Abstract
α-Defensins are proteins exhibiting in vitro anti-HIV-1 activity that may protect against mother-to-child transmission of HIV-1 via breast milk. Correlates of α-defensins in breast milk and transmission risk were determined in a cohort of HIV-1-infected pregnant women in Nairobi followed for 12 months postpartum with their infants. Maternal blood was collected antenatally and at delivery for HIV-1 viral load and infant HIV-1 infection status was determined <48 h after birth and at months 1, 3, 6, 9, and 12. Breast milk specimens collected at month 1 were assayed for α-defensins, HIV-1 RNA, subclinical mastitis, and CC and CXC chemokines. We detected α-defensins in breast milk specimens from 108 (42%) of 260 HIV-1-infected women. Women with detectable α-defensins (≥50 pg/ml) had a median concentration of 320 pg/ml and significantly higher mean breast milk HIV-1 RNA levels than women with undetectable α-defensins (2.9 log10 copies/ml versus 2.5 log10 copies/ml, p = 0.003). Increased α-defensins concentrations in breast milk were also associated with subclinical mastitis (Na+/K+ ratio > 1) and increased breast milk chemokine levels. Overall, 40 (15%) infants were HIV-1 uninfected at birth and subsequently acquired HIV-1. There was no significant association between month 1 α-defensins and risk of HIV-1 transmission. In conclusion, α-defensins were associated with breast milk HIV-1 viral load, chemokine levels, and subclinical mastitis, all of which may alter risk of infant HIV-1 acquisition. Despite these associations there was no significant relationship between breast milk α-defensins and mother-to-child transmission, suggesting a complex interplay between breast milk HIV-1, inflammation, and antiinfective factors.
INTRODUCTION
More than two-thirds of mother-to-child human immunodeficiency virus type 1 (HIV-1) transmission occurs intrapartum or in the postpartum period as a result of infant mucosal exposure to maternal cervicovaginal fluids, blood, and breast milk.1,2 Studies suggest that breastfeeding alone increases the risk of vertical transmission by ~10–14% above baseline among women with established HIV-1 infection and by ~30% for women who become acutely infected postpartum.3–5 Interventions to protect the infant against HIV-1 acquisition during the breastfeeding period could greatly reduce HIV-1 infections in children born in settings where replacement feeding may not be affordable, feasible, or safe.6
Both cell-free and cell-associated virus have been associated with risk of breast milk transmission and the risk varies with the amount of virus in breast milk, the duration of breastfeeding, and the presence of subclinical and clinical mastitis.7–14 The probability of breast milk HIV-1 transmission may also be modified by local immune responses in the breast and antiinfective substances in breast milk, including defensins and chemokines.12,15 We recently documented the relationship between CCR5 (MIP-1α, MIP-1β, and RANTES) and CXCR4 (SDF-1α) chemokines and breast milk transmission of HIV-1 showing that high levels of MIP-1β and SDF-1α were associated with reduced risk, whereas high levels of RANTES were associated with increased transmission.16 α, β, and θ-defensins possess potent antimicrobial activity and contribute to innate immunity as well as regulation of adaptive immune responses by induction of cytokine production and chemotactic effects on leukocytes and dendritic cells.17–20 α-defensins 1–3 have recently been the focus of much research due to their potential for utilization as antimicrobial agents and immunomodulators.
α-defensins which are found in large quantities in the storage granules of neutrophils as well as in natural killer (NK) cells, B and T lymphocytes, macrophages, and epithelial cells have been detected in breast milk.15,21,22 Increasing evidence supports a role for α-defensins in inhibiting HIV-1 infection,23–28 and several different mechanisms have been proposed, including direct activity on HIV-1 virions, inhibition of viral replication following HIV-1 entry into cells, and upregulation of CC chemokines.25–27 A recent study among 23 HIV-1-infected infants suggested that increased α-defensins in breast milk collected at 1 week postpartum were associated with reduced risk of infant HIV-1 acquisition after adjusting for breast milk HIV-1 RNA.22 To further examine associations between α-defensins and vertical HIV-1 transmission, we measured α-defensins in breast milk specimens collected from HIV-1–infected women and defined correlates of elevated α-defensins and the relationship between defensins and infant HIV-1 acquisition during 1 year of follow-up.
MATERIALS AND METHODS
Recruitment and follow-up
This study was nested in a prospective cohort study examining immune responses in infants born to HIV-1–infected women. Between July 1999 and December 2003, pregnant women attending antenatal clinics in Nairobi were offered HIV-1 counseling and testing, and those found to be HIV-1 infected were invited to consent for study participation. All enrollees received short-course zidovudine (AZT) 300 mg twice daily beginning at 34–36 weeks gestation through delivery.29 Women were counseled regarding infant feeding options and self-selected whether to breastfeed or practice replacement feeding. In this study, we focus on the women who opted to breastfeed their infants.
Mother–infant pairs were seen within 48 h of delivery, at 2 weeks postpartum, and monthly thereafter until 12 months postpartum. Maternal blood was obtained at 32 weeks gestation, at delivery, and at 1 month postpartum. Breast milk was obtained by manual self-expression at 2 weeks and 1 month postpartum. To determine infant HIV-1 infection status, peripheral blood was obtained from infants at birth and at 1, 3, 6, 9, and 12 months of age for HIV-1 DNA and HIV-1 RNA assays.
Laboratory assays
Infant and maternal plasma HIV-1 viral loads were determined using the Gen-Probe HIV-1 viral load assay (Gen-Probe Incorporated, San Diego, CA).30,31 Infant plasma specimens were defined as positive if >100 HIV-1 RNA copies/ml and >50 HIV-1 RNA copies/reaction were detected. In addition, infant blood blotted onto filter paper specimens was assayed for HIV-1 gag DNA using a nested PCR method.31 Infants were considered to be infected if they had (1) a positive filter paper DNA or plasma RNA assay on two consecutive visits, or (2) a single filter paper or plasma RNA assay if this was the last available sample.
Breast milk was fractionated when fresh into supernatant and cellular components using centrifugation at 710 g for 20 min and the fractions were frozen for future assays. The breast milk supernatant was stored at −80°C until assayed for α-defensins 1–3 using a commercial enzyme-linked immunosorbent assay (ELISA) kit (Hycult Biotechnology b.v., Netherlands). Validation assays conducted yielded an intraassay coefficient of variation (CV) of 9%.
Breast milk supernatant was tested for CC (MIP-1α, MIP-1β, RANTES) and CXC (SDF-1) chemokines using a commercial ELISA (R&D Systems, Minneapolis, MN). Breast milk HIV-1 RNA levels were determined on a different aliquot of breast milk supernatant using the Gen-Probe HIV-1 viral load assay.32 Samples of whole breast milk were also stored at −80°C and these were analyzed for sodium (Na+) and potassium (K+) using ion-selective electrodes (Olympus Diagnostica GMBH Olympus AU400 analyzer, Hamburg, Germany). Severe subclinical mastitis was defined as an Na+/K+ ratio >1, as previously described.14
Statistical analysis
Statistical analyses were conducted using Stata 8.2 (Stata Corporation, College Station, TX). Variables that were not normally distributed were log10 transformed and geometric means and 95% confidence intervals (CI) reported. Univariate and multivariate analyses were carried out using linear regression for continuous variables and logistic regression for categorical variables to investigate factors associated with α-defensins levels. To determine whether detection of α-defensins in breast milk obtained at month 1 was associated with subsequent infant HIV-1 infection logistic regression was performed with infant infection after birth as the outcome. A second analysis with Cox proportional hazards regression for interval-censored data and time to HIV-1 infection as the outcome was also performed. In this model, we included breastfeeding duration to account for varying breast milk transmission risk to the infants. Infants who died during follow-up were censored at the last visit with known HIV-1 status.
RESULTS
Cohort characteristics and follow up
Of 280 breastfeeding women who provided a breast milk sample at the month 1 postpartum visit, 20 women were excluded from the analysis because their infants had positive HIV-1 DNA or RNA assays <48 h after delivery. Thus, 260 women whose infants were HIV-1 uninfected at birth formed the study population (Fig. 1). Women had a median age of 24 years (range 18–39), 215 (83%) were in monogamous marital relationships, and the median duration of the current partnership was 3 years (range 1–23 years). Mean duration of AZT use prior to delivery was 29 days, and 227 (87%) women used AZT antenatally for 14 days or more. Two hundred and fifteen (83%) women had spontaneous vaginal deliveries, 45 (17%) delivered by cesarean section, with only 8 (18%) among these performed prior to the onset of labor. Twenty-seven (10%) women were diagnosed as having clinical mastitis between delivery and month 1, and 92 (37%) had subclinical mastitis at the month 1 visit.
FIG. 1.
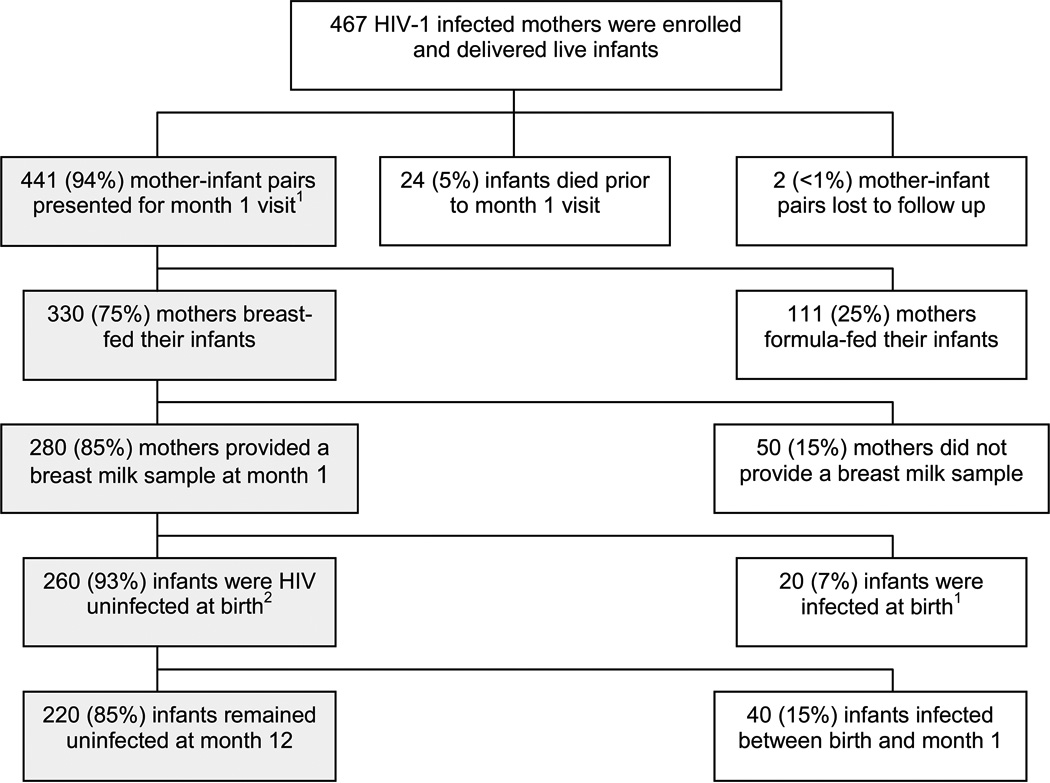
Study population. 1Shading indicates population of interest. 2Infants with positive HIV-1 DNA or RNA assays at <48 h were considered infected in utero and defined as being HIV-1 infected at birth.
Forty (15%) of the 260 infants acquired HIV-1 over the course of follow-up. Twenty-nine (73%) became infected between birth and 1 month of age, and 11 (27%) became infected between the ages of 1 and 12 months. Twenty-four (9%) infants died during follow-up with 13 (54%) among these being HIV-1 infected and 11 (46%) HIV-1 uninfected. Excluding infants who died during follow-up, the mean duration of follow-up was 10 months and 173 (73%) were followed up to the age of 12 months.
Maternal immune status and viral load
At 1 month postpartum, mean maternal CD4+ T cell count was 600 cells/µl and mean maternal CD4 percent was 24%. Thirty-one (14%) women were severely immunosuppressed with CD4 count <200 and/or CD4 percent less than 15. Virus was undetectable in the breast milk of 35 (13%) women and plasma and breast milk viral loads were positively correlated (R = 0.6; p < 0.0001). Mean maternal plasma viral load was 4.7 log10 copies/ml and mean breast milk viral load was 2.9 log10 copies/ml among women with detectable breast milk virus.
Correlates of α-defensins in breast milk
We detected α-defensins in breast milk specimens from 108 (42%) of the 260 women. Among women with detectable α-defensins (≥41 pg/ml), the median α-defensins concentration was 320 pg/ml (range 44–9992 pg/ml) and 13 (12%) women had α-defensins levels >2500 pg/ml.
Levels of breast milk α-defensins were positively correlated with breast milk HIV-1 RNA concentrations (p < 0.001). For every one log increase in α-defensins, breast milk viral load increased 0.2-fold (Table 1). Other correlates of elevated α-defensins in breast milk were subclinical mastitis, lower parity, and higher CD4 percent (Table 1). In multivariate analysis adjusting for HIV-1 RNA in breast milk, subclinical mastitis, lower parity, and higher CD4 percent remained independently associated with α-defensins levels (p = 0.006, p = 0.01, and p < 0.001, respectively) (Table 1).
Table 1.
Correlates of α-Defensins in Month 1 Breast Milk Specimens Obtained from HIV-1-Infected Mothers
| Variable | Regression coefficient |
p value |
|---|---|---|
| Univariate analysis | ||
| Breast milk HIV-1 RNA (log10 copies/ml) | 0.17 | <0.001 |
| Maternal CD4 percent | 0.01 | 0.02 |
| Maternal age | −0.02 | 0.13 |
| Parity | −0.08 | 0.01 |
| Subclinical mastitis at month 1 | 0.22 | 0.01 |
| Multivariate analysis | ||
| Breast milk HIV-1 RNA (log10 copies/ml) | 0.18 | 0.001 |
| Maternal CD4 percent | 0.02 | <0.001 |
| Parity | −0.09 | 0.01 |
| Subclinical mastitis at month 1 | 0.25 | 0.006 |
When women with detectable breast milk α-defensins were compared with women in whom defensins were not detected, we found higher mean breast milk HIV-1 RNA among those with detectable versus those with undetectable levels of α-defensins (2.9 log10 copies/ml versus 2.5 log10 copies/ml, p = 0.003) (Table 2). CC chemokines (MIP-1α, MIP-1β, and RANTES) and the CXC chemokine (SDF-1) were also significantly elevated in breast milk specimens with detectable α-defensins (Table 2). The strongest associations were found between α-defensins and the CC chemokines, especially MIP-1α and MIP-1β (Table 2).
Table 2.
Comparison between Women with Detectable and Undetectable α-Defensins Levels at 1 Month Postpartum
| Breast milk defensins (N = 260) |
Detectable [N = 108 (42%)] |
Undetectable [N = 152 (58%)] |
p value |
|---|---|---|---|
| Mean (95% confidence interval) | |||
| Breast milk HIV-1 RNA (log10 copies/ml) | 2.9 (2.7–3.1) | 2.5 (2.4–2.7) | 0.003 |
| CD4 percent | 26.2 (24.6–27.9) | 23.1 (21.5–24.8) | 0.01 |
| RANTES (log10 pg/ml) | 2.3 (2.2–2.4) | 2.1 (2.0–2.2) | 0.004 |
| MIP-1α (log10 pg/ml) | 1.5 (1.4–1.6) | 1.2 (1.2–1.3) | <0.001 |
| MIP-1β (log10 pg/ml) | 1.9 (1.8–2.0) | 1.6 (1.5–1.6) | <0.001 |
| SDF-1 (log10 pg/ml) | 2.4 (2.1–2.5) | 2.1 (2.0–2.20) | 0.01 |
HIV-1 transmission risk and breast milk α-defensins
Both maternal breast milk HIV-1 RNA and plasma viral load were independently associated with HIV-1 transmission to infants after birth (p < 0.01 for both) (Farquhar et al., in preparation). Thus, in our analysis of the role of α-defensins in breast milk HIV-1 transmission, we adjusted for breast milk HIV-1 viral load. We did not observe a significantly protective effect of α-defensins against infant HIV-1 acquisition (HR 0.7; 95% CI 0.5–1.2; p = 0.2). However, we did observe that when women with detectable α-defensins were compared with women with undetectable α-defensins, the effect of breast milk HIV-1 on transmission risk was attenuated in women with detectable α-defensins. The hazard of infection increased more than 2-fold for every one log increase in breast milk viral load for women with undetectable α-defensins (HR 2.9; 95% CI 1.9–4.4; p < 0.001), whereas for women with detectable α-defensins the change in infection risk for each one log increase in breast milk HIV-1 RNA was less but remained significant (HR 1.7; 95% CI 1.06–2.8; p = 0.03). There was no difference in breast milk α-defensin levels for infants infected early versus those infected after the first month.
DISCUSSION
α-defensins are known to have significant antiinfective properties but their role in breast milk HIV-1 transmission is less well understood. In this Kenyan cohort, α-defensins were detected in 108 (42%) breast milk samples collected from HIV-1-infected women at 1 month postpartum. Levels were in the range of what has been reported as peak concentrations for chemotactic activity (102–105 pg/ml)33 and lower than those demonstrated to inhibit HIV-1 in vitro (MIC50: 0.28–2 × 108 pg/ml).23,24 We found that women with detectable α-defensins had ~0.5 log10 higher HIV-1 concentrations in breast milk than women with undetectable α-defensins. These observations are consistent with a recent report by Kuhn et al.22 and may be secondary to the chemoattractant properties of α-defensins. Elevations in α-defensins within the breast milk compartment could result in an influx of immunologically active cells that would be more readily infected by HIV-1 and this could in turn result in increased HIV-1 replication. However, a healthier immune system among women with detectable α-defensins, as shown by a higher CD4 percent, resulted in attenuation of transmission risk in spite of higher breast milk HIV-1 levels in these women. This highlights the multifactorial nature of host–viral interactions in determining transmission risk and the importance of holistic approaches to understanding determinants of HIV-1 transmission.
We also found a strong association between breast milk defensins levels and subclinical mastitis after adjusting for breast milk HIV-1 RNA (p = 0.006), suggesting that subclinical mastitis may be one explanation for elevated α-defensins in these women. The association with mastitis is not surprising considering that α-defensins are produced by neutrophils which are present in large numbers as part of the inflammatory response during mastitis. While the nature of subclinical mastitis has not been well defined, a high prevalence in resource-limited settings has been associated with nutritional deficiencies, bacterial infection, and poor lactation practices.14,34–36 Additional investigation into the etiology of subclinical mastitis and associated factors may contribute to mother-to-child HIV-1 transmission prevention efforts and further clarify the role of α-defensins in HIV-1-infected breastfeeding women.
A novel finding in this study was that CC and CXC chemokines were positively associated with α-defensin concentrations in breast milk. Guo et al.27 reported that α-defensins up-regulate expression of CC chemokines in macrophages, the predominant cell type in breast milk, and this could be one mechanism for the strong associations we observed in our study. Alternatively, increases in both α-defensins and chemokines could be manifestations of an immune response to HIV-1 infection as levels of these factors were higher in women with a healthier immune system. Comparing breast milk from women with and without HIV-1 infection as well as prospectively following women and assessing clinical or subclinical mastitis may help answer these questions. Studies aimed at defining the direction of the association are important given our recently published study reporting that breast milk MIP-1β and SDF-1 are associated with significant reductions in HIV-1 transmission risk postpartum.16
A limitation of our study was that some of the infants diagnosed to be HIV-1 uninfected at birth and infected at 1 month postpartum may have been infected intrapartum. Our inability to identify these infections may have resulted in misclassification of breast milk exposure, and may have reduced our ability to detect a protective effect for α-defensins. Additionally, we assayed specimens collected at month 1, which may be less relevant than earlier time points for early breast milk transmission events. In the study by Kuhn et al.22 where specimens were collected at week 1 postpartum, a much greater proportion of women (79% versus 42%) had detectable defensins, which also suggests that there are differences in breast milk α-defensin levels during the first month postpartum. In-depth examination of α-defensin levels in breast milk samples collected very early postpartum, the time when most HIV-1 transmissions occur, could therefore provide additional insight into the role α-defensins play in prevention of HIV-1 transmission via breast milk.
ACKNOWLEDGMENTS
This research was supported by the National Institutes of Health, RO1 Grants HD-23412 and DE-14826 and K23 Grant HD-41879. R. Bosire, D. Wamalwa, and B. Lohman were scholars in the AIDS International Training and Research Program (AITRP), supported by Fogarty International Center, National Institutes of Health, grant D43-TW00007.
Footnotes
Presented in part at the International Society for Research in Human Milk and Lactation Symposium, Cambridge, September 10–14, 2004 (abstract number 80).
REFERENCES
- 1.Newell M. Mechanisms and timing of of mother-to-child transmission of HIV-1. AIDS. 1998;12(8):831–837. doi: 10.1097/00002030-199808000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA. 2001;285(6):709–712. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- 3.Dunn DT, Newell ML, Ades AE, Peckham CS. Risk of human immunodeficiency virus type 1 transmission through breastfeeding. Lancet. 1992;340(8819):585–588. doi: 10.1016/0140-6736(92)92115-v. [DOI] [PubMed] [Google Scholar]
- 4.Miotti PG, Taha TET, Kumwenda NI, et al. HIV transmission through breastfeeding: A study in Malawi. JAMA. 1999;282(8):744–749. doi: 10.1001/jama.282.8.744. [DOI] [PubMed] [Google Scholar]
- 5.Nduati R, John G, Mbori-Ngacha D, et al. Effect of breastfeeding and formula feeding on transmission of HIV-1: A randomized clinical trial. JAMA. 2000;283(9):1167–1174. doi: 10.1001/jama.283.9.1167. [DOI] [PubMed] [Google Scholar]
- 6.WHO/UNICEF/UNAIDS. Geneva: WHO; 2000. New data on the prevention of mother-to-child transmission of HIV and their policy implications: Conclusions and recommendations. [Google Scholar]
- 7.Coutsoudis A, Pillay K, Kuhn L, Spooner E, Tsai W, Coovadia H. Method of feeding and transmission of HIV-1 from mothers to children by 15 months of age: Prospective cohort study from Durban, South Africa. AIDS. 2001;15(3):379–387. doi: 10.1097/00002030-200102160-00011. [DOI] [PubMed] [Google Scholar]
- 8.Lewis P, Nduati R, Kreiss J, et al. Cell-free human immunodeficiency virus type 1 in breastmilk. J Infect Dis. 1998;177(1):34–39. doi: 10.1086/513816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nduati R, John G, Richardson B, et al. Human immunodeficiency virus type 1-infected cells in breastmilk: Association with immunosuppresion and vitamin A deficiency. J Infect Dis. 1995;172(6):1461–1468. doi: 10.1093/infdis/172.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rousseau CM, Nduati R, Richardson B, et al. Longitudinal analysis of human immunodeficiency virus type 1 RNA in breastmilk and of its relationship to infant infection and maternal disease. J Infect Dis. 2003;187(5):741–747. doi: 10.1086/374273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semba RD. Mastitis and transmission of human immunodeficiency virus through breast milk. Ann NY Acad Sci. 2000;918(1):156–162. doi: 10.1111/j.1749-6632.2000.tb05484.x. [DOI] [PubMed] [Google Scholar]
- 12.Van de Perre P, Simonon A, Hitimana D, et al. Infective and anti-infective properties of breastmilk from HIV-1-infected women. Lancet. 1993;341(8850):914–918. doi: 10.1016/0140-6736(93)91210-d. [DOI] [PubMed] [Google Scholar]
- 13.Semba RD, Kumwenda N, Hoover DR, et al. Human immunodeficiency virus load in breast milk, mastitis, and mother-to-child transmission of human immunodeficiency virus type 1. J Infect Dis. 1999;180:93–98. doi: 10.1086/314854. [DOI] [PubMed] [Google Scholar]
- 14.Willumsen J, Filteau S, Coutsoudis A, et al. Breastmilk RNA viral load in HIV-infected South African women: Effects of subclinical mastitis and infant feeding. AIDS. 2003;17(3):407–414. doi: 10.1097/00002030-200302140-00015. [DOI] [PubMed] [Google Scholar]
- 15.Armogida SA, Yannaras NM, Melton AL, Srivastava MD. Identification and quantification of innate immune system mediators in human breastmilk. Allergy Asthma Proc. 2004;25(5):297–304. [PubMed] [Google Scholar]
- 16.Farquhar C, Mbori-Ngacha D, Redman R, et al. CC and CXC chemokines in breastmilk are associated with mother to child HIV-1 transmission. Curr HIV Res. 2005;3(4):361–369. doi: 10.2174/157016205774370393. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Biragyn A, Kwak LW, Oppenheim JJ. Mammalian defensins in immunity: More than just microbicidal. Trends Immunol. 2002;23(6):291–296. doi: 10.1016/s1471-4906(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 18.Lehrer RI, Ganz T. Defensins of vertebrate animals. Curr Opin Immunol. 2002;14(1):96–102. doi: 10.1016/s0952-7915(01)00303-x. [DOI] [PubMed] [Google Scholar]
- 19.Ganz T. Defensins: Antimicrobial peptides of innate immunity. Nat Rev Immunol. 2003;3(9):710–720. doi: 10.1038/nri1180. [DOI] [PubMed] [Google Scholar]
- 20.Yang D, Chen Q, Chertov O, Oppenheim JJ. Human neutrophil defensins selectively chemoattract naive T and immature dendritic cells. J Leukoc Biol. 2000;68(1):9–14. [PubMed] [Google Scholar]
- 21.Agerberth B, Charo J, Werr J, et al. The human antimicrobial and chemotactic peptides LL-37 and alpha-defensins are expressed by specific lymphocyte and monocyte populations. Blood. 2000;96(9):3086–3093. [PubMed] [Google Scholar]
- 22.Kuhn L, Trabbatoni D, Kankasa C, et al. α-Defensins in the prevention of HIV transmission among breastfed infants. J Acquir Immune Defic Syndr. 2005;39(2):138–142. [PMC free article] [PubMed] [Google Scholar]
- 23.Mackewicz C, Yuan J, Tran P, et al. alpha-Defensins can have anti-HIV activity but are not CD8 cell anti-HIV factors. AIDS. 2003;17(14):F23–F32. doi: 10.1097/00002030-200309260-00001. [DOI] [PubMed] [Google Scholar]
- 24.Nakashima N, Yamamoto N, Masuda M, Fujii N. Defensins inhibit HIV replication in vitro. AIDS. 1993;7(8):1129. doi: 10.1097/00002030-199308000-00019. [DOI] [PubMed] [Google Scholar]
- 25.Chang TL-Y, Mosoian A, Pine R, Klotman ME, Moore JP. A soluble factor(s) secreted from CD8+ T lymphocytes inhibits human immunodeficiency virus type 1 replication through STAT1 activation. J Virol. 2002;76(2):569–581. doi: 10.1128/JVI.76.2.569-581.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115(3):765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo C, Tan N, Song L, Douglas S, Ho W. Alpha-defensins inhibit HIV infection of macrophages through upregulation of CC-chemokines. AIDS. 2004;18(8):1217–1218. doi: 10.1097/00002030-200405210-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oppenheim JJ, Biragyn A, Kwak LW, Yang D. Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Ann Rheum Dis. 2003;62(Suppl. 2):ii17–ii21. doi: 10.1136/ard.62.suppl_2.ii17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaffer N, Chuachoowong R, Mock PA, et al. Short-course zidovudine for perinatal HIV-1 transmission in Bangkok, Thailand: A randomised controlled trial. Lancet. 1999;353(9155):773–780. doi: 10.1016/s0140-6736(98)10411-7. [DOI] [PubMed] [Google Scholar]
- 30.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A ,C, and D isolates from Kenya. J Clin Microbiol. 2000;38(7):2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panteleeff DD, John G, Nduati R, et al. Rapid method for screening dried blood samples on filter paper for human immunodeficiency virus type 1 DNA. J Clin Microbiol. 1999;37(2):350–353. doi: 10.1128/jcm.37.2.350-353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Panteleeff DD, Emery S, Richardson B, et al. Validation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay with genital swabs and breastmilk samples. J Clin Microbiol. 2002;40(11):3929–3937. doi: 10.1128/JCM.40.11.3929-3937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J Biol Chem. 1996;271(6):2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 34.Flores M, Filteau S. Effect of lactation counselling on subclinical mastitis among Bangladeshi women. Ann Trop Paediatr. 2002;22:85–88. doi: 10.1179/027249302125000210. [DOI] [PubMed] [Google Scholar]
- 35.Nussenblatt V, Lema V, Kumwenda N, et al. Epidemiology and microbiology of subclinical mastitis among HIV-infected women in Malawi. Int J STD AIDS. 2005;16:227–232. doi: 10.1258/0956462053420248. [DOI] [PubMed] [Google Scholar]
- 36.Semba RD, Neville M. Breastfeeding, mastitis, and HIV transmission: Nutritional implications. Nutr Rev. 1999;57:146–153. doi: 10.1111/j.1753-4887.1999.tb01795.x. [DOI] [PubMed] [Google Scholar]


