Abstract
Methylmalonic acidemia (MMA), an inherited metabolic disorder caused by deficient activity of methylmalonyl-CoA mutase (MUT), carries a poor prognosis for long-term survival. While administration of a recombinant adeno-associated virus serotype 8 vector (rAAV8) can rescue Mut-/- mice from neonatal lethality and provide sustained phenotypic correction, translation of gene therapy to human subjects will likely require multiple rounds of systemic administration and ideally, the use of a vector that transduces the kidney. To examine the effectiveness of alternative rAAVs in the treatment of MMA, a serotype 9 rAAV expressing the Mut cDNA was constructed and delivered to newborn Mut-/- mice (n=11). rAAV9 gene therapy directed hepatic transgene expression within 24 hours and effectively rescued the Mut-/- mice from lethality, conferred long-term survival, markedly improved metabolism and resulted in striking preservation of renal function and histology. Systemic re-administration of the vector at a dose similar to that used in human clinical trials (2.5×109 GC rAAV9 per gram) to older, treated Mut-/- mice (n=5) lowered circulating metabolites, increased in vivo propionate oxidative capacity and produced transgene expression in the kidney and liver. Our data support the use of an rAAV9 vector in the acute and chronic treatment of MMA, and highlight the renotropism afforded by this novel serotype.
Introduction
Methylmalonic acidemia (MMA) is an autosomal recessive inborn error of organic acid metabolism characterized by severe metabolic instability, multiorgan pathology, and a poor prognosis for long-term survival [1-7]. This disorder is most commonly caused by deficient activity of methylmalonyl-CoA mutase (MUT) [1], the 5′-deoxyadenosylcobalamin-dependent enzyme that isomerizes L-methylmalonyl-CoA into succinyl-CoA in the mitochondrial inner space.
Current management approaches for vitamin B12 (hydroxocobalamin) non-responsive MMA patients include dietary restriction of propiogenic amino acids, nutritional supplement administration and vigilant monitoring. Liver or combined liver/kidney transplantation have been used to treat those with the most severe clinical manifestations [8-17]. Patients with MMA, even those who have received liver transplants, can develop progressive renal dysfunction [11], pathologically characterized by tubulointerstitial nephritis [18, 19], and eventually require kidney transplantation [20]. Whether circulating metabolite(s) and/or a cell intrinsic defect underlies the renal pathology of MMA remains unknown [21]. The disease manifestations seen in the patient population, even those who have been intensively treated, demonstrate the need for new therapies, ideally ones that could target both the liver and kidney, to increase stability and protect from renal insufficiency.
We have previously demonstrated that a recombinant adeno-associated virus serotype 8 (rAAV8) vector expressing the murine Mut gene under the control of the cytomegalovirus enhancer/chicken β-actin promoter could rescue newborn Mut-/- mice from neonatal lethality and provide long-term phenotypic correction [22]. Although neonatal rAAV8 gene therapy effectively targeted numerous tissues in Mut-/- mice, particularly the liver and skeletal muscle, transgene expression was not detectable in the kidney and decreased in other tissues over time. This suggests that translation to human subjects would likely require multiple rounds of gene delivery throughout life. Alternative rAAV vectors that could provide enhanced renal tropism with the potential to be administered in the setting of an immune response to rAAV8 may be required for effective gene therapy application in humans.
AAV serotypes are numerous, exhibit distinct tissue tropism, and offer the potential for optimization of gene delivery to specific target tissues and/or cell types [23, 24]. Among the naturally occurring adeno-associated viruses isolated from simian and human tissues, serotype 9 has recently been recognized as superior in the ability to direct hepatic and renal gene transfer in a mouse model of renal tubulointerstitial fibrosis [25]. These properties, coupled with previous studies showing that rAAV9 vectors exhibit rapid and widespread transgene expression in mice and larger animals [26-28], suggest that a serotype 9 rAAV vector would be promising in gene therapy applications for MMA [25-31]. To explore the use of this novel serotype in the treatment of mice with methylmalonic acidemia, we developed and characterized an AAV serotype 9 vector, rAAV9-CBA-mMut, to express Mut from the enhanced, chicken β-actin promoter [32]. Intrahepatic delivery of rAAV9-CBA-mMut effectively rescued Mut-/- mice from neonatal lethality, provided immediate and persistent expression of the transgene, and after low-dose systemic re-injection at 1 year, directed expression of the transgene in the liver and kidney. These studies highlight the novel characteristics of rAAV9 mediated gene delivery as a treatment for MMA, particularly rapid transgene expression in vivo and tropism for the kidney, an organ involved in the pathophysiology of many hereditary and acquired disorders.
Results
Gene therapy with low dose AAV9 rescues the lethal Mut-/- phenotype
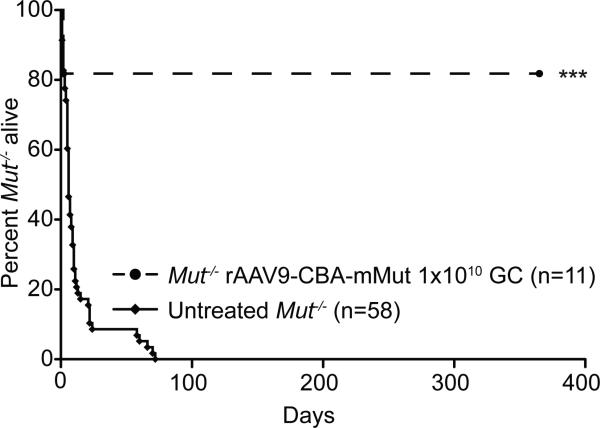
Mut-/- mice received a single dose of 1×1010 vector genome copies of rAAV9-CBA-mMut by intrahepatic injection at birth (n=11). While two treated Mut-/- mice died within the first 48 hours, 82% of the mice that received rAAV9-CBA-mMut gene therapy survived 1 year post treatment (Figure 1). All of the untreated Mut-/- mice died by day of life (DOL) 72, with more than 90% mortality occurring by DOL 24 (Figure 1). In contrast, at 1 year, 89% of the injected heterozygous control littermates were alive. Deceased mice were autolysed, cannibalized or entirely missing from their cages.
Figure 1.
Survival of Mut-/- mice after rAAV9-CBA-mMut gene therapy treatment. Survival is shown in days between untreated Mut-/- mice (n=58) and Mut-/- mice treated with a single intrahepatic dose of 1×1010 GC of rAAV9-CBA-mMut (n=11)., 82% (n=9) of treated Mut-/- mice were alive at day of life (DOL) 365. All untreated Mut-/- mice perished by DOL 72, with the majority of deaths occurring before DOL 24. *** rAAV9-CBA-mMut treated Mut-/- mice survive significantly longer than untreated Mut-/- mice (p<0.001).
rAAV9 gene therapy vector leads to rapid and high level expression of the mutase transgene
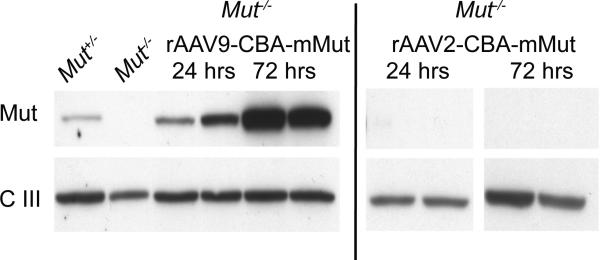
To examine the expression kinetics of the viral transgene in the immediate peri-injection period, livers from treated newborn Mut-/- mice were collected at 24 and 72 hours after treatment with either 1×1010 GC of rAAV9-CBA-mMut or rAAV2-CBA-mMut, a serotype that has well-studied uncoating properties in the murine liver [33]. Untreated Mut+/- and Mut-/- littermate livers were harvested in parallel and served as controls. There was no detectable level of immunoreactive mutase enzyme in untreated Mut-/- mice (Figure 2). However, as early as 24 hours after injection, rAAV9-CBA-mMut treated Mut-/- mice demonstrated hepatic Mut protein at levels similar to those seen in untreated Mut+/- animals (Figure 2). By 72 hours, Mut expression continued to increase in the rAAV9-CBA-mMut treated Mut-/- mice (Figure 2). In contrast, Mut was absent in Mut-/- mice similarly treated with rAAV2-CBA-mMut (Figure 2) at both times, demonstrating the influence of the serotype and tropism on early transgene expression in the liver.
Figure 2.
Mut expression in liver tissues 1 and 3 days after neonatal intrahepatic injection of 1×1010 GC rAAV9-CBA-mMut or rAAV2-CBA-mMut. Immunoreactive Mut enzyme (labeled Mut) is present in the untreated Mut+/- extract and in Mut-/- mice that were treated with rAAV9-CBA-mMut, but not in the untreated Mut-/- mouse or Mut-/- mice that received rAAV2-CBA-mMut. The mitochondrial loading control (labeled complex III) shows approximately the same intensity in each sample.
Growth improvement after gene therapy
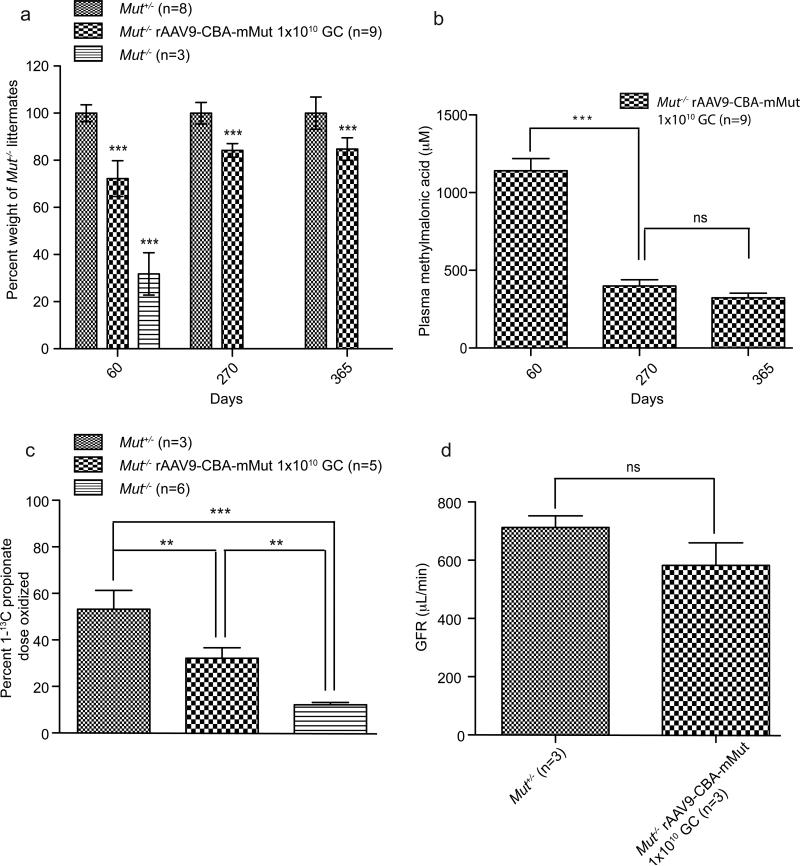
Although untreated Mut-/- and Mut+/- mice are indistinguishable at birth, the rare Mut-/- animals that survive neonatal lethality present signs of severe growth retardation [34]. By 2 months of age, untreated Mut-/- mice weigh less than one third as much as sex-matched Mut+/- littermates, appear grossly abnormal, and uniformly perish by 72 days [22]. In contrast, Mut-/- mice treated with rAAV9-CBA-mMut were significantly larger than untreated Mut-/- mice (Figure 3a) (*** p< 0.001). They appeared and behaved in an identical fashion as treated control littermates, but were slightly smaller than heterozygous littermates, even after vector readministration (Supplemental Figure 1). Furthermore, treated Mut-/- mice thrive on a regular diet, unlike untreated Mut-/- mice that have to be maintained on a high fat and carbohydrate diet.
Figure 3.
Growth and metabolic effects after rAAV9 gene therapy. (a) Percent weight at DOL 60, 270 and 365 between Mut+/- diet and gender matched littermates (n=8,9) compared to untreated Mut-/- mice on DOL 60 (n=3) or Mut-/- mice treated via an intrahepatic injection of rAAV9-CBA-mMut 1×1010 GC at birth (n=9) is depicted. The rAAV9-CBA-mMut treated Mut-/- mice showed significant growth improvement compared to the untreated Mut-/- mice (*** p< 0.001) but were smaller than Mut+/- diet and gender-matched littermates. Error bars represent plus and minus one standard deviation. (b) Plasma methylmalonic acid levels (μM) were measured at DOL of 60, 270 and 365 in the rAAV9-CBA-mMut treated Mut-/- mice (n=9) as a reflection of Mut activity. Error bars represent plus and minus one standard deviation. The rAAV9-CBA-mMut treated mutant mice have increased plasma methylmalonic acid concentrations that are reduced on DOL 270 (399 μM (+/- 40 μM)) and 365 (323 μM (+/- 30 μM)) compared to the values at DOL 60 (1196 μM (+/- 78 μM)). Untreated and treated Mut+/- mice have plasma methylmalonic acid levels between 5-10 μM and are not depicted in this graph. (*** p< 0.001; p=0.2, ns) Error bars represent plus and minus one standard deviation. All untreated Mut-/- mice perished by DOL 72. (c) 1-13C-propionate oxidation 1 year after rAAV9-CBA-mMut treatment. 200 μg of 1-13C-sodium propionate was injected IP into Mut+/- (n=3), 1 × 1010 GC rAAV9-CBA-mMut treated Mut-/- (n=5) or untreated Mut-/- (n=6) mice. 13C enrichment in expired CO2 was measured and used to determine the percent of the administered 1-13C-propionate dose that was oxidized. Error bars surround the ninety five percent confidence intervals. The rAAV9-CBA-mMut treated Mut-/- mice show a significant increase in the ability to oxidize 1-13C-propionate compared to the untreated Mut-/- mice at 25 minutes post-injection (** p< 0.01). (d) Glomelular filtration rate measured by FITC-inulin clearance at 1 year of life between rAAV9-CBA-mMut treated Mut+/- (n=3) and treated Mut-/- mice (n=3). There is no significant difference between treated Mut-/- mice and heterozygous littermates. Error bars represent plus and minus one standard deviation.
Restoration of Mut function and activity
In order to study the efficacy of gene therapy, we analyzed various parameters that reflect the Mut enzymatic activity and function, including metabolite concentrations and ability of the treated Mut-/- mice to oxidize 1-13C-sodium propionate into 13CO2. Compared to controls, plasma MMA levels remained significantly elevated at all times in the treated Mut-/- mice, and stabilized at 323 μmol/L (+/- 30 μmol/L) by 1 year of life (Figure 3b).
Mice with functional hepatic Mut activity can oxidize 1-13C-sodium propionate into 13CO2 [22]. This activity is markedly reduced in untreated Mut-/- mice (Figure 3c). At 9 months, rAAV9-CBA-mMut treated Mut-/- mice and heterozygous littermates were injected with 1-13C-sodium propionate and breath samples were collected while the CO2 production rate was measured. Mut+/- mice convert ~50% of 1-13C-sodium propionate into 13CO2 in 25 minutes (n=3) while untreated Mut-/- mice (n=6) oxidize only ~10% of the injected dose (Figure 3c). At 9 months post treatment, 1-13C-propionate oxidation was significantly increased above the untreated state in the rAAV9-CBA-mMut treated Mut-/- mice (n=5) (Figure 3c), demonstrating that the Mut transgene was persistently expressed and functional in vivo.
rAAV9 gene therapy preserves the glomerular filtration rate and kidney cytoarchitecture
Because human MMA patients suffer from kidney failure and previous studies have established that older, untreated Mut-/- mice exhibit severe and widespread tubulointerstitial changes[35], we examined the effect of gene therapy on kidney function and histology in the rAAV9-CBA-mMut treated Mut-/- mice. The measured glomerular filtration rate (GFR) did not significantly differ between the rAAV9-CBA-mMut treated Mut-/- (n=3) and Mut+/- mice (n=3) (Figure 3d). Because the untreated Mut-/- littermates perish, GFR measurements values for untreated, matched mutant controls are not available for comparison.
Kidney histology was next examined in rAAV9-CBA-mMut treated Mut-/- mice (n=6) and compared to Mut+/- littermates (n=3). Consistent with the GFR measurements, renal histology in the rAAV9-CBA-mMut treated Mut-/- mice was unremarkable, without characteristic tubulointerstitial changes, and appeared identical to the rAAV9-CBA-mMut treated Mut+/- control mice (Supplemental Figure 2).
Low dose systemic reinjection of rAAV9-CBA-mMut at 1 year results in metabolic improvements and transgene expression in the kidney
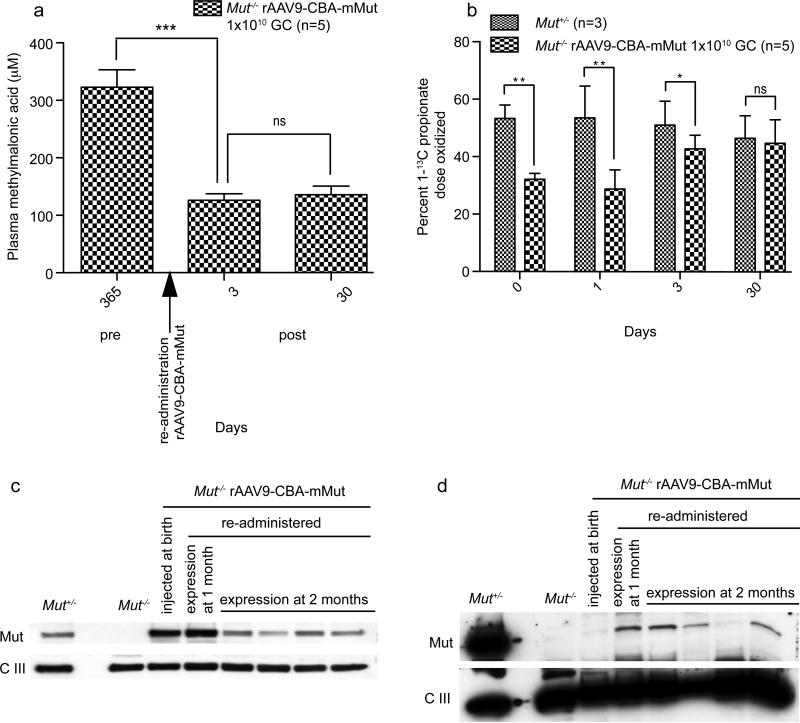
One year after neonatal injection, a set of treated rAAV9-CBA-mMut Mut-/- mice (n=5) were reinjected with a low dose of rAAV9-CBA-mMut (2.5×109 GC/gram) delivered by the retro-orbital route. Measuring the concentration of methylmalonic acid in the plasma and whole-body 1-13C-sodium propionate oxidative capacity monitored the metabolic effects of reinjection. Within 3 days after readministration of rAAV9-CBA-mMut, the plasma methylmalonic acid concentrations significantly decreased to 126 μM (+/- 11 μM) (p<0.001) compared to pre-treatment levels of 323 μM (+/- 30 μM) (Figure 4a) and 1-13C-sodium propionate oxidative capacity increased (Figure 4b). The extent of metabolic improvement remained stable 1 month after reinjection, the plasma methylmalonic acid was unchanged (Figure 4a) and 1-13C-sodium propionate oxidation was equivalent between the re-injected rAAV9-CBA-mMut Mut-/- mice (n=5) and heterozygote controls (n=3) (Figure 4b).
Figure 4.
Metabolic improvement after systemic rAAV9-CBA-mMut readministration. 2.5 × 109 GC rAAV9-CBA-mMut per gram body weight was delivered by retro-orbital injection to Mut+/- (n=3) and Mut-/- mice (n=5) that had previously received rAAV9-CBA-mMut by intrahepatic delivery on DOL 1. (a) Methylmalonic acid concentrations in the rAAV9-CBA-mMut treated Mut-/-mice on DOL 365 were 323 μM (+/- 30 μM) (n=5) and dropped to 126 μM (+/- 11 μM) (n=5) 3 days after readministration of rAAV9-CBA-mMut, and remained at approximately the same level 30 days later (136 μM (+/- 16 μM), n=5). Error bars represent plus and minus one standard deviation. (*** p< 0.001; p= 0.6, ns) (b) 1-13C-propionate oxidation of treated rAAV9-CBA-mMut Mut-/- mice (n=5) and Mut+/- littermates (n=3) before and at 1, 3, and 30 days after systemic re-administration of rAAV9-CBA-mMut. 13C enrichment in expired CO2 was measured and used to determine the percent of the administered 1-13C-propionate dose that was oxidized. Error bars surround the ninety five percent confidence intervals. The rAAV9-CBA-mMut re-treated Mut-/- mice had diminished oxidative capacity at 25 minutes on day 1 after re-injection compared to treated heterozygous controls (**p< 0.01) that increased to heterozygote levels by day 30 (p=0.3, ns) (c) Mut expression in liver extracts prepared from Mut+/- and Mut-/- mice after systemic re-administration of rAAV9-CBA-mMut. 30 μg of clarified whole liver extracts were analyzed for transgene expression by western blotting. The same membranes were probed with either anti-methylmalonyl-CoA mutase antibody (labeled Mut) or an anti-ubiquinol-cytochrome c oxidoreductase antibody (labeled complex III) to control for loading and mitochondrial content. Immunoreactive Mut enzyme is present in the untreated Mut+/- extract, and in Mut-/- mouse that was treated with rAAV9-CBA-mMut 1 year after neonatal injection (lane 4), 1 month after re-injection (lane 5) and 2 months after reinjection (lanes 6 through 9). The mitochondrial loading control shows approximately the same intensity in each sample. (d) Mut expression in kidney extracts prepared from Mut+/- and Mut-/- mice after systemic re-administration of rAAV9-CBA-mMut at 1 year of life. The animals are the same group studied in (c). 30 μg of clarified whole kidney extracts were analyzed for transgene expression by western blotting in exactly the same fashion as for the liver extracts. Immunoreactive Mut enzyme is present in the untreated Mut+/- extract, but not in an untreated Mut-/- animal (lane 3) or Mut-/- mouse that was studied 1 year after receiving a single neonatal injection of rAAV9-CBA-mMut (lane 4). However, in the Mut-/- mice that received a second injection of rAAV9-CBA-mMut 1 year after birth, immunoreactive enzyme was detected 1 month (lane 5) and 2 months (lanes 6, 7 and 9) after retreatment. The mitochondrial loading control shows approximately the same intensity in each sample.
Expression of Mut after reinjection with rAAV9-CBA-mMut
A set of rAAV9-CBA-mMut Mut-/- mice (n=2) were sacrificed 1 month after reinjection to examine transgene expression compared to a rAAV9-CBA-mMut Mut-/- mouse injected at birth and sacrificed at 1 year of age. After 1 year, the level of immunoreactive Mut in the liver of a rAAV9-CBA-mMut treated Mut-/- mouse was similar to that seen in an untreated Mut+/- control (Figure 4c) and showed persistent expression at 1 and 2 months after reinjection with rAAV9-CBA-mMut (Figure 4c). Mut was not present in a kidney extract prepared from a Mut-/- mouse that was treated only with a single neonatal injection of rAAV9-CBA-mMut, but was readily detected 1 month after systemic reinjection (Figure 4d). Further analysis using qPCR confirmed that Mut mRNA was present in the kidney of the re-treated rAAV9-CBA-mMut Mut-/- mice, but at levels well below those measured in heterozygous littermates (data not shown). Western analysis using liver and kidney extracts from re-injected rAAV9-CBA-mMut Mut-/- mice were also examined 2 months after reinjection and yielded similar results to those seen at the earlier 1 month time point. This supports the observation that there is Mut expression in the kidney following systemic gene therapy with rAAV9-CBA-mMut (Figure 4d).
Discussion
In this study, we demonstrate the efficacy of an rAAV9 vector to treat methylmalonic acidemia in a mouse model that replicates the features of severe human disease. Consistent with reports showing that serotype 9 AAV vectors are highly efficacious gene delivery agents in mice[26], we have achieved long-term correction of the neonatal lethal phenotype seen in the untreated Mut-/- mice using a 10-fold lower dose than that previously used with rAAV8 vectors[22, 36]. Early and long-term transgene expression, proven by expression of Mut gene in the liver of Mut-/- mice as early as 24 hours post-treatment with persistence to 1 year and beyond, likely contributes to the biological efficacy of this gene therapy approach. The rescued Mut-/- mice were vigorous and appeared well, tolerated a non-restricted diet, and had markedly improved metabolic parameters compared to the untreated state. Like patients who receive liver and combined liver/kidney transplants[8], the circulating metabolites were not normalized in the treated mice. However, the clinical effects of rAAV9-CBA-mMut gene therapy endured throughout life as the animals appeared and acted in a fashion identical to control littermates.
Recently, the potential of rAAV9 to transduce the kidney and provide biological efficacy in a genetic mouse model of tubulointerstitial renal disease has been established[28]. Because kidney failure is a well-recognized and devastating complication of methylmalonic acidemia, we also examined FITC-inulin clearance in the treated Mut-/- mice to measure the glomelular filtration rate (GFR), a sensitive measure of renal function. No significant differences were observed between Mut+/- and rAAV9-CBA-mMut treated Mut-/- mice in our GFR study. Although the manifestations of renal disease in mouse models of MMA are not fully explored, previous studies have established that tubulointersitial nephritis, similar to what has been documented in MMA patients, does occur in older, untreated Mut-/- mice that escape lethality [35]. In the small number of rAAV9 treated Mut-/- mice that were sacrificed after GFR studies were performed at 1 year of age, renal histology was similar to that seen in treated controls and showed no tubulo-interstitial changes (Figure 3d and Supplemental Figure 2). That the treated Mut-/- mice studied here tolerated a non-restricted diet, have normal glomelular kidney function and no evidence of tubulointerstital changes suggests that rAAV9 gene therapy has ameliorated the progression of MMA associated kidney damage, either by transduction of a critical renal cellular element, such as the proximal tubule, or by reducing the “nephrotoxic” metabolite load.
In light of the published data showing that rAAV9 can be re-administered and still mediate high levels of transgene expression, even in the presence of neutralizing antibodies [37], a subset of treated Mut-/- mice were re-injected at 1 year of age. A dose of rAAV9-CBA-mMut similar to that used in human clinical trials with other rAAVs (2.5×109 GC/gram) [38] was systemically delivered. As early as 3-days post-injection, the re-injected Mut-/- mice exhibited improved metabolism and the response persisted for at least 1 month post-treatment, despite the fact that the circulating metabolites remained substantially increased in re-treated Mut-/- mice. These findings demonstrate that a second round of gene delivery can substantially replenish Mut enzymatic activity in vivo, and be readily measured using a combination of circulating and whole body metabolic parameters.
In order to determine if a systemic rAAV9-CBA-mMut re-administration resulted in a greater transgene expression in the liver and kidney, tissues were harvested 1 or 2 months after re-injection, analyzed by western blotting and qPCR, and compared to mice that had received only a single rAAV9-CBA-mMut treatment at birth. Mut-/- mice that received only a single injection at birth had hepatic Mut protein levels comparable to treated heterozygous littermates. In Mut-/- mice that received a second injection of rAAV9-CBA-mMut at 1 year, transgene expression further increased in the liver (Figure 4c). Although one otherwise asymptomatic treated Mut-/- mouse had elevated LFTs prior to vector re-administration (Supplemental Figure 3) for an unknown reason, liver function tests in the multiply treated animals did not reveal AAV9 associated-transaminitis. Because rAAV9 has been reported to transduce the kidney in mice [25, 26], we next examined whole-kidney extracts prepared from mice at 1 month and 2 months after systemic re-injection. Immunoreactive enzyme was apparent in the Mut-/- kidneys studied after re-treatment, compared to low levels present before re-injection. These data establish that a second injection of rAAV9-CBA-mMut, systemically delivered at a dose equal to that used in human trials for other AAV serotypes, resulted in significantly increased Mut expression in both the liver and the kidney.
The studies presented here clearly demonstrate the therapeutic potential of a serotype 9 rAAV vector to effectively deliver the Mut gene in a mouse model of MMA that faithfully replicates the severe human phenotype. The rapid hepatic transgene expression we have documented in rAAV9-CBA-mMut treated neonatal Mut-/- mice suggests that a rapidly uncoating rAAV vector [33] might be used to treat MMA patients acutely in the setting of metabolic decompensation, perhaps as early as the newborn period. Transduction of the liver, and especially the kidney, further supports the consideration of AAV serotype 9 gene therapy vectors for future use in for the treatment of patients with methymalonic acidemia.
Materials and Methods
Murine model of MMA
Mut-/- mice contain a deletion of exon 3 in the methylmalonyl-CoA mutase gene, display neonatal lethality, and exhibit massive elevations of methylmalonic acid in the blood, urine, and tissues [39]. Mut RNA, protein, and enzymatic activity are undetectable in Mut-/- mice. Mut+/- mice have biochemical parameters identical to Mut+/+ mice and were used as controls throughout the study [22, 39].
rAAV9 construction, production, and delivery
The murine Mut cDNA was cloned into the pAAV2-CI-CB7-RBG expression plasmid to generate rAAV9-CBA-mMut. pAAV2-CI-CB7-RBG contains the transcriptional control elements from the cytomegalovirus enhancer/chicken β-actin promoter, cloning sites for the insertion of a complementary DNA, and the rabbit β-globin polyA signal flanked by AAV2 terminal repeats [32]. rAAVs were subsequently packaged with either AAV9 or AAV2 capsid, purified by cesium chloride centrifugation or column chromatography, and titered by qPCR as previously described [40]. rAAV9-CBA-mMut had a titer of 1.38×1012 genome copies GC/mL; rAAV2-CBA-mMut had a titer of 5.52×1012 GC/mL. 1×1010 GC of the viral vector were delivered to neonatal mice via intrahepatic injection immediately after birth [22]. At 1 year of age, a subset of mice that had been injected in the neonatal period were retreated with 2.5×109 GC/gram body weight rAAV9-CBA-mMut delivered via retroorbital injection. The National Human Genome Research Institute Animal User Committee approved all animal studies.
Western blotting
Tissue samples were homogenized in T-PER tissue protein extraction buffer (Pierce Biotechnology, Rockford, IL) in the presence of Halt protease inhibitor cocktail (Pierce Biotechnology) using a 2-ml Tenbroeck tissue grinder (Wheaton, Millville, NJ). Protein extracts were analyzed by western blot and probed with rabbit polyclonal antibodies against the Mut enzyme [22]. Mitochondrial loading control was detected with mouse monoclonal antibodies against complex III core II (SKU# A-11143, invitrogen, CA). The anti-Mut antibodies were used at a dilution of 1:1000 and the anti-Complex III Core II antibody was used at a dilution of 1:3000. Horseradish peroxidase-conjugated anti-rabbit IgG (NA934; GE Healthcare Life Sciences, Piscataway, NJ) or anti-mouse IgG (NA931; GE Healthcare Life Sciences, Piscataway, NJ) were used as secondary antibodies and visualized after chemiluminescence detection (Pierce Biotechnology, Rockford, IL).
Metabolic studies
Blood samples were collected from treated mice by orbital bleeding. Plasma was isolated by centrifugation, diluted in water, and stored at –80 °C in a screw-top tube. Methylmalonic acid concentrations in plasma were measured by gas chromatography-mass spectrometry [41, 42]. To determine 1-13C-propionate oxidation in vivo, mice were placed into a respiratory chamber after intraperitoneal injection of 200 μg of 1-13C-sodium propionate [22, 43]. Breath samples from mutant, control, and treated mice were collected at 5, 15, and 25 minutes after injection and the isotope ratio (13C/12C) of the expired gas was measured with a gas isotope ratio mass spectrometer (Metabolic Solutions, Nashua, NH). The percent dose oxidized at each time point was calculated as:
Glomerular filtration rate
The glomerular filtration rate (GFR) was measured using the FITC-labeled inulin clearance method [44]. Experiments were performed on age and sex matched Mut+/- (n=3) and Mut-/- mice (n=3) at 1 year of age. All of the mice had received a single neonatal intrahepatic injection of rAAV9-CBA-mMut. Under isoflurane anesthesia, mice were given a single retro-orbital bolus injection of 5% FITC-labeled inulin (3.74 μl/gram body weight). Heparinized serial blood collections were performed from tail cuts at 3, 7, 10, 15, 35, 55, and 75 minutes after inulin administration and the plasma was subsequently removed. Since pH affects FITC fluorescence values, each plasma sample was buffered by mixing 1 μl plasma with 9 μl of 500 mM HEPES solution (pH 7.4). The amount of FITC label present in the samples was then measured using a fluorospectrometer at 538-nm emission (Thermo Scientific, NanoDrop 3300). A two-compartment clearance model was used to calculate the GFR. Plasma fluorescence data were fit to a two-phase exponential decay curve using nonlinear regression (GraphPad Prism, GraphPad Software, San Diego, CA). GFR (μl/minute) was calculated using the equation: GFR= I/(A/α + B/β). I is the amount of FITC-inulin delivered by injection, A and B are the y-intercept values of the two decay rates, and α and β are the decay constants for the distribution and elimination phases, respectively.
Statistical analyses
Differences in the survival between treated and untreated groups were analyzed using a chi-squared test. Differences in weight, metabolite levels, and glomelular filtration rates were assessed using a Student's t-test. The Kruskal-Wallis test was used to determine the statistical significance in measured propionate oxidation rates between groups at 25 minutes after injection. P values less than 0.05 were considered significant.
Supplementary Material
Supplemental figures:
Figure S1: Growth after systemic re-administration.
rAAV9-CBA-mMut treated Mut-/- mice were reinjected at 1 year of age (n=8) and compared to similarly treated Mut+/- diet and gender-matched littermates (n=9). The graph depicts the percent weight at day 14 and 60 post rAAV9 re-administration. Treated Mut-/- mice re-injected with rAAV9-CBA-mMut did not show significant growth improvement compared to Mut+/- diet and gender-matched littermates (*** p< 0.001). Error bars represent plus and minus one standard deviation.
Figure S2: Renal histology.
Histology (hematoxylin and eosin stain) of kidney from a Mut+/- control animal (a) and an rAAV9-CBA-mMut treated Mut-/- knockout littermate sacrificed at 12 months of age (b). Both had received a single intrahepatic injection in the neonatal period. Histological findings were also normal in the other rAAV9-CBA-mMut treated Mut-/- mice studied 1 month after re-administration of rAAV9-CBA-mMut (n=4, data not presented). Renal histology from older, untreated Mut-/- animals were not available for this study but have been previously described (see Reference 34, Figure 2)
Figure S3: Liver function tests before and after readministration.
The level of plasma alanine transaminase (ALT) (a) and aspartate transaminase (AST) (b) concentrations were measured to ascertain whether rAAV9 gene delivery was associated with transaminitis. The horizontal line represents the mean ALT or AST plasma concentration for each group. rAAV9-CBA-mMut Mut-/- mice displayed similar ALT and AST concentrations at 1 year of life and 2 months after re-administration of rAAV9-CBA-mMut. These concentrations were not significantly different between untreated or treated Mut+/- mice. One rAAV9-CBA-mMut treated Mut-/- mouse in this study displayed high ALT and AST concentrations that increased after reinjection. Results are reported as units per liter (U/L).
Acknowledgements
JSS, RJC, JRS and CPV were supported, in part, by the Intramural Research Program of the National Human Genome Research Institute, National Institutes of Health. LL was supported, in part, by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health. JRS also received support from the Angels for Alyssa MMA Research Foundation.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.Fenton WA, Gravel RA, Rosenblatt DS. Disorders of Propionate and Methylmalonate Metabolism. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The Metabolic and Molecular Bases for Inhertited Disease. 8th edn. McGraw-Hill, Inc.; New York: 2001. pp. 2165–2192. [Google Scholar]
- 2.Matsui SM, Mahoney MJ, Rosenberg LE. The natural history of the inherited methylmalonic acidemias. N Engl J Med. 1983;308(15):857–61. doi: 10.1056/NEJM198304143081501. [DOI] [PubMed] [Google Scholar]
- 3.van der Meer SB, et al. Clinical outcome of long-term management of patients with vitamin B12-unresponsive methylmalonic acidemia. J Pediatr. 1994;125(6 Pt 1):903–8. doi: 10.1016/s0022-3476(05)82005-0. [DOI] [PubMed] [Google Scholar]
- 4.Nicolaides P, Leonard J, Surtees R. Neurological outcome of methylmalonic acidaemia. Arch Dis Child. 1998;78(6):508–12. doi: 10.1136/adc.78.6.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Baulny HO, et al. Methylmalonic and propionic acidaemias: management and outcome. J Inherit Metab Dis. 2005;28(3):415–23. doi: 10.1007/s10545-005-7056-1. [DOI] [PubMed] [Google Scholar]
- 6.Horster F, et al. Long-term outcome in methylmalonic acidurias is influenced by the underlying defect (mut0, mut-, cblA, cblB). Pediatr Res. 2007;62(2):225–30. doi: 10.1203/PDR.0b013e3180a0325f. [DOI] [PubMed] [Google Scholar]
- 7.Cosson MA, et al. Long-term outcome in methylmalonic aciduria: a series of 30 French patients. Mol Genet Metab. 2009;97(3):172–8. doi: 10.1016/j.ymgme.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 8.van 't Hoff WG, et al. Combined liver-kidney transplantation in methylmalonic acidemia. J Pediatr. 1998;132(6):1043–4. doi: 10.1016/s0022-3476(98)70407-x. [DOI] [PubMed] [Google Scholar]
- 9.Chakrapani A, et al. Metabolic stroke in methylmalonic acidemia five years after liver transplantation. J Pediatr. 2002;140(2):261–3. doi: 10.1067/mpd.2002.121698. [DOI] [PubMed] [Google Scholar]
- 10.Kayler LK, et al. Long-term survival after liver transplantation in children with metabolic disorders. Pediatr Transplant. 2002;6(4):295–300. doi: 10.1034/j.1399-3046.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- 11.Nyhan WL, et al. Progressive neurologic disability in methylmalonic acidemia despite transplantation of the liver. Eur J Pediatr. 2002;161(7):377–9. doi: 10.1007/s00431-002-0970-4. [DOI] [PubMed] [Google Scholar]
- 12.Hsui JY, et al. Living-related liver transplantation for methylmalonic acidemia: report of one case. Acta Paediatr Taiwan. 2003;44(3):171–3. [PubMed] [Google Scholar]
- 13.Nagarajan S, et al. Management of methylmalonic acidaemia by combined liver-kidney transplantation. J Inherit Metab Dis. 2005;28(4):517–24. doi: 10.1007/s10545-005-0517-8. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan P, et al. Liver transplantation is not curative for methylmalonic acidopathy caused by methylmalonyl-CoA mutase deficiency. Mol Genet Metab. 2006;88(4):322–6. doi: 10.1016/j.ymgme.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Kasahara M, et al. Current role of liver transplantation for methylmalonic acidemia: a review of the literature. Pediatr Transplant. 2006;10(8):943–7. doi: 10.1111/j.1399-3046.2006.00585.x. [DOI] [PubMed] [Google Scholar]
- 16.Morioka D, et al. Efficacy of living donor liver transplantation for patients with methylmalonic acidemia. Am J Transplant. 2007;7(12):2782–7. doi: 10.1111/j.1600-6143.2007.01986.x. [DOI] [PubMed] [Google Scholar]
- 17.Mc Guire PJ, et al. Combined liver-kidney transplant for the management of methylmalonic aciduria: a case report and review of the literature. Mol Genet Metab. 2008;93(1):22–9. doi: 10.1016/j.ymgme.2007.08.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oberholzer VG, et al. Methylmalonic aciduria. An inborn error of metabolism leading to chronic metabolic acidosis. Arch. Dis. Child. 1967;42(225):492–504. doi: 10.1136/adc.42.225.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walter JH, et al. Chronic renal failure in methylmalonic acidaemia. Eur J Pediatr. 1989;148(4):344–8. doi: 10.1007/BF00444131. [DOI] [PubMed] [Google Scholar]
- 20.Van Calcar SC, et al. Renal transplantation in a patient with methylmalonic acidaemia. J Inherit Metab Dis. 1998;21(7):729–37. doi: 10.1023/a:1005493015489. [DOI] [PubMed] [Google Scholar]
- 21.Kolker S, Okun JG. Methylmalonic acid--an endogenous toxin? Cell Mol Life Sci. 2005;62(6):621–4. doi: 10.1007/s00018-005-4463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chandler RJ, Venditti CP. Long-term rescue of a lethal murine model of methylmalonic acidemia using adeno-associated viral gene therapy. Mol Ther. 2010;18(1):11–6. doi: 10.1038/mt.2009.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu Z, Asokan A, Samulski RJ. Adeno-associated virus serotypes: vector toolkit for human gene therapy. Mol Ther. 2006;14(3):316–27. doi: 10.1016/j.ymthe.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Gao G, Vandenberghe LH, Wilson JM. New recombinant serotypes of AAV vectors. Curr Gene Ther. 2005;5(3):285–97. doi: 10.2174/1566523054065057. [DOI] [PubMed] [Google Scholar]
- 25.Schievenbusch S, et al. Combined paracrine and endocrine AAV9 mediated expression of hepatocyte growth factor for the treatment of renal fibrosis. Mol Ther. 2010;18(7):1302–9. doi: 10.1038/mt.2010.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zincarelli C, et al. Analysis of AAV serotypes 1-9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16(6):1073–80. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 27.Yue Y, et al. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol Ther. 2008;16(12):1944–52. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yue Y, Shin JH, Duan D. Whole body skeletal muscle transduction in neonatal dogs with AAV-9. Methods Mol Biol. 2011;709:313–29. doi: 10.1007/978-1-61737-982-6_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki K, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14(1):45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sarkar R, et al. Long-term efficacy of adeno-associated virus serotypes 8 and 9 in hemophilia a dogs and mice. Hum Gene Ther. 2006;17(4):427–39. doi: 10.1089/hum.2006.17.427. [DOI] [PubMed] [Google Scholar]
- 31.Prasad KM, et al. Robust cardiomyocyte-specific gene expression following systemic injection of AAV: in vivo gene delivery follows a Poisson distribution. Gene Ther. 2010 doi: 10.1038/gt.2010.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daly TM, et al. Neonatal intramuscular injection with recombinant adeno-associated virus results in prolonged beta-glucuronidase expression in situ and correction of liver pathology in mucopolysaccharidosis type VII mice. Hum Gene Ther. 1999;10(1):85–94. doi: 10.1089/10430349950019219. [DOI] [PubMed] [Google Scholar]
- 33.Thomas CE, et al. Rapid uncoating of vector genomes is the key to efficient liver transduction with pseudotyped adeno-associated virus vectors. J Virol. 2004;78(6):3110–22. doi: 10.1128/JVI.78.6.3110-3122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chandler RJ, P.M.Z., Shanske S, Sloan J, Hoffmann V, DiMauro S, Venditti CP. Mitochondrial dysfunction in mut methylmalonic acidemia. The FASEB Journal. 2009;23:1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chandler RJ, P.M.Z., Shanske S, Sloan J, Hoffmann V, DiMauro S, Venditti CP. Mitochondrial dysfunction in mut methylmalonic acidemia. The FASEB Journal. 2009;23:1252–1261. doi: 10.1096/fj.08-121848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carrillo-Carrasco N, et al. Liver-directed recombinant adeno-associated viral gene delivery rescues a lethal mouse model of methylmalonic acidemia and provides long-term phenotypic correction. Hum Gene Ther. 2010;21(9):1147–54. doi: 10.1089/hum.2010.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Limberis MP, Wilson JM. Adeno-associated virus serotype 9 vectors transduce murine alveolar and nasal epithelia and can be readministered. Proc Natl Acad Sci U S A. 2006;103(35):12993–8. doi: 10.1073/pnas.0601433103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manno CS, et al. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–7. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 39.Chandler RJ, et al. Metabolic phenotype of methylmalonic acidemia in mice and humans: the role of skeletal muscle. BMC Med Genet. 2007;8:64. doi: 10.1186/1471-2350-8-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao GP, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci U S A. 2002;99(18):11854–9. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Marcell PD, et al. Quantitation of methylmalonic acid and other dicarboxylic acids in normal serum and urine using capillary gas chromatography-mass spectrometry. Anal Biochem. 1985;150(1):58–66. doi: 10.1016/0003-2697(85)90440-3. [DOI] [PubMed] [Google Scholar]
- 42.Allen RH, et al. Elevation of 2-methylcitric acid I and II levels in serum, urine, and cerebrospinal fluid of patients with cobalamin deficiency. Metabolism. 1993;42(8):978–88. doi: 10.1016/0026-0495(93)90010-l. [DOI] [PubMed] [Google Scholar]
- 43.Barshop BA, et al. Metabolism of 1-13C-propionate in vivo in patients with disorders of propionate metabolism. Pediatr Res. 1991;30(1):15–22. doi: 10.1203/00006450-199107000-00004. [DOI] [PubMed] [Google Scholar]
- 44.Qi Z, et al. Serial determination of glomerular filtration rate in conscious mice using FITC-inulin clearance. Am J Physiol Renal Physiol. 2004;286(3):F590–6. doi: 10.1152/ajprenal.00324.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figures:
Figure S1: Growth after systemic re-administration.
rAAV9-CBA-mMut treated Mut-/- mice were reinjected at 1 year of age (n=8) and compared to similarly treated Mut+/- diet and gender-matched littermates (n=9). The graph depicts the percent weight at day 14 and 60 post rAAV9 re-administration. Treated Mut-/- mice re-injected with rAAV9-CBA-mMut did not show significant growth improvement compared to Mut+/- diet and gender-matched littermates (*** p< 0.001). Error bars represent plus and minus one standard deviation.
Figure S2: Renal histology.
Histology (hematoxylin and eosin stain) of kidney from a Mut+/- control animal (a) and an rAAV9-CBA-mMut treated Mut-/- knockout littermate sacrificed at 12 months of age (b). Both had received a single intrahepatic injection in the neonatal period. Histological findings were also normal in the other rAAV9-CBA-mMut treated Mut-/- mice studied 1 month after re-administration of rAAV9-CBA-mMut (n=4, data not presented). Renal histology from older, untreated Mut-/- animals were not available for this study but have been previously described (see Reference 34, Figure 2)
Figure S3: Liver function tests before and after readministration.
The level of plasma alanine transaminase (ALT) (a) and aspartate transaminase (AST) (b) concentrations were measured to ascertain whether rAAV9 gene delivery was associated with transaminitis. The horizontal line represents the mean ALT or AST plasma concentration for each group. rAAV9-CBA-mMut Mut-/- mice displayed similar ALT and AST concentrations at 1 year of life and 2 months after re-administration of rAAV9-CBA-mMut. These concentrations were not significantly different between untreated or treated Mut+/- mice. One rAAV9-CBA-mMut treated Mut-/- mouse in this study displayed high ALT and AST concentrations that increased after reinjection. Results are reported as units per liter (U/L).