Human blood-derived macrophages are non-permissive for influenza virus propagation, and fail to elicit inflammatory and antiviral responses upon infection with high pathogenic avian influenza viruses.
Keywords: innate immunity, inflammasome
Abstract
Systemic infections with HPAIVs, such as H5N1, are characterized by cytokine burst and sepsis. We investigated the role of human monocyte-derived macrophages in these events after infection with different influenza virus strains. Macrophages were infected with low pathogenic H1N1 (PR8) or high pathogenic H7N7 (FPV) and H5N1 (KAN-1) subtypes. Macrophages were found to be nonpermissive for influenza virus propagation. Surprisingly, transcriptome analysis revealed an insufficient innate immune response of macrophages only to HPAIV infections. Induction of inflammatory cytokines, as well as type I IFNs, was significantly attenuated in H5N1- and H7N7-infected cells, contradicting a primary role of macrophages for the cytokine burst. Furthermore, inflammasome activation was impaired significantly in HPAIV-infected macrophages. Interestingly, this finding correlated with a complete suppression of viral protein M2 expression after HPAIV infection, which is known to be involved in influenza viral inflammasome activation. In summary, our data provide first evidences for a strategy of how HPAIVs avoid initial inflammatory responses of macrophages facilitating virus spreading and progression to the systemic stage of disease.
Introduction
HPAIV of the H5N1 subtype can cause severe infections of human hosts with high mortality. Such severe human H5N1 infections are characterized by SIRS, as well as multiorgan failure, leading to ∼60% of fatal cases [1–5]. Thereby, patients show an extremely strong release of cytokines, a so-called cytokine burst, which is supposed to be at least partially responsible for fatal outcomes [6, 7]. However, the source of uncontrolled cytokine production is not understood completely.
The neuro and EC tropism is known to play a critical role in fatal HPAIV infections [8–11]. Recently, specific response patterns of ECs upon H5N1 infections have been shown to contribute to the overwhelming proinflammatory response compared with low pathogenic influenza strains [12, 13]. Nevertheless, a more complex interplay of various innate immune cells is likely to explain the magnitude and rapid kinetics of cytokine burst in HPAIV infections promoting the characteristic picture of HPAIV-induced SIRS.
Basically, macrophages are known to be important amplifiers of antiviral responses [14]. In the lung, H5N1 virus encounters two types of macrophages: resident alveolar macrophages as well as tissue macrophages. Whereas resident alveolar macrophages are reported to produce low levels of cytokines and to be less phagocytic than other tissue macrophages, they get activated during influenza infections and produce higher amounts of cytokines [15]. Furthermore, infiltrating macrophages differentiated from circulating blood monocytes also play a role for the first antiviral response during local infection of the lung [15]. These cells get even more important once infection is spreading, leading to viral sepsis. The exact role of different types of macrophages involved in the initial antiviral response is still not known [15]. After infection with the low pathogenic H1N1 influenza virus PR8, human macrophages have been shown to produce chemokines such as CCL5 (RANTES), CCL3 (MIP-1α), and CCL2 (MCP-1), as well as TNF-α [7, 16–21].
In the present study, we analyzed the response of primary human blood-derived macrophages in a comparative approach after infection with low and high pathogenic influenza viruses to elucidate their contribution to virus propagation and development of a cytokine burst. Contrary to expectation and in contrast to low pathogenic H1N1 influenza virus, we uncover a HPAIV-specific, thorough impairment of the inflammatory and antiviral immune response in which a specific suppression of viral protein M2 expression is likely involved.
MATERIALS AND METHODS
Cell preparation and culture
Human monocytes were isolated from buffy coats of unrelated, healthy German blood donors, as described earlier [22, 23]. For microarray experiments, homogenous cell populations are necessary, as for these experiments, monocytes were obtained by cell apheresis in three single donors. Upon approval of the local ethics committee, human blood samples were taken from healthy blood donors, who provided written, informed consent. Both isolation procedures yielded purities of >90% of monocytes. Human macrophages were obtained by cultivating isolated monocytes in Teflon bags in RPMI-1640 medium (Biochrom AG, Berlin, Germany), supplemented with 1% glutamine, 1% penicillin-streptomycin, and 10% human AB serum provided by the Department of Transfusion Medicine, University of Muenster (Germany). Medium was substituted every 3 days, and cells were used in experiments on Day 7. Purity of macrophages was determined by flow cytometric analysis of monocyte/macrophage markers RM3/1 and 25F9 and DC marker, DC sign. Cells were negative for DC sign and RM3/1 and positive for 25F9, excluding a possible differentiation of cells into DCs (Supplemental Fig. 1).
Ethics statement
Taking of blood samples from humans and cell isolation were conducted with the approval of the local ethics committee (ethics advisory board of the Ärztekammer Westfalen-Lippe and Medical Faculty of Westfaelische Wilhelms-Universitaet Muenster, Germany). Human blood samples were taken from healthy blood donors, who provided written, informed consent for the collection of samples and subsequent cell isolation and analysis. Experiments were performed according to the Declaration of Helsinki's protocols.
Virus preparation and exposure to human macrophages
The HPAIV strain KAN-1 (H5N1), isolated from a fatal human case, was used with permission from Dr. Pilaipan Puthavathana (Bangkok, Thailand). The avian influenza virus FPV (H7N7) and the human influenza virus strain PR8 (Giessen variant) were taken from the strain collection of the Institute of Molecular Virology in Muenster, Germany, and were provided initially by the Institute of Virology in Giessen, Germany. Viruses were propagated on MDCKII cells, which were cultured in MEM (PAA Laboratories, Austria) containing 10% v/v FCS and 100 U/ml penicillin/0.1 mg/ml streptomycin (Gibco, Grand Island, NY, USA).
Human macrophages were infected with five MOIs of virus. For infection with influenza viruses, macrophages were transferred to culture dishes containing 1 million cells/mL. Infection took place in serum-free medium containing BSA. Viruses were added to the culture medium and incubated with the cells for different times, as indicated for the respective experiments. After incubation, cells were washed twice in PBS and used for further experiments.
Plaque assay
We performed the plaque assays as described earlier [13]. Briefly, MDCKII cells were incubated for 30 min with cell supernatants of infected macrophages. The cell monolayers were covered with 2 ml agar, and the cells were incubated for 2 days at 37°C. Staining with neutral red enabled us to count plaques formed by infectious viral particles.
qRT-PCR
Total cellular RNA from 1 × 107 macrophages was isolated using the RNeasy kit (Qiagen, Hilden, Germany). cDNA was synthesized from 1 μg total RNA using RevertAid H Minus Moloney Murine Leukemia Virus RT (Fermentas, St.Leon-Rot, Germany). Specific primers were designed using Primer Express software package (Applied Biosystems, Foster City, CA, USA) and obtained from MWG Biotech (Ebersberg, Germany; Supplemental Table 1). qRT-PCR was performed using the QuantiTect SYBR Green PCR kit (Qiagen) and data acquired with the ABI PRISM 7900 (Applied Biosystems, Darmstadt, Germany). Gene expression was normalized to the endogenous housekeeping control gene GAPDH, and relative expression of respective genes was calculated using the comparative threshold cycle method [24].
DNA microarray hybridization and statistical data analyses
Total cellular RNA was isolated from three independent experiments with 2 × 107 primary human blood-derived macrophages, which were infected for 5 h with three influenza strains: H1N1, H7N7, and H5N1 (RNeasy kit, Qiagen).
Samples were processed for microarray hybridization using Affymetrix Human Genome U133 Plus 2.0 gene arrays, according to the manufacturer's instructions (Affymetrix, Santa Clara, CA, USA). Fluorescent signals were detected by the GeneChip Scanner 3000 and recorded and computed by GeneChip Operating Software (GCOS) version 1.4 (Affymetrix), applying the MAS5 algorithm for normalization. For a more sophisticated data analysis, we used the Expressionist Suite software from GeneData (Basel, Switzerland) as described [25]. Genes with a FC of >2.0 and a P value ≤0.05 (paired t-test) were considered. Indicated is the mean of FC of three sample pairs. “On/off”-regulated genes were evaluated as described [25], considering genes with on:off ratios of 0:3, 0:2, 1:3, 3:0, 2:0, and 3:1, respectively. From this group of on/off-regulated genes, we only included regulations with a high FC of ≥5 and a P value of <0.05 to exclude on/off phenomenons occurring around the background threshold. Microarray data are Minimum Information about a Microarray Experiment (MIAME) compliant and deposited in Gene Expression Omnibus (GEO; link: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?token=rjgjduqgoqqycxy&acc=GSE27702).
We applied PCAs to reduce mathematically the dimensionality of the entire spectrums of gene expression values of a microarray experiment to three components [26]. To identify over-represented, functional categories of genes, we compared the distribution of GO annotations on the Affymetrix U133 Plus 2.0 array with the gene group of interest, applying Fisher's exact test. In the case of genes that are represented by two or more probe sets, only one transcript was taken into account to avoid potential bias.
Immunofluorescence
The viral NP was detected in 5 × 105-infected macrophages using mouse anti-influenza NP mAb (MCA400, clone AA5H, Serotec, Duesseldorf, Germany) and Alexa 488 chicken anti-mouse IgG (H+L) fluorescence secondary antibody (Invitrogen, Grand Island, NY, USA). Cells were fixed with 4% formaldehyde and permeabilized by acetone at −20°C. Blocking was performed with 1% BSA, and incubation with antibodies was carried out for 1 h at room temperature. Between incubation steps, cells were washed with PBS. Analysis of fluorescent stainings was performed by using a Zeiss Axiovert 200M microscope.
Western blot
Cells (1×107) were lysed in RIPA buffer containing protease and phosphatase inhibitors [27]. For detection of secreted proteins, cell supernatants were mixed 1:1 with TCA and incubated on ice for 30 min and pellets lysed in SDS buffer. SDS-PAGE and Western blot staining were performed as described earlier [25] using mouse mAb M1 (GA2B, AbD Serotec, Oxford, UK), rabbit pAb NS1 (gift from the Institute of Molecular Virology), mouse mAb M2 (14C2), goat pAb PB1 (vK20; both from Santa Cruz Biotechnology, Heidelberg, Germany), rabbit pAb IL-1β (Cell Signaling Technologies, Danvers, MA, USA), mouse mAb α-tubulin (ICN, Solon, OH, USA), and rabbit pAb ERK2 (C-14; Santa Cruz Biotechnology). Protein bands were visualized using the ECL (200 μl 250 mM Luminol, 90 μl 90 mM p-cumar acid, 2 ml 1 M Tris, pH 8.5, and 7.1 μl 35% H2O2) system.
Flow cytometry
For intracellular determination of viral NP, 5 × 105 cells were harvested, washed, and fixed with 4% paraformaldehyde at room temperature for 20 min. Pellets were treated with permeabilization buffer (0.1% saponin, 1% FCS, PBS) for 10 min at room temperature, followed by incubation with FITC-labeled NP antibody (mouse mAb, MCA400, clone AA5H, Serotec, Oxford, UK) for 1 h at room temperature. Samples were washed once with permeabilization buffer, and fluorescence was determined by using a FACSCalibur (Becton Dickinson, Heidelberg, Germany), running CellQuest Pro software.
PI staining
For analysis of apoptosis, 106 cells were fixed in 3% paraformaldehyde for 15 min at room temperature and then washed with PBS. RNaseA (0.1 mg/mL) was added and samples incubated at 37°C for 30 min. PI (50 μg/mL) was added for a further 30 min at 37°C. Samples were analyzed at FACSCalibur (Becton Dickinson, Heidelberg, Germany). Apoptotic cells were defined as the cells that were not detected to be in any stage of cell cycle (G0/G1, S, G2/M).
CBA
A CBA was purchased from Becton Dickinson to determine the amount of IL-1β in cell supernatants of 1 × 106 cells. The assay was performed according to the manufacturer's instructions.
Statistical analyses
Results of experiments were assessed by Student's t-test and are expressed as means ± sem. In the case of qRT-PCR data (see Fig. 5), the Rank Sum test was used.
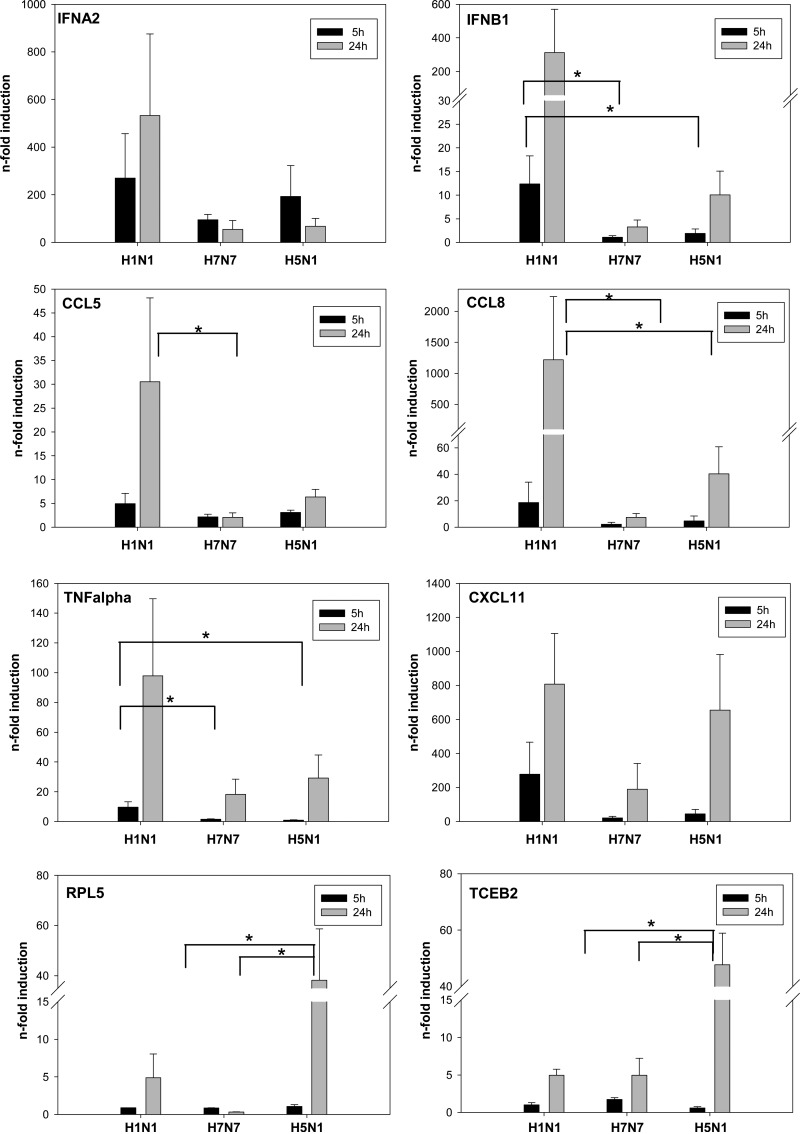
Figure 5. Cyto- and chemokine patterns in influenza-infected macrophages.
Human macrophages were infected with H1N1, H7N7, and H5N1 for 5 h and 24 h. Gene transcription of IFNA2, IFNB1, CCL5, CCL8, TNF-α, CXCL11, RPL5, and TCEB2 was determined by qRT-PCR and plotted as n-fold induction of mRNA. Values were normalized to uninfected control macrophages. Boxes represent means + sem (n=5); *P < 0.05.
RESULTS
Primary human blood macrophages are susceptible to influenza infection
Primary human blood-derived macrophages were infected with three different influenza virus strains: PR8 (H1N1; a low pathogenic human influenza reference strain), FPV (H7N7; a highly pathogenic strain for chicken and mice), and KAN-1 (H5N1; a HPAIV strain isolated from a fatal human case). The infectibility of macrophages with these viruses was confirmed by intracellular flow cytometric analysis of viral NP expression. Efficient viral infection after 5 h was achieved using a viral dose of five MOI. These conditions led to ∼80% of infected macrophages. A higher MOI did not result in further increase of infection rates (Fig. 1A). Based on these results, we chose a dosage of five MOI of virus and an infection period of 5 h for the expression profile experiments.
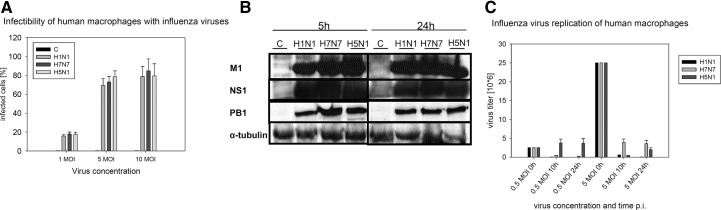
Figure 1. Infectibility of human macrophages with influenza viruses and virus propagation.
(A) In three independent experiments, human macrophages were infected with one, five, and 10 MOI of H1N1, H7N7, and H5N1. Expression of viral NP was determined by flow cytometry, 5 h p.i. Boxes show means + sd of percent NP-positive cells corresponding to the amount of infected cells [control (C)]. (B) Lysates of uninfected control macrophages and H1N1-, H7N7-, and H5N1-infected macrophages were immunoblotted 5 h and 24 h p.i. for the viral proteins M1 (18 kD), NS1 (26 kD), and PB1 (86 kD). Immunostaining of α-tubulin served as a protein-loading control. The blot is representative of three independent experiments. (C) Replication of viral particles within human macrophages was analyzed by plaque assays performed with supernatants of H1N1-, H7N7-, and H5N1-infected macrophages, 10 h and 24 h p.i. Cells were infected with 0.5 or five MOI. Boxes represent means +sd of virus titer measured in three independent experiments. The input viral load is shown at 0 h.
Translation of viral proteins was detected by immunoblotting of M1, NS1, and PB1 after 5 h and 24 h p.i. (Fig. 1B). Concerning the expression of M1, a slight difference in protein accumulation was observed after H1N1 infection and infection with H7N7 and H5N1. However, after 24 h, protein levels were the same after infection with all three virus strains. NS1, as well as PB1, showed a constant protein accumulation, independent of the virus strain used for infection. Results suggest an equal ability of viruses to infect macrophages and to efficiently translate viral proteins in these cells.
Influenza viral propagation is blocked in human macrophages
To analyze viral propagation, supernatants of HPAIV-infected macrophages were analyzed using a plaque assays (Fig. 1C). The reduction of infectious particles in the supernatants, 10 h and 24 h p.i., reflected an efficient endocytosis of viral particles by human macrophages and underpinned their infectibility by influenza virus. However, in contrast to ECs, in which influenza viruses nicely replicate [12, 13], no efficient influenza virus propagation could be detected in human macrophages independent of the strain used for infection (Fig. 1C).
Distribution of the viral NP within the host cell
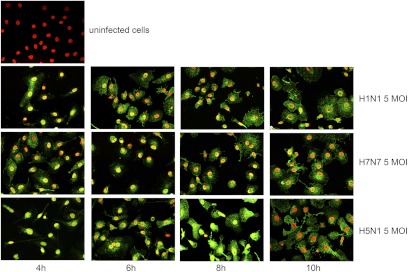
To study the cellular localization of viral NP, the major component of the vRNP complex, immunofluorescence analysis of human blood-derived macrophages at different time-points after infection (4, 6, 8, and 10 h p.i.) was performed. In cells infected with H1N1, we observed an accumulation of NP in the cell nucleus, 4 h p.i., indicating effective infection of the cells and efficient nuclear transport of the RNPs (Fig. 2). Six hours p.i., NP was released from the nucleus into the cytosol and after 8 h, correlating with the first replication cycle, nearly all viral protein could be found in the cytosol. H7N7 and H5N1 showed similar kinetics of NP accumulation and translocation as H1N1, although a slight more intense nuclear staining as well as higher accumulation of NP in the cytosol were observed within the first 8 h in H5N1 virus-infected cells (Fig. 2). At least 10 h p.i., cell nuclei were nearly all NP-free, independent of the viral strain used for infection, pointing to a similar capability to produce viral NP and to release their vRNPs out of the cell nucleus.
Figure 2. Propagation of Influenza A virus in human macrophages.
Immunofluorescence staining of viral NP (green), showing uninfected and H1N1-, H7N7-, and H5N1-infected human macrophages, 4, 6, and 10 h p.i.Cell nuclei were counterstained by DAPI (blue)-presented, false-colored red. NP is first detectable in cell nuclei and released into the cytosol during the time course of infection. The experiment shown is representative of three independent experiments. The same settings were used for taking each picture to guarantee comparability of NP amounts.
Gene expression profiling in influenza-infected human macrophages
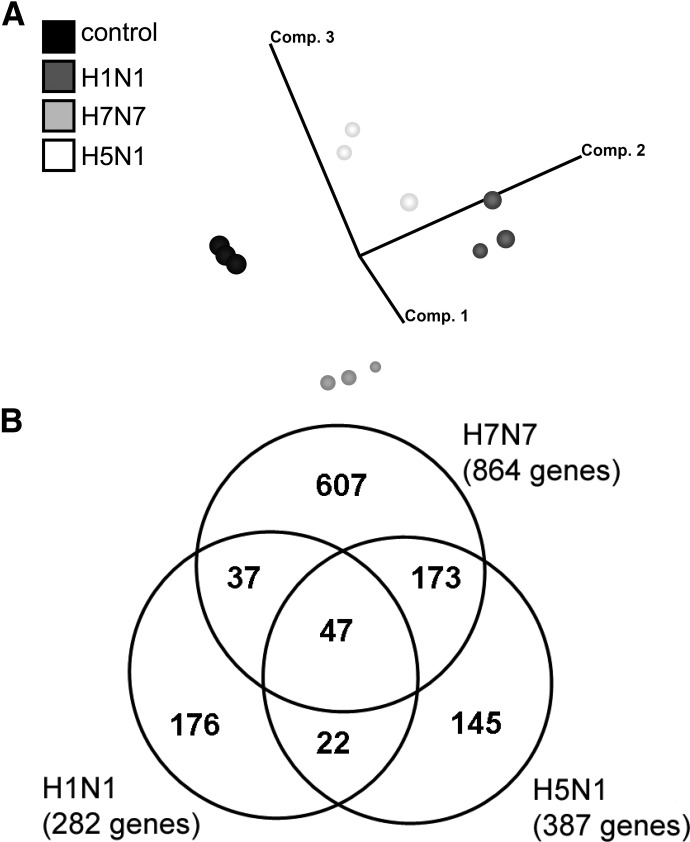
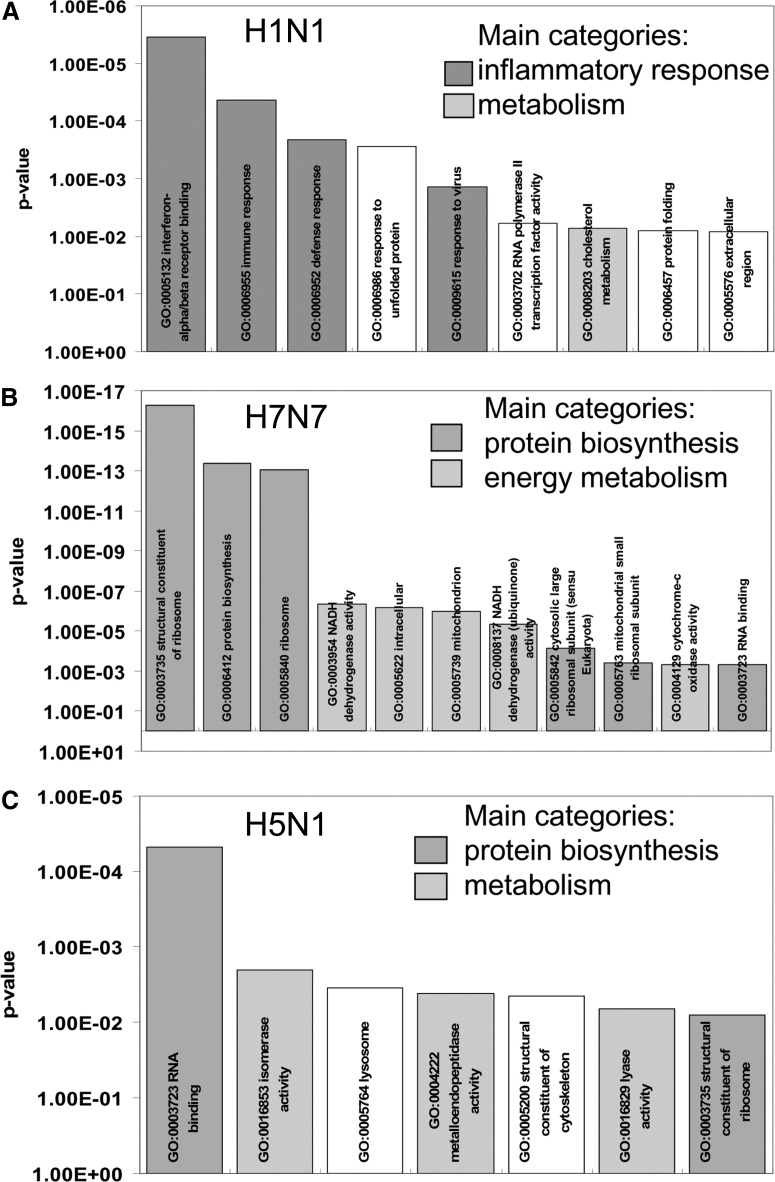
After having learned that H5N1 virus infects human macrophages but does not propagate efficiently in this cell type, we were interested in validating the virus-elicited orchestration of immune response in macrophages in comparison with H1N1 and H7N7. For efficient analysis, we chose a genome-wide comparative gene expression approach and analyzed human macrophages derived from monocytes of three independent blood donors. Cells were infected with five MOI of each virus strain for 5 h. Total RNA from infected and uninfected primary human macrophages was processed for microarray hybridization. As down-regulation of genes is significantly influenced by “cap-snatching” mechanisms [28, 29], not representative of a specific antiviral host response, we restricted our analyses to up-regulated genes. PCA of array data allowed the display of the gene expression profiles in a three-dimensional vector space, computed on the basis of all gene expression changes detectable. The unbiased display of the gene expression patterns of up-regulated genes in uninfected control cells and cells infected with H1N1, H7N7, and H5N1 virus demonstrated nice reproducibility of gene expression changes in human macrophages of different donors (Fig. 3A). Furthermore, PCA disclosed gene expression patterns induced to be distinct and specific, with H1N1 and H7N7 profiles lying closer to each other compared with H1N1 profiles, which are almost oppositely located, relating to the control profiles (Fig. 3A). We found a total of 282 genes induced by H1N1, 176 of them specifically induced by H1N1 and not by H7N7 and H5N1. Eight hundred sixty-four genes were induced by H7N7, and 607 of them were H7N7-specific. H5N1 led to up-regulation of 387 genes, out of which 145 were H5N1-specifically induced (Fig. 3B). Functional clustering, according to GO annotations, revealed that genes belonging to IFN response, immune defense, and antiviral response were induced primarily during H1N1 infection of macrophages (Fig. 4A). In contrast, infection with the HPAIVs did not lead to an over-representation of antiviral or immune response genes but a rather, unspecific over-representation of genes involved in metabolism and protein biosynthesis. Thereby, this pattern of over-represented functional gene groups is virtually the same in the entire set of up-regulated genes (data not shown) and in the group of specifically induced genes, respectively (Fig. 4B and C). These analyses indicate an insufficient activation of immune and antiviral response mechanisms in human macrophages during HPAIV infections compared with H1N1 infections.
Figure 3. Gene expression analysis of HPAIV-infected human macrophages.
(A) PCA displaying gene expression profiles of uninfected macrophages (control) and H1N1-, H7N7-, and H5N1-infected macrophages of three independent experiments. Vector clouds represent gene profiles of individual experiments and are positioned in a three-dimensional vector space, according to variance to each other. The analysis was restricted to genes that are up-regulated by the different strains. (B) Venn diagram indicates the number and overlap of genes induced by H1N1, H7N7, and H5N1, according to microarray analyses of three independent experiments.
Figure 4. Over-representation of functional gene groups.
Functional gene groups according to GO annotations were analyzed for over-representation in the group of genes up-regulated by (A) H1N1, (B) H7N7, and (C) H5N1. Plotted is the statistical significance (y-axis) of over-representation compared with the distribution of functional gene groups on the whole microarray, according to Fisher's exact test. Related GO groups are displayed by color identical bars and summarized into main categories of over-represented functional groups.
Different patterns of cytokine and chemokine induction after infection with H1N1 (PR8) compared with infection with HPAIV
To explore the differential antiviral and inflammatory response patterns indicated by microarray analyses in more detail, qRT-PCR experiments were conducted in H1N1- and HPAIV-infected macrophages, 5 h and 24 h p.i. We evaluated IFNA, IFNB1, and CXCL11, representing antiviral genes, and TNF-α, CCL5, and CCL8, representing proinflammatory genes. Almost all of these genes were virtually induced by H1N1 specifically but only barely by H7N7 and H5N1 (Fig. 5; notice y-axis breaks for extended scaling of H1N1-induced mRNA inductions). This induction pattern was found 5 h p.i. and became even more clear 24 h p.i. Only the induction of CXCL11 did not differ between H1N1 and H5N1, 24 h p.i. Concisely, these experiments confirmed the impaired induction of antiviral and inflammatory genes upon HPAIV infection compared with the strong induction after H1N1 infection, as observed in microarray analysis (Fig. 4). A comparative depiction of FCs received from microarray analysis and qRT-PCR is provided in Supplemental Table 2. To also confirm HPAIV-specific gene regulations by qRT-PCR, we oppose exemplarily two H5N1-specific genes involved in metabolism and transcription—TCEB2 and RPL5 (Fig. 5).
Increased cell death during HPAIV infection compared with H1N1 infection could be excluded as reason for the reduced inflammatory and antiviral gene induction. Within 5 –24 h p.i., apoptosis rates of HPAIV- and H1N1-infected human macrophages were comparable (Supplemental Fig. 2).
Inhibited expression of the influenza A virus M2 protein in human macrophages during HPAIV infection
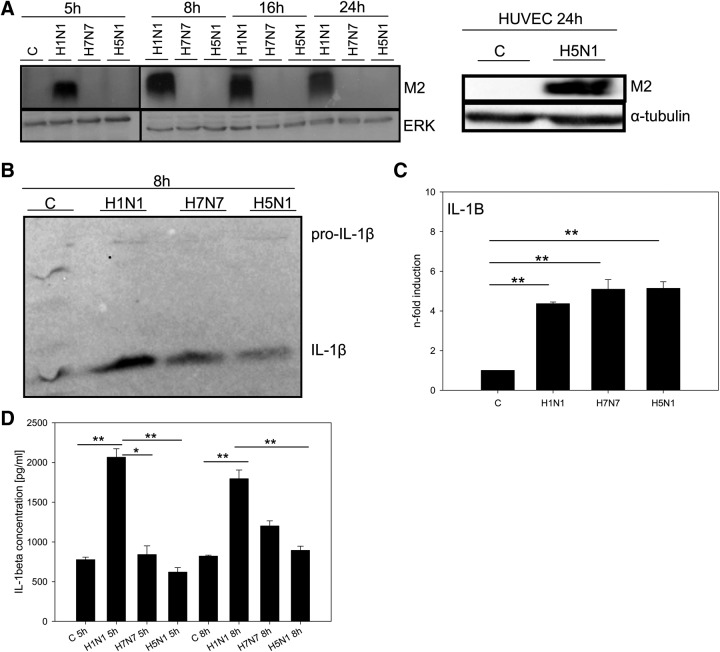
To obtain clues for molecular mechanisms involved in the suppression of the immune response after infection of macrophages with HPAIV, we screened for further specific differences between HPAIV and H1N1 influenza virus infections. Surprisingly, the analysis of macrophage cell lysates over a period of three replication cycles revealed a total lack of viral M2 protein expression in the case of H7N7 and H5N1 infection (Fig. 6A). On the contrary, M2 protein strongly accumulated in H1N1-infected macrophages (Fig. 6A). Interestingly, in HUVECs, in which we previously showed significant H5N1 propagation with strong proinflammatory immune responses [12, 13], viral M2 expression is readily detectable upon H5N1 infection (Fig. 6A).
Figure 6. Impaired protein M2 expression and inflammasome activation in HPAIV-infected macrophages.
(A) Lysates of uninfected and H1N1-, H7N7-, and H5N1-infected human macrophages as well as lysates of uninfected and H5N1-infected HUVECs were immunoblotted for viral M2 protein (15 kDa), 5 h, 8 h, 16 h, and 24 h p.i. Immunostaining of ERK2 served as a protein-loading control. Blot is representative of three independent experiments. (B) Cell supernatants of uninfected and H1N1-, H7N7-, and H5N1-infected human macrophages, 8 h p.i., were immunoblotted for IL-1β protein (17 kDa), also detecting its precursor, pro-IL-1β (35 kDa). Blot is representative of three independent experiments. The protein amount has been normalized to the cell number. (C) The amount of IL-1β was analyzed in cell supernatants of uninfected and H1N1-, H7N7-, and H5N1-infected human macrophages by CBA. Presented are mean levels of IL-1β in pg/mL + sem (n=3); *P < 0.05; **P < 0.01.
Impaired inflammasome activation upon HPAIV infection of human macrophages
M2 protein has been identified as a crucial molecular principle of inflammasome activation by influenza viruses [30]. We hypothesized that the lack of M2 expression in HPAIV-infected macrophages could impede inflammasome activation and IL-1β processing. Therefore, we infected human macrophages with all three influenza strains, respectively, and determined the amount of IL-1β in cell culture supernatants, 5 h and 8 h p.i. by immunoblotting (Fig. 6B) and CBA analyses (Fig. 6D). Upon H5N1 and H7N7 infection, processing and secretion of IL-1β by macrophages were decreasedsignificantly compared with infection with H1N1 virus (Fig. 6B and D). Immunoblotting also allowed weak staining of pro-IL-1β—obviously originating from macrophages dying 8 h p.i.—and displayed no significant differences among the three viral strains (Fig. 6B). Additionally, qRT-PCRs confirmed even induction of IL-1β transcription by all three viruses (Fig. 6C), providing evidence that inflammasome activation in human macrophages is impaired significantly on the level of pro-IL-1β processing upon HPAIV infection.
Summarized, these results suggest that impaired inflammasome activation might be related to suppressed M2 expression. Next to the inhibited induction of inflammatory and antiviral genes, impaired inflammasome activation can be revealed as a second HPAIV-specific mechanism affecting the primary and early immune response of human macrophages.
DISCUSSION
Human macrophages are an integral part of the innate immune system and one of the primary cell types first involved in the antiviral response. These cells have been shown to be susceptible to influenza infections and to produce cytokines and chemokines in response to viral infections [18, 31, 32]. In this study, we aimed to identify specific response patterns of inflammatory blood-derived macrophages to infections with high or low pathogenic influenza virus strains and to further characterize their role in systemic spreading of HPAIV infection. One has to keep in mind that monocytes and macrophages are not a homogeneous population but rather, represent distinct phenotypes that may exhibit even opposite functions. Different subpopulations of blood monocytes are precursors of classical inflammatory macrophages (e.g., CD14+/CD16− monocytes in man or Ly6C+/CD43+ monocytes in mice), whereas alveolar macrophages descend from functionally different subsets, primarily involved in tissue homeostasis (Ly6C−) [33, 34]. Intending to elucidate the systemic nature of H5N1 infections, we focused on the inflammatory subtype of macrophages rather than on locally acting alveolar macrophages.
Comparing the low pathogenic PR8 (H1N1) strain with the HPAIV strains FPV (H7N7) and KAN-1 (H5N1), we found that all strains efficiently infected macrophages and produced viral proteins with similar kinetics. In addition, the RNPs of all viruses were similarly released from the cell nucleus within the first replication cycle. However, no infectious virus particles were produced, indicating that macrophages in general are nonpermissive for influenza viruses.
We further used a genome-wide systems biology approach to disclose virus strain-specific gene expression patterns. Surprisingly, our data suggested a much stronger inflammatory response of macrophages to infections with a low pathogenic virus than to infections with HPAIV. Gene profiles showed an insufficient expression of genes belonging to a proinflammatory and antiviral response program during H7N7 and H5N1 infections, whereas these kinds of genes are well induced after H1N1 infections. As influenza viruses have, in general, a strong influence on the regulation of genes involved in metabolism and protein biosynthesis [29], the lacking induction of inflammatory/antiviral genes consequently results in an over-representation of metabolistic genes in HPAIV-induced gene profiles. However, the induction of metabolistic genes is not a HPAIV-specific process [29], and we could not confirm metabolistic genes by qRT-PCR that are overexpressed significantly in HPAIV- but not in H1N1-infected macrophages. However, qRT-PCR experiments could well confirm microarray data concerning the significantly impaired induction of IFNs and several chemokines in H7N7- and H5N1-infected macrophages compared with H1N1 infections.
The group around Peiris [7] published data on H5N1-infected human macrophages, showing an effective cytokine induction. They concluded from gene profiles that H1N1 and H5N1 viruses qualitatively seem to activate similar signaling pathways but differ quantitatively with a stronger inflammatory and antiviral response after H5N1 infection [35]. They used other human isolates of H5N1—A/HK/483/97 and A/HK/486/97 [7] or A/Vietnam [35]—than we used in our study, infected with lower viral doses, and above all, allowed viruses only 30 min to adsorb to the cells. Indeed, the detailed pattern of cytokine responses elicited in human macrophages might be quite dependent on the viral strain and isolate used. Also Sakabe et al. [36] demonstrated the extent of cytokine induction in human macrophages to vary among H5N1 strains and found a pandemic H1N1 virus to induce higher levels of several cytokines than some H5N1 strains.
Apparently, the differences in the amount of virus used for infection and the infection time are likewise responsible for the differences in cytokine responses determined by the group around Peiris [7, 35] compared with our results. Moreover, the cell population infected in these studies [7, 35] might differ from the well-characterized macrophages we used with respect to the purity and expression of macrophage markers.
Macrophages have been shown to be critical for clearance of viruses and initiation of antiviral adaptive immunity in the response to VSV [37–39]. Ineffective primary inflammatory responses of infiltrating macrophages might be the key to the inadequate local restriction of HPAIV infections in vivo. Whether macrophages stimulated by secondary mechanisms, e.g., via interaction with activated ECs, contribute to a H5N1-induced cytokine storm in the systemic phase of infection cannot be excluded. Initially, they rather seem to fail to initiate an adequate antiviral response. For the cytokine burst, other cell types might play a more important role. We have recently identified ECs to be very important sources of cytokine production in H5N1 infections, activating specific signaling pathways and allowing effective viral propagation [12, 13, 40]. Also, alveolar macrophages have been shown to respond with a significant cytokine production upon influenza infection [6, 15].
To find mechanisms explaining the suppression of the immune response of HPAIV- compared with H1N1-infected, blood-derived macrophages, we searched for specific differences between infection characteristics. Surprisingly, we found a complete lack of viral M2 expression in HPAIV-infected macrophages in contrast to H1N1-infected macrophages. Interestingly, M2 protein has been found to be required for the activation of the NLRP3 inflammasome by influenza virus-infected macrophages [30]. Following studies indeed showed that IL-1β secretion is impaired substantially in HPAIV-infected macrophages but not in H1N1-infected macrophages, suggesting that the suppression of M2 production is critically involved in the abrogation of inflammasome activation. Inflammasome activation plays an important role in the antiviral response to influenza infections [41–44]. As a matter of principle, inflammasome activation and IL-1β secretion lead to the production of chemokines, recruiting inflammatory cells, controlling influenza infections [43]. Levels of IL-6, TNF-α, CXCL1, and IFN-α were reported to be decreased in NLRP3-deficient mice, which were infected with influenza A virus. NLRP3-deficient mice showed increased necrosis in the lung. IL-1β addition to cell cultures reversed these effects, showing that damages caused by influenza A are mediated directly by IL-1β [41, 42].
In conclusion, we were able to demonstrate for the first time that inflammatory macrophages are nonpermissive for influenza infections. The inflammatory and antiviral response of human macrophages is specifically impaired upon HPAIV infections. Additionally, inflammasome activation is HPAIV-specifically affected and apparently related to a specific suppression of Influenza A virus M2 protein expression. Both mechanisms affect the primary and early immune response of human macrophages and might confer HPAIV the ability to escape the primary immune response of macrophages, facilitating systemic spreading and initiation of a cytokine burst at later systemic stages of infection.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the German Federal Ministry of Education and Research (BMBF, FluResearchNet, Grant 01KI07130) and the Interdisciplinary Center of Clinical Research of the University of Muenster. We thank Ursula Nordhues for technical assistance during microarray experiments and the Department of Integrated Functional Genomics (IFG) of the University of Muenster for microarray hybridization as well as for supply of technical equipment.
SEE CORRESPONDING EDITORIAL ON PAGE 1
- EC
- endothelial cell
- FC
- fold change
- FPV
- fowl plague virus (A/FPV/Bratislava/79)
- GO
- gene ontology
- HPAIV
- highly pathogenic avian influenza viruses
- KAN-1
- A/Thailand/KAN-1/2004
- M1/2
- matrix 1/2
- MDCK
- Madin Darby canine kidney
- NLRP3
- nucleotide-binding oligomerization-like receptor family, pyrin domain-containing 3
- NP
- nucleoprotein
- NS1
- nonstructural 1
- PB1
- polymerase basic 1
- PCA
- principal component analyses
- p.i.
- postinfection
- PR8
- A/Puerto Rico/8/34
- qRT-PCR
- quantitative real-time RT-PCR
- RPL5
- 60S ribosomal protein L5
- SIRS
- systemic inflammatory response syndrome
- TCEB2
- transcription elongation factor B, polypeptide 2
- vRNP
- viral RNP
AUTHORSHIP
J.F. performed experiments, analyzed data, and wrote the manuscript. Y.B. and E. H. performed experiments. S.L. designed experiments and wrote the manuscript. J.R. and D.V. designed experiments, analyzed data, and wrote the manuscript.
DISCLOSURES
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
REFERENCES
- 1. Writing Committee of the Second World Health Organization Consultation on Clinical Aspects of Human Infection with Avian Influenza A (H5N1) Virus, Abdel-Ghafar A. N., Chotpitayasunondh T., Gao Z., Hayden F. G., Nguyen D. H., de Jong M.D., Naghdaliyev A., Peiris J. S., Shindo N., Soeroso S., Uyeki T. M. (2008) Update on avian influenza A (H5N1) virus infection in humans. N. Engl. J. Med. 358, 261–273 [DOI] [PubMed] [Google Scholar]
- 2. Beigel J. H., Farrar J., Han A. M., Hayden F. G., Hyer R., de Jong M. D., Lochindarat S., Nguyen T. K., Nguyen T. H., Tran T. H.., et al. (2005) Avian influenza A (H5N1) infection in humans. N. Engl. J. Med. 353, 1374–1385 [DOI] [PubMed] [Google Scholar]
- 3. Lee N., Wong C. K., Chan P. K., Lun S. W., Lui G., Wong B., Hui D. S., Lam C. W., Cockram C. S., Choi K. W.., et al. (2007) Hypercytokinemia and hyperactivation of phospho-p38 mitogen-activated protein kinase in severe human influenza A virus infection. Clin. Infect. Dis. 45, 723–731 [DOI] [PubMed] [Google Scholar]
- 4. Wong S. S., Yuen K. Y. (2006) Avian influenza virus infections in humans. Chest 129, 156–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yuen K. Y., Chan P. K., Peiris M., Tsang D. N., Que T. L., Shortridge K. F., Cheung P. T., To W. K., Ho E. T., Sung R.., et al. (1998) Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet 351, 467–471 [DOI] [PubMed] [Google Scholar]
- 6. Chan M. C., Cheung C. Y., Chui W. H., Tsao S. W., Nicholls J. M., Chan Y. O., Chan R. W., Long H. T., Poon L. L., Guan Y.., et al. (2005) Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir. Res. 6, 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cheung C. Y., Poon L. L., Lau A. S., Luk W., Lau Y. L., Shortridge K. F., Gordon S., Guan Y., Peiris J. S. (2002) Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet 360, 1831–1837 [DOI] [PubMed] [Google Scholar]
- 8. Klenk H. D. (2005) Infection of the endothelium by influenza viruses. Thromb. Haemost. 94, 262–265 [DOI] [PubMed] [Google Scholar]
- 9. Ocana-Macchi M., Bel M., Guzylack-Piriou L., Ruggli N., Liniger M., McCullough K. C., Sakoda Y., Isoda N., Matrosovich M., Summerfield A. (2009) Hemagglutinin-dependent tropism of H5N1 avian influenza virus for human endothelial cells. J. Virol. 83, 12947–12955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Perkins L. E., Swayne D. E. (2001) Pathobiology of A/chicken/Hong Kong/220/97 (H5N1) avian influenza virus in seven gallinaceous species. Vet. Pathol. 38, 149–164 [DOI] [PubMed] [Google Scholar]
- 11. Szretter K. J., Gangappa S., Lu X., Smith C., Shieh W. J., Zaki S. R., Sambhara S., Tumpey T. M., Katz J. M. (2007) Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J. Virol. 81, 2736–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schmolke M., Viemann D., Roth J., Ludwig S. (2009) Essential impact of NF-κB signaling on the H5N1 influenza A virus-induced transcriptome. J. Immunol. 183, 5180–5189 [DOI] [PubMed] [Google Scholar]
- 13. Viemann D., Schmolke M., Lueken A., Boergeling Y., Friesenhagen J., Wittkowski H., Ludwig S., Roth J. (2011) H5N1 virus activates signaling pathways in human endothelial cells resulting in a specific imbalanced inflammatory response. J. Immunol. 186, 164–173 [DOI] [PubMed] [Google Scholar]
- 14. Nichols J. E., Niles J. A., Roberts N. J., Jr., (2001) Human lymphocyte apoptosis after exposure to influenza A virus. J. Virol. 75, 5921–5929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. McGill J., Heusel J. W., Legge K. L. (2009) Innate immune control and regulation of influenza virus infections. J. Leukoc. Biol. 86, 803–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bussfeld D., Kaufmann A., Meyer R. G., Gemsa D., Sprenger H. (1998) Differential mononuclear leukocyte attracting chemokine production after stimulation with active and inactivated influenza A virus. Cell. Immunol. 186, 1–7 [DOI] [PubMed] [Google Scholar]
- 17. Hinder F., Schmidt A., Gong J. H., Bender A., Sprenger H., Nain M., Gemsa D. (1991) Influenza A virus infects macrophages and stimulates release of tumor necrosis factor-α. Pathobiology 59, 227–231 [DOI] [PubMed] [Google Scholar]
- 18. Hofmann P., Sprenger H., Kaufmann A., Bender A., Hasse C., Nain M., Gemsa D. (1997) Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J. Leukoc. Biol. 61, 408–414 [DOI] [PubMed] [Google Scholar]
- 19. Hui K. P., Lee S. M., Cheung C. Y., Ng I. H., Poon L. L., Guan Y., Ip N. Y., Lau A. S., Peiris J. S. (2009) Induction of proinflammatory cytokines in primary human macrophages by influenza A virus (H5N1) is selectively regulated by IFN regulatory factor 3 and p38 MAPK. J. Immunol. 182, 1088–1098 [DOI] [PubMed] [Google Scholar]
- 20. Kaufmann A., Salentin R., Meyer R. G., Bussfeld D., Pauligk C., Fesq H., Hofmann P., Nain M., Gemsa D., Sprenger H. (2001) Defense against influenza A virus infection: essential role of the chemokine system. Immunobiology 204, 603–613 [DOI] [PubMed] [Google Scholar]
- 21. Lehmann C., Sprenger H., Nain M., Bacher M., Gemsa D. (1996) Infection of macrophages by influenza A virus: characteristics of tumour necrosis factor-α (TNF α) gene expression. Res. Virol. 147, 123–130 [DOI] [PubMed] [Google Scholar]
- 22. Barczyk K., Ehrchen J., Tenbrock K., Ahlmann M., Kneidl J., Viemann D., Roth J. (2010) Glucocorticoids promote survival of anti-inflammatory macrophages via stimulation of adenosine receptor A3. Blood 116, 446–455 [DOI] [PubMed] [Google Scholar]
- 23. Ehrchen J., Steinmuller L., Barczyk K., Tenbrock K., Nacken W., Eisenacher M., Nordhues U., Sorg C., Sunderkotter C., Roth J. (2007) Glucocorticoids induce differentiation of a specifically activated, anti-inflammatory subtype of human monocytes. Blood 109, 1265–1274 [DOI] [PubMed] [Google Scholar]
- 24. Liu W., Saint D. A. (2002) A new quantitative method of real time reverse transcription polymerase chain reaction assay based on simulation of polymerase chain reaction kinetics. Anal. Biochem. 302, 52–59 [DOI] [PubMed] [Google Scholar]
- 25. Viemann D., Goebeler M., Schmid S., Klimmek K., Sorg C., Ludwig S., Roth J. (2004) Transcriptional profiling of IKK2/NF-κ B- and p38 MAP kinase-dependent gene expression in TNF-α-stimulated primary human endothelial cells. Blood 103, 3365–3373 [DOI] [PubMed] [Google Scholar]
- 26. Alter O., Brown P. O., Botstein D. (2000) Singular value decomposition for genome-wide expression data processing and modeling. Proc. Natl. Acad. Sci. USA 97, 10101–10106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Viemann D., Strey A., Janning A., Jurk K., Klimmek K., Vogl T., Hirono K., Ichida F., Foell D., Kehrel B.., et al. (2005) Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105, 2955–2962 [DOI] [PubMed] [Google Scholar]
- 28. Engelhardt O. G., Fodor E. (2006) Functional association between viral and cellular transcription during influenza virus infection. Rev. Med. Virol. 16, 329–345 [DOI] [PubMed] [Google Scholar]
- 29. Katze M. G., Krug R. M. (1984) Metabolism and expression of RNA polymerase II transcripts in influenza virus-infected cells. Mol. Cell. Biol. 4, 2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ichinohe T., Pang I. K., Iwasaki A. (2010) Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gong J. H., Sprenger H., Hinder F., Bender A., Schmidt A., Horch S., Nain M., Gemsa D. (1991) Influenza A virus infection of macrophages. Enhanced tumor necrosis factor-α (TNF-α) gene expression and lipopolysaccharide-triggered TNF-α release. J. Immunol. 147, 3507–3513 [PubMed] [Google Scholar]
- 32. Salentin R., Gemsa D., Sprenger H., Kaufmann A. (2003) Chemokine receptor expression and chemotactic responsiveness of human monocytes after influenza A virus infection. J. Leukoc. Biol. 74, 252–259 [DOI] [PubMed] [Google Scholar]
- 33. Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. (2010) Development of monocytes, macrophages, and dendritic cells. Science 327, 656–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ziegler-Heitbrock L., Ancuta P., Crowe S., Dalod M., Grau V., Hart D. N., Leenen P. J., Liu Y. J., MacPherson G., Randolph G. J.., et al. (2010) Nomenclature of monocytes and dendritic cells in blood. Blood 116, e74–e80 [DOI] [PubMed] [Google Scholar]
- 35. Lee S. M., Gardy J. L., Cheung C. Y., Cheung T. K., Hui K. P., Ip N. Y., Guan Y., Hancock R. E., Peiris J. S. (2009) Systems-level comparison of host-responses elicited by avian H5N1 and seasonal H1N1 influenza viruses in primary human macrophages. PLoS One 4, e8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sakabe S., Iwatsuki-Horimoto K., Takano R., Nidom C. A., Le M. Q., Nagamura-Inoue T., Horimoto T., Yamashita N., Kawaoka Y. (2011) Cytokine production by primary human macrophages infected with highly pathogenic H5N1 or pandemic H1N1 2009 influenza viruses. J. Gen. Virol. 92, 1428–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carrasco Y. R., Batista F. D. (2007) B cells acquire particulate antigen in a macrophage-rich area at the boundary between the follicle and the subcapsular sinus of the lymph node. Immunity 27, 160–171 [DOI] [PubMed] [Google Scholar]
- 38. Iannacone M., Moseman E. A., Tonti E., Bosurgi L., Junt T., Henrickson S. E., Whelan S. P., Guidotti L. G., von Andrian U. H. (2010) Subcapsular sinus macrophages prevent CNS invasion on peripheral infection with a neurotropic virus. Nature 465, 1079–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Junt T., Moseman E. A., Iannacone M., Massberg S., Lang P. A., Boes M., Fink K., Henrickson S. E., Shayakhmetov D. M., Di Paolo N. C.., et al. (2007) Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature 450, 110–114 [DOI] [PubMed] [Google Scholar]
- 40. Pauli E. K., Schmolke M., Wolff T., Viemann D., Roth J., Bode J. G., Ludwig S. (2008) Influenza A virus inhibits type I IFN signaling via NF-κB-dependent induction of SOCS-3 expression. PLoS Pathog. 4, e1000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kanneganti T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N.., et al. (2006) Critical role for Cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281, 36560–36568 [DOI] [PubMed] [Google Scholar]
- 42. Kanneganti T. D. (2010) Central roles of NLRs and inflammasomes in viral infection. Nat. Rev. Immunol. 10, 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pang I. K., Iwasaki A. (2011) Inflammasomes as mediators of immunity against influenza virus. Trends Immunol. 32, 34–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thomas P. G., Dash P., Aldridge J. R., Jr., Ellebedy A. H., Reynolds C., Funk A. J., Martin W. J., Lamkanfi M., Webby R. J., Boyd K. L.., et al. (2009) The intracellular sensor NLRP3 mediates key innate and healing responses to influenza A virus via the regulation of caspase-1. Immunity 30, 566–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.