Abstract
Objective
Tumours are commonly hypoxic and this can be associated with aggressive tumour type, metastasis and resistance to therapy. Heat shock proteins (hsps) are induced in response to hypoxia, provide cancer cells with protection against tumour-associated stressors and chaperone oncoproteins that drive tumour proliferation. This study examined the effect of different oxygen concentrations on the expression of hsps in melanoma cell lines.
Methods
Melanoma cell lines were cultured in 2% and 20% O2. Expression of Hsp90, Hsp70, Hsp60, Hsp40 and Hsp32 proteins were determined by flow cytometry.
Results
Growth rates and viability were reduced in the majority of cell lines by culture in 2% O2. Hsp expression was different in 2% compared to 20% O2 and changes in Hsp90 expression correlated with cell line generation time (P<0.005) and viability (P<0.01). Greater total hsp expression correlated with improved viability in 2% but not 20% O2 (P<0.05). Relative expression of the different hsps was consistent across cell lines and each correlated with the others (P = 0.0001) but not with Hsp32. Hsp expression was inversely correlated with cell line adhesion to laminin as well as collagen type IV and Breslow depth of the original primary tumour tissue (P<0.05), but not with Clark level or patient survival. All five hsps were identified on the cell surface.
Conclusion
Culture in 2% O2 variably altered hsp expression in a panel of melanoma cell lines. Hsp expression was associated with certain cell line characteristics and clinical parameters of the originating tumour.
Introduction
It is well established that hypoxia is a feature of human tumours [1], [2], [3]. The hypoxic state is the result of the combined effects of rapid proliferation of malignant cells and abnormal behaviour of blood vessels, resulting in insufficient blood supply to the tumour mass [1], [2], [3], [4]. Hypoxia contributes to the biology of tumours through multiple mechanisms including the promotion of genetic instability, contributing to immune evasion and assisting in the selection of cells more resistant to apoptosis and the harsh tumour microenvironment [2], [5], [6], [7], [8], [9]. Further, hypoxia has been associated with resistance to therapy, more aggressive tumours, tumour invasion, poor prognosis and patient death [5], [8], [10], [11]. Despite typically being a relatively small tumour frequently found on the skin, hypoxia is nevertheless a feature of human melanoma [1], [12], [13]. In melanoma, hypoxia is associated with tumour metastasis and may serve to enhance metastatic spread [14], [15], [16], [17].
The major mechanism by which cells respond to hypoxic stress is by rapid modulation of the expression of the HIF transcription factor [18]. HIF directly regulates the expression of heat shock proteins (hsps) and heat shock protein 90 (Hsp90) has been shown to assist in the stabilisation of HIF under hypoxic conditions in melanoma cells [19], [20]. Other studies suggest Hsp90 is involved in HIF expression and transactivation under hypoxia [21]. Hsps are an essential group of proteins that function as molecular chaperones and play a multitude of roles in eukaryotic cells [22]. Many of their functions contribute to the survival of tumour cells; accordingly, hsps have been shown to be abnormally expressed in a range of human cancers [23]. They promote the growth of cancer cells through multiple mechanisms such as inhibiting apoptosis, enhancing angiogenesis and providing protection against tumour-associated stressors such as hypoxia [23], [24], [25], [26], [27]. Hsps perform these roles in addition to chaperoning overexpressed oncoproteins that drive the proliferation of tumour cells [23], [28]. Consequently, hsps have been identified as valid targets in the treatment of cancer and are currently being evaluated in clinical trials in a number of cancer types including melanoma [29]. Hsp expression has been shown to be important in melanoma and relevant to patient clinical parameters such as Breslow depth, Clark level and survival [30], [31], [32], [33].
The relevance of hypoxia and hsps to cancer is well documented, but has rarely been studied in the context of human cancer cell cultures. Laboratory cell culture is routinely performed under hyperoxic conditions (i.e. in air) and this may be a limitation of this model for the study of human cancer cells which frequently experience hypoxia in vivo. Hsps have been investigated in melanoma tumour tissue and play multiple roles important for cancer growth, but have not been extensively studied in melanoma cell lines. Here, we present a preliminary study that sought to assess hsp expression in relation to melanoma cell line characteristics, patient clinical parameters and low and high oxygen culture conditions in a relatively large panel of melanoma cell lines.
Methods
Cell lines: Melanoma cell lines were selected from the European Searchable Tumour Line Database (ESTDAB; http://www.medizin.uni-tuebingen.de/estdab/) which is the largest existing collection of melanoma cell lines. The ESTDAB cell lines have been thoroughly characterised and controlled with mycoplasma testing and DNA finger printing [34]. The sample cohort used in this study consisted of 40 cell lines of metastatic tumour origin and two of primary tumour origin (EST 66 and 83). For a full list of the cell lines used, see Fig. 3.
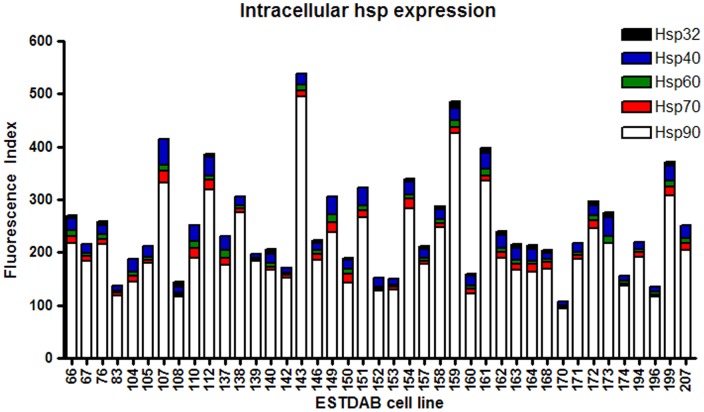
Figure 3. Hsp expression across melanoma cell lines.
Forty two melanoma cell lines were cultured 20% O2 for five days. Following the culture period cell lines were harvested, stained with PE-conjugated antibodies to Hsps 90, 70, 60, 40 and 32 and protein expression assessed by flow cytometry. A Fluorescence Index was calculated (fold increase in mean fluorescence of the stained cells compared with the unstained cells) and was used as a comparative measure of protein expression across the cell lines.
Cell culture: Cell lines were cultured with 35 ml RPMI 1640 medium (Life Technologies, Darmstadt, Germany) supplemented with 10% heat inactivated Foetal Calf Serum (FCS) (Sigma, Munich, Germany) in 75 cm2 cell culture flasks (Greiner Bio-One, Frickenhausen, Germany) for five days in an incubator (37°C, 5% CO2, 95% humidity) in air (20% O2) and in 2% O2 using the Concept 1000 Invivo2 (Ruskinn Technology, Pencoed, UK). Seeding cell number was adjusted according to the generation time (time required for one population doubling) of each cell line in order to avoid confluence and to obtain similar cell numbers at the end of the culture period. Following the culture period cells were washed with Hank's Buffered Salt Solution (HBSS) (PAA, Pasching, Austria) and detached from culture flasks by incubation with Trypsin-EDTA (Cambrex, Wiesbaden, Germany) (200 mg/l EDTA, 500 mg/l Trypsin 1∶250) for approximately three minutes. Trypsin was inactivated by adding of an equal volume of RPMI medium containing 10% FCS.
Determination of cell number and viability: The trypan blue exclusion method was used to determine the number of living and dead cells. Melanoma cells were suspended in 10 ml of HBSS and mixed thoroughly. Equal volumes of a 0.4% trypan blue solution (Sigma) and the cell sample were mixed and applied to a Neubauer haemocytometer. Phase contrast microscopy was used to distinguish between stained (dead) and unstained (living) cells.
Flow cytometry: Following trypsinisation, 3.0×105 cells were washed with PFEA (phosphate buffered saline, 2% FCS, 2 mM EDTA, and 0.01% azide) and Fc receptors blocked with GAMUNEX (Bayer, Leverkusen, Germany) on ice for ten minutes. Cells were washed with PFEA before being permeablised and fixed with Cytofix/Cytoperm and Perm/Wash (BD Biosciences, Heidelberg, Germany) according to the manufacturer’s instructions. Single fluorochrome staining was performed where antibody was diluted in Perm/Wash and incubated for 30 minutes on ice. PE-conjugated antibodies were used at the following concentrations: Hsp90 1:300 (product #SPA-830PE), Hsp70 1:100 (product #SPA-820PE), Hsp60 1:100 (product #SPA-807PE), Hsp40 1:100 (product #SPA-400PEF), Hsp32 1:100 (product #OSA-111PE) (all from Enzo Life Sciences, Lörrach, Germany). Following incubation cells were washed twice with Perm/Wash before being suspended in 120 µL of 1% formaldehyde solution (Merck, Darmstadt, Germany) in PFEA and stored on ice in a darkened container. For cell surface staining, PFEA was used in place of Cytofix/Cytoperm and Perm/Wash. Cells were immediately analysed on an LSR II flow cytometer with FACSDiva software (BD Biosciences). Data was analysed using FlowJo software (Tree Star, Ashland, USA). To perform data analysis, the main population of cells was gated on a forward scatter versus side scatter dot plot according to size and granularity. For each sample a fluorescence index (FI) was calculated in order to allow the comparison of fluorescence values for each cell line. The FI is the fold increase of the mean fluorescence intensity of the main population of cells in the stained cell sample by comparison with the corresponding unstained sample. FI values of less than two were considered negative.
Statistical analysis: Statistical analysis was performed using Prism software version 4.02 (GraphPad, San Diego, USA). Cell line ligand adhesion data was obtained from ESTDAB. Within matching data sets, changes of less than 5.0% were not considered to be different.
Paired two-tailed non-parametric t tests (Wilcoxon matched pairs tests) were used to assess significance between matching data points between two conditions.
Significance between two groups was assessed with two-tailed non-parametric (Mann-Whitney U) tests.
Correlations were assessed with non-parametric two-tailed (Spearman) correlation tests.
Trends across four grouping variables were assessed with two-tailed chi-squared contingency tests.
Results
Low Oxygen Tension Reduces Viability and Retards Growth in the Majority of Melanoma Cell Lines
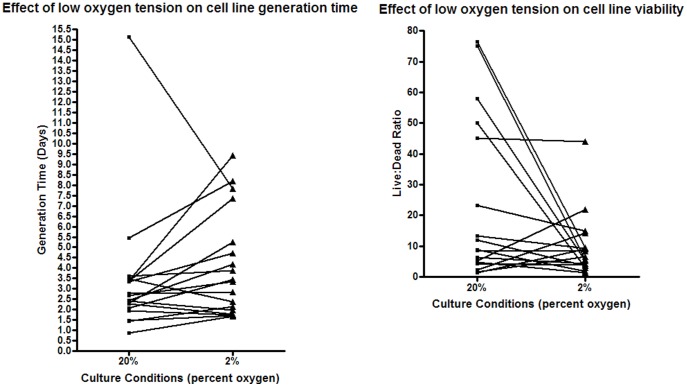
Eighteen melanoma cell lines were selected for culture in 2% O2. Twelve of 18 cell lines showed a mean increase of 70% in generation time when cultured in 2% O2 compared with the 20% O2 condition (P = 0.054). Five of the remaining six cell lines showed reduced generation times (mean decrease 26%), while the remaining cell line did not change (Fig. 1 A). Cell viability was accordingly reduced in 11 of 17 cell lines (P = 0.096), while four showed improved viability and two did not change (Fig. 1 B) (no data for 1 cell line). Four of the five cell lines that showed faster generation times in the 2% O2 condition also displayed improved viability in 2% O2 (EST 174, 194, 196 and 207), while one cell line showed no change for both generation time and viability (EST 143).
Figure 1. Effect of low oxygen culture on melanoma cell line generation time and viability.
Eighteen melanoma cell lines were cultured under identical conditions in 2% and 20% O2 for five days. Following the culture period, the cell lines were harvested and living and dead cells enumerated using trypan blue. Total number of living cells was used to calculate generation time, and ratio of live to dead cells was used as a marker of cell viability.
Low Oxygen Tension Results in Differential Heat Shock Protein Expression
Comparing hsp expression between the 2% and 20% O2 conditions showed that low oxygen culture resulted in up- or down-regulation of hsp expression (Table 1). For each of the four hsps examined, approximately half of the cell lines showed decreased expression in the 2% O2, while a smaller proportion showed increases. Six cell lines showed a reduction in the expression of all four hsps in the 2% O2 condition (EST 66, 76, 105, 108, 138 and 153), but the remaining cell lines up- and down-regulated these four hsps in response to low oxygen tension. Of note is that the four cell lines that showed faster generation times and improved viability in the 2% O2 condition showed a corresponding increase in Hsp90 expression in the 2% O2 culture condition.
Table 1. Change in hsp expression in response to low oxygen tension.
| Hsp | Decrease of hsp expressionin 2% O2 (% of cell lines) | Mean decrease (%) | Increase of hsp expressionin 2% O2 (% of cell lines) | Mean increase (%) |
| 90 | 50.0 | 19.5 | 38.9 | 13.9 |
| 70 | 44.4 | 27.3 | 44.4 | 15.0 |
| 60 | 50.0 | 20.4 | 33.3 | 16.8 |
| 40 | 61.1 | 18.3 | 16.7 | 16.8 |
Eighteen melanoma cell lines were cultured under identical conditions in 2% and 20% O2. After five days the cell lines were harvested, stained with PE-conjugated hsp antibodies and protein expression assessed by flow cytometry. A Fluorescence Index was calculated (fold increase in mean fluorescence of the stained cells compared with the unstained cells) and was used as a comparative measure of protein expression.
Heat shock Protein Expression is Associated with Melanoma Cell Line Generation Time and Viability
Because low oxygen tension resulted in altered hsp expression and influenced generation time and viability in these cell lines, the association of hsp expression with generation time and viability was investigated in the 2% and 20% O2 culture conditions. With the exception of Hsp60 in 20% O2 (P<0.03), total hsp expression was not associated with generation time in either condition (data not shown). When examining the increase or decrease in hsp expression in response to low oxygen tension, associations between the change in Hsp90 expression with cell line generation time (P<0.005, Table 2) were observed, but not for the other hsps (data not shown).
Table 2. Association between change in Hsp90 expression and cell line generation time between 2% and 20% O2 culture.
| DecreasedHsp90 in 2% | IncreasedHsp90 in 2% | |
| Decreased generation time in 2% | 0 | 5 |
| Increased generation time in 2% | 8 | 2 |
P<0.005.
Changes in cell line generation time were correlated with changes in Hsp90 expression between the 2% and 20% O2 culture conditions.
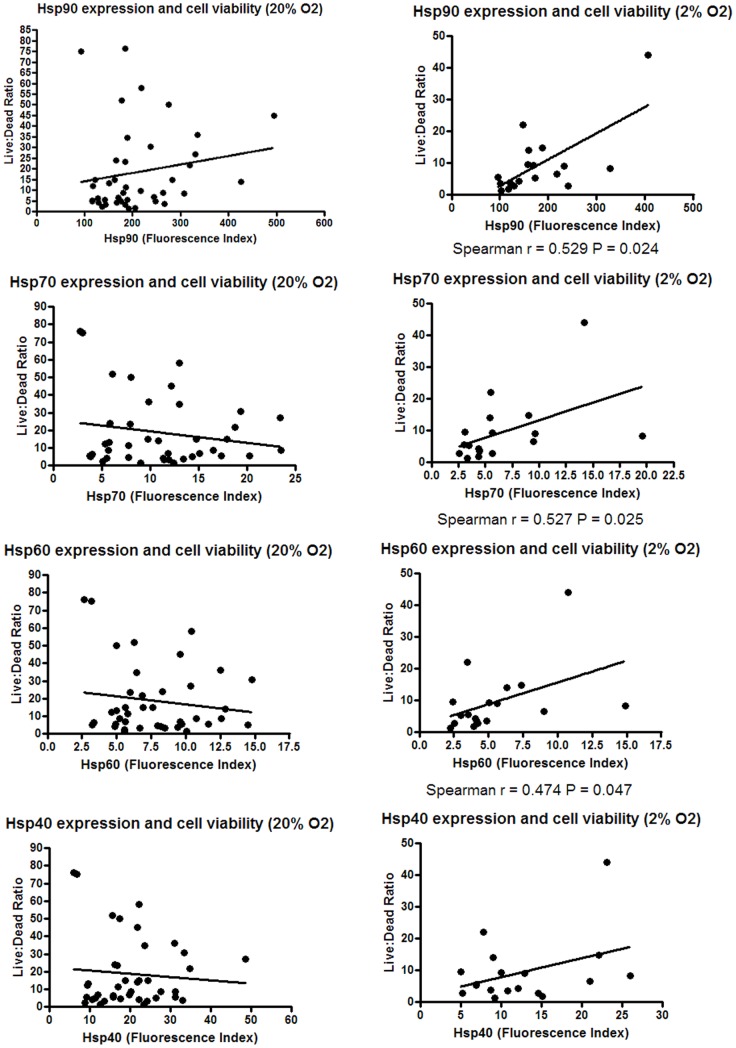
The relationship between hsp expression and viability in the 2% and 20% O2 culture conditions was then assessed. Significant relationships were found between higher expression of Hsp90, Hsp70 and Hsp60 and improved cell viability in the 2% (P<0.05) but not in the 20% O2 culture condition (Fig. 2). Examining the change in hsp expression between the 2% and 20% O2 culture conditions indicated that changes in Hsp90 expression were associated with cell line viability (P<0.01, Table 3).
Figure 2. Effect of low oxygen culture on hsp expression.
Forty two melanoma cell lines were cultured in 20% O2 and 18 of these cell lines additionally in 2% O2 for five days. Following the culture period, the cell lines were harvested and the number of living and dead cells determined using trypan blue. The ratio of live to dead cells was used as a marker of cell viability.
Table 3. Association between change in Hsp90 expression and cell line viability between 2% and 20% O2 culture.
| DecreasedHsp90 in 2% | IncreasedHsp90 in 2% | |
| Decreased viability in 2% | 7 | 2 |
| Increased viability in 2% | 0 | 4 |
P<0.01.
Changes in cell line viability were correlated with changes in Hsp90 expression between the 2% and 20% O2 culture conditions.
The Expression of the Different Heat Shock Proteins Correlates with one Another and with Melanoma Cell Line Adhesion
Hsp expression was compared throughout the cohort of melanoma cell lines cultured in 20% O2 (Fig. 3). Hsp90 was observed to account for the vast majority of total hsp expression in every cell line. The next most abundantly expressed hsps were Hsp40 and Hsp70, while Hsps 60 and 32 were similarly expressed at relatively low levels.
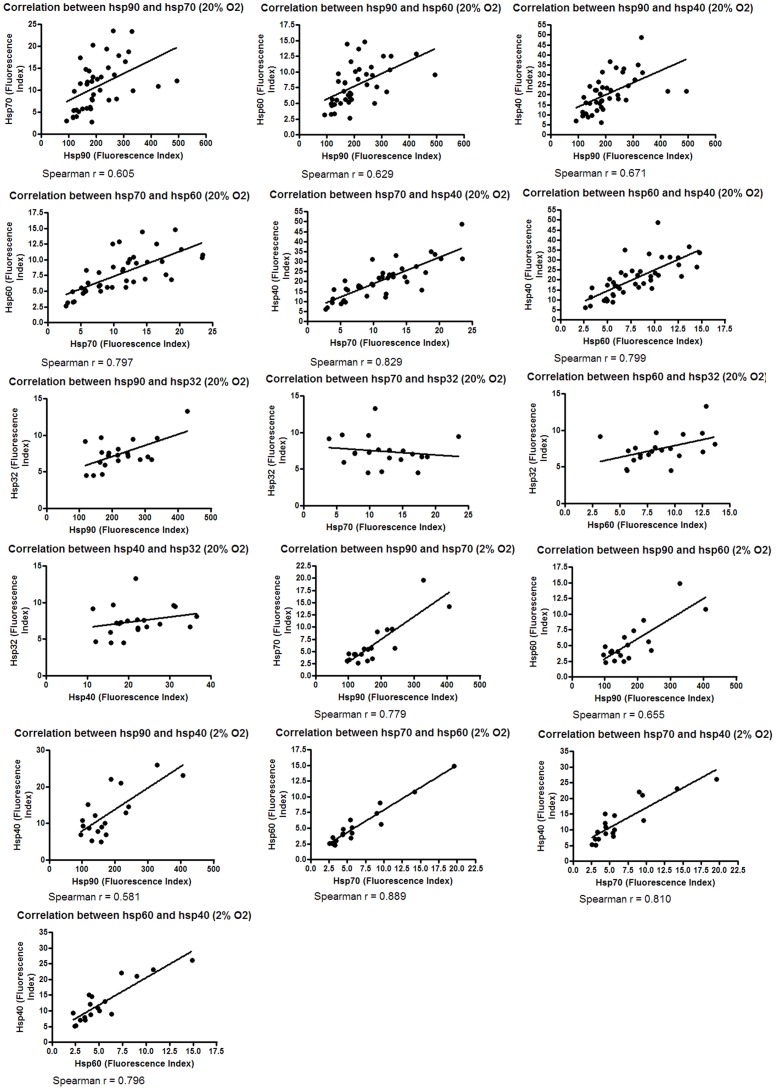
Given that the relative expression of hsps was observed to be consistent, relationships between the expression of these hsps were investigated. The expression of Hsp90, Hsp70, Hsp60 and Hsp40 was found to correlate, but not Hsp32 (in 2% (P = 0.015) and 20% O2 (P = 0.0001)) (Fig. 4, Hsp32 not tested in 2% O2).
Figure 4. Correlation between the expression of different hsps.
Melanoma cell lines were cultured in 2% O2 and 20% O2 for five days. Following the culture period cell lines were harvested, stained with PE-conjugated antibodies to Hsps 90, 70, 60, 40 and 32 and protein expression assessed by flow cytometry.
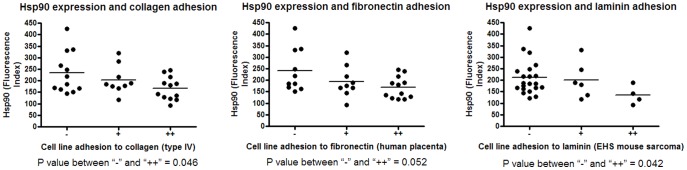
Hsp90 is involved in cell adhesion [35] and for this reason a relationship between Hsp90 expression and adhesion to collagen type IV, fibronectin and laminin was investigated. An inverse correlation was found between the expression of Hsp90 and adhesion to these ligands (Fig. 5).
Figure 5. Correlation between Hsp90 expression and cell line ligand adhesion.
Hsp expression was tested for correlation with the adhesion ability of these cell lines to the collagen type IV, fibronectin and laminin ligands.
Heat Shock Protein Expression Correlates with Breslow Depth but not Clark Level or Patient Survival
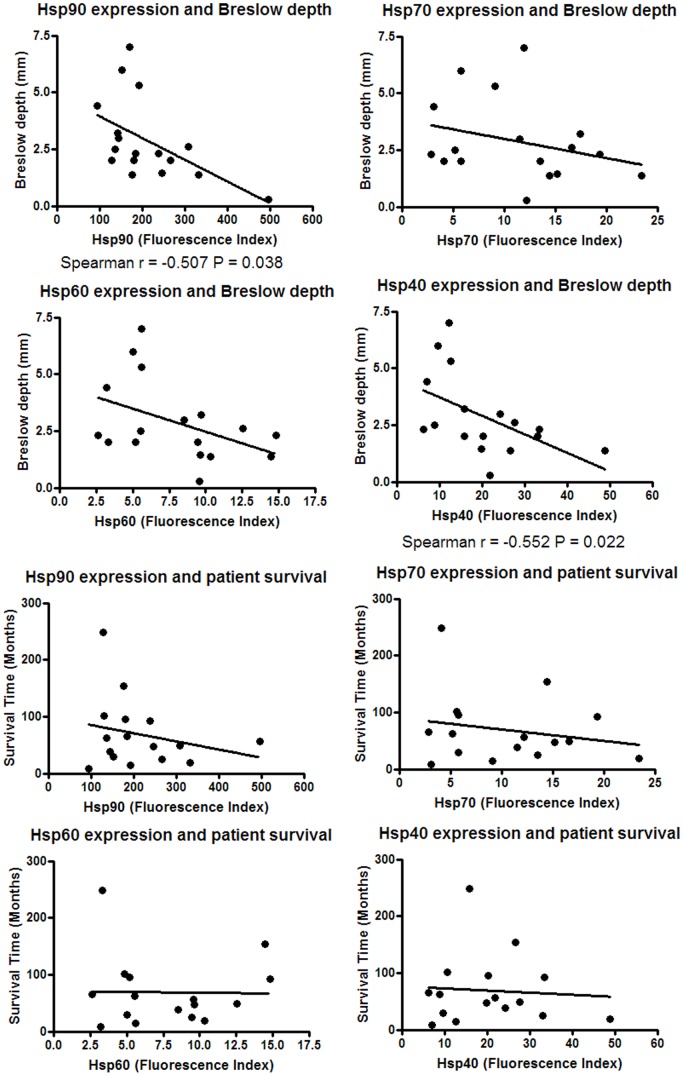
The expression of hsps in melanoma tissue has been shown to correlate with clinical parameters, but few, if any, studies have been performed on hsp expression in melanoma cell lines in this context. Patient survival time, Clark level and Breslow depth of the primary tumour tissue was known for a limited number of the metastasis-derived cell lines in the biobank used here, and were tested for a correlation with the expression of hsps. The expression of Hsp90 and Hsp40 was found to inversely correlate with Breslow depth, but no relationships were found with patient survival or Clark level (Fig. 6, Clark level data not shown).
Figure 6. Relationship between hsp expression and patient clinical parameters.
Hsp expression was investigated for association with melanoma patient clinical parameters.
Hsps are Located on the Surface of Melanoma Cells as Well as Intracellularly
Cancer cells have previously been reported to express hsps on the cell surface and for this reason ten melanoma cell lines were screened for expression of cell surface hsps (Fig. 7). Hsps 90, 70, 60, 40 and 32 were observed to be expressed on all ten cell lines with the exception of three cell lines being negative for Hsp32 (EST 83, 108, 152) while one was negative for Hsp90 (EST 108). In comparison to intracellular expression, cell surface hsp expression was low. Of note is that Hsp90 was not the most highly expressed hsp on the cell surface, in contrast to its intracellular expression level. The expression of cell surface hsps did not correlate with intracellular hsp expression (P>0.35) (data not shown).
Figure 7. Cell surface expression of hsps on melanoma cell lines.
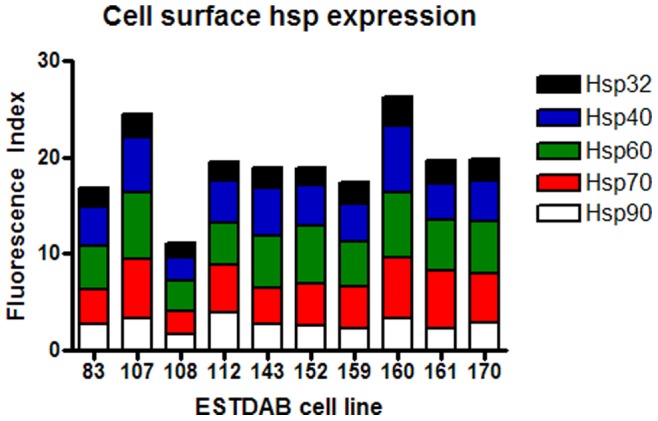
Ten melanoma cell lines were cultured in 20% O2 for five days. Following the culture period cell lines were harvested, stained for cell surface Hsp90, Hsp70, Hsp60, Hsp40 and Hsp32 and protein expression assessed by flow cytometry. A Fluorescence Index was calculated (fold increase in mean fluorescence of the stained cells compared with the unstained cells) and was used as a comparative measure of protein expression.
Discussion
Cancer cell lines are widely used models in cancer studies, but standard practice dictates culture under hyperoxic conditions (i.e. in air). Since hypoxia is a common and important feature of cancer, culture of these cells under high oxygen conditions may contribute to the generation of misleading results. For this reason we investigated viability, generation time and hsp expression in order to assess melanoma cell line response to low oxygen tension. Although hypoxia is a feature of human melanoma, the majority of the cell lines tested here displayed retarded growth and reduced viability under low oxygen conditions. This may be due to the fact that they have been generated and cultured under hyperoxic conditions which have prevented the selection of hypoxia-resistant cells that would normally occur [36]. Hypoxia is a known inducer of the hsp response, but to the best our knowledge, no studies have examined the effect of low oxygen on hsp expression in melanoma or indeed in any other cancer cell lines in the manner performed here [37]. Unexpectedly, a widespread induction of hsp expression did not occur in response to low oxygen tension. Despite this, changes in Hsp90 expression were associated with tolerance to low oxygen as measured by generation time and viability. These data suggest that improved hypoxic tolerance is associated with the induction of hsps in melanoma cells. Further, higher total hsp expression was associated with improved viability in low but not high oxygen conditions, perhaps reflecting the anti-apoptotic role that hsps play in cancer [24], [38]. The greater demand placed on the hsp chaperone system under low oxygen stress when the level of hsp expression is more likely to be below the threshold required for cellular survival may account for the observation that hsp expression was not associated with viability under high oxygen levels. If these observations are at all reflective of in vivo conditions then it follows that anti-neoplastic hsp inhibiting drugs may be relatively more effective in hypoxic tumours in which a range of standard therapies are known to be less effective [5]. Indeed, previous reports have shown that melanoma cells require hsps in order to proliferate [39], [40]. Thus, in vivo under hypoxic conditions their effectiveness may be enhanced. Models that allow regions of variable oxygen concentration, such as three dimensional culture models or in vivo xenografts, could be used to confirm or refute the results presented in this study.
Relative hsp expression was observed to be consistent across the cohort of cell lines and levels of Hsp 90, 70, 60 and 40 expression were shown to correlate with one another but not with Hsp32. Hsp90 is essential for eukaryotic cell viability and it chaperones a large number of overexpressed client proteins in cancer. This may explain why Hsp90 was expressed at a level many fold greater than the other hsps examined [22], [41]. However hsps most often function in coordination with other molecular chaperones [22], [42]. The other hsps examined in this study are major Hsp90 co-chaperones, consistent with the notion that hsps cooperate closely in their role as molecular chaperones [22]. The intimacy of their association may explain the observation that their expression correlated with one another but not with Hsp32, because this protein is not a molecular chaperone and its function is less related to that of the other hsps [43].
Hsp expression was observed to correlate with Breslow depth but not Clark level or patient survival. Studies using primary melanoma tissue have shown increased Hsp90 expression to be associated with greater Breslow depth and Clark level [33], but in the present study with metastatic melanoma cell lines an inverse relationship was observed. This relationship was observed even though these lines have been cultured for several generations under in vitro conditions. Similar reports exist in other contexts [44]. For example, Walter et al. demonstrated MMP-2 mRNA levels in renal cancer cell lines to inversely correlate with patient survival [45]. A potential disadvantage of using cultured cells as models for in vivo processes is that it is not known how reflective the parameters measured under these conditions are of in vivo conditions. The data presented here coupled with at least one previous report may suggest that cancer cell lines maintain a degree of relevance to patient clinical parameters and thus may be of use as models for cancer. This relevance may be maintained indirectly, since results from previous studies using melanoma tissue suggest that in contrast to the results of this study using cell lines, that Breslow depth is positively associated with hsp expression. Since in these cell lines hsp expression was relevant to viability in 2% but not 20% O2, culture under low oxygen tension may be more relevant to in vivo growth conditions, but further studies are needed to confirm these preliminary findings. Of note is that the inverse relationship between hsp expression and Breslow depth was observed in 20% O2, and it is not known if hsp expression in 2% O2 also correlates with Breslow depth. No remarkable differences were observed between the primary-derived and metastasis-derived cell lines in this study.
It should be noted that the observation of an inverse correlation between hsp expression and Breslow depth does not imply that this relationship is present in tumours in vivo, as the level of hsp expression observed in these cell lines may not be reflective of the level in vivo. This could be due to the influence of the artificial cell culture environment. Follow-up studies in tumours and cell lines will confirm or refute the preliminary findings presented here. If this observation is confirmed, further studies could examine other clinical and biological features such as the expression of inflammatory cytokines, tumour ulceration, regional differences in the distribution of hsp expression (for example, proximity to the invasive front, necrotic core or blood vessels), histological sub-type or other factors that may interact with or separately explain this observation. If the level of hsp expression observed in this study is reflective of in vivo expression, then studies using melanoma tissue that have shown higher hsp expression to be associated with greater Breslow depth [33] contradicts the observations here that higher hsp expression correlates with reduced Breslow depth. This contradiction might be explained by hsps influencing the immunogenicity of these cells, producing a more effective immune response and a smaller resultant tumour [46], [47]. Supporting this is a study in which expression of Hsp70 was found to correlate with improved survival in melanoma patients [31]. Further, immune recognition is a potential function for the hsps identified on the cell surface as membrane-located hsps have been shown to act as targets for T cells and NK cells [48], [49]. The finding here that Hsp90 and Hsp70 are expressed on the surface of melanoma cells confirms previous studies, but to the best of our knowledge the expression of Hsp60, Hsp40 or Hsp32 has not previously been reported [50], [51].
Although this investigation is not an extensive study into the effects of low oxygen tension on cancer cell cultures, it does present novel findings with respect to the potential role of hsps and demonstrates that further investigation into the effects of oxygen tension on cancer cell cultures is warranted. These data suggest that hsps associate closely in melanoma cells and that their expression is associated with tolerance to low oxygen tension and patient clinical parameters. The in vivo importance of hypoxia coupled with the finding that hsp expression is relevant to melanoma cell viability in low but not high oxygen tension suggests cancer cell cultures should more frequently be subjected to oxygen tension that more closely resembles in vivo levels in order to cast more light on the largely unknown influence that this condition has on the myriad of facets investigated in modern cancer research.
Acknowledgments
We are grateful to Lilly Öttinger for assistance with harvesting cultured cell lines used in this study. The laboratory of Dirk Schadendorf provided the melanoma patient clinical data. Christopher Shipp is the recipient of an Australian Postgraduate Award and a University of New England Strategic Doctoral Scholarship.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: These authors have no support or funding to report.
References
- 1.Murphy M, Carlson JA, Keough MP, Claffey KP, Signoretti S, et al. Hypoxia regulation of the cell cycle in malignant melanoma: putative role for the cyclin-dependent kinase inhibitor p27Kip1. Journal of Cutaneous Pathology. 2004;31:477–482. doi: 10.1111/j.0303-6987.2004.00205.x. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Harrison L. Tumor Hypoxia: Causative Factors, Compensatory Mechanisms, and Cellular Response The Oncologist 9. 2004. [DOI] [PubMed]
- 3.Kerbel RS. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000;21:505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 4.Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, et al. Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro-Oncology. 2005;7:134–153. doi: 10.1215/S1152851704001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weinmann M, Belka C, Plasswilm L. Tumour Hypoxia: Impact on Biology, Prognosis and Treatment of Solid Malignant Tumours. Onkologie. 2004;27:83–90. doi: 10.1159/000075611. [DOI] [PubMed] [Google Scholar]
- 6.Kim CY, Tsai MH, Osmanian C, Graeber TG, Lee JE, et al. Selection of human cervical epithelial cells that possess reduced apoptotic potential to low-oxygen conditions. Cancer Research. 1997;57:4200–4204. [PubMed] [Google Scholar]
- 7.Lukashev D, Ohta A, Sitkovsky M. Hypoxia-dependent anti-inflammatory pathways in protection of cancerous tissues. Cancer and Metastasis Reviews. 2007;26:273–279. doi: 10.1007/s10555-007-9054-2. [DOI] [PubMed] [Google Scholar]
- 8.Semenza GL. Hypoxia and cancer. Cancer and Metastasis Reviews. 2007;26:223–224. doi: 10.1007/s10555-007-9058-y. [DOI] [PubMed] [Google Scholar]
- 9.Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88–91. doi: 10.1038/379088a0. [DOI] [PubMed] [Google Scholar]
- 10.Brown JM. Tumor hypoxia in cancer therapy. Methods in Enzymology. 2007;435:297–321. doi: 10.1016/S0076-6879(07)35015-5. [DOI] [PubMed] [Google Scholar]
- 11.Höckel M, Vaupel P. Tumor Hypoxia: Definitions and Current Clinical, Biologic, and Molecular Aspects. Journal of the National Cancer Institute. 2001;93:266–276. doi: 10.1093/jnci/93.4.266. [DOI] [PubMed] [Google Scholar]
- 12.Lartigau E, Randrianarivelo H, Avril MF, Margulis A, Spatz A, et al. Intratumoral oxygen tension in metastatic melanoma. Melanoma Research. 1997;7:400–406. doi: 10.1097/00008390-199710000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Rofstad EK, Måseide K. Radiobiological and immunohistochemical assessment of hypoxia in human melanoma xenografts: acute and chronic hypoxia in individual tumours. International Journal of Radiation Oncology. 1999;75:1377–1393. doi: 10.1080/095530099139250. [DOI] [PubMed] [Google Scholar]
- 14.Rofstad EK, Rasmussen H, Galappathi K, Mathiesen B, Nilsen K, et al. Hypoxia promotes lymph node metastasis in human melanoma xenografts by up-regulating the urokinase-type plasminogen activator receptor. Cancer Research. 2002;62:1847–1853. [PubMed] [Google Scholar]
- 15.Rofstad EK, Halsør EF. Hypoxia-associated spontaneous pulmonary metastasis in human melanoma xenografts: involvement of microvascular hot spots induced in hypoxic foci by interleukin 8. British Journal of Cancer. 2002;86:301–308. doi: 10.1038/sj.bjc.6600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rofstad EK, Danielsen T. Hypoxia-induced metastasis of human melanoma cells: involvement of vascular endothelial growth factor-mediated angiogenesis. British Journal of Cancer. 1999;80:1697–1707. doi: 10.1038/sj.bjc.6690586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Victor N, Ivy A, Jiang BH, Agani FH. Involvement of HIF-1 in invasion of Mum2B uveal melanoma cells. Clinical and Experimental Metastasis. 2006;23:87–96. doi: 10.1007/s10585-006-9024-z. [DOI] [PubMed] [Google Scholar]
- 18.Guillemin K, Krasnow MA. The Hypoxic Response: Huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- 19.Baird NA, Turnbull DW, Johnson EA. Induction of the Heat Shock Pathway during Hypoxia Requires Regulation of Heat Shock Factor by Hypoxia-inducible Factor-1. The Journal of Biological Chemistry. 2006;281:38675–38681. doi: 10.1074/jbc.M608013200. [DOI] [PubMed] [Google Scholar]
- 20.Trisciuoglio D, Gabellini C, Desideri M, Ziparo E, Zupi G, et al. Bcl-2 regulates HIF-1alpha protein stabilization in hypoxic melanoma cells via the molecular chaperone HSP90. Public Library of Science One. 2010;5:e11772. doi: 10.1371/journal.pone.0011772. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 21.Le NL, Katschinski DM, Buhr HJ. Role of heat shock protein (HSP) 90 on the stabilization and the activation of the hypoxia-inducible factor (HIF) 1: HSP90-Inhibitors as the potential molecules of anti-HIF-1 tumor therapy. Deutsche Gesellschaft für Chirurgie. 2005;34:115–118. [Google Scholar]
- 22.Zhao R, Houry WA. Molecular Interaction Network of the Hsp90 Chaperone System. Adv Exp Med Biol. 2007;594:27–36. doi: 10.1007/978-0-387-39975-1_3. [DOI] [PubMed] [Google Scholar]
- 23.Bagatell R, Whitesell L. Altered Hsp90 function in cancer: A unique therapeutic opportunity. Mol Cancer Ther. 2004;3:1021–1030. [PubMed] [Google Scholar]
- 24.Gibbons NB, Watson RWG, Coffey RNT, Brady HP, Fitzpatrick JM. Heat-shock proteins inhibit induction of prostate cancer cell apoptosis. The Prostate. 2000;45:58–65. doi: 10.1002/1097-0045(20000915)45:1<58::aid-pros7>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Sun J, Liao JK. Induction of Angiogenesis by Heat Shock Protein 90 Mediated by Protein Kinase Akt and Endothelial Nitric Oxide Synthase. Arterioscler Thromb Vasc Biol. 2004;24:2238–2244. doi: 10.1161/01.ATV.0000147894.22300.4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–172. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 27.Goel G, Guo M, Ding J, Dornbos D, Ali A, et al. Combined effect of tumor necrosis factor (TNF)-alpha and heat shock protein (HSP)-70 in reducing apoptotic injury in hypoxia: a cell culture study. Neuroscience Letters. 2010;483:162–166. doi: 10.1016/j.neulet.2010.07.069. [DOI] [PubMed] [Google Scholar]
- 28.Okui T, Shimo T, Hassan NM, Fukazawa T, Kurio N, et al. Antitumor Effect of Novel HSP90 Inhibitor NVP-AUY922 against Oral Squamous Cell Carcinoma. Anticancer Research. 2011;31:1197–1204. [PubMed] [Google Scholar]
- 29.A Study Evaluating the Safety and Antitumor Activity of IPI-504, in Patients With Metastatic Melanoma. NIH. 2011.
- 30.Kalogeraki A, Garbagnati F, Darivianaki K, Delides GS, Santinami M, et al. HSP-70, C-myc and HLA-DR Expression in Patients with Cutaneous Malignant Melanoma Metastatic in Lymph Nodes. Anticancer Res. 2006;26:3551–3554. [PubMed] [Google Scholar]
- 31.Ricaniadis N, Kataki A, Agnantis N, Androulakis G, Karakousis CP. Long-term prognostic significance of HSP-70, c-myc and HLA-DR expression in patients with malignant melanoma. Eur J Surg Oncol. 2001;27:88–93. doi: 10.1053/ejso.1999.1018. [DOI] [PubMed] [Google Scholar]
- 32.Deichmann M, Polychronidis M, Benner A, Kleist C, Thome M, et al. Expression of the heat shock cognate protein HSP73 correlates with tumour thickness of primary melanomas and is enhanced in melanoma metastases. International Journal of Oncology. 2004;25:259–268. [PubMed] [Google Scholar]
- 33.McCarthy MM, Pick E, Kluger Y, Gould-Rothberg B, Lazova R, et al. HSP90 as a marker of progression in melanoma. Ann Oncol. 2008;19:590–594. doi: 10.1093/annonc/mdm545. [DOI] [PubMed] [Google Scholar]
- 34.Pawelec G, Marsh SG. ESTDAB: a collection of immunologically characterised melanoma cell lines and searchable databank. Cancer Immunology, Immunotherapy. 2006;55:623–627. doi: 10.1007/s00262-005-0117-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsutsumi S, Beebe K, Neckers L. Impact of Heat-shock Protein 90 on Cancer Metastasis. Future Oncology. 2009;5:679–688. doi: 10.2217/fon.09.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dong Z, Wang J. Hypoxia selection of death-resistant cells. A role for Bcl-X(L). The Journal of Biological Chemistry. 2004;279:9215–9221. doi: 10.1074/jbc.M312225200. [DOI] [PubMed] [Google Scholar]
- 37.Hammerer-Lercher A, Mair J, Bonatti J, Watzka SB, Puschendorf B, et al. Hypoxia induces heat shock protein expression in human coronary artery bypass grafts. Cardiovascular Research. 2001;50:115–124. doi: 10.1016/s0008-6363(01)00198-5. [DOI] [PubMed] [Google Scholar]
- 38.Abarzua F, Sakaguchi M, Tanimoto R, Sonegawa H, Li DW, et al. Heat shock proteins play a crucial role in tumor-specific apoptosis by REIC/Dkk-3. Int J Mol Med. 2007;20:37–43. [PubMed] [Google Scholar]
- 39.Babchia N, Calipel A, Mouriaux F, Faussat A-M, Mascarelli F. 17-AAG and 17-DMAG–Induced Inhibition of Cell Proliferation through B-Raf Downregulation in WTB-Raf–Expressing Uveal Melanoma Cell Lines. Invest Ophthalmol Vis Sci. 2008;49:2348–2356. doi: 10.1167/iovs.07-1305. [DOI] [PubMed] [Google Scholar]
- 40.Yerlikaya A, Okur E, Şeker S, Erin N. Combined effects of the proteasome inhibitor bortezomib and Hsp70 inhibitors on the B16F10 melanoma cell line. Molecular Medicine Reports. 2010;3:333–339. doi: 10.3892/mmr_00000262. [DOI] [PubMed] [Google Scholar]
- 41.Neckers L. Heat shock protein 90: the cancer chaperone. J Biosci. 2007;32:517–530. doi: 10.1007/s12038-007-0051-y. [DOI] [PubMed] [Google Scholar]
- 42.Whitesell L, Lindquist SL. HSP90 and the chaperoning of cancer. Nat Rev Cancer. 2005;5:761–772. doi: 10.1038/nrc1716. [DOI] [PubMed] [Google Scholar]
- 43.Ryter SW, Alam J, Choi AM. Heme Oxygenase-1/Carbon Monoxide: From Basic Science to Therapeutic Applications. Physiological Reviews. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 44.Lee JE, Nam HY, Shim SM, Bae GR, Han BG, et al. Expression phenotype changes of EBV-transformed lymphoblastoid cell lines during long-term subculture and its clinical significance. Cell Proliferation. 2010;43:378–384. doi: 10.1111/j.1365-2184.2010.00687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Walther MM, Kleiner DE, Lubensky IA, Pozzatti R, Nyguen T, et al. Progelatinase A mRNA expression in cell lines derived from tumors in patients with metastatic renal cell carcinoma correlates inversely with survival. Urology. 1997;50:295–301. doi: 10.1016/s0090-4295(97)00220-3. [DOI] [PubMed] [Google Scholar]
- 46.Wells AD, Rai SK, Salvato MS, Band H, Malkovsky M. Hsp72-mediated augmentation of MHC class I surface expression and endogenous antigen presentation. Int Immunol. 1998;10:609–617. doi: 10.1093/intimm/10.5.609. [DOI] [PubMed] [Google Scholar]
- 47.Lukacs KV, Lowrie DB, Stokes RW, Colston MJ. Tumor cells transfected with a bacterial heat-shock gene lose tumorigenicity and induce protection against tumors. J Exp Med 178. 1993. [DOI] [PMC free article] [PubMed]
- 48.Harada M, Kimura G, Nomoto K. Heat shock proteins and the antitumor T cell response. Biotherapy. 1998;10:229–235. doi: 10.1007/BF02678301. [DOI] [PubMed] [Google Scholar]
- 49.Multhoff G, Botzler C, Jennen L, Schmidt J, Ellwart J, et al. Heat shock protein 72 on tumor cells: a recognition structure for natural killer cells. J Immunol. 1997;158:4341–4350. [PubMed] [Google Scholar]
- 50.Becker B, Multhoff G, Farkas B, Wild PJ, Landthaler M, et al. Induction of Hsp90 protein expression in malignant melanomas and melanoma metastases. Exp Dermatol. 2004;13:27–32. doi: 10.1111/j.0906-6705.2004.00114.x. (26). [DOI] [PubMed] [Google Scholar]
- 51.Farkas B, Hantschel M, Magyarlaki M, Becker B, Scherer K, et al. Heat shock protein 70 membrane expression and melanoma-associated marker phenotype in primary and metastatic melanoma. Melanoma Res. 2003;13:147–152. doi: 10.1097/00008390-200304000-00006. [DOI] [PubMed] [Google Scholar]