Abstract
Omalizumab, a humanized monoclonal antibody that binds circulating IgE antibody, is a treatment option for patients with moderate to severe allergic asthma whose asthma is poorly controlled with inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators. This review considers the mechanism of action, pharmacokinetics, efficacy, safety and place in management of omalizumab in asthma and focuses particularly on key articles published over the last three years. Omalizumab reduces IgE mediated airway inflammation and its effect on airway remodeling is under investigation. Recent long-term clinical trials confirm the benefits of omalizumab in reducing exacerbations and symptoms in adults and in children with moderate to severe allergic asthma. No clinical or immunological factor consistently predicts a good therapeutic response to omalizumab in allergic asthma. In responders, the duration of treatment is unclear. The main adverse effect of omalizumab is anaphylaxis, although this occurs infrequently. Preliminary data from a five-year safety study has raised concerns about increased cardiovascular events and a final report is awaited. Clinical trials are in progress to determine whether omalizumab has efficacy in the treatment of non-allergic asthma.
Keywords: IgE, severe asthma, allergic asthma, omalizumab
Introduction
Over 300 million individuals worldwide have asthma1 of whom the majority have mild or moderate disease that can be controlled by inhaled corticosteroids, either alone or in combination with inhaled long-acting β2 agonist bronchodilators.1–3 Nevertheless a considerable proportion of patients with asthma,4 particularly those with severe disease5 have poorly controlled symptoms and are at increased risk of exacerbations. In some patients inadequately controlled asthma is due to poor adherence with treatment, untreated co-morbidities, dysfunctional breathing or psychological problems.6,7 For others, there is a need for additional or new therapies.8 Severe asthma occurs in 5% to 10% of the asthmatic population and in this group it is estimated that over 50% have allergic IgE-mediated asthma.9 Omalizumab, a recombinant humanized monoclonal antibody that binds circulating IgE antibody, is a treatment option for moderate to severe allergic asthma in patients whose asthma is not well controlled with inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators.1–3 This review considers the mechanism of action, pharmacokinetics, efficacy, safety and place in management of omalizumab in asthma and focuses particularly on key articles published over the last three years.
Mechanism of Action
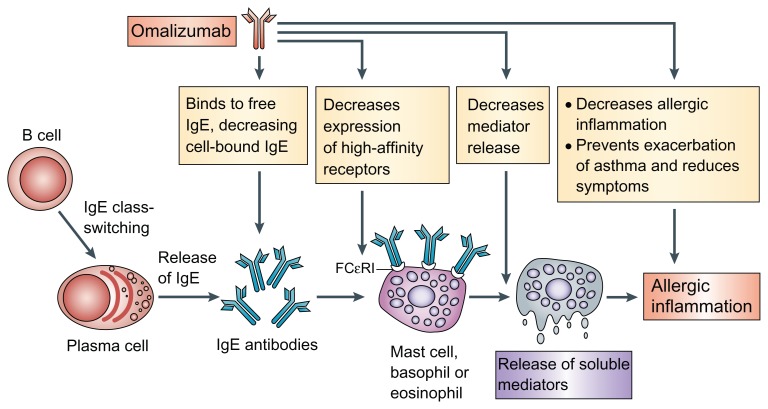
Immunoglobulin E (IgE) is considered to play a key role in the pathogenesis of asthma10 and the level of circulating IgE to common inhalant allergens is a strong risk factor for emergency admissions with asthma.11 Allergen specific IgE binds to high affinity receptors (FCɛRI) on mast cells and basophils to induce an allergic reaction through the release of a wide range of inflammatory mediators such as histamine, tryptase and arachidonic acid metabolites.12,13 High affinity receptors are also expressed on other inflammatory cells including dendritic cells, monocytes and eosinophils. Omalizumab is a recombinant humanized monoclonal antibody that binds to the FC portion of the IgE antibody. By forming complexes with circulating IgE antibody it reduces the levels of free IgE and prevents the binding of IgE to high-affinity IgE receptors on mast cells and basophils and the subsequent release of inflammatory mediators induced by allergen exposure14 (Fig. 1). Omalizumab cannot however, bind to IgE that has already attached to FCɛRI receptors. Omalizumab treatment also down-regulates the expression of FCɛRI receptors on mast cells and basophils.15
Figure 1.
Mechanisms of action of omalizumab in allergic asthma.
Reprinted by permission from Macmillan Publishers Ltd: Nat Rev Immunol,14 copyright 2008.
Abbreviation: Fc RI, high-affinity IgE receptor.
There is considerable interest in determining the effects of omalizumab treatment on chronic airway inflammation and remodeling in asthma.16–19 A placebo controlled study of 16 weeks treatment with omalizumab in forty-five patients with mild to moderate persistent asthma with sputum eosinophilia of 2% or more found that active treatment resulted in a reduction of bronchial biopsy IgE+ cells in the airway mucosa, tissue eosinophil numbers and CD3+, CD4+, and CD8+ T lymphocytes.16 Omalizumab treatment was not associated however, with improvement in airway hyperresponsiveness to methacholine. In addition to reducing the early and late bronchoconstrictor response to inhaled allergen, omalizumab reduces induced sputum eosinophils and bronchial biopsy eosinophil numbers and IgE-bearing cells, but does not attenuate airway responsiveness to methacholine post-allergen challenge.17 A pooled analysis of data from five randomized controlled studies in 2236 patients with moderate to severe persistent allergic asthma receiving moderate to high-dose inhaled corticosteroids found a pattern of improved clinical outcomes, associated with decreased peripheral blood eosinophils and worse clinical outcomes associated with increased peripheral blood eosinophils, in keeping with the efficacy of omalizumab being due, at least in part, with an inhibitory effect on eosinophils.20 Treatment of allergic subjects with omalizumab also reduces the release of Th2 cytokines from peripheral blood basophils.13 Taken together these studies suggest that treatment with omalizumab reduces eosinophilic airway inflammation and IgE-bearing cells, but does alter airway responsiveness. Possible mechanisms for the reduction in eosinophil numbers, although not established,21 could be explained by a reduction in high affinity IgE receptor numbers on dendritic cells causing less antigen presentation to T cells, by decreased release of eosinophilic chemotactic factors from mast cells and basophils and by the induction of eosinophil apoptosis.22 In addition, omalizumab blocks low affinity IgE receptors on epithelial cells and airway smooth muscle, which may decrease the release of eosinophil chemokines such as eotaxin.21
There is limited information on the effects of omalizumab on airway remodeling.19 Prevention of degranulation of allergen bound mast cells by omalizumab prevents the release of pro-inflammatory cytokines, including IL-4, IL-13 and IL-5 that are associated with airway remodeling. The exhaled breath condensate levels of endothelin-1, a proinflammatory mediator also implicated in asthmatic airway inflammation and remodeling23 are reduced following omalizumab, suggesting a potential mechanism by which this therapy could reduce structural changes to the airways.24 A 16 week study of 30 patients with severe persistent asthma, which used computed tomography (CT) to assess airway dimensions, found that treatment with omalizumab compared to usual care was associated with a reduction in indices of airway wall thickness.18 Interestingly, the decrease in airway wall thickness was associated with improvements in lung function and decrease in sputum eosinophil count. A long-term randomized controlled trial is underway in patients with moderate to severe allergic asthma to examine the effects of 78 weeks treatment with omalizumab on bronchial biopsy sub-epithelial eosinophils, mast cell and CD4+ T-lymphocytes as well as thickness of the lamina reticularis, as an index of airway remodeling (The EXPLORE study, www.clinicaltrials.gov NCT00670930). The findings of this study should provide insights into the effects of long-term treatment with omalizumab on airway inflammation and remodeling.
In summary, omalizumab decreases allergic airway inflammation by reducing the expression of high affinity IgE receptors on inflammatory cells and eosinophil numbers within the airways. Research is underway to help establish whether long-term treatment with omalizumab also reduces airway remodeling.
Metabolism and Pharmacokinetic Profile
The metabolism and pharmacokinetic profile of omalizumab has been previously reviewed in detail.25 After a single subcutaneous dose in adult and adolescents with asthma, omalizumab is absorbed slowly over several days, reaching peak serum concentrations after an average of seven to eight days.25,26 The pharmacokinetics of omalizumab are linear at doses above 0.5 mg per kg. Measurements of omalizumab serum concentrations reflect the combination of free omalizumab and that bound to IgE. Serum free IgE levels reduce in a dose-dependent manner within one hour of the first dose of omalizumab and levels are maintained between doses, whereas concentrations of total IgE (ie, the sum of free and omalizumab bound IgE) are increased. Information about drug distribution is limited to reports from intravenous administration of radiolabeled omalizumab in cynomolgus monkeys.27 These studies suggest that most of the drug remains in the central intravascular compartment, with little accumulation in tissues. Elimination of omalizumab appears to involve IgG clearance processes as well as clearance via specific binding and complex formation with its target ligand, IgE. Liver elimination of IgG includes degradation in the reticulo-endothelial system and endothelial cells. Intact IgG is also excreted in bile. In patients with asthma, the omalizumab serum elimination half-life averages 26 days, with apparent clearance rates averaging 2.4 ± 1.1 mL per kg per day. In addition, doubling of body weight approximately doubles clearance rates. The half-life of omalizumab is variable, with values ranging from one to four weeks.25 After discontinuation of omalizumab, free and total IgE concentrations approach baseline slowly. In a dose-ranging study involving patients with ragweed seasonal allergic rhinitis, average free IgE concentrations returned to baseline within eight weeks of the last omalizumab infusion.28 Total IgE concentrations generally take longer to approach baseline after stopping therapy.28,29 One year after discontinuation of omalizumab dosing, circulating IgE levels return to baseline pre-treatment levels with no observed rebound.26 Analyses of population pharmacokinetics of omazulamab suggest that no dose adjustments are necessary for the age range 6 to 76 years, race, ethnicity, gender or Body Mass Index. There are no pharmacokinetic or pharmacodynamic data in patients with renal or hepatic impairment.26
Efficacy
The main evidence for the efficacy of omalizumab in the treatment of patients with allergic asthma is summarized in two systematic reviews.30,31 A Cochrane review published in 2006 of 14 randomized controlled trials in 3143 children and adults with mild to severe allergic asthma found that treatment with omalizumab reduced asthma exacerbations (OR 0.52, 95% CI, 0.41 to 0.65) and increased the proportion of patients who were able to reduce or withdraw inhaled corticosteroids.30 A systematic review published in 2011 of 8 randomized controlled trials in 3,429 children and adults with moderate to severe allergic asthma taking inhaled corticosteroids, including two trials published after 2006,32,33 concluded that omalizumab treatment resulted in a higher proportion of subjects stepping-down and stopping inhaled corticosteroids (relative risk [RR] = 1.80; 95% CI, 1.42–2.28) and reduced the risk of asthma exacerbations (RR = 0.57; 95% CI, 0.48–0.66).31 Data from the systematic review published in 201131 suggested that the number needed to treat for benefit (NTTB) in reducing the rate of exacerbations was 10. A post-hoc analysis suggested that the beneficial effects of omalizumab were not dependent on the age, duration of treatment, or disease severity.31 A study included in the systematic review that provided evidence for the efficacy of omalizumab in children was a 52 week randomized placebo-controlled trial in 627 children aged 6 to <12 years with perennial allergen asthma and a history of exacerbations and poor symptom treatment with medium-dose or high-dose inhaled corticosteroids with or without other controller medications.32 Omalizumab treatment reduced asthma exacerbations by 31% compared to placebo during the first 24-weeks of the study, when the inhaled corticosteroid dose remained stable, and by 43% over a period of 52 weeks, which included a 28-week adjustable-corticosteroid phase.32 In this study the secondary outcomes including symptoms, reliever bronchodilator use and reduction in inhaled corticosteroid dose were not significantly improved by omalizumab treatment.
Recent publications have provided important new information on the use of omalizumab as an add-on treatment to high dose inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators in severe allergic asthma34,35 and in children and young adults with allergic asthma36 (Table 1). A randomized controlled trial in 850 patients who had inadequately controlled asthma despite treatment with high dose inhaled corticosteroids (≥500 mcg of fluticasone inhaler twice daily or equivalent) and inhaled long-acting β2 agonist bronchodilators, with or without other controllers assessed the benefits of the addition of omalizumab over a 48 week period.34 At the end of the treatment period omalizumab produced a 25% relative reduction in the rate of asthma exacerbations (0.66 omalizumab vs. 0.88 placebo per patient) (Table 1). This study demonstrates clinical benefits obtained from the addition of omalizumab to patients with poorly controlled severe allergic asthma despite treatment with high dose inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators, although the magnitude of benefit is relatively small. Interestingly there was a large placebo effect observed in the control group, a finding which has been noted in previous studies with omalizumab.30,35 At baseline, seventeen percent of participants were receiving either chronic oral corticosteroids or an oral corticosteroid course at least 4 times per year, and in this sub-group there was no clinical benefit although it should be noted that the study was not powered to detect a treatment effect.
Table 1.
Summary of recent key randomized placebo-controlled clinical trials of omalizumab as an add-on treatment in children and young adults with allergic asthma.
| Author | Participants | Duration | Main outcomes |
|---|---|---|---|
| Hanania et al, Ann Int Med34 | 850 patients aged 12 to 75 years who had inadequately controlled asthma despite treatment with high dose inhaled corticosteroids (≥500 mcg of fluticasone inhaler twice daily or equivalent) and inhaled long-acting β2 agonist bronchodilators, with or without other controllers | 48 weeks | At the end of the treatment period omalizumab produced a 25% relative reduction in the primary outcome, rate of asthma exacerbations (0.66 omalizumab vs. 0.88 placebo per patient, P = 0.006), as well as producing improvements in several secondary efficacy end-points including an non-clinically significant increase in the mean asthma quality of life questionnaire (AQLQ) score (0.29 points) and a decrease in mean daily number of puffs of albuterol (−0.27 puffs per day) and in mean total asthma symptom score (−0.26) compared with placebo. |
| Bardelas et al, J Asthma35 | 271 patients aged 12 years or older with physician diagnosed persistent allergic asthma | 24 weeks | There was no significant difference with omalizumab treatment in the change from baseline in the primary outcome, the Asthma Control Test (ACT) total score, compared with placebo. In a subgroup of patients with very poorly controlled asthma (ACT ≤ 15) at baseline however, significant benefits were observed for omalizumab compared with placebo for change in ACT score. |
| Busse et al, N Eng J Med36 | 419 inner-city children, adolescents, and young adults with persistent allergic asthma. Almost three quarters of participants had moderate or severe asthma | 60 weeks | At the end of the treatment period omalizumab, compared to placebo, produced a 25% relative reduction in the primary outcome, number of days with asthma symptoms, from 1.96 to 1.48 days per 2 week interval (P < 0.001) as well as producing a reduction in the proportion of subjects who had one or more exacerbations from 48.8% in the placebo group to 30.3% in the omalizumab group and in the hospital admissions because of asthma from 6.3% to 1.5%. In addition, the omalizumab treatment group reduced the use of inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators. |
Abbreviation: RCT, Randomized placebo controlled trial.
A recent double-blind, placebo-controlled study in 271 patients aged 12 years or older with physician diagnosed persistent allergic asthma compared the efficacy of omalizumab with placebo over 24 weeks period.35 There was no significant difference with omalizumab treatment in the change from baseline in the primary outcome measure, the Asthma Control Test (ACT) total score, compared with placebo. In a subgroup of patients with very poorly controlled asthma (ACT ≤ 15) at baseline however, significant benefits were observed for omalizumab compared with placebo for change in ACT score. The results of this study suggest that omalizumab has little impact on ACT scores, except in those patients with very poorly controlled asthma.35
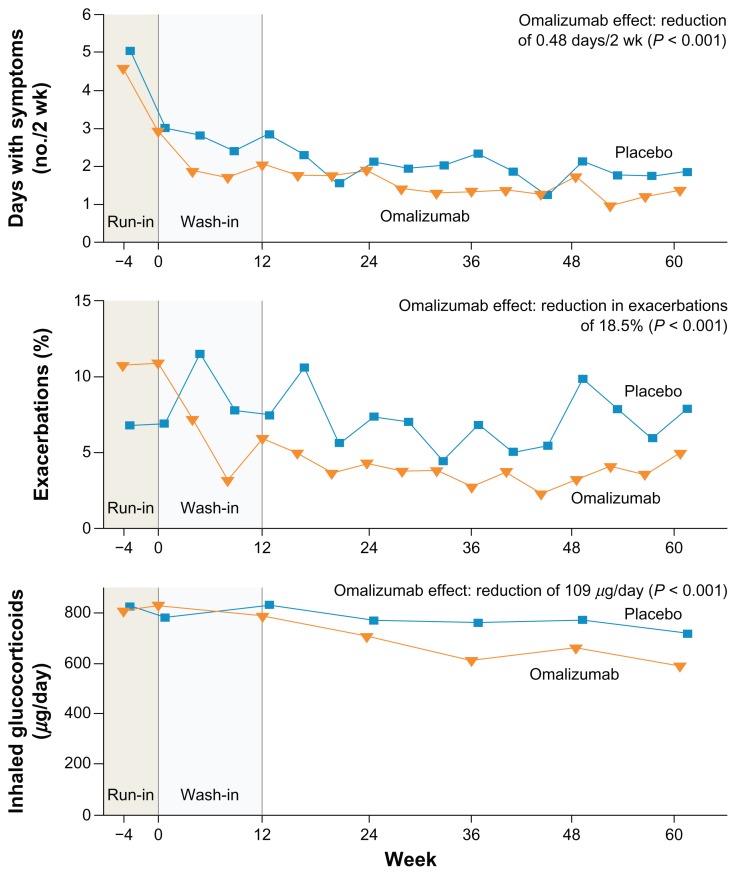
A randomized controlled trial in 419 inner-city children, adolescents, and young adults with persistent allergic asthma examined the benefits of the addition of omalizumab to guideline based treatment over a 60 week period.36 Almost three quarters of participants had moderate or severe asthma. At the end of the treatment period omalizumab, compared to placebo, produced a 25% relative reduction in the number of days with asthma symptoms (Table 1, Fig. 2) as well as producing a reduction in the proportion of subjects who had one or more exacerbations from 48.8% in the placebo group to 30.3% in the omalizumab group and in the hospital admissions because of asthma from 6.3% to 1.5%. A post hoc analysis revealed a marked reduction in seasonal exacerbation with omalizumab. In addition to confirming the clinical benefits from omalizumab treatment in children and young adults with allergic asthma, this study provides evidence for the importance of sensitization to cockroach allergen and house dust mites in an inner-city US population of individuals with allergic asthma.
Figure 2.
Omalizumab in inner-city children, adolescents, and young adults with persistent allergic asthma.
Note: The figure shows the number of days with symptoms (per 2-week interval), frequency of exacerbations, and dose of inhaled glucocorticoid over the duration of the study.
Reproduced from Busse et al with permission.36 Copyright (c) Massachusetts Medical Society.
Several observational studies have reported on ‘real life’ experience with omalizumab in treating patients with allergic asthma in France,37 Germany,38 Belgium,39 United Kingdom,40 Italy,41 South-Eastern Mediterranean centers,42 Israel43 and Spain.44 The most recent and largest study reported on the efficacy, tolerability and IgE entry criteria of omalizumab treatment of 266 patients with uncontrolled severe asthma receiving high dose inhaled corticosteroid and inhaled long-acting β2 agonist bronchodilators.44 The main efficacy outcome of exacerbations, ACT score and global evaluation of treatment effectiveness all improved at 4 months and at 2 years including patients with “off label” IgE levels. Omalizumab treatment was stopped in a minority of patients because of a lack of efficacy. The study provides data from a longer follow-up period (over 2 years) than previous observational studies. Taken together, the results of these studies suggest that omalizumab is an effective treatment for patients with poorly controlled allergic asthma and that efficacy outcomes in a ‘real life’ setting are similar to those reported in clinical trials.
Omalizumab reduces the severity of the acute bronchoconstrictor response to environmental exposure to cat allergen.45 A randomized controlled trial undertaken in 69 patients who gave a history of cat allergen–induced asthma examined the effects of 16 weeks treatment with omalizumab or placebo on controlled exposure to cat allergen.45 After cat allergen exposure the area under the curve for percentage decrease in FEV1 was 15.2% per hour for omalizumab-treated patients compared with 27.3% per hour for patients who received placebo. Symptoms caused by exposure to cat allergens were also reduced by treatment with omalizumab. These findings suggest that omalizumab may have a role in the treatment of patients with cat allergen–induced asthma when avoidance measures and/or drug therapy are not effective in controlling the severity of acute asthma symptoms during environmental exposure to cat allergen. A large proportion of patients with severe allergic asthma also have chronic allergic rhinitis and in this group of patients, omalizumab treatment over a 26 week period improves both asthma and rhinitis quality of life questionnaire scores.46
There is interest in whether omalizumab may be of value in the treatment of other forms of asthma including non-allergic asthma. A case report of a patient with severe persistent asthma, who had elevated total IgE levels and negative specific IgE and skin-prick test results, noted a good clinical response to an open trial of omalizumab treatment, which was followed by a deterioration after its withdrawal.47 Several randomized controlled clinical trials are underway to assess the clinical and immunological effects of omalizumab in severe non-atopic asthma. The primary outcomes in one study are lung function and exacerbations (www.clinicaltrials.gov NCT01113437), and in another study the expression of FcɛRI receptors on blood basophils and dendritic cells (www.clinicaltrials.gov NCT01007149). Hopefully the results of these clinical trials will help clarify the role of omalizumab therapy in the treatment of non-atopic asthma. A study to evaluate the effect of omalizumab on improving the tolerability of specific allergen immunotherapy in 248 patients with at least moderate persistent allergic asthma, with the aim of reducing systemic allergic reactions, reported that the number of participants with systemic allergic reactions to specific immunotherapy was numerically lower after 26 weeks treatment with omalizumab (13.5%) compared to placebo (26.2%).48 A observational study of 18 patients with aspergillus-associated airway diseases recruited from 11 centers in Spain reported on the clinical benefits of omalizumab treatment for a median follow-up of 36 weeks.49 The treatment was discontinued in five patients due to a lack of efficacy and one because of a positive pregnancy test. In the remaining 12 patients there were improvements in symptoms, exacerbations and lung function. Based on these preliminary findings in patients with aspergillus sensitization associated asthma, prospective placebo controlled trials are indicated.
In summary, recent long-term clinical trials confirm the benefits of omalizumab in reducing exacerbations and symptoms in adults and in children with moderate to severe allergic asthma.
Safety
There is increasing data on the safety of the short and long term use of omalizumab, as numbers of patients in clinical trials and clinical use has expanded. An analysis of over 7,500 patients recruited to clinical trials of omalizumab and of 57,300 patients included in post-marketing safety follow-up monitoring revealed a generally favorable safety profile.50 The major adverse effect associated with the use of omalizumab is anaphylaxis. In addition concerns have been raised about an increase in malignancy rates and cardiovascular and cerebrovascular events (Table 2).
Table 2.
Adverse effects of omalizumab.†
Common [<1/10]
|
Notes:
Adapted from Summary of Product Characteristics;26
very common in children 6 to <12 years of age [≥1/10].
The incidence of anaphylaxis reported in clinical trials is 0.14% in omalizumab treated patients and 0.07% in control patients in clinical trials and 0.2% with omalizumab treatment from post-marketing data.50 Nearly 60% of anaphylactic episodes developed in the first two hours after the administration of omalizumab; 39% occurred after the first dose, 19% following the second and 10% with the third dose and a previous history of anaphylaxis was reported in 24% of subjects.51 Recommendations of the US Omalizumab Joint Task Force guideline published in 2007 include informed consent, patient education about anaphylaxis symptoms, supply of an epinephrine auto-injector and a waiting period of two hours following the first 3 injections and 30 minutes for subsequent injections.52 According to the European Medicines Agency (EMA) licence, medications for the treatment of anaphylactic reactions should be available in areas where omalizumab is administered and patients should be informed of the potential for such reactions and the need for prompt medical attention should reactions occur.53
The overall incidence of observed malignancy associated with omalizumab use is rare and comparable to that in the general population.26,51 In clinical trials in adults and adolescents, there was a numerical imbalance in cancers arising in the omalizumab group (0.5%) compared with the control group (0.18%).26 A causal relationship was considered unlikely given the diversity in the type of cancers, the relatively short duration of exposure and the clinical features of the individual cases.26 There were no cases of malignancy with omalizumab in the clinical trials in children 6 to <12 years of age; there was a single case of malignancy in the control group.26 In a recent pooled analysis from 67 clinical trials including 11,459 patients of whom 7789 received omalizumab, 25 malignancies [14 and 11 in omalizumab and placebo treated patients respectively] were identified. Incidence rates per 1,000 patient-years of observation time for omalizumab- and placebo-treated patients were 4.14 (95% CI, 2.26–6.94) and 4.45 (95% CI, 2.22–7.94), respectively. No association was observed between omalizumab treatment and risk of malignancy suggesting that a causal relationship between omalizumab therapy and malignancy is not likely.54
Recently concerns about the cardiovascular and cerebrovascular safety of omalizumab have been raised by the United States Food and Drug Administration (FDA), based on preliminary data from a five year epidemiological study designed to evaluate the clinical effectiveness and long-term safety in patients with moderate to severe asthma (EXELS).55 Detailed results from this study are expected later this year. The numerical imbalance of arterial thrombotic events (ATEs) observed in clinical trials and in the EXCELS cohort, included stroke, transient ischemic attack, myocardial infarction, unstable angina, and cardiovascular death (including death from unknown cause). The rate of ATE in patients in the controlled clinical trials was 6.29 for omalizumab treated patients and 3.42 for control patients. In a Cox proportional hazards model, omalizumab was not associated with ATE risk (hazard ratio 1.86; 95% confidence interval 0.73–4.72). In the observational study, the rate of ATE was 5.59 for omalizumab treated patients and 3.71 for control patients. In a multivariate analysis controlling for baseline cardiovascular risk factors, omalizumab was not associated with ATE risk (hazard ratio 1.11; 95% confidence interval 0.70–1.76).26
An exploratory study in Brazil studied whether anti-IgE treatment of patient with allergic asthma and/or rhinitis would increase the risk of infections with intestinal helminths.56 Following a course of antihelminthic treatment, all subjects received omalizumab or placebo for 52 weeks. Half the omalizumab treated subjects experienced at least one intestinal helminth infection compared with 41% of placebo subjects, suggesting a slight increase in the incidence of helminth infection, although the difference was nonsignificant. The current drug summary of product characteristics advises caution in patients at high risk of helminth infection, in particular when travelling to areas where these infections are endemic, and if patients do not respond to recommended anti-helminth treatment, discontinuation of omalizumab should be considered.26
Adverse events do not appear to occur more frequently in older people. An observational study in a ‘real life’ population of 280 people reported adverse events in 35.5% of patients 50 years or older and, at a similar frequency, in 32.1% of younger patients.57 Omalizumab is designated a Pregnancy Category B drug and animal studies have not demonstrated a risk to the foetus.51 The EXPECT registry, an observational registry following women who have received omalizumab during pregnancy or prior to conception will provide further information. In the 27 cases of pregnancy occurring in patients receiving omalizumab in clinical trials, no significant issues were observed in abortion rates or abnormal deliveries.51 The current opinion is that due to lack of adequate data, omalizumab should not be used during pregnancy unless clearly necessary and women should not breast feed during therapy.26
In summary, the main adverse effect of omalizumab is anaphylaxis, although this occurs infrequently. Preliminary data from a five-year safety study has raised concerns about increased cardiovascular events and a final report is awaited.
Place in Therapy
In 2003, omalizumab was approved by the FDA for the treatment of adults and adolescents aged 12 years and above with moderate to severe persistent allergic asthma whose symptoms are poorly controlled with inhaled corticosteroids.58 A Black Boxed adverse effects warning issued in 2007 advising of the risk of the delayed development of anaphylaxis after administration of omalizumab and a further warning issued in 2009 notified of interim safety findings from the EXCELS study of a possible increase in the number of cardiovascular and cerebrovascular adverse events in treated patients. The European Medicines Agency (EMA) licensed omalizumab in 2005 for the treatment of severe allergic asthma in patients aged 12 years or older in whom symptoms are poorly controlled, including a history of multiple exacerbations, despite inhaled high dose corticosteroids and inhaled long-acting β2 agonist bronchodilators and in whom the FEV1 < 80%. In 2009 the licence was extended to include children aged 6 to <12 years as an add-on treatment for poorly controlled asthma in patients with severe persistent allergic asthma.59 In addition to the US and Europe, omalizumab is licensed for treating patients with moderate to severe allergic asthma in a large number of other countries worldwide including Australia, Canada and Brazil.
Current Guidelines
The US National Asthma Education and Prevention Expert Panel Report 3 (EPR-3) guideline,3 the international Global Initiative for Asthma (GINA) guideline1 and the British guideline on the management of asthma2 have similar recommendations on the management of chronic asthma, with step-wise treatment aimed at controlling symptoms. Steps 4 to 6 of the EPR-3 guideline and Steps 4 and 5 of the GINA and British guidelines are the stage at which asthma is considered severe. Anti-IgE therapy is recommended by the EPR-3 guidelines at steps 5 and 63 and the GINA1 and British2 guidelines for patients at step 5.
Indications
For the purpose of using omalizumab the term allergic is defined as a positive skin test or in vitro reactivity to a perennial aeroallergen and in addition the serum total IgE levels should be in the range 30 to 700 IU/mL in the US. In Europe the serum total IgE ranges are ≥30 to ≤1500 IU/mL in adults and children >12 years and <1300 IU/mL for children over 6 years. The dose (mg) of omalizumab and dose frequency is based on the serum total IgE level (IU/mL) and the patient’s body weight (kg). Based on this calculation, omalizumab is given at a dose of 150 to 375 mg by subcutaneous injection every 2 or 4 weeks. The main contraindication is a past history of a severe hypersensitivity reaction to omalizumab. There is limited data on the efficacy of omalizumab in active cigarette smokers with allergic asthma, as this group is usually excluded from clinical trials of omalizumab, although it is likely that the efficacy will be similar to that reported in non-smokers with allergic asthma.60
Assessment of Therapeutic Response
Several studies have tried to identify clinical or immunological factors that predict a good therapeutic response to omalizumab.61–63 Based on a pooled analysis from seven randomized controlled omalizumab trials, Bousquet and colleagues62 concluded that treatment benefits were not related to baseline total IgE levels. However, in the INvestigatioN of Omalizumab in seVere Asthma TrEatment (INNOVATE) study undertaken in patients with severe allergic asthma and included in the pooled analysis, baseline total IgE was the only predictor of efficacy64 and levels of specific IgE for individual allergens were of no additional benefit in predicting a positive response.63 In inner-city US children, adolescents, and young adults with persistent allergic asthma the presence of sensitization and exposure to cockroach allergen predicted a very good response to omalizumab.36 It has been reported that patients receiving long-term oral corticosteroids or frequent courses of oral corticosteroids respond poorly to omalizumab,34 although there is some limited evidence to support an oral corticosteroid sparing effect of omalizumab.65 An open-label study of 82 patients with severe allergic asthma, found the addition of omalizumab therapy resulted in lower mean (SD) percent maintenance oral corticosteroid use after 32 weeks of treatment (−45.0%) compared with that in 23 patients who received usual care (18.3%).65
It is recommended that a decision is made whether to continue long-term treatment based on assessment of response made after 16 weeks of treatment,61 although a recent study reported that the maximum effect of omalizumab occurred within 4 weeks of starting treatment.36 In several studies of omalizumab, the physician’s overall assessment at 16 weeks following a course of treatment was the best predictor of a continuing beneficial response.62,66 Nevertheless the combination of objective measurements of efficacy, such as ACQ, as well as the physicians’ and patients’ overall assessment are often used to assess response, although these tools have not been validated. Cardiopulmonary exercise testing has been advocated as an additional method to assess and confirm a clinical response to omalizumab in patients with severe asthma although to date the utility of exercise testing as not been investigated in a large number of patients.67
Duration of treatment
Once a patient is considered to respond to omalizumab, treatment is then continued often for several years. It remains uncertain when treatment can be discontinued or whether prolonged treatment is required. Certainly a dose reduction at 6 months, in patients who were responders at 16 weeks, is associated with a recurrence of symptoms.68 A small study of patients with severe cat allergen-induced asthma reported good symptom control and reduced basophil allergen sensitivity three years following completion of six years treatment with omalizumab,69 suggesting that treatment may not need to be indefinite, although whether this conclusion applies to patients who do not have cat allergen-induced asthma is not known.
Special populations
There is no clear indication on the safety of omalizumab in pregnancy, although animal studies in monkeys have not reported adverse effects. Physicians need to assess the risk and benefits of treatment with omalizumab during pregnancy on an individual basis. In the US, a pregnancy exposure register has been established to help provide observational data on the use of omalizumab. There is limited data in the efficacy and safety of omalizumab in older patients over 65 years. Since omalizumab prevents seasonal peaks in asthma exacerbations it has been suggested that a seasonal course of treatment in individuals at high risk should be studied, as this approach would reduce the cost of treatment.36 A recommendation for the use of omalizumab in the treatment of nonallergic asthma and other sub-groups of asthma will be informed by the results of clinical trials underway.
In summary, omalizumab is a treatment option for patients with moderate to severe allergic asthma whose asthma is poorly controlled with inhaled corticosteroids and inhaled long-acting β2 agonist bronchodilators. No clinical or immunological factors consistently predict a good therapeutic response to omalizumab in allergic asthma. In responders, the duration of treatment is unclear. Clinical trials are underway to determine whether omalizumab has efficacy in the treatment of non-allergic asthma.
Cost
An assessment of the cost effectiveness of omalizumab in adults and adolescents with moderate-to-severe allergic asthma concluded that treatment should be reserved for patients with poorly controlled symptoms despite maximal therapy, given the high cost and modest efficacy of omalizumab.70 A cost effectiveness analysis relevant to the US population suggests that adding omalizumab to usual care improves quality-adjusted survival (QALYs), but at an increase in direct medical costs; the incremental cost-effectiveness ratio (ICER) of omalizumab compared to usual care was $172 300/QALY in responders.71 Similar conclusions were reported from a cost effectiveness analysis based on a ‘real-life’ 1-year randomized open-label study using costs in Canada.72 Uncertainty has been expressed over the excess mortality rates applied to severe exacerbations used in models in the UK to assess the cost- effectiveness of omalizumab.73 Due to the high cost of treatment, individual countries have issued specific criteria for use of omalizumab to maximise benefit in selected populations of patients. For example, in the UK, the National Institute of Health and Clinical Excellence (NICE) recommends that omalizumab should only be used in adult patients who give a history of two or more severe exacerbations of asthma requiring admission to hospital within the previous year, or three or more severe exacerbations within the previous year including at least one of which required hospital admission.74 NICE does not recommended omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years based on a review that concluded that omalizumab in addition to standard therapy compared with standard therapy alone did not appear cost-effective in either the overall population or a subgroup of patients hospitalised in the year prior to enrolment.75 In Scotland, the Scottish Medicines Consortium (SMC) restricts its use to patients with chronic oral corticosteroid dependent severe allergic asthma.76
Conclusions
Omalizumab is a recombinant humanized monoclonal antibody that binds circulating IgE antibody. It is approved in the US and Europe, as well as many other countries, for the treatment of adults and adolescents aged 12 years and above with moderate to severe persistent allergic asthma, whose symptoms are poorly controlled with inhaled corticosteroids, plus in Europe patients should also be receiving inhaled long-acting β2 agonist bronchodilators. In Europe, the licence also includes children aged 6 to <12 years as an add-on treatment for poorly controlled asthma in patients with severe persistent allergic asthma. Both national and international guidelines recommend omalizumab for patients with severe allergic asthma that is not controlled with other therapies. Omalizumab decreases allergic airway inflammation by reducing the expression of high affinity IgE receptors on inflammatory cells and eosinophil numbers within the airways. Research is underway to help establish whether long-term treatment with omalizumab also reduces airway remodeling. Recent long-term clinical trials confirm the benefits of omalizumab in reducing exacerbations and symptoms in adults and in children with moderate to severe allergic asthma. No clinical or immunological factors consistently predict a good therapeutic response to omalizumab in allergic asthma. In responders, the duration of treatment is unclear. The main adverse effect of omalizumab is anaphylaxis, although this occurs infrequently. Preliminary data from a five year safety study raised concerns about increased cardiovascular events and a final report is awaited. Clinical trials are in progress to determine whether omalizumab has efficacy in the treatment of non-allergic asthma.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: NCT, RC. Contributed to the writing of the manuscript: NCT, RC. Agree with manuscript results and conclusions: NCT, RC. Jointly developed the structure and arguments for the paper: NCT, RC. Made critical revisions and approved final version: NCT, RC. All authors reviewed and approved of the final manuscript.
Competing Interests
RC has received a grant from Novartis and ERS meeting expenses. NCT is a board member for Asmacure, Chiesi, and Respivert and received speakers fee from AstraZenica, Boston Scientific, Chiesi, GlaxoSmithKline, Novartis and speakers fee and travel expenses from Novartis. His institution received grants from Aerovance, Asthmax, AstraZenica, Centocor, GlaxoSmithKline, Medimmune, Novartis, and Synairgen.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest. Provenance: the authors were invited to submit this paper.
References
- 1.GINA report global strategy for asthma management prevention. 2010. [Accessed February 25, 2012]. http://www.ginasthma.com.
- 2.British guideline on the management of asthma. British Thoracic Society Scottish Intercollegiate Guidelines Network. Thorax. 2008;63:iv, 1–121. doi: 10.1136/thx.2008.097741. [DOI] [PubMed] [Google Scholar]
- 3.Expert Panel Report 3 (EPR-3): Guidelines for the Diagnosis and Management of Asthma-Summary Report 2007. J Allergy Clin. Immunol. 2007;120(5 Suppl 1):S94–138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 4.Partridge M, van der Molen T, Myrseth S-E, Busse W. Attitudes and actions of asthma patients on regular maintenance therapy: the INSPIRE study. BMC Pulm Med. 2006;6(1):13. doi: 10.1186/1471-2466-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dockrell M, Partridge M, Valovirta E. The limitations of severe asthma: the results of a European survey. Allergy. 2007;62(2):134–41. doi: 10.1111/j.1398-9995.2006.01304.x. [DOI] [PubMed] [Google Scholar]
- 6.Heaney LG, Brightling CE, Menzies-Gow A, Stevenson M, Niven RM on behalf of the British Thoracic Society Difficult Asthma N. Refractory asthma in the UK: cross-sectional findings from a UK multicentre registry. Thorax. 2010;65:787–94. doi: 10.1136/thx.2010.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bel EH, Sousa A, Fleming L, Bush A, Chung KF, Versnel J, et al. Diagnosis and definition of severe refractory asthma: an international consensus statement from the Innovative Medicine Initiative (IMI) Thorax. 2011;66(10):910–7. doi: 10.1136/thx.2010.153643. [DOI] [PubMed] [Google Scholar]
- 8.Thomson N, Chaudhuri R, Spears M. Emerging therapies for severe asthma. BMC Medicine. 2011;9(1):102. doi: 10.1186/1741-7015-9-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.The ENFUMOSA Study Group. The ENFUMOSA cross-sectional European multicentre study of the clinical phenotype of chronic severe asthma. Eur Respir J. 2003;22(3):470–7. doi: 10.1183/09031936.03.00261903. [DOI] [PubMed] [Google Scholar]
- 10.Holgate S, Casale T, Wenzel S, Bousquet J, Deniz Y, Reisner C. The anti-inflammatory effects of omalizumab confirm the central role of IgE in allergic inflammation. J Allergy Clin Immunol. 2005;115(3):459–65. doi: 10.1016/j.jaci.2004.11.053. [DOI] [PubMed] [Google Scholar]
- 11.Pollart SM, Chapman MD, Fiocco GP, Rose G, Platts-Mills TAE. Epidemiology of acute asthma: IgE antibodies to common inhalant allergens as a risk factor for emergency room visits. J Allergy Clin Immunol. 1989;83(5):875–82. doi: 10.1016/0091-6749(89)90100-0. [DOI] [PubMed] [Google Scholar]
- 12.Platts-Mills T. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001;164(8 Pt 2):S1–5. doi: 10.1164/ajrccm.164.supplement_1.2103024. [DOI] [PubMed] [Google Scholar]
- 13.Oliver J, Tarleton C, Gilmartin L, Archibeque T, Qualls C, Diehl L, et al. Reduced FcepsilonRI-mediated release of asthma-promoting cytokines and chemokines from human basophils during omalizumab therapy. Int Arch Allergy Immunol. 2010;151(4):275–84. doi: 10.1159/000250436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holgate ST, Polosa R. Treatment strategies for allergy and asthma. Nat Rev Immunol. 2008;8(3):218–30. doi: 10.1038/nri2262. [DOI] [PubMed] [Google Scholar]
- 15.Chanez P, Contin-Bordes Cc, Garcia G, Verkindre C, Didier A, De Blay Fdr, et al. Omalizumab-induced decrease of FcɛRI expression in patients with severe allergic asthma. Respir Med. 2010;104(11):1608–17. doi: 10.1016/j.rmed.2010.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Djukanović R, Wilson SJ, Kraft M, Jarjour NN, Steel M, Chung KF, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170(6):583–93. doi: 10.1164/rccm.200312-1651OC. [DOI] [PubMed] [Google Scholar]
- 17.Van Rensen ELJ, Evertse CE, Van Schadewijk WAAM, Van Wijngaarden S, Ayre G, Mauad T, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy. 2009;64(1):72–80. doi: 10.1111/j.1398-9995.2008.01881.x. [DOI] [PubMed] [Google Scholar]
- 18.Hoshino M, Ohtawa J. Effects of Adding Omalizumab, an Anti-Immunoglobulin E Antibody, on Airway Wall Thickening in Asthma. Respiration. 2012 Jan 11; doi: 10.1159/000334701. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 19.Rabe KF, Calhoun WJ, Smith N, Jimenez P. Can anti-IgE therapy prevent airway remodeling in allergic asthma? Allergy. 2011;66(9):1142–51. doi: 10.1111/j.1398-9995.2011.02617.x. [DOI] [PubMed] [Google Scholar]
- 20.Massanari M, Holgate ST, Busse WW, Jimenez P, Kianifard F, Zeldin R. Effect of omalizumab on peripheral blood eosinophilia in allergic asthma. Resp Med. 2009;104(2):188–96. doi: 10.1016/j.rmed.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Fahy JV. Anti-IgE: Lessons learned from effects on airway inflammation and asthma exacerbation. J Allergy Clin Immunol. 2006;117(6):1230–2. doi: 10.1016/j.jaci.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 22.Noga O, Hanf G, Brachmann I, Klucken AC, Kleine-Tebbe J, Rosseau S, et al. Effect of omalizumab treatment on peripheral eosinophil and T-lymphocyte function in patients with allergic asthma. J Allergy Clin Immunol. 2006;117(6):1493–9. doi: 10.1016/j.jaci.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 23.Xu J, Zhong S. Mechanisms of bronchial hyperresponsiveness: The interaction of endothelin-1 and other cytokines. Respirology. 1999;4(4):413–7. doi: 10.1046/j.1440-1843.1999.00214.x. [DOI] [PubMed] [Google Scholar]
- 24.Zietkowski Z, Skiepko R, Tomasiak-Lozowska M, Bodzenta-Lukaszyk A. Anti-IgE therapy with omalizumab decreases endothelin-1 in exhaled breath condensate of patients with severe persistent allergic asthma. Respiration. 2010;80(6):534–42. doi: 10.1159/000317137. [DOI] [PubMed] [Google Scholar]
- 25.Belliveau P. Omalizumab: a monoclonal anti-IgE antibody. Med Gen Med. 2005;7(1):27. [PMC free article] [PubMed] [Google Scholar]
- 26.SMPC. Xolair 150 mg powder and solvent for solution for injection. 2011. [Accessed February 25, 2012]. http://www.medicines.org.uk/emc/medicine/17029.
- 27.Fox JA, Hotaling TE, Struble C, Ruppel J, Bates DJ, Schoenhoff MB. Tissue distribution and complex formation with IgE of an anti-IgE antibody after intravenous administration in cynomolgus monkeys. J Pharmacol Exp Therap. 1996;279(2):1000–8. [PubMed] [Google Scholar]
- 28.Casale T, Bernstein I, Busse W, LaForce C, Tinkelman D, Stoltz R, et al. Use of an anti-IgE humanized monoclonal antibody in ragweed-induced allergic rhinitis. J Allergy Clin Immunol. 1997;100(1):110–21. doi: 10.1016/s0091-6749(97)70202-1. [DOI] [PubMed] [Google Scholar]
- 29.Casale TB, Condemi J, LaForce C, Nayak A, Rowe M, Watrous M, et al. Effect of Omalizumab on Symptoms of Seasonal Allergic Rhinitis. JAMA. 2001;286(23):2956–67. doi: 10.1001/jama.286.23.2956. [DOI] [PubMed] [Google Scholar]
- 30.Walker S, Monteil M, Phelan K, Lasserson T, Walters E. Anti-IgE for chronic asthma in adults and children. Cochrane Database of Systematic Reviews. 2006;2:CD003559. doi: 10.1002/14651858.CD003559.pub3.. [DOI] [PubMed] [Google Scholar]
- 31.Rodrigo GJ, Neffen H, Castro-Rodriguez J. Efficacy and safety of subcutaneous omalizumab vs. placebo as add-on therapy to corticosteroids for children and adults with asthma. Chest. 2011;139(1):28–35. doi: 10.1378/chest.10-1194. [DOI] [PubMed] [Google Scholar]
- 32.Lanier B, Bridges T, Kulus M, Taylor AF, Berhane I, Vidaurre CF. Omalizumab for the treatment of exacerbations in children with inadequately controlled allergic (IgE-mediated) asthma. J Allergy Clin Immunol. 2009;124(6):1210–6. doi: 10.1016/j.jaci.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 33.Ohta K, Miyamoto T, Amagasaki T, Yamamoto M on behalf of the Study G. Efficacy and safety of omalizumab in an Asian population with moderate-to- severe persistent asthma. Respirology. 2009;14(8):1156–65. doi: 10.1111/j.1440-1843.2009.01633.x. [DOI] [PubMed] [Google Scholar]
- 34.Hanania NA, Alpan O, Hamilos DL, Condemi JJ, Reyes-Rivera I, Zhu J, et al. Omalizumab in Severe Allergic Asthma Inadequately Controlled With Standard Therapy. Ann Intern Med. 2011;154(9):573–82. doi: 10.7326/0003-4819-154-9-201105030-00002. [DOI] [PubMed] [Google Scholar]
- 35.Bardelas J, Figliomeni M, Kianifard F, Meng X. A 26-Week, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Evaluate the Effect of Omalizumab on Asthma Control in Patients with Persistent Allergic Asthma. J Asthma. 2012;49(2):144–52. doi: 10.3109/02770903.2011.648296. [DOI] [PubMed] [Google Scholar]
- 36.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized Trial of Omalizumab (Anti-IgE) for Asthma in Inner-City Children. N Eng J Med. 2011;364(11):1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Molimard M, Gros VL. Impact of Patient-Related Factors on Asthma Control. J Asthma. 2008;45(2):109–13. doi: 10.1080/02770900701815727. [DOI] [PubMed] [Google Scholar]
- 38.Korn S, Thielen A, Seyfried S, Taube C, Kornmann O, Buhl R. Omalizumab in patients with severe persistent allergic asthma in a real-life setting in Germany. Resp Med. 2009;103(11):1725–31. doi: 10.1016/j.rmed.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 39.Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. ‘Real-life’ effectiveness of omalizumab in patients with severe persistent allergic asthma: The PERSIST study. Resp Med. 2009;103(11):1633–42. doi: 10.1016/j.rmed.2009.06.014. [DOI] [PubMed] [Google Scholar]
- 40.Niven R, Chung KF, Panahloo Z, Blogg M, Ayre G. Effectiveness of omalizumab in patients with inadequately controlled severe persistent allergic asthma: An open-label study. Resp Med. 2008;102(10):1371–8. doi: 10.1016/j.rmed.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Cazzola M, Camiciottoli G, Bonavia M, Gulotta C, Ravazzi A, Alessandrini A, et al. Italian real-life experience of omalizumab. Resp Med. 2010;104(10):1410–6. doi: 10.1016/j.rmed.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: The South- Eastern Mediterranean real-life experience. Pulm Pharmacol Therapeut. 2012;25(1):77–82. doi: 10.1016/j.pupt.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 43.Rottem M. Omalizumab reduces corticosteroid use in patients with severe allergic asthma: real-life experience in Israel. J Asthma. 2012;49(1):1–5. doi: 10.3109/02770903.2011.637598. [DOI] [PubMed] [Google Scholar]
- 44.Vennera M, Pérez De, Llano L, Bardagí S, Ausin P, Sanjuas C, González H, et al. Omalizumab Therapy in Severe Asthma: Experience from the Spanish Registry-Some New Approaches. J Asthma. 2012;0(0):1–7. doi: 10.3109/02770903.2012.668255. [DOI] [PubMed] [Google Scholar]
- 45.Corren J, Wood RA, Patel D, Zhu J, Yegin A, Dhillon G, et al. Effects of omalizumab on changes in pulmonary function induced by controlled cat room challenge. J Allergy Clin Immunolv. 2011;127(2):398–405. doi: 10.1016/j.jaci.2010.09.043. [DOI] [PubMed] [Google Scholar]
- 46.Vignola AM, Humbert M, Bousquet J, Boulet LP, Hedgecock S, Blogg M, et al. Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy. 2004;59(7):709–17. doi: 10.1111/j.1398-9995.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 47.van den Berge M, Pauw RG, de Monchy JGR, van Minnen CA, Postma DS, Kerstjens HAM. Beneficial effects of treatment with anti-IgE antibodies (omalizumab) in a patient with severe asthma and negative skin-prick Test Results. Chest. 2011;139(1):190–3. doi: 10.1378/chest.10-0128. [DOI] [PubMed] [Google Scholar]
- 48.Massanari M, Nelson H, Casale T, Busse W, Kianifard F, Geba GP, et al. Effect of pretreatment with omalizumab on the tolerability of specific immunotherapy in allergic asthma. J Allergy Clin Immunol. 2010;125(2):383–9. doi: 10.1016/j.jaci.2009.11.022. [DOI] [PubMed] [Google Scholar]
- 49.Pérez-de-Llano LA, Vennera MC, Parra A, Guallar J, Marin M, Asensio O, et al. Effects of omalizumab in Aspergillus-associated airway disease. Thorax. 2011;66(6):539–40. doi: 10.1136/thx.2010.153312. [DOI] [PubMed] [Google Scholar]
- 50.Corren J, Casale TB, Lanier B, Buhl R, Holgate S, Jimenez P. Safety and tolerability of omalizumab. Clin Exp Allergy. 2009;39(6):788–97. doi: 10.1111/j.1365-2222.2009.03214.x. [DOI] [PubMed] [Google Scholar]
- 51.Tan RA, Corren J. Safety of omalizumab in asthma. Expert Opinion on Drug Safety. 2011;10(3):463–71. doi: 10.1517/14740338.2011.563840. [DOI] [PubMed] [Google Scholar]
- 52.Cox L, Platts-Mills TAE, Finegold I, Schwartz LB, Simons FER, Wallace DV. American Academy of Allergy, Asthma and Immunology/American College of Allergy, Asthma and Immunology Joint Task Force Report on omalizumab-associated anaphylaxis. J Allergy Clin Immunol. 2007;120(6):1373–7. doi: 10.1016/j.jaci.2007.09.032. [DOI] [PubMed] [Google Scholar]
- 53.Holgate S, Buhl R, Bousquet J, Smith N, Panahloo Z, Jimenez P. The use of omalizumab in the treatment of severe allergic asthma: A clinical experience update. Respir Med. 2009;103(8):1098–113. doi: 10.1016/j.rmed.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Busse W, Buhl R, Vidaurre CF, Blogg M, Zhu J, Eisner MD, et al. Omalizumab and the risk of malignancy: Results from a pooled analysis. J Allergy Clin Immunol. 2012 Feb 22; doi: 10.1016/j.jaci.2012.01.033. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 55.Aidan AL, James EF, Abdelkader R, Mary KM, Mary SB, Hassan NT, et al. Baseline characteristics of patients enrolled in EXCELS: a cohort study. Ann Allergy Asthma Immunol. 2009;103(3):212–9. doi: 10.1016/S1081-1206(10)60184-6. [DOI] [PubMed] [Google Scholar]
- 56.Cruz AA, Lima F, Sarinho E, Ayre G, Martin C, Fox H, et al. Safety of anti-immunoglobulin E therapy with omalizumab in allergic patients at risk of geohelminth infection. Clin Exp Allergy. 2007;37(2):197–207. doi: 10.1111/j.1365-2222.2007.02650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korn S, Schumann C, Kropf C, Stoiber K, Thielen A, Taube C, et al. Effectiveness of omalizumab in patients 50 years and older with severe persistent allergic asthma. Annals of Allergy, Asthma, and Immunology. 2010;105(4):313–9. doi: 10.1016/j.anai.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 58.US Food and Drug Administration. Omalizumab approval history. 2011. [Accessed February 25, 2012]. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Label_ApprovalHistory#apphist.
- 59.European Medicines Agency. Summary of the European public assessment report (EPAR) for Xolair. 2009. [Accessed February 25, 2012]. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000606/human_med_001162.jsp&mid=WC0b01ac058001d124&jsenabled=true.
- 60.Spears M, Cameron E, Chaudhuri R, Thomson NC. Challenges of treating asthma in people who smoke. Expert Rev Clin Immunol. 2010;6(2):257–68. doi: 10.1586/eci.09.85. [DOI] [PubMed] [Google Scholar]
- 61.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125(4):1378–86. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 62.Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Resp Med. 2007;101(7):1483–92. doi: 10.1016/j.rmed.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Wahn U, Martin C, Freeman P, Blogg M, Jimenez P. Relationship between pretreatment specific IgE and the response to omalizumab therapy. Allergy. 2009;64(12):1780–7. doi: 10.1111/j.1398-9995.2009.02119.x. [DOI] [PubMed] [Google Scholar]
- 64.Humbert M, Beasley R, Ayres J, Slavin R, Hebert J, Bousquet J, et al. Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy. 2005;60(3):309–16. doi: 10.1111/j.1398-9995.2004.00772.x. [DOI] [PubMed] [Google Scholar]
- 65.Siergiejko Z, Åšwiebocka E, Smith N, Peckitt C, Leo J, Peachey G, et al. Oral corticosteroid sparing with omalizumab in severe allergic (IgE-mediated) asthma patients. Curr Med Res Opinion. 2011;27(11):2223–8. doi: 10.1185/03007995.2011.620950. [DOI] [PubMed] [Google Scholar]
- 66.Bousquet J, Siergiejko Z, Œwiebocka E, Humbert M, Rabe KF, Smith N, et al. Persistency of response to omalizumab therapy in severe allergic (IgE-mediated) asthma. Allergy. 2011;66(5):671–8. doi: 10.1111/j.1398-9995.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 67.Schäper C, Gläser S, Felix SB, Gogolka A, Koch B, Krüll M, et al. Omalizumab treatment and exercise capacity in severe asthmatics: Results from a pilot study. Resp Medv. 2011;105(1):3–7. doi: 10.1016/j.rmed.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 68.Slavin RG, Ferioli C, Tannenbaum SJ, Martin C, Blogg M, Lowe PJ. Asthma symptom re-emergence after omalizumab withdrawal correlates well with increasing IgE and decreasing pharmacokinetic concentrations. J Allergy Clin Immunol. 2009;123(1):107–13. doi: 10.1016/j.jaci.2008.09.050. [DOI] [PubMed] [Google Scholar]
- 69.Nopp A, Johansson SGO, Adédoyin J, Ankerst J, Palmqvist M, Öman H. After 6 years with Xolair; a 3-year withdrawal follow-up. Allergy. 2010;65(1):56–60. doi: 10.1111/j.1398-9995.2009.02144.x. [DOI] [PubMed] [Google Scholar]
- 70.Oba Y, Salzman GA. Cost-effectiveness analysis of omalizumab in adults and adolescents with moderate-to-severe allergic asthma. J Allergy Clin Immunol. 2004;114(2):265–9. doi: 10.1016/j.jaci.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 71.Campbell JD, Spackman DE, Sullivan SD. The costs and consequences of omalizumab in uncontrolled asthma from a USA payer perspective. Allergy. 2010;65(9):1141–8. doi: 10.1111/j.1398-9995.2010.02336.x. [DOI] [PubMed] [Google Scholar]
- 72.Brown R, Turk F, Dale P, Bousquet J. Cost-effectiveness of omalizumab in patients with severe persistent allergic asthma. Allergy. 2007;62(2):149–53. doi: 10.1111/j.1398-9995.2006.01310.x. [DOI] [PubMed] [Google Scholar]
- 73.Jones J, Shepherd J, Hartwell D, Harris P, Cooper K, Takeda A, et al. Omalizumab for the treatment of severe persistent allergic asthma. Health Technol Assess. 2009;13(Suppl 2):31–9. doi: 10.3310/hta13suppl2/05. [DOI] [PubMed] [Google Scholar]
- 74.National Institute of Health and Clinical Excellence. Omalizumab for severe persistent allergic asthma. 2007. [Accessed February 25, 2012]. http://www.nice.org.uk/nicemedia/pdf/TA133Guidance.pdf.
- 75.Walker S, Burch J, McKenna C, Wright K, Griffin S, Woolacott N. Omalizumab for the treatment of severe persistent allergic asthma in children aged 6–11 years. Health Technol Assess. 2009;15(Suppl 1):13–21. doi: 10.3310/hta15suppl1/02. [DOI] [PubMed] [Google Scholar]
- 76.Scottish Medicines Consortium. Omalizumab 150 mg powder solvent for injection (Xolair®) 2007. [Accessed February 25, 2012]. http://www.scottishmedicines.org.uk/files/259_06_omalizumab_Xolair_2ndResub_Sept07.pdf.