Abstract
Infectious complications, predominantly pneumonia, are the most common cause of death in the postacute phase of stroke, although the mechanisms underlying the corresponding immunosuppression are not fully understood. We tested the hypothesis that activation of the α7 nicotinic acetylcholine receptor (α7nAChR) pathway is important in the stroke-induced increase in lung injury caused by Pseudomonas aeruginosa pneumonia in mice. Prior stroke increased lung vascular permeability caused by P. aeruginosa pneumonia and was associated with decreased lung neutrophil recruitment and bacterial clearance in mice. Pharmacologic inhibition (methyllycaconitine IC50: 0.2-0.6 nM) or genetic deletion of the α7nAChR significantly (P<0.05) attenuates the effect of prior stroke on lung injury and mortality caused by P. aeruginosa pneumonia in mice. Finally, pretreatment with PNU-282987, a pharmacologic activator of the α7nAChR (EC50: 0.2 μM), significantly (P<0.05) increased lung injury caused by P. aeruginosa pneumonia, significantly (P<0.05) decreased the release of KC, a major neutrophil chemokine, and significantly (P<0.05) decreased intracellular bacterial killing by a mouse alveolar macrophage cell line and primary mouse neutrophils. In summary, the α7 nicotinic cholinergic pathway plays an important role in mediating the systemic immunosuppression observed after stroke and directly contributes to more severe lung damage induced by P. aeruginosa.—Lafargue, M., Xu, L., Carles, M., Serve, E., Anjum, N., Iles, K. E., Xiong, X., Giffard, R., Pittet, J.-F. Stroke-induced activation of the α7 nicotinic receptor increases Pseudomonas aeruginosa lung injury.
Keywords: vagus nerve, pneumonia
An estimated 780,000 Americans experience a new stroke or recurrence of stroke each year. It is the third leading cause of death in the United States and one of the leading causes of disability (1). Infectious complications, predominantly pneumonia, are the most common cause of death in patients in the postacute phase of stroke. Patients hospitalized for stroke have a higher incidence of pneumonia (5–22%) than patients hospitalized for nonstroke diagnoses (3–5%). Pneumonia is responsible for more than 30% of deaths in patients treated for stroke, indicating that nosocomial lung infection is one of the most serious medical complications after stroke (2–4). The reasons for the higher incidence of pneumonia after stroke are multifactorial. First, the neurological consequences of stroke, including impairment of reflexes that protect the airway, decreased level of consciousness, dysphagia, and the need for mechanical ventilation (5) are predisposing factors for the development of pneumonia. Second, there is recent experimental and clinical evidence that stroke induces a secondary immunosuppressed state that may, in part, explain the higher incidence of nosocomial infections (6–9). Stroke has a profound effect on brain and systemic immune response in both experimental and clinical studies. Focal cerebral ischemia results in a biphasic effect on the peripheral immune system. The initial phase is characterized by a local and systemic activation of the immune system within hours after onset of stroke in mice (9). This response is necessary to remove cellular debris and allow the regenerative/repair process after stroke. However, inflammation may exacerbate damage and is implicated in secondary brain injury (10). Thus, the initial inflammatory response is followed by immunosuppression that reduces secondary neurological damage (9). However, poststroke systemic immunosuppression is associated with an increased risk for infection (11) and is characterized in humans by reduced peripheral blood lymphocyte counts, impaired T- and natural killer cell activity and reduced cytokine production (6–8). Possible effects of stroke on neutrophil migration into the lung in response to bacterial infection is currently unknown.
The mechanisms involved in stroke-induced systemic immunosuppression are not fully understood. Recent experimental work has shown increased levels of acetylcholine after cerebral ischemia, consistent with activation of the vagal cholinergic anti-inflammatory pathway (12, 13), which ameliorates some ischemia-related cerebral damage (14, 15) and via the vagus nerve activates peripheral nicotinic receptors located in endothelial and inflammatory cells of the reticuloendothelial system (16). A large body of work from the laboratory of Dr. K. J. Tracey (e.g., ref. 17) has shown that innate immunity is modulated by a neural reflex comprised of an afferent arm that senses inflammation and an efferent arm, which is the cholinergic anti-inflammatory pathway that inhibits innate immune responses. This efferent arm is dependent on the activation of the α7 nicotinic acetylcholine receptor (α7nAChR), which inhibits nuclear factor-κB (NF-κB) nuclear translocation and suppresses cytokine release by monocytes and macrophages (17). Thus, in the present study, we tested the hypothesis that activation of the α7nAChR signaling pathway is central to the stroke-induced increase in lung injury caused by airspace instillation of P. aeruginosa in mice. The results demonstrate an important role for the α7nAChR signaling pathway in mediating poststroke systemic immunosuppression that results in more severe lung damage induced by P. aeruginosa.
MATERIALS AND METHODS
Reagents
All cell culture media were prepared by the University of California–San Francisco Cell Culture Facility using deionized water and analytical grade reagents. The protein concentration of cell lysates was determined using the Bio-Rad protein assay kit (Bio-Rad, Hercules, CA, USA). The ELISA kit for mouse keratinocyte-derived chemokine (KC) was purchased from R&D Systems (Minneapolis, MN, USA). N-(3R)-1-azabicyclo[2,2,2]oct-3-yl-(4-chlorobenzamide) (PNU-282987), a specific agonist of the α7nAChR, was purchased from Tocris Bioscience (Ellisville, MO, USA). Methyllycaconitine (MLA), a specific inhibitor of the α7nAChR, was obtained from Sigma (St. Louis, MO, USA). 125I-labeled human serum albumin (jeanatope; Iso-Tex Diagnostics, Friendswood, TX, USA) was used as radioactive tracer. Custom antibody mixtures and negative selection columns for neutrophil isolation were from StemCell Technologies (Vancouver, BC, Canada). The α7nAChR antibody (sc-5544) was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). ELISA to measure phospho-p65 protein was obtained from eBioscience, (San Diego, CA, USA). All other reagents were purchased from Sigma.
Cell culture
A mouse alveolar macrophage cell line (MH-S cells, no. CRL-2019; American Type Culture Collection, Manassas, VA, USA) was used for the in vitro experiments. Cells were cultured in RPMI medium supplemented with 10% FBS and 1% penicillin and streptomycin and were maintained at 37°C with 5% CO2.
Preparation of P. aeruginosa
The wild-type (WT) PAK strain of P. aeruginosa was a kind gift from Dr. Stephen Lory (Department of Microbiology and Molecular Genetics, Harvard Medical School, Boston MA, USA). For each experiment, frozen bacteria were inoculated into Luria-Bertani (LB) broth (Invitrogen, Carlsbad, CA, USA), incubated for 6 h at 37°C on a rotating platform, and then diluted 1:100 in fresh LB broth. After 16–18 h of incubation at 37°C, the stationary-phase bacteria were pelleted, washed 3 times in PBS, and suspended in PBS to a concentration adjusted by optical density at 600 nm, as 1 × 109 colony forming units (CFU)/ml for in vitro experiments or 2 × 108 CFU/ml for instillation in mice (50 μl/mouse).
Neutrophil isolation
Mouse neutrophils were purified from bone marrow cell suspensions, as described previously (18). Bone marrow cells were incubated with 30 μl of antibody cocktail specific to the cell surface markers F4/80, CD4, CD45R, CD5, and TER119 for 15 min at 4°C. Antibiotin tetrameric antibody complexes (100 μl) were then added to the cells and incubated for 15 min at 4°C, followed by incubation with 60 μl of colloidal magnetic dextran iron particles for 15 min at 4°C. The cell suspension was then placed into a column surrounded by a magnet. The T cells, B cells, red blood cells, monocytes, and macrophages were captured in the column, allowing the neutrophils to pass through as a result of negative selection. Cells were then washed with RPMI 1640 with 5% FBS. Neutrophil purity, as determined by Wright-Giemsa-stained cytospin preparations, was consistently >98%.
Bacterial killing assay
MH-S cells (1×106) or primary mouse neutrophils (1×106) were incubated with 10−5 M PNU-282987 or its vehicle for 30 min before adding 107 CFU/ml of P. aeruginosa for 45 min at 37°C. Gentamicin (150 μg/ml) was added, and cells were incubated for 1 or 3 h at 37°C. The media were removed, the cells were washed twice with sterile PBS, then lysed by adding 200 μl of hypotonic buffer (pH 7.2), and then incubated on ice for 10 min. After adding 800 μl of sterile water, the cell suspension was serially diluted onto agar plates, incubated for 24 h at 37°C, and the resulting colonies counted. Percent survival of intracellular bacteria was calculated using the following equation: percent killing = (1 − number of bacteria after 3 h/number of bacteria after 1 h) × 100. Preliminary data indicate that lysates from uninfected macrophages did not reveal any bacterial colonies on LB plates and that bacteria alone incubated for 1 h with gentamicin were completely killed (data not shown).
Keratinocyte-derived chemokine (KC) measurement
MH-S cells were seeded in 48-well tissue culture dishes, and after 24 h, they were exposed to PNU (10−5 M) or vehicle for 30 min, then to P. aeruginosa [2×107 CFU/ml; multiplicity of infection (MOI)=10:1] for 4 h. Cell medium was collected and centrifuged, and ELISA assay for KC was carried out according to the manufacturer's protocol (R&D Systems).
Measurement of NF-κB pathway activation
NF-κB pathway activation was determined by the phosphorylation of p65 protein after exposure of mouse neutrophils to P. aeruginosa by ELISA. Briefly, mouse neutrophils were incubated with 10−5 M PNU-282987 or its vehicle for 30 min before adding 107 CFU/ml of P. aeruginosa for 45 min at 37°C. ELISA was carried out according to the manufacturer's protocol (eBioscience).
Expression of the α7nAChR in MH-S cells and mouse neutrophils
Expression of the α7nAChR in MH-S cells and mouse neutrophils was determined by Western blot analysis. Western blot analysis was performed as described previously (19). After equal amounts of protein were loaded in each lane and separated by 10% SDS-PAGE, proteins were transferred using the Bio-Rad transfer cell. Membranes were blocked with blocking buffer (927-40010; LI-COR Bioscience, Lincoln, NE, USA) and incubated with the primary antibody (sc-5544; Santa Cruz Biotechnology). Secondary antibodies from LI-COR Bioscience (0.5 mg each; IRDye 680 goat anti-rabbit IgG, 926-32221; IRDye 800CW goat anti-mouse IgG, 926-32210) were used, and proteins were visualized using the Odyssey infrared imaging system (LI-COR Bioscience). Quantification was done using the Odyssey digital image analysis system.
Cell viability assay
MH-S cell viability was measured by the Alamar Blue assay after exposure to the various experimental conditions. The cell medium was replaced with medium containing 10% Alamar Blue and allowed to incubate at 37°C for 2 h. The media were collected, and absorbance at 530 nm was read on a spectrophotometric plate reader (Biotek, Winooski, VT, USA). In pilot studies, we found that MLA, PNU, propranolol, and terbutaline did not affect the viability of MH-S cells or P. aeruginosa bacteria (data not shown).
Mice
C57BL/6 mice were purchased from Charles River Laboratories (Wilmington, MA, USA) for all experiments except those with the α7-knockout (KO) mice. The α7 nicotinic receptor-KO mice, strain C57BLB/6.129s7-Chrna7tm1Bay/J (stock no. 003232) and control C57BL/6J (000664) mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA). Mice were housed in either the Stanford Medical School animal care facilities or the University of California–San Francisco (UCSF) animal care facilities. All use of animals was according to protocols approved by the Stanford and the UCSF Animal Care and Use Committees and conducted according to the U.S. National Institutes of Health Guide for Care and Use of Laboratory Animals.
Middle cerebral artery occlusion (MCAO) model of stroke
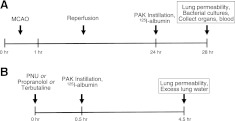
Male mice aged 10- to 14-wk-old were anesthetized with isoflurane and maintained at 1.5-2% isoflurane in O2 during surgical procedures with spontaneous respiration by face mask. Under the operating microscope, the left common carotid artery and external carotid artery were exposed through a midline neck incision. Left MCAO was performed using a silicone-coated 6-0 monofilament suture (Doccol Corp., Redlands, CA, USA) inserted through the common carotid artery, as we have previously described (20). A rectal temperature probe and controlled heating pad (Harvard Apparatus, Holliston, MA, USA) were used to maintain the rectal temperature at 37.0 ± 1°C. Respiratory rate and temperature were monitored. Animals were allowed to awaken from anesthesia during MCAO and return to their cages. After 60 min of occlusion, mice were reanesthetized, the suture was withdrawn to allow reperfusion, and the common carotid was ligated. Sham-operated mice underwent isolation and ligation of the common carotid artery without placing the suture (Fig. 1).
Figure 1.
Schematics of the in vivo mouse experiments. A) Timeline of stroke and pneumonia. In some experiments, mice were treated with drugs prior to MCAO. In all cases, MCAO was for 1 h duration, followed by 24 h of reperfusion. Airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle was performed and animals sacrificed 4 h later for analysis, as described in Materials and Methods. B) Timeline for effects of α7nAChR or β-adrenergic receptors on P. aeruginosa-induced lung injury. Mice were pretreated with PNU-282987 (0.4 mg/kg i.p.), propranolol (3 mg/kg i.p. + 50 μl 10−4 M i.t.) or terbutaline (1 mg/kg i.p + 50 μl 10−4 M i.t.) or their respective vehicles 30 min before the airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU). Mice were euthanized 4 h later, and lung vascular permeability and excess water were measured, as described in Materials and Methods.
Pneumonia model
The mouse pneumonia model treatment was performed as we have previously reported (21). Briefly, mice were anesthetized with tribromoethanol (250 mg/kg i.p.). The mouse was laid on a board with its head elevated at 45°. Then, 50 μl of PBS containing 1 × 107 CFU of P. aeruginosa was instilled into both lungs through the trachea via the mouth by using a 27-gauge gavage needle. The mouse was allowed to recover for 15 min prior to being returned to the cage. Mice were active and appeared normal after 30 min. At 4 h after the bacterial instillation, mice were euthanized with a larger dose of tribromoethanol (500 mg/kg i.p.). Blood samples were collected in a sterile fashion through puncture of the inferior vena cava after laparotomy, and bilateral thoracotomies were performed. The mouse lungs were removed, weighed, and homogenized for lung vascular permeability measurements. Bacterial concentration was determined by quantitative culture of homogenized lung, blood, and spleen tissue (Fig. 1).
Some mice were injected with MLA (10 mg/kg i.p.) or its vehicle 1 h before MCAO or with PNU-282987 (0.4 mg/kg i.p.) 30 min before the airspace instillation of P. aeruginosa (22). Other mice were pretreated with either propranolol (3 mg/kg i.p. and 50 μl 10−4 M i.t.) or terbutaline (1 mg/kg i.p. and 50 μl 10−4 M i.t.) at 1 h before the airspace instillation of P. aeruginosa. Propranolol or terbutaline were also added to the instilled solution containing P. aeruginosa or its vehicle at a concentration of 10−5 M (23). Pilot studies demonstrated that PNU-282987, propranolol, and terbutaline did not affect the viability of P. aeruginosa bacteria (data not shown).
Evaluation of neurological deficit score
Neurological status was evaluated at 24 h, according to a neurological grading score (24). The neurological deficit score grades are as follows: 0, no observable neurological deficit; 1, failure to extend right forepaw; 2, circling to the right; 3, falling to the right; 4, inability to walk spontaneously and a depressed level of consciousness. The evaluator was blinded to experimental group.
Measurement of cerebral infarction area
Infarction area was assessed at 24 h after MCAO. For mice treated with MLA, infarct area was assessed by 2,3,5-triphenyletrazolium chloride (TTC) staining. After 24 h of reperfusion, mice were given an overdose of isoflurane and decapitated. Brains were removed rapidly and sectioned coronally with a rodent brain slicer matrix (Zivic Instruments, Pittsburgh, PA, USA). Sections were incubated in 2% TTC (Sigma), and infarction volume was determined using 4 slices/mouse (25). For α7-KO mice and C57BL6J WT mice, cresyl violet staining was performed 24 h after MCAO to assess infarct area. Mice were perfused with chilled normal saline and 4% paraformaldehyde (PFA) after anesthetic overdose. Brains were removed and put in 4% PFA for 48 h, then coronal sections of 50 μm were made with a Vibratome (Leica VT 1000S; Heidelberger, Nussloch, Germany). The sections were mounted on gelatin-coated microscope slides. After staining with 1.0% cresyl violet acetate (Sigma) for 10 min, sections were dehydrated in 70, 90, and 100% ethanol and then placed in xylene baths at room temperature. Infarction area was determined on coronal sections at rostral 2.34, 1.34, and 0.26 mm, and caudal −0.70, −1.70, and −2.70 mm relative to bregma, as described previously (20). The infarction area was determined by an observer in a blinded procedure using digital imaging analysis software (Photoshop CS3; Adobe Systems, San Jose, CA, USA), extended and corrected for edema, as described previously (26).
Lung vascular permeability measurement
Lung endothelial permeability to protein (%) and excess lung water (μl) were measured as described previously (21). Briefly, 0.5 μCi of 125I-albumin was injected intraperitoneally 4 h before sacrificing the animals to ensure adequate tracer distribution. The blood was collected through puncture of the inferior vena cava. Then, the lungs were removed, counted in a Wizard γ counter (Perkin-Elmer, Waltham, MA, USA), weighed, and homogenized. The homogenate was weighed, and a fraction was centrifuged (12,000 g, 8 min) to assay the hemoglobin concentration in the supernatant. Another fraction of homogenate, supernatant, and blood were weighed and then dried in an oven (60°C for 48 h) for gravimetric determination of the extravascular lung water. The lung wet weight (W) to dry weight (D) ratio W/D was determined by the following standard formula: extravascular lung water = [(W/D)exp × Dexp − (W/D)norm × Dnorm] × 1000 (μl), where exp is experimental and norm is normal. Endothelial permeability was calculated as the counts of 125I-albumin in the blood free lung tissue divided by the counts of 125I-albumin in the plasma.
Bacteria cultures from the lungs, spleen, and blood
Mouse blood, spleen, and lungs were collected in a sterile fashion. The lungs and spleen were homogenized in sterile containers and the homogenates were serially diluted and plated in triplicate on sheep-blood agar plates. Blood was collected in sterile tubes containing 10% sodium citrate prior to serial dilution and plating in triplicate on sheep-blood agar plates for bacterial colony counts.
Bronchoalveolar lavage (BAL), cell count, and protein measurement
BAL fluid was collected by infusing 1 ml of sterile PBS (containing 5 mM EDTA) into the lungs of the mice after tracheal cannulation, as described previously (21). Gentle suction was applied, and ∼85% of the fluid was withdrawn from the lungs. The collected fluid was centrifuged at 6000 rpm for 5 min. The supernatant (100 μl) was immediately used for cytospin preparation. Cytospin preparations were made on glass slides, and differential cell counts were performed by two independent operators using Diff-Quik-stained slides. Mean counts from duplicate slides were obtained and expressed as the number of cells per milliliter of BAL fluid recovered. The remaining supernatant was stored immediately at −80°C for protein measurement.
Statistical analysis
All data are summarized as means ± sd. For statistical analysis, we used StatView 5.0 (SAS Inc., Cary, NC, USA) and MedCalc 7.2.0.2 (MedCalc Software Inc., Mariakerke, Belgium). Normal distribution was verified using the Kolmogorov-Smirnov test. For normally distributed series, unpaired Student's t test or 1-way ANOVA and Fisher's protected least significant difference for post hoc comparisons were used to determine differences between experimental and control groups. For non-normally distributed series, the Mann-Whitney U test was used to compare 2 experimental conditions. A Kaplan-Meier analysis followed by a log rank (Mantel-Cox) test was used to compare the survival between the 4 experimental groups of mice at 24 h. A value of P < 0.05 was considered statistically significant.
RESULTS
Prior stroke increases pulmonary edema and decreases neutrophil recruitment and bacterial clearance following airspace instillation of P. aeruginosa
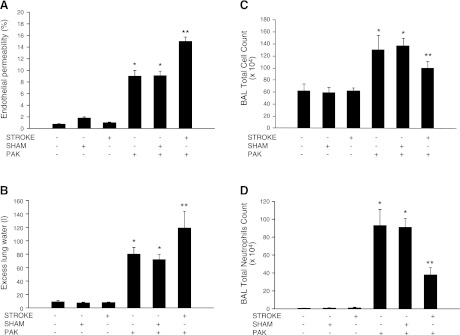
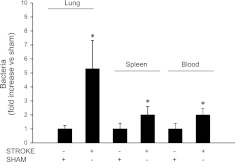
Stroke was performed 24 h before induction of bacterial pneumonia. Airspace instillation of P. aeruginosa caused the development of pulmonary edema that was significantly increased by prior MCAO (Fig. 2A, B). The increase in the severity of pulmonary edema was associated with inhibition of neutrophil recruitment into the airspaces (Fig. 2C, D). Furthermore, there was a decrease in pulmonary bacterial clearance and an increase in the systemic dissemination of P. aeruginosa (Fig. 3). Finally, mice that underwent MCAO and airspace instillation with vehicle 24 h later did not have any bacterial growth from their lung homogenates (data not shown).
Figure 2.
Prior stroke increases lung injury and attenuates neutrophil recruitment in a mouse model of P. aeruginosa pneumonia. C57BL/6 mice underwent transient MCAO or sham surgery, which was followed 24 h later with airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle. Mice were euthanized 4 h later, and BAL was performed, lung vascular permeability (A) and excess lung water (B) were measured, and total cells (C) and neutrophils (D) were counted. Results are shown as means ± sd (n=8 mice/group); *P ≤ 0.05 vs. controls; **P ≤ 0.05 vs. sham-treated mice instilled with P. aeruginosa.
Figure 3.
Prior stroke decreases lung bacterial clearance and increases bacterial dissemination in P. aeruginosa pneumonia. C57BL/6 mice underwent transient MCAO or sham surgery, followed 24 h later by airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle. Mice were euthanized 4 h later, and bacteria were counted in lungs, spleen, and blood. Results are shown as means ± sd (n=8 mice/group). *P ≤ 0.05 vs. sham-treated mice instilled with P. aeruginosa.
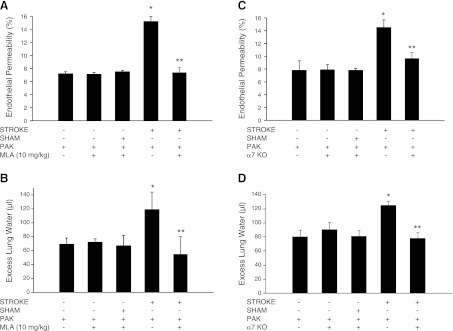
Exacerbation of P. aeruginosa-induced lung injury and mortality by prior stroke is reduced by loss of α7 nicotinicα7nAChR receptors
Prior work demonstrated increased levels of acetylcholine after cerebral ischemia, consistent with activation of the vagal cholinergic anti-inflammatory pathway (12, 13). Furthermore, activation of acetylcholine nicotinic receptors worsened lung injury in some experimental models of bacterial pneumonia (27). To determine whether inhibition of the α7nAChR would inhibit the increased lung injury induced by stroke and P. aeruginosa pneumonia, we used pharmacologic inhibition or genetic deletion of the α7nAChR. Both of these strategies completely suppressed the stroke-induced increase in lung vascular permeability (Fig. 4) and the development of alveolar edema (BAL fluid protein concentration: 1421±122 vs. 805±112 μg/ml for WT and α7-KO mice, respectively; P<0.05). In addition, these strategies prevented the inhibition of lung bacterial clearance, the dissemination of P. aeruginosa to the systemic circulation and spleen (Fig. 5), and the reduction of neutrophil airspace infiltration in response to bacteria instillation (Fig. 6). Notably, pretreatment with MLA, a specific inhibitor of the α7nAChR, or the genetic deletion of that receptor did not decrease the brain infarct area or significantly modify the neurological score after MCAO (Fig. 7). These results are relevant because a direct correlation between the size of the stroke and the risk for developing poststroke nosocomial lung infection has been reported (28). Furthermore, pharmacologic blockade or deletion of the α7nAChR did not increase the lung injury in sham-treated mice that had similar airspace instillation with P. aeruginosa (Fig. 4). Finally, survival studies demonstrated that genetic deletion of the α7nAChR was associated with a significant increase in survival of mice subjected to MCAO followed 24 h later by airspace instillation with P. aeruginosa (Fig. 8).
Figure 4.
Pharmacologic blockade or genetic deletion of α7nAChR attenuates the effect of prior stroke on lung injury induced by P. aeruginosa. C57BL/6 WT (A, B) or α7-KO (C, D) mice underwent MCAO or sham surgery followed 24 h later by airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle and were studied after 4 h. WT mice were pretreated with MLA (10 mg/kg i.p.), a specific pharmacologic inhibitor of the α7nAChR, or vehicle 1 h before MCAO (A, B). Mice were euthanized 4 h later, and excess lung water (B, D) and lung vascular permeability to protein (A, C) were measured. Results are shown as means ± sd (n=8 mice/group). *P ≤ 0.05 vs. controls; **P ≤ 0.05 vs. WT mice that underwent MCAO and P. aeruginosa instillation.
Figure 5.
Genetic deletion of the α7nAChR attenuates the effect of stroke on bacterial clearance in P. aeruginosa pneumonia. C57BL/6 WT or α7-KO mice underwent MCAO or sham surgery, followed 24 h later with airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle. Mice were euthanized 4 h later, and bacteria were counted in lungs (A), spleen (B), and blood (C). Results are shown as means ± sd (n=8 mice/group). *P ≤ 0.05 vs. sham mice instilled with P. aeruginosa; **P ≤ 0.05 vs. WT mice that underwent MCAO and P. aeruginosa instillation.
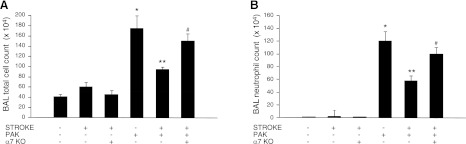
Figure 6.
Genetic deletion of the α7nAChR attenuates the effect of stroke on lung neutrophil recruitment in P. aeruginosa pneumonia. C57BL/6 WT or α7-KO mice underwent MCAO or sham surgery, and 24 h later, mice underwent airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle. Mice were euthanized 4 h later; BAL was performed, and total cells (A) and neutrophils (B) were counted. Results are shown as means ± sd (n=6 mice/group). *P ≤ 0.05 vs. sham mice; **P ≤ 0.05 vs. sham mice instilled with P. aeruginosa; #P ≤ 0.05 vs. WT mice that underwent MCAO and P. aeruginosa instillation.
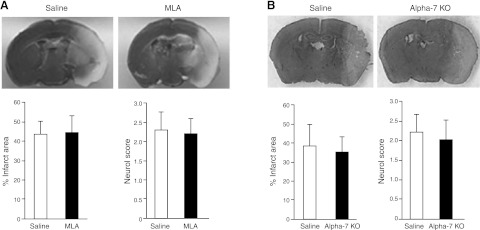
Figure 7.
Pharmacologic blockade or genetic deletion of the α7nAChR does not affect stroke outcome. A) Representative TTC-stained brain sections from a saline-treated (top left panel) and an MLA-treated mouse (top right panel) subjected to focal ischemia. Dead or injured tissue is pale, while healthy tissue is dark. Quantitation (bottom panel) shows that percentage of infarct area (as a percentage of the hemisphere after correction for edema) and neurological score did not differ between drug and vehicle treated groups. B) Representative cresyl violet-stained brain sections from WT (top left panel) and α7-KO mouse (top right panel) subjected to stroke. Neither percentage of infarct area nor neuroscore differed between the groups (bottom panel). Results are shown as means ± sd (n=8 mice/group).
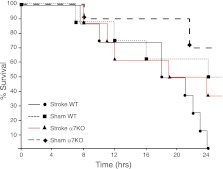
Figure 8.
Genetic deletion of the α7nAChR decreases mortality caused by stroke followed 24 h later by P. aeruginosa pneumonia. C57BL/6 WT or α7-KO mice underwent MCAO or sham surgery, followed 24 h later by airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU). Kaplan-Meier survival analysis was performed (n=10 mice/group). WT mice that underwent MCAO died significantly earlier than the WT mice that underwent sham surgery or the α7-KO mice that underwent MCAO followed by airspace instillation of P. aeruginosa.
Activation of the α7nAChR or inhibition of the β-adrenergic receptor increases P. aeruginosa-induced lung injury
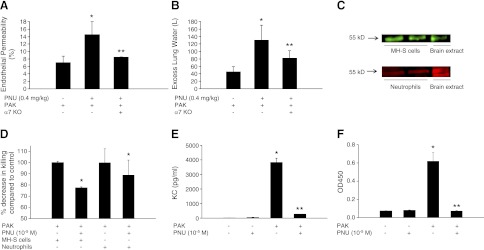
To test the hypothesis that activation of the α7nAChR receptor worsens lung injury, and to eliminate an effect due to developmental adaptation to the chronic absence of the receptor in the KO mice, we pretreated WT mice with PNU-282987 to activate the α7nAChR, and observed a 2- to 3-fold increase in lung vascular permeability caused by P. aeruginosa, an effect not observed in mice lacking the α7nAChR receptor (Fig. 9A, B). Studies were then performed with MH-S mouse macrophage cells and with primary mouse neutrophils, which both express the α7nAChR receptor (Fig. 9C). PNU-282987 significantly decreased intracellular bacterial killing by both macrophages and neutrophils (Fig. 9D) and inhibited the release of KC, a major lung neutrophil chemokine, from MH-S cells (Fig. 9E). Furthermore, PNU-282987 also significantly decreased P. aeruginosa-mediated activation of NF-κB in mouse neutrophils (Fig. 9F). These effects could contribute to increased injury in response to bacterial challenge.
Figure 9.
Pharmacologic activation of the α7nAChR increases lung injury induced by P. aeruginosa. A, B) C57BL/6 WT or α7-KO mice were pretreated with PNU-282987 (0.4 mg/kg i.p.), an activator of the α7nAChR, or its vehicle 30 min before airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU). Mice were euthanized 4 h later, and lung vascular permeability to protein (A) and excess lung water (B) were measured as described in Materials and Methods (n=8 mice/group). *P ≤ 0.05 vs. WT mice instilled with P. aeruginosa; **P ≤ 0.05 vs. WT mice pretreated with PNU then instilled with P. aeruginosa. C) α7nAChR is expressed by MH-S cells and mouse neutrophils. Expression of the α7nAChR was demonstrated on MH-S cells and mouse neutrophils by Western blot analysis. D) MH-S cells and mouse neutrophils were exposed to PNU or vehicle for 30 min, then to P. aeruginosa [2×107 CFU/ml; MOI = 10:1] for 1 h, and percentage decrease in killing was determined compared to vehicle control. *P ≤ 0.05 vs. cells (MH-S or neutrophils) pretreated with PNU vehicle. E) MH-S mouse macrophage cells were pretreated with PNU (10−5 M) or its vehicle for 30 min, then exposed to P. aeruginosa [2×107 CFU/ml; MOI = 10:1] for 4 h. ELISA assay for KC was carried out according to the manufacturer's protocol. F) Mouse neutrophils were pretreated with PNU (10−5 M) or its vehicle for 30 min, then exposed to P. aeruginosa [2×107 CFU/ml; MOI = 10:1] for 30 min. NF-κB activation was determined by the phosphorylation of p65 protein measured by ELISA. Experiments D–F were performed in triplicate and repeated 3 times. *P ≤ 0.05 vs. PNU vehicle; **P ≤ 0.05 vs. cells instilled with P. aeruginosa (E, F). Results are shown as means ± sd.
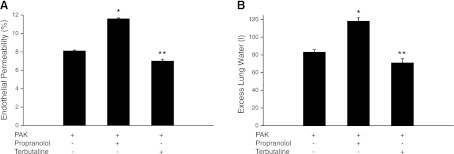
Previous studies have implicated the β-adrenergic signaling pathway in the development of spontaneous lung infection after stroke in mice (29). We found that inhibition, but not activation, of the β-adrenergic signaling pathway was associated with a significant increase in bacterial lung injury compared to vehicle-pretreated mice subjected to P. aeruginosa instillation (Fig. 10).
Figure 10.
Inhibition of the β-adrenergic receptor increases injury induced by P. aeruginosa in mice. C57BL/6 mice were pretreated with propranolol (3 mg/kg i.p. and 50 μl 10−4 M i.t.), a β-adrenergic receptor inhibitor C57BL/6 or terbutaline (1 mg/kg i.p. and 50 μl 10−4 M i.t.) a β-adrenergic receptor agonist or vehicle 1 h before airspace instillation of P. aeruginosa (PAK strain, 1×107 CFU) or vehicle. Mice were euthanized 4 h later, and lung vascular permeability to protein (A) and excess lung water (B) were measured. Results are shown as means ± sd (n=6 mice/group). *P ≤ 0.05 vs. mice pretreated with vehicle (PBS) then instilled with P. aeruginosa; **P ≤ 0.05 vs. mice pretreated with propranolol then instilled with P. aeruginosa.
DISCUSSION
Brain vascular injury is one of the leading causes of death and disability in the United States. Infectious complications, predominantly lung infection, are the most common cause of death in the postacute phase of stroke. Herein, we report 3 novel findings: prior left MCAO is associated with a significant increase in lung injury induced by P. aeruginosa; prior MCAO decreases neutrophil-mediated bacterial clearance from the lung; and activation of the α7nAChR pathway is essential for this immune suppression and increased lung injury. Preventing α7nAChR activation restores bacterial clearance, while activation of these receptors without stroke causes a similar deficit in lung innate immunity.
Our results indicate that MCAO activates the efferent cholinergic arc of the neural reflex that results in an important defect in lung innate immunity readily detected 24 h later. Mice that underwent a stroke but were not instilled with bacteria 24 h later had no bacterial growth from their lungs, excluding a major role for bronchoaspiration in the increased lung injury observed in this model. Furthermore, pharmacologic inhibition or genetic deletion of the α7nAChR did not affect the severity of lung injury caused by P. aeruginosa in sham-treated mice, indicating that the cholinergic pathway does not play a major role in the innate immune response to P. aeruginosa in the absence of stroke.
Activation of the cholinergic anti-inflammatory pathway is protective in some settings, but deleterious in others. In experimental models of sterile lung inflammation, such as acid aspiration or ventilator-induced lung injury, the activation of the α7nAChR pathway significantly attenuated the development of acute lung injury (30, 31). Comparable results were reported in several experimental models of systemic inflammation-endotoxemia, peritonitis-induced lung injury or Escherichia coli-induced gram-negative sepsis (22, 32, 33). In all of these models, the innate immune response contributes to the acute lung injury, so it is not surprising that activation of the anti-inflammatory α7nAChR pathway reduces these types of lung injury. In contrast, it has been shown that activation of the α7nAChR is deleterious in experimental bacterial pneumonia caused by Streptococcus pneumoniae or Legionella pneumophila (27, 34) though not by E. coli (22). To confirm the role of the α7nAChR pathway in modulating lung vascular permeability induced by P. aeruginosa, mice were pretreated with a specific activator of the α7nAChR, PNU-282987. Pretreatment with this agent caused a 3-fold increase in lung vascular permeability caused by P. aeruginosa, while α7nAChR-KO mice were not affected by this treatment, demonstrating the specificity of PNU-282987.
Because the α7nAChR is expressed on alveolar macrophages, neutrophils, and lung epithelial and endothelial cells (30), we tested PNU-282987 on MH-S cells, a mouse alveolar macrophage cell line. Pretreatment with PNU-282987 significantly decreased the release of KC, a major mouse neutrophil chemokine, on exposure to P. aeruginosa and reduced intracellular killing of bacteria, an effect to date only reported in lung epithelial cells (35). These in vitro results are consistent with our in vivo studies demonstrating a severely reduced ability to clear P. aeruginosa and a defect in neutrophil lung infiltration a day after a stroke.
Are there other mechanisms that may explain the suppressive effect of stroke on the lung innate immunity? Previously published studies from the laboratory of Dr. A. Meisel (e.g., ref. 29) have reported that pretreatment and posttreatment with propranolol, a nonspecific inhibitor of β-adrenergic signaling, significantly attenuated the development of spontaneous pneumonia after MCAO in mice. A more recent study from the same investigators showed that MCAO is associated with progression of bacterial aspiration to pneumonia in mice (36). The interpretation of these results is complex. First, we have recently reported that the pharmacologic inhibition or genetic deletion of the β2-adrenergic receptor significantly improved brain injury and neurological outcome (20). Because in the Meisel team's studies, propranolol, which crosses the blood-brain barrier, was administered in most of the experiments before the onset of MCAO, it is possible that some of the protection reported was due to reduced brain injury. Second, recent work from the laboratory of Dr. L. Ulloa (37) has shown that the α7nAChR mediates vagal induction of norepinephrine, providing for the first time a direct link between the sympathetic and parasympathetic system. This study showed that vagal and splenic nerve stimulation or administration of α7nAChR agonists induced the release of epinephrine by the spleen and inhibited the systemic inflammatory response to E. coli endotoxemia (37). In contrast, in the present studies, we found that inhibition, but not activation, of the β-adrenergic receptor was associated with a significant increase in bacterial lung injury compared with mice pretreated with vehicle and then instilled with P. aeruginosa. These results are consistent with previous work in another rodent model of bacterial pneumonia caused by E. coli (38). This study demonstrated that the endogenous β-adrenoreceptor tone has a protective effect in limiting lung inflammation and the accumulation of extravascular lung water by reducing lung vascular injury and increasing the resolution of alveolar edema (38). The difference between the results reported by the Ulloa team and our study may be explained, at least in part, by the fact that β-adrenergic agonists have a direct protective effect on the lung endothelial barrier and stimulate alveolar epithelial fluid transport via a c-AMP-dependent mechanism that limits the development of pulmonary edema after onset of lung injury. Importantly, this mechanism is independent of the modulation of innate immunity by β-adrenergic agonists (39).
We acknowledge that the present study has some limitations. First, we used a double-hit model of stroke followed by airspace instillation of P. aeruginosa 24 h later. Because of the complexity of this animal model, we did not determine the effect of time between MCAO and onset of pneumonia. Second, we studied the effect of MCAO on the lung innate immune response to P. aeruginosa. Although previous studies have shown that the activation of the α7nAChR increased lung injury induced by other bacteria (27, 34), the purpose of the present study was to examine the role of this signaling pathway on the severity of lung injury caused by P. aeruginosa after stroke. Thus, the interpretation of the present results should not be extended to the effect of other bacterial species without additional studies. Finally, we performed these experiments in C57BL/6 male mice. Both strain and sex are known to influence both stroke and susceptibility to infection (40, 41) Several clinical studies have reported that postmenopausal women have poorer long-term functional outcome after ischemic stroke than men (42, 43). Thus, additional studies may be warranted to determine the effect of gender on the innate immune response of the lung to bacterial infection after severe ischemic stroke.
In summary, the α7nAChR cholinergic pathway plays an important role in mediating the systemic immunosuppression observed after stroke that results in more severe lung damage by P. aeruginosa. Thus, modulating the activity of the α7nAChR cholinergic pathway may provide a new therapeutic approach to decrease the incidence and severity of nosocomial pneumonia after stroke.
Acknowledgments
This work was supported, in part, by the U.S. National Institutes of Health, grants RO1 GM-62188 (J.F.P.) and RO1 GM-49831 (R.G.).
Footnotes
- α7nAChR
- α7 nicotinic acetylcholine receptor
- BAL
- bronchoalveolar lavage
- CFU
- colony forming unit
- KC
- keratinocyte-derived chemokine
- KO
- knockout
- LB
- Luria-Bertani
- MCAO
- middle cerebral artery occlusion
- MH-S
- mouse alveolar macrophage cell line
- MLA
- methyllycaconitine
- MOI
- multiplicity of infection
- NF-κB
- nuclear factor-κB
- PNU-282987
- N-(3R)-1-azabicyclo[2,2,2]oct-3-yl-(4-chlorobenzamide)
- TTC
- 2,3,5-triphenyletrazolium chloride
- WT
- wild type
REFERENCES
- 1. Rothwell P. M., Algra A., Amarenco P. (2011) Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet 377, 1681–1692 [DOI] [PubMed] [Google Scholar]
- 2. Henon H., Godefroy O., Leys D., Mounier-Vehier F., Lucas C., Rondepierre P., Duhamel A., Pruvo J. P. (1995) Early predictors of death and disability after acute cerebral ischemic event. Stroke 26, 392–398 [DOI] [PubMed] [Google Scholar]
- 3. Kalra L., Yu G., Wilson K., Roots P. (1995) Medical complications during stroke rehabilitation. Stroke 26, 990–994 [DOI] [PubMed] [Google Scholar]
- 4. Langhorne P., Stott D. J., Robertson L., MacDonald J., Jones L., McAlpine C., Dick F., Taylor G. S., Murray G. (2000) Medical complications after stroke: a multicenter study. Stroke 31, 1223–1229 [DOI] [PubMed] [Google Scholar]
- 5. Hilker R., Poetter C., Findeisen N., Sobesky J., Jacobs A., Neveling M., Heiss W. D. (2003) Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke 34, 975–981 [DOI] [PubMed] [Google Scholar]
- 6. Czlonkowska A., Cyrta B., Korlak J. (1979) Immunological observations on patients with acute cerebral vascular disease. J. Neurol. Sci. 43, 455–464 [DOI] [PubMed] [Google Scholar]
- 7. Chamorro A., Amaro S., Vargas M., Obach V., Cervera A., Torres F., Planas A. M. (2006) Interleukin 10, monocytes and increased risk of early infection in ischaemic stroke. J. Neurol. Neurosurg. Psychiatry 77, 1279–1281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chamorro A., Horcajada J. P., Obach V., Vargas M., Revilla M., Torres F., Cervera A., Planas A. M., Mensa J. (2005) The early systemic prophylaxis of infection after stroke study: a randomized clinical trial. Stroke 36, 1495–1500 [DOI] [PubMed] [Google Scholar]
- 9. Offner H., Subramanian S., Parker S. M., Wang C., Afentoulis M. E., Lewis A., Vandenbark A. A., Hurn P. D. (2006) Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J. Immunol. 176, 6523–6531 [DOI] [PubMed] [Google Scholar]
- 10. Brea D., Sobrino T., Ramos-Cabrer P., Castillo J. (2009) Inflammatory and neuroimmunomodulatory changes in acute cerebral ischemia. Cerebrovasc. Dis. 27(Suppl. 1), 48–64 [DOI] [PubMed] [Google Scholar]
- 11. Chamorro A., Urra X., Planas A. M. (2007) Infection after acute ischemic stroke: a manifestation of brain-induced immunodepression. Stroke 38, 1097–1103 [DOI] [PubMed] [Google Scholar]
- 12. Bertrand N., Ishii H., Beley A., Spatz M. (1993) Biphasic striatal acetylcholine release during and after transient cerebral ischemia in gerbils. J. Cereb. Blood Flow Metab. 13, 789–795 [DOI] [PubMed] [Google Scholar]
- 13. Bertrand N., Ishii H., Spatz M. (1996) Cerebral ischemia in young and adult gerbils: effects on cholinergic metabolism. Neurochem. Int. 28, 293–297 [DOI] [PubMed] [Google Scholar]
- 14. Cheyuo C., Wu R., Zhou M., Jacob A., Coppa G., Wang P. (2011) Ghrelin suppresses inflammation and neuronal nitric oxide synthase in focal cerebral ischemia via the vagus nerve. Shock 35, 258–265 [DOI] [PubMed] [Google Scholar]
- 15. Giuliani D., Ottani A., Mioni C., Bazzani C., Galantucci M., Minutoli L., Bitto A., Zaffe D., Botticelli A. R., Squadrito F., Guarini S. (2007) Neuroprotection in focal cerebral ischemia owing to delayed treatment with melanocortins. Eur. J. Pharmacol. 570, 57–65 [DOI] [PubMed] [Google Scholar]
- 16. Ottani A., Giuliani D., Mioni C., Galantucci M., Minutoli L., Bitto A., Altavilla D., Zaffe D., Botticelli A. R., Squadrito F., Guarini S. (2009) Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J. Cereb. Blood Flow Metab. 29, 512–523 [DOI] [PubMed] [Google Scholar]
- 17. Rosas-Ballina M., Tracey K. J. (2009) The neurology of the immune system: neural reflexes regulate immunity. Neuron 64, 28–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tadie J. M., Bae H. B., Banerjee S., Zmijewski J. W., Abraham E. (2012) Differential activation of RAGE by HMGB1 modulates neutrophil-associated NADPH oxidase activity and bacterial killing. Am. J. Physiol. Cell Physiol. 302, C249–C256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roux J., Carles M., Koh H., Goolaerts A., Ganter M. T., Chesebro B. B., Howard M., Houseman B. T., Finkbeiner W., Shokat K. M., Paquet A. C., Matthay M. A., Pittet J. F. (2010) Transforming growth factor beta1 inhibits cystic fibrosis transmembrane conductance regulator-dependent cAMP-stimulated alveolar epithelial fluid transport via a phosphatidylinositol 3-kinase-dependent mechanism. J. Biol. Chem. 285, 4278–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Han R. Q., Ouyang Y. B., Xu L., Agrawal R., Patterson A. J., Giffard R. G. (2009) Postischemic brain injury is attenuated in mice lacking the beta2-adrenergic receptor. Anesth. Analg. 108, 280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carles M., Lafargue M., Goolaerts A., Roux J., Song Y., Howard M., Weston D., Swindle J. T., Hedgpeth J., Burel-Vandenbos F., Pittet J. F. (2010) Critical role of the small GTPase RhoA in the development of pulmonary edema induced by Pseudomonas aeruginosa in mice. Anesthesiology 113, 1134–1143 [DOI] [PubMed] [Google Scholar]
- 22. Su X., Matthay M. A., Malik A. B. (2010) Requisite role of the cholinergic alpha7 nicotinic acetylcholine receptor pathway in suppressing gram-negative sepsis-induced acute lung inflammatory injury. J. Immunol. 184, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robriquet L., Kipris E., Guery B. (2011) Beta-adrenergic modulation of lung fluid balance in acute P. aeruginosa pneumonia in rats. Exp. Lung Res. 37, 453–460 [DOI] [PubMed] [Google Scholar]
- 24. Yang G., Chan P. H., Chen J., Carlson E., Chen S. F., Weinstein P., Epstein C. J., Kamii H. (1994) Human copper-zinc superoxide dismutase transgenic mice are highly resistant to reperfusion injury after focal cerebral ischemia. Stroke 25, 165–170 [DOI] [PubMed] [Google Scholar]
- 25. Xiong X., Barreto G. E., Xu L., Ouyang Y. B., Xie X., Giffard R. G. (2011) Increased brain injury and worsened neurological outcome in interleukin-4 knockout mice after transient focal cerebral ischemia. Stroke 42, 2026–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swanson R. A., Morton M. T., Tsao-Wu G., Savalos R. A., Davidson C., Sharp F. R. (1990) A semiautomated method for measuring brain infarct volume. J. Cereb. Blood Flow Metab. 10, 290–293 [DOI] [PubMed] [Google Scholar]
- 27. Giebelen I. A., Leendertse M., Florquin S., van der Poll T. (2009) Stimulation of acetylcholine receptors impairs host defence during pneumococcal pneumonia. Eur. Respir. J. 33, 375–381 [DOI] [PubMed] [Google Scholar]
- 28. Minnerup J., Wersching H., Brokinkel B., Dziewas R., Heuschmann P. U., Nabavi D. G., Ringelstein E. B., Schabitz W. R., Ritter M. A. (2010) The impact of lesion location and lesion size on poststroke infection frequency. J. Neurol. Neurosurg. Psychiatry 81, 198–202 [DOI] [PubMed] [Google Scholar]
- 29. Prass K., Meisel C., Hoflich C., Braun J., Halle E., Wolf T., Ruscher K., Victorov I. V., Priller J., Dirnagl U., Volk H. D., Meisel A. (2003) Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. J. Exp. Med. 198, 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Su X., Lee J. W., Matthay Z. A., Mednick G., Uchida T., Fang X., Gupta N., Matthay M. A. (2007) Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am. J. Respir. Cell Mol. Biol. 37, 186–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dos Santos C. C., Shan Y., Akram A., Slutsky A. S., Haitsma J. J. (2011) Neuroimmune regulation of ventilator-induced lung injury. Am. J. Respir. Crit. Care Med. 183, 471–482 [DOI] [PubMed] [Google Scholar]
- 32. Giebelen I. A., van Westerloo D. J., LaRosa G. J., de Vos A. F., van der Poll T. (2007) Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock 28, 700–703 [DOI] [PubMed] [Google Scholar]
- 33. Giebelen I. A., Le Moine A., van den Pangaart P. S., Sadis C., Goldman M., Florquin S., van der Poll T. (2008) Deficiency of alpha7 cholinergic receptors facilitates bacterial clearance in Escherichia coli peritonitis. J. Infect. Dis. 198, 750–757 [DOI] [PubMed] [Google Scholar]
- 34. Matsunaga K., Klein T. W., Friedman H., Yamamoto Y. (2001) Involvement of nicotinic acetylcholine receptors in suppression of antimicrobial activity and cytokine responses of alveolar macrophages to Legionella pneumophila infection by nicotine. J. Immunol. 167, 6518–6524 [DOI] [PubMed] [Google Scholar]
- 35. Yamaguchi H., Friedman H., Yamamoto Y. (2003) Involvement of nicotinic acetylcholine receptors in controlling Chlamydia pneumoniae growth in epithelial HEp-2 cells. Infect. Immun. 71, 3645–3647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Prass K., Braun J. S., Dirnagl U., Meisel C., Meisel A. (2006) Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke 37, 2607–2612 [DOI] [PubMed] [Google Scholar]
- 37. Vida G., Pena G., Deitch E. A., Ulloa L. (2011) Alpha7-cholinergic receptor mediates vagal induction of splenic norepinephrine. J. Immunol. 186, 4340–4346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Su X., Robriquet L., Folkesson H. G., Matthay M. A. (2006) Protective effect of endogenous beta-adrenergic tone on lung fluid balance in acute bacterial pneumonia in mice. Am. J. Physiol. Lung Cell. Mol. Physiol. 290, L769–L776 [DOI] [PubMed] [Google Scholar]
- 39. McAuley D. F., Matthay M. A. (2005) Is there a role for beta-adrenoceptor agonists in the management of acute lung injury and the acute respiratory distress syndrome? Treatments Respir. Med. 4, 297–307 [DOI] [PubMed] [Google Scholar]
- 40. Schulte-Herbruggen O., Klehmet J., Quarcoo D., Meisel C., Meisel A. (2006) Mouse strains differ in their susceptibility to poststroke infections. Neuroimmunomodulation 13, 13–18 [DOI] [PubMed] [Google Scholar]
- 41. Gibson C. L., Coomber B., Murphy S. P. (2011) Progesterone is neuroprotective following cerebral ischaemia in reproductively ageing female mice. Brain 134, 2125–2133 [DOI] [PubMed] [Google Scholar]
- 42. Kim J. S., Lee K. B., Roh H., Ahn M. Y., Hwang H. W. (2010) Gender differences in the functional recovery after acute stroke. J. Clin. Neurol. 6, 183–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fukuda M., Kanda T., Kamide N., Akutsu T., Sakai F. (2009) Gender differences in long-term functional outcome after first-ever ischemic stroke. Intern. Med. 48, 967–973 [DOI] [PubMed] [Google Scholar]