Abstract
Genetically engineered mice have been generated to model cerebral β-amyloidosis, one of the hallmarks of Alzheimer disease (AD) pathology, based on the overexpression of a mutated cDNA of the amyloid-β precursor protein (AβPP) or by knock-in of the murine Aβpp gene alone or with presenilin1 mutations. Here we describe the generation and initial characterization of a new mouse line based on the presence of 2 copies of the human genomic region encoding the wild-type AβPP and the L166P presenilin 1 mutation. At ∼6 mo of age, double-mutant mice develop amyloid pathology, with signs of neuritic dystrophy, intracellular Aβ accumulation, and glial inflammation, an increase in AβPP C-terminal fragments, and an 8 times increase in Aβ42 levels with a 40% decrease in Aβ40 levels, leading to a significant increase (14 times) of Aβ42/Aβ40 ratios, with minimal effects on presenilin or the Notch1 pathway in the brain. We conclude that in mice, neither mutations in AβPP nor overexpression of an AβPP isoform are a prerequisite for Aβ pathology. This model will allow the study of AD pathogenesis and testing of therapeutic strategies in a more relevant environment without experimental artifacts due to the overexpression of a single-mutant AβPP isoform using exogenous promoters.—Vidal, R., Sammeta, N., Garringer, H. J., Sambamurti, K., Miravalle, L., Lamb B. T., Ghetti, B. The Psen1-L166P-knock-in mutation leads to amyloid deposition in human wild-type amyloid precursor protein YAC transgenic mice.
Keywords: presenilin, AβPP processing, animal model
A hallmark of Alzheimer disease (AD) pathology is the accumulation in brain parenchyma and in vessel walls of the insoluble 4-kDa amyloid-β (Aβ) peptide and the intracellular accumulation of neurofibrillary tangles (NFTs) composed of tau paired helical filaments (PHFs) (1). Aβ is a 40/42-43-aa peptide generated by the successive proteolysis of the amyloid-β precursor protein (AβPP) by the β-site AβPP-cleaving enzyme 1 (BACE1) and the γ-secretase complex, whereas cleavage by α-secretase (ADAM, a disintegrin and metalloproteinase) and γ-secretase prevents Aβ generation (1–3). After α- or β-cleavage, the carboxyl-terminal fragments (CTFs) of AβPP known as CTFα and CTFβ, respectively, remain membrane associated and are further cleaved by γ-secretase. The γ-secretase complex is a heterotetrameric aspartyl membrane-bound protease complex comprising 4 interacting molecules: presenilin (PSEN or PS), nicastrin, anterior pharynx defective 1 (APH1), and PS enhancer 2 (Pen2) (1, 4, 5). In addition to AβPP, this complex interacts with a number of different substrates, including Notch, thus participating in a wide range of cellular functions (1, 6). More than 30 mutations in AβPP and ∼200 in the presenilin genes have been found to cause early-onset familial AD (FAD; http://www.molgen.ua.ac.be/ADMutations). The identification of genetic defects in AβPP, PSEN1, and PSEN2 has allowed a better understanding of the molecular mechanisms involved in the processing of AβPP and the generation of Aβ peptides, as well as the development of animal models. Murine models based on gene targeting or transgenic expression of mutant forms of AβPP, PSEN1, and PSEN2 have proven valuable for the study of different aspects of amyloid pathology and cognitive changes and to test therapies (7–10). However, no mouse model recapitulates the neuropathological hallmarks of AD, e.g., amyloid deposition, NFTs, and neuronal cell loss.
Herein we report the generation of Psen1-L166P (PS1-L166P)-knock-in mice to assess the pathological significance of the mutation found in patients with FAD (11). Patients with the PSEN1-L166P mutation have onset in the second decade of life, clinically characterized by progressive ataxia and spasticity soon followed by cognitive deficits. Neuropathologic findings include prominent Aβ deposition as neuritic plaques, cotton wool plaques, severe cerebral amyloid angiopathy (CAA), and NFTs. Psen1-L166P-knock-in mice do not develop amyloid pathology. These mice were crossed with APP yeast artificial chromosome (YAC) transgenic mice, which were generated using a YAC containing the whole human wild-type AβPP gene (without FAD-associated mutations) and having regulatory elements in its chromosomal environment (12). Similar to Aβpp-knock-in models (13), this model has the advantage of achieving correct tissue-specific expression of AβPP throughout the body, showing the presence of AβPP695, AβPP751, and AβPP770 isoforms (695, 751, and 770 aa, respectively) generated by alternative splicing of AβPP. Unlike AβPP cDNA-based transgenic mice, AβPP expression in APP YAC transgenic mice is also relatively independent of the chromosomal integration site (7–10, 12). APP YAC × Psen1-L166P mice develop compact and diffuse Aβ deposits and CAA, accumulate intracellular Aβ, and show a significant increase in AβPP CTFs and Aβ42 peptides and a significant decrease in Aβ40 peptides, leading to an elevation of the Aβ42/Aβ40 ratio, with minimal effects on PS1 endoproteolysis or the Notch pathway in the brain.
MATERIALS AND METHODS
Generation of Psen1-L166P-knock-in mice and APP YAC × Psen1-L166P mice
A presenilin targeting vector was constructed using a mouse genomic fragment obtained from a bacterial artificial chromosome library from the 129/Sv mouse genome. The fragment contained exon 6 of the murine Psen1 gene and included portions of the surrounding introns. A SacI-PstI subclone was mutagenized to change leucine 166 to proline (CTT to CCT) with the creation of a HaeIII site. The AvaI-BsaI (540 bp) fragment containing the L166P mutation was sequenced in both directions to confirm that no other changes were introduced. This fragment was used to replace the original sequence of the Psen1 gene. In addition, a pgk-neo gene cassette was inserted for positive selection at the KpnI-BamHI site. The assembled vector was linearized with NotI and electroporated into C56Bl/6J embryonic stem (ES) cells. After screening and selection of neomycin-resistant clones, supercoiled plasmid encoding Cre was transfected into clones and screened for excision of the selectable marker cassette by PCR. DNA samples were screened by Southern hybridization using Psen1 probes flanking the 5′ arm of homology and the 3′ arm of homology. Chimeric founder mice that exhibited germline transmission of the mutant Psen1 allele were produced by embryo aggregation of the targeted ES cell clones. Embryos were transferred to pseudopregnant recipient females and allowed to develop to term. Male chimeras were mated with C57Bl/6J females to produce heterozygous Psen1-L166P (+/−) mice. DNA was extracted from the biopsied tail of mouse pups, and the F1 generation of the mutant animals was further identified by Southern blot analysis with the 5′ and 3′ external probes. Heterozygous mice were mated to generate homozygous Psen1-L166P (+/+) knock-in mice. The presence of the mutation at codon 166 was confirmed by restriction enzyme analysis and DNA sequencing. To genotype the Psen1-L166P-knock-in mouse, tail DNA was isolated and subjected to PCR analysis using oligonucleotides 5′-CATGGGCTAGAACCAGTAATGTAGCAG-3′ and 5′-GAAACTAACCTCAACTCTTAGCTAGGTACG-3′. Heterozygous females and homozygous males were cross-bred with APP YAC transgenic mice (line Py8.9) containing a single copy of unarranged wild-type human AβPP gene (12) and already in C57Bl/6J background. To determine the presence of the human AβPP allele, tail DNA was isolated and subjected to PCR analysis using 5′-GTGGATAACCCTCCCCCAGCCTAGACCA-3′ and 5′-CTGACCACTCGACCAGGTTCTGGGT-3′ as primers. For all of the experiments reported here, mice were interbred to generate homozygous APP YAC transgenic mice [AβPP (+/+)] with a wild-type (WT) Psen1 murine allele [Psen1-L166P (−/−)], a single copy of the knock-in L166P allele [heterozygous Psen1-L166P (+/−)], and a double copy of the knock-in L166P allele [homozygous Psen1-L166P (+/+)]. PCR was used for genotyping analyses. AβPP homozygosity was determined by cross-breeding founder mice with WT C57Bl/6J mice. All mice used in these experiments were housed under standard conditions in the Research Animal Facility at Indiana University School of Medicine (IUSM) and provided food and water ad libitum. The Institutional Animal Care and Use Committee of IUSM approved all animal procedures.
RNA isolation and multiplex expression analysis
Nine-month-old male (n=3) and female (n=3) Psen1-L166P (+/+) mice and identical age, sex, and number of Psen1-L166P (−/−) mice were anesthetized and transcardially perfused with 0.9% saline, and the brains were removed. The cerebral cortex and hippocampus were placed in 500 μl of RNA later (Qiagen, Valencia, CA, USA) and frozen at −20°C. RNA was isolated using RNeasy Lipid Tissue Mini Kit (Qiagen) according to the manufacturer's protocol. Samples were treated on column with the RNase-free DNase Kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed on 50 ng of total RNA for each sample followed by multiplex PCR, and fragment separation was carried out by capillary electrophoresis using the GeXP Chemistry Protocol (Beckman Coulter, Fullerton, CA, USA) as described previously (14). The following gene-specific primer pairs (without universal tags) were used in RT-PCR. Gene name is followed by accession number, size of amplified fragment, and oligonucleotides (5′–3′): Psen1 (NM_008943; 190 bp) 5′-TTCGGGCTCGTGTTCTACTT-3′ and 5′-GGGCTTGCTCTCTGTTTTTG-3′; Hes3 (NM_008237; 183 bp) 5′-CCTCTGTTCTCAACCCTTGC-3′ and 5′-GTTTGATGCAGGATGTGGTG-3′; Hes5 (NM_010419; 233 bp) 5′-ATGCTCAGTCCCAAGGAGAA-3′ and 5′-AGGCTTTGCTGTGTTTCAGG-3′. Fragments were separated using a CEQ 8000 Automated Capillary DNA sequencer/Genetic Analysis Systems (Beckman Coulter) and analyzed using the GenomeLab GeXP Genetic Analysis System (Beckman Coulter) using the following fragment analysis parameters: slope threshold = 0.9999, peak height threshold = 800 rfu, peak size < 375, peak size > 150, dye = D4. Multiplex-specific fragments were selected by applying exclusion filters, and the data were exported to eXpress Analysis software (Beckman Coulter), where they were normalized against the mouse polymerase II polypeptide A (Polr2a) gene as described previously (14). Relative mRNA level values for each of the triplicates for each sample were averaged, and the means for the replicates were compared between Psen1-L166P (+/+) and (−/−) mice by an unpaired 2-tailed t test. Differences in relative mRNA levels with values of P < 0.05 were considered statistically significant. Data are reported as means ± sd. Statistical analysis was done using GraphPad Prism 5.04 (GraphPad, San Diego, CA, USA).
Hormone assays
Serum levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) were measured by a multiplex assay using a Milliplex map kit from Millipore (RPT-86K; Millipore, Bedford, MA, USA) at the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA, USA). Statistical analysis was done using GraphPad Prism 5.04.
Histology and immunohistochemistry
For these studies, (−/−), (+/−), and (+/+) male and female APP YAC × Psen1-L166P mice were analyzed between 1 and 24 mo of age (n=40). After anesthesia, animals were perfusion fixed with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.2 (Sigma-Aldrich, St. Louis, MO), after which brains and ovaries were removed, embedded in paraffin, and sectioned. Sections (8 μm) were cut and mounted on poly-l-lysine-coated slides and stained with hematoxylin and eosin as described previously (15, 16). Thioflavin S (Th-S) was used to show the presence of amyloid deposits in the brain, as described previously (16). Immunohistochemical stainings were performed by using primary antibodies (Abs) directed against the Aβ peptide (the epitope recognized by the Ab is indicated) as described previously (17). These included monoclonal 3D6 (Aβ1–5; Elan Corp., Dublin, Ireland), monoclonal 10D5 (Aβ3–6; Elan Corp.), monoclonal 4G8 (Aβ18–22; Signet, Dedham, MA, USA), polyclonal anti-Aβ with a pyroglutamyl residue at the amino-terminal position Glu-3 (AβN3pE; IBL, Gunma, Japan), and monoclonal 6E10 (Aβ3–8; Signet). Aβ species ending at residue 42 (AβN–42) were recognized using monoclonal 21F12 (Elan Corp.). To detect AβPP, we used polyclonal Ab ZMD.316, against a synthetic peptide derived from the C-terminal region of the human and mouse AβPP (Zymed/Invitrogen, San Francisco, CA, USA). Polyclonal Abs against glial fibrillary acidic protein (GFAP; Dako, Copenhagen, Denmark) for the detection of astrocytes, and keratan sulfate (5D4; Seikagaku Kogyo, Tokyo, Japan) for the detection of activated microglia, were also used as described previously (16). We also used antibodies against tau (d29) and antibodies specific for hyperphosphorylated tau (AT8; Polymedco, Cortlandt Manor, NY, USA) and PHFs (AT100; Thermo Scientific, Waltham, MA, USA). Ab d29 is an Ab raised in rabbits using a synthetic peptide homologous to residues 42–54 (GLKAEEAGIGDTC) of 0N3R or 0N4R tau coupled to keyhole limpet hemocyanin through a C-terminal Cys as immunogen. The presence of specific antibodies was tested by ELISA and dot blot analysis. Monoclonal AT8 is an anti-phosphorylated tau at Ser202/Thr205, and monoclonal AT100 is an anti-PHF-tau phosphorylated at Ser212 and Thr214.
Electrophoresis and Western blotting
Mice were anesthetized and flush-perfused transcardially with 0.9% saline. Brains were removed, snap-frozen, and stored at −70°C for protein analysis, as described previously (15, 16). Tissue was homogenized in radioimmunoprecipitation assay buffer (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 1 mM EDTA; 1 mM Na3VO4; 50 mM NaF; 1.0% Triton X-100; 0.5% deoxycholate; and 0.1% SDS; all from Sigma), with a protease inhibitor mixture (Complete; Roche Applied Science, Indianapolis, IN, USA). Protein estimation was done using a BCA protein estimation kit (Pierce, Rockford, IL, USA). Lysates containing equal amounts of protein (100 μg) were separated on 10% NuPAGE Bis-Tris gels (Invitrogen, Carlsbad, CA, USA) and transferred to Immobilon-P membrane (Millipore), as described previously (17). Rabbit primary Ab against APP (ZMD.316), PS1 (P5110) (Sigma), Notch intracellular domain (NICD; 07-1232; Millipore), BACE1 (195111; Calbiochem, La Jolla, CA, USA), and monoclonal anti-β-actin (Sigma) were used. As secondary Abs, horseradish peroxidase-conjugated goat Abs (Amersham, Arlington Heights, IL, USA) were used (17). Immunoblots were visualized by chemiluminescence (Pierce) according to the manufacturer's specifications. Blots were scanned and quantified using Image J software (U.S. National Institutes of Health, Bethesda, MD, USA). Statistical analysis was done using GraphPad Prism 5.04.
Quantitation of Aβ
Snap-frozen hippocampi and cerebral cortices were homogenized in guanidine buffer, and human Aβ peptides were quantitated by ELISA as described previously (18) using ELISA kits from Invitrogen (KHB3481 and KHB3544). Aβ levels were expressed as picomoles per gram of wet tissue. Statistical analysis was done using GraphPad Prism 5.04.
RESULTS
Generation of Psen1-L166P-knock-in mice and APP YAC × Psen1-L166P mice
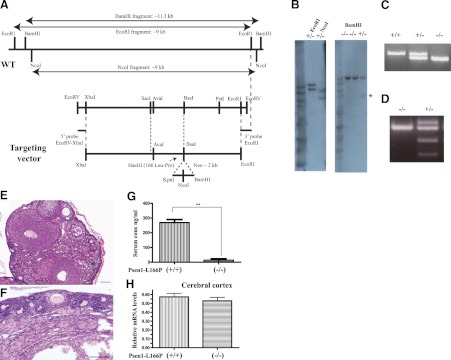
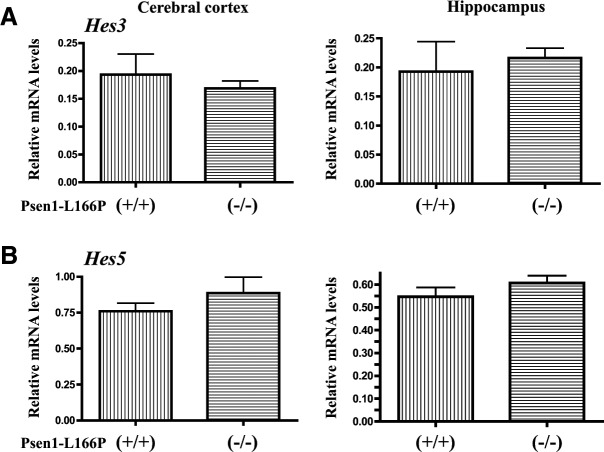
An exon replacement strategy was used to generate mouse lines carrying the L166P mutation in the murine Psen1 gene. We constructed a targeting vector carrying a point mutation that resulted in the replacement of leucine 166 to proline (L166P) in exon 6 of the murine Psen1 gene (Fig. 1A). The assembled vector was linearized with NotI and electroporated into C56Bl/6J ES cells. Following selection, the neomycin-resistance gene was removed by Cre-loxP recombination. Correctly targeted clones were expanded and transiently transfected with CMV-Cre plasmid DNA to remove the pgk-neo gene cassette. Homologous recombination germline transmission and genotype were confirmed by Southern blot and PCR (Fig. 1B, C). Male chimeras were mated with C57Bl/6J females to produce heterozygous Psen1-L166P (+/−) mice. Heterozygous mice were mated to generate homozygous (+/+) knock-in mice. The insertion of the mutation at codon 166 was confirmed by restriction enzyme analysis and DNA sequencing (Fig. 1D). No obvious differences were observed between WT, heterozygous, and homozygous Psen1-L166P-knock-in mice. Mice presented with no growth abnormalities and thrived at appropriate age, like their WT littermates. Male Psen1-L166P (+/+) mice were fertile and had no problems reproducing. Female Psen1-L166P (+/+) mice were infertile, with ovaries that were morphologically different from ovaries from WT mice, showing primordial follicles near the ovarian cortex and consisting largely of ovarian stromal elements (Fig. 1E, F). The serum concentration of FSH was found significantly (P<0.001) elevated in female Psen1-L166P (+/+) mice (∼20 times) compared to age-matched female controls (Fig. 1G). Serum levels of LH were also elevated in the (+/+) group; however, the difference did not reach statistical significance. Gene expression analysis did not show any significant difference in the expression of the Psen1 gene between WT and homozygous Psen1-L166P (+/+) mice in the cerebral cortex (Fig. 1H). No significant differences in the expression of the Notch target genes Hes3 and Hes5 in the cerebral cortex and in the hippocampus were observed between WT [Psen1-L166P (−/−)] and homozygous Psen1-L166P (+/+) mice (Fig. 2). Heterozygous Psen1-L166P (+/−) females and homozygous (+/+) males were mated with APP YAC transgenic mice (line Py8.9) containing a single copy of unarranged human AβPP gene (12). Mice were interbred to generate homozygous APP YAC [AβPP (+/+)] mice with WT Psen1 [APP YAC×Psen1-L166P (−/−)], a single copy of the knock-in L166P allele [APP YAC×Psen1-L166P (+/−)], and a double copy of the knock-in L166P allele [APP YAC×Psen1-L166P (+/+)].
Figure 1.
Generation and characterization of Psen1-L166P-knock-in mice. A) Schematic representation of the Psen1 WT allele and targeting vector. The codon encoding the L166P mutation is located in exon 6. A SacI-PstI (2.1 kb) subclone was mutagenized to change Leu to Pro, which created a HaeIII site. The AvaI-BsaI (540 bp) fragment containing the mutation was sequenced to ensure that no PCR error was introduced. This fragment was used in the final targeting vector construction. The targeting vector was linearized with NotI and purified before C57Bl/6J ES cells were electrophorated. Neomycin-resistant ES clones were selected. B) Southern blot analysis shows homologous recombination in both arms in DNA from selected ES cells digested with EcoRI and NcoI and hybridized with the 5′ EcoRV-XbaI (∼0.3 kb) probe. The WT allele is ∼9 kb, and the knock-in (KI) allele is ∼11 kb in EcoRI-digested DNA and ∼9 and ∼6 kb in NcoI-digested DNA, respectively. Tail genomic DNA was digested with BamHI and hybridized with the 3′EcoRI probe. The WT allele is ∼11 kb, and the KI allele is ∼5 kb (asterisk). C) Genotyping by PCR. The top band is amplified from the mutant allele (670 bp), and the bottom band from the wild-type allele (558 bp). A single band (670 bp) is seen in homozygous Psen1-L166P (+/+) mice, and a single band (558 bp) is seen in WT Psen1-L166P (−/−) mice. D) Restriction enzyme analysis of PCR-amplified genomic DNA using HaeIII. WT allele (−/−) cannot be digested. Digestion is only observed when the mutant allele (+/−) is present, confirming the presence of the L166P mutation. E, F) Hematoxylin and eosin-stained sections of ovaries of 9-mo-old female normal (−/−) (E) and homozygous Psen1-L166P (+/+) (F) mice. Numerous follicles at different stages can be seen throughout the normal ovary (E), but not in (+/+) mice (F). Scale bars = 100 μm. G) Serum concentrations of FSH in 3–4-mo-old female homozygous Psen1-L166P (+/+) mice and normal Psen1-L166P (−/−) mice. **P < 0.001. H) Multiplex RT-PCR expression analysis. Bar graphs depict differential gene expression levels between homozygous Psen1-L166P (+/+) mice and normal Psen1-L166P (−/−) mice. Multiplex RT-PCR analysis was performed on mRNA isolated from the cerebral cortex. Analysis was performed in triplicate and normalized to the Polr2a gene. Group averages are reported as relative mRNA levels (means±sd). No significant expression differences were found (P<0.05) by 2-tailed t test.
Figure 2.
Expression of Notch target genes Hes3 and Hes5 in vivo in Psen1-L166P-knock-in mice. Analysis of the expression of Hes3 (A) and Hes5 (B) genes was performed by multiplex RT-PCR, as described in Material and Methods, on mRNA isolated from the cerebral cortex and hippocampus. Bar graphs depict differential gene expression levels between Psen1-L166P (+/+) and (−/−) mice. Analysis was performed in triplicate and normalized to the Polr2a gene. Group averages are reported as relative mRNA levels (means±sd). No significant expression differences were found (P<0.05) by 2-tailed t test.
Aβ pathology in APP YAC × Psen1-L166P mice
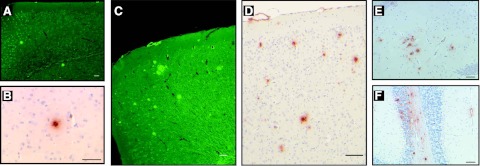
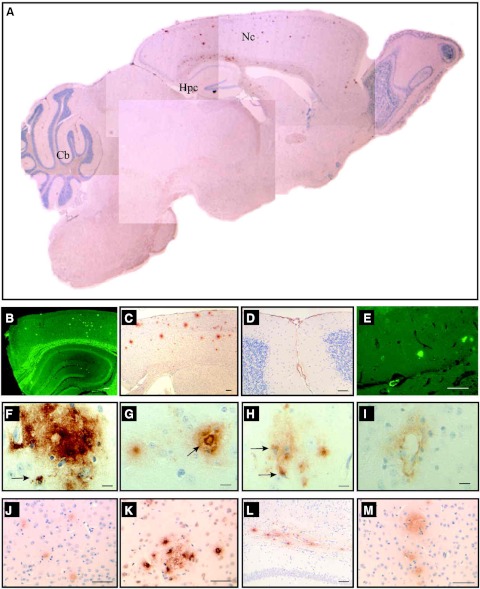
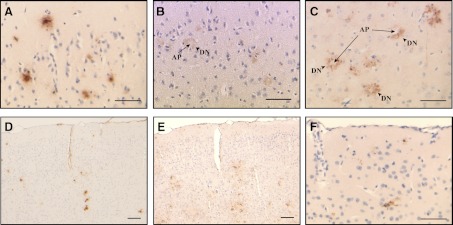
Expression of the entire WT human AβPP gene in APP YAC transgenic mice does not lead to amyloid deposition (12). In these animals, human AβPP transcript and protein are present at levels similar to the endogenous mouse products in the brain and other organs, with relative levels of alternatively spliced products of human AβPP transcripts (695, 751, and 770) paralleling control mouse Aβpp transcripts in the brain (12). Between 1 and ∼5 mo of age, no obvious brain pathology was detected in APP YAC × Psen1-L166P mice. At ∼6 mo of age, we detected in APP YAC × Psen1-L166P (+/+) mice the presence of small, scattered, Th-S-positive plaques in the cerebral cortex (Fig. 3A, B), whereas APP YAC × Psen1-L166P (−/−) and APP YAC × Psen1-L166P (+/−) mice did not show signs of such deposition, even at the oldest age analyzed (24 mo). With age, Aβ deposition in APP YAC × Psen1-L166P (+/+) mice became prominent in the neocortex and leptomeninges (Fig. 3C, D). These results suggest that there is an age-related, regional dependence of Aβ deposition in APP YAC × Psen1-L166P (+/+) mice. Amyloid plaques and diffuse deposits were immunolabeled using a panel of antibodies against different epitopes of the Aβ peptide, including 4G8 (Figs. 3, 4, and 5), 10D5 (Fig. 4), 3D6 (Fig. 4), 6E10 (Fig. 4), 21F12 (Figs. 3 and 4), and Np3E (not shown). In the cerebral cortex, many large confluent Aβ plaques and many small and moderate size plaques were seen (Figs. 3C, D and 4A, C, F, G, K, M). Many of the deposits had amyloid cores and stained with Th-S, but others were diffuse and did not stain with Th-S (Figs. 3 and 4). High magnification demonstrated significant Aβ intracellular localization (Fig. 4F, H). Scarce small plaques were observed in the hippocampus of young animals, becoming more prominent with age (Figs. 3 and 4). Plaques varied in size, with some plaques having haloes of radiating amyloid. Amyloid was observed in the walls of vessels of the hippocampal fissure (Fig. 4E). Vascular Aβ deposits (which stained with Th-S) were also observed in penetrating vessels of the neocortex and in leptomeninges of the cerebellum (Figs. 3 and 4). The cerebellum also showed parenchymal amyloid deposition in older mice (Fig. 3F). As in patients with CAA (19), individual vessels had a varying extent of amyloid deposition, sometimes seen in the form of focal globular clumps. Some clusters of dystrophic neurites associated to an amyloid core could be identified by immunohistochemistry. Dystrophic neurites were made up of large and rounded processes, strongly immunolabeled with an Ab against the C terminus of human AβPP and against the microtubule associated protein tau (d29; Fig. 5). These two immunoreactivities were generally colocalized; however, these deposits were not recognized using an Ab against tau phosphorylated at Ser202/Thr205 (AT8) and PHF-tau phosphorylated at Ser212 and Thr214 (AT100). Clusters of dystrophic neurites in the absence of noticeable amyloid plaques could also be occasionally observed. Amyloid deposits were surrounded by GFAP-immunopositive reactive astroglia (Fig. 5E) and keratan sulfate-positive-activated microglia (5D4-immunopositive; Fig. 5F). 5D4-immunopositive microglia was seen only in the brain of APP YAC × Psen1-L166P (+/+) mice and not in the brain of age/sex matched APP YAC × Psen1-L166P (+/−) or (−/−) mice.
Figure 3.
Histochemical and immunocytochemical detection amyloid deposits. A, B) Only few amyloid deposits were detected in the neocortex of young mice by Th-S (A) or immunohistochemistry (B). C–E) As animals aged, abundant parenchymal Aβ deposition was observed in the cerebral cortex (C, D) and hippocampus (E). In addition to amyloid plaques, leptomeninges also showed the presence of fibrillar Aβ (C, D). F) In the cerebellum, amyloid deposition was observed in leptomeningeal vessels and in the parenchyma. Brain sections from 5 (A), 6 (B), 15 (C, D), and 20-mo-old (E, F) APP YAC × Psen1-L166P (+/+) mice. Th-S (A, C). Immunohistochemistry using Ab 4G8 (B, D, F) and 21F12 (E). Scale bars = 100 μm (A, C, D); 50 μm (B, E, F).
Figure 4.
Distribution of amyloid deposits in APP YAC × Psen1-L166P mice. Sagittal section of an APP YAC × Psen1-L166P (+/+) mouse (A) shows the regional distribution of amyloid deposits in the brain. Numerous amyloid deposits were detected in the neocortex (Nc) by immunohistochemistry using antibodies against different epitopes of the Aβ peptide (A, C, F–H, J, K, M). In the cerebellum (Cb), amyloid deposition was observed in pial (leptomeningeal) vessels (A, D). In the hippocampus (Hpc), Th-S fluorescent plaques were observed in addition to amyloid deposits in the walls of vessels of the hippocampal fissure (A, E). Severe amyloid deposition is observed in the corpus callosum (L). In the neocortex, different varieties of amyloid deposits were observed, including diffuse (F, G), dense-cored (arrow, G), and vascular (I). Some deposits were clearly intracellular (arrows, F, H). Brain sections from an APP YAC × Psen1-L166P (+/+) mouse (15-mo-old). Immunohistochemistry using Abs 4G8 (A, D, F–I), 6E10 (C, L), 10D5 (J), 21F12 (K), and 3D6 (M). Th-S (B, E). Scale bars = 100 μm (B, C); 50 μm (D, E, J–M); 10 μm (F–I).
Figure 5.
Neuritic dystrophy associated with Aβ deposits and inflammatory response. In the periphery of Aβ amyloid plaques (AP, arrows), we observed clusters of swollen and abnormally distorted neuritic profiles. Dystrophic neurites (DN, arrowheads) were immunoreactive with antibodies against tau (B) and APP (C). In the cerebral cortex, amyloid deposition (A, D) was seen accompanied by an increase in the number of reactive astrocytes (E). Keratan sulfate-positive-activated microglia (F) was seen only in the brain of APP YAC × Psen1-L166P (+/+) mice. Brain sections from an APP YAC × Psen1-L166P (+/+) mouse (15 mo old). Immunohistochemistry using Abs 4G8 (A, D), d29 (against nonphosphorylated tau; B), ZMD.316 (against the C terminus of human APP; C), anti-GFAP (E), and 5D4 (F). Scale bars = 50 μm (A–C, F); 100 μm (D, E).
PS1, Notch, and APP endoproteolysis in APP YAC × Psen1-L166P mice
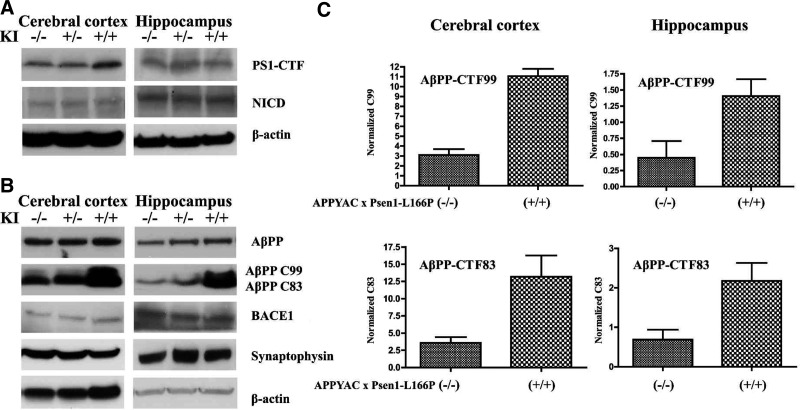
The L166P mutation is upstream of the PS1 endoproteolysis site and is not expected to disrupt the cleavage event. PS1 is endoproteolytically cleaved into 2 polypeptides, an N-terminal fragment (NTF) and a CTF, forming a functional PS heterodimer (3, 4). Western blot analysis of brain tissues documented normal PS1 endoproteolytic processing, producing a CTF of ∼18 kDa in APP YAC × Psen1-L166P (+/+) mice (Fig. 6A). CTFs could be also detected in APP YAC × Psen1-L166P (−/−) and heterozygous APP YAC × Psen1-L166P (+/−) mice, without significant differences in the amounts (Fig. 6A). In addition, no significant differences in NICD levels in the cerebral cortex and hippocampus were observed by Western blot analysis between APP YAC × Psen1-L166P (−/−), (+/−), and (+/+) mice (Fig. 6A). The presence of full-length AβPP and AβPP CTFs, CTFβ and CTFα, which are the substrates of γ-secretase, were characterized by Western blot analysis. The levels of high-molecular-weight AβPP species between the 3 genotypes of mice were similar without reaching statistically significant differences (Fig. 6B). However, the levels of CTFβ (or CTF99), a CTF of AβPP cleaved by BACE1, were significantly increased in the cerebral cortex (P<0.001) and hippocampus (P<0.05) of APP YAC × Psen1-L166P (+/+) mice as compared to APP YAC × Psen1-L166P (−/−) mice (Fig. 6B, C). The levels of CTFα (or CTF83), a CTF of AβPP following cleavage by α-secretase within the Aβ domain, were also significantly increased in the cerebral cortex (P<0.05) and hippocampus (P<0.05) of APP YAC × Psen1-L166P (+/+) mice (Fig. 6B, C). The CTFα band may also contain CTF89, a CTF of AβPP following cleavage by BACE1 at the β′ site (2). When the ratio of CTFβ over AβPP was calculated, there was a >3-fold increase in CTFβ levels in the cerebral cortex and the hippocampus of APP YAC × Psen1-L166P (+/+) mice. The ratio of CTFα over AβPP was also significantly different between the genotypes. We observed a >3-fold increase in CTFα levels in the cerebral cortex and the hippocampus of APP YAC × Psen1-L166P (+/+) mice. Western blot analysis showed that BACE1 levels were not noticeably different between APP YAC × Psen1-L166P (−/−), (+/−), and (+/+) mice (Fig. 6B). In addition, we analyzed levels of synaptophysin in the cerebral cortices and hippocampus of APP YAC × Psen1-L166P (−/−), (+/−), and (+/+) mice by Western blot. Progressive loss of the presynaptic marker synaptophysin is well established in transgenic mice models overexpressing mutant AβPP (20) and correlates well with cognitive decline in AD (21). No significant differences were observed in synaptophysin levels between APP YAC × Psen1-L166P (−/−), (+/−), and (+/+) mice at the young age analyzed, before amyloid deposition can be detected (Fig. 6).
Figure 6.
Biochemical characterization of APP YAC × Psen1-L166P mice. A) No significant differences were observed in the processing of PS1 using an anti-PS1-CTF Ab and in the generation of NICD by Western blot analysis. B, C) No significant differences were observed in the levels of full-length AβPP between the different genotypes (B); however, both CTFα (CTF83) and β (CTF99) accumulated at significant levels in the homozygous mice but not in heterozygous mice in the cerebral cortex and hippocampus (B, C). No significant changes were observed in the levels of BACE1 and synaptophysin between the 3 phenotypes.
Aβ production in APP YAC × Psen1-L166P mice
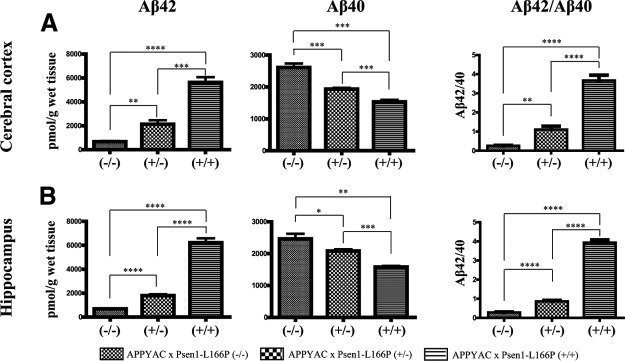
To determine the role of gene-dose effect of the Psen1 targeted mutation L166P in Aβ generation, Aβ deposition, and neuropathology, we measured levels of Aβ40 and Aβ42 in mouse brain of APP YAC × Psen1-L166P (−/−), (+/−), and (+/+) mice by ELISA. Cerebral Aβ levels were evaluated at 6–8 mo of age, an age at which amyloid deposition may be observed in the form of some scattered, small Aβ plaques only in the cerebral cortex of APP YAC × Psen1-L166P (+/+) mice. The presence of the Psen1-L166P allele led to a significant increase in Aβ42 levels in the cerebral cortex (P<0.0001; Fig. 7A) and in the hippocampus (P<0.0001; Fig. 7B) as a function of the number of mutant Psen1 alleles. Compared to APP YAC × Psen1-L166P (−/−) mice, Aβ42 levels in the cerebral cortex were 3 times higher in APP YAC × Psen1-L166P (+/−) (P<0.01) and more than 8 times higher in APP YAC × Psen1-L166P (+/+) mice (P<0.0001). In the hippocampus, Aβ42 levels were ∼2.5 times higher in APP YAC × Psen1-L166P (+/−) mice (P<0.0001) and 9 times higher in APP YAC × Psen1-L166P (+/+) mice (P<0.0001). A statistically significant reduction in Aβ40 levels in the cerebral cortex (P<0.0001; Fig. 7A) and in the hippocampus (P<0.0001; Fig. 7B) paralleled the increased in Aβ42 levels. The knock-in of one Psen1 allele caused an ∼25% decrease in brain Aβ40 in the cerebral cortex and an ∼15% decrease in brain Aβ40 in the hippocampus. The knock-in of both Psen1 alleles caused an ∼40% decrease in brain Aβ40 content in the cerebral cortex and the hippocampus. A statistically significant increase in Aβ42/Aβ40 ratios in the cerebral cortex (P<0.0001) and hippocampus (P<0.0001) was observed (Fig. 7). Compared to APP YAC × Psen1-L166P (−/−) mice, Aβ42/Aβ40 ratios were increase between 3 to 4 times in APP YAC × Psen1-L166P (+/−) mice (P<0.005) and ∼14 times in APP YAC × Psen1-L166P (+/+) mice (P<0.0001) in both the cerebral cortex and hippocampus. Importantly, the increase in the Aβ42/Aβ40 ratios observed in heterozygous and homozygous Psen1 mutant mice appeared to be primarily driven by the significant increase in Aβ42 levels and not by the much smaller decrease in Aβ40 levels. Compared to APP YAC × Psen1-L166P (−/−) mice, we did not observe a significant increase in total Aβ (Aβ40+Aβ42) in APP YAC × Psen1-L166P (+/−) mice, but the increase was highly significant (between 2 and 2.5 times) in APP YAC × Psen1-L166P (+/+) mice. Taken together, these findings indicate that the Psen1-L166P mutation gives rise to a significant in vivo effect in terms of the levels of Aβ42 species in homozygous mice. Using unpaired tests, the levels of Aβ40 and Aβ42 were not found to differ significantly between males and females mice of the same age.
Figure 7.
Aβ levels in APP YAC × Psen1-L166P mice. Comparison of human Aβ40 and Aβ42 levels in cerebral cortices (A) and hippocampi (B) of mice expressing WT (−/−) or mutant Psen1. Aβ40 and Aβ42 levels were quantitated by ELISA in mice from the 3 genotypes (n=3–5 mice/group) at 6–8 mo of age. Values represent group means ± sd broken after the analysis was complete. Statistical analyses were performed with GraphPad Prism 5.04. Differences among means were assessed by 1-way ANOVA followed by Dunnett's or Tukey-Kramer post hoc test. *P < 0.05, **P < 0.01; ***P < 0.001; ****P < 0.0001.
DISCUSSION
Here we describe a novel knock-in model based on a Leu to Pro mutation at codon 166 in PSEN1 that causes a very aggressive form of FAD at an early age (11, 22), leading to a significant increase in Aβ production in cell culture (22, 23) and very strong amyloid deposition in a double-transgenic model (24). As reported for other Psen1-knock-in models, heterozygous and homozygous Psen1-L166P-knock-in mice are viable and overtly healthy for up to 24 mo of age (6–10), although embryonic lethality has recently been reported in a homozygous Psen1-knock-in model [R278I (+/+)] at embryonic day 15–18, similarly to what has been reported for Psen1-knockout mice. The lethality observed in Psen1-R278I (+/+) mice may be due to the abnormal PS1 endoproteolysis seen in these mice, probably leading to problems with Notch1 processing and Notch1 signaling (25). Although the L166P mutation is very aggressive in humans, homozygous Psen1-L166P (+/+) mice did not show any significant changes in PS1 endoproteolysis in the brain, in agreement with previous cell work on the L166P mutation (22, 23). We did not observe significant changes in Notch1 processing or the expression of Notch1 target genes Hes3 and Hes5 that encode the Hes/Hey family of transcription factors (26) in the brain, in contrast with previous findings in transfected cell lines (22, 23). However, abnormal Notch1 signaling may be involved in the lack of follicular development observed in female Psen1-L166P (+/+) mice. Suppression of Notch signaling in the presence of γ-secretase inhibitors in the neonatal ovary has been reported to decrease follicle formation (27).
To further understand the mechanisms involved in Aβ generation, deposition, and clearance, we generated a unique model by crossing Psen1-L166P-knock-in mice with APP YAC transgenic mice (APP YAC × Psen1-L166P) that contain the entire unarranged WT human AβPP gene (12). APP YAC transgenic mice express human AβPP mRNA and protein at levels comparable to endogenous AβPP in the brain and other tissues, with similar relative levels of alternatively spliced human AβPP and murine Aβpp products (12); however, expression of WT human AβPP alone in APP YAC transgenic mice does not lead to amyloid deposition. In fact, amyloid deposition in transgenic mice expressing a WT human AβPP isoform driven by a strong exogenous promoter has rarely been reported (28, 29), and it has never been reported in mice expressing the entire WT AβPP gene (13). APP YAC × Psen1-L166P (+/+) mice develop cerebral parenchymal amyloid deposition with Th-S positive cores of Aβ surrounded by numerous dystrophic neurites and glial inflammation represented by reactive astrocytes and activated microglia. In addition, we observed the presence of diffuse Aβ deposits and CAA. We also observed the presence of intracellular Aβ accumulation, which has been associated with neuronal cell loss in amyloid transgenic models (20, 30). As with other animal models based on the expression of a mutant AβPP allele, introduction of a Psen1-knock-in mutation seems to affect the process of amyloid deposition (13). In these models, onset of amyloid pathology and amyloidogenic processing was somewhat enhanced by the presence of a mutant PSEN1 (7, 8, 13, 30, 31). In APP YAC × Psen1-L166P (+/+) mice, plaque deposition starts at ∼6 mo of age and is not seen in heterozygous APP YAC × Psen1-L166P (+/−) or in APP YAC × Psen1-L166P (−/−) mice at the oldest age analyzed (24 mo). Amyloid deposition predominantly involves the neocortex, the hippocampus, and the corpus callosum. In addition to CAA, the cerebellum shows parenchymal amyloid deposition in aged mice. As in other amyloid models based on the expression of mutant AβPP alone or in combination with presenilin mutations, we did not observe the presence of NFTs (6–10, 31, 32). Despite the absence of neurofibrillary pathology, a neuritic dystrophy was, however, present around Aβ deposits in APP YAC × Psen1-L166P (+/+) mice. The dystrophic processes were labeled by abs against tau (but not hyperphosphorylated tau or PHFs) and against AβPP. At young age (∼5 mo), Aβ42 constitutes the predominant Aβ species in homozygous as well as heterozygous APP YAC × Psen1-L166P mice; however, APP YAC × Psen1-L166P (+/+) mice showed severalfold higher levels of Aβ42, which may account for the amyloid deposition starting at around that age in APP YAC × Psen1-L166P (+/+) mice, without significant changes in the levels of the presynaptic marker synaptophysin. Accordingly, a higher ratio of Aβ42 over Aβ40 was also observed in APP YAC × Psen1-L166P (+/+) mice, in line with data obtained from patients with PSEN1 mutations (33). In homozygous mice, a higher Aβ42/40 ratio occurs in the absence of WT Psen1, and are thus intrinsic to the mutated Psen1 allele. The FAD L166P mutation in Psen1 elevates the production of Aβ42, normally a minor Aβ peptide species, while decreasing the production of Aβ40 in vivo in a gene dose-dependent manner, in agreement with in vitro work reported on this mutation (22). This process seems to occur without significantly altering PS1 endoproteolysis, but in the presence of a significant increase in the levels of α- and β-secretase-mediated proteolytic fragments of AβPP. Increased levels of AβPP CTFs were reported in cells transfected with PSEN1-L166P (22, 23) and may result from a reduced γ-secretase activity rather than from an increase in α- and β-secretase processing of AβPP since both AβPP CTFs increase approximately in the same extent, in both the cerebral cortex and hippocampus (34, 35). The pathological consequence of the accumulation of AβPP CTFs (observed in this model and in patients with AD) is still not completely understood, since accumulation of CTFs may lead to abnormal intracellular signaling and synapse function (6). In addition, accumulated AβPP CTFβs could later be processed by γ-secretase generating Aβ leading to additional aggregation (36) or generate CTFγ (AβPP intracellular domain), which can cause neuronal dysfunction and tau hyperphosphorylation (37, 38).
Current β-amyloid models are based on the expression (or overexpression) of a mutant AβPP sequence within the murine brain; however, in humans the brain and also several tissues in the periphery express AβPP isoforms and harbor substantial levels of Aβ peptides, with unknown implications for a therapeutic approach for AD, most significantly immunotherapy (9). Moreover, all AβPP cDNA-based transgenic mouse models engineered thus far express human FAD mutations associated with early-onset FAD. The success of these models is based on the finding of amyloid deposition, which is driven by the use of strong neuronal promoters and the presence of mutations in the AβPP cDNA sequence. This, in term, may lead to an abnormal pattern of expression and proteolytic cleavage of the mutant AβPP, with the generation of amyloid peptide species that may be peculiar for the FAD mutation (9). The APP YAC × Psen1-L166P model will allow testing of agents aimed at reducing amyloid deposition in a more appropriate setting since the model express alternatively spliced WT AβPP in the brain and other tissues and develop cerebral amyloidosis. Moreover, it will allow the study of the role of different AβPP isoforms in the pathogenesis of AD and to test drugs aimed at inhibiting expression of the human AβPP gene or at altering splicing of human AβPP, which cannot be tested in transgenic models or in murine Aβpp-knock-in models (13, 32). AβPP695 is the isoform that is predominantly expressed in neurons, whereas AβPP751 and AβPP770 isoforms are expressed in most tissues. In Down syndrome and a familial form of AD, there is evidence for a gene-dose effect leading to an increase in AβPP expression (1). However, this has not been seen in sporadic AD, where it has been reported a shift in the ratio between AβPP770 and AβPP751 to AβPP695. These 2 isoforms seem to be elevated in AD brain and may be associated with increased Aβ deposition (39), suggesting that dysregulation of AβPP splicing may have a role in the pathogenesis of AD.
In summary, we have established a new animal model that more faithfully resembles FAD and sporadic AD by having only 2 copies of the entire human WT AβPP gene in combination with the murine Psen1-L166P mutation. Since the model shows high levels of the highly amyloidogenic Aβ42 peptide and develops amyloid deposition, it will allow a more systematic study of Aβ levels, plaque formation, and neurodegeneration in a more relevant environment, without artifacts due to overexpression or use of exogenous promoters. These characteristics may be of critical importance in order to assess the effectiveness of therapeutic strategies.
Acknowledgments
The authors thank Debra Lucas and Rose Richardson for technical assistance and Dr. Jose Bonnin for helpful comments. The authors acknowledge the help of the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core (Charlottesville, VA, USA) for the determination of FSH and LH levels in serum.
This study was supported by U.S. National Institute on Neurological Disorders and Stroke grants NS050227 (R.V.) and NS14426 (B.G.) and U.S. National Institute on Aging grants AG037338 (R.V.) and AG10133 (B.G.).
Author contributions: R.V. and B.G. conceived and designed the experiments; R.V., N.S., L.M., and H.J.G. performed the experiments; R.V., N.S., H.J.G., K.S., B.T.L., and B.G. analyzed the data; R.V. and B.G. wrote the paper.
Footnotes
- Aβ
- amyloid-β
- AβPP
- amyloid-β precursor protein
- Ab
- antibody
- AD
- Alzheimer disease
- APH1
- anterior pharynx defective 1
- BACE1
- β-site AβPP-cleaving enzyme 1
- CAA
- cerebral amyloid angiopathy
- CTF
- carboxyl-terminal fragment
- ES
- embryonic stem
- FAD
- familial Alzheimer disease
- FSH
- follicle-stimulating hormone
- GFAP
- glial fibrillary acidic protein
- LH
- luteinizing hormone
- NFT
- neurofibrillary tangle
- NICD
- Notch intracellular domain
- NTF
- N-terminal fragment
- Pen2
- PS enhancer 2
- PHF
- paired helical filament
- PS
- presenilin
- PSEN
- presenilin
- Th-S
- thioflavin S
- WT
- wild-type
- YAC
- yeast artificial chromosome
REFERENCES
- 1. Holtzman D. M., Morris J. C., Goate A. M. (2011) Alzheimer's disease: the challenge of the second century. Sci. Transl. Med. 3(77), 77sr1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cole S. L., Vassar R. (2008) The role of amyloid precursor protein processing by BACE1, the beta-secretase, in Alzheimer disease pathophysiology. J. Biol. Chem. 283, 29621–29625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang Y. W., Thompson R., Zhang H., Xu H. (2011) APP processing in Alzheimer's disease. Mol. Brain. 4, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wolfe M. S. (2006) The gamma-secretase complex: membrane-embedded proteolytic ensemble. Biochemistry 45, 7931–7939 [DOI] [PubMed] [Google Scholar]
- 5. Hebert S. S., Serneels L., Dejaegere T., Horre K., Dabrowski M., Hébert S. S., Serneels L., Dejaegere T., Horré K., Dabrowski M., Baert V., Annaert W., Hartmann D., De Strooper B. (2004) Coordinated and widespread expression of gamma-secretase in vivo: evidence for size and molecular heterogeneity. Neurobiol. Dis. 17, 260–272 [DOI] [PubMed] [Google Scholar]
- 6. Parent A. T., Thinakaran G. (2010) Modeling presenilin-dependent familial Alzheimer's disease: emphasis on presenilin substrate-mediated signaling and synaptic function. Int. J. Alzheimers Dis. 2010, pii: 825918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crews L., Rockenstein E., Masliah E. (2010) APP transgenic modeling of Alzheimer's disease: mechanisms of neurodegeneration and aberrant neurogenesis. Brain Struct. Funct. 214, 111–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Götz J., Ittner L. M. (2008) Animal models of Alzheimer's disease and frontotemporal dementia. Nat. Rev. Neurosci. 9, 532–544 [DOI] [PubMed] [Google Scholar]
- 9. Kokjohn T. A., Roher A. E. (2009) Amyloid precursor protein transgenic mouse models and Alzheimer's disease: understanding the paradigms, limitations, and contributions. Alzheimers Dement. 5, 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ashe K. H., Zahs K. R. (2010) Probing the biology of Alzheimer's disease in mice. Neuron 66, 631–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Murrell J., Evans R. M., Boyer P. J., Piccardo P., Towfighi J., Ghetti B. (2000) Alzheimer disease with onset in adolescence due to a novel mutation in presenilin 1 (L166P). J. Neuropathol. Exp. Neurol. 59, 187 (abstr.) [Google Scholar]
- 12. Lamb B. T., Sisodia S. S., Lawler A. M., Slunt H. H., Kitt C. A., Kearns W. G., Pearson P. L., Price D. L., Gearhart J. D. (1993) Introduction and expression of the 400 kilobase amyloid precursor protein gene in transgenic mice. Nat. Genet. 5, 22–30 [DOI] [PubMed] [Google Scholar]
- 13. Guo Q., Wang Z., Li H., Wiese M., Zheng H. (2012) APP physiological and pathophysiological functions: insights from animal models. Cell Res. 22, 78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Barbeito A. G., Garringer H. J., Baraibar M. A., Gao X., Arredondo M., Núñez M. T., Smith M. A., Ghetti B., Vidal R. (2009) Abnormal iron metabolism and oxidative stress in mice expressing a mutant form of the ferritin light polypeptide gene. J. Neurochem. 109, 1067–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vidal R., Miravalle L., Gao X., Barbeito A. G., Baraibar M. A., Hekmatyar S. K., Widel M., Bansal N., Delisle M B., Ghetti B. (2008) Expression of a mutant form of the ferritin light chain gene induces neurodegeneration and iron overload in transgenic mice. J. Neurosci. 28, 60–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vidal R., Barbeito A. G., Miravalle L., Ghetti B. (2009) Cerebral amyloid angiopathy and parenchymal amyloid deposition in transgenic mice expressing the Danish mutant form of human BRI2. Brain Pathol. 19, 58–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Miravalle L., Calero M., Takao M., Roher A. E., Ghetti B., Vidal R. (2005) Amino-terminally truncated Aβ peptide species are the main component of cotton wool plaques. Biochemistry 44, 10810–10821 [DOI] [PubMed] [Google Scholar]
- 18. Johnson-Wood K., Lee M., Motter R., Hu K., Gordon G., Barbour R., Khan K., Gordon M., Tan H., Games D., Lieberburg I., Schenk D., Seubert P., McConlogue L. (1997) Amyloid precursor protein processing and A β42 deposition in a transgenic mouse model of Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 94, 1550–1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vinters H. V. (1987) Cerebral amyloid angiopathy: a critical review. Stroke 18, 311–324 [DOI] [PubMed] [Google Scholar]
- 20. Mucke L., Masliah E., Yu G. Q., Mallory M., Rockenstein E. M., Tatsuno G., Hu K., Kholodenko D., Johnson-Wood K., McConlogue L. (2000) High-level neuronal expression of Aβ 1-42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J. Neurosci. 20, 4050–4058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. (1991) Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 30, 572–580 [DOI] [PubMed] [Google Scholar]
- 22. Moehlmann T., Winkler E., Xia X., Edbauer D., Murrell J., Capell A., Kaether C., Zheng H., Ghetti B., Haass C., Steiner H. (2002) Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Aβ 42 production. Proc. Natl. Acad. Sci. U. S. A. 99, 8025–8030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bentahir M., Nyabi O., Verhamme J., Tolia A., Horré K, Wiltfang J., Esselmann H., De Strooper B. (2006) Presenilin clinical mutations can affect gamma-secretase activity by different mechanisms. J. Neurochem. 96, 732–742 [DOI] [PubMed] [Google Scholar]
- 24. Radde R., Bolmont T., Kaeser S. A., Coomaraswamy J., Lindau D., Stoltze L., Calhoun M. E., Jäggi F., Wolburg H., Gengler S., Haass C., Ghetti B., Czech C., Hölscher C., Mathews P. M., Jucker M. (2006) Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 7, 940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Saito T., Suemoto T., Brouwers N., Sleegers K., Funamoto S., Mihira N., Matsuba Y., Yamada K., Nilsson P., Takano J., Nishimura M., Iwata N., Van Broeckhoven C., Ihara Y., Saido T. C. (2011) Potent amyloidogenicity and pathogenicity of Aβ43. Nat. Neurosci. 14, 1023–1032 [DOI] [PubMed] [Google Scholar]
- 26. Kopan R., Ilagan M. X. (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Trombly D. J., Woodruff T. K., Mayo K. E. (2009) Suppression of Notch signaling in the neonatal mouse ovary decreases primordial follicle formation. Endocrinology 150, 1014–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Herzig M. C., Winkler D. T., Burgermeister P., Pfeifer M., Kohler E., Schmidt S. D., Danner S., Abramowski D., Stürchler-Pierrat C., Bürki K., van Duinen S. G., Maat-Schieman M. L. C., Staufenbiel M., Mathews P. M., Jucker M. (2004) Aβ is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat. Neurosci. 7, 954–960 [DOI] [PubMed] [Google Scholar]
- 29. Higgins L. S., Catalano R., Quon D., Cordell B. (1993) Transgenic mice expressing human β-APP751, but not mice expressing β-APP695, display early Alzheimer's disease-like histopathology. Ann. N. Y. Acad. Sci. 695, 224–227 [DOI] [PubMed] [Google Scholar]
- 30. Casas C., Sergeant N., Itier J. M., Blanchard V., Wirths O., van der Kolk N., Vingtdeux V., van de Steeg E., Ret G., Canton T., Drobecq H., Clark A., Bonici B., Delacourte A., Benavides J., Schmitz C., Tremp G., Bayer T. A., Benoit P., Pradier L. (2004) Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Aβ42 accumulation in a novel Alzheimer transgenic model. Am. J. Pathol. 165, 1289–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Flood D. G., Reaume A. G., Dorfman K. S., Lin Y. G., Lang D. M., Trusko S. P., Savage M. J., Annaert W. G., De Strooper B., Siman R., Scott R. W. (2002) FAD mutant PS-1 gene-targeted mice: increased Aβ42 and Aβ deposition without APP overproduction. Neurobiol. Aging 23, 335–348 [DOI] [PubMed] [Google Scholar]
- 32. Duyckaerts C., Potier M. C., Delatour B. (2008) Alzheimer disease models and human neuropathology: similarities and differences. Acta Neuropathol. 115, 5–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Scheuner D., Eckman C., Jensen M., Song X., Citron M., Suzuki N., Bird T. D., Hardy J., Hutton M., Kukull W., Larson E., Levy-Lahad E., Viitanen M., Peskind E., Poorkaj P., Schellenberg G., Tanzi R., Wasco W., Lannfelt L., Selkoe D., Younkin S. (1996) Secreted amyloid beta-protein similar to that in the senile plaques of Alzheimer's disease is increased in vivo by the presenilin 1 and 2 and APP mutations linked to familial Alzheimer's disease. Nat. Med. 2, 864–870 [DOI] [PubMed] [Google Scholar]
- 34. De Jonghe C., Esselens C., Kumar-Singh S., Craessaerts K., Serneels S., Checler F., Annaert W., Van Broeckhoven C., De Strooper B. (2001) Pathogenic APP mutations near the γ-secretase cleavage site differentially affect Aβ secretion and APP C-terminal fragment stability. Hum. Mol. Genet. 10, 1665–1671 [DOI] [PubMed] [Google Scholar]
- 35. Qi Y., Morishima-Kawashima M., Sato T., Mitsumori R., Ihara Y. (2003) Distinct mechanisms by mutant presenilin 1 and 2 leading to increased intracellular levels of amyloid β-protein 42 in Chinese hamster ovary cells. Biochemistry 42, 1042–1052 [DOI] [PubMed] [Google Scholar]
- 36. Sambamurti, Greig K. N. H., Utsuki, Barnwell T. E. L., Sharma, Mazell E., Bhat C. N. R., Kindy M. S., Lahiri D. K., Pappolla M. A. (2011) Targets for AD treatment: conflicting messages from gamma-secretase inhibitors. J. Neurochem. 117, 359–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ghosal K., Vogt D. L., Liang M., Shen Y., Lamb B. T., Pimplikar S. W. (2009) Alzheimer's disease-like pathological features in transgenic mice expressing the APP intracellular domain. Proc. Natl. Acad. Sci. U. S. A. 106, 18367–18372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pimplikar S. W. (2009) Reassessing the amyloid cascade hypothesis of Alzheimer's disease. Int. J. Biochem. Cell Biol. 41, 1261–1268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Menéndez-González M., Pérez-Pinera P., Martínez-Rivera M., Calatayud M. T., Blázquez Menes B. (2005) APP processing and the APP-KPI domain involvement in the amyloid cascade. Neurodegener. Dis. 2, 277–283 [DOI] [PubMed] [Google Scholar]