Abstract
MicroRNA-224 (miR-224) is one of the most commonly up-regulated microRNAs in hepatocellular carcinoma (HCC), which affects crucial cellular processes such as apoptosis and cell proliferation. In this study, we aim to elucidate the molecular mechanism that leads to the overexpression of miR-224 in HCC. We examined the transcript expression of miR-224 and neighboring miR-452 and genes on chromosome Xq28 in tumor and paired adjacent nontumorous tissues from 100 patients with HCC and found that miR-224 is coordinately up-regulated with its neighboring microRNA (miRNA) and genes. This coordinated up-regulation of miRNAs and genes at the Xq28 locus can be mimicked in nontransformed immortalized human liver cells by the introduction of histone deacetylase (HDAC) inhibitors, which resulted in a corresponding increase in histone H3 acetylation in this region. This miR-224-residing locus in Xq28 is reciprocally regulated by HDAC1, HDAC3, and histone acetylase protein, E1A binding protein p300 (EP300). Notably, in HCC tumors that significantly overexpress microRNA-224, EP300 is also overexpressed and displays increased binding to the Xq28 locus. In transformed HCC cells, high miR-224 expression can be attenuated through the inhibition of EP300, using either siRNA or the specific drug C646. In summary, overexpression of EP300 may account, in part, for the up-regulation of miR-224 expression in patients with HCC.—Wang, Y., Toh, H. C., Chow, P., Chung, A. Y. F., Meyers, D. J., Cole, P. A., Ooi, L. L. P. J., Lee, C. G. L. MicroRNA-224 is up-regulated in hepatocellular carcinoma through epigenetic mechanisms.
Keywords: miR-224, histone acetylation, HDAC, EP300
Hepatocellular carcinoma (HCC) is the fifth most common cancer and third leading cause of cancer-related deaths worldwide, with a global annual mortality of ∼600,000 deaths (1, 2). Like other cancers, microRNA (miRNA) deregulation features significantly in HCC (3). To date, at least 14 publications have reported miRNA deregulation in HCC through the systematic profiling of miRNA expression in HCC tumors vs. either paired adjacent nontumorous tissues or unpaired normal liver tissues (4–17). Further functional characterization of selected miRNAs has provided some insights into how these miRNA deregulations can contribute to HCC. For example, the loss of microRNA (miR)-122 in HCC correlates with the suppression of hepatic phenotype and the gain of metastatic properties (18). The down-regulation of lethal-7 (let-7) miRNAs have been shown to increase cell proliferation by relieving its suppression on signal transducer and activator of transcription 3 (19), while miR-21 overexpression increases cell proliferation migration, invasion, and metastatic properties of the cell and decreases apoptotic cell death (20).
miR-224 is one of the most commonly up-regulated miRNAs in HCC (21). Overexpression of miR-224 in liver cells was reported to increase cell proliferation and apoptosis (8, 22) as well as cell migration and invasion (22). In nonliver cells, miR-224 was also found to be involved in transforming growth factor-β-mediated mouse granulosa cell proliferation (23) as well as increased anchorage independent growth of nontransformed mammary cells (24). Hence, miR-224 is a potential oncogenic miRNA that can impact multiple crucial cellular processes and warrants further investigation on its potential role as a clinically relevant target for HCC.
Notably, miR-224 expression was found to be moderately elevated in chronic hepatitis and liver cirrhosis (4). Its expression is further elevated in benign hepatocellular adenoma and most highly overexpressed in HCC (11). This graded increase of miR-224 expression with respect to liver disease progression suggests the potential of miR-224 as a useful biomarker for liver diseases (21).
To date, it remains unclear how the expression of miR-224 is up-regulated in patients with HCC. Hence, in this study, we aim to study the molecular mechanism responsible for miR-224 overexpression in HCC. Elucidating the mechanism of miR-224 regulation will offer valuable insights in evaluating the clinical significance of miR-224 as a potential therapeutic target and a potential diagnostic biomarker for HCC.
MATERIALS AND METHODS
Cell lines and patient samples
The immortalized untransformed human neonatal liver NeHepLxHT cells and the human hepatocellular carcinoma HepG2 cells were purchased from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured according to ATCC-recommended conditions. All cells were incubated at 37°C in a humidified atmosphere with 5% CO2.
Paired tumorous and adjacent nontumorous liver tissues from 100 patients with HCC were obtained from the National Cancer Centre of Singapore (NCCS)/SingHealth Tissue Repository with prior approval from the SingHealth Centralized Institutional Review Board (CIRB; approval 2008/440/B). As it is ethically not feasible to obtain normal livers from healthy individuals, the “normal ” livers in this study were obtained from the nontumorous sections of the livers from 40 patients with colorectal cancer and metastasis to the liver who underwent surgery to remove the metastatic colorectal tumor in the liver. These tissues were also obtained from the NCCS/SingHealth Tissue Repository with prior approval from the SingHealth CIRB (2005/421/B).
Total RNA extraction and reverse transcription quantitative polymerase chain reaction (RT-qPCR)
Total RNA, including low-molecular-weight RNAs, was extracted from patient samples and cell lines using the mirVana miRNA isolation kit (Ambion; Life Technologies, Carlsbad, CA, USA) following manufacturer's instructions. Transcript expression of specific miRNAs and genes was measured by RT-qPCR. Reverse transcription was performed with the High Capacity cDNA Archive Kit (Applied Biosystems; Life Technologies) and ABI Taqman individual miRNA assays (Applied Biosystems) following manufacturers' instructions. The RT products were 2× diluted and stored at −20°C. qPCR was performed to examine mature miRNA expression using Taqman MicroRNA Individual Assays (Applied Biosystems) and the transcript expression of specific genes of interest using QuantiTect SYBR Green PCR Kit (Qiagen, Hilden, Germany), following the respective manufacturer's instructions.
The sequences of the specific primers or catalog IDs of miRNA assays are listed in Table 1.
Table 1.
Taqman assays, qPCR primers, siRNAs, and antibodies used for this study
| miRNA or gene | Catalog no. or sequence |
|---|---|
| Taqman assay | |
| hsa-miR-224 | Applied Biosystems: 002099 |
| hsa-miR-452 | Applied Biosystems: 002329 |
| hsa-miR-16 | Applied Biosystems: 000391 |
| hsa-miR-100 | Applied Biosystems: 000437 |
| RNU48 | Applied Biosystems: 001006 |
| qPCR primer | |
| U6 | F:5′-CTCGCTTCGGCAGCACA-3′ |
| R:5′-AACGCTTCACGAATTTGCGT-3′ | |
| MAGEA4 | F:5′-CTATAAGGAGACAAGGTTCTGAG-3′ |
| R:5′-AGAAGACATGATGACTCTGG-3′ | |
| MAGEA5 | F:5′-TCTGTGAGGAGGCAAGGTTC-3′ |
| R:5′-CAGGCAGGAGTGTGGGCAG-3′ | |
| GABRE | F:5′-CCTATCCTGAGAATGAGATG-3′ |
| R:5′-GCCTGCTCACATTGAAGAAA-3′ | |
| HDAC1 | F:5′-TAAATTCTTGCGCTCCATCC-3′ |
| R:5′-AACAGGCCATCGAATACTGG-3′ | |
| HDAC2 | F:5′-CGTGTAATGACGGTATCATTCC-3′ |
| R:5′-ACCAGATAATGAGTCTGCACC-3′ | |
| HDAC3 | F:5′-CTTTGCCCCAGACTTCACAC-3′ |
| R:5′-CACAGCATCCCAAGCCACTC-3′ | |
| HDAC8 | F:5′-GATGCTGGACATACTTGACC-3′ |
| R:5′-ACCACATGCTTCAGATTCCC-3′ | |
| CREBBP | F:5′-CGGGATGAAGTCACGGTTTG-3′ |
| R:5′-GTAAACGGCTGTGCGGAGGC-3′ | |
| KAT2B | F:5′-AATACATCACACGGCTCGTC-3′ |
| R:5′-ATATGTGAGGAAGTTCAGGA3′ | |
| EP300 | F:5′-GTATCTAACGACCTCTCACA-3′ |
| R:5′-TACACAAGTCATAATCCTCACA-3′ | |
| β-Actin | F:5′-ATGTTTGAGACCTTCAACACC-3′ |
| R:5′-AGGTAGTCAGTCAGGTCCCGGCC-3′ | |
| MAGEA4 promoter | F:5′-GCTATGCCAGGAATCAAAGGAC-3′ |
| R:5′-CGCAAGTCAGGGATGTCACA-3′ | |
| MAGEA5 promoter | F:5′-ACATTTGAGGTCGGAGAGAAGC-3′ |
| R:5′-GGCACTGGATTATTTGGGGTC-3′ | |
| GABRE promoter | F:5′-CTGAATGAGCGACTGAATCC-3′ |
| R:5′-AGCCTCCACAAATCCTCACA-3′ | |
| GABRE intron 6 | F:5′-CTGGCTCTGGGGCTTTGTAG-3′ |
| R:5′-GACTCTGGGGAAGCGGTTTTA-3′ | |
| siRNA | |
| HDAC1 | 5′-GUUAGGUUGCUUCAAUCUA-3′ |
| HDAC2 | 5′-GUGUAAUGACGGUAUCAUU-3′ |
| HDAC3 | 5′-GAUCGAUUGGGCUGCUUUA-3′ |
| HDAC8 | 5′-CCUUCAAUGGCAGUUGGCA-3′ |
| EP300 | 5′-CUAGAGACACCUUGUAGUA-3′ |
| CREBBP | 5′-CCUACUACAGCUAUCAGAA-3′ |
| KAT2B | 5′-CUCUAAUCCUCACUCAUUU-3′ |
| Antibody | |
| β-Actin | Santa Cruz Biotechnology: sc-1616 |
| EP300 | Santa Cruz Biotechnology: sc-584 |
| Pan-histone H3 | Millipore: 06-755 |
| Acetylated H3K9 | Millipore: 352 |
| Acetylated H3K14 | Millipore: 353 |
| HDAC1 | Santa Cruz Biotechnology: sc-7872 |
Genomic DNA extraction and copy number analysis
Genomic DNA was extracted from the tumor and the paired adjacent nontumor samples from 40 patients with HCC using the QIAAmp DNA Mini Kit (Qiagen, Hilden, Germany). For copy number analysis, the DNA was dissolved at 50 ng/μl. Copy number variation analysis was performed using qPCR with specific primers spanning the 200-kbp region of interest along chromosome Xq28. These primers target the promoter regions of melanoma-associated antigens 4 and 5 (MAGEA4 and MAGEA5) and γ-aminobutyric acid receptor subunit ε (GABRE), as well as the intron 6 region of GABRE, where the miR-224/452 cluster resides. The individual raw data were normalized against the genomic DNA content of β-actin.
Treatment of liver cell lines with epigenetic drugs
NeHepLxHT and HepG2 cells were treated with 20 μM of 5-aza-2′-deoxycytidine (5-Aza; Sigma-Aldrich, St. Louis, MO, USA), 0.5 μM of trichostatin A (TSA; Sigma-Aldrich) or 5 μM of suberoylanilide hydroxamic acid (SAHA, Selleck Chemicals, Houston, TX, USA) for 72 h. HepG2 cells were treated with 2 μM, 5 μM or 10 μM of the EP300 inhibitor drug, C646 (25), for 24 h.
Chromatin immunoprecipitation (ChIP)
ChIP was performed on cells treated with the various drugs as well as patient samples using the ChIP Assay Kit (Millipore, Billerica, MA, USA) with ChIP-grade antibodies against acetylated form of histone H3 at lysine 9 (H3K9) or lysine 14 (H3K14) (Millipore), histone deacetylase 1 (HDAC1), or E1A binding protein p300 (EP300) (Santa Cruz, Santa Cruz, CA, USA). These antibodies (listed in Table 1) were verified to recognize their specific targets using Western blot analysis (Supplemental Fig. S1). The ChIP-enriched DNAs were quantitated using qPCR with primers targeting the promoter regions of MAGEA4, MAGEA5, and GABRE, as well as intron 6 of GABRE (GABRE Intron6) where miR-224 and miR-452 reside. These DNAs were normalized against the respective input DNA.
Statistical analyses of experimental data
Unpaired 2-tailed t test was performed to analyze the significance of differences between sample means obtained from ≥3 independent experiments. Pearson correlation was used to analyze the relationship between the expressions of Xq28 genes and miRNAs in samples from patients with HCC. Statistically significant tests were identified as having values of P < 0.05.
RESULTS
miR-224 is coordinately up-regulated with neighboring miR-452 and genes at Xq28 in HCC tumor
As illustrated in Fig. 1A, miR-224, together with miR-452, resides within intron 6 of GABRE and is flanked by two cancer and testis antigens, MAGEA4 and MAGEA5, in a 200-kb region near the end of the q arm of chromosome X, Xq28. In this study, MAGEA4, MAGEA5, GABRE, and miR-452 are collectively designated as miR-224-associated Xq28 genes. To evaluate whether the expression of this cluster of miRNAs and genes is coordinately regulated, RT-qPCR was employed to examine the expression of miR-224 and miR-224-associated Xq28 genes in 40 “normal ” liver samples, 100 tumor and paired adjacent nontumorous liver samples from patients with HCC.
Figure 1.
miR-224 is coordinately up-regulated with neighboring miRNAs and genes on Xq28 in hepatocellular carcinoma patients. A) Genomic structure of 200-kb region of Xq28 consisting of miR-224/miR-452 cluster residing in intron 6 of GABRE gene, flanked by two cancer and testis antigens, MAGEA4 and MAGEA5. B) Relative expression of miR-224 associated miRNAs and genes on Xq28 and unrelated miR-16, miR-100 and U6, in 40 normal liver samples (open box), 100 paired adjacent nontumor samples (light shaded box), and 100 HCC tumor samples (dark shaded box), measured using RT-qPCR and normalized against β-actin (for genes) or RNU48 (for miRNAs) as endogenous control. Data were normalized against the median expression of each miRNA or gene in normal liver samples and presented as box-and-whisker plots with the box spanning the 25th to 75th percentile and the solid line representing the median. Outliers were removed from the plots. *P < 0.05, **P < 0.01, ***P < 0.001. C) Scatterplots showing the representative correlation of relative fold change of transcript expression (in log2 scale) between miR-224 and miR-452 (I) and miR-224 and GABRE (II), in tumor vs. paired adjacent nontumor tissues from 100 patients with HCC. Each spot represents data from one patient with HCC; solid line is the linear regression line. See Table 2 for pairwise Pearson correlation of the relative fold change of Xq28 genes/miRNAs between tumor vs. paired adjacent nontumorous tissues. D) Box-and-whisker plots showing the relative genomic DNA content of Xq28 measured with qPCR using specific primers targeting promoters of MAGEA4, MAGEA5, and GABRE and the miR-224- and mir-452-residing GABRE intron 6, on tumor vs. paired adjacent nontumor samples from 40 patients with HCC.
As shown in Fig. 1B, compared to normal liver samples, miR-224 expression is significantly increased in the nontumor liver tissues of HCC (P<0.001). Its expression is increased even further in the tumors of patients with HCC (P<0.001). A similar trend of expression, whereby normal liver < nontumor of patients with HCC < tumor of patients with HCC, was also observed for miR-452, GABRE, MAGEA4, and MAGEA5. This progressive increase in transcript expression is specific to the miR-224-residing Xq28 locus and not observed in unrelated miRNAs (miR-16 at chromosomes 3 and 13 as well as miR-100 at chromosome 11) and U6 small RNA (in chromosome 15).
Pairwise Pearson correlation between relative transcript expression of miR-224 and its associated Xq28 genes in HCC tumor vs. nontumor tissues was performed to evaluate whether there is coordinate regulation of this cluster of genes and miRNAs. As evident in Fig. 1C and Table 2, there is strong statistical significant correlation (P<0.001) in the expression of miR-224, miR-452, and GABRE, the gene that this miRNAs reside in. A correlation was also found between the expression of the flanking genes (MAGEA4 and A5) and GABRE and miRNAs, although this corelationship is less clearcut. Hence, the expression of miR-224 and miR-224-associated Xq28 genes is correlated in samples from HCC patients. This progressive increase in transcript expression from normal liver to nontumor to tumor tissues of patients with HCC for genes and miRNAs within this 200-kb region of Xq28, as well as the correlated expression within this cluster of genes and miRNAs, suggests that a common mechanism may account for the coregulation of this cluster of genes.
Table 2.
Pairwise Pearson correlation of the relative fold change of Xq28 genes and miRNAs between tumor vs. paired adjacent nontumorous tissues
| Gene or miRNA |
r/P |
||||
|---|---|---|---|---|---|
| MAGEA4 | GABRE | miR-224 | miR-452 | MAGEA5 | |
| MAGEA4 | |||||
| GABRE | 0.160/0.086 | ||||
| miR-224 | 0.175/0.059 | 0.379/0.000* | |||
| miR-452 | 0.172/0.063 | 0.433/0.000* | 0.874/0.000* | ||
| MAGEA5 | 0.606/0.000* | 0.062/0.506 | 0.226/0.014* | 0.207/0.025* | |
Listings show corresponding Pearson correlation coefficient r and the P value.
P <0.05.
We hypothesized that this coordinated overexpression of miR-224 and its associated Xq28 genes may either be due to regional copy number changes due to genomic amplification or epigenetically-mediated transcription activation at this genomic locus. To determine whether changes in copy number could account for the differences in gene expression between the nontumorous and the tumor tissues at this locus, qPCR using primers spanning this region (including the promoters of MAGEA4, MAGEA5 and GABRE, as well as the intron 6 region of GABRE where miR-224 and miR-452 reside) was performed on genomic DNA extracted from tumor and paired adjacent nontumor tissues from 40 patients with HCC. As evident in Fig. 1D, no significant difference in the relative genomic content of the various regions in this miR-224-associated Xq28 cluster was observed. Hence, copy number variation at the genomic DNA level cannot account for the coordinated increase in transcript expression of miR-224 and associated genes at Xq28 in HCC tumor samples. An alternative hypothesis is that this cluster of genes/miRNAs may be regulated epigenetically.
Increased histone acetylation correlates with increased expression of miR-224 and miR-224-associated miRNAs and Xq28 genes
As it is impractical to perform experiments on human liver and HCC samples, we employed an in vitro model to evaluate whether this cluster of genes and miRNAs, including miR-224 at Xq28, is epigenetically regulated. We first evaluated whether NeHepLxHT, an hTERT immortalized human liver cell line, and HepG2, a transformed human HCC cell line, can be used to mimic normal liver cells and HCC tumor cells, respectively, for this study. As shown in Fig. 2A, the transcript expression of miR-224 and its associated Xq28 miRNAs and genes was ∼30-170-fold higher in HepG2 cells than NeHepLxHT cells (P<0.001), although the expression of the U6 control remained similar between these two cell lines. The genomic DNA content at the Xq28 locus in these two cell lines was also not significantly different (Fig. 2B). This is consistent with our observation that the expression of miR-224 and its associated Xq28 miRNAs and genes was significantly higher in the tumor vs. paired adjacent nontumorous tissues of patients with HCC or normal liver tissues, validating the relevance of our in vitro model.
Figure 2.
miR-224 expression positively correlates with histone H3 acetylation status in immortalized human liver cells. A) Relative transcript expression of miR-224 and associated Xq28 miRNAs and genes measured using RT-qPCR in immortalized human liver NeHepLxHT cells and human hepatocellular carcinoma HepG2 cells. B) Relative genomic DNA content of Xq28 in NeHepLxHT and HepG2 cells, measured using qPCR. C) Relative transcript expression of miR-224 and associated Xq28 miRNAs and genes in NeHepLxHT (left panel) and HepG2 (right panel) cells treated with 5-Aza (light shaded bars), TSA (dark shaded bars) and SAHA (solid bars), compared to the respective untreated cells (open bars). D) Relative histone H3 acetylation in NeHepLXT cells, measured as relative abundance of chromatin DNA immunoprecipitated with specific antibodies against H3K9 (left panel) and H3K14 (right panel), quantitated with qPCR and normalized against the respective amount of input DNA. E) Relative H3K9 and H3K14 acetylation in NeHepLxHT and HepG2 cells. Data are presented as means ± se from ≥3 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
To evaluate whether DNA methylation or histone acetylation play a role in influencing the expression of miR-224 and its associated Xq28 genes and miRNAs, small-molecule inhibitor drugs, including the DNA methyltransferase inhibitor drug 5-Aza, or histone deacetylase inhibitor (HDACi) drugs TSA or SAHA, were introduced into either NeHepLxHT or HepG2 cells. As shown in Fig. 2C, in NeHepLxHT cells, where the expression of these miRNAs and genes at the miR-224-associated Xq28 locus were very low, both HDACi drugs enhanced the expression of all the miRNAs and genes at the miR-224 associated Xq28 locus by 2- to 18-fold (P<0.05), while the DNA methyltransferase inhibitor 5-Aza only significantly enhanced the expression of MAGEA4 (P<0.05; Fig. 2C). In contrast, in the transformed HepG2 cells, which already expressed these miRNAs and genes at high levels, treatment with any of the drugs that inhibits the epigenetic machinery did not significantly increase the expression of miR-224 and its associated Xq28 miRNAs and genes. In fact, treatment of HepG2 cells with TSA at 0.5 μM concentration produced significant amount of cell death (data not shown) and inhibited the expression of miR-224, miR-452, and MAGEA5 by ∼2 fold (P<0.05). However, this unusual inhibition on miR-224 expression by TSA may be due to the non-HDACi effect of TSA at high concentration, as the other HDACi drug, SAHA, did not produce similar inhibition on miR-224 expression. Curiously, the control U6 expression was also increased by <1.5-fold (P<0.05) when transformed HepG2 cells were treated with 5-Aza and SAHA. Taken together, these data suggest that histone acetylation/deacetylation may play a role in regulating the transcript expression of miR-224 and associated Xq28 genes in untransformed NeHepLxHT liver cells. MAGEA4 gene expression is likely to be regulated by both histone acetylation/deacetylation and DNA methylation. The relatively little change in miRNA and gene expression on various epigenetic drug treatment in HepG2 cells could be due to epigenetic changes having already taken place during the process of transformation in these cells.
To ascertain the acetylation status of miR-224-associated Xq28 locus in NeHepLxHT cells exposed to the various epigenetic drugs, ChIP was performed using specific antibodies targeting H3K9 and H3K14 positions, which are the two most well-studied histone acetylation marks that were associated with active gene transcription (26, 27). As evident in Fig. 2D, DNA methyltransferase inhibitor 5-Aza had minimal effect on histone H3 acetylation, although there is a slight increase of H3K14 acetylation at the MAGEA4 promoter region, which is likely due to promoter DNA demethylation at the MAGEA4 gene locus. In contrast, the HDACis TSA and SAHA significantly increased histone H3 acetylation at both H3K9 and H3K14 in the entire miR-224-associated Xq28 locus. This positively correlated with the increase in transcript expression of miR-224 and its associated Xq28 miRNAs and genes on treatment with TSA and SAHA in these cells (Fig. 2C).
We then compared the acetylation status at the miR-224-associated Xq28 locus of NeHepLxHT cells with HepG2 cells. Consistent with our above findings, HepG2 cells, which mimic HCC tumor cells with high expression of miRNAs and genes at this locus (Fig. 2A) showed significantly greater H3K9 as well as H3K14 acetylation compared to NeHepLxHT cells, which mimic normal liver cells where the expression of miRNAs and genes in this region is very low (Fig. 2E). Our data thus demonstrate a strong positive correlation between the transcript expression of miR-224 and associated Xq28 gene and miRNAs with the histone acetylation status at the miR-224-associated Xq28 genomic locus.
miR-224 expression is regulated reciprocally by HDAC1, HDAC3, and EP300
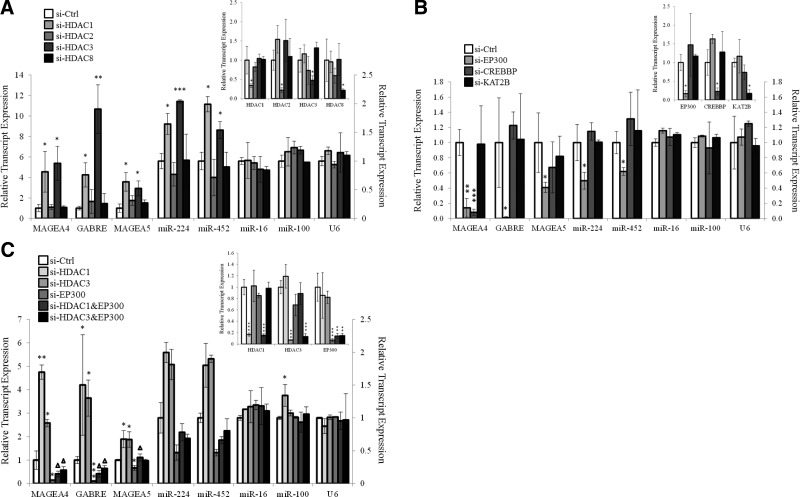
Since histone acetylation/deacetylation was found to play important roles in the regulation of the expression of miR-224 and its associated Xq28 miRNAs and genes, siRNAs against class I HDACs were employed to delineate the specific HDAC that may regulate miR-224-associated Xq28 miRNAs and genes in NeHepLxHT cells. Class I HDACs, comprising HDACs 1, 2, 3, and 8, were selected because both the HDACi drugs used in this study, TSA and SAHA, target class I and II HDACs and because class I HDAC mainly regulate histone deacetylation within the nucleus, which is the site of transcription (28). As shown in Fig. 3A (inset), the HDAC siRNAs specifically inhibited the intended HDAC by >50% (P<0.05) with minimal off-target effect on other nonintended members of class I HDACs. The inhibition of HDACs 1 and 3 significantly enhanced the transcript expression of miR-224 and its associated Xq28 genes by ∼1.5–10-fold (P<0.05) while the inhibition of HDACs 2 or 8 had no significant effects (Fig. 3A). Inhibition of class I HDACs did not significantly affect the expression of unrelated miR-16, miR-100, or control U6 small RNA, suggesting that the observed increase in transcript expression was specific to genes and miRNAs at Xq28 loci.
Figure 3.
miR-224 expression is reciprocally regulated by HDAC1, HDAC3, and EP300. A) Relative transcript expression of miR-224 and associated miRNAs and genes in Xq28 when NeHepLxHT cells were transfected with 50 nM of control siRNA (si-Ctrl), individual siRNAs targeting HDAC1 (si-HDAC1), HDAC2 (si-HDAC2), HDAC3 (si-HDAC3), or HDAC8 (si-HDAC8) with miR-16, miR-100, and U6 as unrelated controls. Inset: specific inhibition of each individual HDAC by its intended siRNA. B) Relative transcript expression of miR-224 and associated miRNAs and genes in Xq28 when NeHepLxHT cells were transfected with 50 nM of si-Ctrl, individual siRNAs targeting EP300 (si-EP300), CREBBP (si-CREBBP), or KAT2B (si-KAT2B). Inset: specific inhibition of the intended target genes by the respective siRNAs. C) Relative transcript expression of miR-224 and associated miRNAs and genes in Xq28 when NeHepLxHT cells were transfected with 50 nM of si-Ctrl, si-HDAC1, si-HDAC3, si-EP300, or combination of siRNAs targeting HDAC1 and EP300 (si-HDAC1 and EP300) or HDAC3 and EP300 (si-HDAC3 and EP300). Inset: specific inhibition of the intended target genes by the respective siRNAs. Data are presented as means ± se from ≥3 independent experiments. ΔP < 0.05 vs. si-EP300; *P < 0.05, **P < 0.01, ***P < 0.001 vs. control; t test.
The acetylation and deacetylation of histones are reversible processes regulated by HDACs and histone acetylases (HATs). Specific siRNAs against EP300, CREB-binding protein (CREBBP), or lysine acetyltransferase 2B (KAT2B) were employed to evaluate whether any of these HATs also play a role in the regulation of the expression of miR-224 and its associated Xq28 miRNAs and genes. As shown in Fig. 3B, inhibition of EP300 resulted in significant reduction in the transcript expression of miR-224 and its associated Xq28 miRNAs and genes (P<0.05). Inhibition of CREBBP resulted in significant reduction only in MAGEA4 transcript levels but not in miR-224 or other associated miRNAs and genes, while inhibition of KAT2B did not affect the transcript expression of miR-224 and associated miRNAs and genes in Xq28. Futhermore, the inhibition of both EP300 and HDACs 1 and 3 could statistically significantly rescue the EP300-associated reduction in miR-224 expression (P<0.05; Fig. 3C). The expression of unrelated miR-16, miR-100, or U6 small RNAs were not affected by the inhibition of either EP300 or HDACs 1 and 3 or both (Fig. 3C), which suggests that the observation is unlikely due to off-target effects of the siRNAs. Taken together, these in vitro data suggests that both EP300 and HDACs 1 and 3 play important roles in the regulation of the expression miR-224 and its associated Xq28 miRNAs and genes in NeHepLxHT cells.
EP300 Up-regulation may account for miR-224 overexpression in HCC tumors
To evaluate the clinical relevance of the in vitro observation, the histone H3 acetylation status, as well as HDAC1 and EP300 binding to the miR-224 associated Xq28 locus, was determined in 3 pairs of representative samples from patients with HCC, which showed increased miR-224 expression in HCC tumors (Supplemental Table S1). Compared to the adjacent nontumorous liver, a significant increase was found in H3K9 and H3K14 acetylation at the miR-224-associated Xq28 locus in the HCC tumors (Fig. 4A), consistent with the in vitro observation that miR-224 and its associated Xq28 miRNAs and genes can be positively regulated by histone acetylation in this locus. In these patients, together with the increase in histone acetylation at the miR-224-associated Xq28 locus, a significant increase was found in EP300 (P<0.05) but not HDAC1 binding at this locus in the HCC tumor tissues compared to the paired adjacent nontumorous liver (Fig. 4B). As shown in Fig. 4C, the increase in EP300 but not HDAC1 binding at the miR-224 associated Xq28 locus is likely due to significant increase in EP300 (P<0.01) but not HDAC1 and HDAC3 expression in the tumors of 100 patients with HCC. EP300 expression is also up-regulated progressively from normal liver, to HCC nontumor to HCC tumor samples (P<0.001), positively correlating with the similar expression pattern in miR-224 locus. Moreover, in patients where the miR-224 expression is >5-fold higher in the tumors compared to the adjacent nontumorous tissues (P<0.05), a significant correlation was found between the expression of miR-224 and the expression of EP300 (r2=0.14, P<0.05; Fig. 4D). Hence, in patients with HCC, the HAT protein, EP300, may play a more important role in the regulation of miR-224 expression than HDACs, which suggests that it may be worthwhile to explore the targeting of EP300 to suppress miR-224 expression in patients with HCC. The HepG2 cells, which express miR-224 at high levels (Fig. 2A), were thus employed as an in vitro model to explore the feasibility of suppressing miR-224 expression through the inhibition of EP300. In HepG2 cells, when EP300 was inhibited either using siRNA against EP300 (Fig. 4D) or an EP300-specific drug, C646 (Fig. 4E), the miR-224 expression was significantly inhibited (P<0.05).
Figure 4.
Increased histone H3 acetylation and EP300 binding at the miR-224 associated Xq28 locus in samples from patients with HCC. A) Relative histone H3 acetylation in HCC tumors vs. nontumors, measured as relative abundance of chromatin DNA immunoprecipitated with specific antibodies againstH3K9 (left panel) and H3K14 (right panel), quantitated using qPCR and normalized against the respective amount of input DNA. B) Relative enrichment of HDAC1 (left panel) and EP300 (right panel) binding along Xq28 in HCC tumor vs. nontumor samples. Data are presented as means ± sd from 3 patient samples (A, B). C) Relative transcript expression of HDAC1, HDAC3, and EP300 in normal liver (open bars), HCC nontumor tissues (light shaded bars), and HCC tumor (dark shaded bars), measured with RT-qPCR and normalized against β-actin as endogenous control. Data were normalized against the median expression of each respective gene in normal liver samples and presented as box-and-whisker plots with the box spanning the 25th to 75th percentile and the solid line representing the median. D) Scatterplot showing the correlation, expressed as relative fold change, of transcript expression (in log2 scale) between miR-224 and EP300 in tumor vs. paired adjacent nontumor tissues from 28 patients with HCC with significantly high miR-224 expression in tumor (>5-fold higher than the paired adjacent nontumor tissues). Each spot represents data from 1 patient; solid line is the linear regression line. E) Relative transcript expression of EP300, miR-224, and U6 when HepG2 cells were transfected with 50 nM of si-Ctrl or si-EP300. F) Relative transcript expression of miR-224 and U6 when HepG2 cells were treated with specified concentration of EP300-specific inhibitor drug C646. E, F) Data are presented as means ± se from ≥3 independent experiments. *P < 0.05. **P < 0.01, ***P < 0.001.
DISCUSSION
miR-224 is one of the most commonly overexpressed miRNAs in HCC (21). This miRNA resides within a 200-kb region of Xq28 in proximity to miR-452 within the GABRE gene and flanked by MAGEA4 and MAGEA5 cancer antigens (Fig. 1A). The expression of miR-224 (11) and the host gene, GABRE (Supplemental Fig. S2), were previously reported to be progressively increased from the normal liver to benign hepatocellular adenoma to malignant HCC. The MAGEA4 cancer antigen was also found previously to be overexpressed in HCC tumors (29). We thus hypothesized that the expression of this cluster of miRNAs and genes may be coordinately regulated. Consistent with the previous reports, these 2 miRNAs and 3 genes at this locus were found to be expressed at low levels in normal liver samples, intermediate levels in the nontumorous tissues of HCC, and high levels in HCC tumors (Fig. 1B). Their expression was also generally correlated with each other (Fig. 1C and Table 2). This progressive increase in miR-224 expression was also observed in the sera of patients with HCC, where there is a 14-fold enhancement of miR-224 expression in patients with liver cirrhosis and 87-fold increase in miR-224 in patients with HCC (30). Taken together, miR-224 may thus represent a promising biomarker to assess the liver status for liver disease and HCC.
We hypothesized that the coordinate regulation of this cluster of miRNAs and genes could either be due to genomic amplification or epigenetic regulation at this locus. As evident in Fig. 1D, the up-regulation of this cluster of miRNAs and genes is unlikely to be due to genomic amplification of this locus. To evaluate the roles of epigenetics in regulating this cluster of genes, in vitro models of nontransformed NeHepLXT and transformed HepG2 liver cells were employed. Histone acetylation rather than DNA methylation was found to play more important roles in regulating the expression of miRNAs and genes at this Xq28 locus, although MAGEA4 expression seems also to be regulated by DNA methylation (Fig. 2). It is likely that epigenetic reprogramming involving histone acetylation may represent early events in HCC tumorigenesis, since on HDACi drug administration, no further increase in the expression of miR-224 and its associated Xq28 miRNAs and genes was observed in the transformed HepG2 cells, which naturally expresses these miRNAs and genes at high levels. Furthermore, as the up-regulation of miR-224 expression precedes cellular transformation, the progressive increase in miR-224 expression may thus be due to the accumulation of epigenetic changes during the course of hepatocarcinogenesis.
In this in vitro model, HDAC1, HDAC3, and EP300 were found to reciprocally regulate the expression of the miRNAs and genes at this locus (Fig. 3). We thus propose the following model, as summarized in Fig. 5. In nontransformed cells, the miR-224 locus is probably maintained by HDAC1 and HDAC3 in a compact heterochromatin state that is transcriptionally quiescent. On cellular transformation, miR-224 expression is activated through histone acetylation, mediated by the overexpression of EP300. Interestingly, in patients with HCC, EP300 rather than HDAC seems to play a more important role in the regulation of miR-224 expression (Fig. 4). Similar to miR-224, EP300 expression is also found to be progressively increased from normal liver to HCC nontumor to HCC tumors, suggesting that EP300 elevation may account for the accumulated epigenetic changes at miR-224 locus for its progressive activation. Furthermore, high EP300 expression was recently reported to correlate with aggressive features and poor prognosis of HCC (31). Nonetheless, histone acetylation may not fully account for the increase in miR-224 expression in patients with HCC, because, although median ∼4- to 8-fold increase in miR-224 expression occurred in the tumors of patients with HCC (Fig. 1B), interfering with histone acetylation only changed miR-224 expression by ∼2 fold (Fig. 3B, D). Thus, it is likely that other additional factors are also involved in the regulation of miR-224 expression after epigenetic events render miR-224 permissible for transcription. miR-224 expression was recently reported to be activated by the Src tyrosine kinase pathway in mammary carcinoma cells (24) and the TGF-β pathway in mouse granulosa cells (23). It is thus possible that, since histone acetylation may result in chromatin remodeling of Xq28 to a more transcriptionally active state (32), it facilitates further regulation of miR-224 by specific transcription factors. Therefore, it may be worthwhile to investigate whether the Src or TGF-β pathway can further activate miR-224 expression in liver cells under epigenetically activated state (8, 22).
Figure 5.
Schematic diagram illustrating regulation of the chromatin state at the miR-224-associated Xq28 locus by HDAC1, HDAC3, and EP300. In nontransformed liver cells, miR-224 locus is kept in compact transcriptionally quiescent heterochromatin state by HDAC1 and HDAC3. In transformed liver cells, miR-224 is activated through increased histone acetylation mediated by EP300.
In summary, histone acetylation was found to play a partial role in the regulation of not only miR-224 but also other miRNAs and genes at this Xq28 locus in patients with HCC. It may thus be worthwhile to also evaluate whether the associated miRNAs and genes in this locus (miR-452, GABRE, MAGEA4, and MAGEA5) are also functionally important in hepatocarcinogenesis. While HDAC1, HDAC3, and EP300 were found to reciprocally regulate miR-224 and its associated Xq28 miRNAs and genes in the cell-culture model, only EP300 seems to play an important role in the regulation of miRNAs and genes at this locus in patients with HCC. Inhibiting EP300 using siRNA or a small-molecule inhibitor drug can significantly reduce miR-224 expression in HepG2, an HCC cell line, which suggests the feasibility of EP300 as a potential druggable target to reverse miR-224 overexpression in patients with HCC. This study thus provided some insights into the molecular mechanism underlying miR-224 overexpression in HCC and its possible therapeutic intervention.
Supplementary Material
Acknowledgments
This work is supported by grants from the National Medical Research Council of Singapore (NMRC/1131/2007), the Biomedical Research Council (BMRC06/1/21/19/449), and the Singapore Millennium Foundation, as well as block funding from National Cancer Center Singapore and Duke–National University of Singapore Graduate Medical School to C.L. This work is also supported by grants from the U.S. National Institutes of Health to P.C. and D.M.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- 5-Aza
- 5-aza-2′-deoxycytidine
- ChIP
- chromatin immunoprecipitation
- CREBBP
- CREB-binding protein
- EP300
- E1A binding protein p300
- GABRE
- γ-aminobutyric acid receptor subunit ε
- H3K9
- acetylated form of histone H3 at lysine 9
- H3K14
- acetylated form of histone H3 at lysine 14
- HAT
- histone acetylase
- HCC
- hepatocellular carcinoma
- HDAC
- histone deacetylase
- HDACi
- histone deacetylase inhibitor
- KAT2B
- lysine acetyltransferase 2B
- MAGEA4
- melanoma-associated antigen 4
- MAGEA5
- melanoma-associated antigen 5
- miR
- microRNA
- miRNA
- microRNA
- qPCR
- quantitative polymerase chain reaction
- RT
- reverse transcription
- SAHA
- suberoylanilide hydroxamic acid
- TSA
- trichostatin A
REFERENCES
- 1. Boyle P., Levin B. (2008) World Cancer Report 2008, International Agency for Research on Cancer, Lyon, France [Google Scholar]
- 2. Parkin D. M., Bray F., Ferlay J., Pisani P. (2005) Global cancer statistics, 2002. CA Cancer J. Clin. 55, 74–108 [DOI] [PubMed] [Google Scholar]
- 3. Wang Y., Lee C. G. (2009) MicroRNA and cancer—focus on apoptosis. J. Cell. Mol. Med. 13, 12–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murakami Y., Yasuda T., Saigo K., Urashima T., Toyoda H., Okanoue T., Shimotohno K. (2006) Comprehensive analysis of microRNA expression patterns in hepatocellular carcinoma and non-tumorous tissues. Oncogene 25, 2537–2545 [DOI] [PubMed] [Google Scholar]
- 5. Gramantieri L., Ferracin M., Fornari F., Veronese A., Sabbioni S., Liu C. G., Calin G. A., Giovannini C., Ferrazzi E., Grazi G. L., Croce C. M., Bolondi L., Negrini M. (2007) Cyclin G1 is a target of miR-122a, a microRNA frequently down-regulated in human hepatocellular carcinoma. Cancer Res. 67, 6092–6099 [DOI] [PubMed] [Google Scholar]
- 6. Meng F., Henson R., Wehbe-Janek H., Ghoshal K., Jacob S. T., Patel T. (2007) MicroRNA-21 regulates expression of the PTEN tumor suppressor gene in human hepatocellular cancer. Gastroenterology 133, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang Y. S., Dai Y., Yu X. F., Bao S. Y., Yin Y. B., Tang M., Hu C. X. (2008) Microarray analysis of microRNA expression in hepatocellular carcinoma and non-tumorous tissues without viral hepatitis. J. Gastroenterol. Hepatol. 23, 87–94 [DOI] [PubMed] [Google Scholar]
- 8. Wang Y., Lee A. T., Ma J. Z., Wang J., Ren J., Yang Y., Tantoso E., Li K. B., Ooi L. L., Tan P., Lee C. G. (2008) Profiling microRNA expression in hepatocellular carcinoma reveals microRNA-224 up-regulation and apoptosis inhibitor-5 as a microRNA-224-specific target. J. Biol. Chem. 283, 13205–13215 [DOI] [PubMed] [Google Scholar]
- 9. Jiang J., Gusev Y., Aderca I., Mettler T. A., Nagorney D. M., Brackett D. J., Roberts L. R., Schmittgen T. D. (2008) Association of MicroRNA expression in hepatocellular carcinomas with hepatitis infection, cirrhosis, and patient survival. Clin. Cancer Res. 14, 419–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Varnholt H., Drebber U., Schulze F., Wedemeyer I., Schirmacher P., Dienes H. P., Odenthal M. (2008) MicroRNA gene expression profile of hepatitis C virus-associated hepatocellular carcinoma. Hepatology 47, 1223–1232 [DOI] [PubMed] [Google Scholar]
- 11. Ladeiro Y., Couchy G., Balabaud C., Bioulac-Sage P., Pelletier L., Rebouissou S., Zucman-Rossi J. (2008) MicroRNA profiling in hepatocellular tumors is associated with clinical features and oncogene/tumor suppressor gene mutations. Hepatology 47, 1955–1963 [DOI] [PubMed] [Google Scholar]
- 12. Connolly E., Melegari M., Landgraf P., Tchaikovskaya T., Tennant B. C., Slagle B. L., Rogler L. E., Zavolan M., Tuschl T., Rogler C. E. (2008) Elevated expression of the miR-17–92 polycistron and miR-21 in hepadnavirus-associated hepatocellular carcinoma contributes to the malignant phenotype. Am. J. Pathol. 173, 856–864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Su H., Yang J. R., Xu T., Huang J., Xu L., Yuan Y., Zhuang S. M. (2009) MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 69, 1135–1142 [DOI] [PubMed] [Google Scholar]
- 14. Huang X. H., Wang Q., Chen J. S., Fu X. H., Chen X. L., Chen L. Z., Li W., Bi J., Zhang L. J., Fu Q., Zeng W. T., Cao L. Q., Tan H. X., Su Q. (2009) Bead-based microarray analysis of microRNA expression in hepatocellular carcinoma: miR-338 is downregulated. Hepatol. Res. 39, 786–794 [DOI] [PubMed] [Google Scholar]
- 15. Ura S., Honda M., Yamashita T., Ueda T., Takatori H., Nishino R., Sunakozaka H., Sakai Y., Horimoto K., Kaneko S. (2009) Differential microRNA expression between hepatitis B and hepatitis C leading disease progression to hepatocellular carcinoma. Hepatology 49, 1098–1112 [DOI] [PubMed] [Google Scholar]
- 16. Pineau P., Volinia S., McJunkin K., Marchio A., Battiston C., Terris B., Mazzaferro V., Lowe S. W., Croce C. M., Dejean A. (2010) miR-221 overexpression contributes to liver tumorigenesis. Proc. Natl. Acad. Sci. U. S. A. 107, 264–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wong Q. W., Ching A. K., Chan A. W., Choy K. W., To K. F., Lai P. B., Wong N. (2010) MiR-222 overexpression confers cell migratory advantages in hepatocellular carcinoma through enhancing AKT signaling. Clin. Cancer Res. 16, 867–875 [DOI] [PubMed] [Google Scholar]
- 18. Coulouarn C., Factor V. M., Andersen J. B., Durkin M. E., Thorgeirsson S. S. (2009) Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 28, 3526–3536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Y., Lu Y., Toh S. T., Sung W. K., Tan P., Chow P., Chung A. Y., Jooi L. L., Lee C. G. (2010) Lethal-7 is down-regulated by the hepatitis B virus x protein and targets signal transducer and activator of transcription 3. J. Hepatol. 53, 57–66 [DOI] [PubMed] [Google Scholar]
- 20. Selcuklu S. D., Donoghue M. T., Spillane C. (2009) miR-21 as a key regulator of oncogenic processes. Biochem. Soc. Trans. 37, 918–925 [DOI] [PubMed] [Google Scholar]
- 21. Wang Y., Lee C. G. (2011) Role of miR-224 in hepatocellular carcinoma: a tool for possible therapeutic intervention? Epigenomics 3, 235–243 [DOI] [PubMed] [Google Scholar]
- 22. Li Q., Wang G., Shan J. L., Yang Z. X., Wang H. Z., Feng J., Zhen J. J., Chen C., Zhang Z. M., Xu W., Luo X. Z., Wang D. (2010) MicroRNA-224 is upregulated in HepG2 cells and involved in cellular migration and invasion. J. Gastroenterol. Hepatol. 25, 164–171 [DOI] [PubMed] [Google Scholar]
- 23. Yao G., Yin M., Lian J., Tian H., Liu L., Li X., Sun F. (2010) MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol. Endocrinol. 24, 540–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X., Shen Y., Ichikawa H., Antes T., Goldberg G. S. (2009) Regulation of miRNA expression by Src and contact normalization: effects on nonanchored cell growth and migration. Oncogene 28, 4272–4283 [DOI] [PubMed] [Google Scholar]
- 25. Bowers E. M., Yan G., Mukherjee C., Orry A., Wang L., Holbert M. A., Crump N. T., Hazzalin C. A., Liszczak G., Yuan H., Larocca C., Saldanha S. A., Abagyan R., Sun Y., Meyers D. J., Marmorstein R., Mahadevan L. C., Alani R. M., Cole P. A. (2010) Virtual ligand screening of the p300/CBP histone acetyltransferase: identification of a selective small molecule inhibitor. Chem. Biol. 17, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kratz A., Arner E., Saito R., Kubosaki A., Kawai J., Suzuki H., Carninci P., Arakawa T., Tomita M., Hayashizaki Y., Daub C. O. (2010) Core promoter structure and genomic context reflect histone 3 lysine 9 acetylation patterns. BMC Genomics 11, 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Johnsson A., Durand-Dubief M., Xue-Franzen Y., Ronnerblad M., Ekwall K., Wright A. (2009) HAT-HDAC interplay modulates global histone H3K14 acetylation in gene-coding regions during stress. EMBO Rep. 10, 1009–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dokmanovic M., Clarke C., Marks P. A. (2007) Histone deacetylase inhibitors: overview and perspectives. Mol. Cancer Res. 5, 981–989 [DOI] [PubMed] [Google Scholar]
- 29. Fu X. Y., Wang H. Y., Tan L., Liu S. Q., Cao H. F., Wu M. C. (2002) Overexpression of p28/gankyrin in human hepatocellular carcinoma and its clinical significance. World J. Gastroenterol. 8, 638–643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gui J., Tian Y., Wen X., Zhang W., Zhang P., Gao J., Run W., Tian L., Jia X., Gao Y. (2010) Serum microRNA characterization identifies miR-885-5 p as a potential marker for detecting liver pathologies. Clin. Sci. (Lond.) 120, 183–193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li M., Luo R. Z., Chen J. W., Cao Y., Lu J. B., He J. H., Wu Q. L., Cai M. Y. (2011) High expression of transcriptional coactivator p300 correlates with aggressive features and poor prognosis of hepatocellular carcinoma. J. Transl. Med. 9, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Baylin S. B., Ohm J. E. (2006) Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat. Rev. Cancer 6, 107–116 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.