Abstract
Background
Efforts to predict psychosis in individuals at high risk for schizophrenia have focused on the identification of sub-threshold clinical criteria and neurobiological markers, including neuropsychological assessment, structural and functional brain imaging, and psychophysiological testing. We sought to evaluate the relative utility of psychosis proneness measures for prospective prediction of psychotic disorders in a group of young relatives at familial risk for schizophrenia.
Methods
We examined the receiver operating characteristics of sub-threshold symptoms in predicting conversion to psychosis in a group of 97 young first- and second-degree relatives of persons with schizophrenia over a 2-year period. Towards this end, we utilized the Structured Interview of Prodromal Symptoms to derive measures of two of the four Scale of Prodromal Symptoms subscales (positive and disorganized) and the Chapman Magical Ideation and Perceptual Aberration scales. These four measures were, together, taken to reflect a putative index of psychosis proneness.
Results
Eleven of the 97 subjects developed a psychotic disorder over 2 years of follow-up. Seventeen of the 97 subjects tested positive on this index of psychosis proneness at baseline and of these 10 converted to psychosis. The sensitivity and specificity of the test were 91 percent and 92 percent respectively. The positive predictive value of the test was 59 percent and its negative predictive value was 99 percent. Addition of measures of cognitive or social function to the index decreased its predictive ability, reducing its specificity and/or sensitivity.
Conclusions
A relatively simple set of clinical measures can be utilized to prospectively identify familial high-risk individuals who convert to psychosis with high specificity and sensitivity. Implications for the proposed addition of an “Attenuated Psychosis Syndrome” in DSM-5 are discussed.
INTRODUCTION
Schizophrenia and related psychotic disorders typically begin in adolescence, often progress, and can cause considerable disability (Harrison et al., 2001; Tandon et al., 2010). There has been renewed interest in preventing or delaying onset of psychosis in those at high risk for developing the illness (Lee and McGlashan, 2005; McGorry, 2008). Accurate prediction algorithms have faced a number of hurdles, including non-specific early psychopathology and the multifactorial etiology of psychotic illness. Despite these challenges, studies over the past decade have generated optimism about identifying individuals at high risk. Thus far, tools to identify individuals at high risk for developing a psychotic disorder have included extensive clinical testing, neuropsychological testing, structural and functional brain imaging, and psychophysiological testing (Correll et al., 2010; Keshavan et al., 2011; Lawrie et al., 2008).
Identification of these individuals is crucial for preventing or delaying the onset of psychosis through a range of interventional approaches (Correll et al., 2010; Liu et al., 2010). At the same time, more precise risk stratification holds the potential to reduce risks of intervention in individuals who are not destined to cross the psychosis threshold. Reflecting this promise, the fifth revision of the Diagnostic and Statistical Manual for Mental Disorders (DSM-5) is considering inclusion of a risk syndrome for psychosis (Bruijnzeel and Tandon, 2011; Carpenter, 2009) based on the presence of attenuated psychotic symptoms. Although the proposal is controversial (Corcoran et al., 2010; Drake and Lewis, 2010; Woods et al., 2010), its utility rests in part on the availability of reliable and valid clinical measures with high predictive value. It is worth mentioning here the distinction between familial high risk and ultra-high risk (clinical high risk). While there has been evidence to support the accuracy of attenuated psychotic symptoms as a predictor of future conversion to psychosis in the clinical high risk population (McGorry, Nelson et al., 2009), relatively few studies have examined this question in the familial high risk group.
We therefore sought to utilize a simple clinical measure of psychosis proneness and evaluate its utility in prospectively predicting individuals at familial high risk for developing a psychotic disorder. We studied 97 first- and second-degree nonpsychotic relatives of individuals with a diagnosis of schizophrenia or schizoaffective disorder over a 2-year period to evaluate whether baseline clinical characteristics would effectively identify those who develop a psychotic disorder over this period. Results of the Structured Interview for Prodromal Symptoms (SIPS, Miller et al., 2002) and the Wisconsin Schizotypy scales (Chapman et al., 1978) at baseline were utilized to define the index of psychosis proneness.
MATERIALS AND METHODS
Subjects
The study was conducted at the Western Psychiatric Institute and Clinic, Pittsburgh. Participants were 199 first- and second-degree relatives of probands with a diagnosis of schizophrenia or schizoaffective disorder. Relatives were recruited by first approaching patients via their treating clinicians or directly through advertisements. Diagnoses of schizophrenia or schizoaffective disorder were confirmed in the index relatives using the Structured Clinical Interview for DSM Disorders (SCID, Spitzer et al., 1992). Relatives were assessed at baseline, year 1, and year 2 using the SCID for the possible emergence of psychopathology. All participants signed informed consent following a full explanation of the study. For participants < 18 years of age, the consent was provided by the parent or guardian and the subjects provided informed assent. The study was approved by the University of Pittsburgh Institutional Review Board.
For subjects to be included in the final sample, they needed to have been free of psychotic illness at baseline, received both the SIPS and the Chapman Wisconsin Schizotypy scales assessments at baseline, and be followed up for two years. Two of the 199 relatives had a prior psychotic illness at baseline and were consequently excluded from the final sample. Subjects without baseline SIPS (18) or Chapman schizotypy (9) asssessments, or neither (40), were excluded from the final sample. Thirty three of the remaining subjects were not followed up for two years, leading to the final sample size of 97 familial high-risk subjects who had complete follow-up data. Conversion to a psychotic disorder was confirmed by repeat SCID interviews, and consensus conferences with senior clinicians (MSK, DM) using all available clinical data, obtained after the conversion.
Measures
Subjects received the Structured Interview for Prodromal Symptoms (SIPS) and the Chapman Schizotypy Scales at baseline. The 19-item SIPS assesses symptoms in four domains (positive, negative, disorganized, and general symptoms) and rates symptom severity on a 0-6 scale on the Scale of Prodromal Symptoms (SOPS), with scores of 3-5 indicating prodromal or attenuated psychotic symptoms. Positive and disorganization symptoms are considered putative psychotic symptoms (Tandon et al., 2009) and are included in the proposed DSM-5 definition of “Attenuated Psychosis Syndrome” whereas negative and general symptoms are not (American Psychiatric Association, 2011). The four Chapman Schizotypy Scales include perceptual aberration, magical ideation, revised social anhedonia, physical anhedonia,. Of these, perceptual aberration and magical ideation define a positive schizotypy factor which has found to predict likelihood of psychosis in longitudinal studies of psychosis-prone subjects (Chapman et al., 1978; Kwapil et al., 2008; Lenzenweger, 1994). Conversion to a DSM-IV psychotic disorder was assessed by annual psychiatric assessments including SCID interviews over the 2-year period (American Psychiatric Association, 2000).
Both attenuated psychotic symptoms and positive schizotypy are found to indicate psychosis-proneness in high risk individuals. Even though they are correlated, the SIPS is a clinician rated measure and the Chapman scales are self-rated, and are therefore likely to provide somewhat unique, though overlapping indices of psychosis proneness. We therefore combined these measures to create a composite index of psychosis-proneness on the basis of four equally weighted items: two SOPS subscales (positive and disorganization) and the two Chapman scales (perceptual aberration and magical ideation), each with thresholds normed for the sample. The thresholds were determined by plotting the sample and calculating the minimum score for the top quartile of scores in that test (Table 2). For each test, a score of 0 was given if the individual scored in the bottom three quartiles and a score of 1 was assigned if the individual tallied in the top quartile of scores on that test. The sum of these 0/1 scores were taken for each subject as the total “psychosis proneness score”.
Table 2.
| Range Of Scores Observed |
Mean | Standard Deviation |
Upper QuartiLe (Threshold score) |
|
|---|---|---|---|---|
| SOPS | ||||
| Positive | 0-16 | 1.6 | 3.1 | 2 |
| Disorganized | 0-8 | 1.1 | 2.0 | 2 |
| Chapman | ||||
| Perceptual Aberration |
0-21 | 3.0 | 4.0 | 4 |
| Magical Ideation |
0-21 | 5.0 | 4.0 | 7 |
Data Analysis
We evaluated basic receiver operating characteristics of the proposed criteria, including sensitivity and specificity of the psychosis-proneness index, as well as its positive (PPV) and negative (NPV) predictive values for predicting conversion to psychosis in an individual at familial high-risk.
The positive and negative likelihood ratios (LR+ and LR− respectively), which are a means of using sensitivity and specificity data to calculate the direct implications of the index in changing psychosis-risk estimates in individual subjects were also assessed (Sackett et al., 2000). In addition, predictive values and likelihood ratios, unlike sensitivity and specificity, reflect the prevalence of the illness in the population under study, which is of particular importance in samples with a low base-rate of illness.
RESULTS
Of the 97 familial high-risk individuals with complete data, 11 developed a psychotic disorder over the 2-year period. Of the 11 converters, one developed schizophreniform disorder (295.4), four developed schizoaffective disorder (295.7), two developed schizophrenia undifferentiated type (295.9), and four developed psychosis not otherwise specified (298.8) (American Psychiatric Association, 2000). The characteristics on the 11 converters and 86 non-converters are compared in Table 2. There were no differences in average age, gender distribution, or race between the two groups.
We decided to begin with a total score of ≥ 2 on the 0-4 index as an indicator of psychosis-proneness. Seventeen of the 97 individuals scored ≥ 2 on the psychosis-proneness index at baseline: of these, 10 converted to psychosis over the 2-year period and 7 were non-converters. Of the 80 individuals scoring < 2, 1 converted and 79 did not.
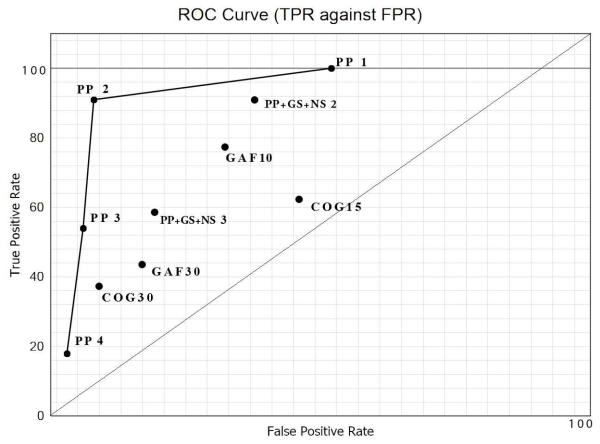
Sensitivity and specificity are statistical measures of the performance of a binary classification test, also known in statistics as classification function. Sensitivity (also called recall rate in some fields) measures the proportion of actual positives which are correctly identified as such. Specificity measures the proportion of negatives which are correctly identified. These two measures are closely related to the concepts of type I and type II errors. For any test, there is usually a trade-off between the measures. This trade-off can be represented graphically as an Receiver Operative Characteristics (ROC) curve (Newman et al., 2001). An ROC curve demonstrates several things: a) It shows the tradeoff between sensitivity and specificity (any increase in sensitivity will be accompanied by a decrease in specificity); b) the closer the curve follows the left-hand border and then the top border of the ROC space, the more accurate the test; c) the closer the curve comes to the 45-degree diagonal of the ROC space, the less accurate the test; d) the slope of the tangent line at a cut-off point gives the likelihood ratio (LR) for that value of the test; e) the area under the curve is a measure of test accuracy (Newman et al., 2001).
The sensitivity of the psychosis-proneness index in predicting likelihood of conversion to psychosis was 91 percent (10/11) and its specificity was 92 percent (79/86). The positive predictive value of the psychosis-proneness index was found to be 59 percent and its negative predictive value found to be 99 percent. The likelihood ratio positive was found to be 11.4 and the likelihood ratio negative was found to be 0.2. The formulae for these measures can be seen in Table 3.
Table 3.
| Psychosis Positive | Psychosis Negative | ||
|---|---|---|---|
|
Psychosis
Proneness Positive |
True Positive: 10 |
False Positive: 7 |
Positive Predictive Value:
|
|
Psychosis
Proneness Negative |
False Negative: 1 |
True Negative: 79 |
Negative Predictive Value:
|
We then compared the utility of our test to that of other indices employed to predict likelihood of converting to psychosis in familial high-risk samples. When we utilized the 30 percent decline in GAF from baseline criterion for predicting conversion to psychosis in a genetic high-risk sample (Woods et al., 2009; Yung et al., 2008), we found both a lower sensitivity [45 percent (5/11)] and a lower specificity [87 percent (75/86)] of the test in predicting conversion to psychosis. When we utilized the 15 percent decline in GAF from baseline criterion for predicting conversion to psychosis in a genetic high-risk sample (Keshavan et al., 2011), we found both a lower sensitivity [72 percent (8/11)] and a lower specificity [73 percent (63/86)] of the test in predicting conversion to psychosis. This might be because the GAF has been shown to have poor inter-rater reliability (Grootenboer et al., 2011). When we utilized the 15 percent decline in cognitive function (i.e. increase in perseverative error score) criterion based on performance on the Wisconsin Card Sorting test (Bhojraj et al., 2011), we also found both a lower sensitivity [63 percent (7/11)] and a lower specificity [59 percent (50/86)] of the test. If we utilized a cut-off score of ≥ 3 on the 0-4 psychosis proneness index, the sensitivity of the test in predicting conversion to psychosis decreased to 64 percent (7/11) whereas its specificity increased to 96 percent (83/86). If we added the negative and general SOPS scales and utilized a cut-off score of ≥ 2, the sensitivity of the test in predicting conversion to psychosis remains 91 percent (10/11) whereas its specificity decreased to 64 percent (55/86). If instead we added the negative and general SOPS scales and utilized a cut-off score of ≥ 3, both the sensitivity of the test in predicting conversion to psychosis decreased to 64 percent (7/11) and its specificity decreased to 85 percent (73/86). Using Chapman scores as a predictor independently, we found a sensitivity of 55 percent (6/11) and a specificity of 70 percent (60/86); if we applied SOPS as a predictor independently, we found a sensitivity of 45 percent (5/11) and a specificity of 59 percent (51/86) (Table 4). All of these data were plotted on an ROC Curve (Figure 1) to evaluate the effect of utilizing different criteria for psychosis proneness.
Table 4.
| Test | True Positive | TPR | True Negative | FPR |
|---|---|---|---|---|
| Psychosis Proneness 1 | 11 | 100 | 45 | 52 |
| Psychosis Proneness 2 | 10 | 91 | 79 | 8 |
| Psychosis Proneness 3 | 6 | 54 | 81 | 6 |
| Psychosis Proneness 4 | 2 | 18 | 83 | 3 |
| Psychosis Proneness + GS + NS 2 |
10 | 91 | 55 | 36 |
| Psychosis Proneness + GS + NS 3 |
7 | 63 | 73 | 15 |
| GAF 10 | 8 | 73 | 61 | 29 |
| GAF 30 | 5 | 45 | 75 | 13 |
| COG 15 | 7 | 63 | 50 | 41 |
| COG 30 | 4 | 36 | 78 | 9 |
Figure 1.
DISCUSSION
The emergence of the early intervention model in psychotic disorders has appropriately generated optimism about the ability to “reshape the course of psychotic disorder” (McGorry, 2009; National Research Council, 2009) because it is founded on a new understanding of psychotic vulnerability in conjunction with practical preventive strategies already established in general health care (Keshavan et al., 2011; McGorry, 2010; Ruhrmann et al., 2009). The success of this strategy is, however, predicated upon the ability to precisely identify individuals at significantly elevated risk of developing a psychotic disorder and the availability of safe and effective interventions that can be specifically provided to those who are likely to benefit. Our study addressed the possibility of predicting the likelihood of developing psychosis in a group of 97 individuals already at elevated risk of such a transition because they were first- or second-degree relatives of persons with a diagnosed psychotic disorder. We found our “psychosis-proneness” index to have high specificity and sensitivity in predicting the likelihood of developing a psychotic disorder over a 2-year period in this group, with a likelihood ratio of a positive test=11.4, which indicates a test of high utility (Sackett et al., 2000). This index was found to provide higher specificity and sensitivity than other tests hitherto utilized to predict likelihood of conversion to psychosis in familial high-risk individuals and clinical high risk samples (Table 5). Additionally, the index which we utilized has the virtue of simplicity and relative ease of clinical application. There are, however, three major caveats about the applicability and potential utility of the “psychosis-proneness” index evaluated in this study.
Table 5.
| Study and Authors | Sample Characteristics APS(Attenuated Psychotic Symptoms) BLIPS(Brief Limited Psychotic Symptoms) Trait (Genetic risk) |
Duration and Instruments |
Rates of Conversion to Psychosis |
|---|---|---|---|
| Personal Assessment and Crisis Evaluation Clinic (PACE) Nelson et al., 2011 |
Sample size=817 Help- seeking 83 percent with APS 27 percent with Trait |
6 months Comprehensive Assessment for At Risk Mental State (CAARMS) |
72/817 = 9 percent APS alone=9.4 percent APS + Trait = 8.7 percent |
| North America Prodrome Longitudinal Study (NAPLS) Cannon et al., 2008 |
Sample size=291 Help- seeking 97 percent with APS |
30 months Structured Interview for Prodromal Symptoms (SIPS) |
82/291 = 28 percent Conversion rate increased to 52 percent among 118 individuals with APS+Genetic Risk |
| Outreach and Support in South London (OASIS) Fusar-Poli et al., 2010 |
Sample size=152 Help- seeking Ultra-High Risk (all three groups) |
24 months CAARMS |
24/152 = 15.6 percent |
| European Prediction of Psychosis Study (EPOS) Ruhrmann et al., 2010 |
Sample size=245 Help-seeking Ultra-High Risk (all 3 groups) and Basic- Symptom based COGDIS |
18 months SIPS and Bonn Scalefor the Assessment of Basic Symptoms (BSABS) |
37/183 = 20 percent By UHR criteria 21 percent By COGDIS criteria 19 percent |
| Edinburgh High-Risk Study (EHRS) Johnstone et al., 2005 |
Sample size = 163 Young adults with family history of schizophrenia (two affected relatives) |
30 months | 20/147 = 13 percent Presence of schizotypy increased likelihood of conversion |
| UCLA Study Schlosser et al., 2011 |
Sample size=84 Help- seeking Ultra-High Risk |
24 months SIPS |
27/84 = 32 percent |
| Orygen Youth Health (OHY) Yung et al., 20008 |
Sample size=119 Help- seeking Ultra-High Risk |
24 months CAARMS |
19/119 = 16 percent |
First, in the general population, there are several groups of individuals who demonstrate an increased liability to develop a psychotic disorder: these include (i) relatives of persons with established schizophrenia or other psychotic disorders, with the degree of increased risk dependent upon the genetic proximity to the affected individual; (ii) help-seeking individuals with brief limited intermittent psychotic symptoms (“BLIPS”) which do not meet criteria for an established psychotic disorder; (iii) help-seeking individuals with attenuated ‘subthreshold’ psychotic symptoms (“APS”), which again do not meet threshold criteria; (iv) non help-seeking individuals with “BLIPS” and “APS”; and (v) individuals with schizotypal personality disorder. It is unclear if the index evaluated in our study, in this case in the first group, would have been found to be equally useful in other groups. Whereas this is an empirical question that needs to be examined in future studies, it argues for the need to carefully examine any set of “psychosis risk” criteria separately in these groups (Cannon et al., 2008; Thompson et al., 2011; Woods et al., 2009; Yung et al., 2008).
Second, thresholds to convert scores on the six tests utilized to estimate a psychosis proneness index were derived from our sample itself: the third quartile score on each of these tests was employed as the cut-off for a 0-1 mark on that test. Whether the specific scores utilized on each of these four tests (Table 1) might generalize to other familial and clinical high-risk samples remains to be evaluated. Again, the success of our approach argues for the utility of customizing the development of psychosis-proneness indices for different populations.
Table 1.
| Converters (N=11) | Nonconverters (N=86) | |
|---|---|---|
| Gender | ||
| Male | 5 | 42 |
| Female | 6 | 44 |
| Age (Mean years, SD) | (16.1, 3.2) | (15.5, 4.6) |
| First-Degree Relatives | ||
| Offspring | 6 | 40 |
| Siblings | 3 | 39 |
| Second-Degree Relatives | 2 | 7 |
| Ethnicity | ||
| Caucasian | 4 | 52 |
| Latino/Hispanic | 0 | 0 |
| African-American | 7 | 33 |
| Other | 0 | 1 |
Both these potential constraints suggest the utility of considering a “progressive closing-in” strategy along a continuum of risk, with additional assessments and interventions based upon the location of the individual along that risk continuum (Nelson et al., 2011; Ruhrmann et al., 2010; Tandon et al., 2009). In our study, the 10 percent likelihood of developing a psychotic disorder (based on familial risk) was “enriched” six-fold (to 59 percent) among those who additionally screened positive on the psychosis-proneness index. Subjects meeting these criteria are 59 times more likely to develop psychosis than someone in the general population. It is conceivable that additional cross-sectional and longitudinal psychophysiological and neuropsychological tests and brain imaging (Bhojraj et al., 2011 and 2011; Koutsouleris et al., 2009; Takahashi et al., 2009) as well as more distal measures of risk (such as early premorbid deficits and environmental risk factors) might add predictive value that could be utilized. Data suggest that such enrichment strategies to identify individuals at progressively higher risk of conversion to psychosis may need to be different depending upon the initial basis for their categorization as a person at higher probability for developing a psychotic disorder than the general population (Corcoran et al., 2008; McIntosh et al., 2011; Smieskova et al., 2010; Sun et al., 2009; Welch et al., 2011)
Third, it is unclear if available interventional approaches could have altered the risk of conversion to psychosis among individuals whose greater vulnerability was identified by our index. Whereas our study does not address this issue, results from other investigations generate optimism about the possibility of specifically modifying the risk of conversion in individuals at high risk (Correll et al., 2010; Dragt et al., 2011; GROUP, 2011; Preti and Cella, 2010; Schlosser et al., 2010). However, many such interventions carry substantial risk and their long-term effectiveness remains inconclusive.
One may argue that predicting psychosis by psychosis proneness scores derived from prodromal symptoms should come as no surprise. However, current efforts in predicting psychosis by prodromal characterization have typically used criteria that define the “late” prodromal phase (Yung et al 1998; Miller et 2002). It is to be noted that very few of our familial high risk subjects met the formal COPS criteria, based on SIPS ratings, for ultra-high risk (Miller et al 2002). However, the SIPS and Chapman scale scores were helpful in predicting psychosis among our familial high risk population. This suggests the value of combining sub-threshold (“early”) prodromal and premorbid indices of psychosis proneness in psychosis prediction.
Even as debates about the propriety of including an “Attenuated Psychosis Syndrome” in DSM-5 continue (Woods et al., 2010; Yung et al., 2010,) it is nonetheless prudent to assess the ability of our tools to better identify those at greater risk for developing a psychotic disorder and evaluate monitoring and interventional strategies to safely and effectively reduce the likelihood of adverse health outcomes in these individuals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Revised 4th ed. American Psychiatric Association; Washington, DC: 2000. DSM-IV-TR. [Google Scholar]
- American Psychiatric Association DSM-5 Progress. 2011 www.DSM5.org.
- Bhojraj TS, Diwadkar VA, Sweeney JA, et al. Gray matter loss in young relatives at risk for schizophrenia: Relation with prodromal symptomatology. Neuroimage. 2011;54(Suppl. 1):S272–S279. doi: 10.1016/j.neuroimage.2010.04.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhojraj TS, Sweeney JA, Prasad KM, et al. Longitudinal alterations of executive function in non-psychotic adolescents at familial risk for schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;34:469–474. doi: 10.1016/j.pnpbp.2010.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruijnzeel D, Tandon R. The concept of schizophrenia: From the 1850s to the DSM-5. Psychiatric Annals. 2011;41:289–295. [Google Scholar]
- Cannon TD, Cadenhead K, Cornblatt B, et al. Prediction of psychosis in youth at high clinical risk: a multisite longitudinal study in North America. Arch. Gen. Psychiatry. 2008;65:28–37. doi: 10.1001/archgenpsychiatry.2007.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter WT. Anticipating DSM-V: Should psychosis risk become a diagnostic class? Schizophr. Bull. 2009;35:841–843. doi: 10.1093/schbul/sbp071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body image aberration in schizophrenia. Abnorm. Psychol. J. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Corcoran CM, Kimhy D, Stanford A, et al. Temporal association of cannabis use with symptoms in individuals at clinical high risk for psychosis. Schizophr. Res. 2008;106:286–293. doi: 10.1016/j.schres.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran CM, First MB, Cornblatt B. The psychosis risk syndrome and its proposed inclusion in the DSM-V: A risk-benefit analysis. Schizophr. Res. 2010;120:16–22. doi: 10.1016/j.schres.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correll CU, Hauser M, Auther AM, Cornblatt BA. Research in people with psychosis risk syndrome: A review of the current evidence and future directions. J. Child Psychol. Psychiatr. 2010;51:390–431. doi: 10.1111/j.1469-7610.2010.02235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragt S, Nieman DH, Veltman D, et al. Environmental factors and social adjustment as predictors of a first psychosis in subjects at ultra high risk. Schizophr. Res. 2011;125:69–76. doi: 10.1016/j.schres.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Drake RJ, Lewis SW. Valuing prodromal psychosis: What do we get and what is the price? Schizophr. Res. 2010;120:38–41. doi: 10.1016/j.schres.2010.03.013. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Byrne M, Valmaggia L, et al. Social dysfunction predicts two years clinical outcome in people at ultra high risk for psychosis. J. Psychiatr. Res. 2010;44:294–301. doi: 10.1016/j.jpsychires.2009.08.016. [DOI] [PubMed] [Google Scholar]
- Genetic Risk and Outcome in Psychosis (GROUP) Investigators Evidence that familial liability for psychosis is expressed as differential sensitivity to cannabis. Arch. Gen. Psychiatry. 2011;68:138–147. doi: 10.1001/archgenpsychiatry.2010.132. [DOI] [PubMed] [Google Scholar]
- Grootenboer EM, Giltay EJ, van der Lem R, van Veen T, van der Wee NJ, Zitman FG. Reliability and validity of the Global Assessment of Functioning Scale in clinical R outpatients with depressive disorders. J Eval Clin Pract. 2011 Jan 11; doi: 10.1111/j.1365-2753.2010.01614.x. doi: 10.1111/j.1365-2753.2010.01614.x. [Epub ahead of print] PubMed PMID: 21223457. [DOI] [PubMed] [Google Scholar]
- Harrison G, Hopper K, Craig T, et al. Recovery from psychotic illness: a 15- and 25-year international follow-up study. Br. J. Psychiatry. 2001;178:506–517. doi: 10.1192/bjp.178.6.506. [DOI] [PubMed] [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, et al. Predicting schizophrenia: findings from the Edinburgh high-risk study. Br. J. Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, DeLisi LE, Seidman LJ. Early and broadly defined psychosis risk states. Schizophr. Res. 2011;126:1–10. doi: 10.1016/j.schres.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutseleris N, Meisenzahl EM, Davatzikos C, et al. Use of neuroanatomical pattern transition. Arch. Gen. Psychiatry. 2009;66:700–712. doi: 10.1001/archgenpsychiatry.2009.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwapil TR, Barrantes-Vidal N, Silvia PJ. The dimensional structure of the Wisconsin Schizotypy Scales: Factor identification and construct validity. Schizophr. Bull. 2008;34:444–457. doi: 10.1093/schbul/sbm098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, McIntosh AM, Hall J, et al. Brain structure and function changes during the development of schizophrenia: The evidence from studies of subjects at increased genetic risk. Schizophr. Bull. 2008;34:330–340. doi: 10.1093/schbul/sbm158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, McGlashan TM, Woods SW. Prevention of schizophrenia: can it be achieved? CNS Drugs. 2005;19:193–206. doi: 10.2165/00023210-200519030-00002. [DOI] [PubMed] [Google Scholar]
- Lenzenweger MF. Psychometric high-risk paradigm, perceptual aberrations, and schizotypy: An update. Schizophr. Bull. 1994;20:121–135. doi: 10.1093/schbul/20.1.121. [DOI] [PubMed] [Google Scholar]
- Liu P, Parker AG, Hetrick SE, et al. An evidence map of interventions across premorbid, ultra high-risk, and first episode phases of psychosis. Schizophr. Res. 2010;123:37–44. doi: 10.1016/j.schres.2010.05.004. [DOI] [PubMed] [Google Scholar]
- McGorry PD. Back to the future. Predicting and reshaping the course of psychotic disorder. Arch. Gen. Psychiatry. 2008;65:25–27. doi: 10.1001/archgenpsychiatry.2007.9. [DOI] [PubMed] [Google Scholar]
- McGorry PD, Nelson B, Amminger GP, et al. Intervention in individuals at ultra-high risk for psychosis: a review and future directions. J Clin Psychiatry. 2009;70:1206–1212. doi: 10.4088/JCP.08r04472. [DOI] [PubMed] [Google Scholar]
- McGorry PD. Risk syndromes, clinical staging and DSM-V: New diagnostic infrastructure for early intervention in psychiatry. Schizophr. Res. 2010;120:49–53. doi: 10.1016/j.schres.2010.03.016. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Owens DC, Moorhead WJ, et al. Longitudinal volume reductions in people at high genetic risk of schizophrenia as they develop psychosis. Biol. Psychiatry. 2011;69:953–958. doi: 10.1016/j.biopsych.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Miller TJ, McGlashan TH, Rosen JL. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of inter-rater reliability and predictive validity. Am. J. Psychiatry. 2002;159:863–865. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- National Research Council and Institute of Medicine . Preventing Mental., Emotional, and Behavioral Disorders among young people: Progress and possibilities. National Acadamies Press; Washington, D.C.: 2009. [PubMed] [Google Scholar]
- Nelson B, Yuen K, Yung AR. Ultra high risk (UHR) for psychosis criteria: Are there different levels of risk for transition to psychosis? Schizophr. Res. 2011;125:62–68. doi: 10.1016/j.schres.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Newman Thomas B., Warren Browner S., Cummings Steven R. Designing Clinical Research. Lippincott Williams & Wilkins; Philadelphia, PA: 2001. Chapter 12 - Designing Studies of Medical Tests; pp. 175–193. [Google Scholar]
- Preti A, Cella M. Randomized-controlled trials in people at ultra high risk of psychosis: A review of treatment effectiveness. Schizophr. Res. 2010;123:30–36. doi: 10.1016/j.schres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Klosterkotter J. Intervention in the at-risk state to D TE prevent transition to psychosis. Curr. Opin. Psychiatry. 2009;22:177–183. doi: 10.1097/YCO.0b013e328324b687. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Salakongas RKR, et al. Prediction of psychosis in adolescents and young adults at high risk: results from the European prediction of psychosis study. Arch. Gen. Psychiatry. 2010;67:241–251. doi: 10.1001/archgenpsychiatry.2009.206. [DOI] [PubMed] [Google Scholar]
- Ruhrmann S, Schultze-Lutter F, Klosterkotter J. Probably at-risk but certainly ill - Advocating the introduction of a psychosis spectrum disorder in DSM-V. Schizophr. Res. 2010;120:23–27. doi: 10.1016/j.schres.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Sackett DL, Strauss SE, Richardson WS, et al. Evidence-based medicine: How to practice and teach EBM. Churchill Livingstone; Edinburgh: 2000. [Google Scholar]
- Schlosser DA, Zinberg JL, Loewy RL, et al. Predicting the longitudinal effects of the family environment on prodromal symptoms and functioning in patients at-risk for psychosis. Schizophr. Res. 2010;118:69–75. doi: 10.1016/j.schres.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlosser DA, Jacobson S, Chen Q, et al. Recovery from at-risk state. Schizophr. Bull. 2011 doi: 10.1093/schbul/sbr098. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smieskova R, Fusar-Poli P, Allen P, et al. Neuroimaging predictors of transition to psychosis-A systematic review and meta-analysis. Neurosci. Behav. Reviews. 2010;34:1207–1222. doi: 10.1016/j.neubiorev.2010.01.016. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbons M, First MB. The Structured Clinical Interview for DSM (SCID). 1. History, rationale, and description. Arch. Gen. Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr. Res. 2009;108:85–92. doi: 10.1016/j.schres.2008.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Wood SJ, Yung AR, et al. Progressive gray matter reduction of the superior temporal gyrus during transition to psychosis. Arch. Gen. Psychiatry. 2009;66:366–376. doi: 10.1001/archgenpsychiatry.2009.12. RIP. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 5. Treatment and Prevention. Schizophr Res. 2010;122:1–23. doi: 10.1016/j.schres.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Thompson A, Nelson B, Yung A. Predictive validity of clinical variables in the “at risk” for psychosis population: International comparison with results from the North American Prodrome Longitudinal Study. Schizophr. Res. 2011 doi: 10.1016/j.schres.2010.09.024. In Press. [DOI] [PubMed] [Google Scholar]
- Welch KA, McIntosh AM, Job DE, et al. The impact of substance abuse on brain structure in people at high risk of developing schizophrenia. Schizophr. Bull. 2011 doi: 10.1093/schbul/sbq013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Addington J, Cadenhead KS, et al. Validity of the prodromal risk syndrome for first psychosis: Findings from the North American Prodrome Longitudinal Study. Schizophr. Bull. 2009;35:894–908. doi: 10.1093/schbul/sbp027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW, Walsh BC, Saksa JR, McGlashan TH. The case for including Attenuated Psychotic Symptoms Syndrome in DSM-5 as a psychosis risk syndrome. Schizophr. Res. 2010;123:199–207. doi: 10.1016/j.schres.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, McGorry PD, McFarlane CA, Francey S, Harrigan S, Patton GC, Jackson HJ. Prediction of psychosis. A step towards indicated prevention of schizophrenia. Br J Psychiatry Suppl. 1998;172(33):14–20. [PubMed] [Google Scholar]
- Yung AR, Nelson B, Stanford C, et al. Validation of “prodromal” criteria to detect individuals at ultra-high risk of psychosis: 2 year follow-up. Schizophr. Res. 2008;105:10–17. doi: 10.1016/j.schres.2008.07.012. [DOI] [PubMed] [Google Scholar]
- Yung AR, Nelson B, Thompson AD, Wood SJ. Should a “risk syndrome for psychosis” be included in the DSM-V? Schizophr. Res. 2010;120:7–15. doi: 10.1016/j.schres.2010.03.017. [DOI] [PubMed] [Google Scholar]