Abstract
To investigate the role(s) of protein-tyrosine sulfation in the retina and to determine the differential role(s) of tyrosylprotein sulfotransferases (TPST) 1 and 2 in vision, retinal function and structure were examined in mice lacking TPST-1 or TPST-2. Despite the normal histologic retinal appearance in both Tpst1−/− and Tpst2−/− mice, retinal function was compromised during early development. However, Tpst1−/− retinas became electrophysiologically normal by postnatal day 90 while Tpst2−/− mice did not functionally normalize with age. Ultrastructurally, the absence of TPST-1 or TPST-2 caused minor reductions in neuronal plexus. These results demonstrate the functional importance of protein-tyrosine sulfation for proper development of the retina and suggest that the different phenotypes resulting from elimination of either TPST-1 or -2 may reflect differential expression patterns or levels of the enzymes. Furthermore, single knock-out mice of either TPST-1 or -2 did not phenocopy mice with double-knockout of both TPSTs, suggesting that the functions of the TPSTs are at least partially redundant, which points to the functional importance of these enzymes in the retina.
Introduction
Protein-tyrosine sulfation is one of many post-translational modifications that proteins can undergo in the cell. Although protein-tyrosine sulfation is utilized from plants to animals [1], our understanding of the functional role of protein-tyrosine sulfation is still in its infancy. Protein-tyrosine sulfation is performed in the trans Golgi network [2] by one of two independent tyrosylprotein sulfotransferases (TPST, 3′-phosphoadenylyl-sulfate:protein-tyrosine O-sulfotransferase, EC 2.2.8.20), TPST-1 and TPST-2 [3]. The TPSTs catalyze protein-tyrosine sulfation through the covalent transfer of a sulfate group from the universal sulfate donor, 3′-phosphoadenosine 5′-phophosulfate (PAPS) to a tyrosine on the nascent protein [4]. The two TPSTs differ in their kinetics [5]–[7].
Among the tissues in which protein-tyrosine sulfation has been investigated is the retina. Multiple sulfated proteins are present in the retinas of different species and the transcripts for both Tpst1 and Tpst2 are expressed [8]. Moreover, sulfated proteins are found in both the neural retina and the retinal pigment epithelium (RPE) [8], [9].
Complete lack of sulfation causes a drastic reduction in scotopic and photopic electroretinographic (ERG) responses and ultrastructural abnormalities in the rod outer segment (OS) characterized by membrane evulsions into the extracellular space, irregular disc membrane spacing, and expanded intradiscal space [10]. Surprisingly, rod photoreceptors continue to show normal function in single cell recordings in the absence of sulfation [10]. The complete absence of sulfation also affects establishment of neuronal circuits and may have effects on long-term synaptic maintenance [10].
To investigate the functional significance of protein-tyrosine sulfation by each of the two TPSTs, Tpst1−/− and Tpst2−/− mice were used. Here, we show that the absence of either TPST caused early functional deficits in the retina as assessed by ERG analysis. However, the ERG responses from Tpst1−/− retinas eventually attained amplitudes comparable to those observed in wildtype (wt, Tpst1&2+/+) mice by postnatal day (P) 50, then remained comparable to wt for the rest of the ages tested. The Tpst2−/− mice displayed early ERG deficits that, unlike Tpst1−/− mice, did not normalize with age. Although the complete lack of protein-tyrosine sulfation disrupts rod OS ultrastructure [10], the presence of either one of the two TPSTs was sufficient to maintain normal OS ultrastructure and retinal histology. Despite the functional impairments caused by the lack of either TPST, deficiency in either TPST-1 or TPST-2 did not result in any large-scale disruption of neuronal architecture or cell types, although somewhat diminished neuronal plexus in the inner retina were noted. These results suggest that the two TPSTs have some level of functional redundancy, although the distinct phenotypes of the Tpst1−/− and Tpst2−/− mice indicate that some retinal proteins must be selectively sulfated by either TPST-1 or -2.
Materials and Methods
Animals
Tpst1−/− (Tpst1tm1Klm, MGI:2183366) and Tpst2−/− (Tpst2tm1Klm, MGI:3512111) were generated, characterized, housed, and fed as previously described [11]–[13]. This study was performed in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institute of Health and the Association for Research in Vision and Ophthalmology Resolution on the Use of Animals in Research. The protocol was approved by the Institutional Animal Care and Use Committees at the University of Oklahoma Health Sciences Center (IACUC 09-024). In all animal experiments, every effort was made to minimize suffering.
Electroretinography
Electroretinograms (ERGs) were recorded from the corneal surface of mice as described in detail previously [10].
Light and Electron Microscopy
Methods used for tissue collection, processing for Spurr’s resin-embedment, microtomy and subsequent examination by light (LM) and electron microscopy (EM) were as previously described [10], [14].
Immunohistochemistry and Lectin Cytochemistry
Immunohistochemistry (IHC) and lectin cytochemistry analyses were performed on tissue sections from frozen or paraffin-embedded eyes as described elsewhere [10]. Analyses were performed on retinas from 9–10 animals for each genotype at 1 to 2 months of age. The panel of well-characterized cell- and synapse-specific marker antibodies used in the present studies is described in Table 1 .
Table 1. Primary antibodies and lectins used for tissue labeling.
| Antigen | Host | Dilution | Source (catalog #; clone #) | Reference |
| Calbindin | Mouse | 1∶300 | Sigma Chemical Company, St. Louis, MO (C9848; clone CB955) | – |
| Calbindin | Rabbit | 1∶1000–1∶5000 | SWANT, Bellinzona, Switzerland (CB38) | – |
| Calretinin | Rabbit | 1∶1000–1∶2000 | Chemicon International, Temecula, CA (AB5054) | – |
| Chx-10 | Sheep | 1∶50 | ExAlpha Biologicals, Inc., Watertown, MA (X1180P) | – |
| Goα | Mouse | 1∶500–1∶1,000 | Chemicon International, Temecula, CA (MAB3073; clone 2A) | [19] |
| Gγ13 | Rabbit | 1∶500 | Dr. R. Margolskee Mount Sinai School of Medicine, New York, NY | [20], [21] |
| Glial Fibrillary Acidic Protein (GFAP) | Mouse | 1∶500 | Chemicon International, Temecula, CA (MAB360; Clone GA5) | [22] |
| Glutamic Acid Decarboxylase, 65 kDa (GAD-65) | Mouse | 1∶500–1∶1000 | Chemicon International, Temecula, CA (MAB351; clone GAD-6) | [23] |
| Glutamine synthetase | Mouse | 1∶1000 | Chemicon International, Temecula, CA (MAB302; clone GS6) | [24] |
| Islet-1 | Mouse | 1∶100–1∶200 | Developmental Studies Hybridoma Bank (clone 39.4D5) | [18] |
| Microtubule AssociatedProtein 1 (MAP-1) | Mouse | 1∶300–1∶500 | Sigma Chemical Company, St. Louis, MO (M4278; clone HM-1) | [25] |
| Peanut Agglutinin (PNA) | – | 1∶10–1∶20 | Molecular Probes, Eugene, OR (L21409) | [26] |
| Protein Kinase C (PKC) | Mouse | 1∶25–1∶100 | BD Transduction Labs, San Jose, CA (610108; clone 3) | – |
| Protein Kinase C (PKC) | Rabbit | 1∶1000–1∶2000 | Sigma Chemical Company, St. Louis, MO (P-4334) | – |
| Sulfotyrosine | Human | 1∶100–1∶1000 | Dr. Kevin Moore, Oklahoma Medical Research Foundation, Oklahoma City OK. Antibody clone PSG-2 | [9], [27] |
| Synapsin I | Rabbit | 1∶500 | Chemicon International, Temecula, CA (AB1543P) | [28] |
| Synaptic Vesicle Protein 2B (SV2B) | Rabbit | 1∶500 | Dr. Roger Janz, University of Texas Houston Medical School, Houston, TX | [29] |
| Synaptotagmin 2 | Mouse | 1∶200 | Zebrafish International Resource Center, Eugene, OR (Clone ZNP-1) | [17], [30] |
| Syntaxin 3 | Rabbit | 1∶750–1∶1000 | Novus Biologicals, Littleton, CO (NB100–1644) | [31]–[33] |
| Tyrosine hydroxylase (TH) | Mouse | 1∶500 | Chemicon International, Temecula, CA (MAB318; clone LNC1) | – |
| Vesicular glutamate transporter 1 (VGLUT1) | Guinea pig | 1∶2500–1∶5000 | Chemicon International, Temecula, CA (AB5905) | [34] |
| Vesicular glutamate transporter 3 (VGLUT3) | Guinea pig | 1∶2500 | Chemicon International, Temecula, CA (AB5421) | [35] |
| Wheat germ agglutinin (WGA) | – | 1∶20 | Molecular Probes, Eugene, OR (W11261) | [26] |
Immunoblotting (Western Analysis)
Immunoblotting was performed using the PSG2 antibody as described previously [15] and images were acquired using a Kodak image station (Carestream Molecular Imaging, Rochester, NY).
Statistical Analysis
Statistical significance was determined using ANOVA with Bonferroni post hoc multiple pairwise comparison tests (PRISM™; GraphPad® Software, San Diego, CA).
Results
Distribution of Tyrosine-sulfated Proteins in Retinas of TPST-1 and TPST-2 Knockout Mice
Previous immunoblotting of extracts from wt retinas using the PSG2 anti-sulfotyrosine antibody showed that the retina contains several tyrosine-sulfated proteins with some of these proteins being specific to certain cell types [8]. To determine how the pattern observed on the immunoblots was affected by the elimination of either TPST, retinal extracts obtained from Tpst1−/− and Tpst2−/− mice were analyzed independently and compared to the pattern observed for wt retinal extracts. As shown in Figure 1 , variations in 5 bands were observed on the immunoblots. In Tpst1−/− retinas, bands 2 and 3 were reduced, and bands 4 and 5 were absent. However, in Tpst2−/− retinas, bands 2, 3 and 4 were reduced in intensity, while bands 1 and 5 were absent. In DKO retinas, all bands were absent, confirming the specificity of PSG2 for sulfated proteins.
Figure 1. Immunoblot (Western) analysis of sulfated proteins in retinas of wildtype, Tpst1−/− and Tpst2−/− mice.
Fifty micrograms of retinal protein extracts were loaded in each lane and 10% non-reducing SDS-PAGE was performed. Proteins were transferred to a nitrocellulose membrane and probed with the PSG2 antibody as described before [8]. The black arrows point to five bands of tyrosine sulfated proteins that show differential sensitivity to elimination of TPST-1 or TPST-2.
Table 3. Number of animals tested in Figure 3D, E & F.
| Age/days | Wildtype | Tpst1−/− | Tpst2−/− |
| 30 | 28 | 7 | 5 |
| 60 | 28 | 7 | 13 |
| 90 | 24 | 8 | 9 |
| 120 | 28 | 12 | 8 |
| 150 | 21 | 3 | 0 |
| 240–300 | 9 | 9 | 12 |
| 360–600 | 9 | 12 | 3 |
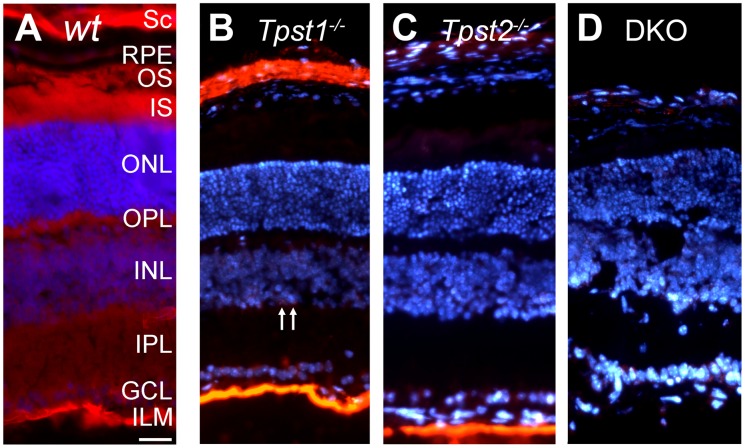
Immunohistochemical (IHC) analysis of sections of wt retina using the PSG2 antibody showed that tyrosine-sulfated proteins were present in all retinal layers and sclera ( Figure 2A ), consistent with previous findings [8]. To obtain clues as to the differential contributions of the two TPSTs to sulfation of retinal proteins, IHC analysis of tyrosine-sulfated proteins was performed on retinas of TPST-1 and TPST-2 knockout mice. In the Tpst1−/− retina, detection of tyrosine-sulfated proteins was observed in the ganglion cell layer (GCL), the inner limiting membrane (ILM), and a subset of cells in the proximal inner nuclear layer (INL) ( Figure 2B ) in addition to the expected strong immunolabeling of the sclera. Although the lack of TPST-2 ( Figure 2C ) did not affect immunolabeling in the ILM, it reduced labeling in the GCL, INL and sclera. Elimination of both enzymes [10] abolished anti-sulfotyrosine immunolabeling in all retinal layers ( Figure 2D ).
Figure 2. Immunohistochemical localization of sulfated proteins.
Retinas from (A) wildtype (wt), (B) Tpst1−/−, (C) Tpst2−/− and (D) Tpst DKO mice were labeled using the PSG2 antibody. Labeling of wt retina showed signal in the sclera (SC), retinal pigment epithelium (RPE), photoreceptor outer segments (OS), inner segments (IS), outer nuclear layer (ONL), outer plexiform layer (OPL), inner nuclear layer (INL), inner plexiform layer (IPL) and ganglion cell layer (GCL). Arrows in panel B indicate cells in the proximal INL that retained sulfated proteins in absence of TPST-1. Retinas were from 30-day old wt, Tpst1−/− and Tpst2−/− mice while the DKO retina was from a 21-day old mouse. Scale bar = 50 µm.
These results indicate that TPST-1 and -2 differentially sulfate some retinal proteins, while there may be some redundancy between the two enzymes for other substrates. Additionally, there could be multiple proteins in the ILM that are differentially sulfated by both TPSTs.
Retinal Function and Structure in Tpst1−/− and Tpst2−/− Mice
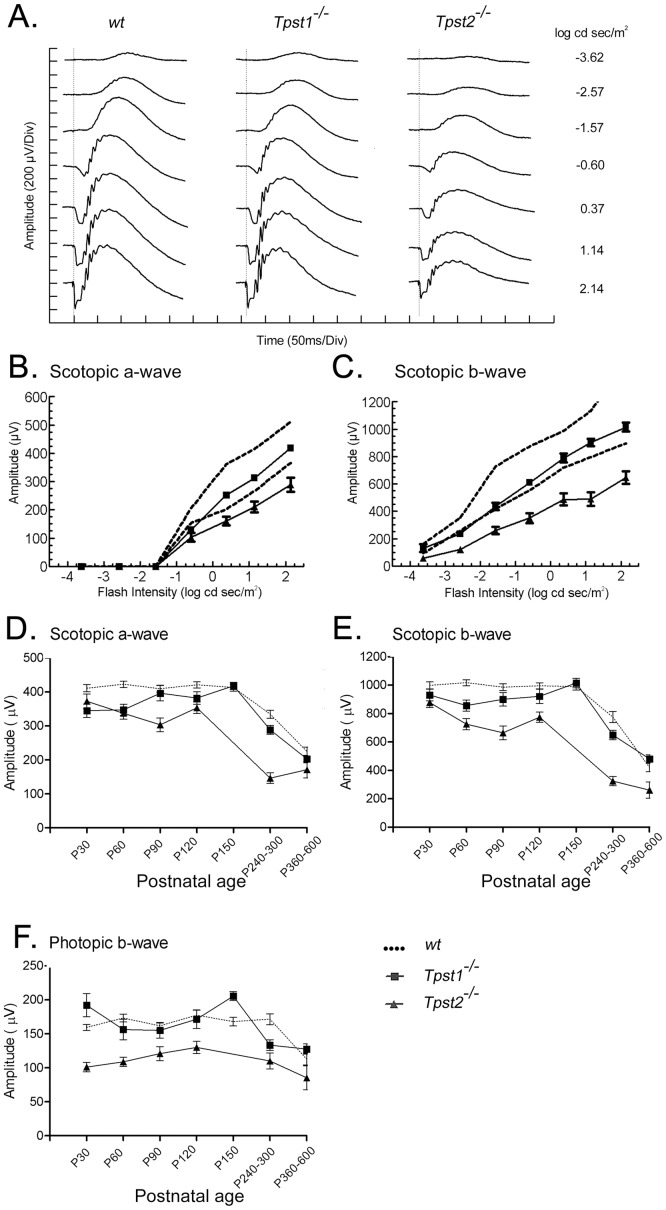
We have previously shown that transcripts for both Tpst1 and Tpst2 are expressed in human and mouse retina [8]. Both transcripts are expressed in the mouse retina as early as P1, although transcript levels fluctuate over the course of retinal development [10]. To determine the effect of the absence of either of the two TPSTs on retinal function, ERG analysis was performed. Figure 3 presents dark-adapted ERGs obtained from representative wt, Tpst1−/− and Tpst2−/− mice to flash stimuli that span an intensity range of several log units, including the range from purely rod-driven (scotopic) responses, to mixed rod- and cone-driven responses, to purely cone-driven (photopic) responses ( Figure 3A ). At the lowest flash intensity, the response of wt mice was dominated by the positive-going b-wave, reflecting the activity of rod bipolar cells and other cells in the inner retina. With increasing flash intensities, the b-wave was preceded by the negative-going a-wave, representing the mass response of the rod photoreceptors. In Tpst1−/− mice, the a-wave was reduced in amplitude compared to wt at low stimulus intensities, but fell within the normal range for higher light intensities ( Figure 3B&C ). In contrast, in Tpst2−/− mice, both the a- and b-wave were reduced in amplitude at all stimulus intensities. While the average intensity-response functions for the a- and b-waves in Tpst1−/− mice fell within the 95% confidence interval defined in wt mice, the a- and b-wave amplitudes for Tpst2−/− fell outside the 95% confidence interval (dashed lines, Figures 3B&C ).
Figure 3. Electroretinographic responses from Tpst1−/− and Tpst2−/− retinas.
A. Representative waveforms recorded at different light intensities from wt, Tpst1−/− or Tpst2−/− retinas under scotopic conditions. B & C, in each panel, the dashed line represents the 95% confidence interval for responses obtained from 3 150-day-old wt littermate controls. The a- and b-waves obtained from 3 150-day-old Tpst1−/− mice (squares) fell near the lower limit of this interval at lower light intensities, but were well within the range at higher light intensities. In contrast, a- and b-waves recorded from 3 123-day-old Tpst2−/− mice (triangles) were reduced in amplitude and fell outside the 95% confidence interval at all light intensities tested. Development of the (D) scotopic a-wave, (E) scotopic b-wave, and (F) photopic b-wave responses for wt (dotted line), Tpst1−/− (squares), and Tpst2−/− (triangles) mice. Note that all responses from Tpst2−/− mice were lower than those for Tpst1−/− and wt mice at all ages. Error bars represent standard error of the mean. The differences between wt and Tpst2−/− presented in D are statistically significant between P30 and P300 (P<0.05–0.001) while the differences between wt and Tpst1−/− are only statistically significant at P30 and P60 (P<0.05–0.001). The differences between wt and Tpst2−/− presented in E are statistically significant between P60 and P600 (P<0.01–0.001) while the differences between wt and Tpst1−/− are only statistically significant at P60 (P<0.05). The differences between wt and Tpst2−/− presented in F are statistically significant for all time points tested (P<0.05–0.001) while the differences between wt and Tpst1−/− are statistically insignificant for all time points tested. Number of animals tested in D, E & F are presented in Table 3.
Developmentally, the scotopic a- and b-wave amplitudes of Tpst1 −/− mice started significantly below those observed in age-matched wt mice, but increased to wt levels by P90 ( Figure 3D&E ). In contrast, the amplitude of the photopic b-wave from Tpst1−/− retinas was comparable to wt retinas even at early ages ( Figure 3F ). Tpst2 −/− mice showed a more severe functional impairment, with the scotopic a- and b-wave amplitude starting smaller than the wt level and never achieving wt levels until after P360 ( Figure 3D, E&F ), although there was a slight, but significant (P≤0.0163), improvement in amplitudes between P90 and P120. The insignificant difference in ERG responses between wt and Tpst2−/− mice after P360 is mostly due to reduced responses in aging wt mice. However, it is possible that lack of TPST-2 may provide some protective effects against age-related changes. There also was a small increase in photopic b-wave amplitudes between P30 and P120 for Tpst2−/− mice. Nevertheless, the responses never reached the amplitudes observed for Tpst1−/− or wt mice. The decline in photopic ERG responses for both Tpst1−/− and Tpst2−/− mice past P150 paralleled that exhibited by wt mice. Together, these data suggest that TPST-2 is more important to retinal function than is TPST-1.
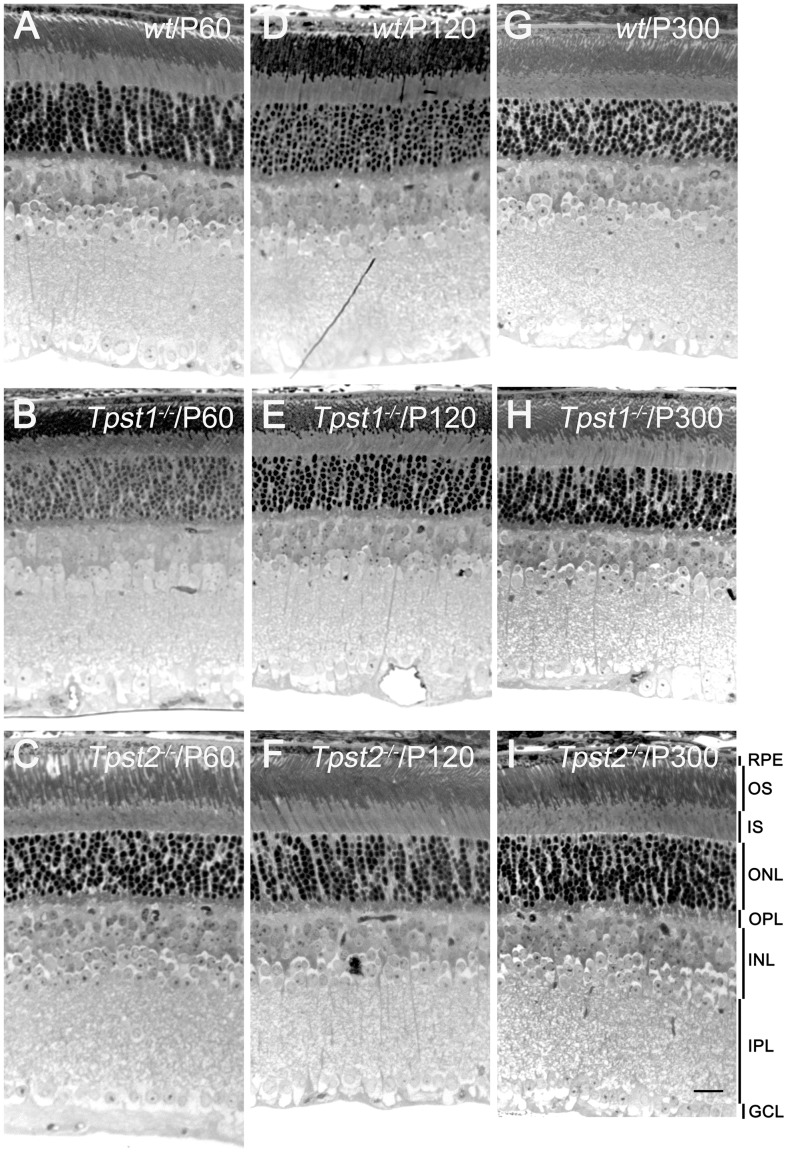
The reduction in both scotopic and photopic function in Tpst2−/− retinas could potentially arise from degenerative changes in rods and cones, in second-order neurons, or both. Alternatively, reduced function might reflect subtle functional or anatomical changes independent of degeneration. However, examination of wt, Tpst1−/−, and Tpst2−/− retinas at the light microscopic levels revealed no obvious histologic anomalies ( Figure 4 ).
Figure 4. Histologic analysis in absence of either TPST-1 or TPST-2.
A, D &G, histological appearance of wt retina at P60, P120 and P300, respectively; B, E & H, Tpst1−/− retina; C, F, & I, Tpst2−/− retina. Abbreviations as in Fig. 1. Scale Bar = 50 µm.
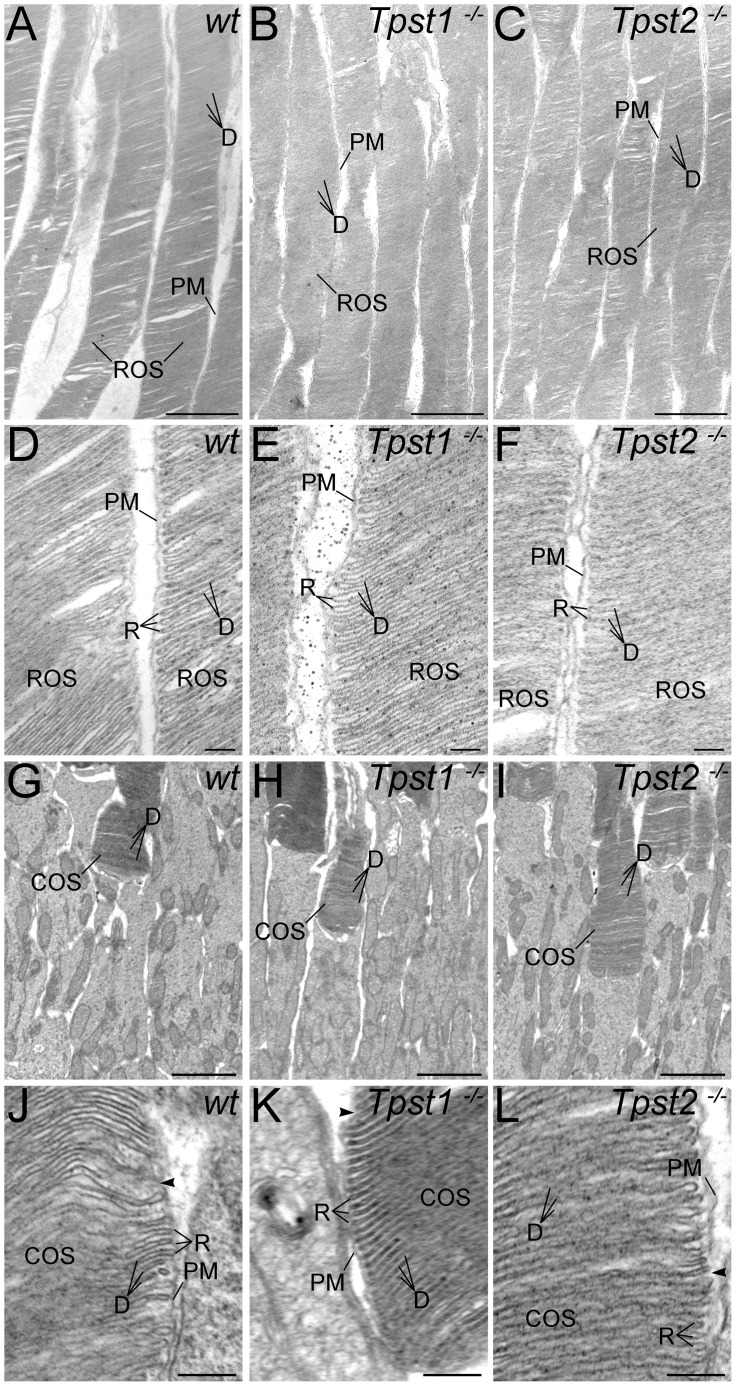
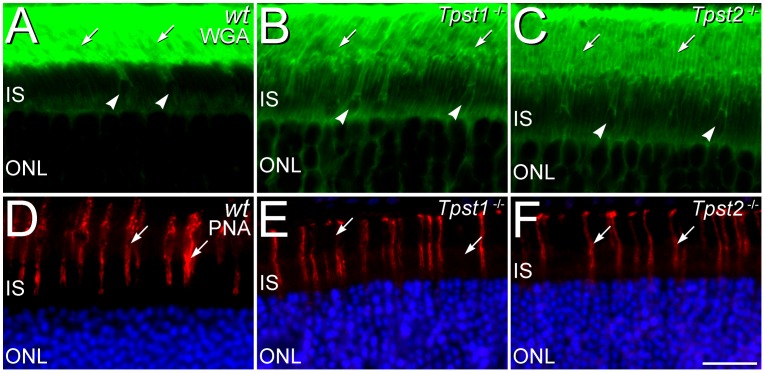
Although double knockout (DKO) of TPST-1 and TPST-2 causes a profound disruption of rod OS (ROS) [10], ROS ultrastructure was unaffected by knockout of either Tpst1−/− or Tpst2−/− and was comparable to that of wt ROS ( Figure 5 ). Cone OS (COS) ultrastructure also was unaffected in either Tpst1−/− and Tpst2−/− retinas, consistent with the normal COS structure previously observed in the Tpst DKO retina [10]. Similarly, the rod- and cone-specific domains in the interphotoreceptor matrix (IPM) around the outer segments of rods and cones also appeared unaffected by elimination of either TPST-1 or TPST-2, as assessed by lectin cytochemistry ( Figure 6 ).
Figure 5. Knockout of TPST-1 or TPST-2 does not disrupt rod or cone outer segment ultrastructure.
A–C: Rod outer segments (ROSs) in (A) wt, (B) Tpst1−/−, and (C) Tpst2−/− retinas. Wildtype, Tpst1−/−, and Tpst2−/− ROSs all show normal organization with closely stacked discs (D) surrounded by the plasma membrane (PM). D–F: ROS ultrastructure at higher magnification in (D) wt, (E) Tpst1−/−, and (F) Tpst2−/− retinas. ROSs in the retinas of mice of all three genotypes show normal flattened discs (D) with the typical hairpin organization of the disc rim (R). Discs are separate from the surrounding plasma membrane (PM) and have little intradiscal space. G–I: Cone outer segments (COSs) in (G) wt, (H) Tpst1−/−, and (I) Tpst2−/− retinas. COSs in the wildtype, Tpst1−/−, and Tpst2−/− retina all show the normal tapered shape, tightly packed, flattened discs, and positioning among the inner segments of neighboring rods. J–L: COS ultrastructure at higher magnification in (J) wt, (K) Tpst1−/−, and (L) Tpst2−/− retinas. COSs in the retinas of mice of all three genotypes show normal flattened discs, hairpin organization of the disc rim, and tight packing. The space between disks is continuous with the extracellular space (arrowheads). Scale bars = 2 µm for A–C and G–I; 0.25 µm for D–F and J–L.
Figure 6. Knockout of TPST-1 or TPST-2 does not disrupt rod and cone-specific domains in the interphotoreceptor matrix (IPM).
A–C. In the retinas of (A) wt, (B) Tpst1−/−, and (C) Tpst2−/− mice, the IPM surrounding rod outer segments (arrows) is labeled by wheat germ agglutinin (WGA; green). The IPM surrounding the inner and outer segments of cones (arrowheads) also shows labeling by WGA. D–F: Peanut agglutinin (PNA) specifically labels the IPM surrounding cones (arrows) in the retinas of (D) wt, (E) Tpst1−/−, and (F) Tpst2−/− mice, but, as appropriate, does not label the IPM surrounding rods. Photoreceptor nuclei in the outer nuclear layer (ONL) are counterstained with DAPI (blue) in panels D–F. IS, inner segment layer. Scale bars = 20 µm for all panels.
Single Knockout of TPST-1 or TPST-2 has Minimal Effects on Retinal Cell Populations, Morphology, or Organization of Retinal Neurons and Synapses
To test whether the deficits in retinal function in Tpst1−/− and Tpst2−/− mice might arise from aberrant neuronal morphology or synaptic organization, we examined the expression and localization of a number of cell- and synapse-specific markers in the retinas of Tpst1−/− and Tpst2−/− mice ( Table 1 ). All populations of retinal neurons examined were present in the Tpst1−/− and Tpst2−/− retinas and showed appropriate cell-specific morphology (summarized in Table 2 ; data shown in Figures S1 and S2). Although all cell types were present in the Tpst1−/− and Tpst2−/− retina, in some cases the extent of their plexes were slightly diminished compared to wt, with knockout of TPST-2 having a slightly stronger effect than knockout of TPST-1. The Type 6 ON-cone bipolar cell was an exception to this pattern as it showed a small expansion of its narrow plexus in the inner plexiform layer (IPL) in both Tpst1−/− and Tpst2−/− retinas (Figure S2), consistent with findings in the Tpst DKO retina [10]. Although elimination of either TPST-1 or TPST-2 affected retinal function, the structural organization of retinal circuits, which reflects their function, in the Tpst1−/− and Tpst2−/− retina was unaffected ( Table 2 ; Figures S1&S2). Retinal neurons projected to only appropriate regions of the outer plexiform layer (OPL) and inner plexiform layer (IPL), with the IPL retaining its normal ON-OFF segregation. Similarly, knockout of either TPST-1 or TPST-2 did not affect synapse-specific expression of presynaptic proteins associated with vesicular neurotransmitter release or the expression of G-protein subunits associated with transmission from photoreceptors to rod or ON-type cone bipolar cells ( Table 2 ). Müller glial cells, the principal glial cell of the retina, showed normal morphology with no elevated expression of glial fibrillary acid protein (GFAP) (Figure S3), a sensitive indicator of retinal stress or degeneration [16]. Thus, the deletion of TPST-1 or -2 did not disrupt cell populations, cell-specific projection patterns or the structural or functional organization of the retina.
Table 2. Effects of TPST-1 and TPST-2 knockout on neuronal populations, morphology and synaptic protein expression.
| Cell- or synapse-specific marker | Wildtype | TPST-1 knockout | TPST-2 knockout | Tpst DKO * |
| Photoreceptors | ||||
| PNA | Cone IPM and flat contacts with OFF-cone bipolar cells | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| WGA | IPM surrounding rod outer segments | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| VGLUT1 | All rod and cone terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| SV2B | All rod and cone terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| Syntaxin 3 | All rod and cone terminals | Present, but labeling may be reduced | Present, but labeling may be reduced | Present, but labeling may be reduced |
| Bipolar cells | ||||
| Synaptotagmin 2 | Type 2 OFF-cone bipolar cells and Type 6 ON-cone bipolar cells | Normal Type 2 OFF-cone bipolar cells; expanded Type 6 ON-cone bipolar cell plexus in IPL | Normal Type 2 OFF-cone bipolar cells; expanded Type 6 ON-cone bipolar cell plexus in IPL | Normal Type 2 OFF-cone bipolar cells; expanded Type 6 ON-cone bipolar cell plexus in IPL |
| PKC-α | Rod bipolar cells and terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| Gγ13 | All rod and ON-cone bipolar cell bodies and terminals | Similar to wildtype | Similar to wildtype | Labeling present in rod bipolar and ON-cone bipolar cells, but reduced. |
| Goα | All ON-type bipolar cell bodies. Other processes in IPL | Similar to wildtype | Similar to wildtype | Labeling present in rod bipolar and ON-cone bipolar cells, and in processes in IPL but reduced. |
| Chx-10 | All bipolar cell nuclei | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| Islet-1 | All rod and ON-cone bipolar cell nuclei | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| VGLUT1 | All bipolar cell terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| SV2B | All bipolar cell terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| Syntaxin 3 | All bipolar cell terminals | Similar to wildtype | Similar to wildtype | Similar to wildtype, but labeling may be reduced |
| Horizontal cells | ||||
| Calbindin | Horizontal cells | Similar to wildtype | Similar to wildtype, but plexus in OPL reduced | Similar to wildtype, but plexus in OPL reduced |
| Amacrine cells | ||||
| Calretinin | Starburst amacrine cells, TH2 amacrine cells (and ganglion cells) | Similar to wildtype | Similar to wildtype | Starburst and TH2 amacrine cell plexes severely reduced. (Ganglion cells similar to wildtype) |
| VGLUT3 | Putative glutamatergic amacrine cells | Similar to wildtype | Process stratification similar to wildtype, but plexus reduced | VGLUT3 cells and plexus severely reduced |
| Tyrosine hydroxylase | Type 1 dopaminergic amacrine cells | Similar to wildtype | Process stratification similar to wildtype, but plexus reduced | Process stratification similar to wildtype, but plexus reduced |
| GAD-65 | GABAergic amacrine cells and processes in IPL | Similar to wildtype | Process stratification similar to wildtype, but labeling reduced | Process stratification similar to wildtype, but labeling strongly reduced |
| Ganglion cells | ||||
| MAP-1 | Ganglion cell dendrites and axons | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| Calretinin | Ganglion cell bodies and axons (and starburst and TH2 amacrine cells) | Ganglion cell labeling similar to wildtype | Ganglion cell labeling similar to wildtype | Ganglion cell labeling similar to wildtype (amacrine cell labeling reduced) |
| Glial cells | ||||
| Glutamate synthetase | Müller cells and astrocytes | Similar to wildtype | Similar to wildtype | Similar to wildtype |
| GFAP | Astrocytes and Müller cell endfeet | Similar to wildtype | Similar to wildtype | Similar to wildtype |
Tpst DKO data from Sherry et al., 2010.
Electron microscopy confirmed that all types of retinal neurons established ultrastructurally appropriate synapses in the Tpst1−/− and Tpst2−/− retina (Figure S4). The synaptic terminals of rods and cones showed normal synaptic ultrastructure, with synaptic ribbon complexes showing the classic post-synaptic triadic organization of horizontal cell and bipolar cell processes aligned with the synaptic ribbon apparatus in the presynaptic photoreceptor terminal. Flat contacts between cones and the dendrites of OFF-cone bipolar cells were also present. Bipolar cell synapses showed the normal diadic arrangement of two post-synaptic processes apposed to a small presynaptic ribbon in the bipolar cell terminal, and amacrine cells established conventional vesicular synapses.
Altogether, these results indicate that retinal neurons differentiate normally in the absence of either TPST-1 or TPST-2. Furthermore, these findings suggest that the functional deficits observed in the ERGs recorded from the Tpst1−/− and Tpst2−/− retinas do not originate from large-scale defects in retinal cell populations, the loss of basic cellular and synaptic organization or defects in the ability to establish synapses.
Discussion
In a previous study, we showed that the complete absence of protein tyrosine sulfation due to knockout of both TPST-1 and TPST-2 severely disrupted the integrity of rod OS, resulting in abnormal disc morphology and protrusion of rod outer segment membranes into the subretinal space, while leaving cone outer segment ultrastructure intact [10]. To investigate the specific roles of TPST-1 and TPST-2 in sulfating retinal proteins and retinal development and homeostasis, mice with deletion of either TPST-1 or TPST-2 were used.
Tpst1−/− mice exhibited a transient developmental delay of ERG responses that reached normal levels by P90. Normalization of the ERG suggests that redundancy exists in the functions of TPST-1 and TPST-2, whereby TPST-2 may compensate to some degree for the absence of TPST-1 during retinal development. This is supported by the fact that in absence of both TPSTs, there is a clear functional and structural phenotype [10]. Since the functional recovery in the absence of TPST-1 is slow, it is likely that the TPST-2 exhibits differential affinity for TPST-1 substrates. However, the differential effects on protein sulfation observed by western blots and the differential distribution of sulfated proteins in retinas of Tpst1−/− and Tpst2−/− mice suggests that the two enzymes are not fully redundant in their functions.
Tpst2−/− mice displayed reduced scotopic and photopic ERG responses at P30; however, these functional deficits showed little or no improvement with age, in contrast to the ERG deficits of Tpst1−/− mice. The persistence of the functional defects in Tpst2−/− mice suggests that TPST-1 cannot fully compensate adequately for the absence of TPST-2 and vice versa. This also implies that some sulfated retinal proteins can only be sulfated by one of the two enzymes and supports the notion that these two enzymes are only partially redundant.
A key finding of these studies is that single knockout of either TPST-1 or TPST-2 results in a primarily functional phenotype, with little effect on retinal structure. Single knockout of either TPST-1 or TPST-2 had no effect on rod or cone outer segment ultrastructure. Similarly, the rod- and cone-specific domains within the IPM, as visualized by lectin cytochemistry, appeared normal in single knockout retinas. Although some very subtle structural changes in retinal neurons and their synaptic plexus were noted in the retinas of mice with single knockout of either TPST-1 or TPST-2, the resulting deficits in protein tyrosine sulfation did not disrupt retinal cell populations, neuronal morphology, organization, or their ability to form ultrastructurally appropriate synapses to any substantial degree. The principal effect on retinal structure and organization was a subtle, generalized reduction in the extent of neuronal plexus, which was more pronounced in the Tpst2−/− than in the Tpst1−/− retina, but less extensive than the reduction of neuronal plexus observed in the retinas of Tpst DKO mice [10].
One intriguing finding from our prior studies on the Tpst DKO mouse was the ability of rod photoreceptor cells to generate normal responses to light when determined by suction electrode recordings of isolated rod photoreceptor cells; in contrast, the global rod response (as recorded by the scotopic ERG a-wave) was significantly depressed and rod OSs showed severe disorganization at the ultrastructural level in vivo [10]. By extension, it is reasonable to suggest that single rod photoreceptors from Tpst1−/− or Tpst2−/− retinas should exhibit normal responses in suction electrode recordings. Taken together, the differences in rod responses in vivo and in vitro suggest that sulfation of protein(s) in the IPM is a critical determinant of the generation of normal rod responses in vivo.
The ERG b-wave deficits present in both Tpst1−/− and Tpst2−/− mice indicate that sulfation deficits impair synaptic transmission by photoreceptors to the inner retina and/or processing within the inner retina. Our studies indicate that the absence of sulfation by TPST-1 or TPST-2 does not cause large aberrations in retinal cell populations, morphology or neuronal architecture, although the neuronal plexus in the retina of sulfation-deficient mice tended to be diminished slightly compared to wt mice. The slightly more pronounced reduction of neuronal plexus in Tpst2−/− mice correlated with the severity of the ERG deficits, consistent with the more obvious reduction of neuronal plexus and retinal function seen in the Tpst DKO mouse [10]. Our ultrastructural studies confirmed that the functional deficits observed in the ERG did not arise from the failure of synapse formation per se, although the possibility remains that sulfation deficits could affect the numbers or specificity of synaptic connections. These findings support the notion that TPST-1 and TPST-2 have somewhat redundant functions and can compensate for the loss of a single isoform.
The current studies, together with our previous studies of the Tpst DKO mouse [10], indicate that sulfation by both TPST-1 and TPST-2 can affect retinal circuitry, although the effects of sulfation deficiency manifest themselves more clearly at the functional level than at the anatomical or ultrastructural levels. The mechanisms by which sulfation might regulate neuronal development and synaptic transmission are not known currently. Given that sulfation is a common modification of secreted proteins, one attractive possibility is that sulfated proteins secreted into the extracellular space or incorporated into the soluble and insoluble fractions of the extracellular matrix [8], provide signals controlling synaptic maturation and/or function.
The retinal phenotypes in Tpst1−/− or Tpst2−/− mice described above could result from global lack of tyrosine sulfation rather than lack of sulfation of retinal proteins. However, since the developmental functional deficit in the Tpst2−/− retina never recovers, it is most likely that these phenotypes are due to lack of sulfation of specific retinal proteins. Nevertheless, the ultimate answer will require tissue and/or cell type-specific conditional knockout experiments.
In summary, the data presented here in conjunction with our previous findings [10] demonstrate that protein-tyrosine sulfation is a key determinant in the development and maintenance of retinal function with more subtle effects on retinal structure. Studies are underway to identify tyrosine sulfated retinal proteins in order to better understand the role of sulfation in retinal function and to delineate how the lack of sulfation affects the functions of specific retinal proteins.
Supporting Information
Lack of TPST-1 or TPST-2 does not induce any large scale disruption of retinal horizontal, amacrine or ganglion cells. A–C: Horizontal cells labeled for calbindin in the wt, Tpst1−/− and Tpst2−/− retina show normal placement in the inner nuclear layer (INL) and project normally to the outer plexiform layer (OPL), although the extent of their plexus in the Tpst2−/− retina is slightly reduced. D–F: Starburst, TH2, and ganglion cell populations label for calretinin and form three distinct projections (1,2,3) in the inner plexiform layer (IPL) as appropriate in the wt, Tpst1−/− and Tpst2−/− retina. G–I: GABAergic amacrine cells and their processes in the IPL of the wt, Tpst1−/− and Tpst2−/− retina, show normal labeling for the 65 kDa form of glutamic acid decarboxylase (GAD-65). Lamination of GABAergic amacrine cell processes in the IPL is also normal (arrowheads). J–L: A small population of amacrine cells (arrowheads) shows appropriate labeling for vesicular glutamate transporter 3 (VGLUT3) in the wt, Tpst1−/− and Tpst2−/− retina. M–O: Ganglion cells, their axons (Ax) and their dendrites in the wt, Tpst1−/− and Tpst2−/− retina show labeling for microtubule-associated protein 1 (MAP-1) as appropriate. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Elimination of TPST-1 or TPST-2 does not induce any large scale disruption of retinal bipolar cells. (A–D) wt retina; (E–H) Tpst1−/−; (I–L) Tpst2−/− retina. The projections of rod bipolar cells (labeled for protein kinase C (PKC), green; panels A, E, I) and Type 2 and Type 6 Cone bipolar cells labeled for synaptotagmin 2 (blue; panels C, G, K) show appropriate morphology and project appropriately to the ON and OFF sublayers of the inner plexiform layer (IPL). The Type 6 cone bipolar cell plexus in the ON sublayer of the IPL (arrowheads) is slightly expanded in TPST-1 and TPST-2 knockout retinas. The terminals of photoreceptors in the outer plexiform layer (OPL) and bipolar cell terminals in the inner plexiform layer (IPL) express vesicular glutamate transporter 1 (VGLUT1, red; panels B,F,J) as appropriate. Labeling of blood vessels (bv) in panels C,G,K is non-specific. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Absence of TPST-1 or TPST-2 does not disrupt Müller glial cells. A–C: Müller cells in wt, Tpst1−/− and Tpst2−/− retina show normal morphology and express glutamine synthetase (GS) as appropriate. Labeling of blood vessels (bv) is non-specific. D–F: Müller cells in the wt, Tpst1−/− and Tpst2−/− retina are not reactive and show normal localization of glial fibrillary acidic protein (GFAP, red) to the end feet (arrowheads) along the inner retinal margin. Nuclei are labeled with DAPI (white) to illustrate retinal layering. Labeling of blood vessels (bv) is non-specific. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Development of normal synaptic ultrastructure in Tpst1−/− and Tpst2−/− retinas. A–C: Rod terminals in (A) wt, (B) Tpst1−/−, and (C) Tpst2−/− retinas show normal ultrastructural organization. Post-synaptic triads comprised of horizontal cell processes (h) in the lateral position and a rod bipolar cell dendrite (b) in the central position are arranged around a synaptic ribbon (r) attached to the presynaptic membrane. D–F: Cone terminals in (D) wt, (E) Tpst1−/− and (F) Tpst2−/− retinas show normal ultrastructural organization. Cones from mice of all three genotypes made multiple synaptic ribbon complexes arranged around a synaptic ribbon attached to the plasma membrane of the cone terminal with the normal triad of two horizontal cell processes and a bipolar cell dendrite. In addition, cones also made flat contacts (fc, and inset in panel E) with bipolar cell dendrites as appropriate. G–I: Bipolar cell terminals in (G) wt, (H) Tpst1−/−, and (I) Tpst2−/− retinas show normal ultrastructural organization. Bipolar cells from mice of all three genotypes made normal synaptic complexes arranged around a short synaptic ribbon attached to the plasma membrane of the bipolar terminal with a dyad of post-synaptic processes (post 1 and post 2) arising from amacrine and ganglion cells. J–L: Conventional synapses made by amacrine cells in (J) wt, (K) Tpst1−/−, and (L) Tpst2−/− retinas show normal ultrastructure with synaptic vesicles (SV) presynaptically, a widened synaptic cleft, and densification of the pre- and post-synaptic membranes. Scale bars = 0.5 µm for A–F; 0.2 µm for G–L.
(TIF)
Acknowledgments
We thank Barbara A. Nagel and Eileen Parks for technical assistance and Drs. Roger Janz and Robert Margolskee for their generous gifts of antisera. The synaptotagmin 2 monoclonal antibody generated by Trevarrow [17] was obtained from the Zebrafish International Resource Center, which is supported by grant P40 RR012546 from the NIH-NCRR. The Islet-1 monoclonal antibody developed by Ericson [18] was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICDH and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: The project described was partly supported by funds from the National Center For Research Resources (P20RR017703), the National Eye Institute (P30EY12190), Oklahoma Center for the Advancement of Science and Technology (OCAST) (DMS), R01 EY007361 (SJF), R01 HD056022 (KLM), R01 EY018137 (MRA), R01 EY10609 (MIN), the Foundation Fighting Blindness (MRA), and by an Unrestricted Grant from Research to Prevent Blindness (SJF). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources, the National Eye Institute, or the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. SJF is the recipient of an RPB Senior Scientific Investigator Award.
References
- 1.Moore KL. Protein tyrosine sulfation: a critical posttranslation modification in plants and animals. Proc Natl Acad Sci U S A. 2009;106:14741–14742. doi: 10.1073/pnas.0908376106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle PA, Huttner WB. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987;105:2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore KL. The biology and enzymology of protein tyrosine O-sulfation. J Biol Chem. 2003;278:24243–24246. doi: 10.1074/jbc.R300008200. [DOI] [PubMed] [Google Scholar]
- 4.Lee RW, Huttner WB. Tyrosine-O-sulfated proteins of PC12 pheochromocytoma cells and their sulfation by a tyrosylprotein sulfotransferase. J Biol Chem. 1983;258:11326–11334. [PubMed] [Google Scholar]
- 5.Ouyang YB, Moore KL. Molecular cloning and expression of human and mouse tyrosylprotein sulfotransferase-2 and a tyrosylprotein sulfotransferase homologue in Caenorhabditis elegans. J Biol Chem. 1998;273:24770–24774. doi: 10.1074/jbc.273.38.24770. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang Y, Lane WS, Moore KL. Tyrosylprotein sulfotransferase: purification and molecular cloning of an enzyme that catalyzes tyrosine O-sulfation, a common posttranslational modification of eukaryotic proteins. Proc Natl Acad Sci U S A. 1998;95:2896–2901. doi: 10.1073/pnas.95.6.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danan LM, Yu Z, Hoffhines AJ, Moore KL, Leary JA. Mass spectrometric kinetic analysis of human tyrosylprotein sulfotransferase-1 and -2. J Am Soc Mass Spectrom. 2008;19:1459–1466. doi: 10.1016/j.jasms.2008.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanan Y, Hoffhines A, Rauhauser A, Murray A, Al-Ubaidi MR. Protein tyrosine-O-sulfation in the retina. Exp Eye Res. 2009;89:559–67. doi: 10.1016/j.exer.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanan Y, Hamilton RA, Moore KL, Al-Ubaidi MR. Protein tyrosine-O-sulfation in bovine ocular tissues. Adv Exp Med Biol. 2012;723:835–841. doi: 10.1007/978-1-4614-0631-0_107. [DOI] [PubMed] [Google Scholar]
- 10.Sherry DM, Murray AR, Kanan Y, Arbogast KL, Hamilton RA, et al. Lack of protein-tyrosine sulfation disrupts photoreceptor outer segment morphogenesis, retinal function and retinal anatomy. Eur J Neurosci. 2010;32:1461–1472. doi: 10.1111/j.1460-9568.2010.07431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang YB, Crawley JT, Aston CE, Moore KL. Reduced body weight and increased postimplantation fetal death in tyrosylprotein sulfotransferase-1-deficient mice. J Biol Chem. 2002;277:23781–23787. doi: 10.1074/jbc.M202420200. [DOI] [PubMed] [Google Scholar]
- 12.Borghei A, Ouyang YB, Westmuckett AD, Marcello MR, Landel CP, et al. Targeted disruption of tyrosylprotein sulfotransferase-2, an enzyme that catalyzes post-translational protein tyrosine O-sulfation, causes male infertility. J Biol Chem. 2006;281:9423–9431. doi: 10.1074/jbc.M513768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westmuckett AD, Hoffhines AJ, Borghei A, Moore KL. Early postnatal pulmonary failure and primary hypothyroidism in mice with combined TPST-1 and TPST-2 deficiency. Gen Comp Endocrinol. 2008;156:145–153. doi: 10.1016/j.ygcen.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stricker HM, Ding XQ, Quiambao A, Fliesler SJ, Naash MI. The C214S mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice. Biochem J. 2005;388:605–13. doi: 10.1042/BJ20041960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tan E, Wang Q, Quiambao AB, Xu X, Qtaishat NM, et al. The relationship between opsin overexpression and photoreceptor degeneration. Invest Ophthalmol Vis Sci. 2001;42:589–600. [PubMed] [Google Scholar]
- 16.Lewis GP, Fisher SK. Up-regulation of glial fibrillary acidic protein in response to retinal injury: its potential role in glial remodeling and a comparison to vimentin expression. Int Rev Cytol. 2003;230:263–290. doi: 10.1016/s0074-7696(03)30005-1. [DOI] [PubMed] [Google Scholar]
- 17.Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–679. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- 18.Ericson J, Thor S, Edlund T, Jessell TM, Yamada T. Early stages of motor neuron differentiation revealed by expression of homeobox gene Islet-1. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 19.Li X, Mumby SM, Greenwood A, Jope RS. Pertussis toxin-sensitive G protein alpha-subunits: production of monoclonal antibodies and detection of differential increases on differentiation of PC12 and LA-N-5 cells. J Neurochem. 1995;64:1107–1117. doi: 10.1046/j.1471-4159.1995.64031107.x. [DOI] [PubMed] [Google Scholar]
- 20.Huang L, Shanker YG, Dubauskaite J, Zheng JZ, Yan W, et al. Ggamma13 colocalizes with gustducin in taste receptor cells and mediates IP3 responses to bitter denatonium. Nat Neurosci. 1999;2:1055–1062. doi: 10.1038/15981. [DOI] [PubMed] [Google Scholar]
- 21.Huang L, Max M, Margolskee RF, Su H, Masland RH, et al. G protein subunit G gamma 13 is coexpressed with G alpha o, G beta 3, and G beta 4 in retinal ON bipolar cells. J Comp Neurol. 2003;455:1–10. doi: 10.1002/cne.10396. [DOI] [PubMed] [Google Scholar]
- 22.Debus E, Weber K, Osborn M. Monoclonal antibodies specific for glial fibrillary acidic (GFA) protein and for each of the neurofilament triplet polypeptides. Differentiation. 1983;25:193–203. doi: 10.1111/j.1432-0436.1984.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 23.Chang YC, Gottlieb DI. Characterization of the proteins purified with monoclonal antibodies to glutamic acid decarboxylase. J Neurosci. 1988;8:2123–2130. doi: 10.1523/JNEUROSCI.08-06-02123.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kentroti S, Baker R, Lee K, Bruce C, Vernadakis A. Platelet-activating factor increases glutamine synthetase activity in early and late passage C-6 glioma cells. J Neurosci Res. 1991;28:497–506. doi: 10.1002/jnr.490280406. [DOI] [PubMed] [Google Scholar]
- 25.Huber G, Matus A. Differences in the cellular distributions of two microtubule-associated proteins, MAP1 and MAP2, in rat brain. J Neurosci. 1984;4:151–160. doi: 10.1523/JNEUROSCI.04-01-00151.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blanks JC, Johnson LV. Specific binding of peanut lectin to a class of retinal photoreceptor cells. A species comparison. Invest Ophthalmol Vis Sci. 1984;25:546–557. [PubMed] [Google Scholar]
- 27.Hoffhines AJ, Damoc E, Bridges KG, Leary JA, Moore KL. Detection and purification of tyrosine-sulfated proteins using a novel anti-sulfotyrosine monoclonal antibody. J Biol Chem. 2006;281:37877–37887. doi: 10.1074/jbc.M609398200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith TW, Nikulasson S, De Girolami U, De Gennaro LJ. Immunohistochemistry of synapsin I and synaptophysin in human nervous system and neuroendocrine tumors. Applications in diagnostic neuro-oncology. Clin Neuropathol. 1993;12:335–342. [PubMed] [Google Scholar]
- 29.Janz R, Sudhof TC. SV2C is a synaptic vesicle protein with an unusually restricted localization: anatomy of a synaptic vesicle protein family. Neurosci. 1999;94:1279–1290. doi: 10.1016/s0306-4522(99)00370-x. [DOI] [PubMed] [Google Scholar]
- 30.Fox MA, Sanes JR. Synaptotagmin I and II are present in distinct subsets of central synapses. J Comp Neurol. 2007;503:280–296. doi: 10.1002/cne.21381. [DOI] [PubMed] [Google Scholar]
- 31.Calakos N, Bennett MK, Peterson KE, Scheller RH. Protein-protein interactions contributing to the specificity of intracellular vesicular trafficking. Science. 1994;263:1146–1149. doi: 10.1126/science.8108733. [DOI] [PubMed] [Google Scholar]
- 32.Galli T, Zahraoui A, Vaidyanathan VV, Raposo G, Tian JM, et al. A novel tetanus neurotoxin-insensitive vesicle-associated membrane protein in SNARE complexes of the apical plasma membrane of epithelial cells. Mol Biol cell. 1998;9:1437–1448. doi: 10.1091/mbc.9.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sherry DM, Mitchell R, Standifer KM, du PB. Distribution of plasma membrane-associated syntaxins 1 through 4 indicates distinct trafficking functions in the synaptic layers of the mouse retina. BMC Neurosci. 2006;7:54. doi: 10.1186/1471-2202-7-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sherry DM, Wang MM, Bates J, Frishman LJ. Expression of vesicular glutamate transporter 1 in the mouse retina reveals temporal ordering in development of rod vs. cone and ON vs. OFF circuits. J Comp Neurol. 2003;465:480–498. doi: 10.1002/cne.10838. [DOI] [PubMed] [Google Scholar]
- 35.Johnson J, Sherry DM, Liu X, Fremeau RT, Jr, Seal RP, et al. Vesicular glutamate transporter 3 expression identifies glutamatergic amacrine cells in the rodent retina. J Comp Neurol. 2004;477:386–398. doi: 10.1002/cne.20250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lack of TPST-1 or TPST-2 does not induce any large scale disruption of retinal horizontal, amacrine or ganglion cells. A–C: Horizontal cells labeled for calbindin in the wt, Tpst1−/− and Tpst2−/− retina show normal placement in the inner nuclear layer (INL) and project normally to the outer plexiform layer (OPL), although the extent of their plexus in the Tpst2−/− retina is slightly reduced. D–F: Starburst, TH2, and ganglion cell populations label for calretinin and form three distinct projections (1,2,3) in the inner plexiform layer (IPL) as appropriate in the wt, Tpst1−/− and Tpst2−/− retina. G–I: GABAergic amacrine cells and their processes in the IPL of the wt, Tpst1−/− and Tpst2−/− retina, show normal labeling for the 65 kDa form of glutamic acid decarboxylase (GAD-65). Lamination of GABAergic amacrine cell processes in the IPL is also normal (arrowheads). J–L: A small population of amacrine cells (arrowheads) shows appropriate labeling for vesicular glutamate transporter 3 (VGLUT3) in the wt, Tpst1−/− and Tpst2−/− retina. M–O: Ganglion cells, their axons (Ax) and their dendrites in the wt, Tpst1−/− and Tpst2−/− retina show labeling for microtubule-associated protein 1 (MAP-1) as appropriate. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Elimination of TPST-1 or TPST-2 does not induce any large scale disruption of retinal bipolar cells. (A–D) wt retina; (E–H) Tpst1−/−; (I–L) Tpst2−/− retina. The projections of rod bipolar cells (labeled for protein kinase C (PKC), green; panels A, E, I) and Type 2 and Type 6 Cone bipolar cells labeled for synaptotagmin 2 (blue; panels C, G, K) show appropriate morphology and project appropriately to the ON and OFF sublayers of the inner plexiform layer (IPL). The Type 6 cone bipolar cell plexus in the ON sublayer of the IPL (arrowheads) is slightly expanded in TPST-1 and TPST-2 knockout retinas. The terminals of photoreceptors in the outer plexiform layer (OPL) and bipolar cell terminals in the inner plexiform layer (IPL) express vesicular glutamate transporter 1 (VGLUT1, red; panels B,F,J) as appropriate. Labeling of blood vessels (bv) in panels C,G,K is non-specific. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Absence of TPST-1 or TPST-2 does not disrupt Müller glial cells. A–C: Müller cells in wt, Tpst1−/− and Tpst2−/− retina show normal morphology and express glutamine synthetase (GS) as appropriate. Labeling of blood vessels (bv) is non-specific. D–F: Müller cells in the wt, Tpst1−/− and Tpst2−/− retina are not reactive and show normal localization of glial fibrillary acidic protein (GFAP, red) to the end feet (arrowheads) along the inner retinal margin. Nuclei are labeled with DAPI (white) to illustrate retinal layering. Labeling of blood vessels (bv) is non-specific. Abbreviations as in Fig. 1. Scale bars = 50 µm.
(TIF)
Development of normal synaptic ultrastructure in Tpst1−/− and Tpst2−/− retinas. A–C: Rod terminals in (A) wt, (B) Tpst1−/−, and (C) Tpst2−/− retinas show normal ultrastructural organization. Post-synaptic triads comprised of horizontal cell processes (h) in the lateral position and a rod bipolar cell dendrite (b) in the central position are arranged around a synaptic ribbon (r) attached to the presynaptic membrane. D–F: Cone terminals in (D) wt, (E) Tpst1−/− and (F) Tpst2−/− retinas show normal ultrastructural organization. Cones from mice of all three genotypes made multiple synaptic ribbon complexes arranged around a synaptic ribbon attached to the plasma membrane of the cone terminal with the normal triad of two horizontal cell processes and a bipolar cell dendrite. In addition, cones also made flat contacts (fc, and inset in panel E) with bipolar cell dendrites as appropriate. G–I: Bipolar cell terminals in (G) wt, (H) Tpst1−/−, and (I) Tpst2−/− retinas show normal ultrastructural organization. Bipolar cells from mice of all three genotypes made normal synaptic complexes arranged around a short synaptic ribbon attached to the plasma membrane of the bipolar terminal with a dyad of post-synaptic processes (post 1 and post 2) arising from amacrine and ganglion cells. J–L: Conventional synapses made by amacrine cells in (J) wt, (K) Tpst1−/−, and (L) Tpst2−/− retinas show normal ultrastructure with synaptic vesicles (SV) presynaptically, a widened synaptic cleft, and densification of the pre- and post-synaptic membranes. Scale bars = 0.5 µm for A–F; 0.2 µm for G–L.
(TIF)