CD154 is expressed by radioresistant cells in the bone marrow, and plays a role in fine-tuning B cell hematopoiesis.
Keywords: ontogeny, bone marrow, B lymphocyte
Abstract
Interactions between CD40 and CD154 play a very important role in control of immune responses, including the delivery of T cell help to B cells and other APCs. Thus far, there has been no role postulated for CD40-CD154 interactions in hematopoiesis. We show here that CD40 is expressed on murine pro-B cells and that its ligation enhances pro-B cell proliferation in vitro and in vivo. In addition, CD154 mRNA is present in the BM. Moreover, we show that a deficiency in CD154 expression has effects on B cell hematopoiesis. Aged, CD154-deficient mice have significantly lower levels of B hematopoietic subsets downstream of pro-B cells in the BM. In addition, B lineage cells reconstitute more slowly following BMT into CD154-deficient recipients. We hypothesize that CD154 is expressed by radio-resistant cells in the BM and plays a role in fine-tuning B cell hematopoiesis.
Introduction
Adult murine B cell hematopoiesis takes place largely in the BM. Progenitor cells progress through well-defined stages from common lymphoid progenitors, through the earliest committed B lineage cells, followed by pro-B cells, pre-B cells, immature, and finally, mature B lymphocytes. The phenotypes of these various stages have been delineated by cell surface staining, as well as by varying degrees of Ig gene rearrangement. For the sake of simplicity, we will use the Hardy nomenclature here, wherein pro-B cells with germ-line Ig genes are designated Fr A, and Fr B and C are pro-B cells with increasing D-J rearrangement but no V-D-J rearrangement, which does not occur until Fr D, pre-B cells [1]. Fr A–C (pro-B cells) are CD19+/B220+/CD43+/IgM–, and Fr D is CD19+/B220+/CD43–/IgM–. Immature B cells (Fr E) are CD19+/B220+/IgM+/IgD–, and the mature population (Fr F) is CD19+/B220hi/IgM+/IgD+. Fr A–C can be differentiated based on expression of heat stable antigen and BP-1 [1].
B cell hematopoiesis depends, in the initial stages, on cell contact between hematopoietic precursor cells and BM stromal cells. One function of stromal cells is the production of IL-7, to which the early progenitor cells can respond by proliferating. A lack of IL-7 [2] or its high-affinity receptor [3] leads to a block in B cell development at the pro-B/pre-B transition.
The interaction between CD40 and its cognate ligand CD154 plays a major role in the control of humoral and cellular immune responses. Ligation of CD40 on mature B cells is pivotal in the delivery of T cell “help” to B cells [4–6]. Similarly, ligation of CD40 on DCs induces DC maturation and can “license” these DCs to deliver enhanced antigen presentation to CTLs [7–9]. Mice deficient in CD40 or CD154 expression exhibit a lack of class-switched antibody responses to T-dependent antigens, as well as deficiencies in cell-mediated immunity [4, 10, 11]. They have not been reported to exhibit hematopoietic deficiencies, having normal numbers of mature B and T cells [4, 12].
It has been reported extensively and is generally accepted that expression of CD40 on B cells correlates with a more advanced state of maturation and that the role of CD40 on B lineage cells is focused on the receipt of T cell help by mature B cells. Consistent with this theory, CD40 was found not to be expressed on mouse pro- or pre-B cell lines [13] and was expressed only on approximately 30% of murine BM pre-B cells and not at all on pro-B cells ex vivo [14, 15]. In contrast, studies about human cells had shown some expression on pro-B cells of normal and malignant origin [16], and indeed, there have been some reports in mice and humans of functional effects of CD40 antibody or CD154 on early B hematopoietic populations [17–19].
In contrast to most reported findings about CD40 expression, preliminary studies in our laboratory indicated expression of CD40 on earlier murine BM cells, including pro-B cells. We hypothesized that CD40-CD154 interactions, in addition to their established role in the control of immune responses, might play a role in the control of B cell hematopoiesis. We investigate this hypothesis further here and demonstrate a role for CD154-CD40 interactions in controlling murine B cell hematopoiesis.
MATERIALS AND METHODS
Mice
BALB/c, SCID/BALB/c, or C57Bl/6 female mice aged 6–8 weeks (unless stated otherwise) were obtained from Harlan (UK). CD154-deficient mice [4] were bred in-house from stock kindly provided by Dr. David Gray (University of Edinburgh, UK) and used at the ages stated. All experiments were performed under a UK Home Office-approved project license in compliance with strict Home Office regulations.
Antibodies
To determine CD40 expression by different stage B cells, the following mouse mAb were used: anti-IgD-eFluor450 (eBioscience, San Diego, CA, USA), anti-CD43-FITC (Southern Biotechnology, Birmingham, AL, USA), anti-CD19-PE (BD Biosciences, San Jose, CA, USA), anti-CD40-allophycocyanin (Southern Biotechnology), anti-IgM-PECy7 (BD Biosciences), and anti-B220-Alexa700 (BD Biosciences). To determine CD40 expression by pro-B cell, Fr B and C antibodies used were CD40-FITC and BP-1 (Ly-51)-PE (BD Biosciences), CD19-TC, and CD43-allophycocyanin. Matching IgG2a isotype control for CD40 staining was from BD Biosciences. To determine CD154 expression by lineage-negative cells in the BM, antibodies against CD154-FITC and CD45-PE (both BD Biosciences) were used.
Immunofluorescent staining of BM cells
Single-cell suspensions of BM cells were prepared, and typically, 1 × 106 cells were used per test carried out. Briefly, BM cells were preincubated with fragment crystalline block (CD16/CD32, BD Biosciences) before addition of staining mAb, which were added at 1 μg/test (in PBS, 0.1% BSA) and incubated 20 min on ice. A UV live/dead discrimination dye (Invitrogen, Carlsbad, CA, USA) was used to exclude dead cells. After three subsequent washes, cells were resuspended in PBS, 0.1% BSA. Stained cells were analyzed by flow cytometry using FACSCalibur, FACSAria, or LSRII analyzers (BD Biosciences). Further analysis was performed using CellQuest™ software (BD Biosciences) or FlowJo (Tree Star, Inc., Ashland, OR, USA).
In vitro coculture of pro-B cells with fibroblast cells
Femurs were removed from a number of BALB/c mice, and BM cells were flushed out aseptically and washed in PBS. BM cells from all mice were pooled and stained with fluorescently labeled antibodies enabling sorting pro-B cells (CD19+/CD43+/IgM–/IgD–) using a FACSAria (BD Biosciences). Pro-B or fraction pro-B cells (25,000) were cocultured with 50,000 irradiated (30 Gy, Cs137 source) WT L929 cells or CD154-expressing L929 cells for 5 days in a humidified atmosphere at 37°C, 5% CO2. Stimuli included anti-CD40 mAb (clone 1C10) at 10 μg/ml and anti-rat IgG2a isotype control mAb (GL117) at 10 μg/ml, both in the presence or absence of murine rIL-7 (R&D Systems, Minneapolis, MN, USA) at 50 ng/ml.
In vitro SCID proliferation
Femurs were removed from SCID mice, and BM cells were flushed out aseptically, washed with PBS, and resuspended at 1 × 107 cells/ml PBS. CFSE (Invitrogen) was added to a final concentration of 1 μM. Following mixing, cells were incubated at 37°C for 10 min. CFSE was quenched by addition of an equal amount of FCS and incubated in the dark for 10 min. Following a wash at 400 g for 5 min, cells were washed a further three times at 400 g for 5 min in RPMI containing 10% FCS. Cells were seeded at 1 × 106 cells/well in a 24-well plate. Controls included unstimulated cells and cells stimulated with the following: 10 μg/ml anti-CD40 mAb (clone 1C10 at 10 μg/ml) or plate-bound anti-CD3 mAb at 0.1 μg/ml and anti-CD28 mAb (BD Biosciences) at 0.5 μg/ml. Cells were incubated for 72 h in a humidified atmosphere at 37°C, 5% CO2. Proliferation in response to CD40 ligation was assessed by determination of the median fluorescence intensity for CFSE following 1C10 stimulation of samples from the three mice.
In vivo SCID pro-B proliferation
Six- to 8-week-old BALB/SCID mice were injected i.p. with 500 μg agonistic anti-CD40 antibody 1C10 [20], and 24 h later, BM was removed, and the percentage of pro-B cells was determined by flow cytometry.
Analysis of CD154 mRNA expression by RT-PCR
RNA extraction.
Whole RNA extraction from spleen and BM was performed using a Qiagen RNeasy mini kit, and RNA was quantified using A260/A280 and stored at –20°C. RT was performed on extracted RNA samples using a Protoscript II RT-PCR kit (New England Biolabs, Beverly, MA, USA), following kit instructions.
PCR primers, all 5′–3′, were as follows: CD154, forward (exon 2) TCG AAG AGG AAG TAA ACC TTC ATG, reverse (exon 4) CTGCATTACTGTTGGCTTCGCTTA, reverse (exon 5) GAG TAA GCC AAA AGA TGA GAA GCC, expected PCR product sizes: Exons 2–4, 243 bp; Exons 2–5, 617 bp; actin, forward TGG AAT CCT GTG GCA TCC ATG AAA, reverse TAA AAC GCAGCTCAGTAACAGTCC, expected product size: 348 bp; CD3 eta, forward CCT TTT CTC CTC ATC CTC CC, reverse TGC ACT CCT GCT GAA TTT TG, expected product size: 250 bp. PCR reaction mixtures were prepared following Protoscript II kit instructions by adding a 2× Taq master mix, 100 pmoles sense and antisense primer, and cDNA derived from the RT-PCR step. PCR cycling conditions were optimized previously for these primers at 94°C, 55°C, and 68°C denaturation, annealing, and extension steps for 30 cycles.
BMT
Recipient mice received two doses of 5.5 Gy irradiation from a Cs137 source, spaced 4 h apart. Mice were kept in high-efficiency particulate air-filtered air with sterile bedding, food, and water from this point on. Antibiotics [neomycin sulfate (Sigma-Aldrich, St. Louis, MO, USA) at 1 mg/ml and polymyxin B (Sigma-Aldrich) at 1000 U/ml] were added to the drinking water. Donor cells were prepared as aseptic single-cell suspensions from the femurs of donor mice, and recipients were injected i.v. with 106 donor cells immediately after the second dose of radiation. A group of naïve, nonirradiated mice served as a control for any alterations of lymphocyte numbers as a result of the weekly tail vein bleeds and not because of the irradiation and BMT itself.
Peripheral blood and BM cell counts
Following transplantation experiments, BM was extracted from both femurs of the mice, and single-cell lymphocyte suspensions were prepared as described above. Cells were counted using a hemocytometer using trypan blue (Sigma-Aldrich) for dead cell discrimination. Following immunofluorescent labeling of the cells, an equal amount of FlowCount™ fluorospheres (Beckman Coulter, Brea, CA, USA) was added. Following the manufacturer's instructions, subsequent gating on these beads and collection of 5000 bead events allowed the absolute number of cells/mouse to be determined. For blood analysis, 100 μl blood was removed from the tail vein into 10 U/ml heparin (Multiparin, CP Pharmaceuticals, UK), and the same procedure followed.
Statistical analysis
Statistical analysis was performed by two-tailed Student's t test where appropriate (parametric data) or by Mann Whitney test (nonparametric data), as described in Results and figure legends. Analyses were performed using Microsoft Excel or GraphPad Prism software.
RESULTS
CD40 is expressed on a proportion of murine pro-B cells in the BM
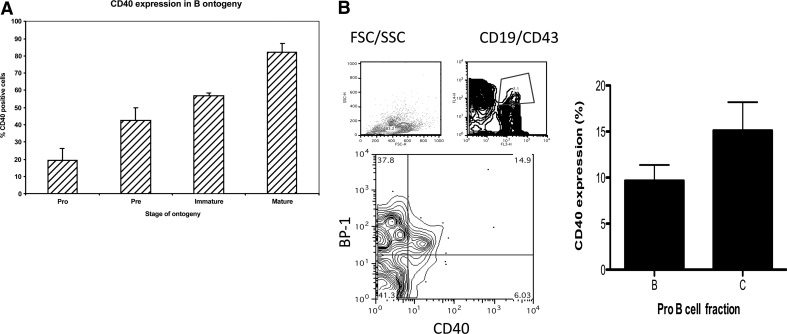
Using multiparametric flow cytometric analysis of murine BM cells (n=3), we showed initially that ∼20% of murine pro-B cells (CD43+/CD19+/ B220lo/IgM–/IgD–) express CD40. Expression rises to 43% on pre-B cells (CD43–/CD19+/ B220lo/IgM–/IgD–), 57% on immature (CD43–/CD19+/B220lo/IgM+/ IgD–), and 82% of mature (CD43–/CD19+/B220lo/IgM+/ IgD+) BM B cells (Fig. 1A). Fig. 1A is a representative plot of several independent experiments.
Figure 1. Expression of CD40 on BM pre-B cells.
(A) CD40 expression on various pre-B cell populations from BALB/c BM was assessed by flow cytometric staining for CD40 in conjunction with B220, CD43, and IgM staining to identify pro-, pre-, immature, and mature populations, as described in Materials and Methods. The bar chart shows the percentages of cells from each population expressing CD40. (B) Expression of CD40 on Fr B and C of pro-B cells defined on the basis of CD19, BP-1, and CD43 staining using the gating strategy shown and as described in Materials and Methods (n=7). SSC/FSC-H, Side/forward-scatter-height; FL4/3, fluorescence 4/3.
Pro-B cells have been subdivided into a number of different fractions based on cell surface phenotype [1]. The two CD19-positive fractions, B (CD19+, CD43+, BP-1–) and C (CD19+, CD43+, BP-1+), were identified phenotypically by flow cytometry as described in Materials and Methods, and CD40 expression was assessed. On average, 9.7% of Fr B cells and 15.1% of Fr C cells expressed CD40. Fig. 1B shows a representative plot of the gating strategy used (left), and pooled data from three independent experiments are shown on the right (n=7).
Function of CD40 expressed by BM pro-B cells
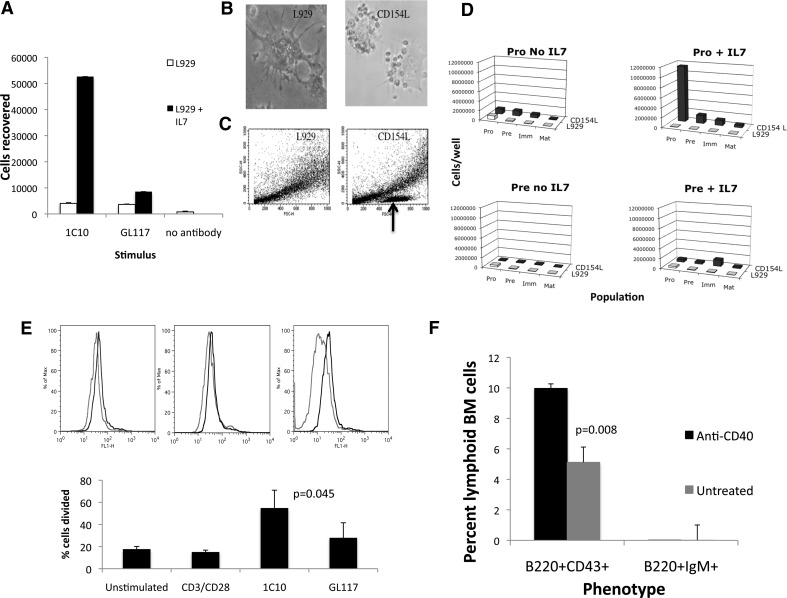
In an initial experiment, we assessed the effect of the agonistic CD40 antibody 1C10 on sorted CD19+/B220+/CD43+ pro-B cells and found a pronounced effect of the antibody on pro-B cell survival/proliferation. Sorted pro-B cells (25,000) were incubated in the presence of L929 fibroblast cells, plus IL-7 and 1C10 or its isotype control antibody GL117 at 10 μg/ml 1C10 for 5 days, as described in Materials and Methods. Double the input cell number was recovered from the wells stimulated with CD40 mAb and IL-7, and there was no evidence of proliferation in the other wells, indicating a likely synergy between the CD40 stimulation and IL-7 in the induction of pro-B cell proliferation (Fig. 2A). Cells remained phenotypically pro-B (not shown).
Figure 2. Effects of CD40 ligation on BM pro-B cells.
(A) Numbers of cells recovered 5 days after sorting of pro-B cells onto L929 fibroblasts in the presence of anti-CD40 (1C10), isotype control mAb (GL117), or no antibody. (B and C) Lymphoid populations in the pro-B cell cultures with CD154-transfected (CD154L; right) or nontransfected (left) L929 cells, as visualized microscopically, and by forward- and side-angle light scatter (lymphoid population marked with an arrow; n=3). Clustering of sorted pro-B cells around CD154-transfected but not normal L929 cells after 5 days culture. (D) Numbers of pro-, pre-, immature, and mature B cells retrieved from cultures of sorted pro (upper)- or pre (lower)-B cells after 5 days incubation ± IL-7 or CD154-transfected fibroblasts. (E) Proliferation of CFSE-labeled, SCID-derived BM after incubation with anti-CD40 mAb (1C10) versus other stimuli. The upper panel shows actual CFSE fluorescence of 1C10-stimulated cells (red) versus cells stimulated with the isotype control mAb GL117 (black). The lower panel shows the mean percent cells divided in each culture as determined using FloJo software (n=3). (F) Effect of the injection of an agonistic CD40 antibody into SCID mice on BM B cell populations at 24 h postinjection (n=3).
It was remotely possible that the CD40 mAb might recognize a CD40 cross-reactive epitope on pro-B cells. We therefore repeated the study using normal L929 fibroblast cells and CD154 stably transfected fibroblasts [21]. After 5 days incubation with CD154-expressing fibroblasts, pro-B cells or their descendants were seen clustered closely with the fibroblast cells but not with normal L929 cells (Fig. 2B). In the presence of IL-7, lymphoid cell numbers in the CD154 cultures were much greater (Fig. 2B and C) when pro-B cells (Fig. 2D, upper panels) were seeded into wells but not when pre-B cells (Fig. 2D, lower panels) were seeded or when the L929 cells were not CD154-transfected (Fig. 2B–D). Seeded pro-B cells cultured with CD154L cells and IL-7 proliferated but remained largely, phenotypically undifferentiated (Fig. 2D).
As mentioned above, there is often redundancy within the TNF/TNFR superfamilies, and several ligands have more than one receptor or vice versa, although CD40 is the only ligand characterized for CD154. To confirm that the CD154-induced pro-B proliferation was acting via CD40 and to exclude contamination by more mature B cell types, we used BM from three SCID mice, which lack mature B and T cells. B cell development in SCIDs is blocked at the pro-B stage. SCID BM was labeled separately with CFSE [22, 23], incubated with CD40 mAb, 1C10 [20], or a combination of CD3 and CD28 antibodies capable of stimulating mature T cells. Percent cells divided (as determined using FloJo software) were significantly higher after CD40 stimulation than after control antibody stimulation, indicating proliferation of SCID BM cells, which we assume to be pro-B cells in response to CD40 stimulation (Fig. 2E). As further confirmation of the role of CD40 ligation in the induction of pro-B cell survival/proliferation, we injected three SCID mice with 500 μg agonistic anti-CD40 antibody 1C10 and removed BM 24 h later for flow cytometric assessment of pre-B cells. Treatment with agonistic CD40 antibody significantly increased pro-B cell (B220+CD43+) proportions 24 h later (Fig. 2F). As expected, there were no immature/mature phenotype cells (B220+IgM+) present in treated or untreated SCID mice.
Evidence for CD154 expression in BM
It is clear from the experiments described above that CD40 is expressed on the B and C fractions of pro-B cells derived from mouse BM and that ligation of CD40 induces proliferation and differentiation of these cells. These effects of CD40 ligation could, however, simply be an in vitro artifact. If CD40 expressed by pro-B cells were to have an important role in the control of B cell ontogeny in vivo, it would be expected that a ligand for CD40 would be detectable in the BM. Although there have been some reports of molecules derived from pathogens having CD40-binding ability [24, 25], CD154 is the only host molecule with recognized CD40-binding ability. We therefore assessed CD154 mRNA expression in the BM by RT-PCR. CD154 mRNA was reproducibly detectable in BM RNA extracts using the 2–4 and 2–5 primer pairs. An example of the 617-bp CD154 band given using the 2–5 primer pair is shown in Fig. 3A. In this example, there is little or no message for CD3 eta, which was included as a marker of T and NK cells; however, this lack of CD3 eta message was not consistent, and in three other PCR experiments, CD3 eta message was also readily detectable along with CD154. From these data then, it was not possible to determine with certainty whether the CD154 message was derived from stromal cells, from activated T or NK cells, or from another source such as activated platelets [26].
Figure 3. Expression of CD154 in the BM.
(A) RT-PCR analysis of BM derived from C57/bl6 (C) or BALB/c mice (B) or from spleens of BALB/c mice (S). Primers for Exons 2–5 were used to amplify CD154 cDNA. (B) Flow cytometric analysis of CD154 expression on CD45-negative cells using MR1 antibody; P = 0.033 versus CD154−/− control cells. Inset shows gating strategy; cells were gated as live and lymphoid using UV live/dead stain as singlets using forward-scatter-height versus forward-scatter-area (FSC-A) and as CD45-negative (CD45 vs. a FITC isotype control; n=3).
In an attempt to confirm expression of CD154 protein in the BM and also to determine the source of CD154 expression, we performed flow cytometric analysis using anti-CD154 antibody and CD45 as a marker of hematopoietic cells and precursors in WT C57/Bl6 and CD154−/− mice. CD154 was expressed by roughly 1% of CD45-negative BM cells of WT mice, indicating a likely stromal origin for CD154 (P=0.033; Fig. 3B). As expected, we did not detect CD154 expression in CD154−/− mice.
Evidence for CD154 function in BM hematopoiesis
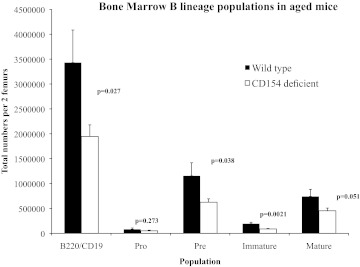
As mentioned above, neither CD154- nor CD40-deficient mice exhibit any gross hematopoietic defects [4, 12]. It is the case though that phenotypes caused by many artificial or natural genetic defects are often not manifest until a particular “challenge” arises. As it has been reported that a hematopoietic defect as a result of CD28 deficiency only became evident on aging [27], we assessed the effect of CD154 deficiency on hematopoietic populations of aged mice. Fig. 4 shows the absolute number of B lineage cells of different hematopoietic stages derived from two femurs of WT C57/Bl6 or CD154−/− mice of age 11–12 months. The figure shows combined results from two experiments with a total of nine WT and 10 CD154−/− mice/group. The results are consistent with those found in a preliminary experiment with three mice/group. There were significantly fewer B lineage cells overall in the BM of CD154−/− mice, and the deficiency was evident in every population downstream of pro-B cells, consistent with an effect of CD154 manifest at the pro-B cell stage. When a similar experiment was performed on young mice (aged 6–8 weeks), there was a significantly lower number of pro-B, pre-B, and immature B cells in the CD154−/− BM in one experiment but not in a second, and no significant difference was found when these data were combined (data not shown).
Figure 4. B lineage deficiencies in the BM of CD154−/− mice.
Total cell numbers of all B lineage cells (CD19+B220+) and B lineage subpopulations were determined in BM of aged (11–13 months) CD154−/− or C57/Bl6 mice by flow cytometry using markers described in Materials and Methods. Results derived from nine C57/Bl6 and 10 CD154−/− mice; P values determined by Student's t test.
Transplantation
We considered that CD154 might play a role in boosting B cell hematopoiesis at times of particular demand for B cells. One such experimental situation might be following lethal irradiation and BMT. We hypothesized that irradiation and transplantation using CD154-deficient donors or recipients might illuminate a potential role for CD154-CD40 interactions in driving B cell hematopoiesis and repopulation of the recipient with B lineage cells. It might also shed some light on the potential cellular source of CD154.
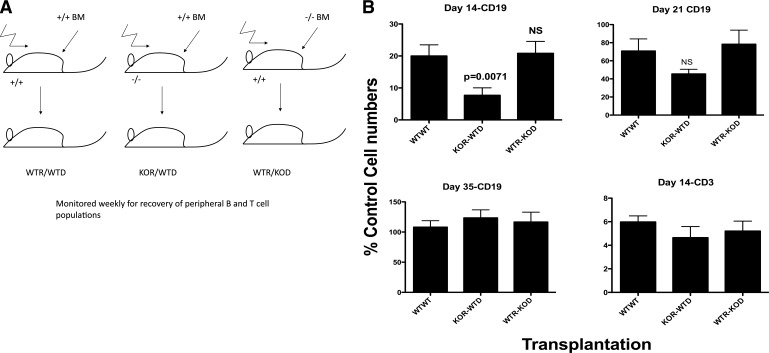
Normal C57/Bl6 (WT) or CD154−/− (KO) mice were used as BM donors or recipients following lethal irradiation. Thus, a mouse labeled KOR-WTD is a CD154−/− recipient that has received BM cells from a normal donor.
Fig. 5 shows the repopulation of peripheral blood with CD19 (B lineage) and CD3 (T lineage) cells at various time-points post-transplantation. The figure was derived by combining data from three separate transplantation experiments, such that the numbers of mice in each group are now WTR/WTD (n=12), KOR/WTD (n=13), and WTR/KOD (n=8). CD19 and CD3 numbers in the blood were calculated/μL and then normalized as a percentage of the CD19 or CD3 numbers found in normal control mouse blood taken and analyzed on the same day. Normal controls were of the same age as the experimental mice and were bled at the same time, as the process of bleeding itself could of course affect cell numbers. Reconstitution of the B cell population in the blood was significantly slower in the CD154−/− recipients given normal BM cells, as compared with WTRs receiving WT or CD154−/− cells, as manifest by the Day 14 figure (Fig. 5; KOR/WTD vs. WTR/WTD, P=0.007; KOR/WTD vs. WTR/KOD, P=0.0042; Mann Whitney test). T cell repopulation was not affected by the origin of donor or recipient cells (Fig. 5B, lower right). By Day 35 postirradiation/transplantation, all groups had normal numbers of B cells in the blood.
Figure 5. Defective B lineage recovery following irradiation and BMT of CD154−/− donor mice.
(A) WT (+/+) or CD154-deficient (−/−) mice were irradiated and used as recipients for +/+ or −/− donor BM. (B) CD19+ or CD3+ lymphoid cells were assessed at various time-points post-transplantation in the blood. Total cell counts of CD19 or CD3 cells were normalized as a percentage of those in untransplanted +/+ mice bled at the same time. Data derived from n = 12 WTR/WTD, n = 13 KOR/WTD, and n = 8 WTR/KOD mice. (B) Day 14, CD19 numbers; P = 0.0071 KOR/WTD versus WTR/WTD, and P = 0.0042 KOR/WTD versus WTR/KOD; Mann Whitney U test.
Similar experiments were performed wherein the numbers of B and T lineage cells present in the BM of transplanted mice were determined at 1 and 3 weeks post-transplantation. At 3 weeks post-transplantation, combining experiments to give 10 KOR/WTD and 10 WTR/KOD mice, mean B lineage numbers per two femurs were 1.82 × 107 and 2.52 × 107, respectively (P=0.21). Although this is not a statistically significant difference, the trend, again, is toward fewer B lineage cells in the BM of CD154−/− recipients in comparison with WTRs. There was no significant difference between the groups at 1 week post-transplantation.
Radio-resistant cells or their progeny in the BM express CD154 message
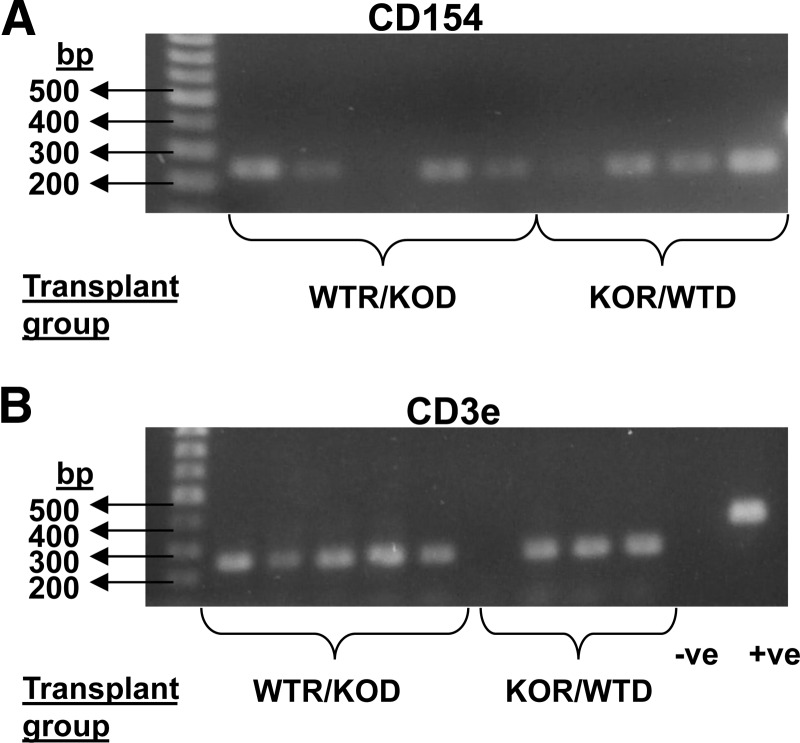
It appeared from the experiments above that CD154 expression by recipient mice was important in speeding B cell reconstitution. As most activated T cells would be removed by lethal irradiation, we decided to assess whether CD154 message could still be detected in irradiated recipients transplanted with CD154−/− donor marrow. BM was extracted from the transplant recipients described above 3 weeks after transplantation. Extracted RNA from these chimeric mice was subjected to RT-PCR for CD154 using the Exon 2–4 primer pair and CD3 eta, and CD154 message was detected in four out of five WTRs that had received BM from a CD154−/− donor. This was seen as a 234-bp band using the 2–4 primer pair (Fig. 6). As CD154 is not expressed in KO mice, the CD154 message detected must have been derived from the WTR mice and therefore, must be expressed in cells that have survived the lethal irradiation or progeny of these cells.
Figure 6. Presence of CD154 and CD3 eta message in BM of transplant recipients.
One percent agarose gel electrophoresis for PCR product from BM of WTR/KOD (n=5) and KOR/WTD (n=4) chimeric mice groups generated by irradiation and BMT. (A) Using CD154 primers (Exons 2–4). (B) Using primers for CD3e; +ve is a positive control using actin primers. Negative control was the reaction in the absence of cDNA template.
DISCUSSION
The CD40/CD154 receptor-ligand pair is pivotal in the control of immune responses, particularly against T-dependent antigens. The interaction also plays a role in T cell priming, including priming of CTLs, in the activation of APCs such as macrophages and maturation of DCs. Until now, however, the importance of this receptor-ligand pair in the control of hematopoiesis was only guessed at as a result of largely circumstantial evidence. For instance, it was known that ligation of CD40 on different human pre-B cells could have biological effects [16, 18] and even that multilineage CD34+ precursor cells express CD40 and differentiate into DCs in response to its ligation [28], but no likely source of CD154 had been identified in the BM, and CD40 expression on murine progenitors was still more controversial.
We show here that in contrast to the findings in many previous studies but in agreement with the study of Martínez-Barnetche et al. [19], CD40 is expressed on murine B cells from the earliest progenitors (pro-B cells) onward. More exactly, CD40 is expressed on the Fr B pro-B cells, which is the first population to express rag 1 and rag 2 gene transcripts and is the first population to have undergone some heavy chain rearrangement. CD40 expression is increased on the Fr C cells.
This CD40 expression by pro-B cells was found to be functional in that an agonistic CD40 antibody was able to boost pro-B cell proliferation, and incubation of pro-B cells with CD154-transfected fibroblasts and IL-7 gave rise to a clustering of the lymphoid cells around the CD154-expressing fibroblasts and enhanced survival of the pre-B cells in comparison with cells incubated with the parent L929 fibroblasts (Fig. 2A–D). SCID BM hematopoiesis is arrested at the pro-B stage, and SCID BM cells proliferated in response to CD40 ligation in vitro, in further confirmation of a functional role for CD40 on pro-B cells. Furthermore, in vivo administration of an agonistic CD40 antibody to SCID mice led to a rise in the percentage of pro-B cells in the BM 24 h later (Fig. 2F).
If CD40 ligation were to play a role in the control of hematopoiesis, it might be expected that CD154-expressing cells would be found in the BM. Activated T cells and platelets are the two main identified sources of CD154, and neither is normally present in large numbers in the BM.
RNA was extracted from BM cells, and RT-PCR was performed for the presence of CD154, CD3 eta, and actin (internal control) message. CD154 message was detected in many experiments, although usually also in the presence of CD3 eta message; on occasion, CD3 eta was not detectable, and CD154 message was. From these data, it was clear that CD154 is expressed in the BM, although it was impossible to rule out a T or NK cell source. This finding contradicts the statement from Martínez-Barnetche et al. [19], who reported that there was no CD154 expression in the BM, as CD154 message was not detected by PCR. However, a careful examination of their figure shows that CD154 message was not detected even in the spleen or LN samples from normal mice, where clearly, at least some CD154 expression might be expected. Consistent with the detection of CD154 mRNA in the BM, we also detected expression of CD154 protein on a small proportion of CD45-negative (nonhematopoietic) cells in the BM.
Having identified expression of CD40 on pro-B Fr B and C cells, along with CD154 expression in the BM and in vitro effects of CD40 ligation on Fr B and C pro-B cells, we attempted to determine whether we could identify a functional effect of CD154 deficiency on B cell hematopoiesis in vivo. In neither CD154 nor CD40 KO mice have any gross defects in hematopoiesis been reported [4, 12]. Obviously, B cell hematopoiesis is able to proceed in the absence of CD40 or CD154. We hypothesized that the CD154-CD40 system may be used as a boost to “emergency” B cell hematopoiesis. The G-CSF plays a similar role in emergency granulopoiesis [29].
A defect in T cell hematopoiesis has been described in CD28-deficient mice, but this only becomes evident on aging [27]. We therefore assessed BM B lineage numbers in CD154−/− and normal controls of approximately 1 year old. There were significantly lower numbers of BM B lineage cells in CD154-deficient mice at 1 year old in comparison with normal control mice (Fig. 4). These deficiencies were manifest on all populations downstream of pro-B cells, consistent with an effect of BM CD154 expression on pro-B populations and also indicating an effect in addition to any possible effect that CD154 expression, outside of the BM, might have on mature B cell numbers via induction of proliferation in lymphoid germinal centers, followed by migration of mature B cells to the BM.
Aged mice might be considered to be under stress, and another potential stress, wherein B cell hematopoiesis might naturally be boosted, would be following irradiation-induced lymphopenia. CD154-deficient or WT mice were used as donors or recipients of BMT following lethal irradiation of the recipients. B and T lineage reconstitution was assessed in peripheral blood, and there were significantly fewer B lineage cells (CD19+) in CD154-deficient recipients at 14 days post-BMT than were found in WTRs, even when donor marrow was from CD154-deficient mice. No such effect on CD3 numbers was seen (Fig. 5), indicating a degree of specificity for B lineage cells and also acting as an internal control showing that experimental variation in donor cell numbers injected was not responsible for variable rates of reconstitution. Although eventually, all mice achieved normal levels of B and T lineage cells, the relative delay in recovery in CD154-deficient recipients points not only to a role of CD154 and presumably CD40 interactions in boosting B cell hematopoiesis post-transplantation but also suggests that the CD154 playing this role is of recipient origin and therefore, expressed by radio-resistant cells. Consistent with this hypothesis, CD154 message was identified in the BM of WTR mice that had been transplanted 3 weeks earlier with CD154−/− marrow after lethal irradiation (Fig. 6). This CD154 must therefore have been of recipient origin and must have been expressed by cells that had survived the lethal irradiation or their progeny. Although some CD154−/− recipients that had received WT marrow also expressed CD154 message, it appears likely that this was donor T cell-derived, as CD154 bands coincided with message for CD3 eta (Fig. 6).
Stromal cells are known to provide support to B hematopoietic cells and are highly radio-resistant, and activated platelets and T cells are known to express CD154. It is unlikely that any platelets of recipient origin would remain at 3 weeks postirradiation; platelet counts drop rapidly in the first week after irradiation and BMT and then recover over the next 2 weeks as reconstitution when donor platelets occur [30]. Thus, most platelets in the irradiated recipients would be of donor origin at 3 weeks post-BMT. Although T cells in the periphery are also severely depleted following irradiation, some radio-resistant T cells remain and can indeed have functional effects following BMT [31]. T cells are therefore a possible source of the CD154 expressed in the BM and appear to play a role in boosting B cell hematopoiesis. In most RT-PCR experiments, CD3 eta message was identified as well as message for CD154, even after adherence steps had been used to attempt to enrich stromal cells or after cell sorting to remove CD3-expressing cells (not shown). In one experiment, however, we identified CD154 message in the absence of detectable CD3 eta message (Fig. 3). From RT-PCR data, it is difficult to conclude that CD154 message was derived from stromal cells or cells of other lineages, although flow cytometry did identify CD154 expression on CD45-negative cells, consistent with stromal expression (Fig. 3B). We are currently working to identify the cellular source of CD154 involved in boosting B cell hematopoiesis in the BM, with stromal cells and possibly T cells as the prime suspects.
We are not alone in identifying functional effects of CD154 on B cell hematopoiesis, although other groups have used more artificial systems. Our data overall agree with and in part contradict the findings of the Moreno group [19]. This group showed, like us, that CD40 expression on murine pre-B cells was not restricted to pre-B cells onward but that some expression could be found on pro-B cells. They also showed that transgenic expression of CD154 by B cells under the control of an Ig κ promoter gave rise to a progressive loss of peripheral B cell numbers from 2 weeks onward, along with the arrest of B cell development in the BM but enhancement of development in the liver at the pro-B cell stage. More specifically, they found an absence of Fr C of pro-B cells in the BM. Of course, there are differences between our two systems. The extent of CD40 cross-linking by CD154 expressed by transgenic B cells might differ considerably from that naturally achieved by CD154-expressing BM cells. The location, the cell types acted upon, and the duration of the effect would likely be quite different. Indeed, the effects of CD154 stimulation on more mature cells can vary considerably, depending on intensity and duration of signaling. For instance, it has been shown that sustained, as opposed to transient stimulation through CD154, can inhibit B cell terminal differentiation [21, 32], and we have shown that stable transfection of allogeneic donor cells with CD154 can have inhibitory effects on alloantibody responses against the transfected cells following transplantation [33], and in most instances, CD154 expression has been shown to have stimulatory effects on immune responses [5, 10, 26]. Whether the loss of Fr C in the BM of the transgenic mice produced by the Moreno group [19] represented an arrest of differentiation or an exit of precursor cells to other sites was not addressed specifically by this group, although notably, the authors did observe hematopoietic foci in the spleens of their mice, suggesting perhaps that the loss of Fr C in BM was at least partially a result of migration. The Moreno group concluded [19] that CD40 ligation, during B cell ontogeny, had a negative regulatory effect on hematopoiesis in the marrow. In contrast, we believe that in normal animals, CD40 ligation during ontogeny has a positive role to play in enhancing proliferation and subsequent differentiation of pro-B cells at times of increased need. As mentioned above, this positive role would be partially consistent with the findings of the Moreno group [19] in that pro-B cell numbers in newborn livers were clearly enhanced by CD154 transgenic expression. In addition, our data are consistent with the findings of Funakoshi et al. [17], who found that administration of soluble rCD154 after BMT in mice was able to accelerate not only pre-B cell development and peripheral B cell numbers but also T cell, granulocyte, and megakaryocyte numbers. The Funakoshi et al. data [17] would point to a role for CD40-CD154 interactions in control of ontogeny of various hematopoietic populations. Although we saw no difference in the speed of T cell (Fig. 5) or granulocyte (not shown) reconstitution in CD154−/−-deficient BMT recipients, we did not assay for other hematopoietic populations. Of course, as with the Moreno work [19], the soluble CD40L, which was injected by Funakoshi et al. [17], could have effects on more cells and on cells at different locations from any effects of natural, BM-expressed CD154. It is likely that some of the effects were secondary effects (induction of cytokine and CSF secretion, etc.), which would not be mediated by localized, BM-expressed CD154. Consistent with this possibility, small doses of CD40L or CD40 antibody can be lethal in irradiated mice, probably because of the induction of IFN production and the lack of a “sink” for the cytokine [34].
Allogeneic BMT is an effective therapy for XHIM syndrome [35–40], the majority of cases of which is caused by CD154 mutations, leading to a lack of binding to CD40. It might be predicted that should CD154-CD40 interactions play the same role in human B cell ontogeny that we believe they play in the mouse, B cell reconstitution after BMT would be slower in these patients than in those with normal CD154 expression and protein. Unfortunately, although there are several published reports of successful treatment of XHIM by BMT, most do not detail B cell reconstitution. We could find only two reports describing B cell reconstitution after BMT of these patients. Duplantier et al. [37] found that peripheral B cell numbers took some 20 months to return to normal following fully matched sibling BMT of an 8-month-old boy with XHIM, and Bordigoni et al. [39] found that in a 10-year-old boy, B cell levels were normal 10 months after BMT. Looking at a large number of older, non-XHIM patients, Storek et al. [41] reported that on average, B cell numbers were normal by 1 year after transplantation. From these data, therefore, it is not possible to determine whether there is, in general, a slower recovery of B cell numbers after irradiation of XHIM patients in comparison with non-XHIM patients.
Overall, our data strongly support a role for CD154-CD40 interactions in the control of murine B cell hematopoiesis. The precise cellular source of CD154 and the mechanism by which its expression might be regulated are currently under investigation.
ACKNOWLEDGMENTS
J.C. was supported by a Wellcome Trust grant (061268 and Yorkshire Cancer Research S286), and S.C. and T.J. were supported by a grant from the Biotechnology and Biological Sciences Research Council (BBSRC; UK; 50-S12061). Much of the flow cytometry was performed in the Flow Cytometry Core Facility of the University of Sheffield Medical School. This paper is in memory of Anne-Marie Buckle who sadly died before submission.
Footnotes
- A260/A280
- absorbance at 260/280 nm
- BM
- bone marrow
- BMT
- bone marrow transplantation
- CD40L/CD154L
- CD40/CD154 ligand
- Cs
- cesium
- Fr
- fraction
- Gy
- gray
- KO
- knockout
- KOD
- knockout donor
- KOR
- knockout recipient
- pre-B cell
- precursor B cell
- pro-B cell
- progenitor B cell
- V-D-J
- variable-diversity-joining
- WTD
- WT donor
- WTR
- WT recipient
- XHIM
- X-linked hyper IgM
AUTHORSHIP
All authors performed experiments to provide data for the manuscript. A.W.H. and J.C. wrote the manuscript.
REFERENCES
- 1. Hardy R. R., Carmack C. E., Shinton S. A., Kemp J. D., Hayakawa K. (1991) Resolution and characterization of pro-B and pre-pro-B cell stages in normal mouse bone marrow. J. Exp. Med. 173, 1213–1225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. von Freeden-Jeffry U., Vieira P., Lucian L. A., McNeil T., Burdach S. E., Murray R. (1995) Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J. Exp. Med. 181, 1519–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Peschon J. J., Morrissey P. J., Grabstein K. H., Ramsdell F. J., Maraskovsky E., Gliniak B. C., Park L. S., Ziegler S. F., Williams D. E., Ware C. B., Meyer J. D., Davison B. L. (1994) Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J. Exp. Med. 180, 1955–1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu J., Foy T. M., Laman J. D., Elliott E. A., Dunn J. J., Waldschmidt T. J., Elsemore J., Noelle R. J., Flavell R. A. (1994) Mice deficient for the CD40 ligand. Immunity 1, 423–431 [DOI] [PubMed] [Google Scholar]
- 5. Armitage R. J., Fanslow W. C., Strockbine L., Sato T. A., Clifford K. N., Macduff B. M., Anderson D. M., Gimpel S. D., Davis-Smith T., Maliszewski C. R., et al. (1992) Molecular and biological characterization of a murine ligand for CD40. Nature 357, 80–82 [DOI] [PubMed] [Google Scholar]
- 6. Heath A. W., Chang R., Harada N., Santos-Argumedo L., Gordon J., Hannum C., Campbell D., Shanafelt A. B., Clark E. A., Torres R., et al. (1993) Antibodies to murine CD40 stimulate normal B lymphocytes but inhibit proliferation of B lymphoma cells. Cell. Immunol. 152, 468–480 [DOI] [PubMed] [Google Scholar]
- 7. Bennett S. R., Carbone F. R., Karamalis F., Flavell R. A., Miller J. F., Heath W. R. (1998) Help for cytotoxic-T-cell responses is mediated by CD40 signaling. Nature 393, 478–480 [DOI] [PubMed] [Google Scholar]
- 8. Ridge J. P., Di Rosa F., Matzinger P. (1998) A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature 393, 474–478 [DOI] [PubMed] [Google Scholar]
- 9. Schoenberger S. P., Toes R. E., van der Voort E. I., Offringa R., Melief C. J. (1998) T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature 393, 480–483 [DOI] [PubMed] [Google Scholar]
- 10. Grewal I. S., Flavell R. A. (1998) CD40 and CD154 in cell-mediated immunity. Annu. Rev. Immunol. 16, 111–135 [DOI] [PubMed] [Google Scholar]
- 11. Stout R. D., Suttles J., Xu J., Grewal I. S., Flavell R. A. (1996) Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J. Immunol. 156, 8–11 [PubMed] [Google Scholar]
- 12. Kawabe T., Naka T., Yoshida K., Tanaka T., Fujiwara H., Suematsu S., Yoshida N., Kishimoto T., Kikutani H. (1994) The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity 1, 167–178 [DOI] [PubMed] [Google Scholar]
- 13. Santos-Argumedo L., Gordon J., Heath A. W., Howard M. (1994) Antibodies to murine CD40 protect normal and malignant B cells from induced growth arrest. Cell. Immunol. 156, 272–285 [DOI] [PubMed] [Google Scholar]
- 14. Castigli E., Young F., Carossino A. M., Alt F. W., Geha R. S. (1996) CD40 expression and function in murine B cell ontogeny. Int. Immunol. 8, 405–411 [DOI] [PubMed] [Google Scholar]
- 15. Hasbold J., Johnson-Leger C., Atkins C. J., Clark E. A., Klaus G. G. (1994) Properties of mouse CD40: cellular distribution of CD40 and B cell activation by monoclonal anti-mouse CD40 antibodies. Eur. J. Immunol. 24, 1835–1842 [DOI] [PubMed] [Google Scholar]
- 16. Uckun F. M., Gajl-Peczalska K., Myers D. E., Jaszcz W., Haissig S., Ledbetter J. A. (1990) Temporal association of CD40 antigen expression with discrete stages of human B-cell ontogeny and the efficacy of anti-CD40 immunotoxins against clonogenic B-lineage acute lymphoblastic leukemia as well as B-lineage non-Hodgkin's lymphoma cells. Blood 76, 2449–2456 [PubMed] [Google Scholar]
- 17. Funakoshi S., Taub D. D., Anver M. R., Raziuddin A., Asai O., Reddy V., Rager H., Fanslow W. C., Longo D. L., Murphy W. J. (1997) Immunologic and hematopoietic effects of CD40 stimulation after syngeneic bone marrow transplantation in mice. J. Clin. Invest. 99, 484–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saeland S., Duvert V., Moreau I., Banchereau J. (1993) Human B cell precursors proliferate and express CD23 after CD40 ligation. J. Exp. Med. 178, 113–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Martínez-Barnetche J., Madrid-Marina V., Flavell R. A., Moreno J. (2002) Does CD40 ligation induce B cell negative selection? J. Immunol. 168, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 20. Heath A. W., Wu W. W., Howard M. C. (1994) Monoclonal antibodies to murine CD40 define two distinct functional epitopes. Eur. J. Immunol. 24, 1828–1834 [DOI] [PubMed] [Google Scholar]
- 21. Randall T. D., Heath A. W., Santos-Argumedo L., Howard M. C., Weissman I. L., Lund F. E. (1998) Arrest of B lymphocyte terminal differentiation by CD40 signaling: mechanism for lack of antibody-secreting cells in germinal centers. Immunity 8, 733–742 [DOI] [PubMed] [Google Scholar]
- 22. Foster R. A., Carlring J., McKendrick M. W., Lees A., Borrow R., Read R. C., Heath A. W. (2009) Evidence of a functional B-cell immunodeficiency in adults who experience serogroup C meningococcal disease. Clin. Vaccine Immunol. 16, 692–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lyons A. B., Parish C. R. (1994) Determination of lymphocyte division by flow cytometry. J. Immunol. Methods 171, 131–137 [DOI] [PubMed] [Google Scholar]
- 24. Imai S., Tezuka H., Furuhashi Y., Muto R., Fujita K. (2001) A factor of inducing IgE from a filarial parasite is an agonist of human CD40. J. Biol. Chem. 276, 46118–46124 [DOI] [PubMed] [Google Scholar]
- 25. Pido-Lopez J., Whittall T., Wang Y., Bergmeier L. A., Babaahmady K., Singh M., Lehner T. (2007) Stimulation of cell surface CCR5 and CD40 molecules by their ligands or by HSP70 up-regulates APOBEC3G expression in CD4(+) T cells and dendritic cells. J. Immunol. 178, 1671–1679 [DOI] [PubMed] [Google Scholar]
- 26. Henn V., Slupsky J. R., Grafe M., Anagnostopoulos I., Forster R., Muller-Berghaus G., Kroczek R. A. (1998) CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 391, 591–594 [DOI] [PubMed] [Google Scholar]
- 27. Gray Parkin K., Stephan R. P., Apilado R. G., Lill-Elghanian D. A., Lee K. P., Saha B., Witte P. L. (2002) Expression of CD28 by bone marrow stromal cells and its involvement in B lymphopoiesis. J. Immunol. 169, 2292–2302 [DOI] [PubMed] [Google Scholar]
- 28. Flores-Romo L., Bjorck P., Duvert V., van Kooten C., Saeland S., Banchereau J. (1997) CD40 ligation on human cord blood CD34+ hematopoietic progenitors induces their proliferation and differentiation into functional dendritic cells. J. Exp. Med. 185, 341–349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basu S., Hodgson G., Zhang H. H., Katz M., Quilici C., Dunn A. R. (2000) “Emergency” granulopoiesis in G-CSF-deficient mice in response to Candida albicans infection. Blood 95, 3725–3733 [PubMed] [Google Scholar]
- 30. Molineux G., Hartley C. A., McElroy P., McCrea C., McNiece I. K. (1996) Megakaryocyte growth and development factor stimulates enhanced platelet recovery in mice after bone marrow transplantation. Blood 88, 1509–1514 [PubMed] [Google Scholar]
- 31. Bagley J., Tian C., Sachs D. H., Iacomini J. (2002) T cells mediate resistance to genetically modified bone marrow in lethally irradiated recipients. Transplantation 74, 1454–1460 [DOI] [PubMed] [Google Scholar]
- 32. Lee B. O., Haynes L., Eaton S. M., Swain S. L., Randall T. D. (2002) The biological outcome of CD40 signaling is dependent on the duration of CD40 ligand expression: reciprocal regulation by interleukin (IL)-4 and IL-12. J. Exp. Med. 196, 693–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCormick A. L., Santos-Argumedo L., Thomas M. S., Heath A. W. (1999) Cell surface expression of CD154 inhibits alloantibody responses: a mechanism for the prevention of autoimmune responses against activated T cells? Cell. Immunol. 195, 157–161 [DOI] [PubMed] [Google Scholar]
- 34. Hixon J. A., Blazar B. R., Anver M. R., Wiltrout R. H., Murphy W. J. (2001) Antibodies to CD40 induce a lethal cytokine cascade after syngeneic bone marrow transplantation. Biol. Blood Marrow Transplant. 7, 136–143 [DOI] [PubMed] [Google Scholar]
- 35. Kato T., Tsuge I., Inaba J., Kato K., Matsuyama T., Kojima S. (1999) Successful bone marrow transplantation in a child with X-linked hyper-IgM syndrome. Bone Marrow Transplant. 23, 1081–1083 [DOI] [PubMed] [Google Scholar]
- 36. Kawai S., Sasahara Y., Minegishi M., Tsuchiya S., Fujie H., Ohashi Y., Kumaki S., Konno T. (1999) Immunological reconstitution by allogeneic bone marrow transplantation in a child with the X-linked hyper-IgM syndrome. Eur. J. Pediatr. 158, 394–397 [DOI] [PubMed] [Google Scholar]
- 37. Duplantier J. E., Seyama K., Day N. K., Hitchcock R., Nelson R. P. J., Ochs H. D., Haraguchi S., Klemperer M. R., Good R. A. (2001) Immunologic reconstitution following bone marrow transplantation for X-linked hyper IgM syndrome. Clin. Immunol. 98, 313–318 [DOI] [PubMed] [Google Scholar]
- 38. Leone V., Tommasini A., Andolina M., Runti G., De Vonderweid U., Campello C., Notarangelo L. D., Ventura A. (2002) Elective bone marrow transplantation in a child with X-linked hyper-IgM syndrome presenting with acute respiratory distress syndrome. Bone Marrow Transplant. 30, 49–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bordigoni P., Auburtin B., Carret A. S., Schuhmacher A., Humbert J. C., Le Deist F., Sommelet D. (1998) Bone marrow transplantation as treatment for X-linked immunodeficiency with hyper-IgM. Bone Marrow Transplant. 22, 1111–1114 [DOI] [PubMed] [Google Scholar]
- 40. Scholl P. R., O'Gorman M. R., Pachman L. M., Haut P., Kletzel M. (1998) Correction of neutropenia and hypogammaglobulinemia in X-linked hyper-IgM syndrome by allogeneic bone marrow transplantation. Bone Marrow Transplant. 22, 1215–1218 [DOI] [PubMed] [Google Scholar]
- 41. Storek J., Dawson M. A., Storer B., Stevens-Ayers T., Maloney D. G., Marr K. A., Witherspoon R. P., Bensinger W., Flowers M. E., Martin P., Storb R., Appelbaum F. R., Boeckh M. (2001) Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood 97, 3380–3389 [DOI] [PubMed] [Google Scholar]