Abstract
Cultured human cells are invaluable biological models for mechanistic studies of genotoxic chemicals and drugs. Continuing replacement of animals in toxicity testing will further increase the importance of in vitro cell systems, which should accurately reproduce key in vivo characteristics of toxicants such as their profiles of metabolites and DNA lesions. In this work, we examined how a common severe deficiency of cultured cells in ascorbate (Asc) impacts the formation of oxidative DNA damage by hexavalent chromium (chromate). Cr(VI) is reductively activated inside the cells by both Asc and small thiols but with different rates and spectra of intermediates and DNA adducts. We found that Cr(VI) exposure of H460 human lung epithelial cells in standard culture (<0.01 mM cellular Asc) induced biologically significant amounts of oxidative DNA damage. Inhibition of oxidative damage repair in these cells by stable XRCC1 knockdown strongly enhanced cytotoxic effects of Cr(VI) and led to depletion of cells from G1 and accumulation in S and G2 phases. However, restoration of physiological levels of Asc (∼1 mM) completely eliminated Cr(VI) hypersensitivity of XRCC1 knockdown. The induction of chromosomal breaks assayed by the micronucleus test in Asc-restored H460, primary human lung fibroblasts, and CHO cells was also unaffected by the XRCC1 status. Centromere-negative (clastogenic) micronuclei accounted for 80–90% of all Cr(VI)-induced micronuclei. Consistent with the micronuclei results, Asc-restored cells also showed no increase in the levels of poly(ADP-ribose), which is a biochemical marker of single-stranded breaks. Asc had no effect on cytotoxicity of O6-methylguanine, a lesion produced by direct DNA alkylation. Overall, our results indicate that the presence of physiological levels of Asc strongly suppresses pro-oxidant pathways in Cr(VI) metabolism and that the use of standard cell cultures creates a distorted profile of its genotoxic properties.
Introduction
Ascorbate (Asc) is a major water-soluble radical scavenger, which also regulates the activity of the growing number of enzymes impacting diverse cellular functions. It acts as a cofactor for a large family of dioxygenases that control activity of transcriptional factors such as HIF1α (1,2) and chromatin remodelling enzymes establishing short- and long-term gene expression patterns (histone and 5 methyl-dC demethylases) (3,4). Asc is also a cofactor for two hydroxylases in biosynthesis of carnitine, which is important in ATP generation through delivery of fatty acids into mitochondria (5). Cultured cells are widely used biological models for mechanistic studies of genotoxic agents; however, they typically contain only low micromolar concentrations of Asc (1,6–9) in comparison to 1–3 mM levels of this vitamin in major tissues (10,11). Severe Asc deficiency of cells in culture is a result of the absence of Asc in the commonly used synthetic media and its limited supply through a typical addition of only 10% serum. This problem is further exacerbated by the instability of vitamin C in cell culture with t 1/2 = 6–7 h (12). A near absence of Asc in cultured cells raises concerns regarding the ability of in vitro models to accurately recapitulate many critical aspects of genotoxicity in vivo, such as the relative role of oxidative versus nonoxidative DNA damage, gene expression responses, defence mechanisms, and others.
Hexavalent Cr is a widespread occupational carcinogen whose genotoxicity results from its metabolic activation inside the cells. Mutagenicity and genotoxicity of Cr(VI) have been attributed to the induction of two classes of DNA damage: Cr–DNA adducts and oxidative DNA damage (13–15). Induction of all forms of DNA damage by Cr(VI) requires its activation through reduction, ultimately yielding thermodynamically stable Cr(III). Cr(VI) reduction in tissues is primarily nonenzymatic and driven by Asc and small thiols such as glutathione and cysteine. In the lung, the main target tissue of toxic and carcinogenic effects of Cr(VI), Asc accounts for ∼95% reduction of Cr(VI) (16). Depending on the nature of the reducer and its concentration, reduction reactions can display different ratios of one- and two-electron transfers generating variable amounts of Cr(V) and Cr(IV) intermediates and sulphur- and carbon-based radicals (17–20). Oxidative DNA damage has largely been attributed to the production of Cr(V) intermediate (13), which in complexes with selected synthetic ligands was capable of direct DNA oxidation (21,22). In the presence of H2O2 in vitro, Cr(V) can form Cr(V)–peroxo complexes or catalyse Fenton-type reactions generating a potent OH• radical and both processes lead to DNA oxidation (23–25). The importance of H2O2 in oxidative DNA damage by Cr(VI) in cells is supported by protective effects of elevated catalase levels (26).
Two characteristics distinguish reduction of Cr(VI) by Asc from that by small thiols: much higher rate [t 1/2 = 1 min for Asc versus 13.3 min for Cys and 60.7 min for glutathione at 1 mM concentrations (27)] and the absence of detectable Cr(V) at the biologically relevant stoichiometry of the reactants (19,20). Considering that Cr(V) was implicated in the generation of oxidative DNA damage via both direct and reactive oxygen species (ROS)-mediated mechanisms, it is possible that Asc deficiency of cultured cells overestimates the oxidation-based component of Cr(VI) genotoxicity. However, approximately a 10-fold elevation of cellular Asc to 80 μM resulted in the increased oxidation of two redox-sensitive dyes, which was interpreted as evidence for a pro-oxidant role of Asc in Cr(VI) metabolism (7). Restoration of physiological levels of Asc in human and rodent cells increased cytotoxicity and particularly, clastogenicity and mutagenicity of Cr(VI) (8,28). Potentiating effects of Asc on Cr(VI) genotoxicity are at least in part caused by the formation of mutagenic and genotoxic Asc-Cr-DNA cross link (6,29). Overall, it is unknown how Asc restoration in cells impacts DNA oxidation by Cr(VI), which is important for a mechanistic understanding of its genotoxicity and the development of chemopreventive strategies and useful biomarkers of exposure and susceptibility. In this work, we examined the role of Asc in the formation of oxidative DNA damage by analysing cytotoxic and clastogenic effects of Cr(VI) in cells with normal and defective DNA repair.
Materials and methods
Materials
Potassium chromate (K2CrO4, ACS reagent, >99% pure) was from Aldrich (Milwaukee, WI, USA). L-ascorbic acid (99.9% pure), dehydro-l-(+)-ascorbic acid dimer (DHA), glutathione (>98% pure), monobromobimane, ascorbate oxidase, and all salts and solvents were from Sigma (St Louis, MO, USA). 1,2-Diamino-4,5-dimethoxybenzene dihydrochloride was from Molecular Probes (Eugene, OR, USA). Foetal bovine serum was from Gemini (Sacramento, CA, USA).
Cells and treatments
Human lung epithelial H460 cells and primary human lung IMR90 fibroblasts were obtained from ATCC. Empty vector- and XRCC1-complemented CHO-EM9 cells were provided by Dr K. Caldecott. IMR90 cells were used at passages 8–12. H460, IMR90 and EM9 derivatives were grown in RPMI-10% serum, Dulbecco's modified Eagle medium-15% serum and Minimum Essential Medium-10% serum media, respectively. Growth medium for EM9 lines additionally contained 1.5 mg/ml G418. For Asc loading, cells were incubated for 90 min with DHA in Krebs–HEPES buffer [30 mM HEPES (pH 7.5), 130 mM NaCl, 4 mM KH2PO4, 1 mM MgSO4, 1 mM CaCl2, 0.5 mM glucose]. H460 and CHO cells were pre-incubated with 2 mM DHA and IMR90 with 5 mM DHA. Exposures to K2CrO4 [Cr(VI)] were in cell type-specific media for the indicated times.
High-performance liquid chromatography measurements of cellular Asc and glutathione
Asc concentrations were measured by high-performance liquid chromatography (HPLC) detection of a specific fluorescent conjugate with 1,2-diamino-4,5-dimethoxybenzene dihydrochloride (28). Trypsinised cells were washed three times with cold phosphate-buffered saline (PBS) and re-suspended in a cold solution containing 50 mM methanesulfonic acid and 5 mM diethylenetriaminepentaacetic acid. Samples were subjected to two cycles of freezing/thawing, and Asc-containing extracts were collected by centrifugation at 12 000 × g for 20 min at 4°C. Cellular extracts were reacted with 0.5 mM 1,2-diamino-4,5-dimethoxybenzene dihydrochloride in the presence of 50 mM Na-acetate buffer (pH 6.2) and 0.5 units ascorbate oxidase. HPLC separation was performed by isocratic elution with 75% 50 mM phosphoric acid (pH 2.0) and 25% acetonitrile. The detection limit for cellular Asc was 0.1 μM. Cellular volumes were determined from forward scattering data measured by flow cytometry.
Measurements of cellular glutathione (GSH) were performed by an HPLC-based procedure as described previously (30). In brief, trypsinised cells were re-suspended in a cold solution containing 40 mM methanesulfonic acid–1 mM diethylenetriaminepentaacetic acid, lysed by two cycles of freezing/thawing and then spun down at 12 000 × g for 10 min at 4°C. The cell extracts were reacted with 2 mM monobromobimane and the fluorescent GSH conjugates were measured by HPLC equipped with the Ultrasphere ODS column (5 μm, 250 × 4.6 mm).
Retroviral infections with short hairpin RNA
XRCC1 knockdown was created by expression of short hairpin RNA (shRNA) from the pSUPER-RETRO retroviral vector. The shRNA vectors were constructed by linearisation with HindIII and BglII followed by ligation of the oligonucleotides containing targeting sequences for firefly luciferase (shLuc, control vector) and human XRCC1: 5′-GCGACCAACGCCTTGATTG-3′ and 5′-AGGGAAGAGGAAGTTGGAT-3′, respectively. Cells were infected twice over 24 h and then selected with puromycin as previously described (31). Selected cells were frozen and each experiment was done with a freshly thawed out cells.
Western blotting
For measurements of protein expression and apoptotic poly (ADP-ribose) polymerase (PARP) cleavage, cells were collected by scraping, washed twice with cold PBS and extracted with a cold lysis buffer [50 mM Tris (pH 8.0), 250 mM NaCl, 1% NP40, 0.1% sodium dodecyl sulphate (SDS), 5 mM EDTA] supplemented with protease and phosphates inhibitors. Proteins were separated by 10% SDS–polyacrylamide gel electrophoresis (PAGE) and electrotransferred onto ImmunoBlot polyvinylidene fluoride membrane (Bio-Rad). PARP1 was detected with rabbit polyclonal antibodies from Cell Signaling and XRCC1 with mouse monoclonal antibodies from Abcam. For detection of poly(ADP-ribose)-modified proteins, cell lysates were obtained by boiling cell pellets in 2% SDS for 5 min, run on 8% SDS-PAGE, transferred onto nitrocellulose membrane and probed with rabbit polyclonal anti-poly(ADP-ribose) antibodies from BD Biosciences.
Cell cycle analysis
Cells were collected by trypsinisation, washed twice with cold PBS and then fixed in cold 70% ethanol for 20 min. Cells were pelleted at 800× g for 5 min at 4°C and washed twice with cold PBS containing 3% foetal bovine serum. Chromosomal DNA was stained in a solution containing 0.45 mg/ml propidium iodide and 0.5 mg/ml ribonuclease A for 40 min at 37°C. Cell cycle profiles were analysed on Becton Dickinson FACSCaliber using Cell Quest software.
Cr uptake
Total cellular Cr was measured by graphite furnace atomic absorption spectroscopy using Zeeman background correction (26). Cells were collected by trypsinisation, washed twice with cold PBS and then extracted with hot nitric acid. Cr concentrations were determined using Perkin-Elmer 4100ZL GF-AAS.
Micronuclei
Cells were grown on Superfrost Plus slides, exposed to Cr(VI) for 3 h and allowed to divide for 48 h. Slides were washed twice with PBS, fixed with 2% paraformaldehyde in PBS for 15 min and permeabilised with 1% Triton X-100 for 15 min at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole and centromere-positive micronuclei were detected by immunostaining with anti-kinetochore antibody (8). All slides were coded and scored in a blind manner using a Nikon Eclipse E800 digital microscope. At least 500 cells were analysed for each slide.
Clonogenic survival
Cells were seeded onto 60-mm dishes (200–500 cells per dish), allowed to attach overnight and treated with Cr(VI) for 3 h and H2O2 for 1 h. Exposure to N-methyl-N’-nitrosoguanidine (MNNG) was done in the presence of 10 μM O6-benzylguanine. MNNG is rapidly inactivated in aqueous solutions and media was not changed after its addition. Cells were fed fresh medium every 4–5 days and grown for 8–10 days without replating. Colonies were stained with Giemsa solution.
Statistics
Statistical significance was evaluated by the Student’s t-test. Differences with P < 0.05 were considered statistically significant. All data are presented as means ± SD.
Results
Characterisation of Asc-restored H460 cells
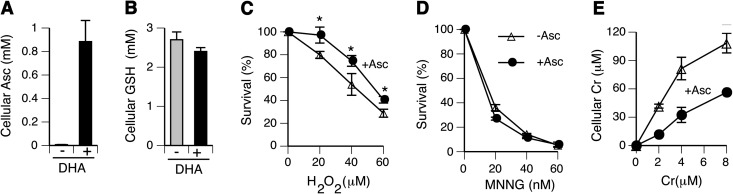
Lung tissue is the main site of malignant and other pathological changes found among Cr(VI)-exposed workers (32,33). Therefore, we chose human lung epithelial H460 cells as our primary cellular model. This cell line accurately recapitulates stress responses of primary cells to DNA damage by ionising radiation (34) and cytotoxic and genotoxic effects of Cr(VI) (28,29). H460 cells retain wild-type p53 tumour suppressor, which integrates numerous stress signals and controls cellular fate after oxidative DNA injury (35). The predominant mechanism for accumulation of vitamin C by human cells is uptake of its oxidised form DHA via glucose transporters (36,37). Figure 1A shows that a 90-min-long incubation of H460 cells with 2 mM DHA was sufficient to raise cellular levels of vitamin C to 0.9 mM from 7 μM in control. Restoration of physiological Asc levels did not lead to significant changes in cellular GSH (Figure 1B), which is another major cellular antioxidant and the second most important reducer of Cr(VI) after Asc (15). Thus, the increase in Asc levels resulted in the overall higher cellular antioxidant capacity. As expected, Asc-restored cells exhibited a significantly increased resistance to clonogenic toxicity by H2O2 (Figure 1C). Mismatch repair plays a major role in cytotoxic and clastogenic effects of Cr(VI) through processing of ternary Cr–DNA adducts into DNA double-stranded breaks and activation of apoptosis (29,38). A full activity of mismatch repair is also required for the induction of cell death by O6-methylguanine (39), a highly toxic DNA lesion produced by Sn1-methylating agents such as MNNG. Elevation of Asc levels showed no impact on mismatch repair functioning, as evidenced by a similar clonogenic survival of standard and Asc-supplemented H460 cells treated with MNNG in the presence of O6-benzylguanine that blocks DNA repair activity of O6-methylguanine DNA methyltransferase (Figure 1D). Control H460 cells showed a very efficient uptake of Cr(VI), resulting in up to 20-fold accumulation of Cr inside the cells relative to its extracellular concentrations (Figure 1E). Asc-restored cells also displayed a dose-dependent uptake of Cr(VI); however, it was on average 2.3 times lower than that found in standard cultures. Leakage of Asc by cells in mass cultures during prolonged incubations creates sufficient concentrations around the cells to inhibit uptake of Cr(VI) through its extracellular conversion to impermeable Cr(III) (8,28).
Fig. 1.
Characterisation of Asc-restored H460 cells. (A) Asc concentrations in control and 2 mM DHA-treated H460 cells. (B) GSH levels in control and Asc-restored H460 cells. (C) Clonogenic toxicity of hydrogen peroxide. (D) Clonogenic toxicity of MNNG. Cells were pre-incubated for 1 h with 10 μM O6-benzylguanine prior to the addition of MNNG. (E) Cr(VI) uptake by control and Asc-restored H460 cells. Cr(VI) treatments were for 3 h. When not visible, error bars were smaller than the graph symbol. Statistics: *P < 0.05.
Toxic responses to Cr(VI) in cells with a deficient repair of oxidative DNA damage
DNA oxidation induces a broad spectrum of lesions, which vary for different oxidants. This diversity complicates the assessment of the significance of oxidative DNA damage based on analytical measurements of specific products as it is hard to know which lesion(s) is the most frequent or important for a particular oxidant. Single-strand breaks (SSB) are one of the most common products of oxidative insult on DNA by ROS, and the presence of these lesions in Cr(VI)-treated cells was detected by the standard alkali-based assays (alkaline elution, alkaline unwinding, alkali Comet assay) (32). However, as common for all DNA phosphotriesters, DNA phosphate-based Cr adducts catalyse strand breakage at high pH (40), complicating the assessment of the true extent of directly induced SSB for Cr(VI). Exposure of Cr-adducted DNA to high pH in aerobic solutions during purification or assay conditions can also cause reoxidation of Cr(III) to higher valent states including Cr(VI), which would result in secondary oxidation processes and base damage. Therefore, we focused on a genetic approach for the evaluation of the role of oxidative DNA damage in human cells with physiological levels of Asc.
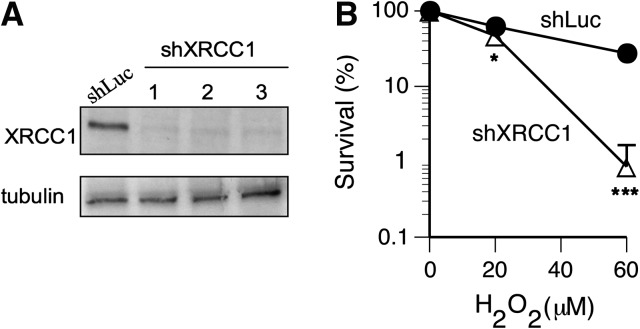
XRCC1 plays a critical role in protection of cells against oxidative DNA damage due to its importance for repair of SSB and oxidised DNA bases (41,42). Loss of XRCC1 strongly increases susceptibility of cells to killing by H2O2 and to a lesser extent, by gamma radiation (43), which reflects different yields of DNA double-strand breaks whose repair is XRCC1 independent. Hamster CHO-EM9 cells lacking XRCC1 protein were also hypersensitive to clonogenic toxicity by Cr(VI) under standard tissue culture conditions (26,44). The hypersensitivity of EM9 cells to Cr(VI) is linked to the formation of oxidative DNA damage by H2O2, as increasing cellular antioxidant potential by elevation of catalase or GSH levels eliminated survival differences between XRCC1-null and XRCC1+ cells (26). Cr–DNA adducts, another form of Cr(VI)-induced DNA damage, are removed by nucleotide excision repair (31,45), a genome defence mechanism in which XRCC1 is not involved. Therefore, to test the role of DNA oxidation in toxic effects of Cr(VI), we decided to construct a histologically relevant human genetic model of oxidant hypersensitivity based on stable XRCC1 knockdown in H460 human lung cells. Infections of H460 cells with the pSUPER-RETRO vector expressing targeting shRNA produced a very efficient and reproducible depletion of XRCC1 protein in three independent populations (Figure 2A). XRCC1-depleted cells displayed strongly diminished clonogenic viability after exposure to H2O2 (Figure 2B), providing a functional validation of the oxidant-hypersensitive phenotype of our cellular model.
Fig. 2.
Validation of XRCC1 deficiency in H460 cells. (A) Western blot demonstrating efficiency of XRCC1 knockdowns in three independently infected H460 populations. (B) Increased clonogenic toxicity of hydrogen peroxide in H460 cells with XRCC1 knockdown. Statistics: *P < 0.05, ***P < 0.001.
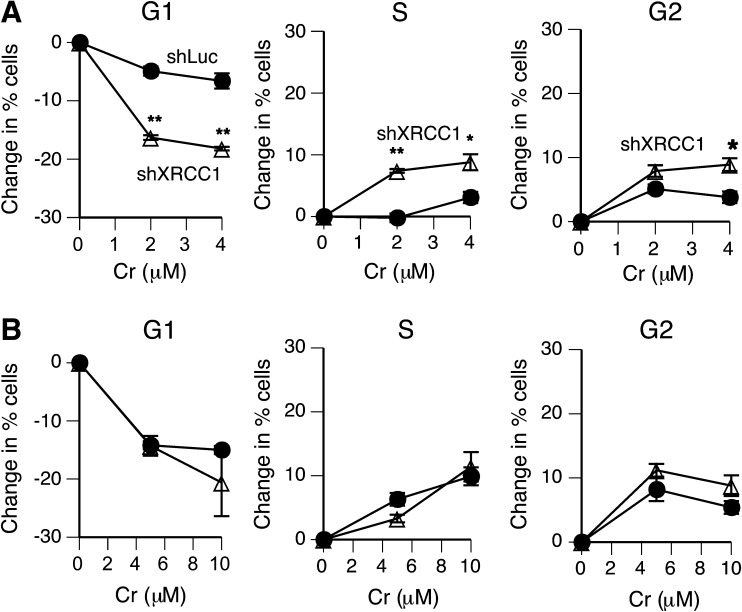
The inability to repair SSB and polymerase-blocking base oxidation products is expected to alter cell cycle distribution of XRCC1-deficient cells experiencing oxidative stress. FACS analysis showed that exposure to 0–4 μM Cr(VI) induced only minor cell cycle changes in control shLuc-H460 cells grown under standard culture conditions (Figure 3A). In shXRCC1 cells, Cr(VI) caused a pronounced loss of cells from G1 phase accompanied by significantly increased percentages of cells in S and G2 phases. These results provide genetic evidence for the induction of biologically significant amounts of oxidative DNA damage by Cr(VI) in human lung cells containing low Asc levels. To study cell cycle effects in Asc-supplemented cells, Cr(VI) concentrations were increased by 2.5-fold to deliver the same intracellular Cr doses as in standard H460 populations (Figure 1E). We found that cell cycle distribution of Asc-restored H460 cells did not differ between shLuc control and shXRCC1 knockdown (Figure 3B). Overall, cell cycle disturbances in Asc-supplemented shLuc-H460 cells were more severe in comparison to those in Asc-deficient cells, likely reflecting a higher formation of DNA double-stranded breaks due misprocessing of Asc–Cr–DNA adducts by mismatch repair (8,29).
Fig. 3.
Cell cycle effects of Cr(VI) in XRCC1-depleted H460 cells. Cells were treated with Cr(VI) for 3 h and cell cycle distribution was determined 24 h later. Statistics: *P < 0.05, **P < 0.01. (A) Changes in percentage of cells among cell cycle phases in standard H460 cultures. (B) Changes in cell cycle phases in Asc-restored H460 cells.
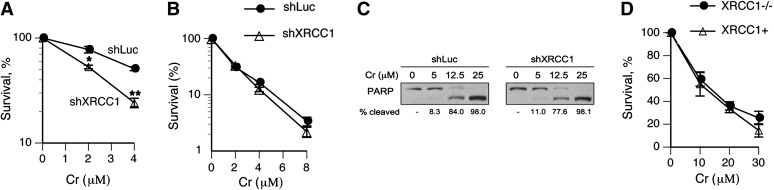
Next, we examined the effects of XRCC1 deficiency on cytotoxic effects of Cr(VI). In agreement with cell cycle results, XRCC1 depletion in H460 cells containing low Asc levels significantly increased clonogenic toxicity of Cr(VI) (Figure 4A). In contrast, Asc-restored control and XRCC1 knockdown cells showed identical clonogenic viability following Cr(VI) treatment (Figure 4B). As observed earlier (28), Cr(VI) was more cytotoxic in Asc-restored cells. To further test the impact of XRCC1 deficiency on cell death, we measured the induction of apoptosis using a classic marker of caspase-mediated PARP cleavage. Figure 4C shows that Asc-restored shLuc and shXRCC1 cells had very similar levels of PARP cleavage, indicating lack of detectable contribution of oxidative DNA damage to Cr(VI)-induced apoptosis. To extend our observations to other cells, we examined Cr(VI) cytotoxicity in CHO-EM9 cells that have been previously found by us and others to be hypersensitive to clonogenic killing by Cr(VI) in standard cultures (26,44). In agreement with results in H460 cells, clonogenic toxicity of Cr(VI) in Asc-restored XRCC1-null and isogenic XRCC1-complemented EM9 cells was similar within 0–80% cell killing range (Figure 4D).
Fig. 4.
Effects of restored Asc levels on cytotoxicity by Cr(VI). (A) Clonogenic toxicity of Cr(VI) in control (shLuc) and XRCC1-depleted (shXRCC1) H460 cells containing low Asc levels (standard tissue culture conditions). Statistics: *P < 0.05, **P < 0.01. (B) Clonogenic toxicity of Cr(VI) in Asc-restored H460 cells. (C) Western blot demonstrating PARP cleavage in Asc-restored H460 cells expressing nonspecific (shLuc) and targeting (shXRCC1) shRNA. Cells were treated with Cr(VI) for 3 h and protein extracts from combined attached and floating cells were prepared 24 h later. (D) Clonogenic toxicity of Cr(VI) in XRCC1-null and XRCC1-complemented CHO-EM9 cells. Both cell lines were pre-incubated with 2 mM DHA prior to Cr(VI) exposure, which resulted in 1.4 mM Asc concentrations inside the cells.
Formation of DNA breaks in cells with restored Asc levels
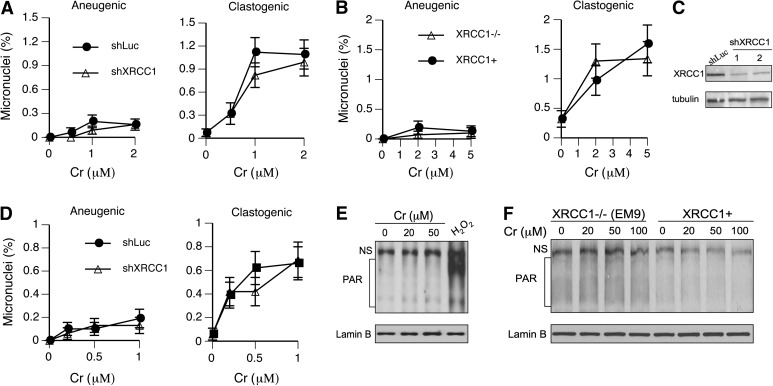
Formation of micronuclei is a very sensitive marker of chromosomal breaks by Cr(VI) in human lung cells containing physiological levels of Asc (8). The inability to repair SSB in XRCC1-deficient cells has been found to result in the increased formation of clastogenic micronuclei (46,47). Thus, the assessment of chromosomal breaks by the micronucleus assay in cells with different XRCC1 levels can serve as a test for the role of oxidative DNA damage in clastogenic effects of Cr(VI). As XRCC1 is expected to impact only the production of acentric (clastogenic) micronuclei, we immunostained cells with anti-centromeric antibody and scored two categories of micronuclei: centromere positive (aneugenic micronuclei containing intact chromosomes) and centromere negative (clastogenic micronuclei containing chromosomal fragments). We found that XRCC1 deficiency had no significant effect on the levels of either clastogenic or aneugenic micronuclei in human H460 or hamster CHO-EM9 cells treated with Cr(VI) (Figure 5A and B). The majority of Cr(VI)-induced micronuclei were centromere negative, accounting on average for 85.2 ± 2.4% and 88.3 ± 4.7% of total micronuclei in repair-proficient H460 and CHO cells, respectively (91.7 ± 6.0 and 93.0 ± 1.8% for XRCC1-deficient counterparts). To further test a role of oxidative DNA damage in chromosomal breakage by Cr(VI), we constructed a stable XRCC1 knockdown in IMR90 primary human lung fibroblasts (Figure 5C). Similarly to established cell lines, primary IMR90 cells with depleted XRCC1 also showed no changes in the frequency of clastogenic and aneugenic micronuclei (Figure 5D). Clastogenic micronuclei made up 81.2 ± 3.6 and 82.1 ± 4.1% of total Cr(VI)-induced micronuclei in control and XRCC1-depleted IMR90 cells, respectively. Thus, despite robust 10-fold increases in the frequency of clastogenic micronuclei by Cr(VI) in three types of Asc-restored cells, inhibition of oxidative DNA damage repair by XRCC1 deficiency had no impact on the formation of chromosomal breaks detectable by the micronucleus assay. These negative findings were not due to the insufficient sensitivity of our shRNA-based models, as we have already established that XRCC1 knockdown increases the formation of clastogenic micronuclei in response to oxidative DNA damage by H2O2 and other oxidants (47). XRCC1-null EM9 cells are a well-characterised model of hypersensitivity to oxidative DNA damage (46).
Fig. 5.
Formation of micronuclei and PAR by Cr(VI) in Asc-restored cells. Data are from 3 (EM9 cells) or 6 slides (H460, IMR90) with at least 500 cells scored for each slide. Shown is the frequency of cells containing micronuclei. (A) Frequency of centromere-positive (aneugenic) and -negative (clastogenic) micronuclei in human H460 cells expressing control (shLuc) and targeting (shXRCC1) shRNA. (B) Micronuclei in XRCC1-null and XRCC1-expressing CHO-EM9 cells with restored Asc levels to 1.4 mM. (C) Western blot demonstrating XRCC1 knockdown in IMR90 primary human lung fibroblasts. For XRCC1-tageting shRNA, extracts from two independently infected populations are shown. (D) Micronuclei in IMR90 cells expressing nonspecific and XRCC1-targeting shRNA. Asc levels in IMR90 cells were restored to 1.2 mM. (E) Western blot for PAR-modified proteins in Asc-restored H460 cells. Cells were treated for 1 h with Cr(VI) and 30 min with 100 μM H2O2. (F) Absence of PAR formation in Asc-restored XRCC1−/− and XRCC1+ CHO cells treated with Cr(VI) for 1 h. PAR, poly(ADP-ribose); NS, nonspecific band.
Generation of SSB rapidly activates PARP1 that forms (ADP-ribose) polymers covalently attached to proteins in the vicinity of DNA breakage (48). The formation of poly(ADP-ribose) is a commonly used biochemical marker for the presence of SSB. Therefore, we further tested a potential induction of SSB by Cr(VI) using western blotting with anti-poly(ADP-ribose) antibodies. Because of a limited persistence of SSB (49), we employed shorter (1 h long) treatments with Cr(VI) to avoid problems with repair during longer incubations. We found that our positive control with H2O2 generated a very strong poly(ADP-ribose) signal but Cr(VI) treatments of Asc-restored H460 cells were completely ineffective (Figure 5E). Clonogenic survival of H460 cells treated with the highest concentration of 50 μM Cr(VI) was only 12.5 ± 1.4% (n = 3), indicating that our negative findings were not due to insufficiently high doses. To enhance the detection sensitivity of poly(ADP-ribose), we further examined the formation of this SSB-induced product in XRCC1−/− and XRCC1+ CHO cells (Figure 5E). Indicative of their greater sensitivity to SSB, XRCC1−/− cells had higher background levels of poly(ADP-ribose) relative to XRCC1+ cells. However, both cell lines showed no changes in poly(ADP)-ribosylated proteins after Cr(VI) exposure.
Discussion
Cr(VI) by itself is completely unreactive towards DNA under physiological conditions and induction of genotoxic effects by Cr(VI) requires its cellular activation via reduction (13–15). Although the final product of Cr(VI) reduction is always Cr(III), the formation of specific intermediates and ternary Cr–DNA adducts is dependent on the nature of the reducing agent. The initial step in Cr(VI) reduction can proceed through either one- or two-electron transfer, which determines the yield of organic radicals and the presence or absence of Cr(V). Kinetic studies predicted that >90% of Cr(VI) reduction by cysteine involves one-electron transfer (30), which is consistent with a strong Cr(V) signal in these reactions (17). The first step in Cr(VI) reduction by glutathione can involve one- or two-electron transfers, with a shift at higher reducer concentrations to a greater role of two-electron transfers with the accompanying loss of Cr(V) intermediate (17,18). Asc is a two-electron reducer and its reactions with Cr(VI) at biological relevant concentrations do not generate detectable Cr(V) (18–20). All three biological reducers form L–Cr–DNA adducts where L is Asc, Cys or glutathione (6,50). Cr(V) intermediate is considered a major source of oxidative DNA damage by Cr(VI) (13). Thus, significant variations in the ratio of the main cellular reducers are expected to change genotoxic properties of Cr(VI) as a result of different yields of reactive intermediates and specific forms of DNA damage.
In this work, we found that restoration of physiological levels of Asc in cultured cells altered the genotoxic profile of Cr(VI) by blocking DNA oxidation-dependent responses. Consistent with earlier reports in mutant hamster cells (26,44), XRCC1-deficient human cells in standard cultures were hypersensitive to cell cycle perturbations and other toxic effects of Cr(VI); however, this oxidant-sensitive phenotype was lost when Asc levels were restored to normal levels. XRCC1 participates in repair of SSB and oxidised base products and its absence sensitises cells to different oxidants (41,42). Thus, XRCC1 deficiency can be used for the assessment of the overall oxidative insult on DNA. The use of this genetic model also avoids pitfalls associated with the detection of SSB and other oxidation products by alkali-based assays due to chemical instability of Cr-bound phosphodiester bonds and reoxidation of Cr(III) to higher oxidative states under conditions of high pH (40). Although repair of some oxidised bases can likely proceed without XRCC1 and not all DNA oxidation products are necessarily replication blocking (toxic) to be detected by our cytotoxic and clastogenic readouts; nevertheless, our results clearly indicate that the overall level of oxidative DNA damage induced by Cr(VI) in human cells is strongly suppressed at physiological concentrations of Asc. This conclusion is consistent with in vivo findings on the absence of detectable amounts of 8-oxo-dG in the liver and kidney of rats after intraperitoneal administration of Cr(VI) (51). Freshly isolated human lymphocytes contain millimolar Asc concentrations (12) and the induction of SSB in these cells required a massive exposure to 1 mM dichromate for 3 h (52), which also points to potent antioxidant effects of high cellular Asc.
The protective role of Asc against oxidative DNA damage by Cr(VI) likely results from a combination of its radical-scavenging activity and rapid Cr(VI) reduction skipping Cr(V) formation. Since Cr(V) is considered as the most important Cr intermediate for both direct and peroxo/ROS-mediated DNA oxidation (13), the elimination of Cr(V) alone in Asc-restored cells should exert a potent antioxidant effect. Even a modest 1.7-fold elevation of Asc concentrations was sufficient to inhibit Cr(V) formation in V79 cells (53). Increased oxidation of redox-sensitive dyes in Cr(VI)-treated A549 cells containing 80 μM Asc relative to more severely Asc-deficient controls (7) probably reflected a more robust reduction of Cr(VI). Intermediate Cr species produced in the in vitro Cr(VI)-Asc reactions with purified reagents were capable of direct oxidising the redox-sensitive probe 2′,7′-dichlorfluorescein without a concomitant induction of oxidative DNA damage, as assayed by the measurements of SSB, abasic sites and mutagenic and replication-blocking activities in shuttle-vector plasmids (27,54). Formation of oxidative DNA damage in Cr(VI) reactions with unpurified Asc was suppressed by catalase and required the presence of molecular oxygen (24), which was necessary for the Fe-dependent production of H2O2 during Asc auto-oxidation (∼50% yield of H2O2 from each oxidised Asc) (20).
Conclusions
Restoration of physiological concentrations of Asc in cultured cells led to the suppression of oxidative DNA damage by Cr(VI) to the levels that were undetectable by cytotoxic and clastogenic readouts. These results taken together with the unique metabolism of Cr(VI) by Asc (18–20) and the previous findings on the ability of elevated cellular Asc to block epigenetic changes by Cr(VI) (55) and to increase the formation of DNA–protein cross links (56) and mutagenic DNA damage (8) demonstrate that Asc deficiency of standard cell cultures leads to a distorted genotoxic profile of Cr(VI). Inhibition of oxidative DNA damage and the production of Asc–Cr–DNA adducts, which are high-affinity substrates for recognition by mismatch repair proteins (29), are likely responsible for a shift of Cr(VI) cytotoxicity and clastogenicity in Asc-restored cells towards mismatch repair-dependent mechanisms (8,28). Another implication of our work is that measurements of DNA oxidation products are not likely to serve as good biomarkers of genetic damage by cellular Cr(VI). Although inflammation could serve as another source of oxidative DNA damage in Cr(VI)-exposed lungs (57), it is a mechanistically distinct route resulting from systemic responses to tissue injury by toxic doses.
Funding
National Institute of Environmental Health Sciences (R01 ES008786 and P42 ES013660).
Acknowledgments
Conflict of interest statement: None declared.
References
- 1.Salnikow K, Donald SP, Bruick RK, Zhitkovich A, Phang JM, Kasprzak KS. Depletion of intracellular ascorbate by the carcinogenic metals nickel and cobalt results in the induction of hypoxic stress. J. Biol. Chem. 2004;279:40337–40344. doi: 10.1074/jbc.M403057200. [DOI] [PubMed] [Google Scholar]
- 2.Flashman E, Davies SL, Yeoh KK, Schofield CJ. Investigating the dependence of the hypoxia-inducible factor hydroxylases (factor inhibiting HIF and prolyl hydroxylase domain 2) on ascorbate and other reducing agents. Biochem. J. 2010;427:135–142. doi: 10.1042/BJ20091609. [DOI] [PubMed] [Google Scholar]
- 3.Hou H, Yu H. Structural insights into histone lysine demethylation. Curr. Opin. Struct. Biol. 2010;20:739–748. doi: 10.1016/j.sbi.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–935. doi: 10.1126/science.1170116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strijbis K, Vaz FM, Distel B. Enzymology of the carnitine biosynthesis pathway. IUBMB Life. 2010;62:357–362. doi: 10.1002/iub.323. [DOI] [PubMed] [Google Scholar]
- 6.Quievryn G, Messer J, Zhitkovich A. Carcinogenic chromium(VI) induces cross-linking of vitamin C to DNA in vitro and in human lung A549 cells. Biochemistry. 2002;41:3156–3167. doi: 10.1021/bi011942z. [DOI] [PubMed] [Google Scholar]
- 7.Martin BD, Schoenhard JA, Hwang JM, Sugden KD. Ascorbate is a pro-oxidant in chromium-treated human lung cells. Mutat. Res. 2006;610:74–84. doi: 10.1016/j.mrgentox.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 8.Reynolds M, Stoddard L, Bespalov I, Zhitkovich A. Ascorbate acts as a highly potent inducer of chromate mutagenesis and clastogenesis: linkage to DNA breaks in G2 phase by mismatch repair. Nucleic Acids Res. 2007;35:465–476. doi: 10.1093/nar/gkl1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miranda CL, Reed RL, Kuiper HC, Alber S, Stevens JF. Ascorbic acid promotes detoxification and elimination of 4-hydroxy-2(E)-nonenal in human monocytic THP-1 cells. Chem. Res. Toxicol. 2009;22:863–874. doi: 10.1021/tx900042u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slade R, Stead AG, Graham JA, Hatch GE. Comparison of lung antioxidant levels in humans and laboratory animals. Am. Rev. Respir. Dis. 1985;131:742–746. doi: 10.1164/arrd.1985.131.5.742. [DOI] [PubMed] [Google Scholar]
- 11.Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr. Med. Chem. 2004;11:1041–1064. doi: 10.2174/0929867043455567. [DOI] [PubMed] [Google Scholar]
- 12.Bergsten P, Amitai G, Kehrl J, Dhariwal KR, Klein HG, Levine M. Millimolar concentrations of ascorbic acid in purified human mononuclear leukocytes. Depletion and reaccumulation. J. Biol. Chem. 1990;265:2584–2587. [PubMed] [Google Scholar]
- 13.Sugden KD, Stearns DM. The role of chromium(V) in the mechanism of chromate-induced oxidative DNA damage and cancer. J. Environ. Pathol. Toxicol. Oncol. 2000;19:215–230. [PubMed] [Google Scholar]
- 14.O'Brien TJ, Ceryak S, Patierno SR. Complexities of chromium carcinogenesis: role of cellular response, repair and recovery mechanisms. Mutat. Res. 2003;533:3–36. doi: 10.1016/j.mrfmmm.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 15.Zhitkovich A. Importance of chromium-DNA adducts in mutagenicity and toxicity of chromium(VI) Chem. Res. Toxicol. 2005;18:3–11. doi: 10.1021/tx049774+. [DOI] [PubMed] [Google Scholar]
- 16.Standeven AM, Wetterhahn KE. Ascorbate is the principal reductant of chromium(VI) in rat lung ultrafiltrates and cytosols, and mediates chromium-DNA binding in vitro. Carcinogenesis. 1992;13:1319–1324. doi: 10.1093/carcin/13.8.1319. [DOI] [PubMed] [Google Scholar]
- 17.Borges KM, Boswell JS, Liebross RH, Wetterhahn KE. Activation of chromium(VI) by thiols results in chromium(V) formation, chromium binding to DNA and altered DNA conformation. Carcinogenesis. 1991;12:551–561. doi: 10.1093/carcin/12.4.551. [DOI] [PubMed] [Google Scholar]
- 18.Bose RN, Moghaddas S, Gelerinter E. Long-lived chromium(IV), chromium(V) metabolites in the chromium(VI)-glutathione reaction: NMR, ESR, HPLC, and kinetic characterization. Inorg. Chem. 1992;31:1987–1994. [Google Scholar]
- 19.Stearns DM, Wetterhahn KE. Reaction of Cr(VI) with ascorbate produces chromium(V), chromium(IV), and carbon-based radicals. Chem. Res. Toxicol. 1994;7:219–230. doi: 10.1021/tx00038a016. [DOI] [PubMed] [Google Scholar]
- 20.Lay PA, Levina A. Activation of molecular oxygen during the reactions of chromium(VI/V/IV) with biological reductants: implications for chromium-induced genotoxicities. J. Am. Chem. Soc. 1998;120:6704–6714. [Google Scholar]
- 21.Levina A, Barr-David G, Codd R, Lay PA, Dixon NE, Hammershoi A, Hendry P. In vitro plasmid DNA cleavage by chromium(V) and -(IV) 2-hydroxycarboxylato complexes. Chem. Res. Toxicol. 1999;12:371–381. doi: 10.1021/tx980229g. [DOI] [PubMed] [Google Scholar]
- 22.Sugden KD, Campo CK, Martin BD. Direct oxidation of guanine and 7,8-dihydro-8-oxoguanine in DNA by a high-valent chromium complex: a possible mechanism for chromate genotoxicity. Chem. Res. Toxicol. 2001;14:1315–1322. doi: 10.1021/tx010088+. [DOI] [PubMed] [Google Scholar]
- 23.Molyneux MJ, Davies MJ. Direct evidence for hydroxyl radical-induced damage to nucleic acids by chromium(VI)-derived species: implications for chromium carcinogenesis. Carcinogenesis. 1995;16:875–882. doi: 10.1093/carcin/16.4.875. [DOI] [PubMed] [Google Scholar]
- 24.da Cruz Fresco P, Kortenkamp A. The formation of DNA cleaving species during the reduction of chromate by ascorbate. Carcinogenesis. 1994;15:1773–1778. doi: 10.1093/carcin/15.9.1773. [DOI] [PubMed] [Google Scholar]
- 25.Sugden KD, Wetterhahn KE. Direct and hydrogen peroxide-induced chromium(V) oxidation of deoxyribose in single-stranded and double-stranded calf thymus DNA. Chem. Res. Toxicol. 1997;10:1397–1406. doi: 10.1021/tx970135r. [DOI] [PubMed] [Google Scholar]
- 26.Messer J, Reynolds M, Stoddard L, Zhitkovich A. Causes of DNA single-strand breaks during reduction of chromate by glutathione in vitro and in cells. Free Radic. Biol. Med. 2006;40:1981–1992. doi: 10.1016/j.freeradbiomed.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 27.Quievryn G, Peterson E, Messer J, Zhitkovich A. Genotoxicity and mutagenicity of chromium(VI)/ascorbate-generated DNA adducts in human and bacterial cells. Biochemistry. 2003;42:1062–1070. doi: 10.1021/bi0271547. [DOI] [PubMed] [Google Scholar]
- 28.Reynolds M, Zhitkovich A. Cellular vitamin C increases chromate toxicity via a death program requiring mismatch repair but not p53. Carcinogenesis. 2007;28:1613–1620. doi: 10.1093/carcin/bgm031. [DOI] [PubMed] [Google Scholar]
- 29.Reynolds MF, Peterson-Roth EC, Johnston T, Gurel VM, Menard HL, Zhitkovich A. Rapid DNA double-strand breaks resulting from processing of Cr-DNA crosslinks by both MutS dimers. Cancer Res. 2009;69:1071–1079. doi: 10.1158/0008-5472.CAN-08-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quievryn G, Goulart M, Messer J, Zhitkovich A. Reduction of Cr(VI) by cysteine: significance in human lymphocytes and formation of DNA damage in reactions with variable reduction rates. Mol. Cell. Biochem. 2001;222:107–118. [PubMed] [Google Scholar]
- 31.Reynolds M, Peterson E, Quievryn G, Zhitkovich A. Human nucleotide excision repair efficiently removes chromium-DNA phosphate adducts and protects cells against chromate toxicity. J. Biol. Chem. 2004;279:30419–30424. doi: 10.1074/jbc.M402486200. [DOI] [PubMed] [Google Scholar]
- 32.Salnikow K, Zhitkovich A. Genetic and epigenetic mechanisms in metal carcinogenesis and cocarcinogenesis: nickel, arsenic and chromium. Chem. Res. Toxicol. 2008;21:28–44. doi: 10.1021/tx700198a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barchowsky A, O'Hara KA. Metal-induced cell signaling and gene activation in lung diseases. Free Radic. Biol. Med. 2003;34:1130–1135. doi: 10.1016/s0891-5849(03)00059-5. [DOI] [PubMed] [Google Scholar]
- 34.Zhang D, Zaugg K, Mak TW, Elledge SJ. A role for the deubiquitinating enzyme USP28 in control of the DNA-damage response. Cell. 2006;126:529–542. doi: 10.1016/j.cell.2006.06.039. [DOI] [PubMed] [Google Scholar]
- 35.Lowe SW, Schmitt EM, Smith SW, Osborne BA, Jacks T. p53 is required for radiation-induced apoptosis in mouse thymocytes. Nature. 1993;362:847–849. doi: 10.1038/362847a0. [DOI] [PubMed] [Google Scholar]
- 36.Rumsey SC, Daruwala R, Al-Hasani H, Zarnowski MJ, Simpson IA, Levine M. Glucose transporter isoforms GLUT1 and GLUT3 transport dehydroascorbic acid. J. Biol. Chem. 1997;272:18982–18989. doi: 10.1074/jbc.272.30.18982. [DOI] [PubMed] [Google Scholar]
- 37.Montel-Hagen A, Kinet S, Manel N, Mongellaz C, Prohaska R, Battini JL, Delaunay J, Sitbon M, Taylor N. Erythrocyte Glut1 triggers dehydroascorbic acid uptake in mammals unable to synthesize vitamin C. Cell. 2008;132:1039–1048. doi: 10.1016/j.cell.2008.01.042. [DOI] [PubMed] [Google Scholar]
- 38.Peterson-Roth E, Reynolds M, Quievryn G, Zhitkovich A. Mismatch repair proteins are activators of toxic responses to chromium-DNA damage. Mol. Cell. Biol. 2005;25:3596–3607. doi: 10.1128/MCB.25.9.3596-3607.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickman MJ, Samson LD. Apoptotic signaling in response to a single type of DNA lesion, O(6)-methylguanine. Mol. Cell. 2004;14:105–116. doi: 10.1016/s1097-2765(04)00162-5. [DOI] [PubMed] [Google Scholar]
- 40.Zhitkovich A, Messer J, Shrager S. Reductive metabolism of Cr(VI) by cysteine leads to the formation of binary and ternary Cr-DNA adducts in the absence of oxidative DNA damage. Chem. Res. Toxicol. 2000;13:1114–1124. doi: 10.1021/tx0001169. [DOI] [PubMed] [Google Scholar]
- 41.Dianova II, Sleeth KM, Allinson SL, Parsons JL, Breslin C, Caldecott KW, Dianov GL. XRCC1-DNA polymerase beta interaction is required for efficient base excision repair. Nucleic Acids Res. 2004;32:2550–2555. doi: 10.1093/nar/gkh567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Horton JK, Watson M, Stefanick DF, Shaughnessy DT, Taylor JA, Wilson SH. XRCC1 and DNA polymerase beta in cellular protection against cytotoxic DNA single-strand breaks. Cell Res. 2008;18:48–63. doi: 10.1038/cr.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Loizou JI, El-Khamisy SF, Zlatanou A, et al. The protein kinase CK2 facilitates repair of chromosomal DNA single-strand breaks. Cell. 2004;117:17–28. doi: 10.1016/s0092-8674(04)00206-5. [DOI] [PubMed] [Google Scholar]
- 44.Bryant HE, Ying S, Helleday T. Homologous recombination is involved in repair of chromium-induced DNA damage in mammalian cells. Mutat. Res. 2006;599:116–123. doi: 10.1016/j.mrfmmm.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 45.O'Brien TJ, Brooks BR, Patierno SR. Nucleotide excision repair functions in the removal of chromium-induced DNA damage in mammalian cells. Mol. Cell. Biochem. 2005;279:85–95. doi: 10.1007/s11010-005-8225-0. [DOI] [PubMed] [Google Scholar]
- 46.Qu T, Morii E, Oboki K, Lu Y, Morimoto K. Micronuclei in EM9 cells expressing polymorphic forms of human XRCC1. Cancer Lett. 2005;221:91–95. doi: 10.1016/j.canlet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Pietruska JR, Johnston T, Zhitkovich A, Kane AB. XRCC1 deficiency sensitizes human lung epithelial cells to genotoxicity by crocidolite asbestos and Libby amphibole. Environ. Health Perspect. 2010;118:1707–1713. doi: 10.1289/ehp.1002312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Petermann E, Keil C, Oei SL. Importance of poly(ADP-ribose) polymerases in the regulation of DNA-dependent processes. Cell. Mol. Life Sci. 2005;62:731–738. doi: 10.1007/s00018-004-4504-2. [DOI] [PubMed] [Google Scholar]
- 49.Lan L, Nakajima S, Oohata Y, Takao M, Okano S, Masutani M, Wilson H, Yasui A. In situ analysis of repair processes for oxidative DNA damage in mammalian cells. Proc. Natl Acad. Sci. USA. 2004;101:13738–13743. doi: 10.1073/pnas.0406048101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhitkovich A, Voitkun V, Costa M. Glutathione and free amino acids form stable adducts with DNA following exposure of intact mammalian cells to chromate. Carcinogenesis. 1995;16:907–913. doi: 10.1093/carcin/16.4.907. [DOI] [PubMed] [Google Scholar]
- 51.Yuann JM, Liu KJ, Hamilton JW, Wetterhahn KE. In vivo effects of ascorbate and glutathione on the uptake of chromium, formation of chromium(V), chromium-DNA binding and 8-hydroxy-2'-deoxyguanosine in liver and kidney of osteogenic disorder shionogi rats following treatment with chromium(VI) Carcinogenesis. 1999;20:1267–1275. doi: 10.1093/carcin/20.7.1267. [DOI] [PubMed] [Google Scholar]
- 52.Gao M, Binks SP, Chipman JK, Levy LS, Braithwaite RA, Brown SS. Induction of DNA strand breaks in peripheral lymphocytes by soluble chromium compounds. Hum. Exp. Toxicol. 1992;11:77–82. doi: 10.1177/096032719201100203. [DOI] [PubMed] [Google Scholar]
- 53.Sugiyama M, Tsuzuki K, Ogura R. Effect of ascorbic acid on DNA damage, cytotoxicity, glutathione reductase, and formation of paramagnetic chromium in Chinese hamster V-79 cells treated with sodium chromate(VI) J. Biol. Chem. 1991;266:3383–3386. [PubMed] [Google Scholar]
- 54.Quievryn G, Messer J, Zhitkovich A. Lower mutagenicity but higher stability of Cr-DNA adducts formed during gradual chromate activation with ascorbate. Carcinogenesis. 2006;27:2316–2321. doi: 10.1093/carcin/bgl076. [DOI] [PubMed] [Google Scholar]
- 55.Sun H, Zhou X, Chen H, Li Q, Costa M. Modulation of histone methylation and MLH1 gene silencing by hexavalent chromium. Toxicol. Appl. Pharmacol. 2009;237:258–266. doi: 10.1016/j.taap.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Macfie A, Hagan E, Zhitkovich A. Mechanism of DNA-protein cross-linking by chromium. Chem. Res. Toxicol. 2010;23:341–347. doi: 10.1021/tx9003402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beaver LM, Stemmy EJ, Constant SL, et al. Lung injury, inflammation and Akt signaling following inhalation of particulate hexavalent chromium. Toxicol. Appl. Pharmacol. 2009;235:47–56. doi: 10.1016/j.taap.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]