3219. doi: 10.1172/JCI29499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hidalgo A, Chang J, Jang JE, Peired AJ, Chiang EY, Frenette PS. Heterotypic interactions enabled by polarized neutrophil microdomains mediate thromboinflammatory injury. Nat Med. 2009;15:384–391. doi: 10.1038/nm.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvalho-Tavares J, Hickey MJ, Hutchison J, Michaud J, Sutcliffe IT, Kubes P. A role for platelets and endothelial selectins in tumor necrosis factor-alpha-induced leukocyte recruitment in the brain microvasculature. Circ Res. 2000;87:1141–1148. doi: 10.1161/01.res.87.12.1141. [DOI] [PubMed] [Google Scholar]
- 25.Santoso S, Sachs UJ, Kroll H, Linder M, Ruf A, Preissner KT, Chavakis T. The junctional adhesion molecule 3 (JAM-3) on human platelets is a counterreceptor for the leukocyte integrin Mac-1. J Exp Med. 2002;196:679–691. doi: 10.1084/jem.20020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Simon DI, Chen Z, Xu H, Li CQ, Dong J, McIntire LV, Ballantyne CM, Zhang L, Furman MI, Berndt MC, Lopez JA. Platelet glycoprotein ibalpha is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18) J Exp Med. 2000;192:193–204. doi: 10.1084/jem.192.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chavakis T, Santoso S, Clemetson KJ, Sachs UJ, Isordia-Salas I, Pixley RA, Nawroth PP, Colman RW, Preissner KT. High molecular weight kininogen regulates platelet-leukocyte interactions by bridging Mac-1 and glycoprotein Ib. J Biol Chem. 2003;278:45375–45381. doi: 10.1074/jbc.M304344200. [DOI] [PubMed] [Google Scholar]
- 28.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 29.Sheremata WA, Jy W, Horstman LL, Ahn YS, Alexander JS, Minagar A. Evidence of platelet activation in multiple sclerosis. J Neuroinflammation. 2008;5:27. doi: 10.1186/1742-2094-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rose JW, Hill KE, Watt HE, Carlson NG. Inflammatory cell expression of cyclooxygenase-2 in the multiple sclerosis lesion. J Neuroimmunol. 2004;149:40–49. doi: 10.1016/j.jneuroim.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 31.Hill KE, Zollinger LV, Watt HE, Carlson NG, Rose JW. Inducible nitric oxide synthase in chronic active multiple sclerosis plaques: distribution, cellular expression and association with myelin damage. J Neuroimmunol. 2004;151:171–179. doi: 10.1016/j.jneuroim.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 32.Lassmann H, Raine CS, Antel J, Prineas JW. Immunopathology of multiple sclerosis: report on an international meeting held at the Institute of Neurology of the University of Vienna. J Neuroimmunol. 1998;86:213–217. doi: 10.1016/s0165-5728(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 33.Lonsdorf AS, Kramer BF, Fahrleitner M, Schonberger T, Gnerlich S, Ring S, Gehring S, Schneider SW, Kruhlak MJ, Meuth SG, Nieswandt B, Gawaz M, Enk AH, Langer HF. Engagement of alphaIIbbeta3 (GPIIb/IIIa) with alphanubeta3 Integrin Mediates Interaction of Melanoma Cells with Platelets: A CONNECTION TO HEMATOGENOUS METASTASIS. J Biol Chem. 2012;287:2168–2178. doi: 10.1074/jbc.M111.269811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becher B, Durell BG, Noelle RJ. IL-23 produced by CNS-resident cells controls T cell encephalitogenicity during the effector phase of experimental autoimmune encephalomyelitis. J Clin Invest. 2003;112:1186–1191. doi: 10.1172/JCI19079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ponomarev ED, Shriver LP, Maresz K, Dittel BN. Microglial cell activation and proliferation precedes the onset of CNS autoimmunity. J Neurosci Res. 2005;81:374–389. doi: 10.1002/jnr.20488. [DOI] [PubMed] [Google Scholar]
- 36.Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- 37.Kerfoot SM, Kubes P. Overlapping roles of P-selectin and alpha 4 integrin to recruit leukocytes to the central nervous system in experimental autoimmune encephalomyelitis. J Immunol. 2002;169:1000–1006. doi: 10.4049/jimmunol.169.2.1000. [DOI] [PubMed] [Google Scholar]
- 38.Kleinschnitz C, Pozgajova M, Pham M, Bendszus M, Nieswandt B, Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115:2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 39.Ehlers R, Ustinov V, Chen Z, Zhang X, Rao R, Luscinskas FW, Lopez J, Plow E, Simon DI. Targeting platelet-leukocyte interactions: identification of the integrin Mac-1 binding site for the platelet counter receptor glycoprotein Ibalpha. J Exp Med. 2003;198:1077–1088. doi: 10.1084/jem.20022181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y, Sakuma M, Chen Z, Ustinov V, Shi C, Croce K, Zago AC, Lopez J, Andre P, Plow E, Simon DI. Leukocyte engagement of platelet glycoprotein Ibalpha via the integrin Mac-1 is critical for the biological response to vascular injury. Circulation. 2005;112:2993–3000. doi: 10.1161/CIRCULATIONAHA.105.571315. [DOI] [PubMed] [Google Scholar]
- 41.Pluskota E, Woody NM, Szpak D, Ballantyne CM, Soloviev DA, Simon DI, Plow EF. Expression, activation, and function of integrin alphaMbeta2 (Mac-1) on neutrophil-derived microparticles. Blood. 2008;112:2327–2335. doi: 10.1182/blood-2007-12-127183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi EY, Chavakis E, Czabanka MA, Langer HF, Fraemohs L, Economopoulou M, Kundu RK, Orlandi A, Zheng YY, Prieto DA, Ballantyne CM, Constant SL, Aird WC, Papayannopoulou T, Gahmberg CG, Udey MC, Vajkoczy P, Quertermous T, Dimmeler S, Weber C, Chavakis T. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322:1101–1104. doi: 10.1126/science.1165218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi EY, Orlova VV, Fagerholm SC, Nurmi SM, Zhang L, Ballantyne CM, Gahmberg CG, Chavakis T. Regulation of LFA-1-dependent inflammatory cell recruitment by Cbl-b and 14-3-3 proteins. Blood. 2008;111:3607–3614. doi: 10.1182/blood-2007-07-103077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langer H, May AE, Daub K, Heinzmann U, Lang P, Schumm M, Vestweber D, Massberg S, Schonberger T, Pfisterer I, Hatzopoulos AK, Gawaz M. Adherent platelets recruit and induce differentiation of murine embryonic endothelial progenitor cells to mature endothelial cells in vitro. Circ Res. 2006;98:e2–e10. doi: 10.1161/01.RES.0000201285.87524.9e. [DOI] [PubMed] [Google Scholar]
- 45.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, Hoppe K, Schiemann M, Kennerknecht E, Sauer S, Schulz C, Kerstan S, Rudelius M, Seidl S, Sorge F, Langer H, Peluso M, Goyal P, Vestweber D, Emambokus NR, Busch DH, Frampton J, Gawaz M. Platelets secrete stromal cell-derived factor 1{alpha} and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–1233. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doring A, Wild M, Vestweber D, Deutsch U, Engelhardt B. E- and P-selectin are not required for the development of experimental autoimmune encephalomyelitis in C57BL/6 and SJL mice. J Immunol. 2007;179:8470–8479. doi: 10.4049/jimmunol.179.12.8470. [DOI] [PubMed] [Google Scholar]
- 47.Thornton P, McColl BW, Greenhalgh A, Denes A, Allan SM, Rothwell NJ. Platelet interleukin-1alpha drives cerebrovascular inflammation. Blood. 2010;115:3632–3639. doi: 10.1182/blood-2009-11-252643. [DOI] [PubMed] [Google Scholar]
- 48.Basso AS, Frenkel D, Quintana FJ, Costa-Pinto FA, Petrovic-Stojkovic S, Puckett L, Monsonego A, Bar-Shir A, Engel Y, Gozin M, Weiner HL. Reversal of axonal loss and disability in a mouse model of progressive multiple sclerosis. J Clin Invest. 2008;118:1532–1543. doi: 10.1172/JCI33464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 50.O'Connor KC, McLaughlin KA, De Jager PL, Chitnis T, Bettelli E, Xu C, Robinson WH, Cherry SV, Bar-Or A, Banwell B, Fukaura H, Fukazawa T, Tenembaum S, Wong SJ, Tavakoli NP, Idrissova Z, Viglietta V, Rostasy K, Pohl D, Dale RC, Freedman M, Steinman L, Buckle GJ, Kuchroo VK, Hafler DA, Wucherpfennig KW. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 52.Brocke S, Piercy C, Steinman L, Weissman IL, Veromaa T. Antibodies to CD44 and integrin alpha4, but not L-selectin, prevent central nervous system inflammation and experimental encephalomyelitis by blocking secondary leukocyte recruitment. Proc Natl Acad Sci U S A. 1999;96:6896–6901. doi: 10.1073/pnas.96.12.6896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bullard DC, Hu X, Schoeb TR, Axtell RC, Raman C, Barnum SR. Critical requirement of CD11b (Mac-1) on T cells and accessory cells for development of experimental autoimmune encephalomyelitis. J Immunol. 2005;175:6327–6333. doi: 10.4049/jimmunol.175.10.6327. [DOI] [PubMed] [Google Scholar]
- 54.Savage B, Saldivar E, Ruggeri ZM. Initiation of platelet adhesion by arrest onto fibrinogen or translocation on von Willebrand factor. Cell. 1996;84:289–297. doi: 10.1016/s0092-8674(00)80983-6. [DOI] [PubMed] [Google Scholar]
- 55.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Han MH, Hwang SI, Roy DB, Lundgren DH, Price JV, Ousman SS, Fernald GH, Gerlitz B, Robinson WH, Baranzini SE, Grinnell BW, Raine CS, Sobel RA, Han DK, Steinman L. Proteomic analysis of active multiple sclerosis lesions reveals therapeutic targets. Nature. 2008;451:1076–1081. doi: 10.1038/nature06559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.