Abstract
The regulation of the acid-base balance in cells is essential for proper cellular homeostasis. Disturbed acid-base balance directly affects cellular physiology, which often results in various pathological conditions. In every living organism, the protein family of carbonic anhydrases regulate a broad variety of homeostatic processes. Here we describe the identification, mapping and cloning of a zebrafish carbonic anhydrase 5 (ca5) mutation, collapse of fins (cof), which causes initially a collapse of the medial fins followed by necrosis and rapid degeneration of the embryo. These phenotypical characteristics can be mimicked in wild-type embryos by acetazolamide treatment, suggesting that CA5 activity in zebrafish is essential for a proper development. In addition we show that CA5 regulates acid-base balance during embryonic development, since lowering the pH can compensate for the loss of CA5 activity. Identification of selective modulators of CA5 activity could have a major impact on the development of new therapeutics involved in the treatment of a variety of disorders.
Introduction
Maintaining proper homeostasis is essential for every living organism. Homeostatic imbalance directly affects cellular metabolism, which eventually leads to physiological defects and pathologic conditions. Carbonic anhydrases (CA) are zinc metalloenzymes that are present in prokaryotes and eukaryotes. They catalyze the reversible dehydration/hydration reaction of carbon dioxide (CO2 + H2O ↔ HCO3 −+ H+) [1], [2]. CAs are involved in many physiological processes such as transport of carbon dioxide and bicarbonate between tissues, acid-base balance and biosynthetic reactions (glucogenesis, lipogenesis and ureagenesis) [3]. CAs are also important therapeutic targets, because of their involvement in various pathological conditions, such as glaucoma, obesity, some infectious diseases, cancer, epilepsy and osteoporosis [4] Therefore, many CA inhibitors and activators have been developed in order to treat these disorders [4]. Of the five different classes of CAs (α-εCA), vertebrates only express proteins of the α-CA class, which comprises 16 members that differ in their kinetic properties, tissue distribution, subcellular localization and their susceptibility to inhibitors [2], [4]–[12]. Whereas most CA isoforms are localized in the cytosol or associate with the plasma membrane, carbonic anhydrase 5 (CA5) is the only mitochondrial α-CA [13]. In mammals CA5 is encoded by two genes, CA5A and CA5B and whereas CA5A is expressed only in the liver, CA5B is widely expressed in many tissues [14]. Here we describe the mapping, cloning and characterization of a ca5 mutant zebrafish (collapse of fins, cof) and show that CA5 is involved in regulating acid-base balance during embryonic development in zebrafish.
Methods
Zebrafish strains and Forward genetic screening
Adult fish were raised and maintained under standard laboratory conditions. Fish experiments were performed in accordance with institutional guidelines and as approved by the Animal Experimentation Committee of the Royal Netherlands Academy of Arts and Sciences. The cof mutant was identified during a forward genetic screen performed at the Hubrecht Institute, Utrecht, The Netherlands. N-Ethyl-N-nitroso-ureum (ENU) mutagenesis was performed as previously described for the creation of the Hubrecht Institute target selected mutagenesis library [15]. F1 progeny of mutagenised male fish were outcrossed to wild-type fish in order to produce approximately 300 F2 families, which were then intercrossed. F3 progeny were screened for epidermal integrity defects at 2–3 dpf. Meiotic mapping of the collapse of fins mutation was performed using standard simple sequence length polymorphisms (SSLP). SSLP primer sequences can be found on www.ensembl.org. Genotyping PCR and subsequent sequencing of the ca5T839A mutation on finclip DNA or DNA of single embryos was performed with the following primers: F: 5 -cggacagcaagacatctg-3′ and R: 5′-ttgtggatacacatccccatag-3′.
Zebrafish embryo culturing
Embryos were raised in egg medium (60 μg/ml sea salt) pH 7. After 24 hpf dechorionated embryos were collected and placed in agarose-coated culture dishes with egg medium or 1x Danieau's medium (58 mM NaCl, 0.7 mM KCl, 0.4 mM MgSO4, 0.6 mM Ca [NO3]2 buffered at different pH with 10 mM Hepes.
Acetazolamide treatment
Acetazolamide (Sigma) was dissolved in DMSO to a concentration of 0.5 M and diluted to a working concentration of 2.5 mM and 5 mM in egg- or Danieau's medium. Control embryos were treated with the same amount of DMSO solvent.
In situ hybridisation, cDNA constructs and RNA synthesis
Whole mount in situ hybridization (ISH) was performed as described previously [16]. Embryos for ISH were fixed with 4% PFA/PBS and stored in 100% methanol. After ISH, embryos were cleared in methanol and mounted in benzylbenzoate/benzylalcohol (2:1) before images were taken. The following primers were used to produce the ca5 cDNA fragment: F: 5′-tgcatccaatgtggcaggag-3′; R: 5′-ttgtgtctgactgcaggcaagg-3′ and the insulin cDNA fragment: F: 5′-ttggtcgtgtccagtgtaag-3′; R: 5′-tgcctctcttccttatcagc-3′. Fragments were cloned into the pCRII-TOPO vector (Invitrogen) and antisense dig-labelled probes were synthesised according to standard protocols. Full-length zebrafish ca5 cDNA (MGC:171653; IMAGE:7448163) was derived by PCR on cDNA with the primers: F: 5′-gcgaattcaccatggtcacactgacagccat-3′ and R: 5′-gcctcgagttattccttagaggggg-3′ and cloned into the pCS2+ vector with EcoR1/Xho1. RNA was synthesised in vitro by using the SP6 mMessage mMachine kit (Ambion). The ca5T839A mutation was introduced using the QuickChange kit (Stratagene).
Results
A missense mutation in zebrafish carbonic anhydrase 5 leads to collapse of the medial fins, heart failure and rapid degeneration of the zebrafish embryo
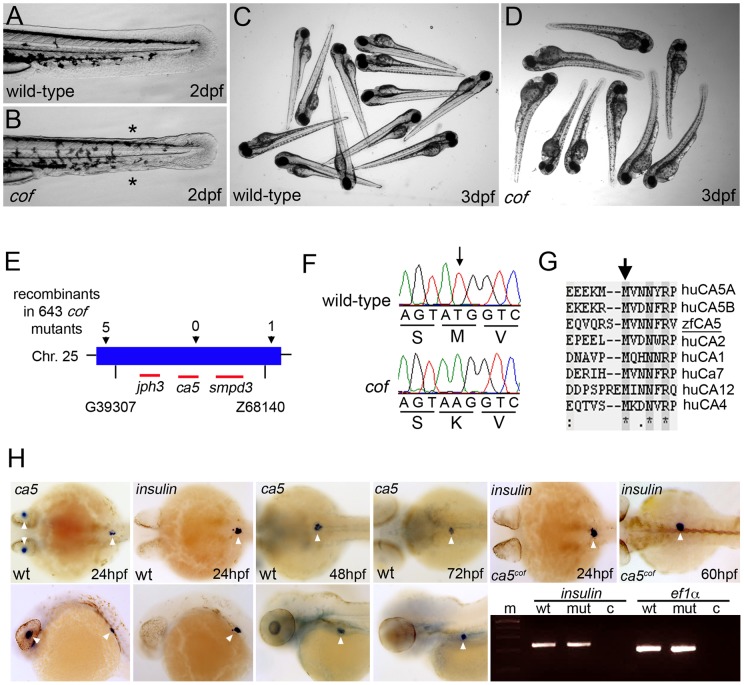
From a forward genetic screen in zebrafish we derived a mutant allele, collapse of fins (cof) that is characterized by defects of epidermal integrity and collapse of the medial fins at 2 days post-fertilization (dpf) (Figure 1A, B). During later stages of development, cardiac failure with edema and necrosis of the yolk-sac can be observed (Figure 1C, D), eventually leading to the rapid degeneration of the complete embryo at 4 dpf. The cof mutant phenotype is not fully penetrant, only 19% (instead of 25%) of the embryos can be phenotypically identified as a mutant in a batch of cof embryos (see Table 1). Meiotic mapping placed the cof allele on chromosome 25 between markers G39307 and z68140 (Figure 1E). Sequencing the open reading frames of the genes within the corresponding genomic interval revealed a T839A mutation in the coding region of the ca5 gene (Figure 1F). ca5 encodes for the zebrafish orthologue of CA5. The ca5T839A mutation results in an amino acid substitution of residue M280 to a lysine (Figure 1F). CA5 protein comparison analyses show that M280 is highly conserved across species and other members of the CA protein family (Figure 1G). The zebrafish genome contains only one ca5 gene and comparison of the amino acid sequences reveals 31% identity between zfCA5 and huCA5A, and 40% between zfCA5 and huCA5B. In order to study the ca5 mRNA expression, whole mount in situ hybridization was performed on wild-type embryos at various stages of development. This revealed ca5 mRNA expression in the lens and in a specific part of the embryo that resembles the developing pancreas at 24 hpf (Figure 1H). Previous studies have identified human CA5B in the insulin-producing β-cells of the pancreas [17]. To verify the mRNA expression of ca5 in the pancreatic β-cells in zebrafish, we compared ca5 expression with the expression of insulin, a marker for the pancreatic β-cells at 24 hpf. Indeed ca5 mRNA is localized at the same position as the insulin expressing cells (Figure 1H). During later stages of development, ca5 remains expressed in the pancreas (Figure 1H). The expression of insulin mRNA in the ca5cof mutants was indistinguishable from that in wild-type embryos (Figure 1H), suggesting that β-cell development is not impaired in ca5cof mutants during development. This was confirmed by determining the level of insulin mRNA expression by PCR on cDNA of wild-type sibling and ca5cof mutant embryos at 60 hpf (Figure 1H). Although we observed a clear morphological defect in the medial fins of the ca5cof mutants, ca5 expression could not be detected in the fin epidermis by in situ hybridisation.
Figure 1. Characterization, mapping and cloning of the cof mutant.
(A–D) Phenotypical comparison of wild-type and cof mutant embryos at 2 dpf (A, B) and 3 dpf (C, D). Asterisks mark the collapse of the medial fins in the cof mutant. (E) Summary of the linkage analysis and mapping of the cof locus at chromosome 25. The arrows mark the direction of the mutation. Red lines indicate the various transcripts in the genomic region. (F) Sequence chromatograms of wild-type and cof mutant cDNA. The corresponding amino acid residues are indicated below. (G) CA5 protein sequence alignment of zebrafish and human and other members of the human CA protein family. Arrow marks residue M280 that is substituted to a lysine in cof mutant embryos. (H) Detection of ca5 mRNA in wild-type embryos at 24, 48 and 72 hpf by in situ hybridization. mRNA expression of insulin at 24 hpf marks the position of pancreatic β-cells. Upper panel shows dorsal view and lower panels lateral view. White arrowheads mark the expression in the lens and the pancreatic β-cells.
Table 1. Quantification of the injection experiments and the various treatments.
| Phenotype (at 3 dpf) | % wild-type | % cof mutant |
| cof batch of embryos (n = 65) | 81 | 19 |
| 100 pg full-length ca5 RNA (n = 98) | 99 | 1 |
| 100 pg ca5T839A RNA (n = 59) | 83 | 17 |
| untreated wild-type embryos (n = 81) | 100 | 0 |
| wild-type embryos treated with 5 mM AZA (n = 73) | 10 | 90 |
| wild-type embryos treated with 2.5 mM AZA (n = 65) | 99 | 1 |
| cof batch of embryos treated with 2.5 mM AZA (n = 58) | 34 | 66 |
| wild-type embryos raised at pH 5 (n = 67) | 100 | 0 |
| wild-type embryos raised at pH 7.6 (n = 67) | 100 | 0 |
| wild-type embryos raised at pH 10 (n = 67) | 100 | 0 |
| wild-type embryos +5mM AZA in pH 5 medium (n = 75) | 77 | 23 |
| wild-type embryos +5mM AZA in pH 7.6 medium (n = 84) | 16 | 84 |
| wild-type embryos +5mM AZA in pH 10 medium (n = 76) | 4 | 96 |
| cof batch of embryos in pH 5 medium (n = 81) | 98 | 2 |
| cof batch of embryos in pH 7.6 medium (n = 77) | 82 | 18 |
| cof batch of embryos in pH 10 medium (n = 68) | 76 | 24 |
Expression of CA5 rescues the ca5cof mutant phenotype and acetazolamide treatment in wild-type embryos phenocopies the ca5cof mutation
To examine whether the ca5T839A mutation in ca5cof mutant embryos causes the ca5cof mutant phenotype, we restored CA5 expression by injecting the full-length zebrafish ca5 RNA. Injecting 100 pg ca5 RNA rescued the ca5cof mutant phenotype completely, whereas it was not rescued after the injection of 100 pg mutant ca5T839A RNA (Figure 2A-F and Table 1).
Figure 2. Rescue of the ca5cof mutant and acetazolamide treatment phenocopies the cof mutation in wild-type embryos.
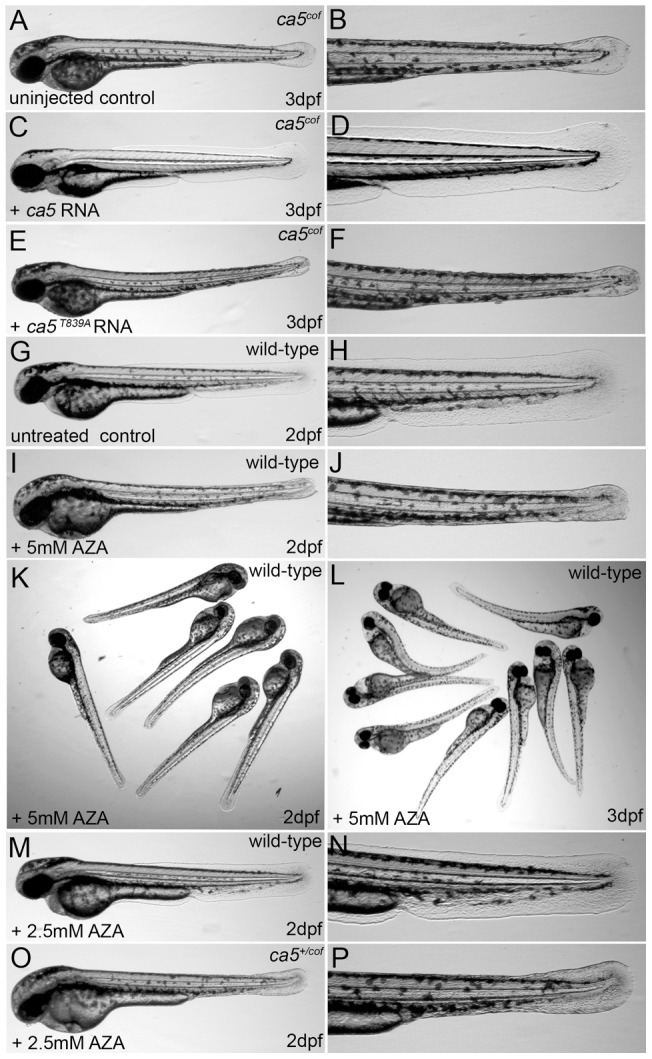
(A, B) ca5cof mutant embryos, (C–F) ca5cof mutant embryos injected with 100 pg full-length wild-type ca5 (C, D) or 100 pg ca5T839A mutant RNA (E, F). (I–L) Wild-type embryos at 2 dpf (I–K) and 3 dpf (L) treated with 5 mM AZA. Treatment of wild-type and cof heterozygous embryos at 2 dpf with 2.5 mM AZA.
In order to determine whether the ca5M280K substitution results in a reduced enzymatic activity of the CA5 protein we treated dechorionated wild-type embryos at 24 hpf with acetazolamide (AZA), a general CA inhibitor. Treatment with 5 mM AZA generated essentially a phenocopy of the ca5cof mutant fish including collapse of the medial fins, cardiac failure and necrosis of the yolk (Figure 2I-L and Table 1), ultimately leading to degeneration of the embryo. We verified the capacity of AZA to inhibit CA5 enzymatic activity by performing synergistic interaction experiments in ca5cof heterozygous sibling embryos. We treated wild-type embryos and a batch of cof embryos with suboptimal concentrations of AZA. The morphology of wild-type embryos treated with 2.5 mM AZA was not altered (Figure 2M, N and Table 1), however in the cof batch of embryos around 66% of the embryos showed the ca5cof mutant phenotype (Table 1). Sequencing revealed that a suboptimal dosage of AZA could induce the cof mutant phenotype in heterozygous embryos (Figure 2O, P and Table 1), whereas all homozygous wild-type sibling embryos were not affected. All this shows that inhibition of CA5 activity by AZA treatment during embryonic development can mimic the ca5cof mutant phenotype. Thus the ca5T839A missense mutation results in a severe reduction or loss of CA5 activity that initially leads to a collapse of the medial fins, followed by complete degeneration of the embryo.
Low pH reduces the effects of acetazolamide treatment in wild-type embryos and rescues the ca5cof mutant phenotype
Carbonic anhydrases are also involved in the regulation of acid-base balance, also in fish [18]. Therefore we examined the effect of altered pH levels on the collapse of the medial fins in AZA-treated wild-type embryos and ca5cof mutant embryos. First, wild-type embryos were raised from 24 hpf onwards in Danieau's medium of pH 5, pH 7.6 or pH 10, containing 5 mM AZA. These experiments show that wild-type embryos are less susceptible to AZA, when cultured in pH 5 medium, compared to embryos cultured in pH 7.6 or pH 10 medium (Figure 3A-L). Furthermore, the ca5cof mutant phenotype can be rescued by raising mutant embryos in Danieau's medium of pH 5 (see Table1), suggesting that normally the increase in cellular pH during embryonic development is compensated by the activity of mitochondrial CA5 (Table 1). We could not observe any significant developmental defects when wild-type embryos were raised in medium of pH 5 or pH 10 (Table 1). All this shows that CA5 is involved in maintaining cellular acid-base balance during zebrafish embryonic development.
Figure 3. Low pH compensates for loss of carbonic anhydrase activity by acetazolamide treatment.
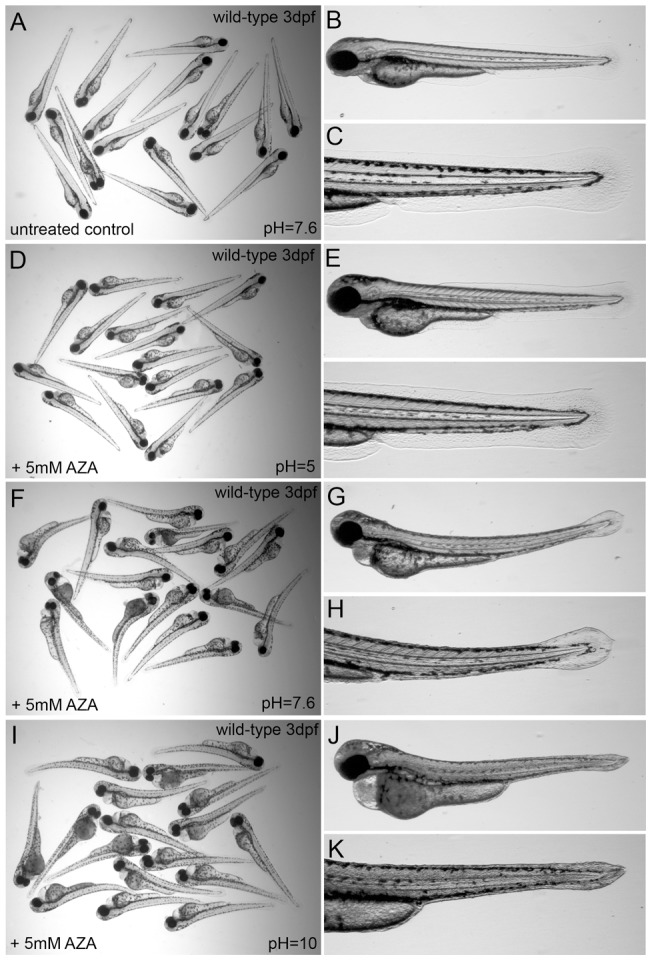
(A–C) Untreated wild-type embryos, (D–L) wild-type embryos at 3 dpf treated in pH 5 (D–F), pH 7.6 (G–I), or pH 10 medium (J–L) with 5 mM AZA.
Discussion
We show that defective CA5 activity in zebrafish results in a disturbed cellular acid-base balance, which leads to the collapse of the medial fins, heart failure and eventually degeneration of the complete embryo. We show that AZA, a general CA inhibitor, can copy the phenotype caused by the ca5cof mutation in wild-type embryos, suggesting that the T839A mutation results in the loss of CA5 enzymatic activity.
Human mitochondrial CA5 activity has been shown to be markedly elevated when the pH increases [19]. Thus loss or a reduced of CA5 activity results in an increase in cellular pH, which eventually leads to defects in cellular homeostasis. This is in accordance with our results in zebrafish that show that lowering the pH of the embryo medium can compensate for the loss of CA5 activity. In addition, the cof mutation is not fully penetrant when cultured in egg medium of pH 7 (∼19%), however an increase of the pH (pH 10) of the medium resulted in full penetrance of the mutation (∼24%) (Table 1), again showing that regulating acid-base balance is the major function of CA5 during zebrafish development.
Although the initial phenotypical defect is observed in the medial fins, ca5 mRNA expression could only be detected in the pancreatic β-cells at 2 and 3 dpf. Defective CA5 function in the pancreatic β-cells cannot explain the medial fin defects and the rapid degeneration of ca5cof mutants. First of all, the level of insulin mRNA in the mutant embryos is not altered, suggesting that β-cell development is not impaired in ca5cof mutants. In addition, zebrafish mutants that lack pancreatic β-cells do not develop the phenotypical characteristics that we observe in the ca5cof mutant [20]. A plausible explanation for the severe medial fin defect and the rapid degeneration of the ca5cof mutant would be that CA5 is expressed at low levels in the epidermis. The defective epidermal acid-base balance, severely affects the epidermal barrier function, which results in rapid necrosis and degeneration of the embryo, especially in an aquatic environment. In fish several of the CA isoforms have been implicated in regulating physiological processes of the skin. For example, in a subtype of ionocytes of the skin and gills cytoplasmic CA regulates ionic exchange and acid-base balance [21]. However, knockdown of these cytoplasmic CA isoforms did not result in obvious morphological defects [21]. Here we observe a rapid degeneration of the complete embryo upon defective CA5 function, revealing that CA5 fulfils a major role in the regulation of cellular epidermal homeostasis, during development in zebrafish.
Although we did not see any effect on pancreatic β-cell development, we cannot rule out that defective CA5 function affects insulin secretion or could affect pancreatic β-cell development during later stages of development. Human CA5B is expressed in pancreatic β-cells and has been shown to provide bicarbonate for the first step of gluconeogenesis. It is therefore implicated in insulin secretion [17], [22]. Furthermore, inhibition of CA activity with AZA resulted in a strong inhibition of glucose-stimulated insulin secretion [17]. In the light of our findings, inhibition of insulin secretion in pancreatic β-cells after AZA treatment could be a secondary effect: defective acid-base balance causes impaired cellular homeostasis which leads to impaired insulin secretion.
Because CA5 is the only mitochondrial CA, it is an excellent pharmaceutical target. Currently many CA inhibitors and activators have been developed in order to treat a range of disorders [4]. Some of these compounds have been shown to inhibit or activate also the mitochondrial CA5 and are used in the clinic as anti-obesity or anti-epileptic drug [23]–[26]. However, pharmacological inhibitors that are selective for CA5 are currently not available.
In conclusion, in this study we report the identification of the first vertebrate in vivo model in which defective CA5 activity results in imbalanced cellular acid-base homeostasis. The fact that AZA treatment in wild-type embryos mimics the ca5cof mutant phenotype shows that zebrafish can be used as an easy and inexpensive in vivo model for screening and validating the functionality of novel CA5 modulators as potential therapeutics for a variety of diseases.
Acknowledgments
We thank Dr. J. Bakkers, Dr. S. Schulte-Merker, S. Chocron and M. Witte for organizing the forward genetic mutagenesis screen at the Hubrecht Institute. We thank Rabab Charafeddine, Valentine Arendsen and Sanne van den Hout for technical assistance.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This research was supported by a grant from the Dystrophic Epidermolysis Bullosa Research Association (UK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lindskog S. Structure and mechanism of carbonic anhydrase. Pharmacol Ther. 1997;74:1–20. doi: 10.1016/s0163-7258(96)00198-2. [DOI] [PubMed] [Google Scholar]
- 2.Sly WS, Hu PY. Human carbonic anhydrases and carbonic anhydrase deficiencies. Annu Rev Biochem. 1995;64:375–401. doi: 10.1146/annurev.bi.64.070195.002111. [DOI] [PubMed] [Google Scholar]
- 3.Geers C, Gros G. Carbon dioxide transport and carbonic anhydrase in blood and muscle. Physiol Rev. 2000;80:681–715. doi: 10.1152/physrev.2000.80.2.681. [DOI] [PubMed] [Google Scholar]
- 4.Supuran CT. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 5.Chegwidden WR, Carter ND. Introduction to the carbonic anhydrases. EXS. 2000;90:14–28. [PubMed] [Google Scholar]
- 6.Hewett-Emmett D. Evolution and distribution of the carbonic anhydrase gene families. EXS. 2000;90:29–76. doi: 10.1007/978-3-0348-8446-4_3. [DOI] [PubMed] [Google Scholar]
- 7.Sly WS. The membrane carbonic anhydrases: from CO2 transport to tumor markers. EXS. 2000;90:95–104. doi: 10.1007/978-3-0348-8446-4_5. [DOI] [PubMed] [Google Scholar]
- 8.Tashian RE. Keeping pace with a fast enzyme: steps and missteps. EXS. 2000;90:569–596. doi: 10.1007/978-3-0348-8446-4_30. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ. Physiology and molecular biology of renal carbonic anhydrase. J Nephrol. 2002;15:S61–S74. [PubMed] [Google Scholar]
- 10.Purkerson J M, Schwartz GJ. Expression of membrane-associated carbonic anhydrase isoforms IV, IX, XII, and XIV in the rabbit: induction of CA IV and IX during maturation. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1256–1263. doi: 10.1152/ajpregu.00735.2004. [DOI] [PubMed] [Google Scholar]
- 11.Hilvo M, Supuran CT, Parkkila S. Characterization and inhibition of the recently discovered carbonic anhydrase isoforms CA XIII, XIV and XV. Curr Top Med Chem. 2007;7:893–899. doi: 10.2174/156802607780636672. [DOI] [PubMed] [Google Scholar]
- 12.Hilvo M, Baranauskiene L, Salzano AM, Scaloni A, Matulis D, et al. Biochemical characterization of CA IX, one of the most active carbonic anhydrase isozymes. J Biol Chem. 2008;283:27799–27809. doi: 10.1074/jbc.M800938200. [DOI] [PubMed] [Google Scholar]
- 13.Nagao Y, Srinivasan M, Platero JS, Svendrowski M, Waheed A, et al. Mitochondrial carbonic anhydrase (isozyme V) in mouse and rat: cDNA cloning, expression, subcellular localization, processing, and tissue distribution. Proc Natl Acad Sci USA. 1994;91:10330–10334. doi: 10.1073/pnas.91.22.10330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shah GN, Hewett-Emmett D, Grubb JH, Migas MC, Fleming RE, et al. Mitochondrial carbonic anhydrase CA VB: differences in tissue distribution and pattern of evolution from those of CA VA suggest distinct physiological roles. Proc Natl Acad Sci USA. 2000;97:1677–1682. doi: 10.1073/pnas.97.4.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wienholds E, Plasterk RH. Efficient target-selected mutagenesis in zebrafish. Genome Res. 2003;13:2700–2707. doi: 10.1101/gr.1725103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thisse C, Thisse B, Schilling TF, Postlethwait JH. Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. Development. 1993;119:1203–1215. doi: 10.1242/dev.119.4.1203. [DOI] [PubMed] [Google Scholar]
- 17.Parkkila AK, Scarim AL, Parkkila S, Waheed A, Corbett JA, et al. Expression of carbonic anhydrase V in pancreatic beta cells suggests role for mitochondrial carbonic anhydrase in insulin secretion. J Biol Chem. 1998;273:24620–24623. doi: 10.1074/jbc.273.38.24620. [DOI] [PubMed] [Google Scholar]
- 18.Gilmour KM, Perry SF. Carbonic anhydrase and acid-base regulation in fish. J Exp Biol. 2009;212:1647–1661. doi: 10.1242/jeb.029181. [DOI] [PubMed] [Google Scholar]
- 19.Dodgson SJ, Forster RE, Storey BT, Mela L. Mitochondrial carbonic anhydrase. Proc Natl Acad Sci USA. 1980;77:5562–5566. doi: 10.1073/pnas.77.9.5562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim HJ, Sumanas S, Palencia-Desai S, Dong Y, Chen JN, et al. Genetic analysis of early endocrine pancreas formation in zebrafish. Mol Endocrinol. 2006;20:194–203. doi: 10.1210/me.2005-0189. [DOI] [PubMed] [Google Scholar]
- 21.Gilmour KM, Thomas K, Esbaugh AJ, Perry SF. Carbonic anhydrase expression and CO2 excretion during early development in zebrafish Danio rerio. J Exp Biol. 2009;212:3837–3845. doi: 10.1242/jeb.034116. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald MJ. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. J Biol Chem. 1995;270:20051–20058. [PubMed] [Google Scholar]
- 23.Vullo D, Nishimori I, Innocenti A, Scozzafava A, Supuran CT. Carbonic anhydrase activators: An activation study of the human mitochondrial isoforms VA and VB with amino acids and amines. bioorg Med Chem. 2007;17:1336–1340. doi: 10.1016/j.bmcl.2006.11.075. [DOI] [PubMed] [Google Scholar]
- 24.Davis RH, Innocenti A, Poulsen SA, Supuran CT. Carbonic anhydrase inhibitors. Identification of selective inhibitors of the human mitochondrial isozymes VA and VB over the cytosolic isoforms I and II from a natural product-based phenolic library. bioorg Med Chem. 2010;18:14–18. doi: 10.1016/j.bmc.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 25.Nishimori I, Innocenti A, Vullo D, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: The inhibition profiles of the human mitochondrial isoforms VA and VB with anions are very different. Bioorg Med Chem. 2007;15:6742–6747. doi: 10.1016/j.bmc.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 26.De Simone G, Supuran CT. Antiobesity carbonic anhydrase inhibitors. Curr Top Med Chem. 2007;7:879–884. doi: 10.2174/156802607780636762. [DOI] [PubMed] [Google Scholar]