Abstract
Oxidation can decrease or increase the Ca2+ sensitivity of the contractile apparatus in rodent fast-twitch (type II) skeletal muscle fibres, but the reactions and molecular targets involved are unknown. This study examined whether increased Ca2+ sensitivity is due to S-glutathionylation of particular cysteine residues. Skinned muscle fibres were directly activated in heavily buffered Ca2+ solutions to assess contractile apparatus Ca2+ sensitivity. Rat type II fibres were subjected to S-glutathionylation by successive treatments with 2,2′-dithiodipyridine (DTDP) and glutathione (GSH), and displayed a maximal increase in pCa50 (−log10µCa2+½ at half-maximal force) of ∼0.24 pCa units, with little or no effect on maximum force or Hill coefficient. Partial similar effect was produced by exposure to oxidized gluthathione (GSSG, 10 mm) for 10 min at pH 7.1, and near-maximal effect by GSSG treatment at pH 8.5. None of these treatments significantly altered Ca2+ sensitivity in rat type I fibres. Western blotting showed that both the DTDP–GSH and GSSG–pH 8.5 treatments caused marked S-glutathionylation of the fast troponin I isoform (TnIf) present in type II fibres, but not of troponin C (TnC) or myosin light chain 2. Both the increased Ca2+ sensitivity and glutathionylation of TnIf were blocked by N-ethylmaleimide (NEM). S-Nitrosoglutathione (GSNO) also increased Ca2+ sensitivity, but only in conditions where it caused S-glutathionylation of TnIf. In human type II fibres from vastus lateralis muscle, DTDP–GSH treatment also caused similar increased Ca2+ sensitivity and S-glutathionylation of TnIf. When the slow isoform of TnI in type I fibres of rat was partially substituted (∼30%) with TnIf, DTDP–GSH treatment caused a significant increase in Ca2+ sensitivity (∼0.08 pCa units). TnIf in type II fibres from toad and chicken muscle lack Cys133 present in mammalian TnIf, and such fibres showed no change in Ca2+ sensitivity with DTDP–GSH nor any S-glutathionylation of TnIf (latter examined only in toad). Following 40 min of cycling exercise in human subjects (at ∼60% peak oxygen consumption), TnIf in vastus lateralis muscle displayed a marked increase in S-glutathionylation (∼4-fold). These findings show that S-glutathionylation of TnIf, most probably at Cys133, increases the Ca2+ sensitivity of the contractile apparatus, and that this occurs in exercising humans, with likely beneficial effects on performance.
Key points
Reactive oxygen-based molecules generated within muscle fibres in both exercise and pathological conditions can greatly affect muscle function. These and consequent reactions can lead to either decreased or increased force response by the contractile proteins, but the mechanisms are unknown.
This study demonstrates that the increase in force response appears to be due to a specific chemical process, known as S-glutathionylation, of a particular cysteine residue present on the troponin I molecule in fast-twitch muscle fibres, which is involved in sensing and responding to changes in intracellular calcium levels.
S-Glutathionylation can occur when glutathione, the primary cellular anti-oxidant, reacts with oxidized cysteine residues.
S-Glutathionylation of troponin I not only helps protect the molecule from oxidative stress, but evidently also makes the contractile apparatus much more sensitive to calcium ions.
This process seemingly occurs in exercising humans and is likely to be an important mechanism helping delay onset of muscle fatigue.
Introduction
Reactive oxygen and nitrogen species are generated in skeletal muscle with normal activity and also in pathological conditions, and affect many aspects of muscle function both in the short and long term (Smith & Reid, 2006; Supinski & Callahan, 2007; Allen et al. 2008; Powers & Jackson, 2008; Lamb & Westerblad, 2011). Application of hydrogen peroxide (H2O2) to intact fast-twitch muscle fibres of the mouse was found to initially cause an increase in force production by increasing the Ca2+ sensitivity of the contractile apparatus, with prolonged exposure leading to a subsequent net decrease in Ca2+ sensitivity (Andrade et al. 1998, 2001). These effects were fully reversible by application of the reducing agent, dithiothreitol (DTT), suggestive that the changes involved reversible oxidation of cysteine residues. Using skinned muscle fibres, we have previously shown that the increase in Ca2+ sensitivity is evidently not due to a direct effect of H2O2 on the contractile apparatus, and instead possibly results from the H2O2 interacting with myoglobin and reduced glutathione (GSH) within the fibre (Lamb & Posterino, 2003; Murphy et al. 2008), resulting in S-glutathionylation of unknown cysteine residues on the contractile proteins (i.e. RSH → RSSG). This could come about by generation in the cytoplasm of the reactive thiyl radical (GS·) and/or S-nitrosoglutathione (GSNO) (Dutka et al. 2011b), or simply by the GSH present reacting with oxidized cysteine residues on the contractile proteins (see Lamb & Westerblad, 2011). S-Glutathionylation is now recognized as an important mechanism that not only helps prevent disruption of protein function by oxidative stress but also can itself regulate protein function (Klatt & Lamas, 2000; Dalle-Donne et al. 2007).
In our previous studies, the presumed S-glutathionylation of the contractile apparatus was mostly elicited by treating the skinned fibre with the reactive disulphide 2,2′-dithiodipyridine (DTDP) to oxidize cysteine residues and then exposing the fibre to GSH for a relatively short period (Lamb & Posterino, 2003). The resulting large increase in Ca2+ sensitivity only occurred in fast-twitch (type II) fibres and was reversed by DTT or relatively prolonged exposure to GSH. The DTDP treatment itself causes a moderate decrease in Ca2+ sensitivity (decrease in pCa50 of ∼0.065 pCa units), and the brief subsequent GSH exposure causes a very large increase in sensitivity (by ∼0.28 pCa units), resulting in a net increase by ∼0.23 pCa units, which is no different from the outcome if the two treatments are applied in immediate succession without examining the shift occurring with the DTDP treatment alone. Following reversal by DTT, a repeat of the DTDP–GSH treatment elicits virtually identical results. Similar DTDP–GSH treatment in type I (slow-twitch) rat fibres caused no increase in Ca2+ sensitivity whatsoever (Lamb & Posterino, 2003).
The aim of the present study was to identify the protein(s) in type II fibres responsible for the increased Ca2+ sensitivity to DTDP–GSH treatment, verifying by Western blotting that this indeed involves S-glutathionylation, and if possible also identifying the specific cysteine residues involved. The experiments were performed predominantly using skinned muscle fibres, with the first goal being to determine whether treatment with oxidized glutathione (GSSG) produced effects similar to DTDP–GSH treatment, which would verify that the effects of the latter were not the result of some unintended action of DTDP. As a low concentration of the alkylating agent N-ethylmaleimide (NEM) has been shown to specifically block the increase in Ca2+ sensitivity to applied H2O2 in intact fibres (Andrade et al. 2001), we examined the ability of NEM to block both the increased Ca2+ sensitivity and S-glutathionylation of specific proteins. As the fast isoform of troponin I (TnIf) was found to undergo S-glutathionylation in tight accord with the Ca2+ sensitivity increase, we examined whether substituting TnIf into type I fibres altered their response to DTDP–GSH treatment. We also examined whether the DTDP–GSH treatment affected type II fibres from toad and chicken muscle, as the TnIf present in such fibres (Wilkinson & Grand, 1978) lacks one particular highly reactive and accessible cysteine residue present in mammalian TnIf, Cys133 (Chong & Hodges, 1982; Tao et al. 1990; Park et al. 1994). Finally, we examined the effect of DTDP–GSH treatment in type I and type II fibres from human vastus lateralis muscle and whether the glutathionylation state of TnIf was affected in exercise.
Methods
Muscle fibres and samples, and ethical approval
All animal experiments were carried out in accordance with the Australian National Health & Medical Research Council's ‘Australian code of practice for the care and use of animals for scientific purposes’, and with approval of the La Trobe University Animal Ethics Committee. Male Long–Evans hooded rats (42 in total, ≥5 months old) were killed by an overdose of isoflurane (4% vol/vol) in a glass chamber, and then the EDL and soleus muscles removed by dissection. Two tropical cane toads (Bufo marinus) that had been maintained at 15°C to lower their activity, were stunned and then killed by pithing, and the iliofibularis muscle removed. Two chickens were killed by overdose of intravenous phenobarbitone and a segment of pectoralis major muscle removed.
All protocols and procedures performed with human subjects were approved by the appropriate Human Research Ethics Committees at Victoria University, University of Melbourne and La Trobe University. Informed consent was obtained in writing from all subjects and the studies conformed to the standards set by the Declaration of Helsinki. The experiments on the effects of DTDP–GSH treatment on human skinned fibres were performed on fibres obtained by muscle biopsy in three subjects (two males and one female, 19–27 years old, all healthy and recreationally fit). After injection of a local anaesthetic (1% lidocaine) into the skin and fascia, a small incision was made in the middle third of the vastus lateralis muscle of each subject and a muscle sample taken using a Bergstrom biopsy needle (McKenna et al. 2006). An experienced medical practitioner took all biopsies at approximately constant depth. The excised muscle sample was rapidly blotted on filter paper to remove excess blood and placed in paraffin oil (Ajax Chemicals, Sydney, Australia) at 10°C for 45 min before individual muscle fibres were dissected. The effect of exercise on S-glutathionylation in muscle of human subjects was performed using tissue available from a previously published study (Merry et al. 2010). Briefly, tissue samples were obtained from five healthy, recreationally active male subjects (age 23 ± 2 years) participating in a double-blind randomised cross-over design exercise study with counterbalanced testing order. A vastus lateralis muscle biopsy was obtained as above with the subjects rested, and also on a different date at least 2 weeks apart after the subjects had completed 40 min of cycling exercise at ∼60% of peak oxygen consumption (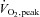 ) (see Merry et al. 2010). Muscle samples were frozen in liquid nitrogen while still in the biopsy needle within 6–12 s following the cessation of exercise. Muscle samples were stored in liquid nitrogen for later analysis and were obtained from the contralateral leg during the second trial.
) (see Merry et al. 2010). Muscle samples were frozen in liquid nitrogen while still in the biopsy needle within 6–12 s following the cessation of exercise. Muscle samples were stored in liquid nitrogen for later analysis and were obtained from the contralateral leg during the second trial.
Preparations and force recording
Rat and toad whole muscles, and muscle biopsies from human and chicken muscle, were pinned at approximately resting length under paraffin oil (Ajax Chemicals, Sydney, Australia) in a petri dish, and kept cool (∼10°C) on an icepack. Individual fibre segments were mechanically skinned with jeweller's forceps and then mounted at 120% of resting length on a force transducer (AME801, SensoNor, Horten, Norway) with a resonance frequency >2 kHz. The skinned fibre segment was then equilibrated for at least 2 min in a Perspex bath containing 2 ml of relaxing solution (see below). Force responses were recorded using a Bioamp pod and Powerlab 4/20 series hardware (ADInstruments, Sydney, Australia). All experiments were performed at room temperature (∼23 ± 2°C).
Skinned fibre solutions
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless otherwise stated. As previously described (Lamb & Posterino, 2003), the ‘relaxing’ solution contained (in mm): EGTA, 50; total ATP, 8; creatine phosphate (CrP), 10; Na+, 36; K+, 126; total Mg2+, 8.5; Hepes, 90; pH 7.1 and pCa >9. The maximum Ca2+-activating solution ‘max’ contained 50 mm CaEGTA and had a pCa of ∼4.7, with total Mg2+ adjusted to maintain 1 mm free (see (Stephenson & Williams, 1981) for apparent affinity constants). These two solutions were mixed in appropriate ratio to produce solutions with pCa in the range 6.7 to 4.7. All solutions had an osmolality of 295 ± 5 mosmol kg−1. Similar strontium-based solutions (with pSr 5.2 =−log10µSr2+½ in the range >9 to 4.0) were made by mixing relaxing solution with a Sr-EGTA solution similar to the maximum Ca2+-activating solution. Exposure to a solution at pSr 5.2 was used to ascertain the predominant troponin C (TnC) isoform present (see Results, and O’Connell et al. 2004; Trinh & Lamb, 2006). Subsequent Western blotting was used to confirm the TnC isoform present in all human, chicken, toad and some rat fibres.
A 100 mm stock of reduced glutathione (GSH) was made in a potassium HDTA (hexa-methylene-diamine-tetraacetate) solution similar to the relaxing solution but with all EGTA replaced with HDTA; the pH of the stock was re-adjusted to 7.10 with KOH, and then diluted 20-fold to give 5 mm in the final solution. A 100 mm stock solution of 2,2′-dithiodipyridine (DTDP) was made in absolute ethanol and diluted 1000-fold in the final solution to 100 μm; matching control solutions with the same amount of ethanol (0.1%) had no noticeably different effect than controls without ethanol. Similarly, NEM was made as a 200 mm or 25 mm stock in ethanol and diluted 1000-fold in the final solution. DTT was added to relaxing solution at 10 mm final concentration from a 1 m stock made in double-distilled water. S-Nitrosoglutathione (GSNO) can cause either S-nitrosylation or S-glutathionylation of a protein thiol. GSNO was dissolved in solution and either applied immediately to the fibre (i.e. within 30 s, termed ‘GSNOimm’) or applied ∼10 min later (i.e. a 10 min delay, termed ‘GSNOdel’), always with an exposure period of 2 min; it was found that the effects of the GSNO treatment was quite different in the two cases. SNAP (S-nitroso-N-acetyl-penicillamine, 5 mm) was applied immediately after addition to solution (i.e. within 30 s).
Except where stated, fibres were never activated in the presence of any of the treatments and were simply exposed to each treatment in relaxing solution, washed in relaxing solution, and then transferred back into the solutions in which the force responses were elicited.
Contractile apparatus experiments and analysis
The force–Ca2+ relationship was determined in each fibre as previously described (Lamb & Posterino, 2003; Murphy et al. 2008) by exposing the skinned fibre segment to a sequence of solutions heavily buffered at progressively higher free µCa2+½ (pCa >9 to 4.7, the latter eliciting maximum force), and then the fibre was fully relaxed again in the relaxing solution. This procedure was performed twice before (‘control’) and twice after each treatment to verify reproducibility and also gauge any small changes occurring with repeated activation and over time. Force produced at each µCa2+½ within a given sequence was expressed relative to maximum force generated in that same sequence, and analysed by individually fitting a Hill curve to each sequence, for each fibre segment, using GraphPad Prism 4 software, yielding separate pCa50 and h values (pCa at half-maximum force and Hill coefficient, respectively) for every case. Maximum force reached in each force–µCa2+½ sequence was expressed relative to the control level before any treatment in the given fibre, after correcting for the small decline occurring with each repetition of the force staircase (typically ∼2 to 3% in EDL fibres), as guaged from the initial control repetitions in the given fibre (see also Murphy et al. 2008).
Western blotting
Non-reducing SDS-PAGE was used for the determination of S-glutathionylation and biotin labelling in rat, human and toad fibres, whereas reducing SDS-PAGE was used for the other analyses of rat fibres following troponin exchange experiments and other experiments with human, chicken and toad fibres. The S-glutathionylation experiments on rat and toad fibres was based on our previously described small-sample methodology (Murphy et al. 2009; Dutka et al. 2011a) with each sample consisting of four or five skinned fibre segments (∼60 μg wet wt). Western blotting of human vastus lateralis muscle fibres was performed using the individual skinned fibre segments in which force measurements had been made (e.g. Fig. 8). In all cases the entire fibre constituents were examined without discarding any fraction. Where multiple skinned fibre segments were examined for S-glutathionylation, they were tied together with a silk suture, washed in relaxing solution for at least 5 min and then transferred successively to the various treatment solutions as required. For biotin-labelling experiments, following an initial 5 min wash in relaxing solution, individual mechanically skinned EDL fibre segments were treated for either 1 or 5 min with 100 μm EZ-Link Biotin-HPDP (ThermoScientific). Treatment with EZ-Link-Biotin-HPDP results in the formation of a disulphide bond between free sulfhydryl (-SH) groups in the fibres and the reagent, resulting in a biotin tag on any reacted proteins. As the last step for all of the above, fibre samples were placed in relaxing solution with 5 mm NEM for 5 min to block free sulfhydryl sites and then placed in non-reducing buffer for SDS-PAGE (final concentration: 125 mm Tris pH 6.8, 10% glycerol, 4% SDS, 0.01% bromophenol blue, 5 mm NEM). With the troponin exchange experiments (see below) (e.g. Fig. 7), the individual soleus skinned fibre segments were mounted on the force transducer and force–pCa measurements made before and after the troponin exchange procedure, and then the fibre was collected into reducing SDS-PAGE buffer, which was similar to the non-reducing buffer described above except the NEM was replaced with the reducing agents urea (4 m) and mercaptoethanol (10%). Total proteins in fibres collected into either buffer were separated on various SDS-PAGE gels (details provided in relevant figure legends, Criterion gels were from BioRad, Hercules, CA, USA) and then wet-transferred to nitrocellulose for 60 min at 100 V in a circulating ice-cooled bath with transfer buffer containing 140 mm glycine, 37 mm Tris base and 20% methanol. Membranes were then variously probed with anti-GSH (mouse monoclonal, 1 in 1000, Cat. No. 101-A, Virogen, Cincinnati, OH, USA), anti-TnI (rabbit polyclonal, 1 in 1000, Cat. No. 4002, Cell Signalling Technology, Danvers, MA, USA), TnC (rabbit polyclonal, 1 in 400, Cat. No. sc-20642, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-myosin light chain 2 (rabbit polyclonal, 1 in 200, Cat. No. sc-15370, Santa Cruz Biotechnology) or anti-actin (rabbit affinity isolated, Cat. No. A2066, Sigma), all diluted in 1% bovine serum albumin in phosphate-buffered saline with 0.025% Tween. Following exposure to relevant secondary antibodies (or in the case of biotin labelling, to streptavidin (1 in 20,000 in 1% BSA)) and a series of washes in Tris-buffered saline with Tween, chemiluminescent substrate (SuperSignal West Femto, Pierce) was applied to membranes and Western blot images taken with ChemiDoc XRS fitted with a charge-coupled device (CCD) camera using Quantity One software (Bio-Rad). With the membrane position unchanged, the white light source was switched on in order to obtain an image of the pre-stained molecular weight markers on the membrane.
Figure 8. Glutathionylation treatment causes increased Ca2+ sensitivity in human type II fibres but not type I fibres.
Segments of individual fibres from vastus lateralis muscle biopsy from a rested human subject were mounted on the force transducer and force–pCa staircases recorded. A, Hill curves for human type II fibre subjected to standard DTDP–GSH treatment and then DTT treatment. B, Western blots of human single fibres, probed first for TnI, then TnC, MHCII and finally MHCI. Fibre in A run in Lane 1. Force response of fibres to pSr 5.2 in accord with TnC isoform in every case (see text). 4–15% Criterion TGX Stain Free gel. C, Hill curves for human type I fibre subjected to successive treatments with DTDP (100 μm, 5 min), GSH (5 mm, 2 min), and finally DTT (10 mm, 10 min).
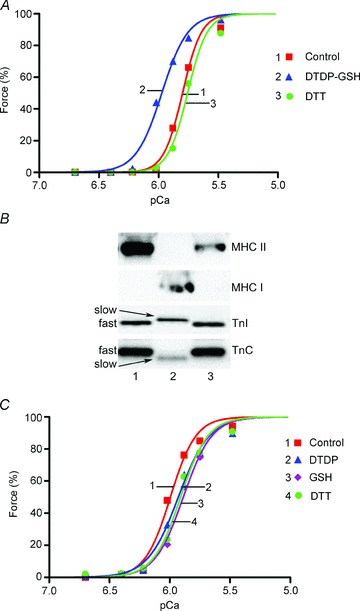
Figure 7. Effect of S-glutathionylation following partial TnIf exchange into a type I fibre.
A, Ca2+-activated force responses in skinned segment of a rat soleus type I fibre following exchange of porcine fast-twitch troponin (see Methods). µCa2+½ initially pCa > 9 and raised progressively (small ticks) in order: pCa 6.70, 6.40, 6.22, 6.02, 5.88, 5.75, 5.48, 4.7, >9). Fibre treated successively with DTDP (100 μm, 5 min), GSH (5 mm, 2 min) and DTT (10 mm, 10 min). µCa2+½ sequence repeated twice after each treatment, giving very similar force responses; only second of each pair shown. Horizontal arrows indicate force level at pCa 6.40 in each case. B, Hill fits to force–pCa data in A. C, Western blotting for TnI, TnC and actin in soleus single fibre segments with (Exch) and without (Con) troponin exchange. The segment producing the force responses in A and B was run in Lane 2; virtually all of the endogenous TnC, and ∼35% of the TnI, was replaced with the respective fast isoform (see text). Lane 4: another fibre following troponin exchange. Lanes 1 and 3: untreated control fibres. Lane 5: 0.5 pmol of the exogenous porcine troponin complex (TnX). 8–16% Criterion Stain Free gel.
In order to determine the level of S-glutathionylation in whole muscle before and after exercise in humans, two to four 10 μm cryosections were cut from each biopsy (Leica 1500 CM1950) and placed into microfuge tubes pre-equilibrated to −20°C (number of slices varied to keep total amount of tissue approximately the same in all cases). Cold physiological-based solution containing 5 mm NEM was added (10 μl) and the samples kept on ice for 2 min, before non-reducing buffer (see above) was added. Samples were stored at −20°C until analysed by Western blotting, as described above. To confirm that the S-glutathionylation effect could be induced in human muscle and measured biochemically, 10 μm sections were cut with the cryostat and placed onto a microscope slide. A circle was drawn around the section with a water-repelling ink (Dako, Glostrup, Denmark) and the section was then treated with solutions as described for the rat fibres. Solutions were aspirated from the sections prior to the addition of the next solution. Following all treatment steps, the section was collected from the slide in the non-reducing buffer by sucking up into a pipette and kept at −20°C until Western blotting.
Troponin extraction and exchange
All skinned fibres used in the troponin extraction and exchange experiments were first mounted on the transducer and then treated with Triton-X 100 in relaxing solution (1% vol/vol) for 10 min (and then washed in relaxing solution) in order to remove membranous structures and possibly aid exchange rates. Troponin C extraction was achieved by bathing the skinned fibre in a K-EGTA rigor solution with 68 mm EGTA, 5 mm EDTA and 90 mm Hepes (pH to 7.1 with KOH) and no added Ca2+ or Mg2+, plus 0.5 mm trifluoperazine (TFP) (adapted from Morris et al. 2001). The fibre was repetitively cycled for 6 min periods between the TFP-rigor solution and relaxing solution, for a total exposure to the TFP-rigor solution of 30 min. This was sufficient to abolish >99% of active force to the maximal Ca2+-activating solution in type II fibres. Extraction of both troponin C and troponin I was achieved by bathing the skinned fibre in relaxing solution with 10 mm vandate for 10 min (see Kogler et al. 1998). Following troponin extraction, the skinned fibre was bathed for 15 min in relaxing solution with 2 mm DTT and fast troponin either from porcine muscle (Sigma, T2275) or rabbit muscle (Ocean Biologicals Inc., Seattle, WA, USA), the latter applied as TnC alone, or equal part mixtures of TnI and TnT, or TnC, TnI and TnT (3 to 10 mg ml−1). Troponin exchange (without initial troponin extraction) was achieved by bathing the skinned fibre segment for 1 h in a low ionic strength rigor solution with zero Ca2+ and Mg2+ (mm: EGTA, 2.5; EDTA, 2.5; Hepes, 10, pH 7.1 with KOH) with porcine or rabbit fast troponin (10 mg ml) and 2 mm DTT.
Statistics
Values are presented as mean ± standard error of the mean (SEM) (or mean ± SD, the sample standard deviation, where n = 2), with n denoting the number of fibres examined. Statistical significance (P < 0.05) was determined with Student's two-tailed paired t test, or where values were not normally distributed by the Wilcoxin signed rank test. Data where n= 2 were not examined statistically.
Results
Effects of treatment with DTDP–GSH or GSSG
In agreement with our previous findings (Lamb & Posterino, 2003), when a skinned type II fibre from rat EDL muscle was treated sequentially with DTDP (100 μm) for 5 min and then GSH (5 mm) for 2 min (denoted as the ‘standard’ DTDP–GSH treatment, all applied in relaxing solution, pCa > 9), the Ca2+ sensitivity of the contractile apparatus was greatly increased, as seen with the final treatment in Fig. 1A. In the 18 EDL fibres examined, the pCa50 was increased on average by ∼0.24 pCa units (row 1 in Table 1), with only a slight decrease in the Hill coefficient (h) (–11 ± 4%, from ∼5.5 to 5.0) and no detectable change in maximum Ca2+-activated force. The effects of the DTDP–GSH treatment were fully reversed with the reducing agent DTT (Table 1). The DTDP–GSH treatment had exactly the same effects in fibres treated with Triton-X 100 (not shown), indicating that it acted directly on the contractile apparatus. Type II fibres obtained from rat soleus muscle, which are almost exclusively type IIA fibres (Bortolotto et al. 2000; O’Connell et al. 2004), showed exactly the same effects to DTDP–GSH as the EDL type II fibres (not shown).
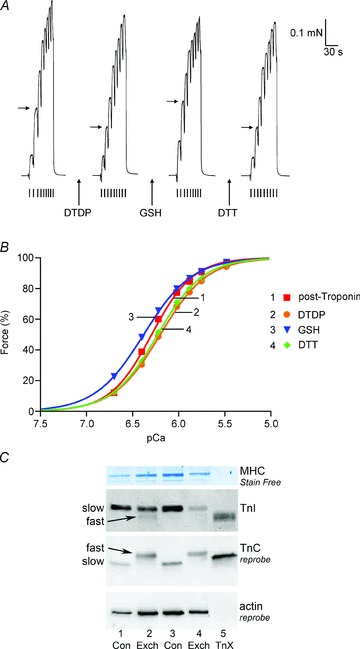
Figure 1. Treatment with GSSG–pH 8.5 increases Ca2+ sensitivity of contractile apparatus.
A, isometric force production in a skinned EDL fibre to solutions with successively higher free µCa2+½, starting at pCa >9 (small ticks in order: pCa 6.40, 6.22, 6.02, 5.88, 5.75, 5.48, 4.7, >9). Sequence repeated twice after each treatment, giving very similar results; only the second of each pair of force–pCa staircases is shown for each treatment. Maximum Ca2+-activated force reached at pCa 4.7. Horizontal arrows mark force level achieved at pCa 5.75. Treatments (all applied at pCa > 9): 10 mm DTT for 10 min; pH 8.5 for 10 min; 10 mm GSSG at pH 8.5 for 10 min; 100 μm DTDP for 5 min followed by 5 mm GSH for 2 min (i.e. ‘standard DTDP–GSH treatment’). B, Hill fits to force–pCa data following each indicated treatment; data from A, with force responses in each ‘staircase’ normalised to their own maximum. C, pCa50 values after indicated treatment for Hill fits in B.
Table 1.
Changes in Ca2+ sensitivity with glutathionylation treatments in type II and type I fibres
| Treatment | ΔpCa50 after treatment | ΔpCa50 after DTT |
|---|---|---|
| Rat type II | ||
| 1. DTDP-GSH | +0.238 ± 0.005 (18)* | −0.240 ± 0.004 (11)* |
| 2. DTDP-GSH (Post-NEM) | +0.030 ± 0.007 (8)* | −0.045 ± 0.004 (5)* |
| 3. GSSG pH 7.1 | +0.055 ± 0.010 (3)* | N.D. |
| 4. GSSG pH 8.5 | +0.191 ± 0.009 (7)* | −0.218 ± 0.003 (2) |
| 5. GSSG pH 8.5 (Post-NEM) | −0.003 ± 0.004 (2) | N.D. |
| Rat type I | ||
| 6. DTDP-GSH | −0.018 ± 0.008 (7) | −0.009 ± 0.003 (6)* |
| 7. GSSG pH 8.5 | −0.023 ± 0.010 (2) | −0.015 ± 0.001 (2) |
| Human type II | ||
| 8. DTDP-GSH | +0.171 ± 0.009 (3)* | −0.196 ± 0.012 (3)* |
| Toad type II | ||
| 9. DTDP-GSH | −0.010 ± 0.006 (5) | +0.006 ± 0.008 (4) |
| Chicken type II | ||
| 10. DTDP-GSH | −0.051 ± 0.013 (3) | +0.001 ± 0.009 (2) |
Data are mean ± SEM (or SD, where n= 2) of the change in pCa50 caused by indicated treatment, and by subsequent DTT treatment in a subset of those fibres. Each treatment applied with skinned fibre in the reduced state, except for ‘Post-NEM’ cases, where the fibre was pre-treated with 25 μm NEM for 2 min. Treatments (all applied at pCa 9): 100 μm DTDP for 5 min (or 1 min in chicken and toad) followed by 5 mm GSH for 2 min; or 10 mm GSSG at pH 7.1 for 10 min; or 10 mm GSSG at pH 8.5 for 10 min. DTT treatment: 10 mm for 10 min in all cases. Number of fibres shown in brackets. N.D., not determined. Fibres classified as type I or II by Sr2+ activation properties, and verified by Western blotting. *Significantly different from zero (P < 0.05); samples with n= 2 not statistically compared.
The effect of the DTDP–GSH treatment was not noticeably different with DTDP (100 μm) exposures of between 30 s and 5 min, and with GSH (5 mm) exposures of between 30 s and 2 min (all applied at pCa > 9 without fibre activation). If the exposure time to DTDP was made even shorter, the increase in pCa50 was smaller, with a 5 s exposure causing only ∼38 and 43% of the maximal shift, and a 10 s exposure causing ∼51 and 57%, in the four EDL fibres examined. Sr2+ ions activate the TnC isoform present in fast-twitch fibres, but only at ∼20-fold higher concentration than for Ca2+ (Lynch et al. 1995; O’Connell et al. 2004; Trinh & Lamb, 2006), and it was found that the DTDP–GSH treatment increased the Sr2+ sensitivity to a similar extent as the Ca2+ sensitivity (maximal increase in pSr50+0.280 ± 0.013, and in pCa50+0.241 ± 0.012 in the above four fibres, and with similar proportional shift in pSr50 with the shorter DTDP exposures). At much higher concentration (e.g. 1 mm) DTDP treatment results in reduction in maximal force and Ca2+ sensitivity in both type II and type I fibres (Lamb & Posterino, 2003), probably by oxidizing cysteine residues on additional types of proteins.
In order to examine whether the effects of the standard DTDP–GSH treatment were attributable to S-glutathionylation, and not dependent on some additional effect of the initial DTDP oxidizing step, we compared the effects of oxidized glutathione (GSSG) as an alternative method of producing S-glutathionylation. We previously found that a 5 min exposure to 2.5 mm GSSG produced no significant change in Ca2+ sensitivity (average +0.004 pCa units in two EDL fibres) (Lamb & Posterino, 2003), and consequently here examined the effects of a longer exposure at higher concentration. In the three EDL fibres examined, a 10 min exposure to 10 mm GSSG at pH 7.1 increased pCa50 by a mean of +0.055 ± 0.010, which is ∼25% of the maximal DTDP–GSH effect. The ability of GSSG to S-glutathionylate a protein cysteine (RSH) is considerably enhanced if the latter is in its thiolate anion form (RS−) (Klatt & Lamas, 2000; Dalle-Donne et al. 2007), which is favoured by increasing the solution pH, and so we next tested the effect of applying GSSG at pH 8.5 for 10 min. As such exposure at pH 8.5 itself causes a ‘once-off’ reduction in Ca2+ sensitivity and maximum force in rat soleus fibres (Dutka et al. 2011a), each EDL fibre was first subjected to the pH 8.5 conditions without GSSG, which resulted in reductions in pCa50 (by –0.079 ± 0.009 pCa units), h (by 34 ± 5%, n= 12) and maximum force (by 6 ± 1%) (e.g. see Fig. 1). A repeated exposure to pH 8.5 conditions had virtually no further effect (not shown). However, as seen in Fig. 1, when 10 mm GSSG was present in the pH 8.5 solution, there was a very marked increase in the Ca2+ sensitivity (mean increase in pCa50∼+0.19, row 4 in Table 1), with little or no change in either h or maximum force. This increase in Ca2+ sensitivity was fully reversed by the standard DTT treatment (10 mm, 10 min), returning the level to approximately that present after the initial pH 8.5 exposure (Table 1).
When the standard DTDP–GSH treatment was applied after the 10 min GSSG at pH 8.5 treatment, it resulted in little further increase in Ca2+ sensitivity, but if the sensitivity increase was first reversed with DTT, DTDP–GSH treatment caused its usual large effect (see Fig. 1). If, however, the DTDP–GSH treatment was applied following a submaximal GSSG treatment (i.e. 10 mm GSSG at pH 7.1, or 2.5 or 5 mm GSSG at pH 8.5), it caused a further increase in Ca2+ sensitivity, but the net effect of the two sequential treatments (total shift of pCa50∼0.23) was still just the same as occurred with the DTDP–GSH treatment alone (Fig. 2). All effects were fully reversed by DTT. These data strongly suggest that the DTDP–GSH and GSSG treatments increase Ca2+ sensitivity by the same mechanism, that presumably being by S-glutathionylation.
Figure 2. Constancy of net increase in Ca2+ sensitivity with sequential GSSG and DTDP-GSH treatments.

Increase in pCa50 when individual EDL fibres were first subjected to a submaximal or near-maximal GSSG treatment (pCa50 shift plotted as abscissa value) and then afterwards subjected to the standard DTDP–GSH treatment (additional increase in pCa50 plotted as ordinate value); the total overall increase in pCa50 was similar irrespective of the initial GSSG treatment. The different first treatments (all lasting 10 min) were: 10 mm GSSG at pH 7.1 (•); 2.5 mm GSSG at pH 8.5 (▾); 5 mm GSSG at pH 8.5 (◆); and 10 mm GSSG at pH 8.5 (▴). The value on the ordinate axis (▪) (i.e. +0.238 pCa units) represents the effect of DTDP–GSH treatment without any first treatment (see Table 1). In all cases subsequent DTT treatment (10 mm, 10 min) fully reversed Ca2+ sensitivity to its original level (i.e. by ∼−0.24 pCa units in all cases) (not shown).
Effects of treatments in type I fibres
Skinned fibres obtained from rat soleus muscle were designated as type I or type II according to their response to Sr2+ (see Methods); type I fibres contain almost exclusively only the slow isoforms of troponin (e.g. see Fig. 7 later) and are relatively sensitive to activation by Sr2+, whereas the type II fibres contain fast troponin isoforms and are relatively insensitive to Sr2+ (Lynch et al. 1995; O’Connell et al. 2004). In contrast to all rat type II fibres examined, rat type I fibres showed no significant change in sensitivity with either the DTDP–GSH treatment or the GSSG–pH 8.5 treatment (rows 6 and 7 in Table 1). Table 1 shows the changes in pCa50 occurring with the various treatments, as well as the effect of subsequent DTT treatment. It should be borne in mind that subjecting skinned fibres to repeated force–pCa measurements itself results in a small progressive decrease in Ca2+ sensitivity (and maximum force) even without any treatment, with the shift being on average ∼–0.012 pCa units per pair of force–pCa ‘staircases’ (see also Lamb & Posterino, 2003; Murphy et al. 2008). It is apparent that the DTDP–GSH and GSSG–pH 8.5 treatments had no detectable effect on pCa50 in rat type I fibres.
Effect of DTDP–GSH treatment during activation in type II fibres
All of the above treatments were applied with the fibres in the relaxed state (at pCa > 9), and it was important to also consider whether the effect was any different in an activated fibre. Applying DTDP to type II fibres during submaximal activation affects Ca2+ sensitivity (increase in pCa50 by ∼0.05 pCa units, and decrease in h) in a different manner to that when applying the DTDP with the fibre relaxed (decrease in pCa50 by ∼0.065 pCa units) (Lamb & Posterino, 2003), possibly because the activation allows access to additional cysteine residues or interaction between relocated proteins. This activation-dependent effect appeared to be independent of the action of DTDP–GSH (Lamb & Posterino, 2003), but the combination of actions potentially complicates interpretation. Importantly, it was found here that when DTDP was applied (for 15 s) whilst the fibre was maximally activated (in pCa 4.7), it had effects similar to those in a resting fibre, decreasing pCa50 by ∼0.08 pCa units. This made it possible to readily compare the effects of DTDP–GSH treatment in the rested and maximally activated states. DTDP was applied for only 15 s and then GSH for only 30 s in order to minimize the time the fibre was maintained in the maximally activated state (which leads to irreversible decrement in force if very prolonged). It was found that such DTDP–GSH treatment produced virtually the same increase in Ca2+ sensitivity irrespective of whether it was applied with the fibre relaxed (+0.206 ± 0.020 pCa units) or maximally activated (+0.197 ± 0.018 pCa units) (both treatments examined in same three type II EDL fibres).
Block by NEM
Andrade et al. (2001) found in intact fast-twitch fibres that a 3 min exposure to 25 μm of NEM blocked the increase in contractile sensitivity that otherwise occurs with H2O2 application, without the reductions in maximal force that occur with higher µNEM½ or longer exposure (Perkins et al. 1997). It was found here that exposing type II EDL fibres for 2 min to 25 μm NEM (at pCa > 9) blocked >85% of the increase in Ca2+ sensitivity occurring with the DTDP–GSH and GSSG–pH 8.5 treatments (Table 1, rows 2 and 5) (e.g. Fig. 3). The NEM treatment itself caused only a minor decrease in maximum force (–2.3 ± 0.7%) and Ca2+ sensitivity (ΔpCa50−0.047 ± 0.008 pCa units) (n= 7). As expected, none of the effects of the alkylating agent NEM were reversed by DTT.
Figure 3. Pre-treatment with NEM blocks action of DTDP–GSH on Ca2+ sensitivity.
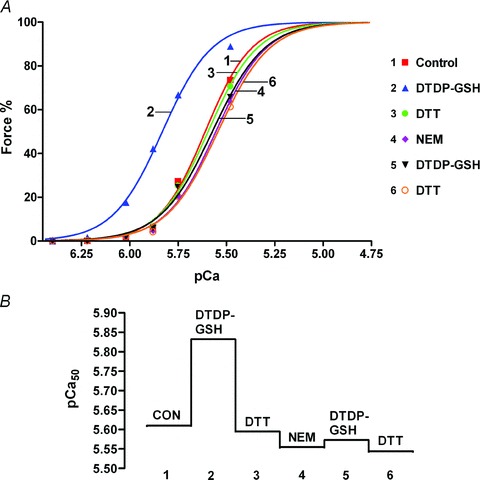
A, Hill fits for force–pCa data for a rat EDL fibre given the standard DTDP–GSH treatment both before and after treatment with NEM (25 μm, 2 min, at pCa > 9). Two successive force–pCa staircases examined after each treatment, with very similar results; data only for second in each pair plotted here. B, pCa50 values following indicated treatment for data in A. Note that there is a small progressive decline in pCa50 with each force–pCa staircase, which occurs even with no treatment (∼0.012 pCa units per staircase pair) (here compare ‘CON’ (control) and subsequent post ‘DTT’ responses).
Western blotting for S-glutathionylation
Western blotting under non-reducing conditions was performed using groups of five EDL fibre segments rather than single segments (see Methods), owing to the low sensitivity of anti-GSH antibody used to detect S-glutathionylation. An anti-GSH signal for a protein running at ∼23 kDa was observed for all fibre samples subjected to the standard DTDP–GSH treatment, with little matching signal apparent in untreated (control) fibres or in fibres treated with DTT (e.g. Fig. 4A and B). Reprobing the membranes with anti-TnI showed the 23 kDa band corresponded with the position of the fast isoform of TnI (TnIf) in every case. The density of the anti-GSH signal for TnIf (normalized to the corresponding TnIf density) was ∼4 times greater in the DTDP–GSH-treated fibres relative to the control fibres (Fig. 4C); these data were obtained from a total of 11 independent gels and DTDP–GSH-treated samples, comparing matching control samples collected in the same experiments in 10 of these cases. The anti-GSH/TnIf ratios for other treatment samples run on a subset of the same gels, expressed relative to DTDP–GSH case (defined as ‘1’), were 0.38 ± 0.26 (SD, n= 2) for samples treated with just DTT, and 0.53 ± 0.07 (n= 4) for fibres treated with DTDP–GSH and then DTT, and 0.31 ± 0.18 (n= 3) for fibres pre-treated with NEM and DTT before DTDP–GSH. The latter two cases were significantly lower than for the DTDP–GSH treatment alone (Fig. 4C), consistent with DTT reversing the effect of DTDP–GSH treatment and with NEM blocking its action. Treatment of fibres with GSSG–pH 8.5 also increased the anti-GSH signal relative to control. In the two cases examined, the anti-GSH/TnIf ratio expressed relative to the DTDP–GSH case run on the same gel was 0.3 ± 0.1 for the control treatment and 2.0 ± 0.2 for the GSSG–pH 8.5 treatment.
Figure 4. DTDP–GSH treatment causes S-glutathionylation of troponin I fast isoform (TnIf).
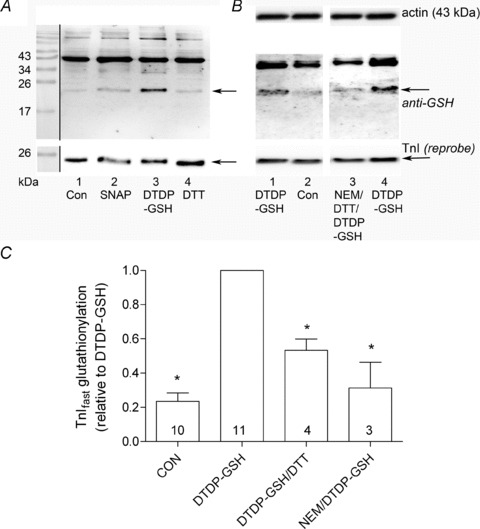
A, upper panel: Western blot with anti-GSH antibody in EDL fibre samples (5 fibre segments per sample) subjected to indicated treatment (all at pCa > 9) (15% SDS-PAGE; non-reducing). In all cases, fibres washed initially for at least 5 min in pCa > 9. Lane 1, control treatment (Con), 10 min washing at pCa > 9; Lane 2, 5 mm SNAP for 5 min; Lane 3, 100 μm DTDP for 5 min, 5 mm GSH for 2 min (i.e. ‘standard DTDP–GSH treatment’); Lane 4, 10 mm DTT for 10 min. All samples blocked with 5 mm NEM for 5 min before adding SDS (see Methods). Arrows indicate bands corresponding to TnI. The bands at ∼43 kDa correspond to actin. Lower panel: subsequent reprobe of same membrane for troponin I (TnI); EDL fibres contain only the fast isoform, TnIf. Positions of molecular weight markers shown on left (see Methods). B, middle panel: anti-GSH blot of EDL fibres given indicated treatments as in A (12.5% SDS-PAGE). Lane 3: fibres treated with NEM (200 μm, 2 min), followed by DTT (10 mm, 10 min), then standard DTDP–GSH treatment. Membrane reprobed for TnI (bottom) and then actin (top). C, mean +SEM ratio of band density for anti-GSH signal relative to corresponding TnIf signal for indicated treatments, expressed relative to DTDP–GSH case on same gel. Number of independent gels shown in each bar. *Significantly different (P < 0.001) from DTDP–GSH case.
Most membranes were also reprobed for actin, and it was found that the TnIf signal expressed relative to either the actin signal or the MHC signal (see Methods) was not significantly altered by any of the treatments. The anti-GSH blots invariably also showed strong signals corresponding to actin (e.g. Fig. 4A), but these signals were not noticeably different between the control and treatment cases. The only other band where the anti-GSH signal was often evidently more intense for the DTDP–GSH-treated samples relative to control was seen at ∼100 kDa and possibly corresponded to the SR Ca-ATPase, which is known to undergo S-glutathionylation (Viner et al. 1999).
Additional experiments were also performed that avoided any possible problems arising with reprobing of membranes (e.g. probing first with anti-GSH and then with anti-TnI, as in Fig. 4). In these experiments, treated EDL fibre samples were divided into three equal parts, which were run on three separate gels, with one each being first probed for either anti-GSH, anti-TnI or anti-TnC. These experiments (not shown) gave results indistinguishable from those obtained by the reprobing procedure, demonstrating that the anti-TnIf signal was accurately assessed by the reprobing method and further verifying that the DTDP–GSH treatment increases the anti-GSH/TnIf ratio. Importantly too, neither type of blotting procedure (e.g. Fig. 4A and B) found any apparent anti-GSH signal corresponding to either TnCf or myosin light chain 2 (MLC2), which both run at ∼18 kDa. (Note that fast TnT isoforms were not examined as they do not contain cysteine residues and hence cannot undergo S-glutathionylation.) The experiments also found no evidence of any appreciable cross-linking of TnIf and TnCf either to each other or to actin, as only single bands were found when probing for TnI or TnC (at ∼23 and 18 kDa, respectively). In summary, both Western blotting methods indicated that the DTDP–GSH and GSSG–pH 8.5 treatments cause S-glutathionylation of TnIf.
Biotin labelling of reactive cysteine residues
The reactivity of the free cysteine groups present in an EDL muscle fibre in relaxing conditions was examined using a reactive thiopyridine reagent (Biotin-HPDP) with a biotin tag that can be detected with high sensitivity. As seen in Fig. 5, a 1 min exposure to the reagent produced appreciable biotin labelling of TnIf as well as labelling of actin and a protein running at ∼105 kDa (possibly the SR Ca-ATPase). Longer exposure to the reagent gave stronger labelling of these proteins as well as of MHC and some other proteins. Taking into account the relative amounts of each protein present in a muscle fibre, the comparatively strong labelling to TnIf seen with the 1 min exposure to Biotin-HPDP confirms that one or more cysteine residues present on TnIf in situ on the thin filament in relaxing conditions is exposed and relatively reactive to thiopyridine reagents. Furthermore, the absence of any biotin labelling at ∼18 kDa even with the more prolonged reagent exposure indicates that the cysteine residues present in TnCf and MLC2 are inaccessible or comparatively unreactive in a fibre in relaxing conditions.
Figure 5. Biotin labelling of reactive cysteines.
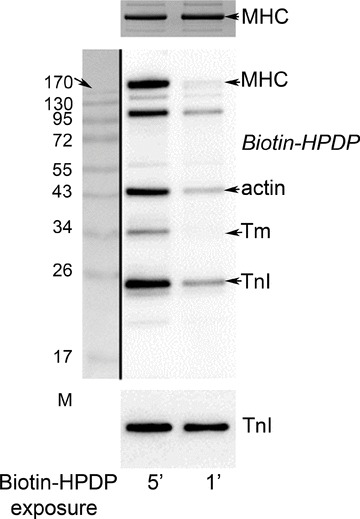
Western blot of single skinned EDL fibre segments treated in relaxing solution with a biotin-tagged thiopyridine reagent (Biotin–HPDP) for either 1 or 5 min, and probed for biotin with strepatividin (see Methods). The 1 min exposure to the reagent produced clear labelling of the TnI isoform present (TnIf), but an even more prolonged (5 min) exposure produced no detectable labelling of either TnC or MLC2 (both run at ∼18 kDa). 12.5% SDS-PAGE Tropomyosin (Tm). Bottom panel: reprobe for TnI.
S-glutathionylation of TnIf by GSNO
GSNO can potentially cause either S-glutathionylation or S-nitrosylation of a given cysteine residue. We have previously reported (Dutka et al. 2011b) that when GSNO (2 mm) is applied to a skinned EDL fibre immediately after being prepared in solution (termed GSNOimm) it causes a large increase in Ca2+ sensitivity (∼+0.14 pCa units), seemingly by the same mechanism as occurs with DTDP–GSH treatment. In contrast, if GSNO is applied to the fibre ∼10 min after preparation (termed GSNOdel) it instead causes a decrease in Ca2+ sensitivity (∼−0.06 pCa units), seemingly in a manner similar to the S-nitrosylating agent, SNAP. As seen in Fig. 6, Western blotting showed that GSNOimm caused S-glutathionylation of TnIf, whereas GSNOdel treatment did not. Very similar results were found in all three independent examinations conducted.
Figure 6. Freshly prepared GSNO produces S-glutathionylation of TnIf.
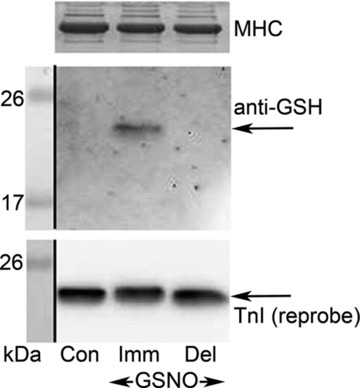
EDL type II fibres (4 fibre segments per sample) were subjected to control treatment (‘Con’, 10 min wash only) or to a 2 min treatment with GSNO (2 mm) either immediately after it was prepared (‘Imm’) or 10 min after it was prepared (delayed, ‘Del’). Fibres first washed for 5 min before either GSNO treatment. All solutions were at pCa > 9. Middle panel: Western blot with anti-GSH antibody. Lower panel: reprobe of membrane for TnI. Top panel: MHC band in coomassie-stained gel post-transfer. 15% SDS-PAGE.
Troponin exchange experiments
To further examine whether S-glutathionylation of TnIf is a mechanism underlying the increase in Ca2+ sensitivity, we tested whether substituting fast troponin isoforms into slow-twitch (type I) muscle fibres made them responsive to the DTDP–GSH treatment. The procedures attempted on rat skinned fibres were (a) to extract just the endogenous TnC and then to add exogenous fast TnC from either porcine or rabbit muscle, (b) to extract both TnC and TnI and then to add the exogenous fast isoforms or (c) to substitute the endogenous TnC and TnI (and probably TnT) without first going through a specific extraction step (see Methods).
TnC extraction and re-addition
TnC extraction was achieved by exposing a skinned fibre for 30 min to a rigor solution of normal ionic strength with TFP and no Ca2+ or Mg2+. This resulted in complete loss of Ca2+-activated force in the eight EDL type II fibres and its reduction to between 1 to 4% of the original level in the two soleus type I fibres examined, with no change in baseline Ca2+-independent force. Western blotting in a subset of these and other fibres confirmed extraction of 80–100% of the TnC, with relatively little if any change in TnI (not shown). After the fibres had been bathed for 15 min in relaxing solution containing exogenous fast troponins (i.e TnC, TnI and TnT) from either rabbit or porcine muscle, maximum force recovered to 56 ± 3% and 39 ± 1% of its original value in type II (n= 6) and type I (n= 4) fibres, respectively. Almost exactly the same level of recovery was seen in two type II fibres when adding TnCf alone, indicating that the reduction in maximum force had been primarily due to the loss of TnC. Following the extraction and replacement of TnC, the Ca2+ sensitivity of the fibres was substantially altered: pCa50 decreased by ∼0.21 to 0.26 pCa units in the type II fibres and by ∼0.35 to 0.44 pCa units in the type I fibres, and h decreased ∼50% in all cases, with no apparent difference between the porcine and rabbit TnCf. The pCa50 is normally ∼0.16 pCa units higher in rat type I fibres compared with type II fibres (Trinh & Lamb, 2006), but after substituting TnCf into both fibres types this difference disappeared.
Following the TnCf substitution, DTDP–GSH treatment increased pCa50 by +0.187 ± 0.026 pCa units in the four type II fibres examined, demonstrating that its normal action still occurred. In contrast, in two other type II fibres where the endogenous TnC was extracted and the fibre exposed to 25 μm NEM for 2 min before washing and adding exogenous TnCf, subsequent DTDP–GSH treatment caused only ∼5% of its usual increase in Ca2+ sensitivity shift (+0.011 ± 0.002 SD), indicating that type II fibres were affected by NEM treatment even when TnCf was largely absent. In the type I fibres following TnCf substitution, DTDP–GSH treatment still failed to cause any significant change in pCa50 (+0.021 ± 0.007, n= 4, P > 0.05); Western blotting of one of these type I fibres verified that most (though not all) of the slow TnC had indeed been replaced with the fast isoform.
TnI and TnC extraction and re-addition
Combined extraction of both TnC and TnI (and possibly some TnT) was achieved by bathing a fibre in relaxing solution with 10 mm vanadate for 10 min (Kogler et al. 1998). No force was produced in the presence of the vanadate, but upon washout with relaxing solution force developed over several minutes in a Ca2+-independent manner, reaching a peak of 31 ± 3% of the original Ca2+-activated maximum force in type II fibres (n= 12) and 62 ± 6% in type I fibres (n= 7). This Ca2+-independent force was suggestive of at least partial TnI extraction, and Western blotting of some fibres confirmed such partial extraction (not shown). Subsequent exposure of the fibre either to a mixture of TnC, TnI and TnT (fast isoforms), or to just TnI and TnT, resulted in the Ca2+-independent force declining to zero in the type II fibres and to ∼2–6% of the original maximum Ca2+-activated force in the type I fibres. Ca2+-activated force only recovered if TnC was added with the TnI and TnT or alternatively added subsequently. Maximum force recovered to ∼55–60% of the original level in the three type II fibres examined, but to only 17 ± 3% in the type I fibres (n= 7), which along with the partial nature of the troponin exchange, precluded meaningful examination of the effects of DTDP–GSH treatment in such fibres.
Troponin substitution without prior extraction
As extraction and subsequent replacement of the troponins appeared to lead to poor recovery of maximum force, troponin exchange was instead performed by exposing the skinned fibres to the exogenous troponins in the presence of a low ionic strength solution with zero Ca2+ and Mg2+ (see Methods); such low ionic strength probably aids unbinding of TnT, allowing it to also be substituted, and possibly even allowing exchange of the entire endogenous slow troponin trimer complex, with the exogenous fast troponin complex. Following 60 min of such exposure, maximum Ca2+-activated force back in standard conditions was still 92–96% of the original maximum in the five type I rat fibres examined (3 with porcine and 2 with rabbit troponin exposure).
However, in contrast to what occurred when exchanging just TnCs with TnCf (where pCa50 was decreased after extraction and addition using either rabbit or porcine TnCf (see above)), exchanging the TnI/TnT by either the vanadate treatment–replacement protocol or the low ionic strength exchange, resulted in a decrease in Ca2+ sensitivity when adding rabbit troponins (pCa50–0.12 ± 0.03 in 5 type I fibres and –0.20 ± 0.02 in 11 type II fibres) but a large increase when adding porcine troponins (pCa50+0.47 ± 0.03 in 5 type I fibres and +0.17 ± 0.04 (SD) in 2 type II fibres). Such data are consistent with the fact that TnCf is virtually identical in rat, rabbit and pig, whereas the TnIf isoforms present differ slightly between the three species, as well as the fact that at least three distinct variants of fast TnT are found in type II fibres even in a single species (Brotto et al. 2006).
Most importantly, following substitution of TnIf into soleus type I fibres, DTDP–GSH treatment resulted in an increase in Ca2+ sensitivity, as seen in Fig. 7A and B. In this experiment the force–pCa characteristics were examined after applying the DTDP and then again after applying GSH, in order to remove the confounding effects of the DTDP treatment itself and directly determine whether the GSH exposure caused a reversible increase in sensitivity. In the three type I fibres examined following porcine TnIf substitution, GSH treatment caused a substantial increase in Ca2+ sensitivity (∼+0.126 pCa units), which was fully reversed by DTT (Table 2), whereas normal type I fibres show no significant effect to such treatment (results here and also Lamb & Posterino (2003); see Table 2), with one of these control cases being one of the three fibres subsequently shown to undergo a large increase in Ca2+ sensitivity after troponin exchange. Western blotting of the type I fibre shown in Fig. 7A indicated that ∼35% of TnIs originally present in the fibre had indeed been substituted with TnIf (lane 2 in Fig. 7C). This estimate of percentage exchange was based on the evident reduction in the intensity of the TnIs band in lane 2 relative to the bracketing untreated fibres run on either side (lanes 1 and 3) (taking into account the sample mass in each lane indicated by the MHC bands shown at the top), together with the fact that non-linearity in the signal detection of the bands evidently resulted in disproportionately weak signals for smaller TnI amounts (which also probably precluded detection of any substituted TnIf in the relatively small sample in lane 4) (see also Murphy et al. 2009). The reversible increase in Ca2+ sensitivity with DTDP–GSH treatment also appeared to occur in the two type I fibres examined following exchange with rabbit troponin (Table 2), albeit to a smaller extent than with the porcine troponin exchange.
Table 2.
Effect of glutathionylation on pCa50 after substituting fast troponin isoforms into slow-twitch fibres
| Treatment | ||||
|---|---|---|---|---|
| Troponin exchange | DTDP | GSH | DTT | |
| 1. Control soleus fibres. (L & P, 2003) (n= 5) | N.A. | −0.059 ± 0.0027 | +0.001 ± 0.002 | +0.023 ± 0.011 |
| 2. Control soleus fibres. This study (n= 2) | N.A. | −0.028 ± 0.001 | +0.012 ± 0.004 | −0.004 ± 0.017 |
| 3. Soleus fibres with porcine troponin. This study (n= 3) | +0.455 ± 0.030* | −0.094 ± 0.016* | +0.126 ± 0.027* | −0.134 ± 0.022* |
| 4. Soleus fibres with rabbit troponin. This study (n= 2) | −0.047 ± 0.001 | −0.006 ± 0.028 | +0.031 ± 0.006 | −0.069 ± 0.043 |
Mean ± SEM (or SD, where n= 2) of change in pCa50 occurring with the indicated treatment in slow-twitch (i.e. type I) fibres from rat soleus muscle. n is the number of fibres examined. Treatments applied sequentially in order left to right across table. Substituted fast troponins were from porcine or rabbit muscle as indicated. N.A., not applicable. ‘L& P, 2003’ denotes data from Lamb & Posterino (2003), carried out under same conditions as this study. *Significantly different from zero (P < 0.05).
Effects of DTDP–GSH treatment in human muscle fibres
The effect of DTDP–GSH treatment was also examined in human vastus lateralis muscle fibres. Each fibre was classified as either type I or II both by its response to Sr2+ (see Methods) and by subsequent Western blotting for MHC, TnC and TnI (e.g. Fig. 8B), the results of which were in complete accord for each of the five fibres examined. All three of the human type II fibres examined (obtained from three different subjects) showed a large increase in Ca2+ sensitivity with DTDP–GSH treatment (∼+0.171 pCa units, row 8 in Table 1), which was fully reversed by DTT (e.g. Fig. 8A). In contrast, none of the three type I human fibres examined showed any increase in Ca2+ sensitivity to DTDP–GSH, instead displaying a decrease in every case (mean change in pCa50 of −0.111 ± 0.006 pCa units), with subsequent DTT treatment increasing Ca2+ sensitivity (+0.053 ± 0.014 pCa units); this effect of the DTDP–GSH treatment was largely or entirely attributable to the effects of the DTDP treatment alone, as seen in Fig. 8C. The 5 min DTDP treatment appeared to have greater effects in human fibres than it did in rat fibres, reversibly decreasing maximum force by 5–15%, which was similar to that seen in rat fibres with more prolonged treatments or higher µDTDP½ (see Lamb & Posterino, 2003). In summary, the results with human muscle closely resemble those in rat, with only the type II fibres showing increased Ca2+ sensitivity to the DTDP–GSH treatment.
TnIf in human muscle is highly homologous to that in other mammals and, as seen in Fig. 9A, it also undergoes S-glutathionylation upon DTDP–GSH treatment. The slow isoform of TnI, TnIs, is also S-glutathionylated by the DTDP–GSH treatment in both human muscle (Fig. 9A, see presence of two bands with anti-GSH) and rat soleus muscle (not shown). Figure 9A also illustrates two other important points. Firstly, the TnIf in rested human muscle shows some base level of S-glutathionylation (see lane 3). Secondly, S-glutathionylation of TnIf causes the TnIf to run slightly slower (i.e. at slightly higher apparent molecular mass) in both rat (lane 2 versus 1) and human muscle (lane 4 versus 3). Close inspection of all gels where TnIf underwent S-glutathionylation consistently showed this small effect (e.g. Figs 4, 6 and 10), though it was most apparent when precisely matched samples were compared as in Fig. 9A. One result of this is that when only a portion of the total TnIf in a sample is S-glutathionylated, the latter forms the trailing (or upper) part of the TnIf band seen with anti-TnI (e.g. lower panel in Fig. 9A), and because only it is seen with anti-GSH (middle panel in Fig. 9A) the anti-GSH bands for TnIf and TnIs appear closer together than do the anti-TnI bands for the two proteins. (Note that this effect is in part due to the fact that TnIs evidently does not run any slower when glutathionylated.) Importantly, the fact that the TnIf band can be seen to run slower following the DTDP–GSH treatment is further strong evidence that the TnIf is indeed affected by the treatment and is correctly identified as being the S-glutathionylated protein apparent on the anti-GSH blots.
Figure 9. TnIf runs more slowly when S-glutathionylated.
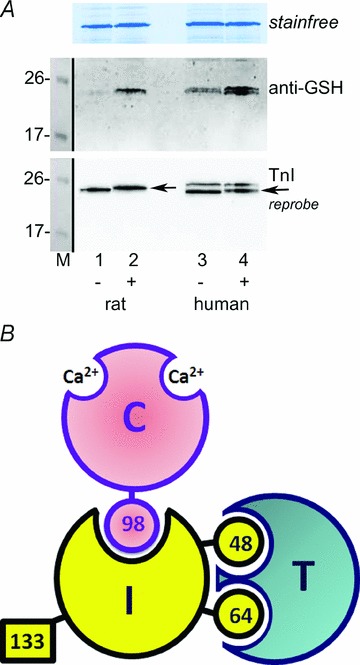
A, Western blots of tissue sections from rat EDL and human vastus lateralis muscles subjected either to a wash only (lanes 1 and 3, ‘–’) or to wash with DTDP (100 μm) and then GSH (5 mm) (lanes 2 and 4, ‘+’). (Adjacent transverse sections of frozen muscles used; treated at room temperature, all in K-HDTA ‘intracellular’ solution at pCa > 9.) Middle panel: anti-GSH antibody shows DTDP–GSH treatment increased glutathionylation of TnIf in rat EDL muscle and of both TnIf and TnIs in human muscle (lower and upper bands, respectively). Bottom panel: reprobe with TnI antibody: only fast isoform (TnIf) is present in rat EDL muscle, whereas both TnIf and TnIs are present in the human muscle. Note that TnIf runs at a slightly higher molecular weight following treatment with DTDP–GSH in both the rat and human muscle. 8–16% Criterion Stain Free gel. B, diagrammatic representation of exposed and hidden cysteine residues on the troponin complex in mammalian fast-twitch (type II) muscle fibres (modified from Chong & Hodges, 1982; © 1982 The American Society for Biochemistry and Molecular Biology). Letters I, C and T indicate TnIf, TnCf and TnTf subunits, and numbers indicate cysteine residues on TnIf and TnCf (there are none on TnTf). Only Cys133 on TnIf is readily accessible to cysteine reagents. TnIs (not shown) lacks an equivalent of Cys133, having Cys38, Cys65 and Cys85, with Cys65 being homologous to Cys 64 on TnIf.
Figure 10. No S-glutathionylation of TnIf in toad fibres.
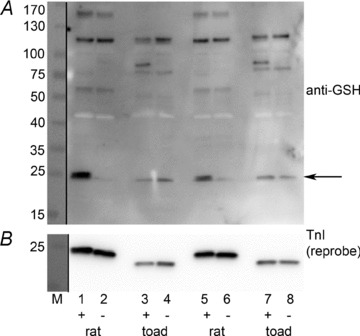
Western blot with anti-GSH (upper panel) and subsequent reprobe for TnI (lower panel). Rat EDL fibres in lanes 1 & 2 and 5 & 6, and toad iliofibularis fibres in lanes 3 & 4 and 7 & 8 (4 fibre segments in each sample). Fibres in left lane of each pair (i.e. in lanes 1, 3, 5 and 7) given standard DTDP–GSH treatment (+), and fibres in adjacent lanes (2, 4, 6 and 8) washed in same solution without DTDP and GSH (–). In the rat EDL fibres the DTDP–GSH treatment caused a very marked increase in the anti-GSH signal corresponding to TnIf (arrow) (compare lanes 1 & 3 with 2 & 6), but no other proteins in the rat fibres showed much increase with treatment. (Note that the anti-GSH signal at ∼43 kDa arising from actin is overexposed and appears white.) TnIf in the toad fibres displayed no anti-GSH signal, either with or without the treatment; note that the anti-GSH signal seen in the toad fibres running similar to the rat TnIf signal does not arise from the toad TnIf, which runs at an appreciably lower molecular weight, as seen in the TnI reprobe below. M, molecular markers (see Methods). 4–15% Criterion TGX Stain Free gel.
Effect of DTDP–GSH treatment in toad and chicken type II fibres
TnIf in most, if not all, mammalian species has three cysteine residues, Cys48, Cys64 and Cys133, and in the troponin complex only Cys133, which is highly reactive, is accessible to applied reactants (Chong & Hodges, 1982), as illustrated in Fig. 9B. Therefore it can be presumed that the S-glutathionylation of TnIf occurs at Cys133. Consistent with this, mammalian TnIs lacks an equivalent of Cys133; it has a Cys65 residue homologous to Cys64 in TnIf, as well as two other cysteine residues (Cys38 and Cys85) that are quite distinct from those in TnIf. The reactivity and accessibility of the cysteine residues in TnIs have not been described, but the results here indicate that at least one of the residues, possibly Cys38 or Cys85, is accessible and becomes S-glutathionylated with DTDP–GSH treatment.
TnIf in chicken and toad muscle are highly homologous to mammalian TnIf except that they both lack the equivalent of Cys133 (Wilkinson & Grand, 1978). Type II fibres were obtained from iliofibularis muscle of the toad and the pectoralis major muscle of the chicken, and all showed the expected low sensitivity of Sr2+ (O’Connell et al. 2006) and fast TnC and TnI bands on Western blotting (not shown). Importantly, DTDP–GSH treatment caused no significant change in Ca2+ sensitivity in either the chicken or toad type II fibres (rows 9 and 10 in Table 1), consistent with the increased Ca2+ sensitivity occurring in mammalian muscle being due to S-glutathionylation of Cys133. (In the chicken and toad fibre experiments listed in Table 1, the DTDP exposure was reduced from 5 min to 1 min, because the longer exposure to DTDP itself resulted in a moderate reduction in maximum force and Ca2+ sensitivity particularly in the chicken fibres; importantly, GSH exposure following the longer DTDP treatment still did not cause any increase in Ca2+ sensitivity in any of the fibres examined.)
Furthermore, DTDP–GSH treatment did not cause any S-glutathionylation of TnIf in toad type II fibres, as seen in Fig. 10. Note that the anti-GSH band detected in the toad fibres in a similar position to TnIf in the rat fibres is an unknown protein and does not correspond to the TnIf in the toad fibres, which was shown on the TnI reprobe to run somewhat lower. The full anti-GSH blot presented in Fig. 10 shows that there are a number of S-glutathionylated proteins present in the toad fibres that are not present in the rat fibres. Importantly, it also shows that the TnIf was the only protein present in these rat type II fibres with a noticeable change in S-glutathionylation upon DTDP–GSH treatment.
S-Glutathionylation of TnIf in exercising humans
Finally, we examined whether the level of S-glutathionylation of TnIf in human muscle was affected by exercise (see Methods). We found that there was a ∼4-fold increase in the level of S-glutathionylation of TnIf in vastus lateralis muscle of five human subjects following 40 min of cycling exercise at ∼60% (Fig. 11).
(Fig. 11).
Figure 11. Increased S-glutathionylation of TnIf in human muscle with prolonged exercise.
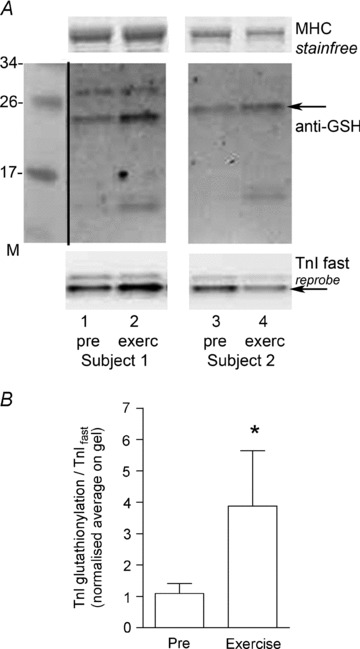
A, Western blot with anti-GSH, and subsequent reprobe for TnI, of transverse sections from vastus lateralis muscle biopsies taken from two human subjects both before (‘pre’) and after 40 min cycling exercise at ∼60% of  (‘exerc’). The anti-GSH signal indicated by arrow overlaid with the TnIf bands (lower of the two TnI bands). Increased glutathionylation was also evident at ∼14 kDa in these two post-exercise cases. Top panel shows corresponding MHC bands imaged in stain-free gel before protein transfer. 4–20% Criterion Stain Free gel. B, mean data (+SEM) from 5 subjects for the TnIf
S-glutathionylation signal before and after exercise (all samples run on same gel, repeated three times). The density of each TnIf glutathionylation signal was first normalised by the corresponding TnIf band density, and then each value was re-expressed relative to the mean of the pre-exercise cases on that gel (effectively declaring latter as ‘1’); values from 3 repetitions averaged to yield a single value for each pre- and post-exercise sample for each subject. *Significantly greater than ‘pre’ (paired t test, n= 5 subjects, P < 0.05, one-sided Wilcoxin signed rank test).
(‘exerc’). The anti-GSH signal indicated by arrow overlaid with the TnIf bands (lower of the two TnI bands). Increased glutathionylation was also evident at ∼14 kDa in these two post-exercise cases. Top panel shows corresponding MHC bands imaged in stain-free gel before protein transfer. 4–20% Criterion Stain Free gel. B, mean data (+SEM) from 5 subjects for the TnIf
S-glutathionylation signal before and after exercise (all samples run on same gel, repeated three times). The density of each TnIf glutathionylation signal was first normalised by the corresponding TnIf band density, and then each value was re-expressed relative to the mean of the pre-exercise cases on that gel (effectively declaring latter as ‘1’); values from 3 repetitions averaged to yield a single value for each pre- and post-exercise sample for each subject. *Significantly greater than ‘pre’ (paired t test, n= 5 subjects, P < 0.05, one-sided Wilcoxin signed rank test).
Discussion
This study provides compelling evidence that TnIf in mammalian mammalian fast-twitch (type II) fibres can be readily S-glutathionylated and that this results in a relatively large increase in Ca2+ sensitivity of the contractile apparatus with no detectable change in maximum force. The maximal effect was a ∼+0.24 pCa unit increase in pCa50 (Table 1), which corresponds to a 1.7-fold decrease in µCa2+½ needed for half-maximal force. We previously showed that treating rat type II fibres with DTDP and then briefly (1 to 2 min) with GSH produces this increase in Ca2+ sensitivity, and that this could be reversed by DTT or much longer (>20 min) exposure to GSH, effects strongly suggesting that the key process involved S-glutathionylation of some unidentified contractile apparatus protein (Lamb & Posterino, 2003). It was found here that a 10 min exposure to oxidized glutathione (GSSG) had the same action, and produced approximately the same maximal effect if applied at pH 8.5 (Figs 1 and 2, Table 1), the latter being used as a means of increasing the reactivity of the cysteine residues (Klatt & Lamas, 2000; Dalle-Donne et al. 2007). Western blotting showed that both the DTDP–GSH and GSSG–pH 8.5 treatments caused S-glutathionylation of TnIf in every instance examined (e.g. Figs 4, 9 and 10), without having any apparent effect on the other possible target proteins, in particular TnCf or myosin light chain 2. The only other protein seen in many instances to display increased S-glutathionylation with the DTDP–GSH treatment was a protein of ∼100 kDa, which was likely to be the SR Ca2+ pump (Viner et al. 1999). Effects on the Ca2+ pump could not account for the increased sensitivity of the contractile apparatus, particularly given that the increased Ca2+ sensitivity still occurred even after removal of all membranes with the detergent Triton-X 100. Western blotting further demonstrated that there was no appreciable level of cross-linking of TnIf with TnCf or other proteins upon DTDP–GSH treatment. Treatment with GSNO can cause either increased or decreased Ca2+ sensitivity depending on how it is applied (Dutka et al. 2011b), and it was found here that the increase in Ca2+ sensitivity occurred only when TnIf was S-glutathionylated (Fig. 6). Finally, DTT treatment reversed both the increase in Ca2+ sensitivity and the S-glutathionylation of TnIf, and NEM pre-treatment blocked both effects (Figs 3 and 4, and Table 1).
Type I fibres lack TnIf, instead having the slow isoform TnIs, and these fibres show no increase in Ca2+ sensitivity to either DTDP–GSH treatment or GSSG–pH 8.5 treatment (Table 1). Crucially, after TnIs was partially substituted with TnIf, the type I fibres did show increased Ca2+ sensitivity upon DTDP–GSH treatment (Fig. 7 and Table 2), strongly implicating TnIf as the crucial mediator of the effect. The effects could not have been mediated by TnTf because there are no cysteine residues in any of the three mammalian variants of TnTf and hence they cannot undergo S-glutathionylation. It was also apparent that the effects were not mediated by TnCf because (i) as mentioned above, TnCf did not become S-glutathionylated with DTDP–GSH treatment, (ii) the Ca2+ sensitivity increase with DTDP–GSH treatment takes place even when a fibre is maximally activated by Ca2+, which is known to make the one cysteine residue on TnCf (Cys98) inaccessible (Park et al. 1994), (iii) specific extraction of TnCs and replacement with TnCf did not cause type I fibres to display increased Ca2+ sensitivity with DTDP–GSH, and (iv) type II fibres treated with NEM whilst largely devoid of TnCf showed virtually no Ca2+ sensitivity increase to DTDP–GSH following re-insertion of TnCf. Furthermore, the biotin–HPDP labelling experiments (e.g. Fig. 5) indicated that the cysteine on TnCf was inaccessible or poorly reactive to thiopyridine reagents.
Cysteine target on TnIf
Mammalian TnIf has three cysteine residues (see Fig. 9B), but when TnIf is in the troponin complex with TnC and TnT only Cys133 is accessible and reactive, and this is the case in both the presence and absence of Ca2+ (Chong & Hodges, 1982). Thus, the S-glutathionylation of TnIf observed here is only attributable to an action on Cys133. Furthermore, the finding here that the increase in Ca2+ sensitivity with DTDP–GSH treatment was the same in both resting and activated fibres, is fully consistent with the known accessibility of Cys133. The fact that the sensitivity shift to DTDP–GSH treatment remained the same after replacement of TnIf in rat type II fibres with either porcine or rabbit TnIf is also consistent with Cys133 being the key target, as it is present in all these variants of TnIf. This cysteine residue is likewise present in the TnIf of human type II fibres, which also show the Ca2+ sensitivity increase to DTDP–GSH (Fig. 8A). Type II fibres from toad and chicken, on the other hand, have fast isoforms of TnI and TnC that are highly homologous to mammalian TnIf and TnCf, having matching cysteine residues to Cys98 in TnCf, and to Cys 48 and Cys64 in TnIf, but they lack the equivalent of Cys133, having an asparagine residue in its place (Wilkinson & Grand, 1978). Significantly, neither toad nor chicken type II fibres showed any change in Ca2+ sensitivity with DTDP–GSH treatment (Table 1), and Western blotting of TnIf in toad fibres showed no detectable S-glutathionylation, either with or without DTDP–GSH treatment (Fig. 10). These findings together strongly implicate S-glutathionylation of Cys133 on TnIf as being the process responsible for the increased Ca2+ sensitivity occurring with DTDP–GSH treatment.
Cys133 on TnIf is located in a highly flexible and mobile domain of the protein which can oscillate back and forth between actin and TnC (Aihara et al. 2006, 2010). The segment on TnIf adjacent to Cys133 binds to the hydrophobic pocket in the N-lobe of TnC in the Ca2+-bound state, and swings back to be frequently near actin in the absence of Ca2+. With S-glutathionylation, the glutamate residue of glutathione adds a negative charge at the cysteine residue and this, together with the accompanying steric effects, is thought to exert an action similar to or even greater than occurs with protein phosphorylation (Klatt & Lamas, 2000; Dalle-Donne et al. 2007). It seems that the net effect of this is to bias the movements of the flexible Cys133 region of TnIf more towards the TnC bound state, so that the interaction between TnI and TnC that controls contractile activation occurs at lower cytoplasmic µCa2+½.
Interestingly, although TnIs does not have a cysteine residue matching Cys133 in TnIf, it does have a serine residue in the corresponding position (Ser134), raising the possibility that phosphorylation of TnIs at Ser134 may play a similar role in modifying the Ca2+ sensitivity of contraction in type I fibres. Furthermore, cardiac TnI (TnIc) also has a serine residue in the homologous location (Ser166 in rat and Ser165 in human), adjacent to region binding in the hydrophobic pocket of cardiac TnCc, and phosphorylation of this residue on TnIc impedes binding of TnIc to TnCc (Ward et al. 2001). However, such phosphorylation does not occur when TnIc is in the normal troponin complex (Ward et al. 2001). In view of the major effects on Ca2+ sensitivity found here with S-glutathionylation of Cys133 in TnIf, the intriguing structural parallels with TnIs and TnIc suggest that a detailed investigation of the occurrence and possible functional changes occurring with phosphorylation at these sites may be warranted.
Functional relevance of S-glutathionylation of TnIf
S-Glutathionylation of TnIf was produced in the present study primarily by unphysiological means, in particular by applying DTDP–GSH or a very high level of GSSG that would never be reached in a muscle cell. However, the same basic process could be expected to quite readily occur in muscle fibres. Cys133 of TnIf is evidently a highly reactive and accessible cysteine residue (results here and Chong & Hodges, 1982; Tao et al. 1990; Park et al. 1994) and hence could be expected to readily react with many of the variety of reactive oxygen and nitrogen species, and other related reactive species, generated during muscle activity (Reid et al. 1992; Allen et al. 2008; Powers & Jackson, 2008; Lamb & Westerblad, 2011), leading by various means to its S-glutathionylation in the presence of cytoplasmic GSH (see Introduction). The rate at which the cytoplasmic GSH subsequently reduces the glutathionylated cysteine residue on TnIf back to a free sulphydryl was found previously to be relatively slow, with only ∼50% reversal occurring in 20 min in the presence of 5 mm GSH (Lamb & Posterino, 2003). This would help favour accumulation of TnIf in the S-glutathionylated state. However, reversal may occur faster in vivo at higher temperature and with the presence of glutaredoxins and thioredoxins (Dalle-Donne et al. 2007).
Most importantly, the present study found that moderate-intensity cycling exercise for 40 min led to a ∼4-fold increase in S-glutathionylation of TnIf in the vastus lateralis muscle of human subjects (Fig. 11). Given that S-glutathionylation of TnIf of human type II fibres was found, like in rat type II fibres, to result in a large increase in Ca2+ sensitivity (Figs 8 and 9), it seems reasonable to conclude that S-glutathionylation of TnIf is likely to be a significant factor influencing muscle performance in exercising humans. Increasing the Ca2+ sensitivity of the contractile apparatus in this way could be of great benefit in countering factors occurring with normal exercise which decrease contractile sensitivity, such as the build-up of inorganic phosphate which is thought to be a major factor in causing muscle fatigue (Allen et al. 2008).
S-Glutathionylation of TnIf not only increases contractile Ca2+ sensitivity considerably but also increases the peak twitch force and rate of force development to action potential stimulation (see Fig. 4 in Dutka et al. 2011b). Thus, the overall effects of S-glutathionylation of TnIf are highly comparable to those occurring with phosphorylation of myosin light chain 2 (Szczesna et al. 2002; Stull et al. 2011), with both causing similar size increases in Ca2+ sensitivity and similar twitch force potentiation in fast-twitch muscle fibres, though by distinctly different mechanisms. It is interesting that the potentiating effects of myosin light chain phosphorylation are seen within a matter of seconds from onset of intense muscle stimulation (Stull et al. 2011) and that the extent of MLC2 phosphorylation is greatly reduced after more prolonged fatiguing stimulation even though twitch potentiation still persists (Tubman et al. 1996). The latter finding, together with the fact that fatiguing stimulation causes twitch potentiation even in myosin light chain kinase knockout mice, has led to the suggestion that other mechanisms can also seemingly cause such post-activation potentiation when muscle has undergone a period of fatiguing stimulation (Tubman et al. 1996; Stull et al. 2011). Clearly, the findings of this study suggest that S-glutathionylation of TnIf would fulfill such a role, especially given that its effects would be expected to increase with the duration and extent of muscle activity owing to the likely increased total generation of reactive oxygen and related species. However, if the activity continued for too long it is possible that other deleterious effects of the oxidants could antagonize and counter the potentiating effects of the S-glutathionylation of TnIf (Andrade et al. 1998; Lamb & Westerblad, 2011).
Concluding remarks
This study has shown that S-glutathionylation of TnIf readily occurs in mammalian fast-twitch skeletal muscle fibres, including in humans during prolonged strenuous cycling exercise. Such S-glutathionylation greatly increases the rate of force development and the Ca2+ sensitivity of the contractile apparatus, and such effects could be expected to substantially influence muscle performance, in particular helping counter the effects of other factors that decrease contractile sensitivity or sarcoplasmic reticulum Ca2+ release that if unchecked potentially lead to reduced force responses and ‘muscle fatigue’. Additionally, the findings implicate Cys133 on TnIf as the site of the S-glutathionylation, and this seemingly fits well with its location in the flexible region of TnIf adjacent to the region binding to TnC during Ca2+ activation. The identification of this molecular process could well provide new avenues for understanding muscle dysfunction in particular situations and possibly also for the development of novel therapeutic strategies for countering such dysfunction.
Acknowledgments
We thank Maria Cellini and Heidy Latchman for technical assistance and the National Health & Medical Research Council of Australia for financial support (Grant number 541938).
Glossary
Abbreviations
- CaEGTA
calcium bound to EGTA
- CrP
creatine phosphate
- DTDP
2,2′-dithiodipyridine
- EDL
extensor digitorum longus
- GSH
reduced glutathione
- GSNO
S-nitrosoglutathione
- GSSG
oxidized gluthathione
- HDTA
hexa-methylene-diamine-tetraacetate
- h
Hill coefficient
- n
number of fibres
- NEM
N-ethylmaleimide
- pCa
–log10µCa2+½
- pCa50
pCa producing half-maximal force
- pSr50
–log10µSr2+½
- pSr50
pSr producing half-maximal force
- ROS
reactive oxygen species
- SNAP
S-nitroso-N-acetyl-penicillamine
- TFP
trifluoperazine
- Tm
tropomyosin
- TnC
troponin C
- TnI
troponin I
- TnT
troponin T
peak oxygen consumption
Author contributions
J.P.M. and R.M.M. designed, carried out and analysed the Western blotting procedures, and prepared the related figures and methods. T.L.D. helped conceive and carry out some of the physiological studies on single fibres. C.R.L. and M.J.M. were responsible for subject care and obtaining the muscle biopsies for the human skinned fibre data. T.L.M. and G.K.M. were responsible for the human exercise experiments and related muscle biopsies. G.D.L. conceived and designed the overall project and the specific physiological and biochemical experiments, analysed the physiological data and drafted the manuscript. All authors approved the final version of the submitted manuscript. Biochemical and physiological measurements were performed at La Trobe University and the human exercise procedures and muscle biopsies performed at Victoria University and University of Melbourne.
References
- Aihara T, Nakamura M, Ueki S, Hara H, Miki M, Arata T. Switch action of troponin on muscle thin filament as revealed by spin labeling and pulsed EPR. J Biol Chem. 2010;285:10671–10677. doi: 10.1074/jbc.M109.082925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara T, Ueki S, Nakamura M, Arata T. Calcium-dependent movement of troponin I between troponin C and actin as revealed by spin-labeling EPR. Biochem Biophys Res Commun. 2006;340:462–468. doi: 10.1016/j.bbrc.2005.12.030. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Allen DG, Westerblad H. Effect of hydrogen peroxide and dithiothreitol on contractile function of single skeletal muscle fibres from the mouse. J Physiol. 1998;509:565–575. doi: 10.1111/j.1469-7793.1998.565bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311. doi: 10.1096/fj.00-0507fje. [DOI] [PubMed] [Google Scholar]
- Bortolotto SK, Cellini M, Stephenson DG, Stephenson GM. MHC isoform composition and Ca2+- or Sr2+-activation properties of rat skeletal muscle fibers. Am J Physiol Cell Physiol. 2000;279:C1564–C1577. doi: 10.1152/ajpcell.2000.279.5.C1564. [DOI] [PubMed] [Google Scholar]
- Brotto MA, Biesiadecki BJ, Brotto LS, Nosek TM, Jin JP. Coupled expression of troponin T and troponin I isoforms in single skeletal muscle fibers correlates with contractility. Am J Physiol Cell Physiol. 2006;290:C567–C576. doi: 10.1152/ajpcell.00422.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong PC, Hodges RS. Proximity of sulfhydryl groups to the sites of interaction between components of the troponin complex from rabbit skeletal muscle. J Biol Chem. 1982;257:2549–2555. [PubMed] [Google Scholar]
- Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation. Free Radic Biol Med. 2007;43:883–898. doi: 10.1016/j.freeradbiomed.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Mollica JP, Lamb GD. Differential effects of peroxynitrite on contractile protein properties in fast- and slow-twitch skeletal muscle fibers of rat. J Appl Physiol. 2011a;110:705–716. doi: 10.1152/japplphysiol.00739.2010. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Mollica JP, Posterino GS, Lamb GD. Modulation of contractile apparatus Ca2+ sensitivity and disruption of excitation–contraction coupling by S-nitrosoglutathione in rat muscle fibres. J Physiol. 2011b;589:2181–2196. doi: 10.1113/jphysiol.2010.200451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P, Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem. 2000;267:4928–4944. doi: 10.1046/j.1432-1327.2000.01601.x. [DOI] [PubMed] [Google Scholar]
- Kogler H, Plathow C, Al-Hillawi E, Trayer IP, Ruegg JC. Replacement of troponin-I in slow-twitch skeletal muscle alters the effects of the calcium sensitizer EMD 53998. Pflugers Arch. 1998;436:398–406. doi: 10.1007/s004240050649. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Posterino GS. Effects of oxidation and reduction on contractile function in skeletal muscle fibres of the rat. J Physiol. 2003;546:149–163. doi: 10.1113/jphysiol.2002.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Westerblad H. Acute effects of reactive oxygen and nitrogen species on the contractile function of skeletal muscle. J Physiol. 2011;589:2119–2127. doi: 10.1113/jphysiol.2010.199059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Stephenson DG, Williams DA. Analysis of Ca2+ and Sr2+ activation characteristics in skinned muscle fibre preparations with different proportions of myofibrillar isoforms. J Muscle Res Cell Motil. 1995;16:65–78. doi: 10.1007/BF00125311. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-Acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merry TL, Wadley GD, Stathis CG, Garnham AP, Rattigan S, Hargreaves M, McConell GK. N-Acetylcysteine infusion does not affect glucose disposal during prolonged moderate-intensity exercise in humans. J Physiol. 2010;588:1623–1634. doi: 10.1113/jphysiol.2009.184333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris CA, Tobacman LS, Homsher E. Modulation of contractile activation in skeletal muscle by a calcium-insensitive troponin C mutant. J Biol Chem. 2001;276:20245–20251. doi: 10.1074/jbc.M007371200. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Dutka TL, Lamb GD. Hydroxyl radical and glutathione interactions alter calcium sensitivity and maximum force of the contractile apparatus in rat skeletal muscle fibres. J Physiol. 2008;586:2203–2216. doi: 10.1113/jphysiol.2007.150516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast- and slow-twitch fibres of rat. J Physiol. 2009;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell B, Blazev R, Stephenson GM. Electrophoretic and functional identification of two troponin C isoforms in toad skeletal muscle fibers. Am J Physiol Cell Physiol. 2006;290:C515–C523. doi: 10.1152/ajpcell.00307.2005. [DOI] [PubMed] [Google Scholar]
- O’Connell B, Nguyen LT, Stephenson GM. A single-fibre study of the relationship between MHC and TnC isoform composition in rat skeletal muscle. Biochem J. 2004;378:269–274. doi: 10.1042/BJ20031170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HS, Gong BJ, Tao T. A disulfide crosslink between Cys98 of troponin-C and Cys133 of troponin-I abolishes the activity of rabbit skeletal troponin. Biophys J. 1994;66:2062–2065. doi: 10.1016/S0006-3495(94)81000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins WJ, Han YS, Sieck GC. Skeletal muscle force and actomyosin ATPase activity reduced by nitric oxide donor. J Appl Physiol. 1997;83:1326–1332. doi: 10.1152/jappl.1997.83.4.1326. [DOI] [PubMed] [Google Scholar]
- Powers SK, Jackson MJ. Exercise-induced oxidative stress: cellular mechanisms and impact on muscle force production. Physiol Rev. 2008;88:1243–1276. doi: 10.1152/physrev.00031.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid MB, Haack KE, Franchek KM, Valberg PA, Kobzik L, West MS. Reactive oxygen in skeletal muscle. I. Intracellular oxidant kinetics and fatigue in vitro. J Appl Physiol. 1992;73:1797–1804. doi: 10.1152/jappl.1992.73.5.1797. [DOI] [PubMed] [Google Scholar]
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stull JT, Kamm KE, Vandenboom R. Myosin light chain kinase and the role of myosin light chain phosphorylation in skeletal muscle. Arch Biochem Biophys. 2011;510:120–128. doi: 10.1016/j.abb.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supinski GS, Callahan LA. Free radical-mediated skeletal muscle dysfunction in inflammatory conditions. J Appl Physiol. 2007;102:2056–2063. doi: 10.1152/japplphysiol.01138.2006. [DOI] [PubMed] [Google Scholar]
- Szczesna D, Zhao J, Jones M, Zhi G, Stull J, Potter JD. Phosphorylation of the regulatory light chains of myosin affects Ca2+ sensitivity of skeletal muscle contraction. J Appl Physiol. 2002;92:1661–1670. doi: 10.1152/japplphysiol.00858.2001. [DOI] [PubMed] [Google Scholar]
- Tao T, Gong BJ, Leavis PC. Calcium-induced movement of troponin-I relative to actin in skeletal muscle thin filaments. Science. 1990;247:1339–1341. doi: 10.1126/science.2138356. [DOI] [PubMed] [Google Scholar]
- Trinh HH, Lamb GD. Matching of sarcoplasmic reticulum and contractile properties in rat fast- and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol. 2006;33:591–600. doi: 10.1111/j.1440-1681.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Tubman LA, MacIntosh BR, Maki WA. Myosin light chain phosphorylation and posttetanic potentiation in fatigued skeletal muscle. Pflugers Arch. 1996;431:882–887. doi: 10.1007/s004240050081. [DOI] [PubMed] [Google Scholar]
- Viner RI, Williams TD, Schoneich C. Peroxynitrite modification of protein thiols: oxidation, nitrosylation, and S-glutathiolation of functionally important cysteine residue(s) in the sarcoplasmic reticulum Ca-ATPase. Biochemistry. 1999;38:12408–12415. doi: 10.1021/bi9909445. [DOI] [PubMed] [Google Scholar]
- Ward DG, Ashton PR, Trayer HR, Trayer IP. Additional PKA phosphorylation sites in human cardiac troponin I. Eur J Biochem. 2001;268:179–185. doi: 10.1046/j.1432-1327.2001.01871.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson JM, Grand RJ. Comparison of amino acid sequence of troponin I from different striated muscles. Nature. 1978;271:31–35. doi: 10.1038/271031a0. [DOI] [PubMed] [Google Scholar]


