Abstract
The calcineurin–NFAT (nuclear factor of activated T-cells) signalling pathway is involved in the regulation of activity-dependent skeletal muscle myosin heavy chain (MHC) isoform type expression. Emerging evidence indicates that nitric oxide (NO) may play a critical role in this regulatory pathway. Thus, the purpose of this study was to investigate the role of NO in activity-induced calcineurin–NFATc1 signalling leading to skeletal muscle faster-to-slower fibre type transformations in vivo. Endogenous NO production was blocked by administering l-NAME (0.75 mg ml−1) in drinking water throughout 0, 1, 2, 5 or 10 days of chronic low-frequency stimulation (CLFS; 10 Hz, 12 h day−1) of rat fast-twitch muscles (L+Stim; n= 30) and outcomes were compared with control rats receiving only CLFS (Stim; n= 30). Western blot and immunofluorescence analyses revealed that CLFS induced an increase in NFATc1 dephosphorylation and nuclear localisation, sustained by glycogen synthase kinase (GSK)-3β phosphorylation in Stim, which were all abolished in L+Stim. Moreover, real-time RT-PCR revealed that CLFS induced an increased expression of MHC-I, -IIa and -IId(x) mRNAs in Stim that was abolished in L+Stim. SDS-PAGE and immunohistochemical analyses revealed that CLFS induced faster-to-slower MHC protein and fibre type transformations, respectively, within the fast fibre population of both Stim and L+Stim groups. The final fast type IIA to slow type I transformation, however, was prevented in L+Stim. It is concluded that NO regulates activity-induced MHC-based faster-to-slower fibre type transformations at the transcriptional level via inhibitory GSK-3β-induced facilitation of calcineurin–NFATc1 nuclear accumulation in vivo, whereas transformations within the fast fibre population may also involve translational control mechanisms independent of NO signalling.
Key points
Exercise is known to trigger skeletal muscle structural and functional adaptations.
Control of these adaptive alterations is a complex process involving multiple signalling pathways and levels of regulation.
The well-characterized calcineurin–nuclear factor of activated T-cells (NFATc1) signalling pathway is involved in the regulation of activity-dependent alterations in skeletal muscle myosin heavy chain expression. Myosin heavy chain is a contractile protein that largely dictates a muscle's speed of contraction.
We show that a signalling molecule called nitric oxide may be regulating alterations in myosin heavy chain expression via activity-modulated calcineurin–NFATc1 signalling.
These findings increase our understanding of how skeletal muscle adaptive alterations are regulated.
Introduction
Mammalian skeletal muscle is a heterogeneous tissue comprised of diverse fibre populations. This fibre type diversity is, in part, attributed to the various myosin heavy chain (MHC) protein isoforms that largely dictate the rate of force development, maximum sarcomeric shortening velocity and rate of cross-bridge cycling (Bottinelli et al. 1991; Pette & Staron, 1997). Fibre types range from slow (type I) to fast (type IIA, IID(X) and IIB) in adult rodent skeletal muscle that contain the correspondingly named MHC isoforms listed in increasing order of shortening velocity: MHCI, MHCIIa, MHCIId(x) and MHCIIb (Pette & Staron, 1997). In response to various contractile demands such as exercise, skeletal muscle demonstrates remarkable adaptability or plasticity that is largely dictated by changes in motor neuron activity. For example, chronic low-frequency electrical stimulation (CLFS; 10 Hz) of the motor nerve mimics the tonic firing pattern typical of slow motor neurons (Hennig & Lomo, 1985) and induces maximal faster-to-slower fibre type transformations in the absence of skeletal muscle injury in the rat model (Putman et al. 1999, 2000, 2001; Martins et al. 2006; LaFramboise et al. 2009). This fibre type transformation generally follows the ‘next nearest-neighbour’ rule where fibre types undergo a predictable pattern of transformation in the direction of fast type IIB→IID(X)→IIA→ slow type I (Pette & Vrbová, 1999; Pette & Staron, 2000). The specific signalling pathways that transduce motor neuron firing patterns into shifts in fibre-specific gene expression, however, remain to be fully elucidated.
The mechanism by which increased levels of tonic firing of motor neurons induce transcription of slower, more energy-efficient, fibre-specific genes involves sustained elevations in low-amplitude intracellular µCa2+½ oscillations, which in turn stimulate a number of key downstream signalling pathways (for reviews see Michel et al. 2004, 2007; Bassel-Duby & Olson, 2006). Calcineurin–NFAT (nuclear factor of activated T-cells) is one of the best characterised of these signalling pathways (Chin et al. 1998; Dunn et al. 1999, 2000, 2001; Liu et al. 2001). Calcineurin is a Ca2+–calmodulin-dependent protein phosphatase that dephosphorylates the four muscle-localised transcription factor isoforms of the NFAT family, NFATc1–c4. NFAT dephosphorylation results in its nuclear translocation and binding to specific sequences on the promoters of target genes that induce slower, more oxidative fibre-specific phenotypes (Hogan et al. 2003; Rana et al. 2005, 2008; Meissner et al. 2007; Calabria et al. 2009), and repress expression of fast contractile protein isoforms, such as TnIf, at least in slow fibres (Rana et al. 2008). Although this pathway describes the activity-induced activation of NFAT, regulation of this transcription factor is complex, being subject to dynamic cycles of activation (i.e. dephosphorylation and nuclear import) and deactivation (i.e. phosphorylation and nuclear export) that results in nuclear-cytoplasmic shuttling (Dunn et al. 2000, 2001; Liu et al. 2001, 2005). Skeletal muscle NFAT phosphorylation can occur by several protein kinases, such as glycogen synthase kinase-3β (GSK-3β), which has been identified as an important promoter of NFAT nuclear export (Shen et al. 2007) and an inhibitor of NFAT-mediated increases in slow MHC gene expression (Jiang et al. 2006).
Other activity-dependent signalling pathways can also co-regulate the transition of fast-twitch fibres toward slower more energy-efficient phenotypes, as demonstrated by the expression dependence of slow (TnIs) and fast (TnIf) isoforms of Troponin-I to patterned electrical activity (Nakayama et al. 1996; Rana et al. 2005). Another signalling intermediate involved in fibre remodelling is the transcriptional co-activator peroxisome proliferator-activated receptor gamma co-activator 1-alpha (PGC1α), which is highly expressed in slow type I fibres (Wu et al. 1999; Lin et al. 2002) and displays considerable plasticity by increasing its expression levels in response to endurance exercise (Baar et al. 2002; Terada et al. 2002; Russell et al. 2003). PGC1α is induced by various upstream signals, such as p38-MAPK (Akimoto et al. 2005; Wright et al. 2007), CaMK and calcineurin (Handschin et al. 2003), and possibly MEF2 (Czubryt et al. 2003; Vissing et al. 2008). PGC1α has also been shown to be induced by AMPK, but signals transmitted through this mechanism are restricted to metabolic genes (Terada et al. 2002; Zong et al. 2002; Putman et al. 2003; Suwa et al. 2006), and do not display regulatory control over expression of contractile proteins such as myosin heavy chains (MHC) (Putman et al. 2003).
Nitric oxide (NO) is a ubiquitous signalling molecule that is controlled at the synthesis level by NO synthase (NOS), which is, in turn, regulated by Ca2+–calmodulin binding (Stamler & Meissner, 2001). Increased NOS activity and resultant NO production occur in response to muscle contraction, as well as CLFS, and are involved in a number of important regulatory processes within this tissue (Reiser et al. 1997; Stamler & Meissner, 2001; McConell & Wadley, 2008). It has, for example, been demonstrated that NO production is reduced in Duchenne muscular dystrophy patients (Grozdanovic & Baumgarten, 1999) but exposure to NO donors improves muscle myoblast differentiation and regeneration within dystrophic muscle fibres (Pisconti et al. 2006; Brunelli et al. 2007; Colussi et al. 2008, 2009). The AKT pathway has also been shown to be important for NO synthesis (Dimmeler & Zeiher, 1999), and NO seems to be a requirement for increased activity of histone deacetylases, which in turn regulate activation of the myogenic transcription factors MEF2 and MyoD (Sartorelli et al. 1999; Lu et al. 2000; Naya et al. 2000).
NO has also been directly linked to mitochondrial biogenesis, and the increased potential for terminal substrate oxidation. Exercise, for example, is known to increase eNOS, which in turn induces the expression of PGC1α, an important intermediary signal leading to mitochondrial biogenesis (see review by Nisoli & Carruba, 2006). Likewise, nNOS activity and NO production are known to increase in response to electrical stimulation (Reiser et al. 1997; McConell & Wadley, 2008). Recently, Drenning et al. (2008, 2009) showed that NO is also associated with inhibitory phosphorylation of GSK-3β, facilitation of NFATc1 nuclear accumulation, and increased slow MHCI mRNA expression in response to Ca2+-ionophore treatment in vitro. Collectively, these observations suggest that CLFS-induced faster-to-slower fibre type transformations may be regulated by both calcineurin and NO working synergistically to promote NFATc1 nuclear accumulation in vivo. Therefore, the purpose of the present study was to test the hypothesis that pharmacological inhibition of NOS activity would prevent CLFS-induced skeletal muscle NFATc1 nuclear accumulation and subsequent faster-to-slower fibre type transformations in vivo. Our findings indicate that NO regulates MHC-related faster-to-slower fibre type conversions at the transcriptional level via neural activity-modulated nuclear accumulation of NFATc1 in vivo involving both calcineurin and GSK-3β whereas transformations within the fast fibre population may also involve translational control mechanisms independent of NO signalling.
Methods
Ethical approval
All animal procedures were carried out in accordance with the guidelines of the Canadian and UK Councils for Animal Care and received ethical approval from the University of Alberta and Concordia University.
Experimental design and use of animals
Sixty adult male Wistar rats (Charles River Laboratories, Montreal, PQ, Canada) were individually housed under controlled environmental conditions (22°C with 12:12 h light–dark cycle) and consumed standard rat chow and water or an aqueous solution of l-NAME ad libitum, which was measured and replaced daily. Animals in the experimental groups received l-NAME (0.75 mg ml−1) in drinking water throughout 0, 1, 2, 5 or 10 days of CLFS. CLFS (10 Hz, 12 h day−1) was applied across the peroneal nerve thereby stimulating the fast-twitch tibialis anterior and extensor digitorum longus (L+Stim; n= 6 animals per group). Outcome measures were compared with control rats receiving only CLFS of matched time points (Stim; n= 6 animals per group). The application of CLFS has been shown to elicit a compensatory effect in the contralateral control muscles due to increased weight bearing (Putman et al. 2000); therefore comparisons were made to animals receiving only a sham operation of the left leg (Control). As a post hoc consideration, mixed fast-twitch plantaris muscles of wild type (WT-C57/BL6) and transgenic mice that over-express constitutively active calcineurin (MCK-CnA*-Tg) were collected and served as positive controls for NFATc1 nuclear localization experiments (Dunn et al. 2000; Chakkalakal et al. 2004).
Systemic inhibition of nitric oxide synthase activity
The pharmacological inhibition of NOS was achieved by administering the competitive non-isoform-specific NOS inhibitor l-NAME (Sigma-Aldrich, Oakville, ON, Canada) daily in the drinking water of animals starting 2 days prior to the onset of stimulation and continuing for the duration of the study. An l-NAME concentration of 0.75 mg ml−1 was used that resulted in a daily dose of ∼100 mg (kg body mass)−1. Body mass was recorded daily throughout the experimental period. This dose has been shown to effectively inhibit NOS activity in the rat (Smith et al. 2002; Sellman et al. 2006).
Chronic low-frequency stimulation
CLFS (10 Hz, impulse width 380 μs, 12 h day−1) was applied across the left common peroneal nerve as previously described (Simoneau & Pette, 1988). Briefly, while animals were under general anaesthesia (75 mg (kg body wt)−1 ketamine, 10 mg (kg body wt)−1 xylazine and 0.5 mg (kg body wt)−1 acepromazine maleate via intraperitoneal injection), bipolar electrodes were implanted lateral to the common peroneal nerve of the left hind limb, externalised at the dorsal intrascapular region, and connected to a small, portable stimulator. Animals were allowed to recover for 7 days before the onset of stimulation.
Muscle sampling
Upon completion of the stimulation period, rats were anaesthetised as before and the tibialis anterior and extensor digitorum longus were excised from both hind limbs and frozen in melting isopentane cooled in liquid N2 (–159°C). Muscles were subsequently stored in liquid N2 (–196°C). The anaesthetised animals were then killed after all muscles were collected with an overdose of Euthanyl (100 mg (kg body wt)−1 via intraperitoneal injection) (Bimedia-MTC Animal Health Inc., Cambridge, ON, Canada), followed by exsanguination. For mice, all surgical procedures were performed under sterile conditions on animals anaesthetized (1.2 μl (g)−1i.m.) with 100 mg (ml)−1 ketamine hydrochloride and 20 mg ml−1 xylaxine in a volume ratio of 1.6:1. Mice were killed by cervical dislocation.
Western blot analyses
Western blot analyses were performed as previously described (Dunn et al. 2000, 2001). Briefly, for extraction of whole cell protein, rat extensor digitorum longus samples were homogenized in 1 ml of RIPA buffer (1% (v/v) Nonidet P-40, 0.5% (w/v) sodium deoxycholate, 0.1% (w/v) SDS, 10 μg (ml)−1 aprotinin, 10 μg (ml)−1 leupeptin, 1 mm phenylmethylsulfonyl fluoride, 10 mm sodium fluoride, 1 mm sodium orthovanadate in PBS, pH 7.4). Homogenates were centrifuged at 20,000 g for 30 min at 4°C. Protein concentrations were determined in order to ensure equal loading of samples between lanes (Bradford, 1976). Samples were subsequently diluted in modified Laemmli lysis buffer and boiled for 5 min (Laemmli, 1970). Each sample (150 μg of total protein) was subjected to 6% SDS–PAGE electrophoresis (Mini-PROTEAN 3 system; Bio-Rad Laboratories, Mississauga, ON, Canada) and then transferred to a polyvinylidene fluoride membrane (Millipore, Bedford, MA, USA). Transfer efficiency and a second evaluation of equal sample loading were confirmed by Ponceau-S staining of membrane-bound proteins. Membranes were incubated in blocking solution (5% powdered milk, 0.1% Tween-20 in TBS, pH 8.0) for 1 h and then incubated overnight at 4°C with rabbit monoclonal anti-phospho-GSK-3β (Ser9) (clone 5B3; Cell Signalling Technologies, Beverly, MA, USA) that was diluted in blocking solution (1:2000), or rabbit monoclonal anti-GSK-3β (clone 27C10; Cell Signalling Technologies) diluted in blocking dilution (1:2000), or with mouse monoclonal anti-NFATc1 (clone 7A6; Santa Cruz Biotechnology, Santa Cruz, CA, USA) also diluted in blocking solution (1:200). Membranes were washed with 0.1% Tween-20 in TBS and then incubated for 1 h with anti-mouse monoclonal (1:1000; Sigma-Aldrich) or anti-rabbit polyclonal (1:2000; Cell Signalling Technologies) horseradish peroxidase secondary antibody conjugates diluted in blocking solution and washed as before. The protein–antibody complex was revealed by chemiluminescence using the Immobilon Western Chemiluminescence Substrate kit (Millipore). Membranes were reprobed with polyclonal rabbit anti-α-tubulin (1:2000; Cell Signalling Technologies), which served as the internal control and further confirmed equal loading. Immunoreactivity of tubulin was visualized as described above after incubation with anti-rabbit horseradish peroxidase secondary antibody conjugates in blocking solution (1:2000; Cell Signalling Technologies).
Quantification of NFATc1
Consistent with previous findings in rodent skeletal muscle (Dunn et al. 2000, 2001), multiple NFATc1 bands ranging from 80–100 kDa in size were detected on Western blots of rat extensor digitorum longus whole cell extracts (Fig. 1A). Pretreatment of samples with alkaline phosphatase (AP) increased the prevalence of only one lower molecular weight species of NFATc1 (∼85 kDa; Fig. 1A, see +AP lane), corresponding to the most dephosphorylated form of this protein. The density of the most-dephosphorylated (de-PO4; ∼85 kDa) and most phosphorylated (PO4; ∼95 kDa) bands of NFATc1 were determined using Fluorchem software (Cell Biosciences, Santa Clara, CA, USA) and expressed as a ratio for each sample.
Figure 1. NFATc1 phosphorylation status in rat fast-twitch muscle.
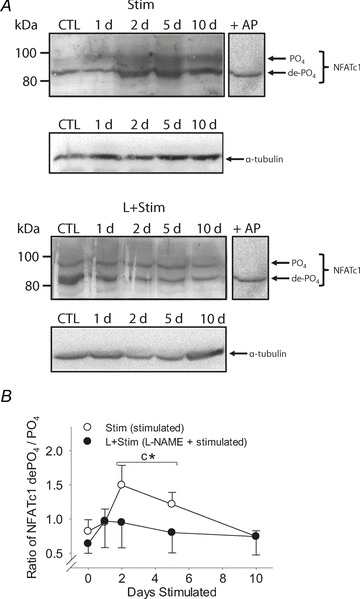
A, Western blots of NFATc1 in whole cell extracts prepared from rat extensor digitorum longus muscles. α-Tubulin served as a loading control for total protein. B, ratio of the most de-PO4-to-most-PO4 NFATc1 protein bands. Values are means ± SEM; n= 6 animals per group. Bracket indicates groups within each treatment condition that were pooled; n= 12 animals per group. Statistical symbols indicate: Cdifferent from Control (i.e. 0 day (d) Stim); *different from L+Stim of the matched time point of stimulation (P < 0.05).
Immunofluorescent detection of NFATc1
Rat tibialis anterior muscles were mounted in embedding medium (Tissue-Tek O.C.T. Compound, Sakura Finetek, Torrance, CA, USA) and 10-μm-thick transverse frozen sections were collected from the mid-belly of each muscle and transferred onto Superfrost Plus microscope slides (Fisher Scientific, Ottawa, ON, Canada) at –20°C. The immunostaining protocol was modified from Xiao et al. (2008). Specifically, sections were fixed with 2% (v/v) paraformaldehyde for 20 min (Sigma-Aldrich) and washed three times in PBS. Sections were then blocked and permeablized, with 2% (v/v) normal goat serum (Sigma-Aldrich) and 0.2% (v/v) Triton X-100 (Sigma-Aldrich), respectively, both in PBS for 1 h each. Sections were incubated overnight at 4°C with monoclonal mouse anti-NFATc1 primary antibody (Santa Cruz Biotechnology) at a 1:200 dilution in PBS containing 1% (v/v) normal goat serum and 0.05% (v/v) Triton X-100. Slides were then washed as before and goat anti-mouse Alexa Fluor 488 secondary antibody (Invitrogen, Life Sciences, Burlington, ON, Canada) was applied for 1 h at room temperature in PBS containing 1% (v/v) normal goat serum and 0.05% (v/v) Triton X-100. Slides were washed as before, air-dried and mounted in Vectashield containing 1.43 nm 4′,6-diamidino-2-phenylindole (DAPI; Vector Laboratories, Burlington, ON, Canada). Control experiments omitting primary antibodies revealed absent or very low-level background staining. Frozen sections of plantaris obtained from WT and MCK-CnA*-Tg were stained in parallel, and served as positive controls (Dunn et al. 2000). Images were acquired on an Olympus BX60 fluorescent microscope (Olympus, Centre Valley, PA, USA) using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA). A total area of 0.08 mm2 was analysed in each rat.
Myosin heavy chain mRNA analyses by real-time reverse transcriptase-polymerase chain reaction
Patterns of MHC isoform expression in tibialis anterior muscles were analysed at the mRNA level using real-time RT-PCR (Martins et al. 2009). TRIzol RNA extraction was performed according to manufacturer's instructions (Invitrogen). The concentrations and purity of RNA extracts were evaluated by measuring the absorbance at 260 and 260/280 nm, respectively, using a NanoDrop ND 1000 system (Rose Scientific Ltd, Edmonton, AB, Canada). Synthesis of cDNA was performed according to an established procedure (Martins et al. 2009). Samples were diluted to 1 μg (μl)−1 and reverse transcription was performed for 1 h at 37°C with oligo (dT12-18) primers (Invitrogen) and moloney murine leukemia virus DNA polymerase (Invitrogen). Primers (Invitrogen) and Taqman-MGB probes (Applied Biosystems, Foster City, CA, USA) were designed with the European Molecular Biology Laboratory–European Bioinformatics Institute and aligned using Clustal W for rat MHCI (X15939), MHCIIa (L13606), MHCIId(x) (XM 213345) and MHCIIb (L24897) (Table 1). Real-time PCR was performed on 1 μl cDNA samples, in duplicate, using an ABI 7900HT thermocycler (Applied Biosystems). 18S rRNA (Applied Biosytems) was used as the endogenous control. Relative changes in MHC isoform gene expression were determined using the 2−ΔΔCt method of analysis (Livak & Schmittgen, 2001). Inter-assay variation was evaluated by repeated analysis of a known sample on each 96-well plate and was confirmed to be negligible. Additionally, the amplification efficiencies of the MHC isoforms and 18S were similar.
Table 1.
Rat specific real-time reverse-transcriptase polymerase chain reaction primers and probes
| Target | Forward primer | Reverse primer | Probe |
|---|---|---|---|
| MHCI | 5′-GCAGTTGGATGAGCGACTCA-3′ | 5′-TCCTCAATCCTGGCGTTGA-3′ | 5′-AGAAGGACTTTGAGTTAAAT-3′ |
| MHCIIa | 5′-GGCGGCAAGAAGCAGATC-3′ | 5′-TTCCGCTTCTGCTCACTCTCT-3′ | 5′-AGGCCAGAGTGCGTG-3′ |
| MHCIId(x) | 5′-GGCGGCAAGAAGCAGATC-3′ | 5′-TTCGTTTTCAACTTCTCCTTCAAGT-3′ | 5′-AGGCCAGGGTCCG-3′ |
| MHCIIb | 5′-GGCGGCAAGAAGCAGATC-3′ | 5′-TTTTCCACCTCGTTTTCAAGCT-3′ | 5′-TGGAGGCCAGAGTGA-3′ |
Electrophoretic analyses of myosin heavy chain protein isoforms
Quantitative MHC protein isoform analyses were completed as previously described (Hämäläinen & Pette, 1996; Putman et al. 2004). Briefly, frozen powdered tibialis anterior muscles were homogenised in an ice-cold buffer containing 100 mm NaP2O7 (pH 8.5), 5 mm EGTA, 5 mm MgCl2, 0.3 mm KCl, 10 mm DTT (Sigma-Aldrich) and 5 mg ml−1 of a protease inhibitor cocktail (Complete, Roche Diagnostics, Indianapolis, IN, USA). Samples were then centrifuged at 12,000 g for 5 min at 4°C; supernatants were diluted 1:1 with glycerol and stored at –20°C until analysed. Prior to gel loading, muscle extracts were diluted in modified Laemmli lysis buffer to a concentration of 0.2 μg (μl)−1 and boiled for 6 min (Laemmli, 1970). Samples (1 μg total protein per lane) were electrophoresed (275 V for 24 h at 8°C) in duplicate on 7% (w/v) SDS-PAGE gels containing glycerol, under denaturing conditions. Gels were then fixed and MHC isoforms were detected by silver staining and evaluated by integrated densitometry (ChemiGenius, GeneSnap and GeneTools, Syngene, UK).
Immunohistochemistry for myosin heavy chain protein isoforms
Tibialis anterior muscles were mounted as before and 10-μm-thick transverse frozen sections were collected from the mid-belly of each muscle at –20°C. Immunostaining was completed according to an established protocol (Putman et al. 2001, 2003). Briefly, sections were washed once in PBS with 0.1% (v/v) Tween-20 (PBS-T), twice in PBS and then incubated for 15 min in 3% (v/v) H2O2 in methanol. Serial sections stained for BA-D5, SC-71 or BF-35 were incubated for 1 h in a blocking solution (BS-1: 1% (w/v) bovine serum albumin and 10% (v/v) horse serum in PBS-T, pH 7.4) containing avidin-D blocking reagent (Vector Laboratories). Serial sections stained for BF-F3 were incubated in a similar blocking solution, with the exception that goat serum was replaced with horse serum (BS-2). Sections were probed with monoclonal antibodies directed against adult MHC isoforms (Schiaffino et al. 1988, 1989) harvested from the supernatants of hybridoma cell lines obtained from the American Type Culture Collection (Manassas, VA, USA): BA-D5 (IgG, anti-MHCI), SC-71 (IgG, anti-MHCIIa) and BF-F3 (IgM, anti-MHCIIb). Clone BF-35 (purified IgG, staining all MHCs except MHCIId(x)) was a generous gift from Dr S. Schiaffino (Padova, Italy). Sections were incubated overnight at 4°C with a primary antibody that was diluted in its corresponding blocking solution containing a biotin blocking reagent (Vector Laboratories). Biotinylated horse anti-mouse-IgG (BA-D5; SC-71; BF-35) or biotinylated goat anti-mouse-IgM (BF-F3) was applied for 1 h. Sections were again washed and incubated with Vectastain ABC reagent according to the manufacturer's instructions (Vector Laboratories) and reacted with 0.07% (w/v) diaminobenzidine, 0.05% (v/v) H2O2 and 0.03% (w/v) NiCl2 in 50 mm Tris-HCl (pH 7.5). All sections were subsequently dehydrated, cleared and mounted in Entellan (Merck, Darmstadt, Germany). Analysis was completed with a Leitz Diaplan microscope (Ernst Leitz Wetzlar GmbH, Germany) fitted with a Pro-Series high performance charge-coupled digital camera (Cohu, San Diego, CA, USA), Image-Pro Plus software (Media Cybernetics) and a custom-designed analytical program (Putman et al. 2000). A similar number of fibres were examined from three representative areas (i.e. deep, middle and superficial regions) in each rat (i.e. 1345 fibres per rat). Type I, IIA and IIB fibres were identified by positive staining, and type IID(X) fibres were identified by the absence of staining.
Statistical analyses
Data are summarised as means ± SEM. Differences between group means were assessed using a two-way ANOVA (i.e. treatment (Stim or L+Stim) × days of stimulation (0, 1, 2, 5 or 10 days)). When a significant F ratio was found, mean values were compared using the Newman–Keuls post hoc analysis. Data in which a priori hypotheses were established and the direction of changes predicted in advance were analysed by the Student's one-tailed t test. Differences were considered significant at P < 0.05. There were no differences between the left and right legs of Control, as determined by the t test for dependent samples, therefore left and right leg data were pooled.
Results
Animal weights
Animals initially weighed 318 ± 3 g and gained 29 ± 6 g during 10 days of stimulation. Additionally, animal weights did not differ between Stim and L+Stim groups at matched time points (i.e. 0, 1, 2, 5 and 10 days).
NFATc1 phosphorylation status and localisation
To test the hypothesis that NOS inhibition prevents activity-induced skeletal muscle NFATc1 nuclear accumulation in vivo, we measured both the phosphorylation status and nuclear localisation of NFATc1 in rat fast-twitch muscles exposed to CLFS. Western blots of rat extensor digitorum longus extracts showed a multiple banding pattern for NFATc1 ranging from 80 to 100 kDa, reflecting the phosphorylation status and post-translational modifications of this transcription factor (Fig. 1A). CLFS induced a marked increase in total NFATc1 protein after 2 days of stimulation compared with Control which was not observed in L+Stim animals. We then identified the most dephosphorylated (de-PO4) and phosphorylated (PO4) forms of this protein and compared the ratio of their expression across experimental conditions (Fig. 1B). CLFS induced a rapid yet transient dephosphorylation of NFATc1 as shown by a 40–50% increase in the ratio of NFATc1 de-PO4-to-PO4 in 2- and 5-day-stimulated animals, respectively, returning to Control levels by day 10. This effect was abrogated in animals that received l-NAME treatment throughout the 10 day time course of stimulation. Likewise, as detected by immunohistochemistry in rat tibialis anterior tissue sections (Fig. 2A and B), CLFS induced a 2.6-fold increase in the proportion of NFATc1-positive nuclei by 5 days of stimulation, returning to baseline Control values by 10 days, while l-NAME treatment blocked this effect (Fig. 2C). Taken together, these results suggest that NOS activity is required for CLFS-induced increases in NFATc1 nuclear accumulation. Analysis of frozen tissue cross-sections of the fast plantaris obtained from WT and transgenic mice overexpressing constitutively active CnA* (Dunn et al. 2000; Chakkalakal et al. 2004) served as a comparative positive control for NFATc1 nuclear localization experiments (Fig. 2D). Note the peak proportion of NFATc1-positive nuclei after 10 days of Stim was comparable to levels observed in CnA* mice, as were baseline levels in their respective controls.
Figure 2. Immunofluorescent analysis of NFATc1 localisation in rat fast-twitch muscle.
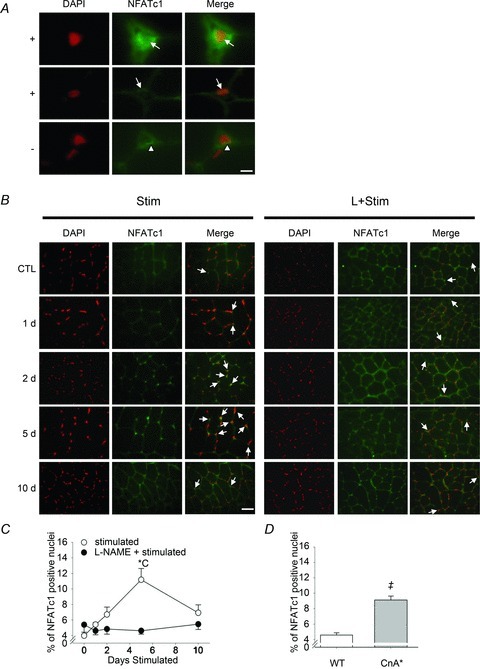
A, representative high magnification Z-stacked photomicrographs of NFATc1-positive (arrows in ‘+’ panel rows) or NFATc1-negative (arrowheads in ‘–’ panel row) nuclei. Sections of rat tibialis anterior muscles were stained for NFATc1 (green) and nuclei visualized with DAPI (red). Note the perinuclear staining of NFATc1 in all panels. Bar represents 6.25 μm. B, representative photomicrographs of Stim- and L+Stim-treated rat tibialis anterior muscles at 0, 1, 2, 5 and 10 days of stimulation. Sections were stained as in A. Arrows point to nuclei identified as NFATc1-positive from high magnification images. Bar represents 50 μm. C, proportion of NFATc1-positive nuclei in Stim- and L+Stim-treated rat tibialis anterior muscles. D, proportion of NFATc1-positive nuclei in plantaris muscles of wild type (WT) and transgenic mice overexpressing constitutively active calcineurin (CnA*) as a comparative control (see Methods). Values are means ± SEM; n= 6 animals per group for Stim and L+Stim and n= 3 animals per group for WT and CnA*. Statistical symbols indicate: Cdifferent from Control (i.e. 0 days Stim); *different from L+Stim of the matched time point of stimulation; ‡CnA* different from wild type (P < 0.05).
GSK-3β phosphorylation status
To better understand the involvement of NOS activity in the regulation of activity-induced NFATc1 nuclear accumulation and MHC mRNA expression in vivo, we measured phosphorylated and total GSK-3β protein by Western blot analysis in rat fast-twitch muscles exposed to CLFS (Fig. 3A). Quantification of Western blots revealed that CLFS induced a rapid increase in both phosphorylated GSK-3β (Fig. 3B) and the ratio of phosphorylated to total GSK-3β (Fig. 3C) from day 1 of stimulation that remained sustained to beyond 5 days, compared with Control levels. l-NAME treatment abrogated this effect throughout the stimulation period (Fig. 3B). These results suggest that NOS activity may be involved in promoting CLFS-induced increases in NFATc1 nuclear accumulation via inhibitory phosphorylation of GSK-3β, thus suppressing NFATc1 phosphorylation and subsequent nuclear export.
Figure 3. GSK-3β phosphorylation status in rat fast-twitch muscle.
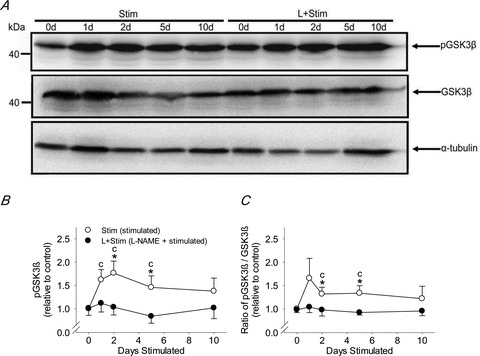
A, representative Western blot of phosphorylated GSK-3β and GSK3b in whole cell extracts prepared from rat extensor digitorum longus muscles; α-tubulin served as a loading control for total protein. B, phosphorylated GSK-3β expressed relative to control. C, ratio of phosphorylated GSK-3β/GSK3b expressed relative to control. Values are means ± SEM; n= 6 animals per group. Statistical symbols indicate: Cdifferent from Control (i.e. 0 days Stim; P < 0.05); *different from L+Stim of the matched time point of stimulation (P < 0.05).
Myosin heavy chain mRNA isoform expression
To test the hypothesis that NOS inhibition prevents activity-induced faster-to-slower fibre type transformations in vivo, we first measured MHC isoforms at the mRNA level in rat fast-twitch muscles exposed to CLFS. As detected by real-time PCR in rat tibialis anterior extracts, CLFS induced an increase in the expression of most MHC mRNAs after 5 days of stimulation, with further increases attaining values that were 16.0-fold (MHCI) (Fig. 4A), 25.1-fold (MHCIIa) (Fig. 4B) and 8.6-fold (MHCIId(x)) (Fig. 4C) by 10 days of stimulation compared with Control. However, MHCIIb mRNA levels were not changed by the 10 day stimulation protocol (Fig. 4D). l-NAME treatment blocked the CLFS-induced increases in MHC mRNA isoform expressions throughout the 10 days of stimulation (Fig. 4A and C). Collectively, these data indicate that NOS activity is essential for activity-induced increases in relatively slower MHC mRNA isoforms that are up-regulated in response to CLFS in rat fast-twitch muscles.
Figure 4. Patterns of MHC mRNA isoform expression in rat fast-twitch muscle.
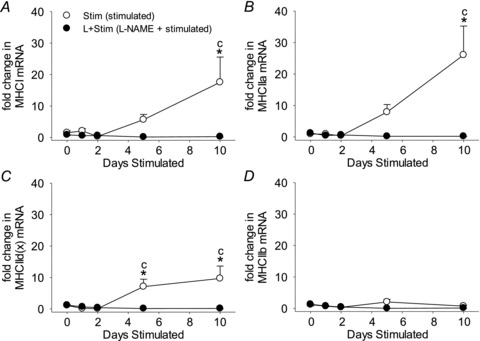
Fold changes in MHCI (A), MHCIIa (B), MHCIId(x) (C) and MHCIIb (D) mRNA expression levels in rat tibialis anterior muscles as determined by the 2−ΔΔCt method of analysis. Values are means ± SEM; n= 6 animals per group. Statistical symbols indicate: Cdifferent from Control (i.e. 0 days Stim); *different from L+Stim of the matched time point of stimulation (P < 0.05).
Myosin heavy chain protein isoform and fibre type expression
To further investigate the effects of NOS inhibition on activity-induced changes in MHC expression in vivo, we measured MHC isoforms at the protein level as well as fibre type proportions in rat fast-twitch muscles exposed to CLFS. Quantitative MHC protein content of whole muscle extracts was measured by SDS-PAGE (Fig. 5A) and detailed fibre type analysis was assessed by immunohistochemistry specific for the various MHC isoforms (Figs 6 and 7). Regardless of treatment condition, the first significant changes in the whole muscle MHC protein isoform pattern were detected by 5 days of stimulation where the relative contents of MHCIIa and MHCIIb increased and decreased, respectively (Fig. 5B). These reciprocal changes progressed with continued stimulation such that by 10 days of stimulation the relative content of MHCIIa had increased 1.9-fold, with a concomitant 1.3-fold decrease in MHCIIb, compared to Control (Fig. 5B; main effects P= 0.0002). Similarly, by 10 days of stimulation, the proportion of fibres expressing MHCIIa was 1.2-fold greater compared with Control (Fig. 7B; main effect P= 0.03). More importantly, 10 days of stimulation induced 2.0-fold increases in the relative content of MHCI and in the proportion of fibres expressing MHCI compared with Control, a response that was blocked by l-NAME treatment (Figs 5B and 7A). Taken together, these results suggest that NOS activity appears necessary for activity-induced increases in MHCI protein expression and type I fibre transformation. On the other hand, NOS activity does not appear to be required for CLFS-induced MHC protein isoform or fibre type conversions within the fast fibre population over the 10 day duration of the present study.
Figure 5. Myosin heavy chain protein isoform distribution in rat fast-twitch muscle.
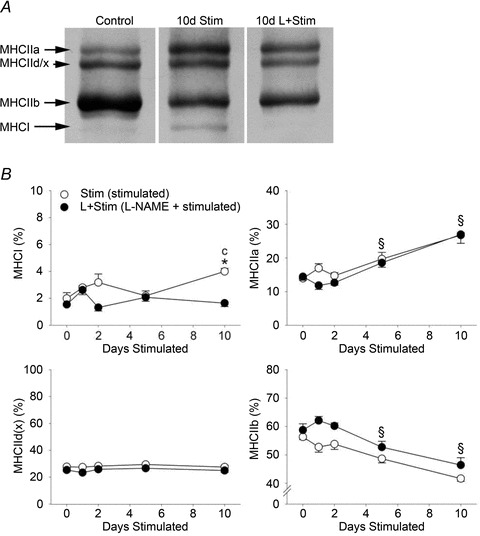
A, example of the electrophoretic method used to quantify MHC isoform composition of rat tibialis anterior muscles. B, percentage of MHCI, MHCIIa, MHCIId(x) and MHCIIb protein isoform content in rat tibialis anterior muscles, as determined by densitometric evaluation of duplicate gels. Values are means ± SEM; n= 6 animals per group. Statistical symbols indicate: Cdifferent from Control (i.e. 0 days Stim); *different from L+Stim of the matched time point of stimulation; §main effect of days of stimulation (P < 0.05).
Figure 6. Immunohistochemical detection of myosin heavy chain isoforms in serial cross-sections of rat fast-twitch muscle.
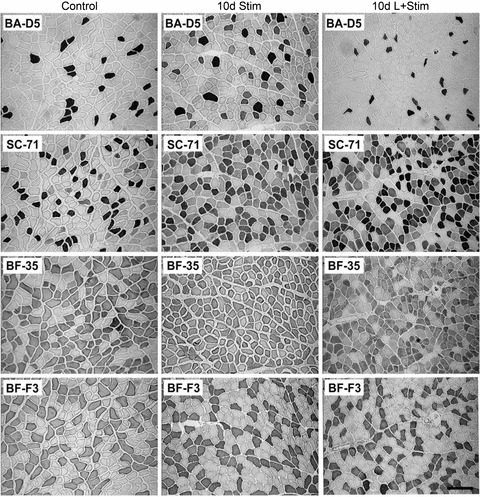
Photomicrographs of representative MHC isoform immunohistochemistry of rat tibialis anterior muscle. Immunostains for MHCI (clone BA-D5), MHCIIa (clone SC-71), MHCIId(x) (clone BF-35; staining all MHCs except MHCIId(x)) and MHCIIb (clone BF-F3). The bar located lower right represents 100 μm.
Figure 7. The proportion of fibres expressing the various adult MHC isoforms in rat fast-twitch muscle.
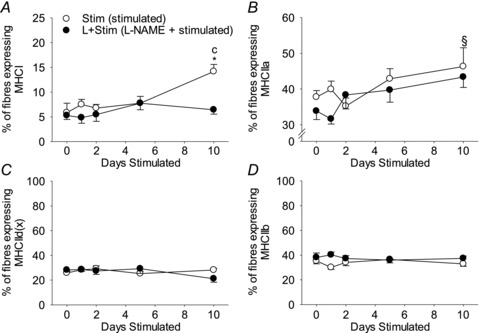
The proportion of fibres expressing MHCI (A), MHCIIa (B), MHCIId(x) (C) and MHCIIb (D). Values are means ± SEM; n= 6 animals per group. Statistical symbols indicate: Cdifferent from Control (i.e. 0 days Stim); *different from L+Stim of the matched time point of stimulation; §main effect of days of stimulation (P < 0.05).
Discussion
NO has been established as an important signalling molecule in skeletal muscle (Stamler & Meissner, 2001). In response to increased nerve-mediated muscle contraction, such as is induced by CLFS, Ca2+–calmodulin-dependent NOS activity and resultant NO production increases (Stamler & Meissner, 2001). It has been reported recently that NOS activity is also required for Ca2+ ionophore-induced NFATc1 nuclear accumulation and MHCI mRNA expression in vitro (Drenning et al. 2008, 2009). Therefore, the purpose of the present study was to investigate the involvement of NOS activity in CLFS-induced NFATc1 nuclear accumulation and subsequent faster-to-slower skeletal muscle fibre type transformations in vivo. Multiple early time points of stimulation were chosen because CLFS-induced faster-to-slower MHC-based isoform transformations are shown to begin at the mRNA level after 3 days and at the protein level after 5 days, responses that continue to change through 10 days of stimulation (Jaschinski et al. 1998). Our study reports the novel finding that nerve activity-induced faster-to-slower MHC isoform transformations are transcriptionally regulated by NOS activity. Specifically, we show that the NOS inhibitor l-NAME prevented CLFS-induced increases in slow MHCI and fast MHCIIa and IId(x) mRNAs that are typically observed during fibre conversions (Fig. 4). On the other hand, we found that l-NAME only inhibited activity-induced accumulation of slow MHCI protein isoforms and type I fibre conversions (Figs 5–7, respectively), suggesting that MHC transformations within the fast fibre population also involve translational control mechanisms independent of NO signalling. In association with these changes, we found that NOS activity is necessary for activity-induced inhibitory phosphorylation of GSK-3β (Fig. 3), and increases in calcineurin-dependent NFATc1 dephosphorylation (Fig. 1), both contributing to the nuclear localisation of this transcription factor (Fig. 2). Collectively, the current data extend previous in vitro findings (Drenning et al. 2008, 2009) and denote the importance of NO as a regulator of MHC-based faster-to-slower fibre type conversions at the transcriptional level via nerve activity-modulated NFATc1 nuclear accumulation in vivo.
Chronic low-frequency stimulation and calcineurin–NFATc1 signalling
In order to elicit a pronounced stimulus for faster-to-slower fibre type transformations in the absence of muscle fibre injury and regeneration, CLFS was employed using the rat model (Putman et al. 1999). CLFS is a model of muscle training that mimics the electrical discharge pattern of slow motor neurons innervating slow-twitch muscles (Hennig & Lomo, 1985), causing Ca2+–calcineurin-dependent NFAT dephosphorylation and nuclear translocation (Dunn et al. 2000, 2001; Liu et al. 2001; Tothova et al. 2006; Shen et al. 2007; Drenning et al. 2008; Calabria et al. 2009), and faster-to-slower fibre type transformations, as exemplified in the current study. Unlike other rodent exercise models, however, CLFS synchronously recruits all targeted motor units, including those not normally activated during sub-maximal exercise training (Pette & Staron, 2000). In doing so, the adaptive potential of CLFS-targeted muscles is maximally challenged. Also, the standardised and highly reproducible conditions of CLFS allows for activity-induced faster-to-slower phenotypic changes to occur in a well-defined time-dependent manner (Pette & Staron, 1997; Jaschinski et al. 1998). Nonetheless, despite these unique properties of CLFS, the extent and time course of neural activation-dependent NFATc1 dephosphorylation and nuclear localisation that we report here were almost identical to those observed after compensatory overload of mouse plantaris muscle, another model effecting major fibre conversions towards slower, more highly oxidative, phenotypes (Dunn et al. 1999, 2000, 2001). Indeed, under physiological overload conditions, NFATc1 dephosphorylation was marked as early as 1 day and peaked between 5–7 days post-overload, with nuclear NFATc1 peaking at 5 days (Dunn et al. 2001). This neural activation-dependent dephosphorylation and nuclear localisation of NFATc1 was found to be calcineurin dependent since it was abolished in mice administrated the calcineurin blockers cyclosporine A or FK506 (Dunn et al. 2001).
Nitric oxide involvement in faster-to-slower fibre type transformations
Several lines of evidence show that the calcineurin–NFAT signalling pathway is involved in the promotion and maintenance of a slower, more energy-efficient, fibre-specific phenotype (as reviewed by Michel et al. 2004, 2007; Liu et al. 2005). Emerging evidence indicates that NO may also be involved in this regulatory pathway. To our knowledge, only the Criswell laboratory has investigated the involvement of NO in faster-to-slower fibre type transformations to date (Smith et al. 2002; Sellman et al. 2006; Drenning et al. 2008, 2009). They initially reported that l-NAME prevented the up-regulation of MHCI mRNA and protein in rat plantaris muscles following 5 and 14 days of functional overload, respectively (Smith et al. 2002; Sellman et al. 2006). More recently, their laboratory has demonstrated that NOS activity is necessary for Ca2+-induced NFATc1 nuclear accumulation and increased MHCI mRNA expression in vitro (Drenning et al. 2008, 2009). Our present results further these observations in vivo by showing that NOS activity is not only required for CLFS-induced increases in NFATc1 dephosphorylation (Fig. 1) and nuclear localisation (Fig. 2), but also plays a critical role in the expression of both slow and fast MHC transcript levels during activity-induced faster-to-slower fibre transformations (i.e. MHCIIb→MHCIId(x)→MHCIIa→MHCI) (Fig. 4).
The temporal relationship between CLFS and activation of target genes in our study followed a pattern typically observed in excitable cells, whereby waves of increased expression are decoded by downstream elements and are often subjected to inhibitory feedback loops, in order to prevent sustained pathway activation (Michel et al. 2004). The time course of signalling changes by CLFS that we report is gradual, with averages reaching significance in the order of NFATc1 dephosphorylation (at day 2), NFATc1 nuclear import and localisation (at day 5) and activation of NFACTc1 MHC gene targets (at day 10). The apparent lag between the events of NFATc1 dephosphorylation and NFATc1 nuclear import is consistent with the former being a prerequisite for the latter, but is also influenced by dynamic nuclear–cytoplasmic NFATc1 shuttling, albeit at a much slower rate due to inhibition of nuclear GSK-3β. The timely progressive activation of MHC gene targets by NFATc1, from day 5 to day 10, thus represents a key event towards fast-to-slow fibre type transformations. Further evidence indicates such signalling is associated with enhanced mRNA stability of the MHC target genes (Chakkalakal et al. 2008). Thus, it is probable that the faster-to-slower fibre adaptive response continues for a period of time well after the initial signalling events are no longer detectable. The mechanisms regulating adaptive changes in MHC mRNA beyond 10 days (Martins et al. 2006) most certainly continue to involve the low-amplitude intracellular µCa2+½ oscillations responsible for sustained calcineurin–NFAT signalling (Chin et al. 1998; Dunn et al. 1999, 2000, 2001; Liu et al. 2001; Michel et al. 2004, 2007). We cannot, however, preclude the possibility that NO may also continue to influence those same gene targets during longer periods of CLFS, and in other models of increased muscle activity.
Calcineurin–NFAT signalling has also been implicated in the shift from the fastest MHCIIb and IId(x) mRNA isoforms to the slower fast MHCIIa mRNA isoform (Dunn et al. 1999, 2001; Allen et al. 2001; Allen & Leinwand, 2002; Calabria et al. 2009). For example, administration of the calcineurin inhibitors cyclosporine A and FK506 is shown to prevent overload-induced IIb→IId(x)→IIa→I fibre conversions at the mRNA and protein levels, as well as to prevent dephosphorylation of NFATc1, in the fast plantaris muscle of mice (Dunn et al. 1999, 2001). Furthermore, activated calcineurin or NFAT overexpression preferentially activated the MHCIIa promoter to a greater extent than the other two fast MHC isoforms (i.e. MHCIIb and MHCIId(x)) (Allen et al. 2001; Allen & Leinwand, 2002). This is consistent with our finding that an effect of Stim was not observed on the fast MHCIIb mRNA, whilst the MHCIIa isoform displayed the greatest increase in mRNA expression. Collectively, our findings suggest that NO may be regulating stimulation-induced fibre type conversions of at least three MHC isoforms at the transcriptional level via neural activity-modulated calcineurin–NFATc1 nuclear accumulation in vivo.
The mechanisms by which NO may be promoting CLFS-induced NFAT nuclear accumulation and increases in slower MHC mRNA isoforms remain to be elucidated. Given we found l-NAME treatment prevented activity-induced increases in the phosphorylation of GSK-3β and NFATc1 nuclear accumulation, it is possible that NO may be regulating the transcriptional activity of MHC isoforms by GSK-3β inhibition, thus suppressing NFATc1 phosphorylation and nuclear export, in response to in vivo stimulation. Our results are supported by the recent in vitro findings that showed NO-donor treatment induced increased phosphorylation of GSK-3β and augmented NFAT-dependent transcriptional activity, while l-NAME prevented this effect in C2C12 myotubes that were exposed to a Ca2+ ionophore (Drenning et al. 2008). Conversely, we cannot rule out the possibility that NO could also be involved in calcineurin-dependent NFAT dephosphorylation and nuclear import during in vivo stimulation. While GSK-3β inhibition does not cause NFATc1 nuclear accumulation in resting fibres (Shen et al. 2006), NO donor treatment has been shown to increase NFATc1 nuclear accumulation and MHCI mRNA expression in control fibres to the same extent as Ca2+ ionophore treatment in primary myotube cultures (Drenning et al. 2009). Thus, although NO is associated with inhibitory phosphorylation of GSK-3β, it appears that NO may also promote NFATc1 nuclear import. It should be noted that NFATc1 and GSK-3β exhibited transient increased activity in the early phase of CLFS, returning to control levels by 10 days of stimulation, during which time the up-regulation of slower MHC isoform mRNAs was still occurring. Previous studies have also reported similar transient increases in calcineurin downstream signalling factors and gene targets during the early phase of functional overload-induced faster-to-slower fibre type transitions (Dunn et al. 1999, 2001; Miyazaki et al. 2004). Therefore, since l-NAME treatment prevented CLFS-induced up-regulation of MHC mRNA isoforms throughout the 10 day stimulation period, NO may be regulating additional downstream targets and signalling pathways during the later phase of faster-to-slower fibre type transitions. Further studies are required to fully delineate the pathway by which NO facilitates activity-induced NFATc1 nuclear accumulation and MHC isoform expression.
Our data also suggest that nerve activity-dependent MHC-based transformations may not be limited to the transcriptional regulation of these contractile proteins. Even though inhibition of NOS activity prevented the activity-induced up-regulation of slower MHC mRNA isoforms, conversions within the fast fibre population still occurred at the protein level in stimulated l-NAME-treated muscles (Figs 5–7). Therefore, activity-induced transformations within the fast fibre population may be further subjected to translational control mechanisms independent of NO signalling via the NFATc1 pathway.
Conclusions
Control of adult skeletal muscle phenotype and adaptive plasticity is a complex process involving multiple signalling pathways and levels of regulation. Our novel findings provide further insight into the mechanisms underlying MHC-based faster-to-slower phenotypic transformations in vivo. Results of the current study show that NFATc1 dephosphorylation, nuclear accumulation and subsequent up-regulation of relatively slower, more energy-efficient, MHC mRNA isoforms involved in CLFS-induced fibre transformations are dependent on NOS activity in rat fast-twitch skeletal muscles. These results support the hypothesis that NO contributes to regulation of activity-induced MHC-based faster-to-slower fibre type transformations at the transcriptional level by acting synergistically with calcineurin and GSK-3β to promote NFATc1 nuclear accumulation in vivo. Additionally, activity-induced transformations within the fast fibre population may be further subjected to translational control mechanisms independent of NO signalling upon NFATc1.
Acknowledgments
This study was funded by research grants from the Natural Sciences and Engineering Council of Canada (NSERC; C.T.P. and R.N.M.), the Alberta Heritage Foundation for Medical Research (AHFMR; C.T.P.), the Alberta Agricultural Research Institute (W.T.D.), the Canadian Institutes of Health Research (CIHR; R.N.M.), the Canada Research Chairs Program (CRC; R.N.M.) and the Canadian Foundation for Innovation (CFI; R.N.M.). K.J.B.M. was supported by NSERC and AHFMR graduate scholarships. R.N.M. is a Canada Research Chair Tier 1 in Cellular and Molecular Neuromuscular Physiology. C.T.P. is a Senior Scholar of AHFMR. The authors thank Dr Tessa Gordon from the University of Alberta for use of laboratory space. We thank Drs Richard Schultz (Univ. of Alberta), Eva R. Chin (Univ. of Maryland), and Alisa Piekny (Concordia Univ.) for their expert advice.
Glossary
Abbreviations
- CnA
calcineurin
- CLFS
chronic low-frequency stimulation
- DAPI
4′,6-diamidino-2-phenylindole
- de-PO4
dephosphorylated
- eNOS
endothelial nitric oxide synthase
- GSK-3β
glycogen synthase kinase-3β
- MCK
muscle creatinine kinase
- MHC
myosin heavy chain
- NFAT
nuclear factor of activated T-cells
- nNOS
neuronal nitric oxide
- NO
nitric oxide
- NOS
nitric oxide synthase
- PGC1α
peroxisome proliferator-activated receptor gamma co-activator 1-alpha
- PO4
phosphorylated
Author contributions
K.J.B.M. and R.N.M. conceived the conceptual framework of the study and designed the experiments. Experiments were performed by K.J.B.M. and M.St-L. Animal surgeries and tissue extractions were performed by K.J.B.M., M.St-L. and R.N.M. Biochemical and immunocytochemical analyses were performed by K.J.B.M., M.St-L., G.K.M., I.M.M. and P.M. K.J.B.M., M.St-L., W.T.D, C.T.P. and R.N.M. contributed to the interpretation of data. K.J.B.M. wrote the manuscript with M.St-L., C.T.P. and R.N.M. All authors approved the final version of the manuscript.
References
- Akimoto T, Pohnert SC, Li P, Zhang M, Gumbs C, Rosenberg PB, et al. Exercise stimulates PGC-1a transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- Allen DL, Leinwand LA. Intracellular calcium and myosin isoform transitions. Calcineurin and calcium-calmodulin kinase pathways regulated preferential activation of the IIa myosin heavy chain promoter. J Biol Chem. 2002;277:45323–45330. doi: 10.1074/jbc.M208302200. [DOI] [PubMed] [Google Scholar]
- Allen DL, Sartorius CA, Sycuro LK, Leinwand LA. Different pathways regulate expression of the skeletal myosin heavy chain genes. J Biol Chem. 2001;276:43524–43533. doi: 10.1074/jbc.M108017200. [DOI] [PubMed] [Google Scholar]
- Baar K, Wende AR, Jones TE, Marison M, Nolte LA, Chen M, et al. Adaptations of skeletal muscle to exercise: rapid increase in the transcriptional coactivator PGC-1. FASEB J. 2002;16:1879–1886. doi: 10.1096/fj.02-0367com. [DOI] [PubMed] [Google Scholar]
- Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006;75:19–37. doi: 10.1146/annurev.biochem.75.103004.142622. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force–velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol. 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quatitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brunelli S, Sciorati C, D’Antona G, Innocenzi A, Covarello D, Galvez BG, et al. Nitric oxide release combined with nonsteroidal antiinflammatory activity prevents muscular dystrophy pathology and enhances stem cell therapy. Proc Natl Acad Sci U S A. 2007;104:264–269. doi: 10.1073/pnas.0608277104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabria E, Ciciliot S, Moretti I, Garcia M, Picard A, Dyar KA, et al. NFAT isoforms control activity-dependent muscle fiber type specification. Proc Natl Acad Sci U S A. 2009;106:13335–13340. doi: 10.1073/pnas.0812911106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakkalakal JV, Harrison MA, Carbonetto S, Chin E, Michel RN, Jasmin BJ. Stimulation of calcineurin signaling attenuates the dystrophic pathology in mdx mice. Hum Mol Genet. 2004;13:379–388. doi: 10.1093/hmg/ddh037. [DOI] [PubMed] [Google Scholar]
- Chakkalakal JV, Miura P, Belanger G, Michel RN, Jasmin BJ. Modulation of utrophin A mRNA stability in fast versus slow muscles via an AU-rich element and calcineurin signaling. Nucleic Acids Res. 2008;36:826–838. doi: 10.1093/nar/gkm1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin ER, Olson EN, Richardson JA, Yang Q, Humphries C, Shelton JM, et al. A calcineurin-dependent transcriptional pathway controls skeletal muscle fiber type. Genes Dev. 1998;12:2499–2509. doi: 10.1101/gad.12.16.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colussi C, Gurtner A, Rosati J, Illi B, Ragone G, Piaggio G, et al. Nitric oxide deficiency determines global chromatin changes in Duchenne muscular dystrophy. FASEB J. 2009;23:2131–2141. doi: 10.1096/fj.08-115618. [DOI] [PubMed] [Google Scholar]
- Colussi C, Mozzetta C, Gurtner A, Illi B, Rosati J, Straino S, et al. HDAC2 blockade by nitric oxide and histone deacetylase inhibitors reveals a common target in Duchenne muscular dystrophy treatment. Proc Natl Acad Sci U S A. 2008;105:19183–19187. doi: 10.1073/pnas.0805514105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czubryt MP, McAnally J, Fishman GI, Olson EN. Regulation of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α) and mitochondrial function by MEF2 and HDAC5. Proc Natl Acad Sci U S A. 2003;100:1711–1716. doi: 10.1073/pnas.0337639100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimmeler S, Zeiher AM. Nitric oxide – an endothelial cell survival factor. Cell Death Differ. 1999;6:964–968. doi: 10.1038/sj.cdd.4400581. [DOI] [PubMed] [Google Scholar]
- Drenning JA, Lira VA, Simmons CG, Soltow QA, Sellman JE, Criswell DS. Nitric oxide facilitates NFAT-dependent transcription in mouse myotubes. Am J Physiol Cell Physiol. 2008;294:C1088–C1095. doi: 10.1152/ajpcell.00523.2007. [DOI] [PubMed] [Google Scholar]
- Drenning JA, Lira VA, Soltow QA, Canon CN, Valera LM, Brown DL, Criswell DS. Endothelial nitric oxide synthase is involved in calcium-induced Akt signaling in mouse skeletal muscle. Nitric Oxide. 2009;21:192–200. doi: 10.1016/j.niox.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Burns JL, Michel RN. Calcineurin is required for skeletal muscle hypertrophy. J Biol Chem. 1999;274:21908–21912. doi: 10.1074/jbc.274.31.21908. [DOI] [PubMed] [Google Scholar]
- Dunn SE, Chin ER, Michel RN. Matching of calcineurin activity to upstream effectors is critical for skeletal muscle fiber growth. J Cell Biol. 2000;151:663–672. doi: 10.1083/jcb.151.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn SE, Simard AR, Bassel-Duby R, Williams RS, Michel RN. Nerve activity-dependent modulation of calcineurin signaling in adult fast and slow skeletal muscle fibers. J Biol Chem. 2001;276:45243–45254. doi: 10.1074/jbc.M105445200. [DOI] [PubMed] [Google Scholar]
- Grozdanovic Z, Baumgarten HG. Nitric oxide synthase in skeletal muscle fibers: a signaling component of the dystrophin-glycoprotein complex. Histol Histopathol. 1999;14:243–256. doi: 10.14670/HH-14.243. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. Slow-to-fast transitions in myosin expression of rat soleus muscle by phasic high-frequency stimulation. FEBS Lett. 1996;399:220–222. doi: 10.1016/s0014-5793(96)01325-7. [DOI] [PubMed] [Google Scholar]
- Handschin C, Rhee J, Lin J, Tarr PT, Spiegelman BM. An autoregulatory loop controls peroxisome proliferator-activated receptor gamma coactivator 1α expression in muscle. Proc Natl Acad Sci U S A. 2003;100:7111–7116. doi: 10.1073/pnas.1232352100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig R, Lomo T. Firing patterns of motor units in normal rats. Nature. 1985;314:164–166. doi: 10.1038/314164a0. [DOI] [PubMed] [Google Scholar]
- Hogan PG, Chen L, Nardone J, Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Genes Dev. 2003;17:2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- Jaschinski F, Schuler MJ, Peuker H, Pette D. Changes in myosin heavy chain mRNA and protein isoforms of rat muscle during forced contractile activity. Am J Physiol Cell Physiol. 1998;274:C365–C370. doi: 10.1152/ajpcell.1998.274.2.C365. [DOI] [PubMed] [Google Scholar]
- Jiang H, Li H, DiMario JX. Control of slow myosin heavy chain 2 gene expression by glycogen synthase kinase activity in skeletal muscle fibers. Cell Tissue Res. 2006;323:489–494. doi: 10.1007/s00441-005-0007-1. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- LaFramboise WA, Jayaraman RC, Bombach KL, Ankrapp DP, Krill-Burger JM, Sciulli CM, et al. Acute molecular response of mouse hindlimb muscles to chronic stimulation. Am J Physiol Cell Physiol. 2009;297:C556–C570. doi: 10.1152/ajpcell.00046.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J, Wu H, Tarr PT, Zhang CY, Wu Z, Boss O, et al. Transcriptional co-activator PGC-1α drives the formation of slow-twitch muscle fibres. Nature. 2002;418:797–801. doi: 10.1038/nature00904. [DOI] [PubMed] [Google Scholar]
- Liu Y, Cseresnyes Z, Randall WR, Schneider MF. Activity-dependent nuclear translocation and intranuclear distribution of NFATc in adult skeletal muscle fibers. J Cell Biol. 2001;155:27–39. doi: 10.1083/jcb.200103020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shen T, Randall WR, Schneider MF. Signaling pathways in activity-dependent fiber type plasticity in adult skeletal muscle. J Muscle Res Cell Motil. 2005;26:13–21. doi: 10.1007/s10974-005-9002-0. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J, McKinsey TA, Zhang CL, Olson EN. Regulation of skeletal myogenesis by association of the MEF2 transcription factor with class II histone deacetylases. Mol Cell. 2000;6:233–244. doi: 10.1016/s1097-2765(00)00025-3. [DOI] [PubMed] [Google Scholar]
- McConell GK, Wadley GD. Potential role of nitric oxide in contraction-stimulated glucose uptake and mitochondrial biogenesis in skeletal muscle. Clin Exp Pharmacol Physiol. 2008;35:1488–1492. doi: 10.1111/j.1440-1681.2008.05038.x. [DOI] [PubMed] [Google Scholar]
- Martins KJ, Gordon T, Pette D, Dixon WT, Foxcroft GR, MacLean IM, Putman CT. Effect of satellite cell ablation on low-frequency-stimulated fast-to-slow fibre-type transitions in rat skeletal muscle. J Physiol. 2006;572:281–294. doi: 10.1113/jphysiol.2005.103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins KJ, Murdoch GK, Shu Y, Harris RL, Gallo M, Dixon WT, et al. Satellite cell ablation attenuates short-term fast-to-slow fibre type transformations in rat fast-twitch skeletal muscle. Pflugers Arch. 2009;458:325–335. doi: 10.1007/s00424-008-0625-z. [DOI] [PubMed] [Google Scholar]
- Meissner JD, Umeda PK, Chang KC, Gros G, Scheibe RJ. Activation of the beta myosin heavy chain promoter by MEF-2D, MyoD, p300, and the calcineurin/NFATc1 pathway. J Cell Physiol. 2007;211:138–148. doi: 10.1002/jcp.20916. [DOI] [PubMed] [Google Scholar]
- Michel RN, Chin ER, Chakkalakal JV, Eibl JK, Jasmin BJ. Ca2+/calmodulin-based signalling in the regulation of the muscle fibre phenotype and its therapeutic potential via modulation of utrophin A and myostatin expression. Appl Physiol Nutr Metab. 2007;32:921–929. doi: 10.1139/H07-093. [DOI] [PubMed] [Google Scholar]
- Michel RN, Dunn SE, Chin ER. Calcineurin and skeletal muscle growth. Proc Nutr Soc. 2004;63:341–349. doi: 10.1079/PNS2004362. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Hitomi Y, Kizaki T, Ohno H, Haga S, Takemasa T. Contribution of the calcineurin signaling pathway to overload-induced skeletal muscle fiber-type transition. J Physiol Pharmacol. 2004;55:751–764. [PubMed] [Google Scholar]
- Nakayama M, Stauffer J, Cheng J, Banerjee-Basu S, Wawrousek E, Buonanno A. Common core sequences are found in skeletal muscle slow- and fast-fiber-type-specific regulatory elements. Mol Cell Biol. 1996;16:2408–2417. doi: 10.1128/mcb.16.5.2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naya FJ, Mercer B, Shelton J, Richardson JA, Williams RS, Olson EN. Stimulation of slow skeletal muscle fiber gene expression by calcineurin in vivo. J Biol Chem. 2000;275:4545–4548. doi: 10.1074/jbc.275.7.4545. [DOI] [PubMed] [Google Scholar]
- Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119:2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Myosin isoforms, muscle fiber types, and transitions. Microsc Res Tech. 2000;50:500–509. doi: 10.1002/1097-0029(20000915)50:6<500::AID-JEMT7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbová G. What does chronic electrical stimulation teach us about muscle plasticity? Muscle Nerve. 1999;22:666–677. doi: 10.1002/(sici)1097-4598(199906)22:6<666::aid-mus3>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Pisconti A, Brunelli S, Di PM, De PC, Deponti D, Baesso S, et al. Follistatin induction by nitric oxide through cyclic GMP: a tightly regulated signaling pathway that controls myoblast fusion. J Cell Biol. 2006;172:233–244. doi: 10.1083/jcb.200507083. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Putman CT, Dixon WT, Pearcey J, MacLean IM, Jendral MJ, Kiricsi M, et al. Chronic low-frequency stimulation up-regulates uncoupling protein-3 in transforming rat fast-twitch skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1419–R1426. doi: 10.1152/ajpregu.00421.2004. [DOI] [PubMed] [Google Scholar]
- Putman CT, Düsterhöft S, Pette D. Changes in satellite cell content and myosin isoforms in low-frequency- stimulated fast muscle of hypothyroid rat. J Appl Physiol. 1999;86:40–51. doi: 10.1152/jappl.1999.86.1.40. [DOI] [PubMed] [Google Scholar]
- Putman CT, Düsterhöft S, Pette D. Satellite cell proliferation in low-frequency stimulated fast muscle of hypothyroid rat. Am J Physiol Cell Physiol. 2000;279:C682–C690. doi: 10.1152/ajpcell.2000.279.3.C682. [DOI] [PubMed] [Google Scholar]
- Putman CT, Kiricsi M, Pearcey J, MacLean IM, Bamford JA, Murdoch GK, et al. AMPK activation increases uncoupling protein-3 expression and mitochondrial enzyme activities in rat muscle without fibre type transitions. J Physiol. 2003;551:169–178. doi: 10.1113/jphysiol.2003.040691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Sultan KR, Wassmer T, Bamford JA, Skorjanc D, Pette D. Fiber-type transitions and satellite cell activation in low-frequency-stimulated muscles of young and aging rats. J Gerontol A Biol Sci Med Sci. 2001;56:B510–B519. doi: 10.1093/gerona/56.12.b510. [DOI] [PubMed] [Google Scholar]
- Rana ZA, Gundersen K, Buonanno A. Activity-dependent repression of muscle genes by NFAT. Proc Natl Acad Sci U S A. 2008;105:5921–5926. doi: 10.1073/pnas.0801330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana ZA, Gundersen K, Buonanno A, Vullhorst D. Imaging transcription in vivo: distinct regulatory effects of fast and slow activity patterns on promoter elements from vertebrate troponin I isoform genes. J Physiol. 2005;562:815–828. doi: 10.1113/jphysiol.2004.075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiser PJ, Kline WO, Vaghy PL. Induction of neuronal type nitric oxide synthase in skeletal muscle by chronic electrical stimulation in vivo. J Appl Physiol. 1997;82:1250–1255. doi: 10.1152/jappl.1997.82.4.1250. [DOI] [PubMed] [Google Scholar]
- Russell AP, Feilchenfeldt J, Schreiber S, Praz M, Crettenand A, Gobelet C, et al. Endurance training in humans leads to fiber type-specific increases in levels of peroxisome proliferator-activated receptor-gamma coactivator-1 and peroxisome proliferator-activated receptor-alpha in skeletal muscle. Diabetes. 2003;52:2874–2881. doi: 10.2337/diabetes.52.12.2874. [DOI] [PubMed] [Google Scholar]
- Sartorelli V, Puri PL, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, et al. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Pitton G, Saggin L, Ausoni S, Sartore S, Lomo T. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, et al. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Sellman JE, Deruisseau KC, Betters JL, Lira VA, Soltow QA, Selsby JT, Criswell DS. In vivo inhibition of nitric oxide synthase impairs upregulation of contractile protein mRNA in overloaded plantaris muscle. J Appl Physiol. 2006;100:258–265. doi: 10.1152/japplphysiol.00936.2005. [DOI] [PubMed] [Google Scholar]
- Shen T, Cseresnyes Z, Liu Y, Randall WR, Schneider MF. Regulation of the nuclear export of the transcription factor NFATc1 by protein kinases after slow fibre type electrical stimulation of adult mouse skeletal muscle fibres. J Physiol. 2007;579:535–551. doi: 10.1113/jphysiol.2006.120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen T, Liu Y, Cseresnyes Z, Hawkins A, Randall WR, Schneider MF. Activity- and calcineurin-independent nuclear shuttling of NFATc1, but not NFATc3, in adult skeletal muscle fibers. Mol Biol Cell. 2006;17:1570–1582. doi: 10.1091/mbc.E05-08-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoneau J-A, Pette D. Species-specific effects of chronic nerve stimulation upon tibialis anterior muscle in mouse, rat, guinea pig and rabbit. Pflügers Arch. 1988;412:86–92. doi: 10.1007/BF00583735. [DOI] [PubMed] [Google Scholar]
- Smith LW, Smith JD, Criswell DS. Involvement of nitric oxide synthase in skeletal muscle adaptation to chronic overload. J Appl Physiol. 2002;92:2005–2011. doi: 10.1152/japplphysiol.00950.2001. [DOI] [PubMed] [Google Scholar]
- Stamler JS, Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- Suwa M, Egashira T, Nakano H, Sasaki H, Kumagai S. Metformin increases the PGC-1α protein and oxidative enzyme activities possibly via AMPK phosphorylation in skeletal muscle in vivo. J Appl Physiol. 2006;101:1685–1692. doi: 10.1152/japplphysiol.00255.2006. [DOI] [PubMed] [Google Scholar]
- Terada S, Goto M, Kato M, Kawanaka K, Shimokawa T, Tabata I. Effects of low-intensity prolonged exercise on PGC-1 mRNA expression in rat epitrochlearis muscle. Biochem Biophys Res Commun. 2002;296:350–354. doi: 10.1016/s0006-291x(02)00881-1. [DOI] [PubMed] [Google Scholar]
- Tothova J, Blaauw B, Pallafacchina G, Rudolf R, Argentini C, Reggiani C, Schiaffino S. NFATc1 nucleocytoplasmic shuttling is controlled by nerve activity in skeletal muscle. J Cell Sci. 2006;119:1604–1611. doi: 10.1242/jcs.02875. [DOI] [PubMed] [Google Scholar]
- Vissing K, McGee SL, Roepstorff C, Schjerling P, Hargreaves M, Kiens B. Effect of sex differences on human MEF2 regulation during endurance exercise. Am J Physiol Endocrinol Metab. 2008;294:E408–E415. doi: 10.1152/ajpendo.00403.2007. [DOI] [PubMed] [Google Scholar]
- Wright DC, Geiger PC, Han DH, Jones TE, Holloszy JO. Calcium induces increases in peroxisome proliferator-activated receptor gamma coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, et al. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- Xiao L, Coutu P, Villeneuve LR, Tadevosyan A, Maguy A, Le BS, et al. Mechanisms underlying rate-dependent remodeling of transient outward potassium current in canine ventricular myocytes. Circ Res. 2008;103:733–742. doi: 10.1161/CIRCRESAHA.108.171157. [DOI] [PubMed] [Google Scholar]
- Zong H, Ren JM, Young LH, Pypaert M, Mu J, Birnbaum MJ, Shulman GI. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc Natl Acad Sci U S A. 2002;99:15983–15987. doi: 10.1073/pnas.252625599. [DOI] [PMC free article] [PubMed] [Google Scholar]


