Abstract
Recent evidence suggests that the macrophage scavenger receptor class A (SR-A, aka, CD204) plays a role in the induction of innate immune and inflammatory responses. We investigated whether SR-A will cooperate with Toll-like receptors (TLRs) in response to TLR ligand stimulation. Macrophages (J774/a) were treated with Pam2CSK4, (TLR2 ligand), Poly I:C (TLR3 ligand), and LPS (TLR4 ligand) for 15 min in the presence or absence of fucoidan (the SR-A ligand). The levels of phosphorylated IκBα (p-IκBα) were examined by Western blot. We observed that Poly I:C and LPS alone, but not Pam2CSK4 or fucoidan increased the levels of p-IκBα. However, LPS-induced increases in p-IκBα levels were further enhanced when presence of the fucoidan. Immunoprecipitation and double fluorescent staining showed that LPS stimulation promotes SR-A association with TLR4 in the presence of fucoidan. To further confirm our observation, we isolated peritoneal macrophages from SR-A deficient (SR-A−/−), TLR4−/− and wild type (WT) mice, respectively. The peritoneal macrophages were treated with LPS for 15 min in the presence and absence of fucoidan. We observed that LPS-stimulated TNFα and IL-1β production was further enhanced in the WT macrophages, but did not in either TLR4−/− or SR-A−/− macrophages, when fucoidan was present. Similarly, in the presence of fucoidan, LPS-induced IκBα phosphorylation, NF-κB binding activity, and association between TLR4 and SR-A were significantly enhanced in WT macrophages compared with LPS stimulation alone. The data suggests that SR-A is needed for LPS-induced inflammatory responses in macrophages.
Keywords: SR-A, TLR4, LPS, Macrophages, NF-κB
Introduction
Toll-like receptors (TLRs) play an important role in the regulation of innate immune and inflammatory responses by recognition of pathogen associated molecular patterns (PAMPs) that are not present in the host[1]. To date, more than ten TLRs have been identified in mammals[1]. TLRs recognize cell wall products from various pathogens and transduce an activation signal into the cell[1]. TLRs (TLR1, TLR2, TLR4, TLR5 and TLR6), which are expressed on the cell surface are involved in the recognition of structures unique to bacteria or fungi, while TLRs that are localized in intracellular compartments (TLR3, TLR7, TLR8, and TLR9) recognize viral or bacterial nucleic acids. After recognition of PAMPs, TLR-mediated cellular signaling predominately activates NF-κB which is an important transfection factor regulating a group of gene expression involved in innate immune and inflammatory responses [1].
The macrophage scavenger receptor class A (SR-A, aka CD204) was initially discovered due to its ability to bind and internalize modified low-density lipoprotein by macrophages [10,16,32]. Subsequently, numerous studies have reported that SR-A can recognize and clear modified host components, apoptotic cells, and pathogens [11]. Recent evidence suggests that SR-A plays a critical role in the induction of innate immune and inflammatory responses by recognition of exogenous PAMPs and endogenous ligands [7]. However, the mechanisms by which SR-A may regulate the innate immune and inflammatory responses have not been entirely elucidated.
It is well known that both SR-A and TLR4 express on the cell surface of macrophages and dendritic cells [16,27,30,32]. Recent studies have reported that there is an interaction between SR-A and TLR4 in response to microbial challenge [3,5,23,31]. For example, SR-A acts as a co-receptor for TLRs to facilitate innate immune recognition and response, resulting in an over exuberant response [28]. On the other hand, TLR ligands synergize with SR-A to mediate bacterial phagocytosis [2], induce SR-A expression [31], and promote SR-A binding to LPS which is the TLR4 ligand[31]. However, it is still unclear whether SR-A is required for TLR ligand-induced inflammatory responses.
In the present study, we examined whether SR-A plays a role in LPS-induced inflammatory response in macrophages. We observed that LPS-induced NF-κB activation and inflammatory cytokine production were significantly enhanced in the wild type macrophages when fucoidan, the SR-A ligand, was present. In the absence of SR-A, LPS-stimulated NF-κB binding activity and inflammatory cytokine production were significantly reduced. The data suggests that SR-A is required for LPS-induced inflammatory response through TLR4-mediated NF-κB activation pathway.
Materials and Methods
Animal
Breeding pairs of SR-A−/− mice on the C57BL/6J background were provided by Dr. Siamon Gordon, Sir William Dunn School of Pathology, Oxford University [6,21]. A small breeding colony was established at East Tennessee State University (ETSU). The TLR4−/− mice (C57BL/10ScCr) and wild type (WT, C57BL/10ScSn) mice were obtained from Jackson Laboratory (Bar Harbor, ME) as described previously[15]. Mice were maintained and or bred in the Division of Laboratory Animal Resources at ETSU. The experiments outlined in this manuscript conform to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85–23, revised 1996). The animal care and experimental protocols for this study were approved by the ETSU Committee on Animal Care.
Cell culture and treatment
The murine macrophage cell line J774 A.1 was obtained from ATCC (ATCC, Manassas, VA, USA). The cells were cultured at 37°C in a humidified incubator (95% air with 5% CO2) in RPMI-1640 medium with 10% fetal bovine serum (FBS), 10 U/ml penicillin and 10 mg/ml streptomycin. Cells (2×106/ml) were cultured in 100 mm tissue culture dishes in RPMI-1640 medium overnight and treated with fucoidan (50 µg/ml, Sigma, St. Louis, MO), Pam2CSK4 (10 ng/ml, InvivoGen, San Diego, CA), Poly I: C (1 µg/ml, InvivoGen), and LPS (1 µg/ml, from E. coli 055:B5, Catalog No: L6529, Sigma), respectively, for the indicated times. The cells were harvested for isolation of the nuclear and cytoplasmic proteins as described previously[12,18].
We also isolated peritoneal macrophages from WT and SR-A−/− and TLR4−/− mice. Briefly, thioglycollate-elicited peritoneal macrophages were prepared by using a standard protocol[9]. In brief, the mice were injected intrapertoneally with 1.5 ml of 3% sterile Brewer thioglycollate medium (Becton-Dickinson Microbiology Systems, Sparks, MD). Three days after injection, macrophages were harvested from the peritoneal cavity by lavaging sterile phosphate buffered saline. The macrophages were centrifuged at 400 × g for 10 min resuspended in RPMI 1640 medium supplemented with 10% fetal calf serum, streptomycin (0.1 mg/ml), and penicillin (100 U/ml). The cells (2×106/ml) were cultured in 100 mm tissue culture dishes (Corning, Inc, Corning, NY) for 2 hrs at 37°C in a humidified incubator with 5% CO2. After rinsing with PBS, adherent macrophages were cultured in RPMI 1640 medium at 37°C with 5% CO2 overnight. The cells were treated with fucoidan (50 µg/ml), and LPS (1 µg/ml), respectively, for the indicated times. There were 3 replicates in each group. The cells were harvested and the nuclear and cytoplasmic proteins were isolated as described previously [12,18]. The supernatants were collected for analysis of inflammatory cytokines (TNF-α and IL-1β).
Western blot
Western blot was performed as described previously[12,17–19]. Briefly, the cellular proteins were separated by SDS-PAGE and transferred onto Hybond ECL membranes (Amersham Biosciences, Amersham place, Little Chalfon, England). The ECL membranes were incubated with the appropriate primary antibody (anti-phospho-IκBα, anti-IκBα (Cell Signaling Technology Inc. Beverly, MA), anti-TLR4 (a gift from Dr. Ruslan Medzhitov at Yale University) and anti-SR-A (Santa Cruz Biotechnology, Santa Cruz, CA), respectively, followed by incubation with peroxidase-conjugated secondary Abs (Cell Signaling Technology). The same membranes were probed with anti-GAPDH (BioDesign, Saco, ME) after being washed with stripping buffer. The signals were detected and quantified with a G: Box system (Syngene, Frederick, MD).
Immunoprecipitation
Immunoprecipitation was performed as described previously [12,18]. Briefly, approximately 800 µg of cellular proteins were immunoprecipitated with 2 µg of antibody to SR-A (Santa Cruz Biotechnology) for 1 hr at 4°C followed by the addition of 20 µl of protein A/G-agarose beads (Santa Cruz Biotechnology). The precipitates were washed four times with lysis buffer and subjected to immunoblotting with anti-TLR4 and anti-SR-A antibodies, respectively [12, 18].
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins were isolated from heart samples as previously described[12,15,18]. NF-κB binding activity was examined by Light Shift Chemiluminescent EMSA kit (Thermo Scientific) according to the instructions of the manufacturer.
ELISA
The levels of inflammatory cytokines; TNFα and IL-1β, were measured in the supernatants from cultured peritoneal macrophages using commercially available ELISA kits (PeproTech, Rocky Hill, NJ) according to the instructions provided by the manufacturer.
Double fluorescent staining
Macrophages were incubated with blocking buffer prior to incubation with a specific anti-SR-A (from goat, 1:100, Santa Cruz, CA) at 4°C overnight. After washing, the cells were incubated with Alexa 555 conjugated anti-goat IgG (1:100, GeneTex, San Antonio, TX) for 1 h at 25°C. The cells were washed again before incubation with anti-TLR4 (rabbit, 1:100, a gift from Dr. Ruslan Medzhitov at Yale University) at 4°C overnight. After washing, the cells were incubated with FITC conjugated anti-rabbit (1:100, GeneTex, San Antonio, TX) for 1 h at 25°C. The cells were covered with fluorescence mounting medium (Vector labs, Burlingame, CA). Images were captured with an EVOS digital inverted fluorescence microscope (Advanced Microscopy Group. Bothell, WA).
Statistical analysis
Data are expressed as mean ± SE. Comparisons of data between groups were made using one-way analysis of variance, and Tukey's procedure for multiple range tests was performed. P < 0.05 was considered significant.
Results
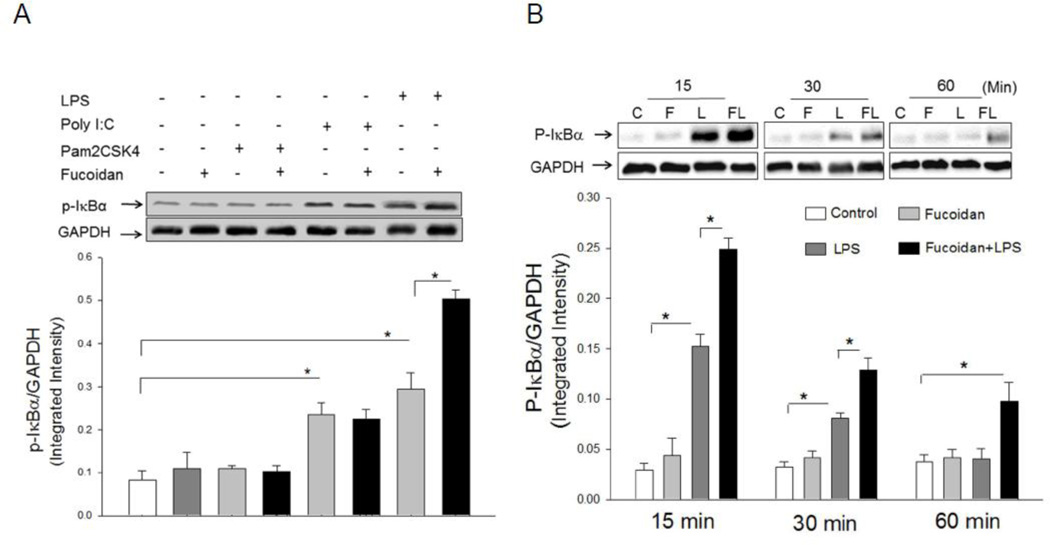
Fucoidan treatment enhances LPS-induced IκBα phosphorylation in macrophages
Recent studies have shown that there is a cooperative effect of scavenger receptors and TLRs on the response to the stimulation with PAMPs [7]. We examined whether SR-A will be required for TLR ligand-induced NF-κB activation. IκBα phosphorylation is the first step for NF-κB nuclear translocation and activation of inflammatory gene expression [1], therefore, we examined IκBα phosphorylation (p-IκBα) as an indicator for NF-κB activation. The macrophage cell line J774a.1 were treated with Pam2CSK4 (TLR2 ligand), Poly I:C (TLR3 ligand) and LPS (TLR4 ligand), respectively, in the presence and absence of fucoidan (SR-A ligand). IκBα phosphorylation was examined. As shown in Figure 1A, Poly I:C and LPS, but not Pam2CSK4, significantly increased the levels of p-IκBα, respectively, compared with untreated control. Fucoidan treatment alone did not induce IκBα phosphorylation in J774a.1 cells. Interestingly, LPS-induced IκBα phosphorylation was significantly enhanced when fucoidan was present. However, fucoidan treatment did not affect IκBα phosphorylation in the macrophages treated with either Poly I:C or Pam2CSK4.The data suggests that fucoidan synergizes with LPS to enhance NF-κB activation.
Figure 1.
A. Fucoidan, the SR-A ligand, enhanced LPS-induced IκBα phosphorylation in macrophages. Macrophages (J774a.1) were treated with LPS (1 µg/ml), Poly I:C (1 µg/ml), Pam2CSK4 (10 ng/ml) and fucoidan (50 µg/ml), individually and in combinations as shown, for 15 min. Cells were harvested and the cytoplasmic proteins were prepared for Western blot analysis of IκBα phosphorylation. There were three replicates in each group. A representative Western blot is shown above the graph. * P<0.05 compared with indicated groups.
B. Fucoidan enhanced LPS-induced IκBα phosphorylation in macrophages in a time dependent manner. Macrophages (J774 A.1) were stimulated with LPS (1 ug/ml) and/or fucoidan (50 µg/ml), for 15, 30, and 60 min, respectively. Cells were harvested and the cytoplasmic proteins were isolated for analysis of IκBα phosphorylation by Western blot. There were three replicates in each group. A representative Western blot is shown above the graph. * P<0.05 compared with indicated groups. C = control, F = fucoidan, L = LPS, and FL = fucoidan + LPS.
Fucoidan enhances LPS-induced IκBα phosphorylation in a time-dependent manner
Next, we examined whether the synergistic effect of fucoidan on LPS-induced IκBα phosphorylation is time-dependent. We treated J774a.1 cells with LPS for 0, 15, 30, 60 and 120 min, respectively, in the presence and absence of fucoidan and measured IκBα phosphorylation. Figure 1B shows that, following LPS stimulation, the levels of p-IκBα peaked at 15 min (5.7 fold) and gradually decreased but remained higher than untreated controls at 30 and 60 min after treatment. Treatment of macrophages with fucoidan alone did not increase the levels of p-IκBα. However, the levels of IκBα phosphorylation in fucoidan + LPS treated cells were significantly higher than that of LPS treatment alone with the peak response occurring at 15 min. The data suggests that fucoidan synergistically enhances the effect of LPS on IκBα phosphorylation in a time-dependent manner.
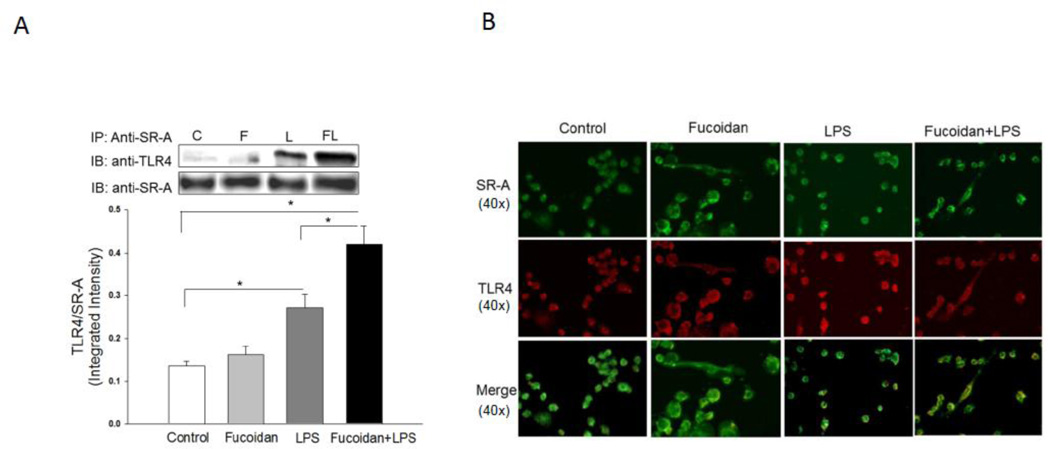
Fucoidan enhances LPS-induced co-association of SR-A and TLR4
To explore potential mechanisms, we examined whether there was an association between SR-A and TLR4 following LPS stimulation in the presence or absence of fucoidan. Macrophages were treated with LPS for 15 min in the presence or absence of fucoidan. The cells were harvested and the cellular proteins were isolated for immunoprecipitation with specific anti-SR-A followed by immunoblot with specific anti-TLR4. Figure 2A shows that LPS stimulation induced an association between SR-A and TLR4 as demonstrated by the presence of TLR4 in SR-A immunoprecipitates. Fucoidan treatment enhanced LPS-induced association of SR-A with TLR4 (Figure 2A). Double fluorescent staining using specific antibodies to SR-A and TLR4 showed that there was no detectable co-localization between SR-A and TLR4 in both untreated control cells and fucoidan-treated cells. LPS stimulation induced co-localization of SR-A with TLR4 as shown by the yellow color in the merged double fluorescent staining image (Figure 2B). Importantly, in the presence of fucoidan, LPS-induced co-localization between SR-A and TLR4 was further increased. The data suggest that presence of fucoidan enhanced LPS-induced IκBα phosphorylation (Figure 1B) through promotion of the interaction between SR-A and TLR4.
Figure 2.
Fucoidan enhanced LPS-induced association between SR-A and TLR4 in macrophages. Macrophages (J774 A.1) were stimulated with LPS (1 µg/ml), fucoidan (50 µg/ml) and fucoidan + LPS, respectively, for 15 min. Cells were harvested and cellular proteins were immunoprecipitated with anti-SR-A followed by immunoblot with anti-TLR4 (A). Double fluorescent staining shows the co-localization of SR-A and TLR4 (B). There were three replicates in each group. Magnification was 40×. A representative immunoblot is shown above the graph. * P<0.05 compared with indicated groups. C = control, F = fucoidan, L = LPS, and FL = fucoidan + LPS.
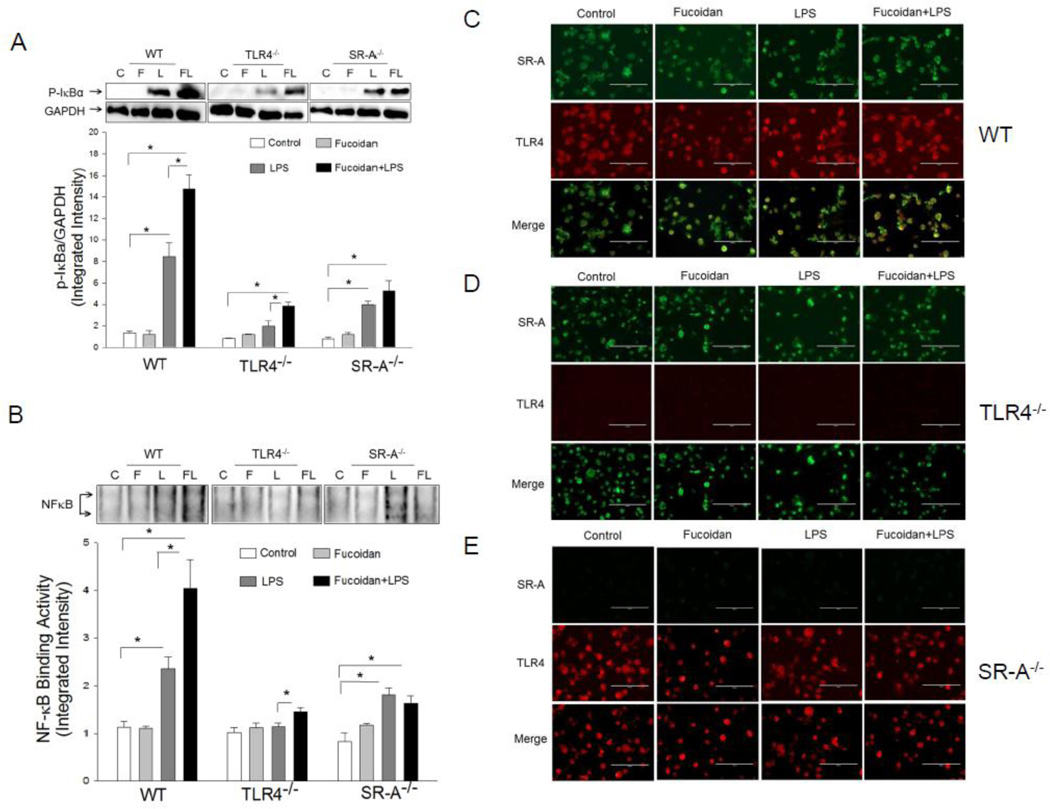
SR-A is required for LPS-induced NF-κB activation in macrophages
To determine whether SR-A is required for the fucoidan-enhanced LPS-induced NF-κB activation in macrophages, we isolated peritoneal macrophages from WT, TLR4−/− and SR-A−/− mice, respectively. The cells were stimulated with LPS in the presence and absence of fucoidan. NF-κB binding activity was measured. Figure 3 shows that LPS stimulation increased the levels of p-IκBα (A) and NF-κB binding activity (B) in both WT and SR-A−/− macrophages compared with untreated control. In the presence of fucoidan, LPS-increased levels of p-IκBα and NF-κB binding activity were further enhanced in WT macrophages but not in SR-A−/− macrophages. LPS or fucoidan treatment alone did not induce increases in IκBα phosphorylation and NF-κB binding activity in TLR4−/− macrophages (Figure 3A, B). However, the levels of p-IκBα and NF-κB binding activity were significantly enhanced when the TLR4−/− macrophages were treated with LPS + fucoidan. The data suggests that SR-A is needed for maximal LPS-induced response in macrophages.
Figure 3.
Fucoidan enhanced LPS-induced NF-κB activation and co-association of SR-A and TLR4 in macrophages. Peritoneal macrophages were isolated from WT, TLR4−/− and SR-A−/− mice, respectively. The cells were stimulated with LPS (1 µg/ml), fucoidan (50 µg/ml), and fucoidan + LPS, respectively, for 15 min. Cells were harvested. The cytoplasmic and the nuclear proteins were prepared for Western blot analysis of IκBα phosphorylation (A) and NF-κB binding activity by EMSA (B). Fucoidan enhanced LPS-induced association of SR-A and TLR4 in WT macrophages (C), but not in TLR4−/− (D), or SR-A−/− (E). The cells were treated with LPS (1 µg/ml), fucoidan (50 µg/ml), and fucoidan + LPS, respectively, for 15 min. The cells were fixed and subjected to double fluorescent staining of co-localization of SR-A (green) and TLR4 (red). Magnification was 40×. There were three replicates in each group. * P<0.05 compared with indicated groups. C = control, F = fucoidan, L = LPS, and FL = fucoidan + LPS.
Double fluorescent staining showed that there was no detectable co-localization of SR-A with TLR4 in both untreated-control and fucoidan-treated WT macrophages. LPS treatment induced a co-localization of SR-A with TLR4 in WT macrophages. When fucoidan was present, LPS-induced co-localization of SR-A and TLR4 was further enhanced (Figure 3C). There was no detectable co-localization of TLR4 and SR-A in TLR4−/− or SR-A−/− macrophages, as expected, after treatment with fucoidan, LPS, or LPS + fucoidan (Figure 3D, 3E). The data suggests that fucoidan-enhanced the effect of LPS on NF-κB activation may be mediated via co-association between SR-A and TLR4.
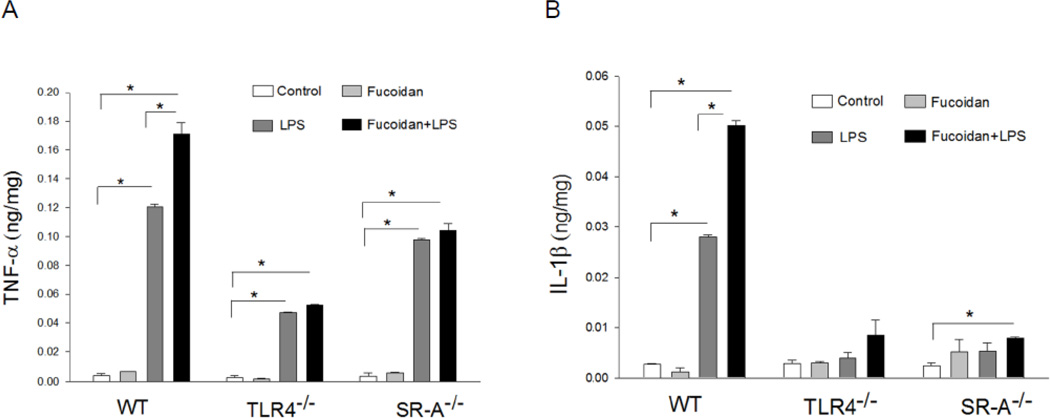
Fucoidan synergizes with LPS to increase inflammatory cytokine secretion in macrophages
TLR4-mediated signaling predominantly activates NF-κB which is an important transcription factor controlling inflammatory cytokine gene expression [1]. We examined whether fucoidan treatment will enhance LPS-induced inflammatory cytokine production. Peritoneal macrophages were isolated from WT, TLR4−/− and SR-A−/− mice, respectively and treated with LPS for 24 hrs in the presence and absence of fucoidan. The supernatants were harvested and the levels of TNFα and IL-1β were measured by ELISA kits. As shown in Figure 4 LPS stimulation increased the secretion of TNFα and IL-1β in WT macrophages. In the presence of fucoidan, however, LPS-increased TNFα and IL-1β production was further enhanced in WT macrophages. LPS also stimulated production of TNF-α in both TLR4−/− and SR-A−/− macrophages but the levels were significantly lower than that in WT macrophages. Fucoidan treatment did not enhance LPS-stimulated production of TNFα and IL-1β in either TLR4−/− or SR-A−/− macrophages.
Figure 4.
SR-A and TLR4 are required for fucoidan-enhanced LPS-induced cytokine production in macrophages. Peritoneal macrophages were isolated from WT, TLR4−/− and SR-A−/− mice, respectively. The cells were stimulated with LPS (1 µg/ml), fucoidan (50 µg/ml), and fucoidan + LPS, respectively, for 24 hrs. Supernatants were harvested for analysis of TNF-α and IL-β production by ELISA. There were three replicates in each group. * P<0.05 compared with indicated groups.
Discussion
The major finding in the present study was that SR-A is needed for LPS-stimulation of NF-κB activation and inflammatory cytokine production in macrophages. LPS stimulation induced NF-κB activation and an association of TLR4 with SR-A in WT macrophages which were further enhanced when the SR-A ligand, fucoidan, was present. Deficiency of either SR-A or TLR4 significantly reduced the response of macrophages to LPS stimulation. These data indicate that presence of SR-A is an essential for LPS-induced TLR4-mediated NF-κB activation and inflammatory cytokine production in macrophages.
SR-A is a trimeric membrane glycoprotein that is expressed on macrophages and dendritic cells [11, 24, 26]. Initial studies have reported that SR-A expressed on macrophages to bind and internalize modified LDL [10]. Recently, SR-A has been shown to mediate phagocytosis, promote cell adhesion and recognize several PAMPs, including LPS, lipoteichoic acid (LTA), bacterial CpG DNA, double–stranded RNA, and yeast zymosan/β-glucans [4,14,20,22,34]. Interestingly, these PAMPs can also be recognized by TLRs [1], indicating there is a possible cooperation between SR-A and TLRs in response to the stimulation with the PAMPs. Indeed, recent studies have shown that SR-A acts as a pattern recognition receptor (PRR) and cooperates with other PRRs to facilitate innate immune recognition and inflammatory responses [14, 20, 28, 34].
In the present study, we observed that stimulation of macrophages with the TLR3 ligand, Poly I:C, and the TLR4 ligand, LPS, significantly increased the levels of IκBα phosphorylation. Interestingly, in the presence of fucoidan, the SR-A ligand, TLR4 ligand, but not TLR2 or TLR3 ligand, induced-IκBα phosphorylation in macrophages was further enhanced. The data suggests that SR-A could synergistically cooperate with TLR4 in response to LPS stimulation. Recently, Seimon et al have reported that SR-A can act cooperatively with TLR4 to facilitate innate immune recognition and response [28]. These authors have shown that stimulation of SR-A with its ligand promotes a TLR4-mediated pro-inflammatory and pro-apoptotic responses in LPS exposed macrophages [28]. SR-A ligands trigger apoptosis in endoplasmic reticulum (ER)-stressed macrophages by cooperating with TLR4 [28]. Other studies have also shown that stimulation of SR-A resulted in the induction of innate immune and inflammatory responses [14, 20, 34]. On the other hand, TLR ligands synergize with SR-A to mediate bacterial phagocytosis [2], induce SR-A expression [31], and promote SR-A binding to the TLR4 ligand, LPS [31]. Collectively, these data suggest that SR-A and TLR4 cooperatively stimulate the innate immune and inflammatory responses. However, it is unclear how SR-A cooperates with TLR4 in the induction of innate immune and inflammatory responses.
To address whether SR-A synergistically cooperates with TLR4 in response to LPS stimulation in macrophages, we performed immunoprecipitation with anti-SR-A followed by immunoblot with anti-TLR4. We observed that LPS stimulation alone induced an association between SR-A and TLR4 in macrophages as evidenced by showing the presence of TLR4 in the immunoprecipitation with anti-SR-A. Importantly, the LPS-induced association of SR-A with TLR4 was significantly greater than that of LPS stimulation alone when the SR-A ligand, fucoidan, was present. Double fluorescent staining showed a consistent result demonstrating increased co-localization of SR-A with TLR4 following LPS + fucoidan stimulation. The data suggest that the presence of SR-A is essential for maximal LPS-induced response in macrophages. Indeed, LPS-induced NF-κB binding activity and inflammatory cytokine production was further increased in WT macrophages when fucoidan was present, indicating that SR-A synergistically cooperates with TLR4 in response to LPS stimulation in macrophages. In contrast, SR-A deficiency resulted in significantly reduced NF-κB binding activity and inflammatory cytokine production even in the presence of both LPS and fucoidan, suggesting that SR-A is required for maximal LPS-induced activation of the TLR4-mediated NF-κB pathway. At present, we do not understand the mechanisms by which LPS promoted SR-A interaction with TLR4 when the SR-A ligand was present. However, recent studies have shown that SR-A interacts with Mer receptor tyrosine kinase [29] and Lyn kinase [24]. Fong et al [8] have reported phosphorylation of SR-A intracellular domains may facilitate the interaction of the SR-A transmembrane domain with intracellular signaling components. LPS stimulation also induced TLR4 tyrosine phosphorylation [13, 25]. Therefore, it is possible that tyrosine kinases may be involved in the interaction between SR-A and TLR4 following their ligand stimulation. Recently, Yu et al reported that LPS stimulation of bone marrow dendritic cells (DCs) induced an interaction between SR-A and TRAF6, resulting in limiting the response of DCs to LPS stimulation[33]. However, Seimon et al [28] have shown that stimulation of SR-A with its ligand promoted activation of the TLR4-mediated NF-κB pathway in response to LPS stimulation and that SR-A could serve as a co-receptor for TLR4 in response to LPS challenge. The difference between our result and those of Yu et al may reflect differences in cells types and readouts employed.
In summary, the present study has shown that SR-A is essential for maximal LPS-induced activation of the TLR4-mediated NF-κB signaling pathway in macrophages. Deficiency of either SR-A or TLR4 reduced the response of macrophages to LPS stimulation. The data indicates that both SR-A and TLR4 are targets for controlling endotoxin-induced inflammatory responses.
We highlight the role of SR-A in TLR4-mediated inflammatory response to LPS challenge in macrophages. We observed that presence of SR-A is essential for LPS-activation of Toll-like receptor 4-mediated NF-κB signaling pathway. The data suggests that both SR-A and TLR4 are targets for controlling endotoxin-induced inflammatory responses.
Acknowledgments
This work was supported, in part, by NIH HL071837 to C.L., NIH GM083016 to C.L. and D.L.W., NIH GM53552 to D.L.W., NIH GM093878 to RLK
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
There was no conflict of interest for the authors in the present study.
References
- 1.Aderem A, Ulevitch RJ. Toll-like receptors in the induction of the innate immune response. Nature. 2000;406:782–787. doi: 10.1038/35021228. [DOI] [PubMed] [Google Scholar]
- 2.Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B. Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J. Leukocyte Biol. 2009;85 doi: 10.1189/jlb.1008631. p.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amiel E, Alonso A, Uematsu S, Akira S, Poynter ME, Berwin B. Pivotal Advance: Toll-like receptor regulation of scavenger receptor-A-mediated phagocytosis. J Leukoc Biol. 2009;85:595–605. doi: 10.1189/jlb.1008631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Areschoug T, Gordon S. Pattern recognition receptors and their role in innate immunity: focus on microbial protein ligands. Contrib Microbiol. 2008;15:45–60. doi: 10.1159/000135685. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Wermeling F, Sundqvist J, Jonsson A-B, Tryggvason K, Pikkarainen T, Karlsson MCI. A regulatory role for macrophage class A scavenger receptors in TLR4-mediated LPS responses. Eur J Immunol. 2010;40:1451–1460. doi: 10.1002/eji.200939891. [DOI] [PubMed] [Google Scholar]
- 6.Cotena A, Gordon S, Platt N. The class A macrophage scavenger receptor attenuates CXC chemokin production and the early infiltration of neutrophils in sterile peritonitis. J. Immunol. 2004;173:6427–6432. doi: 10.4049/jimmunol.173.10.6427. [DOI] [PubMed] [Google Scholar]
- 7.De Winther MP, van Dijk KW, Havekes LM, Hofker MH. Macrophage scavenger receptor class A: A multifunctional receptor in atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:290–297. doi: 10.1161/01.atv.20.2.290. [DOI] [PubMed] [Google Scholar]
- 8.Fong LG, Li D. The Processing of Ligands by the Class A Scavenger Receptor Is Dependent on Signal Information Located in the Cytoplasmic Domain. Journal of Biological Chemistry. 1999;274:36808–36816. doi: 10.1074/jbc.274.51.36808. [DOI] [PubMed] [Google Scholar]
- 9.Fortier AH, Falk LA. Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W. Current protocols in immunology. New York: John Wiley and Sons; 1994. Isolation of Murine Macrophages; pp. 14.1.1–14.1.9. [Google Scholar]
- 10.Goldstein JL, Ho YK, Basu SK, Brown MS. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci. 1979;76:333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J. Lipid Res April Supplement. 2009:S282–S286. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ha T, Hu Y, Liu L, Lu C, McMullen JR, Shioi T, Isumo S, Kelley J, Kao RL, Williams DL, Gao X, Li C. TLR2 ligands induce cardioprotection against ischemia/reperfusion injury through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2010;87:694–703. doi: 10.1093/cvr/cvq116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ha T, Hua F, Liu X, Ma J, McMullen JR, Shioi T, Izumo S, Kelley J, Gao X, Browder W, Williams DL, Kao RL, Li C. Lipopolysaccharide-induced myocardial protection against ischemia/reperfusion injury is mediated through a PI3K/Akt-dependent mechanism. Cardiovascular Research. 2008;78:546–553. doi: 10.1093/cvr/cvn037. [DOI] [PubMed] [Google Scholar]
- 14.Hollifield M, Bou Ghanem E, de Villiers WJ, Garvy BA. Scavenger receptor A dampens induction of inflammation in response to the fungal pathogen Pneumocystis carinii. Infect Immun. 2007;75:3999–4005. doi: 10.1128/IAI.00393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hua F, Ha T, Ma J, Li Y, Kelley J, Gao X, Browder IW, Kao RL, Williams DL, Li C. Protection against Myocardial Ischemia/Reperfusion Injury in TLR4 Deficient Mice is Mediated through a Phosphoinositide 3-Kinase Dependent Mechanism. J. Immunol. 2007;178:7317–7324. doi: 10.4049/jimmunol.178.11.7317. [DOI] [PubMed] [Google Scholar]
- 16.Krieger M. The other side of scavenger receptors: pattern recognition for host defense. Curr Opin Lipidol. 1997;8:275–280. doi: 10.1097/00041433-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Li C, Browder W, Kao RL. Early activation of transcription factor NF-κB during ischemia in perfused rat heart. Am J Physiol. 1999;276:H543–H552. doi: 10.1152/ajpheart.1999.276.2.H543. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Ha T, Kelley J, Gao X, Qiu Y, Kao RL, Browder W, Williams DL. Modulating Toll-like receptor mediated signaling by (1-->3)-β-D-glucan rapidly induces cardioprotection. Cardiovascular Research. 2003;61:538–547. doi: 10.1016/j.cardiores.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Kao RL, Ha T, Kelley J, Browder IW, Williams DL. Early activation of IKKκ during in vivo myocardial ischemia. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H1264–H1271. doi: 10.1152/ajpheart.2001.280.3.H1264. [DOI] [PubMed] [Google Scholar]
- 20.Limmon GV, Arredouani M, McCann KL, Minor RAC, Kobzik L, Imani F. Scavenger receptor class-A is a novel cell surface receptor for double-stranded RNA. The FASEB Journal. 2008;22:159–167. doi: 10.1096/fj.07-8348com. [DOI] [PubMed] [Google Scholar]
- 21.Lu C, Hua F, Liu L, Ha T, Kalbfleisch J, Schweitzer J, Kelley J, Kao R, Williams D, Li C. Scavenger receptor class-A has a central role in cerebral ischemia/reperfusion injury. J Cereb Blood Flow Metab. 2010;30:1972–1981. doi: 10.1038/jcbfm.2010.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mukhopadhyay S, Gordon S. The role of scavenger receptors in pathogen recognition and innate immunity. Immunobiol. 2004;209:39–49. doi: 10.1016/j.imbio.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Mukhopadhyay S, Varin A, Chen Y, Liu B, Tryggvason K, Gordon S. SRA/MARCO-mediated ligand delivery enhances intracellular TLR and NLR function, but ligand scavenging from cell-surface limits TLR-4 response to pathogens. Blood. 2011;117:1319–1328. doi: 10.1182/blood-2010-03-276733. [DOI] [PubMed] [Google Scholar]
- 24.Nikolic DM, Cholewa J, Gass C, Gong MC, Post SR. Class A scavenger receptor-mediated cell adhesion requires the sequential activation of Lyn and PI3-kinase. Am J Physiol Cell Physiol. 2007;292:1450–1458. doi: 10.1152/ajpcell.00401.2006. [DOI] [PubMed] [Google Scholar]
- 25.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 26.Platt N, Gordon S. Is the class A macrophage scavenger receptor (SR-A) multifunctional? -- The mouse's tale. The Journal of Clinical Investigation. 2001;108:649–654. doi: 10.1172/JCI13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Seimon TA, Obstfeld A, Moore KJ, Golenbock DT, Tabas I. Combinatorial pattern recognition receptor signaling alters the balance of life and death in macrophages. PNAS. 2006;103:19794–19799. doi: 10.1073/pnas.0609671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todt JC, Hu B, Curtis JL. The scavenger receptor SR-A I/II (CD204) signals via the receptor tyrosine kinase Mertk during apoptotic cell uptake by murine macrophages. J. Leukocyte Biol. 2008;84:510–518. doi: 10.1189/jlb.0307135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tomokiyo R, Jinnouchi K, Honda M, Wada Y, Hanada N, Hiraoka T, Suzuki H, Kodama T, Takahashi K, Takeya M. Production, characterization, and interspecies reactivities of monoclonal antibodies against human class A macrophage scavenger receptors. Atherosclerosis. 2002;161:123–132. doi: 10.1016/s0021-9150(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 31.Xu WY, Wang L, Wang HM, Wang YQ, Liang YF, Zhao TT, Wu YZ. TLR2 and TLR4 agonists synergistically up-regulate SR-A in RAW264.7 through p38. Molecular Immunology. 2007;44:2315–2323. doi: 10.1016/j.molimm.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Yi H, Yu X, Gao P, Wang Y, Baek S-H, Chen X, Kim HL, Subjeck JR, Wang X-Y. Pattern recognition scavenger receptor SRA/CD204 down-regulates Toll-like receptor 4 signaling-dependent CD8 T-cell activation. Blood. 2009;113:5819–5828. doi: 10.1182/blood-2008-11-190033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu X, Yi H, Guo C, Zuo D, Wang Y, Kim HL, Subjeck JR, Wang X-Y. Pattern recognition scavenger receptor CD204 attenuates toll-like receptor 4 induced NF-κB activation by directly inhibiting ubiquitination of TNF receptor-associated factor 6. Journal of Biological Chemistry. 2011;286:18795–18806. doi: 10.1074/jbc.M111.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu FG, Reich CF, Pisetsky DS. The role of the macrophage scavenger receptor in immune stimulation by bacterial DNA and synthetic oligonucleotides. Immunobiol. 2001;103:226–234. doi: 10.1046/j.1365-2567.2001.01222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]