Abstract
Diverse subsets of endothelial progenitor cells (EPCs) are used for the treatment of ischemic diseases in clinical trials and circulating EPCs levels are considered as biomarkers for coronary and peripheral artery disease. However, despite significant steps forward in defining their potential for both therapeutic and diagnostic purposes, further progress has been mined by unresolved questions around the definition and the mechanism of action of EPCs. Diverse culturing methods and detection of various combinations of different surface antigens were used to enrich and identify EPCs. These attempts were particularly challenged by the close relationship and overlapping markers of the endothelial and hematopoietic lineages. This article will critically review the most commonly used protocols to define EPCs by culture assays or by FACS in the context of their therapeutic or diagnostic use. We also delineate new research avenues to move forward our knowledge on EPC biology.
Keywords: progenitor cells, stem cells, angiogenesis, endothelial progenitor cells, risk factor
Introduction
Since the initial discovery of endothelial progenitor cells (EPCs),1 significant steps forward have been taken in order to reach a better definition and a detailed functional characterization of these cells. However, the outcome and success of several studies have been limited by the lack of unambiguous and consistent definitions of EPCs.2 EPCs have been effectively used to stimulate angiogenesis and vascular repair in several experimental settings. Moreover, human autologous cell therapies using EPC-containing products (such as bone marrow or mobilized peripheral blood) are feasible and effective in the treatment of coronary and peripheral ischemic syndromes.3, 4 Despite these undisputable evidences, the translation of basic research into the clinical practice has been dampened by unresolved questions around EPC definition and functions. In parallel, it has been recognized that measurement of circulating EPC levels can provide clinical information on the atherosclerotic burden and even on the future cardiovascular risk. Despite multiple studies showing associations of circulating EPC phenotypes with patient characteristics and prognosis, the pathophysiological impact of circulating EPC levels is still unclear. In this article, we critically review the most commonly used culture methods and surface antigen-based definitions of EPCs in the context of their use as a therapy or diagnostic aid.
2. Characterisation of EPCs
The identification and characterization of EPC has been most challenging and controversial. In general, two approaches have been used to isolate EPCs: a) culture and colony assays and b) selection of subpopulations based on surface markers. Despite the fact that multiple cultured or selected subpopulations improve neovascularization in animal models of ischemia, the true nature and mechanism of action may differ between the various cell populations. Of note, all current methods for identifying or quantifying the endothelial lineage potential of circulating cells suffer from limitations in that none has been shown to reliably predict the behavior of the circulating cells in a relevant in vivo context. Furthermore, it is not known whether cultured cells exist in the bloodstream as such or whether they mainly represent an artificial phenotype generated by specific culture conditions.5 Furthermore, as we learn more about the plasticity of the cell phenotype, earlier statements indicating that the expression of one or another particular marker “proves” that a cell in culture is not an EPC in vivo, seem to be stronger than the evidence.
2.1. Cultured endothelial progenitor cells
2.1.1. In vitro culture assays
Most culture assays were used to obtain circulating EPCs from peripheral blood for identification of EPCs as biomarkers for cardiovascular disease, for analysis of intracellular signaling pathways, or for enriching cells for therapeutic angiogenesis.6 Overall, the protocols differ mainly regarding the culture time and most of the short term protocols (4–7 days) yield cells with myeloid/hematopoietic characteristics. Particularly, the “early EPCs” which are generated by culturing peripheral blood mononuclear cells on fibronectin for 4 days in VEGF containing medium7–9 express CD45 and typical myeloid markers such as CD14 and CD11b. Although several groups reported the co-expression of endothelial markers by these cells, it has been debated whether the detection of endothelial markers might result from a contamination with microparticles deriving from other elements in the culture (such as platelets) leading to false positive events in the FACS analysis.10 Recent studies show that transfer of epigenetic material, including proteins and nucleic acids, is a previously unappreciated way of intercellular communication.11–13 This can occur by direct cell-to-cell contact, as shown by Koyanagi et al., who reported that transdifferentiation of early EPCs co-cultured with neonatal cardiomyocyte was dependent upon transfer of complex cellular material through nanotube connections.11 Moreover, microRNAs were shown to be transported via Gap junctions from cardiomyocytes to cardiac stem cells thereby inducing cardiac commitment.14 Although not proven for EPCs or bone marrow-derived cells, such direct cell-to-cell communication pathways might modulate cell fate decisions. Transfer of information can also be achieved by cell-.-contact independent mechanisms: for instance, microvesicles have been shown to mediate intercellular communications of bone marrow cells with other cell types, with modification of the transcriptional profile mediated by microRNA and mRNA.15 Thus, one may speculate that contaminating cells or microparticles transfer RNA or microRNAs to the cultured cells thereby truly modifying gene expression patterns and the cellular phenotype of “candidate” EPCs. This may even leave open the possibility that cellular reprogramming through transfer of nucleic acids occurs in vivo and accounts for hemato-endothelial “transition” and overlapping phenotypes. However, the lack of a mature endothelial cell phenotype in cultured “early EPCs” is supported by the recent finding that the endothelial gene promoters are silenced.16 Along this line of evidence, we suggest that evaluation of the epigenetic mechanisms governing phenotypic regulation of the cell represents an important area of investigation that can provide incremental knowledge on EPC biology in vitro and in vivo (Table 1).
TABLE 1. New avenues in the study of EPC origin and fate.
In this table, we describe a few relatively novel important concepts that would significantly expand our current knowledge on EPC biology in the next future.
| Current concepts | Future developments |
|---|---|
| In vitro colony assays |
|
| In vivo lineage tracing |
|
| Epigenetic regulation |
|
| Intercellular communications |
|
| Hemato-endothelial transition |
|
Since the proliferative capacity might be one criterion to define a progenitor cell, several groups established colony assays. The most prominent assay was developed by Hill et al, who selected only non-adherent peripheral blood mononuclear cells (with the intention to remove monocyte/macrophages) for 5 days.17 These colony forming units were named CFU-EC and were initially characterized to express endothelial markers. However, similar to “early EPCs”, these cells also express myeloid and hematopoietic markers and subsequent studies have suggested that CD3+ CD31+ CXCR4+ T cells (referred to as angiogenic T cells) are forming the core of these colonies18 and that a combination of purified T cells and monocytes form CFU-EC structures.19 Likewise, isolated CD14+ cells were shown to give rise to “early EPCs”7 and depletion of CD14+ monocytic cells prevented the formation of CFU-ECs.20 Together, these findings document that short term cultured EPCs constitute a heterogenic population that mainly originates from myeloid hematopoietic cells and share features with immune cells, particularly monocyte/macrophages. Therefore, naming these cells “EPCs” has been criticized and we would prefer the term “circulating angiogenic cells” as already suggested, based on their ability to promote angiogenesis in vivo, not necessarily related to endothelial commitment.21, 22
Interestingly, long term culture of “early EPCs” yielded outgrowing cells with a more mature endothelial cell phenotype, which are often referred to as “late” or “outgrowing” EPCs.23 In addition, Ingram, Yoder and coworkers showed that, when culturing peripheral blood mononuclear cells on collagen for >14 days, mature endothelial cells with a high proliferative capacity can be obtained, which were named “endothelial colony forming cells” (ECFCs).24, 25 This colony assay clearly reveals that, among the cells composing the “early EPCs” or CFU-EC culture, a small minority has true endothelial differentiation potential. From a clinical perspective, generation of ECFC in culture seems to be a on/off phenomenon, implying that ECFC cannot be efficiently obtained from all donors, especially in relation to age and presence of CVD.26, 27 However, the protocols to culture ECFCs has been further refined, and humanized larger scale culture assays have been developed.28 These outgrowing cells appear clonally unrelated to CFU-ECs and, due to the absence of hematopoietic and myeloid markers and the capacity to form vascular networks in implanted matrigel plugs in vivo, these long term cultured ECFCs were considered to be the “true” EPCs. ECFCs, however, lack progenitor markers and are (at the level of endothelial marker expression) indistinguishable from mature endothelial cells. The exact origin of these cells remains to be elucidated, but several authors speculate that these cells are deriving from the vascular wall.25 If this hypothesis is true, these cells may not be true progenitor cells but a selection of highly proliferating shed endothelial cells.
To reduce the complexity of the cell composition and to define the origin of the EPCs, several groups have used selected bone marrow-, umbilical cord- or peripheral blood- derived CD34+ or CD133+ hematopoietic cells instead of peripheral blood mononuclear cells as the starting material. Whereas some authors were unable to gain colonies of mature endothelial cells,29 others showed that the culture of selected cells yields co-expression of endothelial cell markers.30–32 Possibly, cells may react differently depending on the culture conditions. For instance, hypoxic stress or pharmacological modulators of epigenetic enzymes were shown to change the epigenetic signature of endothelial marker genes in cultured EPCs.16 Moreover, Asahara’s group recently further refined the colony assays and identified two different types of colonies that can be gained from cultured CD133+ single or bulk cells.30 Cultured adherent CD133+ cells formed CFUs of either small or larger cells; while the small cell-colonies showed a more primitive hematopoietic stage and a highly proliferative activity, the larger cell-colonies exhibited vasculogenic properties. A hierarchical relationship between primitive small cell CFUs and definite large cell CFUs in vitro was also established, indicating that the EPC phenotype in culture is dynamic over time.30 The systematic use of colony assays represents an important methodological clue to dissect the various steps in the process of EPC generation in vitro and to study cell origin, clonal expansion, hierarchical organization, as well as positive and negative selection (Table 1). However, the existence of in vivo counterparts of cell types defined by colony assays is far from being demonstrated. Generally speaking, the definition of EPCs based on culture protocols has some issues that have not been fully addressed. For example, once a culture process is started, the cells derived are probably no longer representative of what is functioning in the body. Although the cultured cells may as well be an useful product and some culture procedures can be even used to engineer blood vessels in vitro, it is unclear whether the cells obtained after the culture are indeed the same cells that are circulating in the body. The cultured cells are clearly derivative of the circulating cells, but what seems to be lacking so far is to show that the circulating cell(s) that lead to these manufactured cells are, more than other cells, functioning as EPCs in vivo, without being cultured. Even the use of single cell cultures with carefully selected cells does not exclude the possibility that the cells are “reprogrammed” in vitro by the artificial environment.
2.1.2. In vivo features of cultured EPC
Overall, most of the short term or long term culture methods yielded cells with the capacity to improve neovascularization in pre-clinical models.23, 24, 33,34 However, the cells obtained by the protocols differ with respect to their capacity to differentiate in endothelial cells and to physically form new blood vessels. Most studies suggest that the cell gained by the short term culture assays (“early EPCs”, CFU-ECs) predominantly enhance vessel formation by providing a potent mixture of growth factors that support angiogenesis.35–38,34 Rather, the cells obtained after long term culture (“outgrowing EPCs”, ECFCs) may generate endothelial cells and thereby physically contribute to formation of new capillaries.23, 34 It should be noted that the functional assessment of ECFCs is more in its early stages than the study of short-term cultured EPC, which have undergone a thorough critique and re-evaluation. The use of advanced 3D confocal imaging has significantly re-dimensioned the extent to which bone marrow derived cells appear to contribute to the peripheral endothelium in different settings.39–42 The ability of ECFC to differentiate into mature endothelium and to replace the peripheral endothelium in vivo should be viewed under the same rigorous scrutiny. Another typical feature of long term outgrowing cells is that they form vascular structures in vitro in the absence of co-culture, whereas the short term cultured cells require the interaction with endothelial cells and particularly promote vascular network formation of mature endothelial cells in vitro.43 Although it appears as if the discrimination of cells in “early” versus “late” EPCs has reached a consensus in the scientific community, the complex mixture of cells gained particularly in the mononuclear cell culture assays and the fact that “late” EPCs originate from a rare population of cells hidden in the “early EPC”, some protocols may yield cells with overlapping activities e.g. some cultured cells may have both activities. Additionally, the environment may influence the cell fate and therapeutic benefit, and the specific culture conditions may promote endothelial differentiation of myeloid cells.19, 20 Moreover, the in vivo environment may influence cell fate and function. Thus, under ischemic conditions, injection of fully mature endothelial cells failed to improve neovascularization,8, 44, 45 although they formed vessels when implanted into Matrigel plugs.46 Additional complexity is added by the finding that bone marrow-derived or circulating cells can be incorporated in the perivascular area thereby indirectly promoting vessel growth and potentially vessel stability without forming new endothelium. This feature has first been demonstrated for hematopoietic cells,47 including the so-called Tie2-expressing monocytes (TEMs),48 and has been confirmed for human EPCs, which can exist in a quiescent perivascular state in the absence of ischemia, whence they are recruited to the intimal layer after an ischemic stimulus.49 Consistently, suicide gene studies documented that cultured early EPCs injected in mice with myocardial infarction physically incorporated in vessels in vivo for several weeks without necessarily forming new endothelial cells, yet being needed to support post-ischemic angiogenesis.50
2.2. Selection of subpopulation by surface markers
Culture assays have the advantage to expand the cells for therapeutic or diagnostic purposes. However, as discussed above, it is unclear to what extent the artificial milieu changes the cell phenotype and, particularly when starting with preparation of total mononuclear cells, the interaction of the different cells in the mixture may influence the cellular phenotype. Therefore, the direct isolation of cell populations by using surface antigens has the advantage to select defined populations of cells without the necessity of ex vivo manipulation. Several surface antigens have been used to enrich EPCs.
2.2.1 CD34+ and CD133+ cells
The scientific foundation of EPCs is based on the use of isolated hematopoietic CD34+ cells that were shown to give rise to endothelial marker expressing cells in vitro and in vivo.1 Since CD34 can be also expressed by endothelial cells, other groups have used the more immature marker CD133 to select for putative EPCs.51 However, these studies have been criticized, and Case et al were unable to confirm that CD34+CD133+KDR+ cells are giving rise to an endothelial progeny and were generally questioning the concept that bone marrow-derived cells can acquire an endothelial cell fate.29 When analyzing the epigenetic status of CD34+ and CD34+KDR+ cells, indeed a high level of DNA methylation of the eNOS promoter and silencing histone modifications of several endothelial marker genes suggests that these cells are not predisposed to acquire an endothelial cell fate, in the absence of adequate reprogramming stimuli.16 However, multiple groups have convincingly documented that peripheral blood-, bone marrow- and umbilical cord blood-derived CD34+ or CD133+ cells are enriched for endothelial lineage potential and can express endothelial marker genes and form endothelial structures in vitro and in vivo.52,45,30,53 Moreover, human CD34+ cells physically contributed to angiogenesis in a zebrafish model.54 The critical role of the CD34+ cells is further supported by the finding that the pro-angiogenic activity is lacking in selected CD34-negative cells.40, 41 Overall, the interpretation of these discrepant findings is difficult and suggest that not all CD34+ cells can act as EPCs and/or the conditions used to isolate the cells may influence their epigenetic state and functional properties. Indeed, some studies suggested that the subpopulation of CD34+ cells which co-express the VEGF-receptor 2 (KDR) is more enriched in endothelial progenitor cells.42 Friedrich et al. reported that CD133+CD34-KDR+ cells (which have a frequency in peripheral blood similar to CD34+KDR+ cells) are more vasoregenerative and represent a more immature EPC phenotype, which further matures into endothelial cells.43 The parallel analysis of CD45 expression has been also proposed to distinguish EPCs. Most (90%) CD34+ progenitor cells express CD45 at low intensity (CD45dim), while less than 10% are CD45-negative. Case et al. showed that cord blood and G-CSF mobilized peripheral blood CD34+KDR+ and CD34+CD133+KDR+ cells develop into hematopoietic but not endothelial colonies and that, rather, the CD34+CD45-population forms endothelial colonies in vitro.29 Given that cord blood and mobilized peripheral blood are enriched in hematopoietic progenitors, it is not clear to what extent these results apply also to the steady state peripheral blood.55 In addition, the relationship between CD34+CD45- cells and mature circulating endothelial cells (CECs) remain to be established; by analyzing blood samples from male-to-female bone marrow transplantation, we found that only 5–10% of circulating CD34+ cells are of non-bone marrow origin, and may correspond to CECs (Fadini GP, unpublished data). Finally, Schmidt-Lucke et al. recently reported that CD34+KDR+ cells showed better relationships with coronary artery disease and response to statin therapy if restricted to the CD45dim gate.45
2.2.2 Other surface antigens
In order to better define and further enrich for EPCs, several groups used other markers or combinations of several antigens. The SDF-1 receptor CXCR4, which is required for homing of hematopoietic cells, was used to isolate cells with a high migration capacity and improved neovascularization capacity.56 However, the improved functional activity was mainly attributed to the enhanced homing of CXCR4+ cells and the release of multiple pro-angiogenic cytokines. In addition, cells expressing CD31, a surface antigen that on present in monocytes and endothelial cells, were isolated from peripheral blood and bone marrow and these cells showed a high pro-angiogenic and vasculogenic activity.57, 58
2.3. Difficulties in defining EPCs
The first evidence supporting the existence of a common precursor to blood and endothelial lineages stems from the last century. However, even in embryonic development it is unclear whether this concept holds true (for review see 59) and formal proof of such a common precursor in adult life is still missing. A bilineage potential of bone marrow-derived cells has been documented by various in vitro and in vivo studies.60 Moreover, by studying patients carrying the BCR/ABL fusion gene in their bone-marrow-derived cells, Gunsilius et al demonstrate that a variable proportions of endothelial cells is generated by bone marrow-derived cells in vitro and in vivo.61 While, as discussed above, some studies failed to demonstrate a clonal contribution of bone marrow-derived cells to the endothelial lineage, accumulating evidence support a new concept that interconnects hematopoietic and endothelial cells:62 at least during development, a hemogenic endothelium was shown to give rise to hematopoietic cells.63 The hemogenic capacity of developmental endothelial cells progressively decreases and ceases as the endothelium matures. Yet, if a specific endothelium had hemogenic potential during development, it may be possible that mature endothelial cells de-differentiate and re-establish an overlapping endothelial-hematopoietic phenotype, including CD45 expression, also in adulthood. Reactivation of antenatal gene expression profiles and cellular phenotypes has been shown in several adult organs and tissues subjected to injury, including the myocardium and blood vessels.64, 65 Therefore, the discovery of the embryonic endothelial-to-hematopoietic transition (EHT) has important implications for interpretation of the EPC phenotype and vascular biology in general.66 Genetic and epigenetic studies of the hemato-endothelial origin and fate of putative EPC phenotypes, as well as of the EHT in vitro are important future challenges in this research area (Table 1). Indeed, since several antigenic combinations have failed to distinguish between hematopoietic and endothelial progenitors, lineage tracing studies in mice are mandatory to determine the lineage relation of endothelial and hematopoietic cells. However, these studies might be complicated by the fact that many marker proteins are expressed by both endothelial and hematopoietic cells; e.g. even VE-cadherin, which is considered as one of the best and most specific markers for mature endothelial cells, can be detected on subpopulations of hematopoietic cells in the bone marrow. Provided a common hemangiogenic progenitor exists in the adult organism, even the expression of CD45, which is generally considered a specific pan-leukocyte marker, might not be a reliable watershed between the hematopoietic and endothelial lineage.62
3. EPCs as biomarkers of cardiovascular disease
Besides their pathophysiological and therapeutic implications, EPCs have been extensively studied as a novel prototype of cardiovascular risk biomarkers. Various biomarkers (such as for instance, C-reactive protein) are not necessarily involved in the ongoing pathologic processes in the cardiovascular (CV) system. In contrast, although the mechanism by which EPC subtypes control neovascularization and vascular repair remain unclear, various studies suggest that the therapeutic application of the cells affects the recovery of blood flow after ischemia and atherosclerosis. These data indicate that EPC are not innocent biomarkers but active players in maintaining a healthy CV system. Cellular biomarkers were previously limited to classic leukocyte subpopulations, namely neutrophils and monocytes, which correlate with the prevalence and incidence of cardiovascular disease (CVD). Although EPCs are several orders of magnitude less frequent in the bloodstream, the study of these cells has widened the spectrum of cellular biomarkers and has supported the concept that circulating EPCs may affect the CV system.
3.1. EPC quantification in the clinical setting
The measurement of EPCs as cardiovascular biomarkers in large clinical trials requires simple, rapid, and reproducible methods. Flow cytometry is the gold standard for this aim but, as discussed above, none of the proposed antigenic combinations can be considered fully specific for EPCs. Based on the definition of EPCs, the minimal antigenic profile should include at least one marker of stemness/immaturity (usually CD34 and/or CD133 in humans; CD34, c-kit, or Sca-1 in mice), plus at least one marker of endothelial commitment (usually KDR [also known as VEGFR-2 and Flk-1]). Following the original characterization by Asahara,1 circulating EPCs have been defined as CD34+KDR+ by several investigators, as it was confirmed that this phenotype identifies cells capable of stimulating angiogenesis in vivo.40 The number of circulating CD34+KDR+ cells is around 50–100/1 million WBC (0.005–0.01%), equal to about 350–700 cells/mL. Subsequently, it has been criticized that this phenotype overlaps in part with hematopoietic stem/progenitor cells (which are CD34+ and can also express KDR) and with CECs (which express KDR and may be CD34+).67 Although the contamination of the CD34+KDR+ population by CECs (defined as CD45-CD31+CD146+Syto16+)46 should be very low or negligible, some authors suggest that the co-expression of the stem cell antigen CD133 increases specificity for EPCs, as it is not expressed by mature endothelial cells.47 Unfortunately, the frequency of CD34+CD133+KDR+ cells in peripheral blood is about 20-fold lower, making quantification less reliable. Indeed, according to the Poisson distribution of rare events, the coefficient of variation is inversely proportional to the number of positive events. Therefore, increasingly complex antigenic phenotypes might be more specific for EPCs, but have lower reproducibility, thus limiting their use in daily clinical practice. One way to circumvent this limitation is to acquire a very large amount of events (1.000.000 to 2.000.000) to gather a higher number of positive events. Some studies used CD31, vWf, or VE-cadherin as markers for the endothelial commitment. While there is no comparative analysis to recommend the use of KDR instead of other markers, it should be noted that CD31, vWf, and particularly VE-cadherin may identify cells in a more advanced stage of maturation along the endothelial differentiation process68. However, increased complexity of the antigenic combination, despite providing additional information about the cells under investigation, does not necessarily improve the performance of the cells as clinical biomarkers. Indeed, a disease biomarker does not necessarily have to be highly biologically informative, but it is required to have strong statistical associations with several clinical aspects of the disease. Instead of widening the antigenic phenotype to increase specificity, some investigators aimed at simplifying the identification and quantification of circulating EPCs. For instance, Povsic et al. showed that cells expressing aldehyde dehydrogenase (ALDH) at high intensity (ALDHbright) are enriched in EPCs and have clinical correlates.49 This strategy may ease to spread the use of EPC-like biomarkers in the clinical practice, especially if the analysis can be performed in a standardized fashion at different laboratories and, possibly, on stored samples. Despite FACS analysis should be regarded as the most appropriate methodology to quantify EPCs in the clinical setting, several studies used culture methods with short-term protocols (early EPCs, CFU-EC, or CACs), which also allowed to study functions of EPCs in vitro. Therefore, the variety of methods used to study EPCs in published clinical studies is so wide that, when it comes to review the literature, specification of the exact method used in each work becomes critical. Indeed, even when looking at the same clinical condition, studies using different methodologies could come to opposite conclusions.69 Overall, the findings should be considered stronger when the results are confirmed by using different methods to identify or isolate EPCs and, possibly, by different investigators.
3.2 Influence of cardiovascular risk factors on circulating EPCs
Several studies have demonstrated that circulating EPCs are reduced in the presence of classic cardiovascular risk factors, independently from established CVD. Even non-modifiable risk factors appear to impact on EPCs: aging subjects and males have lower CD34+KDR+ EPC levels than young individuals70 and females,71 respectively. While this phenomenon reflects the progressive stem cell pauperization occurring with age, the gender difference in EPCs is likely one determinant of the cardiovascular protection of fertile women. It is still not clear whether family history for CVD associates with low EPCs independently of other risk factors,72,73 but the genetic background plays a role.74 Among classic CVD risk factors, smoking,75–79 hypertension, 80–86 hypercholesterolemia,87, 88 obesity89–91 and diabetes92–95 have been consistently associated with reduced circulating EPCs, even when using disparate methodologies, ranging from FACS analysis to cell culture. In several occasions, linear correlations were also found between severity of the risk factor and degree of EPC level that support a causal link between risk factors and EPC reduction (Table 2). Regarding emerging risk factors, associations or correlations have been shown between reduced EPCs and hyperhomocysteinemia,96 microalbuminuria,97 inflammation,98 and insulin resistance.99 In several occasions, the mechanisms whereby a given risk factor affects EPC biology have been identified (reviewed elsewhere, e.g. 100). It should be noted that CV risk factors most often occur in combination in the same patients. Despite several clinical studies showed the independent effect of the risk factor under investigation in multivariable analyses, it is still unclear to what extent the presence of single risk factors or their combinations negatively affect EPCs. There is indeed evidence from multiple studies that EPCs are progressively reduced as the number of risk factors in the same patient increase.72, 95 In the setting of metabolic syndrome, clustering of risk factors synergistically impaired the number of circulating CD34+ cells.101 Importantly, some reports demonstrated that specific treatments of the risk factors are able to restore circulating EPCs toward normal levels. For instance, smoking cessation increased CD45dimCD34+CD133+KDR+ EPCs.75 Blood pressure lowering with different classes of drugs has shown ability to counteract EPC reduction in hypertensive patients.102, 103 Blood glucose lowering with insulin therapy in diabetic patients was able to increase circulating CD133+KDR+ and CD34+CD133+KDR+ EPCs,104 while other anti-diabetic medications may be active on EPCs.105 LDL-apheresis in patients with familial hypercholesterolemia increased CD34+KDR+ EPCs (although CD34+CD133+KDR+ cells remained unchanged),106 while statin therapy is among the best characterized intervention to increase EPCs.107 Finally, weight reduction increased CD34+ and CD34+c-kit+ cells in obese patients, linearly dependent on the degree of lost weight.91 Taken together, these data consistently show that EPC levels are reduced in the presence of CV risk factors, especially when they cluster together, and that this alteration is partly reversible.
TABLE 2.
Summary of clinical studies reporting EPC alterations in relation to classic cardiovascular risk factors.
| Risk factor | Finding/EPCs phenotype | Correlations/Observations |
|---|---|---|
| Smoke | ||
| Kondo et al.75 | Reduced CD45dimCD34+CD133+KDR+ cells. | Inversely correlated with the number of cigarettes smoked. Increase after smoke cessation, and decreased again after resumption. |
| Ludwig et al.77 | Reduced CFU-EC, and CD34+/CD133+ cells in smoking women | Independent of hormone cycle. |
| Yue et al.78 | Reduced CD34+KDR+ and CD133+KDR+ cells | Inversely correlated with pulmonary arterial pressure and vascular resistance. |
| Michaud et al. 79 | Reduced early EPCs in healthy smokers vs controls | Directly correlated with plasma anti-oxidant capacity and nitrite concentrations. |
| Hypertension | ||
| Pirro et al.80 | Reduced levels of CD34+KDR+ cells in patients with essential hypetension | Correlation with expression of the differentiation factor HOXA9 |
| Oliveras et al.81 | Reduced CD45dimCD34+CD133+ cells and early EPCs in patients with refractory hypertension. | Independent of confounding factors. |
| Umemura et al.82 | Reduced CD45dimCD34+CD133+ cells in patients with hypertension | Hypertension and age independent predictors of low EPCs |
| Giannotti et al.83 | Reduced CD34+KDR+ cells in prehypertensive and hypertensive patients | Dysfunction of cultured EPCs. |
| Delva et al.84 | Normal number of CFU-EC in patients with essential hypertension. | No correlation with blood pressure. |
| Yang et al.85 | Normal number of CD34+KDR+ cells in patients with essential hypertension, but reduced function of early EPCs in vitro. | Proliferatory and migratory activities of circulating EPCs closely correlated with arterial elasticity |
| Lee et al.86 | Reduced CD34+KDR+ cells in hypertensive patients with left ventricular hypertrophy | Reduced adhesiveness of EPCs in hypertensive patients with left ventricular hypertrophy |
| Hypercholesterolemia | ||
| Rossi et al.87 | Reduced CD34+CD133+ cells and impaired function of early EPCs in patients with high LDL cholesterol. | Inverse correlation between EPCs and LDL and direct correlation with HDL |
| Chen et al.88 | Reduction of early cultured EPCs in patients with hypercholesterolemia | <retracted> |
| Obesity | ||
| Muller-Ehmsen et al.91 | Reduced CD34+KDR+, CD34+CD133+ and CD34+c-kit+ cells in obese vs overweight subjects. | Negative correlation between EPCs and BMI |
| Tobler et al.90 | Reduction of CD34+KDR+, CD34+CD133+KDR+ cells in obese patients | Negative correlation with BMI. |
| Heida et al.89 | Reduced function of cultured early EPCs in obese patients. | Eversible by inhibition of p38 MAPK |
| Diabetes mellitus | ||
| Fadini et al.95 | Reduced CD34+ and CD34+KDR+ cells in type 2 diabetes | Direct correlation with ABI. |
| Fadini et al.94 | Reduced CD34+KDR+ cells in newly diagnosed diabetes and reduced CD34+ cells in pre-diabetes | Negative correlations with fasting and post-challenge glucose. |
| Loomans et al.93 | Reduced cultured early EPCs in type 1 diabetes | Negative correlation with HbA1c. |
| Egan et al.92 | Reduction of several FACS EPCs phenotypes in type 2 diabetes | Negative correlation with the presence of multiple diabetic complications. |
3.3 EPCs and prevalence of cardiovascular diseases
Currently, EPC reduction is considered one mechanism whereby risk factors negatively affect cardiovascular function and promote CVD. It can be speculated that patients with lower EPC levels, at means of risk factors, are more susceptible to the development or progression of CVD, because of the defective endothelial repair and compensatory angiogenesis. Indeed, several manifestations of CVD are associated with reduced EPCs in the bloodstream, after correction for confounding factors.108 Among the earliest stages of CVD, subclinical signs of vascular damage are indeed marked by further reduced EPCs. Hill et al. were the first to demonstrate a direct correlation between EPCs (CFU-EC) and endothelial function, measured as brachial artery flow mediated dilation (FMD).17 Subsequent studies have confirmed the relationship between FMD and EPCs, identified as either CFU-EC,109 CD34+KDR+,110, 111 CD133+KDR+,112, 113 or CD34+CD133+KDR+ cells114 in different population of subjects. These consistent findings obtained using different methodologies substantiate the concept that EPCs represent a biomarker of endothelial function. Furthermore, the earliest anatomical sign of atherosclerotic remodeling, increased intima-media thickness (IMT), has been associated with reduced CD34+KDR+ EPCs in healthy subjects, independently of CRP and the Framingham risk score;115, 116 similar associations were found for CD34+ cells.117 Importantly, the level of circulating EPCs further decline in the later stages of atherosclerosis in different districts, as demonstrated for CD34+KDR+ cells in coronary, 118–120 carotid and cerebral,116, 121–123 and peripheral atherosclerosis,116,121; again, similar results were obtained with CD34+ cells.117, 124, 125 Correlations were also found between severity of the atherosclerotic burden and EPC levels,119–121 indicating that low EPCs represent a biomarker of the systemic atherosclerotic involvement. In the literature, there are remarkable exceptions to the widespread concept that EPCs are reduced in patients at risk of or with established CVD. For instance, using the ECFC culture protocol, Guven et al. paradoxically reported progressively higher EPC numbers in parallel with increasing severity of CAD.22 In the population-based Bruneck Study, the number of early EPCs in culture was found to be lower in patients with high carotid IMT, but showed a paradoxical direct correlation with the Framingham risk score.126 The extent to which these discrepancies are related to the method used or to the characteristics of the study population remains to be determined. In the setting of acute CV events, such as myocardial infarction127–129 and stroke,130,131,132 CD34+KDR+ EPCs levels and/or CD34+ cells are increased, because they are mobilized from the bone marrow into the bloodstream (for a review of the mechanism see Aicher et al).133 This is supposed to be a compensatory attempt to provide vasoregenerative cells and limit residual ischemia and/or achieve better reperfusion. Indeed, a stronger EPC mobilization response is associated with a better outcome in terms of left ventricular function128, 134 and neurological disability or lesion area/growth123 after acute MI or stroke, respectively. On the opposite, in pathological conditions characterized by impaired EPC mobilization, such as diabetes mellitus,135 the angiogenic response to ischemia may be compromised by the insufficient supply of EPCs to the ischemic tissue. Quantitative EPC alterations have been found also in the presence of heart failure (HF).136 Levels of CFU-EC, CD34+ cells and CD34+CD133+KDR+ EPCs may display a biphasic trend during the various stages of congestive HF, with elevation and depression in the early and advanced phases, respectively.137 However, as HF is characterized by stage-dependent changes in body fluids, it is critical to determine to what extent these changes in circulating progenitor cells are attributable to hemodilution and hemoconcentration. While this may be unmasked by the correlation between EPC levels and brain natriuretic peptides (BNP),137 progenitor cell counts should be expressed as fractional, to avoid this artifact. The fractional count of CD34+CD45dim cells appears to be reduced in HF irrespectively of etiology, while it was found that reduction and dysfunction of CFU-EC is typical of ischemic HF.138 CD34+ and CD34+CD133+ cells were also shown to inversely correlate with NYHA class.139 Interestingly, exercise activity was able to increase CD34+ cells and CD34+KDR+ EPCs in HF patients,140, 141 although HF patients have a reduced ability to mobilized CD34+KDR+ EPCs after physical exercise, compared to control subjects.142
3.4 Prognostic impact of EPCs
Besides correlations with prevalent CVD, one of the most important requirement for a candidate biomarker is to be predictive of future CVD events. Only a few studies have evaluated the independent ability of circulating (endothelial) progenitors to predict incident CV events. Paucity of these studies is partly attributable to the fact that FACS analysis performs much better on fresh samples and there is no standardization of FACS for rare events quantification of frozen blood cells. In a sample of 120 individuals at different CV risk (including 43 control subjects, 44 patients with stable coronary artery disease, and 33 with acute coronary syndromes), Schmidt-Lucke et al. found that a CD34+KDR+ EPC level below the median value was associated with a higher incidence of a composite CV endpoint suggestive of atherosclerotic disease progression.143 Werner et al. enrolled 519 patients with angiographically confirmed CAD, who were followed-up for 12 months: the CD34+KDR+ EPCs, as well as CD133+ cells and CFU-EC were predictive of a first major cardiovascular event, independently of potential confounders.144 In a population of 216 patients with chronic renal failure on hemodialysis followed-up for an average 23 months, CD34+ cell count was an independent determinant of both cumulative cardiovascular event-free survival and all-cause survival;145 this finding in hemodialysis patients was subsequently confirmed using early EPC culture.146 Similarly, low CD34+ cells were predictive of cardiovascular events and total mortality in a population of 214 subjects at different CV risk (including 114 healthy controls), followed for a median of 34 months. Interestingly, the reduced progenitor cell count was associated with increased CV risk especially in patients with metabolic syndrome.147 Despite all these studies attribute prognostic relevance to the level of circulating progenitor cells, it was not determined to what extent this new biomarker could be used to improve cardiovascular risk stratification in the clinical practice. To answer this question, crude data from the 4 aforementioned longitudinal studies have been pooled to generate a cohort of 1,057 patients with an average follow-up of 1.7 years, who were at moderate to high baseline CV risk (Figure 2). Using statistical metrics specifically designed to assess the performance of a candidate risk biomarker, it was found that progenitor cell quantification (either CD34+ or CD34+KDR+) helps identifying more patients at higher risk of future events over the short term.148 While these data provide further support to the role of EPCs as CVD biomarkers, replication is needed, especially in lower-risk populations, because biomarkers always tend to perform better in high-risk subjects.149 Reduced levels of circulating EPC, defined as cultured pro-angiogenic cells (early EPCs) independently predicted cardiovascular deaths and hospitalizations for cardiovascular reasons in a cohort of 111 patients with HF followed for 2.5 years.150 In a comparative study among cell phenotypes, Schwartzenberg et al. reported that CD34+CD133+ cells, but not CD34+KDR+ and CD133+KDR+ EPC phenotypes, were predictive of future adverse cardiovascular outcomes in 76 patients during a 24-months follow-up.151 However, this result cannot be considered definitive, as the cohort was small and all patients had ACS, which is known to mobilize EPCs,152 and can affect the prognostic ability of the measure. Moreover, in the pooled analysis,148 there was no difference between CD34+ cells and CD34+KDR+ EPC in terms of the prognostic capacity in a much larger cohort of patients. Therefore, comparative analyses are needed to define the best progenitor cell phenotype that predicts future CVD. By now, based on a critical review of available studies looking at different EPC phenotypes as disease biomarkers, the CD34+KDR+ antigenic combination (with or without gating on CD45dim events) appears to be the best compromise in terms of sensitivity, specificity and reliability to quantify EPCs in the clinical setting. In addition, the simple quantification of CD34+ cells has proven as a valid alternative CVD biomarker. In a cohort of patients, levels of CD34+ cells were more strongly correlated to the Framingham risk score than of CD34+KDR+ cells,101 and were able to predict incident CV events in the follow-up,147 while CD34+KDR+ cells were not. At present, the pathophysiological meaning of changes in the total CD34+ cell population is not entirely clear, as they contain ~80% HSC, ~15% EPC, plus a small amount of progenitors for other lineages153 and CECs. Additionally, pauperization of circulating CD34+ cells may be more linked to a generalized biological aging process than specifically to CVD. Nonetheless, as quantification of CD34+ cells is already performed in most hematology laboratories in a standardized fashion, it may be more easily introduced in the clinical practice for CVD risk estimation than complex EPC phenotypes. To sum up, (E)PCs represent a valuable biomarker of cardiovascular risk that mirrors the natural history of the entire atherosclerotic process. Even if no study has so far determined the changes in EPC levels over such a long period of time to span the entire atherosclerotic disease, integrating results from multiple studies suggests that EPC levels identify patients at different risk for adverse outcomes (Figure 3). Moreover, clinical association data comparing different EPC phenotypes helps in attributing significance to some antigenic combinations, while some others may not even exist in vivo and represent mainly in vitro artifacts.
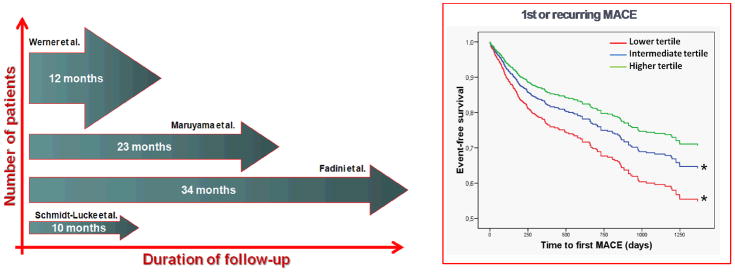
Figure 2.
A pooled analysis of crude data from 4 longitudinal studies of circulating progenitor cells for cardiovascular risk stratification.128–130,132 In the left panel, characteristics of the study are presented: thickness of the arrows are proportional to the number of patients (y-axis), while length of the arrow is proportional to duration of follow-up (x-axis). The right panel shows Kaplan-Meier curves of occurrence of 1st or recurring major adverse cardiovascular event (MACE) in patients categorized as belonging to the higher, intermediate or lower tertile of circulating progenitor cell levels.
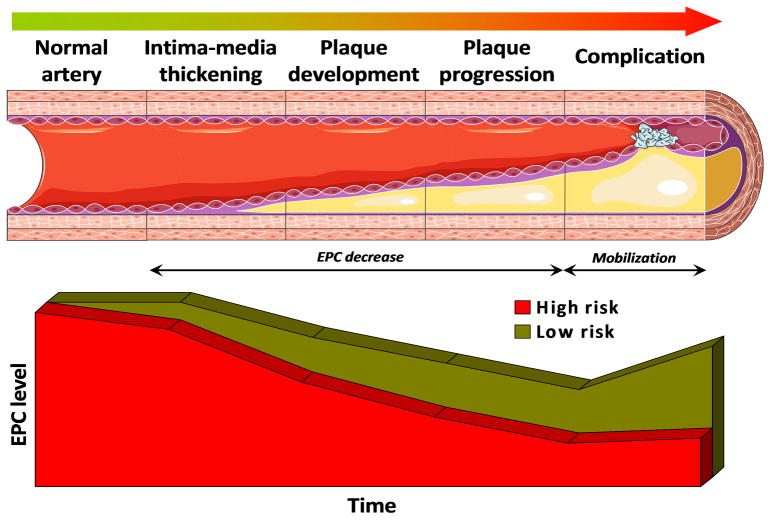
Figure 3.
EPC mirror the natural history of atherosclerosis. The level of EPC (set at maximal in patients with normal arterial anatomy and function) starts to decline when cardiovascular risk factors appear in high risk patients. Further, EPC progressively decline with initial vascular remodeling (IMT), plaque development and progression. Lower EPCs are markers of high risk for future cardiovascular events. Finally, when complications occur (such as AMI or stroke) EPCs should be increased by bone marrow mobilization. When this mechanism is perturbed, a worse outcome can be predicted.
4. Conclusions
This critical re-evaluation of EPC phenotypes for therapeutic and diagnostic purposes reveals that, as our understanding of cellular plasticity improves, the definite EPC identity becomes even more elusive. While this is to some extent attributable to our limited armamentarium for precise lineage tracing analysis, it may also reflect that the endothelial progenitor is a dynamic phenotype in space and time. Indeed, the endothelial differentiation potential of circulating progenitors varies according to the local environment and changes over time, as can be recapitulated in the culture dish. Furthermore, as embryo studies reveal that a EHT is a naturally occurring phenomenon, persistence of hematopoietic feature may not diminish the interest around “circulating angiogenic cells” (CACs, previously termed early EPCs or monocytic EPCs), which may be hierarchically related to ECFC (true EPCs). As long as therapeutic applications are concerned, a detailed functional characterization of the cells under investigation using preclinical models appears to be more relevant than their antigenic phenotype. For diagnostic purposes, clinical biomarker studies should seek a compromise in terms of specificity of the antigenic combination for EPC definition and reliability of their quantification, possibly analyzing multiple phenotypes simultaneously. So far, definition of EPCs is a work-in-progress and remains a challenge that is continuously providing insights on the relationships between the vascular and hematopoietic systems.
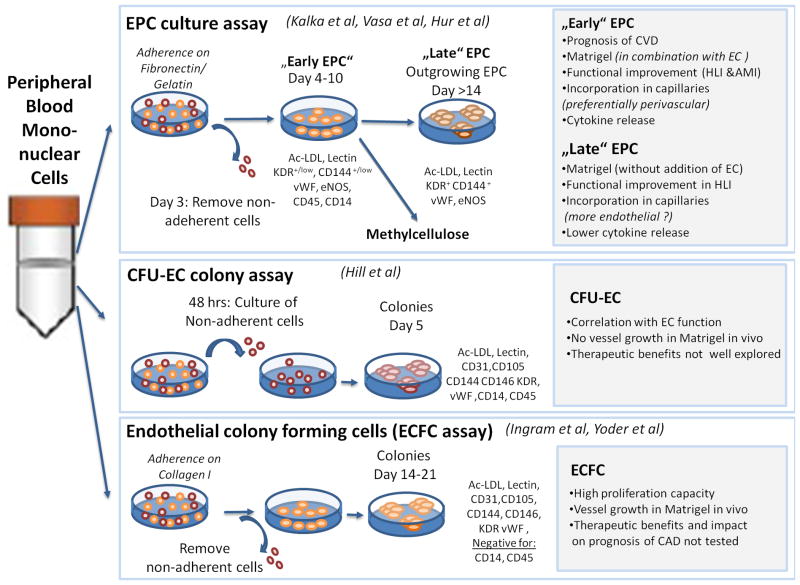
Figure 1.
An overview of the most common methods used to isolate EPCs.
Acknowledgments
Sources of Funding
S.D. is supported by the Excellence Cluster Cardiopulmonary System (Exc 147-1). G.P.F. is supported by the European Foundation for the Study of Diabetes (EFSD).
Non-standard abbreviations and acronyms
- ACS
Acute coronary syndrome
- CEC
Circulating endothelial cells
- CFU
Colony forming unit
- CV
Cardiovascular
- CVD
Cardiovascular disease
- EC
Endothelial cells
- ECFC
Endothelial colony forming cells
- EHT
Endothelial –to-hematopoietic transition
- EPC
Endothelial progenitor cells
- FACS
Fluorescence activated cell sorter
- FMD
Flow mediated dilation
- G-CSF
Granulocyte colony stimulating factor
- KDR
VEGF receptor 2
- TEM
Tie2 expressing monocytes
- VEGF
Vascular endothelial growth factor
- vWF
von willebrand factor
Footnotes
Disclosures
S.D. is founder of t2cure GmbH.
References
- 1.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 2.Ingram DA, Caplice NM, Yoder MC. Unresolved questions, changing definitions, and novel paradigms for defining endothelial progenitor cells. Blood. 2005;106:1525–1531. doi: 10.1182/blood-2005-04-1509. [DOI] [PubMed] [Google Scholar]
- 3.Fadini GP, Agostini C, Avogaro A. Autologous stem cell therapy for peripheral arterial disease meta-analysis and systematic review of the literature. Atherosclerosis. 2010;209:10–17. doi: 10.1016/j.atherosclerosis.2009.08.033. [DOI] [PubMed] [Google Scholar]
- 4.Lipinski MJ, Biondi-Zoccai GG, Abbate A, Khianey R, Sheiban I, Bartunek J, Vanderheyden M, Kim HS, Kang HJ, Strauer BE, Vetrovec GW. Impact of intracoronary cell therapy on left ventricular function in the setting of acute myocardial infarction: A collaborative systematic review and meta-analysis of controlled clinical trials. J Am Coll Cardiol. 2007;50:1761–1767. doi: 10.1016/j.jacc.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Shoji M, Sata M, Fukuda D, Tanaka K, Sato T, Iso Y, Shibata M, Suzuki H, Koba S, Geshi E, Katagiri T. Temporal and spatial characterization of cellular constituents during neointimal hyperplasia after vascular injury: Potential contribution of bone-marrow-derived progenitors to arterial remodeling. Cardiovasc Pathol. 2004;13:306–312. doi: 10.1016/j.carpath.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Fadini GP, Baesso I, Albiero M, Sartore S, Agostini C, Avogaro A. Technical notes on endothelial progenitor cells: Ways to escape from the knowledge plateau. Atherosclerosis. 2008;197:496–503. doi: 10.1016/j.atherosclerosis.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 7.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 8.Kalka C, Masuda H, Takahashi T, Kalka-Moll WM, Silver M, Kearney M, Li T, Isner JM, Asahara T. Transplantation of ex vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci U S A. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Holgado N, Alberca M, Sanchez-Guijo F, Villaron E, Almeida J, Martin A, Armellini A, Garcia C, Blanco B, Sanchez-Abarca I, Martin S, Perez-Simon JA, Garcia-Sanz R, San Miguel JF, del Canizo MC. Short-term endothelial progenitor cell colonies are composed of monocytes and do not acquire endothelial markers. Cytotherapy. 2007;9:14–22. doi: 10.1080/14653240601047726. [DOI] [PubMed] [Google Scholar]
- 10.Prokopi M, Pula G, Mayr U, Devue C, Gallagher J, Xiao Q, Boulanger CM, Westwood N, Urbich C, Willeit J, Steiner M, Breuss J, Xu Q, Kiechl S, Mayr M. Proteomic analysis reveals presence of platelet microparticles in endothelial progenitor cell cultures. Blood. 2009;114:723–732. doi: 10.1182/blood-2009-02-205930. [DOI] [PubMed] [Google Scholar]
- 11.Koyanagi M, Brandes RP, Haendeler J, Zeiher AM, Dimmeler S. Cell-to-cell connection of endothelial progenitor cells with cardiac myocytes by nanotubes: A novel mechanism for cell fate changes? Circ Res. 2005;96:1039–1041. doi: 10.1161/01.RES.0000168650.23479.0c. [DOI] [PubMed] [Google Scholar]
- 12.Rechavi O, Erlich Y, Amram H, Flomenblit L, Karginov FV, Goldstein I, Hannon GJ, Kloog Y. Cell contact-dependent acquisition of cellular and viral nonautonomously encoded small rnas. Genes Dev. 2009;23:1971–1979. doi: 10.1101/gad.1789609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collino F, Deregibus MC, Bruno S, Sterpone L, Aghemo G, Viltono L, Tetta C, Camussi G. Microvesicles derived from adult human bone marrow and tissue specific mesenchymal stem cells shuttle selected pattern of mirnas. PLoS One. 2010;5:e11803. doi: 10.1371/journal.pone.0011803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D’Amario D, del Monte F, Urbanek K, D’Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation. 2011;123:1287–1296. doi: 10.1161/CIRCULATIONAHA.110.982918. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Aliotta JM, Pereira M, Johnson KW, de Paz N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D, Lee D, Ramratnam B, McMillan PN, Hixson DC, Josic D, Quesenberry PJ. Microvesicle entry into marrow cells mediates tissue-specific changes in mrna by direct delivery of mrna and induction of transcription. Exp Hematol. 2010;38:233–245. doi: 10.1016/j.exphem.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohtani K, Vlachojannis GJ, Koyanagi M, Boeckel JN, Urbich C, Farcas R, Bonig H, Marquez VE, Zeiher AM, Dimmeler S. Epigenetic regulation of endothelial lineage committed genes in pro-angiogenic hematopoietic and endothelial progenitor cells. Circ Res. 2011 doi: 10.1161/CIRCRESAHA.111.247304. [DOI] [PubMed] [Google Scholar]
- 17.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 18.Hur J, Yang HM, Yoon CH, Lee CS, Park KW, Kim JH, Kim TY, Kim JY, Kang HJ, Chae IH, Oh BH, Park YB, Kim HS. Identification of a novel role of t cells in postnatal vasculogenesis. Characterization of endothelial progenitor cell colonies. Circulation. 2007 doi: 10.1161/CIRCULATIONAHA.107.694778. [DOI] [PubMed] [Google Scholar]
- 19.Rohde E, Bartmann C, Schallmoser K, Reinisch A, Lanzer G, Linkesch W, Guelly C, Strunk D. Immune cells mimic the morphology of endothelial progenitor colonies in vitro. Stem Cells. 2007 doi: 10.1634/stemcells.2006-0833. [DOI] [PubMed] [Google Scholar]
- 20.Rohde E, Malischnik C, Thaler D, Maierhofer T, Linkesch W, Lanzer G, Guelly C, Strunk D. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 2006;24:357–367. doi: 10.1634/stemcells.2005-0072. [DOI] [PubMed] [Google Scholar]
- 21.Rehman J, Li J, Parvathaneni L, Karlsson G, Panchal VR, Temm CJ, Mahenthiran J, March KL. Exercise acutely increases circulating endothelial progenitor cells and monocyte-/macrophage-derived angiogenic cells. J Am Coll Cardiol. 2004;43:2314–2318. doi: 10.1016/j.jacc.2004.02.049. [DOI] [PubMed] [Google Scholar]
- 22.Guven H, Shepherd RM, Bach RG, Capoccia BJ, Link DC. The number of endothelial progenitor cell colonies in the blood is increased in patients with angiographically significant coronary artery disease. J Am Coll Cardiol. 2006;48:1579–1587. doi: 10.1016/j.jacc.2006.04.101. [DOI] [PubMed] [Google Scholar]
- 23.Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ, Hwang KK, Oh BH, Lee MM, Park YB. Characterization of two types of endothelial progenitor cells and their different contributions to neovasculogenesis. Arterioscler Thromb Vasc Biol. 2004;24:288–293. doi: 10.1161/01.ATV.0000114236.77009.06. [DOI] [PubMed] [Google Scholar]
- 24.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ingram DA, Mead LE, Tanaka H, Meade V, Fenoglio A, Mortell K, Pollok K, Ferkowicz MJ, Gilley D, Yoder MC. Identification of a novel hierarchy of endothelial progenitor cells using human peripheral and umbilical cord blood. Blood. 2004;104:2752–2760. doi: 10.1182/blood-2004-04-1396. [DOI] [PubMed] [Google Scholar]
- 26.Meneveau N, Deschaseaux F, Seronde MF, Chopard R, Schiele F, Jehl J, Tiberghien P, Bassand JP, Kantelip JP, Davani S. Presence of endothelial colony-forming cells is associated with reduced microvascular obstruction limiting infarct size and left ventricular remodelling in patients with acute myocardial infarction. Basic Res Cardiol. 2011 doi: 10.1007/s00395-011-0220-x. [DOI] [PubMed] [Google Scholar]
- 27.Wang CH, Hsieh IC, Su Pang JH, Cherng WJ, Lin SJ, Tung TH, Mei HF. Factors associated with purity, biological function, and activation potential of endothelial colony-forming cells. Am J Physiol Regul Integr Comp Physiol. 2011;300:R586–594. doi: 10.1152/ajpregu.00450.2010. [DOI] [PubMed] [Google Scholar]
- 28.Reinisch A, Hofmann NA, Obenauf AC, Kashofer K, Rohde E, Schallmoser K, Flicker K, Lanzer G, Linkesch W, Speicher MR, Strunk D. Humanized large-scale expanded endothelial colony-forming cells function in vitro and in vivo. Blood. 2009;113:6716–6725. doi: 10.1182/blood-2008-09-181362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Case J, Mead LE, Bessler WK, Prater D, White HA, Saadatzadeh MR, Bhavsar JR, Yoder MC, Haneline LS, Ingram DA. Human cd34+ac133+vegfr-2+ cells are not endothelial progenitor cells but distinct, primitive hematopoietic progenitors. Exp Hematol. 2007;35:1109–1118. doi: 10.1016/j.exphem.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Masuda H, Alev C, Akimaru H, Ito R, Shizuno T, Kobori M, Horii M, Ishihara T, Isobe K, Isozaki M, Itoh J, Itoh Y, Okada Y, McIntyre BA, Kato S, Asahara T. Methodological development of a clonogenic assay to determine endothelial progenitor cell potential. Circ Res. 2011;109:20–37. doi: 10.1161/CIRCRESAHA.110.231837. [DOI] [PubMed] [Google Scholar]
- 31.Gunetti M, Noghero A, Molla F, Staszewsky LI, de Angelis N, Soldo A, Russo I, Errichiello E, Frasson C, Rustichelli D, Ferrero I, Gualandris A, Berger M, Geuna M, Scacciatella P, Basso G, Marra S, Bussolino F, Latini R, Fagioli F. Ex vivo-expanded bone marrow cd34(+) for acute myocardial infarction treatment: In vitro and in vivo studies. Cytotherapy. 2011;13:1140–1152. doi: 10.3109/14653249.2011.597559. [DOI] [PubMed] [Google Scholar]
- 32.Yang J, Ii M, Kamei N, Alev C, Kwon SM, Kawamoto A, Akimaru H, Masuda H, Sawa Y, Asahara T. Cd34+ cells represent highly functional endothelial progenitor cells in murine bone marrow. PLoS One. 2011;6:e20219. doi: 10.1371/journal.pone.0020219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urbich C, Dimmeler S. Endothelial progenitor cells: Characterization and role in vascular biology. Circ Res. 2004;95:343–353. doi: 10.1161/01.RES.0000137877.89448.78. [DOI] [PubMed] [Google Scholar]
- 34.Yoon CH, Hur J, Park KW, Kim JH, Lee CS, Oh IY, Kim TY, Cho HJ, Kang HJ, Chae IH, Yang HK, Oh BH, Park YB, Kim HS. Synergistic neovascularization by mixed transplantation of early endothelial progenitor cells and late outgrowth endothelial cells: The role of angiogenic cytokines and matrix metalloproteinases. Circulation. 2005;112:1618–1627. doi: 10.1161/CIRCULATIONAHA.104.503433. [DOI] [PubMed] [Google Scholar]
- 35.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: In vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, Dimmeler S. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–742. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 37.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–1169. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 38.Heil M, Ziegelhoeffer T, Mees B, Schaper W. A different outlook on the role of bone marrow stem cells in vascular growth: Bone marrow delivers software not hardware. Circ Res. 2004;94:573–574. doi: 10.1161/01.RES.0000124603.46777.EB. [DOI] [PubMed] [Google Scholar]
- 39.Deb A, Patterson C. Hard luck stories: The reality of endothelial progenitor cells continues to fall short of the promise. Circulation. 2010;121:850–852. doi: 10.1161/CIR.0b013e3181d4c360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hagensen MK, Raarup MK, Mortensen MB, Thim T, Nyengaard JR, Falk E, Bentzon JF. Circulating endothelial progenitor cells do not contribute to regeneration of endothelium after murine arterial injury. Cardiovasc Res. 2011 doi: 10.1093/cvr/cvr278. [DOI] [PubMed] [Google Scholar]
- 41.Hagensen MK, Shim J, Falk E, Bentzon JF. Flanking recipient vasculature, not circulating progenitor cells, contributes to endothelium and smooth muscle in murine allograft vasculopathy. Arterioscler Thromb Vasc Biol. 2011;31:808–813. doi: 10.1161/ATVBAHA.110.221184. [DOI] [PubMed] [Google Scholar]
- 42.Wickersheim A, Kerber M, de Miguel LS, Plate KH, Machein MR. Endothelial progenitor cells do not contribute to tumor endothelium in primary and metastatic tumors. Int J Cancer. 2009;125:1771–1777. doi: 10.1002/ijc.24605. [DOI] [PubMed] [Google Scholar]
- 43.Sieveking DP, Buckle A, Celermajer DS, Ng MK. Strikingly different angiogenic properties of endothelial progenitor cell subpopulations: Insights from a novel human angiogenesis assay. J Am Coll Cardiol. 2008;51:660–668. doi: 10.1016/j.jacc.2007.09.059. [DOI] [PubMed] [Google Scholar]
- 44.Kawamoto A, Tkebuchava T, Yamaguchi J, Nishimura H, Yoon YS, Milliken C, Uchida S, Masuo O, Iwaguro H, Ma H, Hanley A, Silver M, Kearney M, Losordo DW, Isner JM, Asahara T. Intramyocardial transplantation of autologous endothelial progenitor cells for therapeutic neovascularization of myocardial ischemia. Circulation. 2003;107:461–468. doi: 10.1161/01.cir.0000046450.89986.50. [DOI] [PubMed] [Google Scholar]
- 45.Kocher AA, Schuster MD, Szabolcs MJ, Takuma S, Burkhoff D, Wang J, Homma S, Edwards NM, Itescu S. Neovascularization of ischemic myocardium by human bone-marrow-derived angioblasts prevents cardiomyocyte apoptosis, reduces remodeling and improves cardiac function. Nat Med. 2001;7:430–436. doi: 10.1038/86498. [DOI] [PubMed] [Google Scholar]
- 46.Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A, Burchfield J, Fox H, Doebele C, Ohtani K, Chavakis E, Potente M, Tjwa M, Urbich C, Zeiher AM, Dimmeler S. Microrna-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science. 2009;324:1710–1713. doi: 10.1126/science.1174381. [DOI] [PubMed] [Google Scholar]
- 47.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P. Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood. 2004;104:2084–2086. doi: 10.1182/blood-2004-01-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 49.Fadini GP, Albiero M, Boscaro E, Agostini C, Avogaro A. Endothelial progenitor cells as resident accessory cells for post-ischemic angiogenesis. Atherosclerosis. 2009;204:20–22. doi: 10.1016/j.atherosclerosis.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 50.Ziebart T, Yoon CH, Trepels T, Wietelmann A, Braun T, Kiessling F, Stein S, Grez M, Ihling C, Muhly-Reinholz M, Carmona G, Urbich C, Zeiher AM, Dimmeler S. Sustained persistence of transplanted proangiogenic cells contributes to neovascularization and cardiac function after ischemia. Circ Res. 2008;103:1327–1334. doi: 10.1161/CIRCRESAHA.108.180463. [DOI] [PubMed] [Google Scholar]
- 51.Gehling UM, Ergun S, Schumacher U, Wagener C, Pantel K, Otte M, Schuch G, Schafhausen P, Mende T, Kilic N, Kluge K, Schafer B, Hossfeld DK, Fiedler W. In vitro differentiation of endothelial cells from ac133-positive progenitor cells. Blood. 2000;95:3106–3112. [PubMed] [Google Scholar]
- 52.Kawamoto A, Iwasaki H, Kusano K, Murayama T, Oyamada A, Silver M, Hulbert C, Gavin M, Hanley A, Ma H, Kearney M, Zak V, Asahara T, Losordo DW. Cd34-positive cells exhibit increased potency and safety for therapeutic neovascularization after myocardial infarction compared with total mononuclear cells. Circulation. 2006;114:2163–2169. doi: 10.1161/CIRCULATIONAHA.106.644518. [DOI] [PubMed] [Google Scholar]
- 53.Ramos AL, Darabi R, Akbarloo N, Borges L, Catanese J, Dineen SP, Brekken RA, Perlingeiro RC. Clonal analysis reveals a common progenitor for endothelial, myeloid, and lymphoid precursors in umbilical cord blood. Circ Res. 2010;107:1460–1469. doi: 10.1161/CIRCRESAHA.110.223669. [DOI] [PubMed] [Google Scholar]
- 54.Pozzoli O, Vella P, Iaffaldano G, Parente V, Devanna P, Lacovich M, Lamia CL, Fascio U, Longoni D, Cotelli F, Capogrossi MC, Pesce M. Endothelial fate and angiogenic properties of human cd34+ progenitor cells in zebrafish. Arterioscler Thromb Vasc Biol. 2011;31:1589–1597. doi: 10.1161/ATVBAHA.111.226969. [DOI] [PubMed] [Google Scholar]
- 55.Fadini GP, Avogaro A, Agostini C. Critical assessment of putative endothelial progenitor phenotypes. Exp Hematol. 2007;35:1479–1480. doi: 10.1016/j.exphem.2007.07.013. author reply 1481–1472. [DOI] [PubMed] [Google Scholar]
- 56.Seeger FH, Rasper T, Koyanagi M, Fox H, Zeiher AM, Dimmeler S. Cxcr4 expression determines functional activity of bone marrow-derived mononuclear cells for therapeutic neovascularization in acute ischemia. Arterioscler Thromb Vasc Biol. 2009 doi: 10.1161/ATVBAHA.109.194688. [DOI] [PubMed] [Google Scholar]
- 57.Kim SW, Kim H, Cho HJ, Lee JU, Levit R, Yoon YS. Human peripheral blood-derived cd31+ cells have robust angiogenic and vasculogenic properties and are effective for treating ischemic vascular disease. J Am Coll Cardiol. 2010;56:593–607. doi: 10.1016/j.jacc.2010.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H, Cho HJ, Kim SW, Liu B, Choi YJ, Lee J, Sohn YD, Lee MY, Houge MA, Yoon YS. Cd31+ cells represent highly angiogenic and vasculogenic cells in bone marrow: Novel role of nonendothelial cd31+ cells in neovascularization and their therapeutic effects on ischemic vascular disease. Circ Res. 2010;107:602–614. doi: 10.1161/CIRCRESAHA.110.218396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldie LC, Nix MK, Hirschi KK. Embryonic vasculogenesis and hematopoietic specification. Organogenesis. 2008;4:257–263. doi: 10.4161/org.4.4.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bailey AS, Jiang S, Afentoulis M, Baumann CI, Schroeder DA, Olson SB, Wong MH, Fleming WH. Transplanted adult hematopoietic stems cells differentiate into functional endothelial cells. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 61.Gunsilius E, Duba HC, Petzer AL, Kahler CM, Grunewald K, Stockhammer G, Gabl C, Dirnhofer S, Clausen J, Gastl G. Evidence from a leukaemia model for maintenance of vascular endothelium by bone-marrow-derived endothelial cells. Lancet. 2000;355:1688–1691. doi: 10.1016/S0140-6736(00)02241-8. [DOI] [PubMed] [Google Scholar]
- 62.Chao H, Hirschi KK. Hemato-vascular origins of endothelial progenitor cells? Microvasc Res. 2010;79:169–173. doi: 10.1016/j.mvr.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G. The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature. 2009;457:892–895. doi: 10.1038/nature07679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puato M, Faggin E, Favaretto E, Bertipaglia B, Rattazzi M, Rizzoni D, Gamba GP, Sartore S, Rosei EA, Pessina AC, Pauletto P. Prevalence of fetal-type smooth muscle cells in the media of microvessels from hypertensive patients. Hypertension. 2004;44:191–194. doi: 10.1161/01.HYP.0000133692.47754.e5. [DOI] [PubMed] [Google Scholar]
- 65.Mercola M, Ruiz-Lozano P, Schneider MD. Cardiac muscle regeneration: Lessons from development. Genes Dev. 2011;25:299–309. doi: 10.1101/gad.2018411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zape JP, Zovein AC. Hemogenic endothelium: Origins, regulation, and implications for vascular biology. Semin Cell Dev Biol. 2011 doi: 10.1016/j.semcdb.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 67.Hirschi KK, Ingram DA, Yoder MC. Assessing identity, phenotype, and fate of endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2008;28:1584–1595. doi: 10.1161/ATVBAHA.107.155960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hristov M, Erl W, Weber PC. Endothelial progenitor cells: Isolation and characterization. Trends Cardiovasc Med. 2003;13:201–206. doi: 10.1016/s1050-1738(03)00077-x. [DOI] [PubMed] [Google Scholar]
- 69.Leor J, Marber M. Endothelial progenitors: A new tower of babel? J Am Coll Cardiol. 2006;48:1588–1590. doi: 10.1016/j.jacc.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 70.Heiss C, Keymel S, Niesler U, Ziemann J, Kelm M, Kalka C. Impaired progenitor cell activity in age-related endothelial dysfunction. J Am Coll Cardiol. 2005;45:1441–1448. doi: 10.1016/j.jacc.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 71.Fadini GP, de Kreutzenberg S, Albiero M, Coracina A, Pagnin E, Baesso I, Cignarella A, Bolego C, Plebani M, Nardelli GB, Sartore S, Agostini C, Avogaro A. Gender differences in endothelial progenitor cells and cardiovascular risk profile: The role of female estrogens. Arterioscler Thromb Vasc Biol. 2008;28:997–1004. doi: 10.1161/ATVBAHA.107.159558. [DOI] [PubMed] [Google Scholar]
- 72.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 73.Bulut D, Tuns H, Mugge A. Cd31+/annexin v+ microparticles in healthy offsprings of patients with coronary artery disease. Eur J Clin Invest. 2009;39:17–22. doi: 10.1111/j.1365-2362.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 74.Humpert PM, Neuwirth R, Battista MJ, Voronko O, von Eynatten M, Konrade I, Rudofsky G, Jr, Wendt T, Hamann A, Morcos M, Nawroth PP, Bierhaus A. Sdf-1 genotype influences insulin-dependent mobilization of adult progenitor cells in type 2 diabetes. Diabetes Care. 2005;28:934–936. doi: 10.2337/diacare.28.4.934. [DOI] [PubMed] [Google Scholar]
- 75.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 76.Di Stefano R, Barsotti MC, Felice F, Magera A, Lekakis J, Leone A, Balbarini A. Smoking and endothelial progenitor cells: A revision of literature. Curr Pharm Des. 2010;16:2559–2566. doi: 10.2174/138161210792062939. [DOI] [PubMed] [Google Scholar]
- 77.Ludwig A, Jochmann N, Kertesz A, Kuhn C, Mueller S, Gericke C, Baumann G, Stangl K, Stangl V. Smoking decreases the level of circulating cd34+ progenitor cells in young healthy women--a pilot study. BMC Womens Health. 2010;10:20. doi: 10.1186/1472-6874-10-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yue WS, Wang M, Yan GH, Yiu KH, Yin L, Lee SW, Siu CW, Tse HF. Smoking is associated with depletion of circulating endothelial progenitor cells and elevated pulmonary artery systolic pressure in patients with coronary artery disease. Am J Cardiol. 2010;106:1248–1254. doi: 10.1016/j.amjcard.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 79.Michaud SE, Dussault S, Haddad P, Groleau J, Rivard A. Circulating endothelial progenitor cells from healthy smokers exhibit impaired functional activities. Atherosclerosis. 2006;187:423–432. doi: 10.1016/j.atherosclerosis.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 80.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and hoxa9 expression in cd34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 81.Oliveras A, Soler MJ, Martinez-Estrada OM, Vazquez S, Marco-Feliu D, Vila JS, Vilaro S, Lloveras J. Endothelial progenitor cells are reduced in refractory hypertension. J Hum Hypertens. 2008;22:183–190. doi: 10.1038/sj.jhh.1002304. [DOI] [PubMed] [Google Scholar]
- 82.Umemura T, Soga J, Hidaka T, Takemoto H, Nakamura S, Jitsuiki D, Nishioka K, Goto C, Teragawa H, Yoshizumi M, Chayama K, Higashi Y. Aging and hypertension are independent risk factors for reduced number of circulating endothelial progenitor cells. Am J Hypertens. 2008;21:1203–1209. doi: 10.1038/ajh.2008.278. [DOI] [PubMed] [Google Scholar]
- 83.Giannotti G, Doerries C, Mocharla PS, Mueller MF, Bahlmann FH, Horvath T, Jiang H, Sorrentino SA, Steenken N, Manes C, Marzilli M, Rudolph KL, Luscher TF, Drexler H, Landmesser U. Impaired endothelial repair capacity of early endothelial progenitor cells in prehypertension: Relation to endothelial dysfunction. Hypertension. 2010;55:1389–1397. doi: 10.1161/HYPERTENSIONAHA.109.141614. [DOI] [PubMed] [Google Scholar]
- 84.Delva P, Degan M, Vallerio P, Arosio E, Minuz P, Amen G, Di Chio M, Lechi A. Endothelial progenitor cells in patients with essential hypertension. J Hypertens. 2007;25:127–132. doi: 10.1097/HJH.0b013e3280109271. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Chen L, Su C, Xia WH, Wang Y, Wang JM, Chen F, Zhang YY, Wu F, Xu SY, Zhang XL, Tao J. Impaired endothelial progenitor cell activity is associated with reduced arterial elasticity in patients with essential hypertension. Clin Exp Hypertens. 2010;32:444–452. doi: 10.3109/10641961003686435. [DOI] [PubMed] [Google Scholar]
- 86.Lee CW, Huang PH, Huang SS, Leu HB, Huang CC, Wu TC, Chen JW, Lin SJ. Decreased circulating endothelial progenitor cell levels and function in essential hypertensive patients with electrocardiographic left ventricular hypertrophy. Hypertens Res. 2011 doi: 10.1038/hr.2011.68. [DOI] [PubMed] [Google Scholar]
- 87.Rossi F, Bertone C, Montanile F, Miglietta F, Lubrano C, Gandini L, Santiemma V. Hdl cholesterol is a strong determinant of endothelial progenitor cells in hypercholesterolemic subjects. Microvasc Res. 2010;80:274–279. doi: 10.1016/j.mvr.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 88.Chen JZ, Zhang FR, Tao QM, Wang XX, Zhu JH. Number and activity of endothelial progenitor cells from peripheral blood in patients with hypercholesterolaemia. Clin Sci (Lond) 2004;107:273–280. doi: 10.1042/CS20030389. [DOI] [PubMed] [Google Scholar]
- 89.Heida NM, Muller JP, Cheng IF, Leifheit-Nestler M, Faustin V, Riggert J, Hasenfuss G, Konstantinides S, Schafer K. Effects of obesity and weight loss on the functional properties of early outgrowth endothelial progenitor cells. J Am Coll Cardiol. 2010;55:357–367. doi: 10.1016/j.jacc.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 90.Tobler K, Freudenthaler A, Baumgartner-Parzer SM, Wolzt M, Ludvik B, Nansalmaa E, Nowotny PJ, Seidinger D, Steiner S, Luger A, Artwohl M. Reduction of both number and proliferative activity of human endothelial progenitor cells in obesity. Int J Obes (Lond) 2010;34:687–700. doi: 10.1038/ijo.2009.280. [DOI] [PubMed] [Google Scholar]
- 91.Muller-Ehmsen J, Braun D, Schneider T, Pfister R, Worm N, Wielckens K, Scheid C, Frommolt P, Flesch M. Decreased number of circulating progenitor cells in obesity: Beneficial effects of weight reduction. Eur Heart J. 2008;29:1560–1568. doi: 10.1093/eurheartj/ehn213. [DOI] [PubMed] [Google Scholar]
- 92.Egan CG, Lavery R, Caporali F, Fondelli C, Laghi-Pasini F, Dotta F, Sorrentino V. Generalised reduction of putative endothelial progenitors and cxcr4-positive peripheral blood cells in type 2 diabetes. Diabetologia. 2008;51:1296–1305. doi: 10.1007/s00125-008-0939-6. [DOI] [PubMed] [Google Scholar]
- 93.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 94.Fadini GP, Pucci L, Vanacore R, Baesso I, Penno G, Balbarini A, Di Stefano R, Miccoli R, de Kreutzenberg S, Coracina A, Tiengo A, Agostini C, Del Prato S, Avogaro A. Glucose tolerance is negatively associated with circulating progenitor cell levels. Diabetologia. 2007;50:2156–2163. doi: 10.1007/s00125-007-0732-y. [DOI] [PubMed] [Google Scholar]
- 95.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 96.Zhu J, Wang X, Chen J, Sun J, Zhang F. Reduced number and activity of circulating endothelial progenitor cells from patients with hyperhomocysteinemia. Arch Med Res. 2006;37:484–489. doi: 10.1016/j.arcmed.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 97.Makino H, Okada S, Nagumo A, Sugisawa T, Miyamoto Y, Kishimoto I, Kikuchi-Taura A, Soma T, Taguchi A, Yoshimasa Y. Decreased circulating cd34+ cells are associated with progression of diabetic nephropathy. Diabet Med. 2009;26:171–173. doi: 10.1111/j.1464-5491.2008.02638.x. [DOI] [PubMed] [Google Scholar]
- 98.George J, Goldstein E, Abashidze S, Deutsch V, Shmilovich H, Finkelstein A, Herz I, Miller H, Keren G. Circulating endothelial progenitor cells in patients with unstable angina: Association with systemic inflammation. Eur Heart J. 2004;25:1003–1008. doi: 10.1016/j.ehj.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 99.Dei Cas A, Spigoni V, Ardigo D, Pedrazzi G, Franzini L, Derlindati E, Urbani S, Monti L, Gnudi L, Zavaroni I. Reduced circulating endothelial progenitor cell number in healthy young adult hyperinsulinemic men. Nutr Metab Cardiovasc Dis. 2011;21:512–517. doi: 10.1016/j.numecd.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Fadini GP, Agostini C, Boscaro E, Avogaro A. Mechanisms and significance of progenitor cell reduction in the metabolic syndrome. Metab Syndr Relat Disord. 2009;7:5–10. doi: 10.1089/met.2008.0067. [DOI] [PubMed] [Google Scholar]
- 101.Fadini GP, de Kreutzenberg SV, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A. Circulating cd34+ cells, metabolic syndrome, and cardiovascular risk. Eur Heart J. 2006;27:2247–2255. doi: 10.1093/eurheartj/ehl198. [DOI] [PubMed] [Google Scholar]
- 102.Bahlmann FH, de Groot K, Mueller O, Hertel B, Haller H, Fliser D. Stimulation of endothelial progenitor cells: A new putative therapeutic effect of angiotensin ii receptor antagonists. Hypertension. 2005;45:526–529. doi: 10.1161/01.HYP.0000159191.98140.89. [DOI] [PubMed] [Google Scholar]
- 103.Benndorf RA, Gehling UM, Appel D, Maas R, Schwedhelm E, Schlagner K, Silberhorn E, Hossfeld DK, Rogiers X, Boger R. Mobilization of putative high-proliferative-potential endothelial colony-forming cells during antihypertensive treatment in patients with essential hypertension. Stem Cells Dev. 2007;16:329–338. doi: 10.1089/scd.2006.0074. [DOI] [PubMed] [Google Scholar]
- 104.Fadini GP, de Kreutzenberg SV, Mariano V, Boscaro E, Bertolini F, Mancuso P, Quarna J, Marescotti M, Agostini C, Tiengo A, Avogaro A. Optimized glycaemic control achieved with add-on basal insulin therapy improves indexes of endothelial damage and regeneration in type 2 diabetic patients with macroangiopathy: A randomized crossover trial comparing detemir versus glargine. Diabetes Obes Metab. 2011;13:718–725. doi: 10.1111/j.1463-1326.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 105.Fadini GP, Avogaro A. Potential manipulation of endothelial progenitor cells in diabetes and its complications. Diabetes Obes Metab. 2010;12:570–583. doi: 10.1111/j.1463-1326.2010.01210.x. [DOI] [PubMed] [Google Scholar]
- 106.Ramunni A, Brescia P, Dambra P, Capuzzimati L, Ria R, De Tullio G, Resta F, Russi G, Vacca A, Coratelli P. Effect of low-density lipoprotein apheresis on circulating endothelial progenitor cells in familial hypercholesterolemia. Blood Purif. 2010;29:383–389. doi: 10.1159/000314650. [DOI] [PubMed] [Google Scholar]
- 107.Vasa M, Fichtlscherer S, Adler K, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 108.Fadini GP, Agostini C, Sartore S, Avogaro A. Endothelial progenitor cells in the natural history of atherosclerosis. Atherosclerosis. 2007;194:46–54. doi: 10.1016/j.atherosclerosis.2007.03.046. [DOI] [PubMed] [Google Scholar]
- 109.Huang PH, Chen YH, Chen YL, Wu TC, Chen JW, Lin SJ. Vascular endothelial function and circulating endothelial progenitor cells in patients with cardiac syndrome x. Heart. 2007;93:1064–1070. doi: 10.1136/hrt.2006.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fadini GP, Pagano C, Baesso I, Kotsafti O, Doro D, de Kreutzenberg SV, Avogaro A, Agostini C, Dorigo MT. Reduced endothelial progenitor cells and brachial artery flow-mediated dilation as evidence of endothelial dysfunction in ocular hypertension and primary open-angle glaucoma. Acta Ophthalmol. 2010;88:135–141. doi: 10.1111/j.1755-3768.2009.01573.x. [DOI] [PubMed] [Google Scholar]
- 111.Esposito K, Ciotola M, Maiorino MI, Giugliano F, Autorino R, De Sio M, Jannini E, Lenzi A, Giugliano D. Circulating cd34+ kdr+ endothelial progenitor cells correlate with erectile function and endothelial function in overweight men. J Sex Med. 2009;6:107–114. doi: 10.1111/j.1743-6109.2008.01042.x. [DOI] [PubMed] [Google Scholar]
- 112.Sibal L, Aldibbiat A, Agarwal SC, Mitchell G, Oates C, Razvi S, Weaver JU, Shaw JA, Home PD. Circulating endothelial progenitor cells, endothelial function, carotid intima-media thickness and circulating markers of endothelial dysfunction in people with type 1 diabetes without macrovascular disease or microalbuminuria. Diabetologia. 2009;52:1464–1473. doi: 10.1007/s00125-009-1401-0. [DOI] [PubMed] [Google Scholar]
- 113.Mok MY, Yiu KH, Wong CY, Qiuwaxi J, Lai WH, Wong WS, Tse HF, Lau CS. Low circulating level of cd133+kdr+cells in patients with systemic sclerosis. Clin Exp Rheumatol. 2010;28:S19–25. [PubMed] [Google Scholar]
- 114.Calo LA, Facco M, Davis PA, Pagnin E, Maso LD, Puato M, Caielli P, Agostini C, Pessina AC. Endothelial progenitor cells relationships with clinical and biochemical factors in a human model of blunted angiotensin ii signaling. Hypertens Res. 2011 doi: 10.1038/hr.2011.72. [DOI] [PubMed] [Google Scholar]
- 115.Fadini GP, Coracina A, Baesso I, Agostini C, Tiengo A, Avogaro A, de Kreutzenberg SV. Peripheral blood cd34+kdr+ endothelial progenitor cells are determinants of subclinical atherosclerosis in a middle-aged general population. Stroke. 2006;37:2277–2282. doi: 10.1161/01.STR.0000236064.19293.79. [DOI] [PubMed] [Google Scholar]
- 116.Chironi G, Walch L, Pernollet MG, Gariepy J, Levenson J, Rendu F, Simon A. Decreased number of circulating cd34+kdr+ cells in asymptomatic subjects with preclinical atherosclerosis. Atherosclerosis. 2007;191:115–120. doi: 10.1016/j.atherosclerosis.2006.02.041. [DOI] [PubMed] [Google Scholar]
- 117.Bielak LF, Horenstein RB, Ryan KA, Sheedy PF, Rumberger JA, Tanner K, Post W, Mitchell BD, Shuldiner AR, Peyser PA. Circulating cd34+ cell count is associated with extent of subclinical atherosclerosis in asymptomatic amish men, independent of 10-year framingham risk. Clin Med Cardiol. 2009;3:53–60. doi: 10.4137/cmc.s2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schmidt-Lucke C, Fichtlscherer S, Aicher A, Tschope C, Schultheiss HP, Zeiher AM, Dimmeler S. Quantification of circulating endothelial progenitor cells using the modified ishage protocol. PLoS One. 2010;5:e13790. doi: 10.1371/journal.pone.0013790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kunz GA, Liang G, Cuculi F, Gregg D, Vata KC, Shaw LK, Goldschmidt-Clermont PJ, Dong C, Taylor DA, Peterson ED. Circulating endothelial progenitor cells predict coronary artery disease severity. Am Heart J. 2006;152:190–195. doi: 10.1016/j.ahj.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 120.Wang HY, Gao PJ, Ji KD, Shen WF, Fan CL, Lu L, Zhu DL. Circulating endothelial progenitor cells, c-reactive protein and severity of coronary stenosis in chinese patients with coronary artery disease. Hypertens Res. 2007;30:133–141. doi: 10.1291/hypres.30.133. [DOI] [PubMed] [Google Scholar]
- 121.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 122.Lau KK, Chan YH, Yiu KH, Li SW, Tam S, Lau CP, Kwong YL, Tse HF. Burden of carotid atherosclerosis in patients with stroke: Relationships with circulating endothelial progenitor cells and hypertension. J Hum Hypertens. 2007;21:445–451. doi: 10.1038/sj.jhh.1002178. [DOI] [PubMed] [Google Scholar]
- 123.Bogoslovsky T, Chaudhry A, Latour L, Maric D, Luby M, Spatz M, Frank J, Warach S. Endothelial progenitor cells correlate with lesion volume and growth in acute stroke. Neurology. 2010;75:2059–2062. doi: 10.1212/WNL.0b013e318200d741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hughes AD, Coady E, Raynor S, Mayet J, Wright AR, Shore AC, Kooner JS, Thom SA, Chaturvedi N. Reduced endothelial progenitor cells in european and south asian men with atherosclerosis. Eur J Clin Invest. 2007;37:35–41. doi: 10.1111/j.1365-2362.2007.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ghani U, Shuaib A, Salam A, Nasir A, Shuaib U, Jeerakathil T, Sher F, O’Rourke F, Nasser AM, Schwindt B, Todd K. Endothelial progenitor cells during cerebrovascular disease. Stroke. 2005;36:151–153. doi: 10.1161/01.STR.0000149944.15406.16. [DOI] [PubMed] [Google Scholar]
- 126.Xiao Q, Kiechl S, Patel S, Oberhollenzer F, Weger S, Mayr A, Metzler B, Reindl M, Hu Y, Willeit J, Xu Q. Endothelial progenitor cells, cardiovascular risk factors, cytokine levels and atherosclerosis--results from a large population-based study. PLoS One. 2007;2:e975. doi: 10.1371/journal.pone.0000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shintani S, Murohara T, Ikeda H, Ueno T, Honma T, Katoh A, Sasaki K, Shimada T, Oike Y, Imaizumi T. Mobilization of endothelial progenitor cells in patients with acute myocardial infarction. Circulation. 2001;103:2776–2779. doi: 10.1161/hc2301.092122. [DOI] [PubMed] [Google Scholar]
- 128.Leone AM, Rutella S, Bonanno G, Abbate A, Rebuzzi AG, Giovannini S, Lombardi M, Galiuto L, Liuzzo G, Andreotti F, Lanza GA, Contemi AM, Leone G, Crea F. Mobilization of bone marrow-derived stem cells after myocardial infarction and left ventricular function. Eur Heart J. 2005;26:1196–1204. doi: 10.1093/eurheartj/ehi164. [DOI] [PubMed] [Google Scholar]
- 129.Massa M, Rosti V, Ferrario M, Campanelli R, Ramajoli I, Rosso R, De Ferrari GM, Ferlini M, Goffredo L, Bertoletti A, Klersy C, Pecci A, Moratti R, Tavazzi L. Increased circulating hematopoietic and endothelial progenitor cells in the early phase of acute myocardial infarction. Blood. 2005;105:199–206. doi: 10.1182/blood-2004-05-1831. [DOI] [PubMed] [Google Scholar]
- 130.Sobrino T, Hurtado O, Moro MA, Rodriguez-Yanez M, Castellanos M, Brea D, Moldes O, Blanco M, Arenillas JF, Leira R, Davalos A, Lizasoain I, Castillo J. The increase of circulating endothelial progenitor cells after acute ischemic stroke is associated with good outcome. Stroke. 2007;38:2759–2764. doi: 10.1161/STROKEAHA.107.484386. [DOI] [PubMed] [Google Scholar]
- 131.Yip HK, Chang LT, Chang WN, Lu CH, Liou CW, Lan MY, Liu JS, Youssef AA, Chang HW. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2008;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 132.Bogoslovsky T, Spatz M, Chaudhry A, Maric D, Luby M, Frank J, Warach S. Stromal-derived factor-1[alpha] correlates with circulating endothelial progenitor cells and with acute lesion volume in stroke patients. Stroke. 2011;42:618–625. doi: 10.1161/STROKEAHA.110.596007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Aicher A, Zeiher AM, Dimmeler S. Mobilizing endothelial progenitor cells. Hypertension. 2005;45:321–325. doi: 10.1161/01.HYP.0000154789.28695.ea. [DOI] [PubMed] [Google Scholar]
- 134.Kuliczkowski W, Derzhko R, Prajs I, Podolak-Dawidziak M, Serebruany VL. Endothelial progenitor cells and left ventricle function in patients with acute myocardial infarction: Potential therapeutic considertions. Am J Ther. 2010 doi: 10.1097/MJT.0b013e3181e0cab3. [DOI] [PubMed] [Google Scholar]
- 135.Fadini GP. Is bone marrow another target of diabetic complications? Eur J Clin Invest. 2011;41:457–463. doi: 10.1111/j.1365-2362.2010.02417.x. [DOI] [PubMed] [Google Scholar]
- 136.Andreou I, Tousoulis D, Tentolouris C, Antoniades C, Stefanadis C. Potential role of endothelial progenitor cells in the pathophysiology of heart failure: Clinical implications and perspectives. Atherosclerosis. 2006;189:247–254. doi: 10.1016/j.atherosclerosis.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 137.Valgimigli M, Rigolin GM, Fucili A, Porta MD, Soukhomovskaia O, Malagutti P, Bugli AM, Bragotti LZ, Francolini G, Mauro E, Castoldi G, Ferrari R. Cd34+ and endothelial progenitor cells in patients with various degrees of congestive heart failure. Circulation. 2004;110:1209–1212. doi: 10.1161/01.CIR.0000136813.89036.21. [DOI] [PubMed] [Google Scholar]
- 138.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 139.Fritzenwanger M, Lorenz F, Jung C, Fabris M, Thude H, Barz D, Figulla HR. Differential number of cd34+, cd133+ and cd34+/cd133+ cells in peripheral blood of patients with congestive heart failure. Eur J Med Res. 2009;14:113–117. doi: 10.1186/2047-783X-14-3-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gatta L, Armani A, Iellamo F, Consoli C, Molinari F, Caminiti G, Volterrani M, Rosano GM. Effects of a short-term exercise training on serum factors involved in ventricular remodelling in chronic heart failure patients. Int J Cardiol. 2010 doi: 10.1016/j.ijcard.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 141.Erbs S, Hollriegel R, Linke A, Beck EB, Adams V, Gielen S, Mobius-Winkler S, Sandri M, Krankel N, Hambrecht R, Schuler G. Exercise training in patients with advanced chronic heart failure (nyha iiib) promotes restoration of peripheral vasomotor function, induction of endogenous regeneration, and improvement of left ventricular function. Circ Heart Fail. 2010;3:486–494. doi: 10.1161/CIRCHEARTFAILURE.109.868992. [DOI] [PubMed] [Google Scholar]
- 142.Van Craenenbroeck EM, Bruyndonckx L, Van Berckelaer C, Hoymans VY, Vrints CJ, Conraads VM. The effect of acute exercise on endothelial progenitor cells is attenuated in chronic heart failure. Eur J Appl Physiol. 2011;111:2375–2379. doi: 10.1007/s00421-011-1843-1. [DOI] [PubMed] [Google Scholar]
- 143.Schmidt-Lucke C, Rossig L, Fichtlscherer S, Vasa M, Britten M, Kamper U, Dimmeler S, Zeiher AM. Reduced number of circulating endothelial progenitor cells predicts future cardiovascular events: Proof of concept for the clinical importance of endogenous vascular repair. Circulation. 2005;111:2981–2987. doi: 10.1161/CIRCULATIONAHA.104.504340. [DOI] [PubMed] [Google Scholar]
- 144.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 145.Maruyama S, Taguchi A, Iwashima S, Ozaki T, Yasuda K, Kikuchi-Taura A, Soma T, Ishii H, Murohara T, Takahashi H, Kasuga H, Kumada Y, Toriyama T, Ito Y, Kawahara H, Yuzawa Y, Matsuo S. Low circulating cd34+ cell count is associated with poor prognosis in chronic hemodialysis patients. Kidney Int. 2008;74:1603–1609. doi: 10.1038/ki.2008.495. [DOI] [PubMed] [Google Scholar]
- 146.Lorenzen J, David S, Bahlmann FH, de Groot K, Bahlmann E, Kielstein JT, Haller H, Fliser D. Endothelial progenitor cells and cardiovascular events in patients with chronic kidney disease--a prospective follow-up study. PLoS One. 2010;5:e11477. doi: 10.1371/journal.pone.0011477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fadini GP, de Kreutzenberg S, Agostini C, Boscaro E, Tiengo A, Dimmeler S, Avogaro A. Low cd34+ cell count and metabolic syndrome synergistically increase the risk of adverse outcomes. Atherosclerosis. 2009;207:213–219. doi: 10.1016/j.atherosclerosis.2009.03.040. [DOI] [PubMed] [Google Scholar]
- 148.Fadini GP, Maruyama S, Ozaki T, Taguchi A, Meigs J, Dimmeler S, Zeiher AM, de Kreutzenberg S, Avogaro A, Nickenig G, Schmidt-Lucke C, Werner N. Circulating progenitor cell count for cardiovascular risk stratification: A pooled analysis. PLoS One. 2010;5:e11488. doi: 10.1371/journal.pone.0011488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 150.Balconi G, Lehmann R, Fiordaliso F, Assmus B, Dimmeler S, Sarto P, Carbonieri E, Gualco A, Campana C, Angelici L, Masson S, Mohammed SA, Dejana E, Gorini M, Zeiher AM, Latini R. Levels of circulating pro-angiogenic cells predict cardiovascular outcomes in patients with chronic heart failure. J Card Fail. 2009;15:747–755. doi: 10.1016/j.cardfail.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 151.Schwartzenberg S, Afek A, Charach G, Rubinstein A, Ben-Shoshan Y, Kissil S, Maisel-Auslender S, Keren G, George J. Comparative analysis of the predictive power of different endothelial progenitor cell phenotypes on cardiovascular outcome. World J Cardiol. 2010;2:299–304. doi: 10.4330/wjc.v2.i9.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Porto I, Leone AM, De Maria GL, Craig CH, Tritarelli A, Camaioni C, Natale L, Niccoli G, Biasucci LM, Crea F. Are endothelial progenitor cells mobilized by myocardial ischemia or myocardial necrosis? A cardiac magnetic resonance study. Atherosclerosis. 2011;216:355–358. doi: 10.1016/j.atherosclerosis.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 153.Schober A, Hristov M, Kofler S, Forbrig R, Lohr B, Heussen N, Zhe Z, Akhtar S, Schumann U, Krotz F, Leibig M, Konig A, Kaczmarek I, Reichart B, Klauss V, Weber C, Sohn HY. Cd34+cd140b+ cells and circulating cxcl12 correlate with the angiographically assessed severity of cardiac allograft vasculopathy. Eur Heart J. 2011;32:476–484. doi: 10.1093/eurheartj/ehq402. [DOI] [PubMed] [Google Scholar]