Abstract
Underlying mechanisms of individual variation in severity of influenza infection and response to vaccination are poorly understood. We investigated the effect of reduced heme oxygenase-1 (HO-1) expression on vaccine response and outcome of influenza infection. HO-1-deficient and wild-type (WT) mice (kingdom, Animalia; phylum, Chordata; genus/species, Mus musculus) were infected with influenza virus A/PR/8/34 with or without prior vaccination with an adenoviral-based influenza vaccine. A genome-wide association study evaluated the expression of single-nucleotide polymorphisms (SNPs) in the HO-1 gene and the response to influenza vaccination in healthy humans. HO-1-deficient mice had decreased survival after influenza infection compared to WT mice (median survival 5.5 vs. 6.5 d, P=0.016). HO-1-deficient mice had impaired production of antibody following influenza vaccination compared to WT mice (mean antibody titer 869 vs. 1698, P=0.02). One SNP in HO-1 and one SNP in the constitutively expressed isoform HO-2 were independently associated with decreased antibody production after influenza vaccination in healthy human volunteers (P=0.017 and 0.014, respectively). HO-1 deficient mice were paired with sex- and age-matched WT controls. HO-1 affects the immune response to both influenza infection and vaccination, suggesting that therapeutic induction of HO-1 expression may represent a novel adjuvant to enhance influenza vaccine effectiveness.—Cummins, N. W., Weaver, E. A., May, S. M., Croatt, A. J., Foreman, O., Kennedy, R. B., Poland, G. A., Barry, M. A., Nath, K. A., Badley, A. D. Heme oxygenase-1 regulates the immune response to influenza virus infection and vaccination in aged mice.
Keywords: single-nucleotide polymorphism, pathogenesis, cytokine
Seasonal influenza virus infection is a major cause of morbidity and mortality worldwide, causing an estimated 36,000 deaths each year in the United States alone, according to the Centers for Disease Control and Prevention. While vaccination is the primary method of preventing morbidity and mortality from influenza virus infection, the response rate is highly variable and is markedly decreased in the older adult population. Similarly, clinical influenza infection ranges from a mild self-limited illness to severe respiratory failure and adult respiratory distress syndrome accompanied by multiorgan system failure and death. The immune response to influenza infection is complex and involves virus recognition by Toll-like receptors (TLRs), activation of innate immune effector mechanisms, production of proinflammatory and anti-inflammatory chemokines and cytokines, and coordinated antigen presentation, leading to adaptive Th1 and Th2 responses (1). The production of protective, influenza-neutralizing antibodies in response to infection or vaccination is regulated by antigen-specific CD4+ T-cell interactions with antigen-presenting cells expressing human leukocyte antigen (HLA) class II molecules in association with viral antigens (1). Known risk factors for both decreased vaccine response and more severe illness include age, chronic illness, pregnancy, and immunosuppression (2). Genetic factors also contribute to both decreased vaccine response and more severe influenza illness, including specific HLA haplotypes, and polymorphisms in critical mediators of the immune response, such as TLRs (TLR3), proinflammatory cytokines (IL-6, IL-12, IL-18, and IFNγ), and cytokine receptors (IL-1R, IL-2R, IL-4R, IL-10R, IL-12R, and TNFR) (1, 3).
Heme oxygenase (HO) is the rate-limiting enzyme involved in the degradation of heme, which yields carbon monoxide (CO), iron, and biliverdin; biliverdin is subsequently converted to bilirubin via the catalytic effects of biliverdin reductase. HO exists in two isoforms. HO-1 is inducibly expressed in response to numerous cellular stressors in virtually all tissues, and HO-2 is constitutively expressed in cells (4, 5). In addition to its cytoprotectant, antioxidant, and antiapoptotic properties, HO-1 is generally anti-inflammatory in nature through effects that reflect its degradation of heme and the products that are generated (6, 7); specifically, heme is recognized as a proinflammatory species, while CO and bile pigments can interrupt multiple steps in inflammatory processes, including the production of proinflammatory Th1 cytokines; activation of leukocytes and the endothelium; the activation of proinflammatory transcription factors; TLR signaling; NADPH oxidase activity and signaling; and the behavior of dendritic cells (5, 6).
HO-1 expression in the immune response to multiple infections, including gram-positive and gram-negative sepsis, tuberculosis, and cerebral malaria, generally dampens pathological consequences of injury, thereby improving host survival, but sometimes with the result of decreasing pathogen clearance (8–10). Intranasal infection of two strains of wild-type (WT) mice with influenza virus has been demonstrated to result in increased HO-1 expression and activity over background in the lungs compared to uninfected mice (11). Other experiments with mice engineered to overexpress HO-1 in the lungs, which are subsequently infected with influenza virus, have demonstrated decreased lung inflammation and mortality compared to mice with normal expression of HO-1, suggesting that HO-1 expression prevents the development of an overactive immune response and collateral tissue damage (12).
Clinical observations suggest that experimental evidence indicating the cytoprotective and anti-inflammatory effects of HO also applies to human disease. Homozygous deletion in the HO-1 gene, for example, leads to death in childhood, while polymorphisms in the human HO-1 promoter region, characterized by long GT repeats accompanied by decreased HO-1 expression and/or HO activity, have been associated with poor outcomes in many inflammatory conditions, including transplant allograft rejection, ischemia/reperfusion injury, cardiovascular disease, and hemorrhagic stroke, among others (5, 13). There are very few studies of human HO-1 single-nucleotide polymorphisms (SNPs) and the propensity to human disease, but one such study suggests that the frequency of multiorgan dysfunction in critically ill patients may be associated with certain HO-1 SNPs (14).
The purpose of the current study is to investigate the effect of reduced HO-1 expression on vaccine response and the outcome of influenza virus infection in a murine model, and the effect of SNPs in HO genes on the generation of protective neutralizing antibodies in human subjects receiving influenza vaccination, in order to discern the clinical relevance of HO-1 on influenza infection and influenza vaccination response.
MATERIALS AND METHODS
Murine model
All animal studies were performed in accordance with federal guidelines and were approved by the Institutional Animal Care and Use Committee at Mayo Clinic (Rochester, MN, USA). HO-1−/− mice, on a predominantly C57BL/6 background, were generated as described previously (24, 25), by breeding HO-1+/− females with HO-1−/− males. Wild-type (WT) controls were generated and selected from the same colony from heterozygote pairings. Genotypes of offspring mice were confirmed by PCR of tail DNA using the following primers: HO-1 WT, forward 5′-AGAGTACACTGACTGTGGGTG-3′ and reverse 5′-AGGGCCGTGTAGATATGGTAC-3′; HO-1 mutant, forward 5′-GAAGGTGAGATGACAGGAGATC-3′ and reverse 5′-GCTTGGGTGGAGAGGCTATTC-3′. Mice between the ages of 50 and 80 wk of age were utilized throughout the study to model effects seen in older humans, who are at increased risk of severe influenza disease and poor vaccine response. Younger mice between the ages of 16 and 20 wk were utilized where indicated. HO-1−/− mice were paired with sex- and age-matched WT controls for each experiment.
Murine vaccination model
First-generation replication defective (E1/E3 deleted) Ad5 vectors that expressed the codon-optimized hemagglutinin (HA) gene of A/PR/8/34 were constructed using the Ad-Easy system (Agilent Technologies, Santa Clara, CA, USA) in 293A cells, as described previously (26). All adenoviruses were purified by CsCl banding and quantitated by optical density (OD260). Mice were restrained using the scruff method and immunized with a total of 1010 vp of adenovirus expressing the A/PR/8/34 HA gene divided in 25 μl of saline injected into each quadriceps muscle. At 3 wk postimmunization, blood was drawn by retroorbital puncture, and serum was collected using Becton Dickinson microtainer tubes with serum separator (Becton Dickinson, Franklin Lakes, NJ, USA). Starting at a dilution of 1:5, sera were diluted 2-fold in 50 μl of DPBS in a 96-well, nonsterile, non-tissue culture-treated, round-bottom microtiter plate. Influenza virus [4 hemagglutination units (HAU)] in 50 μl was added to the diluted sera and incubated at room temperature for 1 h. After incubation, 50 μl of a 1% chicken red blood cell solution was added and incubated at room temperature for 1 h. The hemagglutination inhibition (HI) titer was determined to be the highest serum dilution to inhibit hemagglutination.
Influenza virus infection
Mice were immunized with the Ad-vectored vaccines. At 3 wk after immunization, the mice were anesthetized with ketamine (140 mg/kg)/xylazine (5.55 mg/kg) administered intraperitoneally. The mice were weighed for baseline measurements. The mice were challenged intranasally with 50 times the 50% lethal dose (LD50; Fig. 1A, B) or 100 LD50 (Fig. 1C, D) of influenza A/PR/8/34 virus. The mice were placed on their backs, and 10 μl of A/PR/8/34 virus was pipetted into each nares for a total volume of 20 μl. The mice were then weighed and monitored daily for signs of disease. Mice were humanely euthanized if their body weight dropped to 75% of baseline weights. Where noted, after sacrifice, lungs were dissected, perfused, fixed in 2% formalin, and embedded in paraffin, and hematoxylin and eosin slides were prepared. Microsomal preparations on unfixed lung tissue and subsequent Western blot analysis for HO-1 protein expression using an anti-HO-1 antibody (SPA895; StressGen Biotechnologies, Victoria, BC, Canada) were performed as described previously (7, 16).
Figure 1.
Course of influenza infection in WT and HO-1−/− mice. Age- and sex-matched WT and HO-1−/− aged mice were challenged with 2 doses (see Materials and Methods) of intranasal influenza virus and monitored for weight change and survival for 8 to 10 d. A) Survival was not statistically significantly decreased in HO-1−/− mice compared to WT mice after low-dose infection (P>0.05, log-rank test). B) HO-1−/− mice lost more weight after low-dose infection compared to WT mice. *P = 0.03 at d 5 and 6, Mann-Whitney U test. C) Survival was decreased in HO-1−/− mice compared to WT mice after high-dose infection (P=0.016, log-rank test). D) HO-1−/− mice tended to lose more weight after high-dose infection compared to WT controls (P=0.06 at d 4–6 after infection, Mann-Whitney U test).
Lung viral titers
Mice were infected intranasally with 100 LD50 influenza A/PR/8/34. At 3 d postinfection, the mice were euthanized by ketamine/xylazine overdose. The lungs were aseptically removed and put into 1.0 ml of sterile PBS. The lungs were homogenized using a VWR PowerMax homogenizer (VWR International, Radnor, PA, USA) at full speed for 30 s. The lung homogenate was centrifuged at 200 g for 5 min to pellet debris, and the supernatant was harvested for analysis. The 50% tissue culture infectious dose (TCID50) was determined using Madin-Darby canine kidney (MDCK) cells, as described previously (27). Briefly, the lung homogenates were diluted in DMEM containing 5% FBS, 100 U/ml of penicillin, and 100 μg/ml of streptomycin in a U-bottom tissue culture-treated 96-well plate. MDCK cells (2.5×104) were added to the diluted virus and incubated at 35°C overnight. The following day, the medium was removed, and DMEM containing 0.0002% trypsin and antibiotics was added. The cells were incubated for 4 d at 35°C. Finally, 50 μl of 0.5% chicken red blood cells were added to each well, and the plate was incubated at room temperature for 1 h. The TCID50 was calculated using the Reed and Muench technique.
Multiplex cytokine assay
Serum samples collected 5 d after vaccination or infectious challenge with 100 LD50 influenza virus were assayed for cytokine levels, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-17, eotaxin, granulocyte colony-stimulating factor (G-CSF), granulocyte-monocyte colony-stimulating factor (GM-CSF), interferon-γ, keratinocyte-derived chemokine (KC), monocyte chemotactic protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, regulated on activation normal T cell expressed and secreted (RANTES/CCL5), and tumor necrosis factor α (TNF-α). Serum cytokine concentrations were measured using a multiplex bead suspension array (Bio-Plex Pro Mouse Cytokine 23-plex Assay; cat. no. M60009RDPD; Bio-Rad, Hercules, CA, USA) per manufacturer's protocol.
Genome-wide association study
All human studies were performed according to protocols approved by the Institutional Review Board of the Mayo Clinic, and informed consent was obtained from each subject. All individuals were typed using the Illumina Infinium HumanHap550 BeadChip arrays (Illumina, San Diego, CA, USA). Initial QC steps included genotype reproducibility, inheritance by descent, gender check, SNP call rate, minor allele frequency, Hardy-Weinberg equilibrium (HWE), and subject call rate. We found three pairs of duplicated subjects and removed one data set from each pair. Overall, genotype concordance was 97.9%, indicating good reproducibility. The mean call rate for SNP typing was 98.2% (range: 36.3% to 100%), and the 6.2% of SNPs with call rates <95% were excluded from the analysis, as were monomorphic SNPs and those failing HWE. The mean call rate for individuals was 98.6% (range: 50.5–99.99%), and the 71 subjects with call rates <95% were excluded from the study. Following the QC steps, our cohort included 147 subjects and used data from 526,687 SNPs for the analysis. P values for SNP association analyses were adjusted for age at enrollment, ethnicity, and time since immunization. Smallpox-neutralizing antibody titers were assessed using a high-throughput, reporter-based viral neutralization assay, as described previously (28). Antibody titer is reported as ID50: serum dilution required to inhibit 50% of viral activity.
Statistical analysis
Data are expressed as means ± se or medians [interquartile range (IQR)] for parametric and nonparametric measures, respectively, and were compared between 2 groups using the Student's t test or Mann-Whitney U test when appropriate, or between multiple groups using 1-way analysis of variance. Survival data were compared using Kaplan-Meier curves and the log-rank comparison test. Values of P < 0.05 were considered statistically significant. Statistical analysis was performed on GraphPad Instat software (GraphPad, La Jolla, CA, USA).
RESULTS
Aged HO-1-deficient mice have a worsened outcome compared to WT mice after influenza virus challenge
Because influenza virus infection in mice induces HO-1 expression in the lungs (11), and genetic overexpression of HO-1 in the lungs of mice attenuates the course of experimental influenza viral infection (12), we questioned whether the absence of HO-1 expression would be associated with a more severe illness after influenza virus infection. We chose aged mice because the burden of influenza disease is greatest in the older population. First, we confirmed the presence of HO-1 protein expression in the lungs of WT mice, and absence of HO-1 protein expression in HO-1−/− mice, after influenza virus infection (Supplemental Fig. S1). In unvaccinated animals, HO-1−/− mice had a more severe course of influenza illness, with greater weight loss and reduced survival (Fig. 1), compared to WT mice using two different infectious challenge doses. For mice infected with 50 LD50 influenza virus, HO-1−/− mice compared to WT experienced a nonsignificantly accelerated mortality (Fig. 1A) and a significant increase in weight loss (P=0.03 at d 5 and 6 after infection, Fig. 1B). For mice infected with 100 LD50 influenza virus, HO-1−/− mice tended to lose more weight after infection compared to WT mice, although the difference was not statistically significant (P=0.06 for d 4–6 after infection, Fig. 1D). At the higher dose, median survival in the HO-1−/− mice was 24 h shorter compared to the WT mice, (5.5 vs. 6.5 d, respectively, P=0.016; Fig. 1C). Therefore, the outcome of influenza virus infection is worse in an HO-1-deficient state in aged mice. A mortality difference was not noted in younger HO-1−/− compared to WT mice (aged 16-20 wk; Supplemental Fig. S2), suggesting an acquired impairment in immunity associated with age.
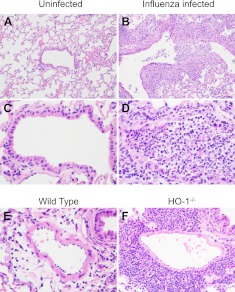
Induction of HO-1 exerts anti-inflammatory effects (6, 7). Therefore, we questioned whether the reduced survival of HO-1−/− mice following influenza virus infection is associated with altered viral replication or altered production of inflammatory cytokines. Viral burden in the lungs measured 3 d after infection was not significantly different between WT and HO-1−/− mice [median (interquartile range) TCID50/ml of 853 (151, 13653) and 427 (213, 2414), respectively, P=0.69]. Similarly, pulmonary lesions in infected WT and HO-1−/− mice were similar and had comparable distribution in the small airways (Fig. 2A, B). All infected mice had necrotizing bronchiolitis with varying degrees of epithelial necrosis and sloughing. Dense sheets of neutrophils often filled the airways, and the adjacent epithelium was variably hyperplastic. Pulmonary interstitium was expanded by mixed inflammatory infiltrates comprised of lymphocytes, plasma cells, and fewer numbers of macrophages and neutrophils (Fig. 2C, D). Alveolar spaces contained proteinaceous fluid admixed with foamy macrophages. The single prominent difference that was evident between the HO-1−/− and WT mice was that HO-1−/− mice had dense lymphoplasmacytic perivascular infiltrates surrounding many of the pulmonary vessels, while in WT mice, these infiltrates were absent or minimal (Fig. 2E, F). These pathology findings suggested enhanced inflammation in the HO-1−/− mice.
Figure 2.
Lung histopathology was examined in aged mice at 5 d after intranasal influenza virus infection. A–D) Representative images of airway inflammation from uninfected (A, C) and infected (B, D) mice. E, F) Representative images of perivascular infiltrates in WT (E) and HO-1−/− (F) mice.
Therefore, serum samples from WT and HO-1−/− mice both prior to and 1, 5, and 7 d after influenza virus infection were analyzed for the expression of 23 cytokines (Supplemental Table S1). At baseline, the serum level of IL-2 was significantly lower in the HO-1−/− mice compared to WT (10.9±12.9 vs. 43.2±13.4 pg/ml, P<0.05), although changes in IL-2 after infection were not significantly different between the knockout and WT mice. However, mirroring the differences seen in lymphoplasmacytic infiltrates in the lung, the HO-1−/− mice had a higher fold change in early RANTES expression compared to WT within the first 24 h after infection (2.4±2.2-fold increase vs. 0.60±0.24 decrease, P=0.04). This difference in RANTES expression did not persist beyond 24 h, (Fig. 3A). Over the course of infection, HO-1−/− mice had an increase in MCP-1 expression compared to a decrease in WT mice (P=0.04 for differences in slope of linear regression, Fig. 3B).
Figure 3.
Systemic cytokine expression after influenza infection. A) Fold change in serum RANTES level was increased in HO-1−/− mice compared to WT mice at 24 h after influenza infection (P=0.04, Mann-Whitney U test), although this did not persist beyond 24 h. B) HO-1−/− mice experienced a continued rise in serum MCP-1 levels up to 7 d after infection compared to WT mice, which experienced a decrease over time (P=0.04, linear regression comparing the slopes of the two trends).
Aged HO-1-deficient mice have impaired antibody production in response to vaccination
Because inflammation contributes to T-cell recruitment and the development of T-cell help, which favors an optimized B-cell response to vaccination, we questioned whether the HO-1-deficient state would result in an altered humoral response to vaccination. WT and HO-1−/− mice (50–80 wk old) were injected intramuscularly with an adenoviral vector expressing the influenza H1 protein or saline vehicle control. HI titers, a measure of protective antibodies against influenza virus, were measured in serum collected 21 d after vaccination. The adenoviral-vaccinated HO-1−/− mice exhibited a 2-fold decrease (P=0.02) in HI titer after immunization compared to age- and sex-matched, vaccinated WT mice (Fig. 4A). The WT and HO-1−/− mice vaccinated with vehicle control did not generate detectable anti-influenza antibodies (data not shown). Because of the high titers of antibody generated in both groups of mice with this vaccine approach, there was no significant difference between immunized WT and immunized HO-1−/− mice in survival (100 vs. 96%, respectively, data not shown) or weight loss (Fig. 4B) after an infectious challenge. However, the decreased antibody production in response to vaccination in HO-1−/− mice was associated with elevated levels of IL-5, IL-6, IL-10, IL-12(p40 subunit), MIP-1α, and MIP-1β in adenoviral-immunized HO-1−/− mice compared to immunized WT mice (Table 1).
Figure 4.
Influenza vaccine response in WT and HO-1 −/− mice. A) HI titers measured in HO-1−/− (n=23) and age- and sex-matched WT controls (n=24) at 21 d after vaccination with an adenoviral vector expressing H1. B) Immunized mice were subsequently challenged with influenza infection, and immunized HO-1−/− mice tended to lose more weight after infectious challenge compared to immunized WT controls, although the difference was not statistically significant (P>0.05).
Table 1.
Serum levels of 23 cytokines in WT or HO-1−/− mice 5 d after vaccination with either Ad5.H1 or vehicle control (n=4/group)
| Cytokine | WT |
HO-1−/− |
P | ||
|---|---|---|---|---|---|
| Vehicle | Vaccine | Vehicle | Vaccine | ||
| IL1a | 50.4 ± 37.8 | 69.6 ± 31.2 | 22.6 ± 6.4 | 30.8 ± 8.8 | 0.07 |
| IL1b | 382.0 ± 57.0 | 387.4 ± 69.7 | 465.4 ± 218.7 | 2454.3 ± 2585.5 | 0.07 |
| IL2 | 43.2 ± 13.4 | 38.0 ± 43.0 | 10.9 ± 12.9 | 15.8 ± 14.2 | 0.22 |
| IL3 | 6.8 ± 4.4 | 8.3 ± 2.4 | 6.0 ± 5.8 | 133.7 ± 236.7 | 0.19 |
| IL4 | 7.5 ± 3.2 | 6.8 ± 0.8 | 4.7 ± 2.4 | 6.9 ± 2.8 | 0.45 |
| IL5 | 38.3 ± 5.0 | 39.5 ± 12.7 | 33.2 ± 8.1 | 227.5 ± 160.1 | 0.03* |
| IL6 | 8.6 ± 1.4 | 13.4 ± 4.2 | 12.4 ± 13.3 | 235.9 ± 411.0 | 0.047* |
| IL9 | 499.5 ± 84.0 | 540.0 ± 50.3 | 467.7 ± 165.7 | 595.3 ± 36.4 | 0.33 |
| IL10 | 19.2 ± 30.5 | 30.7 ± 61.4 | 39.5 ± 39.4 | 135.3 ± 80.6 | 0.047* |
| IL12 ± p40 | 478.7 ± 149.5 | 396.8 ± 115.8 | 1464.9 ± 951.8 | 4162.1 ± 3948.8 | 0.01* |
| IL12 ± p70 | 327.8 ± 103.2 | 166.1 ± 38.2 | 200.4 ± 100.0 | 371.1 ± 40.6 | 0.01* |
| IL13 | 773.7 ± 228.0 | 887.5 ± 102.7 | 634.1 ± 357.9 | 847.8 ± 147.0 | 0.45 |
| IL17 | 91.9 ± 50.6 | 91.4 ± 21.7 | 49.4 ± 33.1 | 184.0 ± 124.2 | 0.19 |
| Eotaxin | 1932.7 ± 348.3 | 1699.6 ± 602.2 | 1648.1 ± 527.1 | 1949.3 ± 1433.4 | 0.93 |
| G-CSF | 120.5 ± 37.2 | 176.8 ± 122.9 | 92.0 ± 44.0 | 653.7 ± 1056.1 | 0.29 |
| GM-CSF | 172.5 ± 7.1 | 126.1 ± 76.5 | 159.7 ± 31.6 | 122.2 ± 65.1 | 0.68 |
| IFNγ | 130.8 ± 46.3 | 107.9 ± 32.3 | 113.0 ± 22.3 | 177.3 ± 42.6 | 0.23 |
| KC | 50.7 ± 11.0 | 56.1 ± 12.2 | 68.3 ± 10.6 | 77.0 ± 43.4 | 0.26 |
| MCP-1 | 411.8 ± 80.5 | 567.3 ± 115.0 | 608.5 ± 266.6 | 942.1 ± 294.8 | 0.25 |
| MIP1α | 403.1 ± 86.8 | 329.1 ± 36.4 | 420.8 ± 102.6 | 580.1 ± 38.8 | 0.003* |
| MIP1β | 118.3 ± 26.3 | 137.6 ± 15.4 | 153.2 ± 30.7 | 919.7 ± 1227.9 | 0.02* |
| RANTES | 79.9 ± 34.8 | 165.3 ± 100.6 | 56.0 ± 28.4 | 252.0 ± 219.8 | 0.13 |
| TNFα | 348.9 ± 38.4 | 476.4 ± 117.4 | 485.8 ± 152.0 | 5728.7 ± 8652.9 | 0.06 |
Serum levels are given as picograms per milliliter. Values are expressed as means ± sd. P values comparing differences across groups were determined by ANOVA with Kruskal-Wallis post test or nonparametric equivalent.
P ≤ 0.05.
Influenza vaccine response in humans is related to polymorphisms in the HO-1 promoter region
In humans, the variable expression of HO-1 in response to inflammatory stimuli is associated with polymorphisms in the HO-1 gene (5). Because of our data linking antibody response to influenza vaccination to HO-1 expression in mice, we questioned whether antibody response to influenza vaccination in humans would be associated with SNPs in the HO-1 gene. In a genome-wide association study of 147 influenza vaccine recipients, one SNP (rs743811) in the HO-1 3′ untranslated region, and one intronic SNP (rs2160567) in HO-2, were independently and significantly associated with decreased H1-specific HI titers after influenza vaccination (Table 2). Subjects with 0, 1, and 2 minor alleles in either SNP had median (IQR) H1-specific HI titers of 160 (80, 320), 80 (60, 160), and 20 (20, 20) respectively, (P=0.017 and P=0.014, respectively, for the HO-1 and HO-2 SNPs). As total HO activity is determined by a combination of basal expression of HO-2 and induced expression of HO-1, this supports our murine data, suggesting that HO expression is a critical determinant of the response to influenza vaccine. In a complementary analysis of healthy recipients of the smallpox vaccine, 2 SNPs in HO-1 (rs743811, rs5755720) and 2 SNPs in HO-2 (rs2160567, rs11866840) were significantly and independently associated with decreases in neutralizing antibody response, (Supplemental Table S2).
Table 2.
SNPs in HMOX1 and HMOX2 associated with decreased influenza vaccine response as measured by median (IQR) HI antibody titer in normal human subjects (n=147 total)
| SNP | Chr | Gene | Major allele | Minor allele | 0 minor alleles | 1 minor alleles | 2 minor alleles | P |
|---|---|---|---|---|---|---|---|---|
| rs743811 | 22 | HMOX1 | A | G | 160 (80, 320) | 80 (60, 160) | 20 (20, 20) | 0.017* |
| [n=77] | [n=58] | [n=12] | ||||||
| rs2160567 | 16 | HMOX2 | A | G | 160 (80, 320) | 80 (60, 160) | 20 (20, 20) | 0.014* |
| [n=62] | [n=71] | [n=14] |
HMOX1, heme oxygenase 1; HMOX2, heme oxygenase 2; chr, chromosome.
P ≤ 0.05; ordinal effects model.
DISCUSSION
It is well recognized that both the severity of influenza infection and the immune response to influenza vaccination are highly variable, yet the mechanisms for such interindividual variability remain elusive. In the current report, we demonstrate for the first time that HO-1 deficiency worsens influenza disease, and reduces vaccine response in an aged murine model, and that SNPs in HO-1 and HO-2 affect human influenza vaccine responses.
HO-1 has been previously investigated in the pathogenesis of influenza. In 2 strains of mice (C57BL/6 and C3H/HeJ) infected with influenza A/PR/8/34 (the same viral strain as was used in the present murine study), an increase in HO-1 message and activity in the lungs of infected mice was associated with improved survival despite similar viral content in the lungs (11). The reciprocal experiment of genetic overexpression of HO-1 in the lungs of WT mice by an adenoviral vector resulted in improved survival, decreased inflammatory cell infiltration of the lung, and decreased apoptosis of respiratory epithelial cells compared to mice with normal HO-1 expression (12). Our study extends these observations to the HO-1-deficient state, which is clinically relevant, given the existence of functional SNPs in the HO-1 gene that lead to variable HO-1 expression in humans.
The exact mechanisms of HO-1-mediated protection in influenza infection were previously unclear. The studies mentioned above suggest that HO-1 expression does not have a direct antiviral effect. Heme, the cellular substrate of HO-1 metabolism, is an essential prosthetic group in many cellular enzymes, but also has multiple cytoxic effects, including oxidative stress, induction of DNA damage, cell cycle arrest, mitochondrial toxicity, and apoptosis, when insufficiently metabolized (15). Toxic intracellular levels of heme also induce expression of MCP-1 through NF-κB signaling (16). In response to influenza infection, respiratory epithelial cells and resident monocytes/macrophages produce chemokines, including RANTES and MCP-1, which preferentially recruit blood mononuclear cells to the site of infection (17, 18). Results of our study suggest that HO-1 may have an important role in the control of this local inflammation and possibly cell migration and recruitment, since lymphoplasmacytic perivascular infiltrates and serum levels of the chemokines RANTES and MCP-1, and not the proinflammatory cytokines IL-1, IL-6 or TNF-α, were higher after influenza infection in HO-1−/− mice compared to WT mice. Increased MCP-1 expression along with heightened activation of its transcription factor, NF-κB, occur in HO-1−/− mice compared to WT in response to other inflammatory stimuli, namely intravenous administration of hemoglobin and ischemia/reperfusion injury of the kidney. Conversely, HO-1 overexpressing renal epithelial cells exhibit less MCP-1 mRNA and less NF-κB activation when exposed to proinflammatory stimuli, such as LPS and albumin. In aggregate, these prior observations support the association seen in our current study (19, 20).
It is notable that the worsened influenza disease severity demonstrated in the HO-1−/− mice compared to WT mice was not demonstrated in younger mice (aged 16–20 wk) in the current study (Supplemental Fig. S2). This finding raises several possibilities worthy of further study, including age-related changes in the expression, regulation, or function of HO-1 regulated processes and immune mediators, or changes in compensatory mechanisms of heme degradation in the setting of insufficient HO-1 induction.
The role of HO-1 in vaccine response, either in humans or in mice, has not been previously reported. Cytokine and chemokine expression plays an important role in the coordinated immune response to the vaccine in order to produce adequate immunological protection (1). Proinflammatory cytokines and chemokines predominate as a primary response early after vaccination, and are followed by secondary anti-inflammatory cytokines after the development of adaptive immune responses in a negative feedback loop. Indeed, in the setting of HO-1 deficiency, our data demonstrate that the serum cytokine profile after vaccination in the HO-1−/− mice encompasses an exaggerated production of both proinflammatory and anti-inflammatory cytokines (see Table 1). Consistent with these observations are our prior studies, which demonstrate that the heightened sensitivity of HO-1−/− mice to lipopolysaccharide is accompanied by an exaggerated production of both proinflammatory and anti-inflammatory cytokines (7). We speculate that the heightened proinflammatory component of the inflammatory response in HO-1−/− mice may underlie the decreased vaccine response: SNPs in genes for both IL-6 and IL-12, both of which were abnormally expressed in the vaccinated HO-1−/− mice, have been previously reported to be associated with suboptimal response to influenza vaccination in humans (1). The finding of a similar association of SNPs with vaccine response in human recipients of the trivalent influenza vaccine increases the applicability of our results. The association between HI titer and HO-2 polymorphisms in humans is also novel and is consistent with our HO-1 data, as HO-2 is the constitutively expressed isoform of heme oxygenase. In fact, the association of single nucleotide polymorphisms in HO-1 and HO-2, with vaccine response is a particular strength of our study, in that relatively few studies on HO-1 polymorphisms have been conducted on SNPs (14, 21, 22), while most have focused on the (GT)n repeat-length promoter polymorphism (5, 13).
In summary, we demonstrate that HO-1 is a regulator of the immune response to both influenza infection and vaccination. These findings assume additional importance as the first demonstration of pharmacologic induction of HO-1 activity in humans has recently been reported (23). In this study, 10 healthy adult volunteers were randomized to receive either hemin intravenously at a dose of 3 mg/kg once or vehicle placebo control and followed for 48 h. HO-1 venous plasma levels and venous leukocyte activity were significantly elevated in 4 of the 5 recipients of hemin up to 48 h after infusion, with no adverse effects noted on routine hematologic and coagulation parameters. HO-1 may, therefore, become a viable, novel target for immunomodulatory therapy to improve clinical outcomes in influenza infection and/or to improve vaccine effectiveness in subgroups of at-risk individuals, both of which have an obvious potential public health effect given the annual morbidity and mortality associated with seasonal or pandemic influenza.
Supplementary Material
Acknowledgments
The authors acknowledge support from the U.S. National Institutes of Health, including grants AI-40384, AI-89859Z, AR-056950-02, and DK-007013-32, for this work.
All authors critically reviewed and approved the final version of the paper. Conflicts of interest: G.A.P. has offered consultative advice on novel influenza vaccine development to Merck & Co., Avianax, Theraclone Sciences (formally Spaltudaq Corporation), MedImmune LLC, Liquidia Technologies, Novavax, Novartis Vaccines and Therapeutics, and PAXVAX. All other authors declare no conflicts of interest. The authors thank Allan Ackerman for technical assistance with the study.
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- HA
- hemagglutinin
- HAU
- hemagglutination unit
- HI
- hemagglutination inhibition
- HO
- heme oxygenase
- LD
- lethal dose
- MCP-1
- monocyte chemotactic protein-1
- MIP-1
- macrophage inflammatory protein-1
- RANTES
- regulated on activation normal T cell expressed and secreted
- SNP
- single-nucleotide polymorphism
- TCID
- tissue culture infective dose
- TLR
- Toll-like receptor
- TNF-α
- tumor necrosis factor α
- WT
- wild type
REFERENCES
- 1. Poland G. A., Ovsyannikova I. G., Jacobson R. M. (2008) Immunogenetics of seasonal influenza vaccine response. Vaccine 26(Suppl. 4), D35–D40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Katz J. M., Plowden J., Renshaw-Hoelscher M., Lu X., Tumpey T. M., Sambhara S. (2004) Immunity to influenza: the challenges of protecting an aging population. Immunol. Res. 29, 113–124 [DOI] [PubMed] [Google Scholar]
- 3. Trammell R. A., Toth L. A. (2008) Genetic susceptibility and resistance to influenza infection and disease in humans and mice. Expert Rev. Mol. Diagn. 8, 515–529 [DOI] [PubMed] [Google Scholar]
- 4. Nath K. A. (2006) Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int. 70, 432–443 [DOI] [PubMed] [Google Scholar]
- 5. Abraham N. G., Kappas A. (2008) Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 60, 79–127 [DOI] [PubMed] [Google Scholar]
- 6. Kapturczak M. H., Wasserfall C., Brusko T., Campbell-Thompson M., Ellis T. M., Atkinson M. A., Agarwal A. (2004) Heme oxygenase-1 modulates early inflammatory responses: evidence from the heme oxygenase-1-deficient mouse. Am. J. Pathol. 165, 1045–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tracz M. J., Juncos J. P., Grande J. P., Croatt A. J., Ackerman A. W., Rajagopalan G., Knutson K. L., Badley A. D., Griffin M. D., Alam J., Nath K. A. (2007) Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1-/- mice. Am. J. Pathol. 170, 1820–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sinnis P., Ernst J. D. (2008) CO-opting the host HO-1 pathway in tuberculosis and malaria. Cell Host Microbe 3, 277–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chung S. W., Liu X., Macias A. A., Baron R. M., Perrella M. A. (2008) Heme oxygenase-1-derived carbon monoxide enhances the host defense response to microbial sepsis in mice. J. Clin. Invest. 118, 239–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chung S. W., Hall S. R., Perrella M. A. (2009) Role of haem oxygenase-1 in microbial host defence. Cell. Microbiol. 11, 199–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Choi A. M., Knobil K., Otterbein S. L., Eastman D. A., Jacoby D. B. (1996) Oxidant stress responses in influenza virus pneumonia: gene expression and transcription factor activation. Am. J. Physiol. 271, L383–L391 [DOI] [PubMed] [Google Scholar]
- 12. Hashiba T., Suzuki M., Nagashima Y., Suzuki S., Inoue S., Tsuburai T., Matsuse T., Ishigatubo Y. (2001) Adenovirus-mediated transfer of heme oxygenase-1 cDNA attenuates severe lung injury induced by the influenza virus in mice. Gene Ther. 8, 1499–1507 [DOI] [PubMed] [Google Scholar]
- 13. Exner M., Minar E., Wagner O., Schillinger M. (2004) The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic. Biol. Med. 37, 1097–1104 [DOI] [PubMed] [Google Scholar]
- 14. Saukkonen K., Lakkisto P., Kaunisto M. A., Varpula M., Voipio-Pulkki L. M., Varpula T., Pettila V., Pulkki K. (2010) Heme oxygenase 1 polymorphisms and plasma concentrations in critically ill patients. Shock 34, 558–564 [DOI] [PubMed] [Google Scholar]
- 15. Tracz M. J., Alam J., Nath K. A. (2007) Physiology and pathophysiology of heme: implications for kidney disease. J. Am. Soc. Nephrol. 18, 414–420 [DOI] [PubMed] [Google Scholar]
- 16. Kanakiriya S. K., Croatt A. J., Haggard J. J., Ingelfinger J. R., Tang S. S., Alam J., Nath K. A. (2003) Heme: a novel inducer of MCP-1 through HO-dependent and HO-independent mechanisms. Am. J. Physiol. Renal Physiol. 284, F546–F554 [DOI] [PubMed] [Google Scholar]
- 17. Julkunen I., Melen K., Nyqvist M., Pirhonen J., Sareneva T., Matikainen S. (2000) Inflammatory responses in influenza A virus infection. Vaccine 19(Suppl. 1), S32–S37 [DOI] [PubMed] [Google Scholar]
- 18. Sladkova T., Kostolansky F. (2006) The role of cytokines in the immune response to influenza A virus infection. Acta Virol. 50, 151–162 [PubMed] [Google Scholar]
- 19. Pittock S. T., Norby S. M., Grande J. P., Croatt A. J., Bren G. D., Badley A. D., Caplice N. M., Griffin M. D., Nath K. A. (2005) MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int. 68, 611–622 [DOI] [PubMed] [Google Scholar]
- 20. Murali N. S., Ackerman A. W., Croatt A. J., Cheng J., Grande J. P., Sutor S. L., Bram R. J., Bren G. D., Badley A. D., Alam J., Nath K. A. (2007) Renal upregulation of HO-1 reduces albumin-driven MCP-1 production: implications for chronic kidney disease. Am. J. Physiol. Renal Physiol. 292, F837–F844 [DOI] [PubMed] [Google Scholar]
- 21. Yun L., Xiaoli L., Qi Z., Laiyuan W., Xiangfeng L., Chong S., Jianfeng H., Shufeng C., Hongfan L., Gu D. (2009) Association of an intronic variant of the heme oxygenase-1 gene with hypertension in northern Chinese Han population. Clin. Exp. Hypertens. 31, 534–543 [DOI] [PubMed] [Google Scholar]
- 22. Sambo M. R., Trovoada M. J., Benchimol C., Quinhentos V., Goncalves L., Velosa R., Marques M. I., Sepulveda N., Clark T. G., Mustafa S., Wagner O., Coutinho A., Penha-Gonçalves C. (2010) Transforming growth factor beta 2 and heme oxygenase 1 genes are risk factors for the cerebral malaria syndrome in Angolan children. PLoS One 5, e11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bharucha A. E., Kulkarni A., Choi K. M., Camilleri M., Lempke M., Brunn G. J., Gibbons S. J., Zinsmeister A. R., Farrugia G. (2010) First-in-human study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin. Pharmacol. Ther. 87, 187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nath K. A., Haggard J. J., Croatt A. J., Grande J. P., Poss K. D., Alam J. (2000) The indispensability of heme oxygenase-1 in protecting against acute heme protein-induced toxicity in vivo. Am. J. Pathol. 156, 1527–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nath K. A., Vercellotti G. M., Grande J. P., Miyoshi H., Paya C. V., Manivel J. C., Haggard J. J., Croatt A. J., Payne W. D., Alam J. (2001) Heme protein-induced chronic renal inflammation: suppressive effect of induced heme oxygenase-1. Kidney Int. 59, 106–117 [DOI] [PubMed] [Google Scholar]
- 26. Mok H., Palmer D. J., Ng P., Barry M. A. (2005) Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol. Ther. 11, 66–79 [DOI] [PubMed] [Google Scholar]
- 27. Cottey R., Rowe C. A., Bender B. S. (2001). Influenza virus. In Current Protocols in Immunology, John Wiley & Sons, New York: [DOI] [PubMed] [Google Scholar]
- 28. Kennedy R., Pankratz V., Swanson E., Watson D., Golding H., Poland G. A. (2009) Statistical approach to estimate vaccinia-specific neutralizing antibody titers using a high-throughput assay. Clin. Vaccine Immunol. 16, 1105–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.