Executive Summary
Objective
An application was received to review the evidence on the ‘The Da Vinci Surgical System’ for the treatment of gynecologic malignancies (e.g. endometrial and cervical cancers). Limitations to the current standard of care include the lack of trained physicians on minimally invasive surgery and limited access to minimally invasive surgery for patients. The potential benefits of ‘The Da Vinci Surgical System’ include improved technical manipulation and physician uptake leading to increased surgeries, and treatment and management of these cancers.
The demand for robotic surgery for the treatment and management of prostate cancer has been increasing due to its alleged benefits of recovery of erectile function and urinary continence, two important factors of men’s health. The potential technical benefits of robotic surgery leading to improved patient functional outcomes are surgical precision and vision.
Clinical Need
Uterine and cervical cancers represent 5.4% (4,400 of 81,700) and 1.6% (1,300 of 81,700), respectively, of incident cases of cancer among female cancers in Canada. Uterine cancer, otherwise referred to as endometrial cancer is cancer of the lining of the uterus. The most common treatment option for endometrial cancer is removing the cancer through surgery. A surgical option is the removal of the uterus and cervix through a small incision in the abdomen using a laparoscope which is referred to as total laparoscopic hysterectomy. Risk factors that increase the risk of endometrial cancer include taking estrogen replacement therapy after menopause, being obese, early age at menarche, late age at menopause, being nulliparous, having had high-dose radiation to the pelvis, and use of tamoxifen.
Cervical cancer occurs at the lower narrow end of the uterus. There are more treatment options for cervical cancer compared to endometrial cancer, however total laparoscopic hysterectomy is also a treatment option. Risk factors that increase the risk for cervical cancer are multiple sexual partners, early sexual activity, infection with the human papillomavirus, and cigarette smoking, whereas barrier-type of contraception as a risk factor decreases the risk of cervical cancer.
Prostate cancer is ranked first in men in Canada in terms of the number of new cases among all male cancers (25,500 of 89,300 or 28.6%). The impact on men who develop prostate cancer is substantial given the potential for erectile dysfunction and urinary incontinence. Prostate cancer arises within the prostate gland, which resides in the male reproductive system and near the bladder. Radical retropubic prostatectomy is the gold standard treatment for localized prostate cancer. Prostate cancer affects men above 60 years of age. Other risk factors include a family history of prostate cancer, being of African descent, being obese, consuming a diet high in fat, physical inactivity, and working with cadium.
The Da Vinci Surgical System
The Da Vinci Surgical System is a robotic device. There are four main components to the system: 1) the surgeon’s console, where the surgeon sits and views a magnified three-dimensional image of the surgical field; 2) patient side-cart, which sits beside the patient and consists of three instrument arms and one endoscope arm; 3) detachable instruments (endowrist instruments and intuitive masters), which simulate fine motor human movements. The hand movements of the surgeon’s hands at the surgeon’s console are translated into smaller ones by the robotic device and are acted out by the attached instruments; 4) three-dimensional vision system: the camera unit or endoscope arm. The main advantages of use of the robotic device are: 1) the precision of the instrument and improved dexterity due to the use of “wristed” instruments; 2) three-dimensional imaging, with improved ability to locate blood vessels, nerves and tissues; 3) the surgeon’s console, which reduces fatigue accompanied with conventional laparoscopy surgery and allows for tremor-free manipulation. The main disadvantages of use of the robotic device are the costs including instrument costs ($2.6 million in US dollars), cost per use ($200 per use), the costs associated with training surgeons and operating room personnel, and the lack of tactile feedback, with the trade-off being increased visual feedback.
Research Questions
For endometrial and cervical cancers,
1. What is the effectiveness of the Da Vinci Surgical System vs. laparoscopy and laparotomy for women undergoing any hysterectomy for the surgical treatment and management of their endometrial and cervical cancers?
2. What are the incremental costs of the Da Vinci Surgical System vs. laparoscopy and laparotomy for women undergoing any hysterectomy for the surgical treatment and management of their endometrial and cervical cancers?
For prostate cancer,
3. What is the effectiveness of robotically-assisted radical prostatectomy using the Da Vinci Surgical System vs. laparoscopic radical prostatectomy and retropubic radical prostatectomy for the surgical treatment and management of prostate cancer?
4. What are the incremental costs of robotically-assisted radical prostatectomy using the Da Vinci Surgical System vs. laparoscopic radical prostatectomy and retropubic radical prostatectomy for the surgical treatment and management of prostate cancer?
Research Methods
Literature Search
Search Strategy
A literature search was performed on May 12, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment for studies published from January 1, 2000 until May 12, 2010. Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with unknown eligibility were reviewed with a second clinical epidemiologist, then a group of epidemiologists until consensus was established. The quality of evidence was assessed as high, moderate, low or very low according to GRADE methodology.
Inclusion Criteria
English language articles (January 1, 2000-May 12, 2010)
Journal articles that report on the effectiveness or cost-effectiveness for the comparisons of interest using a primary data source (e.g. obtained in a clinical setting)
Journal articles that report on the effectiveness or cost-effectiveness for the comparisons of interest using a secondary data source (e.g. hospital- or population-based registries)
Study design and methods must be clearly described
Health technology assessments, systematic reviews, randomized controlled trials, non-randomized controlled trials and/or cohort studies, case-case studies, regardless of sample size, cost-effectiveness studies
Exclusion Criteria
Duplicate publications (with the more recent publication on the same study population included)
Non-English papers
Animal or in-vitro studies
Case reports or case series without a referent or comparison group
Studies on long-term survival which may be affected by treatment
Studies that do not examine the cancers (e.g. advanced disease) or outcomes of interest
Outcomes of Interest
For endometrial and cervical cancers,
Primary outcomes:
-
Morbidity factors
- Length of hospitalization
- Number of complications*
-
Peri-operative factors
Number of lymph nodes recovered
For prostate cancer,
Primary outcomes:
-
Morbidity factors
- Length of hospitalization
- Amount of morphine use/pain*
-
Peri-operative factors
Number of lymph nodes recovered
-
Oncologic factors
- Proportion of positive surgical margins
-
Long-term outcomes
- Urinary continence
- Erectile function
Summary of Findings
-
Robotic use for gynecologic oncology compared to:
-
Laparotomy: benefits of robotic surgery in terms of shorter length of hospitalization and less blood loss. These results indicate clinical effectiveness in terms of reduced morbidity and safety, respectively, in the context of study design limitations.
The beneficial effect of robotic surgery was shown in pooled analysis for complications, owing to increased sample size.
More work is needed to clarify the role of complications in terms of safety, including improved study designs, analysis and measurement.
-
Laparoscopy: benefits of robotic surgery in terms of shorter length of hospitalization, less blood loss and fewer conversions to laparotomy likely owing to the technical difficulty of conventional laparoscopy, in the context of study design limitations.
Clinical significance of significant findings for length of hospitalizations and blood loss is low.
Fewer conversions to laparotomy indicate clinical effectiveness in terms of reduced morbidity.
-
-
Robotic use for urologic oncology, specifically prostate cancer, compared to:
-
Retropubic surgery: benefits of robotic surgery in terms of shorter length of hospitalization and less blood loss/fewer individuals requiring transfusions. These results indicate clinical effectiveness in terms of reduced morbidity and safety, respectively, in the context of study design limitations. There was a beneficial effect in terms of decreased positive surgical margins and erectile dysfunction. These results indicate clinical effectiveness in terms of improved cancer control and functional outcomes, respectively, in the context of study design limitations.
Surgeon skill had an impact on cancer control and functional outcomes.
The results for complications were inconsistent when measured as either total number of complications, pain management or anastomosis. There is some suggestion that robotic surgery is safe with respect to less post-operative pain management required compared to retropubic surgery, however improved study design and measurement of complications need to be further addressed.
Clinical significance of significant findings for length of hospitalizations is low.
-
Laparoscopy: benefits of robotic surgery in terms of less blood loss and fewer individuals requiring transfusions likely owing to the technical difficulty of conventional laparoscopy, in the context of study design limitations.
Clinical significance of significant findings for blood loss is low.
The potential link between less blood loss, improved visualization and improved functional outcomes is an important consideration for use of robotics.
-
All studies included were observational in nature and therefore the results must be interpreted cautiously.
Economic Analysis
The objective of this project was to assess the economic impact of robotic-assisted laparoscopy (RAL) for endometrial, cervical, and prostate cancers in the province of Ontario.
A budget impact analysis was undertaken to report direct costs associated with open surgery (OS), endoscopic laparoscopy (EL) and robotic-assisted laparoscopy (RAL) based on clinical literature review outcomes, to report a budget impact in the province based on volumes and costs from administrative data sets, and to project a future impact of RAL in Ontario. A cost-effectiveness analysis was not conducted because of the low quality evidence from the clinical literature review.
Hospital costs were obtained from the Ontario Case Costing Initiative (OCCI) for the appropriate Canadian Classification of Health Intervention (CCI) codes restricted to selective ICD-10 diagnostic codes after consultation with experts in the field. Physician fees were obtained from the Ontario Schedule of Benefits (OSB) after consultation with experts in the field. Fees were costed based on operation times reported in the clinical literature for the procedures being investigated. Volumes of procedures were obtained from the Ministry of Health and Long-Term Care (MOHLTC) administrative databases.
Direct costs associated with RAL, EL and OS included professional fees, hospital costs (including disposable instruments), radiotherapy costs associated with positive surgical margins in prostate cancer and conversion to OS in gynecological cancer. The total cost per case was higher for RAL than EL and OS for both gynecological and prostate cancers. There is also an acquisition cost associated with RAL. After conversation with the only supplier in Canada, hospitals are looking to spend an initial 3.6M to acquire the robotic surgical system
Previous volumes of OS and EL procedures were used to project volumes into Years 1-3 using a linear mathematical expression. Burden of OS and EL hysterectomies and prostatectomies was calculated by multiplying the number of cases for that year by the cost/case of the procedure.
The number of procedures is expected to increase in the next three years based on historical data. RAL is expected to capture this market by 65% after consultation with experts. If it’s assumed that RAL will capture the current market in Ontario by 65%, the net impact is expected to be by Year 3, 3.1M for hysterectomy and 6.7M for prostatectomy procedures respectively in the province.
RAL has diffused in the province with four surgical systems in place in Ontario, two in Toronto and two in London. RAL is a more expensive technology on a per case basis due to more expensive robot specific instrumentation and physician labour reflected by increased OR time reported in the clinical literature. There is also an upfront cost to acquire the machine and maintenance contract. RAL is expected to capture the market at 65% with project net impacts by Year 3 of 3.1M and 6.7M for hysterectomy and prostatectomy respectively.
Background
Objective of Analysis
The objective of this health technology assessment was to determine the effectiveness and incremental costs of the Da Vinci Surgical System for endometrial, cervical and prostate cancers.
Clinical Need and Target Population
Gynecologic Oncology: Endometrial and Cervical Cancers
Surgery in gynecologic oncology has evolved from techniques that provided access to the reproductive system by large incisions in the abdomen to techniques that provide access by small incisions, referred to as minimally invasive surgery. Laparoscopic surgery, also referred to as minimally invasive surgery provides a number of patient benefits including faster recovery and shorter length of hospitalization, improved cosmesis, decreased blood loss, and reduced post-operative pain. In contrast, the open abdominal approach referred to as laparotomy has a number of patient disadvantages due to the use of a large abdominal incision to reach target reproductive organs such as increased length of hospitalization, increased post-operative analgesic requirements, and higher morbidity. Limitations of laparoscopy as a technique for complex surgeries such as hysterectomy with lymphadenectomy have been a barrier to its use by less skilled surgeons due to the steep learning curve. The limitations include counterintuitive hand movement (e.g. fulcrum effect), an unsteady two-dimensional visual field, restricted instrument motion, ergonomic difficulty, and tremor amplification. Robotic-assisted minimally invasive surgery in the field of gynecologic oncology allows less skilled surgeons to perform complex surgeries by minimally invasive methods rather than by laparotomy. (1)
Endometrial cancer is ranked fourth in women in Canada in terms of the number of new cases among all female cancers after breast, lung and colorectal cancers (% distribution: 5.4%, 27.8%, 13.1%, and 12.1%, respectively), whereas cervical cancer represents 1.6% of new female cancers among all female cancers in Canada. The age-standardized incidence rate in Ontario for endometrial cancer is 21 per 100,000 and for cervical cancer it is 7 per 100,000 indicating that the incidences are low. In comparison, the age-standardized incidence rate in Ontario for breast cancer is 102 per 100,000. The ten year tumour-based prevalence-duration of endometrial cancer is 7.2% and for cervical cancer it is 2.7% among all prevalent female cancers in Canada. The age-standardized mortality rate in Ontario is less than 5 per 100,000 for both cancers indicating that there is also low mortality from these gynecologic cancers. (2) For gynecologic oncology, the limited update of conventional laparoscopy techniques among surgeons in the absence of a reduction in the incidence of these gynecologic cancers suggest that future female cancer populations will be at a disadvantage with respect to treatment and management of their disease. This is a growing concern in light of an alternative robotic technology.
Endometrial cancer predominately affects women over the age of 50 years, at a time when women may be peri-menopausal. (3) Risk factors that increase the risk of endometrial cancer include taking estrogen replacement therapy after menopause, being obese, early age at menarche, late age at menopause, being nulliparous, having had high-dose radiation to the pelvis, and use of tamoxifen. (4) Cervical cancer commonly affects women between the ages of 30 to 59 years. (5) Risk factors that increase the risk for cervical cancer are multiple sexual partners, early sexual activity, infection with the human papillomavirus, and cigarette smoking, whereas barrier-type of contraception as a risk factor decreases the risk of cervical cancer. (6)
Endometrial and cervical cancers arise within the female reproductive system therefore the impact on women who develop these cancers is high, given a woman’s desire for reproduction. This is particularly relevant for cervical cancer, which affects women at a young reproductive age. Endometrial cancer affects the uterus. The uterus is a hollow pear shaped organ that lies beneath the lower abdomen, between the navel and the pubic bone. The uterus is comprised of endometrium type and muscle tissues, and is the organ that contains a developing fetus. Endometrial cancer arises from the endometrium, which forms the inner lining of the uterus. At the lower end of the uterus is the cervix which together forms a continuous body of tissue. (7) The main clinical sign associated with suspected endometrial cancer is abnormal vaginal bleeding, and evaluation of symptoms is performed by dilation and curettage. (8) Surgery is the most common treatment option for endometrial cancer. Surgical options include total hysterectomy and radical hysterectomy. (9) Cervical cancer effects less women in Canada compared to endometrial cancer due to established screening programs. (5) Cervical cancer arises when cells in the cervix change from normal appearance to dysplastic, and over time with additional cell growth, cancer develops. Cervical cancer screening by the Pap test can detect pre-cancerous lesions and is a procedure in which a sample of cells are scraped from the cervix and examined under the microscope for pathological changes. Advanced cervical cancer is associated with vaginal bleeding and discharge. (10) Surgery is sometimes used as a treatment option for cervical cancer. Surgical options also include total hysterectomy and radical hysterectomy. (11)
Treatment by Hysterectomy
Hysterectomy is the surgical removal of the uterus. There are different types of hysterectomies, which are defined in part by the surgical method used to remove the uterus and whether the adjoining cervix or any surrounding organs are also removed. These include: 1) total abdominal hysterectomy, where the uterus and cervix are removed through a large incision in the abdomen; 2) vaginal hysterectomy, where the uterus and cervix are removed through the vagina; 3) total laparoscopic hysterectomy, where the uterus and cervix are removed through a small incision in the abdomen; 4) radical hysterectomy, where the uterus, cervix and part of the vagina are removed. The ovaries, fallopian tubes or nearby lymph nodes may also be removed; 5) partial (or supracervical) hysterectomy, where only the upper part of the uterus is removed and the cervix is left in place; 6) laparoscopic-assisted vaginal hysterectomy, where the uterus is removed through a cut inside the vagina. The surgeon will also insert a laparoscope and other instruments into the abdomen region through two or three small incisions. (10;12) The main differences between radical and simple hysterectomy are the isolation of the uterine vessels at their origin, removal of parametrial tissues lateral to the cervix, and a vaginal margin of 1-2 cm for radical hysterectomy. (13)
In Canada, there are approximately 50,000 hysterectomies performed each year, including benign and oncologic conditions. Canadian guidelines recommend the use of vaginal hysterectomies or laparoscopic-assisted vaginal hysterectomies. However, a majority of hysterectomies are still performed abdominally, a highly invasive procedure. (14) One reason for this is that physicians in Canada operate on a fee-for-service model. Minimally invasive surgery, such as laparoscopic hysterectomy is a technically challenging and laborious procedure. Therefore, surgeons would prefer to perform an abdominal hysterectomy since it can be performed more efficiently. However, from the patient perspective, minimally invasive surgery offers more health benefits in terms of reduced morbidity. From a health care perspective, patients receiving minimally invasive surgery spend fewer post-operative days in hospital. (15) Therefore, there are a number of unresolved issues for the treatment and management of gynecologic conditions in Canada.
In Ontario, total abdominal hysterectomy with the removal of one or both ovaries was performed more often than simple hysterectomy for endometrial cancer (72% vs. 4%), followed by total abdominal hysterectomy with the removal of one or both ovaries and pelvic or para-aortic lymph node excision (19%). (16) Radical hysterectomy occurred most often for cervical cancer (39%), followed by total hysterectomy or cervicectomy with or without lymph node excision (32%), and cone biopsy (27%). (17)
Urologic Oncology: Prostate Cancer
Surgery for prostate cancer advanced with the identification of anatomic and physiologic structures that reduced blood loss, urinary incontinence and impotence when performing retropubic radical prostatectomy (open surgery). The advent of minimally invasive technology resulted in decreased operation time and decreased hospital length of stay while preserving tumour resection, and preservation of continence and potency. Robotically-assisted radical prostatectomy using the Da Vinci Surgical System was introduced to urology in 2000 and has generated interest among surgeons and patients due to its potential benefits. (18)
Prostate cancer is ranked first in men in Canada in terms of the number of new cases among all male cancers (25,500 of 89,300 or % distribution: 28.6%). The age-standardized incidence rate in Ontario for prostate cancer is 149 per 100,000 indicating that the incidence is substantial. In comparison, the age-standardized incidence rate in men in Ontario for lung cancer is 56 per 100,000. The ten year person-based prevalence is 0.8% of the Canadian population, affecting 135,061 men in Canada. The ten year tumour-based prevalence-duration of prostate cancer is 38.2%. The age-standardized mortality rate in Ontario is 23 per 100,000 indicating that death from prostate cancer is not trivial. (2)
Prostate cancer affects men above 60 years of age. Other risk factors include a family history of prostate cancer, being of African descent, being obese, consuming a diet high in fat, physical inactivity, and working with cadium. (19)
The impact on men who develop prostate cancer is substantial given the potential for erectile dysfunction and urinary incontinence. Prostate cancer arises within the prostate gland, which resides in the male reproductive system near the bladder. The prostate is a small body of tissue that surrounds the urethra and it produces fluid that makes up part of the semen. Symptoms of prostate cancer include weak or interrupted flow of urine, frequent or trouble with urination, and painful ejaculation to name a few. There are a number of tests that can be used to diagnose prostate cancer including digital rectal exam, prostate-specific antigen test, transrectal ultrasound, and biopsy. A closely related condition is known as benign prostatic hyperplasia, which describes an enlarged prostate that occurs as men age. Though it is not cancer, it is treated similarly as prostate cancer with surgery. (20;21)
Treatment by Prostatectomy
Four standard treatment options exist for prostate cancer including watchful waiting, surgery, radiation therapy, and hormone therapy. Radical prostatectomy is a surgical procedure to remove the prostate and surrounding tissues and seminal vesicles. (22) A retropubic prostatectomy is where the prostate is removed through an incision in the abdominal wall of 8-10 cm. This report will not review perineal prostatectomy, an alternative surgical method by which to perform a radical prostatectomy. Radical retropubic prostatectomy is the gold standard treatment for localized prostate cancer. However, technological advancements introduced laparoscopic methods for prostate cancer surgery. Therefore, another surgical method to perform a radical prostatectomy is laparoscopic prostatectomy, which removes the prostate and other tissues using minimally invasive surgery. (23) In Canada, the Canadian Institutes of Health Information identified approximately 16,000 prostatectomies that are performed each year however this refers to non-cancerous conditions such as benign enlargement of the prostate. (24) In Ontario, the Institute of Clinical Evaluative Sciences reported that of men with prostate cancer, approximately half had surgery within a year of diagnosis and 59% had a radical prostatectomy. (25)
A recent article in the New England Journal of Medicine (August 19th, 2010) suggests that the number of prostatectomies in the U.S. are increasing despite a decrease in the incidence of prostate cancer, and when men choose therapy for their prostate cancer, they are increasingly choosing a surgical option and the surgical approach of favour is robotic surgery. This has implications both for cost per surgical case and volume of cases treated surgically. Men are in favour of robotic surgery despite evidence for the long-term benefits. Worldwide, the number of robotic-assisted surgeries has tripled since 2007, from 80,000 to 205,000. In the U.S., the number of robotic surgery units increased by 75%, from 800 to 1,400 between the years of 2007 and 2009. (26)
The Da Vinci Surgical System
The Da Vinci Surgical System is a robotic device for the surgical treatment and management of cancer. There are four main components to the system: 1) the surgeon’s console, where the surgeon sits and views a magnified three-dimensional image of the surgical field; 2) patient side-cart, which sits beside the patient and consists of up to three instrument arms and one endoscope arm; 3) detachable instruments (endowrist instruments and intuitive masters), which simulate fine motor human movements. The hand movements of the surgeon’s hands at the surgeon’s console are translated into smaller ones by the robotic device and are acted out by the attached instruments; 4) three-dimensional vision system: the camera unit or endoscope arm. The main advantages of use of the robotic device are: 1) the precision of the instrument and improved dexterity due to the use of “wristed” instruments. The robotic instruments provide 7 degrees of freedom whereas traditional laparoscopy instruments provide 4 degrees of freedom (e.g. open, close, turn clockwise and counterclockwise). The wristed instruments are 5 to 8 mm in diameter. 2) three-dimensional imaging, with improved ability to locate blood vessels, nerves and tissues; 3) the surgeon’s console, which reduces fatigue accompanied with conventional laparoscopy surgery and allows for tremor-free manipulation. The crucial difference between the robotic system and traditional laparoscopy is that the surgeon is not in direct contact with the surgical instruments when performing robotic surgery. For traditional laparoscopy surgery, the surgical instruments are in direct contact with the surgeon’s hands, therefore tremor amplification due to unsteady hands is a drawback of conventional laparoscopy. Whether the term “robotic surgery” or “robotic-assisted laparoscopic surgery” indicating the advancement of laparoscopy and not the creation of a separate technique should be used is not clearly distinguished. (1;27-30) The main disadvantages of use of the robotic device are the costs including instrument costs ($2.6 million in US dollars) (Personal communication, manufacturer, July 21st, 2010), cost per use ($200 per use), the costs associated with training surgeons and operating room personnel (31), and the lack of tactile feedback, with the trade-off being increased visual feedback. (27)
Robotic Surgery for Endometrial and Cervical Cancers
The goal of robotics in gynecologic oncology is to maximize surgeons’ abilities when performing complex minimally invasive techniques. (1) Compared to minimally invasive surgery by conventional laparoscopy, robotic operation times, learning curve, blood loss, complications, and length of hospital stay are less, whereas lymph node recovery is improved. (27) Minimally invasive surgery is a method to reduce the morbidity of surgery including blood loss, complications, post-operative pain, and length of hospital stay compared with open surgery. There are no guidelines on patient selection for robotic surgery in gynecology however a main factor necessary to complete the surgery is the patient’s ability to withstand a steep Trendelenberg position. (31) Additional difficulties that may impede successful endometrial cancer staging using robotic surgery include obesity, adhesive disease and uterine size. Obesity, in addition to older age, diabetes, and hypertension increase surgical risk and have a higher peri-operative morbidity and mortality for abdominal hysterectomy and endometrial cancer. (32) The issue with obesity and other co-morbid conditions such as diabetes is that the presence of these co-morbid conditions increases the risk of minor wound complications when using open surgery. Surgical procedures also tend to be technically challenging when performed on an obese patient due to increased subcutaneous thickness. From a technical perspective, slim or petite patients limit the ability to achieve adequate spacing between the robotic arms compared to larger patients. (29) Challenges using robotic surgery and staging for ovarian cancer ensues due to the restricted ability to perform high para-aortic lymph node dissection, deep pelvis lymph node dissection and ovary resection simultaneously using a single docking approach due to the current design of the robotic system. (30) Novel configurations of the robot are being explored (Personal communication, expert, December 9th, 2010).
Robotic Surgery for Prostate Cancer
The surgical techniques involved in robotically-assisted radical prostatectomy differ from the retropubic approach including patient positioning, where robotic surgery uses a steep Trendelenburg position needed to move the abdominal contents, and general anesthesia, whereas in the retropubic approach, the patient lies flat and undergoes general, epidural or spinal anesthesia. The steep Trendelenburg position maybe associated with head edema, increased intraocular pressure and cardiopulmonary alterations, particularly in obese patients. Robotic surgery involves multiple incisions ranging from 5 to 12 mm in diameter whereas the retropubic approach is performed through an 8 cm incision. (33)
Robotically-assisted radical prostatectomy is indicated for clinically localized prostate cancer in men with a life expectancy of more than 10 years. Contraindications include prior intra-abdominal surgery, obesity and a large prostate. Additional difficulties when performing robotic surgery include a narrow pelvis and large glands or lobes. The extent of surgeon experience may diminish these contraindications and difficulties. The most important outcomes for men and prostate cancer following radical prostatectomy are cancer control, urinary control and erectile function. Cancer control is determined by survival, however in the absence of long-term follow-up studies, surrogate factors such as positive surgical margin rate and prostate-specific antigen (PSA) recurrence may be evaluated in short-term studies. Additional outcome measures include blood loss/transfusion rate, operation time, length of hospitalization, pain, duration of catheterization, and complications. (34) Contraindications such as obesity may increase the likelihood of peri-operative complications during minimally invasive surgery, as well as for retropubic radical prostatectomy. (23) The overall goals of robotic surgery are to maximize cancer control, urinary continence and sexual function while maintaining the benefits of minimally invasive surgery with respect to minimal morbidity. The advanced minimally invasive skills required of laparoscopic prostatectomy have resulted in its limited widespread use.
History of Robotic Surgery
Robotic surgery is a recent technology. One of the original applications was for military surgeons to perform surgery on wounded personnel from a safe and remote location. The technology developed to enhance existing minimally invasive surgery, originally developed for cardiac surgery. A voice-activated robotic arm known as Aesop was introduced to the operating rooms to operate a camera during laparoscopic surgery. A device known as Zeus evolved, which included robotic arms attached to the surgical table and a robotic console. The novelty of the surgeon removed from the operating table was introduced at this time. Currently, there is only one approved device for surgical robotics in Canada. The robotic system known as the Da Vinci Surgical System has been further modified and refined, as discussed above. (35)
Regulatory Status
The Da Vinci Surgical System has been licensed by Health Canada since 2001 as a Class IV device. The device is used in conjunction with endoscopic and endowrist instruments including rigid endoscopes, blunt and sharp endoscopic clip appliers, dissectors, scissors, scalpels, ultrasonic shears, forceps/pick-ups, needle holders, endoscopic retractors, stablizers, and electrocautery instruments and accessories. The Da Vinci Surgical System assists in the accurate control of endoscopic and accessories for endoscopic manipulation of tissue including grasping, cutting, blunt and sharp dissection, and suturing to name a few. The surgical procedures that the Da Vinci Surgical System is used for include urologic surgical procedures, general laparoscopic surgical procedures, gynecologic laparoscopic surgical procedures, general non-cardiovascular thoracoscopic surgical procedures, and thoracoscopically-assisted cardiotomy procedures. It is also used with adjunctive mediastinotomy for coronary anastomosis during cardiac revascularization. It is indicated for adult and pediatric use. It is intended for use by trained physicians in an operating room environment.
There are a total of four devices in Ontario (University Health Network, St. Michael’s Hospital, and London Regional Cancer Centre (2 devices)), and eleven in Canada. This device is not listed in the Schedule of Benefits but its use may be billed according to the service being provided (e.g. hysterectomy). No known add on fees exist in Ontario for robotic devices.
Summary of Background Information
The issue for endometrial cancer is that obesity is a strong risk factor for disease. Laparoscopic procedures, although minimally invasive, pose a technical problem for large-sized patients. (29) Therefore, a robotic-assisted procedure may be the favoured surgical option for endometrial cancer.
The issue for cervical cancer is that the standard treatment of care for early stage cervical cancer (IA2 to IIA) is a radical hysterectomy with pelvic and para-aortic lymphadenectomy, whereas the role of lymphadenectomy remains controversial for endometrial cancer. Advanced stage cervical cancer is treated by chemoradiation. Approximately 7-15% of patients with early invasive cervical carcinoma have lymphatic spread. (32) Removing the pelvic and para-aortic lymph nodes is a complex surgical procedure that is hampered when performed using traditional laparoscopic techniques due to the limitations of the technology, therefore, the robotic-assisted procedure may be the favoured surgical option for cervical cancer.
The issue for prostate cancer is the increased use of PSA testing has led to an increased number of candidates for radical prostatectomy due to prostate cancer. (18) The robotic device would allow surgeons with open surgery skills to use minimally invasive technology more efficiently and satisfy patient preference for high-tech surgery. (36)
Evidence-Based Analysis
Research Questions
For endometrial and cervical cancers,
1) What is the effectiveness of the Da Vinci Surgical System vs. laparoscopy and laparotomy for women undergoing any hysterectomy for the surgical treatment and management of their endometrial and cervical cancers?
2) What are the incremental costs of the Da Vinci Surgical System vs. laparoscopy and laparotomy for women undergoing any hysterectomy for the surgical treatment and management of their endometrial and cervical cancers?
For prostate cancer,
3) What is the effectiveness of robotically-assisted radical prostatectomy using the Da Vinci Surgical System vs. laparoscopic radical prostatectomy and retropubic radical prostatectomy for the treatment and management of prostate cancer?
4) What are the incremental costs of robotically-assisted radical prostatectomy using the Da Vinci Surgical System vs. laparoscopic radical prostatectomy and retropubic radical prostatectomy for the treatment and management of prostate cancer?
Research Methods
Literature Search
Search Strategy
A literature search was performed on May 12, 2010 using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment for studies published from January 1, 2000 to May 12, 2010.
Abstracts were reviewed by a single reviewer and, for those studies meeting the eligibility criteria, full-text articles were obtained. Reference lists were also examined for any additional relevant studies not identified through the search. Articles with an unknown eligibility were reviewed with a second clinical epidemiologist and then a group of epidemiologists until consensus was established.
Inclusion Criteria
English language articles (January 1, 2000-May 12, 2010)
Journal articles that report on the effectiveness or cost-effectiveness for the comparisons of interest using a primary data source (e.g. obtained in a clinical setting)
Journal articles that report on the effectiveness or cost-effectiveness for the comparisons of interest using a secondary data source (e.g. hospital- or population-based registries)
Study design and methods must be clearly described
Health technology assessments, systematic reviews, randomized controlled trials, non-randomized controlled trials and/or cohort studies, case-case studies, regardless of sample size, cost-effectiveness studies
Exclusion Criteria
Duplicate publications (with the more recent publication on the same study population included)
Non-English papers
Animal or in vitro studies
Case reports or case series without a referent or comparison group
Studies on long-term survival which may be affected by treatment
Studies that do not examine the cancers (e.g. advanced disease) or outcomes of interest
Outcomes of Interest
For endometrial and cervical cancers,
Primary outcomes:
-
Morbidity factors
- Length of hospitalization
- Number of complications*
-
Peri-operative factors
Number of lymph nodes recovered
For prostate cancer,
Primary outcomes:
-
Morbidity factors
- Length of hospitalization
- Amount of morphine use/pain*
-
Peri-operative factors
Number of lymph nodes recovered
-
Oncologic factors
- Proportion of positive surgical margins
-
Long-term outcomes
- Urinary continence
- Erectile function
Statistical Analysis
A pooled analysis of individual studies was performed using Review Manager v. 5 (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008). Summary measures were expressed as the weighted mean difference for continuous data and odds ratio for dichotomous data using the Mantel-Haenszel method for unequal groups from observational studies. Statistical heterogeneity was assessed using the chi-square test. A p≤0.10 associated with a chi-square statistic was considered substantial heterogeneity and a random effects model was used. In the absence of heterogeneity, a fixed effects model was used. In the case of zero events, 0.5 was added automatically to all cells. Graphical display of the forest plots was also examined and subgroup analysis was performed where needed to clarify results. Specific details of the subgroup analyses are described separately for gynecologic oncology and prostate cancer in the following sections: Meta-Analysis: Endometrial and Cervical Cancers and Meta-Analysis: Prostate Cancer.
Quality of Evidence
The quality of the body of evidence was assessed as high, moderate, low, or very low according to the GRADE Working Group criteria (37), as presented below.
Quality refers to the criteria such as the adequacy of allocation concealment, blinding and follow-up.
Consistency refers to the similarity of estimates of effect across studies. If there are important and unexplained inconsistencies in the results, our confidence in the estimate of effect for that outcome decreases. Differences in the direction of effect, the magnitude of the difference in effect, and the significance of the differences guide the decision about whether important inconsistency exists.
Directness refers to the extent to which the interventions and outcome measures are similar to those of interest.
As stated by the GRADE Working Group, the following definitions of quality were used in grading the quality of the evidence:
| High | Further research is very unlikely to change confidence in the estimate of effect. |
| Moderate | Further research is likely to have an important impact on confidence in the estimate of effect and may change the estimate. |
| Low | Further research is very likely to have an important impact on confidence in the estimate of effect and is likely to change the estimate. |
| Very Low | Any estimate of effect is very uncertain |
Results of Evidence-Based Analysis
A literature search using OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment yielded 2,712 studies, of which 30 studies met the inclusion criteria. Four health technology assessments and two systematic reviews were also included.
Table 1: Study Design Type of Included Studies (N=30 Studies).
| Study Design | Level of Evidence† |
Number of Eligible Studies | |
|---|---|---|---|
| Gynecologic | Prostate | ||
| Systematic review of RCTs | 1a | - | - |
| Large RCT | 1b | - | - |
| Large RCT unpublished but reported to an international scientific meeting | 1(g) | 0 | 0 |
| Small RCT | 2 | 0 | 0 |
| Small RCT unpublished but reported to an international scientific meeting | 2(g) | 0 | 0 |
| Systematic review of non-RCTs with contemporaneous controls | 3a.1 | - | 1 |
| Non-RCT with contemporaneous controls | 3a | 1 | 5 |
| Systematic review of non-RCTs with historical controls | 3b.1 | 1 | - |
| Non-RCT with historical controls | 3b | 5 | 12 |
| Non-RCT presented at international conference | 3(g) | - | - |
| Surveillance (database or register) | 4a | 1 | 4 |
| Case series (multisite) | 4b | - | - |
| Case series (single site) | 4c | - | - |
| Retrospective review, modelling | 4d | - | - |
| Case series presented at international conference | 4(g) | - | - |
| Expert opinion | 5 | - | - |
| Total | 8 | 22 | |
RCT refers to randomized controlled trial.
Surgical Indications Combined (Endometrial, Cervical and Prostate Cancers)
Health Technology Assessments
A health technology assessment conducted in Belgium (2009) (38) examining the clinical effectiveness of robotic surgical systems compared to conventional laparoscopic or open surgery1 for a number of surgical indications included 18 health technology assessments, systematic reviews or horizon scans, with the remaining studies reviewed comprising observational studies.
For gynecologic oncology, no health technology assessments were reviewed. Three observational studies were reviewed, of which two of the three were comparative studies. Both of these studies showed a favourable peri-operative profile for robotic-assisted hysterectomy compared to conventional laparoscopic hysterectomy. The patient population was described for one study only, and included early-stage cervical cancer. For gynecologic oncology, they concluded that although a more favourable peri-operative profile was shown for robotic-assisted hysterectomy, there is no evidence to suggest that it is superior.
For urology, they analyzed the published literature according to short- and long-term outcomes. For short-term outcomes, a number of studies reviewed included case series, and are not included here for discussion. Three health technology assessments were reviewed with respect to short-term outcomes. However, whether robotic-assisted or conventional laparoscopic radical prostatectomy was considered in those health technology assessments was not clarified. For observational studies that included a comparison group, a recent meta-analysis of pooled data including 19 studies compared robotic-assisted and laparoscopic radical prostatectomy to open retropubic radical prostatectomy (7 studies).2 This study concluded a more favourable peri-operative profile including less blood loss and reduced number of transfusions for the combined laparoscopic surgical procedures compared to open surgery. No differences were shown for the risk of positive surgical margins, and one year urinary continence and erectile function between the two comparison groups. One systematic review was not clearly described with regards to the comparisons that were made and another large observational study was specific to discharge management and therefore not relevant for this discussion. Two smaller observational studies showed discordant results, with one study of individually matched patients showing no statistical differences for peri-operative factors including operation time, blood loss, hospital stay, bladder catheterizations, and positive surgical margin rates, whereas the transfusion rate was less for the laparoscopic group compared to robotic group (3% vs. 9.8%, p=0.03). The other study compared retropubic, laparoscopic and robotic-assisted radical prostatectomy and showed for patients with similar demographics, there were no differences for functional and oncological outcomes but a more favourable peri-operative profile including shortened length of hospital stay, less blood loss, reduced transfusion requirements, and less complications in the robotic group. For the long-term studies, outcomes measured were not relevant for this discussion (e.g. PSA). For urology, they concluded that there is a lack of evidence that supports one method over another. From the health technology assessment overall which included a number of surgical indications, they concluded that there is no evidence to support the benefits or drawbacks of robotic-assisted surgery in the absence of controlled comparative studies due to the heterogeneity in surgeon skill and experience. (38)
A health technology assessment conducted in Australia (2004) (39) examining the safety and efficacy of robotic-assisted surgery showed that a majority of studies were in urology (n=18), cardiovascular (n=19), and general surgery (n=19), with less studies for thoracic (n=7), gynecology (n=2), and pediatric (n=2). Among the two studies in the area of gynecology, one study was on tubal reanastomoses and the other study, although on hysterectomy, included only two cases. Among the 18 studies in the area of urology, 10 studies were on radical prostatectomy however only one of those studies was a comparative study. The results from that single comparative study showed a shorter length of hospital stay, less blood loss, decreased number of transfusions, and increased operative time for robotic-assisted radical prostatectomy compared to the control group (not specified). The overall conclusion from the health technology assessment was that there is a lack of substantive evidence to demonstrate the safety or efficacy of robotic surgery compared with conventional open or laparoscopic surgery for any surgical application. (39)
A health technology assessment conducted in Canada (2004) (40) by the Medical Advisory Secretariat on computer-assisted surgery using telemanipulators for a number of surgical indications included four health technology assessments or systematic reviews and 19 observational studies, of which four studies included a comparison group. For the literature reviewed, only one observational study was specific to prostate cancer. This study compared robotic-assisted radical prostatectomy to retropubic radical prostatectomy and showed a more favourable peri-operative and functional profile for robotic surgery including shorter hospital length of stay, less complications, increased proportion discharged within 24 hours, and return to continence and erectile function, which were statistically significant. Overall, they concluded that the technology is experimental, more research is needed, and its usefulness is not yet clear. (40)
A health technology assessment conducted in Spain (2007) (41) examined robotic-assisted radical prostatectomy compared with radical retropubic prostatectomy including two systematic reviews and six original papers. One additional paper included used laparoscopic radical prostatectomy as the reference procedure. The two systematic reviews included have been described above. (39;40) Among the six original papers, a number of study design limitations were described. The endpoints examined included operation time, estimated blood loss, proportion and total number of blood transfusions, pathologic parameters including proportion of positive margins, functional endpoints such as urinary continence and erectile function, morbidity factors such as length of hospitalizations, duration of bladder catheterization, dosage of analgesia, and rate of complications. Their assessment showed that a majority of the results favored robotic-assisted radical prostatectomy compared to radical retropubic prostatectomy, a majority of which were significant. However, no differences between surgical procedures were also common. When laparoscopic radical prostatectomy was considered as the comparator, no differences were shown for pathological and functional endpoints. Estimated blood loss was reduced for robotic-assisted radical prostatectomy compared to laparoscopic radical prostatectomy. Overall, they concluded that there is not sufficient evidence regarding the safety and efficacy of robotic-assisted radical prostatectomy compared to radical retropubic prostatectomy or laparoscopic radical prostatectomy. (41)
Endometrial and Cervical Cancers
Systematic Reviews
One systematic review was identified for endometrial and cervical cancers. A systematic review that included 27 papers on cervical cancer (n=18), endometrial cancer (n=11) and ovarian cancer (n=7) [not discussed] were evaluated for use of robotic-assisted surgery in gynecologic oncology and outcomes including estimated blood loss, number of lymph nodes extracted, operation time, length of hospital stay, and complications. The literature search was from 1950 to 2008. Comparative and non-comparative studies were included in the systematic review. Study designs included case series, case reports, and prospective and retrospective cohort studies. Multiple types of surgeries were reviewed in addition to hysterectomy for endometrial and cervical cancers. (42) Only studies and outcomes relevant to this report are summarized below.
For endometrial cancer and studies that included a comparison group for radical hysterectomy and staging, results showed reduced blood loss and shorter hospital stay for the robotic group compared to the laparotomy group. The operation time was longer in the robotic group and three procedures were converted to laparotomy. There was no difference in post-operative complications. (43) Three studies compared all three surgical procedures and showed a more favourable profile for the minimally invasive groups compared to laparotomy for the outcomes of blood loss, hospital stay and lymph nodes retrieved. Operation time was longer in the minimally invasive groups compared to laparotomy. (44-46) One additional study compared robotic surgery to laparoscopy among obese women and showed a shorter operation time, reduced blood loss and increased lymph node retrieval for robotic surgery. (47)
For cervical cancer and studies that included a comparison group for radical hysterectomy, the results for the robotic group compared to the laparoscopy group showed no differences for operation time, pelvic lymph node retrieval, estimated blood loss, length of hospital stay, conversions to laparotomy, and complications (48). When robotic surgery was compared to laparotomy, a more favourable profile was shown for the robotic group with respect to operation time, pelvic lymph node retrieval, estimated blood loss, and length of hospital stay. There was no difference in the overall number of post-operative complications. (49) One additional comparative study showed similar trends in results, except for operation time and lymph node retrieval (50), and another study showed similar trends in results except for operation time (51). Another study in the review with laparotomy as the comparison group was based on advanced cervical cancer. (52) An additional study on cervical cancer compared all three surgical procedures and showed reduced blood loss and shorter length of hospital stay for the minimally invasive surgical groups compared to laparotomy, whereas there were no differences between the three surgical groups for the number of complications and number of lymph nodes retrieved. (53)
The overall conclusions from the systematic review were minimally invasive surgery including robotic surgery and laparoscopy were equivalent for outcomes such as operation time, estimated blood loss, length of hospital stay, and number of complications (endometrial cancer). Specific trends in the type of complications were also noted. The equivalent surgical outcomes between the two minimally invasive techniques may be due to highly specialized and skilled surgeons in minimally invasive procedures. However, surgeons comfortable with robotic surgery and not laparoscopy may not afford the same benefits to patients with respect to conversions to laparotomy. Methodological limitations included temporal bias when groups of patients within studies are compared at two different time periods. (42)
A systematic review identified from the literature search included only four studies, two of which did not include a comparison group. The two studies that included a comparison group have been previously discussed. (54) A systematic review as an update to the abovementioned systematic review (54), included 11 studies of mixed case reports, case series and comparative studies. (55) The comparative studies have been previously discussed, except for one study, which will be discussed below. (56) A recent systematic review was identified however included benign conditions in addition to cervical and endometrial cancers and the relevant studies have already been identified. (57)
Randomized Controlled Trials
There are no published randomized controlled trials comparing robotically-assisted minimally invasive surgery for hysterectomy with either laparoscopy or laparotomy for endometrial or cervical cancers examining standard cancer outcomes. Currently, there is a Phase III randomized controlled trial underway comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. The primary outcome is disease-free survival at 4.5 years post-surgery. It is a worldwide study that will evaluate the equivalence between the technologies having 370 patients per arm. The Canadian site is at the Princess Margaret Hospital. The estimated study completion date is 2017. (58;59)
Non-Randomized Controlled Trials
There is some evidence that robotic surgery is equivalent in terms of progression-free and overall survival to that of open surgery for radical hysterectomy for cervical cancer. (60) A summary of the study characteristics of the observational studies identified from the systematic literature search are shown in Tables 1 and 3, including the individual studies reviewed from the only included systematic review. (42)
A comparative case-case retrospective medical record review study examined morbidity and peri-operative factors including lymph node recovery for robotic-assisted surgery (RB) compared to laparoscopic surgery (LP) for endometrial cancer requiring surgical staging including hysterectomy. Surgeries were predominately performed by a single experienced surgeon. Operation time was well-defined. Groups were comparable with respect to age, body mass index, history of abdominal surgery, pre-existing co-morbid conditions, FIGO surgical stage, and tumour type. Less estimated mean blood loss was shown for the robotic group compared to the laparoscopy group (RB: 109, standard deviation [SD]: 83.3 vs. LP: 187, SD: 187 ml, p<0.0001). In contrast, longer operation times were shown for the robotic group compared to the laparoscopy group (RB: 237, SD: 57 vs. LP: 178, SD: 58.9 min, p<0.0001). No differences were shown for lymph nodes recovered, blood transfusions and length of hospital stay. There was one conversion to laparotomy in the robotic group (1%), whereas there were 9 conversions in the laparoscopy group (5.2%). There were two major post-operative complications in the robotic group and none in the laparoscopy group. (61)
A comparative case-case retrospective study using a historical referent group examined morbidity and peri-operative factors including lymph node recovery for robotic-assisted surgery compared to open surgery (OS) for cervical cancer requiring type III radical hysterectomy and bilateral pelvic lymphadenectomy. The robotic surgeries were performed by two experienced robotic surgeons. Groups were comparable with respect to age and body mass index. Less estimated mean blood loss was shown for the robotic group compared to the open surgery group (RB: 165 vs. OS: 323 ml, p=0.001) and decreased hospital length of stay was shown for the robotic group compared to the open surgery group (RB: 1.4 vs. OS: 2.8 days, p<0.001). No differences were shown for operation time and pelvic lymph node yield. (62)
A comparative case-case retrospective study using information extracted from a database examined morbidity and peri-operative factors including lymph node recovery for robotic-assisted surgery compared to laparoscopic and open surgery for endometrial cancer requiring surgical staging including hysterectomy. All surgeries were performed by two qualified surgeons. Patient assignment was based upon uterine size and financial capacity. Groups were comparable with respect to age, body mass index, proportion with co-morbidities, proportion having an abdomino-pelvic surgery history, pre-operative haemoglobin, cell type, and FIGO stage of disease. When differences were examined across the three surgical groups, there were no differences shown for operation time and para-aortic lymph node recovery. There were no conversions to open surgery for either the robotic or laparoscopy surgical groups. Significant differences were shown for median post-operative hospital stay (RB: 7.9 vs. LP: 7.7 vs. OS: 10.8 days, p<0.001), the proportion of overall complications (RB: 7.1 vs. LP: 8 vs. OS: 25%, p=0.049), and the proportion of transfusions (RB: 14.3 vs. LP: 16 vs. OS: 42.9%, p=0.006), where the open surgery group had more detrimental levels compared to both minimally-invasive surgical groups. In contrast, the open surgery group showed an increased number of pelvic lymph nodes recovered, followed by the robotic surgery group, and then the laparoscopy group (RB: 21.1, SD: 9.3 vs. LP: 18.4, SD: 7.3 vs. OS: 24.4, SD: 10.1, p=0.024). Only the proportion of transfusions was examined to estimate patient blood loss because it is difficult to measure blood loss accurately. (63)
A comparative case-case prospective study examined morbidity and peri-operative factors including lymph node recovery for robotic surgery compared to laparoscopic surgery and open surgery for cervical cancer. Individuals were matched for cancer stage and type. A single surgeon performed the laparoscopic and open surgeries. Two surgeons performed the robotic surgeries. Surgeons had advanced robotic and laparoscopic training (Personal communication, author, July 16th, 2010). Groups were comparable with respect to body mass index and FIGO stage of disease. The robotic group was slightly older than the open surgery group with respect to mean age (RB: 55, SD: 12.7 vs. OS: 42, SD: 12 years, p=0.004), whereas there was no difference when compared to the laparoscopy group (LP: 52.8, SD: 14.2 years). Significant differences were shown between the robotic group and the open surgery group with respect to increased mean operation time (RB: 2.4, SD: 0.8 vs. OS: 1.9, SD: 0.6 hours, p=0.05), less estimated mean blood loss (RB: 130, SD: 119.4 vs. OS: 621.4, SD: 294 cm3, p<0.0001), increased mean number of lymph nodes recovered (RB: 32.4, SD: 10 vs. OS: 25.7, SD: 11.5, p<0.05), a fewer proportion of blood transfusions (RB: 3.1 vs. OS: 35.7%, p=0.007), and shorter length of hospital stay (RB: 2.6, SD: 2.1 vs. OS: 4, SD: 1.7, p=0.03) for the robotic group. There were no differences for post-operative complications. Significant differences were also shown between the robotic group and the laparoscopy group with respect to an increased mean number of lymph nodes recovered for the robotic group (RB: 32.4, SD: 10 vs. LP: 18.6, SD: 5.3, p<0.001). There were no differences for operation time, estimated blood loss, number of blood transfusions, number of post-operative complications, and length of hospital stay. The matched design of cancer stage and type helped to examine differences between surgical procedures and not severity of disease or surgical complexity. (56)
A comparative prospective study examined morbidity and peri-operative factors including lymph node recovery for robotic-assisted surgery, laparoscopic surgery and open surgery for endometrial cancer requiring surgical staging including hysterectomy. Up to five surgeons performed each surgical technique, with experience for minimally invasive techniques ranging from novice to expert. Patient assignment was based upon informed decision. Groups were comparable with respect to age and tumour grade. Median body mass index was higher in the open surgery group compared to the other two groups (RB: 29 vs. LP: 31 vs. OS: 37 kg/m2, p=0.03), and an increased number of stage II cancers were shown among individuals undergoing open surgery (RB: 9.4 vs. LP: 0 vs. OS: 19.2%, p=0.025), compared to the other two groups.
Significant differences were shown for median operation time and median estimated blood loss, favouring robotic surgery (Time, RB: 195 vs. LP: 270 vs. OS: 202 min, p=0.023; Blood, RB: 50 vs. LP: 150 vs. OS: 500 ml, p<0.0001). The minimally invasive groups had shorter median hospital stays compared to the open surgery group (1 vs. 3 days, p<0.0001), and the robotic group had a borderline lower proportion of complications than the laparoscopic and open surgery groups (RB: 19 vs. LP: 29 vs. OS: 42%, p=0.05). There was one conversion to open surgery in the robotic group and two conversions to open surgery in the laparoscopy group. Overall, when continuous variables were compared across the three surgical groups, the statistics used was not clear. (64)
A comparative case-case prospective study examined morbidity and peri-operative factors including lymph node recovery for robotic surgery compared to open surgery for cervical cancer. A majority of the robotic surgeries were performed by two senior surgeons who had never performed laparoscopic procedures. Operation time and estimated blood losses were well-defined. Groups were comparable with respect to body mass index, the number of co-morbidities, a history of abdominal surgery, neoadjuvant chemotherapy, and FIGO stage of disease. Individuals in the robotic group were slightly younger than in the open surgery group with respect to mean age (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035). Significant differences were shown between the robotic group and the open surgery group with respect to increased mean operation time (RB: 272.3, SD: 42.3 vs. OS: 199.6, SD: 65.6 min, p<0.001), less estimated mean blood loss (RB: 78, SD: 94.8 vs. OS: 221.8, SD: 132.4 ml, p<0.001), shorter mean hospital stay (RB: 3.7, SD: 1.2 vs. OS: 5, SD: 2.4 days, p<0.01), and fewer mean lymph nodes recovered (RB: 20.4, SD: 6.9 vs. OS: 26.2, SD: 11.7, p<0.05) for the robotic group when controlled for age. There were zero conversions to open surgery and no differences between groups for post-operative complications when complications were examined individually and according to time after surgery. (65)
A comparative case-case prospective study examined morbidity and peri-operative factors including lymph node recovery for robotic surgery compared to laparoscopic surgery for endometrial cancer requiring surgical staging including hysterectomy. The minimally invasive techniques were performed by one of two surgeons. Operation time was well-defined. Groups were comparable with respect to age, number of co-morbidities, and tumour grade and stage (FIGO unknown?). Individuals in the robotic group had an increased mean body mass index (RB: 34.2, SD: 9 vs. LP: 28.7, SD: 6.9 kg/m2, p<0.001), compared to the laparoscopy group. The robotic group showed a more favourable profile compared to laparoscopy with respect to a decreased mean operation time (RB: 242, SD: 53 vs. LP: 287, SD: 55 min, p<0.001), shorter median length of hospital stay (RB: 1 vs. LP: 2 nights, p<0.001), reduced median estimated blood loss (RB: 88 vs. LP: 200 ml, p<0.001), a lowered proportion of transfusions (RB: 3 vs. LP: 18%, p=0.002), and lowered proportion of conversions (RB: 12.4 vs. LP: 26.3%, p=0.017).3 There were no differences for the number of lymph nodes recovered and complications. (66)
Meta-Analysis: Endometrial and Cervical Cancers
Studies with data in a format suitable for meta-analysis are shown below for length of hospitalization, complications, operation time, blood loss, conversions, and lymph node recovery. Studies were grouped according to level of surgeon skill or experience as indicated in the original paper (i.e. experience with robotics (experience), or initial experience with robotic surgery (learning curve)). The specific details from the original papers used to categorize the surgeons in the studies as experienced or part of the learning curve are shown below each forest plot. A cut point for determining what constitutes the learning curve was not examined in this report. Approximately 20-75 procedures are suggested as the point by which the learning curve has been overcome, at least with respect to operation time. (1) For the comparisons of interest (e.g. robotic surgery vs. abdominal surgery and robotic surgery vs. laparoscopy), groups were comparable for age and stage of disease, unless otherwise indicated based on review of the original papers. For particular studies, when the stage of disease was different between the two surgical groups being compared then these studies were examined separately within the level of surgeon skill or experience (e.g. learning curve, stage difference). This was done to examine the extent of bias when examining the effect of the level of surgeon skill or experience on the outcomes for the comparisons of interest. A tumour with more advanced stage may require more extensive surgery, and this may lead to an unfavourable surgical profile (Personal communication, expert, July 22nd, 2010). Pathological stage of disease was reviewed in the original papers. Pathological stage of disease was reported for all included studies, except for two studies, which reported on uterine weight. (44;46) Other subgroup analyses were to analyze the data by sample size when the heterogeneity of the summary estimate was high, and body mass index. Body mass index was reported for all included studies, except for one study. (48) For particular studies, when body mass index differed between surgical groups being compared, then this was reported. An obese patient may have an increased surgical risk and peri-operative morbidity (29;32), therefore differences in body mass index between surgical groups may lead to biased estimates. Other comorbidities that may influence the outcomes under study were not consistently reported in the included studies, and therefore were not considered for this meta-analysis. There were a total of 15 studies included in the meta-analysis.
1. Length of Hospitalization (days)
a) Robotic vs. Abdominal
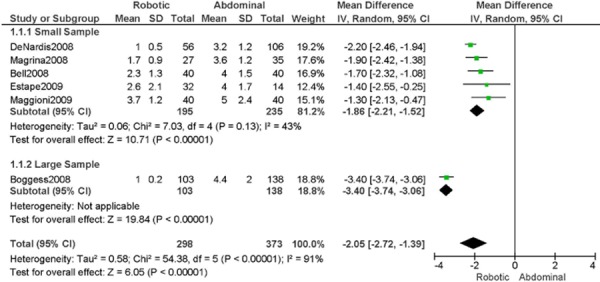
Experience was variably defined as familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); advanced training in robotics. (56)
Learning curve was variably defined as during the learning curve of our robotics program (43); the surgeon started performing robotic hysterectomies in 2005 (44); all robotic hysterectomies were performed by senior surgeons who had never performed the procedure laparoscopically (65); implementation of the robotics program. (45)
Age difference was shown for DeNardis 2008 (RB: 58.9, SD: 10.3 vs. OS: 62.5, SD: 10.8 years, p=0.05); for Bell 2008 (RB: 63.0, SD: 10.1 vs. OS: 72.3, SD: 12.5 years, p=0.0005); for Estape 2009 (RB: 55.0, SD: 12.7 vs. OS: 42.0, SD: 12.0 years, p=0.004); and for Maggioni 2009 (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in OS (significance not given), and DeNardis 2008, favouring stage III tumours in OS (significance not given).
Body mass index difference was shown for DeNardis 2008 (RB: 28.5, SD: 6.4 vs. OS: 34.0, SD: 9.3 kg/m2, p=0.0001), with a higher body mass index in OS.
There was no stage information in Bell 2008. There was no difference in uterine weight.
b) Robotic vs. Laparoscopy
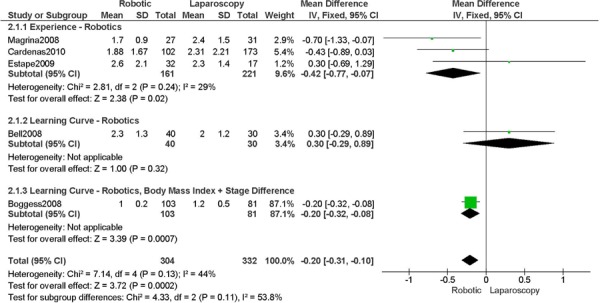
All studies used surgeons that were experienced in laparoscopy, except one study (Bell 2008).
Experience was variably defined as familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); experienced in robot-assisted approaches (61); advanced training in robotics. (56)
Learning curve was variably defined as the surgeon started performing robotic hysterectomies in 2005 (44); implementation of the robotics program. (45)
Age difference was shown for Bell 2008 (RB: 63.0, SD: 10.1 vs. LP: 68.4, SD: 11.8 years, p=0.03).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB.
2. Total Number of Complications (number of events)
a) Robotic vs. Abdominal
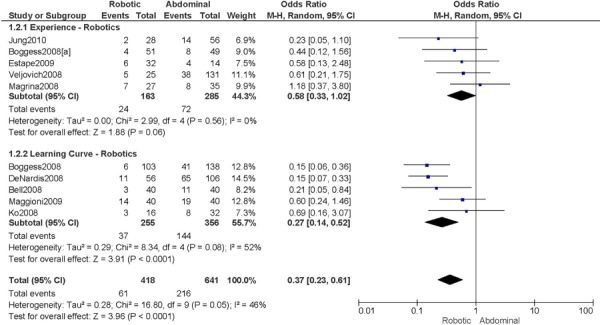
Complications defined as intra-/peri-operative and post-operative complications (<30 days), except for Bell 2008 (peri-operative only), Veljovich 2008 (major and minor complications), Boggess 2008 (post-operative only), Maggioni 2009 (early (<1 month) and late (>1 month) complications), and DeNardis 2008 (peri-operative and delayed (1-6 weeks post-operative) complications) (5 studies).
Experience was variably defined as qualified surgeons (63); as >50 robotic surgeries (49); advanced training in robotics (56); familiarity with the use of the robotic system for benign and other malignant pelvic conditions. (53)
Learning curve was variably defined as implementation of the robotics program (45); during the learning curve of our robotics program (43); the surgeon started performing robotic hysterectomies in 2005 (44); all robotic hysterectomies were performed by senior surgeons who had never performed the procedure laparoscopically (65); inception of the robotic program. (50)
Age difference was shown for Boggess 2008a (RB: 47.4, SD: 12.9 vs. OS: 41.9, SD: 11.2 years, p=0.029); for Estape 2009 (RB: 55.0, SD: 12.7 vs. OS: 42.0, SD: 12.0 years, p=0.004); for DeNardis 2008 (RB: 58.9, SD: 10.3 vs. OS: 62.5, SD: 10.8 years, p=0.05); for Bell 2008 (RB: 63.0, SD: 10.1 vs. OS: 72.3, SD: 12.5 years, p=0.0005); and for Maggioni 2009 (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in OS (significance not given), and DeNardis 2008, favouring stage III tumours in OS (significance not given).
Body mass index difference was shown for Veljovich 2008 (RB: 27.6, Range (18.7-49.4) vs. OS: 32.2, Range (16.4-65.8) kg/m2), with higher body mass index in OS; DeNardis 2008 (RB: 28.5, SD: 6.4 vs. OS: 34.0, SD: 9.3 kg/m2, p=0.0001), with a higher body mass index in OS.
There was no stage information in Bell 2008. There was no difference in uterine weight.
b) Robotic vs. Laparoscopy
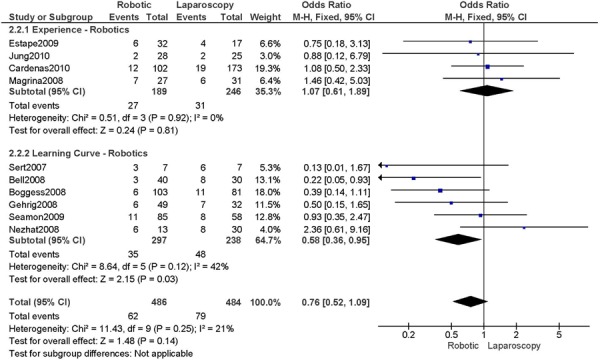
All studies used surgeons that were experienced in laparoscopy, except for two studies (Bell 2008; Gehring 2008).
Complications defined as intra-/peri-operative and post-operative complications (<30 days), except for Cardenas 2010 (late post-operative complications, up to day 10), for Bell 2008 (peri-operative only), Boggess 2008 (post-operative only), Seamon 2009 (peri-operative only) (4 studies).
Experience was variably defined as advanced training in robotics (56); qualified surgeons (63); experienced in robot-assisted approaches (61); familiarity with the use of the robotic system for benign and other malignant pelvic conditions. (53)
Learning curve was variably defined as no mention otherwise (51); the surgeon started performing robotic hysterectomies in 2005 (44); implementation of the robotics program (45); entire experience from initiation (Personal communication, author, July 19th, 2010) (47); as prior to robotic experience (66); the approach was offered. (48)
Age difference was shown for Bell 2008 (RB: 63.0, SD: 10.1 vs. LP: 68.4, SD: 11.9 years, p=0.03).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB. Body mass index difference for Seamon 2009 (RB: 34.2, SD: 9.0 vs. LP: 28.7, SD: 6.9 kg/m2, p<0.001), with a higher body mass index in RB.
There was no stage information in Bell 2008. There was no difference in uterine weight.
3. Operation Time (hours)
a) Robotic vs. Abdominal
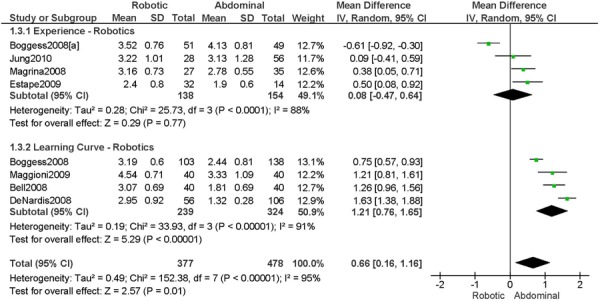
Experience was variably defined as >50 robotic surgeries (49); qualified surgeons (63); familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); advanced training in robotics. (56).
Learning curve was variably defined as the implementation of the robotics program (45); all robotic hysterectomies were performed by senior surgeons who had never performed the procedure laparoscopically (65); the surgeon started performing robotic hysterectomies in 2005 (44); as during the learning curve of our robotics program. (43)
Age difference was shown for Boggess 2008a (RB: 47.4, SD: 12.9 vs. OS: 41.9, SD: 11.2 years, p=0.029); for Estape 2009 (RB: 55.0, SD: 12.7 vs. OS: 42.0, SD: 12.0 years, p=0.004); for Maggioni 2009 (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035); for Bell 2008 (RB: 63.0, SD: 10.1 vs. OS: 72.3, SD: 12.5 years, p=0.0005); and for DeNardis 2008 (RB: 58.9, SD: 10.3 vs. OS: 62.5, SD: 10.8 years, p=0.05).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in OS (significance not given), and DeNardis 2008, favouring stage III tumours in OS (significance not given).
Body mass index difference was shown for DeNardis 2008 (RB: 28.5, SD: 6.4 vs. OS: 34.0, SD: 9.3 kg/m2, p=0.0001), with a higher body mass index in OS.
There was no stage information in Bell 2008. There was no difference in uterine weight.
b) Robotic vs. Laparoscopy
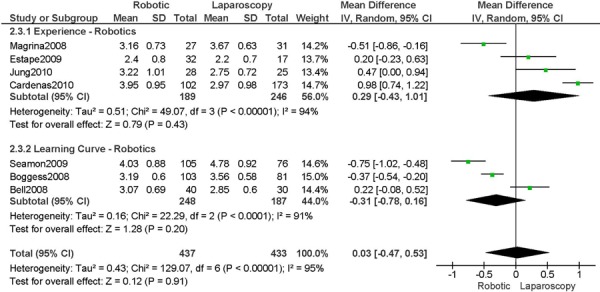
All studies used surgeons that were experienced in laparoscopy, except one study (Bell 2008).
Experience was variably defined as familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); advanced training in robotics (56); qualified surgeons (63); experienced in robot-assisted approaches. (61)
Learning curve was variably defined as prior to robotic experience (66); implementation of the robotics program (45); the surgeon started performing robotic hysterectomies in 2005. (44)
Age difference was shown for Bell 2008 (RB: 63.0, SD: 10.1 vs. LP: 68.4, SD: 11.9 years, p=0.03).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB. Body mass index difference for Seamon 2009 (RB: 34.2, SD: 9.0 vs. LP: 28.7, SD: 6.9 kg/m2, p<0.001), with a higher body mass index in RB.
There was no stage information in Bell 2008. There was no difference in uterine weight.
4. Blood Loss (ml)
a) Robotic vs. Abdominal
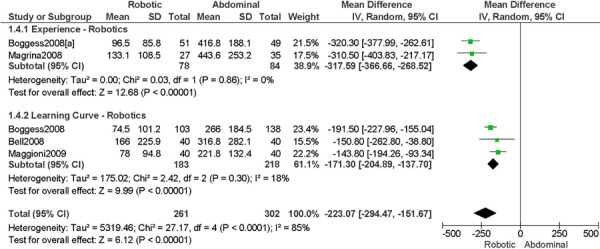
Experience was variably defined as >50 robotic surgeries (49); familiarity with the use of the robotic system for benign and other malignant pelvic conditions. (53)
Learning curve was variably defined as implementation of the robotics program (45); the surgeon started performing robotic hysterectomies in 2005 (44); all robotic hysterectomies were performed by senior surgeons who had never performed the procedure laparoscopically. (65)
Age difference was shown for Boggess 2008a (RB: 47.4, SD: 12.9 vs. OS: 41.9, SD: 11.2 years, p=0.029); for Bell 2008 (RB: 63.0, SD: 10.1 vs. OS: 72.3, SD: 12.5 years, p=0.0005); and for Maggioni 2009 (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in OS (significance not given).
There was no stage information in Bell 2008. There was no difference in uterine weight.
b) Robotic vs. Laparoscopy
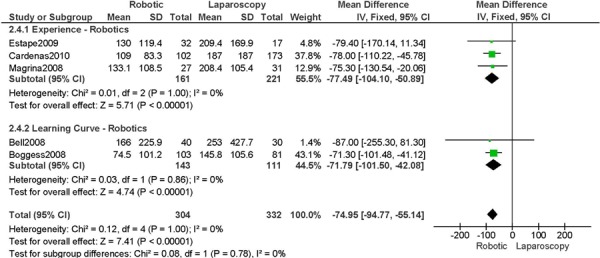
All studies used surgeons that were experienced in laparoscopy, except one study (Bell 2008).
Experience was variably defined as advanced training in robotics (56); experienced in robot-assisted approaches (61); familiarity with the use of the robotic system for benign and other malignant pelvic conditions. (53)
Learning curve was variably defined as the surgeon started performing robotic hysterectomies in 2005 (44); implementation of the robotics program. (45)
Age difference was shown for Bell 2008 (RB: 63.0, SD: 10.1 vs. LP: 68.4, SD: 11.9 years, p=0.03).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB.
There was no stage information in Bell 2008. There was no difference in uterine weight.
5. Conversions (number of events)
a) Robotic vs. Abdominal (not applicable)
b) Robotic vs. Laparoscopy
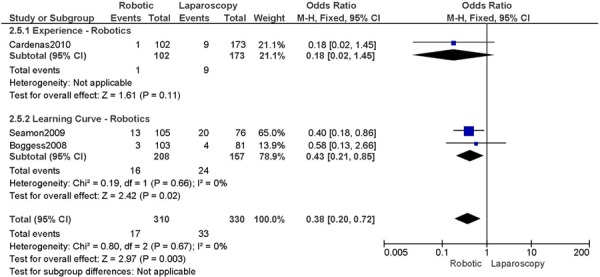
All studies used surgeons that were experienced in laparoscopy.
Experience was variably defined as experienced in robot-assisted approaches. (61)
Learning curve was variably defined as prior to robotic experience (66); implementation of the robotics program. (45)
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB. Body mass index difference for Seamon 2009 (RB: 34.2, SD: 9.0 vs. LP: 28.7, SD: 6.9 kg/m2, p<0.001), with a higher body mass index in RB.
6. Overall Lymph Node Recovery (total number of nodes)
a) Robotic vs. Abdominal
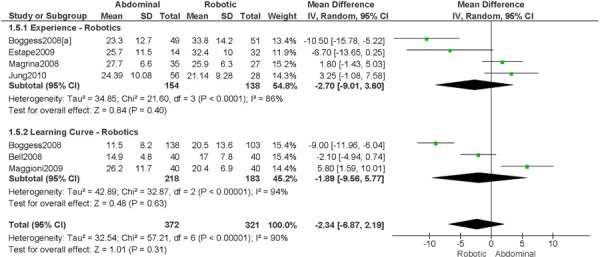
Total number of nodes refers to pelvic nodes, except for Estape 2009, Magrina 2008, Boggess 2008 and Bell 2008 which also include (para) aortic nodes (4 studies). Where pelvic and para-aortic node data was reported separately, information on pelvic lymph node recovery was used (67), which included one study (Jung 2010).
Experience was variably defined as >50 robotic surgeries (49); advanced training in robotics (56); familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); qualified surgeons. (63)
Learning curve was variably defined as implementation of the robotics program (45); the surgeon started performing robotic hysterectomies in 2005 (44); all robotic hysterectomies were performed by senior surgeons who had never performed the procedure laparoscopically. (65)
Age difference was shown for Boggess 2008a (RB: 47.4, SD: 12.9 vs. OS: 41.9, SD: 11.2 years, p=0.029); Estape 2009 (RB: 55.0, SD: 12.7 vs. OS: 42.0, SD: 12.0 years, p=0.004); for Bell 2008 (RB: 63.0, SD: 10.1 vs. OS: 72.3, SD: 12.5 years, p=0.0005); and for Maggioni 2009 (RB: 44.1, SD: 9.1 vs. OS: 49.8, SD: 14.1 years, p=0.035).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in OS (significance not given).
There was no stage information in Bell 2008. There was no difference in uterine weight.
b) Robotic vs. Laparoscopy
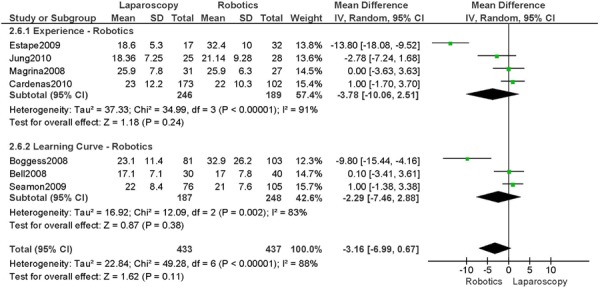
All studies used surgeons that were experienced in laparoscopy, except one study (Bell 2008).
Total number of nodes refers to pelvic and para-aortic nodes, except for Jung 2010 and Seamon 2009 which refer to pelvic nodes only (2 studies). Where pelvic and para-aortic node data was reported separately, information on pelvic lymph node recovery was used (67), which included two studies (Jung 2010; Seamon 2009).
Experience was variably defined as advanced training in robotics (56); qualified surgeons (63); familiarity with the use of the robotic system for benign and other malignant pelvic conditions (53); experienced in robot-assisted approaches. (61)
Learning curve was variably defined as implementation of the robotics program (45); the surgeon started performing robotic hysterectomies in 2005 (44); as prior to robotic experience. (66)
Age difference was shown for Bell 2008 (RB: 63.0, SD: 10.1 vs. LP: 68.4, SD: 11.9 years, p=0.03).
Stage difference was shown for Boggess 2008, favouring IIB, IIIA/IIIB/IIIC and IVA/IVB tumours in LP (significance not given) and body mass index difference (RB: 32.9, SD: 7.6 vs. LP: 29.0, SD: 6.5 kg/m2, p=0.0008), with a higher body mass index in RB. Body mass index difference for Seamon 2009 (RB: 34.2, SD: 9.0 vs. LP: 28.7, SD: 6.9 kg/m2, p<0.001), with a higher body mass index in RB.
There was no stage information in Bell 2008. There was no difference in uterine weight.
Summary of Results of the Meta-Analysis
Results from the meta-analysis show a favourable profile for robotic surgery compared to abdominal surgery with respect to a shorter length of hospitalization (Mean difference [MD]: -2.05, 95% Confidence interval [CI]: -2.72, -1.39 days, p<0.00001), an approximately 60% reduced risk of complications (Odds ratio [OR]: 0.37, 95% CI: 0.23, 0.61, p<0.0001), and less blood loss (MD: -223.07, 95% CI: -294.47, -151.67 ml, p<0.00001). In contrast, robotic surgery did not show a favourable profile compared to abdominal surgery with respect to operation time, showing longer operation times (MD: 0.66, 95% CI: 0.16, 1.16 hours, p=0.01) for robotic surgery. There was no difference between robotic surgery and abdominal surgery for lymph node recovery (MD: -2.34, 95% CI: -6.87, 2.19, p=0.31). The role of surgeon skill or experience was shown for operation time, with institutions using robotic surgery at the time of the learning curve having increased operation times compared to abdominal surgery (MD: 1.21, 95% CI: 0.76, 1.65 hours, p<0.00001). There were no clear effects for age, stage of disease, or obesity.
When robotic surgery was compared to laparoscopy, a more favourable profile was shown for length of hospitalization, with robotic surgery requiring a shorter length of hospital stay (MD: -0.21, 95% CI: -0.31, -0.10 days, p=0.0002), less blood loss (MD: -74.95, 95% CI: -94.77, -55.14 ml, p<0.00001), and for conversions, with robotic surgery showing an approximately 60% reduced risk of conversions (OR: 0.38, 95% CI: 0.20, 0.72, p=0.003). There was one study for length of hospitalization that favoured robotic surgery during the learning curve however the effect could have been driven by different patient populations between surgical procedures (e.g. stage of disease). No differences were shown for complications (OR: 0.76, 95% CI: 0.52, 1.09, p=0.14), operation time (MD: 0.03, 95% CI: -0.47, 0.53 hours, p=0.91), and lymph nodes (MD: -3.16, 95% CI: -6.99, 0.67, p=0.11). There was some suggestion that even during the learning curve, robotic surgery was less likely to be related to patient complications compared to laparoscopy (OR: 0.58, 95% CI: 0.36, 0.95, p=0.03). Also, stage differences between surgical groups may have contributed to a favourable profile for robotic surgery in one study for overall lymph node recovery during the learning curve.
Where stage differences were shown, there were 20.3%, 18.5% and 11.7% of stage IIB, IIIA/IIIB/IIIC and IVA/IVB endometrial tumours in the open surgery, laparoscopy and robotic surgery groups, respectively. When restricted to stage III disease or above, there were 14.5%, 18.5% and 9.7% of tumours in the open surgery, laparoscopy and robotic surgery groups, respectively. (45) Overall, the stage difference between the laparoscopy and robotic surgery groups was approximately 2-fold.
Prostate Cancer
Systematic Reviews
There was one systematic review identified and included for prostate cancer. A systematic review and cumulative analysis comparing retropubic (RP), laparoscopic (LP) and robotically-assisted radical prostatectomy (RB) was conducted to examine a number of morbidity, peri-operative, oncological, and long-term outcomes. This systematic review is the largest synthesis of comparative studies on the topic to date. The literature search included published papers since 1999. For the review, 37 studies were identified, of which 10 (27%) compared robotically-assisted radical prostatectomy with retropubic radical prostatectomy and 4 (11%) compared robotically-assisted radical prostatectomy with laparoscopic radical prostatectomy. The remaining studies compared retropubic radical prostatectomy with laparoscopic radical prostatectomy [not discussed]. Individual studies were shown by level of evidence according to Phillips and Sackett, and data abstracted from individual studies were detailed. None of the studies discussed below were graded higher than level 2b evidence for low-quality observational studies. No randomized controlled trials were included for the comparisons of interest. Only studies and outcomes relevant to this report are summarized below.
Among five studies for duration of in-hospital stay in days, robotically-assisted radical prostatectomy showed a more favourable profile compared to retropubic radical prostatectomy regardless of the level of evidence, and a majority of the results were statistically significant. When comparing robotically-assisted radical prostatectomy to laparoscopic radical prostatectomy in two studies for duration of in-hospital stay in days, the results were less consistent, showing favourable results in both directions, although neither was significant. Cumulative analysis was not possible for either comparison. One study examined the amount of morphine use in mg after either robotically-assisted radical prostatectomy or retropubic radical prostatectomy and showed that less morphine was needed after robotically-assisted prostatectomy (RB: 21 vs. RP: 24.4 mg, p<0.05). Cumulative analysis was not possible. There were no studies on morphine use comparing robotically-assisted radical prostatectomy to laparoscopic radical prostatectomy.
Up to seven studies examined peri-operative outcomes and showed robotically-assisted radical prostatectomy to have a more favourable profile compared to retropubic radical prostatectomy regardless of the level of evidence. The results were consistent for median/mean amount of blood loss in ml (5/5 studies), transfusion rate as a proportion (6/6 studies), duration of catheterization in days (3/3 studies), and for the most part, overall complication rate as a proportion (5/6 studies), of which a majority of the results were statistically significance except complication rate. Results from the cumulative analysis showed an increased risk of transfusions for retropubic surgery compared to robotic surgery. Cumulative analysis showed no difference for complication rates, and was not possible for blood loss, duration of catheterization and operation time. Operation time in minutes as a median or mean value was increased for robotically-assisted radical prostatectomy compared to retropubic radical prostatectomy (4/5 studies). Among four studies, when robotically-assisted radical prostatectomy was compared to laparoscopic radical prostatectomy, the results were less consistent for operation time (RB: 2/4 studies) and blood loss (RB: 2/4 studies) showing favourable results in both directions, where a majority of favourable results were significant. Three studies showed lower transfusion rates for robotically-assisted radical prostatectomy of which 1/3 studies had significant results. The remaining study showed no difference. One study examined catheterization duration and showed a slightly longer duration for robotic surgery (RB: 9.2 vs. LP: 9%), though the results were similar and not significantly different. The results for complication rates were in both directions; one study favouring robotic surgery and the other not (RB: 1/2 studies), and both findings were not significant. Cumulative analysis showed no difference between retropubic surgery and laparoscopy for operation time, blood loss, transfusion rate, and complication rate. Cumulative analysis was not possible for duration of catheterization. Among the few studies that examined anastomotic stricture as a proportion, there was one study that favoured robotically-assisted radical prostatectomy compared to retropubic radical prostatectomy, and the evidence was divided among two studies when robotically-assisted radical prostatectomy was compared to laparoscopic prostatectomy. None of the differences were significant.
For long-term health outcomes, there were three studies that examined time to urinary continence after either 3, 6 or 12 months comparing robotically-assisted radical prostatectomy to retropubic radical prostatectomy. Two studies showed a favourable profile for robotically-assisted radical prostatectomy of decreased time to urinary continence as a median time in days at 6 months or as a proportion at 3 months, and one study showed the reverse at 12 months. One of the favourable studies had a slight advantage only (RB: 76 vs. RP: 75% at 3 months), with the other favourable study being significant. Cumulative analysis was not possible. There was only one study on urinary continence that compared robotically-assisted radical prostatectomy to laparoscopic prostatectomy, which showed a slightly less favourable profile for robotic surgery (RB: 90 vs. LP: 92% at 6 months), again, the results were not that different and not significant. None of the studies used a validated questionnaire. There was only one study that examined time to erectile function as median time in days comparing robotically-assisted radical prostatectomy to retropubic radical prostatectomy. Two additional studies examined intercourse as either time in days or as a proportion at 12 months. All three studies favoured robotic surgery. Cumulative analysis was not possible. Only one study examined the proportion of patients having an erection sufficient for intercourse comparing robotically-assisted radical prostatectomy to laparoscopic prostatectomy at 3 months, and favoured robotically-assisted radical prostatectomy. This study included a validated questionnaire to assess erectile function, known as the International Index of Erectile Function. Cumulative analysis was not possible.
For oncological factors, there were up to six studies that examined the positive surgical margin rate as a proportion comparing robotically-assisted radical prostatectomy to retropubic radical prostatectomy. Five of the six studies showed a lower positive surgical margin rate for robotically-assisted radical prostatectomy, of which two studies showed statistically significant differences in positive surgical margin rates (RB: 6 vs. RP: 23% and RB: 15 vs. RP: 35.7%, p<0.05 for both) with adequate sample sizes (n=100 or 200 for each group). This trend held when limited to patients with organ confined (stage 2) prostate cancer in three studies, although differences did not reached statistical significance. Cumulative analysis showed an increased risk of positive surgical margins for retropubic surgery compared to robotic surgery. The evidence was less consistent and not significant for overall positive surgical margin rate when examined for robotically-assisted radical prostatectomy compared to laparoscopic prostatectomy in 3 of 3 studies. Cumulative analysis showed no differences.
The overall conclusions from the systematic review from the author’s perspective are blood loss and transfusion rates benefit from minimally invasive procedures however functional and oncological outcomes were not able to be adequately assessed. Factors that are dependent on the level of expertise of the surgeon or health care system may be variable. Further prospective, multicentre, comparative studies are needed. (18)
A number of reviews were identified that were not included for a number of reasons. Two were non-systematic (68;69), one was a comparison of case studies (70), three included a reduced series of studies that were also included in a more recent systematic review that is discussed (39;71-73), and one was described in detail in a more recent health technology assessment that has been discussed. (74) A recent study not identified in the systematic review discussed above, was additionally reviewed. (75)
In one systematic review, a number of study design limitations were described and are worth noting including: (1) the absence of consensus within the surgical community on the best way to report complications limiting comparisons across studies and between series, (2) the influence of surgeon experience on operation time, positive surgical margin rate, urinary continence, and potency, (3) variations in definitions, data collection methods and length of follow-up for urinary continence, (4) difficulty measuring potency including patient age, type and quality of nerve sparing procedure, and use of medications, and lack of a standardized and valid measure of assessment. (71)
Randomized Controlled Trials
There are no published randomized controlled trials comparing robotically-assisted radical prostatectomy with either laparoscopic prostatectomy or retropubic radical prostatectomy for prostate cancer examining standard cancer outcomes (e.g. disease-free survival).
Non-Randomized Controlled Trials
A summary of the study characteristics are shown in Table 2.
Outcomes Combined
A comparative case-case prospective study examined morbidity, peri-operative, functional, and oncological factors for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Robotic surgeries were performed by experienced surgeons. There were no differences between the two groups for age or stage of disease. There were no differences in operation time, proportion of positive surgical margins and proportion with urinary continence defined as 0-1 pads per day, although, the length of follow-up differed between the groups (RB: 6 vs. RP: 42 months as a mean). Differences favouring robotic surgery were shown for the proportion requiring blood transfusion (RB: 5 vs. RP: 65%, p<0.001), mean length of hospital stay (RB: 8, SD: 8 vs. RP: 17, SD: 7 days, p<0.001) and mean catheter duration (RB: 12, SD: 7 vs. RP: 18, SD: 7 days, p=0.004). (76)
A comparative case-case retrospective study using a peri-operative database examined morbidity and peri-operative factors for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. A majority of the robotic surgeries were performed by a single surgeon (77%) and a majority of the retropubic surgeries were performed by two surgeons (91%). The surgical experience is that of an inception program. Operation time and pain management were well-defined. When a subset of the patient population was matched (RB: 219 of 256 and RP: 251 of 280), there were no differences for age, weight and height. Individuals in the open surgery group had a slightly higher mean Gleason sum score (RB: 6.5 vs. RP: 6.7, p=0.03). Mean operation time was higher in the robotic group (RB: 296, SD: 76 vs. RP: 193, SD: 69 min, p<0.0001), whereas mean estimated blood loss (RB: 287, SD: 317 vs. RP: 1087 ml, SD: 853, p<0.0001), proportion requiring a blood transfusion (RB: 0.4 vs. OS: 24%, p<0.0001), mean post-operative morphine equivalent dose (RB: 11, SD: 7.7 vs. RP: 15, SD: 9.8 mg, p<0.0001), and mean hospital stay (RB: 44, SD: 77 vs. RP: 56, SD: 26 hours, p=0.009) was less for the robotic group compared to the open surgery group. The results from this study are consistent with respect to the robotic group having less blood loss and fewer patients requiring transfusions, and also reduced morphine requirements together with a shorter length of hospital stay for the robotic group. (77)
A comparative case-case retrospective study using a surgical database examined peri-operative and oncological factors for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy and laparoscopic radical prostatectomy. Each procedure was performed by a single experienced surgeon and surgical choice was at the clinician’s discretion. There were no differences in age and body mass index between the three groups. The stages of disease also appeared evenly distributed. The statistical analysis in terms of the groups being compared was not clear. In general, there was a trend of a more favourable profile for the minimally invasive procedures, with mean operation time (minutes), mean estimated blood loss (ml), proportion of blood transfusions, and mean hospital stay (days) showing the greatest benefits for robotic surgery. The proportion with positive surgical margins was comparable across the three groups. (78)
A prospective cohort study examined morbidity, peri-operative, functional, and oncological factors for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. The robotic surgeries were performed by two surgeons and the retropubic surgeries were performed by four surgeons, all experienced surgeons. Surgical procedures were determined by patients and physicians. Complications were recorded by the Clavien system, and urinary continence and erectile function were well-defined using validated measures: the International Consultation of Incontinence Questionnaire – Urinary Incontinence (ICIQ-UI) short-form and the International Index of Erectile Function (IIEF) 5-item respectively. The groups were comparable for body mass index, co-morbidities and stage of disease. Individuals in the retropubic group were slightly older. Results showed a more favourable profile for the robotic group compared to the retropubic group for median intraoperative blood loss (data not shown, p<0.001), proportion requiring blood transfusions (RB: 1.9 vs. RP: 14%, p<0.01), median catheter duration (RB: 5 vs. RP: 6 days, p<0.001), median length of hospital stay (RB: 6 vs. RP: 7 days, p=0.01), proportion that were continent after 12 months (RB: 97 vs. RP: 88%, p=0.01), mean time to continence (RB: 25, SD: 39 vs. RP: 75, SD: 116 days, p<0.001), and the proportion potent after 12 months (RB: 81 vs. RP: 49%, p<0.001). There were slightly more severe complications in the robotic group although for complications overall, there was no difference (RB: 10 vs. RP: 13%, p=0.854). The median operation time was longer (data not shown, p<0.001). There were no differences in peri-operative complications, and the proportion of positive surgical margins overall and for localized disease. This study is the first prospective comparative study to use validated questionnaires to assess urinary continence and erectile function. Statistically significant differences in peri-operative outcomes may not be clinically significant if the costs of the Da Vinci are not justified. Further work is needed to examine functional and oncological outcomes. (79)
A comparative case-case retrospective study using a database examined morbidity, peri-operative, functional, and oncological factors for robotically-assisted radical prostatectomy compared to laparoscopic radical prostatectomy. Procedures were performed by a single surgeon experienced with laparoscopic surgery. The robotic surgeries were part of an initial program. Continence was well-defined and potency was defined according to the International Index of Erectile Function and International Prostate Symptom Score (robotics only). Complications were recorded according to the Clavien system. The results showed differences favouring robotic surgery including decreased mean operation time (RB: 199 vs. LP: 232, p<0.001), less estimated blood loss (RB: 230 vs. LP: 311, p=0.004), and shorter length of hospital stay (RB: 1.95 vs. LP: 3.4 days, p<0.0001). There were an overall of 10.7% of complications in the robotics group and 14.7% in the laparoscopic group (no p-value given). There was no difference in the proportion of positive margins overall and for localized disease, and urinary continence and potency up to 12 months after surgery. There was one conversion to open surgery in the robotic group and none in the laparoscopic group. (80)
A comparative case-case retrospective study examined morbidity, peri-operative, functional, and oncological outcomes for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. All surgeries were performed by a single surgeon and robotic surgeries were based on the initial cases of a new robotics program (e.g. during the learning curve). Patient preference determined surgical assignment. Urinary continence was well-defined. There were no differences for body mass index, anesthetic/surgical risk class and clinical stage of disease between surgical groups. Individuals in the retropubic group were slightly older than individuals in the robotic group (RB: 67.3, SD: 6.2 vs. RP: 70, SD: 6.1 years, p<0.05). A more favourable profile for the robotic surgery group was shown for blood loss (RB: 314, SD: 284 vs. RP: 912, SD: 370 ml, p<0.0001), proportion requiring transfusions (RB: 13.3 vs. RP; 60%, p<0.0001), catheter duration (RB: 7.7, SD: 2.1 vs. RP: 9.2, SD: 2.9 days, p<0.05), length of hospital stay (RB: 7.3, SD: 2.3 vs. RP: 8.4, SD: 2.2 days, p<0.05), and proportion with urinary continence at three months (RB: 76.7 vs. OS: 36.7%, p=0.04). The proportion of overall positive surgical margins (RB: 50 vs. RP: 20%, p<0.05) and anastomosis time (RB: 43.9, SD: 11.4 vs. RP: 17.7, SD: 3.5 min, p<0.0001) was increased for the robotic group compared to the retropubic group. There were no differences for operation time, proportion of complications and urinary continence at 12 months. There was some suggestion of differences in potency at 12 months (RB: 87.5 vs. RP: 50%). The factors that influence erectile function include previous level of sexual function, age and intra-operative injury of the neurovascular bundle. (81)
A comparative case-case prospective study examined morbidity, peri-operative, functional, and oncological outcomes for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Case groups were matched however matching factors were not provided. The role of consecutive cases in the referent group was also not clear. The matched-paired analysis was also not clear. All surgeries were performed by three surgeons, all of which were not experienced with minimally invasive surgery. Urinary continence and potency were well-defined. Data were collected by a third-party interviewer. Groups were comparable with respect to age and clinical stage. Pathology staging showed an increased number of stage pT3/pT4 tumours in the retropubic group. In adjusted analysis for age, pathology, and surgical factors, the robotic group showed a more favourable profile for median blood loss, catheterization duration and hospital stay (p<0.001 for most). Median operation time was increased in the robotic group (p<0.001). In unadjusted analysis, urinary continence was achieved in a shorter duration by an increased number of men in the robotic group (p=0.007). At 12 months, there were an increased proportion of men with urinary continence in the robotic group (RB: 97 vs. RP: 88%, p=0.014). Potency was achieved by an increased proportion of men at 3, 6 and 12 months (RB, 3 mo: 31, 6 mo: 43, 12 mo: 61 vs. RP, 3 mo: 18, 6 mo: 31, 12 mo: 41%, p=0.006, p=0.045, p=0.003). There was no difference in the proportion of positive surgical margins for organ-confined disease. (75)
A comparative case-case retrospective study using a prostatectomy database examined peri-operative factors and lymph node recovery for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. The robotic surgeries were performed by three surgeons and the retropubic surgeries by a single surgeon. All patients underwent a standard-template pelvic lymphadenectomy using similar anatomic boundaries. Patients had increased pre-operative risk. Results showed no difference between the surgery groups for operation time, whereas mean estimated blood loss (RB: 206 vs. RP: 1399 ml, p<0.0001) (SDs not given) and mean lymph node number retrieved was less for the robotic group (RB: 12.5 vs. RP: 15, p<0.0001), compared to the retropubic group. Whether the difference in nodal yield is clinically significant is not clear. Pelvic lymphadenectomy in high-risk patients using the robotic approach should not be avoided. (82)
A prospective cohort study examined morbidity, peri-operative and oncological factors for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. There was no age difference between the two groups. Results showed increased mean operation time for the robotic group (RB: 210, SD: 41.3 vs. RP: 163, SD: 163, SD: 29 min, p<0.001), whereas mean estimated blood loss (RB: 151, SD: 96.5 vs. RP: 707, SD: 415.3 ml, p<0.001), number requiring blood transfusions (RB: 0 vs. RP: 4, p=0.03), in-hospital narcotic use (RB: 32, SD: 14.8 vs. RP: 52, SD: 23.4 mg, p=0.001), mean length of hospital stay (RB: 1.2, SD: 0.8 vs. RP: 1.3, SD: 1 days, p=0.049), and proportion of complications (RB: 21.4 vs. RP: 41.6%, p=0.002) was decreased for the robotic group compared to the retropubic group, although only slightly for length of hospital stay. There was no difference for the proportion of positive surgical margins between the groups. (83)
A comparative case-case prospective study examined urinary and sexual function for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy and laparoscopic radical prostatectomy. There were no age and clinical stage differences between the three groups. The retropubic surgeries were performed by one of three surgeons, the laparoscopic surgeries were performed by one of two surgeons and the robotic surgeries were performed by one of two surgeons, the same potential surgeons as for laparoscopy. Urinary and sexual functions were assessed using the University of California Los Angeles, Prostate Cancer Index (UCLA PCI). (84) Results showed a more favourable profile for the retropubic and robotic groups at 1 month (RB: 33 vs. LP: 25 vs. RP: 38%, p=0.03), as a percent of baseline. A more favourable profile for robotic surgery was shown at 3 months for sexual function (RB: 35 vs. LP: 21 vs. RP: 24%, p=0.03), as a percent of baseline. (85)
A comparative case-case retrospective medical record review study examined hospital length of stay for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. There were no differences for age and stage between groups. There were a decreased number of obese patients in the robotic group (RB: 7.1 vs. 16.3%, p=?). Results showed a decreased median number of hospital nights in the robotic group (RB: 1 vs. RP: 3). (86)
A prospective cohort study examined morbidity and peri-operative factors for robotically-assisted radical prostatectomy compared to laparoscopic radical prostatectomy. The laparoscopic surgeries were performed by two experienced surgeons and the robotic surgeries by one surgeon and included the learning curve. There were no differences between groups for age, body mass index and clinical stage. Results showed a more favourable profile for robotic surgery and less blood loss (RB: 469, SD: 380 vs. LP: 889, SD: 531 ml, p<0.01), and a decreased operation time (RB: 145.6, SD: 34.4 vs. 164.7, SD: 49.1 minutes, p<0.01). There were no differences between groups for the proportion of transfusions, length of hospital stay, bladder catheterization, and total number of complications. Operation time was examined in detail. (87)
A prospective cohort study examined morbidity and peri-operative factors for robotically-assisted radical prostatectomy compared to laparoscopic radical prostatectomy. There were no differences between groups for age. Results showed a more favourable profile for robotic surgery and a decreased catheter duration (RB: 7.6, SD: 2.8 vs. LP: 8.8, SD: 3.1 days, p<0.05). There was an approximately 3-fold difference between groups for the percent of positive surgical margins favouring robotic surgery (RB: 30.0 vs. LP: 11.8%, p=?). There was less of an apparent difference for overall positive surgical margins between groups, with a tendency to favour robotic surgery and a decreased overall proportion of positive surgical margins (RB: 41.2 vs. LP: 50.0%, p=?). It appeared that there were a decreased number of complications in the robotic group (RB: 2.9 vs. LP: 17.6%, p=?). There were no differences between groups for operation time, blood loss, hospital length of stay, and urinary continence at 1 month. (88)
Complications
A prospective cohort study examined complications for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Surgical method was determined by the physician. Nine surgeons performed the open surgery and six of these surgeons performed the robotic surgery. Results showed an increased proportion of blood transfusions in the radical retropubic prostatectomy group compared to the robotic surgery group (RB: 23 vs. RP: 4.6%, p<0.05). There were no differences for anastomotic leakage between the surgery groups. When detailed categorization of complications was examined using the Clavien grading system, there was an increased proportion of grade I, II, IIIb, and IV level complications for the radical retropubic prostatectomy surgery group compared to the robotic surgery group (p<0.001 for all). For functional outcomes, there was an increased proportion of surgeries for urinary incontinence IIIb in the radical retropubic prostatectomy group compared to the robotic surgery group (RB: 2.2 vs. RP: 0.5%, p<0.001). Prostate volume and length of follow-up did not contribute to the results. (89)
Lymph Node Recovery
A comparative case-case retrospective medical record review study examined standard lymphadenectomy for robotically-assisted radical prostatectomy compared to open radical prostatectomy. A third group of 43 patients underwent extended lymphadenectomy. There was no difference in the mean number of lymph nodes obtained per patient for robotic surgery compared to open surgery using standard lymphadenectomy (RB: 8.1 vs. RP: 7.6, p=0.839). The open surgery group with extended lymphadenectomy (OSE) showed an increased number of mean lymph nodes obtained compared to the standard approach (RB: 8.1 vs. RP: 7.6 vs. OSE: 14.8, p=0.001). (90)
A comparative case-case retrospective study examined pelvic lymph node yield from pathology records for robotically-assisted radical prostatectomy compared to retropubic radical prostatectomy. Two surgeons performed the robotic surgeries and two surgeons performed the retropubic surgeries. A standard lymphadenectomy template was used. There were no differences in age and Gleason scores between the two surgery groups. The results showed a lower mean number of lymph nodes obtained in the robotic group compared to the retropubic group (RB: 3.3 vs. RP: 7.3, p<0.001). Pelvic lymph node dissection occurred at the same time as the prostatectomy, which may have caused technical limitations for robotic surgery and reduced lymph node yield due to the placement of the robotic arms to maximize prostate dissection and not access to the pelvic lymph nodes. (91)
Positive Surgical Margins
A comparative case-case retrospective medical record review study examined the proportion of surgical margins for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Procedures were performed by a single surgeon. Robotic surgeries reflect the initial learning experience. Analysis was restricted to low and intermediate risk patients. There was no age difference between the groups (nRB=94, nRP=98). Positive surgical margins were assessed in detail and processed according to a standard protocol. There was no difference in the proportion of positive surgical margins between the surgery groups (RB: 13 vs. RP: 14%, p=0.5) for a reduced sample size (nRB=88, nRP=84). For stage T2 tumours, there was also no difference between the surgery groups (RB: 10 vs. RP: 15%, p=0.5). (92)
A comparative case-case prospective study examined the proportion of positive surgical margins for robotically-assisted radical prostatectomy compared to laparoscopic radical prostatectomy. Robotic surgeries represent the initial experience of the robotics program and the laparoscopic surgeries represent the final experience of the institution. Patients elected treatment options after consultation. There were no differences for age, estimated blood loss and prostate size between the surgical groups (p>0.05). Individuals in the robotic group were slightly larger sized than those in the laparoscopic group (Mean body mass index, RB: 28.4 vs. LP: 26.8 kg/m2, p=0.036). A majority of patients had localized disease (pT2 of ∼85%). Results showed a lower proportion of positive surgical margins for the robotic group compared to the laparoscopic group (RB: 6 vs. LP: 18%, p=0.032). Differences were absent when examined by stage of disease. The technical advantages of robotic surgery are likely the reason for the favourable oncological outcome. (93)
A comparative case-case retrospective medical record review study examined the proportion of surgical margins for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Procedures were performed by the same single experienced open surgeon. Robotic surgeries reflect the learning curve. Groups were comparable with respect to age and clinical stage. Positive surgical margin was defined and specimens were processed according to a given technique. Results showed that there were an increased proportion of patients with positive surgical margins in the radical retropubic prostatectomy surgery group compared to the robotic surgery group (RB: 22 vs. RP: 36%, p=0.007). This trend held when examined by pathological stage of disease. The strength of this study was that a more favourable profile for the robotic surgery group with respect to lower positive surgical margins was attained during the learning curve of a robotics program. (94)
A comparative case-case prospective study examined the proportion of surgical margins for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. Two surgeons performed the robotic surgeries, beyond their learning curve. There were no differences in patient characteristics (data not shown). Results showed no difference in the overall proportion of positive surgical margins between groups and no difference in the proportion of positive surgical margins for stage pT2 disease. The authors suggest that although the groups being compared may be similar on known characteristics, it is the unknown characteristics that render the groups being compared as less than ideal. (95)
A comparative case-case prospective study examined margin positivity for robotically-assisted radical prostatectomy compared to radical retropubic prostatectomy. One senior surgeon performed both types of surgeries. There was no difference in age and perhaps some differences in pathological stage of disease although p-values were not given. Results showed a more favourable profile for robotic surgery and a decreased overall proportion of positive surgical margins (RB: 14 vs. RP: 27, p=0.05). Among pT2 disease, there appeared to be a similar proportion of positive surgical margins between the two groups (pT2a, RB: 1 vs. RP: 1 and pT2b, RB: 12 vs. RP: 9). (96)
Meta-Analysis: Prostate Cancer
A meta-analysis was performed to examine the research questions for prostate cancer. Studies with data in a format suitable for meta-analysis are shown below. For positive surgical margins, primary data shown in Ficarra et al. (2009) (18) were incorporated with the studies identified from the systematic search, and limited to localized, stage II prostate cancer. For transfusions, primary data shown in Ficarra et al. (2009) (18) were incorporated with the studies identified from the systematic search and limited to prospective studies when examining retropubic surgery as the comparison group. For urinary continence, studies included were limited to those with 12 months of follow-up. (34) For anastomosis and laparoscopy, data from Ficarra et al. (2009) (18) were reported since there was only one study with useable data. A meta-analysis of studies on lymph node recovery for robotic surgery compared to laparoscopy was excluded since there were no studies with useable data and of the three studies comparing robotic surgery to retropubic surgery, no studies had data in a suitable format.
Studies were grouped according to level of surgeon skill or experience as indicated in the original paper (i.e. experience with robotics (experience), or initial experience with robotic surgery (learning curve)). Specific details from the original papers were used to categorize the surgeons in the studies as experienced or part of the learning curve, and are footnoted below each forest plot. A cut point for determining what constitutes the learning curve was not examined in this report. Approximately 25 cases are suggested for which minimal competence is achieved, whereas to perform a standard robotic-assisted radical prostatectomy approximately 50-120 cases are suggested for a trained laparoscopic surgeon. The learning curve for laparoscopically-trained and laparoscopically-naïve surgeons is suggested to be similar, though prior experience with open surgery and extent of training may influence the ability of the surgeon to achieve adequate peri-operative, functional and oncological outcomes. The learning curve should incorporate the surgeon’s ability to achieve adequate peri-operative, functional and oncological outcomes in addition to being able to safely perform the robotic surgery with reduced operation times. (97)
For the comparisons of interest (e.g. robotic surgery vs. retropubic surgery and robotic surgery vs. laparoscopy), groups were comparable for age and stage of disease, unless otherwise indicated based on review of the original papers. For particular studies, when the stage of disease was different between the two surgical groups being compared then these studies were examined separately within the level of surgeon skill or experience (e.g. learning curve, stage difference). This was done as a subgroup analysis to examine the extent of bias when examining the effect of the level of surgeon skill or experience on the outcomes for the surgical comparisons of interest. A tumour with more advanced stage may require more extensive surgery, and this may lead to an unfavourable surgical profile (Personal communication, expert, July 22nd, 2010). Pathological stage of disease was reviewed in the original papers. When pathological stage of disease was not shown, then the clinical stage of disease was reviewed. For stage of disease, only differences between localized disease and disease that has spread was reviewed and reported. In the absence of stage information, the pathological Gleason score was reviewed and reported. Six studies had information on clinical stage only (76;81;87;89;94;98-101), and three studies had information on pathological Gleason score (77;83;102). Other subgroup analyses were to analyze the data by sample size when the heterogeneity of the summary estimates was high, and body mass index. Body mass index was not reported in 12 studies (75-77;80;83;88;89;92;94-96;102-105), with the remaining studies except one study showing no differences in body mass index between surgical groups. Only one study showed a higher body mass index in the robotic surgery group compared to the laparoscopy group, although the maximum value was higher in the laparoscopy group. (93) There were studies included in the meta-analysis that were part of the published systematic review (18), and identified in the systematic search. Their study designs are summarized in Table 6. There were a total of 26 studies included in the meta-analysis.
1. Positive Surgical Margins (number of events, localized prostate cancer – stage pT2)
a) Robotic vs. Retropubic
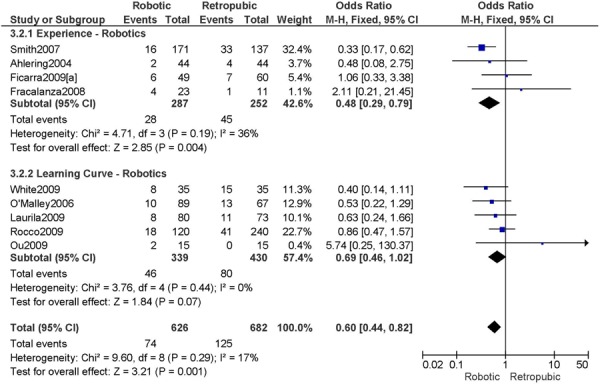
Experience was variably defined as the last 200 of 1,238 robotic surgeries (106); after having completed 45 robotic surgeries (107); having had completed at least 50 robotic surgeries (79); experience of more than 50 robotic surgeries. (108;108)
Learning curve was variably defined as the early experience, excluding the first 20 robotic surgeries (92); as initial cases (96); as a laparoscopically naïve/no previous experience with pure laparoscopic operation (75); initiation of a robotics program/first 50 robotic surgeries (94); initial experience during the learning curve/30 initial robotic surgeries. (81)
Age difference shown for Ficarra 2009 (median, interquartile range [IQR], RB: 61, IQR: 57-67 vs. RP: 65, IQR: 61-69, p<0.001); Francalanza 2008 (RB: 62, IQR: 56-68 vs. RP: 68.5, IQR: 59-71 years, p=0.009); Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05).
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
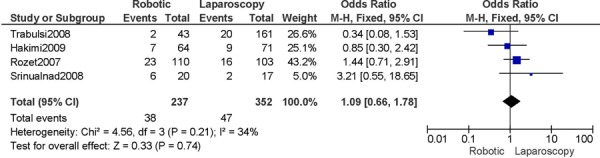
2. Erectile Dysfunction (number of events)
a) Robotic vs. Retropubic
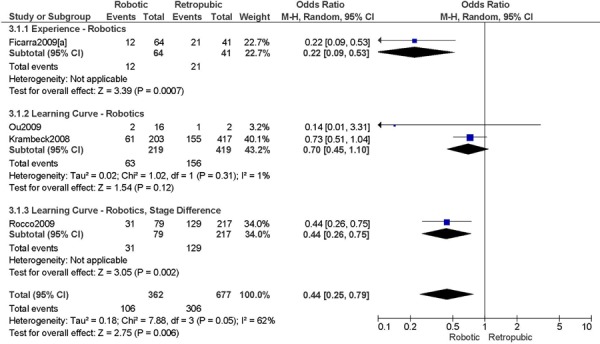
Experience was variably defined as having had completed at least 50 robotic surgeries. (79)
Learning curve was variably defined as the initial experience during the learning curve/30 initial robotic surgeries (81); as laparoscopically naïve/no previous experience with pure laparoscopic operation (75); initial experience. (103)
Age difference shown for Ficarra 2009 (RB: 61, IQR: 57-67 vs. RP: 65, IQR: 61-69, p<0.001) and Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05). Stage difference shown for Rocco 2009, favouring pT3/pT4 in RP, p=0.041.
Data collection using a third-party interviewer (Rocco 2009).
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
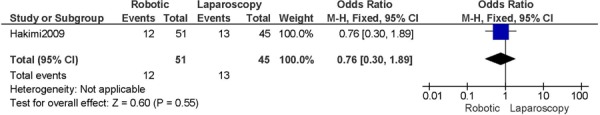
Study used surgeons that were experienced in laparoscopy.
Learning curve was variably defined as learning curve with robotic surgery. (80)
3. Urinary Incontinence (number of events, 12 months of follow-up)
a) Robotic vs. Retropubic
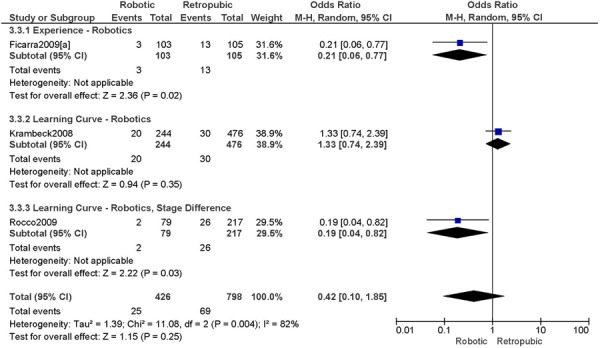
Experience was variably defined as having had completed at least 50 robotic surgeries. (79)
Learning curve was variably defined as the initial experience (103); as laparoscopically naïve/no previous experience with pure laparoscopic operation. (75)
Age difference shown for Ficarra 2009 (RB: 61, IQR: 57-67 vs. RP: 65, IQR: 61-69, p<0.001). Stage difference shown for Rocco 2009, favouring pT3/pT4 in RP, p=0.041.
Ou et al. (2009) (81) was excluded since the 12 month urinary continence data was not clear.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
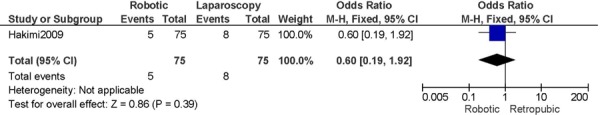
Study used surgeons that were experienced in laparoscopy.
Learning curve was variably defined as learning curve with robotic surgery. (80)
4. Length of Hospitalization (days)
a) Robotic vs. Retropubic – Learning Curve - Robotics
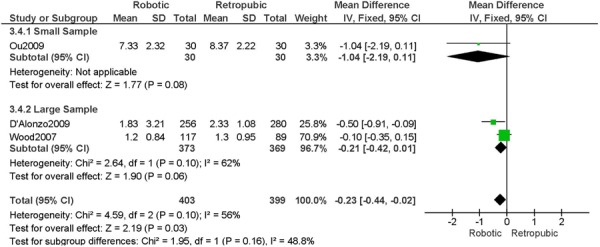
Learning curve was variably defined as the initial experience during the learning curve/30 initial robotic surgeries (81); inception program (77); no robotic experience. (83)
Age difference shown for Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05). Stage information not known for D’Alonzo 2009 and Wood 2007. Slight difference shown for Gleason score (RB: 6.5, SD: 0.7 vs. RP: 6.7, SD: 0.9, p=0.03) in D’Alonzo 2009.
No difference for Gleason score in Wood 2007.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
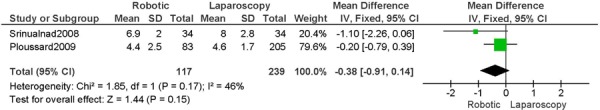
5. Blood Loss (ml)
a) Robotic vs. Retropubic – Learning Curve - Robotics
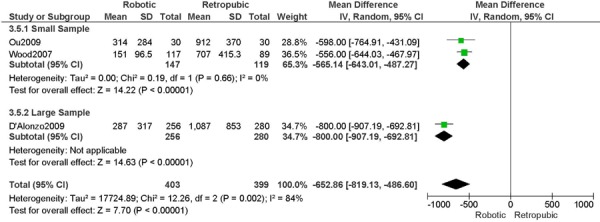
Age difference shown for Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05). Stage information not known for D’Alonzo 2009. Slight difference shown for Gleason score (RB: 6.5, SD: 0.7 vs. RP: 6.7, SD: 0.9, p=0.03) in D’Alonzo 2009.
No difference for Gleason score in Wood 2007.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
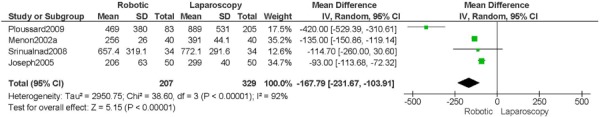
One study had no previous laparoscopic experience. (101) All other studies were experienced in laparoscopy.
Learning curve was variably defined as learning curve (87); initial experience with robotic technique (101); early experience (88); transition to robotic surgery. (104)
Mean difference: 19.45, 95% CI: -112.53, 106.73, p=0.96 (taken from Ficarra et al., 2009). (18)
6. Transfusions (number of events)
a) Robotic vs. Retropubic (prospective studies only)
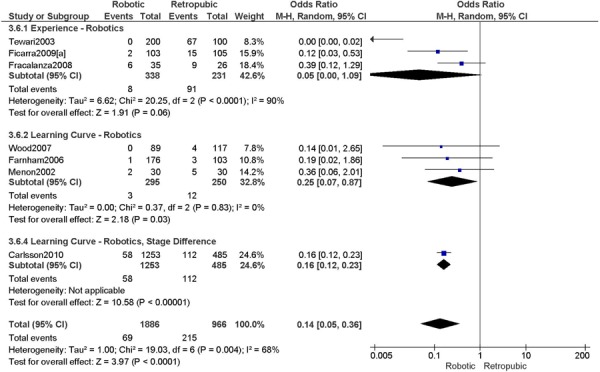
Experience was variably defined as having had performed 400 robotic surgeries between 2000 and 2002 (109); having had completed at least 50 robotic surgeries (79); experience of more than 50 robotic surgeries. (108)
Learning curve was variably defined as no robotic experience (83); the initial experience (102); initial patients/mentored (100); includes the initial learning curve. (89)
Age difference shown for Francalanza 2008 (RB: 62, IQR: 56-68 vs. RP: 68.5, IQR: 59-71 years, p=0.009) and Ficarra 2009 (RB: 61, IQR: 57-67 vs. RP: 65, IQR: 61-69, p<0.001). For Carlsson 2010, there were age (RB: 62, IQR: 35-78 vs. RP: 63, IQR: 47-77, p<0.001) and stage differences, favouring cT3 tumours in RP, p<0.001.
For Tewari 2003, the OR estimate is 0.001.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
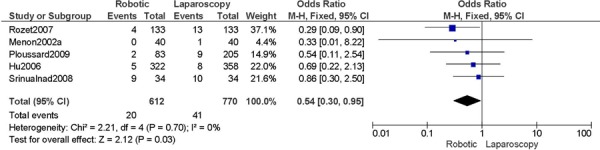
7. Operation Time (hours)
a) Robotic vs. Retropubic
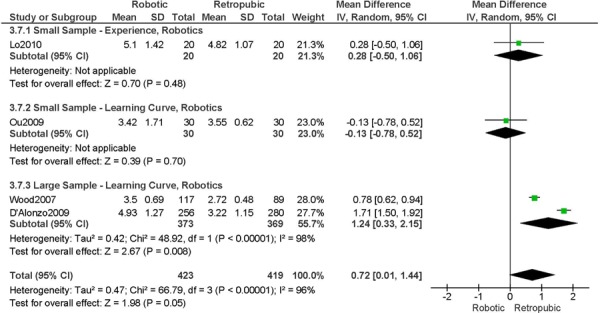
Experience was variably defined as prior experience. (76)
Learning curve was variably defined as the initial experience during the learning curve/30 initial robotic surgeries (81); no robotic experience (83); inception program. (77)
Age difference shown for Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05). Stage information not known for D’Alonzo 2009. Slight difference shown for Gleason score (RB: 6.5, SD: 0.7 vs. RP: 6.7, SD: 0.9, p=0.03) in D’Alonzo 2009.
No difference for Gleason score in Wood 2007.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
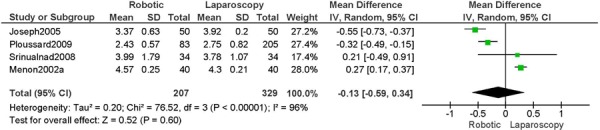
One study had no previous laparoscopic experience. (101) All other studies were experienced in laparoscopy.
Learning curve was variably defined as initial experience with robotic technique (101); learning curve (87); early experience (88); transition to robotic surgery. (104)
Mean difference: -19.39, 95% CI: -49.34, 88.13, p=0.58 (taken from Ficarra et al., 2009). (18)
8. Total Number of Complications (number of events)
a) Robotic vs. Retropubic
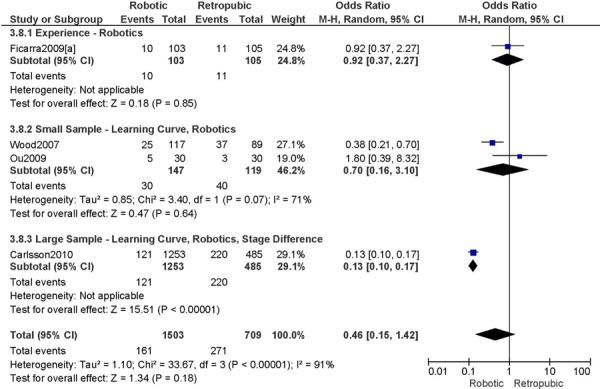
Post-operative complications (83); Peri-operative complications, not clear (81); Peri-operative, early post-operative (<30 days) and late postoperative (>30 days-15 months) complications (89); peri-operative complications. (79)
Experience was variably defined as having had completed at least 50 robotic surgeries. (79)
Learning curve was variably defined as no robotic experience (83); the initial experience during the learning curve/30 initial robotic surgeries (81); the initial learning curve. (89)
Age difference shown for Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05) and Ficarra 2009 (RB: 61, IQR: 57-67 vs. RP: 65, IQR: 61-69, p<0.001). Carlsson 2010, there were age (RB: 62, IQR: 35-78 vs. RP: 63, IQR: 47-77, p<0.001) and stage differences, favouring cT3 tumours in RP, p<0.001.
One death in each surgical group in Carlsson 2010.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
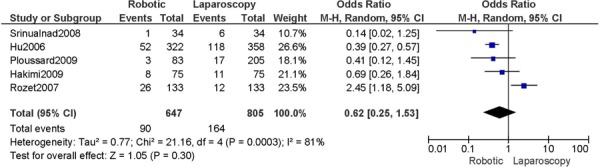
Peri-operative and post-operative complications (88); Intra-operative and peri-operative complications (99); short-term post-operative complications (87); peri-operative and post-operative complications (80); overall complications. (98)
One study was experienced in laparoscopy (88); one study adopted the laparoscopic technique (99); the remaining studies were also experienced in laparoscopy. One study was part of a high volume laparoscopic referral centre. (98)
Learning curve was variably defined as early experience (88); acquiring a robot (99); learning curve (87); learning curve with robotic surgery (80); initial robotic surgeries. (98)
No deaths in either group in Rozet 2007.
No conversions to open surgery in Srinualnad 2008 and Ploussard 2009.
9. Post-Operative Pain (morphine equivalent, mg)
a) Robotic vs. Retropubic – Learning Curve - Robotics
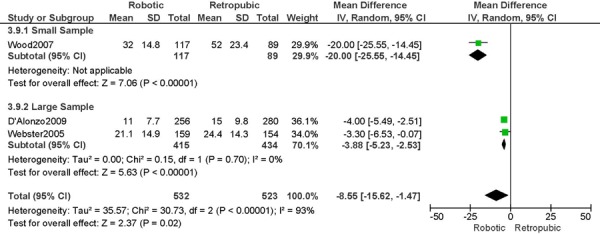
Learning curve was variably defined as no robotic experience (83); inception program (77); initial experience. (105)
Stage information not known for D’Alonzo 2009 and Webster 2005. Slight difference shown for Gleason score (RB: 6.5, SD: 0.7 vs. RP: 6.7, SD: 0.9, p=0.03) in D’Alonzo 2009. Inclusion criteria of patients having clinically localized prostate cancer in Webster 2005.
No difference for Gleason score in Wood 2007.
b) Robotic vs. Laparoscopy (no studies)
10. Catheterization Duration (days)
a) Robotic vs. Retropubic – Learning Curve - Robotics
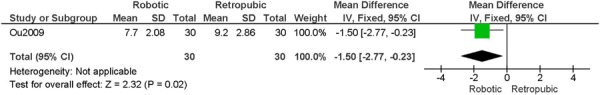
Learning curve was variably defined as the initial experience during the learning curve/30 initial robotic surgeries. (81)
Age difference shown for Ou 2009 (RB: 70, SD: 6.1 vs. RP: 67.3, SD: 6.2, p<0.05).
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
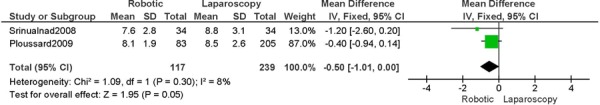
11. Anastomotic Stricture (number of events)
a) Robotic vs. Retropubic – Learning Curve - Robotics
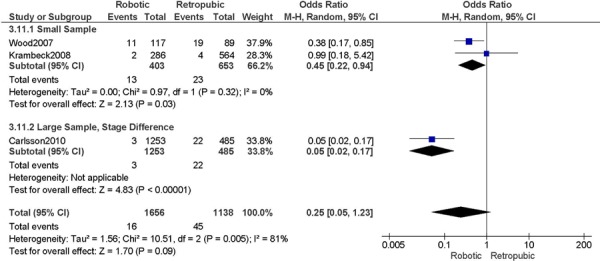
Learning curve was variably defined as no robotic experience (83); the initial experience (103); the initial learning curve. (89)
Refers to post-operative complications (83); early peri-operative complications, within the first month after surgery (103); late post-operative complications (>30 days-15 months) (89).
Refers to early urinary retention (83); bladder neck contracture in addition to stricture (103); bladder neck contracture alone. (89)
For Carlsson 2010, there were age (RB: 62, IQR: 35-78 vs. RP: 63, IQR: 47-77, p<0.001) and stage differences, favouring cT3 tumours in RP, p<0.001.
b) Robotic vs. Laparoscopy – Learning Curve - Robotics
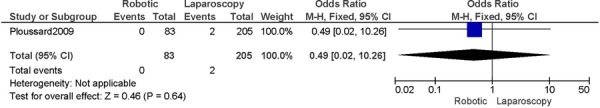
Study used surgeons that were experienced in laparoscopy.
Learning curve was variably defined as the learning curve. (87)
Based upon anastomotic leakage.
Relative risk: 1.42, 95% CI: 0.40, 5.06, p=0.59 (taken from Ficarra et al., 2009). (18)
[Based upon two studies: bladder neck contracture up to 30 days after surgery (104); unknown. (99)]
Summary of Results of the Meta-Analysis
Results from the meta-analysis show a favourable profile for robotic surgery compared to retropubic surgery with respect to positive surgical margins, where the presence of positive surgical margins in localized prostate cancer was less likely for robotic surgery (OR: 0.60, 95% CI: 0.44, 0.82, p=0.001). This effect was more marked for institutions experienced with robotic surgery (OR: 0.48, 95% CI: 0.29, 0.79, p=0.004). There was no difference between surgical procedures during the learning curve. For erectile dysfunction, there was a 56% reduced risk of erectile dysfunction for robotic surgery compared to retropubic surgery (OR: 0.44, 95% CI: 0.25, 0.79, p=0.006), an effect which was more marked for institutions experienced with robotic surgery (OR: 0.22, 95% CI: 0.09, 0.53, p=0.0007). There was no difference during the learning curve, except where stage difference between surgical groups may account for a beneficial effect of robotic surgery. Overall, there was no difference between robotic surgery and retropubic surgery for urinary incontinence however when stratified by surgeon experience, there was a 0.21 times decreased risk for urinary incontinence for robotic surgery compared to retropubic surgery (OR: 0.21, 95% CI: 0.06, 0.77, p=0.02). There was also no difference during the learning curve, except where stage difference between surgical groups may account for a beneficial effect of robotic surgery. Robotic surgery was also favoured with respect to a shorter length of hospitalization (MD: -0.23, 95% CI: -0.44, -0.02 days, p=0.03) and less blood loss (MD: -652.86, 95% CI: -819.13, -486.60 ml, p<0.00001), with heterogeneity of the pooled estimate reduced by stratifying by sample size. Overall, robotic surgery was less likely to require a transfusion (OR: 0.14, 95% CI: 0.05, 0.36, p<0.0001), a trend which was shown among studies during the learning curve of robotic surgery (OR: 0.25, 95% CI: 0.07, 0.87, p=0.03). Stage difference between surgical groups contributed to a further reduced effect during the learning curve (OR: 0.16, 95% CI: 0.12, 0.23, p<0.00001), whereas there was no difference between surgical procedures for institutions experienced with robotic surgery. In contrast, robotic surgery did not show a favourable profile compared to retropubic surgery with respect to operation time, showing longer operation times with borderline significance (MD: 0.72, 95% CI: 0.01, 1.44 hours, p=0.05). This effect was increased during the learning curve for large samples (MD: 1.24, 95% CI: 0.33, 2.15 hours, p=0.008). Overall, there was no difference between robotic surgery and retropubic surgery when examined for total number of complications (OR: 0.46, 95% CI: 0.15, 1.42, p=0.18). Stage difference between surgical procedures accounted for a favourable effect of robotic surgery during the learning curve. For robotic surgery, there was decreased morphine requirements (MD: -8.55, 95% CI: -15.62, -1.47 mg, p=0.02) and a shorter catheter duration compared to retropubic surgery (MD: -1.50, 95% CI: -2.77, -0.23 days, p=0.02). No differences were shown for anastomotic stricture (OR: 0.25, 95% CI: 0.05, 1.23, p=0.09) however in small studies, there is some suggestion of a favourable profile for robotic surgery (OR: 0.45, 95% CI: 0.22, 0.94, p=0.03). The beneficial effect shown in the large study may be accounted for by stage difference. When the summary estimate was significant favouring robotic surgery, such as for positive surgical margins and erectile dysfunction, there was also a significant effect among experienced surgeons. There were no clear effects of age. When an effect was shown for studies during the learning curve favouring robotic surgery, stage differences between the surgical populations being compared may have played a role (e.g. urinary incontinence, complications). There were no studies with suitable data for lymph node recovery.
Where stage differences were shown, there were 37% of pT3/pT4 tumours in the retropubic group compared to 27% of pT3/pT4 tumours in the robotic group (erectile dysfunction, urinary incontinence) (75), and 10.4% of cT3 tumours in the retropubic group compared to 3.8% of cT3 tumours in the robotic group (transfusions, total number of complications, anastomotic stricture). (89) Overall, when stage differences appeared to have an effect on the estimate, there was an approximately 5-10% difference between the proportions of advanced tumours in the surgical groups being compared.
When robotic surgery is compared to laparoscopy surgery, a more favourable profile is shown for blood loss (MD: -167.79, 95% CI: -231.67, -103.91 ml, p<0.00001) and the number requiring a transfusion, with an approximately 50% reduced risk (OR: 0.54, 95% CI: 0.30, 0.95, p=0.03). No differences were shown for positive surgical margins (OR: 1.09, 95% CI: 0.66, 1.78, p=0.74), erectile dysfunction (OR: 0.76, 95% CI: 0.30, 1.89, p=0.55), urinary incontinence (OR: 0.60, 95% CI: 0.19, 1.92, p=0.39), operation time (MD: -0.13, 95% CI: -0.59, 0.34 hours, p=0.60), and total number of complications (OR: 0.62, 95% CI: 0.25, 1.53, p=0.30). All of the studies analyzed in the meta-analysis included surgeons that were inexperienced with robotic surgery, e.g. learning curve. Data taken from Ficarra et al., 2009 (18) suggest no difference between surgical procedures for anastomotic stricture. One study individually analyzed also showed no difference for anastomotic stricture. There was no difference between surgical procedures for length of hospitalization and catheterization duration, although the effect was borderline for catheterization duration (MD: -0.50, 95% CI: -1.01, 0, p=0.05). There were no studies on post-operative pain or lymph node recovery.
Summary of Evidence-Based Analysis
Endometrial and Cervical Cancers
The evaluation of robotically-assisted hysterectomy for endometrial and cervical cancers from previous health technology assessments showed that there had been limited work to date and concluded there is no evidence to support that robotic surgery is superior to laparoscopy or laparotomy.
A qualitative assessment of the evidence was performed to compare robotic surgery to abdominal surgery and separately, robotic surgery compared to laparoscopy. For robotic surgery vs. abdominal surgery considering endometrial and cervical cancer studies combined, in 11 of 11 studies, robotic surgery was associated with a decreased length of hospitalization indicating superior clinical effectiveness with respect to reduced morbidity. Patients were comparable with respect to body mass index in a majority of studies, a factor that may influence the success of hysterectomy surgery by either approach. Differences in body mass index may also indicate a selection bias, where in two studies (43;46), patients in the open surgery group had a higher body mass index than the robotic group suggesting a slightly healthier patient population in the robotic group (RB: overweight vs. OS: obese). Given the minimally invasive nature of robotic surgery and that robotic surgeries were performed by experienced surgeons, except in 5 of 11 studies (43-45;50;65), decreased morbidity in terms of reduced length of hospitalization was expected compared to open surgery. A similar explanation is consistent for the benefits of robotic surgery compared to open surgery with respect to decreased blood loss. The evidence for the benefits of a reduced number of complications for robotic surgery compared to open surgery was shown in 3 of 10 studies, with the remaining studies showing no difference, a trend due in part to the lack of a comprehensive surgical outcome assessment. (110) For example, in the study by Jung et al. (2009) (63) and Boggess et al. (2008) (49), a large proportion of overall complications were shown for open surgery due to a number of wound dehiscence complications not accounted for in some of the other studies. (53;56;65) This trend has also been identified in a recent systematic review. (57) Improved assessment of the safety of robotic surgery would be facilitated with standardized complication reporting. The evidence for the benefits of robotic surgery was weak for operation time and lymph node recovery.
For robotic surgery vs. laparoscopy and considering endometrial and cervical cancer studies combined, a majority of the evidence indicated no difference between the two minimally invasive procedures. There may be some advantage of robotic surgery with respect to a shorter length of hospitalization, reduced operation time and blood loss, and a fewer number of conversions to laparotomy. Future studies using advanced analysis may uncover more subtle differences between procedures. For example, multivariable analysis would help clarify the results when comparing two minimally invasive procedures, in that, the number of conversions to laparotomy may be accounted for at the patient-level when examining length of hospitalization, operation time or blood loss. Small differences in effect may be missed using aggregate-level data when comparing two minimally invasive procedures.
Overall, the benefits of robotic surgery were highlighted and the results were more consistent when open surgery was considered as the comparison group and not laparoscopy perhaps owing to the substantial procedural differences between robotic surgery and open surgery, and this was demonstrated using group-level data and analysis.
The major limitations of the study designs are selection bias and temporal bias. All of the studies were observational studies. Therefore, none of the studies randomized patients to one of the surgical procedures. In the absence of randomization, observational studies may randomly select patients. However, for rare diseases and surgeries such as the ones under assessment, random sampling may not be feasible due to too few patients and the need for lengthy recruitment periods. To minimize selection bias, where some patients enter the study or undergo a procedure and other patients do not that is not due to random selection, a number of studies included consecutive cases. The group of patients being compared between surgical procedures may be imbalanced on a number of factors that may affect the outcomes. For the most part, surgical options were offered to patients therefore patients self-selected themselves to one of the surgical procedures. Healthier patients may choose robotically-assisted surgery and therefore study results may show equivalent or better outcomes compared to open surgery and laparoscopy owing to favourable patient characteristics and less complicated surgeries, given experienced open or laparoscopic surgeons. Although selection bias cannot be rule out in the absence of random sampling, a majority of studies examined age and body mass index and showed that groups were comparable on at least body mass index, a potentially major selection factor. Differences in pathology-related factors between surgical groups may have occurred and contributed to differences in results, which may have been known or unknown to patients. For temporal bias, surgical procedures may only be available at a given time point, such as before or after the introduction of a robotics program at an institution. Any factors that may be different between the two time periods may have contributed to differences in results, such as the patients that may have been included in the studies, operation room factors or health care system factors.
The outcomes were not always defined in all studies, however measurement error is likely minimal due to the nature of the outcomes (e.g. time, millilitres, count data etc.), except for the measurement of complications as mentioned above. What constitutes adequate lymphadenectomy is not yet clear for endometrial cancer, with patient, surgical and pathological variations contributing to variation. (111;112) A factor that may influence the length of hospitalization is the health care system however a shorter length of hospitalization and reduced blood loss was consistently favoured for robotic surgery compared to open surgery across different health care systems (e.g. USA, Europe). A summary of the quality of the evidence is shown in Appendix 2 (GRADE Evidence), in which a majority of the studies were graded as low quality of evidence.
Results from the meta-analysis confirmed the results from the qualitative assessment, although not all studies had usable data. The benefits of robotic surgery vs. abdominal surgery were shown for length of hospitalization, blood loss, and complications. The role of surgeon skill had little impact, except for operation time, where robotic surgeries performed during the learning curve had increased operation times compared to abdominal surgery. Patient characteristics had no impact. The benefits of robotic surgery vs. laparoscopy surgery were shown for length of hospitalization, blood loss, and conversions. The clinical significance of significant findings for length of hospitalization and blood loss is low. The role of surgeon skill had little impact, except for length of hospitalization, where experienced robotic surgeons showed a reduced length of hospitalization compared to laparoscopy. Where stage differences existed, they had an impact irrespective of outcome (length of hospitalization, lymph nodes). Overall, the beneficial effects of robotic surgery were shown despite the learning curve (e.g. conversions). An overall summary of the comparisons in the meta-analysis is shown in Table 7.
In summary, the results of this evidence-based analysis show that robotic surgery has a more favourable profile with respect to a reduced length of hospitalization and less blood loss compared to laparotomy for women undergoing any hysterectomy surgery for the surgical treatment and management of endometrial and cervical cancers. In light of the study design limitations, the benefits of a reduced length of hospitalization and less blood loss are consistent with the substantial differences in surgical procedures between robotic-assisted minimally invasive surgery and laparotomy. For robotic surgery compared to laparoscopy, the greatest benefit of robotic surgery was shown for the reduced number of conversions owing to the established technical difficulties of laparoscopy. Given that the comparison between robotic surgery and laparoscopy already involves two minimally invasive procedures, any additional benefit of robotic surgery would be considered substantial. An overall summary of the technology is shown in Table 8 and a summary of the quality of the evidence is shown in Appendix 2 (GRADE Evidence).
Prostate Cancer
The evaluation of robotically-assisted radical prostatectomy for prostate cancer from previous health technology assessments concluded that there is no evidence to support that robotic surgery is superior to laparoscopic radical prostatectomy or retropubic radical prostatectomy.
A qualitative assessment of the evidence was performed to compare robotic surgery to retropubic surgery and separately, robotic surgery compared to laparoscopy. Considering oncological outcome a priority, in 6 of 10 studies comparing robotic surgery to retropubic surgery for positive surgical margins, there was no difference in surgical procedures. In 3 of 10 studies, robotic surgery was more favourable with respect to a lower proportion of positive surgical margins, and in 1 of 8 studies, robotic surgery was less favourable. Factors which may affect the proportion of positive surgical margins include: 1) surgeon skill and experience, 2) stage of disease, with lower stage, less advanced tumours having a lower proportion of positive surgical margins, 3) whether stage migration to lower stage, less advanced tumours has occurred as a result of prostate cancer screening, and 4) variations in specimen sampling. (28) When the cancer is organ-confined as in stage II prostate cancer, any positive surgical margin is attributed to surgeon error during surgery. Surrounding organs complicate the accurate removal of the prostate during surgery. (113) Among the three studies with favourable results was the systematic review. (18;94;96) In the systematic review, when the data and study designs were examined more closely, an increased risk of positive surgical margins was consistently shown across study designs and when examined for localized prostate cancer in pooled analysis. Studies showing a favourable profile for robotic surgery compared to retropubic surgery included positive surgical margin status overall and when stage of disease was considered, and for which detailed pathology specimen handling was described. (94;114) Considering that only one study included experienced surgeons and accounted for stage of disease, favourable results supporting robotic surgery with respect to superior oncological outcomes may have occurred due to improved vision and dexterity of robotic surgery. Contributing to the mixed results are too few patients with localized disease for meaningful comparisons. (18;79;92;94)
For erectile function, in 5 of 5 studies, recovery of erectile function was more favourable for robotic surgery compared to retropubic surgery. Factors to consider when assessing erectile function include: 1) the definition of potency, 2) pre-operative erectile function, 3) patient age, 4) whether nerve-sparing procedures were used, 5) whether a validated questionnaire was used, 5) physician bias (34), and 6) the presence of co-morbidities. (115) Of the five studies that showed favourable results for robotic surgery, one was the systematic review. When the two individual studies in the systematic review were examined more closely, together with the four studies identified from the literature search, important methodological differences were identified. One study used a validated questionnaire, and although demographic characteristics were accounted for and whether a nerve-sparing procedure was performed, pre-operative erectile function was not assessed. (79) Conversely, in the other four studies, potency was measured using a non-validated tool (75;81;103;109), despite other study strengths. Erectile function/sexual function/potency was measured as: 1) the ability to achieve erections and having an erection strong enough for intercourse (109), 2) erections satisfactory for intercourse with or without the use of phosphodiesterase inhibitors (103), and 3) as the ability to have complete sexual intercourse with or without oral pharmacological agents. (75) It was not defined for one study. (81) The validated questionnaire addresses the multidimensional nature of sexual function. (116) Sildenafil (Viagara) use was reported in one study only. (109) Two studies collected data using a third-party interviewer. (75;109) One other study used a validated questionnaire (University of California Los Angeles, Prostate Cancer Index) and baseline information. (85) Increased accuracy afforded by robotic surgery likely contributed to a superior functional outcome with respect to erectile function, however only a limited number of heterogeneous studies have been conducted.
For urinary continence, among the four studies that showed a favourable profile for robotic surgery compared to retropubic surgery, each study used a different definition of urinary continence (34) including: 1) no pad use or only one safety pad (75); 2) the need for surgery for urinary continence using the Clavien grading system (89); 3) responses of ‘no leak’ or ‘leak about once a week or less often’ to the question of ‘how often do you leak urine’ according to the International Consultation of Incontinence Questionnaire – Urinary Continence short-form (79); and 4) pad-free status. (81) Among the remaining studies, including the two individual studies from the systematic review that showed no differences between surgical procedures, methodological reasons may include shortened length of follow-up (107), different length of follow-up between comparison groups (76), and different characterization of the outcome variable. (75;103) One additional study from the systematic review examined time to urinary continence and showed a reduced time to urinary continence for robotic surgery compared to retropubic surgery. (109) There were three studies that had 12 months of follow-up. (75;79;103). One other study used a validated questionnaire (University of California Los Angeles, Prostate Cancer Index) and baseline information but showed a less favourable profile for robotic surgery, which was not statistically tested. (85) Overall, the methodology is too variable to conclude whether there is a beneficial effect of robotic surgery for the functional outcome of urinary continence compared to retropubic surgery.
For morbidity and peri-operative factors, there appears to be robust evidence favouring robotically-assisted radical prostatectomy compared to retropubic radical prostatectomy for length of hospitalization (8/8 studies), blood loss (7/7 studies), transfusions (7/7 studies), and catheterization duration (5/5 studies) despite differences in health care system factors, and to a lesser extent, complications (3/5 studies), given different methods of reporting complications. There were three studies on post-operative pain and all three of them favoured robotic surgery with respect to a decreased amount of morphine required (3/3 studies). Less morphine requirements indicates less pain and less narcotic-related morbidity including impaired pulmonary function, decreased mental status and intestinal ileus. (105) There were too few studies with conflicting results for anastomotic stricture (3 studies) and lymph node recovery (3 studies) to suggest that one surgical method is more superior to another. The systematic review contributed 5 studies for length of hospitalization and blood loss, 6 studies for transfusions and complications, 3 studies for catheterization duration, 1 study for morphine use and anastomotic stricture, and no studies for lymph node recovery. (18) For operation time, there was a trend for increased operation time for robotic surgery compared to retropubic surgery. For operation time, there were no clear trends with respect to surgeon skill or experience, pathology factors or other peri-operative factors across studies. More advanced analyses which accounts for factors which may influence operation time may help to uncover consistent trends. The systematic review contributed 5 studies for operation time. (18)
For robotic surgery compared to laparoscopy, there were fewer studies with conflicting results. For positive surgical margins, two of three studies contributed by the systematic review showed a favourable profile for robotic surgery and decreased positive surgical margins. The one study that did not favour robotic surgery for positive surgical margins overall and for stage II cancer was performed at an institution highly proficient in pure laparoscopy surgery. (98) Though, none of the results were significant. Two additional individual studies identified from the systematic search showed conflicting results, however when stage II cancer was considered for both studies, they were consistent in showing no difference between the two surgical techniques likely owing to established laparoscopy programs. (80;93) One additional study favoured robotic surgery. (88) Urinary continence was difficult to assess. Some studies used a strict definition of continence (e.g. no leak and no pad use), had different lengths of follow-up (3 months vs. 3, 6, 12 months), and included experienced laparoscopic surgeons. (80;104) One study only assessed urinary continence after 1 month of follow-up. (88) One study used a validated questionnaire and assessed a number of components of urinary function, and favoured robotic surgery. (85) Erectile function was similarly difficult to assess since only three studies had been conducted and they were heterogeneous with respect to the definition of potency, the use and score of the IIEF, and whether potency was cross-classified with the use of a nerve-sparing operation, in addition to experienced laparoscopic surgeons. (80;104) An additional study used a validated questionnaire (85), however there was some suggestion of a more favourable profile for robotic surgery and erectile function.
For morbidity and peri-operative factors when comparing robotic surgery to laparoscopy, there were few studies with inconsistent results despite highly experienced laparoscopic surgeons and comparable surgical groups with respect to age and tumour stage (80;87;93;104), in addition to body mass index (87;101) and risk score. (98;99) In one study, there was a difference in body mass index, with the robotic group having a slightly higher body mass index. (93) The natural link between morbidity and peri-operative outcomes (97), and the inconsistency in the findings suggest that aggregate-level data analysis may not have been sufficient to uncover trends between two minimally invasive techniques. There were no studies on post-operative pain management and lymph node recovery.
There were three prospective cohort studies (79;83;89) identified from the systematic literature search, in addition to seven studies that were examined as part of the systematic review by Ficarra et al. (2009). (18) Although the prospective design is favoured with respect to establishing temporality compared to retrospective studies, the main limitation of the prospective studies included in this review is that patients self-select themselves to one of the surgical options. Therefore, imbalances may occur between surgical groups on factors that may affect the outcomes, therefore biasing the results. This may be most relevant for functional outcomes and morbidity factors (34), as well as pathology-related factors. (117) In contrast, the benefit of a randomized controlled trial is the random assignment of patients to one of the surgical procedures which ensures that the groups being compared are similar with respect to known and unknown factors that may affect the outcomes of interest. Despite that randomized controlled trials may enrol patient volunteers the comparison of the surgical interventions is valid due to the process of randomization. Therefore, the prospective studies in this review were not analyzed separately, except for transfusions. Results of both retrospective and prospective observational studies should be interpreted with caution. Other study design limitations include measurement error, limitations of sample size and analysis, as discussed above. The link between recovery of erectile function, urinary continence, and positive surgical margins (118) makes aggregate-level data difficult to interpret. A summary of the quality of the evidence is shown in Appendix 2 (GRADE Evidence).
Results from the meta-analysis confirmed the results from the qualitative assessment, although not all studies had usable data and the meta-analysis highlighted important differences when surgical experience was taken into account. The benefits of robotic surgery vs. retropubic surgery were shown for positive surgical margins (overall, experience), erectile dysfunction (overall, experience), urinary incontinence (experience), length of hospitalization (overall), blood loss (overall), transfusions (overall, learning), and post-operative pain management (overall). For catheter duration, there was one study that showed a beneficial effect of robotic surgery, despite the learning curve. For transfusions, it also appears there is a beneficial effect of robotic surgery despite the learning curve. The clinical significance of significant findings for length of hospitalization is low. The benefits of robotic surgery vs. laparoscopy surgery were fewer compared to when retropubic surgery was the comparison group and included blood loss and transfusions, as shown in the meta-analysis. The clinical significance of significant findings for blood loss is low. An overall summary of the comparisons in the meta-analysis is shown in Table 7.
In summary, the results of this evidence-based analysis show that robotic surgery has a more favourable profile with respect to important cancer control and functional outcomes compared to retropubic surgery, particularly when the robotic experience of the surgeon is taken into account. Given that the comparison between robotic surgery and laparoscopy already involves two minimally invasive procedures, any additional benefit of robotic surgery would be considered substantial. An overall summary of the technology is shown in Table 8 and a summary of the quality of the evidence is shown in Appendix 2 (GRADE Evidence).
Conclusion
-
Robotic use for gynecologic oncology compared to:
-
Laparotomy: benefits of robotic surgery in terms of shorter length of hospitalization and less blood loss. These results indicate clinical effectiveness in terms of reduced morbidity and safety, respectively, in the context of study design limitations.
The beneficial effect of robotic surgery was shown in pooled analysis for complications, owing to increased sample size.
More work is needed to clarify the role of complications in terms of safety, including improved study designs, analysis and measurement.
-
Laparoscopy: benefits of robotic surgery in terms of shorter length of hospitalization, less blood loss and fewer conversions to laparotomy likely owing to the technical difficulty of conventional laparoscopy, in the context of study design limitations.
Clinical significance of significant findings for length of hospitalizations and blood loss is low.
Fewer conversions to laparotomy indicate clinical effectiveness in terms of reduced morbidity.
-
-
Robotic use for urologic oncology, specifically prostate cancer, compared to:
-
Retropubic surgery: benefits of robotic surgery in terms of shorter length of hospitalization and less blood loss/fewer individuals requiring transfusions. These results indicate clinical effectiveness in terms of reduced morbidity and safety, respectively, in the context of study design limitations. There was a beneficial effect in terms of decreased positive surgical margins and erectile dysfunction. These results indicate clinical effectiveness in terms of improved cancer control and functional outcomes, respectively, in the context of study design limitations.
Surgeon skill had an impact on cancer control and functional outcomes.
The results for complications were inconsistent when measured as either total number of complications, pain management or anastomosis. There is some suggestion that robotic surgery is safe with respect to less post-operative pain management required compared to retropubic surgery, however improved study design and measurement of complications needs to be further addressed.
Clinical significance of significant findings for length of hospitalizations is low.
-
Laparoscopy: benefits of robotic surgery in terms of less blood loss and fewer individuals requiring transfusions likely owing to the technical difficulty of conventional laparoscopy, in the context of study design limitations.
Clinical significance of significant findings for blood loss is low.
The potential link between less blood loss, improved visualization and improved functional outcomes is an important consideration for use of robotics.
-
All studies included were observational in nature and therefore the results must be interpreted cautiously.
Existing Guidelines for the Da Vinci Surgical System
There are no widely accepted guidelines for the Da Vinci Surgical System.
A task force was established in 2006 by the U.S. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) and the Minimally Invasive Robotic Association (MIRA). (119) Four areas were addressed:
1) Training and credentialing: How should training for robotic surgery be accomplished? What is the appropriate process for credentialing robotic surgery?
2) Clinical applications of robots in surgery: What are the appropriate clinical applications for robotic surgery; has efficacy been demonstrated for these applications?
3) Risks of surgery and cost-benefit analysis: What are the physical risks of robotic surgery to the patient? What financial costs are involved in robotic surgery and are these costs justified?
4) Research: What are the important unanswered questions in robotic surgery? What direction should future research take?
In summary, the following conclusions were formulated in response to the four areas above:
1) Training and credentialing includes training, judgment about safety and mentored clinical experience.
2) As of June 2006, there is no level 1 evidence. Benefits and limitations are described, including difficulty with multi-quadrant surgery. Many surgical specialties may benefit from robotics including pediatric surgery, gynecology, general surgery, urology, thoracic surgery, and head and neck surgery. Main limitations to its clinical use include cost, training issues and lack of outcome data.
3) A cost-analysis is complex and includes team training, instrument set-up and maintenance costs as well as the cost of operating time, patient complications, length of hospital stay, or length of patient convalescence/recovery period compared to alternative technologies.
4) At the time of these guidelines, there was reported to be 1 commercially available general surgery robot. Details of the evolution or advancement of robotics and patient care, clinical outcomes and safety were described. The need for outcome registries for robotic surgery was emphasized.
Economic Analysis
Disclaimer: The Medical Advisory Secretariat uses a standardized costing method for its economic analyses of interventions. The main cost categories and the associated methods from the province’s perspective are as follows:
Hospital: Ontario Case Costing Initiative cost data are used for in-hospital stay, emergency visit and day procedure costs for the designated International Classification of Diseases (ICD) diagnosis codes and Canadian Classification of Health Interventions procedure codes. Adjustments may be required to reflect accuracy in estimated costs of the diagnoses and procedures under consideration. Due to the difficulties of estimating indirect costs in hospitals associated with a particular diagnosis or procedure, the secretariat normally defaults to considering direct treatment costs only.
Nonhospital: These include physician services costs obtained from the Ontario Schedule of Benefits, laboratory fees from the Ontario Schedule of Laboratory Fees, drug costs from the Ontario Drug Benefit Formulary, and device costs from the perspective of local health care institutions whenever possible or its manufacturer.
Discounting: For cost-effectiveness analyses, a discount rate of 5% is applied as recommended by economic guidelines.
Downstream costs: All numbers reported are based on assumptions on population trends (i.e. incidence, prevalence and mortality rates), time horizon, resource utilization, patient compliance, healthcare patterns, market trends (i.e. rates of intervention uptake or trends in current programs in place in the Province), and estimates on funding and prices. These may or may not be realized by the system or individual institutions and are often based on evidence from the medical literature, standard listing references and educated hypotheses from expert panels. In cases where a deviation from this standard is used, an explanation is offered as to the reasons, the assumptions, and the revised approach. The economic analysis represents an estimate only, based on the assumptions and costing methods that have been explicitly stated above. These estimates will change if different assumptions and costing methods are applied to the analysis.
Study Question
The objective of this project was to assess the economic impact of robotic-assisted laparoscopy (RAL) for endometrial, cervical and prostate cancers in the province of Ontario.
Economic Analysis Method
A budget impact analysis was undertaken to report direct costs associated with open surgery (OS), endoscopic laparoscopy (EL) and robotic-assisted laparoscopy (RAL) based on clinical literature review outcomes, to report a budget impact in the province based on volumes and costs from administrative data sets, and to project a future impact of RAL in Ontario. A cost-effectiveness analysis was not conducted because of the low quality evidence from the clinical literature review.
The following significant, short-term outcomes from the clinical literature review were costed:
Hospitalization – assumed it accounted for complications, blood loss and transfusions
Operation time – assumed it accounted for professional fees
Radiotherapy cost due to positive surgical margins in prostate cancer
Conversions to OS in gynecology
Hospital costs were obtained from the Ontario Case Costing Initiative (OCCI) (120) – accessed July 2010 for the appropriate Canadian Classification of Health Intervention (CCI) codes (121) restricted to selective ICD-10 diagnostic codes (121) after consultation with experts in the field.
Physician fees were obtained from the Ontario Schedule of Benefits (OSB) (122) – accessed July 2010 after consultation with experts in the field. Fees were costed based on operation times reported in the clinical literature for the procedures being investigated. A description of the calculation of professional cost per case is described in Appendix 3.
Volumes of procedures were obtained from Ministry of Health and Long-Term Care (MOHLTC) administrative databases (123) – accessed July 2010.
The following is a list of the CCI and ICD-10 codes used in the analysis:
ICD-10 - Uterus and Cervix Diagnosis
C53 Malignant neoplasm of cervix uteri
C53.0 Malignant neoplasm of endocervix
C53.1 Malignant neoplasm of exocervix
C53.8 Overlapping malignant lesion of cervix uteri
C53.9 Malignant neoplasm cervix uteri, unspecified
C54 Malignant neoplasm of corpus uteri
C54.0 Malignant neoplasm of isthmus uteri lower uterine segment
C54.1 Malignant neoplasm of endometrium
C54.2 Malignant neoplasm of myometrium
C54.3 Malignant neoplasm of fundus uteri
C54.8 Overlapping malignant lesion of corpus uteri
C54.9 Malignant neoplasm corpus uteri NOS
C55 Malignant neoplasm of uterus, part unspecified
CCI Interventions – Uterus and Cervix
1.RM.89.^^ Excision total, uterus and surrounding structures
Includes: Hysterectomy; panhysterectomy; total hysterectomy
Code also: Any bladder neck suspension (see 1.PL.74.^^); any concomitant removal of ovaries and fallopian tubes (see 1.RD.89.^^); any concomitant suspension of vaginal vault (see 1.RS.74.^^); any pelvic floor repair (see 1.RS.80.^^)
1.RM.89.DA using endoscopic (laparoscopic) approach
1.RM.89.LA using open approach
ICD-10 - Prostate Diagnosis
C61 Malignant neoplasm of prostate
CCI - Interventions - Prostate
1.QT.91.^^Excision radical, prostate
Includes: Prostatectomy, radical; prostatovesiculectomy; radical nerve-sparing prostatectomy
Excludes: Cystoprostatectomy (see 1.PM.91.^^); prostatectomy with (sub)total bladder resection (see 1.PM.91.^^)
Code Also: Any concomitant orchidectomy (see 1.QM.89.^^)
1.QT.91.DA using endoscopic (laparoscopic) approach
1.QT.91.PK using open retropubic approach
The following Ontario Health Insurance Plan (OHIP) codes were used in the analysis:
OHIP – Professional fees - Hysterectomy
S757 Total hysterectomy
S763 Radical hysterectomy
E862 When performed laparoscopically
OHIP – Professional fees - Prostatectomy
S651 Prostatectomy - open retropubic surgery
S653 Prostatectomy - endoscopic laparoscopy
Economic Literature Review
Two literature searches were conducted on June 1st and June 8th, 2010 and the following databases were searched:
OVID MEDLINE
MEDLINE In-Process and Other Non-Indexed Citations
OVID EMBASE
Wiley Cochrane
CINAHL
Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
EconLit
The search strategy is presented in Appendix 1. We reviewed published articles that fit the following inclusion criteria:
Full economic evaluations (cost-effectiveness analysis (CEA), cost-utility analysis (CUA), cost-benefit analysis (CBA), cost-minimization analysis (CMA))
Economic evaluations reporting Incremental Cost-Effectiveness Ratios (ICER), i.e. cost per quality adjusted life year (QALY)/life years gained (LYG), or cost per event avoided, or studies reporting total costs
Studies in patients with endometrial, cervical, and prostate cancers
Studies reporting on RAL
Studies in English
No CEA was identified in the literature as a result of poor quality clinical evidence. Several cost analyses reporting the direct costs associated with RAL, EL and OS were identified. Table 9 describes the differences in costs.
The economic literature was consistent in reporting higher direct costs, i.e. hospital and professional costs for RAL versus other comparative strategies, i.e. EL and OS. Bell et al. (2008) (44) reported a higher average direct cost for OS than RAL because they reported higher room and board costs for OS than RAL however professional fees and robotic specific instrumentation were considerably higher for RAL than OS which is consistent with the economic literature and our data. Camberlin et al. (2009) (38) (Belgium HTA group) also reviewed the economic literature and reported only cost comparisons for prostatectomy and hysterectomy strategies.
Target Population
The target population of this economic analysis was patients with endometrial, cervical, and prostate cancer.
Perspective
The primary analytic perspective was that of the Ministry of Health and Long-Term Care (MOHLTC).
Comparators & Effect Estimates
The significant clinical outcomes were costed to reflect incurred benefits. Please see the clinical part of EBA for further details on studies included in the analysis and discussion of relevant outcomes.
Table 10 describes the average length of stay (LOS) in-hospital for RAL, EL and OS for endometrial, cervical and prostate cancers. Weighted averages were calculated because in some instances standard deviations were not reported in the included studies therefore for consistency, weighted averages were used to cost outcomes from the clinical literature review. RAL had a lower weighted average LOS in-hospital compared to EL and OS for both gynecological and prostate cancers also reflected in the lower number of complications, episodes of blood loss and transfusions.
Table 11 describes the weighted average operation time to conduct RAL, EL and OS for endometrial, cervical, and prostate cancers. Weighted averages were calculated because in some instances standard deviations were not reported in the included studies therefore for consistency, weighted averages were used to cost outcomes from the clinical literature review. RAL had a higher weighted average operation time compared to EL and OS for both gynecological and prostate cancers. This may be a reflection of the fact that RAL is a relatively new technology versus EL and OS. This incurred time translated to higher professional fees.
Table 12 describes the weighted average proportion of positive surgical margins after RAL, EL and OS for prostate cancer. RAL had a lower rate of positive surgical margins compared to EL and OS for prostate cancer. This translated to lower radiotherapy cost for RAL versus EL and OS.
Table 13 describes the weighted average proportion of conversion to OS after RAL and EL for gynecological cancer. RAL had a lower rate of conversion to OS compared to EL. This translated to lower OS conversion cost for RAL versus EL.
Resource Use and Costs
Direct costs associated with RAL, EL and OS included professional fees, hospital costs, radiotherapy cost associated with positive surgical margins in prostate cancer and conversion to OS in gynecological cancer. Table 14 describes the individual and total cost associated with each procedure.
Professional fees were obtained from the OSB and were costed based on the weighted average operation time of each procedure from the clinical literature (see Appendix 3). There is no fee associated with RAL therefore it was assumed that the professional fees were comparable to that of EL. The professional cost per case was higher for RAL because as per evidence in the clinical literature the operation time was longer for RAL compared to EL and OS therefore reflecting the higher cost per case for RAL.
The hospital cost was obtained from the OCCI data set for the appropriate codes mentioned above for the past five fiscal years (FY) and weighted average costs were calculated across all diagnoses for each procedure. There is no experience with RAL in the province therefore it was assumed that the hospital cost per case was comparable to EL. The cost of RAL however, is higher because it requires robot specific instrumentation which is more costly than the instrumentation associated with EL and OS. A cost per case for disposables for RAL was reported to be $2,500 (Personal communication, manufacturer, July 2010) per patient which was added to the hospital cost per case. Even though RAL had a lower LOS in-hospital than EL and OS, the hospital cost per case was higher for RAL because it requires robot specific instrumentation which is more costly than the instrumentation associated with EL and OS.
Radiotherapy was costed for prostate cancer based on a typical regimen in Ontario of 30 fractions of radiotherapy, one radiation oncologist consult, physician assessments every six weeks and physician visit follow-up every three months after findings of positive surgical margins (Personal communication, clinical expert, July 2010). There was a higher cost of radiotherapy for EL and OS versus RAL because there were a higher proportion of positive surgical margins reported in the clinical literature for EL and OS.
Conversion to OS was costed for gynecological cancer based on evidence from the clinical literature. There was a higher cost attached to EL because more patients converted to OS with EL than with RAL.
Therefore the total cost per case was higher for RAL than EL and OS for both gynecological and prostate cancers.
There is also an acquisition cost associated with RAL. After conversation with the only supplier in Canada, hospitals are looking to spend an initial cost of 3.6M to acquire the robotic surgical system. Table 15 describes the breakdown associated with the acquisition cost of the robotic surgical system.
Ontario Perspective
Volumes of hysterectomies and prostatectomies were abstracted from MOHLTC administrative data sets with appropriate codes after consultation with clinical experts. Table 16 describes the volumes of hysterectomies and prostatectomies in the province in the past five FYs.
Figures 1 and 2 describe these data diagrammatically.
Figure 1. Volumes of Hysterectomy in Ontario – FYs 2004-2008.
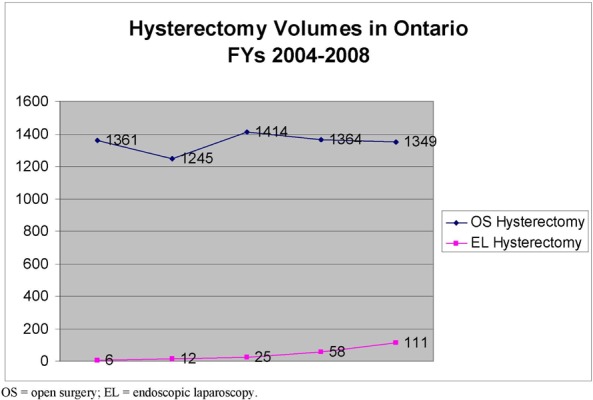
Figure 2. Volumes of Prostatectomy in Ontario – FYs 2004-2008.
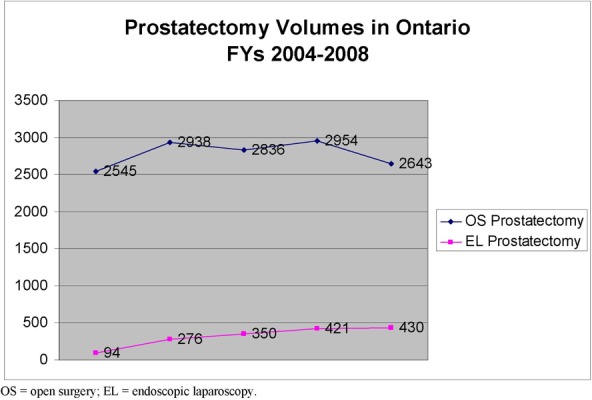
Burden of OS and EL hysterectomies and prostatectomies was calculated by multiplying the number of cases for that year by the cost/case of the procedure which included professional fee, hospital cost including all necessary instrumentation cost to perform surgery, radiotherapy cost and conversion to OS cost associated with that procedure for that specific year. Table 17 describes the average burden to the province.
As reflected in the data, surgeons are not performing conventional laparoscopic procedures but are opting to perform open surgeries. In FY 08/09, the province spent 39M on prostatectomies and 14M on hysterectomies out of which a small proportion of cases were ELs (6M for prostatectomies and 1M for hysterectomies). Figure 3 describes the data diagrammatically.
Figure 3. Costs of Prostatectomy and Hysterectomy in Ontario – FYs 2004-2008.
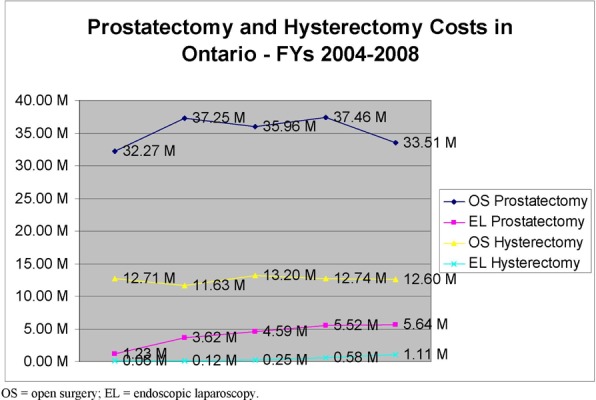
Budget Impact Analysis of Robotic-Assisted Laparoscopy
Previous volumes of OS and EL procedures were used to project volumes into Years 1-3 using a linear mathematical expression. Table 18 describes the projected volumes.
Number of procedures is expected to increase in the next three years based on historical data. RAL is expected to capture this market. It was first assumed to mimic the American experience that RAL would capture 100% of the market share. Table 19 describes the net impact that 100% uptake by RAL of the OS + EL market would have in the province.
If it’s assumed that RAL will completely replace the current situation in Ontario, the net impact is expected to be by Year 3 4.7M for hysterectomy and 10.2M for prostatectomy procedures respectively in the province.
A more realistic uptake rate in the province, after consultation with experts is 65%. Table 20 describes the net impact to Ontario with this uptake rate.
If it’s assumed that RAL will capture the current market in Ontario by 65%, the net impact is expected to be by Year 3, 3.1M for hysterectomy and 6.6M for prostatectomy procedures respectively in the province.
Conclusion
RAL has diffused in the province with four surgical systems in place in Ontario, two in Toronto and two in London. RAL is a more expensive technology on a per case basis due to more expensive robot specific instrumentation and physician labour reflected by increased OR time reported in the clinical literature. There is also an upfront cost to acquire the machine and maintenance contract. RAL is expected to capture market at 65% with project net impacts by Year 3 of 3.1M and 6.6M for hysterectomy and prostatectomy respectively.
Glossary
- Anastomotic stricture
The surgical union of parts, e.g. to repair a bladder neck contracture
- C-C (historical)
A description of the study design where both case groups are ascertained historically, typically at two different points in time, and the data source is not clear (e.g. clinical charts, research study) [C-C is for case-case comparisons]
- Cervicectomy
Removal of the cervix with preservation of the uterus
- Dilation and curettage
A medical procedure in which the uterine cervix is dilated and a surgical instrument is inserted into the uterus to sample the endometrium
- Endoscope
A flexible or rigid tubular instrument for visualizing internal organs for diagnosis or treatment purposes. Typically it has one or more channels to enable passage of instruments (e.g. forceps or scissors). A device with a light attached to the end of it to look inside the body. An endoscope is passed through a natural body opening or through a small incision.
- Estimated blood loss
The difference between the total amount of suctioned and irrigation fluids, or sum of suctioned fluids and weighed sponges
- FIGO
International Federation of Gynecologic Oncology (staging)
- Gleason score
A grading system by microscopic evaluation of how similar the tumour tissue resembles normal tissue. Scores range from 2-10 and indicate how likely the tumour will spread (e.g. higher scores and more likely to spread)
- Laparoscope
A rigid endoscope. A way of examining the ovaries, appendix or other abdominal organs (e.g. a laparoscope is inserted through small surgical cuts in the pelvic or belly area)
- Laparotomy
Surgery that opens the abdomen, also referred to as open surgery or abdominal surgery in this report
- Operation time
From the beginning of skin incision to completion of skin closure
- Parametrium
Connective tissue around the portion of the uterus closest to the cervix
- Positive surgical margin
Tumour extending to the inked surface of the prostate examined under pathology review, or accidental incision into the prostate and a site of tumour, or capsular incision
- Post-operative complications
Complications occurring after surgery, within 30 days
- Prospective C-C
A description of the study design where the cases are ascertained prospectively and compared to a historical referent groups(s) [C-C is for case-case comparisons] (e.g. before and after the introduction of a robotics program)
- Prospective cohort
A description of the study design where one or more surgical procedures are compared in a longitudinal manner
- Retropubic radical prostatectomy
A surgical procedure to remove the prostate, surrounding tissue and seminal vesicles through an incision in the abdominal wall. Removal of nearby lymph nodes may also occur
- Retrospective, database
A description of the study design where case groups are ascertained retrospectively from a pre-existing database that collects patient information on an ongoing, prospective nature. A case-case comparison is typically examined
- Retrospective MRR
A description of the study design where the case groups are ascertained from preexisting clinical charts by medical record review. A case-case comparison is typically examined
- Stage
The extent of cancer based on tumour size, lymph node involvement and spread
- Stage II Prostate Cancer
Cancer that has not spread outside the prostate, otherwise referred to as localized disease. Can be assessed clinically (e.g. stage cT2), or by pathology review (e.g. stage pT2)
- Stage III Prostate Cancer
Cancer that has spread beyond the outer layer of the prostate to nearby tissues. The seminal vesicles may also be involved. Can be assessed clinically (e.g. stage cT3), or by pathology review (e.g. stage pT3)
- Stage IV Prostate Cancer
Cancer has metastasized to the lymph nodes or to other parts of the body, often to the bones. Can be assessed clinically (e.g. stage cT4), or by pathology review (e.g. stage pT4)
- Trendelenburg
An elevated pelvic position for operations within the abdominal cavity, where the patient is placed head down on a table inclined at 45 degrees from the floor with the knees uppermost and the legs hanging over the end of the table
- Tumour-based prevalence
Refers to the number of previously diagnosed cancer cases who have survived to a specified date (person-based prevalence is the number of people)
- Wound dehiscence
The parting of a sutured wound resulting from infection
Appendices
Appendix 1: Literature Search Strategies
Clinical Literature
Search date: May 12, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, CINAHL, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment
Database: Ovid MEDLINE® <1950 to April Week 4 2010>
Search Strategy:
_________________________________________________________________
exp Uterine Neoplasms/ (88099)
((gynecologic* or cervi* or uterine or uterus or endomet*) adj2 (malignanc* or cancer* or neoplas* or dysplas* or tumo?r* or carcinoma*)).ti,ab. (55819)
exp Hysterectomy/ (21078)
exp Prostatic Neoplasms/ (70800)
(prostat* adj2 (cancer* or carcinoma* or neoplas* or tumo?r* or adeno* or malignan*)).ti,ab. (62823)
exp Prostatectomy/ (18551)
or/1-6 (203766)
exp Robotics/ (7295)
exp Surgery, Computer-Assisted/ (5433)
(davinci or da vinci or robot*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (10595)
or/8-10 (15011)
7 and 11 (967)
limit 12 to (english language and humans and yr=“2000 –Current”) (871)
Database: EMBASE <1980 to 2010 Week 21>
Search Strategy:
__________________________________________________________________
exp Uterus Tumour/ (74216)
((gynecologic* or cervi* or uterus or uterine or endomet*) adj2 (malignanc* or cancer* or neoplas* or dysplas* or tumo?r* or carcinoma*)).ti,ab. (46015)
exp hysterectomy/ (22321)
exp prostate tumour/ (74749)
(prostat* adj2 (cancer* or neoplas* or tumo?r* or adeno* or malignan*)).ti,ab. (52296)
exp prostatectomy/ (15662)
or/1-6 (177528)
exp robotics/ or exp computer assisted surgery/ (9301)
(davinci or da vinci or robot*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (9614)
8 or 9 (11243)
7 and 10 (1160)
limit 11 to (human and english language and yr=“2000–Current”) (970)
Economic Literature
Prostate Cancer
Search date: June 1, 2010
Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment, EconLit
Database: Ovid MEDLINE(R) <1950 to May Week 3 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Prostatic Neoplasms/ (71146)
(prostat* adj2 (cancer* or carcinoma* or neoplas* or tumo?r* or adeno* or malignan*)).ti,ab. (63163)
exp Prostatectomy/ (18614)
exp Robotics/ (7375)
exp Surgery, Computer-Assisted/ (5516)
(davinci or da vinci or robot*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (10687)
exp Economics/ (421620)
exp Models, Economic/ (7181)
exp Resource Allocation/ (13274)
exp “Value of Life”/ or exp “Quality of Life”/ (86633)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (187886)
ec.fs. (269512)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or “value of life” or “willingness to pay”).ti,ab. (63578)
or/1-3 (89254)
or/4-6 (15163)
or/7-13 (717730)
14 and 15 and 16 (81)
limit 17 to (english language and yr=“2000 -Current”) (77)
Database: EMBASE <1980 to 2010 Week 21>
Search Strategy:
--------------------------------------------------------------------------------
exp prostate tumour/ (74749)
(prostat* adj2 (cancer* or neoplas* or tumo?r* or adeno* or malignan*)).ti,ab. (52296)
exp prostatectomy/ (15662)
exp robotics/ or exp computer assisted surgery/ (9301)
(davinci or da vinci or robot*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (9614)
or/1-3 (83365)
4 or 5 (11243)
6 and 7 (923)
exp “Health Care Cost”/ (117665)
exp Health Economics/ (260232)
exp Resource Management/ (15844)
exp Economic Aspect/ or exp Economics/ or exp Quality Adjusted Life Year/ or exp Socioeconomics/ or exp Statistical Model/ or exp “Quality of Life”/ (550696)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (118745)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or “value of life” or “willingness to pay”).ti,ab. (59515)
or/9-14 (630043)
8 and 15 (210)
Gynecological Cancer
Search date: June 8, 2010
Databases searched: Databases searched: OVID MEDLINE, MEDLINE In-Process and Other Non-Indexed Citations, OVID EMBASE, Wiley Cochrane, Centre for Reviews and Dissemination/International Agency for Health Technology Assessment, EconLit
Database: Ovid MEDLINE(R) <1996 to May Week 4 2010>
Search Strategy:
--------------------------------------------------------------------------------
exp Uterine Neoplasms/ (38266)
((gynecologic* or cervi* or uterine or uterus or endomet*) adj2 (malignanc* or cancer* or neoplas* or dysplas* or tumo?r* or carcinoma*)).ti,ab. (31940)
exp Hysterectomy/ (8781)
exp Robotics/ (6762)
exp Surgery, Computer-Assisted/ (5305)
(davinci or da vinci or robot*).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] (9251)
or/1-3 (52886)
or/4-6 (13581)
7 and 8 (174)
exp Economics/ (217305)
exp Models, Economic/ (6032)
exp Resource Allocation/ (7410)
exp “Value of Life”/ or exp “Quality of Life”/ (69435)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (84893)
ec.fs. (171798)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or “value of life” or “willingness to pay”).ti,ab. (48108)
or/10-16 (395586)
9 and 17 (13)
Database: EMBASE <1980 to 2010 Week 22>
Search Strategy:
--------------------------------------------------------------------------------
exp Uterus Tumour/ (74446)
((gynecologic* or cervi* or uterus or uterine or endomet*) adj2 (malignanc* or cancer* or neoplas* or dysplas* or tumo?r* or carcinoma*)).ti,ab. (46164)
exp robotics/ or exp computer assisted surgery/ (9366)
(davinci or da vinci or robot*).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] (9685)
1 or 2 (84404)
3 or 4 (11322)
5 and 6 (180)
exp “Health Care Cost”/ (118051)
exp Health Economics/ (261115)
exp Resource Management/ (15880)
exp Economic Aspect/ or exp Economics/ or exp Quality Adjusted Life Year/ or exp Socioeconomics/ or exp Statistical Model/ or exp “Quality of Life”/ (552641)
(econom$ or cost$ or budget$ or pharmacoeconomic$ or pharmaco-economic$ or valu$).ti. (119009)
((cost$ adj benefit$) or costbenefit$ or (cost adj effective$) or costeffective$ or econometric$ or life value or quality-adjusted life year$ or quality adjusted life year$ or quality-adjusted life expectanc$ or quality adjusted life expectanc$ or sensitivity analys$ or “value of life” or “willingness to pay”).ti,ab. (59682)
or/8-13 (632174)
7 and 14 (32)
limit 15 to (english language and yr=“2000 -Current”) (32)
Appendix 2: GRADE Evidence Tables
| Robotics Compared to Abdominal: Gynecologic Oncology | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments** | |
| Assumed risk | Corresponding risk | |||||
| Abdominal | Robotic | |||||
| Length of Hospitalization | The mean length of hospitalization in the intervention groups was 2.05 lower (2.72 to 1.39 lower) | 671 (6 studies) | □□⊖⊖ low1 |
RB > OS | ||
| Complications | Study population | OR 0.37 (0.23 to 0.61) | 1059 (10 studies) | □□⊖⊖ low1,2 |
RB > OS | |
| 337 per 1000 | 158 per 1000 (105 to 237) | |||||
| Medium risk population | ||||||
| 280 per 1000 | 126 per 1000 (82 to 192) | |||||
| Operation Time | The mean operation time in the intervention groups was 0.66 higher (0.16 to 1.16 higher) | 855 (8 studies) | □□⊖⊖ low3 |
RB < OS | ||
| Blood Loss | The mean blood loss in the intervention groups was 223.07 lower (294.47 to 151.67 lower) | 563 (5 studies) | □□⊖⊖ low1 |
RB > OS | ||
| Lymph Nodes | The mean lymph nodes in the intervention groups was 2.34 lower (6.87 lower to 2.19 higher) | 693 (7 studies) | □⊖⊖⊖ very low4 |
n/a | ||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
RB > OS indicates that robotics had a more favourable profile for the specific outcome; RB < OS indicates robotics had a less favourable profile for the specific outcome.
CI: Confidence interval; OR: Odds ratio; RB: Robotics; OS: Open surgery.
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Level of surgeon skill differed across studies.
Complications were reported differently across studies.
Surgeons experienced in robotics had a more favourable operating time.
Inconsistency not explained by level of surgeon skill or patient characteristics
| Robotics Compared to Laparoscopy: Gynecologic Oncology | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopy | Robotic | |||||
| Length of Hospitalization | The mean length of hospitalization in the intervention groups was 0.2 lower (0.31 to 0.1 lower) | 636 (5 studies) | □□⊖⊖ low1 |
RB > LP | ||
| Complications | Study population | OR 0.76 (0.52 to 1.09) | 970 (10 studies) | □□⊖⊖ low1,2 |
n/a | |
| 163 per 1000 | 129 per 1000 (92 to 175) | |||||
| Medium risk population | ||||||
| 206 per 1000 | 165 per 1000 (119 to 220) | |||||
| Operation Time | The mean operation time in the intervention groups was 0.03 higher (0.47 lower to 0.53 higher) | 870 (7 studies) | □⊖⊖⊖ very low1,3 |
n/a | ||
| Blood Loss | The mean blood loss in the intervention groups was 74.95 lower (94.77 to 55.14 lower) | 636 (5 studies) | □□⊖⊖ low1 |
RB > LP | ||
| Conversions | Study population | OR 0.38 (0.2 to 0.72) | 640 (3 studies) | □□⊖⊖ low1 |
RB > LP | |
| 100 per 1000 | 41 per 1000 (22 to 74) | |||||
| Medium risk population | ||||||
| 52 per 1000 | 20 per 1000 (11 to 38) | |||||
| Lymph Nodes | The mean lymph nodes in the intervention groups was 3.16 lower (6.99 lower to 0.67 higher) | 870 (7 studies) | □□⊖⊖ low4 |
n/a | ||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
RB > LP indicates that robotics had a more favourable profile for the specific outcome; RB < LP indicates robotics had a less favourable profile for the specific outcome.
CI: Confidence interval; OR: Odds ratio; RB: Robotics; LP: Laparoscopy.
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Level of surgeon skill differed across studies.
Complications were reported differently across studies.
Inconsistency not explained by level of surgeon skill or patient characteristics
Extensive laparoscopic experience may have facilitated the uptake of robotic surgery.
| Robotics Compared to Retropubic: Prostate Cancer | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments** | |
| Assumed risk | Corresponding risk | |||||
| Retropubic | Robotic | |||||
| Erectile Dysfunction | Study population | OR 0.44 (0.25 to 0.79) |
1039 (4 studies) | □□⊖⊖ low1,2 |
RB > RP | |
| 452 per 1000 | 266 per 1000 (171 to 395) | |||||
| Medium risk population | ||||||
| 506 per 1000 | 311 per 1000 (204 to 447) | |||||
| Positive Surgical Margins | Study population | OR 0.60 (0.44 to 0.82) |
1308 (9 studies) | □□⊖⊖ low3 |
RB > RP | |
| 183 per 1000 | 118 per 1000 (90 to 155) | |||||
| Medium risk population | ||||||
| 104 per 1000 | 65 per 1000 (49 to 87) | |||||
| Urinary Incontinence | Study population | OR 0.42 (0.1 to 1.85) |
1224 (3 studies) | □⊖⊖⊖ low4,5,6 |
n/a | |
| 86 per 1000 | 38 per 1000 (9 to 148) | |||||
| Medium risk population | ||||||
| 120 per 1000 | 54 per 1000 (13 to 201) | |||||
| Length of Hospitalization | The mean length of hospitalization in the intervention groups was 0.23 lower (0.44 to 0.02 lower) | 802 (3 studies) | □□⊖⊖ low |
RB > RP | ||
| Blood Loss | The mean blood loss in the intervention groups was 652.86 lower (819.13 to 486.6 lower) | 802 (3 studies) | □□⊖⊖ low |
RB > RP | ||
| Transfusions | Study population | OR 0.14 (0.05 to 0.36) |
2852 (7 studies) | □□⊖⊖ low2 |
RB > RP | |
| 223 per 1000 | 39 per 1000 (14 to 94) | |||||
| Medium risk population | ||||||
| 167 per 1000 | 27 per 1000 (10 to 67) | |||||
| Operation Time | The mean operation time in the intervention groups was 0.72 higher (0.01 to 1.44 higher) | 842 (4 studies) | □□⊖⊖ low2 |
RB < RP | ||
| Complications | Study population | OR 0.46 (0.15 to 1.42) | 2212 (4 studies) | □⊖⊖⊖ very low2,7 |
n/a | |
| 382 per 1000 | 221 per 1000 (85 to 467) | |||||
| Medium risk population | ||||||
| 260 per 1000 | 139 per 1000 (50 to 333) | |||||
| Post-Operative Pain | The mean post-operative pain in the intervention groups was 8.55 lower (15.62 to 1.47 lower) | 1055 (3 studies) | □□⊖⊖ low |
RB > RP | ||
| Catheterization Duration | The mean catheterization duration in the intervention groups was 1.5 lower (2.77 to 0.23 lower) | 60 (1 study) | □□⊖⊖ low |
RB > RP | ||
| Anastomotic Stricture | Study population | OR 0.25 (0.05 to 1.23) |
2794 (3 studies) | □□⊖⊖ low |
n/a | |
| 40 per 1000 | 10 per 1000 (2 to 49) | |||||
| Medium risk population | ||||||
| 45 per 1000 | 12 per 1000 (2 to 55) | |||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
RB > RP indicates that robotics had a more favourable profile for the specific outcome; RB < RP indicates robotics had a less favourable profile for the specific outcome.
CI: Confidence interval; OR: Odds ratio; RB: Robotics; RP: Retropubic.
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Measurement of erectile dysfunction was not consistent across studies.
Level of surgeon skill differed across studies.
Differences in pathology review across studies, some of which are unknown.
Measurement of urinary continence was measured and characterized inconsistently.
Experienced surgeons had a more favourable profile for robotic surgery.
Stage difference in one study, with more advanced tumours in the retropubic surgery group.
Differences in the reporting of complications may have contributed to the inconsistency.
| Robotics Compared to Laparoscopy: Prostate Cancer | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Illustrative comparative risks* (95% CI) |
Relative effect (95% CI) | No of Participants (studies) |
Quality of the evidence (GRADE) | Comments** | |
| Assumed risk | Corresponding risk | |||||
| Laparoscopy | Robotic | |||||
| Transfusions | Study population | OR 0.54 (0.3 to 0.95) |
1382 (5 studies) | □□⊖⊖ low |
RB > LP | |
| 53 per 1000 | 29 per 1000 (17 to 50) | |||||
| Medium risk population | ||||||
| 25 per 1000 | 14 per 1000 (8 to 24) | |||||
| Complications | Study population | OR 0.62 (0.25 to 1.53) |
1452 (5 studies) | □□⊖⊖ low1 |
n/a | |
| 204 per 1000 | 137 per 1000 (60 to 282) | |||||
| Medium risk population | ||||||
| 147 per 1000 | 97 per 1000 (41 to 209) | |||||
| Positive Surgical Margins | Study population | OR 1.09 (0.66 to 1.78) |
572 (4 studies) | □□⊖⊖ low |
n/a | |
| 134 per 1000 | 144 per 1000 (93 to 216) | |||||
| Medium risk population | ||||||
| 127 per 1000 | 137 per 1000 (88 to 206) | |||||
| Erectile Dysfunction | Study population | OR 0.76 (0.3 to 1.89) |
96 (1 study) | □□⊖⊖ low |
n/a | |
| 289 per 1000 | 236 per 1000 (109 to 434) | |||||
| Medium risk population | ||||||
| 289 per 1000 | 236 per 1000 (109 to 434) | |||||
| Urinary Incontinence | Study population | OR 0.6 (0.19 to 1.92) |
150 (1 study) | □□⊖⊖ low |
n/a | |
| 107 per 1000 | 67 per 1000 (22 to 187) | |||||
| Medium risk population | ||||||
| 107 per 1000 | 67 per 1000 (22 to 187) | |||||
| Blood Loss | The mean blood loss in the intervention group was 167.79 lower (231.67 to 103.91 lower) | 536 (4 studies) | □□⊖⊖ low |
RB > LP | ||
| Operation Time | The mean operation time in the intervention group was 0.13 lower (0.59 lower to 0.34 higher) | 536 (4 studies) | □□⊖⊖ low2 |
n/a | ||
| Length of Hospitalization | The mean operation time in the intervention group was 0.38 lower (0.91 lower to 0.14 higher) | 356 (2 studies) | □□⊖⊖ low |
n/a | ||
| Catheterization Duration | The mean operation time in the intervention group was 0.50 lower (1.01 lower to 0 higher) | 356 (2 studies) | □□⊖⊖ low |
Borderline effect | ||
| Anastomotic Stricture | Study population | OR 0.49 (0.02 to 10.26) |
288 (1 study) | □□⊖⊖ low |
n/a | |
| 10 per 1000 | 5 per 1000 (0 to 94) | |||||
The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI).
RB > LP indicates that robotics had a more favourable profile for the specific outcome; RB < LP indicates robotics had a less favourable profile for the specific outcome.
CI: Confidence interval; OR: Odds ratio; RB: Robotics; LP: Laparoscopy.
GRADE Working Group grades of evidence
High quality: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality: We are very uncertain about the estimate.
Odds ratios cover <1 and >1, with previous surgical skill likely contributing.
Mean differences cover negative and positive values, with previous surgical skill likely contributing.
Appendix 3: Professional Costs
| Cost per Unit | Basic Units | Reference | Average Duration of Surgery |
Reference | Total Cost | Assumptions | |
|---|---|---|---|---|---|---|---|
| Radical Prostatectomy - Robotic Assisted Laparoscopy | |||||||
| Physician | $1,411.70 | OSB - S653 | $1,411.70 | Assumed same fees as conventional laparoscopy | |||
| Anaesthesia | $14.54 | 8 | OSB - S653 | 4.34 | EBA 2010 | $786.68 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 10 | OSB - S653 | 4.34 | EBA 2010 | $392.89 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $2,591.27 | ||||||
| Radical Prostatectomy - Endoscopic Laparoscopic Approach | |||||||
| Physician | $1,411.70 | OSB - S653 | $1,411.70 | ||||
| Anaesthesia | $14.54 | 8 | OSB - S653 | 4.09 | EBA 2010 | $742.51 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 10 | OSB - S653 | 4.09 | EBA 2010 | $357.89 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $2,512.10 | ||||||
| Radical Prostatectomy - Open Retropubic Approach | |||||||
| Physician | $1,000.35 | OSB - S651 | $1,000.35 | ||||
| Anaesthesia | $14.54 | 10 | OSB - S651 | 3.21 | EBA 2010 | $618.90 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 6 | OSB - S651 | 3.21 | EBA 2010 | $190.83 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,810.09 | ||||||
| Hysterectomy - Robotic Assisted Laparoscopy | |||||||
| Physician | $578.75 | OSB - E862 | $578.75 | Assumed same fees as conventional laparoscopy | |||
| Anaesthesia | $14.54 | 6 | OSB - E862 | 4.09 | EBA 2010 | $713.43 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 6 | OSB - E862 | 4.09 | EBA 2010 | $311.81 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,603.99 | ||||||
| Total Hysterectomy - Endoscopic Laparoscopic Approach | |||||||
| Physician | $578.75 | OSB - E862 | $578.75 | Add 25% premium to physician fee S757 for laparoscopic procedure | |||
| Anaesthesia | $14.54 | 6 | OSB - E862 | 3.40 | EBA 2010 | $592.70 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 6 | OSB - E862 | 3.40 | EBA 2010 | $216.15 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,387.60 | ||||||
| Total Hysterectomy - Open Approach | |||||||
| Physician | $463.00 | OSB - S757 | $463.00 | ||||
| Anaesthesia | $14.54 | 6 | OSB - S757 | 2.48 | EBA 2010 | $432.11 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 6 | OSB - S757 | 2.48 | EBA 2010 | $251.28 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,146.39 | ||||||
| Radical Hysterectomy - Vaginal Approach | |||||||
| Physician | $893.55 | OSB - S763 | $893.55 | ||||
| Anaesthesia | $14.54 | 8 | OSB - S763 | 2.48 | EBA 2010 | $461.19 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 8 | OSB - S763 | 2.48 | EBA 2010 | $274.32 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,629.06 | ||||||
| Radical Hysterectomy - Abdominal Approach | |||||||
| Physician | $893.55 | OSB - S763 | $893.55 | ||||
| Anaesthesia | $14.54 | 8 | OSB - S763 | 2.48 | EBA 2010 | $461.19 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour up to and including first 1.5 hours = 2 units; after 1.5 hours = 3 units |
| Surgical Assistance | $11.52 | 8 | OSB - S763 | 2.48 | EBA 2010 | $274.32 | Assumed basic units + time units = 1st hour = 1 unit; after 1st hour = 2 units; after 3rd hour = 3 units |
| TOTAL/PROCEDURE | $1,629.06 |
Summary Tables
Table 1: Summary of Study Characteristics by Cancer Type for Gynecological Cancers (N=17 Studies).
| Author [Year] | Study Location | Cancer Type | Study Design | Cases/Referent* (no.) |
|---|---|---|---|---|
| Cardenas- [2010] | Pennsylvania, USA | Endometrial | Retrospective MRR±Cases,Ref | 102/173 |
| Geisler [2010] | Ohio, USA | Cervical | C-C (historical)±Cases,Ref | 15/30§ |
| Jung [2010] | Seoul, Korea | Endometrial | Retrospective, database | 28/25/56 |
| Estape [2009] | Florida, USA | Cervical | Prospective C-C±Cases | 32/17/14 |
| Hoekstra [2009] | Chicago, USA | Endometrial | Prospective cohort | 32/7/26 |
| Maggioni [2009] | Milan, Italy | Cervical | Prospective C-C¥,† | 40/40§ |
| Seamon [2009] | Ohio, USA | Endometrial | Prospective C-C±Cases,Ref | 105/76 |
| Bell [2008]‡ | South Dakota, USA | Endometrial | Retrospective MRR | 40/30/40 |
| Boggess [2008]‡ | UNC, USA | Cervical | C-C (historical)±Cases,Ref | 51/49§ |
| Boggess [2008]‡ | UNC, USA | Endometrial | Prospective C-C±Cases,Ref | 103/81/138 |
| DeNardis [2008]‡ | Florida, USA | Endometrial | Retrospective MRR)±Ref | 56/106§ |
| Gehrig [2008]‡ | UNC, USA | Endometrial | C-C (historical)±Cases | 49/32 |
| Ko [2008]‡ | Boston, USA | Cervical | C-C (historical)±Cases,Ref | 16/32§ |
| Magrina [2008]‡ | Arizona, USA | Cervical/Endometrial | Prospective C-C¥ | 27/31/35 |
| Nezhat [2008]‡ | MSMC, USA | Cervical | Retrospective MRR** | 13/30 |
| Veljovich [2008]‡ | Sweden | Endometrial | Retrospective MRR** | 25/4/131 |
| Sert [2007]‡ | Oslo, Norway | Cervical | C-C (historical) | 7/7 |
Sample sizes according to: robotic/laparoscopic, or robotic/laparoscopic/abdominal after reported exclusions.
Robotics/laparotomy.
Consecutive patients.
(Individually)-matched study design.
Matching factors were not provided. Data for the referent group was ascertained by retrospective chart review.
Systematic review.
Robotic surgeries were ascertained prospectively.
Note: MRR, medical record review; C-C, case-case comparison; Ref, referent; UNC, University of North Carolina; MSMC, Mount Sinai Medical Center.
Table 2: Summary of Study Characteristics for Prostate Cancer (N=21 Studies).
| Author [Year] | Study Location | Study Design | Length of Follow-up* | Cases/Referent* (no.) |
|---|---|---|---|---|
| Carlsson [2010] | Karolinska University Hospital, Sweden | Prospective cohort± | 19/30 months | 1253/485 |
| Lo [2010] | Prince of Whales Hospital, Hong Kong | Prospective C-C±Cases,Ref | 6/42 months | 20/20 |
| D’Alonzo [2009] | Duke University Medical Center, USA | Retrospective, database | - | 256/280 |
| Drouin [2009] | Paris, France | Retrospective, database | - | 71/85/83 |
| Ficarra [2009a] | Padua, Italy | Prospective cohort± | ≥12 months | 103/105 |
| Hakimi [2009] | New York, USA | Retrospective, database¥ | 12 months | 75/75§ |
| Laurila [2009] | UWHC, USA | Retrospective MRR±Ref | - | 88/84 |
| Ou [2009] | Taichung, Taiwan | C-C (historical)±Cases,Ref | Up to 12 months | 30/30 |
| Polcari [2009] | Illinois, USA | Retrospective MRR± | - | 60/64 |
| Rocco [2009] | Milan, Italy | Prospective C-C | Up to 12 months | 120/240 |
| White [2009] | Michigan, USA | Retrospective MRR±Cases,¥ | - | 50/50 |
| Yates [2009] | Providence, USA | C-C (historical) | 62/61 | |
| Zorn [2009] | Chicago, USA | Retrospective, database±Cases | - | 296/471 |
| Trabulsi [2008] | Thomas Jefferson University Hospital, USA | Prospective C-C±,† | - | 50/190§ |
| Wood [2007] | Michigan, USA | Prospective cohort± | 2 and 6 weeks‡ | 117/89 |
| Ball [2006] | Virginia, USA | Prospective C-C† | Up to 6 months | 82/124/135 |
| Hohwu [2009] | Karolinska University Hospital, Sweden Aarhus University Hospital Skejby, Denmark | Retrospective MRR± | - | 127/147 |
| Ploussard [2009] | Creteil, France | Prospective cohort | - | 83/205§ |
| Weizer [2010] | Michigan, USA | Prospective C-C | - | 515/118 |
| O’Malley [2006] | University of Melbourne, Australia | Prospective C-C± | - | 102/102 |
| Srinualnad [2008] | Mahidol University, Bangkok | Prospective cohort | Up to 1 month | 34/34§ |
Sample sizes according to: robotic/retropubic prostatectomy or robotic/laparoscopic/retropubic prostatectomy.
Robotic/laparoscopic.
Consecutive patients.
Individually-matched study design.
Data were collected Prospectively, before and after the introduction of a robotics program.
Follow-up refers to health-related quality of life questionnaire [not discussed here].
Note: C-C, case-case comparison; Ref, referent group; UWHC, University of Wisconsin Hospital and Clinics; MRR, medical record review.
Table 3: Summary of Type of Hysterectomy and Surgery by Cancer Type (N=17 Studies).
| Type of Hysterectomy | Surgery Comparisons | |||||
|---|---|---|---|---|---|---|
| Author [Year] | Radical | Total | Partial/Vaginal | Abdominal | Laparoscopic | Robotic |
| Endometrial Cancer | ||||||
| Cardenas- [2010] | - | X† | - | - | X | X |
| Jung [2010] | - | X† | - | X | X | X |
| Hoekstra [2009] | - | X† | - | X | X | X |
| Seamon [2009] | - | X† | - | - | X | X |
| Bell [2008]‡ | - | X† | - | X | X | X |
| Boggess [2008]‡ | - | X† | - | X | X | X |
| DeNardis [2008]‡ | - | X | - | X | - | X |
| Gehrig [2008]‡ | - | X† | - | - | X | X |
| Veljovich [2008]‡ | - | X† | - | X | X | X |
| Cervical Cancer | ||||||
| Geisler [2010] | X | - | - | X | - | X |
| Estape [2009] | X | - | - | X | X | X |
| Maggioni [2009] | X | - | - | X | - | X |
| Boggess [2008]‡ | X | - | - | X | - | X |
| Ko [2008]‡ | X | - | - | X | - | X |
| Magrina [2008]‡ | X | - | - | X | X | X |
| Nezhat [2008]‡ | X | - | - | - | X | X |
| Sert [2007]‡ | X | - | - | - | X | X |
Indicates that the necessary information was not examined.
Hysterectomy, with surgical staging.
Systematic review.
Table 4: Consistency of Results for Outcomes of Gynecologic Oncology: Qualitative Assessment.
| Technology | Outcomes (No. Studies)*,§ | ||||||
|---|---|---|---|---|---|---|---|
| Morbidity Factors | Peri-Operative Factors | Lymph | |||||
| Length of Hospitalization | Complications | Operation Time | Blood Loss | Conversions | Lymph Node Recovery | ||
| Endometrial cancer | |||||||
| Robotic vs. Laparoscopy | + (7) | = (6) | + or = (7) | + (7) | + (3) | = (7) | |
| Cardenas- [2010] | = | -¥ | - | + | +¥ | = | |
| Jung [2009] | + | + | = | +† | n/a | + | |
| Seamon [2009] | + | = | + | + | + | = | |
| Bell [2008]‡ | = | + | = | = | ? | = | |
| Boggess [2008]‡ | + | = | + | + | = | + | |
| Gehrig [2008]‡ | + | = | + | + | ? | + | |
| Veljovich [2008]‡ | = | ? | = | = | ? | = | |
| Robotic vs. Abdominal | + (5) | + (5) | - (5) | + (5) | n/a | = (5) | |
| Jung [2009] | + | + | = | +† | n/a | - | |
| Bell [2008]‡ | + | + | - | + | n/a | = | |
| Boggess [2008]‡ | + | + | - | + | n/a | + | |
| DeNardis [2008]‡ | + | = | - | + | n/a | = | |
| Veljovich [2008]‡ | + | = | - | + | n/a | = | |
| Cervical cancer | |||||||
| Robotic vs. Laparoscopy | + or = (4) | = (4) | = (4) | + or = (4) | = (1) | = (4) | |
| Estape [2009] | = | = | = | = | ? | + | |
| Magrina [2008]‡ | +¥ | = | + | +¥ | ? | = | |
| Nezhat [2008]‡ | = | = | = | = | n/a | = | |
| Sert [2007]‡ | + | +¥ | = | + | ? | = | |
| Robotic vs. Abdominal | + (6) | = (6) | - (6) | + (6) | n/a | = (6) | |
| Geisler [2009] | + | ? | = | + | n/a | = | |
| Estape [2009] | + | = | - | + | n/a | + | |
| Maggioni [2009] | + | = | - | + | n/a | - | |
| Boggess [2008]‡ | + | = | + | + | n/a | + | |
| Ko [2008]‡ | + | = | - | + | n/a | = | |
| Magrina [2008]‡ | + | = | - | + | n/a | = | |
+/- evidence favouring both technologies;
evidence not provided;
evidence that showed no difference between technologies;
evidence favouring the technology;
evidence not supportive of the technology compared to the referent technology, e.g. laparoscopy or abdominal as the referent group.
Results refer to those that were reported as a result of a statistical test of difference.
Systematic review.
For study completeness, outcome information was also included without a statistical test for difference, especially if the magnitude of the difference was substantial.
Blood transfusions.
Note: Hoekstra et al. (2009) was excluded; Magrina et al. (2008) is based on pairwise comparisons; Jung et al. (2010) and Nezhat et al. (2008), there were zero conversions in both surgical groups.
Table 5: Consistency of Results for Outcomes of Prostatectomy: Qualitative Assessment.
| Technology | Outcomes (No. Studies)*,§ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Morbidity Factors | Peri-Operative Factors | Lymph | ||||||||
| Length of Hospitalization | Post-Operative Pain | Operation Time | Blood Loss | Transfusions | Catheterisation Duration | Complications | Anastomotic Stricture | Lymph Node Recovery | ||
| Robotic vs. Laparoscopy | + or +/- (4) | ? | + (4) | + (5) | + (2) | = (3) | + or +/- (4) | +/- (1) | ? | |
| Ficarra [2009]± | +/- | ? | +/- | +/- | + | = | +/- | +/- | ? | |
| Hakimi [2009] | + | ? | + | + | ? | ? | +¥ | ? | ? | |
| Trabulsi [2008] | ? | ? | ? | = | ? | ? | ? | ? | ? | |
| Ploussard [2009] | = | ? | + | + | + | = | = | ? | ? | |
| Srinualnad [2008] | = | ? | = | = | ? | + | +¥ | ? | ? | |
| Ball [2006] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
| Robotic vs. Retropubic | + (8) | + (3) | - (8) | + (7) | + (7) | + (5) | + (5) | +/- or = (3) | - (3) | |
| Carlsson [2010] | ? | ? | ? | ? | + | ? | + | = | ? | |
| Lo [2010] | + | ? | = | ? | + | + | ? | ? | ? | |
| D’Alonzo [2009] | + | + | - | + | + | ? | ? | ? | ? | |
| Ficarra [2009]± | + | + | - | + | + | + | + | + | ? | |
| Ficarra [2009a] | + | ? | - | + | + | + | -¥ or = | ? | ? | |
| Laurila [2009] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
| Ou [2009] | + | ? | = | + | + | + | = | - | ? | |
| Polcari [2009] | ? | ? | ? | ? | ? | ? | ? | ? | = | |
| Rocco [2009] | + | ? | - | + | ? | + | ? | ? | ? | |
| White [2009] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
| Wood [2007] | + | + | - | + | + | ? | + | ? | ? | |
| Yates [2009] | ? | ? | ? | ? | ? | ? | ? | ? | - | |
| Zorn [2009] | ? | ? | = | + | ? | ? | ? | ? | - | |
| Weizer [2010] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
| Hohwu [2009] | +¥ | ? | ? | ? | ? | ? | ? | ? | ? | |
| O’Malley [2006] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
| Ball [2006] | ? | ? | ? | ? | ? | ? | ? | ? | ? | |
+/- evidence favouring both technologies.
evidence not provided.
evidence that showed no difference between technologies.
evidence favouring the technology.
evidence not supportive of the technology compared to the referent technology, e.g. laparoscopy or retropubic as the referent groups.
Results refer to those that were reported as statistically significant.
Systematic review. Refers to the overall trend in results, regardless of statistical significance.
For study completeness, outcome information was also included without a statistical test for difference, especially if the magnitude of the difference was substantial.
Positive surgical margins is not specific to tumour stage, except for Hakimi et al. (2009) which refers to stage II disease.
Note: Drouin et al. (2009) was excluded. Anastomotic data for Ploussard was excluded as small difference between groups and no statistical test was given.
Table 5: Consistency of Results for Outcomes of Prostatectomy (cont’d).
| Technology | Outcomes (No. Studies)*,§ | |||
|---|---|---|---|---|
| Oncological Factors | Long-Term Outcomes | |||
| Positive Surgical Margins | Urinary Continence | Erectile Function | ||
| Robotic vs. Laparoscopy | + or +/- (4) | + or = (4) | + or = (3) | |
| Ficarra [2009]± | +/- | = | + | |
| Hakimi [2009] | = | = | = | |
| Trabulsi [2008] | + | ? | ? | |
| Ploussard [2009] | ? | ? | ? | |
| Srinualnad [2008] | + | +¥ | ? | |
| Ball [2006] | ? | +¥ | +¥ | |
| Robotic vs. Retropubic | = (10) | + (7) | + (5) | |
| Carlsson [2010] | ? | + | ? | |
| Lo [2010] | = | = | ? | |
| D’Alonzo [2009] | ? | ? | ? | |
| Ficarra [2009]± | + | +/- | + | |
| Ficarra [2009a] | = | + | + | |
| Laurila [2009] | = | ? | ? | |
| Ou [2009] | - | + | +¥ | |
| Polcari [2009] | ? | ? | ? | |
| Rocco [2009] | = | + | + | |
| White [2009] | + | ? | ? | |
| Wood [2007] | = | ? | ? | |
| Yates [2009] | ? | ? | ? | |
| Zorn [2009] | ? | ? | ? | |
| Weizer [2010] | = | ? | ? | |
| Hohwu [2009] | ? | ? | ? | |
| O’Malley [2006] | + | ? | ? | |
| Ball [2006] | ? | -¥ | +¥ | |
+/- evidence favouring both technologies.
evidence not provided.
evidence that showed no difference between technologies.
evidence favouring the technology.
evidence not supportive of the technology compared to the referent technology, e.g. laparoscopy or retropubic as the referent groups.
Results refer to those that were reported as statistically significant.
Systematic review. Refers to the overall trend in results, regardless of statistical significance.
For study completeness, outcome information was also included without a statistical test for difference, especially if the magnitude of the difference was substantial.
Positive surgical margins is not specific to tumour stage, except for Hakimi et al. (2009), which refers to stage II disease.
Note: Drouin et al. (2009) was excluded.
Table 6: Studies taken from Ficarra et al., 2009 (N=13 Studies).
| Author [Year] | Study Location | Study Design | Length of Follow-up | Cases/Referent* (no.) |
|---|---|---|---|---|
| Ahlering [2004] | California, USA | Prospective C-C± | - | 60/60 |
| Farnham [2006] | Tennessee, USA | Prospective cohort | - | 176/103 |
| Fracalanza [2008] | Italy | Prospective cohort± | - | 35/26 |
| Hu [2006] | California, USA | Prospective C-C | - | 322/358§ |
| Joseph [2005] | Rochester, USA | Retrospective MRR± | 3 months | 50/50§ |
| Krambeck [2009] | Mayo Clinic, USA | Retrospective, database¥ | Up to 1 year | 294/588 |
| Menon [2002] | Michigan, USA | Prospective cohort | ~ 6 weeks | 30/30 |
| Menon [2002a] | Michigan, USA | Prospective cohort | Up to 8.5 months | 40/40§ |
| Nelson [2007] | Tennessee, USA | Prospective cohort± | ? | 629/374 |
| Rozet [2007] | France | Retrospective, database¥ | - | 133/133§ |
| Smith [2007] | Tennessee, USA | C-C (historical)±Ref | - | 200/200 |
| Tewari [2003] | Michigan, USA | Prospective cohort± | Variable | 200/100 |
| Webster [2005] | Tennessee, USA | Prospective cohort | Up to 14 days | 159/154 |
Sample sizes according to: robotic/retropubic prostatectomy.
Robotic/laparoscopic.
Consecutive patients.
Individually-matched study design; 2:1 ratio (Krambeck et al., 2009).
Information not provided.
Note: C-C, Case-case comparison; MRR, medical record review; Ref, referent group.
Table 7: Overall Summary of the Comparisons in the Meta-Analysis.
| Outcomes (units) | Comparison | MD/OR | Pt | 95% CI | ||
|---|---|---|---|---|---|---|
| Gynecology | ||||||
| Length of hospitalization (days) | RB vs. OS | MD | -2.05 | -2.72 | -1.39 | * |
| RB vs. LP | MD | -0.21 | -0.31 | -0.10 | * | |
| Complications | RB vs. OS | OR | 0.37 | 0.23 | 0.61 | * |
| RB vs. LP | OR | 0.76 | 0.52 | 1.09 | ||
| Operation time (hours) | RB vs. OS | MD | 0.66 | 0.16 | 1.16 | |
| RB vs. LP | MD | 0.03 | -0.47 | 0.53 | ||
| Blood loss (ml) | RB vs. OS | MD | -223.07 | -294.47 | -151.67 | * |
| RB vs. LP | MD | -74.95 | -94.77 | -55.14 | * | |
| Lymph nodes (count) | RB vs. OS | MD | -2.34 | -6.87 | 2.19 | |
| RB vs. LP | MD | -3.16 | -6.99 | 0.67 | ||
| Conversions | RB vs. OS | not applicable | ||||
| RB vs. LP | OR | 0.38 | 0.20 | 0.72 | * | |
| Prostate | ||||||
| Positive surgical margins | RB vs. RP | OR | 0.60 | 0.44 | 0.82 | * |
| RB vs. LP | OR | 1.09 | 0.66 | 1.78 | ||
| Erectile dysfunction | RB vs. RP | OR | 0.44 | 0.25 | 0.79 | * |
| RB vs. LP | OR | 0.76 | 0.30 | 1.89 | ||
| Urinary incontinence | RB vs. RP | OR | 0.42 | 0.10 | 1.85 | |
| RB vs. LP | OR | 0.60 | 0.19 | 1.92 | ||
| Length of hospitalization (days) | RB vs. RP | MD | -0.23 | -0.44 | -0.02 | * |
| RB vs. LP | MD | -0.38 | -0.91 | 0.14 | ||
| Blood loss (ml) | RB vs. RP | MD | -652.86 | -819.13 | -486.60 | * |
| RB vs. LP | MD | -167.79 | -231.67 | -103.91 | * | |
| Transfusions | RB vs. RP | OR | 0.14 | 0.05 | 0.36 | * |
| RB vs. LP | OR | 0.54 | 0.30 | 0.95 | * | |
| Operation time (hours) | RB vs. RP | MD | 0.72 | 0.01 | 1.44 | * |
| RB vs. LP | MD | -0.13 | -0.59 | 0.34 | ||
| Complications | RB vs. RP | OR | 0.46 | 0.15 | 1.42 | |
| RB vs. LP | OR | 0.62 | 0.25 | 1.53 | ||
| Pain (mg) | RB vs. RP | MD | -8.55 | -15.62 | -1.47 | * |
| RB vs. LP | not applicable | |||||
| Catheter duration (days) | RB vs. RP | MD | -1.50 | -2.77 | -0.23 | * |
| RB vs. LP | MD | -0.50 | -1.01 | 0 | ||
| Anastomotic stricture | RB vs. RP | OR | 0.25 | 0.05 | 1.23 | |
| RB vs. LP | OR | 0.49 | 0.02 | 10.26 | ||
For statistical significance.
MD, mean difference; OR, odds ratio; Pt, point estimate; CI, confidence interval; RB, robotic surgery; OS, open surgery; LP, laparoscopy; RP, retropubic. Not applicable for studies that did not have useable data, except for conversions.
Table 8: Overall Summary of Technology.
| Technology | Cancer Type | Strong Evidence of Benefits | Moderate Evidence of Benefits | Weaker Evidence of Benefits |
|---|---|---|---|---|
| Robotics |
|
↓ Length of hospitalization (CE) ↓ Blood loss (PO/S) ↓ Conversions (PO) [LP only] |
↓ Complications (S) | ↓ Operation time (PO) ↑ Node recovery (PO) |
|
↓ Length of hospitalization (CE) ↓ Blood loss (PO/S) ↓ Transfusion rate (PO/S) |
↑ Recovery of erectile function/intercourse (CE) ↓ Positive surgical margins (CE) ↓ Catheter duration (PO) ↓ Post-operative pain (CE/S) |
↓ Complications (S) ↑ Urinary continence (CE) ↓ Anastomosis (S) ↓ Operation time (PO) |
CE, clinical effectiveness; PO, peri-operative factors; S, safety; LP, laparoscopy.
Table 9: Direct Costs for Robotic-Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Gynecological and Prostate Cancers from the Economic Literature Review.
| Article | Open Surgery | Endoscopic Laparoscopy | Robotic-Assisted Laparoscopy | Surgery | Setting |
|---|---|---|---|---|---|
| Bell 2008 (44) | $7,404 USD | $5,564 USD | $6,002 USD | Hysterectomy | South Dakota |
| Bolenz 2010 (124) | RRP $4,437 USD | $5,687 USD | $6,752 USD | Prostatectomy | Texas |
| Mouraviev 2007 (125) | RRP $10,704 USD | - | $10,047 USD | Prostatectomy | North Carolina |
| RPP $10,536 USD | |||||
| Burgess 2006 (126) | RRP $16,522 USD | - | $25,443 USD | Prostatectomy | Louisiana |
| RPP $16,320 USD | |||||
| Scales 2005 (127) | RRP $8,146 USD | - | $8,929 USD | Prostatectomy | North Carolina |
| Lotan 2004 (128) | RRP $5,554 USD | $6,041 USD | $7,280 USD | Prostatectomy | Texas |
RRP = radical retropubic prostatectomy;
RPP = radical perineal prostatectomy.
Table 10: Length of Stay (LOS) In-Hospital for Robotic-Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Gynecological and Prostate Cancers from the Clinical Literature Review.
| Cancer Type | Surgical Approach | |||||
|---|---|---|---|---|---|---|
| Gynecology | Robotic-Assisted Laparoscopy | Open Surgery | ||||
| Mean LOS (days) |
SD | Total | Mean LOS (days) |
SD | Total | |
| DeNardis 2008 | 1 | 0.5 | 56 | 3.2 | 1.2 | 106 |
| Magrina 2008 | 1.7 | 0.9 | 27 | 3.6 | 1.2 | 35 |
| Bell 2008 | 2.3 | 1.3 | 40 | 4 | 1.5 | 40 |
| Estape 2009 | 2.6 | 2.1 | 32 | 4 | 1.7 | 14 |
| Maggioni 2009 | 3.7 | 1.2 | 40 | 5 | 2.4 | 40 |
| Boggess 2008 | 1 | 0.2 | 103 | 4.4 | 2 | 138 |
| 298 | 373 | |||||
| Weighted Average | 1.77 | 3.99 | ||||
| Gynecology | Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | ||||
| Mean LOS (days) |
SD | Total | Mean LOS (days) |
SD | Total | |
| Magrina 2008 | 1.7 | 0.9 | 27 | 2.4 | 1.5 | 31 |
| Cardenas 2010 | 1.88 | 1.67 | 102 | 2.31 | 2.21 | 173 |
| Estape 2009 | 2.6 | 2.1 | 32 | 2.3 | 1.4 | 17 |
| Bell 2008 | 2.3 | 1.3 | 40 | 2 | 1.2 | 30 |
| Boggess 2008 | 1 | 0.2 | 103 | 1.2 | 0.5 | 81 |
| 304 | 332 | |||||
| Weighted Average | 1.70 | 2.02 | ||||
| Weighted Average | 1.73 | |||||
| Prostate | Robotic-Assisted Laparoscopy | Open Surgery | ||||
| Mean LOS (days) |
SD | Total | Mean LOS (days) |
SD | Total | |
| Ou 2009 | 7.33 | 2.32 | 30 | 8.37 | 2.22 | 30 |
| D'Alonzo 2009 | 1.83 | 3.21 | 256 | 2.33 | 1.08 | 280 |
| Wood 2007 | 1.2 | 0.84 | 117 | 1.3 | 0.95 | 89 |
| 403 | 399 | |||||
| Weighted Average | 2.06 | 2.55 | ||||
| Prostate | Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | ||||
| Mean LOS (days) |
SD | Total | Mean LOS (days) |
SD | Total | |
| Rozet 2007 | 5.4 | NA | 133 | 4.9 | NA | 133 |
| Hakimi 2009 | 1.95 | NA | 75 | 3.4 | NA | 75 |
| Srinualnad 2008 | 6.9 | 2 | 34 | 8 | 2.8 | 34 |
| Ploussard 2009 | 4.4 | 2.5 | 83 | 4.6 | 1.7 | 205 |
| 325 | 447 | |||||
| Weighted Average | 4.51 | 4.75 | ||||
| Weighted Average | 3.15 | |||||
SD, standard deviation.
Table 11: Operation Time for Robotic-Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Gynecological and Prostate Cancers from the Clinical Literature Review.
| Cancer Type | Surgical Approach | |||||
|---|---|---|---|---|---|---|
| Gynecology | Robotic-Assisted Laparoscopy | Open Surgery | ||||
| Mean (hours) | SD | Total | Mean | SD | Total | |
| Boggess 2008[a] | 3.52 | 0.76 | 51 | 4.13 | 0.81 | 49 |
| Jung 2010 | 3.22 | 1.01 | 28 | 3.13 | 1.28 | 56 |
| Magrina 2008 | 3.16 | 0.73 | 27 | 2.78 | 0.55 | 35 |
| Estape 2009 | 2.4 | 0.8 | 32 | 1.9 | 0.6 | 14 |
| Boggess 2008 | 3.19 | 0.6 | 103 | 2.44 | 0.81 | 138 |
| Maggioni 2009 | 4.54 | 0.71 | 40 | 3.33 | 1.09 | 40 |
| Bell 2008 | 3.07 | 0.69 | 40 | 1.81 | 0.69 | 40 |
| DeNardis 2008 | 2.95 | 0.92 | 56 | 1.32 | 0.28 | 106 |
| 377 | 478 | |||||
| Weighted Average | 3.26 | 2.48 | ||||
| Gynecology | Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | ||||
| Mean (hours) | SD | Total | Mean | SD | Total | |
| Magrina 2008 | 3.16 | 0.73 | 27 | 3.67 | 0.63 | 31 |
| Estape 2009 | 2.4 | 0.8 | 32 | 2.2 | 0.7 | 17 |
| Jung 2010 | 3.22 | 1.01 | 28 | 2.75 | 0.72 | 25 |
| Cardenas 2010 | 3.95 | 0.95 | 102 | 2.97 | 0.98 | 173 |
| Seamon 2009 | 4.03 | 0.88 | 105 | 4.78 | 0.92 | 76 |
| Boggess 2008 | 3.19 | 0.6 | 103 | 3.56 | 0.58 | 81 |
| Bell 2008 | 3.07 | 0.69 | 40 | 2.85 | 0.6 | 30 |
| 437 | 433 | |||||
| Weighted Average | 3.50 | 3.40 | ||||
| Weighted Average | 3.39 | |||||
| Prostate | Robotic-Assisted Laparoscopy | Open Surgery | ||||
| Mean (hours) | SD | Total | Mean | SD | Total | |
| Ou 2009 | 3.42 | 1.71 | 30 | 3.55 | 0.62 | 30 |
| Wood 2007 | 3.5 | 0.69 | 117 | 2.72 | 0.48 | 89 |
| D'Alonzo 2009 | 4.93 | 1.27 | 256 | 3.22 | 1.15 | 280 |
| Lo 2010 | 5.1 | 1.42 | 20 | 4.82 | 1.07 | 20 |
| 423 | 419 | |||||
| Weighted Average | 4.44 | 3.21 | ||||
| Prostate | Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | ||||
| Mean (hours) | SD | Total | Mean | SD | Total | |
| Joseph 2005 | 3.37 | 0.63 | 50 | 3.92 | 0.2 | 50 |
| Menon 2002a | 4.57 | 0.25 | 40 | 4.3 | 0.21 | 40 |
| Ploussard 2006 | 2.43 | 0.57 | 83 | 2.75 | 0.82 | 2.05 |
| Srinualnad 2008 | 3.99 | 1.79 | 34 | 3.78 | 1.07 | 34 |
| 207 | 126.05 | |||||
| Weighted Average | 3.33 | 3.98 | ||||
| Weighted Average | 4.07 | |||||
SD, standard deviation.
Table 12: Positive Surgical Margins for Robotic-Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Prostate Cancer from the Clinical Literature Review.
| Prostate Cancer | Surgical Approach | |||||
|---|---|---|---|---|---|---|
| Robotic-Assisted Laparoscopy | Open Surgery | |||||
| Events | % | Total | Events | % | Total | |
| Smith 2007 | 16 | 9.4% | 171 | 33 | 24.1% | 137 |
| Ahlering 2004 | 2 | 4.5% | 44 | 4 | 9.1% | 44 |
| Ficarra 2009[a] | 6 | 12.2% | 49 | 7 | 11.7% | 60 |
| Fracalanza 2008 | 4 | 17.4% | 23 | 1 | 9.1% | 11 |
| White 2009 | 8 | 22.9% | 35 | 15 | 42.9% | 35 |
| Laurila 2009 | 8 | 10.0% | 80 | 11 | 15.1% | 73 |
| Rocco 2009 | 18 | 15.0% | 120 | 41 | 17.1% | 240 |
| Ou 2009 | 2 | 13.3% | 15 | 0 | 0.0% | 15 |
| O'Malley 2006 | 10 | 11.2% | 89 | 13 | 19.4% | 67 |
| Total events | 74 | 626 | 125 | 682 | ||
| Weighted Average | 11.8% | 18.3% | ||||
| Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | |||||
| Events | % | Total | Events | % | Total | |
| Trabulsi 2008 | 2 | 4.7% | 43 | 20 | 12.4% | 161 |
| Hakimi 2009 | 7 | 10.9% | 64 | 9 | 12.7% | 71 |
| Rozet 2007 | 23 | 20.9% | 110 | 16 | 15.5% | 103 |
| Srinualnad | 6 | 30.0% | 20 | 2 | 11.8% | 17 |
| Total events | 38 | 237 | 47 | 352 | ||
| Weighted Average | 16.0% | 13.4% | ||||
| Weighted Average | 13.0% | |||||
Table 13: Conversions to Open Surgery for Robotic-Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Gynecological Cancer from the Clinical Literature Review.
| Gynecology | Surgical Approach | |||||
|---|---|---|---|---|---|---|
| Robotic-Assisted Laparoscopy | Endoscopic Laparoscopy | |||||
| Events | % | Total | Events | % | Total | |
| Cardenas 2010 | 1 | 0.98% | 102 | 9 | 5.20% | 173 |
| Seamon 2009 | 13 | 12.38% | 105 | 20 | 26.32% | 76 |
| Boggess 2008 | 3 | 2.91% | 103 | 4 | 4.94% | 81 |
| Total | 17 | 310 | 33 | 330 | ||
| Weighted Averages | 5.48% | 10.00% | ||||
Table 14: Direct Resources/Costs Associated with Robotic Assisted Laparoscopy, Endoscopic Laparoscopy and Open Surgery for Gynecological and Prostate Cancers in Ontario.
| Procedure | Professional Fees | Hospital Cost | Radiotherapy Cost | Conversion to OS | Total Cost/Case |
|---|---|---|---|---|---|
| Prostatectomy | |||||
| Robotic-Assisted Laparoscopy | $2,507 | $10,276 | $2,795 | $15,578 | |
| Prostatectomy | |||||
| Endoscopic Laparoscopy | $2,479 | $7,750 | $2,876 | $13,105 | |
| Prostatectomy | |||||
| Open Retropubic Surgery | $1,810 | $6,922 | $3,948 | $12,680 | |
| Hysterectomy | |||||
| Robotic-Assisted Laparoscopy | $1,571 | $10,250 | $512 | $12,333 | |
| Hysterectomy | |||||
| Endoscopic Laparoscopy | $1,388 | $7,724 | $934 | $10,045 | |
| Hysterectomy | |||||
| Open Surgery | $1,146 | $8,191 | $9,338 |
Table 15: Costs Associated with Robotic Surgical System.
| Upfront Costs | USD | Comments |
|---|---|---|
| Da Vinci Si | $2,600,000 | Acquisition cost for a 10 year lifespan; Canada has 11 machines with Ontario having 4, 2 in London (London Health Sciences Centre and St. Joseph’s Health Care) and 2 in Toronto (Toronto General Hospital and St. Michaels Hospital) |
| Initial Instruments and Accessories | $331,177 | Upfront instrumentation required - allows surgery on approx 40-80 patients |
| Service contract for 5 years | $700,000 | 5-year service required with first year on warranty at 175K/year - not mandatory that it's upfront; Service is usually renewed at same cost thereafter |
| Total | $3,631,177 | |
| Disposables | $2,526 | Cost per patient (n=70) - will increase depending on quantity |
Table 16: Volumes of Prostatectomy and Hysterectomy in Ontario – FYs 2004-2008.
| VOLUMES | 2004/2005 | 2005/2006 | 2006/2007 | 2007/2008 | 2008/2009 |
|---|---|---|---|---|---|
| OS Prostatectomies | 2545 | 2938 | 2836 | 2954 | 2643 |
| EL Prostatectomies | 94 | 276 | 350 | 421 | 430 |
| All Prostatectomies | 2639 | 3214 | 3186 | 3375 | 3073 |
| OS Hysterectomies | 1361 | 1245 | 1414 | 1364 | 1349 |
| EL Hysterectomies | 6 | 12 | 25 | 58 | 111 |
| All Hysterectomies | 1367 | 1257 | 1439 | 1422 | 1460 |
OS = open surgery; EL = endoscopic laparoscopy.
Table 17: Costs of Prostatectomy and Hysterectomy in Ontario – FYs 2004-2008.
| COSTS | 2004/2005 | 2005/2006 | 2006/2007 | 2007/2008 | 2008/2009 |
|---|---|---|---|---|---|
| OS Prostatectomies | 32.27 M | 37.25 M | 35.96 M | 37.46 M | 33.51 M |
| EL Prostatectomies | 1.23 M | 3.62 M | 4.59 M | 5.52 M | 5.64 M |
| All Prostatectomies | 33.50 M | 40.87 M | 40.55 M | 42.97 M | 39.15 M |
| OS Hysterectomies | 12.71 M | 11.63 M | 13.20 M | 12.74 M | 12.60 M |
| EL Hysterectomies | 0.06 M | 0.12 M | 0.25 M | 0.58 M | 1.11 M |
| All Hysterectomies | 12.77 M | 11.75 M | 13.45 M | 13.32 M | 13.71 M |
Table 18: Volumes of Prostatectomy and Hysterectomy in Ontario – Projected Estimates for Years 1-3.
| VOLUMES | Year 1 | Year 2 | Year 3 |
|---|---|---|---|
| OS Prostatectomies | 2847 | 2868 | 2889 |
| EL Prostatectomies | 559 | 641 | 723 |
| All Prostatectomies | 3406 | 3509 | 3612 |
| OS Hysterectomies | 1375 | 1385 | 1394 |
| EL Hysterectomies | 119 | 145 | 170 |
| All Hysterectomies | 1494 | 1529 | 1565 |
OS = open surgery; EL = endoscopic laparoscopy.
Table 19: Net Impact with 100% Uptake Rate by Robotic-Assisted Laparoscopy in Ontario in Years 1-3.
| 100% Uptake | |||
|---|---|---|---|
| Hysterectomy | Year 1 | Year 2 | Year 3 |
| OS + EL | 14.0M | 14.4M | 14.7M |
| 100% RAL | 18.5M | 19.0M | 19.4M |
| Net Impact | 4.5M | 4.6M | 4.7M |
| Prostatectomy | Year 1 | Year 2 | Year 3 |
| OS + EL | 43.4M | 44.7M | 46.1M |
| 100% RAL | 53.1 M | 54.7 M | 56.3 M |
| Net Impact | 9.7M | 10.0M | 10.2M |
OS = open surgery; EL = endoscopic laparoscopy; RAL = robotic assisted laparoscopy.
Table 20: Net impact with 65% Uptake Rate by Robotic-Assisted Laparoscopy in Ontario in Years 1-3.
| 65% Uptake | |||
|---|---|---|---|
| Hysterectomy | Year 1 | Year 2 | Year 3 |
| OS + EL | 14.0M | 14.4M | 14.7M |
| 65% RAL + 35% OS + EL | 16.9M | 17.3M | 17.8M |
| Net Impact | 2.9M | 2.9M | 3.1M |
| Prostatectomy | Year 1 | Year 2 | Year 3 |
| OS + EL | 43.4M | 44.7M | 46.1M |
| 65% RAL + 35% OS + EL | 49.7M | 51.2M | 52.7M |
| Net Impact | 6.3M | 6.5M | 6.6M |
OS = open surgery; EL = endoscopic laparoscopy; RAL = robotic assisted laparoscopy.
Suggested Citation
This report should be cited as follows:
Medical Advisory Secretariat. Robotic-assisted minimally invasive surgery for gynecologic and urologic oncology: an evidence-based analysis. Ont Health Technol Assess Ser [Internet]. 2010 December [cited YYYY MM DD]; 10(27) 1-118. Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_robotic_surgery_20101220.pdf.
Permission Requests
All inquiries regarding permission to reproduce any content in the Ontario Health Technology Assessment Series should be directed to MASinfo.moh@ontario.ca.
How to Obtain Issues in the Ontario Health Technology Assessment Series
All reports in the Ontario Health Technology Assessment Series are freely available in PDF format at the following URL: www.health.gov.on.ca/ohtas.
Print copies can be obtained by contacting MASinfo.moh@ontario.ca.
Conflict of Interest Statement
All analyses in the Ontario Health Technology Assessment Series are impartial and subject to a systematic evidence-based assessment process. There are no competing interests or conflicts of interest to declare.
Peer Review
All Medical Advisory Secretariat analyses are subject to external expert peer review. Additionally, the public consultation process is also available to individuals wishing to comment on an analysis prior to finalization. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Contact Information
The Medical Advisory Secretariat
Ministry of Health and Long-Term Care
20 Dundas Street West, 10th floor
Toronto, Ontario
CANADA
M5G 2C2
Email: MASinfo.moh@ontario.ca
Telephone: 416-314-1092
ISSN 1915-7398 (Online)
ISBN 978-1-4435-4452-8 (PDF)
About the Medical Advisory Secretariat
The Medical Advisory Secretariat is part of the Ontario Ministry of Health and Long-Term Care. The mandate of the Medical Advisory Secretariat is to provide evidence-based policy advice on the coordinated uptake of health services and new health technologies in Ontario to the Ministry of Health and Long-Term Care and to the healthcare system. The aim is to ensure that residents of Ontario have access to the best available new health technologies that will improve patient outcomes.
The Medical Advisory Secretariat also provides a secretariat function and evidence-based health technology policy analysis for review by the Ontario Health Technology Advisory Committee (OHTAC).
The Medical Advisory Secretariat conducts systematic reviews of scientific evidence and consultations with experts in the health care services community to produce the Ontario Health Technology Assessment Series.
About the Ontario Health Technology Assessment Series
To conduct its comprehensive analyses, the Medical Advisory Secretariat systematically reviews available scientific literature, collaborates with partners across relevant government branches, and consults with clinical and other external experts and manufacturers, and solicits any necessary advice to gather information. The Medical Advisory Secretariat makes every effort to ensure that all relevant research, nationally and internationally, is included in the systematic literature reviews conducted.
The information gathered is the foundation of the evidence to determine if a technology is effective and safe for use in a particular clinical population or setting. Information is collected to understand how a new technology fits within current practice and treatment alternatives. Details of the technology’s diffusion into current practice and input from practising medical experts and industry add important information to the review of the provision and delivery of the health technology in Ontario. Information concerning the health benefits; economic and human resources; and ethical, regulatory, social and legal issues relating to the technology assist policy makers to make timely and relevant decisions to optimize patient outcomes.
If you are aware of any current additional evidence to inform an existing evidence-based analysis, please contact the Medical Advisory Secretariat: MASinfo.moh@ontario.ca. The public consultation process is also available to individuals wishing to comment on an analysis prior to publication. For more information, please visit http://www.health.gov.on.ca/english/providers/program/ohtac/public_engage_overview.html.
Disclaimer
This evidence-based analysis was prepared by the Medical Advisory Secretariat, Ontario Ministry of Health and Long-Term Care, for the Ontario Health Technology Advisory Committee and developed from analysis, interpretation, and comparison of scientific research and/or technology assessments conducted by other organizations. It also incorporates, when available, Ontario data, and information provided by experts and applicants to the Medical Advisory Secretariat to inform the analysis. While every effort has been made to reflect all scientific research available, this document may not fully do so. Additionally, other relevant scientific findings may have been reported since completion of the review. This evidence-based analysis is current to the date of the literature review specified in the methods section. This analysis may be superseded by an updated publication on the same topic. Please check the Medical Advisory Secretariat Website for a list of all evidence-based analyses: http://www.health.gov.on.ca/ohtas.
List of Abbreviations
- CI
Confidence interval(s)
- ICIQ-UI
International Consultation of Incontinence Questionnaire – Urinary Incontinence
- IIEF
International Index of Erectile Function
- IQR
Inter-quartile range
- LP
Laparoscopy
- MAS
Medical Advisory Secretariat
- OHTAC
Ontario Health Technology Advisory Committee
- OR
Odds ratio
- OS
Open surgery
- PSA
Prostate-specific antigen
- RB
Robotic surgery
- RCT
Randomized controlled trial
- RP
Retropubic surgery
- RR
Relative risk
- SD
Standard deviation
Footnotes
Safety measure
Safety measure
Safety measure
Safety measure
Open surgery, abdominal surgery and laparotomy are used interchangeably. See Glossary for definition.
Open surgery and retropubic radical prostatectomy may be used interchangeably. See Glossary for definition.
The sample size was reduced when examining peri-operative and morbidity factors (nrobotic=92, nlaparoscopy=56), except conversions.
References
- 1.Advincula AP, Wang K. Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol. 2009;16(3):291–301. doi: 10.1016/j.jmig.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 2.Canadian Cancer Society's Steering Committee. Canadian cancer statistics. Toronto, Ontario: Canadian Cancer Society. 2009. 2009. [[cited: 2010 Mar 31]]. [Internet] 124 p. Available from: http://tinyurl.com/cxhqgm .
- 3.Sorosky JI. Endometrial cancer. Obstet Gynecol. 2008;111:436–47. doi: 10.1097/AOG.0b013e318162f690. (2 Pt 1) [DOI] [PubMed] [Google Scholar]
- 4.Canadian Cancer Society. Causes of uterine cancer. [Internet] [updated 2009 Dec 10; cited 2010 Mar 31] Available from: http://www.cancer.ca/Canada-wide/About%20cancer/Types%20of%20cancer/Causes%20of%20uterine%20cancer.aspx?sc_lang=en .
- 5.Public Health Agency of Canada. Cervical cancer facts and figures. [Internet] [updated 2009 Feb 3; cited 2010 Apr 27] Available from: http://www.phac-aspc.gc.ca/cdmc/cancer/cervical_cancer_figures-cancer_du_col_uterus-eng.php .
- 6.Health Canada. Screening for cervical cancer. [Internet] [updated 2006 Dec 6; cited 2010 Mar 31] Available from: http://www.hc-sc.gc.ca/hl-vs/iyh-vsv/diseases-maladies/cervical-uteruseng.php .
- 7.National Cancer Institute. Endometrial cancer treatment: general information about endometrial cancer. [Internet] [updated 2010 Nov 5; cited 2010 Dec 23] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/Patient .
- 8.Humphrey MM. Apte SM. The use of minimally invasive surgery for endometrial cancer. Cancer Control. 2009;16(1):30–7. doi: 10.1177/107327480901600105. [DOI] [PubMed] [Google Scholar]
- 9.National Cancer Institute. Endometrial cancer treatment: treatment option overview. [Internet] [updated 2010 Nov 5; cited 2010 Dec 23] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/endometrial/Patient/page4 .
- 10.National Cancer Institute. Cervical cancer treatment: general information about cervical cancer. [Internet] [updated 2010 Apr 6; cited 2010 Apr 21] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/cervical/Patient .
- 11.National Cancer Institute. Cervical cancer treatment: treatment option overview. [Internet] [updated 2010 Apr 6; cited 2010 Apr 21] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/cervical/Patient/page4 .
- 12.Medline Plus. Medical dictionary. [Internet] [updated 2010 May 6; cited 2010 May 6] Available from: http://www.nlm.nih.gov/medlineplus/ency/article/002915.htm .
- 13.Bandera CA. Magrina JF. Robotic surgery in gynecologic oncology. Curr Opin Obstet Gynecol. 2009;21(1):25–30. doi: 10.1097/GCO.0b013e32831ffe8e. [DOI] [PubMed] [Google Scholar]
- 14.Laberge PY. Singh SS. Surgical approach to hysterectomy: introducing the concept of technicity. J Obstet Gynaecol Can. 2009;31(11):1050–3. doi: 10.1016/S1701-2163(16)34350-X. [DOI] [PubMed] [Google Scholar]
- 15.Lefebvre G. Doing what's best. J Obstet Gynaecol Can. 2009;31(5):397–400. doi: 10.1016/s1701-2163(16)34168-8. [DOI] [PubMed] [Google Scholar]
- 16.Elit L, Kwon J. S, Schultz S. E, Przybysz R, Wilton A. S, Simunovic M, Urbach D. R. Surgery for uterine cancer. Toronto, Ontario: Institute for Clinical Evaluative Sciences. 2008. [[cited: 2010 Apr 26]]. [Internet] 146 p. Available from: http://www.ices.on.ca/file/Cancer%20Surgery%20in%20Ontario,%20Chapter%206,%20Surgery%20for%20Uterine%20Cancer.pdf .
- 17.Elit L, Kwon J. S, Schultz S. E, Przybysz R, Wilton A. S, Simunovic M, Urbach D. R. Surgery for cervical cancer. Toronto, Ontario: Institute for Clinical Evaluative Sciences. 2008. [[cited: 2010 Apr 26]]. [Internet] 188 p. Available from: http://www.ices.on.ca/file/Cancer%20Surgery%20in%20Ontario,%20Chapter%208,%20Surgery%20for%20Cervical%20Cancer.pdf .
- 18.Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, Graefen M, et al. Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 2009;55(5):1037–63. doi: 10.1016/j.eururo.2009.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Public Health Agency of Canada. Prostate cancer. [Internet] [updated 2010 Mar 4; cited 2010 May 3] Available from: http://www.phac-aspc.gc.ca/cd-mc/cancer/prostate_cancer-cancer_prostate-eng.php .
- 20.Nickel JC, Herschorn S, Corcos J, Donnelly B, Drover D, Elhilali M, et al. Canadian guidelines for the management of benign prostatic hyperplasia. Can J Urol. 2005;12(3):2677–83. [PubMed] [Google Scholar]
- 21.National Cancer Institute. Prostate cancer treatment: general information about prostate cancer. [Internet] [updated 2010 Nov 5; cited 2010 Dec 23] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/prostate/Patient .
- 22.National Cancer Institute. Prostate cancer treatment: treatment option overview. [Internet] [updated 2010 Nov 5; cited 2010 Dec 23] Available from: http://www.cancer.gov/cancertopics/pdq/treatment/prostate/Patient/page4 .
- 23.Bivalacqua TJ, Pierorazio PM, Su LM. Open, laparoscopic and robotic radical prostatectomy: optimizing the surgical approach. Surg Oncol. 2009;18(3):233–41. doi: 10.1016/j.suronc.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Canadian Institute for Health Information. Health indicators. Ottawa, Ontario: CIHI. 2009. [[cited: 2010 Apr 9]]. [Internet] 133 p. Available from: http://secure.cihi.ca/cihiweb/products/Healthindicators2009_en.pdf .
- 25.Finelli A, Sharir S, Gunraj N, Simunovic M. Surgery for prostate cancer. Toronto, Ontario: Institute for Clinical Evaluative Sciences. 2008. [[cited: 2010 Aug 9]]. [Internet] 52 p. Available from: http://www.ices.on.ca/file/Cancer%20Surgery%20in%20Ontario,%20Chapter%203,%20Surgery%20for%20Prostate%20Cancer.pdf .
- 26.Barbash GI, Glied SA. New technology and health care costs - the case of robotic-assisted surgery. N Engl J Med. 2010;363(8):701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 27.Mettler L, Schollmeyer T, Boggess J, Magrina JF, Oleszczuk A. Robotic assistance in gynecological oncology. Curr Opin Oncol. 2008;20(5):581–9. doi: 10.1097/CCO.0b013e328307c7ec. [DOI] [PubMed] [Google Scholar]
- 28.Sharma NL, Shah NC. Neal DE. Robotic-assisted laparoscopic prostatectomy. Br J Cancer. 2009;101(9):1491–6. doi: 10.1038/sj.bjc.6605341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin PS, Wakabayashi MT, Han ES. Role of robotic surgery in endometrial cancer. Curr Treat Options Oncol. 2009;10:33–43. doi: 10.1007/s11864-009-0086-4. (1-2) [DOI] [PubMed] [Google Scholar]
- 30.Boggess JF. Robotic surgery in gynecologic oncology: evolution of a new surgical paradigm. J Robotic Surg. 2007;1:31–7. doi: 10.1007/s11701-007-0011-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mendivil A, Holloway RW, Boggess JF. Emergence of robotic assisted surgery in gynecologic oncology: American perspective. Gynecol Oncol. 2009;114 doi: 10.1016/j.ygyno.2009.02.002. (2:Suppl) Suppl-31. [DOI] [PubMed] [Google Scholar]
- 32.Cho JE, Liu C, Gossner G, Nezhat FR. Laparoscopy and gynecologic oncology. Clin Obstet Gynecol. 2009;52(3):313–26. doi: 10.1097/GRF.0b013e3181b088d2. [DOI] [PubMed] [Google Scholar]
- 33.Schaeffer EM, Loeb S, Walsh PC. The case for open radical prostatectomy. Urol Clin North Am. 2010;37(1):49–55. doi: 10.1016/j.ucl.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Borden LS Jr, Kozlowski PM. Robotic-assisted laparoscopic radical prostatectomy: an objective assessment and review of the literature. ScientificWorldJournal. 2006;6:2589–061. doi: 10.1100/tsw.2006.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Visco AG, Advincula AP. Robotic gynecologic surgery. Obstet Gynecol. 2008;112(6):1369–84. doi: 10.1097/AOG.0b013e31818f3c17. [DOI] [PubMed] [Google Scholar]
- 36.Brandina R, Berger A, Kamoi K, Gill IS. Critical appraisal of robotic-assisted radical prostatectomy. Curr Opin Urol. 2009;19(3):290–6. doi: 10.1097/MOU.0b013e328329a356. [DOI] [PubMed] [Google Scholar]
- 37.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Camberlin C, Senn A, Leys M, De Laet C. Robot-assisted surgery: health technology assessment health services research. Brussels, Belgium: Belgian Health Care Knowledge Centre. 2009. [[cited: 2010 Mar 31]]. [Internet] 135 p. Report No. 104C. Available from: http://www.kce.fgov.be/Download.aspx?ID=1462 .
- 39.Tooher R. The da Vinci surgical robotics system: technology overview. Adelaide, South Australia: ASERNIP-S. 2004. Jul 1, [[cited: 2010 Mar 31]]. [Internet] 117 p. Report No. 45. Available from: http://www.surgeons.org/AM/Template.cfm?Section=ASERNIP_S_Publications&Template=/CM/ContentDisplay.cfm&ContentFileID=1887 .
- 40.Medical Advisory Secretariat. Computer-assisted surgery using telemanipulators: an evidence-based analysis. Ont Health Technol Assess Ser. 2004 Feb;4(1):1–37. [Internet] [cited 2010 05 03] Available from: http://www.health.gov.on.ca/english/providers/program/mas/tech/reviews/pdf/rev_teleman_020104.pdf . [cited 2010 Mar 31] [PMC free article] [PubMed] [Google Scholar]
- 41.Llanos, Mendez A, Villegas, Portero R. Robot-assisted surgery using da Vinci robot telemanipulation in prostatectomy. Sevilla: Ministerio de Sanidad y Consumo. 2007 51 p. [Google Scholar]
- 42.Cho JE, Nezhat FR. Robotics and gynecologic oncology: review of the literature. J Minim Invasive Gynecol. 2009;16(6):669–81. doi: 10.1016/j.jmig.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 43.DeNardis SA, Holloway RW, Bigsby GE, Pikaart DP, Ahmad S, Finkler NJ. Robotically assisted laparoscopic hysterectomy versus total abdominal hysterectomy and lymphadenectomy for endometrial cancer. Gynecol Oncol. 2008;111(3):412–7. doi: 10.1016/j.ygyno.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 44.Bell MC, Torgerson J, Seshadri-Kreaden U, Suttle AW, Hunt S. Comparison of outcomes and cost for endometrial cancer staging via traditional laparotomy, standard laparoscopy and robotic techniques. Gynecol Oncol. 2008;111(3):407–11. doi: 10.1016/j.ygyno.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 45.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A comparative study of 3 surgical methods for hysterectomy with staging for endometrial cancer: robotic assistance, laparoscopy, laparotomy. Am J Obstet Gynecol. 2008;199(4):360–9. doi: 10.1016/j.ajog.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 46.Veljovich DS, Paley PJ, Drescher CW, Everett EN, Shah C, Peters WA. III. Robotic surgery in gynecologic oncology: program initiation and outcomes after the first year with comparison with laparotomy for endometrial cancer staging. Am J Obstet Gynecol. 2008;198(6):679. doi: 10.1016/j.ajog.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Gehrig PA, Cantrell LA, Shafer A, Abaid LN, Mendivil A. Boggess JF. What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008;111(1):41–5. doi: 10.1016/j.ygyno.2008.06.030. [DOI] [PubMed] [Google Scholar]
- 48.Nezhat FR, Datta MS, Liu C, Chuang L, Zakashansky K. Robotic radical hysterectomy versus total laparoscopic radical hysterectomy with pelvic lymphadenectomy for treatment of early cervical cancer. J Soc Laparoendosc Surg. 2008;12(3):227–37. [PMC free article] [PubMed] [Google Scholar]
- 49.Boggess JF, Gehrig PA, Cantrell L, Shafer A, Ridgway M, Skinner EN, et al. A case-control study of robot-assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008;199(4):357. doi: 10.1016/j.ajog.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 50.Ko EM, Muto MG, Berkowitz RS, Feltmate CM. Robotic versus open radical hysterectomy: a comparative study at a single institution. Gynecol Oncol. 2008;111(3):425–30. doi: 10.1016/j.ygyno.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 51.Sert B, Abeler V. Robotic radical hysterectomy in early-stage cervical carcinoma patients, comparing results with total laparoscopic radical hysterectomy cases. The future is now? Int J Med Robot. 2007;3(3):224–8. doi: 10.1002/rcs.152. [DOI] [PubMed] [Google Scholar]
- 52.Lambaudie E, Houvenaeghel G, Walz J, Bannier M, Buttarelli M, Gurriet B, et al. Robot-assisted laparoscopy in gynecologic oncology. Surg Endosc. 2008;22(12):2743–7. doi: 10.1007/s00464-008-0116-5. [DOI] [PubMed] [Google Scholar]
- 53.Magrina JF, Kho RM, Weaver AL, Montero RP, Magtibay PM. Robotic radical hysterectomy: comparison with laparoscopy and laparotomy. Gynecol Oncol. 2008;109(1):86–91. doi: 10.1016/j.ygyno.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 54.Pareja R, Ramirez PT. Robotic radical hysterectomy in the management of gynecologic malignancies. J Minim Invasive Gynecol. 2008;15(6):673–6. doi: 10.1016/j.jmig.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 55.Smith AL, Pareja R, Ramirez PT. Robotic radical hysterectomy. A literature review. Minerva Ginecol. 2009;61(4):339–46. [PubMed] [Google Scholar]
- 56.Estape R, Lambrou N, Diaz R, Estape E, Dunkin N, Rivera A. A case matched analysis of robotic radical hysterectomy with lymphadenectomy compared with laparoscopy and laparotomy. Gynecol Oncol. 2009;113(3):357–61. doi: 10.1016/j.ygyno.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 57.Cho JE, Shamshirsaz AH, Nezhat C, Nezhat C, Nezhat F. New technologies for reproductive medicine: laparoscopy, endoscopy, robotic surgery and gynecology. A review of the literature. Minerva Ginecol. 2010;62(2):137–67. [PubMed] [Google Scholar]
- 58.Obermair A, Gebski V, Frumovitz M, Soliman PT, Schmeler KM, Levenback C, et al. A phase III randomized clinical trial comparing laparoscopic or robotic radical hysterectomy with abdominal radical hysterectomy in patients with early stage cervical cancer. J Minim Invasive Gynecol. 2008;15(5):584–8. doi: 10.1016/j.jmig.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 59.U.S. National Institutes of Health. Laparoscopic approach to cervical cancer. [Internet] [updated 2010 Feb 23; cited 2010 May 7] Available from: http://clinicaltrials.gov/ct2/show/NCT00614211?term=laparoscopic+robotic+radical+hysterectomy&rank=1 .
- 60.Cantrell LA, Mendivil A, Gehrig PA, Boggess JF. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a 3-year experience. Gynecol Oncol. 2010;117(2):260–5. doi: 10.1016/j.ygyno.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 61.Cardenas-Goicoechea J, Adams S, Bhat SB, Randall TC. Surgical outcomes of robotic-assisted surgical staging for endometrial cancer are equivalent to traditional laparoscopic staging at a minimally invasive surgical center. Gynecol Oncol. 2010;117(2):224–8. doi: 10.1016/j.ygyno.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geisler JP, Orr CJ, Khurshid N, Phibbs G, Manahan KJ. Robotically assisted laparoscopic radical hysterectomy compared with open radical hysterectomy. Int J Gynecol Cancer. 2010;20(3):438–42. doi: 10.1111/IGC.0b013e3181cf5c2c. [DOI] [PubMed] [Google Scholar]
- 63.Jung YW, Lee DW, Kim SW, Nam EJ, Kim JH, Kim JW, et al. Robot-assisted staging using three robotic arms for endometrial cancer: comparison to laparoscopy and laparotomy at a single institution. J Surg Oncol. 2010;101(2):116–21. doi: 10.1002/jso.21436. [DOI] [PubMed] [Google Scholar]
- 64.Hoekstra AV, Jairam-Thodla A, Rademaker A, Singh DK, Buttin BM, Lurain JR, et al. The impact of robotics on practice management of endometrial cancer: transitioning from traditional surgery. Int J Med Robot. 2009;5(4):392–7. doi: 10.1002/rcs.268. [DOI] [PubMed] [Google Scholar]
- 65.Maggioni A, Minig L, Zanagnolo V, Peiretti M, Sanguineti F, Bocciolone L, et al. Robotic approach for cervical cancer: comparison with laparotomy: a case control study. Gynecol Oncol. 2009;115(1):60–4. doi: 10.1016/j.ygyno.2009.06.039. [DOI] [PubMed] [Google Scholar]
- 66.Seamon LG, Cohn DE, Henretta MS, Kim KH, Carlson MJ, Phillips GS, et al. Minimally invasive comprehensive surgical staging for endometrial cancer: robotics or laparoscopy? Gynecol Oncol. 2009;113(1):36–41. doi: 10.1016/j.ygyno.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 67.bu-Rustum NR, Gomez JD, Alektiar KM, Soslow RA, Hensley ML, Leitao MM Jr, et al. The incidence of isolated paraaortic nodal metastasis in surgically staged endometrial cancer patients with negative pelvic lymph nodes. Gynecol Oncol. 2009;115(2):236–8. doi: 10.1016/j.ygyno.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 68.Orvieto MA, Patel VR. Evolution of robot-assisted radical prostatectomy. Scandinavian Journal of Surgery: SJS. 2009;98(2):76–88. doi: 10.1177/145749690909800203. [DOI] [PubMed] [Google Scholar]
- 69.El-Hakim A, Tewari A. Robotic prostatectomy - a review. MedGenMed. 2004;6(4):20. [PMC free article] [PubMed] [Google Scholar]
- 70.Berryhill J, Jhaveri J, Yadav R, Leung R, Rao S, El-Hakim A, et al. Robotic prostatectomy: a review of outcomes compared with laparoscopic and open approaches. Urology. 2008;72(1):15–23. doi: 10.1016/j.urology.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 71.Coelho RF, Chauhan S, Palmer KJ, Rocco B, Patel MB, Patel VR. Robotic-assisted radical prostatectomy: a review of current outcomes. BJU Int. 2009;104(10):1428–35. doi: 10.1111/j.1464-410X.2009.08895.x. [DOI] [PubMed] [Google Scholar]
- 72.Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic radical prostatectomy for localized prostate cancer: a systematic review of comparative studies. J Urol. 2006;175(6):2011–7. doi: 10.1016/S0022-5347(06)00265-5. [DOI] [PubMed] [Google Scholar]
- 73.Tooher R, Swindle P, Woo H, Miller J, Maddern G. Laparoscopic radical prostatectomy: an accelerated systematic review. South Australia: Australian Safety and Efficacy Register of New Interventional Procedures - Surgical. 2005. p. 118 p. Available from: http://www.surgeons.org/AM/Template.cfm?Section=ASERNIP_S_Publications&Template=/CM/ContentDisplay.cfm&ContentFileID=2681 .
- 74.Parsons JK, Bennett JL. Outcomes of retropubic, laparoscopic, and robotic-assisted prostatectomy. Urology. 2008;72(2):412–6. doi: 10.1016/j.urology.2007.11.026. [DOI] [PubMed] [Google Scholar]
- 75.Rocco B, Matei D-V, Melegari S, Ospina JC, Mazzoleni F, Errico G, et al. Robotic vs open prostatectomy in a laparoscopically naive centre: A matched-pair analysis. BJU Int. 2009;104(7):991–5. doi: 10.1111/j.1464-410X.2009.08532.x. [DOI] [PubMed] [Google Scholar]
- 76.Lo KL, Ng CF, Lam CN, Hou SS, To KF, Yip SK. Short-term outcome of patients with robot-assisted versus open radical prostatectomy: for localised carcinoma of prostate. Hong Kong Med J. 2010;16(1):31–5. [PubMed] [Google Scholar]
- 77.D'Alonzo RC, Gan TJ, Moul JW, Albala DM, Polascik TJ, Robertson CN, et al. A retrospective comparison of anesthetic management of robot-assisted laparoscopic radical prostatectomy versus radical retropubic prostatectomy. J Clin Anesth. 2009;21(5):322–8. doi: 10.1016/j.jclinane.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 78.Drouin SJ, Vaessen C, Hupertan V, Comperat E, Misrai V, Haertig A, et al. Comparison of midterm carcinologic control obtained after open, laparoscopic, and robot-assisted radical prostatectomy for localized prostate cancer. World J Urol. 2009;27(5):599–605. doi: 10.1007/s00345-009-0379-z. [DOI] [PubMed] [Google Scholar]
- 79.Ficarra V, Novara G, Fracalanza S, D'Elia C, Secco S, Iafrate M, et al. A prospective, non-randomized trial comparing robot-assisted laparoscopic and retropubic radical prostatectomy in one European institution. BJU Int. 2009;104(4):534–9. doi: 10.1111/j.1464-410X.2009.08419.x. [DOI] [PubMed] [Google Scholar]
- 80.Hakimi AA, Blitstein J, Feder M, Shapiro E, Ghavamian R. Direct comparison of surgical and functional outcomes of robotic-assisted versus pure laparoscopic radical prostatectomy: singlesurgeon experience. Urology. 2009;73(1):119–23. doi: 10.1016/j.urology.2008.08.491. [DOI] [PubMed] [Google Scholar]
- 81.Ou Y-C, Yang C-R, Wang J, Cheng C-L, Patel VR. Comparison of robotic-assisted versus retropubic radical prostatectomy performed by a single surgeon. Anticancer Res. 2009;29(5):1637–42. [PubMed] [Google Scholar]
- 82.Zorn KC, Katz MH, Bernstein A, Shikanov SA, Brendler CB, Zagaja GP, et al. Pelvic lymphadenectomy during robot-assisted radical prostatectomy: Assessing nodal yield, perioperative outcomes, and complications. Urology. 2009;74(2):296–302. doi: 10.1016/j.urology.2009.01.077. [DOI] [PubMed] [Google Scholar]
- 83.Wood DP, Schulte R, Dunn RL, Hollenbeck BK, Saur R, Wolf JS Jr, et al. Short-term health outcome differences between robotic and conventional radical prostatectomy. Urology. 2007;70(5):945–9. doi: 10.1016/j.urology.2007.06.1120. [DOI] [PubMed] [Google Scholar]
- 84.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Brook RH. The UCLA Prostate Cancer Index: development, reliability, and validity of a health-related quality of life measure. Med Care. 1998;36(7):1002–12. doi: 10.1097/00005650-199807000-00007. [DOI] [PubMed] [Google Scholar]
- 85.Ball AJ, Gambill B, Fabrizio MD, Davis JW, Given RW, Lynch DF, et al. Prospective longitudinal comparative study of early health-related quality-of-life outcomes in patients undergoing surgical treatment for localized prostate cancer: a short-term evaluation of five approaches from a single institution. J Endourol. 2006;20(10):723–31. doi: 10.1089/end.2006.20.723. [DOI] [PubMed] [Google Scholar]
- 86.Hohwu L, Akre O, Pedersen KV, Jonsson M, Nielsen CV, Gustafsson O. Open retropubic prostatectomy versus robot-assisted laparoscopic prostatectomy: a comparison of length of sick leave. Scand J Urol Nephrol. 2009;43(4):259–64. doi: 10.1080/00365590902834802. [DOI] [PubMed] [Google Scholar]
- 87.Ploussard G, Xylinas E, Paul A, Gillion N, Salomon L, Allory Y, et al. Is robot assistance affecting operating room time compared with pure retroperitoneal laparoscopic radical prostatectomy? J Endourol. 2009;23(6):939–43. doi: 10.1089/end.2008.0521. [DOI] [PubMed] [Google Scholar]
- 88.Srinualnad S. Early experience of robotic assisted laparoscopic radical prostatectomy. J Med Assoc Thai. 2008;91(3):377–82. [PubMed] [Google Scholar]
- 89.Carlsson S, Nilsson AE, Schumacher MC, Jonsson MN, Volz DS, Steineck G, et al. Surgeryrelated complications in 1253 robot-assisted and 485 open retropubic radical prostatectomies at the Karolinska University Hospital, Sweden. Urology. 2010;75(5):1092–7. doi: 10.1016/j.urology.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 90.Polcari AJ, Hugen CM, Sivarajan G, Woods ME, Paner GP, Flanigan RC. Comparison of open and robot-assisted pelvic lymphadenectomy for prostate cancer. J Endourol. 2009;23(8):1313–7. doi: 10.1089/end.2009.0109. [DOI] [PubMed] [Google Scholar]
- 91.Yates J, Haleblian G, Stein B, Miller B, Renzulli J, Pareek G. The impact of robotic surgery on pelvic lymph node dissection during radical prostatectomy for localized prostate cancer: the Brown University early robotic experience. Can J Urol. 2009;16(5):4842–6. [PubMed] [Google Scholar]
- 92.Laurila TA, Huang W, Jarrard DF. Robotic-assisted laparoscopic and radical retropubic prostatectomy generate similar positive margin rates in low and intermediate risk patients. Urol Oncol. 2009;27(5):529–33. doi: 10.1016/j.urolonc.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 93.Trabulsi EJ, Linden RA, Gomella LG, McGinnis DE, Strup SE, Lallas CD. The addition of robotic surgery to an established laparoscopic radical prostatectomy program: effect on positive surgical margins. Can J Urol. 2008;15(2):3994–9. [PubMed] [Google Scholar]
- 94.White MA, De Haan AP, Stephens DD, Maatman TK. Maatman TJ. Comparative analysis of surgical margins between radical retropubic prostatectomy and RALP: are patients sacrificed during initiation of robotics program? Urology. 2009;73(3):567–71. doi: 10.1016/j.urology.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 95.Weizer AZ, Strope S, Wood DP Jr. Margin control in robotic and laparoscopic prostatectomy: what are the REAL outcomes? Urol Oncol. 2010;28(2):210–4. doi: 10.1016/j.urolonc.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 96.O’Malley PJ, Van AS, Bouchier-Hayes DM, Crowe H, Costello AJ. Robotic radical prostatectomy in Australia: initial experience. World J Urol. 2006;24(2):165–70. doi: 10.1007/s00345-006-0064-4. [DOI] [PubMed] [Google Scholar]
- 97.Singh I, Hemal AK. Robotic-assisted radical prostatectomy in 2010. Expert Rev Anticancer Ther. 2010;10(5):671–82. doi: 10.1586/era.10.35. [DOI] [PubMed] [Google Scholar]
- 98.Rozet F, Jaffe J, Braud G, Harmon J, Cathelineau X, Barret E, et al. A direct comparison of robotic assisted versus pure laparoscopic radical prostatectomy: a single institution experience. J Urol. 2007;178(2):478–82. doi: 10.1016/j.juro.2007.03.111. [DOI] [PubMed] [Google Scholar]
- 99.Hu JC, Nelson RA, Wilson TG, Kawachi MH, Ramin SA, Lau C, et al. Perioperative complications of laparoscopic and robotic assisted laparoscopic radical prostatectomy. J Urol. 2006;175(2):541–6. doi: 10.1016/S0022-5347(05)00156-4. [DOI] [PubMed] [Google Scholar]
- 100.Menon M, Tewari A, Baize B, Guillonneau B, Vallancien G. Prospective comparison of radical retropubic prostatectomy and robot-assisted anatomic prostatectomy: the Vattikuti Urology Institute experience. Urology. 2002;60(5):864–8. doi: 10.1016/s0090-4295(02)01881-2. [DOI] [PubMed] [Google Scholar]
- 101.Menon M, Shrivastava A, Tewari A, Sarle R, Hemal A, Peabody JO, et al. Laparoscopic and robot assisted radical prostatectomy: establishment of a structured program and preliminary analysis of outcomes. J Urol. 2002;168(3):945–9. doi: 10.1016/S0022-5347(05)64548-X. [DOI] [PubMed] [Google Scholar]
- 102.Farnham SB, Webster TM, Herrell SD, Smith JA Jr. Intraoperative blood loss and transfusion requirements for robotic-assisted radical prostatectomy versus radical retropubic prostatectomy. Urology. 2006;67(2):360–3. doi: 10.1016/j.urology.2005.08.029. [DOI] [PubMed] [Google Scholar]
- 103.Krambeck AE, DiMarco DS, Rangel LJ, Bergstralh EJ, Myers RP, Blute ML, et al. Radical prostatectomy for prostatic adenocarcinoma: a matched comparison of open retropubic and robot-assisted techniques. BJU Int. 2009;103(4):448–53. doi: 10.1111/j.1464-410X.2008.08012.x. [DOI] [PubMed] [Google Scholar]
- 104.Joseph JV, Vicente I, Madeb R, Erturk E. Patel HR. Robot-assisted vs pure laparoscopic radical prostatectomy: are there any differences? BJU Int. 2005;96(1):39–42. doi: 10.1111/j.1464-410X.2005.05563.x. [DOI] [PubMed] [Google Scholar]
- 105.Webster TM, Herrell SD, Chang SS, Cookson MS, Baumgartner RG, Anderson LW, et al. Robotic assisted laparoscopic radical prostatectomy versus retropubic radical prostatectomy: a prospective assessment of postoperative pain. J Urol. 2005;174(3):912–4. doi: 10.1097/01.ju.0000169455.25510.ff. [DOI] [PubMed] [Google Scholar]
- 106.Smith JA Jr, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178(6):2385–9. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 107.Ahlering TE, Woo D, Eichel L, Lee DI, Edwards R, Skarecky DW. Robot-assisted versus open radical prostatectomy: a comparison of one surgeon's outcomes. Urology. 2004;63(5):819–22. doi: 10.1016/j.urology.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 108.Fracalanza S, Ficarra V, Cavalleri S, Galfano A, Novara G, Mangano A, et al. Is robotically assisted laparoscopic radical prostatectomy less invasive than retropubic radical prostatectomy? Results from a prospective, unrandomized, comparative study. BJU Int. 2008;101(9):1145–9. doi: 10.1111/j.1464-410X.2008.07513.x. [DOI] [PubMed] [Google Scholar]
- 109.Tewari A, Srivasatava A, Menon M. Members of the VIP Team. A prospective comparison of radical retropubic and robot-assisted prostatectomy: experience in one institution. BJU Int. 2003;92(3):205–10. doi: 10.1046/j.1464-410x.2003.04311.x. [DOI] [PubMed] [Google Scholar]
- 110.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernardini MQ, Murphy JK. Issues surrounding lymphadenectomy in the management of endometrial cancer. J Surg Oncol. 2009;99(4):232–41. doi: 10.1002/jso.21200. [DOI] [PubMed] [Google Scholar]
- 112.Chan JK, Urban R, Cheung MK, Shin JY, Husain A, Teng NN, et al. Lymphadenectomy in endometrioid uterine cancer staging: how many lymph nodes are enough? A study of 11,443 patients. Cancer. 2007;109(12):2454–60. doi: 10.1002/cncr.22727. [DOI] [PubMed] [Google Scholar]
- 113.Skarecky DW, Brenner M, Rajan S, Rodriguez E Jr, Narula N, Melgoza F, et al. Zero positive surgical margins after radical prostatectomy: is the end in sight. Expert Rev Med Devices. 2008;5(6):709–17. doi: 10.1586/17434440.5.6.709. [DOI] [PubMed] [Google Scholar]
- 114.Smith J, Chan RC, Chang SS, Herrell SD, Clark PE, Baumgartner R, et al. A comparison of the incidence and location of positive surgical margins in robotic assisted laparoscopic radical prostatectomy and open retropubic radical prostatectomy. J Urol. 2007;178(6):2385–90. doi: 10.1016/j.juro.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 115.Patel VR, Shah K, Palmer KJ, Thaly R, Coughlin G. Robotic-assisted laparoscopic radical prostatectomy: a report of the current state. Expert Rev Anticancer Ther. 2007;7(9):1269–78. doi: 10.1586/14737140.7.9.1269. [DOI] [PubMed] [Google Scholar]
- 116.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–30. doi: 10.1016/s0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 117.Sairam K, Dasgupta P. Robot assisted radical prostatectomy: current concepts. Minerva Urol Nefrol. 2009;61(2):115–20. [PubMed] [Google Scholar]
- 118.Orvieto MA, Coelho RF, Chauhan S, Mathe M, Palmer K, Patel VR. Erectile dysfunction after robot-assisted radical prostatectomy. Expert Rev Anticancer Ther. 2010;10(5):747–54. doi: 10.1586/era.10.16. [DOI] [PubMed] [Google Scholar]
- 119.The SAGES-MIRA Robotic Surgery Consensus Group. A consensus document on robotic surgery. Los Angeles: Society of American Gastrointestinal and Endoscopic Surgeons. 2007. [[cited: 2010 Apr 6]]. p. 22 p. [Internet] Available from: http://www.sages.org/publications/publicationpdf.php?id=ROBOT .
- 120.Ontario Case Costing Initiatve. Ontario case costing initiative. [Internet] [updated 2009; cited 2009 Jun 18] Available from: http://www.occp.com/
- 121.Canadian Institute for Health Information. Canadian Classification of Health Interventions - ICD-10-CA/CCI, Version 2006. [Google Scholar]
- 122.Ontario Ministry of Health and Long-Term Care. Ontario schedule of benefits for physician services under the Health Insurance Act. [Internet] [updated 2010 Mar 24; cited 2010 Apr] Available from: http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html .
- 123.Ontario Ministry of Health and Long-Term Care. Intellihealth provincial health planning database. [Internet] [updated 2009; cited 2009 Nov 1] Available from: https://www.intellihealth.moh.gov.on.ca/SASWebReportStudio/logoff.do?forceLogoff=true .
- 124.Bolenz C, Gupta A, Hotze T, Ho R, Cadeddu JA, Roehrborn CG, et al. Cost comparison of robotic, laparoscopic, and open radical prostatectomy for prostate cancer. Eur Urol. 2010;57(3):453–8. doi: 10.1016/j.eururo.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 125.Mouraviev V, Nosnik I, Sun L, Robertson CN, Walther P, Albala D, et al. Financial comparative analysis of minimally invasive surgery to open surgery for localized prostate cancer: a single-institution experience. Urology. 2007;69(2):311–4. doi: 10.1016/j.urology.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 126.Burgess SV, Atug F, Castle EP, Davis R, Thomas R. Cost analysis of radical retropubic, perineal, and robotic prostatectomy. J Endourol. 2006;20(10):827–30. doi: 10.1089/end.2006.20.827. [DOI] [PubMed] [Google Scholar]
- 127.Scales CD Jr, Jones PJ, Eisenstein EL, Preminger GM, Albala DM. Local cost structures and the economics of robot assisted radical prostatectomy. J Urol. 2005;174(6):2323–9. doi: 10.1097/01.ju.0000181830.43340.e7. [DOI] [PubMed] [Google Scholar]
- 128.Lotan Y, Cadeddu JA, Gettman MT. The new economics of radical prostatectomy: Cost comparison of open, laparoscopic and robot assisted techniques. J Urol. 2004;172:1431–5. doi: 10.1097/01.ju.0000139714.09832.47. (4 I) [DOI] [PubMed] [Google Scholar]


