Abstract
Deletion of muscle RING finger 1 (MuRF1), an E3 ubiquitin ligase, leads to sparing of muscle mass following denervation. The purpose of this study was to test the hypothesis that muscle sparing in mice with a deletion of MuRF1 is due to the selective inhibition of the ubiquitin proteasome system. Activities of the 20S and 26S proteasomes, calpain and cathepsin L, were measured in the triceps surae muscles of wild-type (WT) and MuRF1-knockout (KO) mice at 3 and 14 d following denervation. In addition, fractional protein synthesis rates and differential gene expression were measured in WT and KO muscle. The major finding was that 20S and 26S proteasome activities were significantly elevated (1.5- to 2.5-fold) after 14 d of denervation in both WT and KO mice relative to control, but interestingly, the activities of both the 20S and 26S proteasome were significantly higher in KO than WT mice. Further, mRNA expression of MAFbx was elevated after 14 d of denervation in KO, but not WT, mice. These data challenge the conventional dogma that MuRF1 is controlling the degradation of only contractile proteins and suggest a role for MuRF1 in the global control of the ubiquitin proteasome system and protein turnover.—Gomes, A. V., Waddell, D. S., Siu, R., Stein, M., Dewey, S., Furlow, J. D., Bodine, S. C. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation.
Keywords: E3 ubiquitin ligase, ubiquitin proteasome system, MAFbx, muscle atrophy
Loss of skeletal muscle mass is a debilitating response to unloading, nerve injury, and many systemic diseases. Although the molecular mechanisms involved in atrophy are clearly diverse, all forms of atrophy undergo an imbalance between the synthesis and degradation of proteins. The ubiquitin proteasome system (UPS) is the major proteolytic system in skeletal muscle, and activation of the UPS has been observed in many disease models, including denervation (1), disuse (2), diabetes (3), fasting (1), cancer (4, 5), sepsis (6, 7), and metabolic acidosis (8), thus implicating the UPS as a common mechanism responsible for the induction of muscle loss regardless of the trigger.
Differential transcriptional profiling has identified muscle RING finger 1 (MuRF1) and muscle atrophy F-box (MAFbx)/atrogin-1 as excellent markers of muscle atrophy (9, 10). These atrophy-related genes are muscle-specific E3 ubiquitin ligases (9, 10) that are expressed at relatively low levels in resting skeletal muscle and rapidly up-regulated under divergent atrophy-inducing conditions. These observations have led to the theory that MuRF1 and MAFbx are key regulators of the atrophy process through the targeting of specific contractile proteins for degradation by the UPS. Denervation, a well-established model that results in an accumulation of polyubiquitinated proteins and loss of muscle mass, was the first model used to determine whether null deletions of MuRF1 or MAFbx altered the extent of muscle loss during an atrophy-inducing event (9). Following 7 to 14 d of denervation, the hind limb muscles of mice with deletions of either gene had significantly less atrophy than muscles from wild-type littermates (9). Subsequently, MuRF1-null mice have been shown to result in muscle sparing following hind limb unloading (11) and glucocorticoid treatment (12).
The consistent induction of MAFbx and MuRF1 in different models of muscle atrophy suggests that these enzymes are critical regulators of the atrophy process, and thus, identification of the pathways or proteins modified by these enzymes, as well as the mechanisms that regulate their activity and expression, is of great importance. While MuRF1 has been implicated in the control of muscle size via targeted proteasome degradation, additional functions for MuRF1 have been implicated, including regulation of carbohydrate metabolism (13), inhibition of protein synthesis (14), and transcriptional regulation (15).
In addition to MuRF1 and MAFbx, other genes are transcriptionally regulated under atrophy conditions (16). Among the genes strongly induced during atrophy are those involved in ubiquitin-mediated protein degradation, such as polyubiquitins, ubiquitin fusion proteins, and multiple subunits of the 20S proteasome and its 19S regulator. Further, the lysosomal cathepsins and Ca2+-dependent calpains, the other significant proteolytic systems in striated muscle, are implicated in muscle atrophy. Although some studies report increases in cathepsin B and L, and m-calpain enzyme activities during disuse-induced atrophy (2), others do not (17). To our knowledge, measurement of the response of all three degradation systems following disuse atrophy has not been previously reported in normal or MuRFl-null mice.
In the present study, we investigated the effect of the absence of MuRF1 on the UPS and the other major proteolytic systems in skeletal muscle following denervation-induced atrophy. Our analyses focused on mice with a null deletion of MuRF1, since functional sparing of muscle mass was observed in the MuRF1-null mice, but not the MAFbx-null mice, following extended (28 d) denervation. Although it has been suggested that proteasome activity would be affected by the loss of MuRF1, a comprehensive analysis of the proteasome has not previously been performed. Thus, despite considerable interest in MuRF1 as a key regulator of skeletal muscle mass, little is known about the relationship between the lack of MuRF1 and proteolytic pathways involved in skeletal muscle atrophy. The present results suggest that MuRF1 is an important regulator of protein degradation during denervation through regulation of the ubiquitin proteasome pathway. However, counter intuitively, deletion of MuRF1 resulted in an increase, as opposed to a decrease, in proteasome activity following denervation, which was even greater than that seen in denervated wild-type mice. This increase in proteasome activity was related to increases in the amount of 26S proteasome, as well as increases in proteasome activator 28α (PA28α). These results present new insights into the role of MuRF1 in the regulation of muscle mass.
MATERIALS AND METHODS
Chemicals
Calpain inhibitor IV and cathepsin L inhibitor I were purchased from Calbiochem (La Jolla, CA, USA). Epoxomicin and cathepsin L substrate were purchased from Peptides International (Louisville, KY, USA). Proteasome substrates were purchased from Enzo Life Sciences (Farmingdale, NY, USA). All chemicals were of >98% purity unless stated otherwise.
Animal models
All experiments were performed in 6-mo-old female mice with initial mean ± sd body weights of 24 ± 2 g. Animals were assigned to one of two experimental groups (cage control or denervation). Mice with germ line deletions of MuRF1 and MAFbx were investigated and have been previously described (9). Mice are on a 129J/C57BL6 mixed background, and all experiments used age and gender-matched wild-type (WT) littermates as controls. Mice were randomized to treatment, so that mean starting body weights of each group were similar. All animal procedures were approved by the University of California, Davis (UC Davis) Institutional Animal Care and Use Committee and conformed to the American Physiological Society's Guiding Principles in the Care and Use of Animals.
Targeted denervation of the lower limb muscles in the right leg was accomplished through transection of the sciatic nerve. Under isoflurane anesthesia (3% inhalation) and with the use of aseptic surgical techniques, the sciatic nerve was isolated in the midthigh region and cut with sharp scissors. Mice were given an analgesic (buprenorphine, 0.1 mg/kg) immediately following the surgery and returned to their cage following recovery.
Tissue collection
For tissue collection, mice were anesthetized with isoflurane (3% inhalation) and the tibialis anterior (TA) and triceps surae (TS) complex muscles (medial and lateral gastrocnemius, plantaris, and soleus) were dissected free of connective tissue, weighed, frozen in liquid nitrogen, and stored at −80°C for later analysis. On completion of tissue removal, mice were euthanized by exsanguination.
Western blots
Muscles were homogenized at 4°C in proteasome assay lysis buffer (50 mM Tris, 150 mM NaCl, 5 mM MgCl2, and 1 mM EGTA, pH 7.5). Homogenates were clarified by centrifugation at 12,000 g for 30 min before determination of protein concentration by Bradford assay (Bio-Rad, Hercules, CA, USA). SDS-PAGE was performed on 4–20 or 12% gels using 20–30 μg of protein. Western blots were revealed with enhanced chemiluminescence (West Pico; Pierce, Rockford, IL, USA). Antibodies against proteasome subunits were used to detect protein expression levels.
Primary antibodies were obtained from Enzo, Epitomics (Burlingame, CA, USA), Sigma (St. Louis, MO, USA), and Abcam (Cambridge, MA, USA). Secondary HRP-conjugated antibodies were obtained from Sigma (goat anti-mouse and goat anti-rabbit) and Santa Cruz Biotechnology (Santa Cruz, CA, USA; rabbit anti-goat). Enhanced chemiluminescence was carried out using Pierce's West Pico detection reagent.
Microarray analysis
Total RNA from the TS muscles was prepared by polytron homogenization in TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's recommendation. Denaturing agarose gels were used to assess the quality of the isolated RNA with an expected 2:1 28S to 18S ribosomal RNA ratio and with minimal smearing, followed by semiquantitative RT-PCR using gene-specific primers for MuRF1, MAFbx, and HDAC4 to verify both the knockout (KO) phenotype and the expected induction of these genes on denervation.
For microarray analysis, RNA from 3 individual animals in each treatment group (control vs. denervated) were prepared in parallel by conversion to cDNA followed by amplification and labeling of corresponding cRNA with biotin-labeled UTP using an Illumina TotalPrep RNA Amplification Kit (Ambion, Austin, TX, USA). Equal amounts of amplified total, labeled cRNA were hybridized to Illumina Sentrix mouse 6 × 2 v1 BeadChip at the UC Davis Genome Center gene expression facility, using a single-pass photomultiplier tube scan. Data were analyzed using Illumina Beadstudio v3.2.3 software, using quantile normalization. Data were initially filtered to demand that at least one sample group signal was above background (>150 average signal intensity), and the array P value was <0.01. Microarray data have been deposited in GEO with the accession number pending.
Calpain activity measurement
Assays were carried out in a total volume of 200 μl in black 96-well plates. Protein samples (25–50 μg) were incubated with 200 μM of substrate in 50 mM Tris, 1 mM EDTA, 10 mM CaCl2, 150 mM NaCl, and 0.5 mM DTT, at pH 7.5. Released AMC was measured using a Fluoroskan Ascent fluorometer (Thermo Electron, Waltham, MA, USA) at an excitation wavelength of 390 nm and an emission wavelength of 460 nm for up to 120 min. Each assay was conducted in the absence and presence of a specific calpain inhibitor to determine calpain-specific activity (50 μM calpain inhibitor IV). This assay measures both calpain I and II activity.
Cathepsin L activity measurements
Assays were carried out in a total volume of 100 μl in black 96-well plates. Protein samples (25 μg) were incubated with 200 μM of substrate (Z-Phe-Arg-AMC) in 100 mM sodium acetate buffer containing 1 mM EDTA and 1 mM DTT, pH 5.5. Released AMC was measured using a Fluoroskan Ascent fluorometer at an excitation wavelength of 390 nm and an emission wavelength of 460 nm for up to 120 min. Each assay was conducted in the absence and presence of a specific cathepsin inhibitor to determine cathepsin L-specific activity (10 μM cathepsin L inhibitor I).
20S and 26S proteasome activity measurement
Proteins were extracted from muscle homogenates and proteasome activities were assayed, as described previously (18). Briefly, muscles were homogenized with hand-held (Potter-Elvehjem) homogenizers in 1.5 ml buffer containing 50 mM Tris, 150 mM NaCl, 5 mM MgCl2, 1 mM EDTA, and 1 mM DTT, at pH 7.5. Proteasomes were collected in the supernatant after 30 min centrifugation at 12,000 g. The caspase (β1), trypsin (β2), and chymotrypsin (β5)-like activities of proteasomes were assayed using 20–30 μg of protein and fluorescently tagged substrates (Z-LLE-AMC, Boc-LSTR-AMC and Suc-LLVY-AMC). All assays were carried out in a total volume of 100 μl. The ATP-dependent 26S assays were performed in the homogenization buffer after the addition of 100 μM ATP. The ATP-independent 20S assay buffer for the chymotrypsin-like proteasome activity was carried out in 25 mM HEPES (pH 7.5), 0.5 mM EDTA, and 0.03% SDS. For the caspase- and trypsin-like 20S activities, the buffer composition was 25 mM HEPES (pH 7.5), 0.5 mM EDTA, 0.05% Nonidet P-40, and 0.001% SDS. Released AMC was measured using a Fluoroskan Ascent fluorometer at an excitation wavelength of 390 nm and an emission wavelength of 460 nm for up to 120 min. Each assay was conducted in the absence and presence of a specific proteasome inhibitor to determine proteasome-specific activity (10 μM epoxomicin for chymotrypsin-like, 40 μM epoxomicin for trypsin-like, and 40 μM Z-Pro-Nle-Asp-H for caspase-like activity). All samples were assayed in triplicate per animal for ≥4 animals.
Fractional rates of protein synthesis
The fractional rate of protein synthesis in the TS muscles was determined from the incorporation of [2H]alanine using a precursor:product relationship, as described previously (19, 20). The hind limb muscles of WT and MuRF1-KO mice were denervated for 3 or 14 d, as described above. On d 3 or 14 of denervation, an initial blood sample was collected from the tail of each animal. Each animal was then given an intraperitoneal injection of deuterated water (2H2O; Sigma) equivalent to 3% of total body water, which was calculated to be equal to body weight × 0.75 in milliliters. Following a 4-h equilibration time in which food was removed from the cage, mice were anesthetized with 3% isoflurane, and the TA and TS were excised, weighed, and frozen in liquid nitrogen. A final blood sample was collected via cardiac puncture. The TS along with the two blood samples collected from each animal were sent to the Mouse Metabolic Phenotyping Center (Case Western University, Cleveland, OH, USA) to determine the protein fractional synthesis rate by methods described previously (19, 21).
Statistical analysis
A Student's t test was used for comparisons between groups (SigmaPlot 11; OriginLab Corp., Northampton, MA, USA). Statistical significance was set at P < 0.05.
RESULTS
Sparing of muscle fiber integrity in MuRF1-KO, but not MAFbx-KO, following denervation
Muscle sparing has been shown to occur in both MuRF1- and MAFbx-null mice following 14 d of denervation (9). Evaluation of both MuRF1 and MAFbx mice at an extended period of denervation (i.e., 28 d) showed that while muscle mass continues to be preserved in both the MuRF1- and MAFbx-null mice, muscle fiber integrity is preserved only in the MuRF1-KO mice (Fig. 1A). As shown in Fig. 1, following 28 d of denervation there is an increase in the appearance of vacuoles (arrows) within individual muscle fibers and a greater incidence of muscle fiber necrosis in muscles from the MAFbx but not MuRF1 mice. On the basis of these observations, further studies were restricted to the MuRF1-KO mice and involved the examination of proteolytic pathways in the TS of WT and MuRF1-KO mice after 3 and 14 d of denervation to investigate the underlying mechanisms involved in muscle sparing in MuRF1-KO mice. At 3 d of denervation, there was no significant atrophy or difference in the response of WT and MuRF1-KO mice. At 14 d of denervation, however, significant loss of mass was found in both WT (41% loss) and MuRF1-KO (25% loss) mice, with significant muscle sparing apparent in the KO mice relative to WT (Fig. 1C). These results are consistent with previous findings (9).
Figure 1.
Muscle sparing in MuRF1-KO but not MAFbx-KO mice. A) Representative muscle cross section from the TA muscle of WT, MuRF1-KO, and MAFbx-KO mice taken after 28 d of denervation. Muscle cross sections were stained with hematoxylin and eosin and show muscle fiber degeneration (arrows), primarily in the MAFbx-KO mice. B) Muscle mass, expressed as relative wet weight (denervated/control), is plotted for the TS complex (solid bars) and TA (open bars) muscles following 28 d of denervation. Data are expressed as means ± sd of muscles from WT (n=10), MuRF1-KO (n=7), and MAFbx-KO (n=7) mice. C) Muscle mass, expressed as relative wet weight (denervated/control), is plotted for the TS complex following 3 d (solid bars) and 14 d (open bars) of denervation. Data are means ± sd of muscles from WT (n=5–7/time point) and MuRF1-KO (n=7–8/time point) mice. *P < 0.05 vs. WT denervated muscle.
Proteasome activity increased to a greater extent in MuRF1-KO than WT mice following denervation
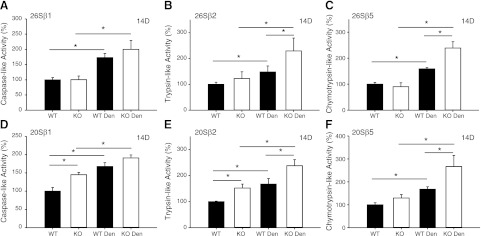
Muscle sparing in the MuRF1-KO mice could be related to a decrease in protein degradation via the UPS. Thus, we measured both ATP-dependent (26S) and ATP-independent (20S) activities in the TS of WT and KO mice following 3 and 14 d of denervation. After 3 d of denervation, there was an increase in β1 and β2 26S activities of WT and β2 26S activity of KO mice (Fig. 2). Prolonged denervation (14 d) resulted in significant increases in all three (β1, β2, β5) ATP-independent and dependent proteasome activities in WT and KO mice; however, the extent of the increase following denervation was significantly greater in KO compared to WT muscle, especially for β2 and β5 20S and 26S proteasome activity (Fig. 3).
Figure 2.
20S and 26S proteasome proteolytic activities in WT and MuRF1-KO mice after 3 d of denervation. ATP-dependent (26S; A–C) and ATP-independent (20S; D–F) activities in the TS muscles of WT (solid bars) and MuRF1-KO (open bars) mice following 3 d of denervation. Individual subunit activities are shown for β1, caspase-like activity (A, D); β2, trypsin-like activity (B, E); and β5, chymotrypsin-like activity (C, F). Data are expressed as means ± sd of muscles (n=4/group). *P < 0.05.
Figure 3.
20S and 26S proteasome proteolytic activities in WT and MuRF1-KO mice after 14 d of denervation. ATP-dependent (26S; A–C) and ATP-independent (20S; D–F) activities in the TS muscles of WT (solid bars) and MuRF1-KO (open bars) mice following 14 d of denervation. Individual subunit activities are shown for β1, caspase-like activity (A, D); β2, trypsin-like activity (B, E); and β5, chymotrypsin-like activity (C, F). Data are means ± sd of muscles (n=4/group). *P < 0.05.
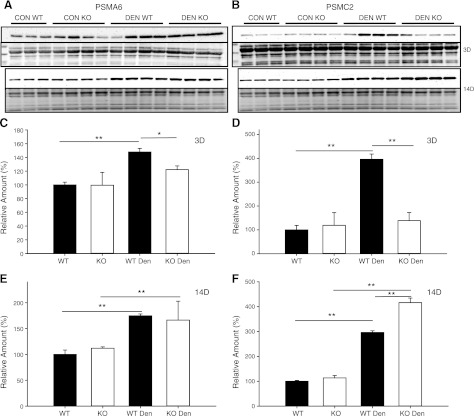
To determine whether the increase in proteasome activity was due to an increase in the amount of proteasome, Western blots were performed for specific 19S (PSMC2) and 20S (PSMA6) proteasome subunits (Fig. 4). Increases in PSMC2 and PSMA6, proteasome amounts were observed after 3 d of denervation in WT mice only (Fig. 4). After 14 d of denervation, significant increases in both PSMC2 and PSMA6 proteasome levels were observed in WT and KO mice; however, PSMC2 (19S) proteasome levels increased to a greater extent in KO compared to WT mice, which could explain the higher proteasome activity in KO mice following denervation.
Figure 4.
Protein expression of 19S and 20S proteasome subunits in control (con) and denervated (den) muscle. A, B) Western blots of lysates from the TS of WT (n=3–4) and MuRF1-KO (n=4) mice following 3 and 14 d of denervation. Immunoblots for PSMA6 (α1, 20S subunit; A, top panel) and Ponceau staining (A, bottom panel) and PSMC2 (Rpt1, 19S subunit; B, top panel) and Ponceau staining (B, bottom panel). C–F) Quantification of immunoblots from WT (solid bars) and MuRF1-KO (open bars) mice; data are expressed as means ± sd. Relative amounts (expressed as a percentage of WT con) of PSMA6 protein (C, E) or PSMC2 protein (D, F) in TS after 3 d (C, D) or 14 d (E, F) of denervation. *P < 0.05, **P < 0.001.
The greater proteasome activity in KO compared to WT mice following denervation could also be related to differences in the expression of proteasome regulatory proteins. Western blot analysis of proteasome activator 28α (PA28α), a known regulator of the 20S proteasome, revealed a significant increase in the amount of protein in both WT and KO mice 14 d following denervation, with KO mice showing higher levels of the activator than the WT mice (Supplemental Fig. S1).
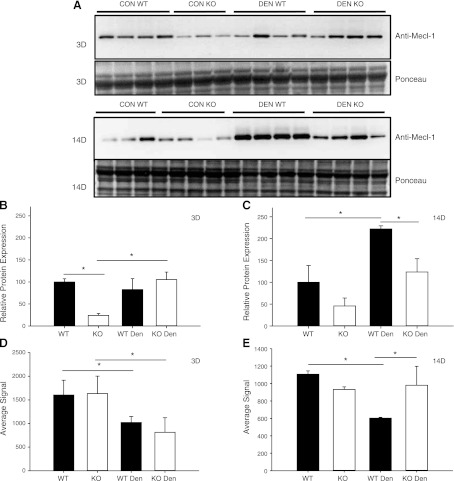
Another possible contributor to differences in proteasome activities could be differential expression of the inducible proteasome subunits. Western blot analysis of PSMB10 (also referred to as Mecl-1 or β2i) showed that under control conditions, KO mice expressed less PSMB10 protein compared to WT mice (Fig. 5). After 3 d of denervation, expression of PSMB10 protein significantly increased in KO, but not WT, mice. After 14 d of denervation, PSMB10 protein levels were significantly elevated in both KO and WT muscles; however, the total amount was significantly greater in WT compared to KO.
Figure 5.
Protein and mRNA expression of the inducible proteasome subunit PSMB10 in control (con) and denervated (den) muscle. A) Western blots of lysates from the TS of WT (n=3–4) and MuRF1-KO (n=3–4) mice following 3 and 14 d of denervation. A) Immunoblot against anti-Mecl-1, antibody for the inducible proteasome subunit PSMB10 (top panel) and Ponceau staining (bottom panel). B, C) Quantification of immunoblots from WT (solid bars) and MuRF1-KO (open bars) mice; data are expressed as means ± sd. Relative amounts (expressed as a percentage of WT con) of PSMB10 protein in TS after 3 d (B) or 14 d (C) of denervation. D, E) Relative mRNA expression of the PSMB10 gene in the TS of WT (solid bars) and KO (open bars) mice after 3 d (D) or 14 d (E) of denervation. *P < 0.05.
To further investigate the effect of the lack of MuRF1 on the ubiquitin system, the amount of polyubiquitinated proteins in muscle lysates was determined by Western blotting using two commonly used antiubiquitin antibodies, FK1 and P4D1. Polyubiquitination was increased only in the TS of WT mice after 3 d of denervation (Fig. 6). After 14 d of denervation, however, the level of polyubiquitination was elevated in both WT (P=0.08) and KO (P=0.03) mice (Fig. 6).
Figure 6.
Polyubiquitination levels in the TS muscle of WT and MuRF1-KO mice following denervation (den). A, B) Western blots of lysates from the TS of WT (n=3–4) and MuRF1-KO (n=4) mice following 3 (A) and 14 (B) d of denervation. Polyubiquitinated proteins were determined by immunoblotting with two antiubiquitin antibodies, FK1 and P4D1. C, D) Quantification of immunoblots from WT (solid bars) and MuRF1-KO (open bars) mice; data are expressed as means ± sd. Relative amounts (expressed as a percentage of control WT) of polyubiquitinated proteins in TS after 3 d (C) or 14 d (D) of denervation. *P < 0.05, **P < 0.001.
Differential expression of genes associated with ubiquitination in WT and MuRF1-KO mice
Gene expression patterns were analyzed in the TS muscle of WT and MuRF1-KO mice by Illumina microarrays at 3 and 14 d following denervation. Differential gene expression analysis revealed that expression levels of the 20S proteasome subunits were all lower for KO muscle relative to WT muscle after 3 d of denervation (Supplemental Table S1). However, after 14 d of denervation, 20S proteasome subunit expression levels were greater in KO relative to WT muscle. In general, changes in protein expression were consistent with the changes in mRNA expression. An exception to the finding that proteasome subunit expression increased in response to denervation was the inducible 20S subunits: PSMB8, PSMB9, and PSMB10. The mRNA expression of the inducible subunits (PSMB8, PSMB9, and PSMB10) decreased in WT mice after 14 d of denervation, while KO muscle showed no change or increased expression of these subunits (Fig. 5 and Supplemental Table S1). In the case of the inducible subunits, at least for PSMB10, protein expression did not follow the mRNA expression changes (Fig. 5).
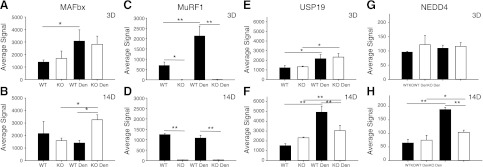
In addition to the proteasome subunits, expression of other components of the ubiquitin system (E1s, E2s, E3s, deubiqutinating enzymes, etc.) was altered following denervation. Further comparison of the response of WT and KO mice to denervation revealed a number of genes that were differentially expressed (Fig. 7 and Supplemental Tables S1 and S2). Interestingly, one gene that was differentially expressed in WT and KO mice was MAFbx. MAFbx (annotated as FBXO32 on the microarray) and MuRF1 (annotated as TRIM63) expression significantly increased after 3 d of denervation in the TS of WT mice; however, after 14 d, the expression of both genes was back to control levels (Fig. 7), consistent with previous reports (9). In contrast, MAFbx expression significantly increased in the KO mice after 3 d of denervation and remained significantly elevated from control after 14 d of denervation (Fig. 7). As expected, the MuRF1 signal in samples from KO mice was undetectable above background.
Figure 7.
Differential mRNA expression in WT and MuRF1-KO mice following denervation (den). mRNA expression (average signal from Illumina microarray) of MAFbx (A, B), MuRF1 (C, D), USP19 (E, F) and NEDD4 (G, H) after 3 d (A, C, E, G) and 14 d (B, D, F, H) of denervation in WT (solid bars) and MuRF1-KO (open bars). Data are expressed as means ± sd from n = 3/group. *P < 0.05, **P < 0.001.
Other differentially expressed genes were USP19, USP14, and NEDD4. USP19 expression, a deubiquitinating enzyme (22), increased 1.7-fold in both WT and KO mice after 3 d of denervation; however, after 14 d, USP19 expression was elevated 3.2-fold in WT mice and only 1.3-fold in the KO mice (Fig. 7 and Supplemental Table S2). USP14 mRNA expression, another deubiquitinating enzyme, was similar to that observed for USP19 (Supplemental Table S2). NEDD4 mRNA expression, an E3 ubiquitin ligase (23), was unchanged in both WT and KO mice after 3 d of denervation but was significantly increased (2.9-fold) in WT, but not KO, mice after 14 d of denervation (Fig. 7 and Supplemental Table S2).
Calpain and cathepsin L activities were elevated in both WT and MuRF1-KO mice in response to denervation
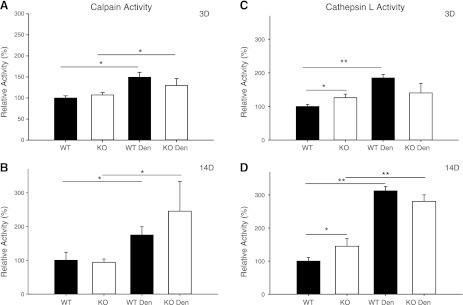
Two other major proteolytic systems, the calcium-dependent calpain I and II proteases and the lysosomal enzyme, cathepsin L, are present in skeletal muscle, and were investigated for comparison with changes in the UPS. Following 3 d of denervation, calpain (I and II) activity increased in both WT and KO mice (Fig. 8). In contrast, the lysosomal enzyme, cathepsin L, increased by >75% in WT mice but was unchanged in KO mice following 3 d of denervation (Fig. 8). After 14 d of denervation, cathepsin L activity significantly increased to the same extent in WT and KO mice. Calpain activity was also elevated in both WT and KO mice following 14 d of denervation (Fig. 8). The expression patterns of selected calpain and cathepsin genes are provided in Supplemental Table S1.
Figure 8.
Calpain and cathepsin L activities in WT and MuRF1-KO mice following denervation (den). A, B) Calcium-dependent calpain activity in the TS muscle of WT (solid bars) and KO (open bars) mice after 3 (A) and 14 (B) d of denervation. C, D) Cathepsin L activity in the TS muscle of WT (solid bars) and KO (open bars) mice after 3 (C) and 14 (D) d of denervation. Data are expressed as means ± sd for n = 3 or 4 mice/group. Enzyme activity is expressed relative to the WT Con group. *P < 0.05, **P < 0.001.
Differential expression of autophagy markers in WT and MuRF1-KO mice in response to denervation
Beclin-1 is an autophagy-related gene that is required for the initiation of the formation of the autophagasome. Western blot analysis revealed that beclin-1 expression increased in WT, but not KO, mice at 3 d of denervation (Supplemental Fig. S1B). After 14 d of denervation, beclin-1 protein expression was significantly elevated in both WT and KO mice; however, the amount of beclin-1 was significantly higher in WT compared to KO muscle, suggesting that lack of MuRF1 may reduce autophagy associated with denervation.
Examination of the gene expression patterns for the autophagy genes (Supplemental Table S1) revealed a number of interesting differences between WT and MuRF1-KO mice after denervation, especially following extended denervation (14 d). At 3 d of denervation, mRNA expression levels were elevated in both WT and KO mice for the ATG8 family members GABARAPL1 and MAP1LC3A, which remained elevated at 14 d. At 14 d, GABARAP, another ATG8 family member, was up-regulated in MuRF1-KO, but not WT mice. Interestingly, at 14 d, other autophagy genes (ATG2a, ATG7, ATG12, and ATG16L1) increased in WT, but not KO, mice.
Protein fractional synthesis rate increases in both WT and MuRF1-KO mice following extended denervation
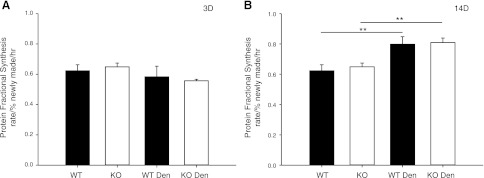
To determine how protein synthesis is affected by denervation and the lack of MuRF1, the protein fractional synthesis rate was determined in the TS following 3 and 14 d of denervation. Fractional synthesis rate was not significantly altered after 3 d of denervation. After 14 d of denervation, however, a significant increase in the fractional synthesis rate of the muscle of WT (28%) and MuRF1-KO (24.7%) mice was observed (Fig. 9).
Figure 9.
Protein fractional synthesis rate (FSR) of the TS muscle of WT and MuRF1-KO mice following denervation (den). Protein FSR (% newly made/h) was measured in TS of WT (solid bars) and MuRF1-KO (open bars) mice after 3 (A) and 14 d of denervation (B). Data are means ± sd for n = 4–10. ** P < 0.001.
Overall protein expression changes in response to denervation
Overall protein expression, as assessed by SDS-PAGE of muscle lysates from WT and KO mice, revealed visible differences in the migration or expression levels of specific proteins after extended denervation (Supplemental Fig. S2). Interestingly, a number of bands appeared to decrease to a greater extent in WT than KO muscle following denervation. These results suggest that the expression pattern of muscle proteins is altered by lack of MuRF1 during denervation.
DISCUSSION
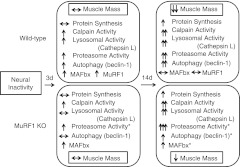
The regulation of skeletal muscle atrophy by proteolytic and protein synthesis pathways remains poorly understood. Thus, we investigated both protein synthesis and multiple degradation pathways following acute (3 d) and extended (14 d) denervation in WT and MuRF1-null mice. The major findings are summarized in Fig. 10. Previously, it was shown that mice with a null deletion of MuRF1 have attenuated muscle loss in response to denervation for durations >7 d (9); however, the mechanisms by which deletion of MuRF1 spares muscle loss following denervation or other atrophy-inducing conditions remains a major question in the field. Given that MuRF1 is an E3 ubiquitin ligase, it has been proposed that sparing in the MuRF1-null mice is related to a suppression of degradation by the ubiquitin proteasome pathway (24); however, our data do not support this hypothesis. At 14 d of denervation, we found that the TS complex from MuRF1-KO mice had 39.4% sparing of muscle mass relative to WT mice, which is consistent with a previous report (9). With respect to degradation systems, we found no suppression of calpain, cathepsin L, or proteasome activities in the MuRF1-KO mice following 14 d of denervation. In fact, we found that MuRF1-KO mice had significantly higher proteasome activity relative to WT, especially with respect to chymotrypsin-like (β5) proteolytic activity, which is the most dominant of the three proteolytic activities of the proteasome. Interestingly, we also found that MAFbx expression remained elevated in the MuRF1-KO mice compared to control, while it returned to baseline in the WT mice after 14 d of denervation. The only degradation system that may be suppressed in the MuRF1-KO following denervation was autophagy, as based on beclin-1 protein expression and the suppression of certain autophagy genes, but this requires further investigation.
Figure 10.
Summary of major findings. Diagrammatic representation of the major changes found in the TS complex of WT and MuRF1-KO mice following 3 and 14 d of denervation. Both proteolytic and synthesis pathways are significantly affected by denervation. Asterisks indicate novel and significant differences that were observed between WT and MuRF1-KO mice following denervation. The increased proteasome activity in MuRF1-KO mice following denervation was unexpected and runs contrary to current dogma. We propose that the increase in protein synthesis in MuRF1-KO mice is through different pathways than occurs in WT mice. Further studies are required to identify the specific protein targets of MuRF1. Note that the most significant differences between the WT and MuRF1-KO mice do not occur at 3 d, when MuRF1 mRNA expression in the WT is at a peak, but at 14 d, after MuRF1 mRNA expression has gone back to baseline.
Protein turnover following denervation: regulation of protein degradation pathways
This study is the first to measure enzyme activities in all three major proteolytic pathways at multiple time points during atrophy, coupled with measurement of fractional protein synthesis rate. Investigation of the ATP-dependent (26S) and ATP-independent (20S) proteasome activities showed that both are increased as a result of denervation. The increases in 20S and 26S proteolytic activities were minimal after 3 d of denervation in both WT and KO mice. In contrast, large increases in all proteasome proteolytic activities occurred by 14 d of denervation, with significantly greater increases in several of the proteolytic subunit activities for MuRF1-KO compared to WT mice. This was unexpected since higher proteasome activities have been associated with greater muscle loss, and the lack of MuRF1 results in muscle sparing following denervation. The higher proteasome activity in MuRF1-KO compared to WT mice after 14 d of denervation is consistent with a trend toward decreased polyubiquitination levels (P=0.077) in the KO mice when compared to WT mice after 14 d of denervation. A previous study reported a lower level of polyubiquitination in the MuRF1-KO mice compared to WT following 10 d of denervation and suggested that this reflects a decrease in proteasome activity due to the absence of MuRF1 (24); however, our data show the opposite.
While the UPS is thought to be the predominant pathway for protein degradation in skeletal muscle, other pathways are involved. Increases in lysosomal proteolysis and calpain activity have been reported following denervation (2), and thus, we measured and compared cathepsin L activity and calpain (combined I and II) activity in muscle lysates from WT and MuRF1-KO mice following 3 and 14 d of denervation. The data show that calpain activity increased in WT mice within 3 d of denervation and remained significantly elevated after 14 d. In the MuRF1-KO mice, cathepsin L activity was elevated compared to WT at rest, and the increase in activity following denervation was initially delayed. These data show that suppression of these pathways is not evident in the MuRF1-KO mice following denervation and cannot explain the sparing of muscle mass. Overall, our data on proteasomal, calpain, and lysosomal pathways conclusively demonstrate that proteolysis is increased in skeletal muscle following denervation, but the quantity and quality of various specific components vary in important ways between the WT and KO mice.
Protein turnover following denervation: regulation of protein synthesis
It has been suggested that in most types of muscle atrophy, the rapid loss of muscle protein is due to suppression of the overall rates of protein synthesis and elevation of protein degradation rates (16). To estimate protein synthesis, we measured the fractional protein synthesis rate (FSR) following denervation using a flooding dose of 2H2O (19). The data revealed that at 3 d of denervation, there was a small decrease in FSR in both WT and KO mice; however, at 14 d of denervation a significant increase in FSR was measured in both WT and KO mice. A significant increase in protein synthesis was previously reported in the rat diaphragm 3 and 14 d following unilateral denervation (25). In that same study, net protein breakdown was observed after 5 d of denervation due to significant increases in protein degradation (25). Another recent study found significant increases in the FSR of the rat gastrocnemius after 9 d in a model that replicates the conditions observed in critically ill, intensive-care patients (mechanical ventilation, sedation, and pharmacological paralysis; ref. 15). The increase in protein synthesis following denervation is likely important for increasing translation of the large number of mRNA species that are transcriptionally up-regulated following inactivity. Although there was a significant increase (>24%) in protein synthesis after 14 d of denervation in both WT and KO mice, proteolysis, as estimated from the chymotrypsin-like activity of the proteasome, more than doubled in WT and tripled in KO skeletal muscle. On the basis of these data, it appears that net protein balance is shifted to protein breakdown in both WT and KO mice. However, the net protein breakdown in the KO mice must be less than that in the WT mice since loss of muscle mass is significantly less in the MuRF1-KO than WT mice. The important remaining question is what specific proteins are being degraded and synthesized in the WT and KO mice? The SDS-PAGE gel of whole muscle lysates (Supplemental Fig. S2) clearly showed differences in protein expression following denervation in the WT and KO mice. On the basis of the present study, the pathways responsible for the increase in protein synthesis following denervation cannot be identified. It is conceivable that different signaling pathways are driving the increase in synthesis in the WT and KO mice following denervation. For example, in a previous study, we showed that rapamycin treatment for 14 d following denervation does not increase muscle loss, suggesting that mTOR may not be driving the increase in synthesis observed in the WT mice (26). A recent study also suggested that mTOR was not driving the increase in synthesis seen in the diaphragm following denervation (25). A critical question, therefore, is whether protein synthesis in the MuRF1-KO mice is increased following denervation as the result of activation of the mTOR pathway, thus providing a mechanism for the selective muscle sparing.
Transcriptional response to denervation
Differential expression studies have been performed previously on denervated hind limb muscles (27). Unique to this study, however, is the comparison of expression patterns in WT and MuRF1-KO mice, with an emphasis on those genes associated with proteolysis. We found that the expression of most 19S and 20S proteasome subunits (with the exception of the inducible subunits, PSMB8, PSMB9, and PSMB10) was increased after 3 and 14 d of denervation. Although increased mRNA expression of proteasome subunits was evident at 3 d of denervation, increases in 26S and 20S proteasome activity were relatively small: 2 of 6 subunit activities for WT and 1 of 6 activities for KO. At 14 d, however, significant increases in the activity of all 20S and 26S proteasome activities were observed in both WT and MuRF1-KO mice. The explanation for the enhanced activity in the MuRF1-KO mice could be related to a number of factors, one being an increase in the number of proteasomes. Another possibility is a differential change in the expression (both mRNA and protein) of the 20S proteasome regulators, PA28α and PA28β. Recently, overexpression of PA28α was shown to increase β1 and β5 proteasome activities and protect against oxidative stress in neonatal rat ventricular myocytes (28). In this study, we found differential mRNA expression of both PA28 subunits in WT and KO mice, and the protein expression of the PA28α subunit increased more in KO mice than WT mice at 14 d of denervation, suggesting that this activator could play a role in the increase in proteasome activity following denervation, and may be partly responsible for the differences observed between WT and KO mice.
Increases in proteasome activities can also be related to changes in the subunit composition of the 20S core. Substitution of the β1, β2, and β5 subunits with β1i (PSMB9 or LMP-2), β2i (PSMB10 or MECL-1), and β5i (PSMB8 or LMP7) forms a special 20S core proteasome known as the immunoproteasome (29). The immunoproteasome may be capable of selective degradation of modified proteins and is reported to have altered activity (30). Interestingly, mRNA expression of three of the inducible proteasome subunits (PSMB8, PSMB9, and PSMB10) was decreased after 14 d of denervation in WT mice, but was unchanged (PSMB10) or increased (PSMB8, PSMB9) in MuRF1-KO mice (Fig. 5 and Supplemental Table S1). For at least one of the inducible subunits (PSMB10); however, changes in protein expression did not follow changes in mRNA expression. Changes in the expression of the inducible subunits has been noted in several diseases, including diabetes (31) and Huntington's disease (30). In the atrophic diabetic heart, decreases in PSMB9 (LMP-2) and increases in PSMB8 (LMP-7) have been reported. In contrast, isoproterenol-induced hypertrophy in mouse heart showed increased expression of the inducible subunits (PSMB9/LMP-2 and PSMB8/LMP-7; ref. 32). Our data suggest that deletion of MuRF1 leads to an increase in proteasome activity that may be protective against the effects of inactivity and unloading. The role of the inducible subunits and the immunoproteasome in muscle atrophy or sparing of muscle loss following denervation is unknown and requires further investigation.
Increased expression of proteasome subunits and proteins associated with ubiquitination has been shown to occur in multiple catabolic conditions, including denervation (33–35). However, only two deubiquitinating enzymes, ubiquitin-specific peptidase 14 (USP14) and ubiquitin-specific peptidase 19 (USP19), have been shown to be both induced and involved in muscle atrophy (16, 36). USP14 is associated with the proteasome, and inhibition of this enzyme increases proteasome activity. While USP14 mRNA expression levels increased in both KO and WT muscle 3 d after denervation, expression of the gene returned to control levels in KO mice, but it remained elevated in WT mice after 14 d of denervation. Expression of USP19 showed a similar trend as USP14 (Supplemental Table S2). Recently, Sundaram et al. (22) showed that reducing USP19 protein levels by 70–90% in L6 myotubes results in a 20% decrease in the rate of proteolysis and an 18% decrease in the rate of protein synthesis, with no significant change in total protein content. These results are important because they suggest that USP19 can affect protein turnover by modulating both protein breakdown and protein synthesis (22). Further, while overall protein content was unchanged by lowering USP19, protein expression of the major myofibrillar proteins, such as myosin heavy chain, actin, troponin T, and tropomyosin were increased in the myotubes (22). Suppression of USP19 expression, as seen in the MuRF1-KO mice, might be important in the sparing of myofibrillar proteins (and fiber size) in the MuRF1-KO mice following denervation. In fact, SDS-PAGE gels of 14-d muscle lysates revealed bands that were decreased in WT mice, but were not in KO mice, suggesting that some myofibrillar proteins are spared in KO muscle (Supplemental Fig. S2). Using mouse skeletal C2C12 cells, Polge et al. (37) recently demonstrated that recombinant GST-MuRF1 physically interacts with actin in vitro and that MuRF1 can polyubiquitinate actin, leading to its degradation. These results suggest that the lack of MuRF1 might spare the degradation of both thick- and thin-filament proteins, as opposed to the selective sparing of only the thick-filament proteins (24). It is possible that the lack of MuRF1 results in lower degradation rates for many myofibrillar proteins (as also suggested by Supplemental Fig. S2). The present findings highlight the need for further investigation into the identification of the specific substrates of MuRF1.
The microarray results also suggest that other deubiquitinating enzymes may be involved in muscle atrophy. Ubiquitin carboxyl-terminal esterase L1 (UCHL1), which cleaves the peptide bonds formed by the C-terminal glycine of ubiquitin, showed both a delayed and suppressed level of expression in the KO mice relative to WT in response to denervation (see Supplemental Table S2). In addition, USP3, USP4, and USP20, were all found to be up-regulated following denervation (Supplemental Table S2). Increased expression of skeletal muscle ubiquitin-conjugating enzymes (such as UBC-E2G) has previously been reported under catabolic conditions (38, 39). While mRNA expression of most conjugating enzymes was unaltered after 3 d of denervation, several conjugating enzymes (such as UBE3B, UBE3C, UBE2E2, UBE2O, and UBAP2) were differentially regulated in WT compared to KO mice after 14 d of denervation. Examination of other ubiquitin-conjugating enzymes showed similar expression patterns in WT and KO mice following denervation (Supplemental Table S2), revealing that the absence of MuRF1 selectively affects some ubiquitin-conjugating enzymes, mainly by lowering their mRNA expression levels after prolonged denervation (14 d).
Two E3 ubiquitin ligases (MAFXb/FBXO32 and NEDD4) also showed interesting expression patterns in the MuRF1-KO mice. NEDD4 is an E3 ubiquitin ligase, which has been shown to be up-regulated under a variety of atrophy-inducing conditions, including denervation, hind limb unloading, and advanced chronic obstructive pulmonary disease (23, 40). In the MuRF1-KO mice, the microarray data show that up-regulation of NEDD4 is significantly suppressed following denervation. Interestingly, another E3 ubiquitin ligase, MAFbx, showed the opposite pattern. Here we show that MAFbx expression was not suppressed, but in fact remained elevated, in the MuRF1-KO mice following denervation. This result shows that suppression of MAFbx is not a requirement to achieve muscle sparing. In fact, the MAFbx-KO mice reveal that complete suppression of MAFbx under denervation conditions is actually deleterious (see Fig. 1). Further, we recently found sparing of muscle mass in the MuRF1-KO, but not the MAFbx-KO mice, following dexamethasone treatment (12).
CONCLUSIONS
The present findings provide new insights into the signaling pathways through which MuRF1 exerts its muscle-sparing effects. These results clearly demonstrate that the absence of MuRF1 results in a paradoxical increase, not decrease, in proteasome activity at the time when muscle sparing is most obvious. Further, the data suggest that there may be alterations in the composition of the proteasomes, which could alter the specific proteins that are degraded. For the first time, we also demonstrate that MuRF1 may be affecting other components of the ubiquitin system, such as proteasome-associating partners (USP14), ubiquitin-conjugating enzymes, ubiquitin ligases, and ubiquitin-specific peptidases, based on differential gene expression patterns in KO mice. Clearly, more research is required to understand the complex interactions between protein synthesis and degradation in response to various stressors in order to understand the regulation of skeletal muscle mass, including the determination of the molecular mode of action of MuRF1, a critical player in multiple atrophy inducing conditions.
Supplementary Material
Acknowledgments
The authors thank their colleagues at the University of California, Davis, for support and technical expertise.
This research was supported by grants from the Muscular Dystrophy Association (S.C.B.) and the U.S. National Institutes of Health (DK75801 to S.C.B. and J.D.F.; HL096819 to A.V.G.), and partly supported by a Howard Hughes Medical Institute basic science research training program (S.D.), and a Kirchstein National Research Service Award (D.S.W.).
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
- FSR
- fractional synthesis rate
- KO
- knockout
- MAFbx
- muscle atrophy F-box
- MuRF1
- muscle RING finger 1
- PA28
- proteasome activator 28
- TA
- tibialis anterior
- TS
- tricep surae
- UPS
- ubiquitin proteasome system
- WT
- wild type
REFERENCES
- 1. Medina R., Wing S. S., Goldberg A. L. (1995) Increase in levels of polyubiquitin and proteasome mRNA in skeletal muscle during starvation and denervation atrophy. Biochem. J. 307, 631–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Taillandier D., Aurousseau E., Meynial-Denis D., Bechet D., Ferrara M., Cottin P., Ducastaing A., Bigard X., Guezennec C. Y., Schmid H. P., Attaix D. (1996) Coordinate activation of lysosomal, Ca2+-activated and ATP-ubiquitin-dependent proteinases in the unweighted rat soleus muscle. Biochem. J. 316, 65–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Price S. R., Bailey J. L., Wang X., Jurkovitz C., England B. K., Ding X., Phillips L. S., Mitch W. E. (1996) Muscle wasting in insulinopenic rats results from activation of the ATP-dependent, ubiquitin–proteasome proteolytic pathway by a mechanism including gene transcription. J. Clin. Invest. 98, 1703–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Temparis S., Asensi M., Taillandier D., Aurousseau E., Larbaud D., Obled A., Bechet D., Ferrara M., Estrela J. M., Attaix D. (1994) Increased ATP-ubiquitin-dependent proteolysis in skeletal muscles of tumor-bearing rats. Cancer Res. 54, 5568–5573 [PubMed] [Google Scholar]
- 5. Baracos V. E., DeVivo C., Hoyle D. H., Goldberg A. L. (1995) Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. 268, E996–E10067539218 [Google Scholar]
- 6. Tiao G., Hobler S., Wang J. J., Meyer T. A., Luchette F. A., Fischer J. E., Hasselgren P. O. (1997) Sepsis is associated with increased mRNAs of the ubiquitin-proteasome proteolytic pathway in human skeletal muscle. J. Clin. Invest. 99, 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Voisin L., Breuille D., Combaret L., Pouyet C., Taillandier D., Aurousseau E., Obled C., Attaix D. (1996) Muscle wasting in a rat model of long-lasting sepsis results from the activation of lysosomal, Ca2+-activated, and ubiquitin-proteasome proteolytic pathways. J. Clin. Invest. 97, 1610–1617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mitch W. E., Medina R., Grieber S., May R. C., England B. K., Price S. R., Bailey J. L., Goldberg A. L. (1994) Metabolic acidosis stimulates muscle protein degradation by activating the adenosine triphosphate-dependent pathway involving ubiquitin and proteasomes. J. Clin. Invest. 93, 2127–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bodine S. C., Latres E., Baumhueter S., Lai V. K., Nunez L., Clarke B. A., Poueymirou W. T., Panaro F. J., Na E., Dharmarajan K., Pan Z. Q., Valenzuela D. M., DeChiara T. M., Stitt T. N., Yancopoulos G. D., Glass D. J. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 [DOI] [PubMed] [Google Scholar]
- 10. Gomes M. D., Lecker S. H., Jagoe R. T., Navon A., Goldberg A. L. (2001) Identification of ubiquitin ligases required for skeletal muscle atrophy. Proc. Natl. Acad. Sci. U. S. A. 98, 14440–14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Labeit S., Kohl C. H., Witt C. C., Labeit D., Jung J., Granzier H. (2010) Modulation of muscle atrophy, fatigue and MLC phosphorylation by MuRF1 as indicated by hindlimb suspension studies on MuRF1-KO mice. J. Biomed. Biotechnol. 2010, 693741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baehr L. M., Furlow J. D., Bodine S. C. (2011) Muscle sparing in muscle RING finger 1 null mice: response to synthetic glucocorticoids. J. Physiol. 589, 4759–4776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hirner S., Krohne C., Schuster A., Hoffmann S., Witt S., Erber R., Sticht C., Gasch A., Labeit S., Labeit D. (2008) J. Mol. Biol. 379, 666–677 [DOI] [PubMed] [Google Scholar]
- 14. Koyama S., Hata S., Witt C. C., Ono Y., Lerche S., Ojima K., Chiba T., Doi N., Kitamura F., Tanaka K., Abe K., Witt S. H., Rybin V., Gasch A., Franz T., Labeit S., Sorimachi H. (2008) MuRF1-dependent regulation of systemic carbohydrate metabolism as revealed from transgenic mouse studies. J. Mol. Biol. 376, 1224–1236 [DOI] [PubMed] [Google Scholar]
- 15. Ochala J., Gustafson A. M., Diez M. L., Renaud G., Li M., Aare S., Qaisar R., Banduseela V. C., Hedstrom Y., Tang X., Dworkin B., Ford G. C., Nair K. S., Perera S., Gautel M., Larsson L. (2011) Preferential skeletal muscle myosin loss in response to mechanical silencing in a novel rat intensive care unit model: underlying mechanisms. J. Physiol. 589, 2007–2026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lecker S. H., Jagoe R. T., Gilbert A., Gomes M., Baracos V., Bailey J., Price S. R., Mitch W. E., Goldberg A. L. (2004) Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 [DOI] [PubMed] [Google Scholar]
- 17. Ikemoto M., Nikawa T., Takeda S., Watanabe C., Kitano T., Baldwin K. M., Izumi R., Nonaka I., Towatari T., Teshima S., Rokutan K., Kishi K. (2001) Space shuttle flight (STS-90) enhances degradation of rat myosin heavy chain in association with activation of ubiquitin-proteasome pathway. FASEB J. 15, 1279–1281 [DOI] [PubMed] [Google Scholar]
- 18. Gomes A. V., Young G. W., Wang Y., Zong C., Eghbali M., Drews O., Lu H., Stefani E., Ping P. (2009) Contrasting proteome biology and functional heterogeneity of the 20 S proteasome complexes in mammalian tissues. Mol. Cell. Proteomics 8, 302–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Anderson S. R., Gilge D. A., Steiber A. L., Previs S. F. (2008) Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metabolism 57, 347–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. McCabe B. J., Bederman I. R., Croniger C., Millward C., Norment C., Previs S. F. (2006) Reproducibility of gas chromatography-mass spectrometry measurements of 2H labeling of water: application for measuring body composition in mice. Anal. Biochem. 350, 171–176 [DOI] [PubMed] [Google Scholar]
- 21. Dufner D. A., Bederman I. R., Brunengraber D. Z., Rachdaoui N., Ismail-Beigi F., Siegfried B. A., Kimball S. R., Previs S. F. (2005) Using 2H2O to study the influence of feeding on protein synthesis: effect of isotope equilibration in vivo vs. in cell culture. Am. J. Physiol. Endocrinol. Metab. 288, E1277–E1283 [DOI] [PubMed] [Google Scholar]
- 22. Sundaram P., Pang Z., Miao M., Yu L., Wing S. S. (2009) USP19-deubiquitinating enzyme regulates levels of major myofibrillar proteins in L6 muscle cells. Am. J. Physiol. Endocrinol. Metab. 297, E1283–E1290 [DOI] [PubMed] [Google Scholar]
- 23. Koncarevic A., Jackman R. W., Kandarian S. C. (2007) The ubiquitin-protein ligase Nedd4 targets Notch1 in skeletal muscle and distinguishes the subset of atrophies caused by reduced muscle tension. FASEB J. 21, 427–437 [DOI] [PubMed] [Google Scholar]
- 24. Cohen S., Brault J. J., Gygi S. P., Glass D. J., Valenzuela D. M., Gartner C., Latres E., Goldberg A. L. (2009) During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell. Biol. 185, 1083–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Argadine H. M., Mantilla C. B., Zhan W. Z., Sieck G. C. Intracellular signaling pathways regulating net protein balance following diaphragm muscle denervation. Am. J. Physiol. Cell Physiol 300, C318–C327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kline W. O., Panaro F. J., Yang H., Bodine S. C. (2007) Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J. Appl. Physiol. 102, 740–747 [DOI] [PubMed] [Google Scholar]
- 27. Batt J., Bain J., Goncalves J., Michalski B., Plant P., Fahnestock M., Woodgett J. (2006) Differential gene expression profiling of short and long term denervated muscle. FASEB J. 20, 115–117 [DOI] [PubMed] [Google Scholar]
- 28. Li J., Powell S. R., Wang X. (2011) FASEB J. 25, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yewdell J. W. (2005) Immunoproteasomes: regulating the regulator. Proc. Natl. Acad. Sci. U. S. A. 102, 9089–9090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diaz-Hernandez M., Hernandez F., Martin-Aparicio E., Gomez-Ramos P., Moran M. A., Castano J. G., Ferrer I., Avila J., Lucas J. J. (2003) Neuronal induction of the immunoproteasome in Huntington's disease. J. Neurosci. 23, 11653–11661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dewey S., Gomes A. V. Non-antigen processing immunoproteasomes in diabetic hearts? J. Mol. Cell. Cardiol. 49, 1–4 [DOI] [PubMed] [Google Scholar]
- 32. Drews O., Tsukamoto O., Liem D., Streicher J., Wang Y., Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ. Res. 107, 1094–1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tiao G., Fagan J., Roegner V., Lieberman M., Wang J. J., Fischer J. E., Hasselgren P. O. (1996) Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J. Clin. Invest. 97, 339–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wing S. S., Haas A. L., Goldberg A. L. (1995) Increase in ubiquitin-protein conjugates concomitant with the increase in proteolysis in rat skeletal muscle during starvation and atrophy denervation. Biochem. J. 307, 639–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baracos V. E., DeVivo C., Hoyle D. H., Goldberg A. L. (1995) Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. Endocrinol. Metab. 268, E996–E1006 [Google Scholar]
- 36. Combaret L., Adegoke O. A., Bedard N., Baracos V. D., Wing S. S. (2005) USP19 is a ubiquitin-specific protease regulated in rat skeletal muscle during catabolic states. Am. J. Physiol. Endocrinol. Metab. 288, E693–E700 [DOI] [PubMed] [Google Scholar]
- 37. Polge C., Heng A. E., Jarzaguet M., Ventadour S., Claustre A., Combaret L., Bechet D., Matondo M., Uttenweiler-Joseph S., Monsarrat B., Attaix D., Taillandier D. (2011) Muscle actin is polyubiquitinylated in vitro and in vivo and targeted for breakdown by the E3 ligase MuRF1. FASEB J. 25, 3790–3802 [DOI] [PubMed] [Google Scholar]
- 38. Chrysis D., Underwood L. E. (1999) Regulation of components of the ubiquitin system by insulin-like growth factor I and growth hormone in skeletal muscle of rats made catabolic with dexamethasone. Endocrinology 140, 5635–5641 [DOI] [PubMed] [Google Scholar]
- 39. Lorite M. J., Smith H. J., Arnold J. A., Morris A., Thompson M. G., Tisdale M. J. (2001) Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Brit. J. Cancer 85, 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Plant P. J., Brooks D., Faughnan M., Bayley T., Bain J., Singer L., Correa J., Pearce D., Binnie M., Batt J. (2010) Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 42, 461–471 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.