Abstract
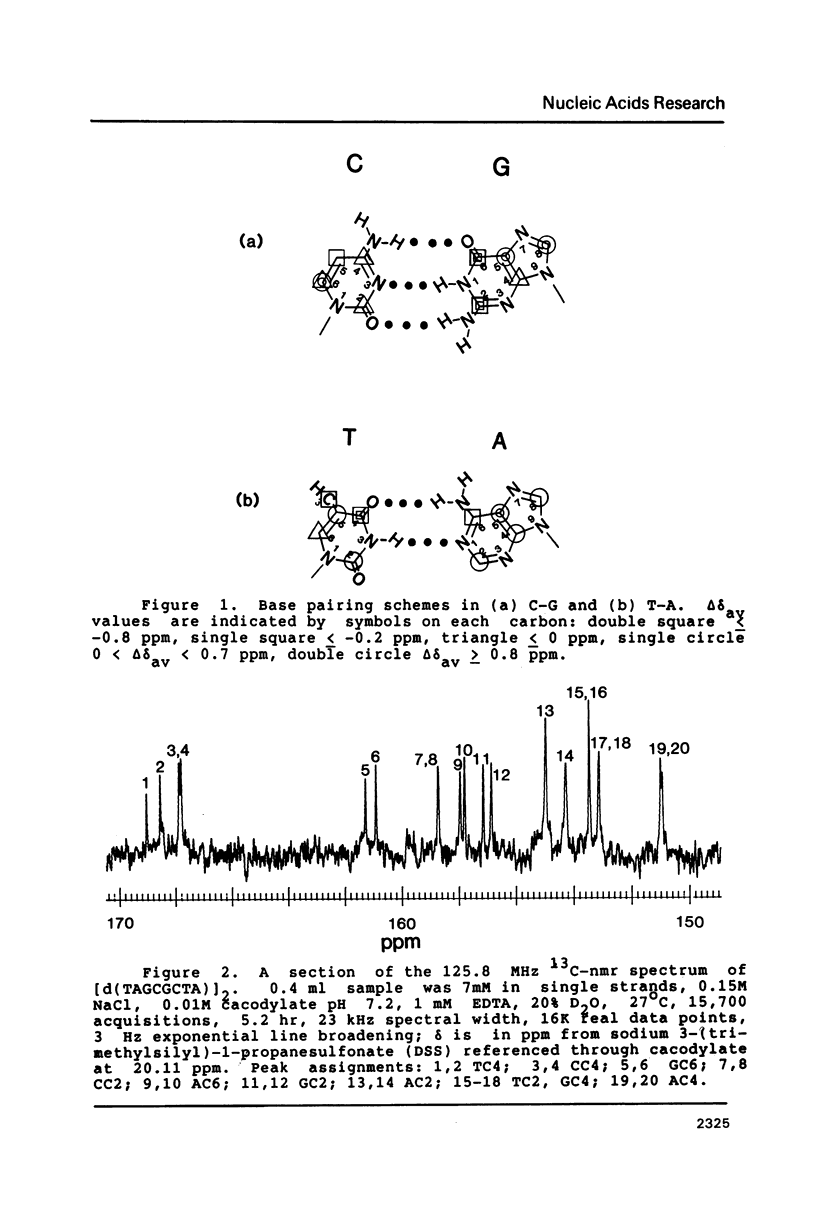
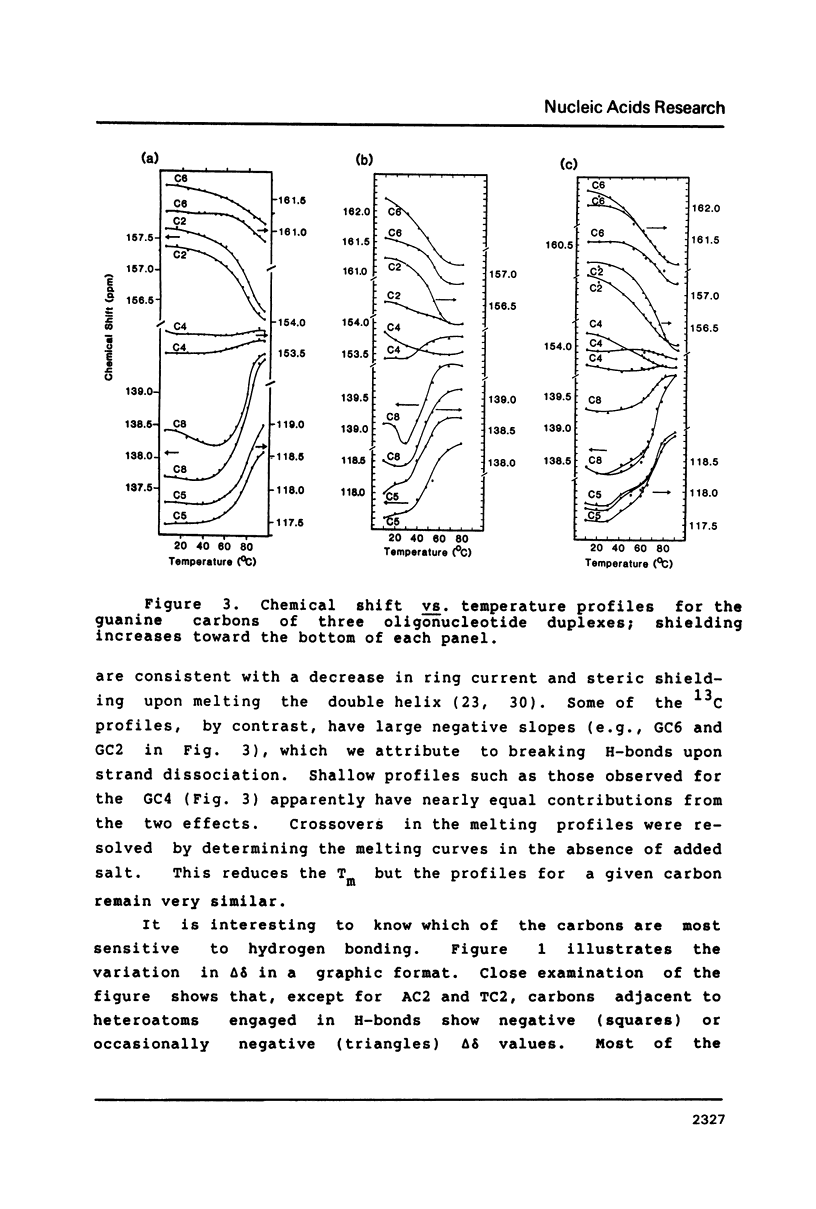
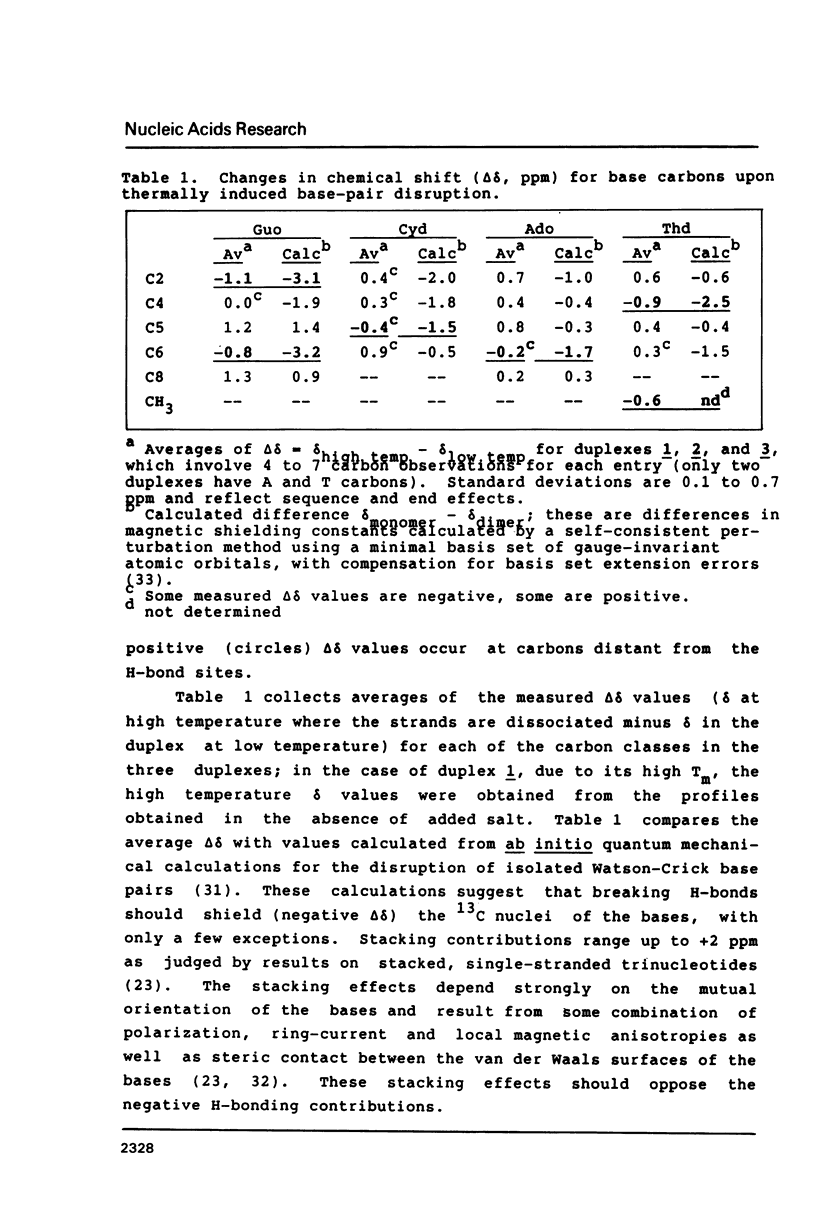
13C-nmr chemical shifts of the nucleotides in DNA are sensitive to hydrogen bonding, especially for three of the carbons immediately bonded to exocyclic oxygen or nitrogen atoms acting as H-bond acceptors or donors. GuoC2, GuoC6 and ThdC4 are strongly deshielded (about 1 ppm) upon Watson-Crick pairing in oligodeoxynucleotide duplexes, regardless of the base sequence. Deshielding at these sites may be useful to distinguish bases involved in Watson-Crick pairs from unpaired bases.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Kan L. S., Ts'o P. O. Conformation and interaction of short nucleic acid double-stranded helices. I. Proton magnetic resonance studies on the nonexchangeable protons of ribosyl ApApGpCpUpU. Biochemistry. 1975 Nov 4;14(22):4847–4863. doi: 10.1021/bi00693a012. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Zanatta N., Holak T. A., Levy G. C., van Boom J. H., Wang A. H. Conformation and dynamics of short DNA duplexes: (dC-dG)3 and (dC-dG)4. J Biomol Struct Dyn. 1984 Jun;1(6):1373–1386. doi: 10.1080/07391102.1984.10507526. [DOI] [PubMed] [Google Scholar]
- Cruz P., Bubienko E., Borer P. N. A model for base overlap in RNA. Nature. 1982 Jul 8;298(5870):198–200. doi: 10.1038/298198a0. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Frederick C. A., Grable J., Melia M., Samudzi C., Jen-Jacobson L., Wang B. C., Greene P., Boyer H. W., Rosenberg J. M. Kinked DNA in crystalline complex with EcoRI endonuclease. Nature. 1984 May 24;309(5966):327–331. doi: 10.1038/309327a0. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C. Ab-initio quantum mechanical calculations of NMR chemical shifts in nucleic acid constituents. I. The Watson-Crick base pairs. J Biomol Struct Dyn. 1984 Aug;2(1):233–248. doi: 10.1080/07391102.1984.10507560. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C. Ab-initio quantum mechanical calculations of NMR chemical shifts in nucleic acids constituents. III. Chemical shift variations due to base stacking. J Biomol Struct Dyn. 1986 Aug;4(1):99–110. doi: 10.1080/07391102.1986.10507648. [DOI] [PubMed] [Google Scholar]
- Giessner-Prettre C., Pullman B. On the atomic or "local" contributions to proton chemical shifts due to the anisotropy of the diamagnetic susceptibility of the nucleic acid base. Biochem Biophys Res Commun. 1976 May 17;70(2):578–581. doi: 10.1016/0006-291x(76)91086-x. [DOI] [PubMed] [Google Scholar]
- Hingerty B., Brown R. S., Jack A. Further refinement of the structure of yeast tRNAPhe. J Mol Biol. 1978 Sep 25;124(3):523–534. doi: 10.1016/0022-2836(78)90185-7. [DOI] [PubMed] [Google Scholar]
- Holbrook S. R., Sussman J. L., Warrant R. W., Kim S. H. Crystal structure of yeast phenylalanine transfer RNA. II. Structural features and functional implications. J Mol Biol. 1978 Aug 25;123(4):631–660. doi: 10.1016/0022-2836(78)90210-3. [DOI] [PubMed] [Google Scholar]
- Jamin N., James T. L., Zon G. Two-dimensional nuclear Overhauser enhancement investigation of the solution structure and dynamics of the DNA octamer [d(GGTATACC)]2. Eur J Biochem. 1985 Oct 1;152(1):157–166. doi: 10.1111/j.1432-1033.1985.tb09176.x. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. Binding of an antitumor drug to DNA, Netropsin and C-G-C-G-A-A-T-T-BrC-G-C-G. J Mol Biol. 1985 Jun 25;183(4):553–563. doi: 10.1016/0022-2836(85)90171-8. [DOI] [PubMed] [Google Scholar]
- Kopka M. L., Yoon C., Goodsell D., Pjura P., Dickerson R. E. The molecular origin of DNA-drug specificity in netropsin and distamycin. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1376–1380. doi: 10.1073/pnas.82.5.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leupin W., Wagner G., Denny W. A., Wüthrich K. Assignment of the 13C nuclear magnetic resonance spectrum of a short DNA-duplex with 1H-detected two-dimensional heteronuclear correlation spectroscopy. Nucleic Acids Res. 1987 Jan 12;15(1):267–275. doi: 10.1093/nar/15.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel D. J. Netropsin . dG-dG-dA-dA-dT-dT-dC-dC complex. Antibiotic binding at adenine . thymine base pairs in the minor groove of the self-complementary octanucleotide duplex. Eur J Biochem. 1979 Sep;99(2):369–378. doi: 10.1111/j.1432-1033.1979.tb13265.x. [DOI] [PubMed] [Google Scholar]
- Patel D. J., Shapiro L., Hare D. Sequence-dependent conformations of DNA duplexes: the TATA segment of the d(G-G-T-A-T-A-C-C) duplex in aqueous solution. Biopolymers. 1986 Apr;25(4):693–706. doi: 10.1002/bip.360250412. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Ughetto G., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986 Jun 6;232(4755):1255–1258. doi: 10.1126/science.3704650. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Ughetto G., van der Marel G., van Boom J. H., Rich A. Molecular structure of an anticancer drug-DNA complex: daunomycin plus d(CpGpTpApCpG). Proc Natl Acad Sci U S A. 1980 Dec;77(12):7204–7208. doi: 10.1073/pnas.77.12.7204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheek R. M., Boelens R., Russo N., van Boom J. H., Kaptein R. Sequential resonance assignments in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Biochemistry. 1984 Mar 27;23(7):1371–1376. doi: 10.1021/bi00302a006. [DOI] [PubMed] [Google Scholar]
- Stone M. P., Winkle S. A., Borer P. N. 13C-NMR of ribosyl ApApA, ApApG and ApUpG. J Biomol Struct Dyn. 1986 Feb;3(4):767–781. doi: 10.1080/07391102.1986.10508460. [DOI] [PubMed] [Google Scholar]
- Tanaka T., Letsinger R. L. Syringe method for stepwise chemical synthesis of oligonucleotides. Nucleic Acids Res. 1982 May 25;10(10):3249–3260. doi: 10.1093/nar/10.10.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Fujii S., van Boom J. H., van der Marel G. A., van Boeckel S. A., Rich A. Molecular structure of r(GCG)d(TATACGC): a DNA--RNA hybrid helix joined to double helical DNA. Nature. 1982 Oct 14;299(5884):601–604. doi: 10.1038/299601a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Wemmer D. E., Chou S. H., Hare D. R., Reid B. R. Sequence-specific recognition of DNA: assignment of nonexchangeable proton resonances in the consensus Pribnow promoter DNA sequence by two-dimensional NMR. Biochemistry. 1984 May 8;23(10):2262–2268. doi: 10.1021/bi00305a027. [DOI] [PubMed] [Google Scholar]
- Williamson M. P., Marion D., Wüthrich K. Secondary structure in the solution conformation of the proteinase inhibitor IIA from bull seminal plasma by nuclear magnetic resonance. J Mol Biol. 1984 Mar 5;173(3):341–359. doi: 10.1016/0022-2836(84)90125-6. [DOI] [PubMed] [Google Scholar]


