Abstract
We sought to evaluate the survival of patients who received breast surgery prior to any other breast cancer therapy following a metastatic diagnosis. Standard treatment for stage IV breast cancer is systemic therapy without resection of the primary tumor. Registry-based studies suggest that resection of the primary tumor may improve survival in stage IV cancer. We performed a retrospective analysis using data from the National Comprehensive Cancer Network (NCCN) Breast Cancer Outcomes Database. Patients were eligible if they had a metastatic breast cancer diagnosis at presentation with disease at a distant site and either received surgery prior to any systemic therapy or received systemic therapy only. Eligible patients who did not receive surgery were matched to those who received surgery based on age at diagnosis, ER, HER2, and number of meta-static sites. To determine whether estimates from the matched analysis were consistent with estimates that could be obtained without matching univariate and multivariable analyses of the unmatched sample were also conducted. There were 1,048 patients in the NCCN database diagnosed with stage IV breast cancer from 1997 to 2007. 609 meta-static breast cancer patients were identified as eligible for the study. Among the 551 patients who had data available for matching, 236 patients who did not receive surgery were matched to 54 patients who received surgery. Survival was similar between the groups with a median of 3.4 years in the nonsurgery group and 3.5 years in the surgery group. The groups were similar after adjusting for the presence of lung metastases and use of trastuzumab therapy (HR = 0.94, CI 0.83–1.08, P = 0.38). When matching for the variables associated with a survival benefit in previous studies, surgery was not shown to improve survival in the stage IV setting for this subset
Keywords: Stage IV breast cancer, Surgery, Survival, Mastectomy
Introduction
Data from the Surveillance, Epidemiology, and End Results (SEER) program suggests that 6% of women diagnosed with breast cancer present with stage IV disease in the United States [1]. The mainstay of treatment for stage IV disease has been systemic therapy in the form of chemotherapy, endocrine therapy, and/or targeted therapy. Radiation has been used to target impending fractures, palliate pain, and for treatment of metastatic disease to the brain. Surgical therapy for women with metastatic breast cancer has been reserved for palliation of symptomatic local breast or chest wall tumor burden [2]. Over the last decade, several retrospective studies have suggested a survival benefit for surgical extirpation of the primary tumor in women with stage IV breast cancer [3–12].
The reviews looking at outcomes for patients undergoing resection of the breast primary in the setting of stage IV disease have largely considered the group as a whole, drawing generalized conclusions about the use of surgery in this population. We hypothesize that other patient and tumor factors rather than surgery account for observed differences in survival. Those patients who are likely to have more favorable outcomes may be those who have lower burden of metastatic disease or who have tumors with favorable response to initial systemic therapy. Selection bias and disease burden may be substantial contributors to improved outcomes seen among women with stage IV breast cancer who undergo breast surgery [13]. In an institutional review from Dana Farber Cancer Institute, Brigham and Women’s Hospital, and the Massachusetts General Hospital, it was noted that the survival benefit associated with surgery was potentially secondary to a phenomenon of stage migration and clinical selection bias of patients. In particular, a survival benefit was noted for women who had surgery prior to diagnosis of metastatic disease, a patient population that is likely to differ substantially from those women who have clinically apparent metastatic breast cancer at the time of presentation with primary in-breast disease, or from women who have metastatic recurrence [13]. A previous case-matching series suggested that survival differences might be accounted for by significant clinical selection biases [14]. If extirpation of the tumor itself is responsible for the survival benefit, we hypothesized that this benefit might also be seen in the subset of patients who first received surgery and then had systemic therapy in the setting of known stage IV disease. Thus, we focused on patients whose first therapy was surgery.
We sought to examine the survival benefit of surgery on the primary tumor in a subset of stage IV breast cancer patients by using the multi-center clinical database for breast cancer treatment and outcomes maintained by the National Comprehensive Cancer Network. This resource has the advantage of well-described clinical treatments and outcomes in the modern era, and includes institutions with varying clinical practices.
Methods
Data source
In 1997, the NCCN Breast Cancer Outcomes Database began collecting prospective clinical, treatment, and outcomes data on women with newly diagnosed breast cancer patients seen at participating member institutions. A description of patient eligibility and data collection methods for the database has been described elsewhere [15–18].
Data on patient and tumor characteristics and treatment information are collected through review of medical records and institutional tumor registries by trained, dedicated abstractors and are subjected to rigorous quality assurance. Data on vital status and cause of death are ascertained from medical records and confirmed using the Social Security Death Index and the National Death Index (NDI). Institutional review boards from each center approved the study, data collection, transmission, and storage protocols. At centers where institutional review boards require signed informed consent for data collection, only patients who provided consent are included in the database.
Patient selection
The following eight participating institutions provided data for this analysis: Arthur G. James Cancer Hospital at Ohio State University (Columbus, OH), City of Hope Comprehensive Cancer Center (Duarte, CA), Dana-Farber Cancer Institute (Boston, MA), Fox Chase Cancer Center (Philadelphia, PA), H. Lee Moffitt Cancer Center (Tampa, FL), Roswell Park Cancer Institute (Buffalo, NY), The University of Texas M.D. Anderson Cancer Center (Houston, TX), and University of Michigan Comprehensive Cancer Center (Ann Arbor, MI). Patients were included for analysis if they presented to a NCCN institution with stage IV breast cancer between July 30, 1997 and December 31, 2007. Patients were deemed stage IV if their metastatic diagnosis occurred at the time of or up to 90 days from date of initial breast cancer diagnosis. Diagnosis of a distant metastasis included any metastasis in the bone, bone marrow, intra-abdominal region, brain, liver, lung, distant lymph nodes, meninges, pleural effusions, other distant visceral or nonvisceral sites, or skin. Patients were identified as having definitive local surgery if the date of breast-conserving surgery or mastectomy was within 1 year of initial breast cancer diagnosis date.
Among the 1,048 potentially eligible patients, we identified a subset of patients who began therapy for their metastatic breast cancer after the diagnosis of a distant metastasis and included patients who first received surgery followed by systemic therapy (surgery group), or patients who received systemic therapy only (nonsurgery group). Of these 609 patients, we excluded those who did not have available data on the four clinical factors used to match the nonsurgery group to the surgery group (n = 58), leaving a final analysis cohort of 551 patients.
Variables of interest
Characteristics included age at diagnosis and race. Age at diagnosis was categorized as less than 55 years old versus ≥55 years old. Race was categorized white (Caucasian nonHispanic) versus nonwhite (Hispanic, African-American nonHispanic, Asian nonHispanic, and other). The database also contained information collected from pathology reports on: tumor subtype (ER, PR, and HER2 status), date of metastatic diagnosis, and site(s) and number of distant metastases. ER status was categorized as positive or negative. HER2 status is coded “high positive” or “positive NOS” if there is a positive result by fluorescence in situ hybridization (FISH) for HER2 amplification or immunohistochemistry (IHC) was performed with quantification of 3+. Patients with a negative result by FISH or those with IHC 2+, 1+, or 0 are coded as HER2 negative. For the purpose of the analysis, number of metastatic sites was dichotomized (1 site vs. >1 site); and the following dichotomous variables were defined based on metastatic site: any liver metastasis, any lung metastasis, any bone metastasis, and any brain metastasis. Treatment information included use of systemic therapy, defined as use of any chemotherapy, any endocrine therapy or any trastuzumab. Use of any radiotherapy was defined as radiotherapy to the primary tumor or to a metastatic site. Overall survival was defined as time from breast cancer diagnosis to death from any cause. Patients’ vital status was reviewed through the NDI for survival up to a cutoff date of December 31, 2006. If the date of last contact and follow-up with a patient preceded the cutoff date, times were censored at the cutoff. Otherwise, times were censored at the date of last contact and follow-up with the patient.
Statistical analysis
Nonsurgery patients were matched to surgery patients on age at diagnosis (<55 vs. ≥55 years), ER status (ER+ vs. ER−), HER2 status (HER2+ vs. HER2−), and number of metastatic sites (1 site vs. >1 site) as these variables were thought be prognostic and related to selection for surgery. A 5:1 matching ratio was planned in order to include as many of the nonsurgery patients as possible. To evaluate the comparability of the matched groups, we inspected distributions of tumor and treatment factors that were not included in the matching in order to identify potential imbalances between the groups. Variables included metastatic site, use of any chemotherapy, use of any endocrine therapy, use of any trastuzumab, and any radiation to the primary tumor or to metastatic sites.
To determine whether covariate adjustments might be needed after matching, variables that were not used in the matching were inspected for differences between the groups. The groups were compared on these variables using a Fisher’s test, or if appropriate, a Chi-square test with a somewhat liberal P value of 0.20 or less considered to be evidence of imbalance between the groups so as not to exclude potentially important group differences. Survival estimates were obtained using the Kaplan–Meier method. Hazard ratios (HR) and 95% confidence intervals were determined using a Cox model including strata defined by the matching variables in order to account for the matching design. Variables having observed imbalances between the groups were included as covariate adjustments in the Cox model.
To determine whether estimates from the matched analysis were consistent with estimates that could be obtained without matching, analyses of the larger unmatched sample were also performed. Analyses of the unmatched sample included univariate analysis of survival with Kaplan–Meier estimates of median survival and group comparisons using the log-rank test. Multivariable analysis using a Cox regression model was also performed. Variables that were statistically significant in univariate analysis at the 0.05 level were included as covariate adjustments in the Cox model. Additional models were fit that also included variables that were known to be associated with survival but not statistically significant in univariate analysis. All analyses were performed using SAS version 9.2.
Results
There were 1,048 patients who were diagnosed with stage IV disease at the time of or up to 90 days from initial presentation, of whom 609 were eligible patients who began therapy after metastatic diagnosis and either received surgery prior to systemic therapy or received systemic therapy alone. There were 439 patients who were not eligible for the study for several reasons. Eighty-four patients with disease in their supraclavicular nodes were excluded since ipsilateral supraclavicular nodes were considered a site of distant metastases only up to January 1, 2003 as a result of changes in the 6th edition AJCC staging rules. Two hundred twenty-one patients had surgery prior to the discovery of a distant metastasis. One hundred thirtyone patients received systemic therapy prior to having surgery. Three patients received no systemic therapy. Of the eligible patients, 551 (90%) had data available for matching (54 surgery patients and 497 nonsugar patients).
Characteristics prior to matching for the 551 eligible patients are shown in Table 1. Patients in the surgery group were more likely to be younger (surgery: 65% < 55 years, nonsurgery: 46% < 55 years), less likely to have more than one distant metastatic site (surgery: 31% vs. nonsurgery: 49%), and somewhat more likely to have received endocrine therapy for their breast cancer (surgery: 74% vs. nonsurgery: 65%). Distributions of ER status, HER2 status, number of metastatic sites, use of chemotherapy, use of radiation therapy, and use of trastuzumab were similar.
Table 1.
Patient characteristics prior to matching
| Variable | Nonsurgery (n = 497) |
Surgery (n = 54) |
P |
|---|---|---|---|
| Vital status | |||
| Alive | 178 (36%) | 16 (30%) | |
| Died | 319 (64%) | 38 (70%) | |
| Age (years) | |||
| Mean (SD) | 56.3 (13.5) | 53.4 (14.0) | |
| <55 | 227 (46%) | 35 (65%) | <0.01 |
| ≥55 | 270 (54%) | 19 (35%) | |
| Race | |||
| White | 386 (78%) | 42 (78%) | 0.97 |
| Nonwhite | 109 (22%) | 12 (22%) | |
| Unknown | 2 (<1%) | 0 (0%) | |
| Year of diagnosis | |||
| 1997–2004 | 279 (56%) | 42 (78%) | <0.01 |
| 2005–2007 | 218 (44%) | 12 (22%) | |
| ER status | |||
| Positive | 353 (71%) | 39 (72%) | 0.85 |
| Negative | 144 (29%) | 15 (28%) | |
| HER2neu status | |||
| Positive | 134 (27%) | 19 (35%) | 0.20 |
| Negative | 363 (73%) | 35 (65%) | |
| Number of metastatic sites | |||
| 1 | 255 (51%) | 37 (69%) | 0.02 |
| >1 | 242 (49%) | 17 (31%) | |
| Metastatic sites | |||
| Any liver metastasis | 169 (34%) | 14 (26%) | 0.23 |
| Any lung metastasis | 146 (29%) | 17 (31%) | 0.75 |
| Any bone metastasis | 340 (68%) | 34 (63%) | 0.42 |
| Any brain metastasis | 16 (3%) | 2 (4%) | 0.69* |
| Systemic therapy | |||
| Any chemotherapy | 418 (84%) | 42 (78%) | 0.23 |
| Any hormonal therapy | 322 (65%) | 40 (74%) | 0.17 |
| Any trastuzumab | 187 (38%) | 16 (30%) | 0.25 |
| Other therapy | |||
| Any RT (primary or metastasis) | 45 (9%) | 7 (13%) | 0.35 |
Based on a Fisher exact test. All other P values are based on a Chi-square test
We were able to match 236 nonsurgery patients to the 54 surgery patients, and achieved a matching ratio close to, but somewhat lower than planned. Patient and tumor characteristics after matching are shown in Table 2. As expected, significant differences were not observed between the groups in terms of the matched characteristics. There were some differences between the groups for factors not used in the matching. Lung metastases were more common in the surgery group (P = 0.08) and use of trastuzumab was more common in the non-surgery group (P = 0.08). Patients in the surgery group were less likely to be recently diagnosed compared with those in the non-surgery group (P < 0.01). Other factors were similar between the groups.
Table 2.
Patient characteristics after matching
| Variable | Nonsurgery (n = 236) |
Surgery (n = 54) |
P |
|---|---|---|---|
| Vital status | |||
| Alive | 94 (40%) | 16 (30%) | |
| Died | 142 (60%) | 38 (70%) | |
| Age (years) | |||
| Mean (SD) | 52.2 (13.6) | 53.4 (14.0) | |
| <55 | 160 (68%) | 35 (65%) | 0.67 |
| ≥55 | 76 (32%) | 19 (35%) | |
| Race | |||
| White | 174 (74%) | 42 (78%) | 0.57 |
| Nonwhite | 61 (26%) | 12 (22%) | |
| Unknown | 1 (<1%) | 0 (0%) | |
| Year of diagnosis | |||
| 1997–2004 | 134 (57%) | 42 (78%) | <0.01 |
| 2005–2007 | 102 (43%) | 12 (22%) | |
| ER status | |||
| Positive | 176 (75%) | 39 (72%) | 0.72 |
| Negative | 60 (25%) | 15 (28%) | |
| HER2neu status | |||
| Positive | 76 (32%) | 19 (35%) | 0.67 |
| Negative | 160 (68%) | 35 (65%) | |
| Number of metastatic sites | |||
| 1 | 151 (64%) | 37 (69%) | 0.53 |
| >1 | 85 (36%) | 17 (31%) | |
| Metastatic sites | |||
| Any liver metastasis | 79 (33%) | 14 (26%) | 0.28 |
| Any lung metastasis | 48 (20%) | 17 (31%) | 0.08 |
| Any bone metastasis | 157 (67%) | 34 (63%) | 0.62 |
| Any brain metastasis | 8 (3%) | 2 (4%) | 1.00* |
| Systemic therapy | |||
| Any chemotherapy | 198 (84%) | 42 (78%) | 0.28 |
| Any hormonal therapy | 158 (67%) | 40 (74%) | 0.31 |
| Any trastuzumab | 100 (42%) | 16 (30%) | 0.08 |
| Other therapy | |||
| Any RT (primary or metastasis) | 22 (9%) | 7 (13%) | 0.42 |
Based on a Fisher exact test. All other P-values are based on a Chi-square test
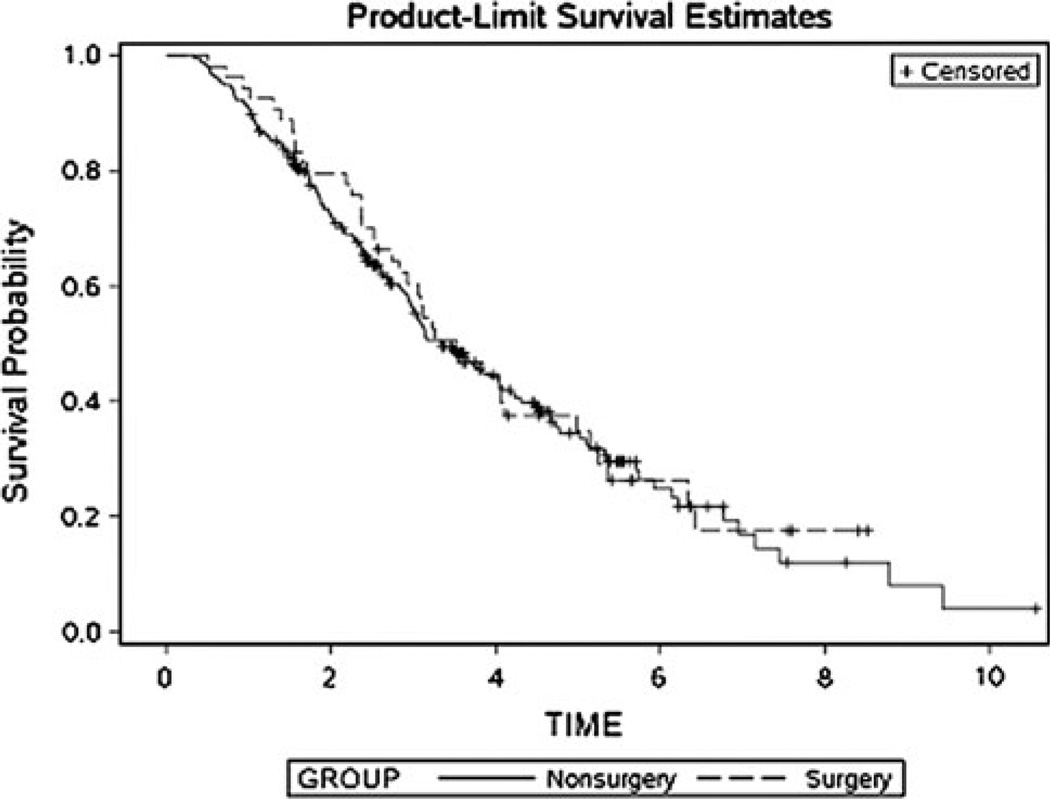
There were a total of 180 deaths in the matched data set. There were 38 (70%) deaths in the surgery group and 142 (60%) in the nonsurgery group. Kaplan–Meier estimates of median survival were similar between the matched groups (Fig. 1). The median survival was 3.5 years (CI 2.7–5.0) in the surgery group and 3.4 years (CI 3.0–4.0) in the nonsurgery group. Survival was similar after adjusting for year of diagnosis, use of trastuzumab and the presence of any lung metastases (HR = 0.94, CI 0.83–1.08, P = 0.38).
Fig. 1.
Kaplan–Meier survival curves for the surgery and nonsurgery groups after matching
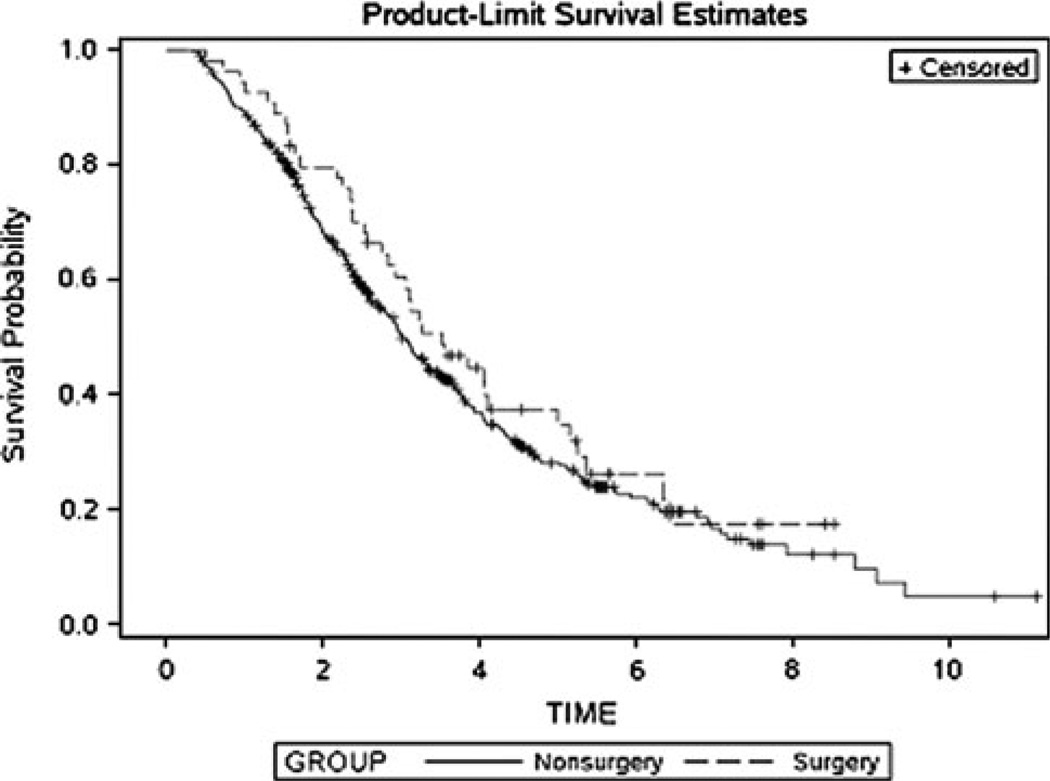
In the unmatched sample, there were a total of 357 (64%) deaths among the 551 patients. There were 38 (36%) deaths among the surgery patients and 319 deaths among the nonsurgery patients. The median survival was 3.5 years in the surgery group (CI 2.7–5.0) and 3.0 years in the nonsurgery group (CI 2.8–3.3) and the survival curves were similar (P = 0.29), as shown in Fig. 2. In univariate analysis, ER+ status (P < 0.0001), fewer metastatic sites (P < 0.0001), use of endocrine therapy (P < 0.0001), and treatment without chemotherapy (P = 0.02) were associated with longer survival and presence of a liver metastasis, presence of a lung metastasis, and presence of a brain metastasis were associated with shorter survival (P = 0.02, P < 0.001, P = 0.02, respectively). Significant associations with patient age, race, year of diagnosis, HER2 status, presence of bone metastasis, use of trastuzumab, use of radiation therapy to the primary tumor or metastasis were not observed. A significant difference between surgery and nonsurgery groups after multivariable adjustment was not observed and the adjusted HR was similar to that of the matched sample (HR = 0.94, CI 0.84–1.05, P = 0.27).
Fig. 2.
Kaplan–Meier survival curves for the surgery and nonsurgery groups prior to matching
Discussion
Using a case-matched analysis from a large multi-institutional data resource, we sought to determine whether surgery of the primary tumor is associated with improved survival in metastatic breast cancer in a subset of patients who received breast surgery followed by systemic therapy. We matched for important clinical factors including age, number of sites of metastatic disease, ER and HER2 status in order to define a group of patients who were comparable to patients in the surgery group but did not receive surgery. Prior to matching, no significant survival benefit was seen in patients who underwent surgery. When matched for these clinical factors, there was still no survival advantage to surgery.
There were several limitations in this study. There is the potential for error in accurate recording of data in large database studies, such as this one. However, the use of these databases leads to the opportunity to study a more extensive population of patients than is possible at a single institution. Also, we were unable to determine the decision-making process for timing and/or performance of surgery from the recorded data. More patients had surgery in the earlier time period of the study (1997–2004), and the significant improvements in systemic treatment including endocrine, chemotherapy, and targeted therapies in the latter portion of the study may make a significant impact on patient survival.
Another one of the limitations of retrospective studies such as ours is that investigators are often unable to determine if the patients’ response to systemic therapy may affect surgeons’ decisions to proceed with surgery. Previous work suggests that women without significant response to therapy may not be offered surgical management [13], whereas patients with a significant clinical response may be offered more aggressive surgical therapy [19]. Women with a favorable response to systemic therapy may indeed have improved survival, but we did not examine this group of women in our dataset. To address this potential limitation, patients who had surgery prior to any other therapy for their MBC were selected in order to reduce the chances that treatment decision about surgery could be influenced by response to systemic therapy and thereby bias our results.
Though there are some specific clinical scenarios in which surgery may provide a survival benefit, many studies to date have drawn this as a broadly applicable conclusion [20, 21]. An analysis of the National Cancer Data Base of the American College of Surgeons who were treated for stage IV breast cancer found improved survival in the 57.2% of these patients who underwent surgery for the primary tumor [3]. A 40% reduction in mortality was also seen with surgery of the primary tumor in 300 stage IV breast cancer patients enrolled in the Geneva Cancer Registry [4]. An analysis of 224 patients treated for stage IV breast cancer at the University of Texas M.D. Anderson Cancer Center found a significant improvement in metastatic progression-free survival and a trend toward improved overall survival in patients who underwent surgery for the primary tumor [5]. A review of the SEER registries also noted improved median survival in women presenting with stage IV disease and treated with surgery [6]. Neuman et al. suggested that there may be a survival benefit for women undergoing surgery with stage IV hormone positive or HER2 positive disease, but not for triple negative disease [7].
Previously, a smaller case-matched series suggested that substantial clinical selection bias could readily account for survival differences seen in women who have breast surgery for stage IV breast cancer [14]. If it is extirpation of the tumor itself that is responsible for the survival benefit, then we assert that this benefit should persist in any patient population. Patients who had surgery prior to any other therapy for their MBC were selected in order to reduce the chances that treatment decision about surgery could be influenced by response to systemic therapy and thereby bias our results. It is difficult to retrospectively evaluate the effect of surgery independent from the effect of systemic therapy in patients who received systemic therapy first, as the response to systemic therapy may predict the likelihood that the patient undergoes surgery. This retrospective review of a matched cohort from a large national database supports that this generalized conclusion cannot be drawn, given that it does not apply to all subsets of patients. Randomized trials will help to determine the benefit of surgery in this setting [22, 23].
Currently, three large international randomized trials are underway to help determine whether surgery portends to an improvement in survival. In India, the TATA (NCT 00193778) medical group offer women with stage IV breast cancer women upfront therapy with induction chemotherapy and then randomize to surgery versus continued drug therapy. The Turkish Federation of Breast Diseases (NCT00557986) trial randomizes women upfront to surgery versus drug therapy. Finally, the ECOG 2108 (NCT01242800) trial allows women with newly diagnosed breast cancer to receive upfront therapy at the discretion of the treating oncologist; women who either show stable disease or response are randomized to definitive local therapy consisting either of breast-conserving therapy with appropriate axillary staging and radiation or mastectomy with or without reconstruction with appropriate axillary staging and the use of radiation similar to women with nonmetastatic disease.
Pending the results of such studies, clinicians must inform treatment decisions based on the best available data. Retrospective reviews are burdened by inherent clinical selection biases. We carefully tried to adjust for as many confounding factors as possible and allowing a comparison of the specific effect of surgery in a subset of stage IV patients. At present, we believe that the impact of surgery on survival in metastatic breast cancer reflects clinical selection biases such that patients with better prognosis are more likely to be offered surgery, rather than a pure benefit of the surgical procedure itself. Present evidence suggests that surgery should be limited to patients where an operation is likely to provide direct palliative benefit to breast or chest wall disease. Other cases where local therapy is discussed with our patients with metastatic breast cancer should be viewed with caution. Upcoming trials will hopefully help define which subsets of women with stage IV breast cancer may most benefit from surgery to remove the primary tumor.
Acknowledgments
Supported in part by Grant No. CA089393 from the National Cancer Institute to Dana-Farber Cancer Institute and by the National Comprehensive Cancer Network.
Footnotes
Conflict of interest None.
Contributor Information
Laura Dominici, Department of Surgical Oncology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA; Dana Farber Cancer Institute, Boston, MA, USA.
Julie Najita, Dana Farber Cancer Institute, Boston, MA, USA.
Melissa Hughes, Dana Farber Cancer Institute, Boston, MA, USA.
Joyce Niland, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Paul Marcom, Duke University, Durham, NC, USA.
Yu-ning Wong, Fox Chase Cancer Center, Philadelphia, PA, USA.
Bradford Carter, Moffitt Cancer Center, Tampa, FL, USA.
Sara Javid, University of Washington, Seattle, WA, USA.
Stephen Edge, Roswell Park Cancer Institute, Buffalo, NY, USA.
Harold Burstein, Dana Farber Cancer Institute, Boston, MA, USA.
Mehra Golshan, Email: mgolshan@partners.org, Department of Surgical Oncology, Brigham and Women’s Hospital, 75 Francis Street, Boston, MA 02115, USA; Dana Farber Cancer Institute, Boston, MA, USA.
References
- 1.Ries LAG, Melbert D, Krapcho M, et al. Bethesda, MD: National Cancer Institute; 2007. SEER cancer statistics review, 1975–2004. http://seer.cancer.gov/csr/1975_2004/, based on November 2006 SEER data submission, posted to the SEER web site. [Google Scholar]
- 2.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–984. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 3.Khan SA, Stewart AK, Morrow M. Does aggressive local therapy improve survival in metastatic breast cancer? Surgery. 2002;132:620–626. doi: 10.1067/msy.2002.127544. [DOI] [PubMed] [Google Scholar]
- 4.Rapiti E, Verkooijen HM, Vlastos G, et al. Complete excision of primary breast tumor improves survival of patients with metastatic breast cancer at diagnosis. J Clin Oncol. 2006;24:2743–2749. doi: 10.1200/JCO.2005.04.2226. [DOI] [PubMed] [Google Scholar]
- 5.Babiera GV, Rao R, Feng L, et al. Effect of primary tumor extirpation in breast cancer patients who present with stage IV disease and an intact primary tumor. Ann Surg Oncol. 2006;13:776–782. doi: 10.1245/ASO.2006.03.033. [DOI] [PubMed] [Google Scholar]
- 6.Gnerlich J, Jeffe DB, Deshpande AD, et al. Surgical removal of the primary tumor increases overall survival in patients with metastatic breast cancer: analysis of the 1988–2003 SEER data. Ann Surg Oncol. 2007;14:2187–2194. doi: 10.1245/s10434-007-9438-0. [DOI] [PubMed] [Google Scholar]
- 7.Neuman HB, Morrogh M, Gonen M, et al. Stage IV breast cancer in the era of targeted therapy: does surgery of the primary tumor matter? Cancer. 2010;116:1226–1233. doi: 10.1002/cncr.24873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singletary SE, Walsh G, Vauthey JN, et al. A role for curative surgery in the treatment of selected patients with metastatic breast cancer. Oncologist. 2003;8:241–251. doi: 10.1634/theoncologist.8-3-241. [DOI] [PubMed] [Google Scholar]
- 9.Dooley WC. A surgical indication in incurable breast cancer. Ann Surg Oncol. 2005;13:759–760. doi: 10.1245/ASO.2006.09.910. [DOI] [PubMed] [Google Scholar]
- 10.Khan SA, Lurie RH. Does resection of an intact breast primary improve survival in metastatic breast cancer? Oncology. 2007;21:924–944. [PubMed] [Google Scholar]
- 11.Khan SA. Primary tumor resection in stage IV breast cancer: consistent benefit, or consistent bias? Ann Surg Oncol. 2007;14:3285–3287. doi: 10.1245/s10434-007-9547-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blanchard DK, Shetty PB, Hilsenbeck SG, et al. Association of surgery with improved survival in stage IV breast cancer patients. Ann Surg. 2008;247:732–738. doi: 10.1097/SLA.0b013e3181656d32. [DOI] [PubMed] [Google Scholar]
- 13.Bafford AC, Burstein HJ, Barkley CR, et al. Breast surgery in stage IV breast cancer: impact of staging and patient selection on overall survival. Breast Cancer Res Treat. 2009;115:7–12. doi: 10.1007/s10549-008-0101-7. [DOI] [PubMed] [Google Scholar]
- 14.Cady B, Nathan NR, Michaelson JS, et al. Matched pair analyses of stage IV breast cancer with or without resection of primary breast site. Ann Surg Oncol. 2008;15:3384–3395. doi: 10.1245/s10434-008-0085-x. [DOI] [PubMed] [Google Scholar]
- 15.Christian C, Edge S, Niland J, et al. A Multi-institutional analysis of the current determinants of breast reconstruction: a study of the National Comprehensive Cancer Network. Ann Surg. 2006;243:241–249. doi: 10.1097/01.sla.0000197738.63512.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edge SB, Niland JC, Bookman MA, et al. Emergence of sentinel lymph node in breast as standard-of-care in academic comprehensive cancer centers. J Natl Cancer Inst. 2001;95:1514–1521. doi: 10.1093/jnci/djg076. [DOI] [PubMed] [Google Scholar]
- 17.Alderman A, Collins ED, Schott A, et al. The impact of breast reconstruction on the delivery of chemotherapy. Cancer. 2010;116:1791–1800. doi: 10.1002/cncr.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hassett MJ, Hughes ME, Niland JC, et al. Chemotherapy use for hormone-receptor positive, node negative breast cancer. J Clin Oncol. 2008;26:5553–5560. doi: 10.1200/JCO.2008.17.9705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hortobagyi GN. Can we cure limited metastatic breast cancer? J Clin Oncol. 2002;20:620–623. doi: 10.1200/JCO.2002.20.3.620. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael AR, Anderson ED, Chetty U, et al. Does local surgery have a role in the management of stage IV breast cancer? Eur J Surg Oncol. 2003;29:17–19. doi: 10.1053/ejso.2002.1339. [DOI] [PubMed] [Google Scholar]
- 21.Fields RC, Jeffe DB, Trinkaus K, et al. Surgical resection of the primary tumor is associated with increased long-term survival in patients with stage IV breast cancer after controlling for site of metastasis. Ann Surg Oncol. 2007;14:3345–3351. doi: 10.1245/s10434-007-9527-0. [DOI] [PubMed] [Google Scholar]
- 22.Pockaj BA, Wasif N, Duceck AC, et al. Metastasectomy and surgical resection of the primary tumor in patients with stage IV breast cancer. Ann Surg Oncol. 2010;17:2419–2426. doi: 10.1245/s10434-010-1016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ruiterkamp J, Voogd AC, Bosscha K, et al. Impact of breast surgery on survival in patients with distant metastases at initial presentation: a systematic review of the literature. Breast Cancer Res Treat. 2010;120:9–16. doi: 10.1007/s10549-009-0670-0. [DOI] [PubMed] [Google Scholar]