Abstract
Erythropoietin acts by binding to its cell surface receptor on erythroid progenitor cells to stimulate erythrocyte production. Erythropoietin receptor expression in nonhematopoietic tissue, including skeletal muscle progenitor cells, raises the possibility of a role for erythropoietin beyond erythropoiesis. Mice with erythropoietin receptor restricted to hematopoietic tissue were used to assess contributions of endogenous erythropoietin to promote skeletal myoblast proliferation and survival and wound healing in a mouse model of cardiotoxin induced muscle injury. Compared with wild-type controls, these mice had fewer skeletal muscle Pax-7+ satellite cells and myoblasts that do not proliferate in culture, were more susceptible to skeletal muscle injury and reduced maximum load tolerated by isolated muscle. In contrast, mice with chronic elevated circulating erythropoietin had more Pax-7+ satellite cells and myoblasts with increased proliferation and survival in culture, decreased muscle injury, and accelerated recovery of maximum load tolerated by isolated muscle. Skeletal muscle myoblasts also produced endogenous erythropoietin that increased at low O2. Erythropoietin promoted proliferation, survival, and wound recovery in myoblasts via the phosphoinositide 3-kinase/AKT pathway. Therefore, endogenous and exogenous erythropoietin contribute to increasing satellite cell number following muscle injury, improve myoblast proliferation and survival, and promote repair and regeneration in this mouse induced muscle injury model independent of its effect on erythrocyte production.—Jia, Y., Suzuki, N., Yamamoto, M., Gassmann, M., Noguchi, C. T. Endogenous erythropoietin signaling facilitates skeletal muscle repair and recovery following pharmacologically induced damage.
Keywords: PI3K/Akt, myoblast, wound healing, transgenic mice
Erythropoietin (EPO), a hypoxia-responsive glycoprotein cytokine produced in the kidney, is the primary regulator for erythroid differentiation and acts by binding to its receptor on the surface of erythroid progenitor cells to promote survival, proliferation, and differentiation. However, EPO receptor (EpoR) expression extends beyond hematopoietic progenitor cells and includes the cardiovascular system, neural progenitors and neurons, and skeletal muscle progenitor cells (1, 2). Mice that lack EpoR die in utero of severe anemia and exhibit other developmental defects in brain and heart, including increased apoptosis and decreased progenitor cell proliferation (3). In rodents, estrogen-stimulated EPO production in the uterus contributes to endometrium angiogenesis during transition from diestrus to proestrus (4).
In differentiating erythroid progenitor cells, EPO induces expression of EpoR that is then down-regulated in erythroid precursor cells with no significant expression on mature erythrocytes. Similarly, EpoR expressed in skeletal myoblasts is down-regulated with differentiation (2). In culture, EPO stimulates myoblast proliferation (2), suggesting that EPO signaling may contribute to muscle development, regeneration, or repair, although no gross morphological abnormalities are observed in unchallenged mice with EpoR restricted to hematopoietic tissue (5). Satellite cells or muscle progenitor cells express the Pax-7 homeobox gene that is critical for satellite cell maintenance and self-renewal (6). The quiescent Pax-7+ adult satellite cells that act as skeletal muscle stem cells during injury give rise to a subpopulation of cells that undergo self-renewal, while others differentiate to myoblasts and contribute to muscle fiber formation. Proliferating progenitor cells express myogenic regulatory factors (MRFs) Myf5 and MyoD, withdraw from the cell cycle, terminally differentiate and express late MRFs myogenin and MRF4, and fuse to form muscle fibers. Pax-7+Myf5− cells contribute to the satellite cell reservoir capable of symmetric cell division and also give rise to Pax-7+Myf5+ satellite cells that lose contact with the basal lamina and become committed myogenic cells (7). In the developing mouse embryo, the pattern of EpoR expression resembles, in part, that of the early MRF Myf5, and EPO stimulates Myf5 expression in myoblast culture (2).
Increased EPO signaling in myoblasts by forced expression of EpoR or exogenous EPO treatment promoted myoblast survival following transplantation and restored dystrophin expression in muscle fibers in muscular dystrophy mice (8). We now make use of two mouse models for EPO signaling, one with restricted expression of EpoR to erythroid cells and the other with high-level expression of transgenic EPO, to determine the role of normal and elevated EPO to promote satellite cell survival and muscle regeneration. We demonstrate that EPO contributes directly to myoblast proliferation and survival, leading to muscle regeneration and repair. We also show that myoblasts produce endogenous EPO that can contribute to myoblast survival. Furthermore, as a proof of concept, EPO treatment in an in vivo mouse model of muscle injury increases the pool of satellite cells available at the site of injury and contributes to muscle regeneration and recovery of maximum load tolerated by isolated muscle.
MATERIALS AND METHODS
Transgenic mice and muscle wound model
Hemizygous transgenic EPO-expressing tg6 mice (PDGF-β promoter/human EPO cDNA; ref. 9), wild-type (WT) littermates (control mice), and TgEpoR mice with EpoR restricted to hematopoietic tissue (erythroid GATA-1 promoter/EpoR cDNA transgene on an EpoR−/− background; ref. 5) were examined. Mice were on C57BL/6 background and were 4 wk old to avoid age-related muscle degeneration observed in older (≥5 mo) tg6 mice (10), and exercise performance in young tg6 mice was comparable to WT mice (11). Animal studies were carried out according to guidelines of the National Institute of Diabetes and Digestive and Kidney Diseases Animal Care and Use Committee. Age- and sex-matched tg6, TgEpoR, and WT mice were randomly divided into three groups for treatment: cardiotoxin (CTX), cardiotoxin plus EPO (CTX+EPO), and untreated control (each, n=6). Body composition and lean body mass were measured using the Brucker model NMR minispec analyzer (Brucker, Milton, ON, Canada). Prior to injection, mice were anesthetized using isoflurane gas, hind limbs were shaved, and skin was dissected to locate the muscle and vessels. CTX (100 μl) in PBS (10 μM; Sigma Chemical, St. Louis, MO, USA) was injected at 2 mm depth into the gastrocnemius muscle, ∼2 mm below the great saphenous vein. For CTX + EPO treatment, EPO (3000 U/kg; Epoetin-Alfa, Amgen, Thousand Oaks, CA, USA) was coadministered with CTX. To measure the damaged muscle, 1% Evans blue dye solution (NBT; Invitrogen, Carlsbad, CA, USA) was intraperitoneally injected with 1% volume relative to body mass 24 h prior to tissue collection (12). At 1, 3, and 14 d after the injection, mice were anesthetized and perfused with 4% paraformaldehyde in PBS. The gastrocnemius muscles were harvested, paraffin embedded, and sectioned for immunochemical staining. The maximum load tolerated by isolated muscle (tension to rupture) was measured after recovery from CTX injury using a force gauge. The gastrocnemius muscle was isolated with tendons at both ends. The proximal tendon was clamped, and the distal tendon was connected to the Compact Force Gauge (RH13; Mecmesin, Sterling, VA, USA). The gauge was pulled, and minimum force that was required to break the muscle was recorded.
Primary myoblast isolation and cell culture
Primary myoblasts were isolated from the gastrocnemius muscles of 4-wk-old mice cut thoroughly and treated with 0.25% trypsin/0.5 mM EDTA (1 h at 37°C), filtered through a 70-μm cell strainer, selected by differential sedimentation, and cultured on gelatin-coated culture dishes in F10 primary myoblast growth medium with 20% FBS (37°C; 5% CO2). C2C12 cells (CRL-1772; American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM with 10% FBS. Cultures were treated with EPO and LY-294002 (50 μM) for signaling experiments. Cell viability was determined through an ATP luminescence assay (PerkinElmer, Boston, MA, USA). Cells were fixed with 4% paraformaldehyde in PBS for terminal transferase dUTP nick end labeling (TUNEL) analysis. For the scrape-wound assay, cell cultures at 80% confluence were scraped with a pipette tip, and the scraped area was analyzed by Image Pro (Media Cybernetics, Inc., Bethesda, MD, USA) after 24 h.
Quantitative real-time reverse transcriptase-polymerase chain reaction (RT-PCR)
Gene expression was quantified by real-time RT-PCR using gene-specific primers for EpoR, mouse, and transgenic EPO and β-actin (7900HT; PE Applied Biosystems, Foster City, CA, USA), as described previously (2, 9), as well as mouse apoptosis array (RT2Profiler PCR array, cat. no. APM-012C; SABiosciences, Frederick, MD, USA).
Western blotting and immunochemical staining
Cell extracts were prepared, and protein was separated by polyacrylamide gel electrophoresis and transferred to nitrocellulose. Specific proteins were detected using primary antibodies for EpoR (M-20), AKT (C-20), p-AKT (sc-7985), Bad (C-7), Bcl-x (S-18), and β-actin (SC-1615) (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA; 4°C, overnight) and corresponding secondary antibody and chemiluminescence (Invitrogen, Carlsbad, CA, USA). Muscle paraffin sections were prepared for immunochemical staining and analyzed using antibodies for Pax-7 (1:200; Developmental Studies Hybridoma Bank, Iowa City, IA; 4°C; overnight) and corresponding fluorescence-conjugated secondary antibody (1:400; SC-2039; Santa Cruz Biotechnology). Cultured cells were fixed with 4% paraformaldehyde in PBS and analyzed with an in situ cell death detection system (TMR red; Roche Diagnostics, Mannheim, Germany). Fluorescence-positive cells were detected by fluorescence microscopy and examined under a Nikon Eclipse TE-2000-U fluorescent microscope (Nikon, Tokyo, Japan), and the images were captured using the PerkinElmer confocal image system.
EPO bioassay
Epo-dependent HCD57 cells (13) were cultured with varying EPO concentrations to create a standard curve and compared with HCD57 cells cultured in conditioned medium of primary myoblasts or C2C12 cells.
Reporter gene assay
The EpoR-luciferase construct contained the EpoR proximal promoter extending 194 bp 5′ from the transcription start site to create the EPOR construct and the GATA-binding site was mutated to create the ΔGATA reporter gene construct (14). Transfection into C2C12 cells was carried out using FuGene 6 (Promega, Madison, WI, USA). Luciferase activity was determined 48 h after transfection. A pIRES2-EGFP-based GATA-3 expression vector (3) was used for overexpression of GATA-3.
Statistical analysis
Microsoft Excel (Microsoft, Redmond, WA, USA) was used to calculate standard deviation and P values by the 1-way ANOVA.
RESULTS
EPO signaling and skeletal muscle
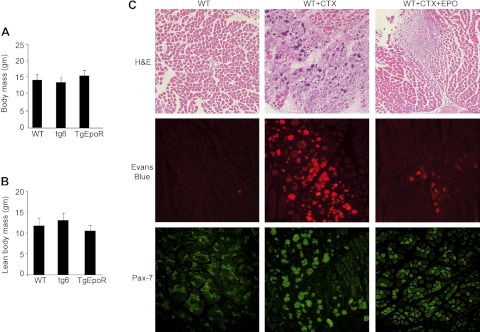
EpoR expression in skeletal myoblasts that is down-regulated with differentiation (2) suggests that endogenous EPO signaling may contribute to skeletal muscle formation, maintenance, or repair. Therefore, we used a mouse injury model and compared 4-wk-old WT mice with mice with high circulating EPO (tg6) and EpoR restricted to hematopoietic tissue (TgEpoR). Young mice were selected prior to age-related changes in body mass readily detected at 4 mo as an increase in TgEpoR mice (15) and a decrease in tg6 mice (16), and the age-related neuromuscular degeneration detected in tg6 mice (5). Body mass at 4 wk was comparable among the WT, tg6, and TgEpoR mice (Fig. 1A). Analysis for lean body mass showed a nonsignificant tendency of tg6 mice to have greater lean mass than TgEpoR mice (Fig. 1B). Inspection of muscle fiber cross sections from slides prepared from the gastrocnemius muscle showed that fiber diameter appeared to be larger in tg6 mice (Supplemental Fig. S1A), but quantification revealed no significant difference in fiber diameter between tg6 and WT mice.
Figure 1.
EPO protection in CTX-induced muscle injury and repair in vivo. A, B) Total body mass (A) and lean body mass (B) were determined for TgEpoR, tg6, and WT littermate mice at 4 wk of age (sex matched, n=6). C) Representative sections of gastrocnemius muscles from WT mice harvested at 7 d after PBS treatment (100 μl), treatment with PBS containing CTX (WT+CTX; 10 μM), and treatment with CTX plus EPO (WT+CTX+EPO; 3000 U/kg), and stained for hematoxylin and eosin (H&E), Evans blue dye (red), and Pax-7 (green). For Evans blue dye uptake, at 24 h prior to tissue collection, Evans blue dye solution (1%) was injected intraperitoneally.
EPO increases Pax7+ satellite cells and muscle repair
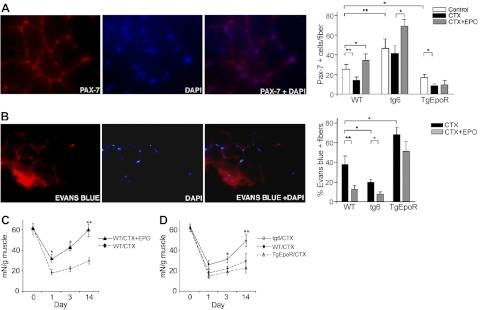
With muscle injury, the number of satellite cells at the site of injury is determined by the loss of satellite cells due to muscle damage, activation of quiescent satellite cells to divide and self-renew or differentiate into proliferating muscle progenitor cells, and recruitment of satellite cells from the surrounding area. As a model of muscle injury, CTX was injected into the gastrocnemius muscle. The resultant damage by CTX injury to muscle fibers and reduction in Pax-7+ cells were minimized by EPO treatment (Fig. 1C). In WT mice, the number of satellite cells quantified by Pax-7 staining at d 3 after CTX injury decreased significantly compared with uninjured muscle (Fig. 2A). To determine the potential for EPO treatment to promote wound healing, as proof of concept, we coinjected EPO at high dose (3000 U/kg) with CTX. We observed that EPO treatment increased the number of Pax-7+ cells compared with injection of CTX alone, and cell numbers became significantly greater than in the absence of muscle injury (Fig. 2A), suggesting that in addition to reduced damage and increased survival of satellite cells, EPO treatment increased recruitment or proliferation of satellite cells in the injured muscle.
Figure 2.
EPO enhanced recovery in CTX-induced muscle injury. A) Three groups of mice each (4 wk in age; sex matched; n=6) from TgEpoR, tg6, and WT littermate mice were treated with 100 μl PBS (control; open bars), PBS containing CTX (10 μM; solid bars) and PBS containing CTX + EPO (3000 U/kg; shaded bars) by injection into the gastrocnemius muscle. Muscles were harvested 3 d after injection, and paraffin sections were stained for Pax-7 (red) and DAPI (blue). Representative sections are shown (left panels), and Pax7+ cells were quantified (right panel). B) Mice were treated as described in A, and at 24 h prior to tissue collection, Evans blue dye solution (1%) was injected intraperitoneally. Muscles were harvested and stained for Evans blue dye (red) and DAPI (blue). Representative sections are shown (left panels), and percentages of Evans blue-positive fibers were quantified and compared for CTX treatment without (solid bars) and with EPO treatment (shaded bars). C) Muscle tension to rupture determined for dissected gastrocnemius muscle was used to assess muscle regeneration before (d 0) or after CTX treatment (d 1, 3, and 14) in WT mice without (solid line) and with EPO treatment (3000 U/kg; dashed line). Mice were 4 wk of age (female; n=6). D) Muscle tension to rupture of the isolated gastrocnemius muscle was determined for TgEpoR (open triangles; dashed-dotted line), tg6 (open circles; solid line) and WT littermate mice (solid circles; dashed line) 4 wk in age (sex matched, n=6) before (d 0) or after CDX treatment (d 1, 3, and 14). *P < 0.05; **P < 0.01.
The tg6 mice represent a model of chronic EPO treatment with plasma EPO level 12-fold greater than WT mice, resulting in hematocrit up to 89% (5). The number of Pax-7+ cells distributed throughout the gastrocnemius muscle in tg6 mice was ∼2-fold greater than in WT mice (Fig. 2A), indicating that chronic EPO treatment, even without muscle injury, increases the number of Pax-7+ satellite cells. With CTX-induced muscle injury in tg6-mice, the marked decrease in Pax-7+ cells in WT mice following muscle injury was not seen, and the number of Pax-7+ cells in tg6 mice was not statistically different from uninjured muscle (Fig. 2A), suggesting that chronic EPO treatment contributes to the pool of satellite cells by providing protection to satellite cells and/or enhancing satellite cell activation and proliferation and recruitment to the site of injury. Coinjection of EPO with CTX in tg6 mice further increased the number of Pax-7+ cells (Fig. 2A), indicating that although circulating EPO level in tg6 mice is high, the protective and stimulating effects of endogenous and transgenic EPO can be further augmented by acute EPO treatment.
In TgEpoR-mice, the number of Pax-7+ cells was two-thirds that of WT mice in the uninjured gastrocnemius muscle (Fig. 2A), suggesting that endogenous EPO may promote satellite cell survival in skeletal muscle. As in WT mice, Pax-7+ cell numbers in TgEpoR mice decreased with CTX treatment and muscle injury; however, in contrast to WT and tg6 mice, TgEpoR mice did not exhibit an increase in the number of Pax-7+ cells with EPO treatment in the CTX-injured muscle (Fig. 2A). This suggests that the increase in satellite cells with EPO treatment in injured skeletal muscle is not due to EPO stimulation of hematopoietic cells but rather is a result of nonhematopoietic response. Furthermore, both endogenous and exogenous EPO can increase the number of Pax-7+ satellite cells, while loss of EpoR in nonhematopoietic cells (TgEpoR mice) decreases the number of Pax-7+ cells and abrogates the Pax-7+ satellite cell response to EPO during muscle injury.
The decrease in ischemic-reperfusion injury in heart by EPO treatment that promotes survival of cardiomyocytes is mediated, in part, by indirect EPO activity on cardiomyocytes (17, 18). Therefore, although mature muscle fibers do not express EpoR, we examined the ability of EPO to promote muscle fiber survival and/or repair and reduce formation of damaged muscle fibers, as assessed by Evan's blue dye uptake (Fig. 2B). We found that compared with CTX treatment alone, coinjection of EPO with CTX in WT mice reduced the number of damaged muscle fibers by one-third at d 3 following injury (Fig. 2B). In high-EPO-expressing tg6 mice, the percentage of damaged muscle fibers was reduced by half compared with WT mice. Coinjection of exogenous EPO with CTX further reduced the percentage of damaged muscle fibers in tg6 mice. With loss of EpoR in nonhematopoietic tissue, TgEpoR mice exhibited the greatest susceptibility to muscle damage, and the percentage of damaged muscle fibers was not changed with EPO coinjection with CTX compared to CTX alone (Fig. 2B). These data suggest that endogenous EPO promotes satellite cell proliferation and possibly survival with CTX-induced muscle damage that is further increased with chronic or acute EPO treatment, while loss of EpoR in nonhematopoietic tissue increases sensitivity to muscle damage and abrogates the protective effect of exogenous EPO treatment. In addition, the effect of EPO in skeletal muscle injury is independent of its erythropoietic activity.
EPO promotes functional recovery after muscle injury
Preliminary assessment of general response to muscle injury was by position-holding time, the length of time that mice are able to grasp onto a wire grid plate in a cage when the cage is inverted. Testing indicated that position-holding time for tg6 mice was about twice that of WT mice, and that EPO treatment in WT mice increased position-holding time at 7 d following CTX treatment, but not immediately following injection of CTX + EPO (at d 1; Supplemental Figs. S1B, C and S3). To determine whether EPO administration could directly affect muscle recovery, quantification of isolated muscle tension to rupture was determined before and after CTX muscle injury. Muscle appeared weakest at d 1 following muscle injury in WT mice, and muscle tension to rupture results gradually improved during the 14 d following injury (Fig. 2C). EPO treatment with CTX markedly reduced the decrease in muscle tension to rupture, returning to preinjury levels by d 14 after injury with EPO treatment (Fig. 2C). In tg6 mice compared with WT mice, the decrease in muscle tension to rupture was less severe following CTX injury, and the recovery was better by d 3 after injury and was even more significant at d 14 (Fig. 2D). TgEpoR mice showed the greatest decrease in muscle tension to rupture and slowest recovery compared with WT and tg6 mice (Fig. 2D). These data suggest that the contribution of endogenous EPO activity to recovery from muscle injury can be further augmented by acute and chronic EPO administration.
Myoblast cultures from WT, tg6, and TgEpoR mice
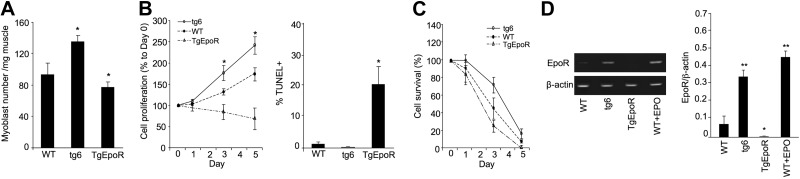
To determine the influence of alterations of EPO signaling on myoblast development, myoblasts isolated from the gastrocnemius muscle of WT, TgEpoR, and tg6 mice were cultured in vitro. Primary myoblasts were more readily recovered from tg6 mice and more difficult to recover from TgEpoR mice (Fig. 3A). Surprisingly, in the absence of exogenous EPO, Tg6 myoblast proliferation was greatest, yielding 40% more cells after 5 d in culture compared with WT myoblasts (Fig. 3B). Furthermore, cultures of myoblasts from TgEpoR mice that lacked EpoR failed to increase in cell numbers. These data suggest that the extent of EPO signaling in vivo during myoblast development may affect the intrinsic property of primary myoblasts to survive and proliferate when cultured ex vivo. Assessment of apoptotic cells after 5 d in undifferentiated culture medium showed significantly more TUNEL+ cells in TgEpoR cultures at 20% compared with WT and tg6 cultures that were 1% or less (Fig. 3B).
Figure 3.
Primary myoblast cultures. Myoblasts from gastrocnemius muscle of TgEpoR mice, tg6 mice, and WT littermates at 4 wk of age were isolated for culture. B) Primary myoblasts were cultured for 5 d, and proliferation was determined for tg6 (open circles; solid line), TgEpoR (open triangles; dashed-dotted line), and WT (solid circles; dashed line) myoblasts (left panel); results are normalized to d 0. Percentage of TUNEL+ stained cells was determined after 5 d of culture (right panel). C) Primary myoblasts from tg6, TgEpoR, and WT mice were cultured under 5% O2 for 5 d; percentage of surviving cells compared to d 0 is shown. D) EpoR and β-actin mRNA expression in primary myoblasts harvested from TgEpoR and tg6 mice, and from WT mice without and with EPO treatment (5 U/ml) primary myoblast by quantitative real-time RT-PCR. EpoR expression is normalized to β-actin as the internal control. *P < 0.05; **P < 0.01.
Endogenous EPO activity and low-oxygen-induced apoptosis in myoblast cultures
We observed that primary myoblast cultures exposed to reduced oxygen tension (5% O2) appeared to cease proliferation and decrease in cell numbers, dropping by 10-fold after 5 d (Fig. 3C). In the absence of exogenous EPO, we found that primary myoblasts isolated from tg6 mice exhibited improved survival at 5% O2 compared with myoblasts isolated from WT mice, suggesting a lasting survival effect in isolated myoblasts by overexpression of the EPO transgene in tg6 mice. In contrast, myoblasts isolated from TgEpoR mice showed increased susceptibility to low oxygen tension, with reduced survival (Fig. 3C). These observations provide further evidence for a survival effect of endogenous EPO and an intrinsic defect in TgEpoR myoblasts with loss of endogenous EPO signaling. These results may relate, in part, to direct EpoR expression in myoblasts. EPO induction of EpoR in WT myoblasts illustrates the link between EpoR expression and EPO response. We found that EpoR in myoblasts increased by 5- to 6-fold in tg6 myoblast cultures compared with WT myoblasts, even in the absence of exogenous EPO, and EpoR was absent in TgEpoR myoblast cultures (Fig. 3D).
Myoblasts and direct dose-dependent EPO response
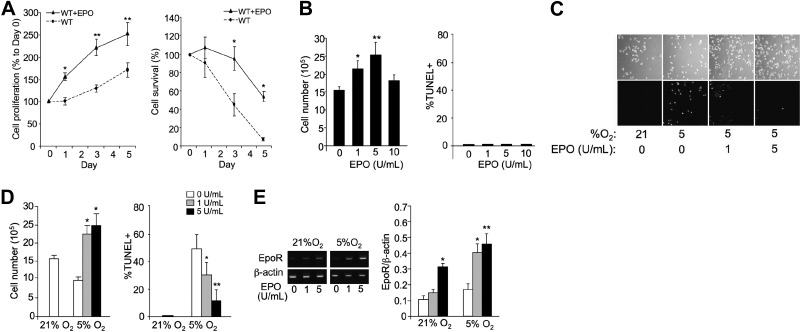
The reduction in damaged muscle fibers with EPO treatment in CTX muscle injury in WT mice suggests an indirect effect of EPO, since EpoR expression is absent in mature muscle fibers. EPO treatment in WT myoblast cultures illustrates the direct effect of EPO in stimulating primary myoblast proliferation (Fig. 4A, left panel). The increased survival of EPO-treated WT myoblast cultures exposed to 5% O2 provided further evidence for the direct effect of EPO (Fig. 4A, right panel). C2C12 myoblasts exposed to increasing EPO concentration showed the dose-dependent response of myoblasts to EPO and an optimum effect on proliferation at 5 U/ml (Fig. 4B). This optimum in EPO concentration is in contrast to erythroid progenitor cells that express higher levels of EpoR and are EPO responsive over a broad range of EPO concentration. In contrast to cultures grown at 21% O2 with no significant apoptosis, at 5% O2 there was a marked decrease in proliferation and increase in apoptosis, as determined by TUNEL+ staining (Fig. 4B–D). EPO treatment exhibited a dose-dependent effect reflecting increased cell survival and decreased percentage of TUNEL+ cells (Fig. 4D). EpoR expression was induced at low oxygen tension, and increasing EPO concentration further increased EpoR expression at both normoxia and reduced oxygen tension (Fig. 4E). These data are indicative of a direct effect of EPO on stimulating myoblast proliferation and survival in culture in a dose-dependent manner.
Figure 4.
EPO protects myoblasts from 5% O2-induced apoptosis. A) Cell proliferation was determined for primary myoblasts from gastrocnemius muscle isolated from 4-wk-old WT mice that were cultured without (circles; dashed line) and with EPO (5 U/ml; triangles; solid line) for 5 d (left panel). Primary myoblast cultures were also exposed to 5% O2 for 5 d; percentage of surviving cells compared to d 0 is shown (right panel). B) Proliferation (left panel) and TUNEL assay (right panel) of C2C12 cells seeded at 1 × 106 cells/well in 6-well pales and treated with EPO (0, 1, 5, and 10 U/ml) for 24 h were determined. C) C2C12 cells seeded at 1 × 106 cells/well in 6-well plates, treated with EPO (0, 1, and 5 U/ml), and cultured at 5% O2 for 24 h were compared with cells cultured at 21% O2. D) Quantification of cell numbers (left panel) and percentage of TUNEL+ cells (right panel) from C. E) EpoR and β-actin mRNA expression in C2C12 cells cultured at 21 and 5% O2 and without and with EPO treatment (5 U/ml) was determined by quantitative real-time RT-PCR. EpoR expression is normalized to β-actin as the internal control. **P < 0.01.
EPO increases myoblast recovery in an in vitro wound assay
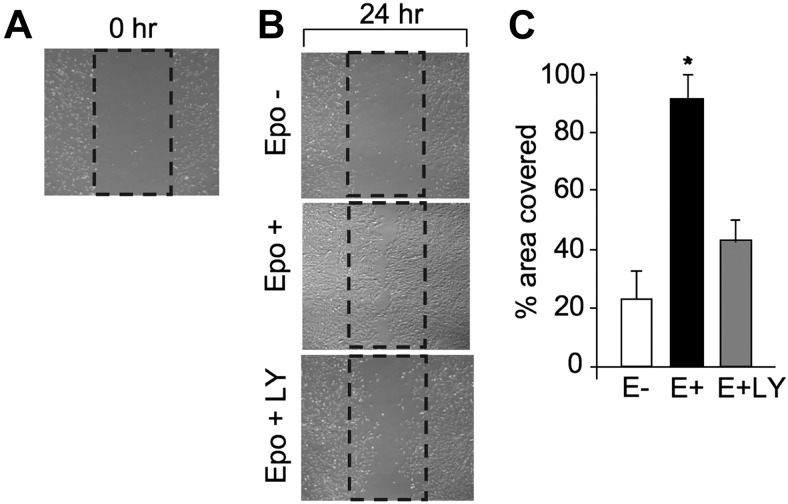
Direct EPO response of myoblasts was further demonstrated by the cell scrape-wound assay used to examine EPO activity in stressed C2C12 myoblast cell cultures (Fig. 5). Cultures were disrupted by mechanical scraping (Fig. 5A), and growth recovery into the scraped area after 24 h was quantified (Fig. 5B). Cultures treated with EPO increased growth recovery by 4-fold (Fig. 5B, C), indicating that EPO facilitated cell migration in addition to promoting myoblast proliferation.
Figure 5.
Cell scrape-wound assay. A) C2C12 cells were cultured in growth medium to ∼80% confluence for the in vitro scratch assay. Percentage of area covered by myoblasts growing back into the scraped area after 24 h was monitored. B, C) Cells were treated without (open bar) and with EPO (5 U/ml; solid bar) and EPO plus LY-294002 (LY; 50 μM; shaded bar) after the cells were scraped. After 24 h, images of the scraped area were captured by Nikon phase-contrast microscope (B) and the percentage of the scraped area covered by myoblasts was determined by the Image Pro program (C). *P < 0.05.
EPO production in myoblasts
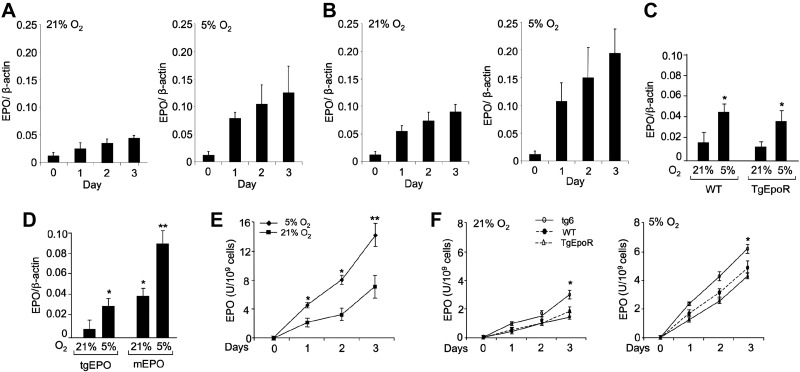
Myoblasts from TgEpoR mice lack EpoR and were unable to proliferate in culture, while myoblasts from tg6 mice exhibited increased EpoR expression and increased proliferation compared with WT myoblasts (Fig. 3C–E). The relative expression of EpoR combined with the possibility that myoblasts express EPO to stimulate an autocrine response would account for variation in proliferation of WT, tg6, and TgEpoR-myoblasts. Therefore, we examined C2C12 cells for EPO expression (Fig. 6A). We found that EPO mRNA was expressed in C2C12 cells and increased with days in culture. EPO is known to be inducible by hypoxia, and C2C12 EPO expression increased at low oxygen tension (Fig. 6B). As with increased EpoR expression with EPO treatment, we also observed that EPO treatment of C2C12 cells increased endogenous EPO production at both 21 and 5% O2.
Figure 6.
EPO production in the C2C12 and primary myoblast cultures from TgEpoR, tg6, and WT mice. A, B) Quantitative real-time RT-PCR of mouse EPO mRNA expression under 21% O2 (left panel) or 5% O2 (right panel) for C2C12 cell cultures without (A) and with (B) EPO treatment (5 U/ml); β-actin mRNA expression was used as the internal control. C) EPO expression was determined for primary myoblasts isolated from WT and TgEpoR mice at 21% O2, as well as the induction of EPO expression at reduced oxygen tension (5% O2). D) Expression of endogenous mouse EPO (mEPO) and human tgEPO was determined for primary myoblasts isolated from tg6 mice at 21% O2, as well as the induction of transgenic and endogenous EPO expression at reduced oxygen tension (5% O2). E) EPO activity in conditioned medium from C2C12 cells cultured at 21% O2 (squares) and 5% O2 (diamonds) was determined by an EPO bioassay. Cell culture supernatants were collected at different time points (d 0, 1, 2, 3). F) Primary myoblasts isolated from WT mice (solid circles; dashed line), tg6 mice (open circles; solid line) and TgEpoR mice (open triangles; solid line) were cultured. EPO bioactivity at 21% O2 and EPO induction at 5% O2 were determined in conditioned medium harvested at d 0, 1, 2, and 3. *P < 0.05.
Primary myoblasts also expressed EPO in WT and TgEpoR myoblasts at comparable levels, and the levels of expression were further increased at low oxygen tension (Fig. 6C). Increased EPO expression at low oxygen tension in TgEpoR myoblasts that lack EpoR indicates that induction of EPO expression at low oxygen tension does not require an EPO response. Increase of endogenous EPO expression by EPO treatment suggests that tg6 myoblasts expressing transgenic EPO would express more endogenous EPO than WT or TgEpoR myoblasts. To distinguish between endogenous mouse EPO expression and transgenic human EPO expression, we designed species-specific primers for EPO. We observed expression of the human transgenic EPO (tgEPO) in tg6 myoblasts (Fig. 6D) and noted that endogenous mouse EPO expression was 2- to 3-fold greater than that of WT and TgEpoR myoblasts (Fig. 6C, D). In tg6 myoblasts, both endogenous mouse EPO and human transgenic EPO were induced by hypoxia. Induction of the human EPO transgene expression is likely due to hypoxia induction of transgene PDGF promoter activity.
To assess EPO activity produced in myoblast cultures, conditioned medium was assayed by an EPO bioassay using EPO-dependent HCD57 cells (Fig. 6E, F). EPO activity in C2C12 culture supernatant was analogous to EPO expression, increased with days in culture, and increased significantly when cells were cultured under 5% O2 compared with 21% O2 (Fig. 6E). Primary myoblast cultures from tg6 mice produced higher EPO activity compared with WT- and TgEpoR-derived cultures (Fig. 6F). The increase in tg6 cultures reached significance at d 3. Hypoxia also induced EPO production in cultures from WT, tg6, and TgEpoR mice, and the greatest production was observed for tg6-derived cultures. Therefore, tg6 myoblasts express the highest level of EPO consistent with the greatest level of proliferation compared with WT and TgEpoR myoblast cultures. Interestingly, TgEpoR myoblasts expressed EPO mRNA and produced EPO activity at levels comparable to WT myoblasts, providing further support for EPO production in myoblasts independent of EpoR expression.
EPO activates the AKT signaling pathway
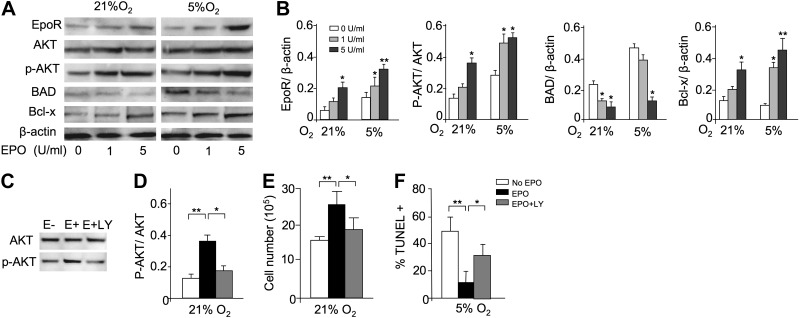
To determine potential signaling pathways involved in the antiapoptotic EPO response of myoblasts, we used a mouse apoptosis RT-PCR array to analyze differences in C2C12 cells cultured without and with EPO treatment for 24 h at 5% O2. The greatest changes in expression (>10-fold) were detected in genes relating to the PI3K-AKT signaling pathway, such as Akt1, Bcl2, Bad, and caspase 9 (data not shown). Changes in genes associated with other signaling pathways, such as NF-κB and RAS/MAPK, were <3-fold, and no change in JAK2 or STAT5 gene expression was detected. To confirm these results, Western blotting of PI3K-AKT signaling pathway proteins in C2C12 cells exposed to 21 and 5% O2 and various concentrations of EPO were performed (Fig. 7A, B). EPO treatment induced AKT protein phosphorylation, while total AKT protein remained unchanged in C2C12 cells cultured at 21 and 5% O2. Decreased expression of downstream apoptotic BAD protein and increased expression of antiapoptotic Bcl-x protein were concomitant with the increase in AKT protein phosphorylation. Reducing the oxygen tension from 21 to 5% O2, particularly with EPO treatment, increased expression of these PI3K-AKT-associated proteins. EpoR protein expression also increased with reduced oxygen tension and with exogenous EPO (Fig. 7A, B). These changes in EpoR protein are analogous to the pattern of EpoR mRNA expression (Fig. 4E). The similarity in the pattern of EpoR protein expression and members of the PI3K-AKT signaling pathway suggests that EPO activity and myoblast protection from apoptosis may be mediated via the PI3K-AKT signaling pathway.
Figure 7.
EPO protection and AKT-signaling pathway in myoblasts. A) To confirm the profile expression array results, Western blot analysis for EpoR, AKT, phosphorylated AKT (p-AKT), BAD, and Bcl-x was performed for C2C12 cells cultured with EPO (0, 1, 5 U/ml) for 24 h at 21 and 5% O2; β-actin protein expression was used as internal control. B) Western blot analysis results were quantified for cells cultured without EPO (open bars) and with EPO at 1 U/ml (shaded bars) and at 5 U/ml (solid bars) and normalized to β-actin. C–F) C2C12 cells were cultured for 24 h without EPO (open bars) and with EPO (5 U/ml; black bars) and with EPO plus LY-294002 (50 μM; shaded bars), an inhibitor of the PI3K-AKT signaling pathway. LY-294002 inhibited the EPO-induced AKT phosphorylation, determined by Western blot analysis for AKT and p-AKT (C, D), and reversed the proliferative effect of EPO at 21% O2 (seeded initially at 1×106 cells/well in 6-well plates; E) and the antiapoptotic EPO effect at 5% O2, as indicated by percentage TUNEL+ (F). Quantification of Western blot analysis results are shown as p-AKT/AKT (D). **P < 0.01.
Treatment with the PI3K-AKT inhibitor LY-294002 greatly diminished C2C12 cell EPO activity (Fig. 7C–F). The EPO effect on cell proliferation and protection from apoptosis was significantly reduced by concomitant treatment with LY-294002. EPO-stimulated AKT phosphorylation was abrogated by LY-294002 (Fig. 7E, F). LY-294002 also affected EPO-stimulated myoblast recovery in the cell scrape-wound assay. The addition of LY-249002 with EPO in this in vitro cell wound assay reduced the area covered by myoblasts by half compared with EPO treatment alone (Fig. 5B, C). Therefore, LY-294002 inhibition of EPO-stimulated proliferation and protection provides further evidence for involvement of PI3K-AKT signaling pathway in EPO activity in myoblast.
EPO induction of GATA-3 contributes to myoblast response
These data suggest that EPO response is determined to some extent by the level of EpoR expression. In hematopoietic cells, the GATA-factor binding motif is critical for a high level of EpoR expression (14, 19). We previously demonstrated that EPO stimulation of myoblasts induces expression of GATA-3 (2). Using reporter gene assays, we found that the GATA-factor binding motif is also important for a high level of EpoR promoter activity in myoblasts (Fig. 8). Activity of the EpoR promoter in C2C12 cells was diminished with mutation of the GATA factor-binding motif (Fig. 8A). Conversely, cotransfection with a GATA-3 expression vector increased EpoR promoter activity, but not if the GATA factor-binding motif was mutated (Fig. 8B). Overexpression of GATA-3 also increased endogenous EpoR gene expression (Fig. 8C, D). EPO stimulation of myoblasts also increased expression of early MRFs Myf5 and MyoD (2). We observed that increased expression of GATA-3 also provided a marked increase in MyoD expression (Fig. 8E). In myoblasts, GATA-3 can also contribute to the proliferative response to EPO. GATA-3 overexpression also increased cell numbers in undifferentiated proliferating C2C12 cultures (Fig. 8F). These data suggest that EPO induction of GATA-3 contributes to EPO response via direct induction of EpoR, affecting the MRF expression program and promoting cell proliferation.
Figure 8.
GATA-3 increases EpoR expression and myoblast proliferation. A) Luciferase reporter gene constructs containing the EpoR promoter, the EpoR promoter with the GATA binding site mutated (ΔGATA), and a promoterless control were assayed in C2C12 myoblasts and normalized to activity of the SV40 promoter construct. B) Luciferase activities of the EpoR and ΔGATA reporter gene constructs with cotransfection of the GATA-3 expression vector are compared with activity of the EpoR construct without GATA-3 overexpression. C–E) GATA-3 (C), EpoR (D), and MyoD (E) expression levels in C2C12 cells without and with overexpression of GATA-3. F) Cell proliferation of C2C12 cells with overexpression of GATA-3 (diamonds; dashed line), with mock transfection (triangles; solid line) and with untransfected cells (control; squares; solid line).
DISCUSSION
The potential for endogenous EPO signaling to contribute to skeletal muscle repair and regeneration is exemplified by the increased susceptibility and delayed recovery in muscle injury of TgEpoR mice with EpoR restricted to hematopoietic tissue. These mice exhibit a reduced pool of Pax-7+ satellite cells in skeletal muscle, increased sensitivity to CTX-induced muscle injury, and slower recovery of the maximum load tolerated by isolated muscle compared with WT mice. Unexpectedly, we found that primary myoblasts isolated from TgEpoR mice exhibited an intrinsic defect: they were unable to proliferate in vitro and showed increased sensitivity to low oxygen compared with WT myoblasts. This intrinsic loss of proliferating ability in culture appears to be directly related, in part, to loss of EPO response due to absence of EpoR, despite myoblast production of EPO. Skeletal muscles have the capacity to regenerate muscle fibers, and under normal physiological conditions, the weekly turnover of myofiber nuclei is 1-2% (20). Normal skeletal muscle development does not appear to be significantly different between WT and TgEpoR mice, and no difference in isolated muscle tension to rupture was observed among uninjured TgEpoR, WT, and tg6 mice. However, the loss of EPO signaling in TgEpoR mice is readily apparent in vivo with muscle damage or injury when satellite cells are activated to generate myoblasts for muscle repair and regeneration. This suggests that in muscle, EPO may contribute more importantly to stress response rather than normal basal processes. For example, studies of ischemic heart failure suggested that EPO improvement of microvascularization is observed only in the presence of ischemia (21). Similarly, although TgEpoR mice show no gross morphological defect in brain, cultures of primary neurons that lack EpoR also exhibit marked increase sensitivity to hypoxia (3). In contrast to this complete lack of EPO response in nonhematopoietic tissue in TgEpoR mice, mice with decreased endogenous EPO production did not exhibit changes in skeletal muscle for EpoR expression or intrinsic functional properties (22).
Chronic and acute EPO treatments are able to augment endogenous EPO activity in the CTX model of muscle injury. Chronic EPO treatment, represented by transgenic EPO-expressing tg6 mice, and acute EPO treatment at the time of CTX injury reduced or eliminated the decrease in the pool of Pax-7+ satellite cells in the injured muscle, significantly decreased the percentage of damaged muscle fibers during recovery, and increased recovery of muscle tension to rupture. In addition, myoblasts can respond directly to EPO, as indicated by cultures of isolated tg6 myoblasts and of EPO-treated WT myoblasts. We observed that elevated EPO increased cell proliferation and cell survival at low O2 in addition to increasing EpoR. The effects of EPO treatment also were suggested in other models of muscle injury, including the prevention of mitochondria degradation in bupivacaine-induced myotoxicity (23). We show that endogenous EPO is expressed in myoblasts and is increased by hypoxia and exogenous EPO, suggesting that an autocrine response to EPO contributes to the intrinsic differences in proliferation and survival associated with myoblast cultures isolated from tg6 and TgEpoR mice. Hypoxia induction of EPO production in human skeletal muscle has also been reported (24). Myoblast production of EPO provides a possible mechanism for improved survival of myoblasts with forced expression of EpoR in vivo following transplantation into muscular dystrophy mice to restore dystrophin expression in muscle fibers (8). The present studies were carried out on young (4 wk old) mice with similar body weight and lean mass, long before the onset of neuromuscular or vascular degeneration associated with older tg6 mice (≥5 mo), attributed to chronic EPO exposure and high hematocrit (>80%), with increased blood viscosity (10, 11).
Pax-7+ satellite cells in skeletal muscle activated during injury give rise to a subpopulation of cells that undergo self-renewal and return to quiescence, while others expressing MRFs, such as Myf5, differentiate to myoblasts and contribute to muscle fiber formation (7). The relative decrease and increase of Pax-7+ cells in uninjured skeletal muscle of TgEpoR mice and tg6 mice, respectively, indicates that the pool size of satellite cells, the main players in regeneration of skeletal muscle damaged by injury or disease (25), is affected by EPO signaling. We previously found that EPO treatment in myoblast cultures induces Myf5 expression and delays myogenin expression (2), suggesting the possibility that EPO may contribute directly to expansion of myoblasts or the Pax-7+/Myf5+ pool of cells following muscle injury. Hence, endogenous EPO shows the potential to contribute to stress or injury by promoting progenitor/precursor cell proliferation and survival in erythroid tissue, as well as in other select nonhematopoietic tissue.
JAK2 signaling is required for early myogenic differentiation, transcription activation, and activity of muscle transcription factors MyoD and MEF2 (26). EPO-EpoR signaling in erythroid cells induces multiple signaling pathways, including JAK2-STAT5, MAPK, and PI3K-AKT (27, 28). Increased human EpoR expression and associated JAK2 activity in the exercising leg were observed, and these changes were accompanied by local EPO release (29). In addition, crosstalk between JAK2 and NF-κB signaling pathways has been suggested in EPO hypoxic brain preconditioning that protects neurons from ischemic and degenerative injury (30). We observed EPO activation of the JAK2-STAT5 pathway in C2C12 cells (2), but we found no evidence of NF-κB activation or changes in protein levels of IκB, which usually decrease with NF-κB activation, or of the NF-κB p65 subunit (data not shown). We identified that EPO-EpoR signaling in myoblasts activates the PI3K-AKT signaling pathway, analogous to the neuroprotective antiapoptotic effect of EPO (31), and increases the antiapoptotic Bcl-x protein and decreases the apoptotic BAD protein. In myoblasts, AKT and mTOR are linked to insulin-like growth factor-1 (IGF-1) stimulation, and the PI3K/AKT pathway has been suggested as a possible mechanism for regulation of survival by the small GTPase RhoA (32, 33). However, gene expression profiling and Western blotting did not reveal changes in mTOR or MAPK, or in expression of RAS or MAPKK with EPO stimulation. Inhibition of PI3K-AKT signaling blocked EPO-proliferative and survival activity in myoblast cultures and EPO activity in the in vitro cell scrape-wound assay. These results suggest that EPO-EpoR-mediated myoblast response in wound repair requires activation of the PI3K-AKT signaling pathway. EPO cardioprotection has also been demonstrated to be mediated via the PI3K-Akt signaling pathway (17, 34).
Although hematopoietic stem cells have been implicated in skeletal muscle fiber formation and skeletal muscle regeneration (35, 36), the data provided here suggest that the contribution of hematopoietic cells to muscle repair in this model of CTX-induced muscle injury in mice is minimal. TgEpoR mice express EpoR only in the hematopoietic lineage and exhibit appropriate EPO response to anemic stress. However, treatment of EPO simultaneously with CTX in TgEpoR mice did not increase the numbers of PAX-7+ cells compared with CTX alone, suggesting that the hematopoietic/erythroid progenitor cell response to this single dose of exogenous EPO does not affect skeletal muscle regeneration.
EPO response beyond increased erythropoiesis and stimulation of muscle progenitor cells may also contribute to EPO-stimulated muscle regeneration and repair. EpoR is expressed on endothelial cells, and EPO treatment can stimulate angiogenesis, endothelial cell mobilization, and endothelial cell production of nitric oxide (9, 37, 38). Activation of the tissue endothelial system and resultant increase in nitric oxide and endothelin-1 is also observed in tg6 mice with high EPO production and high hematocrit (39). In a rat model for traumatized skeletal muscle injury, EPO treatment improved functional capillary density and reduced leukocytic response and leakage of the microvasculature (40, 41). Indeed, in open crush injury to the rat soleus muscle, intramuscular application of EPO increased fast twitch and titanic force recovery (10–20%) during the postinjury healing process of 42 d, and this associated EPO activity was suggested to go along with improvement in overall tissue integrity and microcirculation by EPO, as well as its cellular antiapoptotic and proliferative properties (40). Repeated phlebotomy in WT mice resulting in increased EPO production also increased capillary density and blood flow in the ischemic leg, with increased HIF-1α, VEGF, and circulating endothelial progenitor cells (42). In heart ischemic/reperfusion injury in mice, endothelial response contributes significantly to EPO-stimulated cardioprotection (18). As observed in a rat model for ischemic heart failure, local ischemia may be necessary to promote the EPO angiogenic response in skeletal muscle (21). Of note, EPO administration in normal human subjects did not increase angiogenic activity in muscle during basal conditions (43).
The current study provides evidence that endogenous EPO contributes to repair and regeneration after skeletal muscle injury. Specifically, EPO promotes expansion and/or recruitment of the pool Pax-7+ satellite cells to the site of injury, decreases the number of damaged muscle fibers, and increases recovery of the maximum load tolerated by isolated muscle in this mouse model of CTX-induced injury. Furthermore, this EPO activity appears to require EpoR expression and includes direct EPO response of myoblasts. Loss of EpoR expression beyond hematopoietic tissue compromises skeletal muscle repair and regeneration. Whether EPO is protective against skeletal muscle damage to reduce rupture, z-line streaming, or other morphological changes requires further study. Indeed, for EPO activity in mouse brain, rather than protecting neurons from ischemic reperfusion injury, endogenous EPO signaling contributes to poststroke recovery (44). Finally, muscle regeneration and repair are enhanced by chronic elevated EPO or acute EPO treatment at the site of injury. Therefore, endogenous EPO and exogenous EPO treatment increase recovery in this model of muscle injury and regeneration.
Supplementary Material
Acknowledgments
The authors thank Li Wang, Heather Rogers, Susan E. Ivie, and Xiaobing Yu for their contributions in studies on transcription regulation and position-holding time.
Funding is provided by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases.
Author contributions: Y.J. performed conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, and final approval of manuscript; N.S. provided study material and data analysis and interpretation; M.Y. provided study material and data analysis and interpretation; M.G. provided study material and data analysis and interpretation; and C.T.N. provided conception and design, financial support, data analysis and interpretation, manuscript writing, and final approval of manuscript.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- CTX
- cardiotoxin
- EPO
- erythropoietin
- EpoR
- erythropoietin receptor
- MRF
- myogenic regulatory factor
- WT
- wild type
- RT-PCR
- reverse transcriptase-polymerase chain reaction
- tgEPO
- transgenic EPO
- TUNEL
- terminal transferase dUTP nick end labeling
REFERENCES
- 1. Chen Z. Y., Asavaritikrai P., Prchal J. T., Noguchi C. T. (2007) Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J. Biol. Chem. 282, 25875–25883 [DOI] [PubMed] [Google Scholar]
- 2. Ogilvie M., Yu X., Nicolas-Metral V., Pulido S. M., Liu C., Ruegg U. T., Noguchi C. T. (2000) Erythropoietin stimulates proliferation and interferes with differentiation of myoblasts. J. Biol. Chem. 275, 39754–39761 [DOI] [PubMed] [Google Scholar]
- 3. Yu X., Shacka J. J., Eells J. B., Suarez-Quian C., Przygodzki R. M., Beleslin-Cokic B., Lin C. S., Nikodem V. M., Hempstead B., Flanders K. C., Costantini F., Noguchi C. T. (2002) Erythropoietin receptor signalling is required for normal brain development. Development 129, 505–516 [DOI] [PubMed] [Google Scholar]
- 4. Yasuda Y., Masuda S., Chikuma M., Inoue K., Nagao M., Sasaki R. (1998) Estrogen-dependent production of erythropoietin in uterus and its implication in uterine angiogenesis. J. Biol. Chem. 273, 25381–25387 [DOI] [PubMed] [Google Scholar]
- 5. Suzuki N., Ohneda O., Takahashi S., Higuchi M., Mukai H. Y., Nakahata T., Imagawa S., Yamamoto M. (2002) Erythroid-specific expression of the erythropoietin receptor rescued its null mutant mice from lethality. Blood 100, 2279–2288 [DOI] [PubMed] [Google Scholar]
- 6. Lepper C., Conway S. J., Fan C. M. (2009) Adult satellite cells and embryonic muscle progenitors have distinct genetic requirements. Nature 460, 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kuang S., Kuroda K., Le G. F., Rudnicki M. A. (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jia Y., Warin R., Yu X., Epstein R., Noguchi C. T. (2009) Erythropoietin signaling promotes transplanted progenitor cell survival. FASEB J. 23, 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruschitzka F. T., Wenger R. H., Stallmach T., Quaschning T., de Wit C., Wagner K., Labugger R., Kelm M., Noll G., Rulicke T., Shaw S., Lindberg R. L., Rodenwaldt B., Lutz H., Bauer C., Luscher T. F., Gassmann M. (2000) Nitric oxide prevents cardiovascular disease and determines survival in polyglobulic mice overexpressing erythropoietin. Proc. Natl. Acad. Sci. U. S. A. 97, 11609–11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Heinicke K., Baum O., Ogunshola O. O., Vogel J., Stallmach T., Wolfer D. P., Keller S., Weber K., Wagner P. D., Gassmann M., Djonov V. (2006) Excessive erythrocytosis in adult mice overexpressing erythropoietin leads to hepatic, renal, neuronal, and muscular degeneration. Am. J. Physiol. Regul. Integr. Comp. Physiol. 291, R947–R956 [DOI] [PubMed] [Google Scholar]
- 11. Schuler B., Arras M., Keller S., Rettich A., Lundby C., Vogel J., Gassmann M. (2010) Optimal hematocrit for maximal exercise performance in acute and chronic erythropoietin-treated mice. Proc. Natl. Acad. Sci. U. S. A. 107, 419–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hamer P. W., McGeachie J. M., Davies M. J., Grounds M. D. (2002) Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J. Anat. 200, 69–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng H., Zhang J., Yoon T., Song D., Li D., Lin A. (2011) Phosphorylation of Bcl-associated death protein (Bad) by erythropoietin-activated c-Jun N-terminal protein kinase 1 contributes to survival of erythropoietin-dependent cells. Int. J. Biochem. Cell Biol. 43, 409–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chin K., Oda N., Shen K., Noguchi C. T. (1995) Regulation of transcription of the human erythropoietin receptor gene by proteins binding to GATA-1 and Sp1 motifs. Nucleic Acids Res. 23, 3041–3049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Teng R., Gavrilova O., Suzuki N., Chanturiya T., Schimel D., Hugendubler L., Mammen S., Yver D. R., Cushman S. W., Mueller E., Yamamoto M., Hsu L. L., Noguchi C. T. (2011) Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat. Commun. 2, 520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Katz O., Stuible M., Golishevski N., Lifshitz L., Tremblay M. L., Gassmann M., Mittelman M., Neumann D. (2010) Erythropoietin treatment leads to reduced blood glucose levels and body mass: insights from murine models. J. Endocrinol. 205, 87–95 [DOI] [PubMed] [Google Scholar]
- 17. Fu P., Arcasoy M. O. (2007) Erythropoietin protects cardiac myocytes against anthracycline-induced apoptosis. Biochem. Biophys. Res. Commun. 354, 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Teng R., Calvert J., Sibmooh N., Piknova B., Suzuki N., Sun J., Martinez K., Yamamoto M., Schechter A., Lefer D., Noguchi C. T. (2011) Acute erythropoietin cardioprotection is mediated by endothelial response. Basic Res.Cardiol. 106, 343–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zon L. I., Youssoufian H., Mather C., Lodish H. F., Orkin S. H. (1991) Activation of the erythropoietin receptor promoter by transcription factor GATA-1. Proc. Natl. Acad. Sci. U. S. A. 88, 10638–10641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cosgrove B. D., Sacco A., Gilbert P. M., Blau H. M. (2009) A home away from home: Challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation 78, 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Westenbrink B. D., Oeseburg H., Kleijn L., van der H. P., Belonje A. M., Voors A. A., Schoemaker R. G., de Boer R. A., van Veldhuisen D. J., van Gilst W. H. (2008) Erythropoietin stimulates normal endothelial progenitor cell-mediated endothelial turnover, but attributes to neovascularization only in the presence of local ischemia. Cardiovasc. Drugs Ther. 22, 265–274 [DOI] [PubMed] [Google Scholar]
- 22. Hagstrom L., Agbulut O., Hasnaoui-Saadani R., Marchant D., Favret F., Richalet J. P., Beaudry M., Launay T. (2010) Epo is relevant neither for microvascular formation nor for the new formation and maintenance of mice skeletal muscle fibres in both normoxia and hypoxia. J. Biomed. Biotechnol. 2010, 137817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nouette-Gaulain K., Bellance N., Prevost B., Passerieux E., Pertuiset C., Galbes O., Smolkova K., Masson F., Miraux S., Delage J. P., Letellier T., Rossignol R., Capdevila X., Sztark F. (2009) Erythropoietin protects against local anesthetic myotoxicity during continuous regional analgesia. Anesthesiology 110, 648–659 [DOI] [PubMed] [Google Scholar]
- 24. Ameln H., Gustafsson T., Sundberg C. J., Okamoto K., Jansson E., Poellinger L., Makino Y. (2005) Physiological activation of hypoxia-inducible factor-1 in human skeletal muscle. FASEB J. 19, 1009–1011 [DOI] [PubMed] [Google Scholar]
- 25. Tedesco F. S., Dellavalle A., az-Manera J., Messina G., Cossu G. (2010) Repairing skeletal muscle: regenerative potential of skeletal muscle stem cells. J. Clin. Invest. 120, 11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang K., Wang C., Xiao F., Wang H., Wu Z. (2008) JAK2/STAT2/STAT3 are required for myogenic differentiation. J. Biol. Chem. 283, 34029–34036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pelletier S., Gingras S., Funakoshi-Tago M., Howell S., Ihle J. N. (2006) Two domains of the erythropoietin receptor are sufficient for Jak2 binding/activation and function. Mol. Cell. Biol. 26, 8527–8538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhao W., Kitidis C., Fleming M. D., Lodish H. F., Ghaffari S. (2006) Erythropoietin stimulates phosphorylation and activation of GATA-1 via the PI3-kinase/AKT signaling pathway. Blood 107, 907–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rundqvist H., Rullman E., Sundberg C. J., Fischer H., Eisleitner K., Stahlberg M., Sundblad P., Jansson E., Gustafsson T. (2009) Activation of the erythropoietin receptor in human skeletal muscle. Eur. J. Endocrinol. 161, 427–434 [DOI] [PubMed] [Google Scholar]
- 30. Digicaylioglu M., Lipton S. A. (2001) Erythropoietin-mediated neuroprotection involves cross-talk between Jak2 and NF-κB signalling cascades. Nature 412, 641–647 [DOI] [PubMed] [Google Scholar]
- 31. Um M., Gross A. W., Lodish H. F. (2007) A “classical” homodimeric erythropoietin receptor is essential for the antiapoptotic effects of erythropoietin on differentiated neuroblastoma SH-SY5Y and pheochromocytoma PC-12 cells. Cell. Signal. 19, 634–645 [DOI] [PubMed] [Google Scholar]
- 32. Ren H., Accili D., Duan C. (2010) Hypoxia converts the myogenic action of insulin-like growth factors into mitogenic action by differentially regulating multiple signaling pathways. Proc. Natl. Acad. Sci. U. S. A. 107, 5857–5862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ohanna M., Sobering A. K., Lapointe T., Lorenzo L., Praud C., Petroulakis E., Sonenberg N., Kelly P. A., Sotiropoulos A., Pende M. (2005) Atrophy of S6K1(−/−) skeletal muscle cells reveals distinct mTOR effectors for cell cycle and size control. Nat. Cell Biol. 7, 286–294 [DOI] [PubMed] [Google Scholar]
- 34. Mao W. K., Iwai C. K., Liu J. H., Sheu S. S., Fu M., Liang C. S. (2008) Darbepoetin alfa exerts a cardioprotective effect in autoimmune cardiomyopathy via reduction of ER stress and activation of the PI3K/Akt and STAT3 pathways. J. Mol. Cell. Cardiol. 45, 250–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fukada S., Yamamoto Y., Segawa M., Sakamoto K., Nakajima M., Sato M., Morikawa D., Uezumi A., Miyagoe-Suzuki Y., Takeda S., Tsujikawa K., Yamamoto H. (2008) CD90-positive cells, an additional cell population, produce laminin α2 upon transplantation to dy(3k)/dy(3k) mice. Exp. Cell Res. 314, 193–203 [DOI] [PubMed] [Google Scholar]
- 36. Abedi M., Foster B. M., Wood K. D., Colvin G. A., McLean S. D., Johnson K. W., Greer D. A. (2007) Haematopoietic stem cells participate in muscle regeneration. Br. J. Haematol. 138, 792–801 [DOI] [PubMed] [Google Scholar]
- 37. Anagnostou A., Liu Z., Steiner M., Chin K., Lee E. S., Kessimian N., Noguchi C. T. (1994) Erythropoietin receptor mRNA expression in human endothelial cells. Proc. Natl. Acad. Sci. U. S. A. 91, 3974–3978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nakano M., Satoh K., Fukumoto Y., Ito Y., Kagaya Y., Ishii N., Sugamura K., Shimokawa H. (2007) Important role of erythropoietin receptor to promote VEGF expression and angiogenesis in peripheral ischemia in mice. Circ. Res. 100, 662–669 [DOI] [PubMed] [Google Scholar]
- 39. Quaschning T., Ruschitzka F., Stallmach T., Shaw S., Morawietz H., Goettsch W., Hermann M., Slowinski T., Theuring F., Hocher B., Luscher T. F., Gassmann M. (2003) Erythropoietin-induced excessive erythrocytosis activates the tissue endothelin system in mice. FASEB J. 17, 259–261 [DOI] [PubMed] [Google Scholar]
- 40. Rotter R., Menshykova M., Winkler T., Matziolis G., Stratos I., Schoen M., Bittorf T., Mittlmeier T., Vollmar B. (2008) Erythropoietin improves functional and histological recovery of traumatized skeletal muscle tissue. J. Orthop. Res. 26, 1618–1626 [DOI] [PubMed] [Google Scholar]
- 41. Gassmann M., Manini A., Stallmach T., Saam B., Kuhn G., Grenacher B., Bogdanova A. Y., Vogel J. (2008) Abortion in mice with excessive erythrocytosis is due to impaired arteriogenesis of the uterine arcade. Biol. Reprod. 78, 1049–1057 [DOI] [PubMed] [Google Scholar]
- 42. Kawamura I., Takemura G., Kanamori H., Takeyama T., Kawaguchi T., Tsujimoto A., Goto K., Maruyama R., Watanabe T., Shiraki T., Aoyama T., Fujiwara T., Fujiwara H., Minatoguchi S. (2010) Repeated phlebotomy augments angiogenesis to improve blood flow in murine ischemic legs. Am. J. Physiol. Heart Circ. Physiol. 299, H372–H378 [DOI] [PubMed] [Google Scholar]
- 43. Lundby C., Hellsten Y., Jensen M. B., Munch A. S., Pilegaard H. (2008) Erythropoietin receptor in human skeletal muscle and the effects of acute and long-term injections with recombinant human erythropoietin on the skeletal muscle. J. Appl. Physiol. 104, 1154–1160 [DOI] [PubMed] [Google Scholar]
- 44. Tsai P. T., Ohab J. J., Kertesz N., Groszer M., Matter C., Gao J., Liu X., Wu H., Carmichael S. T. (2006) A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J. Neurosci. 26, 1269–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.