Abstract
This study isolated the effects of maternal hypoxia independent of changes in maternal nutrition on maternal circulatory and placental molecular indices of oxidative stress and determined whether maternal antioxidant treatment conferred protection. Pregnant rats were subjected to normoxic pregnancy or 13% O2 chronic hypoxia for most of gestation with and without maternal treatment with vitamin C in the drinking water. Maternal hypoxia with and without vitamin C did not affect maternal food or water intake and led to a significant increase in maternal and fetal haematocrit. At gestational day 20, maternal plasma urate and l-cysteine concentrations, and placental levels of 4-hydroxynonenal and heat shock protein 70 were increased while placental heat shock protein 90 levels were decreased in hypoxic pregnancy. The induction of maternal circulatory and placental molecular indices of oxidative stress in hypoxic pregnancies was prevented by maternal treatment with vitamin C. Maternal hypoxia during pregnancy with or without vitamin C increased placental weight, but not total or compartmental volumes. Maternal treatment with vitamin C increased birth weight in both hypoxic and normoxic pregnancies. The data show that maternal hypoxia independent of maternal undernutrition promotes maternal and placental indices of oxidative stress, effects that can be prevented by maternal treatment with vitamin C in hypoxic pregnancy. While vitamin C may not be the ideal candidate of choice for therapy in pregnant women, and taking into consideration differences in ascorbic acid metabolism between rats and humans, the data do underlie that antioxidant treatment may provide a useful intervention to improve placental function and protect fetal growth in pregnancy complicated by fetal hypoxia.
Key points
High-altitude pregnancy is associated with reduced oxygenation and placental complications, which can affect maternal and fetal outcome. However, most high-altitude populations are also impoverished and because maternal undernutrition itself is known to promote placental problems, the extent to which complications during high-altitude pregnancy could be due to maternal oxygen and/or nutrient restriction remains unclear.
The aim of the study was to investigate whether reduced placental oxygenation, independent of maternal undernutrition, increases maternal and placental oxidative stress and whether maternal treatment with vitamin C is protective.
The study shows that hypoxic pregnancy increased maternal circulating and placental molecular indices of oxidative stress.
Maternal vitamin C treatment was protective and increased birth weight.
The study offers insight to mechanism and intervention against the effects of high altitude on pregnancy.
Introduction
Over 140 million people live at altitudes greater than 3000 m, comprising the largest single human group at risk for complications during pregnancy due to hypoxia (Moore et al. 2011). Human pregnancy at high altitude has been associated with a reduction in the pregnancy-associated increase in uterine blood flow, thereby increasing the prevalence of complications associated with limited utero-placental perfusion (Zamudio et al. 1995). For instance, pre-eclampsia is 1.7 times (95% CI: 1.3–2.3) more frequent at high altitude and 2.2 times (95% CI: 1.4–3.5) more frequent among primiparous women (Keyes et al. 2003; Zamudio, 2007). Pregnancy at high altitude has also been associated with reduced placental antioxidant enzyme activity and increased placental nitrative stress (Zamudio et al. 2007a,b). However, because most high-altitude populations are also impoverished with a high incidence of maternal malnutrition (Giussani et al. 2001) and because maternal undernutrition itself is known to promote placental oxidative stress (Richter et al. 2009), the extent to which complications during high-altitude pregnancy could be due to maternal oxygen and/or nutrient restriction remains unclear. In experimental animal models, it is also difficult to isolate the effects of maternal oxygen and nutrient deprivation. For example, several independent studies have reported that exposure of pregnant rats to chronic 10% oxygen isobaric hypoxia for the last third of gestation reduces maternal food intake by ca. 40% from baseline (de Grauw et al. 1986; Williams et al. 2005; Camm et al. 2010).
Recently, we have developed a rat model of hypoxic pregnancy that does not affect maternal food intake (Herrera et al. 2011). The model involves exposure of the pregnant dam to 13% oxygenation for most of pregnancy. The partial pressure of oxygen at sea level (21% O2) is 159 mmHg and it decreases by 50% at 5496 m (Mortazavi et al. 2003). At 13% O2, the partial pressure of oxygen equates to an altitude of ca. 3600 m above sea level, the altitude at which pregnancy complications have been demonstrated to be significantly increased in human populations (Keyes et al. 2003; Zamudio, 2007). Therefore, the model permits isolation of the effects of maternal hypoxia at a level of human relevance independent of maternal nutrition on maternal, placental and fetal outcomes. Using this model, the present study determined the effects of maternal hypoxia on molecular indices of oxidative stress in the maternal plasma and placenta, as well as on placental stereology and on birth weight. The study also investigated whether maternal treatment with antioxidants during hypoxic pregnancy would be protective and offer plausible targets for intervention.
The antioxidant chosen was vitamin C, as ascorbate is an endogenous antioxidant and an essential component of the diet in humans and other species (Bánhegyi et al. 1997). Together with reduced glutathione, it is the most important water-soluble antioxidant of mammalian tissues (Baydas et al. 2002). The plasma vitamin C concentrations also increase throughout gestation in several species, consistent with a functional role for this antioxidant in prenatal life (Kolb et al. 1991).
Methods
Animals
All experiments were carried out under the UK Animals (Scientific Procedures) Act 1986, and all procedures were approved by the Local Ethic Review Committee of the University of Cambridge. Virgin, female Wistar rats (3 months old), fed ad libitum on maintenance diet (laboratory rat chow, VRF1, Charles River, UK) were delivered to the Central Biomedical Services (CBS) at the University of Cambridge. At the CBS, rats were mated after a minimum of 10 days acclimatisation. The presence of a copulatory plug in the rat cage was considered to be day 0 of pregnancy. The pregnant rats were housed singly in individual ventilated cages (IVC units, 21% O2, 70–80 air changes per hour) in rooms with controlled humidity (60%), controlled temperature (21°C) and a 12:12 h light–dark cycle (lights on at 0700 h). From the beginning of pregnancy, maternal weight, food and water intake were quantified and documented daily.
Experimental procedures
Pilot experiments revealed that maternal exposure to 13% oxygen chronic hypoxia prior to day 5 of pregnancy in the rat markedly increased the rate of pregnancy loss. Therefore, from day 6 of pregnancy, rats were randomly divided into four groups (n= 7 per group): control and hypoxic pregnancy, with and without vitamin C treatment (5 mg ml−1 maternal drinking water freshly prepared every day). Pregnant rats subjected to hypoxia were placed inside a chamber, which combined a PVC isolator (PFI Plastics Ltd, UK) with a nitrogen generator (N2MID60, Domnick Hunter Ltd, UK). The percentage of oxygen in the chamber was controlled by altering the inflow of air and nitrogen. Oxygen concentration was monitored continuously throughout the treatment period with an oxygen analyser (ICA, UK). Carbon dioxide concentrations inside the chamber were monitored throughout treatment using a portable carbon dioxide analyser, which was calibrated daily (The Electronic Workshop, Department of Physiology, University of Cambridge). The hypoxic chamber contained a separate sealed unit, which could house one rat cage at a time, without alteration to the O2 and CO2 concentrations of the ambient air inside the main chamber. This unit was opened briefly once per day to replace and weigh the food and water containers as well as to clean or replace cages. On day 20 of gestation (term is approximately 21 days), the dams were weighed. Anaesthesia was induced with isoflurane and then maintained by a mixture of ketamine (40 mg kg−1) and xylazine (5 mg kg−1) injected intraperitoneally. Once anaesthetised, a maternal blood sample (1 ml in EDTA plus 0.5 ml in metaphosphoric acid) for measurement of circulatory indices of oxidative stress was taken by cardiac puncture, the pregnant uterus was exposed via a mid-line incision and the anaesthetised pups were killed via spinal transection. Dams that had been housed in the hypoxic chamber underwent the procedure while being ventilated with 13% O2 via a small cone. Fetal blood was collected from a neck incision by capillarity. Maternal and fetal blood was spun in a Hawksley centrifuge for determination of haematocrit. All fetuses and associated placentas were weighed and the placentas were either immediately frozen in liquid nitrogen for protein isolation, or fixed for stereology. In all pups, the ano-genital distance was measured with digital callipers for determination of sex (Franko & Giussani, 2004). At the end of fetectomy, the mothers were killed by cervical dislocation. A separate cohort of dams was allowed to deliver to determine the effects on birth weight of maternal hypoxia with and without vitamin C treatment.
Determination of ascorbic acid, uric acid and l-cysteine
Reversed-phase high-performance liquid chromatography (HPLC) with electrochemical detection was used to analyse uric and ascorbic acid, as well as l-cysteine, based on the method of Iriyama et al. with modifications (Iriyama et al. 1984; Pagano et al. 2004). Maternal plasma previously acidified 1:1 with ice-cold 10% metaphosphoric acid (MPA) was centrifuged and the supernatant stored at −70°C for shipment on dry ice to King's College London. This supernatant was thawed on ice and 50 μl added to 400 μl HPLC grade water, 50 μl of 50% MPA and 200 μl of HPLC grade heptane. The samples were then mixed on a vortex stirrer for 30 s prior to centrifugation at 13,000 r.p.m. for 5 min at 4°C. The lower (aqueous) layer was then removed and transferred to a 0.8 ml HPLC vial. Aliquots of 20 μl were injected onto a 4.6 × 250 mm, 5 μm C18 Apex II column with guard (Jones Chromatography, Glamorgan, UK) and eluted with a 0.2 mol l−1 K2HPO4–H3PO4 (pH 2.1) mobile phase containing 0.25 mmol l−1 octane sulfonic acid at a flow rate of 1.0 ml min−1. An electrochemical detector (EG & G Instruments, Wokingham, UK) was used for detection, with the working electrode set at 800 mV and a sensitivity 0.2 μA. Final concentrations for ascorbic acid, uric acid and l-cysteine were calculated with external standards which were run simultaneously. The coefficient of variation of analysis was <5%, with minimum detection limits of 0.1, 0.05 and 0.1 μm for ascorbic acid, uric acid and l-cysteine, respectively.
Western blot analysis
Tissue homogenization to obtain protein lysates and subsequent sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotting were performed as previously described for human and rat placental tissue (Tjoa et al. 2006; Richter et al. 2009). The specific primary antibodies were: rabbit polyclonal to catalase (1:2500; Abcam, Cambridge, UK, code Ab1877), Mn-SOD (1:1000; Upstate Biotech, NY, USA, code 06-984), 4-HNE (1:1000; Abcam, Cambridge, UK, code 210767) and HSP70 (1:20,000; Stressgen, York, UK, code Spa812C); mouse monoclonal to HSP90 (1:1000; Stressgen, York, UK, code Spa 830F), eNOS (1:2500; BD Transduction Labs, UK, code 610297) and β-actin (1:50,000; Sigma, St Louis, USA, code A5441). The horseradish peroxidase-conjugated secondary antibodies were from GE Healthcare UK Ltd (anti-rabbit and -mouse). Membranes were re-probed with antibody recognising β-actin to control for protein loading and to normalise relative levels of protein expression. The optical density of the immunoreactive bands was analysed using ImageJ software (National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij), and the ratio protein to β-actin was calculated for each sample.
Placental stereology
The placentas were transversally cut into two halves and one half was immersion fixed in 4% paraformaldehyde (PFA) overnight at room temperature. One fixed half was processed into paraffin wax, exhaustively sectioned (Leica RM 2235 microtome, Leica Microsystems, Germany), and then stained with haematoxylin and eosin (H&E). All quantitative analyses of fixed tissue were performed using an Olympus BX-50 microscope, fitted with a motorized specimen stage and microcator. All analyses were performed using the Computer Assisted Stereology Toolbox (CAST) version 2.0 program (Olympus, Denmark), with the observer blind to the treatment groups. For each paraffin-embedded half placenta, fifteen 7 μm sections were selected an equal distance apart throughout the thickness of the half placenta. To determine the absolute volume of placentas, a point grid was superimposed on the sections (×1.25). Points falling on the sample were counted and the Cavalieri Principle was applied in order to reach a volume estimate (Gundersen & Osterby, 1981) using the equation:
where V(obj) is the estimated placental volume, t is the thickness between the sections sampled (number of intervening sections multiplied by section thickness), a(p) is the area associated with each point and ∑P the sum of points on sections. To account for shrinkage due to paraffin processing, the diameter of erythrocytes present in sections of fetal fixed tissue was measured and compared with the diameter of erythrocytes present in fresh blood from fetal rats of the same gestational age (Burton & Palmer, 1988; Ali et al. 1996). All measurements were subsequently corrected using this factor. At ×10, fields of view on the 7 μm sections used for determining the absolute placental volume were selected by meander sampling and measured by point counting to estimate compartment densities of the three zones: labyrinthine zone, junctional zone and decidua basalis using the equation:
where Vv(struct,ref) is the volume fraction of a compartment (e.g. labyrinthine zone) within a reference space (e.g. placenta), P(struct) is the number of points falling on the compartment, and P(total) is the total number of points falling on the reference space (including the component). The volume densities obtained were converted to absolute quantities by multiplying by total placental volume (Gundersen & Osterby, 1981; Mayhew et al. 2003).
Data and statistical analyses
Placental efficiency was calculated as the ratio of fetal body weight to placental weight. The mean ratio of each protein to β-actin was transformed to arcsin (Zar, 1984). Data are expressed as mean ± SEM. Comparisons of variables relating specifically to fetal and placental biometry are best assessed using a Generalised Mixed Linear Model (SPSS). This statistical technique permits the experimental effects to be assessed on all collected pups and associated placentas, taking into account size and sex of the litter, thereby negating the need to pool these variables per litter (West et al. 2007). In contrast, comparisons of variables relating to biochemical, molecular and stereological analyses were performed only using data generated from tissues from two male pups per litter to control for sex and within-litter variation. These data were compared statistically using one-way ANOVA on absolute or ranked data, as appropriate, followed by the Tukey or Dunn's post hoc test, respectively. For all statistical comparison, significance was accepted when P < 0.05.
Results
Effects on food/water intake and haematocrit
In control pregnancies (C), maternal food and water intake did not vary significantly between days 6 and 20 of gestation, and averaged 29.4 ± 0.7 g and 51.9 ± 1.7 ml per day, respectively. Neither maternal exposure to hypoxia (H) nor maternal treatment with vitamin C (CVC and HVC) affected the daily intake of maternal food (H: 26.6 ± 0.5 g; HVC: 27.6 ± 1.0 g; CVC: 28.1 ± 0.7 g) or water (H: 45.7 ± 1.2 ml; HVC: 52.5 ± 1.8 ml; CVC: 52.0 ± 2.5 ml).
Maternal hypoxia significantly increased maternal and fetal haematocrit at day 20 of gestation (Fig. 1A and B). Maternal treatment with vitamin C had no effect on maternal or fetal haematocrit in control or hypoxic pregnancies (Fig. 1A and B).
Figure 1. Effects of maternal hypoxia with or without vitamin C treatment on maternal (A) and fetal haematocrit (B) at gestational day 20.
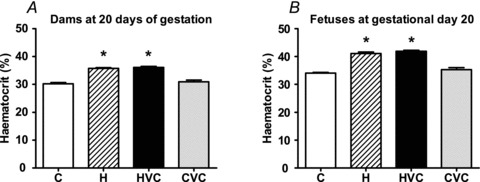
Bars represent mean ± SEM (n= 7 per group). C, control; H, hypoxia; HVC, hypoxia plus vitamin C; CVC, control plus vitamin C. Significant (P < 0.05) differences are: *versus other treatments (one-way ANOVA and Tukey's post hoc test).
Maternal circulating ascorbate, urate and l-cysteine concentrations
Basal concentrations of ascorbate were similar in control and hypoxic pregnancies. Maternal treatment with vitamin C elevated maternal ascorbate concentrations by ca. 70% of baseline in both control and hypoxic pregnancies (Fig. 2A). Maternal exposure to hypoxia increased maternal circulating urate and l-cysteine concentrations, effects which could be prevented by vitamin C. Maternal treatment with vitamin C in control pregnancy did not affect basal maternal urate or l-cysteine (Fig. 2B and C).
Figure 2. Effects of maternal hypoxia with or without vitamin C treatment on maternal circulating levels of ascorbate (A) and the indices of oxidative stress urate (B) and l-cysteine (C).
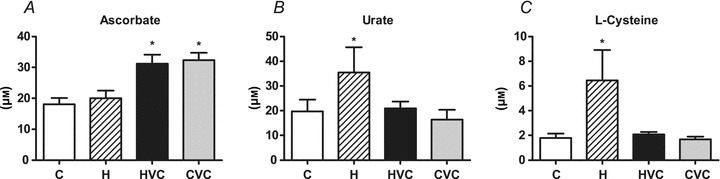
Bars represent mean ± SEM (n= 7 per group). C, control; H, hypoxia; HVC, hypoxia plus vitamin C; CVC, control plus vitamin C. Significant (P < 0.05) differences are: *versus control and hypoxia for plasma ascorbate and versus other treatments for urate and l-cysteine (one-way ANOVA and Tukey's post hoc test).
Western blot analysis of placental tissue
Hypoxia increased the placental levels of heat shock protein 70 (HSP70) and 4-hydroxynonenal (4-HNE), effects which could be prevented by treatment with vitamin C (Fig. 3A and B). Maternal treatment with vitamin C in control pregnancy did not affect placental HSP70 or 4-HNE (Fig. 3A and B). Hypoxia decreased the placental expression of heat shock protein 90 (HSP90), an effect which was restored towards control levels with maternal vitamin C treatment (Fig. 3C). Maternal treatment with vitamin C in control pregnancies did not affect the placental expression of HSP90 (Fig. 3C). Changes in the placental expression of endothelial nitric oxide synthase (eNOS) followed the same pattern as the changes in the placental expression of HSP90. However, all comparisons fell outside statistical significance (Fig. 3D). Hypoxia increased the placental expression of catalase (Fig. 4A) but not manganese-superoxide dismutase (Fig. 4B). Maternal treatment with vitamin C did not affect the placental expression of either antioxidant enzyme in hypoxic or control pregnancies (Fig. 4A and B).
Figure 3. Effects of maternal hypoxia with or without maternal vitamin C treatment on the relative expression of heat shock protein 70 (HSP70; A), 4-hydroxynonenal (HNE; B), heat shock protein 90 (HSP90; C) and endothelial nitric oxide synthase (eNOS; D) in the rat placenta.
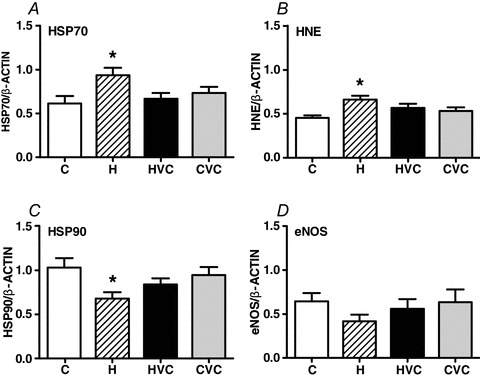
Bars represent mean ± SEM (n= 7 per group). Total protein samples (7.5 μg per lane) were subjected to immunoblotting using commercial polyclonal antibodies. Respective representative immunodetected bands of 72 kDa (HSP70), 35 kDa (HNE; selected band), 90 kDa (HSP90), 140 kDa (eNOS) and 43 kDa (β-actin) are also shown. C, control; H, hypoxia; HVC, hypoxia plus vitamin C; CVC, control plus vitamin C. Significant (P<0.05) differences are: *versus other treatments (one-way ANOVA with Tukey's post hoc test).
Figure 4. Effects of maternal hypoxia with or without maternal vitamin C treatment on the relative expression of catalase (A) and manganese superoxide dismutase (Mn-SOD; B) in the rat placenta.
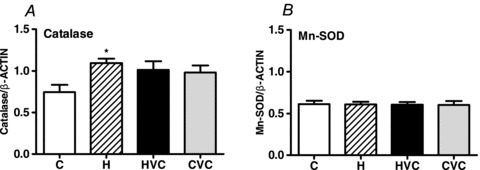
Bars represent mean ± SEM (n= 7 per group). Total protein samples (7.5 μg per lane) were subjected to immunoblotting using commercial polyclonal antibodies. Respective representative immunodetected bands of 59 kDa (catalase), 24 kDa (Mn-SOD) and 43 kDa (β-actin) are also shown. C, control; H, hypoxia; HVC, hypoxia plus vitamin C; CVC, control plus vitamin C. Significant (P<0.05) differences are: *versus control (one-way ANOVA with Tukey's post hoc test).
Placental and fetal biometry and birth weight
At day 20 of gestation, hypoxia with or without vitamin C increased placental weight but not fetal body weight (Fig. 5A and B). Maternal treatment with vitamin C in control pregnancies did not affect placental or fetal body weight (Fig. 5A and B). Consequently, hypoxic pregnancies had significantly lower calculated values for placental efficiency relative to controls (H = 5.59 ± 0.11, n= 49 vs. C = 5.65 ± 0.16, n= 38; P < 0.05). Maternal treatment with vitamin C during hypoxia did not restore placental efficiency to control values (HVC = 5.17 ± 0.09, n= 60), and maternal treatment with vitamin C in control pregnancies did not affect placental efficiency (CVC = 5.87 ± 0.09, n= 60). There were no differences in litter size or any evidence of reabsorbed placentae within the groups.
Figure 5. Effects of maternal hypoxia with or without maternal vitamin C treatment on placental weight (A), fetal body weight (B) and birth weight (C).
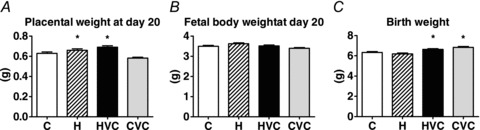
A and B, placental weight and fetal body weight: bars represent mean ± SEM at day 20 of gestation (n= 7 dams per group). C, control (n= 63); H, hypoxia (n= 49); HVC, hypoxia plus vitamin C (n= 60); CVC, control plus vitamin C (n= 60). Significant (P < 0.001) differences are: *versus other treatments (Mixed Linear Model µSPSS½). C, birth weight: bars represent mean ± SEM (n= 7 dams per group). C, control (n= 49); H, hypoxia (n= 43); HVC, hypoxia plus vitamin C (n= 37); CVC, control plus vitamin C (n= 33). Significant (P < 0.05) differences are: *versus other treatments (Mixed Linear Model µSPSS½).
When a separate cohort of animals was allowed to deliver, maternal exposure to hypoxia did not have a significant effect on birth weight. However, maternal treatment with vitamin C with or without hypoxia increased birth weight relative to control pregnancy (Fig. 5C).
Stereological analysis was carried out to determine whether any measured changes in placental weight and placental efficiency may be related to changes in total, or compartmental, placental volumes at gestational day 20. No significant differences occurred between groups for the overall placental volume (Fig. 6A), the compartmental volumes (Fig. 6B), or for the compartment to whole volume ratios (Fig. 6C).
Figure 6. Effects of maternal hypoxia with or without maternal vitamin C treatment on absolute and relative placental volumes.
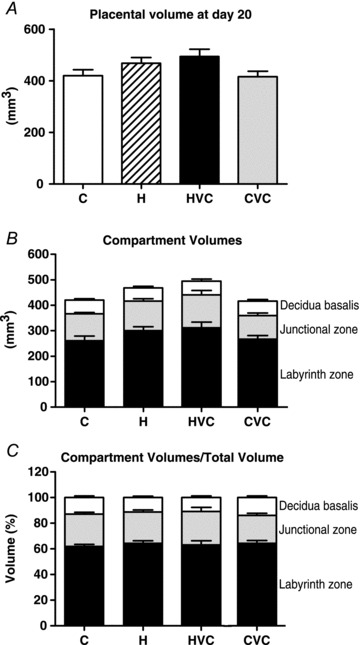
Total placental volume (A), placental compartment volumes (B) and the compartment volumes relative to total placental volume (C), at day 20 of gestation in control (C, n= 9), control plus vitamin C (CVC, n= 8), hypoxia (H, n= 7), and hypoxia plus vitamin C (HVC, n= 6) placentas. One-way ANOVA followed by Dunn's post hoc test.
Discussion
In humans, there has been considerable interest in investigating the effect of vitamin C supplementation in conditions of basal vitamin C depletion, such as during pregnancy when the fetus is dependent on the maternal diet for its own supply. In this study we addressed a separate question and asked whether maternal treatment with excess vitamin C, above basal levels, confers any protective effect against excess free radical synthesis and oxidative stress, as occur in hypoxic pregnancy. Although rats synthesise their own vitamin C and maintain basal circulating vitamin C levels (Bánhegyi et al. 1997), they are a convenient species to investigate the effects of maternal treatment with excess vitamin C above basal levels in pregnancy complicated by oxidative stress. The data show that maternal exposure to a level of chronic hypoxia of human relevance during most of pregnancy and independent of changes in maternal nutrition significantly increased maternal and fetal haematocrit and significantly elevated maternal and placental molecular indices of oxidative stress. Maternal treatment with vitamin C during hypoxic pregnancy did not affect the increase in maternal and fetal haematocrit but it prevented the induction of maternal and placental oxidative stress. Further, maternal treatment with vitamin C increased birth weight in both normoxic and hypoxic pregnancy. Therefore, the data support the idea that the hypoxia of pregnancy at high altitude promotes oxidative stress in the mother and the placenta and that maternal treatment with excess vitamin C during high-altitude pregnancy may be protective on maternal and fetal outcomes.
The dose of vitamin C used in the present investigation was derived from a previous study in our laboratory that achieved elevations in circulating ascorbate concentrations within the required range for it to be able to act as an antioxidant in vivo in ovine pregnancy (Thakor et al. 2010b). In rat pregnancy, this equated to ca. 0.9 mg g−1 day−1 of vitamin C administration. Although this dose of vitamin C far exceeds that given to pregnant women in all reported clinical trials (1000 mg per day per woman; Poston et al. 2006; Spinnato et al. 2007; Kontic-Vucinic et al. 2008; Rumbold et al. 2008; Villar et al. 2009), the increment from baseline in circulating ascorbate concentrations achieved in dams in the present study was ca. 70% and, therefore, similar to the increment achieved in pregnant women in the VIP trial following maternal vitamin C administration (Poston et al. 2006). The similar increment above baseline in circulating plasma ascorbate concentrations, despite the very different dosing regimens between rats and humans, highlights species differences in intestinal absorption, tissue accumulation and renal reabsorption and/or excretion (Corpe et al. 2010). In addition, steady state plasma ascorbate concentrations also depend on distribution, catabolism, reutilisation (vitamin C recycling) and body size (Levine et al. 2001). Humans have also lost gulonolactone oxidase, which catalyses the final step of ascorbate synthesis in the rat and several other species (Bánhegyi et al. 1997). Data derived from the maternal circulation in the present study show significant increases in plasma urate and cysteine levels in hypoxic pregnancies. Although the mitochondrial respiratory chain provides the main source of reactive oxygen species (ROS), they can also arise from other metabolic reactions and enzymes, such as xanthine oxidoreductase, NAD(P)H oxidase, nitric oxide (NO) synthase and lipoxygenase (Freeman & Crapo, 1981; Griendling et al. 2000). During periods of oxygen deprivation or hypoxia, xanthine oxidoreductase, relative to other processes, is the single most important system generating ROS (Halliwell & Gutteridge, 1999; Schachter & Foulds, 1999). The enzymes convert hypoxanthine to xanthine and xanthine to uric acid. Xanthine oxidase conversion of purines is coupled to the generation of •O2−. Consequently, elevations in plasma urate have become an established in vivo bioassay for •O2− generation (Halliwell & Gutteridge, 1999) and of placental and fetal complications in pregnancy (Roberts et al. 2005; Bainbridge & Roberts, 2008). The sulphur amino acid l-cysteine is involved in the regulation of oxidative status and of protein metabolism. It directly scavenges ROS, it forms an integral part of the methionine–cysteine metabolic pathway, and it is a precursor of glutathione and taurine, themselves essential in the host's protection against oxidative stress (Métayer et al. 2008). Combined, therefore, data derived from the maternal circulation in the present study suggest that, in hypoxic pregnancy, elevations in maternal plasma levels of l-cysteine and urate may represent increased protein oxidation, damage and degradation (see Métayer et al. 2008) and an enhanced antioxidant response to vascular oxidative stress. Further, maternal treatment with vitamin C has a protective effect on these circulatory indices of vascular dysfunction in hypoxic pregnancy and is without effect in control pregnancy.
At the level of the placenta, a number of reports support the idea that several indices of oxidative stress are elevated in complicated pregnancy and most of them implicate the hypoxic placenta as a source of ROS (for reviews, see: Sankaralingam et al. 2006; Al-Gubory et al. 2010; Myatt, 2010). For instance, HSP70 protein is a sensitive index of oxidative stress (Papp et al. 2003) and 4-HNE adducts are established biomarkers indicating lipid oxidation (Poli et al. 2008). Indeed, significantly greater levels of HSP70 and 4-HNE have been consistently reported in human placentas stressed with ischaemia-reperfusion-induced oxidative stress (Hung et al. 2001; Cindrova-Davies et al. 2007). It is also well established that the chaperone protein HSP90 plays an integral role in regulating the activation of eNOS and, thereby, NO production. HSP90 achieves this in part by cooperatively enhancing the affinity of eNOS for binding to calmodulin and Akt among other cofactors (Dudzinski & Michel, 2007). In pre-eclampsia, increased superoxide generation is associated with reduced expression of the chaperone protein HSP90 in the fetal vasculature (Gu et al. 2006). In addition to alterations in the expression of proteins associated with oxidative stress and NO bioavailability, other molecular indices of placental oxidative stress include changes in the expression of antioxidant enzymes. It is known that the mammalian placenta expresses all major antioxidant systems, including catalase, Mn-SOD, Cu/Zn-SOD and glutathione peroxidase (Wang & Walsh, 1996). Elevations in the expression of placental antioxidant enzymes are an established compensatory response to challenges inducing oxidative stress (Clifton et al. 2005). The molecular data in the present study show that pregnancy complicated by maternal hypoxia led to a significant increase in the placental expression of HSP70 and 4-HNE, a significant reduction in HSP90 and a significant increase in the expression of catalase. Further, these changes did not occur in normoxic or hypoxic pregnancy treated with vitamin C. Collectively, therefore, these findings are consistent with both molecular evidence of oxidative stress in placentas from pregnancies complicated by hypoxia, and with a protective effect of maternal treatment with vitamin C against placental oxidant stress in hypoxic pregnancy.
The biometric data in the present study show that early onset developmental hypoxia increased placental weight but it did not affect birth weight. Interestingly, maternal treatment with vitamin C significantly enhanced birth weight in both hypoxic and normoxic pregnancy. In rat pregnancy, uterine wall invasion and the establishment of the placenta start after day 9.25 of gestation (Ellington, 1987). Consequently, in this experimental model, placentation occurred during hypoxia. This contrasts with many other studies that have investigated the effects of maternal hypoxia during the last third of gestation in rat pregnancy, modelling late-onset oxygen deprivation in complicated human pregnancy. In those studies, late-onset maternal hypoxia induced overt fetal asymmetric growth restriction (Gilbert & Leturque, 1982; Bae et al. 2003; Williams et al. 2005; Xu et al. 2006; Rueda-Clausen et al. 2009; Camm et al. 2010) without significant effects on placental weight (Gilbert & Leturque, 1982; Camm et al. 2010). In contrast, in the present study, early-onset maternal hypoxia significantly increased placental weight without fetal growth restriction. The differences in the placental and fetal phenotypes between the studies are probably due to the differential temporal growth demands of the placenta and fetus during pregnancy. In the rat, whereas placental growth starts early in gestation and continues throughout pregnancy, fetal growth is exponential and maximal by the end of gestation (Witlin et al. 2002). Therefore, early-onset maternal hypoxia may stimulate greater than normal placental growth and alterations in placental morphology to cushion the adverse effects of the challenge on fetal growth. Accordingly, studies of human pregnancy at high altitude have reported a reduction in the thickness of the villous membrane, improved placental vascularisation with increased placental capillary diameter, capillary length and capillary volume (Reshetnikova et al. 1994; Mayhew, 2003; Cartwright et al. 2007).
The molecular basis underlying the stimulatory effect of vitamin C on fetal growth and birth weight in hypoxic and normoxic pregnancy may reflect alterations in placental and umbilical perfusion. In addition to neural, endocrine and local factors, Chen & Keaney (2004) proposed that the cellular oxidant milieu may also be an important regulator of vascular tone. Vascular endothelial cells generate ROS, such as •O2− (Droge, 2002). Superoxide readily combines with NO, limiting its bioavailability (Kissner et al. 1997). Hence, under physiological conditions, manipulation of the vascular NO:•O2− ratio will modify vascular tone, whereby a fall in the ratio will promote constriction and an increase in the ratio will promote dilatation (Chen & Keaney, 2004). It is likely that modification of this oxidant tone will have a comparatively greater effect on blood flow in circulations that are highly dependent on NO bioavailability and in those that are not innervated, such as the placental and umbilical vascular beds (Vatish et al. 2006). The stimulatory effect of vitamin C on fetal growth may therefore reflect increased blood flow in the placental and umbilical vascular beds, with consequent increased delivery of oxygen and nutrient to the developing fetus. Accordingly, previous studies in our and other laboratories have reported that maternal treatment with the endogenous antioxidant melatonin can protect fetal growth in rat pregnancy complicated by undernutrition (Richter et al. 2009), both melatonin and vitamin C can enhance in vivo umbilical vascular conductance even in healthy ovine pregnancy by a NO-dependent mechanism (Thakor et al. 2010a) and maternal antioxidants can diminish fetal growth restriction in highland and lowland native ewes in pregnancy at high altitude (Parraguez et al. 2011).
Finally, there is evidence for regulation of signal transduction and gene expression by ascorbate, which might help to explain at least some of its beneficial effects on pregnancy reported by us and others. For instance, intracellular vitamin C can directly attenuate basal or hypoxia-induced expression of the master gene hypoxia-inducible factor-1α (HIF-1α). It can also enhance the expression of DNA mismatch repair protein Mut L homologue-1 (MLH1), p73 or alternatively inhibit the activation of nuclear factor kappa B (NFkB), Cdc25C and insulin-like growth factor-I receptor (Li & Schellhorn, 2007). Whether changes in the expression of any of these genes is relevant for placental and/or fetal tissue(s) in the present experimental model remains to be investigated.
In conclusion, maternal hypoxia independent of maternal undernutrition promotes maternal and placental indices of oxidative stress, effects that can be prevented by maternal treatment with vitamin C in hypoxic pregnancy. Although vitamin C may prove an unsuitable candidate for therapy in pregnant women (Poston et al. 2006; Spinnato et al. 2007; Kontic-Vucinic et al. 2008; Rumbold et al. 2008; Villar et al. 2009), treatment with other antioxidants at human tolerable doses, such as with melatonin (Richter et al. 2009; Thakor et al. 2010a) or allopurinol (Derks et al. 2010), may still provide a viable clinical intervention to improve placental function and protect fetal growth in pregnancy complicated by fetal hypoxia.
Acknowledgments
This work was supported by the BBSRC, the British Heart Foundation and The Lister Institute for Preventive Medicine. H.G.R. is a postdoctoral fellow of Beca Presidente de la República (CONICYT) from the Chilean Government. D.A.G. is a Royal Society Wolfson Research Merit Award Holder. The authors declare no conflicts of interest.
Glossary
Abbreviations
- C
control pregnancy
- CVC
control maternal treatment with vitamin C
- eNOS
endothelial nitric oxide synthase
- H
hypoxic pregnancy
- HVC
hypoxia maternal treatment with vitamin C
- 4-HNE
4-hydroxynonenal
- HSP70
heat shock protein 70
- HSP90
heat shock protein 90
- Mn-SOD
manganese superoxide dismutase
- ROS
reactive oxygen species
Author contributions
The experiments in this study were performed in the Department of Physiology, Development and Neuroscience, University of Cambridge, UK; MRC-HPA Centre for Environment and Health, Analytical & Environmental Science Division, King's College London; and Maternal and Fetal Research Unit, Division of Women's Health, King's College London. H.G.R and D.A.G conceived and designed the experiments. H.G.R, E.J.C, B.N.M., F.N., C.M.C., T.C-D., O.S-B., C.D., I.S.M. and D.A.G. collected, analysed and interpreted the experimental data. H.G.R., F.J.K., G.J.B., L.P. and D.A.G. drafted the article and revised it critically for important intellectual content. All authors approved the final version for publication.
References
- Al-Gubory KH, Fowler PA, Garrel C. The roles of cellular reactive oxygen species, oxidative stress and antioxidants in pregnancy outcomes. Int J Biochem Cell Biol. 2010;42:1634–1650. doi: 10.1016/j.biocel.2010.06.001. [DOI] [PubMed] [Google Scholar]
- Ali KZ, Burton GJ, Morad N, Ali ME. Does hypercapillarization influence the branching pattern of terminal villi in the human placenta at high altitude? Placenta. 1996;17:677–682. doi: 10.1016/s0143-4004(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- Bainbridge SA, Roberts JM. Uric acid as a pathogenic factor in preeclampsia. Placenta. 2008;29:S67–S72. doi: 10.1016/j.placenta.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bánhegyi G, Braun L, Csala M, Puskás F, Mandl J. Ascorbate metabolism and its regulation in animals. Free Radic Biol Med. 1997;23:793–803. doi: 10.1016/s0891-5849(97)00062-2. [DOI] [PubMed] [Google Scholar]
- Baydas G, Karatas F, Gursu MF, Bozkurt HA, Ilhan N, Yasar A, Canatan H. Antioxidant vitamin levels in term and preterm infants and their relation to maternal vitamin status. Arch Med Res. 2002;33:276–280. doi: 10.1016/s0188-4409(02)00356-9. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Palmer ME. Eradicating fetomaternal fluid shift during perfusion fixation of the human placenta. Placenta. 1988;9:327–332. doi: 10.1016/0143-4004(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW, Giussani DA. Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol. 2010;203:495.e24–e34. doi: 10.1016/j.ajog.2010.06.046. [DOI] [PubMed] [Google Scholar]
- Cartwright JE, Keogh RJ, Tissot van Patot MC. Hypoxia and placental remodelling. Adv Exp Med Biol. 2007;618:113–126. doi: 10.1007/978-0-387-75434-5_9. [DOI] [PubMed] [Google Scholar]
- Chen K, Keaney J. Reactive oxygen species-mediated signal transduction in the endothelium. Endothelium. 2004;11:109–121. doi: 10.1080/10623320490482655. [DOI] [PubMed] [Google Scholar]
- Cindrova-Davies T, Spasic-Boskovic O, Jauniaux E, Charnock-Jones DS, Burton GJ. Nuclear factor-kappa B, p38, and stress-activated protein kinase mitogen-activated protein kinase signaling pathways regulate proinflammatory cytokines and apoptosis in human placental explants in response to oxidative stress: effects of antioxidant vitamins. Am J Pathol. 2007;170:1511–1520. doi: 10.2353/ajpath.2007.061035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL, Vanderlelie J, Perkins AV. Increased anti-oxidant enzyme activity and biological oxidation in placentae of pregnancies complicated by maternal asthma. Placenta. 2005;26:773–779. doi: 10.1016/j.placenta.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Corpe CP, Tu H, Eck P, Wang J, Faulhaber-Walter R, Schnermann J, et al. Vitamin C transporter Slc23a1 links renal reabsorption, vitamin C tissue accumulation, and perinatal survival in mice. J Clin Invest. 2010;120:1069–1083. doi: 10.1172/JCI39191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Grauw TJ, Myers RE, Scott WJ. Fetal growth retardation in rats from different levels of hypoxia. Biol Neonate. 1986;49:85–89. doi: 10.1159/000242515. [DOI] [PubMed] [Google Scholar]
- Derks JB, Oudijk MA, Torrance HL, Rademaker CM, Benders MJ, Rosen KG, et al. Allopurinol reduces oxidative stress in the ovine fetal cardiovascular system after repeated episodes of ischemia-reperfusion. Pediatr Res. 2010;68:374–380. doi: 10.1203/PDR.0b013e3181ef7780. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247–260. doi: 10.1016/j.cardiores.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington SK. A morphological study of the development of the chorion of rat embryos. J Anat. 1987;150:247–263. [PMC free article] [PubMed] [Google Scholar]
- Franko KL, Giussani DA. Sexual dimorphism in adrenal gland development in the rat fetus (abstract) Reprod Sci. 2004;11:A69. [Google Scholar]
- Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- Gilbert M, Leturque A. Fetal weight and its relationship to placental blood flow and placental weight in experimental intrauterine growth retardation in the rat. J Dev Physiol. 1982;4:237–246. [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S, Barker DJ. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatr Res. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Griendling KK, Sorescu D, Ushio-Fukai M. NAD(P)H oxidase: role in cardiovascular biology and disease. Circ Res. 2000;86:494–501. doi: 10.1161/01.res.86.5.494. [DOI] [PubMed] [Google Scholar]
- Gu Y, Lewis DF, Zhang Y, Groome LJ, Wang Y. Increased superoxide generation and decreased stress protein Hsp90 expression in human umbilical cord vein endothelial cells (HUVECs) from pregnancies complicated by preeclampsia. Hypertens Pregnancy. 2006;25:169–182. doi: 10.1080/10641950600912950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen HJ, Osterby R. Optimizing sampling efficiency of stereological studies in biology: or ‘do more less well!’. J Microsc. 1981;121:65–73. doi: 10.1111/j.1365-2818.1981.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford, UK: Oxford Science Publications; 1999. [Google Scholar]
- Herrera EA, Camm EJ, Cross CM, Mullender JL, Wooding FB, Giussani DA. Morphological and functional alterations in the aorta of the chronically hypoxic fetal rat. J Vasc Res. 2011;49:50–58. doi: 10.1159/000330666. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Burton GJ. In vitro ischemia-reperfusion injury in term human placenta as a model for oxidative stress in pathological pregnancies. Am J Pathol. 2001;159:1031–1043. doi: 10.1016/S0002-9440(10)61778-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iriyama K, Teranishi T, Mori H, Nishiwaki H, Kusaka N. Simultaneous determination of uric and ascorbic acids in human serum by reversed-phase high-performance liquid chromatography with electrochemical detection. Anal Biochem. 1984;141:238–243. doi: 10.1016/0003-2697(84)90451-2. [DOI] [PubMed] [Google Scholar]
- Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young DA, Moore LG. Intrauterine growth restriction, preeclampsia, and intrauterine mortality at high altitude in Bolivia. Pediatr Res. 2003;54:20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- Kissner R, Nauser T, Bugnon P, Lye PG, Koppenol WH. Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol. 1997;10:1285–1292. doi: 10.1021/tx970160x. [DOI] [PubMed] [Google Scholar]
- Kolb E, Wahren M, Leo M, Siebert P, Erices J, Göllnitz L, Völker L. Ascorbic acid concentration in plasma, in amniotic and allantoic fluids, in the placenta and in 13 tissues of sheep fetuses and newborn lambs. Dtsch Tierarztl Wochenschr. 1991;98:424–427. [PubMed] [Google Scholar]
- Kontic-Vucinic O, Terzic M, Radunovic N. The role of antioxidant vitamins in hypertensive disorders of pregnancy. J Perinat Med. 2008;36:282–390. doi: 10.1515/JPM.2008.063. [DOI] [PubMed] [Google Scholar]
- Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci U S A. 2001;98:9842–9846. doi: 10.1073/pnas.171318198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Schellhorn HE. New developments and novel therapeutic perspectives for vitamin C. J Nutr. 2007;137:2171–2184. doi: 10.1093/jn/137.10.2171. [DOI] [PubMed] [Google Scholar]
- Mayhew TM( Changes in fetal capillaries during preplacental hypoxia: growth, shape remodelling and villous capillarization in placentae from high-altitude pregnancies. Placenta. 2003;24:191–198. doi: 10.1053/plac.2002.0895. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Huppertz B, Kaufmann P, Kingdom JC. The ‘reference trap’ revisited: examples of the dangers in using ratios to describe fetoplacental angiogenesis and trophoblast turnover. Placenta. 2003;24:1–7. doi: 10.1053/plac.2002.0878. [DOI] [PubMed] [Google Scholar]
- Métayer S, Seiliez I, Collin A, Duchêne S, Mercier Y, Geraert PA, Tesseraud S. Mechanisms through which sulfur amino acids control protein metabolism and oxidative status. J Nutr Biochem. 2008;19:207–215. doi: 10.1016/j.jnutbio.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Eisenberg MJ, Langleben D, Ernst P, Schiff RL. Altitude-related hypoxia: risk assessment and management for passengers on commercial aircraft. Aviat Space Environ Med. 2003;74:922–927. [PubMed] [Google Scholar]
- Moore LG, Charles SM, Julian CG. Humans at high altitude: hypoxia and fetal growth. Respir Physiol Neurobiol. 2011;178:181–190. doi: 10.1016/j.resp.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myatt L. Review: Reactive oxygen and nitrogen species and functional adaptation of the placenta. Placenta. 2010;31:S66–S69. doi: 10.1016/j.placenta.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano G, Degan P, d’Ischia M, Kelly FJ, Pallardó FV, Zatterale A, et al. Gender- and age-related distinctions for the in vivo prooxidant state in Fanconi anaemia patients. Carcinogenesis. 2004;25:1899–1909. doi: 10.1093/carcin/bgh194. [DOI] [PubMed] [Google Scholar]
- Parraguez VH, Atlagich M, Araneda O, García C, Muñoz A, De Los Reyes M, Urquieta B. Effects of antioxidant vitamins on newborn and placental traits in gestations at high altitude: comparative study in high and low altitude native sheep. Reprod Fertil Dev. 2011;23:285–296. doi: 10.1071/RD10016. [DOI] [PubMed] [Google Scholar]
- Papp E, Nardai G, Söti C, Csermely P. Molecular chaperones, stress proteins and redox homeostasis. Biofactors. 2003;17:249–257. doi: 10.1002/biof.5520170124. [DOI] [PubMed] [Google Scholar]
- Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med Res Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- Poston L, Briley AL, Seed PT, Kelly FJ, Shennan AH. Vitamins in pre-eclampsia (VIP) trial consortium. Vitamin C and vitamin E in pregnant women at risk for pre-eclampsia (VIP trial): randomised placebo-controlled trial. Lancet. 2006;367:1145–1154. doi: 10.1016/S0140-6736(06)68433-X. [DOI] [PubMed] [Google Scholar]
- Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. Am J Obstet Gynecol. 1994;171:1560–1565. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- Richter HG, Hansell JA, Raut S, Giussani DA. Melatonin improves placental efficiency and birth weight and increases the placental expression of antioxidant enzymes in undernourished pregnancy. J Pineal Res. 2009;46:357–364. doi: 10.1111/j.1600-079X.2009.00671.x. [DOI] [PubMed] [Google Scholar]
- Roberts JM, Bodnar LM, Lain KY, Hubel CA, Markovic N, Ness RB, Powers RW. Uric acid is as important as proteinuria in identifying fetal risk in women with gestational hypertension. Hypertension. 2005;46:1263–1269. doi: 10.1161/01.HYP.0000188703.27002.14. [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Morton JS, Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- Rumbold A, Duley L, Crowther CA, Haslam RR. Antioxidants for preventing pre-eclampsia. Cochrane Database Syst Rev. 2008;1:CD004227. doi: 10.1002/14651858.CD004227.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankaralingam S, Arenas IA, Lalu MM, Davidge ST. Preeclampsia: current understanding of the molecular basis of vascular dysfunction. Expert Rev Mol Med. 2006;8:1–20. doi: 10.1017/S1462399406010465. [DOI] [PubMed] [Google Scholar]
- Schachter M, Foulds S. Free radicals and the xanthine oxidase pathway. In: Grace PA, Mathie RT, editors. Ischaemia-Reperfusion Injury. Oxford, UK: Blackwell Science; 1999. pp. 137–147. [Google Scholar]
- Spinnato JA, 2nd, Freire S, Pinto E, Silva JL, Cunha Rudge MV, et al. Antioxidant therapy to prevent preeclampsia: a randomized controlled trial. Obstet Gynecol. 2007;110:1311–1318. doi: 10.1097/01.AOG.0000289576.43441.1f. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Seron-Ferre M, Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010a;49:399–406. doi: 10.1111/j.1600-079X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Richter HG, Kane AD, Dunster C, Kelly FJ, Poston L, Giussani DA. Redox modulation of the fetal cardiovascular defence to hypoxaemia. J Physiol. 2010b;588:4235–4247. doi: 10.1113/jphysiol.2010.196402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjoa ML, Cindrova-Davies T, Spasic-Boskovic O, Bianchi DW, Burton GJ. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am J Pathol. 2006;169:400–404. doi: 10.2353/ajpath.2006.060161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatish M, Randeva HS, Grammatopoulos DK. Hormonal regulation of placental nitric oxide and pathogenesis of pre-eclampsia. Trends Mol Med. 2006;12:223–233. doi: 10.1016/j.molmed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Villar J, Purwar M, Merialdi M, Zavaleta N, Thi Nhu Ngoc N, et al. World Health Organisation multicentre randomised trial of supplementation with vitamins C and E among pregnant women at high risk for pre-eclampsia in populations of low nutritional status from developing countries. BJOG. 2009;116:780–788. doi: 10.1111/j.1471-0528.2009.02158.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Walsh SW. Antioxidant activities and mRNA expression of superoxide dismutase, catalase, and glutathione peroxidase in normal and preeclamptic placentas. J Soc Gynecol Investig. 1996;3:179–184. [PubMed] [Google Scholar]
- West B, Welch K, Galecki A. Linear Mixed Models: A Practical Guide Using Statistical Software. Boca Raton, USA: Chapman & Hall; 2007. [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC, Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R360–R367. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- Witlin AG, Li ZY, Wimalawansa SJ, Grady JJ, Grafe MR, Yallampalli C. Placental and fetal growth and development in late rat gestation is dependent on adrenomedullin. Biol Reprod. 2002;67:1025–1031. doi: 10.1095/biolreprod.101.002196. [DOI] [PubMed] [Google Scholar]
- Xu Y, Williams SJ, O’Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- Zamudio S. High-altitude hypoxia and preeclampsia. Front Biosci. 2007;12:2967–1277. doi: 10.2741/2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Kovalenko O, Vanderlelie J, Illsley NP, Heller D, Belliappa S, Perkins AV. Chronic hypoxia in vivo reduces placental oxidative stress. Placenta. 2007a;28:846–853. doi: 10.1016/j.placenta.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Palmer SK, Stamm E, Coffin C, Moore LG. Uterine blood flow at high altitude. In: Sutton JR, Houston CS, editors. Hypoxia and the Brain. Burlington: VT, Queen City Press; 1995. pp. 112–124. [Google Scholar]
- Zamudio S, Wu Y, Ietta F, Rolfo A, Cross A, Wheeler T, et al. Human placental hypoxia-inducible factor-1alpha expression correlates with clinical outcomes in chronic hypoxia in vivo. Am J Pathol. 2007b;170:2171–2179. doi: 10.2353/ajpath.2007.061185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar JH. Data transformations. In: Kurts B, editor. Biostatistical Analysis. New Jersey: Prentice-Hall; 1984. pp. 236–243. [Google Scholar]


