Abstract
Conventional views of the tunica adventitia as a poorly organized layer of vessel wall composed of fibroblasts, connective tissue and perivascular nerves are undergoing revision. Recent studies suggest that the adventitia has properties of a stem/progenitor cell niche in the artery wall that may be poised to respond to arterial injury. It is also a major site of immune surveillance and inflammatory cell trafficking, and harbors a dynamic microvasculature, the vasa vasorum, that maintains the medial layer and provides an important gateway for macrophage and leukocyte migration into the intima. In addition, the adventitia is in contact with tissue that surrounds the vessel and may actively participate in exchange of signals and cells between the vessel wall and the tissue in which it resides. This brief review highlights recent advances in our understanding of the adventitia and its resident progenitor cells and discusses progress toward an integrated view of adventitial function in vascular development, repair and disease.
Keywords: vascular development, stem cell, restenosis, progenitor cell niche
Introduction
For many years, the tunica adventitia has been defined as the outer layer of blood vessels consisting mainly of fibroblasts and perivascular nerves embedded in a collagen-rich connective tissue matrix (Figure 1A). We now know that that definition is far too limiting. Recent studies show that the adventitia functions as a dynamic compartment for cell trafficking into and out of the artery wall, it participates in growth and repair of the vessel wall, and it mediates communication between vascular endothelial cells and smooth muscle cells and their local tissue environment (Figure 1B).1-3 The adventitia is also where formation and regression of microvessels that penetrate and nourish the media and intima are controlled.4-8 The adventitia contains lymphatic vessels and autonomic nerves and it plays a critical role in control of lumen size by regulation of medial smooth muscle tone and control of inward (negative) and outward (positive) wall remodeling responses.9-13 Moreover, the adventitia contains resident populations of macrophages, T-cells, B-cells, mast cells and dendritic cells that carry out important surveillance and innate immune functions in response to foreign antigens.14-17 Of particular interest is the accumulating evidence that the adventitia functions as a stem/progenitor cell niche in the artery wall that maintains both endothelial and mural cell progenitors that may be poised to respond to arterial injury.18-22 Therefore the adventitia consists of a complex community of interacting cell types and abundant evidence now indicates that a better understanding of molecular mechanisms for homeostasis, repair and disease of the vessel wall will require a greater appreciation of the integrated role of the adventitia with the intimal and medial layers. Toward this goal we review the current literature and highlight recent findings on this important but understudied tissue layer.
Figure 1.
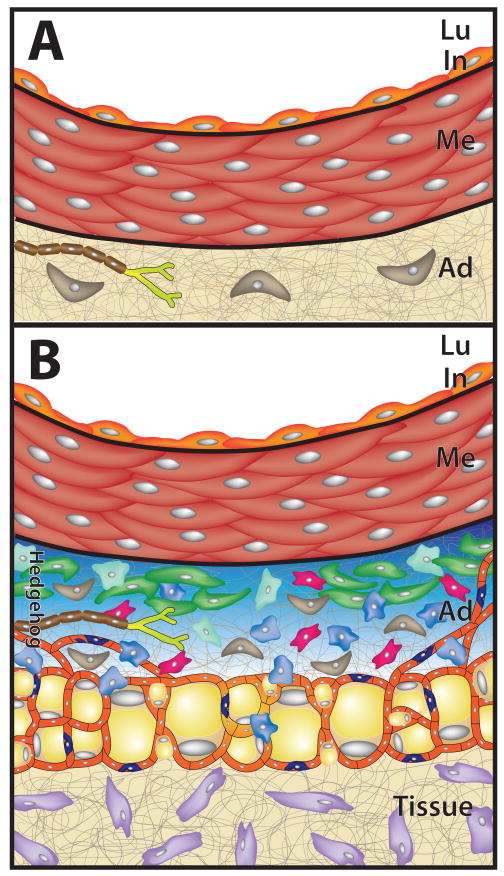
The arterial adventitia. (A) The adventitia (Ad) has traditionally been viewed as a loose collection of fibroblasts (brown) and perivascular nerves (light green) embedded in a collagen-rich extracellular matrix (ECM). (B) Recent studies suggest instead that the adventitia supports a dynamic mixture of interacting cell types. In addition to fibroblasts and perivascular nerves, adventitia contains macrophages (blue), mast cells (pale green), CD34+/Sca1+ progenitor cells (dark green), T cells (red), microvascular endothelial cells (tan), pericytes (dark blue), and adipocytes (yellow). In the mouse, these cells exist in a domain sonic hedgehog signaling (Hedgehog, blue shaded) restricted to the adventitia.20 Lu, lumen; In, intima; Me, media; Ad, adventitia; Tissue, tissue surrounding blood vessel; Hedgehog, domain of sonic hedgehog signaling restricted to the adventitia.
Development of the Adventitia: The End of the Beginning
Major advances have been made in our understanding of the molecular and genetic pathways that control vascular development.23-24 Most of those advances have come from studies focused on the early steps including vasculogenesis, vascular patterning, and formation of venous and lymphatic endothelial cells. However, we still know very little about later stages of vascular development and particularly lacking are studies on formation of the adventitia. In broad outlines development of the vessel wall begins with the formation of angioblasts and their self-assembly into a primitive vascular plexus.25 The vascular plexus then undergoes a complex remodeling process to yield a network of large and small arteries, veins, and lymphatic vessels that constitute a functional embryonic circulatory system.26-28 Vascular plexus remodeling is closely followed by investment of nascent vessel walls with smooth muscle cells (SMCs) and pericytes.29-32 The interaction of endothelial cells with mural cells initiates maturation responses in both cell types32-34 that are coupled with inputs from biomechanical forces acting on these cells to shape continued development of the vessel wall. 35-37
The morphogenetic mechanisms that guide patterning of the medial layers and maturation of the artery wall are poorly understood at this time. For large elastic arteries, the synthesis and assembly of a load-bearing extracellular matrix are essential steps in the late stages of vascular development -- from E15.5 to birth in the mouse.38-43 The classic studies of Wolinsky and Glagov showed that the number of smooth muscle layers present in the media of adult arteries is characteristic of species and vessel type and correlates with normalization of circumferential wall stress to values between 0.5 to 3.0 N/m.44 For example, the thoracic aorta of the mouse contains 6-8 layers of smooth muscle and elastin, while the rat contains 8-10 layers, and humans have around 40-50 layers.44 These smooth muscle layers are separated from the intima by the internal elastic lamina (IEL) and from the adventitia by the external elastic lamina (EEL).
A fundamental question about the morphogenesis of blood vessels that we still have no clear answer to is what mechanisms act to terminate the iterative layering process that drives formation of the tunica media? The answer to that question provides an important starting point to understand how the adventitia is formed. A recent analysis of tunica media formation in mice hypomorphic for elastin sheds some light on this question.38 In elastin (Eln)-deficient mice development of the aortic media is not significantly different from wild type until E18.5, well after the time that the characteristic number of layers of SMCs is laid down (E15.5).38,45 Beginning at E18.5, Eln-/- embryos exhibit elevated rates of SMC proliferation leading to formation of dramatically elongated and tortuous arteries with thickened walls that rapidly progress to vascular occlusion and perinatal death.46 In Eln+/- embryos the aorta develops normally until E18.5 after which additional layers of SMC and elastin are produced ectopically in the inner adventitial layer while lumen diameter is preserved.38,45,47 The elastic lamellae that do form in these mice are thinner than in wild type mice. As Eln+/- mice have a normal life span, the adaptations in arterial wall structure during the late fetal period appear to have distributed wall tension per SMC layer to approximately normal values and to restore arterial compliance to within the physiological range.38,47,48 These studies indicate that the characteristic number of SMC layers in the mouse thoracic aorta is established by E15.5 thus signaling the initiation of development of the aortic adventitia.38,45 What then is the cellular basis of compensatory wall structural changes that occur later in arterial development (E18.5 to birth) in elastin-deficient mice? Is this adaptive mechanism retained in at least some adult vessels and upon injury or pathologic activation could it explain, for example, the dramatic wall remodeling responses described in pulmonary hypertension?49 Interest in these questions has been amplified by recent reports suggesting that the inner adventitia has properties of a progenitor cell niche in the artery wall characterized by a restricted domain of sonic hedgehog (Shh) signaling (Figure 2).18-22 As discussed further below, resident progenitor cells expressing the stem cell antigen-1 marker (Sca1, also called Ly6A/E) have been isolated from the aortic adventitia that exhibit potentials to differentiate to vascular SMCs and pericytes in primary explant culture. Considering the clustering of these resident progenitor cells near the media-adventitia border (Figure 3) and their differentiation to SMCs in vitro18,20,21, it is possible that the ectopic layers of SMC and elastin that appear in the inner adventitia of elastin hypomorphic mice38,45,47are derived from these resident progenitor cells. It will be of interest, therefore, to determine the number, localization and fate of adventitial progenitor cells in elastin-deficient mice.
Figure 2.

Sonic hedgehog signaling in the adventitia. Arterial tissues from postnatal day 2 transgenic mice expressing β-galactosidase from either the Patched-1 (Ptc1-lacZ) or Patched-2 (Ptc2-lacZ) locus in the mouse. Ptc1 and Ptc2 are target genes for sonic hedgehog signaling and are commonly used as reporters of hedgehog signaling activity in vivo. Histological cross-sections through the aorta (Ao, A-B), pulmonary trunk (PT, A-B), and coronary arteries (C-D) at low magnification, and descending thoracic aorta (E, F) at high power, reveal that Hh-responsive Ptc-LacZ-positive cells are restricted to the adventitial layer in the perinatal period.
Figure 3.
Organization of Sca1+ progenitor cells in the arterial adventitia. The left common carotid artery of a postnatal day 9 C57Bl/6 mouse was fixed and stained for Sca1 expression and examined by confocal microscopy. (A) Reconstructed low power view of stacked Z-plane confocal images showing that adventitial Sca1+ progenitor cells organize around the artery wall in clusters within the adventitia. The entire artery was prepared as described above and imaged in whole mount. The stacked Z-plane images that are shown in panel A only reconstruct the ventral adventitial surface of the common carotid artery. Sca1-positive cells are limited to the adventitial layer. (B, C) High power views at different angles of Sca1+ cells in the adventitia showing flattened cells (B) that organize in loose clusters of variable size (C) close to the media-adventitia border.
Response of the Adventitia to Arterial Injury
At this point it is instructive to look back in time to see that these intriguing observations of resident progenitor cells in the adventitia may have been anticipated by earlier reports describing a role for the adventitia in arterial wound healing. The vessel wall has a substantial intrinsic capacity for wound repair. Under normal conditions the artery wall in adult animals has very low to undetectable rates of endothelial cell or SMC proliferation. When subjected to overstretch injury, however, proliferation rates in all layers of artery wall can increase over one hundred fold.50,51 Most animal models of intimal hyperplasia rely on injury to rodent arteries that lack the preexisting subendothelial intimal cells that are commonly present in human arteries.52 In rodent vessels, migration of medial SMCs to the intima is, therefore, thought to be essential to form a neointima during repair of arterial injury. Observations that medial cell proliferation precedes accumulation of intimal cells and that intimal cells express SMC marker genes including SMα-actin and SM22α led to the hypothesis that intimal cells are derived from medial SMCs.50,53-56 This view was challenged by experiments performed in porcine coronary arteries and both canine and rat carotid arteries. In these cases adventitial cells respond to injury initially by increased cell proliferation and then later appear to migrate out of the adventitia and make significant contributions to medial repair and neointimal lesion formation.57-61 For example, Holifield et al showed that controlled enzyme digestion from the adventitial side of uninjured canine carotid arteries produced what they called nonmuscle “type 2” cells in contrast to digestion from the luminal side that produced smooth muscle marker-positive cells.58 Type 2 cells proliferated in serum-containing culture medium and expressed SMα-actin but not SM-MHC. By contrast, SMCs from adult carotid media did not spread or proliferate in vitro but remained viable and maintained expression of SMC marker proteins including SM-MHC at undiminished levels. Of interest, neointimal SMCs from balloon-injured canine carotid arteries were morphologically and immunologically identical to type 2 cells.58
Direct evidence for the capability of adventitial cells to migrate through the media and into the intima has been obtained by transplanting cells onto the adventitial side of an artery and monitoring movement of these cells following arterial injury.18,61-63 For example, adenoviral vectors expressing β-galactosidase were used to label adventitial cells prior to balloon catheter injury of rat common carotid arteries. β-Galactosidase-positive cells were observed within the medial layer at 3 days and in the neointima at 7 and 14 days after injury.64 Adenoviral-mediated delivery of Smad7, an inhibitory Smad that blocks TGFβ signaling, to the adventitia prior to balloon injury resulted in significant reductions in the number of β-galactosidase-positive cells in the neointima concomitant with reduced neointimal thickening. These findings suggest that cells originating in the adventitia migrate to the neointima and contribute to intimal thickening after arterial injury, at least in rodent arteries, and that TGF-β signaling plays an important role in one or more steps in this process.64 One adventitial cell type that may respond to arterial injury in this way is the adventitial fibroblast based on many observations that these cells undergo phenotypic conversion to SMC-like myofibroblasts.2,9,65 In addition, an intriguing possibility is that adventitial Sca1+ progenitor cells (discussed further below) may be stimulated by arterial injury to adopt an SMC-like phenotype, migrate into the media, and possibly into the intima, and thereby participate in neointimal formation.18,20 Evidence to support this possibility was provided by Hu et al who isolated adventitial Sca1+ cells from genetically-marked donor mice and then transplanted those cells onto the outside of a vein graft placed into the arterial circulation.18 Adventitia-derived Sca1+ cells were found in the media at 2 weeks and in the intima at 4 weeks where they no longer expressed Sca1 antigen and became immunopositive for SMC marker proteins. An early event observed following many forms of arterial injury is adventitial inflammation and an accumulation of monocytes and activated macrophages.12,14,16 This local inflammatory response may be necessary for down regulation of the ongoing adventitial niche signaling that maintains Sca1+ cells as progenitors thus resulting in the “release” of these progenitor cells to adopt other cell fates. Inhibition of early leukocyte recruitment decreases injury-induced neointimal formation consistent with this possibility.2,12 Recent studies using inducible SMC-specific cre recombinase-mediated fate mapping approaches show that a majority of neointimal SMCs do indeed arise from medial SMCs in a femoral artery wire injury model in adult mice.66 However, important paracrine contributions to neointimal lesion growth can be made by numerically small or transient cell populations in vivo.67,68 Moreover, the adventitia is a rich source of reactive oxygen species (ROS)3,10,13,69 and of nitric oxide (NO)10,13. ROS production has been associated with increased adventitial fibroblast migration70, extracellular matrix production13,71, medial smooth muscle hypertrophy72,73, and neointimal hyperplasia after arterial injury74. Taken together, the adventitia may have two roles in neointimal lesion formation that are not mutually exclusive; (1) as a hub for recruitment of inflammatory cells that may directly stimulate medial SMC migration and proliferation as well as disrupt ongoing adventitial niche signaling to enable the differentiation of resident progenitor cells towards a SMC-like fate, and (2) as a source of neointimal precursor cells that contribute to the cellular mass of developing neointimal lesions.
The Adventitia as a Stem/Progenitor Cell Niche in the Artery Wall
As mentioned above, in 2004 Hu et al reported that adult ApoE-/- mice harbored progenitor cell populations in the aortic root adventitia that differentiated to SMCs when exposed to PDGF-BB in vitro. These cells had the surface marker profile Sca1+, CD34+, c-kit+, Flk1+, but were negative for the embryonic stem cell marker SSEA-1. When obtained from genetically marked Rosa26 donor mice and transferred onto the outside of an experimental vein graft that was then placed into the arterial circulation, adventitial Sca1-positive cells migrated through the media and were found in the graft neointima at 4 weeks where they had down-regulated Sca1 expression and were now SMC marker positive.18 By comparison, when genetically–marked adventitial Sca1-negative cells (Sca1-, mostly fibroblasts) were grafted instead of adventitial Sca1+ cells the vast majority of Sca1- cells remained clustered in the graft adventitia after 4 weeks and they were only rarely were found in the neointima. Thus, in this model adventitial Sca1+ cells with a potential to form SMCs could migrate from the adventitia to vein graft neointima, differentiate to SMCs and promote neointimal lesion growth.18,75 In 2006, Zengin et al reported a “vasculogenic zone” in adult human arteries that contained CD34+ progenitor cells capable of forming vascular structures in arterial ring explant cultures in vitro and promoting the formation of microvessels in transplantable tumor models in vivo.19 This “vasculogenic zone” was localized to a region between the media and the adventitia in human blood vessels that was reminiscent of the location in which Sca1+ cells were found in the aortic root of wild type and ApoE-/- mice.18-20 Pasquinelli et al, also reported finding CD34+ cells located between the media and the adventitia in human thoracic aortas and femoral arteries.76 Hoshino et al (2008) identified progenitor cells in the adventitia of human pulmonary arteries that expressed mesenchymal stem/progenitor cell markers but were negative for endothelial and hematopoietic cell markers.77 These progenitor cells differentiated to SMCs, osteogenic cells and adipogenic cells in selective culture media in vitro. Campagnolo et al, isolated CD34+/CD31- cells from human saphenous veins that expressed the stem cell marker Sox2 and displayed clonogenic and multilineage differentiation potential in vitro (discussed further below).21 Finally, Zorzi et al demonstrated that rat thoracic aorta contains adventitial macrophage-like cells which function to support angiogenesis in an aortic ring assay, presumably by releasing VEGF.78 A subset of these cells acquired an endothelial cell phenotype when cultured in the presence of VEGF and formed capillary-like structures in a matrigel assay in vitro. These studies raise the possibility that adventitial progenitor cells may play a role in formation and maintenance of a microvascular network in the adventitia called the vasa vasorum (discussed further below).
In 2008, Passman et al reported that CD34+, Sca1+, c-kit-, Flk1+, CD140b+ cells were found clustered in a domain of sonic hedgehog (Shh) signaling that was restricted to the adventitial layer of artery wall (Figure 3).20 In E18.5 Shh-/- embryos the number of adventitial Sca1+ cells (AdvSca1) in the aortic root was greatly diminished.20 Treatment of AdvSca1 cells in primary explant culture with Shh or the Hh signaling antagonist cyclopamine provided evidence that Hh signaling mediates mitogenic and survival responses in these progenitor cells.20,79 Mouse aortic AdvSca1 cells appear to be a heterogeneous population with different potentials for cell differentiation. When freshly isolated from mouse thoracic aorta and placed into serum-containing culture medium, roughly 50% of the isolated AdvSca1 cells differentiated to SMC-like cells and formed pericytes in matrigel assays in vivo, about 25% proliferated and maintained a progenitor cell phenotype (i.e., self-renewal), while the remaining 25% lost expression of Sca1 but did not acquire detectable levels of SMC marker proteins.20 A small percentage of AdvSca1 cells formed osteogenic cells in the presence of BMP2 or adipogenic cells in the presence of dexamethasone, insulin and isobutylmethylxanthine.20,80 Similarly, Campagnolo et al isolated a population of CD34+/CD31- cells from the perivascular zone of adventitial vasa vasorum in human saphenous veins from patients undergoing coronary artery bypass surgery.21 These cells could be cloned in vitro and possessed multilineage potential to form osteoblasts, adipocytes, pericytes and SMCs under selective differentiation-promoting conditions in vitro. When transplanted into ischemic hindlimbs of immunodeficient mice, human saphenous vein-derived progenitor cells adopted a pericyte-like phenotype, formed N-cadherin-mediated physical contacts with endothelial cells, improved hindlimb angiogenesis, and enhanced blood flow recovery.21 In summary, these data suggest that the adventitia harbors multiple types of progenitor cells that appear to act in concert as part of a healing response to vascular injury.
Microvascular Networks in the Adventitia – Vasa Vasorum
The studies described above demonstrate that the adventitia supports a unique progenitor niche-like environment which contains cells that have the potential to self-renew and contribute to arterial wound repair and neointimal growth. Both progenitor cells and niche-like support cells in the adventitia may communicate with medial SMCs and luminal endothelial cells via soluble factors produced within the media and intima and carried by transmural bulk fluid flow driven by steep pressure gradients from the lumen to the adventitia81,82. In addition, the adventitia supports angiogenesis of the vasa vasorum and contributes to the progression of atherosclerotic plaques. The vasa vasorum (“vessels of the vessels”) forms a microvascular network in the adventitia of large arteries (>0.5mm thick) that supplies oxygen and nutrients to the outer layers of the vessel wall.4,83-85 For example, when intercostal arteries, the source of vasa vasorum in the descending aorta, are ligated in dogs, the tunica media undergoes necrosis.4,86 These studies show that whereas proximal SMC layers nearest the lumen are nourished by diffusion from the circulation, the middle and outer layers of the descending aorta are supplied by vessels originating from the adventitia.
Many studies have correlated the presence of atherosclerotic plaque lesions with segments of the vessel wall supplied by vasa vasorum.87-89 Similar to its role to provide oxygen and nutrients to the outer artery wall, the vasa vasorum also extends into the intima and nourishes developing atherosclerotic plaques. Moreover, as the principle route for leukocyte trafficking into the lesion, modulating the number vasa vasorum microvessels may affect plaque growth. For example, treatment with two angiogenesis inhibitors, endostatin and the fumagillin analog TNP-470, inhibited atherosclerotic plaque progression.90-92 Likewise, angiostatin, an inhibitor of endothelial cell migration and proliferation that promotes endothelial apoptosis, was also found to inhibit plaque neovascularization and plaque growth.6,93,94 The antiangiogenic proterties of plasminogen activator inhibitor (PAI) are well established.95,96 In previous studies, Mulligan-Kehoe and colleagues determined that PAI-123, a truncated form of PAI, is also a potent antiangiogenic factor that represses FGF2-stimulated angiogenesis in vitro.97,98 In atherosclerosis-prone LDL receptor-/- /ApoB48-deficient mice, Drinane et al reported that treatment with PAI-123 decreased plaque area, plaque cholesterol composition, and the density of vasa vasorum in the adventitia and perivascular region of the descending aorta.8 Interestingly, an examination of plaques arising in the innominate artery demonstrated that inhibition of angiogenesis in the vasa vasorum with PAI-123 actually promoted outward remodeling and ultimately, plaque regression.8 Therefore, plaque growth is effectively decreased in vivo through the inhibition of vasa vasorum angiogenesis and disruption of blood supply to developing plaque lesions.
The role of hypoxia in mediating angiogenesis in the adventitia is not limited to the descending aorta. For example, the pulmonary artery undergoes adventitial neovascularization in response to hypoxia.99,100 Davie et al examined the effect of cytokines released by adventitial fibroblasts (AdvFBs) upon the proliferation, migration and tubulogenesis of vasa vasorum endothelial cells (VVECs).99 In culture, exposure to conditioned media from AdvFBs cultured under hypoxic conditions increased the proliferation of VVECs and potentiated the formation of tubular endothelial structures. The authors went on to highlight the pro-angiogenic effects of endothelin-1 (ET-1) produced by hypoxic AdvFBs and signaling through ETB receptors in VVECs in vitro. Therefore, in the cases described above, adventitial progenitor cells may serve as a local source of pericytes de novo that stabilize nascent microvessels and thereby promote angiogenesis in the outer layers of artery wall.
Role of Adventitial Inflammation in Vascular Wall Remodeling
Vascular remodeling in response to chronic changes in blood flow depends upon interactions of the endothelium with cells of the medial and adventitial layers.11,101 Using a mouse carotid flow reduction model, Zhou et al showed that inward remodeling required early adventitial accumulation of CXCR3-positive macrophages.16 In this model, the chemokine receptor CXCR3 and its ligands IP10 and Mig were required to recruit monocytes to the adventitia. Both IP10 and Mig transcripts rapidly increased to reach peak levels within 6 hours of carotid flow reduction surgeries.16 The authors found evidence for a unique subset of macrophages accumulating in the adventitia of vessels undergoing inward remodeling responses suggesting either amplification of a preexisting subset of adventitial macrophages or homing of monocytes with particularly high levels of CXCR3 expression to the adventitia, or both.16 Likewise, Tang et al showed that macrophage depletion prevented flow-dependent inward remodeling in mouse carotid artery.12 Inward remodeling was associated with transient adventitial macrophage activation and superoxide-stimulated cytokine production (IL1β, IL6, IP10, and Mig). Both cytokine production and inward remodeling were dependent upon expression of MyD88, an adapter protein that is critical for activation of the transcription factor NF-kB by factors regulating immune responses and inflammation.12 This mechanism is consistent with multiple studies implicating TLR-dependent signaling in the development of intimal hyperplasia and atherosclerosis.102-106
Another example of a role for the adventitia in vessel wall remodeling comes from an analysis of the blunted response to angiotensin II (AngII)-mediated hypertension in RAG1-/- mice lacking B and T lymphocytes. In this model, adoptive transfer of T cells, but not B-cells, restored the AngII-induced hypertensive response.107 Infusion of low doses of AngII stimulated vessel wall cells to produce RANTES and other chemokines that recruited T cells to the aortic adventitia and adjacent periadventitial adipose tissues. In these adventitial and periadventitial sites T cells became activated to produce TNFα, INFγ and p47phox.107 Increased T cell p47phox activity stimulated superoxide production that scavenged locally produced nitric oxide (NO) in the vessel wall. Adipocytes then released TNFα and other cytokines that stimulate NADPH oxidase activity in SMCs further reducing NO levels and increasing vascular smooth muscle contractile tone. These studies highlight the important roles played by the adventitia as a site for T cell homing, activation and superoxide production.3,10,107 They also point to coordinate signaling interactions between adventitial cells and periadventitial adipose tissue as mediators of effector T cell activation, cytokine production and blood pressure regulation. Infusion of AngII also stimulates the production of IL6 and MCP1 by adventitial cells.15 Production of these factors is correlated with adventitial thickening, monocyte recruitment, macrophage differentiation and aortic wall remodeling. When monocytes and aortic adventitial fibroblasts were cocultured in vitro, increased levels of IL6 and MCP1 were found in conditioned medium. This conditioned medium promoted the differentiation of monocytes into macrophages, enhanced MCP1 production and MMP9 expression by adventitial fibroblasts.15 These findings provide further support for the important role of the arterial adventitia as a mediator of inflammatory cell interactions that can initiate physiological flow-dependent remodeling responses as well as predispose the artery wall to vasospasm and pathogenic remodeling of the medial layer.
Interactions of the Adventitia with Surrounding Tissues
Because of its location between the vessel wall and the surrounding tissues in which the vessel is located, adventitial cells could, in principle, participate in tissue repair or disease processes in either the vessel wall itself or in surrounding nonvascular tissues (Figure 4). For example, in an experiment designed to produce pancreatic adenocarcinomas, Tian et al used Pdx-cre to activate expression in pancreatic epithelial cells of a constitutively active form of the Shh signaling protein smoothened (Smo-W535L, also called SmoM2) in transgenic mice.108 Surprisingly, although they could demonstrate expression of the SmoM2 transgene in pancreatic epithelium and localization of SmoM2 protein to primary cilia, a requirement for Hh signal pathway activation in vertebrates109, there was no signaling response detectable in the epithelium and no adenocarcinomas were found. However, expression of Pdx-cre is known to leak into the stromal compartment and activate signaling responses in pancreatic mesenchymal cells. In fact, careful examination of Pdx-cre mice crossed with a cre-activated reporter strain (R26R) reporter mice revealed that in addition to reporter expression in pancreatic epithelium, rare β-galactosidase-positive cells were also detected in the adventitia of pancreatic blood vessels.108 Indeed large mesenchymal tumors were formed in Pdx-Cre/SmoM2 transgenic mice that originated from the adventitial layer of arteries within the pancreas and projected into pancreatic stroma. These mesenchymal tumors were smooth muscle marker-positive suggesting that they either arose from adventitial myofibroblasts or from the resident pool of adventitial Sca1+ progenitor cells. These results raise the possibility that the adventitial progenitor cell niche may be a substrate not only for wound repair or disease processes in the artery wall but also for hyperplastic or neoplastic changes in adjacent tissues as well.108
Figure 4.
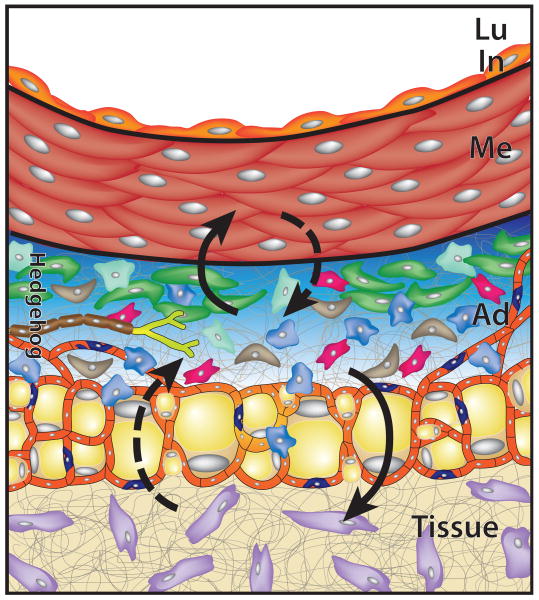
Adventitia interacts with surrounding tissues. The adventitia is positioned between the vessel wall (top) and the surrounding tissues in which the vessel is located (Tissue, bottom). From this position, adventitial cells could participate in development, repair and disease processes in either of these adjacent tissues. In this example, Shh signaling (blue shaded region) maintains Sca1+ adventitial progenitor cells (green) that can differentiate to SMC-like cells to replace lost or damaged myocytes in the media (upward solid arrow). Under other conditions, resident adventitial cells, including Sca1+ progenitors, could respond to injury or disease of surrounding tissues and participate along with cells of the adjacent tissues (purple) to repair tissue damage (downward solid arrow). Dashed arrows indicate hypothetical roles of adventitial cells or adjacent tissue cells in the processes indicated.
Perivascular adipose tissue (PVAT) is often found associated with large arteries and important signaling interactions are thought to occur between PVAT cells and cells of the artery wall. For example, PVAT is a source of mesenchymal progenitor cells that can produce pericyte-like cells and participate in the formation and regression of microvascular networks in the adventitia and perivascular space110. PVAT is also a source of paracrine factors that affect short-term contractile responses of vascular SMCs to vasoactive agonists as well as influence long-term regulation of blood pressure111. These affects may be at least partly dependent on the ratio of brown to white adipocytes that comprise thoracic PVAT111. In addition to medial SMCs, PVAT may also signal to adventitial cells. Using liquid chromatography-tandem mass spectrometry the secretome of PVAT was investigated in rats112. Among the secretory proteins identified was complement 3 (C3), and C3 was subsequently shown to stimulate adventitial fibroblast migration and myofibroblast transition in vitro112. Moreover, increased expression of C3 in PVAT was found to be tightly associated with adventitial thickening and myofibroblast clustering around PVAT in deoxycorticosterone acetate-salt hypertensive rats112. Finally, inflammation and oxidative stress in PVAT may play important roles in the vascular dysfunction associated with metabolic syndrome.113
Summary and New Perspectives
Going forward, the studies described above raise a number of important new questions to be addressed. We still do not know what mechanisms act in developing arteries to terminate tunica media formation at the characteristic number of layers of smooth muscle and elastic fibers identified by Wolinsky and Glagov.44 Does the onset of Shh signaling in the adventitia around E16.5 in the mouse20 reflect formation of a media-adventitia border during vascular development? If so, what is the molecular identity of such a border-forming mechanism? What is the origin of adventitial progenitor cells in the embryo? Hu et al18 showed that these cells do not arise from bone marrow, Passman et al20 reported that they are not derived from cardiac neural crest and Wasteson et al114 showed that they do not originate from somites. What roles do the individual adventitial cell types, including resident tissue macrophages, play in morphogenesis of the adventitia? In adult arteries, it is now well documented that adventitial cells respond rapidly to vascular injury.59,115 Also, inflammation of the adventitia is common in arteries undergoing wound repair, atheroma formation or flow-induced wall remodeling.12,14-16,115,116 What secreted factors recruit monocytes and T cell subsets to the adventitia and what resident cell types in the adventitia produce those factors? Recent reports suggest that the adventitia has properties of a stem/progenitor cell niche in the artery wall.18-21 What functions do adventitial progenitor cells have in maintenance and repair of the artery wall? What cell types interact with resident progenitor cells to produce a progenitor niche-like signaling environment in the adventitia? In addition to Shh, what other secreted factors and extracellular matrix components are critical elements of the adventitial progenitor niche?117,118 What mechanisms act to maintain adequate pool sizes of adventitial progenitor cells in adult vessels? Is depletion of this progenitor pool with age a contributing factor to the degenerative changes in artery walls seen in the elderly or in rapid aging syndromes?119-120 Does arterial injury and accompanying inflammation disrupt signaling interactions that maintain progenitor cells and lead to depletion of endogenous progenitor pools in the adventitia or in perivascular niches found in other tissues?115,116,121-123 Finally, how do adventitial cells contribute to formation of intimal lesions, and do adventitial cells have a role in tissue repair or disease pathogenesis in tissues and organs that normally surround individual arteries? Answers to these and other important questions about the adventitial layer of blood vessels will provide for a better understanding of how all three layers of vessel wall interact to form, maintain and repair a functioning vascular system.
Acknowledgments
We thank Jenna Regan for assistance with Figure 2, Colin Maguire for help with Figure 3, and members of Majesky, Mahoney and Daum laboratories for helpful discussions.
Sources of Funding: The authors were supported by National Institutes of Health Grants HL93594 and HL19242 (to MWM), HL88374 (to GD), HL87513 (to WMM, Jr), an American Heart Association grant 09PRE2060165 (to VJH), the Curriculum in Genetics & Molecular Biology at the University of North Carolina at Chapel Hill, and the Seattle Children's Research Institute.
Non-standard Abbreviations and Acronyms
- AdFibs
adventitial fibroblasts
- AdvSca1
adventitial Sca1-positive progenitor cell
- En
embryonic day n
- EEL
external elastic lamina
- Eln
elastin
- ET1
endothelin-1
- FGF
fibroblast growth factor
- Hh
hedgehog
- IEL
internal elastic lamina
- IFNγ
interferon-γ
- MCP1
monocyte chemotactic protein-1
- NO
nitric oxide
- PAI1
plasminogen activator inhibitor-1
- Ptc
hedgehog receptor patched
- PVAT
perivascular adipose tissue
- Sca1
stem cell antigen-1 also called Ly6A/E
- SM-MHC
smooth muscle myosin heavy chain
- Smo
smoothened
- SmoM2
constitutively active smoothened
- Shh
sonic hedgehog
- SMC
smooth muscle cell
- TgGH
transgenic growth hormone mouse
- TNFα
tumor necrosis factor-α
- VEGF
vascular endothelial cell growth factor
- VVECs
vasa vasorum endothelial cells
Footnotes
Disclosures: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gutterman DD. Adventitia-dependent influences on vascular function. Am J Physiol Heart Circ Physiol. 1999;277:1265–1272. doi: 10.1152/ajpheart.1999.277.4.H1265. [DOI] [PubMed] [Google Scholar]
- 2.Sartore S, Chiavegato A, Faggin E, Franch R, Puato M, Ausoni S, Pauletto P. Contribution of adventitial fibroblasts to neointima formation and vascular remodeling: from innocent bystander to active participant. Circ Res. 2001;81:46–53. doi: 10.1161/hh2401.100844. [DOI] [PubMed] [Google Scholar]
- 3.Haurani MJ, Pagano PJ. Adventitial fibroblast reactive oxygen species as autacrine and paracrine mediators of remodeling: bellwether for vascular disease? Cardiovasc Res. 2007;75:679–689. doi: 10.1016/j.cardiores.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 4.Heistad DD, Marcus ML, Larsen GE, Armstrong ML. Role of vasa vasorum in nourishment of the aortic wall. Am J Physiol Heart Circ Physiol. 1981;240:H781–H787. doi: 10.1152/ajpheart.1981.240.5.H781. [DOI] [PubMed] [Google Scholar]
- 5.Kwon HM, Sangiorgi G, Ritman EL, McKenna C, Holmes DR, Jr, Schwartz RS, Lerman A. Enhanced coronary vasa vasorum neovascularization in experimental hypercholesterolemia. J Clin Invest. 1998;101:1551–1556. doi: 10.1172/JCI1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulton KS, Vakii K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, Lo KM, Gillies S, Javaherin K, Folkman J. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atheroslclerosis. Proc Natl Acad Sci USA. 2003;100:4736–4741. doi: 10.1073/pnas.0730843100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritman EL, Lerman A. The dynamic vasa vasorum. Cardiovasc Res. 2007;75:649–658. doi: 10.1016/j.cardiores.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drinane M, Mollmark J, Zagorchev L, Moodie K, Sun B, Hall A, Shipman S, Morganelli P, Simons M, Mulligan-Kehoe MJ. The antiangiogenic activity of rPAI-1(23) inhibits vasa vasorum and growth of atherosclerotic plaque. Circ Res. 2009;104:337–345. doi: 10.1161/CIRCRESAHA.108.184622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JD, Bryant SR, Couper LL, Cary CPH, Gotwals PJ, Koetlianshy VE, Lindner V. Soluble transforming growth factor-β type II receptor inhibits negative negative remodeling, fibroblast transdifferentiation, and intimal lesion formation but not endothelial growth. Circ Res. 1999;84:1212–2222. doi: 10.1161/01.res.84.10.1212. [DOI] [PubMed] [Google Scholar]
- 10.Rey FE, Pagano PJ. The reactive adventitia: fibroblast oxidase in vascular function. Arterioscler Thromb Vasc Biol. 2002;22:1962–1971. doi: 10.1161/01.atv.0000043452.30772.18. [DOI] [PubMed] [Google Scholar]
- 11.Korshunov VA, Schwartz SM, Berk BC. Vascular remodeling: Hemodynamic and biochemical mechanisms underlying Glagov's phenomenon. Arterioscler Thromb Vasc Biol. 2007;27:1722–1728. doi: 10.1161/ATVBAHA.106.129254. [DOI] [PubMed] [Google Scholar]
- 12.Tang PCY, Qin L, Zielonka J, Zhou J, Matte-Martone C, Bergaya S, van Rooijen N, Shlomchik WD, Min W, Sessa WC, Pober JS, Tellides G. MyD88-dependent superoxide-initiated inflammation is necessary for flow-mediated inward remodeling of conduit arteries. J Exp Med. 2008;205:3159–3171. doi: 10.1084/jem.20081298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang HD, Ratsep MT, Chapman A, Boyd R. Adventitial fibroblasts in vascular structure and function: the role of oxidative stress and beyond. Can J Physiol Pharmacol. 2010;88:177–186. doi: 10.1139/Y10-015. [DOI] [PubMed] [Google Scholar]
- 14.Galkina E, Kadl A, Sanders J, Varughese D, Sarembock IJ, Ley K. Lymphocyte recruitment into the aortic wall before and during development of atherosclerosis is partially L-selectin dependent. J Exp Med. 2006;203:1273–1282. doi: 10.1084/jem.20052205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tieu BC, Lee C, Sun H, LeJeune W, Recinos A, 3rd, Ju X, Spratt H, Guo DC, Milewicz D, Tilton RG, Brasier AR. An adventitial IL6/MCP1 amplification loop accelerates macrophage-mediated vascular inflammation leading to aortic dissection in mice. J Clin Invest. 2009;119:3637–3651. doi: 10.1172/JCI38308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou J, Tang PC, Qin L, Gayed PM, Li W, Skokos EA, Kyriakides TR, Pober JS, Tellides G. CXCR3-dependent accumulation and activation of perivascular macrophages is necessary for homeostatic arterial remodeling to hemodynamic stresses. J Exp Med. 2010;207:1951–1966. doi: 10.1084/jem.20100098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Swedenborg J, Maryanpaa MI, Kovanen PT. Mast cells: important players in the orchestrated pathogenesis of abdominal aortic aneurysms. Arterioscler Thromb Vasc Biol. 2011;31:734–740. doi: 10.1161/ATVBAHA.110.213157. [DOI] [PubMed] [Google Scholar]
- 18.Hu Y, Zhang Z, Torsney E, Afzal AR, Davison F, Metzler B, Xu Q. Abundant progenitor cells in the adventitia contribute to atherosclerosis of vein grafts in ApoE-deficient mice. J Clin Invest. 2004;113:1258–1265. doi: 10.1172/JCI19628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zengin E, Chalajour F, Gehling UM, Ito WD, Treede H, Lauke H, Weil J, Reichenspurner H, Killic N, Ergun S. Vascular wall resident progenitor cells: a source for postnatal vasculogenesis. Development. 2006;133:1543–1551. doi: 10.1242/dev.02315. [DOI] [PubMed] [Google Scholar]
- 20.Passman JN, Dong XR, Wu SP, Maguire CT, Hogan KA, Bautch VL, Majesky MW. A sonic hedgehog signaling domain in the arterial adventitia supports resident Sca1+ smooth muscle progenitor cells. Proc Natl Acad Sci USA. 105:9349–9354. doi: 10.1073/pnas.0711382105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campagnolo P, Cesselli D, Al Haj Zen A, Beltrami AP, Krankel N, Katare R, Angelini G, Emanueli C, Madeddu P. Human adult vena saphena contain perivascular progenitor cells endowed with clonogenic and proangiogenic potential. Circulation. 2010;121:1735–1745. doi: 10.1161/CIRCULATIONAHA.109.899252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Majesky MW, Dong XR, Regan JN, Hoglund VJ. Vascular smooth muscle progenitor cells: Building and repairing blood vessels. Circ Res. 2011;108:365–377. doi: 10.1161/CIRCRESAHA.110.223800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 24.Swift MR, Weinstein BM. Arterial-venous specification during development. Circ Res. 2009;104:576–588. doi: 10.1161/CIRCRESAHA.108.188805. [DOI] [PubMed] [Google Scholar]
- 25.Coultas L, Chawengsaksophak K, Rossant J. Endothelial cells and VEGF in vascular development. Nature. 2005;438:937–945. doi: 10.1038/nature04479. [DOI] [PubMed] [Google Scholar]
- 26.Eichmann A, Yuan L, Moyon D, LeNoble F, Pardanaud L, Breant C. Vascular development: From precursor cells to branched arterial and venous networks. Int J Dev Biol. 2005;49:259–267. doi: 10.1387/ijdb.041941ae. [DOI] [PubMed] [Google Scholar]
- 27.Lucitti JL, Jones EA, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134:3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horowitz A, Simons M. Branching morphogenesis. Circ Res. 2008;103:784–795. doi: 10.1161/CIRCRESAHA.108.181818. [DOI] [PubMed] [Google Scholar]
- 29.Armulik A, Abramsson A, Betscholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97:512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 30.Majesky MW. Developmental basis of vascular smooth muscle diversity. Arterioscler Thromb Vasc Biol. 2007;27:1248–1258. doi: 10.1161/ATVBAHA.107.141069. [DOI] [PubMed] [Google Scholar]
- 31.Gaengel L, Genove G, Armulik A, Betsholtz C. Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler Thromb Vasc Biol. 2009;29:630–638. doi: 10.1161/ATVBAHA.107.161521. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Wada R, Yamashita T, Mi Y, Deng CX, Hobson JP, Rosenfeldt HM, Nava VE, Chae SS, Lee MJ, Liu CH, Hla T, Spiegel S, Proia RL. Edg1, the G protein-coupled receptor for sphingosine-1-phosphate, is essential for vascular maturation. J Clin Invest. 2000;106:951–961. doi: 10.1172/JCI10905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114:5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu H, Kennard S, Lilly B. Notch3 expression is induced in mural cells through an autoregulatory loop that requires endothelial-expressed Jagged 1. Circ Res. 2009;104:466–475. doi: 10.1161/CIRCRESAHA.108.184846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mammoto T, Ingber DE. Mechanical control of tissue and organ development. Development. 2010;137:1407–1420. doi: 10.1242/dev.024166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nat Rev Mol Cell Biol. 2009;10:53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Culver JC, Dickinson ME. The effects of hemodynamic force on embryonic development. Microcirculation. 2010;17:164–178. doi: 10.1111/j.1549-8719.2010.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wagenseil JE, Ciliberto CH, Knutsen RH, Levy MA, Kovacs A, Mecham RP. The importance of elastin to aortic development in mice. Am J Physiol Heart Circ Physiol. 2010;299:H257–H264. doi: 10.1152/ajpheart.00194.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berry CL, Looker T, Germain J. The growth and development of the rat aorta. I. Morphological aspects. J Anat. 1972;113:1–16. [PMC free article] [PubMed] [Google Scholar]
- 40.Gerrity R, Cliff W. The aortic tunica media of the developing rat: I. Quantitative stereologic and biochemical analysis. Lab Invest. 1975;32:585–600. [PubMed] [Google Scholar]
- 41.Clark JM, Glagov S. Transmural organization of the arterial media. The lamellar unit revisited. Arteriosclerosis. 1985;5:19–34. doi: 10.1161/01.atv.5.1.19. [DOI] [PubMed] [Google Scholar]
- 42.Ramirez F, Sakai LY, Dietz HC, Rifkin DB. Fibrillin microfibrils: multipurpose extracellular networks in organismal physiology. Physiol Genomics. 2004;19:151–154. doi: 10.1152/physiolgenomics.00092.2004. [DOI] [PubMed] [Google Scholar]
- 43.Wagenseil JE, Mecham RP. Vascular extracellular matrix and arterial mechanics. Physiol Rev. 2009;89:957–989. doi: 10.1152/physrev.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolinsky H, Glagov S. A lamellar unit of aortic medial structure and function in mammals. Circ Res. 1967;20:99–111. doi: 10.1161/01.res.20.1.99. [DOI] [PubMed] [Google Scholar]
- 45.Kelleher CM, McLean SE, Mecham RP. Vascular extracellular matrix and aortic development. Curr Top Devel Biol. 2004;62:153–188. doi: 10.1016/S0070-2153(04)62006-0. [DOI] [PubMed] [Google Scholar]
- 46.Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E, Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- 47.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest. 1998;102:1783–1787. doi: 10.1172/JCI4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Faury G, Pezet M, Knutsen R, Boyle W, Heximer S, McLean S, Minkes R, Blumer K, Kovacs A, Kelly D, Li DY, Starcher B, Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology. 2006;21:134–145. doi: 10.1152/physiol.00053.2005. PubMed: 16565479. [DOI] [PubMed] [Google Scholar]
- 50.Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- 51.Reidy MA, Clowes AW, Schwartz SM. Endothelial regeneration V: inhibition of endothelial regrowth in arteries of rat and rabbit. Lab Invest. 1983;49:569–575. [PubMed] [Google Scholar]
- 52.Schwartz SM, deBlois D, O'Brien ERM. The intima: Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 53.Baumgartner HR, Studer A. Controlled over-dilatation of the abdominal aorta in normo- and hypercholesterolemic rabbits. Pathol Microbiol (Basel) 1963;26:129–148. [PubMed] [Google Scholar]
- 54.Stemerman MB, Ross R. Experimental arteriosclerosis. I. Fibrous plaque formation in primates, an electron microscope study. J Exp Med. 1972;136:769–789. doi: 10.1084/jem.136.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz SM, Stemerman MB, Benditt EP. The aortic intima. II. Repair of the aortic lining after mechanical denudation. Am J Pathol. 1975;81:15–42. [PMC free article] [PubMed] [Google Scholar]
- 56.Regan CP, Adam PJ, Madsen CS, Owens GK. Molecular mechanisms of decreased smooth muscle differentiation marker expression after vascular injury. J Clin Invest. 2000;106:1139–1147. doi: 10.1172/JCI10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scott NA, Cipolla GD, Ross CE, Dunn B, Martin FH, Simonet L, Wilcox JN. Identification of a potential role for the adventitia in vascular lesion formation after overstretch balloon injury of porcine coronary arteries. Circulation. 1996;93:2178–2187. doi: 10.1161/01.cir.93.12.2178. [DOI] [PubMed] [Google Scholar]
- 58.Holifield B, Helgason T, Jemelka S, Taylor A, Navran S, Allen J, Seidel C. Differentiated vascular myocytes: Are they involved in neointimal formation. J Clin Invest. 1996;97:814–825. doi: 10.1172/JCI118481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shi Y, O'Brien JE, Fard A, Mannion JD, Wang D, Zalewski A. Adventitial myofibroblasts contribute to neointimal formation in injured porcine coronary arteries. Circulation. 1996;94:1655–1664. doi: 10.1161/01.cir.94.7.1655. [DOI] [PubMed] [Google Scholar]
- 60.Oparil S, Chen SJ, Chen YF, Durand JN, Allen L, Thompson JA. Estrogen attenuates the adventitial contribution to neointima formation in injured rat carotid arteries. Cardiovasc Res. 1999;44:608–614. doi: 10.1016/s0008-6363(99)00240-0. [DOI] [PubMed] [Google Scholar]
- 61.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, Hawkins SM, Clowes AW. Matrix metalloproteinase-9-overexpression enhances vascular smooth muscle cells migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–1185. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 62.Li G, Chen SJ, Oparil S, Chen YF, Thompson JA. Direct in vivo evidence demonstrating neointimal migration of adventitial fibroblasts after balloon injury of rat carotid arteries. Circulation. 2000;101:1362–1365. doi: 10.1161/01.cir.101.12.1362. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez-Menocal L, St-Pierre M, Wei Y, Khan S, Mateu S, Calfa M, Rahnemai-Azar AA, Striker G, Pham SM, Vazquez-Padron RI. The origin of post-injury neointimal cells in the rat balloon injury model. Cardiovasc Res. 2009;81:46–53. doi: 10.1093/cvr/cvn265. [DOI] [PubMed] [Google Scholar]
- 64.Mallawaarachchi CM, Weissberg PL, Siow RCM. Smad7 gene transfer attenuates adventitial cell migration and vascular remodeling after balloon injury. Arterioscler Thromb Vasc Biol. 2005;25:1381–1387. doi: 10.1161/01.ATV.0000168415.33812.51. [DOI] [PubMed] [Google Scholar]
- 65.Poole JC, Cromwell SB, Benditt EP. Behavior of smooth muscle cells and formation of extracellular structures in the reaction of arterial walls to injury. Am J Pathol. 1971;62:391–414. [PMC free article] [PubMed] [Google Scholar]
- 66.Nemenoff RA, Horita H, Ostriker AC, Furgeson SB, Simpson PA, VanPutten C, Crossno J, Offermanns S, Weiser-Evans MCM. SDF-1α induction in mature smooth muscle cells by invactivation of PTEN is a critical mediator of exacerbated injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2011;31:00–00. doi: 10.1161/ATVBAHA.111.223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daniel JM, Bielenberg W, Stieger P, Weinert S, Tillmanns H, Sedding DG. Time course analysis on the differentiation of bone marrow-derived progenitor cells into smooth muscle cells during neointima formation. Arterioscler Thromb Vasc Biol. 2010;30:1890–1896. doi: 10.1161/ATVBAHA.110.209692. [DOI] [PubMed] [Google Scholar]
- 68.Yu H, Stoneman V, Clarke M, Figg N, Xin HB, Kotlikoff M, Littlewood T, Bennett M. Bone marrow-derived smooth muscle-like cells are infrequent in advanced primary atherosclerotic plaques but promote atherosclerosis. Arterioscler Thromb Vasc Biol. 2011 doi: 10.1161/ATVBAHA.110.218578. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 69.Wang HD, Pagano PJ, Du Y, Cayatte AJ, Quinn MT, Brecher P, Cohen RA. Superoxide anion from the adventitia of the rat thoracic aorta inactivates nitric oxide. Circ Res. 1998;82:810–818. doi: 10.1161/01.res.82.7.810. [DOI] [PubMed] [Google Scholar]
- 70.Haurani MJ, Cifuentes ME, Shepard AD, Pagano PJ. Nox4 oxidase overexpression specifically decreases endogenous Nox4 mRNA and inhibits angiotensin II-induced adventitial myofibroblast migration. Hypertension. 2008;52:143–149. doi: 10.1161/HYPERTENSIONAHA.107.101667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsuruda T, Kato J, Hatakeyama K, Masuyama H, Cao YN, Imamura T, Kitamura K, Asada Y, Eto T. Antifibrotic effect of adrenomedullin on coronary adventitia in angiotensin II-induced hypertensive rats. Cardiovasc Res. 2005;65:921–929. doi: 10.1016/j.cardiores.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 72.Ushio-Fukai M, Alexander RW, Akers M, Griendling KK. p38 Mitogen-activated protein kinase is a critical component of the redox-sensitive signaling pathways activated by angiotensin II. Role in vascular smooth muscle hypertrophy. J Biol Chem. 1998;273:15022–15029. doi: 10.1074/jbc.273.24.15022. [DOI] [PubMed] [Google Scholar]
- 73.Wang HD, Johns DG, Xu S, Cohen RA. Role of superoxide anion in regulating pressor and vascular hypertrophic response to angiotensin II. Am J Physiol Heart Circ Physiol. 2002;282:H1697–H1702. doi: 10.1152/ajpheart.00914.2001. [DOI] [PubMed] [Google Scholar]
- 74.Weaver M, Liu J, Pimentel D, Reddy DJ, Harding P, Peterson EL, Pagano PJ. Adventitial delivery of dominant-negative p67phox attenuates neointimal hyperplasia of rat carotid artery. Am J Physiol Heart Circ Physiol. 2006;290:H1933–H1941. doi: 10.1152/ajpheart.00690.2005. [DOI] [PubMed] [Google Scholar]
- 75.Torsney E, Xu Q. Resident vascular progenitor cells. J Mol Cell Cardiol. 2011;50:304–311. doi: 10.1016/j.yjmcc.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Pasquinelli G, Tazzari PL, Vaselli C, Foroni L, Buzzi M, Storci G, Alviano F, Ricci F, Bonafe M, Orrico C, Bagnara GP, Stella A, Conte R. Thoracic aortas from multiorgan donors are suitable for obtaining resident angiogenic mesenchymal stromal cells. Stem Cells. 2007;25:1627–1634. doi: 10.1634/stemcells.2006-0731. [DOI] [PubMed] [Google Scholar]
- 77.Hoshino A, Chiba H, Nagai K, Ishii G, Ochiai A. Human vascular adventitial fibroblasts contrain mesenchymal stem/progenitor cells. Biochem Biophys Res Commun. 2008;368:305–310. doi: 10.1016/j.bbrc.2008.01.090. [DOI] [PubMed] [Google Scholar]
- 78.Zorzi P, Aplin AC, Smith KD, Nicosia RF. The rat aorta contains resident mononuclear phagocytes with proliferative capacity and proangiogenic properties. J Leukoc Biol. 2010;88:1051–1059. doi: 10.1189/jlb.0310178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Regan JN. Ph D dissertation. 2010. Regulation of adventitia-resident progenitor cells. [Google Scholar]
- 80.Spiegelman BM, Green H. Control of specific protein biosynthesis during the adipose conversion of 3T3 cells. J Biol Chem. 1980;255:8811–8818. [PubMed] [Google Scholar]
- 81.Zerwes HG, Risau W. Polarized secretion of a platelet-derived growth factor-like chemotactic factor by endothelial cells in vitro. J Cell Biol. 1987;105:2037–2041. doi: 10.1083/jcb.105.5.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alberding JP, Baldwin AL, Barton JK, Wiley E. Effects of pulsation frequency and endothelial integrity on enhanced arterial transmural filtration produced by pulsatile pressure. Am J Physiol Heart Circ Physiol. 2005;289:H931–H937. doi: 10.1152/ajpheart.00775.2004. [DOI] [PubMed] [Google Scholar]
- 83.Wolinsky H, Glagov S. Nature of species differences in the medial distribution of aortic vasa vasorum in mammals. Circ Res. 1967;20:406–421. doi: 10.1161/01.res.20.4.409. [DOI] [PubMed] [Google Scholar]
- 84.Heistad DD, Marcus ML. Role of vasa vasorum in nourishment of the aorta. Blood Vessels. 1979;16:225–238. doi: 10.1159/000158209. [DOI] [PubMed] [Google Scholar]
- 85.Zamir M, Silver MD. Vasculature in the walls of human coronary arteries. Arch Pathol Lab Med. 1985;109:659–662. [PubMed] [Google Scholar]
- 86.Wilens SL, Malcolm JA, Vazquez JM. Experimental infarction (medial necrosis) of the dog's aorta. Am J Pathol. 1965;47:695–711. [PMC free article] [PubMed] [Google Scholar]
- 87.Barger AC, Beeuwkes R, 3rd, Lainey LL, Silverman KJ. Hypothesis: vasa vasorum and neovascularization of human coronary arteries. A possible role in the pathophysiology of atherosclerosis. N Engl J Med. 1984;310:175–177. doi: 10.1056/NEJM198401193100307. [DOI] [PubMed] [Google Scholar]
- 88.Khurana R, Zhuang Z, Bhardwai S, Murakami M, De Muinck E, Yla-Herttuala S, Ferrara N, Martin JF, Zachary I, Simons M. Angiogenesis-dependent and independent phases of intimal hyperplasia. Circulation. 2004;110:2436–2443. doi: 10.1161/01.CIR.0000145138.25577.F1. [DOI] [PubMed] [Google Scholar]
- 89.Moreno PR, Purushothaman KR, Zias E, Sanz J, Fuster V. Neovascularization in human atherosclerosis. Curr Mol Med. 2006;6:457–477. doi: 10.2174/156652406778018635. [DOI] [PubMed] [Google Scholar]
- 90.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Synthetic analogues of fumagillin that inhibit angiogenesis and suppress tumor growth. Nature. 1990;348:555–557. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 91.O'Reilly MS, Boehm T, Shing Y, Fukai N, Vasios G, Lane WS, Flynn E, Birkhead JR, Olsen BR, Folkman J. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell. 1997;88:277–285. doi: 10.1016/s0092-8674(00)81848-6. [DOI] [PubMed] [Google Scholar]
- 92.Moulton KS, Heller E, Konerding MA, Flynn E, Palinski W, Folkman J. Angiogenesis inhibitors endostatin or TNP-470 reduce intimal neovascularization and plaque growth in apolopoprotein E-deficient mice. Circulation. 1999;99:1726–1732. doi: 10.1161/01.cir.99.13.1726. [DOI] [PubMed] [Google Scholar]
- 93.Eriksson K, Magnusson P, Dixelius J, Claesson-Welsh K, Cross MJ. Angiostatin and endostatin inhibit endothelial migration in response to FGF and VEGF without interfering with specific intracellular signal transduction pathways. FEBS Lett. 2003;536:19–24. doi: 10.1016/s0014-5793(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 94.Claesson-Welsh L, Welsh M, Ito N, Anand-Apte B, Soker S, Zetter B, O'Reilly M, Folkman J. Angiostatin induces endothelial cell apoptosis and activation of focal adhesion kinase independently of the integrin-binding motif RGD. Proc Natl Acad Sci USA. 1998;95:5579–5583. doi: 10.1073/pnas.95.10.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bacharach E, Itin A, Keshet E. In vivo patterns of expression of urokinase and its inhibitor PAI-1 suggest a concerted role in regulating physiological angiogenesis. Proc Natl Acad Aci USA. 1992;89:10686–10690. doi: 10.1073/pnas.89.22.10686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bajou K, Masson V, Gerard RD, Schmitt PM, Albert V, Praus M, Lund LR, Frandsen TL, Brunner N, Dano K, Fusenig NE, Weidle U, Carmeliet G, Loskutoff D, Collen D, Carmeliet P, Foidart JM, Noel A. The plasminogen activator inhibitor PAI-1 controls in vivo tumor vascularization by interacting with proteases, not vitronectin. Implications for antiangiogenic strategies. J Cell Biol. 2001;152:777–784. doi: 10.1083/jcb.152.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mulligan-Kehoe MJ, Kleinman HK, Drinane M, Wagner RJ, Wieland C, Powell RJ. A truncated plasminogen activator inhibitor-1 protein blocks the availability of heparin-binding vascular endothelial growth factor-A isoforms. J Biol Chem. 2002;277:49077–49089. doi: 10.1074/jbc.M208757200. [DOI] [PubMed] [Google Scholar]
- 98.Drinane M, Walsh J, Mollmark J, Simons M, Mulligan-Kehoe MJ. The anti-angiogenic activity of rPAI-1(23) inhibits fibroblast growth factor-2 functions. J Biol Chem. 2006;281:33336–33344. doi: 10.1074/jbc.M607097200. [DOI] [PubMed] [Google Scholar]
- 99.Davie NJ, Gerasimovskaya EV, Hofmeister SE, Richman AP, Jones PL, Reeves JT, Stenmark KR. Pulmonary artery adventitial fibroblasts cooperate with vasa vasorum endothelial cells to regulate vasa vasorum neovascularization: a process mediated by hypoxia and endothelin-1. Am J Pathol. 2006;168:1793–1807. doi: 10.2353/ajpath.2006.050754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Meyrick B. The pathology of pulmonary artery hypertension. Clin Chest Med. 2001;22:393–404. doi: 10.1016/s0272-5231(05)70279-3. [DOI] [PubMed] [Google Scholar]
- 101.Langille BL, O'Donnell F. Reductions in arterial diameter produced by chronic dicreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- 102.Edfeldt K, Swedenborg J, Hansson GK, Yan ZQ. Expression of toll-like receptors in human atherosclerotic lesions: a possible pathway for plaque activation. Circulation. 2002;105:1158–1161. [PubMed] [Google Scholar]
- 103.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 104.Niessner A, Shin MS, Pryshchep O, Goronzy JJ, Chaikof EL, Weyand CM. Synergistic proinflammatory effects of the antiviral cytokine interferon-alpha and Toll-like receptor 4 ligands in the atherosclerotic plaque. Circulation. 2007;116:2043–2052. doi: 10.1161/CIRCULATIONAHA.107.697789. [DOI] [PubMed] [Google Scholar]
- 105.Schoneveld AH, Oude Nijhuis MM, van Middelaar B, Laman JD, de Kleijn DP, Pasterkamp G. Toll-like receptor 2 stimulation induces intimal hyperplasia and atherosclerotic lesion development. Cardiovasc Res. 2005;66:162–169. doi: 10.1016/j.cardiores.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 106.Schoneveld AH, Hoefer I, Sluijter JP, Laman JD, de Kleijn DP, Pasterkamp G. Atherosclerotic lesion development and Toll like receptor 2 and 4 responsiveness. Atherosclerosis. 2007;197:95–104. doi: 10.1016/j.atherosclerosis.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Gorozny J, Weyand C, Harrison DG. Role of the T cell in the genesis of angioensin II-induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. doi: 10.1084/jem.20070657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254–4259. doi: 10.1073/pnas.0813203106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huangfu D, Anderson KV. Cilia and hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA. 2005;102:11325–11330. doi: 10.1073/pnas.0505328102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Traktuev DO, Merfeld-Clauss S, Li J, Kolonin M, Arap W, Pasqualini R, Johnstone BA, March KL. A population of multipotent CD34-positive adipose stromal cells share pericyte mesenchymal surface markers, reside in a periendothelial location, and stabilize endothelial networks. Circ Res. 2008;102:77–85. doi: 10.1161/CIRCRESAHA.107.159475. [DOI] [PubMed] [Google Scholar]
- 111.Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656:68–73. doi: 10.1016/j.ejphar.2011.01.023. [DOI] [PubMed] [Google Scholar]
- 112.Ruan CC, Zhu DL, Chen QZ, Chen J, Guo SJ, Li XD, Gao PJ. Perivascular adipose tissue-derived complement 3 is required for adventitial fibroblast functions and adventitial remodeling in deoxycorticosterone acetate-salt hypertensive rats. Arterioscler Thromb Vasc Biol. 2010;30:2568–2574. doi: 10.1161/ATVBAHA.110.215525. [DOI] [PubMed] [Google Scholar]
- 113.Marchesi C, Ebrahimian T, Angulo O, Paradis P, Schiffrin EL. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 114.Wasteson P, Johansson BR, Jukkola T, Breuer S, Akyurek LM, Partanen J, Lindahl P. Developmental origin of smooth muscle cells in the descending aorta in mice. Development. 2008;135:1823–1832. doi: 10.1242/dev.020958. [DOI] [PubMed] [Google Scholar]
- 115.Wilcox JN, Scott NA. Potential role of the adventitia in arteritis and atherosclerosis. Int J Cardiol. 1996;54(Suppl):S21–S35. doi: 10.1016/s0167-5273(96)02811-2. [DOI] [PubMed] [Google Scholar]
- 116.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJR. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 117.Brack AS, Conboy IM, Conboy MJ, Shen J, Rando TA. A temporal switch from notch to wnt signaling in muscle stem cells is necessary for normal adult myogenesis. Cell Stem Cell. 2008;10:50–59. doi: 10.1016/j.stem.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 118.Charles N, Ozawa T, Squatrito M, Bleau A, Brennan CW, Hambardzumyan D, Holland EC. Perivascular nitric oxide activates notch signaling and promotes stem-like character in PDGF-induced glioma cells. Cell Stem Cell. 2010;6:141–152. doi: 10.1016/j.stem.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Capell BC, Collins FS, Nabel EG. Mechanisms of cardiovascular disease in accelerated aging syndromes. Circ Res. 2007;101:13–26. doi: 10.1161/CIRCRESAHA.107.153692. [DOI] [PubMed] [Google Scholar]
- 120.Olive M, Harten I, Mitchell R, Beers JK, Djabali K, Cao K, Erdos MR, Blair C, Funke B, Smoot L, Gerhard-Herman M, Machan JT, Kutys R, Virmani R, Collins FS, Wight TN, Nabel EG, Gordon LB. Cardiovascular pathology in Hutchinson-Gilford progeria: Correlation with the vascular pathology of aging. Arterioscler Thromb Vasc Biol. 2010;30:2301–2309. doi: 10.1161/ATVBAHA.110.209460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- 122.Goldberg JS, Hirschi KK. Diverse roles of the vasculature within the neural stem cell niche. Regen Med. 2009;4:879–897. doi: 10.2217/rme.09.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tavazoie M, Van der Veken L, Silva-Vargas V, Louissant M, Colonna L, Zaidi B, Garcia-Verdugo JM, Doetsch F. A specialized niche for adult neural stem cells. Cell Stem Cell. 2008;3:279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]