Abstract
Fluid and electrolyte homeostasis is integral to blood pressure regulation. However, the central molecular mechanisms regulating the neural control of sodium excretion remain unclear. We have demonstrated that brain Gαi2-subunit protein pathways mediate the natriuretic response to α2-adrenoreceptor activation in vivo. Consequently, we examined the role of brain Gαi2 proteins in the neural mechanisms facilitating fluid and electrolyte homeostasis in response to acute [i.v. volume expansion (VE)] or chronic stressful stimuli (dietary sodium restriction vs. supplementation) in conscious Sprague-Dawley rats. Selective oligodeoxynucleotide (ODN)-mediated down-regulation of brain Gαi2 proteins, but not a scrambled ODN, abolished the renal sympathoinhibitory response and attenuated the natriuresis to VE. In scrambled ODN-treated rats, chronic changes in dietary sodium intake evoked an endogenous, hypothalamic paraventricular nucleus (PVN)-specific, decrease (sodium deficiency) or increase (sodium excess) in PVN Gαi2 proteins; plasma norepinephrine levels were inversely related to dietary sodium content. Finally, in rats treated with an ODN to prevent high salt-induced up-regulation of brain Gαi2 proteins, animals exhibited sodium retention, global sympathoexcitation, and elevated blood pressure. Collectively, these data demonstrate that PVN Gαi2 protein pathways play an endogenous role in maintaining fluid and electrolyte balance by controlling the influence the sympathetic nervous system has on the renal handling of sodium.—Kapusta, D. R., Pascale, C. L., Wainford, R. D. Brain heterotrimeric Gαi2-subunit protein-gated pathways mediate central sympathoinhibition to maintain fluid and electrolyte homeostasis during stress.
Keywords: renal sympathetic nerve activity, RSNA, natriuresis, sodium homeostasis, blood pressure regulation, G-protein-coupled receptors
Fluid and electrolyte homeostasis is essential for life. Changes in plasma and/or cerebrospinal fluid sodium levels, as might occur following chronic changes in dietary sodium intake, are sensed by osmoreceptors or sodium-sensitive receptors present in the hypothalamic paraventricular nucleus (PVN), supraoptic nucleus (SON), and circumventricular organs (1–3), brain sites that have direct neural projections to the PVN (3–5). Further, these integrated neural pathways provide a major influence on the activity of PVN parvocellular neurons and, therefore, central sympathetic outflow (6, 7). It is well established that the renal sympathetic nerves participate in the renal handling of water and sodium and regulation of systemic arterial blood pressure (7–11). In regard to regulatory mechanisms governing these processes, central α2-adrenergic receptors are well established as a prominent system that produces renal sympathoinhibition. Increased plasma and/or cerebrospinal fluid sodium content evokes PVN-mediated sympathoinhibition, particularly to the kidneys to facilitate natriuresis and normotension in salt-resistant subjects (11, 12).
In response to alterations in plasma and cerebrospinal fluid sodium concentration, multiple brain G-protein-coupled receptor (GPCR) systems (e.g., the α2-adrenoreceptor, the GABAB receptor; refs.13–15) are activated to restore fluid and electrolyte balance through the initiation of complex downstream signaling pathways that influence sympathetic outflow. Despite having a direct interaction with GPCRs following ligand binding, the physiological role that different brain Gα-subunit proteins play in mediating the cardiovascular and renal excretory responses to central GPCR activation in vivo remains largely unknown. We have demonstrated that following central α2-adrenoreceptor stimulation in conscious rats, the observed natriuresis is selectively mediated by downstream central Gαi2, but not Gαi1, Gαi3, Gαo, or Gαs, subunit GTP-binding regulatory protein signal transduction pathways (16). The underlying mechanisms by which brain Gαi2-subunit protein-gated pathways produce the α2-adrenoreceptor-evoked natriuresis in vivo are unknown.
The aim of this study was to determine the physiological roles that brain Gαi2-subunit protein-gated signal transduction pathways play in vivo in the neural control of sodium excretion during physiologically relevant stimuli that challenge fluid and electrolyte homeostasis. Studies were performed in Sprague-Dawley (SD) rats to determine the role of brain Gαi2-subunit protein pathways in mediating the renal excretory responses to acute [intravenous (i.v.) isotonic saline VE] and chronic (deficiency or excess of dietary sodium intake) stimuli. These stressors each markedly affect the renal handling of sodium (and water), at least in part, by altering central sympathetic outflow to the kidneys (7, 11, 17–19). The importance of understanding the underlying cellular and signaling pathways involved in the central neural control of sodium excretion in health and disease is highlighted by the multiple pathophysiological disease states that exhibit sodium retention, including heart failure and certain models of hypertension, particularly salt-sensitive hypertension (20, 21).
Our recent findings demonstrated that central nervous system (CNS) Gαi2-subunit proteins mediate the natriuretic response to central administration of the α2-adrenoreceptor agonist guanabenz (16) and that elevated dietary salt intake decreases the endogenous expression of brain Gαq proteins in salt-resistant rats (22). Based on these observations, we hypothesize that in response to an acute sodium and water load, brain Gαi2-subunit protein-gated pathways are activated to mediate renal sympathoinhibition, thereby facilitating the renal excretion of sodium and water. Further, we hypothesize that chronic alterations in dietary sodium intake will lead to endogenous changes in brain Gαi2-subunit protein levels as a mechanism to affect central sympathetic outflow and contribute to daily sodium and water homeostasis.
MATERIALS AND METHODS
Animals
Male SD rats (Harlan Laboratories Inc., Indianapolis, IN, USA), 275–300 g, were housed individually under a 12-h light-dark cycle. For these investigations, rats were randomly assigned to experimental treatment groups in which total body sodium and water homeostasis was challenged by either an acute isotonic saline VE or an alteration in dietary sodium intake for 1 wk. Rats assigned to the acute VE study were allowed tap water and standard rodent diet (TestDiet; Purina Mills, St. Louis, MO, USA) ad libitum. For dietary sodium challenge studies, rats were maintained for 7 d on either a normal, low, or high sodium intake diet as follows: rats fed a normal sodium intake were allowed tap water ad libitum and a standard control rodent diet that contained a total Na content of 0.4% (174 mEq Na+/kg); rats fed a low sodium intake were allowed tap water ad libitum and a modified low-salt diet that contained a total Na content of 0.03% (13 mEq Na+/kg; TestDiet); and rats fed a high sodium intake were allowed 0.9% saline drinking water (154 mEq Na+/L) and standard 0.4% NaCl chow. All procedures were conducted in accordance with the U.S. National Institutes of Health and the Louisiana State University Health Sciences Center Institutional Animal Care and Use Committee guidelines for the care and use of animals.
Surgical procedures
Intracerebroventricular (i.c.v.) cannula implantation
For administration of drugs and vehicle into the brain, all animals used in these investigations were anesthetized [intraperitoneal (i.p.) ketamine, 30 mg/kg in combination with i.p. xylazine, 3 mg/kg] and stereotaxically implanted with a stainless steel cannula into the right lateral cerebral ventricle at least 5–7 d prior to experimentation (16, 22).
Oligodeoxynucleotide administration
At 24 h prior to study, rats were randomly assigned to receive an i.c.v. injection (25 μg/5 μl) of a scrambled (SCR; 5′-GGGCGAAGTAGGTCTTGG-3′) or a Gαi2 (5′-CTTGTCGATCATCTTAGA-3′) phosphodiesterase oligodeoxynucleotide (ODN) probe (Midland Certified Reagent Co., Midland, TX, USA) dissolved in isotonic saline (16, 22). A National Center for Biotechnology Information (NCBI) Basic Local Alignment Search Tool (BLAST) search of the Rattus norvegicus Reference Sequence (RefSeq) protein database confirmed the specificity of the Gαi2 ODN for the Gαi2 rat protein sequence and that the SCR ODN does not match any known rat protein sequence. In addition, our previous studies have demonstrated the selective nature of using an i.c.v. Gαi2 ODN pretreatment in vivo (16).
Acute cardiovascular and renal function studies
On the day of the acute VE experiment (24 h post-i.c.v. ODN pretreatment), animals were anesthetized (i.p. sodium methohexital, 20 mg/kg, supplemented with 10 mg/kg intravenously as required) and were surgically instrumented with catheters in the left femoral artery, left femoral vein, and bladder for measurement of arterial blood pressure, i.v. administration of isotonic saline, and collection of urine samples, respectively (16, 22).
For certain studies, rats (still anesthetized with sodium methohexital) were implanted with a recording electrode on a renal nerve bundle of the left kidney for direct measurement of multifiber renal sympathetic nerve activity (RSNA) in the conscious state using methods previously described (19, 23). Spike potentials were amplified (×10,000–50,000) and filtered (low, 30 Hz; high, 3000 Hz) with a Grass P511 Bandpass Amplifier (Grass Instruments, Quincy, MA, USA), and the signal was continuously recorded using a computer data acquisition system (model MP100; Biopac Systems, Santa Barbara, CA, USA).
The acute isotonic saline VE protocol was repeated in rats having undergone chronic bilateral renal denervation (RDNX). In brief, for RDNX, each kidney was exposed by a dorsal flank incision, and the renal vein and artery were stripped of all visible renal nerve bundles. The renal artery was then coated with a 10% phenol solution in ethanol to destroy any remaining renal nerve fibers (24). Renal denervation was confirmed via HPLC analysis of norepinephrine (NE) content in kidney tissue following completion of the VE protocol using established methodology. In brief, whole kidneys were homogenized in a citrate buffer (0.1 M citrate, 10% ethanol, and 250 μg/ml sodium octyl sulfate) at pH 4. The homogenate was centrifuged at 12 000 g (15 min) and frozen at −80°C until assayed. An internal standard of 3,4-dihydroxybenzylamine hydrobromide (DHBA) was added in a final concentration of 1 ng/100 μl in both unknown samples and NE standards, DHBA recovery was ∼85% in these studies. The chromatographic system consisted of Dual Rainin Rabbit HP pumps (Mettler-Toledo, Inc., Columbus, OH, USA) equipped with a self-washing piston pump head operating at a flow rate of 1.5 ml/min. The HPLC column was a Rainin microsorb C18 column (25 cm×4.6 mm; Mettler-Toledo); a 1.5-cm × 4.6-mm C18 guard column preceded the analytical column. NE was assayed by electrochemical detection utilizing an ESA model 5100 Coulchem multielectrode array (ESA, Bedford, MA, USA). NE content was determined in unknown and standard samples by comparison to retention times and integrated areas of peaks.
After surgical preparation, rats assigned to the acute VE protocol were placed in a rat holder (a chamber of stainless-steel rods connected by Plexiglas ends; the metal rods formed an inverted U shape and flat base in which the rat would sit) to minimize movement and damage to the renal nerve recording electrode preparation and to permit steady-state urine collection. An i.v. infusion of isotonic saline (20 μl/min) was then started, and the rat was allowed 2 h to regain full consciousness and allow cardiovascular and renal excretory function to stabilize (19, 22). Before the start of the experiment, the arterial catheter was flushed and attached to a pressure transducer (model P23XL; Viggo-Spectramed, Oxnard, CA, USA), and the urinary bladder catheter was led to a collection vial. Mean arterial pressure (MAP), heart rate (HR; derived from the pulse pressure), and RSNA were recorded using a computer-driven Biopac data acquisition software (MP100 and AcqKnowledge 3.8.2; Biopac Systems, Inc., Goleta, CA, USA).
Experimental protocols
VE studies
Studies were performed to examine the cardiovascular, renal excretory, and RSNA responses to acute isotonic saline VE in conscious rats pretreated (24 h) with a Gαi2 ODN or SCR ODN (25 μg; n=6/group). After surgical instrumentation and a 2-h stabilization period in which rats were infused i.v. with isotonic saline (20 μl/min), cardiovascular, renal excretory, and RSNA parameters were measured, and urine was collected during a 20-min control period. The infusate was then increased so that rats received an isotonic saline load equivalent to 5% of body weight (BW) over a 30-min period. Continuous 10-min urine samples were collected during the VE period. The isotonic saline infusate was then returned to a rate of 20 μl/min, after which urine samples were collected during a 90-min recovery period (22).
The role of intact renal nerves in mediating the cardiovascular and renal excretory responses to acute VE was examined in animals pretreated with a Gαi2 or scrambled ODN. For these studies, the same protocol as described above was repeated in rats in which the influence of the renal nerves on renal excretory function was removed via chronic RDNX.
Furosemide studies
Studies were performed to determine whether ODN-mediated down-regulation of brain Gαi2 proteins modified the ability of the kidneys to regulate water and sodium excretion during basal conditions, and to a stimulus that alters water and sodium excretion independent of the CNS. For these studies, the effects of central Gαi2 or SCR ODN pretreatment (24 h) on renal excretory function was examined in rats during a continuous infusion of isotonic saline alone (20 μl/min) during a control (20 min) and 120-min experimental period (i.e., basal renal excretory function). In other groups of rats, this protocol was repeated with the exception that following collection of control urine samples, the cardiovascular and renal excretory responses to i.v. bolus injection of the diuretic furosemide (7.5 mg/kg) were measured for 120 min.
Daily water and sodium balance studies
Studies were performed to determine how ODN-mediated down-regulation of brain Gαi2 proteins alters daily water and sodium balance during alterations in sodium intake. For these studies, rats were housed in individual metabolic cages (model 18cv; Fenco, Cataumet, MA, USA) with external food containers and water bottles. Metabolic cages were equipped with a double fine-mesh screen that allowed separation of food and feces contamination from urine, which was collected in vials that contained a layer of mineral oil to prevent urine evaporation. Rats were randomly assigned to receive a normal-, low-, or high-sodium-intake diet (see above) for 7 d. All rats were provided free access to rodent chow and allowed tap water ad libitum via external trays and bottles, respectively, and adapted to the metabolic cages for 3 d before data collection commenced. On the morning of d 7, rats in each dietary sodium group received a single i.c.v. injection of a SCR or a Gαi2 ODN probe (25 μg; n=12/group/diet) dissolved in isotonic saline. Following ODN injection, measurements were then made for BW, food and water intake, and urine output during a 24-h period, enabling calculation of daily sodium and water balance. Following completion of the metabolic balance study on the next day, the same animals were then randomly assigned to a subgroup in which they were sacrificed for measurement of plasma NE and plasma renin activity (PRA; n=6/diet group) or surgically instrumented with arterial, venous, and bladder catheters and subjected to an acute VE study (n=6/diet group; see above). Brains were collected and frozen for measurement of Gα-subunit proteins.
Analytical techniques
Urine volume was determined gravimetrically. Urine sodium (UNa) concentration was measured by flame photometry (model 943; Instrumentation Laboratories, Lexington, MA, USA) and expressed as urinary sodium excretion volume (UNaV). RSNA is expressed as microvolts. For each 10-min control and experimental period, the values for integrated RSNA were sampled over the entire collection period, and the numbers were averaged. Because of the limitations of comparing values for multifiber RSNA between animals, data for RSNA were expressed as the percentage of the control, with the baseline control values for each animal taken as 100% (23). After completion of each experiment, the level of postmortem background noise was measured, and the value was then subtracted from all control and experimental values of RSNA.
CNS tissue collection
In certain experimental groups, following completion of the VE protocol or a 7-d altered sodium challenge study, whole brains were removed and frozen at −80°C. Frontal brain cortex (BC), hypothalamic PVN, SON, posterior hypothalamus (PH) nuclei, and ventrolateral medulla (VLM) samples were extracted from frozen brains cut on a cryostat using a brain punch tool (Stoelting, Wood Dale, IL, USA) as described previously (16, 22, 23).
Gα-protein immunoblotting
Tissue lysates were prepared from frozen brain tissues, and protein levels were quantified. Lysates were resolved on SDS-PAGE gels and transferred to nitrocellulose membrane (GE Healthcare, Piscataway, NJ, USA). Gαi1–3 and Gαo levels were determined using antibodies purchased from Santa Cruz Biotechnologies (Santa Cruz, CA, USA), directed against Gαi1 (1:100; sc-391), Gαi2 (1:200; sc-13534), Gαi3 (1:1000; sc-262), and Gαo (1:200; sc-382); protein levels were normalized to GAPDH (anti-GAPDH 1:1000 ab-9483; Abcam, Cambridge, MA, USA) (16, 22, 23). Chemiluminescent immunoreactive bands were detected by a horseradish peroxidase-conjugated secondary antibody; data were imaged and semiquantified using Bio-Rad Quantity One image analysis software (Bio-Rad Laboratories, Hercules, CA). Probing with each antibody was performed sequentially following stripping of the membrane with a commercially available stripping reagent (Bio-Rad Laboratories) as per the manufacturer's instructions.
PRA and NE assays
Animals were sacrificed by decapitation, and PRA was determined using a GammaCoat PRA 125I radioactive immunoassay kit (DiaSorin, Stillwater, MN, USA), and plasma NE content was determined using NE enzyme-linked immunosorbent assay kit (Immuno-Biological Laboratories, Minneapolis, MN, USA) as per the manufacturers' instructions (n=6/group).
Statistical analysis
All data are expressed as means ± se. For a single treatment group (e.g., SCR or Gαi2 ODN) the magnitude of changes in a specific cardiovascular or renal excretory parameter occurring at different time points after initiation of the isotonic saline VE were compared with the average respective group control value by a 1-way repeated-measure analysis of variance (ANOVA) with subsequent Dunnett's test. Differences occurring between treatment groups (e.g., SCR vs. Gαi2 ODN) were assessed by a 2-way repeated-measure ANOVA, with treatment group being one fixed effect and time the other, with the interaction included. The time (min) was then the repeated factor. Post hoc analysis was performed using Bonferroni's test. Statistical significance was defined as values of probability (P<0.05).
RESULTS
Acute VE studies
ODN-mediated down-regulation of brain Gαi2 proteins attenuates the natriuretic and diuretic responses evoked by an acute i.v. isotonic saline VE
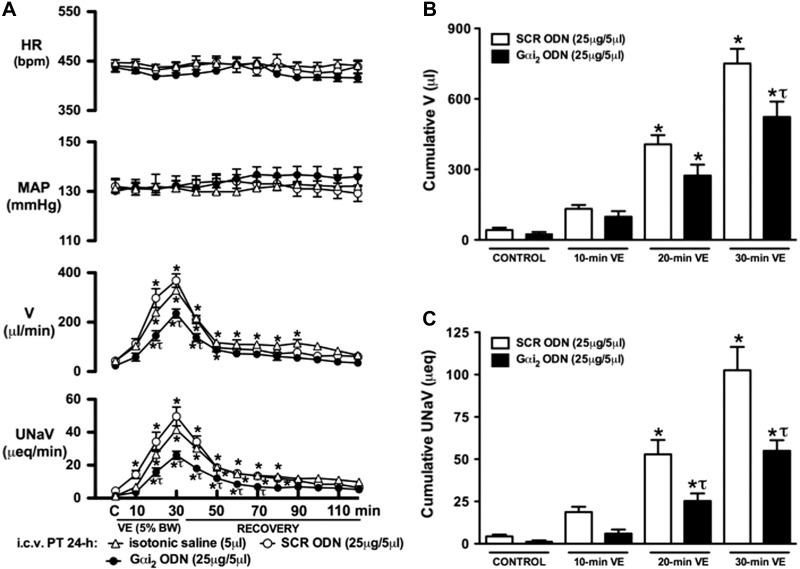
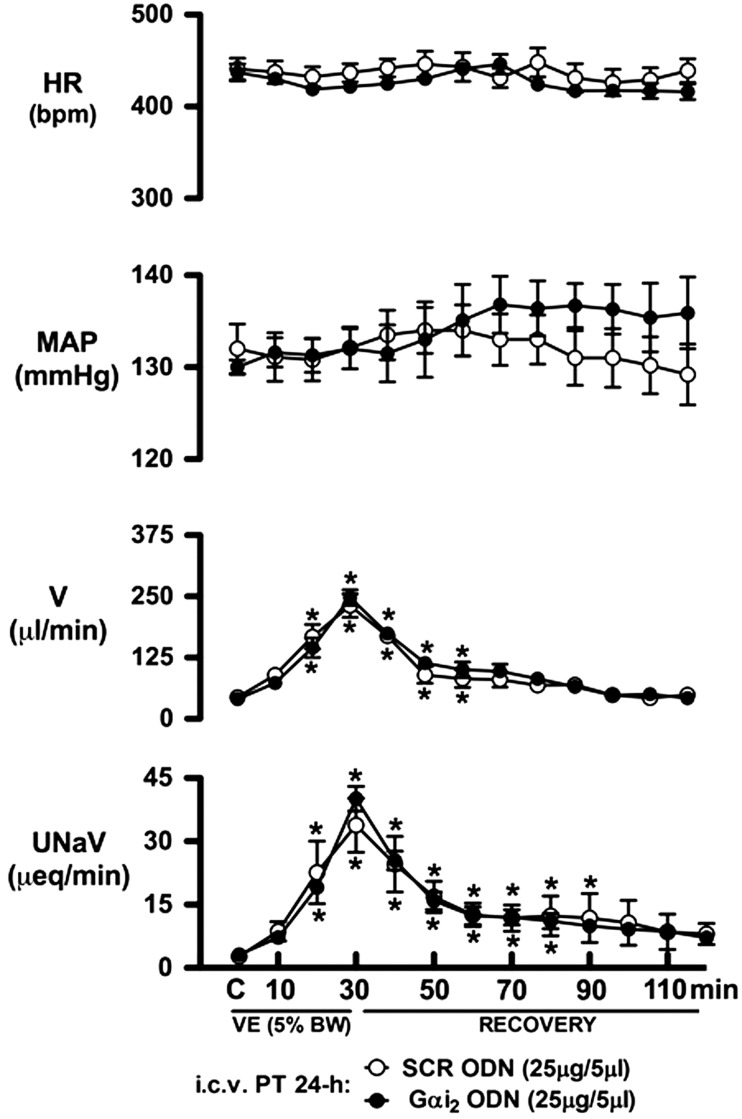
The integrated physiological stimulus of an acute i.v. isotonic saline VE evoked a profound (P<0.05) diuretic and natriuretic response in SD rats pretreated i.c.v. with isotonic saline vehicle (5 μl, 24 h; Fig. 1). As compared to this group, central pretreatment with a SCR or Gαi2 ODN (25 μg/5 μl, 24 h) did not alter the animal's BW (SCR ODN, Δ+5±3 g; Gαi2 ODN, Δ+4±3 g) or baseline cardiovascular or renal parameters during the experimental control period (Fig. 1A). In SCR ODN-pretreated rats, an acute isotonic saline VE evoked significant increases in urine flow rate (V) and urinary sodium excretion (UNaV), which were of comparable magnitude and time course as those observed in saline vehicle pretreated rats (Fig. 1A). In contrast, prior down-regulation of central Gαi2 proteins significantly attenuated the magnitude and duration of both the diuretic and natriuretic responses evoked by the acute isotonic saline load [30-min peak V (μl/min): SCR ODN pretreatment 325±36 vs. Gαi2 ODN pretreatment 211±24, P<0.05; 30-min peak UNaV (μeq/μl): SCR pretreatment 45.2±6.9 vs. Gαi2 ODN pretreatment 24.5±4.1, P<0.05]. The attenuation of these renal excretory responses resulted in significantly reduced cumulative diuretic (−30±4%) and natriuretic (−48±4%), responses during the 30-min VE period in SCR (Fig. 1B, C; open bars) vs. Gαi2 (Fig. 1B, C; solid bars) ODN pretreatment groups. In these studies, during control, 30-min VE, and 90-min recovery periods, arterial blood pressure and HR did not change in rats pretreated with i.c.v.saline vehicle or a SCR/Gαi2 ODN (Fig. 1A).
Figure 1.
Changes in systemic cardiovascular and renal excretory function (A), cumulative urine output (B), and cumulative urinary sodium excretion (C) during an i.v. isotonic saline VE (5% BW over 30 min) in SD rats pretreated with i.c.v. isotonic saline vehicle (5 μl), SCR, or Gαi2 ODN (25 μg/5 μl; 24 h). Values are means ± se (n=6/group). C, control. *P < 0.05 compared with respective control group value. τP < 0.05 compared with respective i.c.v. SCR ODN group value.
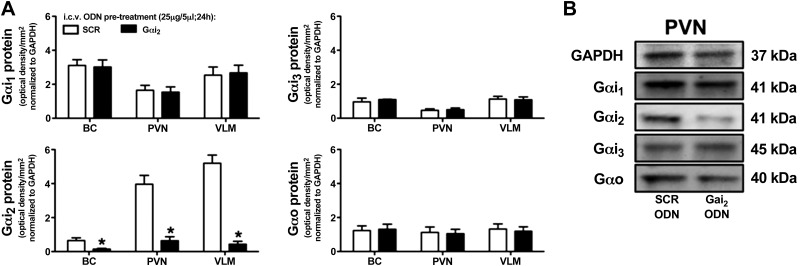
To confirm the selectivity and efficacy of using a single administration of a targeted Gαi2 ODN, we examined the expression of Gαi1-, Gαi2-, Gαi3-, and Gαo-subunit proteins in the BC, PVN, and VLM of SCR vs. Gαi2 ODN-pretreated animals for which the physiological data are presented in Fig. 1. In these animals, central pretreatment with a Gαi2 ODN significantly (P<0.05) and selectively reduced Gαi2 protein expression levels in the BC, PVN, and VLM compared to animals pretreated with a nonspecific control SCR ODN sequence (Fig. 2).
Figure 2.
A) Effect of i.c.v. SCR or Gαi2 ODN pretreatment (25 μg/5 μl; 24 h) on brain Gαi2-subunit protein levels normalized to GAPDH and expressed as optical density units per square millimeter in the BC, hypothalamic PVN, and VLM of male SD rats. Values are means ± se (n=6/group). B) Representative immunoblots illustrating GAPDH and Gα-subunit protein levels in the PVN from male SD rats pretreated with a SCR or Gαi2 ODN. *P < 0.05 vs. i.c.v. SCR ODN group.
ODN-mediated down-regulation of brain Gαi2 proteins does not adversely affect ability of the kidneys to alter water and sodium excretion
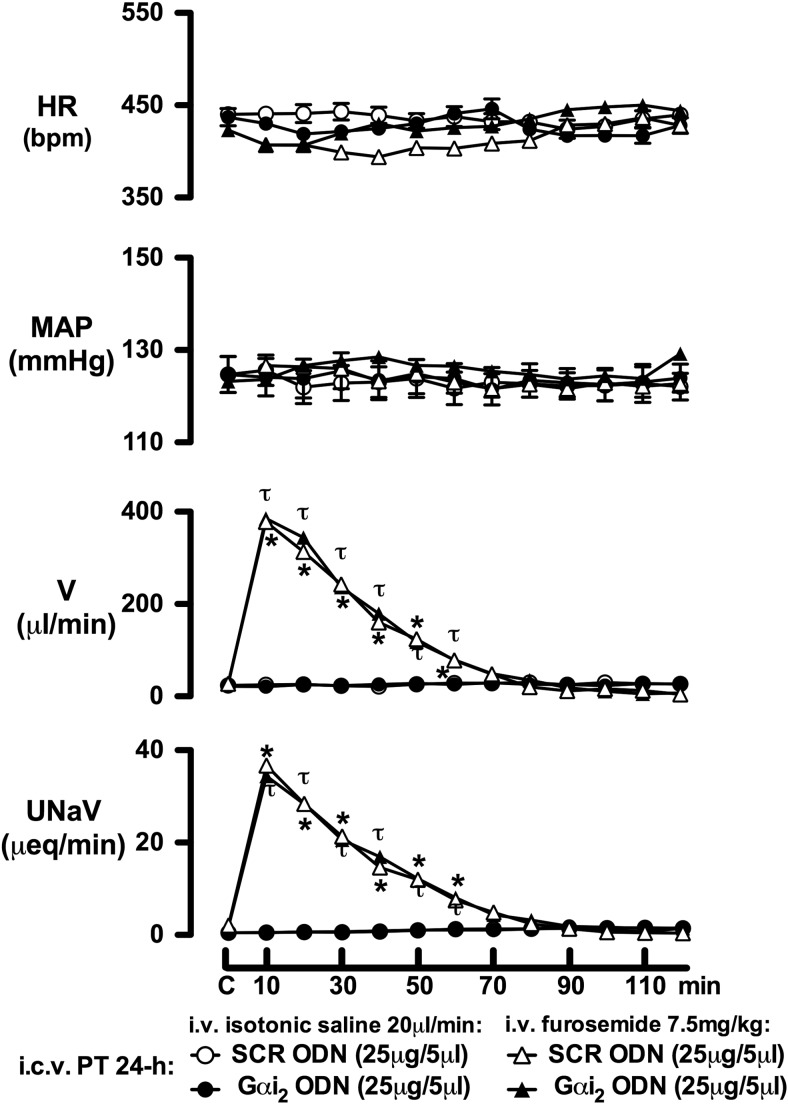
Studies were performed to establish that ODN-mediated down-regulation of brain Gαi2 proteins does not adversely affect the ability of the kidneys to modulate water and sodium excretion. For these studies, separate groups of animals were pretreated (24 h) with either a SCR or Gαi2 ODN, and on the next day, the renal responses to the continuous infusion of isotonic saline alone (20 μl/min) or i.v. bolus injection of the loop diuretic furosemide (7.5 mg/kg) were examined. As illustrated in Fig. 3, down-regulation of central Gαi2 proteins did not alter basal levels for urine flow rate or urinary sodium excretion in rats infused with saline. Further, central Gαi2 ODN pretreatment did not alter the marked diuretic and natriuretic responses to i.v. bolus furosemide compared to those observed in rats pretreated with a SCR ODN.
Figure 3.
Systemic cardiovascular and renal excretory responses produced by i.v. bolus furosemide (7.5 mg/kg) or continuous isotonic saline infusion (20 μl/min) in SD rats. Prior to study, rats were pretreated with either an i.c.v. SCR or Gαi2 ODN (25 μg/5 μl; 24 h). Values are means ± se (n=6/group). C, control. *P < 0.05 vs. respective i.v. furosemide i.c.v. SCR ODN pretreatment group control value; τP < 0.05 vs. respective i.v. furosemide i.c.v. Gαi2 ODN pretreatment group control value.
Activation of brain Gαi2 protein pathways contributes to maximal water and sodium excretion during an isotonic saline load by an inhibitory influence on renal sympathetic nerve activity
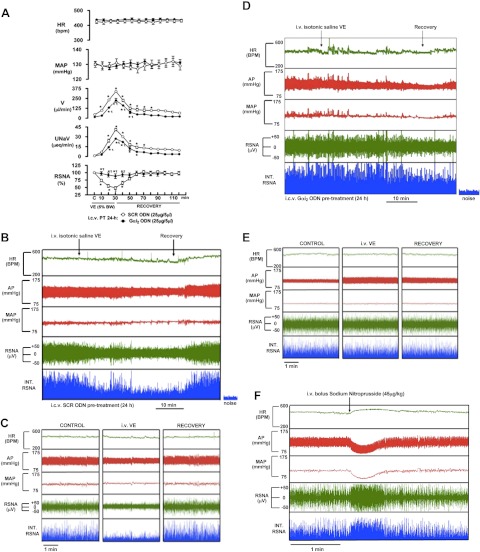
In SCR ODN-treated rats implanted with a renal nerve recording electrode, isotonic saline VE produced a concurrent and significant (P<0.05) increase in urine flow rate and urinary sodium excretion, and decrease in RSNA. The renal sympathoinhibition was maximal after 30 min of the volume load and returned to baseline within 30 min of cessation of this stimulus (Fig. 4A–C). In contrast to these responses, Gαi2 ODN pretreatment (24 h) of rats significantly attenuated the diuretic and natriuretic responses and abolished the suppression of RSNA evoked by the VE [peak ΔRSNA (% control): SCR ODN pretreatment 47±5 vs. Gαi2 93±8, P<0.05; Fig. 4A, D, E]. Indicating the viability of the renal sympathetic nerve recording in the same Gαi2 ODN-pretreated animals, we observed a rapid and profound increase in RSNA in response to an i.v. bolus of sodium nitroprusside (Fig. 4F).
Figure 4.
A) Systemic cardiovascular and renal excretory responses produced by acute isotonic saline VE (5% BW over 30 min) in rats in which RSNA was measured. Prior to start of these studies, rats were pretreated with an i.c.v. SCR or Gαi2 ODN (25 μg/5 μl; 24 h). Values are means ± se (n=6/group). B) Representative tracing obtained from the Biopac data acquisition system illustrating typical HR, arterial pressure (AP), MAP, RSNA, and integrated RSNA (INT. RSNA) responses to an i.v. 5% BW isotonic saline VE in a SCR ODN-pretreated rat. C) Five-minute tracings taken during control, VE, and recovery periods from the tracing shown in panel B in an SCR ODN-pretreated rat. D) Representative tracing obtained from the Biopac data acquisition system illustrating typical HR, AP, MAP, RSNA, and INT. RSNA responses to an i.v. 5% BW isotonic saline VE in a Gαi2 ODN-pretreated rat. E) Five-minute tracings taken during control, VE, and recovery periods from the tracing in D in a Gαi2 ODN-pretreated rat. F) Representative tracing obtained from the Biopac data acquisition system illustrating typical HR, AP, MAP, RSNA, and INT. RSNA responses to an i.v. bolus administration of sodium nitroprusside (45 μg/kg) in the same Gαi2 ODN-pretreated rat as in D and E. *P < 0.05 vs. respective control group; τP < 0.05 vs. respective i.c.v. SCR ODN group.
In rats having undergone chronic bilateral RDNX, central Gαi2 ODN pretreatment failed to alter the pattern, magnitude, or duration of either the natriuretic [peak UNaV (μeq/μl): RDNX SCR ODN pretreatment 38.0±4.6 vs. RDNX Gαi2 ODN pretreatment 41.0±2.9] or diuretic responses [peak V (μl/min): RDNX SCR ODN pretreatment 231±24 vs. RDNX Gαi2 ODN pretreatment 247±16] to acute i.v. isotonic saline VE (Fig. 5). In these studies, efficacy of the RDNX procedure was verified by a significant reduction in norepinephrine content in kidneys from denervated animals [NE (ng/mg wet tissue weight): intact SCR ODN pretreatment 2.04±0.3 vs. RDNX SCR ODN pretreatment 0.09±0.04, RDNX Gαi2 ODN pretreatment 0.06±0.02, P<0.05].
Figure 5.
Systemic cardiovascular and renal excretory responses produced by acute isotonic saline VE (5% BW over 30 min) in rats with chronic RDNX. Prior to start of these studies, rats were pretreated with an i.c.v. SCR or Gαi2 ODN (25 μg/5 μl; 24 h). Values are means ± se (n=6/group). *P < 0.05 vs. respective control group.
Daily water and sodium balance studies
Following 7 d of consumption of normal-, low-, or high-sodium diet, studies were performed to determine the relationship between how alterations in daily salt intake affect Gαi2-subunit protein expression in different brain regions (Fig. 6), and 24-h sodium and water balance (Fig. 7A, B). Following completion of this 24-h metabolic study, subgroups of rats were then sacrificed for measurement of plasma NE (Fig. 7C) and PRA (Fig. 7D) and collection of brain tissue for measurement of protein expression or implanted with instruments for an acute VE study (Fig. 7E, F).
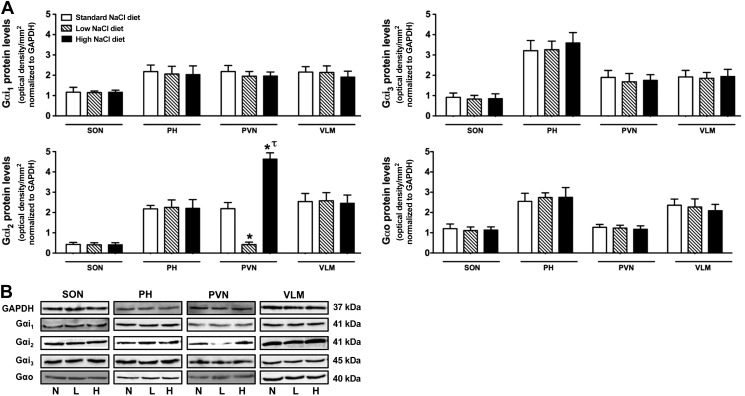
Figure 6.
A) Effects of altered dietary sodium intake on brain Gα-subunit expression in SD rats pretreated with a SCR ODN (25 μg/5 μl; 24 h). For these studies, rats were maintained on either a normal-, low-, or high-sodium diet for 7 d prior to SCR ODN injection. Gα-subunit protein expression was normalized to GAPDH and expressed as optical density units per square millimeter in the SON, PH nuclei, hypothalamic PVN, and VLM. Values are means ± se (n=6/group). B) Representative immunoblots illustrating GAPDH and Gα-subunit protein levels in the SON, PH, PVN, and VLM from male SD rats for which group data are presented in panel A. N, normal salt intake; L, low salt intake; H, high-salt intake. *P < 0.05 vs. respective normal-sodium-intake group; τP < 0.05 vs. respective low-sodium-intake group.
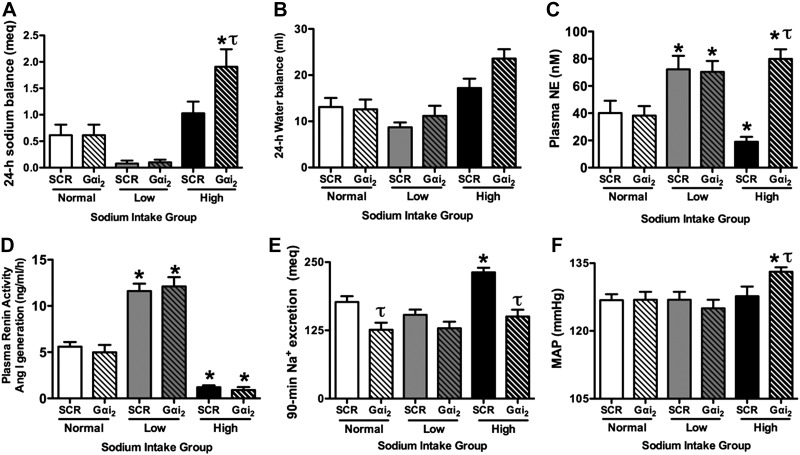
Figure 7.
Twenty-four-hour sodium balance (A), 24-h water balance (B), circulating plasma NE levels (C) PRA (expressed as Ang I generation; D), cumulative 90-min sodium excretion in response to an acute 5% isotonic saline VE (E), and mean arterial pressure (F) in male SD rats maintained for 7 d on a normal-, low-, or high-sodium-intake diet, pretreated with either an i.c.v. SCR or Gαi2 ODN (25 μg/5 μl; 24 h). Values are means ± se (n=6/group). *P < 0.05 vs. respective i.c.v. ODN group on normal salt intake; τP < 0.05 vs. i.c.v. SCR ODN group on same sodium intake.
Alteration in daily salt intake influences endogenous brain Gαi2-subunit protein expression
When maintained on a standard sodium intake (0.4% NaCl), SD rats expressed Gαi2-subunit proteins at comparable levels in the PH, PVN, and VLM, with lower levels of Gαi2 protein observed in the SON (Fig. 6A). In comparison, rats fed a 7-d low- or high-salt diet exhibited an endogenous change in Gαi2-subunit protein expression, which was selective to the PVN. Whereas dietary sodium restriction evoked a 5-fold reduction in PVN Gαi2 protein levels, dietary sodium supplementation evoked an approximate 2-fold increase in PVN Gαi2 protein expression [PVN Gαi2 protein levels (ODU/mm2 normalized to GAPDH): standard NaCl diet 2.2±0.4 vs. low NaCl diet 0.42±0.7 vs. high NaCl diet 4.64±0.4, P<0.05; Fig. 6]. In these studies, dietary sodium restriction or supplementation for 7 d had no detectable effect on the expression of Gαi1-, Gαi3-, or Gαo-subunit proteins in any brain region examined (Fig. 6).
Central Gαi2-subunit protein-gated pathways regulate central sympathetic outflow to influence sodium homeostasis and blood pressure regulation in SD rats
As shown in Fig. 7A, B, following 7 d of a normal or low sodium intake, central Gαi2 ODN treatment did not alter 24-h sodium balance as compared to levels for SCR ODN-treated rats. However, in rats fed a high-sodium diet, central Gαi2 ODN treatment significantly increased 24-h sodium balance [Na+ balance (meq/24 h): SCR ODN high NaCl 1.02±0.2 vs. Gαi2 ODN high NaCl 1.9±0.3, P<0.05; Fig. 7A]. In contrast, 24-h water balance was not significantly different between groups of rats pretreated with a Gαi2 ODN vs. SCR ODN (Fig. 7B). In SCR ODN-treated rats, a low-sodium diet stimulated increases in plasma NE concentration (Fig. 7C) and PRA (Fig. 7D) above respective levels observed in rats fed a normal-sodium diet. The low-salt-induced increase in these neural and humoral pathways, respectively, was not altered in rats pretreated with a Gαi2 ODN (Fig. 7C, D). In contrast, in rats previously fed a diet high in sodium, Gαi2 ODN treatment completely prevented the decrease in plasma NE levels [plasma NE (nM): SCR ODN high salt 19±4 vs. Gαi2 ODN high salt 80±7, P<0.05; Fig. 7C], without preventing the high-salt-induced suppression of PRA (Fig. 7D). As illustrated, Gαi2 ODN treatment significantly impaired the natriuretic response to acute isotonic saline VE in rats fed a normal- and high-sodium intake diet (Fig. 7E). Finally, as compared to levels observed in rats that received an i.c.v. injection of a SCR ODN, basal MAP was significantly (P<0.05) elevated in Gαi2 ODN-treated rats maintained on a high-salt diet (Fig. 7F).
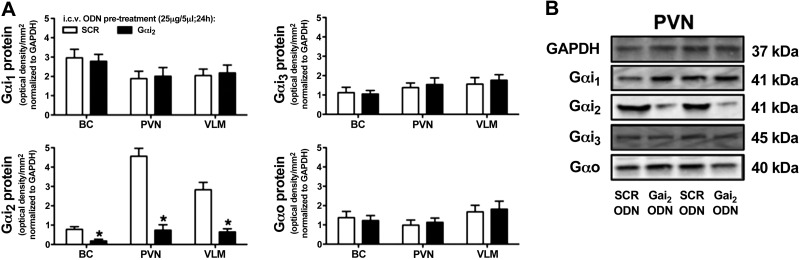
Following the completion of experiments performed in rats fed a high-salt diet (Fig. 7), we examined how pretreatment with either the SCR or Gαi2 ODN altered Gα-subunit expression in different brain regions. As shown in Fig. 8, central pretreatment (25 μg, 24 h) of rats with a Gαi2 ODN significantly (P<0.05) and selectively reduced respective Gαi2 protein expression levels in the BC, PVN, and VLM as compared to animals pretreated with a nonspecific control SCR ODN sequence.
Figure 8.
A) Effect of i.c.v. SCR or Gαi2 ODN pretreatment (25 μg/5 μl; 24 h) on brain Gαi2-subunit protein levels normalized to GAPDH and expressed as optical density units/mm2 in the BC, hypothalamic PVN, and VLM of male SD rats maintained for 7 d on a high-sodium-intake diet. Values are means ± se (n=6/group). B) Representative immunoblots illustrating GAPDH and Gα-subunit protein levels in the PVN from male SD rats pretreated with a SCR or Gαi2 ODN maintained for 7 d on a high-sodium-intake diet. *P < 0.05 vs. i.c.v. SCR ODN group.
DISCUSSION
Changes in central sympathetic outflow to the kidneys play a pivotal role in controlling the renal excretion of sodium and water during stressful conditions that perturb fluid and electrolyte homeostasis (7–11). However, to our knowledge, the current study is the first to show that brain Gαi2-subunit protein signaling pathways are essential for promoting maximal natriuresis and diuresis. The findings in this study demonstrate that in conscious SD rats, ODN-mediated down-regulation of brain Gαi2-subunit proteins significantly attenuated the natriuretic and diuretic responses to an acute i.v. isotonic saline VE. In contrast, the natriuretic and diuretic responses to i.v. bolus administration of furosemide (loop diuretic) were maintained in ODN-treated rats, in which expression levels for brain Gαi2 proteins were decreased. Together, these data reveal the presence of a central molecular pathway, mediated by brain Gαi2-subunit proteins, that directly influences the renal handling of sodium and water.
The physiological stimulus of an i.v. isotonic saline VE increases urinary sodium and water excretion, in part, by the inhibition of RSNA (17, 19, 25). The present results clearly demonstrate that central Gαi2 proteins are involved in mediating the renal excretory responses to this acute stimulus via activating a downstream inhibitory influence on RSNA. Two lines of evidence substantiate this premise. First, in rats implanted with a renal nerve recording electrode, prior ODN-mediated down-regulation of brain Gαi2-subunit proteins concurrently abolished the suppression of RSNA and blunted the natriuresis and diuresis to the i.v. isotonic saline load. Second, chronic bilateral renal denervation, a technique used to remove influence of the renal sympathetic nerves on kidney function, prevented Gαi2 protein down-regulation from altering the renal excretory responses to the i.v. VE. Central α2-adrenoceptors are the principal brain GPCRs involved in producing renal sympathoinhibition, and consequently enhanced sodium and water excretion, in response to acute VE (25). Therefore, our current findings integrate fully with our previous report that brain Gαi2-subunit protein-gated pathways mediate the natriuretic response to central α2-adrenoreceptor activation in conscious rats (16). Although not tested in the present investigations, these findings suggest that during VE central α2-adrenoreceptor pathways may be activated and contribute to increased renal excretion of sodium and water via a renal sympathoinhibitory pathway that is dependent on brain Gαi2-subunit proteins.
Based on the findings noted above, we next examined how brain Gαi2 protein pathways might be involved in regulating the renal excretion of water and sodium during conditions of sustained alterations in dietary sodium intake. In these studies, subchronic (7 d) dietary sodium restriction, or excess, had no effect on expression levels for Gαi1-, Gαi3-, or Gαo-subunit proteins in any brain site examined. This is in agreement with our report that in conscious rats, these specific brain Gα-subunit proteins are not involved in mediating either the hypotension or natriuresis to central α2-adrenoreceptor stimulation (16). However, in these same animals, a sustained alteration in dietary sodium intake caused endogenous changes in Gαi2 protein expression levels, which were specific to the PVN. In response to chronic sodium restriction, PVN Gαi2 protein levels were markedly decreased. In contrast, excess dietary sodium intake triggered a significant up-regulation of Gαi2 protein levels in the PVN. These changes in Gαi2 expression were highly specific to the PVN and did not occur in neighboring hypothalamic regions (i.e., SON, posterior hypothalamus) or other brain sites examined (VLM). The observation that site-specific changes in PVN Gαi2 protein expression occur in response to differences in salt intake is of considerable pathophysiological interest, since this brain site plays a key role in the central control of sympathetic outflow in health, stress, and disease (5, 6, 12).
To examine the potential significance of the above findings, we examined how ODN-mediated down-regulation of brain Gαi2 protein levels would affect 24-h sodium and water balance in rats that had previously adapted (over 7 d) to a change in dietary sodium intake. In rats fed a normal or sodium-restricted diet, ODN-mediated knockdown of brain Gαi2 proteins did not alter daily sodium or water balance or resting MAP from levels observed in SCR ODN-treated animals. Further, in rats placed on a low-sodium diet, down-regulation of brain Gαi2 protein pathways did not prevent the expected increases in plasma NE levels and renin activity, both of which are markers of global sympathoexcitation and activation of sodium-retaining pathways. These findings are not unexpected in light of our finding that Gαi2 protein pathways are instead involved in central pathways that mediate inhibition of RSNA and natriuresis/diuresis (e.g., as to an acute volume load). These findings do, however, highlight the importance of our observation that PVN Gαi2-subunit protein expression is endogenously down-regulated during conditions of a low-salt diet, presumably to maximize sympathoexcitatory and sodium-retaining pathways.
In rats adapted to a high-sodium diet, ODN-mediated down-regulation of central Gαi2 proteins selectively prevented the suppression of circulating NE levels, without altering the characteristic decrease in PRA. In fact, as indicated by significantly increased plasma NE levels, this group of animals actually demonstrated global sympathoexcitation of similar magnitude to that observed in animals maintained on a sodium-restricted intake. Evidence that these animals, which were fed a high-salt diet, exhibited marked sodium retention is also supported by the finding that this group of rats had an impaired ability to excrete an acute sodium load and had a moderate elevation in systemic arterial blood pressure (i.e., conversion to salt-sensitive hypertension). These findings support the long established Guytonion hypothesis of the intimate connection between fluid and electrolyte homeostasis and the long-term regulation of blood pressure (9, 26, 27). However, and as originally proposed by Rodriguez-Iturbe and Vaziri (28), our data indicate that enhanced central sympathetic outflow also plays a role in these regulatory processes. Our current findings demonstrate that PVN Gαi2-subunit protein-gated pathways play a critical role in maintaining fluid and electrolyte homeostasis and normotension in salt-resistant phenotypes by providing a downstream signal transduction pathway in which activation of brain (PVN) GPCRs (e.g., the α2-adrenoreceptor, the GABAB receptor; refs. 29–31) inhibits central sympathetic outflow. As predicted by the hypothesis of Rodriguez-Iturbe and Vaziri (28), the physiological importance of this signal transduction pathway is demonstrated by the observation that in salt-resistant rats, down-regulation of brain Gαi2 protein expression leads to an elevation in sympathetic drive, renal sodium retention, and increased blood pressure. From these findings, it may be speculated that derangements in the ability to enhance brain Gαi2 signaling pathways during consumption of a high-salt diet may contribute to sympathoexcitation and potentially the development of salt-sensitive hypertension.
It should be noted that site-specific PVN microinjection studies, which are beyond the scope of the current investigations, are required to definitively establish the PVN as a brain site in which Gαi2 protein pathways act to produce sympathoinhibitory influences on the renal handling of sodium and water. Further investigations are also needed to explore the potential CNS mechanisms by which alterations (increase or decrease) in dietary sodium intake are translated to endogenous changes in Gαi2 protein expression levels selective to the PVN. While our findings highlight a role for endogenous changes in Gαi2 proteins in the PVN during changes in dietary sodium intake, we acknowledge that Gαi2 protein pathways in other brain regions may also contribute to maintenance of fluid and electrolyte balance under conditions in which Gαi2 expression levels are not altered. For instance, during an acute stimulus such as a VE, it remains possible that GPCR receptor stimulation (e.g., α2-adrenoceptors) and, thus, downstream Gαi2 protein activity (targeted ODN sensitive) is increased in brain regions such as the VLM, which thereby contributes to the observed inhibition of RSNA and natriuresis/diuresis to this physiological stressor.
CONCLUSIONS
These data represent the first report of a central molecular mechanism, in this case involving brain Gαi2-subunit protein-gated signal-transduction pathways, which plays an endogenous role in controlling the level of central sympathetic outflow that affects renal excretory function in vivo. In this regard, we show that brain Gαi2 protein pathways are essential to inhibit RSNA and produce maximal natriuresis and diuresis to an acute sodium and water load. Further, sustained alterations in dietary sodium intake evoke a selective and marked decrease (low-salt diet) or increase (high-salt diet) in endogenous PVN Gαi2 protein expression, which affects central sympathetic outflow and contributes to daily sodium and water homeostasis. Collectively, these data provide a novel integrated central molecular/cellular target (brain Gαi2 subunit proteins) for which new therapies can be directed to alter systemic cardiovascular parameters (e.g., antihypertensive medications) and/or renal excretory function (natriuretic compounds) to treat the multiple disease states that feature sympathoexcitation and the impaired renal handling of sodium, e.g., salt-sensitive hypertension or congestive heart failure.
Acknowledgments
This work was funded by American Heart Association (AHA) Beginning-Grant-in-Aid 0855293E (R.D.W.), AHA Grant-in-Aid 2250585 (D.R.K.), U.S. National Institutes of Health (NIH) National Center for Research Resources (NCRR) grant P20RR018766 (R.D.W. and D.R.K.), and NIH grant R01HL107330 (R.D.W.).
Footnotes
- BC
- brain cortex
- BW
- body weight
- CNS
- central nervous system
- DHBA
- 3,4-dihydroxybenzylamine hydrobromide
- GPCR
- G-protein coupled receptor
- HR
- heart rate
- i.c.v.
- intracerebroventricular
- i.p.
- intraperitoneal
- i.v.
- intravenous
- INT. RSNA
- integrated renal sympathetic nerve activity
- MAP
- mean arterial pressure
- NE
- norepinephrine
- ODN
- oligodeoxynucleotide
- PH
- posterior hypothalamus
- PRA
- plasma renin activity
- PVN
- paraventricular nucleus
- RDNX
- bilateral renal denervation
- RSNA
- renal sympathetic nerve activity
- SCR
- scrambled
- SD
- Sprague-Dawley
- SON
- supraoptic nucleus
- UNaV
- urinary sodium excretion volume
- V
- urine flow rate
- VE
- volume expansion
- VLM
- ventrolateral medulla
REFERENCES
- 1. Weiss M. L., Hatton G. I. (1990) Collateral input to the paraventricular and supraoptic nuclei in rat. I. Afferents from the subfornical organ and the anteroventral third ventricle region. Brain Res. Bull. 24, 231–238 [DOI] [PubMed] [Google Scholar]
- 2. Sly D. J., McKinley M. J., Oldfield B. J. (2001) Activation of kidney-directed neurons in the lamina terminalis by alterations in body fluid balance. Am. J. Physiol. 281, R1637–R1646 [DOI] [PubMed] [Google Scholar]
- 3. Brooks V. L., Haywood J. R., Johnson A. K. (2005) Translation of salt retention to central activation of the sympathetic nervous system in hypertension. Clin. Exp. Pharmacol. Physiol. 32, 426–432 [DOI] [PubMed] [Google Scholar]
- 4. Toney G. M., Chen Q. H., Cato M. J., Stocker S. D. (2003) Central osmotic regulation of sympathetic nerve activity. Acta Physiol. Scand. 177, 43–55 [DOI] [PubMed] [Google Scholar]
- 5. De Warner H. E., He F. J., MacGregor G. A. (2004) Plasma sodium and hypertension. Kidney Int. 66, 2454–2466 [DOI] [PubMed] [Google Scholar]
- 6. Leenen F. H., Ruzicka M., Huang B. S. (2002) The brain and salt sensitive hypertension. Curr. Hypertens. Resp. 4, 129–135 [DOI] [PubMed] [Google Scholar]
- 7. DiBona G. F. (2004) The sympathetic nervous system and hypertension. Hypertension 43, 147–150 [DOI] [PubMed] [Google Scholar]
- 8. May C. N., Frithiof R., Hood S. G., McAllen R. M., McKinley M. J., Ramchandra R. (2009) Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp. Physiol. 95, 34–40 [DOI] [PubMed] [Google Scholar]
- 9. Guyton A. C. (1991) Blood-pressure control: special role of the kidneys and body fluids. Science 252, 1813–1116 [DOI] [PubMed] [Google Scholar]
- 10. Lohmeier T. E., Hildebrandt W., Hood A. (1999) Renal nerves promote sodium excretion during long-term increases in salt intake. Hypertension 33, 487–492 [DOI] [PubMed] [Google Scholar]
- 11. DiBona G. F. (2005) Neural control of the kidney. Am. J. Physiol. 289, R633–R641 [DOI] [PubMed] [Google Scholar]
- 12. He F. J., Markandu N. D., Sagnella G. A., de Wardener H. E., MacGregor G. A. (2005) Plasma sodium: ignored and underestimated. Hypertension 45, 98–102 [DOI] [PubMed] [Google Scholar]
- 13. Ruffolo R. R., Jr., Nichols A. J., Stadel J. N., Hieble J. P. (1991) Structure and function of alpha-receptors. Pharmacol. Rev. 43, 475–595 [PubMed] [Google Scholar]
- 14. Nasman J., Kukkonen J. P., Ammoun S., Akerman K. E. (2001) Role of G-protein availability in differential signaling by alpha-2-adrenoceptors. Biochem. Pharmacol. 62, 913–922 [DOI] [PubMed] [Google Scholar]
- 15. Odagaki Y., Koyama T. (2001) Identification of galpha subtype(s) involved in gamma-aminobutyric acid (B) receptor-mediated high-affinity guanosine triphosphate activity in rat cerebral cortical membranes. Neurosci. Lett. 297, 137–141 [DOI] [PubMed] [Google Scholar]
- 16. Wainford R. D., Kapusta D. R. (2011) Functional selectivity of central Gα-subunit proteins in mediating the cardiovascular and renal excretory responses evoked by central α(2)-adrenoceptor activation in vivo. [E-pub ahead of print] Br. J. Pharmacol. doi: 10.1111/j.1476-5381.2011.01662.x [DOI] [PMC free article] [PubMed]
- 17. Haselton J. R., Goering J., Patel K. P. (1994) Parvocellular neurons in the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J. Auton. Nerv. Syst. 50, 1–11 [DOI] [PubMed] [Google Scholar]
- 18. Kapusta D. R., Obih J. C. (1995) Central kappa opioids blunt the renal excretory responses to volume expansion by a renal nerve-dependent mechanism. J. Pharmacol. Exp. Ther. 273, 199–205 [PubMed] [Google Scholar]
- 19. Gottlieb H. B., Kapusta D. R. (2005) Endogenous central kappa-opioid systems augment renal sympathetic nerve activity to maximally retain urinary sodium during hypotonic saline volume expansion. Am. J. Physiol. 289, R1289–R1296 [DOI] [PubMed] [Google Scholar]
- 20. Bayorh M.A., Ogbolu E.C., Williams E., Thierry-Palmer M., Sanford G., Emmett N., Harris-Hooker S., Socci R.R., Chu T. C., Chenault V. M. (1998) Possible mechanisms of salt-induced hypertension in Dahl salt-sensitive rats. Physiol. Behav. 65, 563–568 [DOI] [PubMed] [Google Scholar]
- 21. Lastra G., Dhuper S., Johnson M. S., Sowers J. R. (2010) Salt, aldosterone, and insulin resistance: impact on the cardiovascular system. Nat. Rev. Cardiol. 7, 577–584 [DOI] [PubMed] [Google Scholar]
- 22. Wainford R. D., Kapusta D. R. (2010) Hypothalamic paraventricular nucleus G alpha q subunit protein pathways mediate vasopressin dysregulation and fluid retention in salt-sensitive rats. Endocrinology 151, 5403–5414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wainford R. D., Kapusta D. R. (2009) Chronic high-NaCl intake prolongs the cardiorenal responses to central N/OFQ and produces regional changes in the endogenous brain NOP receptor system. Am. J. Physiol. 296, R280–R288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kompanowska-Jezierska E., Wolff H., Kuczeriszka M., Gramsbergen J. B., Walkowska A., Johns E. J., Bie P. (2008) Renal nerves and nNOS: roles in natriuresis of acute isovolumetric sodium loading in conscious rats. Am. J. Physiol. 294, R1130–R1139 [DOI] [PubMed] [Google Scholar]
- 25. Patel K. P. (1991) Central alpha-2 adrenergic mechanisms in the renal nerve mediated natriuresis and diuresis produced by acute volume expansion. J. Auton. Nerv. Syst. 36, 47–54 [DOI] [PubMed] [Google Scholar]
- 26. Guyton A. C., Coleman T. G., Cowley A. V., Jr., Scheel K. W., Manning R. D., Norman R. A., Jr. (1972) Arterial pressure regulation. Overriding dominance of the kidneys in the long-term regulation and in hypertension. Am. J. Med. 52, 584–594 [DOI] [PubMed] [Google Scholar]
- 27. Guyton A. C. (1992) Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19, I2–I8 [DOI] [PubMed] [Google Scholar]
- 28. Rodriguez-Iturbe B., Vaziri N. D. (2007) Salt-sensitive hypertension: update on novel findings. Nephrol. Dial. Transplant 22, 992–995 [DOI] [PubMed] [Google Scholar]
- 29. Akine A., Montanaro M., Allen A. M. (2003) Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Autonom. Neurosci. 108, 17–21 [DOI] [PubMed] [Google Scholar]
- 30. Coote J. H. (1995) Cardiovascular functions of the paraventricular nucleus of the hypothalamus. Biol. Signals 4, 142–149 [DOI] [PubMed] [Google Scholar]
- 31. Frithiof R., Ramchandra R., Hood S., May C., Rundgren M. (2009) Hypothalamic paraventricular nucleus mediates sodium induced changes in cardiovascular and renal function in conscious sheep. Am. J. Physiol. 297, R185–R193 [DOI] [PubMed] [Google Scholar]