Executive Summary
Objective
The objective of this health technology policy assessment was to determine the effectiveness of spinal cord stimulation (SCS) to manage chronic intractable neuropathic pain and to evaluate the adverse events and Ontario-specific economic profile of this technology.
Clinical Need
SCS is a reversible pain therapy that uses low-voltage electrical pulses to manage chronic, intractable neuropathic pain of the trunk or limbs. Neuropathic pain begins or is caused by damage or dysfunction to the nervous system and can be difficult to manage.
The prevalence of neuropathic pain has been estimated at about 1.5% of the population in the United States and 1% of the population in the United Kingdom. These prevalence rates are generalizable to Canada.
Neuropathic pain is extremely difficult to manage. People with symptoms that persist for at least 6 months or who have symptoms that last longer than expected for tissue healing or resolution of an underlying disease are considered to have chronic pain. Chronic pain is an emotional, social, and economic burden for those living with it. Depression, reduced quality of life (QOL), absenteeism from work, and a lower household income are positively correlated with chronic pain.
Although the actual number is unknown, a proportion of people with chronic neuropathic pain fail to obtain pain relief from pharmacological therapies despite adequate and reasonable efforts to use them. These people are said to have intractable neuropathic pain, and they are the target population for SCS.
The most common indication for SCS in North America is chronic intractable neuropathic pain due to failed back surgery syndrome (FBSS), a term that describes persistent leg or back and leg pain in patients who have had back or spine surgery. Neuropathic pain due to complex regional pain syndrome (CRPS), which can develop in the distal aspect of a limb a minor injury, is another common indication. To a lesser extent, chronic intractable pain of postherpetic neuralgia, which is a persistent burning pain and hyperesthesia along the distribution of a cutaneous nerve after an attack of herpes zoster, is also managed with SCS.
For each condition, SCS is considered as a pain management therapy only after conventional pain therapies, including pharmacological, nonpharmacological, and surgical treatments, if applicable, have been attempted and have failed.
The Technology
The SCS technology consists of 3 implantable components: a pulse generator, an extension cable, and a lead (a small wire). The pulse generator is the power source for the spinal cord stimulator. It generates low-voltage electrical pulses. The extension cable connects the pulse generator to the lead. The lead is a small, insulated wire that has a set of electrodes at one end. The lead is placed into the epidural space on the posterior aspect of the spinal cord, and the electrodes are positioned at the level of the nerve roots innervating the painful area. An electrical current from the electrodes induces a paresthesia, or a tingling sensation that masks the pain.
Before SCS is initiated, candidates must have psychological testing to rule out major psychological illness, drug habituation, and issues of secondary gain that can negatively influence the success of the therapy. Successful candidates will have a SCS test stimulation period (trial period) to assess their responsiveness to SCS. The test stimulation takes about 1 week to complete, and candidates who obtain at least 50% pain relief during this period are deemed suitable to receive a permanent implantation of a spinal cord stimulator
Review Strategy
The Medical Advisory Secretariat (MAS) reviewed all published health technology assessments of spinal cord stimulation. Following this, a literature search was conducted from 2000 to January, 2005 and a systematic review of the literature was completed. The primary outcome for the systematic review was pain relief. Secondary outcomes included functional status and quality of life. After applying the predetermined inclusion and exclusion criteria, 2 randomized controlled trials (MAS level 2 evidence), and 2 prospective non-randomized controlled trials with a before-and-after-treatment study design (MAS level 3a evidence) were retrieved and reviewed.
Summary of Findings
The authors of 6 health technology assessments concluded that evidence exists to support the effectiveness of SCS to decrease pain in various neuropathic pain syndromes. However, the quality of this evidence varied among reports from weak to moderate.
The systematic review completed by MAS found high quality level 2 evidence that SCS decreases pain and level 3a evidence that it improves functional status and quality of life in some people with neuropathic pain conditions. The rate of technical failures was approximately 11%, which included electrode lead migration and/or malposition. Procedural complications included infection and dural puncture; each occurred at a rate of 1.2%.
Conclusions
SCS may be considered for patients with chronic, neuropathic pain for whom standard pain treatments have failed and when there is no indication for surgical intervention to treat the underlying condition.
Objective
The purpose of this health technology assessment was to determine the effectiveness of spinal cord stimulation (SCS) as a pain management therapy for chronic, intractable neuropathic pain and to evaluate the adverse events and Ontario-specific economic profile of this technology.
Background
Clinical Need: Target Population and Condition
SCS is a form of neuromodulation used to manage chronic, intractable neuropathic pain of the trunk and limbs. (1;2) Pain is defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.” (3) Neuropathic pain is a specific type of pain that is characterized by unique symptoms and initiated or caused by damage or dysfunction to the nervous system. (3-5) Neuropathic pain is often described as shooting, burning, or lancing. (4;6-8). In some cases of neuropathic pain, actual nerve damage is not always apparent, despite symptoms indicating neurological dysfunction. (4;9)
The prevalence of neuropathic pain has been estimated at about 1.5% of the population in the United States and 1% of the population in the United Kingdom. (4;10) Although the actual number is unknown, a proportion of people with chronic neuropathic pain fail to obtain pain relief from pharmacological therapies despite adequate and reasonable efforts to use them. These people are said to have intractable (11) neuropathic pain, and they are the target population for SCS.
Neuropathic pain is extremely difficult to manage. People with symptoms that persist for at least 6 months or who have symptoms that last longer than expected for tissue healing or resolution of an underlying disease are considered to have chronic pain. (4;12;13) Chronic pain is an emotional, social, and economic burden for those living with it. Depression, reduced quality of life (QOL), absenteeism from work, and a lower household income are positively correlated with chronic pain. (13-16)
Meana et al. (16) reported that the prevalence of depression among Canadians with chronic pain was twice that experienced by those without chronic pain. It was twice as high among people younger than 65 years with chronic pain compared with people aged 65 years and older. Currie and Wang (15) reported a more than 6-fold (6.2; 95% confidence interval [CI], 5.2–7.6) increase in depression in Canadians with chronic back pain compared to those without. Moulin et al. (13) found that Canadians missed, on average, 9.3 working days (95% CI, 4.7–13.7) due to chronic pain; 16 days (95% CI, 5.1–26.9) if the pain was severe. Furthermore, people with chronic pain had significantly lower incomes compared with those without chronic pain. (13) Regarding QOL, Moulin et al. (13) found that 49% of Canadians reported great difficulty attending social and family events, 61% were unable to participate in their usual recreational activities, and 58% were unable to carry out their daily activities at home.
Neuropathic pain is associated with medical conditions that are etiologically heterogeneous. Some of these conditions are listed in Table 1. (4) However, each medical condition shares common symptoms associated with neuropathic pain, such as no visible injury, a paradoxical combination of sensory loss and hypersensitivity in the painful area, paroxysms of pain, and a gradual increase of pain following repetitive stimulation. (17) Because of this, it has been proposed that neuropathic pain may be explained by the same or similar mechanisms despite the medical condition. (17)
Table 1: Medical Conditions Associated With Neuropathic Pain.
| Medical Condition |
|---|
| Failed back surgery syndrome |
| Complex regional pain syndrome, Type I and II |
| Postherpetic neuralgia |
| Trigeminal neuralgia |
| HIV-associated pain |
| Pain after amputation |
| Pain after stroke |
| Multiple sclerosis |
| Cancer-related pain |
| Diabetic neuropathy |
| Spinal cord injury |
Indications for Spinal Cord Stimulation
Of the medical conditions listed in Table 1, neuropathic pain from failed back surgery syndrome (FBSS) is the most common indication for SCS in North America. Neuropathic pain due to complex regional pain syndrome (CRPS) is another common indication. To a lesser extent, neuropathic pain due to postherpetic neuralgia, persistent burning pain and hyperesthesia along the distribution of a cutaneous nerve which can occur after an attack of herpes zoster, is also managed with SCS. For each condition, SCS is considered only after conventional pain therapies, including pharmacological, nonpharmacological, and surgical treatments, if applicable, have been tried and have failed.
Less commonly in North America, SCS has been used to manage ischemic pain of peripheral vascular disease and angina.
Failed Back Surgery Syndrome
FBSS is a generalized term used to describe persistent low back pain and leg pain in patients who have not had a successful result with back or spine surgery. (18;19) Those people whose leg pain is greater than their back pain are suitable candidates for SCS. About 15% to 40% of patients will have chronic back and limb pain after undergoing lumbar surgery. (20)
Complex Regional Pain Syndrome
CRPS is a neuropathic pain condition that develops in the distal aspect of a limb, usually after an injury, which may be even minor in nature. However, 6% to 10% of the cases are initiated spontaneously with no precipitating injury. (5;5;21) There are 2 types of the syndrome: I and II. Although the salient criterion differentiating them is a definable nerve injury for Type II, the symptoms of both types are the same. The pathophysiology of this pain syndrome is not well understood; therefore, treatment is focused on managing the symptoms. (22). Diagnostic criteria include these (3;22):
An initiating injury (for example a minor fracture) or cause of immobilization (for example, a stroke) for Type I; and a known nerve injury for Type II
Spontaneous pain or evoked pain (allodynia /hyperalgesia) that is not limited to the area of a single peripheral nerve and is disproportionate to the initiating event
Evidence (past or present) of edema (swelling), skin blood flow abnormality, or abnormal sudomotor (sweat gland) activity in the region of the pain since the initiating event
Exclusion of a medical condition that would explain the pain and dysfunction
Treatment for CRPS is focused on restoring functional capacity through physiotherapy and/or occupational therapy, improving QOL by fostering coping skills through psychological therapy, and managing pain to provide relief and encourage rehabilitation. It has been suggested that if a patient has failed all conservative pain management techniques and is not progressing in rehabilitation by 12 to 16 weeks, then it is reasonable to consider SCS. (5;23)
CRPS most commonly affects people aged 36 to 42 years and is diagnosed more often in women than in men. The upper extremity is involved 44% to 61% of the time, and the lower extremity is affected 39% to 61% of the time. It is estimated that it occurs at a rate of 16% after a fracture, 10% to 29% after a strain or sprain, 3% to 24% after surgery, and 8% after a crash injury. (5;23) The prevalence of CRPS Type I is estimated at 20.57 cases per 100,000 people. The incidence rate is 5.46 per 100,00 person-years at risk. (24)
Postherpetic Neuralgia
Post herpetic neuralgia is persistent pain, which can occur after an attack of the herpes zoster virus. Herpes zoster, also known as shingles, is caused by the reactivation of the varicella zoster virus that has lain latent since primary infection. Antivirals can reduce the pain if they are given early in the course of the illness. (25) Several drugs, including gabapentin, tricyclic antidepressants and opioids, are used to manage chronic pain due to postherpetic neuralgia.
The lifetime risk of herpes zoster is 10% to 30%, and the incidence increases with age. About 20% of those older than 50 years will experience pain (post herpetic neuralgia) 6 months after the onset of a herpes zoster rash. (25). More than 60% of herpes zoster cases in Canada are in adults older than 45 years, and the highest rate is in adults aged 65 years and older. Brisson et al. (26) estimated the incidence of herpes zoster in Canada using physicians’ consultation rates for herpes zoster infections. In adults 45 to 64 years of age, the mean consultation rate was 423 per 100,000 population years, and for adults aged 65 years and older, the rate was 812 per 100,000 population years.
Existing Treatments Other Than Technology Being Reviewed
The goal of pain management is to make pain tolerable and to improve functionality. (27) Pain management includes multiple therapies categorized into pharmacological, nonpharmacological, and surgical. (28) Generally, a treatment progresses from therapies that are less invasive and have minor side effects to those that are more invasive. (29) Often, multiple medications for pain relief will be combined and used with nonpharmacological therapies. (27) The drug therapies for neuropathic pain recommended by the council of the College of Physicians and Surgeons of Ontario (CPSO) and common nonpharmacological therapies are examined in this review.
Pharmacological Therapy for Neuropathic Pain
The CPSO (27) ratified evidence-based recommendations for pharmacological treatment of neuropathic pain on November 3, 2000. (See Appendix 2.) These recommendations included anticonvulsants, antidepressants, oral drugs with local anesthetic type properties, opioids, topical capsaicin, and intravenous regional sympathetic blocks.
The CPSO’s recommendations recognized that neuropathic pain usually requires multidrug therapy and that therapies should be started sequentially not simultaneously. The guidelines suggest that first-line pharmacotherapy may include tricyclic antidepressants and/or anticonvulsants as adjuvant medications. The recommendations also note that opioids may be used in selected patients, but not as a first-line therapy. (27)
Of the pain medications recommended in the CPSO guidelines, only the opioid analgesics and capsaicin are approved as pain treatments by the Health Protection Branch of Health Canada. Anticonvulsants, antidepressants, and oral drugs with local anesthetic properties are considered adjuvant pain therapies. Adjuvant pain therapies are those with a primary treatment indication other than pain management.
Anticonvulsants and Antidepressants
The CPSO (27) has determined that strong evidence from a least 1 systematic review of multiple well-designed randomized controlled trials (RCTs) (CPSO level 1 evidence)(See Appendix 2) exists for anticonvulsants and antidepressants in different neuropathic syndromes (Appendix 2). The mechanism by which anticonvulsants and antidepressants control pain is unknown.(30)
Anticonvulsants
Gabapentin, carbamazepine, clonazepam, sodium valproate, and phenytoin have been evaluated as treatments neuropathic pain. (8) Of these, gabapentin was ranked as a first-line treatment, and carbamazepine as a second-line treatment, by an expert panel at the fourth international conference on the mechanisms and treatment of neuropathic pain. (9) In a systematic review of anticonvulsant drugs for acute and chronic pain, Wiffen et al. (8) estimated that 66% (95% CI 61%–71%) of patients who receive either gabapentin or carbamazepine for neuropathic pain will obtain good pain relief; however, they found no clear therapeutic advantage of gabapentin over carbamazepine.
How gabapentin works to relieve pain has not been established. (31) Common adverse effects of gabapentin include dizziness and, in the elderly, balance and gait problems, and cognitive impairment. Adjusting the dose may be required. (32) Gabapentin has an excellent tolerability and safety profile and a lack of reported drug interactions. (9) It is eliminated solely by renal excretion as an unchanged drug. People with impaired renal function need a lower dose. It is not metabolized in humans; therefore, liver impairment is not an issue. (31)
It would take about 3 to 8 weeks for titration, plus 1 to 2 weeks at a maximum tolerated dose, to determine if adequate pain relief can be obtained with gabapentin.
Carbamazepine is recommended for patients who have not responded to gabapentin and is the drug of choice for trigeminal neuralgia. (9) Common adverse effects of carbamazepine are drowsiness, headache, unsteadiness, diplopia, dizziness, nausea, vomiting, and allergic skin reactions. These often dissipate after the initial phase of therapy. More serious adverse reactions include hematologic, hepatic, cardiovascular, and dermatologic reactions, which require discontinuation of therapy. (32)
Gabapentin is approved in Canada as an anticonvulsant. The United States Food and Drug Administration approved it in May 2002 to treat postherpetic neuralgia. (31) Carbamazepine is also approved in Canada as an anticonvulsant.(32) The United States Food and Drug Administration has approved carbamazepine for the treatment of trigeminal neuralgia. (9)
Table 2 shows the number needed to treat (NNT) for gabapentin and carbamazepine to obtain 1 patient with at least 50% pain relief compared with a placebo. (8)
Table 2: Effectiveness of Anticonvulsants: Number Needed To Treat.
| Diagnosis | Drug | Number of Studies | N | Number Needed To Treat (95% Confidence Interval) |
|---|---|---|---|---|
| Neuropathic pain | Gabapentin* | 2 | 380 | 3.7 (2.6–4.9) |
| Carbamazepine† | 5 | 537 | 2.5 (2.0–3.4) |
This includes diabetic neuropathy and postherpetic neuralgia.
This includes diabetic neuropathy, trigeminal neuralgia, and central stroke pain.
Antidepressants
Two types of antidepressants have been used to treat neuropathic pain: tricyclic antidepressants, which include amitriptyline, clomipramine, desipramine, imipramine and maprotiline; and selective serotonin reuptake inhibitors, which include citalopram, fluoxetine, paroxetine, and tramadol. The usefulness of tricyclic antidepressants is often limited by their adverse effects, which include sedation, blurred vision, dry mouth, constipation, postural hypotension, weight gain, loss of balance, and cognitive impairment in the elderly. (9) They should be used cautiously with patients who have a history of cardiovascular disease, glaucoma, urinary retention, or autonomic neuropathy.
It takes about 6 to 8 weeks, with at least 1 to 2 weeks at the maximum tolerated dosage, to determine if adequate pain relief can be obtained with an antidepressant. (9)
Amitriptyline, clomipramine, desipramine, imipramine, citalopram, fluoxetine, and paroxetine are available in Canada, but Health Canada has not approved these to treat neuropathic pain. (32)
Table 3 shows the NNT for tricyclic antidepressants and selective serotonin reuptake inhibitors to achieve at least 50% pain relief in various neuropathic pain conditions compared with a placebo. (17)
Table 3: Effectiveness of Antidepressants: Number Needed To Treat.
| Diagnosis | Type of Antidepressant |
Number of Studies |
N | Number Needed To Treat (95% Confidence Interval) |
|---|---|---|---|---|
| Painful | ||||
| neuropathy | TCA* | 12 | 276 | 2.4 (2.0–3.0) |
| SSRI* | 3 | 83 | 6.7 (3.4–435) | |
| Postherpetic neuralgia |
TCA | 3 | 77 | 2.3 (1.7–3.3) |
| SSRI | NR* | NR | NR | |
| Peripheral nerve injury |
TCA | 1 | 15 | 2.5 (1.4–10.6) |
| SSRI | NR | NR | NR |
TCA indicates tricyclic antidepressant; SSRI, selective serotonin reuptake inhibitor; NR, not reported.
Drugs with Local Anesthetic Type properties
The CPSO (27) has determined that strong evidence from at least 1 properly designed randomized controlled trial (RCT) of appropriate size (CPSO level 2 evidence)(See Appendix 2) exists for oral drugs with local anesthetic type properties in different neuropathic syndromes. Mexiletine is a Class I, type 1B antiarrhythmic and a drug with local anesthetic-type properties. (7) It is approved in Canada as an antiarrhythmic. (32)
Table 4 shows the NNT for mexiletine at 625 mg per day to obtain 50% pain relief in painful neuropathy compared with a placebo. (17)
Table 4: Effectiveness of Mexiletine: Number Needed To Treat.
| Diagnosis | Drug | Number of Studies |
N | Number Needed To Treat (95 %confidence interval) |
|---|---|---|---|---|
| Painful neuropathy | Mexiletine | 1 | 126 | 10 (3-∞) |
Opioid therapy
The CPSO (27) has determined that strong evidence from at least 1 properly designed RCT of appropriate size (CPSO level 2 evidence) exists for the use of opioids for postherpetic neuralgia. Level 5 evidence, defined as the opinions of respected authorities, based on clinical evidence, descriptive studies, or on reports of an expert committee; exists for the use of opioids for trigeminal neuralgia (see Appendix 2).
The CPSO’s recommendations include managing neuropathic pain with an opioid in accordance with the following guidelines:
An attempt to identify probable pain mechanism is undertaken by the clinician.
Caution, but not contraindication, in patients whose pain is due primarily to psychological factors.
Awareness of risk factors for the development of dependence on prescribed opioids.
In most cases an adequate trial of a nonopioid and adjuvant analgesics should be done first.
Avoid short-acting opioids such as meperidine and anileridine.
The CPSO also recommends that opioid therapy for neuropathic pain should be initiated at a relatively low dose and titrated to the patient’s reports of pain relief and adverse effects. The optimal dose is when the patient reports satisfactory pain relief and no adverse effects. It has been suggested that titration of sustained-release strong opioids should be introduced over 3 to 4 months. (33)
Common adverse effects of opioids are constipation, sedation, and nausea. Cognitive impairment and problems with mobility can also occur. Abruptly discontinuing opioid therapy may cause symptoms of withdrawal. It would take about 4 to 6 weeks to determine if adequate pain relief can be obtained with an opioid. (9)
Codeine, morphine, hydromorphone, oxycodone, and fentanyl are approved analgesics by Health Canada. (32)
The NNT for opioids to obtain at least a 50% reduction in neuropathic pain is about 3. (12)
Topical Capsaicin
The CPSO (27) has determined that strong evidence from at least 1 properly designed RCT of appropriate size (CPSO level 2 evidence)(see Appendix 2) exists for the use of topical capsaicin in diabetic neuropathy and postherpetic neuralgia.
Health Canada has approved capsicin as a topical analgesic (32).
Table 5 shows the NNT for 0.075% topical capsaicin to achieve at least 50% reduction in pain after 8 weeks of use compared with a placebo. (34)
Table 5: Effectiveness of Topical Capsaicin: Number Needed to Treat.
| Diagnosis | Drug | Number Needed To Treat (95% confidence interval) |
|---|---|---|
| Neuropathic pain | Topical capsaicin (0.075%) | 5.7 (4.0–10) |
Intravenous Regional Sympathetic Blocks
The CPSO (27) has determined that evidence from well-designed trials without randomization, single group pre-post, cohort, time series or matched case-controlled studies (CPSO level 3) (see Appendix 2) exists for the use of intravenous regional sympathetic blocks for reflex sympathetic dystrophy (CRPS, Type I). However, the CPSO does not recommend the use of intravenous regional sympathetic blocks for reflex sympathetic dystrophy.
Nonpharmacological Interventions
Nonpharmacological interventions may include physiotherapy, transcutaneous electrical nerve stimulation (TENS), psychological counseling, or acupuncture. Each of these therapies will be briefly described; however, it is beyond the scope of this health technology assessment to complete a full review of the effectiveness of each nonpharmacological therapy.
Physiotherapy and Exercise
Physiotherapy and exercise are used to improve functional status and minimize functional disability of patients with chronic pain. A systematic review by White et al. (35) did not find evidence to support the ability of an exercise program to improve the functional ability of people with peripheral neuropathy. However, van Tulder et al. (36) concluded that there is strong evidence that exercise and conventional physiotherapy are equally effective at improving pain and functional status in people with chronic low back pain (including patients with nerve root pain and sciatica).
Psychologically Based Pain Therapies
The purpose of psychologically based pain therapies is to restore function and psychological integrity despite continuing pain. Various psychological interventions are used with the goal of improving activity level and reducing maladaptive pain behaviours and drug use. (37)
Transcutaneous Electrical Nerve Stimulation
TENS is a noninvasive therapy that is used to relieve pain by electrically stimulating peripheral nerves through electrodes placed on the skin’s surface. (38) Carroll et al. (39) did a systematic review of TENS for chronic pain and concluded it was not possible to provide evidence-based recommendations for its use to manage chronic pain because of the poor quality of the studies. A meta-analysis by Brosseau et al., (40) found that TENS therapy did not significantly relieve pain in people with chronic low back pain.
Acupuncture
In 1998 to 1999, 1% to 2% of Canadians reported receiving acupuncture treatments. (38) Acupuncture involves inserting a needle into a specific site on the body to relieve symptoms of a disease or medical condition. The Alberta Heritage Foundation for Medical Research (38) determined that the evidence on the effectiveness of acupuncture to treat back or chronic pain was inconclusive. Similarly, Linde et al. (41) concluded that the evidence to support the effectiveness of acupuncture to treat chronic back pain was inconclusive.
Surgical Treatments
Reoperation for failed back surgery syndrome
FBSS refers to persistent low back pain and leg pain after lumbar spine surgery. (18;19) Spincemaille et al. (42) have suggested that the population with FBSS can be divided into those with back pain, those with leg pain, and those with back and leg pain. The last 2 groups are classified as persistent neuropathic limb pain secondary to surgery. An estimated 30% to 50% of patients benefit from a second surgical procedure. (43) It has been suggested (5) that reliable indicators for surgery may include recurrent disc herniation or disc herniation de novo with evidence of neural compression on objective imaging studies and physical examination.
Neuroablative Techniques
Many neuropathic pain syndromes are thought to be due to sympathetically maintained pain. Sympathetically maintained pain is defined as pain maintained by sympathetic efferent innervation or by circulating catecholamines. (28) This has led to using therapies that temporarily or permanently interrupt the sympathetic nervous system. Temporary interruption can be performed through injections of alcohol, phenol, or local anesthetics. Permanent interruption can be done either chemically or surgically.
Mailis and Furlan (28) reviewed the effects of chemical and surgical sympathectomies, the surgical interruption of a pathway in the sympathetic nervous system, on neuropathic pain and concluded that both interventions are based on poor-quality evidence, uncontrolled studies, and personal experience. Importantly, the complications of these procedures were considerable and included worsening pain, new pain and abnormal forms of sweating. (28)
Measuring Pain
Valid and reliable measures of pain intensity include the visual analogue scale (VAS) for pain and the McGill Pain Questionnaire (MPQ). A VAS has a 10 cm horizontal or vertical line with a label of “no pain” at one end and “worst pain ever” at the other. (44) The MPQ provides information on the quality and intensity of the pain. (45;46) Farrar et al. (47) determined that a reduction of 2 points, or about 30% on an 11-point pain intensity numeric rating scale, represents a clinically important difference. Collins et al. (48) determined that a VAS score over 3.0 cm would be comparable to moderate pain on a 4-point categorical scale; 5.4 cm would be comparable to severe pain.
New Technology Being Reviewed: Spinal Cord Stimulation
The SCS Device
SCS was first used in 1967 and is a reversible method of managing chronic intractable neuropathic pain of the trunk or limbs. (29;49;50) Pain control with SCS is achieved by the production of an electrical field over segments of the spinal cord that are presumed to be involved in initiating the pain. (29;51) SCS blocks neuropathic pain but not nociceptive pain. (29) Nociceptive pain occurs from the irritation of specialized pain receptors in tissues such as the skin, bones, joints, and viscera and often indicates ongoing tissue damage. (12) Examples of nociceptive pain include pain from a burn and pain due to osteoarthritis.
The precise mechanism of action of SCS is not known; (1) however, it is thought that it modulates the perception of pain by electrically stimulating the large-diameter afferent nerve fibers in the dorsal (toward the back) columns of the spinal cord. (29) This action creates a tingling feeling called paresthesia and at the same time inhibits the transmission of pain to the brain. This results in the paresthesia or tingling feeling replacing or “painting over” the sensation of pain. (52;53)
The SCS technology has 3 implantable components (54):
A pulse generator
An extension cable
A lead
The Pulse Generator
The pulse generator is the battery of the spinal cord stimulator, which generates the low-voltage electrical pulses for stimulation. (29;55) The amplitude, pulse width, and pulse rate are programmed by a physician using a remote-control-like device called a physician programmer. The amplitude is the strength of the stimulation measured in volts (V), and the number of volts used determines the strength of the tingling or paresthesia. The pulse width, which is measured in microseconds (<s), determines how long the stimulation lasts and how wide an area the paresthesia covers. Finally, the pulse rate is the number of electrical pulses per second measured in Hertz (Hz). It determines the speed of the stimulation. Once the optimal stimulating parameters are found, the patient can control the amplitude or strength of the stimulation within the parameters set by the physician by using a remote-control-like device called a patient programmer.
There are 2 types of neurostimulators: an implantable pulse generator (IPG) and a radio frequency neurostimulator. (29;55) Both types are surgically implanted just under the skin in the lower abdomen or in the buttock area. The IPG must be surgically replaced once the battery is depleted. The radio frequency neurostimulator is powered by an external radio frequency power source and is no longer available in Canada.
The Extension Cable
The extension cable connects the pulse generator to the lead and is available in varying lengths. The extension cable can be detached from the lead and the pulse generator. (29;55)
The Lead
The lead is an insulated wire that connects at one end to the extension cable and has at its other end a set of 4 to 8 electrodes. (55) The electrodes deliver the electrical stimulation generated by the IPG (the battery) to the dorsal columns of the spinal cord. The anode is a positive electrode and the cathode is the negative electrode. The physician programs different anode and cathode combinations called arrays to conduct the electrical stimulation to the dorsal columns of the spine.
The lead is positioned within the epidural space on the posterior aspect of the spinal cord. (29) Areas of the body called dermatomes can be mapped to certain segments of the spinal cord, which are closely related to the vertebral levels of the spine. By placing the electrodes over several contiguous vertebral segments, more than one dermatome can be covered with paresthesia when stimulation is activated. This is important because neuropathic pain often involves more than one dermatome. (50) The adequacy of the paresthesia coverage of the painful dermatomes determines successful SCS. (29)
There are 2 types of leads: percutaneous and paddle leads. (29) Both types are inserted into the epidural space. (51) The percutaneous lead is inserted percutaneously (through the skin) and the paddle lead is inserted surgically. Percutaneous insertion involves threading the lead through a hollow needle called a Tuohy needle into the epidural space. (29) Local anesthetic and radiological imaging devices such as fluoroscopy are used to make insertion easier. The advantages of using percutaneously placed leads are that less operating room time is required and it is a less-invasive procedure.(1) However, previous surgery or anatomical changes in the spine may preclude a percutaneous lead insertion.
Surgically placed leads are placed under direct vision through a small laminotomy and tend to move or migrate less often within the epidural space than percutaneously inserted leads. However, the surgical insertion is more invasive than percutaneous insertion.
Before the spinal cord stimulator is permanently implanted, the candidate must have a psychological assessment and then complete a test stimulation period. (See Figure 1.)
Figure 1: Phases of Spinal Cord Stimulation.

Psychological Evaluation
Emotional and behavioural influences can affect the perception of pain and pain relief. (1) Psychiatric disorders, poor comprehension, lack of compliance, drug or alcohol abuse, drug-seeking behaviour, or issues related to secondary gain may interfere with the patient’s commitment to, and the success of, the therapy and are contraindications to SCS. (1;56) For these reasons, patient evaluation by a neuropsychologist is required.
SCS Test Stimulation Phase
If the psychological assessment is favourable, patients have test stimulations to determine if they are responsive to SCS therapy and can tolerate the paresthesia. Generally, only those who obtain at least a 50% reduction in pain intensity during the test stimulation phase and can tolerate the paresthesia should have the SCS device permanently implanted. (1)
Test stimulation starts with the physician percutaneously placing a lead and connecting it to a temporary external pulse generator. The patient is sedated but not unconscious for the lead insertion, which takes between 45 minutes and 2 hours (Personal communication with clinical expert, February 14, 2005). To correctly position the electrodes, the spinal cord stimulator is activated during this procedure and the patient helps guide the electrode placement by reporting to the physician where he or she is feeling the paresthesia.
While the nature of this procedure renders it a day surgery, many patients are admitted overnight for monitoring and patient teaching (Personal communication with clinical expert, February 14, 2005). After discharge from the hospital and over the next 4 to 7 days, the patient with the help of a nurse (neuromodulation nurse)or pain doctor monitors his or her pain intensity. During this period the stimulation parameters may be changed to optimize pain control. A successful test stimulation period is defined as at least a 50% reduction in pain. Successful candidates can then have a permanent spinal cord stimulator implanted. On average, about 70% to 80% of candidates will have a successful SCS trial stimulation. (Personal communication with clinical expert, February 14, 2005) If the trial stimulation phase is unsuccessful, the percutaneously placed lead is removed.
Permanent Implantation Phase
During the implantation phase, a permanent lead is inserted percutaneously. The lead is then attached to the extension cable, which is tunneled under the skin to connect to the IPG. The IPG is implanted just under the skin in the abdomen or gluteal (buttock) area. The insertion of a permanent lead and implantation of a pulse generator takes about 2 to 3 hours, and the patient is admitted overnight for recovery (Personal communication with clinical expert, February 17, 2005).
Patient Follow-up
Several follow-up visits occur in the first year after implantation to adjust stimulation parameters and assess pain control. Follow-up may occur at 1, 3, and 6 weeks after the procedure and then at 3, 6, and 12 months for the first year, but may vary among practitioners. Annual visits are scheduled thereafter to assess for any needed modifications in stimulation parameters to maintain pain control and to make sure the SCS battery is not depleted. (Personal communication with clinical experts on February 17, 2005 and April 13, 2005).
Efficacy of Spinal Cord Stimulation
There have been 2 studies comparing SCS with a placebo. A summary of each study follows.
In 1991, Marchand et al. (57) published a prospective randomized placebo-controlled crossover single-blinded trial on 8 chronic back pain patients who were using SCS and reporting at least a 30% decrease in pain intensity. The patients were told the purpose of the study was to test new parameters of stimulation. Stimulation was discontinued at least 8 hours before the study started. During the study, patients were given either 30 minutes of active SCS with their normal stimulation parameters or 30 minutes of placebo stimulation. For the placebo stimulation, the investigator pretended to manipulate the SCS controls. Patients recorded their perceived pain intensity and the unpleasantness of the pain on a VAS before treatment, every 10 minutes during treatment, and after treatment.
All of the patients reported paresthesia during placebo stimulation. However, the ratings of perceived pain intensity (P = .006) and pain unpleasantness (P = .007) were significantly reduced with the active stimulation compared with the placebo.
The authors concluded that active SCS reduced perceived pain intensity and unpleasantness significantly compared with placebo stimulation.
This study was limited by its small sample size.
In 1996, Tesfaye et al. (58) published a prospective non-randomized placebo-controlled crossover trial of patients during test stimulation. Ten patients with disabling diabetic neuropathy without previous exposure to SCS had a 7-day test stimulation in which they received placebo stimulation for 2 days and active stimulation for 2 days. During each 2-day period, the patients rated their pain level every 4 hours using a VAS of pain.
Results showed the median (interquartile range) baseline VAS score was 62.5 (28.2–71.8), and the median VAS score during placebo stimulation was 33.5 (15.5–56.3). The median VAS score during active stimulation was 15.5 (1.5–31.2). Pain was significantly lower with active stimulation than with placebo stimulation (P = .004).
The authors concluded that, “Spinal cord stimulation offers a new and effective treatment for chronic diabetic neuropathic pain.” (58)
It is unclear if patients in this study were blinded to their treatment allocation. If not, then this is a limitation of the study. This study also had a small sample size.
Complications Associated With Spinal Cord Stimulation
Complications can be divided into procedural complications and technical failures. (59) Procedural complications include wound infection, cerebrospinal fluid leaks, dural puncture headaches, and the inability to thread the lead percutaneously into the epidural space. Technical failures include lead migration and fracturing, unwanted stimulation, inadequate paresthesia coverage and pain over the IPG battery implantation site. Early IPG battery failure can also occur. (49) The longevity of the IPG battery depends on the amplitude use and the pulse width requirements and whether the stimulator is used continuously or intermittently (cycling mode) (personal communication with clinical expert, April 13, 2005)
Infection is the most common procedural complication, with a reported incidence ranging from 1.4% to 11%. (59) North et al. (50) reported an incidence of 5% for superficial surgical wound infections in a cohort of 205 patients followed-up between 2 years and 20 years. Superficial infections may clear with intravenous antibiotics but if it fails to resolve the spinal cord stimulator is removed. The stimulator may be reimplanted once the infection has resolved.
There has been one report of paralysis associated with a bacterial infection located at the tip of the lead with the subsequent development of an epidural and intradural abscess requiring surgical intervention. (60) Four cases of aseptic meningitis have been reported, 2 that resolved spontaneously and 2 that required removal of the spinal cord stimulator. (60)
One hundred and fourteen infections were reported to Medtronic Inc. between September 1, 2000 and July 1, 2002. (61) Bacterial growth was reported in 47% of the cases, and no bacterial growth was reported in 18% of the cases. Eighty-seven percent of cases were treated with antibiotic therapy. The IPG implantation site was the most common site of infection (54%), the electrode lead (17%) was the second most common. (Infection of the electrode lead can occur at the site where the lead and the connector cord join. Personal communication with clinical expert, April 13, 2005). In 94% of the cases, the spinal cord stimulator was removed in whole or in part, and 91% of patients had a successful resolution. There were no infection-related deaths.
There has been one report of relapsing ulcerative colitis approximately 6 weeks after implantation of the spinal cord stimulator and continuous stimulation. Stimulation was discontinued, but the device remained implanted while the ulcerative colitis was treated. Once the ulcerative colitis was in remission, stimulation resumed. However, 2 weeks after the initiation of stimulation the ulcerative colitis symptoms recurred. Stimulation was again stopped, and the device was explanted. Remission returned and was sustained after explantation. (62)
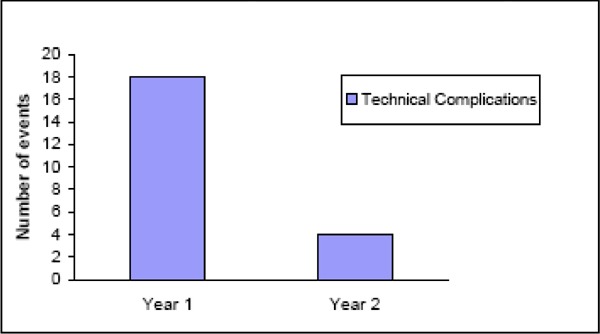
The most common technical failure is lead migration. (49) Lead migration occurs when the lead shifts position longitudinally (up or down) or laterally (side to side) within the epidural place. The leads may also fracture, which impedes proper transmission of the electrical pulses. The result of each of these technical failures is inadequate paresthesia coverage of the painful dermatomes and less pain relief. Often there is an attempt by the clinician to reprogram the stimulation parameters to recapture adequate paresthesia; however, if this fails, then surgical revision of the lead is needed. (29) Kemler et al. (63) reported that the incidence of technical complications is greatest in the first year after implantation and falls markedly thereafter. (See Figure 2.)
Figure 2: Incidences of Technical Complications of Spinal Cord Stimulation at 1 and 2 Years (63).
Painful antenna coupling is a technical failure unique to the radio frequency SCS device. Explanting the device often solves the problem. (64). There has been one report of accidental activation of a radio frequency spinal cord stimulator with an anti-theft device. The patient sustained neurological injury manifested as dysarthria, ataxia, tremor, and prolonged memory impairment. (65)
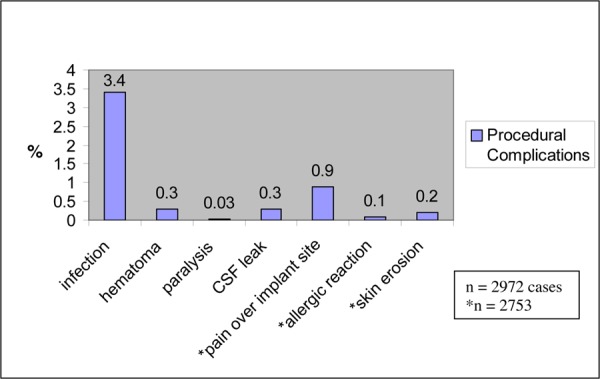
Cameron (49) calculated the incidence of technical failures and procedural complications in 68 studies of more than 2700 patients who were treated with SCS for neuropathic and ischemic pain. These results are shown in Table 6.
Table 6: Spinal Cord Stimulation Technical Failures and Procedural Complications.
| Complication | Incidence, % |
|---|---|
| Lead migration | 13.2 |
| Infection | 3.4 |
| Hematoma | 0.3 |
| Paralysis | 0.03 |
| Cerebrospinal fluid leak | 0.3 |
| Unwanted stimulation | 2.4 |
| Pain over implant | 0.9 |
| Allergic reaction | 0.1 |
| Skin erosion | 0.2 |
| Lead breakage | 9.1 |
| Hardware malfunction | 2.9 |
| Loose connection | 0.4 |
| Battery failure | 1.6 |
| Other | 1.4 |
Contraindications to SCS include these (56):
No partial sparing of the dorsal column fibers (e.g., total paraplegia)
The presence of other stimulation devices with sensing capacities (e.g., pacemakers or implantable cardiac defibrillators are contraindicated to SCS)
Severe diseases likely to interfere with neuromodulation procedures, such as coagulopathies and immunodeficiency diseases
Existing drug habituation problem (should be treated before commencing SCS)
Major psychiatric disorders such as active psychosis, severe depression, or hypochondria and somatization disorder; poor compliance and/or insufficient understanding of the therapy; lack of appropriate social support; and drug and alcohol abuse or drug-seeking behaviour
Regulatory Status
Health Canada (http://www.hc-sc.gc.ca/hpfb-dgpsa/tpd-dpt/index_devices_e.html, accessed January, 2005) licenses 7 spinal cord stimulator devices. However, only 4 are currently available (See Table 7). The Itrel 3 System is a single-lead device, the Synergy Neurostimulator is a dual-lead device, and the Synergy Veristrel is a smaller (with a smaller battery) dual-lead system only available from the manufacturer through special order and rarely used in Canada (Personal communication, Medtronic Inc., January 11, 2005). Health Canada recently approved the Genesis Neurostimulation System in February 2005. Radio frequency spinal cord neurostimulators (X-Trel RF and Mattrix RF) are no longer available in Canada (Table 8).
Table 7: Spinal Cord Stimulation Devices Licensed and Available in Canada.
| Licence Number | Licence Name | Class | Device Name | Purpose |
|---|---|---|---|---|
| 14740 | Itrel System | IV | Itrel 3 System Implantable Pulse Generator | To treat chronic intractable pain and gastroparesis |
| 645 | Synergy Neurostimulator System For Spinal Cord Stimulation |
IV | Synergy Neurostimulator Dual-Channel Itrel IPG For Spinal Cord Stimulation |
To help manage chronic intractable pain |
| 37764 | Synergy Veristrel Implantable Pulse Generator |
IV | Synergy Versitrel IPG |
To help manage chronic intractable pain of the trunk or limbs |
| 67516 | Genesis Neurostimulation System |
IV | Genesis IPG Neurostimulator Power Source |
Indicated as aid in the management of chronic intractable pain of the trunk and/or limbs, including unilateral or bilateral pain associated with any of the following: failed back surgery syndrome, and intractable low back and leg pain. |
Table 8: Spinal Cord Stimulation Devices Licensed but Not Available in Canada.
| Licence Number | Licence Name | Class | Device Name |
|---|---|---|---|
| 871 | X-Trel RF System | IV | X-Trel Receiver |
| 871 | X-Trel RF System | IV | X-Trel RF Transmitter |
| 11115 | Mattrix System | IV | Mattrix Receiver |
| 11115 | Mattrix System | IV | Mattrix Transmitter |
| 14740 | Itrel System | IV | Itrel II IPG |
Literature Review on Effectiveness
Objective
The primary objective was to evaluate the effectiveness and safety of SCS to manage chronic neuropathic pain.
Questions Asked
Does pain management with SCS:
Decrease perceived pain intensity?
Improve functional status?
Improve the QOL of people with neuropathic pain?
Outcome Measures
The primary outcome was pain relief.
The secondary outcomes were as follows:
Functional status
QOL
Technical failures and procedural complications
Methods
Search Strategy
The Medical Advisory Secretariat did a computer-aided search limited to human studies. Case reports, letters, editorials, non-systematic reviews, and comments were excluded. Foreign-language studies were included to determine bias in reviewing only English-language reports. (Appendix 1)
Initial Search
2000 to November week 3, 2004
OVID MEDLINE
EMBASE
Other Non-Indexed Citations
Cochrane Database of Systematic Reviews
Cochrane CENTRAL
INHATA
Updated Search
2000 to January week 3, 2005
OVID MEDLINE
Other Non-Indexed Citations
EMBASE
Inclusion Criteria
Systematic reviews, RCTs, prospective non-RCTs including before-and-after treatment designs
Studies that compared SCS to alternate treatment(s) or treatment states (before-and-after studies)
Adults with neuropathic pain conditions
Patients with FBSS with leg pain equal to or greater than low back pain
Subjects who have had at least one of the following: pain for at least 6 months and/or have failed conservative treatments
Publicly available Health Technology Assessments
Exclusion Criteria
Studies that did not include a subjective measure of pain intensity
Studies that compared technical factors of SCS
Studies that investigated chronic mechanical back pain, ischemic limb or cardiac pain
Studies with a study sample of mixed pain conditions (neuropathic pain and nociceptive pain conditions in same study sample) and separate results for each type of pain were not reported
Multiple reports that include results of same study sample (in these cases the study with the longest follow-up period reported was selected for inclusion in this review)
Intervention
SCS with any of the following techniques:
Percutaneous or paddle electrodes
Implantable pulse generator or radio frequency receiver
Single or dual electrodes
Single- or multi-channel electrodes
Any type of simulation parameters used
Mono-polar or multi-polar
Controls included conventional pharmacological, nonpharmacological, or surgical therapies; or self-controlled (before-and-after study design)
Outcomes of Interest
Subjective measurement of pain intensity with at least one of the following validated pain scales: VAS, or MPQ.
Other measures of pain including a numerical rating scale or medication quantification scale, or the percentage of patients experiencing pain relief.
Functional status
QOL
Assessment of Methodological Quality of Randomized Controlled Trials
Relevant RCTs were assessed using the instrument to measure the likelihood of bias in pain research reports developed by Jadad et al. (66)
-
In addition, each study was evaluated for allocation concealment (67) where:
A = adequate
B = unclear
C = inadequate
D = not done
Description of the Scale by Jadad et al.(66)
Was the study described as randomized?
Was the study described as double blinded?
Was there a description of withdrawals and dropouts?
Score 1 for “Yes” and 0 for “No”
| Give 1 additional point if: | For question 1, the method to generate the sequence of randomization was described and was appropriate. |
| Deduct 1 point if: | For question 1, the method to generate the sequence of randomization was described and it was inappropriate and/or for question 2, the study was described as double blinded but the method of blinding was inappropriate. |
Results of Literature Review
The initial search yielded 311 citations, and the updated search yielded an additional 16 citations, for 327 citations. Twenty-six were foreign-language studies. Of the 301 English-language articles, 20 met the inclusion criteria
The full articles were retrieved for 20 of the citations (Table 9). Of these, 4 health technology assessments were excluded: 3 because they were assessed as non-systematic reviews (lack of clearly defined question, no inclusion/exclusion criteria or clear outcome measures proposed), (68-70) and 1 because it had case control studies only. (71)
Table 9: Results of Literature Search by Medical Advisory Secretariat.
| Type of Trial | Initially Retrieved | Included |
|---|---|---|
| Existing health technology assessments | 10 | 6 |
| Randomized controlled trials | 3 | 2 |
| Non-randomized controlled trials | 7 | 2 |
Six clinical trial reports including 1 RCT and 5 non-randomized controlled trials (non-RCT) were excluded: 1 RCT was a multiple report; (72) 1 non-RCT with a sample comprised of a heterogenous pain population; (73) 2 non-RCTs that included patients with predominately low back (axial) pain; (74;75) 1 non-RCT that compared the effects of different stimulation programs among patients; (64), and 1 non-RCT study that did not report a measure of pain relief. (18). Therefore, 10 reports were excluded, leaving 10 to be reviewed fully (Table 9).
Heath Technology Assessments
Six health technology assessments of small RCTs were reviewed. Five were published in peer-reviewed journals. (19;49;51;59;76) The sixth was completed by the Australian Safety and Efficacy Register of New Interventional Procedures Surgical (ASERNIP-S) (Table 11). (54) Each review is discussed in turn below.
Table 11: Middleton et al.
| Spinal Cord Stimulation (Neurostimulation): An Accelerated Systematic Review | |
|---|---|
| Author | Middleton et al. |
| Agency | Australian Safety and Efficacy Register of New Interventional Procedures Surgical (ASERNIP-S) |
| Date | June 2003 |
| Objective | To assess the effectiveness and safety of spinal cord stimulation by an accelerated systematic review. |
| Search | Up to April 2003, MEDLINE, Pre-MEDLINE, The Cochrane Library, Issue 2, 2003 |
| Inclusion criteria | Randomized controlled trials |
| Outcome | Pain or pain relief |
| Results: Effectiveness | 9 randomized controlled trials including:
Complex regional pain syndrome:
|
| Results: Safety | Failed back surgery syndrome:Complex regional pain syndrome:
Adverse events with spinal cord stimulation:
|
| Conclusion reported in the ASERNIP-S Health Technology Assessment |
|
Taylor et al., 2005(77)
Spinal Cord Stimulation for Chronic Back and Leg Pain and Failed Back Surgery Syndrome: A Systematic Review and Analysis of Prognostic Factors.
Taylor and colleagues (77) used the updated methods guidelines for systematic reviews of the Cochrane Collaboration Back Review Group. They searched the Cochrane Controlled Trials Register, MEDLINE, and EMBASE up to January 2002. The search was not restricted by language and included RCTs and non-RCTs. They retrieved 1 RCT, 1 cohort study, and 72 case series. They pooled the results from the case series and estimated relative risk or risk difference for the before-and-after studies (probability of patient achieving outcome before SCS compared with after SCS).
Results: Randomized Controlled Trial
Taylor et al.(77) report results of a randomized trial by North et al.(77) that were presented at a scientific meeting in 2000. North et al.(77) randomized 50 patients with FBSS to receive either SCS or a reoperation. They found that significantly more patients treated with SCS had at least 50% pain relief compared with the patients that had reoperations (37.5% for SCS vs. 11.5% for reoperation; P = .0475). Taylor et al. gave the study a grade of 4/5 using the Jadad et al. (66) methodological quality scale.
Results: Cohort Study
Dario et al. (18) completed a cohort study that compared people with neuropathic pain treated successfully with medical therapy with people who were treated with SCS because medical therapy had not worked for them. In their assessment, Taylor et al. (77) suggested that a limitation of the study is the imbalance in prognostic variables between groups, because people who failed medical therapy and were treated with SCS may have had more severe disease compared with those that did not fail medical therapy. Dario et al. (18) did not complete a statistical analysis of the VAS pain scores between the spinal cord stimulation treated patients and the medical therapy treated patients because they felt the two treatment groups were not comparable (personal communication with the author, January 21, 2005). While Taylor et al. (77) state that there was no difference in functional capacity between the SCS and medically treated patients as measured by the Pain Disability Index and Oswestry scores this is inconsistent with that reported by Dario et al.(18) Dario et al. (18) report a statistically significant difference (P < .05) in the Owestry scale score between the medically treated patients and those treated with spinal stimulation. The baseline average Owestry scale score in the medically treated group before treatment was 23 (range 10-35) and the average score at 7-year follow-up was 6 (approximate range 3-11). However, the baseline average score before treatment in the spinal cord stimulation group was 12 (range 6-17) and the average score at the 7-year follow up was 9 (range 16-5). There was no adjustment in the statistical analysis to allow for the differences in baseline Owestry scores and this may confound the statistical analysis of the parameter. Taylor et al. (77) gave this study by Dario et al. (18) a grade of 1/5 using the methodological quality scale developed by Jadad et al. (66)
Results: Case Series
The 72 case series comprised 3,427 patients with spinal cord stimulator implants. Sample sizes ranged from 1 to 304, and all patients had received SCS. Follow-up monitoring ranged from 1 to 106 months. Taylor et al.(77) rated the quality of these case series with an assessment tool developed specifically for the systematic review and that had not been validated. Higher scores indicated better-quality studies. The median score was 1 (range, 0—6). There was statistical heterogeneity in the level of pain relief with SCS across studies (Q, 2521.90; df, 64; P < .0001). (77) Despite this, the authors computed a pooled random-effects model for the outcome of at least 50% pain relief (Table 10).
Table 10: Pooled Random-Effects Model for at Least 50% Pain Relief.
| Outcome | Case Series That Reported the Outcome |
Number of Cases/Total Number of Cases |
Pooled Results % (95% CI) |
|---|---|---|---|
| Pain relief of at least 50% | 65 | 1992/3313 | 62 (5669) |
The percentage of patients that obtained at least a 50% reduction of pain intensity after SCS was 15% to 20% lower in the higher-quality studies, compared with lower-quality studies (P = .010). It was also higher in studies that had shorter follow-up periods (P < .0001), in chronic low back pain or FBSS populations (P < .0001), and in multicentre studies (P = .013).
Taylor et al (77) concluded that the level of evidence to support the effectiveness of SCS to treat patients with chronic low back pain or failed back surgical syndrome is moderate. They also concluded that poor-quality studies may exaggerate the estimate of a SCS treatment effect.
The main limitation of this systematic review by Taylor et al. (77) is that results from the case series were pooled statistically despite statistical heterogeneity between studies.
Mailis-Gagnon et al., 2004(51)
Spinal Cord Stimulation For Chronic Pain
This systematic review was published in the Cochrane Database of Systematic Reviews. Mailis-Gagnon and colleagues (51) searched MEDLINE and EMBASE, up to September 2003, and the Cochrane Central Register of Controlled Trials (CENTRAL) up to Issue 3, 2003. They also searched textbooks and reference lists in retrieved articles. They consulted experts in the field of pain and the main manufacturer of the stimulators. They did not impose a language restriction on the search and included RCTs and non-RCTS that evaluated SCS for chronic pain. Their search retrieved 2 RCTs. The heterogeneity of the participants, interventions, and outcome measures precluded statistically pooling the results.
Results: Randomized Controlled Trials
Kemler et al. (72) did an RCT of 54 patients with CRPS Type I treated either with SCS plus physiotherapy (n = 36) or physiotherapy only (n = 18). In the intention to treat analysis pain was significantly lower at 6 months in the patients who had received SCS and physiotherapy, compared with those who received only physiotherapy (P < .001). On health-related QOL, they found no difference between the groups at 6 months. Using the scale develped by Jadad et al. (66) Mailis-Gagnon et al. graded the methodological quality of this study as 3/5.
In the other RCT, North et al. (20) reported the preliminary results of an RCT that compared patients who received SCS with a control group that had reoperations. At 6 months after treatment, 17% (2/12) of patient receiving SCS had crossed over to the reoperation group, while 67% (10/15) of the control group had crossed over to SCS (P = .018). Mailis-Gagnon et al. graded the methodological quality of this study as 1/5.
Mailis-Gagnon et al (51) conclude that there is limited evidence in favour of SCS to treat FBSS and CRPS, but insufficient evidence to determine the benefits and harms of SCS. More trials are needed to assess if SCS effectively treats chronic pain conditions.
Cameron, 2004(49)
Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review
Cameron (49)specified explicit inclusion and exclusion criteria for a literature review of the efficacy and safety of SCS to treat chronic pain, including pain of the trunk and limbs, ischemic pain, and angina pain. He searched MEDLINE from January 1981 to the beginning of 2003, and he hand-searched articles published in the journal Neuromodulation. The search was restricted to English-language articles. He included RCTs, prospective controlled and non-controlled, and retrospective studies.
Cameron retrieved 68 articles:
16 with back and leg articles (2 RCTs, including North 1995, Marchand 1991)
12 with CRPS Types I and II (1 RCT including that by Kemler, 2000)
13 with ischemic limb pain studies (2 RCTs)
11 with angina pain studies (3 RCTs)
16 with studies including various pain diagnoses (0 RCT)
For the data analysis, he pooled outcomes obtained with similar outcome measures and calculated means and standard deviations. The author does not describe methods used to pool data.
Results: Back and Leg Pain Studies
North et al. (20): as reported in the review by Mailis-Gagnon et al. (51)
Cameron (49) classified the study by North et al. (20) as a non-randomized study.
Marchand et al.(57) reported results of a placebo-controlled crossover trial of 8 patients treated with active spinal cord stimulation and placebo spinal cord stimulation. Both the perceived pain intensity (P = .006) and pain unpleasantness (P = .007) were statistically reduced by active SCS but not by placebo stimulation.
Also reviewed by Cameron (49) under the category of back and leg pain studies were 8 prospective studies without matched controls, in which the overall success rate of SCS was 65% (n = 332); and 6 retrospective studies without matched controls, in which the overall success rate of SCS was 64% (n = 232).
Results: Complex Regional Pain Syndrome Type I or Type II Studies
Kemler et al. (72): as reported by Mailis-Gagnon et al. (51)
Also included under the category of complex regional pain studies were 3 prospective studies without matched controls, in which the overall success rate of SCS was 84% (n = 19); and 8 retrospective studies, in which the overall success rate of SCS was 84% (n = 192).
Cameron (49)concludes the review by stating that there is some evidence to indicate that SCS has positive, symptomatic, long-term effects on CRPS Types I and II and pain due to FBSS. However, few large randomized controlled studies examining the efficacy of SCS have been reported for chronic pain conditions including CRPS Types I and II, FBSS, refractory angina pain, severe ischemic limb pain secondary to peripheral vascular disease and peripheral neuropathic pain.
Cameron (49) has completed an exhaustive review comprising a collection of 20 years of clinical research on SCS to manage multiple chronic pain conditions. However, the review did not describe the methods used to pool the data. Treatment effects of SCS reported for the prospective no control studies may be inflated due to the observational study design.
Turner et al., 2004(19)
Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications
Turner et al. (19) used explicit inclusion, exclusion, and outcome criteria. The literature search was completed by an experienced health services librarian who searched these databases: MEDLINE, EMBASE, The Science Citation Index, Cochrane Central Register of Controlled Trials, and Current Contents bibliographic databases up to May 16, 2003. The manufacturer of spinal cord stimulators was consulted for additional references. Finally, the reviewer also searched personal files, journals, and books; and reviewed the bibliographies of relevant articles for additional studies. The search was restricted to English-language articles. Turner et al.(19) included RCTs, prospective matched-group cohort studies, non-matched cohort studies, and case series. They retrieved 7 studies: 1 RCT and 6 prospective case series. The data were analyzed qualitatively.
Results: Randomized Controlled Trial
Kemler et al. (72): as reported by Mailis-Gagnon et al. (51) and Cameron, (49). Turner et al. (19) calculated the NNT for SCS from the results reported by Kemler et al. (72). A NNT of 3 was determined, which indicate that 3 patients need to be given a trial of SCS for 1 patient to report a score of at least 6 or “much pain improvement” on a 7-point Global Perceived Effect Scale at 6 months follow up.
Results: Case Series
5 studies found a mild to moderate improvement in pain.
3 studies reported that SCS was associated with a statistically significant improvement in functional status (P < .05); however, in the absence of a control group, the reviewers concluded that an improvement in functional status due to other events (e.g., natural history) could not be ruled out.
Turner et al. (19) concluded that there is moderate evidence that SCS plus physiotherapy is more effective at relieving pain than physiotherapy only for patients with CRPS Type I at 6 and 12 months.
Turner et al. (19) also concluded that there was inadequate evidence to support the efficacy of SCS to reduce physical disability, work disability, and medication consumption in patients who have FBSS and CRPS Type I.
There were no limitations to this systematic review by Turner et al.
Grabow et al., 2003(59)
Spinal Cord Stimulation for Complex Regional Pain Syndrome: An Evidence-Based Medicine Review of the Literature.
Grabow et al. (59) searched MEDLINE (1966-2002), The Cochrane Library (on-line version 2002), the ISI Web of Science (1954–2002), and WebSPIRS from SilverPlatter (1966–2002), each up to April 2002. The literature search also included personal files, textbooks, bibliographies of retrieved articles, and literature from the manufacturers of spinal cord stimulators. The search was restricted to English-language articles.
They included RCTs, clinical trials, case-control studies, and case reports. They retrieved 15 studies: 1 RCT, 2 prospective studies, and 12 retrospective studies. They did a qualitative analysis of the data.
Results: Randomized Controlled Trial
Kemler et al. (72): as reported by Mailis-Gagnon et al (51), and Cameron, (49) and Turner, (19).
Grabow et al.(59) rated the quality of the study by Kemler et al. (72)a IB using the Oxford Center for Evidenced-Base Medicine: Levels (1a-5) Grade (A-D). (http://www.cebm.net/levels_of_evidence.asp#levels) (accessed April 26, 2005). A grade of 1B is defined as an individual RCT with narrow confidence intervals.
Similar to Turner et al. (19) Grabow et al. (59) calculated a NNT of 3.0 (95% CI, 1.9–7.0) from the results of Kemler et al. (72) using a rating of 6 (much improved) on the Global Perceived Effect scale.
Results: Other Studies
7 studies reported baseline VAS scores, and 5 of these reported VAS scores after SCS. The mean baseline VAS score ranged from 6.7 to 8.3, and the range at follow-up was 1.3 to 4.5. Statistical testing on differences between baseline and follow-up was done in 4 of the 7 studies.
12 studies reported that SCS was a successful and effective therapy for CRPS. Success ranged from 53.7% to 100% in these studies.
1 study reported SCS was unsuccessful (study completed in 1974).
1 study’s conclusions were unclear.
Grabow et al. (59) concluded SCS was effective for the management of pain for patients with CRPS who did not respond to more conservative medical management.
There were no limitations to the systematic review by Grabow et al. (59)
Middleton et al. 2003 (54)
Table 12: Summary of Health Technology Assessments on Spinal Cord Stimulation Effectiveness and Quality of Evidence.
| Author, Year | Population | RCT Included | Comment | SCS Effective?*/Quality of Evidence |
|---|---|---|---|---|
| Taylor et al., 2005 (77) | Chronic back and leg pain FBSS* |
North et al. (77) (full results presented at scientific meeting) | North et al. study was scored as 4/5 on a methodological quality rating scale† | Yes Moderate |
| Mailis-Gagnon et al., 2004 (51) | Chronic pain | Kemler et al.: 2000, 2001, 2002 (72;79;80) North et al. 1995 | Kemler et al. study was rated 3/5, and the North et al. study was rated 1/5 on a methodological quality rating scale† | “Limited evidence in favour of SCS for FBSS and CRPS Type I.” “Insufficient evidence to determine benefits and harms of SCS.” |
| Cameron, 2004 (49) | Chronic pain FBSS CPRPS* Type I |
Kemler et al. 2000(72) North et al. 1995 (20) Marchand et al.1991 (57) | North et al. study considered non-randomized | Yes Weak |
| Turner et al., 2004 (19) | FBSS CRPS Type I |
Kemler et al. 2000 (72) | The study by North et al. in 1994 was not included in this review because an outcome measure of pain was not reported. | Yes Moderate for CRPS only |
| Grabow et al., 2003 (59) | CRPS Type I | Kemler et al. 2000 (72) | Yes 1B‡ |
|
| ASERNIP-S, 2003 (54) | FBSS, diabetic neuropathy CRPS, angina, critical limb ischemia | Kemler et al.: 2000, 2001, 2002 (72;79;80) North: 1994, 1995, 2002 (20;69;78) | YES Not reported |
FBSS indicates failed back surgery syndrome; CRPS, complex regional pain syndrome; SCS, spinal cord stimulation.
By Jadad et al. (66)
Using the Oxford Center for Evidence-Based Medicine rating scale.
Technical Failures and Procedural Complications Reported in the Health Technology Assessments:
Table 13 lists the technical failures and procedural complications reported in each of the 5 systematic reviews published in peer-reviewed journals. Complications reported by the ASERNIP-S review were shown in Table 11.
Table 13: Technical Failures and Procedural Complications Reported in 5 Health Technology Assessments.
| Type of Problem | Taylor et al. 2005 (77) |
Mailis-Gagnon et al. 2004 (51) |
Cameron 2004 (49) |
Turner et al. 2004 (19) |
Grabow et al. 2003 (59) |
|---|---|---|---|---|---|
| Lead problems | 27% | 4% | Migration: 9.7% Breakage: 13.2% |
23.1% | 8.3%–42.8% |
| Generator-related problems | 6% | None reported | 1.6 | 5.8 | None reported |
| Extension cable problems | 10% | None reported | Not reported | None reported | None reported |
| Reoperation | None reported | None reported | None reported | 23.1% | 11.1%–50% |
| Subcutaneous dissection of generator pocket | None reported | 8.3% | None reported | None reported | None reported |
| Infection | 6% | 4% | 3.4% | 4.5% superficial 0.1% deep |
1.4%–11.7% |
| CSF* leak | 7% | Not reported | 0.3% | Not reported | Not reported |
CSF indicates cerebrospinal fluid.
Cameron (49) reported technical failures and procedural complication rates on more than 2700 people treated with SCS. These results are shown in Figures 3 and 4 on the next page.
Figure 3: Technical Failures.
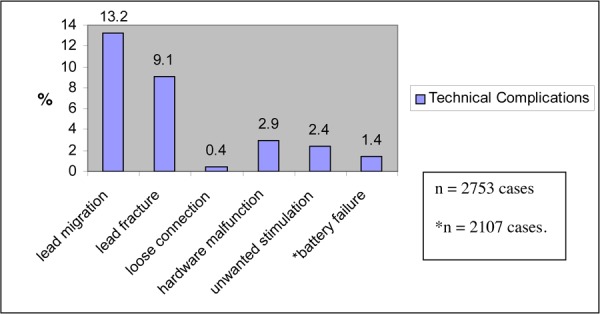
Figure 4: Procedural Complications.
Summary of Existing Health Technology Assessments
The authors of all 6 health technology assessments (19;49;51;54;59;77) concluded that there is evidence to support the effectiveness of SCS to manage pain in various neuropathic pain syndromes. However, the quality of this evidence ranged from very weak to moderate.
Two reviews, including Taylor et al.’s (77) and Cameron’s (49) had pooled study outcome data from non-RCTs. Taylor et al. (77) pooled results from statistically heterogeneous case series studies using a random-effects model. Cameron (49) did not describe the methods they used to pool the data. Therefore, the usefulness of these pooled estimates is questionable.
The other 4 systematic reviews gave qualitative summaries only. Turner et al. (19) and Grabow et al., (59) reported a NNT of 3 for SCS to improve pain relief using the results of the RCT by Kemler et al. (72)
Across studies included in these 6 health technology assessments the rate of technical failures ranged from 1.6% to 42.8%. The rate of infection occurred ranged from 1.4% to 11.7%.
Only 2 RCTs were identified among these 6 health technology assessments: Kemler et al. (72) and North et al. (20;78) However, a published update on 2-year outcomes for each of these studies is now available. These updated results are included and discussed in the Medical Advisory Secretariat systematic review that follows.
Medical Advisory Secretariat Systematic Review
Quality of Evidence
Table 14: Quality of Evidence of Included Studies.
| Study Design | Level of Evidence |
Number of Eligible Studies |
|---|---|---|
| Systematic review(s) of large RCTs | 1a | 0 |
| Large RCT | 1b | 0 |
| Large RCT unpublished but reported to an international scientific meeting | 1(g)† | 0 |
| Small RCT | 2 | 2 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 |
| Non-RCT with contemporaneous controls | 3a | 2 |
| Non-RCT with historical controls | 3b | 0 |
| Non-RCT presented at international conference | 3(g) | 0 |
| Surveillance (database or register) | 4a | n/a |
| Case series (multisite) | 4b | n/a |
| Case series (single site) | 4c | n/a |
| Retrospective review, modeling | 4d | n/a |
| Case series presented at international conference | 4(g) | n/a |
RCT refers to randomized controlled trial.
g indicates grey literature.
The Medical Advisory Secretariat included 2 RCTs and 2 prospective non-RCTs in its systematic review. One is from the United States, 2 are from The Netherlands, and 1 is from Germany. Study characteristics are detailed in Appendix 3.
Quality of Level 2 Small Randomized Controlled Trials
The 2 RCTS (63;81) were graded as 3/5 on the Jadad et al. (66) methodological quality score. Both studies were also given a Cochrane collaboration concealment grade of A, which indicates adequate concealment of the randomization schedule. (82)
In the RCT by North et al., (81) 50 patients with FBSS were randomized to receive either SCS or reoperation. The authors used a 1:1 treatment-to-control allocation ratio. In the other RCT, Kemler et al. (63) randomized 54 patients with CRPS to receive either SCS plus physiotherapy or only physiotherapy. The authors used a 2:1 treatment-to-active control allocation ratio.
North et al.’s (81) primary outcome was a composite of the number of patients that crossed over from the randomized to the active control procedure and the proportion of successes at last follow-up. Success was defined as at least 50% pain relief and patient satisfaction with treatment. North and colleagues did not adjust the level of significance to account for 2 primary outcomes. The primary outcome for Kemler et al. was the change in baseline and post-treatment VAS scores between the treatment and control groups.
North et al. (81) and Kemler et al. (63) each adequately described their sample size calculation and statistical analysis. North et al. (81) calculated their sample size based on the number of expected successes in each treatment, which was based on preliminary data. They used a statistical power of 80% (n = 50). Kemler et al. (63) based their sample size on a projected 2.3 cm difference in VAS scores between the SCS-treated group and the control group. They used a statistical power of 90% (n = 54).
Both groups of authors stated they did an intention-to-treat analysis. However, North et al. analyzed their results using the number of patients randomized and treated, not the number randomized. Kemler et al. did the intention-to-treat analysis at 6 months post-treatment; however, they excluded data from 2 patients from their 2-year analysis, including that for 1 control patient who received a spinal cord stimulator and 1 patient in the SCS treatment group who required a special SCS lead after 6 months.
Both studies accounted for dropouts and or withdrawals. North et al. (81)had 4 withdrawals and 1 death that was unrelated to treatment in the SCS group. No one withdrew from the control group. Kemler et al.(63) had 3 withdrawals at 2 years, 1 in the SCS plus physiotherapy group and 2 in the physiotherapy only group.
Quality of the Level 3a Nonrandomized Controlled Trials
The 2 prospective non-RCTs (42;83) in this review each used before-and-after-treatment study designs. Neither determined sample sizes before doing the study. Both outlined the inclusion and exclusion criteria. Harke et al. (83) stated they enrolled consecutive cases. However, they did not specify a primary outcome. Spincemaille et al. (42) prospectively enrolled eligible patients from 14 centres (personal communication with author, February 16, 2005). Eligible patients were registered with an independent research centre, which assigned the patient a unique study number. They stated that the primary outcome variable was pain reduction measured with VAS, the MPQ, and the Medication Quantification Scale. They adequately described their statistical analysis, whereas Harke and colleagues did not define the level of significance they used. Neither did an intention-to-treat analysis.
Neither the RCTs nor the non-RCTs were double-blinded. Two studies, the RCT by North et al. (81) and the non-RCT by Spincemaille et al., (42) used a disinterested third-party evaluator to collect outcome data. Neither Kemler et al. (63) nor Harke et al. (83) described how they collected outcome data. All studies used the VAS for pain to measure the effectiveness of SCS. Details on outcome are described further in this report.
Blinding is difficult in RCTs of SCS because of the paresthesia that accompanies the test stimulation.(72) Kemler et al. (72) suggest that a placebo effect is unlikely in general because of the recurrence of pain when the electrode position shifts.
Of the 2 RCTs, Kemler et al. (63) included 54 people randomly allocated in a 2:1 ratio to either SCS and physiotherapy (treatment group) or only physiotherapy (control group). They randomized 36 people to the treatment group to undergo the testing phase of SCS. Of these, 24 received a permanently implanted spinal cord stimulator. They randomized 18 people to the control group.
North et al. (81) enrolled 50 people who were randomly allocated in a 1:1 ratio to receive either a reoperation or SCS for FBSS. Of these, 24 received permanent implantation of the spinal cord stimulators and 26 had reoperations.
A total of 133 study subjects were enrolled in the 2 prospective non-RCTs.(42;83) The sample sizes were 28 in the study by Harke et al. (83) and to 105 in the study by Spincemaille et al. (42)
Pain medication was used concurrently with study treatment by people in all 4 studies. However, inclusion criteria in each study required participants to have failed pharmacological therapy before participating in the study. North et al. (81) reported that all subjects were managed with a routine physical therapy protocol. However, all study subjects had previously failed to obtain adequate pain relief with physical therapy treatment.
Table 15 shows the measures of pain intensity, functionality, and QOL in the 4 studies included in the Medical Advisory Secretariat’s systematic review.
Table 15: Outcome Measures Used in the 4 Studies Included in the Medical Advisory Secretariat’s Systematic Review.
| Study, Year | Level of Evidence | Pain | Functionality | Quality of Life |
|---|---|---|---|---|
| North et al. (81) 2005 | 2 | % crossover to alternate treatment % success defined as at least 50% pain relief on VAS* and satisfaction with treatment |
Ability to perform daily activities | Not assessed |
| Kemler et al., (63) 2004 | 2 | VAS: 0 cm = no pain; 10 cm = very severe pain. McGill Pain Questionnaire Global Perceived effect (1, worse ever; 2, much worse; 3, worse; 4, not improved and not worse; 5, improved; 6, much improved, 7, best ever. |
Test of Jebsen et al.* Kemler foot test Goniometry: Range of motion of both ankles or both wrists and all fingers Jamar dynamometer grip strength Hand held myometer strength of foot dorsi and plantar flexion |
Nottingham Health Profile Euroquol-5D Sickness Impact Profile-Short Version The Self-Rating Depression Scale |
| Spincemaille et al., (42) 2004 | 3a | VAS: 0 cm = no pain; 10 cm = worse pain ever. McGill Pain Questionnaire |
ROLAND disability score | Sickness Impact Profile-68 Euroquol-5D |
| Harke et al., (83) 2002 | 3a | VAS: 0 points = no pain; 10 points = unbearable pain | Pain disability index: 0, no disability; 10, total disability | Not assessed |
VAS indicates visual analogue scale of pain.
Outcome Measures
As Table 15 shows, each study used the VAS to measure perceived pain intensity. North et al. (81) reported using a VAS but did not provide details of the VAS scale itself. North et al.(81) and Kemler et al. (63) reported the proportion of study subjects obtaining at least 50% pain relief as measured by a VAS. These data were used to derive the NNT estimates, which are reported in the analysis section of this review.
Population Characteristics
The study populations, sex, average age, average duration of pain before study treatment, and previous pain therapies used are shown in Table 16.
Table 16: Study Population Characteristics.
| Study, Year | Level of Evidence |
N Population |
Average (SD) Age, Years |
Sex, % Male |
Average (SD) Duration of Pain, Months |
Therapies Failed |
|---|---|---|---|---|---|---|
| North et al., (81) 2005 | 2 | 50 Failed back surgery syndrome with radiculopathy |
52.0 (13.5) | 48 | Not reported | Non-invasive medical, physical, and behavioural therapies. |
| Kemler et al., (63) 2004 | 2 | 54 Complex regional pain syndrome, Type I |
Treatment 40.0 (12.0) Control 35.0 (8.0) |
Treatment 39 Control 17 |
Treatment 40 (28) Control 34 (22) |
Physiotherapy, sympathetic blockade, TENS, pain medication |
| Spincemaille et al., (42) 2004 | 3a | 105 Failed back surgery syndrome |
52.5 (9.5) | Not reported | 138 (115) | Physiotherapy, TENS, local infiltration, NSAIDS, tricyclic anti-depressants, morphine or analogues. |
| Harke et al., (83) 2002 | 3a | 28 Postherpetic neuralgia Comorbid conditions: CVS; brain, lung, endocrine disorders; cancer |
71.2 (8.4) | 43 | 41.0 (35.5) | Weak and strong opioids, antidepressants, anticonvulsants, analgesics, and corticosteroids. |
As Table 16 shows, the study populations comprised people diagnosed with FBSS, CRPS, and postherpetic neuralgia. North et al. (81)did not report the ages separately for the treatment and control groups. Patients with postherpetic neuralgia were older compared with patients who had other conditions in the other 3 studies. This is keeping with the incidence pattern for this disease. All patients with postherpetic neuralgia had comorbid conditions. The minimum average duration of pain was 34 months. Patients across all studies had failed to achieve pain relief with standard pharmacological or nonpharmacological therapies before enrolling in the studies.
Treatment Characteristics
Table 17 shows the type of lead used during trial stimulation, the location by vertebral level, the_duration of test stimulation, the type of spinal cord stimulator permanently implanted, the stimulation parameters, and the average duration of follow-up.
Table 17: Spinal Cord Stimulation Treatment Characteristics.
| Study, Year | Test Stimulation Leads |
Electrode Position |
Duration of Test Phase/% Success |
Technology Used for Implantation: Generator/ Leads |
Parameters | Average (SD) Duration of Follow-up, Months |
|---|---|---|---|---|---|---|
| North et al., (81) 2005 | Percutaneous | Not reported | 3 days/70% | IPG* or radio frequency receiver/ Surgically inserted leads | Not reported | 34.8 (13.2) |
| Kemler et al., (63) 2004 | Percutaneous | C4 T12 |
7 days/67% | IPG/Percutaneous leads | 85 Hz 210 μsec 0–10 volts |
24 |
| Spincemaille et al., (42) 2004 | Not described | Not reported | Not reported/78% | Not reported/Not reported | Not reported | 12 |
| Harke et al., (83) 2002 | Percutaneous | Not reported | 5–7 days/100% | IPG/Percutaneous leads | 50—130 Hz 90—450 μsec 1—6 volts |
Median, 29 (range, 9–38.5) |
IPG indicates implantable pulse generator.
As Table 17 shows, percutaneously inserted leads were used for the test stimulation in 3 of the 4 studies. One study did not report the type of lead used. A successful test stimulation period was defined in all studies as at least 50% pain relief, which occurred in 67% to 100% of people tested. Two studies used an IPG, 1 used both an IPG and a radio frequency receiver/transmitter, and 1 did not report the type of device used. The minimum average duration of follow-up was 12 months, and the maximum was approximately 35 months (SD, 13).
Results
VAS Pain Scores
Table 18 shows the VAS pain scores either between treatment and control groups, or before and after receipt of SCS for each study.
Table 18: Visual Analogue Scale Scores for Pain.
| Study, Year | N | N for Analysis |
Average (SD) VAS Score at Follow-up |
Comment | P |
|---|---|---|---|---|---|
| North et al., (81) 2005 | 50 | 45 | Score not reported Number of people achieving at least a 50% decrease in pain intensity on the VAS* Treatment: 9/19 (47.3) Control: 3/26(11.5%) |
< .01 | |
| Kemler et al., (63) 2004 | 54 | 52 | VAS: Treatment: -2.1 (2.8) Control: 0.0 (1.5) |
Results reported as within-group mean change in VAS scores. (Negative value indicates a reduction on the VAS.) Results reported for the intention-to-treat analysis. Comparison of mean change between treatment and control groups is significant. |
.001 |
| Spincemaille et al., (42) 2004 | 105 | 96 | Before: 7.3 (1.2) After: 3.0 (2.4) |
< .05 | |
| Harke et al., (83) | 25 | 23 | Before: median, 9.0 (range, 7.5—10.0) After: median, 1.0 (range, 1.0–2.75) |
< .001 |
VAS indicates visual analogue scale of pain.
As Table 18 shows, 3 studies reported a significant decrease in pain scores with SCS compared with either a control group, or after treatment with SCS compared with baseline scores. (42;63;83) North et al. (81) found significantly more patients who had SCS experienced at least a 50% reduction in their VAS scores compared with people who had reoperations. However, 4 study subjects in the SCS group were lost to follow-up. Because of this, North et al. (81) reported a worse-case-scenario analysis. Thus, assuming that all patients lost to follow-up in the SCS group did not improve, the success rate for SCS would be 9/23 (39%) instead of 9/19 (47.4%). Comparing this to the 11.5% (3/26) success rate in the reoperation group, the difference is statistically significant at the P < .04 level. Harke et al. (83) did not designate pain as a primary outcome measure but completed statistical testing on 7 outcome measures. To correct for multiple comparisons a conservative approach would be to adjust the level of statistical significance using a Bonferroni correction of .05/7. This would yield a statistical significance level of .007. The level of significance for the VAS scores before and after treatment was < .001. Therefore, it is unlikely that this result represents a type I statistical error.
Other Pain Measurements
In addition to using the VAS of pain, all of the studies used other methods to quantify pain relief. Results of these pain measurements are shown in Table 19.
Table 19: Other Pain Measurements Used Across Studies.
| Study, Year | N | N for Analysis | Measurement | Result | P |
|---|---|---|---|---|---|
| North et al., (81) 2005 | 50 | 45 | Opioid intake | For increase in opioid use: Treatment: 3/23 (13%) Control: 11/26 (42%) |
.025 |
| Kemler et al., (63) 2004 | 54 | 52 | Global Perceived Effect of treatment score of 6: “much improved” | Treatment: 15/35 (43%) Control: 1/16 (65%) |
.001 |
| Spincemaille et al., (42) 2004 | 105 | 96 | McGill Pain Questionnaire Medication quantification scale |
Mean (SD) Before: 22.4 (9.4) After: 10.8 (8.0) Before: 11.5 (7.9) After: 6.05 (4.8) |
< .05 < .05 |
| Harke et al., (83) 2002 | 25 | 23 | Analgesic consumption | Needed pain medication during SCS Yes: 10/23 (43.5%) No: 13/23 (56.5%) Opioid used: Before: 19/23 (82.6%) After: 1/23 (4.5%) |
.02 .002 |
As Table 19 shows, both Spincemaille et al. (42) and Harke et al. (83) reported a statistically significant decrease in pain medication consumption after SCS compared with before treatment with SCS. Harke et al. (83) noted that 14 patients in their study continued to take antidepressants for symptoms of depression after SCS; however, they denied an objective effect of antidepressants on pain because of the recurrence of pain when the SCS device was turned off. Kemler et al. (63) reported a significant decrease in pain intensity as measured by the MPQ and also reported that more people in the SCS plus physiotherapy treatment group reported they were “much improved” (score of 6/7) on the Global Perceived Effect Scale compared with people in the control group.
Functional Status
Results of the functional status measurements for each study are reported in Table 20.
Table 20: Functional Status Outcome.
| Study, Year | N | N for Analysis |
Measurement | Functional Status Score, Average (SD) |
P |
|---|---|---|---|---|---|
| North et al., (81) 2005 | 50 | 45 | Not described | Scores not reported | Not significant |
| Kemler et al., (63) 2004 | 54 | 31 (upper extremity) 19 (lower extremity) |
Range-of-motion tests for upper and lower extremities. | All range-of-motion tests not significant except for that for the ankle, measured in degrees: SCS +*PT group (mean change from baseline at 2-year follow up): 0 (16) *PT only group (mean change from baseline at 2-year follow up): 13 (8) |
< .04 (intention-to-treat analysis) |
| Spincemaille et al., (42) 2004 | 105 | 96 | ROLAND Disability Questionnaire | Before: 16.9 (3.5) After: 12.4 (4.8) |
< .05 |
| Harke et al., (83) 2002 | 25 | 23 | Disability Index: 0 = no disability 10 = total disability |
Scores not reported | < .001 |
PT=physiotherapy
All of the functional status outcomes were secondary outcome measures in each of the 4 studies under review. As table 20 shows, different instruments were used to quantify the effect of SCS on functional status. Spinacemaille et al. (42) and Harke et al. (83) reported a statistically significant improvement in functional status measurements after SCS compared with baseline values. Spincemaille et al. (42) reported an improvement at 12 months, and Harke et al. (83) found a significant improvement at a median follow-up time of 29 months in people with postherpetic neuralgia.
However, no improvement in functional status was reported in either of the RCTS at 2-year follow-up. It is likely that because this was a secondary measure in each study, neither RCT had adequate statistical power to detect a difference in this outcome measure. While Kemler et al. (63) reported a significant improvement in the range of motion of the ankle in the control group compared with the SCS treatment group (P < .04), this may have been due to a type I error, because 10 statistical comparisons, excluding the primary end point, were done without statistical adjustment for multiple testing.
Quality of Life
Results of the QOL assessments for each study are shown in Table 21.
Table 21: Results of Quality of Life Assessments Across Studies.
| Study, Year | N | N for Analysis |
Measurement | Average (SD) Quality of Life Score |
P |
|---|---|---|---|---|---|
| North et al., (81) 2005 | 50 | 45 | Not assessed | ||
| Kemler et al., (63) 2004 | 54 | 52 | Nottingham Health Profile Euroquol-5D Sickness Impact Profile-SF Self-Rating Depression Scale |
Not reported Treatment: 7 (20) Control: 12 (18) Not reported Not reported |
Not significant .41 Not significant Not significant |
| Spincemaille et al., (42) 2004 | 105 | 96 | Euroquol-5D Sickness Impact Profile-68 |
Before: 55.2 (14.5) After: 38.2 (19.2) Before: 19.4 (10.1) After: 11.7 (9.4) |
< .05 < .05 |
| Harke et al., (83) 2002 | 25 | 23 | Not assessed | ||
As Table 21 shows, only 2 studies assessed the effect of SCS on QOL, and QOL was a secondary outcome for both studies. Kemler et al. (63) did not find a statistically significant difference in the QOL scores at 2-year follow-up between the SCS treatment group and the physiotherapy control group. However, Spincemaille et al. (42) reported a statistically significant difference in the Euroquol-5D and Sickness Impact Profile-68 scores at 12 months compared with baseline scores.
Numbers Needed to Treat
Two RCTs reported dichotomous outcome data on the proportion of successes defined as achieving at least 50% pain relief. (63;81) The NNT are presented in Table 22.
Table 22: Success at 2-year Follow-Up.
| Study, Year | Group | Success Treatment Group |
Success Control Group |
Number Needed To Treat |
|---|---|---|---|---|
| North et al., (81) 2005 | Test group* Implanted group* |
9/24 (37.5%) 9/17 (52.9%) |
3/26 (11.5%) 3/26 (11.5%) |
3.8 2.4 |
| Kemler et al., (63) 2004 | Test group Implanted group |
13/36 (36.1%) 13/24 (54.2%) |
1/18 (5.6%) 1/18 (5.6%) |
3.3 2.1 |
The test group includes all candidates who underwent a test stimulation phase. The implanted group includes only those candidates that received a permanently implanted spinal cord stimulator. North et al. (81) defined success as at least 50% pain relief and patient satisfaction with treatment Kemler et al.(63;72) defined success as 50% decrease on the VAS after SCS, compared with baseline scores.
As Table 22 shows, Kemler et al. (63) compared a group that received SCS plus physiotherapy with a control group receiving only physiotherapy for neuropathic pain. North et al. (81) compared patients who received SCS to control patients that had reoperations. The NNT for the test group for both studies is between 3 and 4. Therefore, for every 3 to 4 patients who have test stimulations, 1 will be successful, which is defined as having at least 50% pain relief 2 years after permanent implantation. For every 2 people who have a permanent implantation, 1 will be a successful 2 years after implantation.
Technical Failures and Procedural Complications
Technical failures and procedural complications are reported in Tables 23 and 24.
Table 23: Technical Failures With Spinal Cord Stimulation Across Studies.
| North et al., (81) 2005 |
Kemler et al., (63) 2004 |
Spincemaille et al., (42) 2004 |
Harke et al., (83) 2002 |
Total Technical Failures |
Total Technical Failures/166 Cases, % |
|
|---|---|---|---|---|---|---|
| No. of SCS* cases | 19 | 23 | 96 | 28 | ||
| Duration of follow-up, months | 34 | 24 | 12 | 29 | ||
| Lead problems | 3 | 10 | 2 | 3 | 18 | 10.8 |
| IPG* problems | 0 | 8 | 0 | 9 | 17 | 10.2 |
| Explant IPG | 1 | 3 | 0 | 2 | 6 | 3.6 |
| Re-implant IPG | 1 | 1 | 0 | 0 | 2 | 1.2 |
SCS indicates spinal cord stimulation; IPG, implantable pulse generator.
Table 24: Procedural Complications With Spinal Cord Stimulation Across Studies.
| North et al., (81) 2005 |
Kemler et al., (63) 2004 |
Spincemaille et al., (42) 2004 |
Harke et al. (83) 2002 |
Total Complications |
Total Complications/ 166 cases, % |
|
|---|---|---|---|---|---|---|
| No. of SCS* cases | 19 | 23 | 96 | 28 | ||
| Follow-up, months | 34 | 24 | 12 | 29 | ||
| Infection | 1 | 1 | 0 | 0 | 2 | 1.2 |
| Dural puncture | 0 | 2 | 0 | 0 | 2 | 1.2 |
| Dural puncture headache | 0 | 1 | 0 | 0 | 1 | 0.6 |
| Recurrent device rejection | 0 | 1 | 0 | 0 | 1 | 0.6 |
| Relapsing ulcerative colitis | 0 | 1 | 0 | 0 | 1 | 0.6 |
| Death (unrelated to SCS) | 1 | 0 | 1 | 0 | 2 | 1.2 |
SCS indicates spinal cord stimulation.
As Table 23 shows, there were lead problems in approximately 11% of 166 SCS cases. These included lead migration and malposition. The incidence of IPG problems was 10.2%. Problems with the IPG included revision of the IPG implantation site, and replacement of the IPG due to battery failure. The IPG battery was explanted due to infection, recurrent rejection, battery failure, and failed therapy. Harke et al. (83) explanted 2 spinal cord stimulator devices because of progressive dementia most likely related to comorbid illnesses.
Device-related infection has been reported as the most common adverse event associated with SCS. (61) As Table 24 shows, 2 studies included in this systematic review reported infections. The overall infection rate was 1.2%. Kemler et al. (63) reported clinical signs (not culture positive) of infection in 1 subject who required antibiotic treatment and removal of the SCS device. North et al. (81) reported an infection at the implantation site of a neurostimulator radio frequency receiver. The SCS device was explanted and antibiotic therapy was administered.
Two studies each reported 1 death. In the RCT by North et al. (81) one SCS-treated study subject died suddenly of a cardiac event shortly after 6 months of treatment. Spincemaille et al. (42) reported 1 death but did not provide details on the cause.
Summary of Findings of Medical Advisory Secretariat Literature Review
Table 25 summarizes the levels of evidence for the 3 neuropathic pain conditions studied in the clinical trials included in this systematic review.
Table 25: Summary: Levels of Evidence by Neuropathic Condition and Outcome of Interest.
| Primary Outcome for Systematic Review |
Secondary Outcomes for Systematic Review | ||
|---|---|---|---|
| Neuropathic Pain Condition | Pain Relief | Functional Status | Quality of Life |
| Failed back surgery syndrome | Level 2 (1 study) Level 3a (1 study) |
*Level 3a (1 study) | *Level 3a (1 study) |
| Complex regional pain syndrome Type I | Level 2 (1 study) | *Lack of evidence evidence | *Lack of evidence |
| Postherpetic neuralgia | †Level 3a (1 study) | †Level 3a (1 study) | Not assessed |
Secondary measure for study.
A multiple outcome measure for study.
Pain Relief
The following summarizes the level of evidence to support the effectiveness of SCS to relieve pain by at least 50% as measured by the VAS in the 3 main neuropathic medical conditions of interest for this review.
Failed back surgery syndrome: There is level 2 evidence from 1 study of high quality and level 3a evidence from one study of high quality for the use of SCS for neuropathic limb pain secondary to failed back surgery. Pain relief was qualified as a primary outcome in both level 2 and level 3a studies.
Complex regional pain syndrome, Type I: There is level 2 evidence from 1 high-quality study for the use of SCS for neuropathic limb pain associated with this neuropathic condition. Pain relief was qualified as a primary outcome in the study.
Postherpetic neuralgia: There is Level 3a evidence from 1 study with a small sample size (n = 28). Pain relief was a multiple outcome measure in the study.
Functional Status
The following summarizes the evidence supporting the effectiveness of SCS to improve functional status as measured by the ROLAND Disability Questionnaire and the Pain Disability Questionnaire in the 3 main conditions of interest in this review.
Failed back surgery syndrome: There is level 3a evidence from one study of high quality for the use of SCS to improve functional status in people with neuropathic limb pain secondary to failed back surgery. Functional status was a secondary outcome in the study.
Complex regional pain syndrome, Type I: There is a lack of evidence for the use of SCS to improve the functional status of people with neuropathic pain associated with this condition. Functional status was a secondary outcome. A lack of evidence may reflect a type II statistical error.
Postherpetic neuralgia: There is level 3a evidence from 1 study with a small sample size, (n = 28). Functional status was a multiple outcome measure in the study.
Quality of Life
The following summarizes the level of evidence to support the effectiveness of SCS to improve QOL in the 3 main conditions of interest in this review.
Failed back surgery syndrome: There is level 3a evidence from 1 study of high quality for the use of SCS to improve the QOL in people with neuropathic limb pain secondary to failed back surgery. QOL was a secondary outcome in the study.
Complex regional pain syndrome, Type I: There is a lack of evidence for the use of SCS to improve the QOL of people with neuropathic pain associated with this condition. A lack of evidence may reflect a type II statistical error.
Postherpetic neuralgia: QOL was not evaluated in this patient population.
Technical Failures and Complications
The results of the literature review showed that the most common technical failures were lead problems (10.8%) and IPG problems (10.2%). The most common procedural complications were infection (1.2%) and dural puncture (1.2%). None of the studies reviewed reported treatment-related deaths.
Economic Analysis
Ontario-Based Economic Analysis
Disclaimer: This economic analysis represents an estimate only, based on assumptions and costing methodologies that have been explicitly stated. These estimates will change if different assumptions and costing methodologies are applied for the purpose of developing implementation plans for the technology
Hospitalization Costs
Using a combination of International Classification of Disease 10 codes (ICD-10) and Canadian Classification of Intervention (CCI) Codes (Appendix 4) the number of SCS related hospitalizations per fiscal year was estimated from the discharge abstracts database. 53 related hospitalizations were identified for fiscal year 2002 and 32 for fiscal year 2003. Therefore, a range of 32 to 53 SCS related hospitalizations annually was used to calculate hospitalization costs.
To determine the cost in Canadian dollars per SCS case, the prospectively adjusted for complexity resource intensity weights known as PAC-10 weights were used. The PAC-10 weights are based on a weight of 1.0 having a dollar value of $4,505 during 2003 (Personal communication, Ministry of Health and Long-Term Care, May 2005). The average PAC-10 weight for SCS related hospitalizations in fiscal year 2003 is 1.54. The 2002 average PAC-10 weight was within 0.1 of this number. Therefore 1.54 was considered to be the overall average for both fiscal years and the cost per SCS related hospitalizations was estimated at $6,956 (1.54 × $4,505). The associated cost for the annual range of SCS related hospitalizations (32-53) for the past 2 fiscal years is between $223,000 and $369,000. It is important to note that the estimated cost per SCS case of less than $7,000 does not cover the hospital’s cost of purchasing the SCS device.
Device Costs
To obtain the price of the SCS device please consult Medtronic, Inc. http://www.medtronic.com or Advanced Neuromodulation Systems http://www.ans-medical.com.
Professional Costs
This section outlines the Ontario Health Insurance Policy (OHIP) costs for SCS treatment. SCS treatment involves a psychological assessment, then surgery to insert the trial SCS lead (trial phase) which takes approximately 2 hours. 70% of the time this will lead to the insertion of a permanent SCS device (permanent phase). 6 postoperative visits with either a neurosurgeon or a neurologist are estimated after permanent implantation of the SCS device.
All possible candidates for SCS undergo a psychological assessment. However, not all will be successful and proceed to the trial phase. Therefore, more people will have a psychological assessment than will eventual receive treatment with SCS. An estimated 70% of people having a psychological assessment will proceed to the trial phase. Of the patients who undergo the trial phase, about 70% to 80% will have a successful trial course of SCS and will be candidates for permanent SCS implantation. The following fees have been adjusted upward by 2% to reflect the new OMA agreement.
Table 26: Estimated OHIP costs for SCS treatment.
| Treatment Phase | Cost (Canadian Dollars) | Fee Schedule Codes (FSC) and description of code | |
|---|---|---|---|
| A | Total costs for psychological Assessment | $51 | FSCK032: Physician reimbursement for neurocognitive assessment. Ontario fee schedule code |
| Trial phase | $328.24 | FSC244: Physician reimbursement for percutaneous diagnostic stimulation of brain or spinal cord or trigeminal nerve root and/or ganglion (IOP). | |
| $240.11 | Upper limit of expected cost for anesthesia services for the trial phase surgery The Anesthetist costs are the number of units/case and a unit cost of $12.01 for an anesthetist. For the trial phase an upper limit of 20 units are estimated. This includes 8 base units + 1 unit for each 15 minutes in the first hour of treatment + 2 units for every 15 minutes thereafter) |
||
| B | Total professional fees for trial phase | $568 | |
| Permanent insertion phase | $307.38 | FSCZ823: Physician payment for implantation/revision of stimulation pack leads | |
| $328.24 $384.17 |
FSCZ244: Physician reimbursement for percutaneous diagnostic stimulation of brain or spinal cord or trigeminal nerve root and/or ganglion (IOP) Total costs for anesthesia services for permanent insertion phase. Anesthetist costs for permanent insertion phase include 8 base units + 1 unit for each 15 minutes in first hour + 2 units for every15 minutes thereafter. A unit fee for anesthetists is $12.01 Two FSC charges are used for this phase: FSCZ244 charged for 2 hours of service and FSC823 charged for 1 hour of service). Assumption: Base units for each FSC code of 8 units + time units of 32 units = expected number of units (8 base units under FSCZ823 + 8 base units under Z244 + 12 time units under FSCA823 + 4 time units under FSCZ244) |
||
| C | Total Profession medical fees for Permanent insertion phase | $1,020 | |
| D | Follow-up assessments | Any of the following FSC codes are applicable: | |
| $127.50 $25.14 $102.00 $26.52 |
FSCA185: neurology consult FSCA188: neuorology partial assessment FSCA045: neurosurgery consult FSCA044: neurosurgery partial assessment |
||
| Total expected post operative physician costs | $333 | Estimated 1 consult by each of neurosurgery and neurology plus 2 partial assessments by each | |
| Total estimated professional medical fees per SCS case | $2,270 | Use total costs found in row A, B, C, D $51/(70%*70%) + $568/70% + $1,020 + $333 |
|
| Total estimated professional medical fees based on 53 annual permanent insertion phases. | $120,286 | 53 × $2270 |
Downstream Cost Savings
SCS procedures are known to reduce the need for medications to treat neuropathic pain. However, because these medications are prescribed for a number of ailments, and because the patterns of prescribing are not easily assessed specifically for neuropathic pain, it is difficult to quantify the potential cost offsets of SCS. Based on previous experiences the present value of a lifetime use of prescription pain medications would exceed $10,000, which would offset a large portion of the total costs associated with SCS treatment (estimated at approximately $20,000). It is important to note, however, that not all drug costs savings would accrue to the province, because the Ontario Drug Benefit program only covers 23.6% of the residents of Ontario.
Cost-Effectiveness
A number of studies indicate that although SCS has high up-front costs compared with conventional therapy, in the long-term, it saves costs. A study (84) at the Cleveland Clinic in the United States of 222 consecutive patients followed-up for a average of 3.1 years postoperatively found a $17,963 (US dollars) net per-patient per-year savings compared to medical cost before SCS treatment. This was primarily due to a drop in other surgical procedures and medical imaging investigations such as magnetic resonance imaging or computed tomography scans).
Similarly, a prospective matched cohort Canadian study (85) comparing SCS with conventional pain therapy found cost-savings of approximately $11,000 (Canadian dollars) over 5 years postoperatively and a break-even in costs at 2.5 years.
A meta-analysis of 14 cost-effectiveness studies (76) of SCS confirmed a finding of long-term cost-savings associated with this intervention.
Existing Guidelines for Use of Technology
Several professional groups have published guidelines for the use of SCS.
European Task Force
A consensus statement prepared by the Task Force of the European Federation of the International Association for the Study of Pain (IASP) (56) was published in 1998. These guidelines recommend that SCS be used “only in those patients in whom well conducted, more conservative pain treatments have failed, and there is no indication for further surgical intervention to treat the underlying pathology.” SCS was recommended in the following conditions:
Neurogenic pain conditions
Mixed neurogenic and nociceptive pain conditions (FBSS)
Intractable angina pectoris
Peripheral vascular disease
American Society of Anesthesiologists
The American Society of Anesthesiologists (86) has published guidelines for the management of chronic pain. They state: “Spinal cord stimulation should not be a first-line treatment but may be considered after failure of oral medications. Spinal cord stimulation may be effective in the management of patients with peripheral neuropathic pain or with pain arising from the spinal cord (arachnoiditis, syringomyelia, spinal cord injury, and multiple sclerosis). It should be preceded by a trial with a percutaneous electrode system.”
Consensus Statement
Canadian Pain Society
The Canadian Pain Society does not have guidelines for the use of SCS in chronic neuropathic pain. However, a published consensus statement (12) for the use of opioid analgesics in the treatment of chronic non-cancer pain mentions SCS as a palliative surgical procedure.
Policy Considerations
Demographics
The number of people in Ontario with neuropathic pain due to FBSS, CRPS, and postherpetic neuralgia has been estimated as shown below
Failed Back Surgery Syndrome
About 15% to 40% of patients will have chronic back and limb pain after lumbar surgery. Based on fee schedule codes from provider services, about 5343 spine surgeries have been completed yearly between 2001 and 2003. Of these, it is estimated that 15% to 40%, or 801 to 2137, will develop chronic back and limb pain.
Complex Regional Pain Syndrome
The incidence of CRPS is estimated at 5.46 cases per 100,000 people. Using the Ontario Ministry of Finance 2001 census, the number of people between the ages of 15 and 79 with CRPS is estimated at 514 (based on 9,416,627 people between the ages of 15 and 79).
Postherpetic Neuralgia
The incidence of herpes zoster in Canada has been estimated at 423 per 100,000 population-years for people aged 45 to 64, and 812 per 100,000 population-years for people aged 65 years or older. Using the Ontario Ministry of Finance 2001 census, an estimated 12,093 new cases of herpes zoster will occur per year in people aged 45 to 64 (population estimate 2,858,898), and 9373 cases will occur in people aged 65 to 79 (population estimate of 1,154,335). Twenty per cent of people older than 50 years who receive treatment will experience pain 6 months after the onset of the herpes zoster rash. (25)This yields an estimated 4293 people with postherpetic neuralgia.
It has been estimated that approximately 10% and up to 20% of people with neuropathic pain may develop intractable pain (personnel communication, December 14, 2004). Of these, about 70% will proceed to test stimulation after psychological evaluation. As this systematic review shows, 67% to 100% (average, 84%) of people undergoing test stimulation will be successful and proceed to SCS implantation. Using these estimates, and the estimates of the incidence of FBSS, CRPS and postherpetic neuralgia, the number of people in Ontario that would need SCS (target population) has been derived.
Estimate of Target Population
The lower estimate of FBSS, 15% of all spine surgeries, has been used to take into account this unknown estimate. Therefore, if we consider those with FBSS, CRPS, and postherpetic neuralgia, the estimated number of people in Ontario per year that would benefit from SCS is as follows:
| (A) | People with FBSS (15% of spine surgeries) + CRPS + postherpetic neuralgia: 5608 (801 + 514 + 4293) | |
| (B) | 10% to 20% of those in (A) will develop intractable pain: | 561–1122 |
| (C) | 70% of those in (B) will proceed to test stimulation after a psychological evaluation: | 393–785 |
| (D) | On average 84% of those in (C) will have a successful test stimulation and proceed to SCS implantation: | 330–660 |
Therefore it is estimated that 330-660 people per year would benefit from SCS treatment.
Number of Spinal Cord Stimulation Devices Implanted in People in Ontario
The number of SCS devices implanted in people in Ontario in the last 2 years was estimated from the number of SCS related hospitalizations extracted from the Provincial Health Planning Database using the CCI codes for the SCS procedure and the appropriate ICD-10 diagnosis codes. For the 2002 fiscal year, 53 patient separations were captured. For the 2003 fiscal year, 32 were captured. Therefore, about 30 to 50 people in Ontario are receiving SCS per year.
Diffusion of Technology
In 1998, it was estimated (56) that each year 15,000 patients world-wide (5000 in Europe alone) were using SCS.
Number of Sites Offering Spinal Cord Stimulation Therapy
Eight hospitals in Ontario implant spinal cord stimulators in people, but not all are active. One site does approximately 30 SCS implantations per year. Two sites have closed their program due to a lack of infrastructure and funding support, and 5 sites do only a few implantations (approximately fewer than 10 per year).
Health System Considerations
Infrastructure
Neuropsychological resources are required to assess the eligibility of patients for SCS. More patients have psychological testing to determine suitability for SCS than actually receive SCS treatment.
As more patients receive the SCS implants, the number of patients requiring long-term management will increase, which will require more downstream human resources to manage the case load. A dedicated nurse specifically trained in neuromodulation therapy (neuromodulation nurse) would facilitate patient assessment in the operating room during lead insertion and electrode placement, and during the post-operative clinic visits and long-term management. The high incidence (approximately 11% from the systematic review) of technical complications, in particular lead migration, makes patient assessment demanding in the first year.
Need to attract interest from medical specialists, including anesthesiologists and neurosurgeons, to support the program.
Operating room time needed to manage technical failures, which are prevalent in the first year.
Equipment
SCS supplies are required, including electrodes for test stimulation and the full device for patients that proceed to permanent implantation.
Conclusions
Level 2 evidence from 2 studies of high quality supports the effectiveness of SCS to reduce pain in some neuropathic pain conditions.
There is supportive evidence from secondary outcomes from level 3a evidence that treatment with SCS improves functional status and QOL.
The need for SCS services is estimated at 330 to 660 people per year.
Current services provide SCS to 30 to 50 people per year.
Glossary
- Adjuvant therapy
A therapy that is added to a primary therapy to increase the effectiveness of the primary therapy
- Afferent nerves
A nerve that carries impulses toward the central nervous system; the opposite is an efferent nerve
- Analgesia
The relief of pain without loss of consciousness
- Chronic pain
Persistent, long-term pain that cannot be removed
- Complex regional pain syndrome
A chronic pain condition associated with intense, continuous pain that does not improve with time and that most often affects one of the arms, legs, hands, or feet; also called reflex sympathetic dystrophy syndrome and causalgia
- Dermatomes
Localized areas of the body that are supplied with afferent nerves from a single spinal nerve; responsible for pain and other sensations
- Epidural space
The space between the dura mater and the walls of the vertebral canal, containing venous plexuses and fibrous and alveolar tissue
- Failed back surgery syndrome
A generalized term that is often used to describe the condition of patients who have not had a successful result with back surgery or spine surgery
- Herpes zoster
An acute, localized infection caused by the varicella-zoster virus that produces a painful, blistering rash; also called shingles
- Hyperesthesia
Extreme sensitivity to normal touch, pain, or other stimuli that can manifest as a painful sensation
- Incidence
Number of new cases of a disease over time.
- Interquartile range
Used to express the inner 50% of values (the range between the 75th and 25th percentiles)
- Intractable pain
Pain that does not respond to treatment
- Ischemic pain
Pain felt throughout the chest, typically as squeezing, tightness, pressure, or burning
- Laminotomy
Surgery to cut the lamina also called the vetebral arch, which is a thin, flat bony layer of the vertebrae (back bone) that covers the spinal canal
- Median
A distribution’s midpoint at which exactly one-half of the values fall above and one-half fall below
- Neuromodulation
Electrical stimulation of a peripheral nerve, the spinal cord, or the brain for relief of pain
- Neuropathic pain
Pain caused by damage to the tissue of the peripheral or central nervous system (e.g., a pinched nerve); generally felt as burning or tingling and often happening in an area of sensory loss
- Number needed to treat (NNT)
This is how many patients must be treated with an intervention for a certain period to prevent 1 bad outcome or result in 1 good outcome
- Nociceptive pain
Pain caused by injury or disease outside the central nervous system that is often felt as a dull ache (e.g., pain due to arthritis)
- Opioid
A strong drug to treat moderate to severe pain
- Percutaneous
Done through the skin
- Postherpetic neuralgia
Persistent burning pain and hyperesthesia along the distribution of a cutaneous nerve after an attack of herpes zoster; it may last for a weeks to months
- Prevalence
Total number of people with the disease at any one time
- Quality-adjusted Life-year
The number of life years adjusted by the degree of poor health
- Spinal cord stimulation
A reversible pain therapy that uses low-voltage electrical pulses to manage chronic, intractable neuropathic pain of the trunk or limbs
- Sympathectomy
The transection or interruption (chemical or surgical) of any part of the sympathetic nervous system pathways
- Transcutaneous electrical nerve stimulations (TENS)
A therapy that delivers low-voltage electrical stimulation to the nerves to relieve pain
- Type I error
This happens when data show a statistically significant result, although no true difference or association exists; it often happens when multiple comparisons are done
Appendices
Appendix 1: Literature Search Strategy – Spinal Cord Stimulation
Search date: December 3, 2004
Databases searched: OVID Medline, OVID In Process and Other Non-Indexed Citations, Embase, Cochrane database of Systematic Reviews, Cochrane CENTRAL, INAHTA
Database: Ovid MEDLINE(R) <1996 to November Week 3 2004>
Search Strategy:
----------------------------------------------------------------------
1 exp Electric Stimulation Therapy/ (5057)
2 exp Electrodes, implanted/ (7923)
3 exp Electric Stimulation/ (21613)
4 neuromodulation.mp. (556)
5 exp Spinal Cord/ or exp Spine/ (37230)
6 or/1-4 (33105)
7 5 and 6 (1925)
8 spinal cord stimulat$.mp. [mp=title, original title, abstract, name of substance, mesh subject heading] (423)
9 dorsal column stimulat$.mp. (21)
10 7 or 8 or 9 (2059)
11 exp Pain/ (68779)
12 exp Complex Regional Pain Syndromes/ (961)
13 exp Phantom Limb/ (327)
14 neuropathic pain.mp. (2201)
15 exp Peripheral Nervous System Diseases/ (24699)
16 failed back surgery syndrome.mp. (69)
17 exp Treatment Failure/ (8910)
18 chronic pain$.mp. (4151)
19 exp Arterial Occlusive Diseases/ (36685)
20 or/11-19 (135043)
21 10 and 20 (558)
22 limit 21 to human (375)
23 limit 22 to systematic reviews (24)
24 22 (375)
25 limit 24 to (case reports or comment or editorial or letter or “review” or “review literature” or review, multicase or “review of reported cases”) (177)
26 24 not 25 (198)
27 23 or 26 (213)
28 limit 27 to yr=2000-2005 (123)
Similar search strategy employed for Cochrane CENTRAL
Database: EMBASE <1996 to 2004 Week 48>
Search Strategy:
----------------------------------------------------------------------
1 exp electrostimulation therapy/ (33461)
2 exp electrostimulation/ (11012)
3 exp electrode/ (15373)
4 exp neuromodulation/ (6205)
5 exp electroanesthesia/ (9)
6 or/1-5 (59583)
7 exp spinal cord/ (13319)
8 exp SPINE/ (19341)
9 6 and (7 or 8) (1980)
10 spinal cord stimulat$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (897)
11 dorsal column stimulat$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (29)
12 epidural stimulat$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (35)
13 or/10-12 (935)
14 9 or 13 (2707)
15 exp Pain/ (144201)
16 exp Agnosia/ (820)
17 failed back surgery syndrome.mp. (105)
18 exp Neuropathy/ (68937)
19 exp Treatment Failure/ (19357)
20 or/15-19 (212213)
21 14 and 20 (930)
22 limit 21 to (human and yr=2000-2005) (489)
23 exp “Systematic Review”/ or Meta Analysis/ or systematic review$.mp. or systematic overview$.mp. or meta anlys$.mp. or metaanlys$.mp. [mp=title, abstract, subject headings, drug trade name, original title, device manufacturer, drug manufacturer name] (21567)
24 22 and 23 (15)
25 22 (489)
26 Case Report/ (333388)
27 25 not 26 (408)
28 limit 27 to (editorial or letter or note or “review”) (174)
29 27 not 28 (234)
30 24 or 29 (245)
Appendix 2: College of Physicians and Surgeons of Ontario: Interventions for the Treatment of Neuropathic Pain, 2000
Reprinted with Permission from the College of Physicians and Surgeons of Ontario, Evidence-based recommendations for medical management of chronic non-malignant pain. 2000. (27)
| Condition | Anti- convulsants |
Anti- depressants |
Oral Drugs with Local Anesthetic Type Properties |
Opioids | Topical (Capsaicin) |
Intravenous Regional Sympathetic Blocks |
|---|---|---|---|---|---|---|
| Trigeminal neuralgia | Level I | No controlled trials | Level II | Level V | No controlled trials | Not applicable |
| Peripheral nerve injury | No controlled trials | No controlled trials | Level II | No controlled trials | No controlled trials | No controlled trials |
| Postherpetic neuralgia | Level II | Level II | Level II | Level II | Level II | No controlled trials |
| Complex regional pain syndrome Type I | No controlled trials | No controlled trials | No controlled trials | No controlled trials | No controlled trials | Level III |
| Diabetic neuropathy | Level I | Level I | Level II | Refer to comments in guidelines. | Level II | No controlled trials |
| Pain after stroke | Level II | Level II | No controlled trials | No controlled trials | No controlled trials | No controlled trials |
| Spinal cord injury | No controlled trials | No controlled trials | Level II | No controlled trials | No controlled trials | Not applicable |
| Pain after mastectomy | No controlled trials | No controlled trials | No controlled trials | No controlled trials | Level II | Not applicable |
Level legend:
| Level 1: | Strong evidence from at least 1 systematic review of multiple, well-designed RCTs. |
| Level II: | Strong evidence from at least 1 properly designed RCT of appropriate size. |
| Level III: | Evidence from well-designed trials without randomization, single-group pre-post, cohort, time series, or matched-case controlled studies. |
| Level IV: | Evidence from well-designed nonexperimental studies from more than 1 centre or research group. |
| Level V: | Opinions of respected authorities, based on clinical evidence, descriptive studies, or reports of expert committee. |
Appendix 3: Study Characteristics
| Study, Year | Methods | Participants | Interventions | Outcomes | Notes |
|---|---|---|---|---|---|
| North et al., 2005(81) | RCT - Subjects randomized to SCS or back reoperation (re-op.); - Randomization determined by opening sealed opaque envelopes that contained computer-generated random assignments from an outside biostatistician - Non-blinded study. - Withdrawals/dropouts accounted for. - Quality 3/5 (Jadad score) |
50 patients: Patients with failed back surgery syndrome recruited by 8 spine surgeons. Inclusion criteria: -Patients with surgically remediable nerve root compression with complaints of persistent or recurrent radicular pain with or without low back pain after at least 1 lumbosacral spine surgery -Pain refractory to conservative care -Imaging findings of neural compression Exclusion criteria: -Disabling neurological deficit in the distribution of a nerve root(s) caused by surgically remediable compression -Critical cauda equina compression -Gross instability needing fusion -Untreated dependency on narcotics or benzodiazepines -Major untreated psychiatric comorbidity -Unresolved issues of secondary gain -Concurrent or disabling chronic pain problem, low back pain exceeding radicular pain (hip, buttock and leg pain) |
24 patients were randomized to a test period of SCS Test Period: 3 days with a temporary Medtronic Pisces Quad Percutaneous Leads (3487A) Success defined as at least 50% pain relief, stable or improved pain medication intake, improved physical activity. Successful patients proceeded to implantation phase. 17 patients proceeded to implantation phase. Permanent leads were surgically inserted (Medtronic Resume Electrode 3587A or 3487 A-56) along with an Implantable Pulse Generator inserted (Medtronic X-trel or Itrel Pulse Generator) 26 patients were randomized to a reoperation (re-op). Reoperation included laminectomy ± foraminotomy ± discectomy with or without fusion, with or without instrumentation All patients received standard postoperative analgesics, which were tapered as soon as possible, and routine postoperative physical therapy. Follow-up: 0.5, 1, and 2 years by disinterested non-blinded third party evaluator |
Success was defined as at least 50% pain relief and patient satisfaction with treatment. Study end points: -Crossover from the randomized to the alternate procedure. -Success at last follow-up. -Improvement in daily activities, neurological status and medication use. Results: Crossover at 2 years: 5/24 (21%) crossed to re-op. vs. 14/26 (54%) crossed to SCS. (P = .02) Success at 2 years: 9/19 (47%) SCS patients vs. 3/26 (12%) re-op patients (P < .01) Opioid use at 2 years: 3/23 (13%) SCS patients vs. 11/26 (42%) re-op patients had increased opiate use (P = .025). |
4 patients in the SCS group were lost to follow-up. 1 SCS patient died suddenly of a cardiac event at 6 months follow-up. Intention-to-treat analysis included patients randomized and treated (N = 50). 10 patients were randomized & not treated; in 9 patients, Workers’ compensation did not authorize participation, and 1 other patient had a stroke that precluded treatment. |
| Kemler et al., 2004(63) | RCT -Subjects randomized to SCS plus physiotherapy (PT), or standardized physical therapy in a 2:1 allocation ratio in favour of the SCS + PT group; - Table of random numbers used to make randomization schedule - Randomization was stratified by location of pain (hand or foot); - Randomization code was concealed to investigators. -Intention-to-treat analysis. - Non-blinded - Withdrawals/dropouts accounted for. - Quality 3/5 (Jadad score) |
54 Patients Inclusion criteria: -18–65 years old -reflex sympathetic dystrophy (RSD) diagnosed using the International Association for the Study of Pain (IASP) criteria -Patients had impaired function; symptoms beyond the area of trauma; and the disease was restricted to one hand or foot -Disease persisted for at least 6 months; there was no sustained response to standard therapy -Patient had a VAS pain score of at least 5 cm, (0 cm = no pain to 10 cm = very severe pain) Exclusion Criteria: -Raynaud’s disease, current or previous neurologic issue unrelated to RSD -Concurrent condition affecting function of the diseased or contralateral extremity -Blood-clotting disorder -Anticoagulant therapy -Use of a cardiac pace maker-A serious psychiatric disorder. |
Study treatments: SCS + PT vs. PT only. 36 patients were randomized to a test period of SCS Test period: 7 days with a temporary percutaneously inserted electrodes (model 3861; Medtronic) connected to an external simulator (Medtronic Model 3625); Success was defined as at least 50% pain relief on the VAS or a score of at least 6 (much improved) on a 7-point scale of global perceived effect of treatment. Successful patients would proceed to implantation 24 patients proceeded to implantation of SCS device. Permanent leads were inserted percutaneous (model 3487A; Medtronic) along with an implantable pulse generator (Itrel III, model 7425; Medtronic). 18 patients were randomized to receive PT only. -Patients underwent a standardized physical therapy program of graded exercises to improve strength, mobility, and function of affected limb 30 minutes twice per week, with a minimum of 2 days between treatments for 6 months -Physical therapists were trained to provide a standardized program; coordinating physical therapist monitored standardization of treatment. -Continuation with program after 6 months was optional. -SCS trial phase failures received physical therapy. Follow-up at 1, 3, and 6 months, and at 1 and 2 years. |
Outcome measures: Pain, perceived effect of treatment, functional status, quality of life. Results: At 2 years: mean pain intensity was reduced with SCS + PT vs. PT respectively -mean, 2.1 (SD, 2.8) vs. mean, 0 (SD, 1.5) (P < .001) Global Perceived Effect Score: At 2 years: 15/35 (43%) in SCS + PT reported score of ≥ 6 (much improvement) vs.1/16 (6%) PT patients (P = .001) % success: (defined as a 50% decrease in the VAS score at the start of treatment) At 2 years: 13/35 (57%) SCS + PT successfully treated vs. 1/16 (6%) PT patients. Functional status or quality of life was not significantly different between groups at 2 years |
Baseline measurement taken after randomization and before treatment in all groups. |
| Spincemaille et al., 2004(42) | Prospective non-RCT with a before-and-after treatment design 14 centres participated. Eligible subjects were registered with an independent centre and given a unique study number. |
105 patients with failed back surgery syndrome, defined as persistent limb pain with or without concomitant minor back pain after prior surgery for a slipped lumbar disc or spinal. Inclusion criteria: -18 years of age or older -Pain for more than 12 months -Surgical therapy not an option as per surgeon-failed other non-invasive therapies -VAS pain score ≥ 5. Exclusion criteria: -Previous SCS -Drug addiction -Noncompliance -Coagulopathy -Anticoagulation therapy -Immunologically compromised -Life expectancy < 1 year -Secondary nerve entrapment -Pregnant -Cardiac pacemaker -SCL-90 score ≥ 225. |
Study treatments: SCS only: patient was his/her own control before and after SCS. 135 patients were given a test period of SCS. The type of lead used for the test period was not described. Success was defined as patients having at least 50% reduction in pain intensity. Successful patients would proceed to implantation of SCS device. 105 patients proceeded to implantation of SCS device. Type of lead and SCS device not described. Follow-up: 1, 3, 6, 9, 12, 18 months. Data collected by an independent centre. |
Primary outcome: pain reduction. Secondary outcomes: functional status and quality of life scores. Results: mean (S.D.) Pain Visual analogue scale score: Pre: 7.3 (1.3) Post: 3.0 (2.4) McGill Pain Questionnaire: Pre: 22.4 (9.4) Post: 10.8 (8.0) Functionality Roland Disability: Pre: 16.9 (3.5) Post: 12.4 (4.8) Quality of Life: Sickness Impact Profile-68: Pre: 19.4 (10.1) Post: 11.7 (9.4) Euroquol-5D: Pre: 55.2 (14.5) Post: 38.2 (19.2) For all outcomes (P < .05), post-SCS scores vs. pre-SCS scores. |
9 patients were lost to follow-up at 12 months 4 patients had insufficient stimulation. 2 patients stated the therapy was inadequate. 2 were waiting for lead revision. 1 died, but the cause was not stated. |
| Harke et al., 2002(83) | Prospective non-RCT with a before-and-after treatment design. Consecutive enrollment between 1994 and 2000 |
28 Patients with postherpetic neuralgia (PHN); 4 patients with acute herpes zoster pain. Inclusion criteria: -Postherpetic neuralgia or acute herpes zoster -Ineffective medication and increasing pain, diagnosis confirmed by neurologist Exclusion Criteria: -Responsive to selective sympathetic nerve blocks -Strong neurotic disorders |
Study treatments: SCS only: patient was own control before and after SCS. 28 patients with postherpetic neuralgia were given a test period of SCS for 5–7 days using a percutaneously inserted quadripoloar lead. An external pulse generator 3625 Medtronic was used for all patients. 28 patients with postherpetic neuralgia proceeded to implantation of SCS device. Patients kept same electrodes used during trial phase but received an implantable pulse generator to which the lead was then attached. (Medtronic Itrel II or III device) Follow-up: Median, 29 months (range, 9–38.5,] months) |
Results: Of the 28 patients with postherpetic neuralgia, 23 were long-term responders. 5/28 stopped using SCS due to progressive dementia that rendered them unable to comply with therapy. Results from patients with postherpetic neuralgia: Pain: Visual analogue scale scores, median (interquartile range) Pre: 9 (7.5—10.0) Post: 1.0 (1.0–2.75) P < .001 Functionality: Pain Disability Index: function improved significantly after SCS (P < .001) Pain medication: 13/23 patients did not require any pain medication during SCS |
All patients had co-morbid disorders including cardiovascular, brain, lung, and endocrine disorders; or cancer. Periodic SCS inactivation tests were done to test for spontaneous improvement. During SCS inactivation periods, there was an observed reoccurrence of pain. Antidepressants were a comedication in 14 patients because of depressive symptoms. An analgesic effect of antidepressants could not be determined, because all patients had reappearance of pain during the inactivation period of SCS regardless of antidepressant use. |
Appendix 4: ICD-10 and CCI Codes
ICD_10 Codes
B02.2
B54.9
E10.4
E10.6
E11.4
E11.6
G54.0 (.1 to .9 inclusive)
G55.0 (.1 to .9 inclusive)
G56.4
G62.9
G54.4
G57.0
M79.2
M79.6
M54.1
M50.1
M51.1
M54.4
R52.1
R52.2
R52.9
T85.1
T85.5
T85.7
T85.9
G97.0
G97.1
G63
G63.2
M14.6
M890
CCI Codes
1AX53
1YY84
3AW92
1AX54
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Spinal cord stimulation for neuropathic pain: an evidence-based analysis. Ontario Health Technology Assessment Series 2005;5(4).
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2N6
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 1-4249-0129-4 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practicing medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of publication. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
Abbreviations
- CI
Confidence interval
- CPSO
College of Physicians and Surgeons of Ontario
- CRPS
Complex regional pain syndrome
- FBSS
Failed back surgery syndrome
- IPG
Implantable pulse generator
- MPQ
McGill Pain Questionnaire
- NNT
Number needed to treat
- PHN
Postherpetic neuralgia
- QOL
Quality of Life
- QALY
Quality-adjusted Life-year
- RCT
Randomized controlled trial
- SCS
Spinal cord stimulation
- SD
Standard deviation
- SSRI
Selective serotonin reuptake inhibitor
- TCA
Tricyclic antidepressant
- TENS
Transcutaneous electrical nerve stimulation
- VAS
Visual analogue scale
References
- 1.Prager J, Jacobs M. Evaluation of patients for implantable pain modalities: medical and behavioral assessment. Clin J Pain. 2001;17(3):206–14. doi: 10.1097/00002508-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Alo KM, Holsheimer J. New trends in neuromodulation for the management of neuropathic pain. Neurosurgery. 2002 Jan 1;50:690–704. doi: 10.1097/00006123-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Merskey H, Bogduk N. Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms 2nd ed. Seattle, WA, USA. 1994 IASP Press. [Google Scholar]
- 4.Bennett GJ. Neuropathic pain: new insights, new interventions. Hosp Pract. 1998;33:95–114. doi: 10.3810/hp.1998.10.114. [DOI] [PubMed] [Google Scholar]
- 5.Wetzel FT, Hassenbusch S, Oakley JC, Willis KD, Simpson RK Jr, Ross EL. Treatment of chronic pain in failed back surgery patients with spinal cord stimulation: a review of current literature and proposal for future investigation. Neuromodulation. 2000;3(2):59–74. doi: 10.1046/j.1525-1403.2000.00059.x. [DOI] [PubMed] [Google Scholar]
- 6.McQuay HJ. Neuropathic pain: evidence matters. Eur J Pain. 2002;6:11–8. doi: 10.1053/eujp.2001.0316. [DOI] [PubMed] [Google Scholar]
- 7.Martin LA, Hagen NA. Neuropathic pain in cancer patients: mechanisms, syndromes, and clinical controversies. J Pain Symptom Manage. 1997;14(2):99–117. doi: 10.1016/s0885-3924(97)00009-2. [DOI] [PubMed] [Google Scholar]
- 8.Wiffen P, Collins S, McQuay H, Carroll D, Jadad A, Moore A. Anticonvulsant drugs for acute and chronic pain. Cochrane Database Syst Rev 2000. (Issue 3) doi: 10.1002/14651858.CD001133. Art. No.: CD001133. DOI: 10.1002/14651858.CD001133. [DOI] [PubMed] [Google Scholar]
- 9.Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–34. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 10.Chong MS, Bajwa ZH. Diagnosis and treatment of neuropathic pain. J Pain Symptom Manage. 2003;25(5S):S4–S11. doi: 10.1016/s0885-3924(03)00064-2. [DOI] [PubMed] [Google Scholar]
- 11.United States Food and Drug Administration (FDA) Code of federal regulations. 2002 Chapter 11--Drug Enforcement Administration, Department of Justice. Report No.: Title 21. Volume 9. Report No. 21CFR1306.07. [Google Scholar]
- 12.Jovey RD, Ennis J, Gardner-Nix J, Goldman B, Hays H, Lynch M, et al. Use of opioid analgesics for the treatment of chronic noncancer pain--a consensus statement and guidelines from the Canadian Pain Society, 2002. Pain Res Manage. 2003;8:3A–14A. doi: 10.1155/2003/436716. [DOI] [PubMed] [Google Scholar]
- 13.Moulin DE, Clark AJ, Speechley M, Morley-Forster P. Chronic pain in Canada - prevalence, treatment, impact and the role of opioid analgesia. Pain Res Manage. 2002;7(4):179–84. doi: 10.1155/2002/323085. [DOI] [PubMed] [Google Scholar]
- 14.Crook J, Rideout E, Browne G. The prevalence of pain complaints in a general population. Pain. 1984;18:299–314. doi: 10.1016/0304-3959(84)90824-8. [DOI] [PubMed] [Google Scholar]
- 15.Currie SR, Wang J. Chronic back pain and major depression in the general Canadian population. Pain. 2004;107:54–60. doi: 10.1016/j.pain.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Meana M, Desmeules M, Cho R. Chronic pain: the extra burden on Canadian women. BMC Womens Health. 2004;4((Suppl.1)):S17. doi: 10.1186/1472-6874-4-S1-S17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- 18.Dario A, Fortini G, Bertollo D, Bacuzzi A, Grizzetti C, Cuffari S. Treatment of failed back surgery syndrome. Neuromodulation. 2001;4(3):105–10. doi: 10.1046/j.1525-1403.2001.00105.x. [DOI] [PubMed] [Google Scholar]
- 19.Turner JA, Loeser JD, Deyo RA, Sanders SB. Spinal cord stimulation for patients with failed back surgery syndrome or complex regional pain syndrome: a systematic review of effectiveness and complications. Pain. 2004;108(1):137–47. doi: 10.1016/j.pain.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 20.North RB, Kidd DH, Piantadosi S. Spinal cord stimulation versus reoperation for failed back surgery syndrome: a prospective, randomized study design. Act Neurochir [Suppl] 1995;64:106–8. doi: 10.1007/978-3-7091-9419-5_23. [DOI] [PubMed] [Google Scholar]
- 21.Raja SN, Grabow TS. Complex regional pain syndrome I (reflex sympathetic dystrophy) Anesthesiology. 2002;96(5):1254–60. doi: 10.1097/00000542-200205000-00031. [DOI] [PubMed] [Google Scholar]
- 22.Stanton-Hicks M, Janig W, Hassenbusch S, Haddox JD, Boas R, Wilson P. Reflex sympathetic dystrophy: changing concepts and taxonomy. Pain. 1995;63:127–33. doi: 10.1016/0304-3959(95)00110-E. [DOI] [PubMed] [Google Scholar]
- 23.Stanton-Hicks M, Burton AW, Breuhl SP, Carr DB, Harden N, Hassenbusch SJ, et al. An updated interdisciplinary clinical pathway for CRPS: report of an expert panel. Pain Practice. 2002;2(1):1–16. doi: 10.1046/j.1533-2500.2002.02009.x. [DOI] [PubMed] [Google Scholar]
- 24.Sandroni P, Benrud-Larson M, McClelland RL, Low PA. Complex regional pain syndrome type I: incidence and prevalence in Olmsted County; a population-based study. Pain. 2003;103:199–207. doi: 10.1016/s0304-3959(03)00065-4. [DOI] [PubMed] [Google Scholar]
- 25.Thomas SL, Hall AJ. What does epidemiology tell us about risk factors for herpes zoster? Lancet Infect Dis. 2004;4:26–33. doi: 10.1016/s1473-3099(03)00857-0. [DOI] [PubMed] [Google Scholar]
- 26.Brisson M, Edmunds WJ, Law B, Gay NJ, Walld R, Brownell M, et al. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol Infect. 2001;127:305–14. doi: 10.1017/s0950268801005921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.College of Physicians and Surgeons of Ontario (CPSO) Evidence-based recommendations for medical management of chronic non-malignant pain. 2000 [report on the Internet] Ontario. College of Physicians and Surgeons of Ontario. [Google Scholar]
- 28.Mailis A, Furlan A. Sympathectomy for neuropathic pain. Cochrane Database Syst Rev. 2002;(Issue 1) doi: 10.1002/14651858.CD002918. Art. No.: CD002918.DOI:10.1002/14651858.CD002918. [DOI] [PubMed] [Google Scholar]
- 29.Krames E. Implantable devices for pain control: spinal cord stimulation and intrathecal therapies. Best Pract Res Clin Anaesthesiol. 2002;16(4):619–49. doi: 10.1053/bean.2002.0263. [DOI] [PubMed] [Google Scholar]
- 30.Duhmke RM, Cornblath DD, Hollingshead JRF. Tramadol For neuropathic pain. Cochrane Database Syst Rev. 2004;(Issue 2) doi: 10.1002/14651858.CD003726.pub2. Art. No.: CD003726. DOI: 10.1002/14651858.CD003726.pub2. [DOI] [PubMed] [Google Scholar]
- 31.Alberta Heritage Foundation for Medical Research. Gabapentin for non-malignant chronic pain. Edmonton: Alberta Heritage Foundation for Medical Research (AHFMR) 2004 [report on the Internet] Report No.: TN47. [Google Scholar]
- 32.Canadian Pharmacists Association. The compendium of pharmaceuticals and specialties (CPS) 2005 Ottawa. Canadian Pharmacists Association. Ref Type: Serial (Book, Monograph) [Google Scholar]
- 33.Kalso E, Allan L, Dellemijn PLI, Raura CC, Ilias WK, Jensen TS, et al. Recommendations for using opioids in chronic non-cancer pain. Eur J Pain. 2003;7:381–6. doi: 10.1016/S1090-3801(02)00143-X. [DOI] [PubMed] [Google Scholar]
- 34.Mason L, Moore A, Derry S, Edwards JE, McQuay HJ. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 2004;324;((7446)):991. doi: 10.1136/bmj.38042.506748.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White CM, Pritchard J, Turner-Stokes L. Exercise for people with peripheral neuropathy. Cochrane Database Syst Rev. 2004;(Issue:1) doi: 10.1002/14651858.CD003904.pub2. Art No.:CD003904.pub2.DOI:10.10002/14651858.CD003904.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Tulder MW, Malmivaara A, Esmail R, Koes BW. Exercise therapy for low-back pain. Cochrane Database Syst Rev. 2000;(Issue 2) doi: 10.1002/14651858.CD000335. Art. No.: CD000335. DOI: 10.1002/14651858.CD000335. [DOI] [PubMed] [Google Scholar]
- 37.McQuay HJ, Moore AR, Eccleston C, Morley S, Williams A. Systematic review of outpatient services for chronic pain control. Health Technol Assess. 1997;1(6) [PubMed] [Google Scholar]
- 38.Tait PL, Brooks L, Harstall C. Acupuncture: evidence from systematic reviews and meta-analyses. Edmonton: Alberta Heritage Foundation for Medical Research (AHFMR) 2002 [report on the Internet] Report No.: HTA 27: Series A. [Google Scholar]
- 39.Carroll D, Moore RA, McQuay HJ, Fairman F, Tramer M, Leijon G. Transcutaneous electrical nerve stimulation (TENS) for chronic pain. Cochrane Database Syst Rev. 2000;(Issue 4) doi: 10.1002/14651858.CD003222. Art. No.: CD003222. DOI: 10.1002/14651858.CD003222. [DOI] [PubMed] [Google Scholar]
- 40.Brosseau L, Milne S, Robinson V, Marchan S, Shea B, Wells G, et al. Efficacy of the transcutaneous electrical nerve stimulation for the treatment of chronic low back pain. Spine. 2002;27(6):596–603. doi: 10.1097/00007632-200203150-00007. [DOI] [PubMed] [Google Scholar]
- 41.Linde K, Vickers A, Hondras M, ter Riet G, Thormahlen J, Berman B, et al. Systematic reviews of complementary therapies-an annotated bibliography. Part 1: acupuncture. 2001;1(3) doi: 10.1186/1472-6882-1-3. BMC Complement Altern Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spincemaille GH, Beersen N, Dekkers MA, Theuvenet PJ. Neuropathic limb pain and spinal cord stimulation: results of the Dutch prospective study. Neuromodulation. 2004;7(3):184–92. doi: 10.1111/j.1094-7159.2004.04198.x. [DOI] [PubMed] [Google Scholar]
- 43.Hu RW, Jaglal S, Axcell T, Anderson G. A population-based study of reoperations after back surgery. Spine. 1997;22:2265–71. doi: 10.1097/00007632-199710010-00013. [DOI] [PubMed] [Google Scholar]
- 44.Katz J, Melzack R. Pain control in the peroperative period: measurement of pain. Surg Clin North Am. 1999;79(2):231–52. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 45.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–99. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 46.Melzack R. The short-form McGill Pain Questionnaire. Pain. 1987;30:191–7. doi: 10.1016/0304-3959(87)91074-8. [DOI] [PubMed] [Google Scholar]
- 47.Farrar JT, Young JP, LaMoreaux L, Werth JL, Poole RM. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–58. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 48.Collins SL, Moore A, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain. 1997;72:95–7. doi: 10.1016/s0304-3959(97)00005-5. [DOI] [PubMed] [Google Scholar]
- 49.Cameron T. Safety and efficacy of spinal cord stimulation for the treatment of chronic pain: a 20-year literature review. J Neurosurg Spine. 2004 Mar;100(3):254–67. doi: 10.3171/spi.2004.100.3.0254. [DOI] [PubMed] [Google Scholar]
- 50.North RB, Kidd DH, Zahurak M, James C, Long D. Spinal cord stimulation for chronic, intractable pain: experience over two decades. Neurosurgery. 1993;32(3):384–95. doi: 10.1227/00006123-199303000-00008. [DOI] [PubMed] [Google Scholar]
- 51.Mailis-Gagnon A, Furlan AD, Sandoval JA, Taylor R. Spinal cord stimulation for chronic pain. Cochrane Database Syst Rev. 2004;(Issue 3) doi: 10.1002/14651858.CD003783.pub2. Art. No.: CD003783. DOI: 10.1002/14651858.CD003783.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Oakley JC, Prager JP. Spinal cord stimulation: mechanisms of action. Spine. 2002;27(22):2574–83. doi: 10.1097/00007632-200211150-00034. [DOI] [PubMed] [Google Scholar]
- 53.Ubbink DT, Jacobs MJ. Spinal cord stimulation in critical limb ischemia. A review. 2000 Mar;100(2):48–53. Acta Chir Belg. [PubMed] [Google Scholar]
- 54.Middleton P, Simpson B, Maddern G. Spinal cord stimulation (neurostimulation): an accelerated systematic review. Report. 2003 [report on the Internet] Adelaide, South Australia: Australian Safety and Efficacy Register of New Interventional Procedures - Surgical (ASERNIP-S) Report No.: AERSNIP-S R Report No. 43. [Google Scholar]
- 55.Alo KM, Yland MJ, Kramer DL, Charnov JH, Redko V. Computer assisted and patient interactive programming of dual octrode spinal cord stimulation in the treatment of chronic pain. Neuromodulation. 1998;1(1):30–45. doi: 10.1111/j.1525-1403.1998.tb00028.x. [DOI] [PubMed] [Google Scholar]
- 56.Gybels J, Erdine S, Maeyaert J, Meyerson B, Winkelmuller W, Augustinsson L, et al. Neuromodulation of pain. Eur J Pain. 1998;2:203–9. doi: 10.1016/s1090-3801(98)90016-7. [DOI] [PubMed] [Google Scholar]
- 57.Marchand S, Bushnell MC, Molina-Negro P, Martinez SN, Duncan GH. The effects of dorsal column stimulation on measure of clinical and experimental pain in man. Pain. 1991;45:249–57. doi: 10.1016/0304-3959(91)90049-4. [DOI] [PubMed] [Google Scholar]
- 58.Tesfaye S, Watt J, Benbow SJ, Pang KA, Miles J, MacFarlane Electrical spinal-cord stimulation for painful diabetic peripheral neuropathy. Lancet. 1996;348:1696–701. doi: 10.1016/S0140-6736(96)02467-1. [DOI] [PubMed] [Google Scholar]
- 59.Grabow TS, Tella PK, Raja SN. Spinal cord stimulation for complex regional pain syndrome: an evidence-based medicine review of the literature. Clin J Pain. 2003 Nov;19(6):371–83. doi: 10.1097/00002508-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Meglio M, Cioni B, Rossi GF. Spinal cord stimulation in management of chronic pain. J Neurosurg. 1989;70:519–24. doi: 10.3171/jns.1989.70.4.0519. [DOI] [PubMed] [Google Scholar]
- 61.Follett KA, Boortz-Marx RL, Drake JM, DuPen S, Schneider SJ, Turner MS, et al. Prevention and management of intrathecal drug delivery and spinal cord stimulation system infections. Anesthesiology. 2004;100(6):1582–94. doi: 10.1097/00000542-200406000-00034. [DOI] [PubMed] [Google Scholar]
- 62.Kemler MA, Barendse GAM, Van Kleef M. Relapsing ulcerative colitis associated with spinal cord stimulation. Gastroenterology. 1999;117:215–7. doi: 10.1016/s0016-5085(99)70570-6. [DOI] [PubMed] [Google Scholar]
- 63.Kemler MA, De Vet HCW, Barendse GAM, Van Den Wildenberg FAJM, van Kleef M. The effect of spinal cord stimulation in patients with chronic reflex sympathetic dystrophy: two years’ follow-up of the randomized controlled trial. Ann Neurol. 2004;55(1):13–8. doi: 10.1002/ana.10996. [DOI] [PubMed] [Google Scholar]
- 64.Alo KM, Redko V, Charnov J. Four year follow-up of dual electrode spinal cord stimulation for chronic pain. Neuromodulation. 2002;5(2):79–88. doi: 10.1046/j.1525-1403.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- 65.Eisenberg E, Waisbrod H. Spinal cord stimulator activation by an antitheft device. J Neurosurg. 1997;87:961–2. doi: 10.3171/jns.1997.87.6.0961. [DOI] [PubMed] [Google Scholar]
- 66.Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 67.Assessment of study quality. 2004 [monograph on the Internet] In. Alderson P, Green S, Higgins JPT, editors. Cochrane reviewers’ handbook. [Google Scholar]
- 68.Carter ML. Spinal cord stimulation in chronic pain: a review of the evidence. Anaesth Intensive Care. 2004 Feb;32(1):11–21. doi: 10.1177/0310057X0403200102. [DOI] [PubMed] [Google Scholar]
- 69.North RB, Wetzel FT, Straus B, Prager J, Saal J, Slosar P, et al. Spinal cord stimulation for chronic pain of spinal origin: a valuable long-term solution. Spine. 2002;27(22):2584–92. doi: 10.1097/00007632-200211150-00035. [DOI] [PubMed] [Google Scholar]
- 70.Rushton DN. Electrical stimulation in the treatment of pain. Disabil Rehabil. 2002 May 20;24(8):407–15. doi: 10.1080/09638280110108832. [DOI] [PubMed] [Google Scholar]
- 71.Stocks RA, Williams CT. Spinal cord stimulation for chronic pain. In Foxcroft DR, Muthu V (eds) STEER: Succinct and Timely Evaluated Evidence Reviews 201: 1(5) 2001 [report on the Internet] Report. London: Bazian Ltd (Editors), Wessex Institute for Health Research and Development, University of Southampton. [Google Scholar]
- 72.Kemler MA, Barendse GA, van Kleef M, De Vet HC, Rijks CP, Furnee CA, et al. Spinal cord stimulation in patients with chronic reflex sympathetic dystrophy. N Engl J Med. 2000 Aug 31;343(9):618–24. doi: 10.1056/NEJM200008313430904. [DOI] [PubMed] [Google Scholar]
- 73.Allegri M, Arachi G, Barbieri M, Paulin L, Bettaglio R, Bonetti G, et al. Prospective study of the success and efficacy of spinal cord stimulation. Minerva Anestesiol. 2004 Mar;70(3):117–24. [PubMed] [Google Scholar]
- 74.Barolat G, Oakley JC, Law JD, North RB, Ketcik B, Sharan A. Epidural spinal cord stimulation with a multiple electrode paddle lead is effective in treating intractable low back pain. Neuromodulation. 2001;4(2):59–66. doi: 10.1046/j.1525-1403.2001.00059.x. [DOI] [PubMed] [Google Scholar]
- 75.Kavar B, Rosenfeld JV, Hutchinson A. The efficacy of spinal cord stimulation for chronic pain. J Clin Neurosci. 2000 Sep;7(5):409–13. doi: 10.1054/jocn.1999.0225. [DOI] [PubMed] [Google Scholar]
- 76.Taylor RS, Taylor RJ, Van Buyten J-P, Buchser E, North R, Bayliss S. The cost effectiveness of spinal cord stimulation in the treatment of pain: a systematic review of the literature. J Pain Symptom Manage. 2004;27(4):370–8. doi: 10.1016/j.jpainsymman.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 77.Taylor RS, Van Buyten JP, Buchser E. Spinal cord stimulation for chronic back and leg pain and failed back surgery syndrome: a systematic review and analysis of prognostic factors. Spine. 2005 Jan 1;30(1):152–60. doi: 10.1097/01.brs.0000149199.68381.fe. [DOI] [PubMed] [Google Scholar]
- 78.North RB, Kidd DH, Lee DH, Piantadosi S. A prospective, randomized study of spinal cord stimulation versus reoperation for failed back surgery syndrome: initial results. Stereotact Funct Neurosurg. 1994;62:267–72. doi: 10.1159/000098631. [DOI] [PubMed] [Google Scholar]
- 79.Kemler MA, Reulen JPH, Barendse GAM, van Kleef M, De Vet HCW, Van Den Wildenberg FAJM. Impact of spinal cord stimulation on sensory characteristics in complex regional pain syndrome type I: A randomized trial. Anesthesiology. 2001;95(1):72–80. doi: 10.1097/00000542-200107000-00016. [DOI] [PubMed] [Google Scholar]
- 80.Kemler MA, Furnee CA. Economic evaluation of spinal cord stimulation for chronic reflex sympathetic dystrophy. Neurology. 2002;59(8):1203–9. doi: 10.1212/01.wnl.0000028686.74056.e3. [DOI] [PubMed] [Google Scholar]
- 81.North RB, Kidd DH, Farrokhi F, Piantadosi SA. Spinal cord stimulation versus repeated lumbosacral spine surgery for chronic pain: a randomized, controlled trial. Neurosurgery. 2005 Jan;56(1):98–107. doi: 10.1227/01.neu.0000144839.65524.e0. [DOI] [PubMed] [Google Scholar]
- 82.He L, Wu B, Zhou M. Non-antiepileptic drugs for trigeminal neuralgia. Cochrane Database Syst Rev. 2002;(Issue 3) doi: 10.1002/14651858.CD004029.pub2. Art. No.: CD004029. DOI: 10.1002/14651858.CD004029. [DOI] [PubMed] [Google Scholar]
- 83.Harke H, Gretenkort P, Ladleif HU, Koester P, Rahman S. Spinal cord stimulation in postherpetic neuralgia and in acute herpes zoster pain. Anesth Analg. 2002 Mar;94(3):694–700. doi: 10.1097/00000539-200203000-00040. [DOI] [PubMed] [Google Scholar]
- 84.Mekhail NA, Aeschbach A, Stanton-Hicks M. Cost benefit analysis of neurostimulation for chronic pain. Clin J Pain. 2004;20(6):462–8. doi: 10.1097/00002508-200411000-00012. [DOI] [PubMed] [Google Scholar]
- 85.Kumar K, Malik S, Demeria D, Kanpolat Y, Burchiel KJ. Treatment of chronic pain with spinal cord stimulation versus alternative therapies: Cost-effectiveness analysis. Neurosurgery. 2002;51;(1):106–16. doi: 10.1097/00006123-200207000-00016. [DOI] [PubMed] [Google Scholar]
- 86.American Society of Anesthesiologists Task Force on Pain Management CPS. Practice guidelines for chronic pain management. 1997;86:995–1004. Anesthesiology. [PubMed] [Google Scholar]


