Abstract
Purpose
The DNA double-strand break (DSB) damage response induced by high energy charged particles on lung fibroblast cells embedded in a 3-dimensional (3-D) collagen tissue equivalents was investigated using antibodies to the DNA damage response proteins gamma-histone 2AX (γ-H2AX) and phosphorylated DNA-PKcs (p-DNA-PKcs).
Materials and methods
3-D tissue equivalents were irradiated in positions across the linear distribution of the Bragg curve profiles of 307.7 MeV/nucleon, 556.9 MeV/nucleon, or 967.0 MeV/nucleon 56Fe ions at a dose of 0.30 Gy.
Results
Patterns of discrete DNA damage streaks across nuclei or saturated nuclear damage were observed, with saturated nuclear damage being more predominant as samples were positioned closer to the physical Bragg peak. Quantification of the DNA damage signal intensities at each distance for each of the examined energies revealed a biological Bragg curve profile with a pattern of DNA damage intensity similar to the physical Bragg curve for the particular energy. Deconvolution microscopy of nuclei with streaked or saturated nuclear damage pattern revealed more details of the damage, with evidence of double-strand breaks radially distributed from the main particle track as well as multiple discrete tracks within saturated damage nuclei.
Conclusions
These 3-D culture systems can be used as a biological substrate to better understand the interaction of heavy charged particles of different energies with tissue and could serve as a basis to model space-radiation-induced cancer initiation and progression.
Keywords: heavy ion irradiation, DNA damage, DNA double-strand break repair, 3-D tissue equivalents
Introduction
Astronauts on prolonged deep-space missions, such as a voyage to Mars or extended stays on the Moon, will be traversed by a mixed field of particles consisting mainly of intermittent radiation from solar particle events and chronic radiation from high energy and high mass (HZE) charged particles (Schimmerling 1992, White and Averner 2001). Risks to astronauts from exposure to this unique radiation environment are of great concern to the National Aeronautics and Space Administration (NASA) given the potential for protons and HZE particles to contribute to end organ damage such as central nervous system degeneration, cataract formation, and carcinogenesis (Cucinotta et al. 2001a, Rastegar et al. 2002, Raber et al. 2004, Cucinotta and Durante 2006, Durante and Cucinotta 2008). Although they are a small percentage of the overall space radiation spectrum, HZE particles may be the greatest contributors to the risk for both carcinogenic and non-carcinogenic effects. This is thought to be a result of the complex type of DNA damage induced by HZE particles, in which particles traverse cells and tissues and deposit their energy not only through their linear trajectory but also through secondary low linear energy transfer (LET) delta rays that cause indirect ionization of the DNA in cells along the radial distribution to the particle trajectory (Cucinotta et al. 1998). Therefore, the downstream biological effects of an HZE particle can be spread many cell dimensions away from its linear trajectory.
An important component in the planning of a mission to Mars or an extended stay on the Moon is the design of shields that adequately protect the astronauts from cosmic radiation. Conventional aluminum shielding is thought to worsen the radiation effects inside the spaceship hull and recently has been shown to produce DNA lesions that are less amenable to repair compared to more favourable polyethylene shielding (Wilson et al. 2001, Mukherjee et al. 2008). Ideal shielding would be hydrogen-rich compounds that fragment heavy charged particles into less ionising species, therefore decreasing the DNA damage burden and reducing cancer risk. Historically, model calculations have been implemented to design appropriate shielding but it is becoming increasingly accepted that more biological data is needed to validate these mathematical models (George et al. 2003, Shavers et al. 2004, Wilson et al. 2004, Walker et al. 2005). Recent studies (Desai et al. 2005, Wu et al. 2006) describe DNA damage or micronuclei induction, respectively, across the distribution of an iron particle’s physical Bragg curve (300 MeV/nucleon, 600 MeV/nucleon, 1 GeV/nucleon) in order to better understand the biological effects of particles as they stop and release their energy in a living substrate. An important concept described in these studies is that physical Bragg curves do not necessarily represent the biological damage caused by a particle across its trajectory since biological effects are dependent on the track structure of both primary and secondary radiation. The Desai et al. and Wu et al. studies were also performed in monolayers of tissue culture cells, and while more data such as this is needed, examining similar biological endpoints in tissues is warranted. The recent modeling of nuclear fragmentation and HZE particle distributions in 3-dimensional (3-D) simulations of homogenous tissues attests to this concept (Ponomarev and Cucinotta 2006). One of the main concerns that cannot be addressed in 2-dimensional (2-D) cellular models is: Does an HZE particle traversing through a tissue lead to an increased flux of lighter particles that may cause chromosomal alterations or mutagenesis rather than killing a cell outright? This is particularly true for low fluence exposures such as missions to Mars where over a three-year mission every cell in the body would be traversed on average by a single HZE particle (Cucinotta et al. 2001b). Such knowledge will help in the design of spacecraft components and shielding materials to limit such an effect. Furthermore, biological Bragg curves may be more useful to modeling because there may be differences in the shapes of Bragg curves that depend upon the biological endpoint being assayed. This point was recently raised by Wu et al. (2006) who considered bystander effects associated with micronuclei induction after HZE exposure. Modeling bystander effects or the effects of delta rays in a 2-D tissue culture setting may not be an adequate model for tissues. Moreover, the concept of a microlesion, which is a high LET phenomenon (Koniarek and Worgul 1992, Koniarek et al. 2004), cannot be modelled adequately in a 2-D tissue culture setting. Having 3-D tissue models will allow investigators to better model cell death, cellular transformation and microlesions and build mathematical models to infer HZE particle effects in humans.
This study was conceived as an initial step in examining DNA damage induction and repair in a human tissue. While this 3-D tissue equivalent is not a tissue per se, it does allow the examination of DNA double-strand break (DSB) induction and rejoining through a cross-section of human cells. Another unique aspect of this study is the use of a dual marker system for identifying surrogates of DNA DSB. In prior studies γ-H2AX was used to identify DNA DSB repair foci. Recent publications have shown that γ-H2AX activation responds to chromatin relaxation and can spread far beyond the initial lesion in the DNA and is hence, not specific to DNA DSB (Rogakou et al. 1998, Ward and Chen 2001, McManus and Hendzel 2005, Han et al. 2006, Cowell et al. 2007, Asaithamby et al. 2008). Other studies have demonstrated that antibodies to γ-H2AX can give non-specific binding and have used other co-localising proteins markers (such as CDKN1A) to demonstrate the sites of DNA damage after heavy-charged particle irradiation (Jakob et al. 2003). In this study an antibody against phosphorylated DNA-PKcs (pT2609) (p-DNA-PKcs) was used in addition to γ-H2AX to illustrate the DNA damage sites. Co-localised measures of p-DNA-PKcs and γ-H2AX are considered to be highly specific for DNA DSB (Chan et al. 2002, Chen et al. 2005).
The DNA damage examined in our experiments was caused by charged particles impinging upon cells in 3-D tissue equivalents (contracted fibroblast-collagen gels) that have been placed at increasing distance along the Bragg curve profile of 56Fe charge particles of different energies (307.7 MeV/nucleon, 556.9 MeV/nucleon, or 967.0 MeV/nucleon). The intensity of DNA DSB signal in the form of co-localised γ-H2AX and p-DNA-PKcs (pT2609) were obtained by measuring the mean grey scale values in the nuclei of the cells within this 3-D cellular architecture. 56Fe particles were chosen because while it is a particle with a very low fluence, it is predicted to have the highest dose equivalence in deep space and therefore thought to be most potentially damaging to cells (Hartman et al. 2001) A dose of 0.30 Gy was used to limit the number of particle traversals to roughly one or fewer per cell. Previous studies have focused on the biological imaging of heavy-charged particle tracks by demonstrating the various numbers of different proteins localising at sites of DNA damage and illustrate specific patterns of distribution for these proteins (Jakob et al. 2003). In this study, the effect of a tissue structure on particle trajectories, and thus the pattern of DNA damage protein distribution in relation to the particle’s position in the Bragg curve, were studied with finer detail by deconvolving individual cell nuclei groups.
Materials and methods
Cell culture
Lung fibroblasts from a 61-year-old male (61 ML, American Type Tissue Culture collection [ATTC], Manassas, VA, USA) were cultured in mono-layers in X media (4 parts Dulbecco’s Modified Eagle Media [DMEM] to 1 part Medium 199; Hyclone, Logan, UT, USA) with 10% cosmic calf serum (Hyclone) until near confluence then sub-cultured, collected, and mixed with rat tail collagen type 1 (BD Biosciences, San Jose, CA, USA) to make three-dimensional fibroblast-collagen gels at a density of approximately 2.15 × 106 cells per gel. For a period of seven days, the fibroblasts were allowed to contract the collagen gels in 12-well plastic dishes (Falcon, BD Biosciences) to a diameter of 5 mm and thickness of 3–4 mm. These contracted gels (from here on called plugs because of their shape) were then transported to the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory inside tightly capped cryovials filled with serum supplemented media.
Irradiation logistics
Once at the NSRL, plugs were subsequently placed inside new sterile freezer vial plastic caps (containing media and covered with a plastic film to prevent media leakage and to hold the plugs in place), positioned atop of a foam support, and fixed in the path of 56Fe charged particles. The beam size was set to 20 × 20 cm for all irradiations. The foam support with the plugs was placed in the middle of the beam path to ensure proper equal coverage of the plug’s cross-section by the beam. Positioning along the Bragg curve was accomplished by varying the amount of water-equivalent material (variable filter) ahead of the plug. Plugs were linearly distributed at specific positions along the Bragg curve profile for 56Fe of three different energies. These positions along the Bragg curve were based upon predetermined distributions generated by the NSRL Physics Support Team. Plugs exposed to the 307.7 MeV/nucleon beam were placed behind 0.0, 3.55, 3.80, 3.85, and 4.15 cm of variable filter material, 0, 9.5, 11.0, 11.225, 11.25, and 11.5 cm of material for the 556.9 MeV/nucleon beam, and 0, 19.775, 20.775, 24.575, 24.925, and 25.175 cm of material for the 967.0 MeV/nucleon beam. Beam intensity and dose were monitored for each irradiation by observing in real-time the progressive amount of dose being delivered to the target sample. Each plug sample was fixed with 10% neutral buffered formalin 30 min after irradiation.
Histology and staining
Plug samples were sectioned into 5 micron (µ) slices and attached to positively charged glass slides as paraffin embedded sections. Sectioning was performed parallel or transverse to the beam path. Slides were then processed for immunofluorescent staining. Briefly, slides were deparaffinised with xylene and then dehydrated with ethanol series. Antigen retrieval was achieved by boiling slides in sodium citrate solution (10 mM, pH of 6.5) for 20 min in a conventional microwave oven. After a cool-down period of 30 min in the sodium citrate, samples were blocked for one hour with 1% bovine serum albumin (BSA; Sigma, St Louis, MO, USA) for 30 min in a 37°C incubator. Following blocking, slides were incubated for 2 h at ambient temperature in a primary antibody solution consisting of a phosphate buffered solution (PBS) and monoclonal gammahistone 2AX (γ-H2AX) antibody (pS139, ab22551; Abcam, Cambridge, MA, USA) and polyclonal phosphorylated DNA-PKcs (p-DNA-PKcs) antibody (pT2609, ab4194; Abcam). Species-specific fluorochrome-labeled secondary antibodies were used for primary antibody detection (Alexa Fluor 568 [quantum yield = 0.69], Alexa Fluor 488 [quantum yield = 0.92]; Invitrogen, CA, USA). Incubation was then performed for 1 h at ambient temperature. 4′,6 diamidino-2-phenylindole (DAPI; Vector Laboratories, Inc., Burlingame, CA, USA) was used for nuclear counterstaining.
Microscopy
An epifluorescence microscope (Zeiss Axiovert 200 M; Göttingen, Germany) was used to visualise the DNA damage and nuclear staining. Images for relative intensity calculations were taken at 40× objective magnification. All images were taken at the same exposure interval and were taken at random. Four fields of view for each section corresponding to a sample at a specific position along the Bragg peak were imaged. Each field of view contained on average 20–25 cell nuclei. Identification of the spatial location in the Bragg curve for each sample was not made known to the microscopist in order to decrease bias.
A Deltavision RT microscope and SoftWoRx software (Applied Precision; Issaquah, WA) were used to visualise and deconvolve, respectively, individual nuclear images. Briefly, individual nuclei were captured at exposures that were below the saturation limit. Multiple optical sections were obtained for each nuclei in order to create a three dimensional distribution of DNA damage. The raw image was then processed through the deconvolution software to obtain a focused image of DNA damage throughout each section of the nucleus. Imaris software (Bitplane Scientific Solutions; St Paul, MN, USA) was used to make three-dimensional reconstructions of the DNA damage response.
Image processing
All 40× objective magnification immunofluorescent images were processed using Image J (http://rsb.info.nih.gov). Significant damage signal was classified as nuclear co-localisation of γ-H2AX and p-DNA-PKcs pT2609 (γ-H2AX signal = red, pT609 = green, co-localised = yellow). Damage signal intensities were calculated by carefully circling each damage focus or streak within each nucleus with the Image J drawing tool and obtaining the mean grey scale value average for all the circled damage signals within each field of view (between 20 and 25 cell nuclei). DAPI nuclear counterstaining was manually removed from all images before the damage foci intensity calculations to eliminate any additional signal. Four separate intensity measuring experiments (between 80 and 100 total cell nuclei) were performed for each spatial location in the Bragg curve for each particular energy as well for non-irradiated controls. The average signal intensity of the non-irradiated controls was subtracted from the average intensity values corresponding each examined distance. The intensity values for each distance across the Bragg curve were normalised to the 0.0 cm distance. Averages and standard errors of means (SEM) were calculated from the four separate intensity-measuring experiments for each spatial location in the Bragg curve for each energy.
Results
Qualitative and quantitative analysis of DNA damage
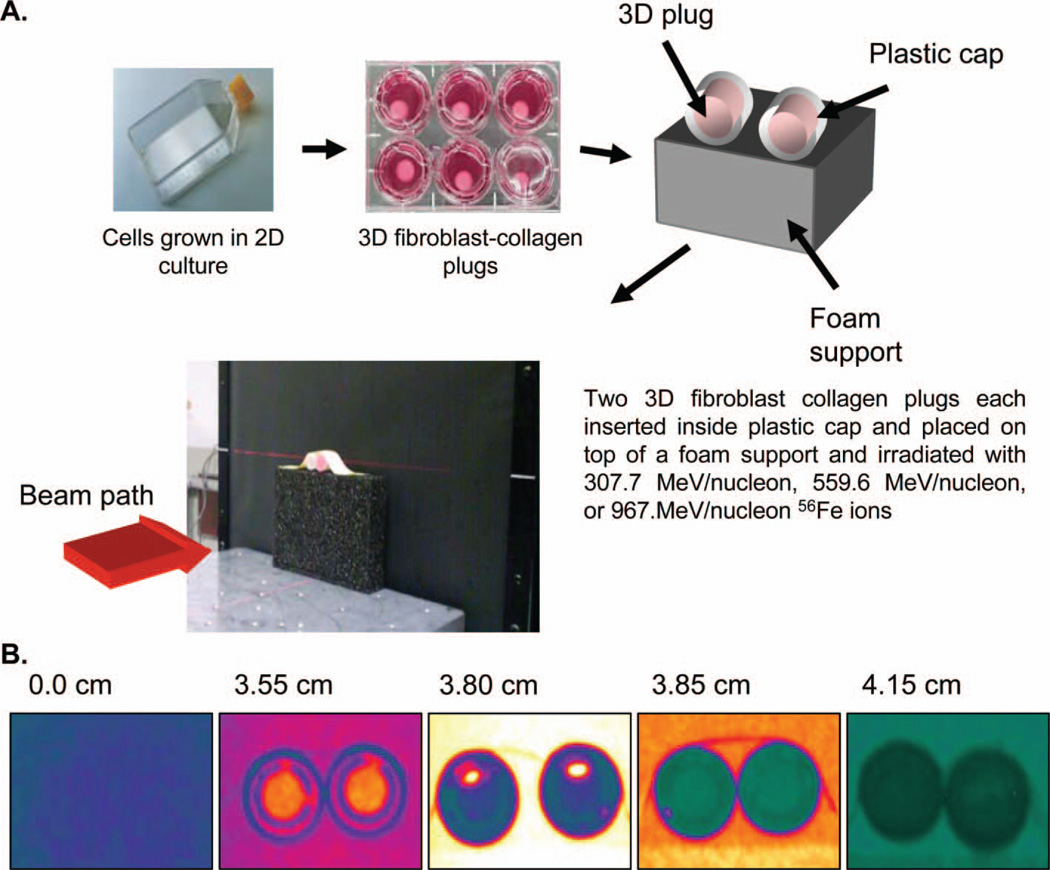
Figure 1A shows the set-up of the contracted collagen gels (plugs) and their positioning in the beam path prior to irradiation, as described in the methods. With the assistance of the NSRL physics support team, the plugs were positioned at specific locations in the particle’s physical Bragg curve. Figure 1B illustrates this process for the 307.7 MeV/nucleon 56Fe beam. Individual images in this panel correspond to media filled plastic support caps used to find the spatial locations before the peak (0.0 and 3.55 cm), near or at the peak (3.80 and 3.85 cm), and after the peak (4.15 cm). The various colours observed represent the relative quantity of energy deposited to the support caps during this calibration process. Blue colouration is equivalent to less particle energy deposition and corresponds to specimens placed at the location where particles begin to first interact with matter (position 0.0 cm). As particles traverse space they decrease their velocity and have more time to interact with matter. Consequently, the amount of energy being deposited increases markedly as particles stop in matter. This larger amount of energy deposition is manifested as the yellow, white, and red colours (in the case of the 307.7 MeV/nucleon energy positions 3.80 and 3.85 cm) and corresponds to the particles at or very near the Bragg peak.
Figure 1.
(A) Collagen-fibroblast plugs are made from lung fibroblast cells grown in conventional monolayer. Once mixed with collagen to make a three dimensional tissue equivalent, they are properly oriented for exposure to high LET radiation at the NASA Space Radiation Laboratory (NSRL) at Brookhaven National Laboratory (BNL). (B) Placement of plugs at different positions in the Bragg curve of a 307.7 MeV/nucleon 56Fe particle shows varying energies deposited to tissues depending on their location in the Bragg curve.
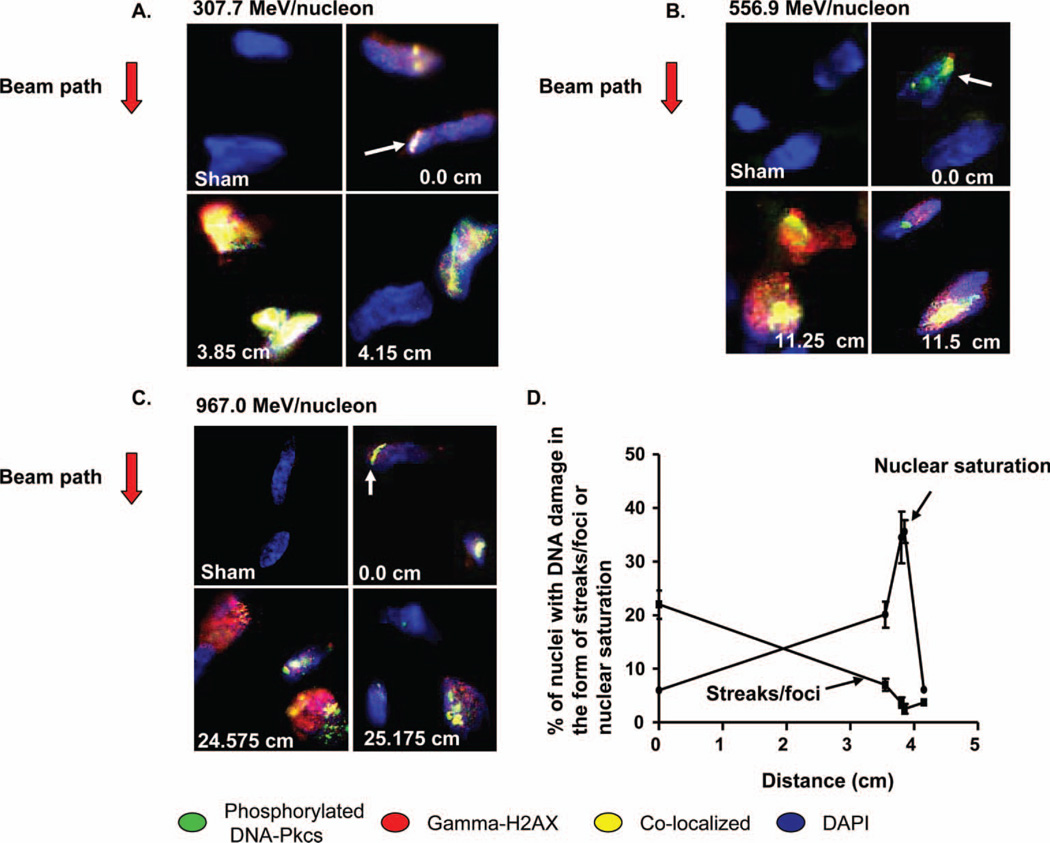
Matter placed after the Bragg peak will experience less energy deposition in general since a significant number of particles will have already stopped (position 4.15 cm). Positioning of the tissue plugs at the desired location in the Bragg curve of the 56Fe particles was accomplished by leaving the location of the plugs fixed relative to the beam port and placing increasing amounts of variable filter material in front of the plugs. This effectively decreases the particle’s velocity and modulates the amount of energy deposited to the tissue equivalent. Tissue fixation was performed thirty minutes after irradiating the tissues at the specific locations of the Bragg curve and the presence of DSB were subsequently assessed via the co-localisation of γ-H2AX and p-DNA-PKcs. Fluorescent images were obtained for multiple cells per distance as detailed in the methods. Figure 2A, 2B and 2C shows sample representative images of the DNA damage imparted to cells in a 3-D tissue equivalent at specific locations of the Bragg curve for the various energies examined. The direction of the beam is indicated to the left of the figure panels. Samples corresponding to a position at or near the Bragg peak (3.85 cm for 307.7 MeV/nucleon, 11.25 cm for 556.9 MeV/nucleon, and 24.575 for 967.0 MeV/nucleon) displayed a more intense DNA damage response, where a near saturation of overlapping signal (yellow) is seen across the entire nucleus of a number of cells, and less discrete streaks and foci are observed. Samples located before the Bragg peak (sample images at 0.0 cm for all energies) in general displayed less nuclei with near saturating signal and instead showed a paucity of damage within the nuclei, with most of the visible damage manifesting as DNA damage streaks or discrete foci (white arrows). Nuclei located after the Bragg peak continued to show evidence of DNA damage, in the form of streaks or foci, although the amount of affected nuclei were less numerous. These findings are quantitatively illustrated in Figure 2D for the 307.7 MeV/nucleon energy where the percentage of nuclei with DNA damage in the form of nuclear saturation or streaks/foci is plotted against the location of the examined sample in the Bragg curve.
Figure 2.
(A), (B), (C) DNA damage in nuclei of specimens placed at various locations along the Bragg peak for 307.7 MeV/nucleon, 556.9 MeV/nucleon, or 967.0 MeV/nucleon 56Fe ions, respectively. The beam direction for each energy is indicated next to the left of each panel. The damage is appreciated as either discrete damage streaks or foci (white arrows) or saturated nuclear damage. All images taken at 40× objective magnification. (D) Percentage of nuclei in a 307.7 MeV/nucleon specimen showing streaks/foci or nuclear saturation damage. Co-localised γ-H2AX and DNA-PKcs nuclear streak damage is more prevalent in distances farther away from the Bragg peak while saturated nuclear damage is more common closer to the peak. Error bars indicate the standard error for the mean (SEM) for three separate counting experiments.
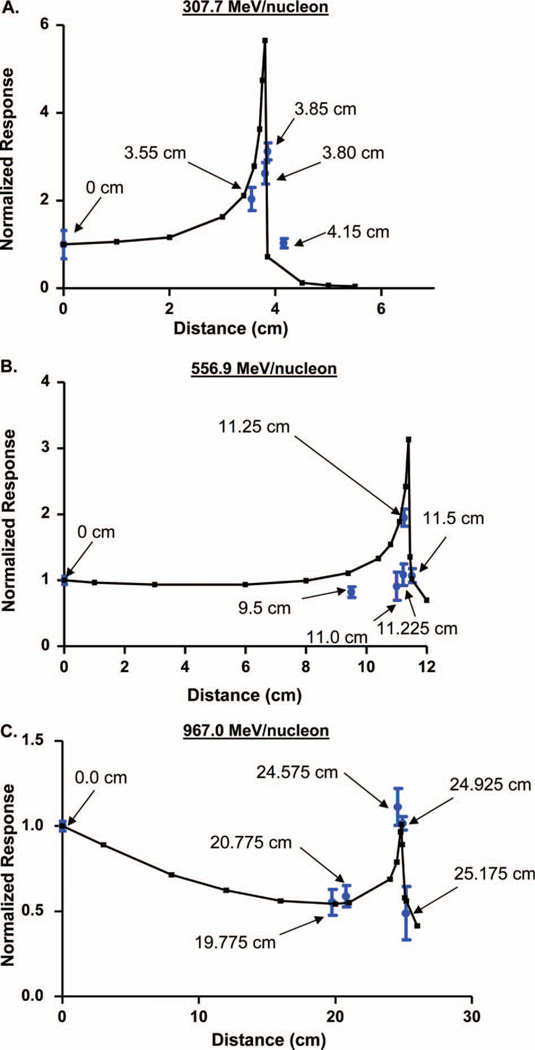
The average mean grey scale values for co-localised DNA damage were calculated from images containing multiple nuclei and averaged to represent the intensity of the acute DNA damage response for each distance along the Bragg curve. Figure 3 shows the average intensity of DNA damage as a ratio to 0.0 cm distance (blue dots) in relation to the corresponding location in the physical Bragg curve (black curve) for the 307.7 MeV/nucleon (Figure 3A), 556.9 MeV/nucleon (Figure 3B), or 967.0 MeV/nucleon (Figure 3C) 56Fe particles. The calculated intensities for the 967.0 MeV/nucleon energy closely match the physical curve. The calculated intensities for the 307.7 MeV/nucleon and 556.9 MeV/nucleon energies, although displaying a fairly accurate correlation with their respective physical curves, exhibit some deviations with respect to the peak intensity and position of some of the non-peak intensity values in relation to the physical curve (generally decreased in comparison to the physical Bragg peak). While several factors could explain some of these deviations (see Discussion), the damage intensity data for each of the examined spatial locations as a particle travels through a tissue equivalent creates a biological Bragg curve that closely matches the physical energy profile for the examined energies since the highest calculated mean grey scale value for samples in each particle energy group falls at a distance that is very near the respective peak of the physical Bragg curve.
Figure 3.
(A), (B), (C) Normalised DNA damage intensities calculated by averaging the mean grey scale values of the DNA damage intensities corresponding to 56Fe ions with 307.7 MeV/nucleon, 556.9 MeV/nucleon, or 967.0 MeV/nucleon energy super-imposed over the physical Bragg profiles for each particle energy. Four separate intensity measuring experiments (between 80 and 100 total cell nuclei) were performed for each spatial location in the Bragg curve for each particular energy as well for non-irradiated controls. Error bars indicate the standard error for the mean (SEM) for these four separate intensity measuring experiments.
Close-up view of the DNA damage by deconvolution microscopy
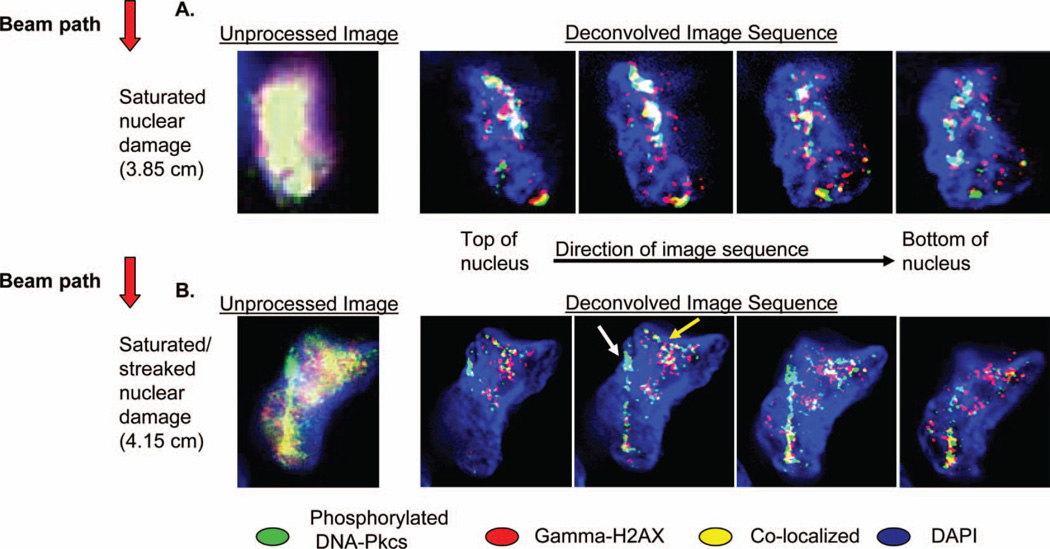
The observed nuclear streaks and saturated damage observed in the samples placed across the Bragg curve for the various particle energies examined contain both in- and out-of-focus light at the focal plane at which the picture was taken (Figure 4A, unprocessed image). The out-of-focus light likely represents DSB residing at different focal planes within the nucleus per tissue-equivalent section thickness (5 µ). Although informative for assessing the intensity of DNA damage response, the out-of-focus light makes it difficult to more closely examine the three-dimensional structure of the various DNA damage response patterns observed (track vs. nuclear saturation pattern damage). Visualising the damage response in all spatial directions can help elucidate the particle track structure as it traverses tissues. To correct the problem of the out-of-focus light, cell nuclei with saturated damage or streaks were imaged at multiple focal planes (z-stacks) and deconvolved. Here we show the results for samples in the 307.7 MeV/nucleon energy group. In contrast to the saturated out of focused damage signal of nuclei at the Bragg peak (3.80 cm position) as seen by epifluorescent microscopy (Figure 4A, unprocessed image), deconvolution brings light into focus at all examined planes (sample deconvolved image sequence, 4A). In this sequence, deconvolution shows that DNA damage is present all throughout the nucleus as one proceeds through the microscopic sections of the sample. The significant amount of damage encompassing a large amount of the nucleus likely results from the increased particle-DNA interaction occurring as an iron particle decreases in velocity and deposits its energy to the surrounding matter. Deconvolution of a nucleus at 4.15 cm (Figure 4B, unprocessed image) showing a combination of mixed streaking and saturating DNA damage response after the Bragg peak reveals a particle track with smaller tangential tracks (white and yellow arrows, respectively, deconvolved image sequence, 4B). This type of DNA damage pattern (most likely caused by a combination of primary and secondary particles as well as spallation products), although most consistent with DNA damage at the Bragg peak where the build-up of secondary particles is the highest, was rarely found in samples placed beyond the Bragg peak. Such a finding may be due to rare ions that had not yet stopped in matter or may be a result of inherent limitations in the experimental design (see Discussion).
Figure 4.
Magnified pictures of representative cell nuclei from specimens with saturated nuclear damage or mixed streaked- saturated nuclear damage for a 307.7 MeV/nucleon 56Fe beam. Both images come from samples sectioned parallel to the beam path. (A). Close up view of the saturated nuclear damage pattern processed with an epifluorescent microscope and corresponding deconvolved image sequences of the saturated damage revealing distinct particle tracks at multiple optical sections. Unprocessed image obtained at 40× objective magnification. Deconvolved images obtained at 60× objective magnification. (B). Sample nucleus with mixed streaked-saturated damage. Deconvolution shows a particle track with small tangential secondary tracks. Unprocessed image obtained at 60× magnification. Deconvolved images obtained at 60× objective magnification.
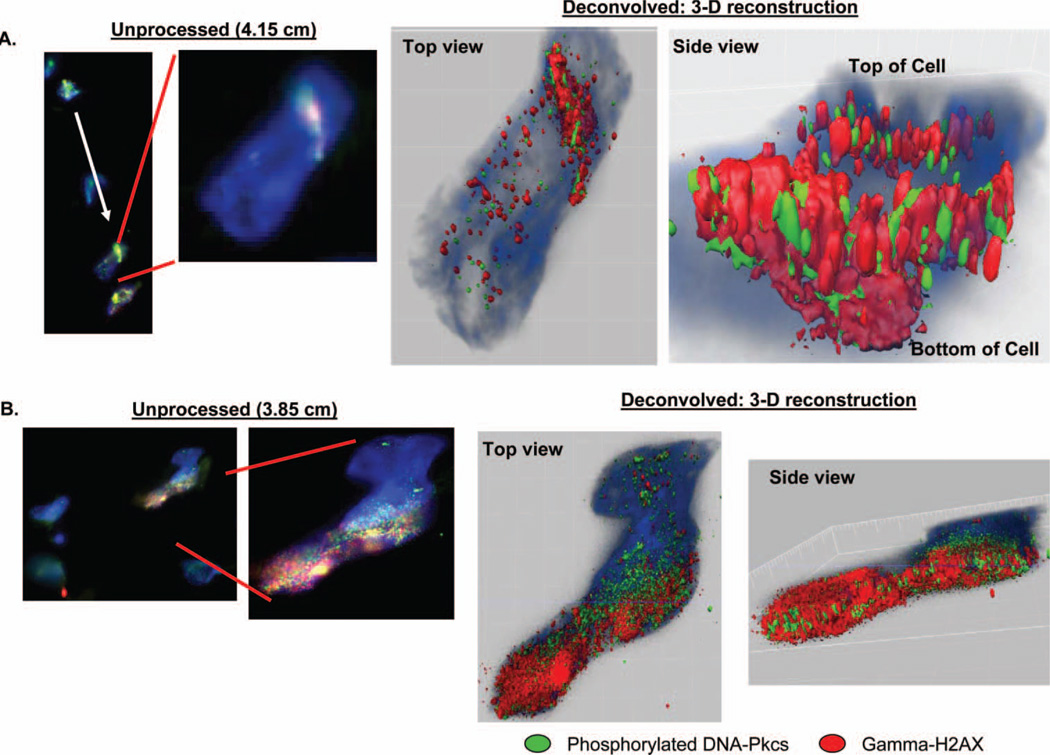
The deconvolved image sequences can also be used to reconstruct a 3-D surface model of the DNA damage to better evaluate the DNA damage response protein distribution in a 3-D architecture. Multiple deconvolved images of a subset of nuclei with representative streaked or saturated damage were stacked to reconstruct the three dimensional distribution of DNA damage proteins in both streaked and saturated damage examples (γ-H2AX, red; p-DNA-PKcs, green) without merging both signals (Figure 5). With this model the signal from each protein at areas of DNA damage can be compared. What becomes immediately apparent is the amount of γ-H2AX response in relation to p-DNA-PKcs: even though the quantum yield (QY) of the secondary antibody to γ-H2AX (Alexa 568 secondary fluorophore, QY 0.69) is lower than the QY of the secondary antibody to p-DNA-PKcs (Alexa 488 secondary fluorophore, QY 0.92) the streaked (Figure 5 panel A) and saturated nuclear damage pattern (Figure 5 panel B) show a predominance of γ-H2AX signal over p-DNA-PKcs at the charged particle’s point of entry into the cell nucleus. This finding correlates with the pattern of γ-H2AX and p-DNA-PKcs expression in the deconvolved image sequences of multiple examined nuclei in where we observe a predominance γ-H2AX over p-DNA-PKcs (data not shown), particularly at the area corresponding to the main particle track. This suggests that γ-H2AX activation can spread beyond the initial lesion and thus may not be specific for the precise location of the DNA DSB, consistent with previously described observations. In a subset of deconvolved nuclei, we observed isolated p-DNA-PKcs signal (no co-localisation with γ-H2AX). This occurred mainly at the periphery of the main particle track, as illustrated in Figure 5 panel B (isolated green p-DNA-PKcs signal peripheral to the concentrated DNA damage signal).
Figure 5.
3D reconstructions of deconvolved images from nuclei with streaked (panel A; sample sectioned parallel to beam path, white arrow illustrating direction of particle path) and nuclear saturation damage (panel B; sample sectioned transverse to the particle path, particle enters cell head-on). Left-most unprocessed epifluorescent images obtained at 40× objective magnification while right-sided unprocessed images were obtained at 60× objective magnification. 3-D reconstructions (right-most images, top and side views of the nuclei) demonstrate a surface rendition of the γ-H2AX and DNA-PKcs damage.
Discussion
This model of a 3-D tissue construct combined with DNA DSB specific markers represents the first-generation analysis of the spatial distribution of DNA damage in a tissue. The data generated suggest that the biological Bragg curve, in this case for DSB induction, follows the Bragg curves for 56Fe particles irrespective of the nominal energy of the particle. This is so even for 56Fe particles accelerated to 1 GeV where the normalised exposure is reduced by roughly 40% as the polyethylene density increases to 20 cm before increasing again at the Bragg peak (Figure 3C). The density and complexity of ionisations caused by HZE particles lead to such a deleterious effect as these high LET radiations leave dense and complex fields of DNA DSB in the process of linearly traversing matter (Asaithamby et al. 2008, Paap et al. 2008). Indeed, while the dose used here was averaged over a cm3, some cells likely absorbed several Gy when they were positioned in the trajectory line of a particle that was decreasing in velocity and subsequently depositing large amounts of energy. Our study supports this notion as it shows that the charged particles cause more intense DNA damage when tissue equivalents are at or near the Bragg peak and complements an important concept in the application of heavy ions to tumor therapy, that tumors can be effectively treated when exposed to the Bragg peak region of a charged particle (an area of increased relative biological effectiveness [RBE]) while decreasing the damage in exposed normal tissues outside of the Bragg peak region (Weyrather and Kraft 2004, Scholz and Elsasser 2007).
Although our experimental model demonstrates a correlation of the DNA damage intensity with the physical Bragg curve generated for a heavy ion of a specific energy, not all of the data points fall exactly in their respective physical Bragg curve profiles (Figure 3, particularly the 307.7 and 556.9 MeV/nucleon energies). Several limitations, inherent to the experimental design, could account for these discrepancies. When the actual plug samples were placed inside the caps for irradiation unpredictable tilting of the plug in the plastic cap could have shifted the position of a portion of the plug by few or a fraction of a millimeter and caused the localisation of the plug in the physical Bragg curve to change slightly. As a result, the final calculated DNA damage intensity could deviate from the expected intensity predicted by the physical profile. Another limitation could be the sample size used to calculate the average DNA damage intensity for each examined distance in the Bragg curve. Images captured for the damage intensity calculations contain a combination of in and out of focus damage signals. The out-of-focus signal for some of the nuclei may have caused an underestimation of the actual damage intensity. Including more nuclei in the analysis (above the 80–100 examined for each distance) could have compensated for this potential underestimation. In addition, the images used to calculate the damage signal intensities (captured from samples 5 µ in thickness) do not contain whole nuclei (average size up to 10 µ) and therefore are not fully representative of the DNA damage taking place in each nucleus. Underestimations of the DNA damage intensity could result from this limitation and thus a larger sample of nuclei or a thicker sample section may have conferred more accurate average signal intensities. Such limitations to the experimental design, particularly potential tilting of the plug inside the plastic cap, could also explain why primary and secondary particle tracks were observed in nuclei belonging to samples beyond the Bragg peak (such as in the 4.15 cm nucleus described in the results) where we would expect a paucity of damage and lack of particle fragmentation. Any displacement of the plugs by a fraction of a millimeter before irradiation could place a small portion of the plug closer to the Bragg peak where the frequency of primary and secondary particles and spallation products are at their highest.
The predominance of γ-H2AX over p-DNA-PKcs signal in areas of particle entry, as observed in the surface renditions of the DNA damage obtained from the deconvolution data, seems to support the observation that γ-H2AX activation may not be specific to DNA damage. Although the presently described deconvolution microscopy data is qualitative, and not based on large amount of individual nuclei analysis, it would be a useful to visualise larger cohorts of cell nuclei at several locations in the Bragg curve of various HZE particles to quantify the γ-H2AX response in relation to p-DNA-PKcs and therefore objectively determine if γ-H2AX activation spreads beyond the DSB site marked by the presence p-DNA-PKcs.
The presence of isolated p-DNA-PKcs signal in the periphery of the main damage site as observed in a subset of cells (representative image Figure 5B) is reminiscent of prior reports demonstrating that DNA-PKcs does not localise to sites of DNA DSB (Jakob et al. 2002, Bekker-Jensen et al. 2006). In these reports, the immunofluorescence analysis for DNA-PKcs targeted the unphosphorylated form. It has been established that DNA-PKcs autophosphorylation is a necessary step for DNA repair, and precise co-localisation with γ-H2AX is dependent on staining for the phosphorylated form of DNA-PKcs (Chan et al. 2002, Uematsu et al. 2007). Consequently, we infer that the isolated p-DNA-PKcs signal observed in our experiment may be due to background signal or potential non-specific binding of the antibody. To determine with more confidence that γ-H2AX activation is more widespread than other protein markers of DSB, additional proteins such as 53BP1 can be examined to determine if similar patterns arise, and therefore support the notion of decreased specificity of γ-H2AX.
Our experimental method describes an initial step to examine the induction and repair of DNA damage in tissues in the context of ionising radiation that varies in LET. Taking into consideration the limitations described above, and improving on these in subsequent investigations, recently developed more complex 3-D tissue equivalents (such as organotypic cultures (OTC) containing both fibroblasts and lung epithelial cells) can be implemented in future experiments to explore the differences in cellular transformation or cell death with respect to the position in the Bragg curve. Since it is recognised that DNA DSB produced by HZE particles in particular are considered to be more complex as the LET increases, and subsequently more difficult to repair (Goodhead 1994, Sutherland et al. 2001), it is likely that cells receiving large amounts of DNA damage, as would be the case when they are located at the Bragg peak, will likely have irreparable damage and not survive. On the other hand, cells at either side of the Bragg peak are more prone to experience non-lethal DNA damage, survive, and have a higher chance of developing mutations as a result of inappropriately repaired DNA lesions. Therefore, using 3-D tissue equivalents models to explore the intensity of DNA damage depending on the region of irradiation in the Bragg curve and correlating these results with the rate of carcinogenesis has direct application to answer NASA’s concerns of developing estimates of risk for carcinogenesis after heavy ion exposure. In addition, 3-D tissue equivalents models may have relevance in the clinical field. Future experiments can be designed where 3-D tissue equivalents irradiated with therapeutically relevant ions, such as carbon at therapeutic dose ranges, can be analysed for the rate of transformation depending on the region of irradiation in the Bragg curve. Recent reports demonstrate an increased chromosomal aberration frequency observed in cells as they are irradiated by 12C charged particles with a higher LET, i.e., particles closer to the Bragg peak (Manti et al. 2007). Despite a reduced chromosomal aberration frequency and increased survival in cells irradiated at distances far away from the peak, there are uncertainties with respect to the transformation rate of the irradiated healthy tissue. Biologically obtained transformation rates using 3-D culture models may complement transformation data obtained from biophysical models for optimal treatment planning and reduction of off-target effects (Scholz and Elsasser 2007).
Conclusions
We have shown how biological Bragg curves can be calculated from the intensity of the DNA damage and response of cells in tissue-like constructs and how these closely match the physical Bragg curves of the tested particle. In addition, with the use of deconvolution microscopy, particle tracks can be monitored through the depth of the surrogate tissue and we can begin to speculate on how HZE particle-induced DNA damage may be manifested in tissue. Since this first- generation attempt to model DNA damage in a tissue was performed, more realistic 3-D models have subsequently been developed. This new model uses normal or genetically modified lung epithelial cells. With appropriate biomarkers for transformation, the opportunity to determine the mechanism by which HZE particles initiate or promote carcinogenesis may soon follow.
Acknowledgements
This research was supported by the Office of Science (BER), U.S. Department of Energy, Grant No. DE-AI02-05ER64048 and NASA, NNJ05HD36G NSCOR. We wish to thank the NSRL physics support team for assistance in the design and execution of the irradiation logistics and Oliver Delgado for transporting and setting up the tissue equivalents for irradiation.
Footnotes
Declaration of interest: The authors do not have any conflict of interests or financial arrangements to disclose.
References
- Asaithamby A, Uematsu N, Chatterjee A, Story MD, Burma S, Chen DJ. Repair of HZE-particle-induced DNA double-strand breaks in normal human fibroblasts. Radiation Research. 2008;169:437–446. doi: 10.1667/RR1165.1. [DOI] [PubMed] [Google Scholar]
- Bekker-Jensen S, Lukas C, Kitagawa R, Melander F, Kastan MB, Bartek J, Lukas J. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. The Journal of Cell Biology. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan DW, Chen BP, Prithivirajsingh S, Kurimasa A, Story MD, Qin J, Chen DJ. Autophosphorylation of the DNA-dependent protein kinase catalytic subunit is required for rejoining of DNA double-strand breaks. Genes and Development. 2002;16:2333–2338. doi: 10.1101/gad.1015202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen BP, Chan DW, Kobayashi J, Burma S, Asaithamby A, Morotomi-Yano K, Botvinick E, Qin J, Chen DJ. Cell cycle dependence of DNA-dependent protein kinase phosphorylation in response to DNA double strand breaks. Journal of Biological Chemistry. 2005;280:14709–14715. doi: 10.1074/jbc.M408827200. [DOI] [PubMed] [Google Scholar]
- Cowell IG, Sunter NJ, Singh PB, Austin CA, Durkacz BW, Tilby MJ. gammaH2AX foci form preferentially in euchromatin after ionising-radiation. PLoS ONE. 2007;2:e1057. doi: 10.1371/journal.pone.0001057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cucinotta FA, Durante M. Cancer risk from exposure to galactic cosmic rays: Implications for space exploration by human beings. The Lancet Oncology. 2006;7:431–435. doi: 10.1016/S1470-2045(06)70695-7. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, Manuel FK, Jones J, Iszard G, Murrey J, Djojonegro B, Wear M. Space radiation and cataracts in astronauts. Radiation Research. 2001a;156:460–466. doi: 10.1667/0033-7587(2001)156[0460:sracia]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Cucinotta FA, Nikjoo H, Goodhead DT. The effects of delta rays on the number of particle-track traversals per cell in laboratory and space exposures. Radiation Research. 1998;150:115–119. [PubMed] [Google Scholar]
- Cucinotta FA, Schimmerling W, Wilson JW, Peterson LE, Badhwar GD, Saganti PB, Dicello JF. Space radiation cancer risks and uncertainties for Mars missions. Radiation Research. 2001b;156:682–688. doi: 10.1667/0033-7587(2001)156[0682:srcrau]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Desai N, Durante M, Lin ZW, Cucinotta F, Wu H. High LET-induced H2AX phosphorylation around the Bragg curve. Advances in Space Research. 2005;35:236–242. doi: 10.1016/j.asr.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Durante M, Cucinotta FA. Heavy ion carcinogenesis and human space exploration. Nature Reviews Cancer. 2008;8:465–472. doi: 10.1038/nrc2391. [DOI] [PubMed] [Google Scholar]
- George K, Durante M, Willingham V, Wu H, Yang TC, Cucinotta FA. Biological effectiveness of accelerated particles for the induction of chromosome damage measured in metaphase and interphase human lymphocytes. Radiation Research. 2003;160:425–435. doi: 10.1667/rr3064. [DOI] [PubMed] [Google Scholar]
- Goodhead DT. Initial events in the cellular effects of ionizing radiations: Clustered damage in DNA. International Journal of Radiation Biology. 1994;65:7–17. doi: 10.1080/09553009414550021. [DOI] [PubMed] [Google Scholar]
- Han J, Hendzel MJ, Allalunis-Turner J. Quantitative analysis reveals asynchronous and more than DSB-associated histone H2AX phosphorylation after exposure to ionizing radiation. Radiation Research. 2006;165:283–292. doi: 10.1667/rr3516.1. [DOI] [PubMed] [Google Scholar]
- Hartman PS, Hlavacek A, Wilde H, Lewicki D, Schubert W, Kern RG, Kazarians GA, Benton EV, Benton ER, Nelson GA. A comparison of mutations induced by accelerated iron particles versus those induced by low earth orbit space radiation in the FEM-3 gene of Caenorhabditis elegans. Mutation Research. 2001;474:47–55. doi: 10.1016/s0027-5107(00)00154-8. [DOI] [PubMed] [Google Scholar]
- Jakob B, Scholz M, Taucher-Scholz G. Characterization of CDKN1A (p21) binding to sites of heavy-ion-induced damage: Colocalization with proteins involved in DNA repair. International Journal of Radiation Biology. 2002;78:75–88. doi: 10.1080/09553000110090007. [DOI] [PubMed] [Google Scholar]
- Jakob B, Scholz M, Taucher-Scholz G. Biological imaging of heavy charged-particle tracks. Radiation Research. 2003;159:676–684. doi: 10.1667/0033-7587(2003)159[0676:biohct]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Koniarek JP, Thomas JL, Vazquez M. Detection of microlesions induced by heavy ions using liposomes filled with fluorescent dye. Advances in Space Research. 2004;34:1373–1377. doi: 10.1016/j.asr.2004.01.012. [DOI] [PubMed] [Google Scholar]
- Koniarek JP, Worgul BV. Do heavy ions cause microlesions in cell membranes? Advances in Space Research. 1992;12:417–420. doi: 10.1016/0273-1177(92)90138-n. [DOI] [PubMed] [Google Scholar]
- Manti L, Durante M, Grossi G, Pugliese M, Scampoli P, Gialanella G. Chromosome aberrations in human lymphocytes from the plateau region of the Bragg curve for a carbon-ion beam. Nuclear Instruments & Methods in Physics Research Section B-Beam Interactions with Materials and Atoms. 2007;259:884–888. [Google Scholar]
- McManus KJ, Hendzel MJ. ATM-dependent DNA damage-independent mitotic phosphorylation of H2AX in normally growing mammalian cells. Molecular Biology of the Cell. 2005;16:5013–5025. doi: 10.1091/mbc.E05-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee B, Camacho CV, Tomimatsu N, Miller J, Burma S. Modulation of the DNA-damage response to HZE particles by shielding. DNA Repair (Amst) 2008;7:1717–1730. doi: 10.1016/j.dnarep.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Paap B, Wilson DM, 3rd, Sutherland BM. Human abasic endonuclease action on multilesion abasic clusters: Implications for radiation-induced biological damage. Nucleic Acids Research. 2008;36:2717–2727. doi: 10.1093/nar/gkn118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev AL, Cucinotta FA. Nuclear fragmentation and the number of particle tracks in tissue. Radiation Protection Dosimetry. 2006;122:354–361. doi: 10.1093/rpd/ncl465. [DOI] [PubMed] [Google Scholar]
- Raber J, Rola R, Lefevour A, Morhardt D, Curley J, Mizumatsu S, Vandenberg SR, Fike JR. Radiation-induced cognitive impairments are associated with changes in indicators of hippocampal neurogenesis. Radiation Research. 2004;162:39–47. doi: 10.1667/rr3206. [DOI] [PubMed] [Google Scholar]
- Rastegar N, Eckart P, Mertz M. Radiation-induced cataract in astronauts and cosmonauts. Graefes Archive for Clinical and Experimental Ophthalmology. 2002;240:543–547. doi: 10.1007/s00417-002-0489-4. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. Journal of Biological Chemistry. 1998;273:5858–5868. doi: 10.1074/jbc.273.10.5858. [DOI] [PubMed] [Google Scholar]
- Schimmerling W. Radiobiological problems in space. An overview. Radiation and Environmental Biophysics. 1992;31:197–203. doi: 10.1007/BF01214827. [DOI] [PubMed] [Google Scholar]
- Scholz M, Elsasser T. Biophysical models in ion beam radiotherapy. Advances in Space Research. 2007;40:1381–1391. [Google Scholar]
- Shavers MR, Zapp N, Barber RE, Wilson JW, Qualls G, Toupes L, Ramsey S, Vinci V, Smith G, Cucinotta FA. Implementation of ALARA radiation protection on the ISS through polyethylene shielding augmentation of the Service Module Crew Quarters. Advances in Space Research. 2004;34:1333–1337. doi: 10.1016/j.asr.2003.10.051. [DOI] [PubMed] [Google Scholar]
- Sutherland BM, Bennett PV, Schenk H, Sidorkina O, Laval J, Trunk J, Monteleone D, Sutherland J. Clustered DNA damages induced by high and low LET radiation, including heavy ions. Physica Medica. 2001;(17) Suppl. 1:202–204. [PubMed] [Google Scholar]
- Uematsu N, Weterings E, Yano K, Morotomi-Yano K, Jakob B, Taucher-Scholz G, Mari PO, Van Gent DC, Chen BP, Chen DJ. Autophosphorylation of DNA-PKCS regulates its dynamics at DNA double-strand breaks. The Journal of Cell Biology. 2007;177:219–229. doi: 10.1083/jcb.200608077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SA, Tweed J, Wilson JW, Cucinotta FA, Tripathi RK, Blattnig S, Zeitlin C, Heilbronn L, Miller J. Validation of the HZETRN code for laboratory exposures with 1A GeV iron ions in several targets. Advances in Space Research. 2005;35:202–207. doi: 10.1016/j.asr.2005.02.077. [DOI] [PubMed] [Google Scholar]
- Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. Journal of Biological Chemistry. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- Weyrather WK, Kraft G. RBE of carbon ions: Experimental data and the strategy of RBE calculation for treatment planning. Radiotherapy and Oncology. 2004;73(Suppl. 2):S161–S169. doi: 10.1016/s0167-8140(04)80041-0. [DOI] [PubMed] [Google Scholar]
- White RJ, Averner M. Humans in space. Nature. 2001;409:1115–1118. doi: 10.1038/35059243. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Shinn JL, Tripathi RK, Singleterry RC, Clowdsley MS, Thibeault SA, Cheatwood FM, Schimmerling W, Cucinotta FA, Badhwar GD, Noor AK, Kim MY, Badavi FF, Heinbockel JH, Miller J, Zeitlin C, Heilbronn L. Issues in deep space radiation protection. Acta Astronautica. 2001;49:289–312. doi: 10.1016/s0094-5765(01)00107-2. [DOI] [PubMed] [Google Scholar]
- Wilson JW, Tripathi RK, Qualls GD, Cucinotta FA, Prael RE, Norbury JW, Heinbockel JH, Tweed J. A space radiation transport method development. Advances in Space Research. 2004;34:1319–1327. doi: 10.1016/j.asr.2003.10.048. [DOI] [PubMed] [Google Scholar]
- Wu H, Hada M, Meador J, Hu X, Rusek A, Cucinotta FA. Induction of micronuclei in human fibroblasts across the Bragg curve of energetic heavy ions. Radiation Research. 2006;166:583–589. doi: 10.1667/RR0535.1. [DOI] [PubMed] [Google Scholar]