Abstract
A major therapeutic target for Parkinson's disease (PD) is providing increased glial-derived neurotrophic factor (GDNF) to dopaminergic neurons. We tested the hypothesis that innate immune activation increases astrocyte GDNF production and that this is regulated by specific eicosanoid receptors. Innate immune-activated primary murine astrocytes were assayed for GDNF expression and secretion. Controls were agent vehicle exposure and wild-type mice. Rank order for up to 10-fold selectively increased GDNF expression was activators of TLR3 > TLR2 or TLR4 > TLR9. TLR3 activator-stimulated GDNF expression was selectively JNK-dependent, followed cyclooxygenase (COX)-2, was coincident with membranous PGE2 synthase, and was not significantly altered by a nonspecific COX- or a COX-2-selective inhibitor. Specific eicosanoid receptors had opposing effects on TLR3 activator-induced GDNF expression: ∼60% enhancement by blocking or ablating of PGE2 receptor subtype 1 (EP1), ∼30% enhancement by activating PGF2α receptor or thromboxane receptor, or ∼15% enhancement by activating EP4. These results demonstrate functionally antagonistic eicosanoid receptor subtype regulation of innate immunity-induced astrocyte GDNF expression and suggest that selective inhibition of EP1 signaling might be a means to augment astrocyte GDNF secretion in the context of innate immune activation in diseased regions of brain in PD.—Li, X., Cudaback, E., Breyer, R. M., Montine, K. S., Keene, C. D., Montine, T. J. Eicosanoid receptor subtype-mediated opposing regulation of Toll-like receptor-stimulated expression of astrocyte glial-derived neurotrophic factor.
Keywords: PGE2, EP1, FP, TP, Parkinson's disease
Innate immunity consists of those aspects of the immune response that are usually defined as local actions and do not require genetic restriction and clonal expansion. Major cellular effectors of the innate immune response are tissue macrophages, although parenchymal cells participate as well. One critical function of innate immunity is surveillance that is accomplished by pattern recognition receptors; key among these are the Toll-like receptors (TLRs), a family of at least 10 receptors that are coupled to signal transduction cascades that lead to transcriptional activation of several genes, importantly including cyclooxygenase (COX)-2. Originally described from the perspective of microorganisms, now a variety of endogenous TLR ligands produced in chronic “sterile” disease states are also recognized. These include some heat-shock proteins, hyaluronan, stathmin (a ubiquitous regulator of microtubule disassembly), oxidized lipoproteins, some aggregated forms of amyloid-β peptide, α-synuclein, and molecules released by degenerating cells (1–3). These latter endogenous activators have led to interest in innate immune activation as a potential pathogenic contributor and therapeutic target in neurodegenerative diseases, especially Alzheimer's disease (AD; ref. 4) and Parkinson's disease (PD; refs. 5–9).
Immune activation in brain can have both beneficial and deleterious effects. For example, microglia express several TLRs, and activation of TLR3 or TLR4 can lead to microglial-mediated neurotoxicity through elaboration of a combination of cytokines, chemokines, and free radical injury, which is dependent on specific prostaglandin E2 (PGE2) receptor subtypes (10). In contrast to neuronal damage from activation of microglial TLRs, activation of astrocyte TLR3 or TLR4 leads to increased secretion of several neurotrophic factors, including brain-derived neurotrophic factor (BDNF) and vascular endothelial growth factor (VEGF) (11); glial-derived neurotrophic factor (GDNF) was not included in these studies. One study has shown increased GDNF mRNA after LPS activation; however, this was not validated with assays of GDNF secretion (12). Indeed, others reported no change in GDNF secretion after activation of astrocyte TLR4 (13). In contrast, with use of an immortalized glioblastoma cell line, PGE2 exposure induced secretion of GDNF (14), an effect that could be mimicked by exposure to phorbol 12,13-didecanoate, okadaic acid, nitric oxide donors, or hydrogen peroxide (15); however, these studies did not assay expression. Thus, it remains unclear whether innate immune activation of astrocytes leads to increased expression or secretion of GDNF and whether these processes are or are not regulated by PGE2-mediated signaling.
Mature nigral dopaminergic neurons, the major target of neurodegeneration in PD and also affected in AD, are exquisitely dependent on GDNF (16–18). Indeed, after extensive successful preclinical testing in experimental toxicant models of PD that included nonhuman primates (19–23), clinical trials using putaminal infusion of GDNF in patients with PD have failed to show consistent therapeutic efficacy (24–26) despite initially promising results (27). The situation is further complicated by untoward effects in some studies on nonhuman primates, one group reporting cerebellar toxicity after striatal targeting of recombinant GDNF infusion (28) and another reporting unexpected weight loss with virally driven GDNF expression in substantia nigra (29). Although great care will be needed going forward because of potential side effects, the compelling preclinical results with GDNF in models of PD continue to motivate investigators to develop alternative approaches for increased local production or optimized delivery of GDNF to diseased regions of brain (30–41). Here we first tested the hypothesis that expression and secretion of GDNF are modulated by activation of different TLRs expressed by astrocytes. Even if innate immune activation increased astrocyte GDNF secretion, it is presumably insufficient in diseased regions of brain of patients with PD. Therefore, we also sought to determine the signaling mechanisms that regulate astrocyte GDNF expression and secretion in the context of innate immune activation.
MATERIALS AND METHODS
Materials
DMEM/F12 medium and FBS were purchased from HyClone Laboratories (Logan, UT, USA). G5 supplement was from Invitrogen (Carlsbad, CA, USA). SC-51089, NS-398, 17-phenyl trinor PGF2α, sulprostone, CAY10598, ZK118182, and U-46619 were from Cayman Chemical Company (Ann Arbor, MI, USA). 2-Aminoethoxy-diphenyl borate (2-APB) was from Tocris Bioscience (Ellisville, MO, USA). Double-stranded polyinosinic-polycytidylic acid (PIC; activates TLR3) was from Sigma-Aldrich (St. Louis, MO, USA). LPS (activates TLR4), SP600125, SB202190, U0126, and BAY11-7085 were from Calbiochem (La Jolla, CA, USA). Pam3Cys-Ser-(Lys)4 (Pam3; activates TLR2) and CpG (activates TLR9) were from Invivogen (San Diego, CA, USA). Papain and DNase I were from Worthington Biochemicals (Lakewood, NJ, USA).
Animals
All procedures were approved by the University of Washington institutional animal care and use committee. C57BL/6 mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). PGE2 receptor subtype 1 (EP1)−/− mice on the C57BL/6 background were generated as described previously (42). The animals were maintained in a specific pathogen-free environment.
Cell culture
Primary glial culture was performed as described previously (43). After the flasks were shaken 3 times to remove the microglia, the underlying astrocytes were subcultured by replating at 4 × 105 cells/well in 6-well plates coated with polyornithine. Cells reaching confluence at 3 d after subculture were treated with individual reagents.
RT-PCR and quantitative real-time PCR
Total RNA extraction, cDNA synthesis, RT-PCR, and quantitative real-time PCR were performed as described previously (43). Primer sequences for RT-PCR are listed in Table 1. Primers and probes for quantitative real-time PCR were purchased from Applied Biosystems (Carlsbad, CA, USA).
Table 1.
Sequences of primers used for RT-PCR
| Gene | Sense primer, 5′-3′ | Antisense primer, 5′-3′ |
|---|---|---|
| COX2 | GCTGTACAAGCAGTGGCAAA | CCCCAAAGATAGCATCTGGA |
| mPGEs | GGCCTTTCTGCTCTGCAGCA | GGAGAACTGGGCCAGGACAT |
| GDNF | AAAGTAGGCCAGGCATGTTG | TTCGCACTGTAGCAGGAATG |
| NGF | CATGGGGGAGTTCTCAGTGT | GCACCCACTCTCAACAGGAT |
| BDNF | TAATGCAGCATGATGGGAAA | TCACAGTGAAAGCACCTTGC |
| VEGF | AGCACAGCAGATGTGAATGC | AATGCTTTCTCCGCTCTGAA |
| PGFS | GTTGGTATCCAGGGGAACCT | CCTCCCACACACCTCTTCAT |
| TXAS | GAGGTGCTGGGACAACGTAT | AAAGGGCAGGTATGTGAACG |
| PAI-1 | AAGTCTTTCCGACCAAGAGCA | ATCACTTGCCCCATGAAGAG |
| GAPDH | GACAAAATGGTGAAGGTCGGTG | TGATGTTAGTGGGGTCTCGCTC |
GDNF ELISA assay
Confluent astrocytes were exposed to reagents in serum-free medium with G5 supplement. Conditioned media were collected for GDNF measurement by ELISA (Promega, Madison, WI, USA) according to the manufacturer's instructions.
Western blot analysis
Cell lysates were prepared in RIPA buffer. Then 15 μg of total protein was loaded and separated by 4-12% Bis-Tris gel (Invitrogen), followed by transfer to PVDF membrane. The membrane was then blotted with phosphorylated (P)-JNK, P-p38, P-ERK, and IκBα antibodies (Cell Signaling, Danvers, MA, USA). The proteins of interest were detected with ECL reagents (Thermo Fisher Scientific, Rockford, IL, USA).
Statistical analyses
Values are expressed as means ± se. Group comparisons with unpaired t tests or ANOVA with Bonferroni-corrected posttests were performed using GraphPad Prism 5 (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
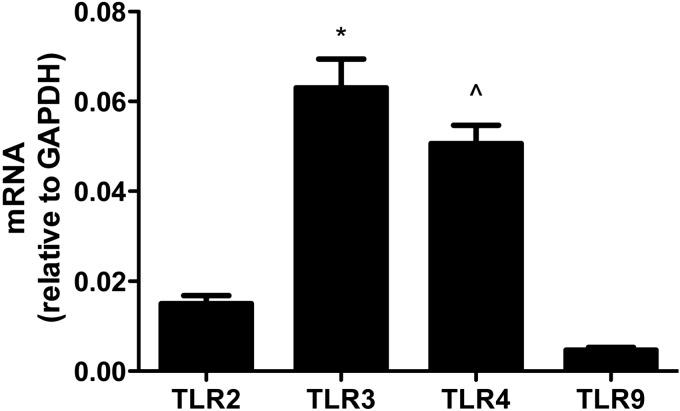
Here we used a panel of TLR activators to model stimulation of innate immune response by astrocytes: Pam3 for TLR2, PIC for TLR3, LPS for TLR4, and CpG for TLR9 (44). We confirmed the results of others (45) by showing that each of these TLRs was expressed by our primary wild-type (WT) murine astrocyte cultures (Fig. 1).
Figure 1.
TLR expression by WT murine primary astrocytes. WT astrocytes (4×105 cells from cultures in 6-well plates) were harvested after 3 d, and RNA was extracted for real-time PCR of the different TLRs shown. TLR mRNA level in each sample was normalized to endogenous GAPDH. Data are average ± se normalized levels (n=9 cultures/TLR). ANOVA had P < 0.0001; Bonferroni-corrected posttests for paired comparisons were significant for TLR3 vs. TLR2 or TLR9 and for TLR4 vs. TLR2 or TLR9, but not for TLR2 vs. TLR9 or TLR3 vs. TLR4. *P < 0.001, ∧P < 0.001 vs. TLR2 and TLR9; Bonferroni posttest.
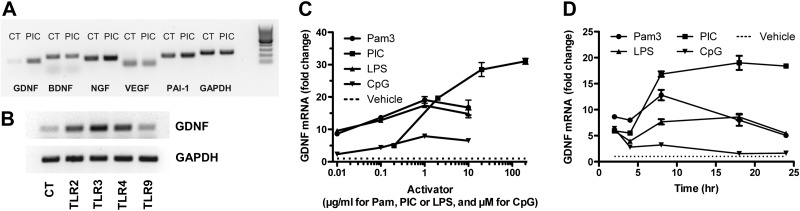
Figure 2 presents results of a survey of TLR activators and expression of astrocyte neurotrophic factors in murine primary cultures. Figure 2A shows a representative gel of PCR-amplified mRNA for GDNF, BDNF, nerve growth factor (NGF), VEGF, and plasminogen activator inhibitor (PAI)-1, a pleiotropic protein with actions that include modulating proteolysis of pro-BDNF to BDNF (46). PIC selectively induced expression of GDNF among this group of astrocyte-derived factors. We next surveyed activation of multiple TLRs expressed by astrocytes and demonstrated that PIC yielded the greatest increase in GDNF expression (Fig. 2B). We then performed concentration (Fig. 2C) and time dependence (Fig. 2D) experiments with commonly used concentration ranges of the four TLR activators. Although direct comparisons of the different TLR activators are limited, our findings extended the results of others who surveyed the effect of TLR activators on expression of different trophic and anti-inflammatory factors and showed that PIC yielded the greatest induction (11).
Figure 2.
Induced expression of neurotrophic factors by TLR activators. A) WT murine primary astrocytes were incubated with 20 μg/ml PIC for 18 h. RNA was isolated, and PCR was performed for expression of GDNF, NGF, BDNF, VEGF, and PAI-1; GAPDH is shown as loading control (CT). B) Example of PCR for GDNF expression after 18 h incubation with 20 μg/ml PIC using GAPDH as a loading control. C) WT astrocytes treated with varying concentrations of an activator of TLR2 (Pam), TLR3 (PIC), TLR4 (LPS), or TLR9 (CpG) for 8 h, after which RNA was extracted for real-time PCR. GDNF mRNA level in each sample was normalized to endogenous GAPDH. Data are presented as average ± se fold change relative to cultures exposed to vehicle without TLR activator (dotted line) vs. concentration of activator (n=3 at each point). D) WT astrocytes were treated with 1 μg/ml Pam, 20 μg/ml PIC, 1 μg/ml LPS, or 1 μM CpG for 2, 4, 8, 18, or 24 h, at which time RNA was extracted for real-time PCR. GDNF mRNA level in each sample was normalized to endogenous GAPDH. Data are presented as the average ± se fold change relative to cultures exposed to vehicle without TLR activator (dotted line) vs. time (n=3 at each point).
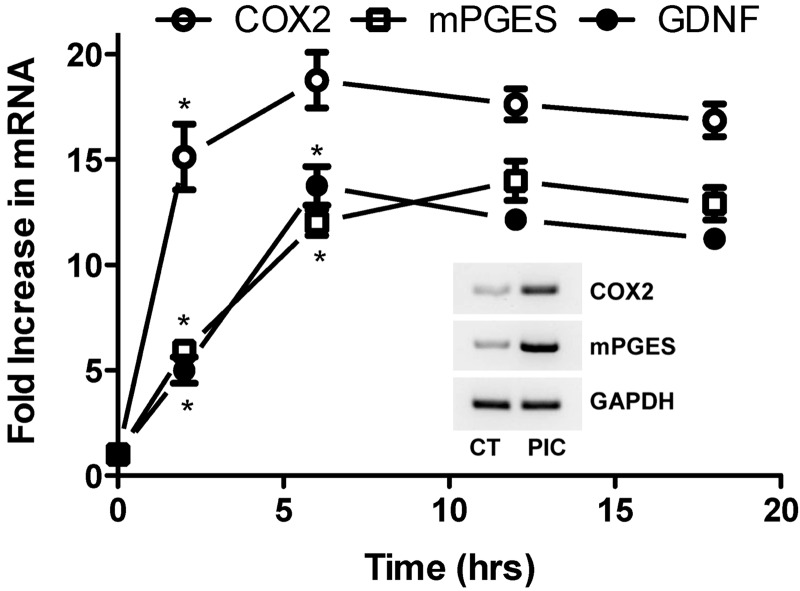
Increased expression of COX-2 and eicosanoid synthases with the resulting production of PGs and thromboxane (Tx) A2 is a critical feature in cellular signaling that follows activation of TLRs. We examined the temporal relationship of PIC-induced expression of GDNF with COX-2 and membranous PGE2 synthase (mPGES) because of the important role of PGE2 in innate immune activation for other cell types (Fig. 3). As expected, PIC exposure led to early and strong induction of COX-2 that peaked at ∼2 h and maintained increased levels up to our last time point at 18 h. A somewhat delayed but similarly strong induction of mPGES peaked at ∼6 h and maintained elevated levels until 18 h, a time course that nearly perfectly mirrored that of GDNF (see Fig. 3 inset for representative RT-PCR).
Figure 3.
Induction of GDNF, COX-2, and mPGES expression induced by TLR3 activation. Time course in WT murine primary astrocytes incubated with 20 μg/ml PIC for indicated times is shown. RNA was isolated, and quantitative real-time PCR was performed for GDNF, COX-2, and mPGES expression. Results were normalized to 18s rRNA and are presented as average ± se (n=3) fold increase relative to values at time 0 for each mRNA. Inset: example of PCR for COX-2 and mPGES expression after an 18-h PIC or vehicle (CT) incubation with GAPDH as the loading control. ANOVA for each mRNA vs. time had P < 0.0001. Bonferroni-corrected posttests for paired comparisons with the previous time point were significant for COX-2 mRNA at 2 h, and mPGES and GDNF mRNA at 2 and 6 h, but P > 0.05 for all other time points. *P < 0.01 vs. previous time point; Bonferroni posttest.
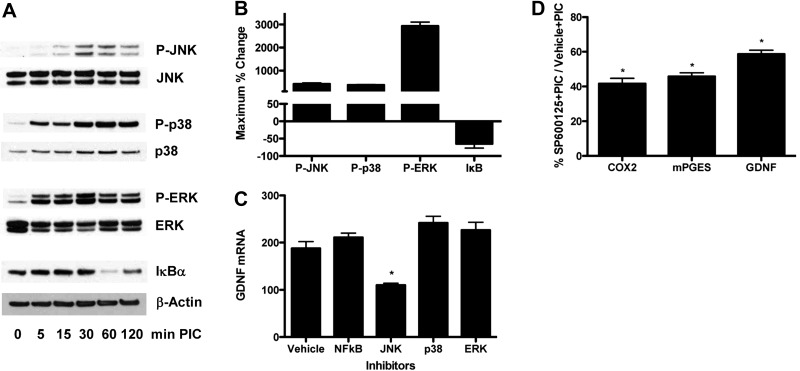
We next surveyed potential second messenger signaling pathways to determine those that may be responsible for increased expression of GDNF in primary astrocytes after TLR3 activator (Fig. 4). Western blots of primary astrocyte extracts showed increased phosphorylation of JNK, p38, and ERK as well as degradation of IκBα, all indicative of activation, albeit with different time courses (Fig. 4A) and extents of phosphorylation (Fig. 4B). We sought to determine cause-and-effect relationships using inhibitors of each of these signaling pathways. Only the inhibitor of JNK signaling (SP600125) partially suppressed PIC induction of astrocyte GDNF expression; inhibition of p38 with SB202190, ERK with U0126, or NF-κB with BAY11-7085 did not alter PIC-induced expression of GDNF (Fig. 4C). We also observed that PIC-induced expression of COX-2 and mPGES was similarly inhibited by 10 μM SP600125 (Fig. 4D; vehicle plus PIC=100%), further correlating PGE2 signaling and increased GDNF expression in primary astrocytes after TLR3 activation. Higher concentrations of SP600125 (20 μM) initiated cell death (data not shown).
Figure 4.
JNK signaling selectively but partially mediates PIC-induced GDNF expression and PG pathway activation. A) WT murine primary astrocytes were incubated with 20 μg/ml PIC for up to 120 min, sampled at the times shown, and analyzed by Western blot for activation of JNK, p38, and ERK by assaying phosphorylation and IκBα by assaying its degradation. Respective blots were stripped and then reprobed with JNK, p38, ERK, or β-actin antibody. B) Average ± se maximum percentage change from experiments in panel A was calculated by densitometric scanning of blots. C, D) WT murine primary astrocytes were pretreated with vehicle or inhibitor of JNK (SP600125, 10 μM), p38 (SB202190, 10 μM), ERK (U0126, 10 μM), or NF-κB (BAY11-7085, 2 μM) for 30 min, followed by addition of 20 μg/ml PIC for an additional 18 h. RNA was isolated, and quantitative real-time PCR was performed, and results were normalized to 18s rRNA. C) Expression of GDNF presented as average ± se (n=6). ANOVA had P < 0.0001. Bonferroni-corrected paired comparison was significant for JNK inhibitor vs. all other inhibitors or vehicle. Vehicle vs. all other inhibitors had P > 0.05. *P < 0.001; Bonferroni posttest. D) Expression of COX-2, mPGES, and GDNF presented as average percentage of cultures exposed to vehicle (100%) rather than JNK inhibitor (SP600125) before TLR3 activation with PIC. Unpaired t tests were performed on raw data rather than percentages. *P < 0.001.
So far, our results showed that TLR3 activation leads to selective JNK-dependent increased expression of GDNF and that increased GDNF expression follows COX-2 induction but is coincident with induced expression of mPGES. Our next step was to investigate cause-and-effect relationships between PIC-induced GDNF expression and PGE2 signaling in murine primary astrocytes. Initial experiments used a nonspecific COX inhibitor (ibuprofen) and a relatively selective COX-2 inhibitor (NS-398). In both cases, COX inhibitors tended to increase TLR3-induced GDNF expression by ∼10−15%; however, this change was not statistically significant.
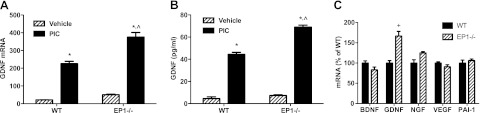
Next, we investigated the contribution of EP1 (Fig. 5). Primary murine astrocytes prepared from EP1−/− mice had an ∼60% increase in TLR3-induced GDNF expression compared with cultures prepared from WT mice (Fig. 5A). Furthermore, ablation of EP1 did not significantly alter baseline GDNF expression or secretion by astrocytes; however, secretion of GDNF was comparably increased in EP1−/− astrocytes exposed to PIC (Fig. 5B). Enhancement of TLR3-induced expression in EP1−/− astrocytes was specific to GDNF and not observed with the other neurotrophic factors investigated: NGF, BDNF, VEGF, or PAI-1 (Fig. 5C). Next, we repeated our experiments with WT primary murine astrocytes using an EP1 antagonist (Table 2). TLR3-induced GDNF expression, and secretion was significantly increased to 193 and 200% of vehicle control (P<0.001 for each) in the presence of EP1 antagonist. EP1 antagonist also suppressed TLR3-induction of COX-2 and mPGES expression to 47 and 62% of vehicle control, respectively (P<0.001 for each). Because EP1 messenger signaling is linked to liberation of intracellular calcium ions, we sought to validate our findings by using 2-APB, an antagonist of the IP3 receptor that also acts to increase intracellular calcium ions (Table 2). Results with 2-APB mirrored those with EP1 antagonist by significantly increasing TLR3-induced GDNF expression and secretion by 186 and 182%, respectively (P<0.001 for each), and suppressing COX-2 and mPGES expression to 56% (P<0.001) and 70% (P<0.01), respectively.
Figure 5.
Genetic ablation of EP1 receptor selectively enhances TLR3-induced GDNF expression and secretion. A, B). WT and EP1−/− murine primary astrocytes were incubated with 20 μg/ml PIC or vehicle for 18 h, and GDNF mRNA (A) and protein (B) levels were determined by quantitative real-time PCR and ELISA (n=6/group, average±se). Two-way ANOVA had P < 0.001 for genotype, exposure, and interaction. Bonferroni-corrected posttests were significant for PIC vs. vehicle for both WT and EP1−/−, and for WT vs. EP1−/− with PIC, but P > 0.05 for WT vs. EP1−/− with vehicle. C) WT and EP1−/− murine primary astrocytes were incubated with 20 μg/ml PIC for 18 h (n=5 or 6/group, average±sem). Two-way ANOVA had P < 0.0001 for astrocyte genotype, trophic factor mRNA, and interaction. Bonferroni-corrected paired comparisons between astrocyte genotypes were significant for GDNF and P > 0.05 for all other trophic factors. *P < 0.001 vs. vehicle, ∧,+P < 0.001 vs. WT, +P < 0.001 vs. WT; Bonferroni posttest.
Table 2.
EP1 antagonist suppresses TLR3-induced expression of COX2 and mPGES and enhances TLR3-induced expression and secretion of GDNF in primary murine astrocytes
| Antagonist | COX-2 mRNA | mPGES mRNA | GDNF mRNA | Secreted GDNF |
|---|---|---|---|---|
| SC51089 (EP1) | 46.9 + 7.6*,∧ | 61.9 + 1.7+,∧ | 193.0 + 12.0*,∧ | 199.8 + 7.6*,∧ |
| 2-APB (IP3 receptor) | 56.2 + 6.7*,∧ | 69.5 + 5.1+,# | 185.7 + 4.2*,∧ | 181.9 + 17.0* |
WT murine primary astrocytes were pretreated with vehicle, EP1 antagonist SC51089 (60 μM), or IP3 receptor antagonist 2-APB (20 μM) for 30 min, followed by incubation with 20 μg/ml PIC in the presence of vehicle or the antagonists for an additional 12 h (COX-2 and mPGES mRNA) or 18 h (GDNF mRNA and secreted protein). All data are average ± se percentage of vehicle-exposed cultures (n=6 cultures/group, including the corresponding 12- and 18-h vehicle-exposed cultures). Levels of mRNA were determined by quantitative real-time PCR and normalized to 18s rRNA. Medium GDNF concentration was determined by ELISA. ANOVA was significant for vehicle control vs. SC51089 and 2-APB groups at each endpoint. Bonferroni-corrected paired comparisons were significant for vehicle vs. SC51089 and 2APB at all endpoints but nonsignifcant for all paired comparisons of SC51089- and 2APB-exposed groups.
P < 0.0001,
P < 0.001 vs. vehicle at endpoint; ANOVA.
P < 0.001,
P < 0.01 vs. vehicle at endpoint; Bonferroni posttest.
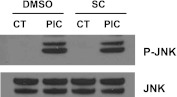
One mechanism by which EP1 signaling might suppress TLR3-induced expression and secretion of GDNF is by altering JNK activation. To test this possibility, we determined levels of P-JNK in WT primary murine astrocytes after TLR3 activation in the presence of EP1 antagonist or vehicle. Figure 6 is a representative Western blot that shows no detectable change in levels of P-JNK caused by EP1 antagonist. Although activation of JNK is necessary for full induction of astrocyte GDNF expression and secretion by TLR3 activator, these results suggest that EP1-mediated suppression of GDNF expression and secretion is downstream of JNK activation.
Figure 6.
EP1 antagonist does not alter PIC-induced JNK activation. WT murine primary astrocytes were pretreated with vehicle (DMSO) or EP1 antagonist SC51089 (SC, 60 μM) for 30 min, followed by addition of 20 μg/ml PIC for an additional 30 min. Immunoblots were performed for total and phosphorylated (P)-JNK.
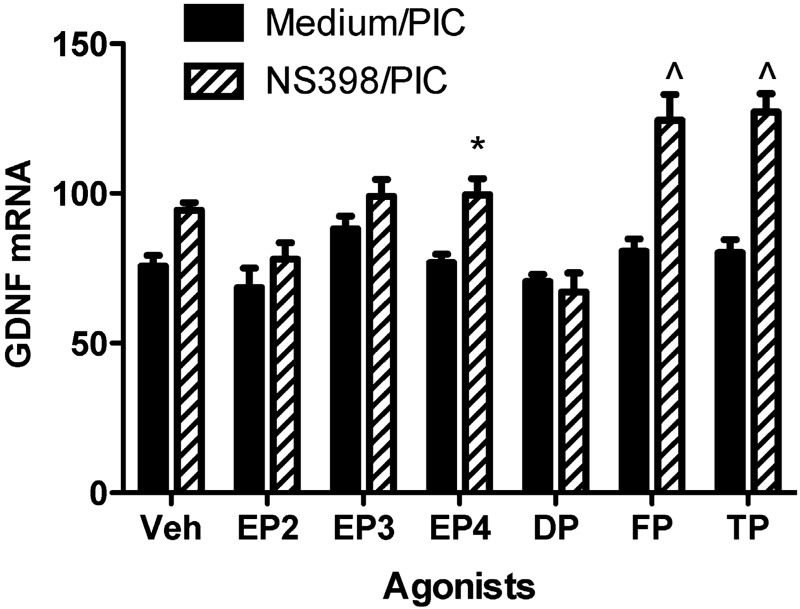
A possible explanation for our results is that COX-2 inhibitors might block both suppressors and enhancers of astrocyte GDNF expression after exposure to TLR3 activator and thereby yield no significant net effect. If true, then EP1 signaling acts as a COX-dependent suppressor of PIC-induced GDNF expression, and blocking EP1 unveiled unknown COX-dependent enhancers. We tested this hypothesis by treating cells with a COX-2-selective inhibitor to block all COX-2-dependent enhancers or suppressors of GDNF expression and then exposed the astrocytes to PIC plus agonists of specific eicosanoid receptors (Fig. 7). As mentioned above, our results showed that COX-2 inhibition yielded an ∼15% increase in PIC-stimulated GDNF expression; however, this was not statistically significant, suggesting that any potential COX-2-dependent enhancers of GDNF expression were roughly balanced with EP1-dependent suppression. Next, we surveyed selective agonists of eicosanoid receptors, including other EP receptor subtypes, PGD2 receptor (DP), PGF receptor (FP), and Tx receptor (TP). We discovered that selective agonists of FP or TP receptors substantially enhanced TLR3-induced GDNF expression by primary murine astrocytes (P<0.001 for each), whereas an agonist of EP4 receptor had a smaller but still significant (P<0.05) enhancement. We repeated these experiments with a nonselective COX inhibitor (indomethacin). Under these conditions, there was again no significant enhancement of TLR3-induced GDNF expression similar to the COX-2 inhibitor (data not shown); however, in contrast to COX-2 inhibition, none of the eicosanoid receptor agonists, including those for FP and TP, significantly enhanced GDNF expression in the presence of a nonselective COX inhibitor. Finally, unlike mPGES, TLR3 activation did not alter expression of synthases for Tx A2 (TXAS)or PGF2α (PGFS) (data not shown).
Figure 7.
Agonists of EP4, FP, or TP receptor selectively enhance PIC-induced GDNF expression. WT murine primary astrocytes were pretreated with medium or COX-2 inhibitor (NS398, 50 μM) for 30 min, followed by incubation with 20 μg/ml PIC in the presence of vehicle (veh) or agonist of EP2 (butaprost, 20 μM), EP3 (sulprostone, 10 μM), EP4 (CAY10598, 10 μM), DP (ZK118182, 10 μM), FP (17-phenyl trinor PGF2α, 10 μM), or TP (U-46619, 10 μM) for an additional 18 h. GDNF mRNA level was quantified by real-time PCR. Results were normalized to 18s rRNA and are presented as average ± se (n=6). Two-way ANOVA had P < 0.0001 for medium/PIC vs. NS398/PIC, for comparison among the agonists and for interaction between these dimensions. Bonferroni-corrected paired comparison posttests were significant for N398/PIC + veh vs. NS398/PIC + FP, N398/PIC + veh vs. NS398/PIC + TP, and NS398/PIC + veh vs. NS398/PIC + EP4, and P > 0.05 for all other paired comparisons, including medium/PIC + veh vs. NS398/PIC + veh. *P < 0.05, ∧P < 0.001 vs. NS398/PIC + veh; Bonferroni posttest.
DISCUSSION
Several lines of investigation have strongly indicated that increasing levels of GDNF in diseased regions of brain might be therapeutically beneficial to patients with PD; however, this approach currently is limited by methods for delivery of cells, viruses, or genes. Here we tested the hypothesis that endogenous GDNF expression and secretion might be modulated by the innate immune response. Indeed, others have shown that TLR activation of astrocytes leads to increased expression and secretion of a variety of anti-inflammatory and neuroprotective factors that together can act to protect neurons in culture from noxious stimuli (11).
It is important to recognize that although TLR activation was identified initially through a focus on infectious diseases, a growing body of research now has validated a large repertoire of endogenous TLR activators that are generated from cellular stress or injury, including endogenous TLR activators produced in neurodegenerative diseases such as PD (47, 48). Indeed, endogenous substances released by degenerating cells activate TLR3 in a mouse model of retinal degeneration, in which TLR3 is necessary for immune activation and retinal cell loss (3). Just like endogenous and exogenous activators of innate immune response, each of the compounds used here to activate specific TLRs also may activate other pathogen recognition receptors; e.g., in addition to TLR3, PIC activates retinoic acid-inducible gene-I-like receptors that are cytosolic RNA helicases (49). This complexity of pathogen receptor activation was not the focus of our work; rather, we used these activators as tools to model innate immune response. Nevertheless, caution should be exercised to not equate PIC exposure exclusively with TLR3 activation. Existing studies have presented conflicting data on TLR-induced GDNF expression and/or secretion in astrocytes or astrocyte-like cells with some reporting increased GDNF expression or secretion, whereas others showed no change in GDNF secretion after TLR activation (12–15). We demonstrated that astrocyte GDNF expression and secretion were induced by several TLR ligands, but the greatest apparent effect was with PIC. Notably, PIC-induced expression of GDNF was relatively selective among a group of astrocyte-derived neurotrophic factors that included BDNF and PAI-1, NGF, and VEGF. Finally, we demonstrated that PIC in turn activated multiple signaling pathways in astrocytes; however, increased expression of GDNF was selectively dependent on the JNK signaling pathway, strengthening the link between TLR3 activation and increased GDNF expression. Taken together, these results demonstrated that activators of astrocyte TLR2, TLR3, TLR4, or TLR9 led to induction of GDNF expression and that this was greatest for the TLR3 activator, restricted to GDNF among multiple neurotrophic factors, and largely mediated through the JNK signaling pathway.
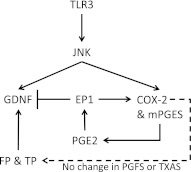
PIC activation of astrocytes led not only to increased GDNF expression but also to expression of COX-2 and mPGES, the enzymes necessary for induced synthesis of PGE2. Increased production of PGE2 is a feature of innate immune activation and is characteristic of the substantia nigra in patients with PD and in animal models of PD (50, 51). Moreover, some (52–54), but not all (55–57), epidemiologic studies suggest that use of COX inhibitors may lower the risk of developing PD. We pursued investigation of cause-and-effect relationships among these associated events by first examining the effect of a nonselective COX inhibitor and a COX-2-selective inhibitor on PIC-induced GDNF expression by astrocytes; somewhat surprisingly, there was no significant effect despite the associated expression of COX-2, mPGES, and GDNF. Because induction of COX-2 initiates a cascade of multiple bioactive eicosanoids, we hypothesized that one explanation for our observations was that some COX-2-derived eicosanoids were suppressors of GDNF expression and other COX-2-derived eicosanoids were enhancers of GDNF expression (Fig. 8). Our primary culture experiments identified one receptor subtype for PGE2, EP1, as a suppressor of GDNF expression in the setting of innate immune activation, apparently through increasing intracellular calcium levels. These results suggest that in the presence of increased PGE2, as occurs in the substantia nigra of patients with PD, local activation of EP1 may dampen GDNF expression and secretion.
Figure 8.
Diagram of proposed model of antagonistic regulation of astrocyte GDNF secretion based on our findings. TLR3 activation results in GDNF expression as well as increased COX-2 and mPGES. Whereas PGE2 production resulting from this activation suppresses GDNF transcription (through EP1 receptor agonism), other eicosanoids, in particular PGF2 and thromboxane, stimulate GDNF secretion. This model highlights the selective, suppressive role EP1 receptors play in balanced control of GDNF synthesis/secretion and emphasizes the therapeutic potential of EP1 receptor antagonists as a neurotrophic therapy for PD.
If our hypothesis about roughly balanced COX-2-dependent enhancers and suppressors of PIC-induced GDNF expression is correct, then there should be eicosanoid products of COX that enhance GDNF expression to counter EP1-mediated suppression and thereby yield no net effect by inhibiting COX-2. Indeed, our experiments demonstrated that in primary astrocyte cultures, selective agonists of TP and FP, the receptors for TxA2 and PGF2α, were strong enhancers of GDNF expression, and an agonist of EP4 was a weaker enhancer of GDNF expression, in the context of PIC exposure. It is important to recognize that EP1-mediated suppression, as well as TP-, FP-, or EP4-mediated enhancement of astrocyte GDNF expression, did not occur in resting astrocytes but rather in those activated by PIC. Moreover, use of a nonselective COX inhibitor suppressed the modulatory effects of PG receptor agonists, suggesting that some COX1-derived products are necessary to manifest this functional antagonism. In summary, our results using pharmacologic agents suggest a roughly balanced suppression by EP1 and enhancement by TP, FP, and EP4 of endogenous astrocyte GDNF expression in the context of innate immune activation.
Because GDNF provides critical neurotrophic support for dopaminergic neurons of the substantia nigra and is a major therapeutic target in PD, our data raise the possibility that selective blockage of EP1 signaling may yield increased GDNF expression and secretion in regions of brain with activated innate immunity, such as the substantia nigra in patients with PD.
Innate immune activation in neurodegenerative diseases probably has beneficial as well as deleterious effects, and the key to harnessing this response to injury for therapeutic benefit probably will be modulation rather than overall suppression. Here we have shown that selective activation or suppression of specific eicosanoid receptors significantly increased GDNF expression and secretion by astrocytes in the context of innate immune activation. Among these functionally antagonistic eicosanoid receptors, suppression of EP1 signaling most robustly increased astrocyte GDNF expression. We already have shown that blockade of EP1 signaling in microglia also has the potentially beneficial effect of selectively decreasing secretion of known neurotoxic cytokines IL-6 and TNF-α (43). EP1 also is expressed on neurons in brain that are relevant to PD, including dopamine D1 receptor- and D2 receptor-expressing neurons, where EP1 facilitates striatal dopaminergic neurotransmission (58, 59), and dopaminergic neurons in substantia nigra pars compacta, where EP1 enhances GABA-mediated inhibition (60). Although the roles of EP1 among parenchymal cells in brain are complex, taken together, the results of these studies raise the possibility that EP1 antagonists, for which there are lead compounds such as the one used here, may be therapeutically beneficial immune modulators for PD to suppress the neurotoxic microglial response and enhance astrocyte GDNF secretion.
Acknowledgments
The authors thank Meilany Wijaya for assistance with animal husbandry, Aimee Schantz and Amy Look for administrative support, and Carol Arnold for managerial support.
This work was funded by the U.S. National Institutes of Health (ES16754, NS62864, AG35681, AG00258, DK37097, and GM15431) and the Nancy and Buster Alvord Endowment.
Footnotes
- 2-ABP
- 2-aminoethoxy-diphenyl borate
- AD
- Alzheimer's disease
- BDNF
- brain-derived neurotrophic factor
- COX
- cyclooxygenase
- DP
- prostaglandin D2 receptor
- EP1-4
- prostaglandin E2 receptor subtype 1–4
- FP
- prostaglandin F receptor
- GDNF
- glial-derived neurotrophic factor
- mPGES
- membranous prostaglandin E2 synthase
- NGF
- nerve growth factor
- PAM3
- Pam3Cys-Ser-(Lys)4
- PAI
- plasminogen activator inhibitor
- PD
- Parkinson's disease
- PGE2
- prostaglandin E2
- PIC
- polyinosinic-polycytidylic acid
- TLR
- Toll-like receptor
- TP
- thromboxane receptor
- Tx
- thromboxane
- VEGF
- vascular endothelial growth factor
- WT
- wild-type
REFERENCES
- 1. Beraud D., Twomey M., Bloom B., Mittereder A., Ton V., Neitzke K., Chasovskikh S., Mhyre T. R., Mhyre, Maguire-Zeiss K. A. (2011) α-Synuclein alters Toll-like receptor expression. Front. Neurosci. 5, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bsibsi M., Bajramovic J. J., Vogt M. H., van Duijvenvoorden E., Baghat A., Persoon-Deen C., Tielen F., Verbeek R., Huitinga I., Ryffel B., Kros A., Gerritsen W.H., Amor S., van Noort J. M. (2010) The microtubule regulator stathmin is an endogenous protein agonist for TLR3. J. Immunol. 184, 6929–6937 [DOI] [PubMed] [Google Scholar]
- 3. Shiose S., Chen Y., Okano K., Roy S., Kohno H., Tang J., Pearlman E., Maeda T., Palczewski K., Maeda A. (2011) Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J. Biol. Chem. 286, 15543–15555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lim J. E., Kou J., Song M., Pattanayak A., Jin J., Lalonde R., Fukuchi K. (2011) MyD88 deficiency ameliorates β-amyloidosis in an animal model of Alzheimer's disease. Am. J. Pathol. 179, 1095–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Roodveldt C., Labrador-Garrido A., Gonzalez-Rey E., Fernandez-Montesinos R., Caro M., Lachaud C. C., Waudby C.A., Delgado M., Dobson C.M., Pozo D. (2010) Glial innate immunity generated by non-aggregated alpha-synuclein in mouse: differences between wild-type and Parkinson's disease-linked mutants. PLoS One 5, e13481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stefanova N., Fellner L., Reindl M., Masliah E., Poewe W., Wenning G. K. (2011) Toll-like receptor 4 promotes α-synuclein clearance and survival of nigral dopaminergic neurons. Am. J. Pathol. 179, 954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stone D. K., Reynolds A. D., Mosley R. L., Gendelman H. E. (2009) Innate and adaptive immunity for the pathobiology of Parkinson's disease. Antioxid. Redox Signal. 11, 2151–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ros-Bernal F., Hunot S., Herrero M. T., Parnadeau S., Corvol J. C., Lu L., Alvarez-Fischer D., Carrillo-de Sauvage M.A., Saurini F., Coussieu C., Kinagawa K., Prigent A., Höglinger G., Hamon M., Tronche F., Hirsch E. C., Vyas S. (2011) Microglial glucocorticoid receptors play a pivotal role in regulating dopaminergic neurodegeneration in parkinsonism. Proc. Natl. Acad. Sci. U. S. A. 108, 6632–6637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fuxe K. G., Tarakanov A. O., Goncharova L. B., Agnati L. F. (2008) A new road to neuroinflammation in Parkinson's disease? Brain Res. Rev. 58, 453–458 [DOI] [PubMed] [Google Scholar]
- 10. Shie F. S., Montine K. S., Breyer R. M., Montine T. J. (2005) Microglial EP2 is critical to neurotoxicity from activated cerebral innate immunity. Glia 52, 70–77 [DOI] [PubMed] [Google Scholar]
- 11. Bsibsi M., Persoon-Deen C., Verwer R. W., Meeuwsen S., Ravid R., van Noort J. M. (2006) Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia 53, 688–695 [DOI] [PubMed] [Google Scholar]
- 12. Tanaka T., Oh-Hashi K., Shitara H., Hirata Y., Kiuchi K. (2008) NF-κB independent signaling pathway is responsible for LPS-induced GDNF gene expression in primary rat glial cultures. Neurosci. Lett. 431, 262–267 [DOI] [PubMed] [Google Scholar]
- 13. Li X. Z., Bai L. M., Yang Y. P., Luo W. F., Hu W. D., Chen J., Mao C., Liu C. (2009) Effects of IL-6 secreted from astrocytes on the survival of dopaminergic neurons in lipopolysaccharide-induced inflammation. Neurosci. Res. 65, 252–258 [DOI] [PubMed] [Google Scholar]
- 14. Verity A. N., Wyatt T. L., Lee W., Hajos B., Baecker P. A., Eglen R. M., Johnson R. M. (1999) Differential regulation of glial cell line-derived neurotrophic factor (GDNF) expression in human neuroblastoma and glioblastoma cell lines. J. Neurosci. Res. 55, 187–197 [DOI] [PubMed] [Google Scholar]
- 15. Verity A. N., Wyatt T. L., Hajos B., Eglen R. M., Baecker P. A., Johnson R. M. (1998) Regulation of glial cell line-derived neurotrophic factor release from rat C6 glioblastoma cells. J. Neurochem. 70, 531–539 [DOI] [PubMed] [Google Scholar]
- 16. Lin L. F., Doherty D. H., Lile J. D., Bektesh S., Collins F. (1993) GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 260, 1130–1132 [DOI] [PubMed] [Google Scholar]
- 17. Granholm A. C., Reyland M., Albeck D., Sanders L., Gerhardt G., Hoernig G., Shen L., Westphal H., Hoffer B. (2000) Glial cell line-derived neurotrophic factor is essential for postnatal survival of midbrain dopamine neurons. J. Neurosci. 20, 3182–3190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pascual A., Hidalgo-Figueroa M., Piruat J. I., Pintado C. O., Gómez-Diaz R., López-Barneo J. (2008) Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 11, 755–761 [DOI] [PubMed] [Google Scholar]
- 19. Kearns C. M., Gash D. M. (1995) GDNF protects nigral dopamine neurons against 6-hydroxydopamine in vivo. Brain Res. 672, 104–111 [DOI] [PubMed] [Google Scholar]
- 20. Kordower J. H., Emborg M. E., Bloch J., Ma S. Y., Chu Y., Leventhal L., McBride J., Chen E. Y., Palfi S., Roitberg B. Z., Brown W. D., Holden J. E., Pyzalski R., Taylor M. D., Carvey P., Ling Z., Trono D., Hantraye P., Déglon N., Aebischer P. (2000) Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson's disease. Science 290, 767–773 [DOI] [PubMed] [Google Scholar]
- 21. Grondin R., Cass W. A., Zhang Z., Stanford J. A., Gash D. M., Gerhardt G. A. (2003) Glial cell line-derived neurotrophic factor increases stimulus-evoked dopamine release and motor speed in aged rhesus monkeys. J. Neurosci. 23, 1974–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Maswood N., Grondin R., Zhang Z., Stanford J. A., Surgener S. P., Gash D. A., Gerhardt G. A. (2002) Effects of chronic intraputamenal infusion of glial cell line-derived neurotrophic factor (GDNF) in aged rhesus monkeys. Neurobiol. Aging 23, 881–889 [DOI] [PubMed] [Google Scholar]
- 23. Tomac A., Lindqvist E., Lin L. F., Ogren S. O., Young D., Hopper B. J., Olson L. (1995) Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 373, 335–339 [DOI] [PubMed] [Google Scholar]
- 24. Nutt J. G., Burchiel K. J., Comella C. L., Jankovic J., Lang A. E., Laws E. R., Jr., Lozano A. M., Penn R. D., Simpson R. K., Jr., Stacy M., Wooten G.F., and ICV GDNF Study Group (2003) Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 60, 69–73 [DOI] [PubMed] [Google Scholar]
- 25. Lang A. E., Gill S., Patel N. K., Lozano A., Nutt J. G., Penn R., Brooks D. J., Hotton G., Moro E., Heywood P., Brodsky M. A., Burchiel K., Kelly P., Dalvi A., Scott B., Stacy M., Turner D., Wooten V. G., Elias W. J., Laws E. R., Dhawan V., Stoessl A. J., Matcham. J., Coffey R. J., Traub M. (2006) Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 59, 459–466 [DOI] [PubMed] [Google Scholar]
- 26. Slevin J. T., Gash D. M., Smith C. D., Gerhardt G. A., Kryscio R., Chebrolu H., Walton A., Wagner R., Young A. B. (2007) Unilateral intraputamenal glial cell line-derived neurotrophic factor in patients with Parkinson disease: response to 1 year of treatment and 1 year of withdrawal. J. Neurosurg. 106, 614–620 [DOI] [PubMed] [Google Scholar]
- 27. Gill S. S., Patel N. K., Hotton G. R., O'Sullivan K., McCarter R., Bunnage M., Brooks D. J., Svendsen C. N., Heywood P. (2003) Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 9, 589–595 [DOI] [PubMed] [Google Scholar]
- 28. Hovland D. N., Jr., Boyd R. B., Butt M. T., Engelhardt J. A., Moxness M. S., Ma M. H., Emery M. G., Ernst N. B., Reed R. P., Zeller J. R., Gash D. M., Masterman D. M., Potter B. M., Cosenza M. E., Lightfoot R. M. (2007) Six-month continuous intraputamenal infusion toxicity study of recombinant methionyl human glial cell line-derived neurotrophic factor (r-metHuGDNF in rhesus monkeys. Toxicol. Pathol. 35, 1013–1029 [DOI] [PubMed] [Google Scholar]
- 29. Su X., Kells A. P., Huang E. J., Lee H. S., Hadaczek P., Beyer J., Bringas J., Pivirotto P., Penticuff J., Eberling J., Federoff H. J., Forsayeth J., Bankiewicz K. S. (2009) Safety evaluation of AAV2-GDNF gene transfer into the dopaminergic nigrostriatal pathway in aged and parkinsonian rhesus monkeys. Hum. Gene Ther. 20, 1627–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lau Y. S., Patki G., Das-Panja K., Le W. D., Ahmad S. O. (2011) Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur. J. Neurosci. 33, 1264–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zigmond M. J., Cameron J. L., Leak R. K., Mirnics K., Russell V. A., Smeyne R. J., Smith A. D. (2009) Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat. Disord. 15(Suppl. 3), S42–S45 [DOI] [PubMed] [Google Scholar]
- 32. Tajiri N., Yasuhara T., Shingo T., Kondo A., Yuan W., Kadota T., Wang F., Baba T., Tayra J.. T., Morimoto T., Jing M., Kikuchi Y., Kuramoto S., Agari T., Miyoshi Y., Fujino H., Obata F., Takeda I., Furuta T., Date I.. (2010) Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 1310, 200–207 [DOI] [PubMed] [Google Scholar]
- 33. Biju K., Zhou Q., Li G., Imam S. Z., Roberts J. L., Morgan W. W., Clark R. A., Li S. (2010) Macrophage-mediated GDNF delivery protects against dopaminergic neurodegeneration: a therapeutic strategy for Parkinson's disease. Mol. Ther. 18, 1536–1544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu J., Yu W., Chen Y., Su Y., Ding Z., Ren H,., Jiang Y., Wang J. (2010) Intrastriatal transplantation of GDNF-engineered BMSCs and its neuroprotection in lactacystin-induced Parkinsonian rat model. Neurochem. Res. 35, 495–502 [DOI] [PubMed] [Google Scholar]
- 35. Thompson L. H., Grealish S., Kirik D., Bjorklund A. (2009) Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur. J. Neurosci. 30, 625–638 [DOI] [PubMed] [Google Scholar]
- 36. Yang X., Mertens B., Lehtonen E., Vercammen L., Bockstael O., Chtarto A., Levivier M., Brotchi J., Michotte Y., Baekelandt V., Sarre S., Tenenbaum L. (2009) Reversible neurochemical changes mediated by delayed intrastriatal glial cell line-derived neurotrophic factor gene delivery in a partial Parkinson's disease rat model. J. Gene Med. 11, 899–912 [DOI] [PubMed] [Google Scholar]
- 37. Eberling J. L., Kells A. P., Pivirotto P., Beyer J., Bringas J., Federoff H. J., Forsayeth J., Bankiewicz K. S. (2009) Functional effects of AAV2-GDNF on the dopaminergic nigrostriatal pathway in parkinsonian rhesus monkeys. Hum. Gene Ther. 20, 511–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Garbayo E., Montero-Menei C. N., Ansorena E., Lanciego J. L., Aymerich M. S., Blanco-Prieto M. J. (2009) Effective GDNF brain delivery using microspheres—a promising strategy for Parkinson's disease. J. Control. Release 135, 119–126 [DOI] [PubMed] [Google Scholar]
- 39. Hong M., Mukhida K., Mendez I. (2008) GDNF therapy for Parkinson's disease. Expert Rev. Neurother. 8, 1125–1139 [DOI] [PubMed] [Google Scholar]
- 40. Emborg M. E., Ebert A. D., Moirano J., Peng S., Suzuki M., Capowski E., Joers V., Roitberg B. Z., Aebischer P., Svendsen C. N. (2008) GDNF-secreting human neural progenitor cells increase tyrosine hydroxylase and VMAT2 expression in MPTP-treated cynomolgus monkeys. Cell Transplant. 17, 383–395 [PubMed] [Google Scholar]
- 41. Lindvall O., Wahlberg L. U. (2008) Encapsulated cell biodelivery of GDNF: a novel clinical strategy for neuroprotection and neuroregeneration in Parkinson's disease? Exp. Neurol. 209, 82–88 [DOI] [PubMed] [Google Scholar]
- 42. Guan Y., Zhang Y., Wu J., Qi Z., Yang G., Dou D., Gao Y., Chen L., Zhang X., Davis L. S, Wei M., Fan X., Carmosino M., Hao C., Imig J. D, Breyer R. M, Breyer M. D. (2007) Antihypertensive effects of selective prostaglandin E2 receptor subtype 1 targeting. J. Clin. Invest. 117, 2496–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Li X., Cudaback E., Keene C. D., Breyer R. M., Montine T. J. (2011) Suppressed microglial E prostanoid receptor 1 signaling selectively reduces tumor necrosis factor α and interleukin 6 secretion from Toll-like receptor 3 activation. Glia 59, 569–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Okun E., Griffioen K. J., Lathia J. D., Tang S. C., Mattson M. P., Arumugam T. V. (2009) Toll-like receptors in neurodegeneration. Brain Res. Rev. 59, 278–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Carpentier P. A., Begolka W. S., Olson J. K., Elhofy A., Karpus W. J., Miller S. D. (2005) Differential activation of astrocytes by innate and adaptive immune stimuli. Glia 49, 360–374 [DOI] [PubMed] [Google Scholar]
- 46. Soeda S., Koyanagi S., Kuramoto Y., Kimura M., Oda M., Kozako T., Hayashida S., Shimeno H. (2008) Anti-apoptotic roles of plasminogen activator inhibitor-1 as a neurotrophic factor in the central nervous system. Thromb. Haemost. 100, 1014–1020 [DOI] [PubMed] [Google Scholar]
- 47. Kariko K., Ni H., Capodici J., Lamphier M., Weissman D. (2004) mRNA is an endogenous ligand for Toll-like receptor 3. J. Biol. Chem. 279, 12542–12550 [DOI] [PubMed] [Google Scholar]
- 48. Ginsberg S. D., Galvin J. E., Chiu T. S., Lee V. M., Masliah E., Trojanowski J. Q. (1998) RNA sequestration to pathological lesions of neurodegenerative diseases. Acta Neuropathol. 96, 487–494 [DOI] [PubMed] [Google Scholar]
- 49. Saito T., Gale M., Jr. (2008) Differential recognition of double-stranded RNA by RIG-I-like receptors in antiviral immunity. J. Exp. Med. 205, 1523–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Teismann P., Tieu K., Choi D. K., Wu D. C., Naini A., Hunot S., Vila M., Jackson-Lewis V., Przedborski S. (2003) Cyclooxygenase-2 is instrumental in Parkinson's disease neurodegeneration. Proc. Natl. Acad. Sci. U. S. A. 100, 5473–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mattammal M. B., Strong R., Lakshmi V. M., Chung H. D., Stephenson A. H. (1995) Prostaglandin H synthetase-mediated metabolism of dopamine: implication for Parkinson's disease. J. Neurochem. 64, 1645–1654 [DOI] [PubMed] [Google Scholar]
- 52. Chen H., Jacobs E., Schwarzschild M. A., McCullough M. L., Calle E. E., Thun M. J., Ascherio A. (2005) Nonsteroidal antiinflammatory drug use and the risk for Parkinson's disease. Ann. Neurol. 58, 963–967 [DOI] [PubMed] [Google Scholar]
- 53. Gagne J. J., Power M. C. (2010) Anti-inflammatory drugs and risk of Parkinson disease: a meta-analysis. Neurology 74, 995–1002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao X., Chen H., Schwarzschild M. A., Ascherio A. (2011) Use of ibuprofen and risk of Parkinson disease. Neurology 76, 863–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Becker C., Jick S. S., Meier C. R. (2011) NSAID use and risk of Parkinson disease: a population-based case-control study. Eur. J. Neurol. 18, 1336–1342 [DOI] [PubMed] [Google Scholar]
- 56. Driver J. A., Logroscino G., Lu L., Gaziano J. M., Kurth T. (2011) Use of non-steroidal anti-inflammatory drugs and risk of Parkinson's disease: nested case-control study. BMJ 342, d198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Bornebroek M., de Lau L. M., Haag M. D., Koudstaal P. J., Hofman A., Stricker B. H., Breteler M. M. (2007) Nonsteroidal anti-inflammatory drugs and the risk of Parkinson disease. Neuroepidemiology 28, 193–196 [DOI] [PubMed] [Google Scholar]
- 58. Furuyashiki T., Narumiya S. (2011) Stress responses: the contribution of prostaglandin E2 and its receptors. Nat. Rev. Endocrinol. 7, 163–175 [DOI] [PubMed] [Google Scholar]
- 59. Kitaoka S., Furuyashiki T., Nishi A., Shuto T., Koyasu S., Matsuoka T., Miyasaka M., Greengard P., Narumiya S. (2007) Prostaglandin E2 acts on EP1 receptor and amplifies both dopamine D1 and D2 receptor signaling in the striatum. J. Neurosci. 27, 12900–12907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tanaka Y., Furuyashiki T., Momiyama T., Namba H., Mizoguchi A., Mitsumori T., Kayahara T., Shichi H., Kimura K., Matsuoka T., Nawa H., Narumiya S.. (2009) Prostaglandin E receptor EP1 enhances GABA-mediated inhibition of dopaminergic neurons in the substantia nigra pars compacta and regulates dopamine level in the dorsal striatum. Eur. J. Neurosci. 30, 2338–2346 [DOI] [PubMed] [Google Scholar]