Abstract
We examined the role of nitric oxide (NO) and prostanoids in the regulation of leg blood flow and systemic blood pressure before and after 8 weeks of aerobic high-intensity training in individuals with essential hypertension (n= 10) and matched healthy control subjects (n= 11). Hypertensive subjects were found to have a lower (P < 0.05) blood flow to the exercising leg than normotensive subjects (30 W: 2.92 ± 0.16 vs. 3.39 ± 0.37 l min−1). Despite the lower exercise hyperaemia, pharmacological inhibition of the NO and prostanoid systems reduced leg blood flow to a similar extent during exercise in the two groups and vascular relaxation to the NO-dependent vasodilator acetylcholine was also similar between groups. High-intensity aerobic training lowered (P < 0.05) resting systolic (∼9 mmHg) and diastolic (∼12 mmHg) blood pressure in subjects with essential hypertension, but this effect of training was abolished when the NO and prostanoid systems were inhibited. Skeletal muscle vascular endothelial NO synthase uncoupling, expression and phosphorylation status were similar in the two groups before and after training. These data demonstrate that a reduction in exercise hyperaemia in hypertensive subjects is not associated with a reduced capacity of the NO and prostanoid systems to induce vasodilatation or with altered acetylcholine-induced response. However, our data suggest that the observed reduction in blood pressure is related to a training-induced change in the tonic effect of NO and/or prostanoids on vascular tone.
Key points
Nitric oxide and prostanoids are substances that dilate the blood vessels. We examined the role of these vasodilators in the regulation of blood flow to contracting muscle and systemic blood pressure before and after a training intervention in subjects with essential hypertension and in healthy controls.
We show that blood flow to the exercising leg is lower in essential hypertension.
Surprisingly, this effect on blood flow is not the result of a reduced capacity of the nitric oxide and prostanoid systems to dilate the blood vessels; however, these systems do appear to play a role in the training induced reduction in blood pressure.
These findings advance our understanding of vascular dysfunction associated with essential hypertension and the mechanisms underlying the blood pressure reducing effect of exercise.
Introduction
Skeletal muscle blood flow is tightly regulated to match tissue oxygen demands (Andersen & Saltin, 1985). Increased peripheral resistance and decreased maximal vasodilatation are, however, well known characteristic features of chronic hypertension (Taddei et al. 1997) and as a consequence this disease state may be associated with reductions in blood flow. This potential impairment in oxygen delivery to contracting muscle could lead to tissue ischaemia and impaired tolerance to physical activity.
Within the vascular system, nitric oxide (NO) and vasodilator prostanoids relax smooth muscle cells, thus resulting in vasodilatation, and although pharmacological inhibition of the synthesis of either does not affect the magnitude of exercise hyperaemia, the ∼30% reduction in blood flow observed during combined blockade (Saunders et al. 2005; Mortensen et al. 2007; Nyberg et al. 2010) suggest that these two compounds act in synergy to contribute to muscle blood flow control during exercise. As the functions of both the NO and prostanoid systems are severely affected in essential hypertension (Taddei et al. 1997), a reduced blood flow to contracting muscle could be linked to an inadequacy of these systems to induce vasodilatation. Exercise training has been shown to lower leg blood flow at the same absolute workload (Kiens et al. 1993; Proctor et al. 2001; Krustrup et al. 2004), but to what extent this is related to alterations in the NO and prostanoid system and whether this adaptation is also present in hypertensive subjects remain unknown.
Training interventions have proved successful in lowering blood pressure in individuals with hypertension. Although training characteristics have been shown not to be predictive of the blood pressure response to aerobic training, the magnitude of blood pressure reduction is associated with the gain in  (Cornelissen & Fagard, 2005). This relationship is intriguing as improvements in
(Cornelissen & Fagard, 2005). This relationship is intriguing as improvements in 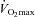 to a large extent are an effect of adaptations in central haemodynamics (Saltin et al. 1968), whereas improved peripheral vascular function (Taddei et al. 1997a) and remodelling (Heerkens et al. 2007) and/or decreases in muscle sympathetic nerve activity (MSNA; Laterza et al. 2007) are likely to explain the reduction in blood pressure. Nevertheless, this association does suggest that high-intensity aerobic training is a powerful stimulus for reducing blood pressure due to its large effect on improvements in
to a large extent are an effect of adaptations in central haemodynamics (Saltin et al. 1968), whereas improved peripheral vascular function (Taddei et al. 1997a) and remodelling (Heerkens et al. 2007) and/or decreases in muscle sympathetic nerve activity (MSNA; Laterza et al. 2007) are likely to explain the reduction in blood pressure. Nevertheless, this association does suggest that high-intensity aerobic training is a powerful stimulus for reducing blood pressure due to its large effect on improvements in  (Helgerud et al. 2007).
(Helgerud et al. 2007).
NO plays an important role in whole body blood pressure regulation in humans (Joyner & Casey, 2009) and the training-induced effect on blood pressure may be related to an improved function of endothelial nitric oxide synthase (eNOS), as evidenced by an augmented vascular relaxation to the NO-dependent vasodilator acetylcholine (ACh) after training (Higashi et al. 1999). However, direct evidence demonstrating a role of NO in the training-induced lowering of blood pressure in humans is lacking.
Putative mechanisms underlying the effect of training on vascular NO function include changes in eNOS protein expression, phosphorylation status of eNOS, and eNOS uncoupling. The latter is linked to an increased monomerization of the homodimeric enzyme and leads to production of superoxide instead of NO (Förstermann & Munzel, 2006). In an animal model of hypertension, uncoupled eNOS has been shown to increase superoxide formation and decrease vascular NO bioavailability (Mollnau et al. 2002), but the extent to which eNOS uncoupling is present in essential hypertension and whether training can affect the degree of uncoupling remain to be determined.
To address these issues, we examined the effect of inhibiting the formation of NO and prostanoids on resting and exercising haemodynamics before and after an 8 week aerobic high-intensity training period in normotensive subjects and individuals diagnosed with essential hypertension. In addition, NO metabolites formed within the vascular network at rest and during acute exercise and muscle enzymes involved in the synthesis of NO were also measured before and after training.
Methods
Ethical approval
Ten hypertensive subjects (systolic and diastolic blood pressure of 183 ± 7 and 101 ± 5 mmHg (± SEM), respectively) and 11 age, weight and height matched normotensive controls (142 ± 5 and 74 ± 3 mmHg) were studied (Table 1). The reported blood pressure values in Table 1 represent an average of readings obtained after 30 min of supine rest at three different time points during experimental day 1. The subjects were also instructed to perform home blood pressure monitoring, and measurements from the normotensive subjects showed values of 119 ± 4 and 70 ± 2 mmHg (average of 2 daily measurements performed for 1 week), thereby confirming that these individuals were normotensive. The study was approved by the Ethics Committee of Copenhagen and Frederiksberg communities (H-2-2009-096) and conducted in accordance with the guidelines of the Declaration of Helsinki. Written informed consent was obtained from all subjects before enrolment into the study.
Table 1.
Baseline characteristics before and after 8weeks of aerobic high-intensity exercise training
| Variable | Normotensive | Hypertensive | ||
|---|---|---|---|---|
| Before (week 0) | After (week 8) | Before (week 0) | After (week 8) | |
| Male/female | 6/5 | 4/6 | ||
| Age (years) | 46 ± 1 | 47 ± 1 | ||
| Systolic blood pressure (mmHg) | 142.1 ± 5.2 | 144.5 ± 4.9 | 182.8 ± 7.3* | 174.0 ± 5.9*† |
| Diastolic blood pressure (mmHg) | 73.6 ± 2.8 | 74.0 ± 2.3 | 101.1 ± 4.7* | 89.0 ± 2.7*† |
| Mean arterial pressure (mmHg) | 98.8 ± 3.4 | 98.6 ± 3.3 | 126.6 ± 5.0* | 117.5 ± 4.2*† |
| Body weight (kg) | 76.7 ± 2.8 | 76.3 ± 3.0 | 80.6 ± 7.7 | 79.8 ± 8.3 |
| Body fat (%) | 29.4 ± 2.5 | 28.1 ± 2.3 | 30.8 ± 2.6 | 30.0 ± 2.9 |
| Experimental leg mass (kg) | 12.2 ± 0.7 | 12.0 ± 0.7 | 12.1 ± 0.9 | 12.1 ± 1.0 |
 (l min−1) (l min−1) |
2.64 ± 0.19 | 2.81 ± 0.18† | 2.49 ± 0.19 | 2.73 ± 0.18† |
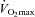 relative to body weight (ml min−1 kg−1) relative to body weight (ml min−1 kg−1) |
34.4 ± 1.6 | 36.9 ± 1.4† | 32.6 ± 2.7 | 36.1 ± 2.7† |
| Total cholesterol (mmol l−1) | 4.4 ± 0.2 | 4.6 ± 0.3 | 4.8 ± 0.2 | 4.9 ± 0.2 |
| HDL (mmol l−1) | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.1 |
| LDL (mmol l−1) | 2.4 ± 0.2 | 2.6 ± 0.3 | 3.0 ± 0.2 | 2.9 ± 0.1 |
| Triglycerides (mmol l−1) | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.2 |
Values are means ± SEM. *Significantly different from normotensive; †significantly different from before training.
Nine out of 10 hypertensive subjects were under treatment with angiotensin-converting enzyme (ACE) inhibitors (n= 4), calcium channel blockers (n= 6) and/or diuretics (n= 4). Potential subjects taking β-blockers were excluded from the study. Two weeks prior to the first experimental day the hypertensive subjects were instructed to stop their medication. Two subjects were subsequently allowed to take a low dose of diuretic medication until 3 days before the experimental day (both before and after training) and at the start of the training period due to developed symptoms (headache). Five of the subjects remained without medication throughout the training period, and the four subjects taking diuretics stopped taking the medication 2–3 weeks into the training period due to a lowering of blood pressure. All subjects were habitually inactive (less than 1 h of moderate intensity exercise per week), had normal resting ECG and no signs of ischaemia or arrhythmias during exercise (ECG), and were non-smokers, and none of the subjects in either group had been diagnosed with cardiovascular disease, renal dysfunction, insulin resistance, diabetes, or hypercholesterolaemia.
Study design
Two experimental days separated by 1 week were performed before and after an 8 week training period. Subjects refrained from caffeine, alcohol and exercise for 24 h before the experimental days and were asked to record their food intake such that the diet was similar before the experimental days. The premenopausal women were tested at the same time point during their menstrual cycle before and after training. Subjects ingested a standardized breakfast 2 h before reporting to the laboratory at 08.00 h.
Before the first experimental day the subjects visited the laboratory to become accustomed to the one-leg knee-extensor model (Andersen & Saltin, 1985) and to perform an incremental bicycle ergometer exercise test in which pulmonary maximal oxygen uptake (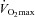 , l min−1) was determined(Quark b2 system, Cosmed, Rome, Italy; Table 1). To evaluate the effect of training,
, l min−1) was determined(Quark b2 system, Cosmed, Rome, Italy; Table 1). To evaluate the effect of training, 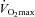 was also measured post-training.
was also measured post-training.
Exercise training programme
Subjects performed supervised aerobic exercise training (cycling ergometer) two to three times a week (Supplemental Table 1). On one additional day subjects performed an independent training session (cycling or running). During all training sessions subjects wore a TEAM2 WearLink+ (Polar, Kempele, Finland) to record heart rate (HR, Supplemental Fig. 1). During the 8 week training intervention both groups showed similar high training compliance.
Experimental days
Day 1
After local anaesthesia, catheters were placed in the femoral artery and vein of the experimental leg and in the femoral artery of the non-experimental leg, and a muscle biopsy was obtained from m. vastus lateralis of the non-experimental leg. ACh was infused into the femoral artery for 2.5 min at three different doses (10, 25 and 100 μg min−1 (kg leg mass)−1). The subjects then completed three steps of 2.5 min of one-leg knee-extensor exercise (10, 20 and 30 W) under the following conditions: infusion of saline (control) or indomethacin (inhibition of prostanoid formation) +NG-mono-methyl-l-arginine (l-NMMA; inhibition of NO formation). Indomethacin (200 μg min−1 (kg leg mass)−1; Confortid, Actavis, Copenhagen, Denmark) and l-NMMA (2.1 mg min−1 (kg leg mass)−1; Clinalfa, Bachem, Bubendorf, Switzerland) were infused into the femoral artery for 5 min before start of exercise and throughout exercise.
Day 2
Catheters for intravascular microdialysis probes were placed in the femoral artery and vein of the experimental leg 4–5 cm below the inguinal ligament and advanced 10 cm in the proximal direction. A microdialysis probe (CMA 70 bolt, CMA microdialysis, Stockholm) with a 10 mm membrane (20 kDa cut-off) was inserted into these two catheters. Correct placement of the probes was verified by ultrasound. Additionally, one catheter (20 Ga) for measurements of intra-arterial pressure was placed into the femoral artery of the non-experimental leg. Microdialysate was collected for 10 min during resting conditions and during one-leg knee-extensor exercise (20 W). The microdialysis probes were perfused at a rate of 5 μl min−1 with Ringer–acetate solution and to determine the relative exchange of the stable metabolites of NO, nitrate and nitrite (NOx), a small amount (2.7 nm) of µ2-3H½ATP (<0.1μCi ml−1) was added to the perfusate for calculation of probe recovery. Dalteparin (25 IE ml−1; Fragmin, Pfizer) was added to the perfusate to avoid blood clotting on the membrane. The molecular probe recovery (PR) was calculated as:
PR = (dpminfusate– dpmdialysate)/dpminfusate, where dpm denotes disintegrations per minute (Scheller & Kolb, 1991; Jansson et al. 1994). The µ3H½ATP activity (in dpm) was measured on a liquid scintillation counter (Tri-Carb 2000; Copenhagen, Denmark) after addition of the perfusate to 3 ml of Ultima Gold scintillation liquid (Perkin Elmer). After collection of samples, the microdialysate was weighed, and the actual flow rate was calculated to estimate any loss of fluid or abnormal decrease in perfusion rate.
Femoral arterial blood flow (LBF) was measured with ultrasound Doppler (Logic E9, GE Healthcare) equipped with a linear probe operating an imaging frequency of 9 MHz and Doppler frequency of 4.2–5.0 MHz. The site of blood velocity measurements in the common femoral artery was distal to the inguinal ligament but above the bifurcation into the superficial and profound femoral branch to avoid turbulence from the bifurcation. All recordings were obtained at the lowest possible insonation angle and always below 60 deg. The sample volume was maximized according to the width of the vessel, and kept clear of the vessel walls. A low-velocity filter (velocities <1.8 m s−1) rejected noises caused by turbulence at the vascular wall. Doppler tracings and B-mode images were recorded continuously and Doppler tracings were averaged over 16 heart cycles at the time of blood sampling. Vessel diameter was determined after each Doppler recording. Arterial diameter measures were assessed during the systole from arterial B-mode images with the vessel parallel to the transducer. Intra-arterial pressure was monitored with transducers (Pressure Monitoring Kit, Baxter Deerfield, IL, USA) positioned at the level of the heart. Blood samples were drawn after 2 min of exercise/infusion and blood gases, haemoglobin and lactate were measured using an ABL725 analyser (Radiometer Copenhagen, Denmark). Leg mass was calculated from whole-body dual-energy X-ray absorptiometry scanning (Prodigy, GE Healthcare).
Analysis of nitrate and nitrite
The stable metabolites of NO, nitrite and nitrate were measured using a fluorometric assay kit (Cayman Chemical Co., Ann Harbor, MI, USA).
Quantification of protein expression
Freeze dried tissue samples of m. vastus lateralis were homogenized in lysis buffer and Western blot analysis was performed as previously described (Bangsbo et al. 2009). Low temperature SDS-PAGE (LT-PAGE) was performed for detection of eNOS monomers using reported procedures (Klatt et al. 1995). Briefly, total proteins were incubated in 1 × Laemmli buffer without dithiothreitol (DTT) and then subjected to SDS-PAGE with 5% gel. Gels and buffers were equilibrated at 4°C before electrophoresis, and the buffer tank was placed in cooling conditions to maintain temperature of the gel <15°C. Subsequent to LT-PAGE, the gels were transferred and the blots probed as routine Western blot.
Antibodies used were: eNOS, eNOS-PThr495, nNOS (BD Transduction Laboratories, USA), and eNOS-PSer1177 (Calbiochem Millipore, Billerica, MA, USA). To control for loading differences the blots were also probed with an antibody against GAPDH (Abcam, Cambridge, UK).
Statistical analysis
A two-way ANOVA was performed to test significance between the normotensive and hypertensive subjects and a two-way repeated measures ANOVA was performed to detect training-induced changes within each group. After a significant F test, pairwise differences were identified using the Student–Newman–Keuls post hoc procedure. Differences in baseline characteristics were assessed with Student's t test for unpaired and paired data when comparing between groups and within each group, respectively. The significance level was set at P < 0.05 and data are means ± SEM.
Results
Subject characteristics
The 8 week training period lowered systolic (P < 0.05) and diastolic (P < 0.05) blood pressure in the hypertensive group, whereas 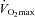 was increased in both normotensive (P < 0.001) and hypertensive (P < 0.05; Table 1) subjects. In the hypertensive group, the improvement in
was increased in both normotensive (P < 0.001) and hypertensive (P < 0.05; Table 1) subjects. In the hypertensive group, the improvement in 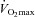 tended (P= 0.084, r2= 0.57) to correlate with the reduction in blood pressure.
tended (P= 0.084, r2= 0.57) to correlate with the reduction in blood pressure.
Haemodynamic responses to ACh infusion
During infusion of ACh, there was no difference in the change in LBF and LVC with ACh infusion between the normotensive and hypertensive group before or after training (Fig. 1). After training, the change in LVC was higher (P < 0.05) in the hypertensive group when compared to before training, whereas it was similar to before training in the normotensive group. There was no correlation between blood pressure reduction and changes in vasodilator response to ACh. Blood variables are presented in Supplemental Table 2.
Figure 1. Change in leg blood flow and vascular conductance with infusion of ACh.
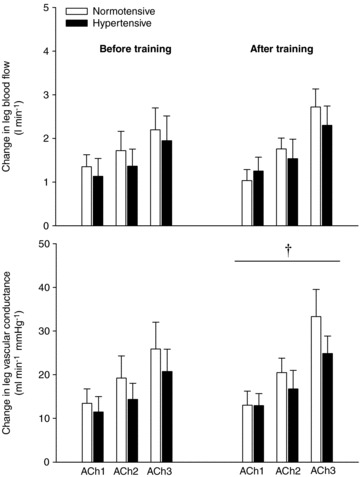
ACh was infused at 10 (ACh 1), 25 (ACh 2), and 100 μg min-1 (kg leg mass)-1 (ACh 3) in normotensive (n = 10) and hypertensive (n= 9) subjects before and after 8 weeks of aerobic exercise training. Values are means ±SEM. †Overall effect of training on leg vascular conductance in the hypertensive group.
Leg and systemic variables at rest and during exercise
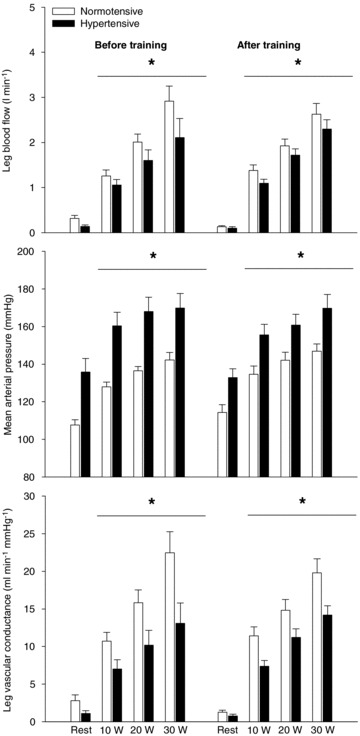
Before training, exercise increased LBF from 0.47 ± 0.07 to 3.39 ± 0.37 l min−1 (30 W) and 0.33 ± 0.06 to 2.92 ± 0.16 l min−1 (30 W) in the normotensive and hypertensive group, respectively, and was lower (P < 0.05) in the hypertensive group compared to the normotensive group (Fig. 2). No difference in arterial O2 content was observed between normotensive and hypertensive subjects and the lower blood flow was therefore paralleled by a lower (P < 0.05) leg O2 delivery (0.33 ± 0.03, 0.47 ± 0.03 and 0.55 ± 0.03 ml O2 min−1 versus 0.36 ± 0.04, 0.52 ± 0.04 and 0.69 ± 0.08 ml O2 min−1 in the hypertensive and normotensive group, respectively, during 10, 20 and 30 W). After training, LBF was lower (P < 0.05) during exercise at the same absolute workload in the normotensive subjects when compared to before training, whereas exercise LBF remained unaltered in the hypertensive subjects. After training exercise LBF was similar in the normotensive and the hypertensive groups.
Figure 2. Leg blood flow, mean arterial pressure, and leg vascular conductance during rest and one-leg knee-extensor exercise.
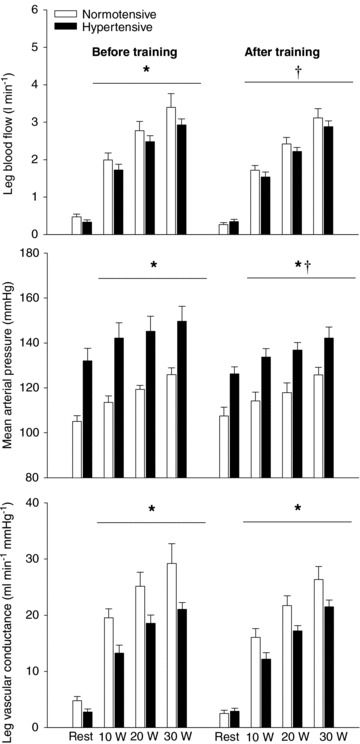
Exercise was performed at 10, 20 and 30 W in normotensive (n = 9) and hypertensive (n = 9) subjects before and after 8 weeks of aerobic high-intensity training. Values are means ± SEM. *Overall difference between groups; †overall effect of training on mean arterial pressure in the hypertensive group and overall effect of training on leg blood flow in the normotensive group.
Exercise training lowered mean arterial pressure (MAP) at rest and during exercise in the hypertensive group (P < 0.05) when compared to before training, whereas it remained similar in the normotensive group. MAP at rest and during exercise was higher (P < 0.001) in the hypertensive compared to the normotensive group before and after training. Both before and after training, leg vascular conductance (LVC) was lower during exercise (P < 0.001) in the hypertensive group compared to the normotensive group. Blood variables are presented in Supplemental Table 3.
Leg and systemic variables at rest and during exercise with inhibition of NO and prostanoid formation
Both before and after the training period, the hypertensive group had a lower LBF (P < 0.05) and LVC (P < 0.001) during infusion of l-NMMA and indomethacin at rest and during exercise compared to the normotensive group (Fig. 3). MAP was higher both at rest and during exercise (P < 0.001) in the hypertensive when compared to the normotensive group. Before training, exercising leg 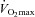 was lower (P < 0.05) in the hypertensive subjects. Blood variables are presented in Supplemental Table 4.
was lower (P < 0.05) in the hypertensive subjects. Blood variables are presented in Supplemental Table 4.
Figure 3. Leg blood flow, mean arterial pressure, and leg vascular conductance during rest and one-leg knee-extensor exercise with infusion of l-NMMA and indomethacin.
Exercise was performed at 10, 20, and 30 W in normotensive (n = 9) and hypertensive (n = 8) subjects before and after 8 weeks of aerobic high-intensity training. Values are means ± SEM. *Overall difference between groups.
The effect of training on leg and systemic variables at rest and during exercise during inhibition of NO and prostanoid formation
Taking into account the change in baseline blood flow with infusion of l-NMMA and indomethacin, the normotensive group had a lower (P < 0.05) change in LBF during exercise at 10 W (0.34 ± 0.08 versus 0.73 ± 0.09 l min−1) and 20 W (0.49 ± 0.08 versus 0.70 ± 0.13 l min−1) after training, when compared to before training (Fig. 4). There was no effect of training on the exercise-induced change in LBF during NO and prostanoid inhibition in the hypertensive group. MAP during resting conditions was lower (P < 0.05) in the hypertensive group after compared to before training (135 ± 7 versus 126 ± 4 mmHg), but this training-induced lowering of MAP was abolished when l-NMMA and indomethacin together were infused due to an increase (P < 0.05) in MAP (Fig. 3). Similarly, the training-induced lowering of MAP during exercise was also abolished when l-NMMA and indomethacin were infused. In the normotensive group, infusion of l-NMMA and indomethacin tended (P= 0.062) to increase MAP after training.
Figure 4. Reduction in LBF during one-leg knee-extensor exercise with infusion of l-NMMA and indomethacin (A) and mean arterial pressure during seated resting conditions (B).
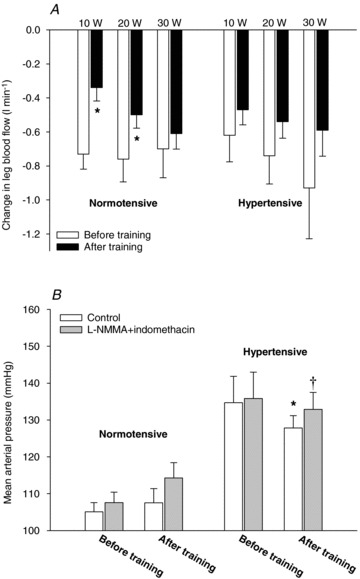
HAemodynamic changes in normotensive (n = 9) and hypertensive (n = 8) subjects before and after 8 weeks of aerobic high-intensity training. Values are means ± SEM. *Significantly different from before training; †significantly different from control.
Plasma NOx and leg NOx uptake at rest and during exercise
Before training, resting femoral arterial plasma NOx concentration tended to be lower (P= 0.063) in the hypertensive group, but as exercise at 20 W induced a ∼30% increase (P < 0.05) in femoral arterial plasma NOx concentration in the hypertensive group, and it did not change in the normotensive group, no difference was detected between the two groups during exercise (Fig. 5). Before training, there was a significant difference (P < 0.05) between the two groups in leg NOx uptake. After training, venous NOx tended (P= 0.073) to be lower during resting conditions in the hypertensives when compared to the normotensives, whereas no difference in resting NOx uptake was detected.
Figure 5. Arterial and venous NOx and leg NOx uptake during one-leg knee-extensor exercise at rest and 20 W.
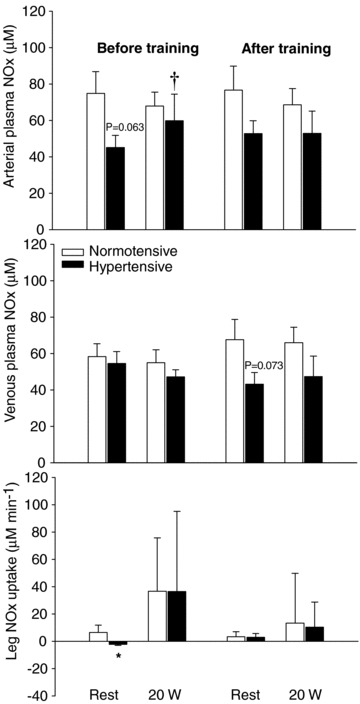
Plasma NOx in normotensive (n = 7) and hypertensive (n = 7) subjects before and after 8 weeks of aerobic high-intensity training. Values are means ± SEM. *Significantly different from normotensive; †significantly different from rest.
Skeletal muscle eNOS and nNOS protein, eNOS uncoupling and eNOS phosphorylation in muscle tissue
No group differences in skeletal muscle protein expression and phosphorylation status in any of the enzymes investigated was observed before or after the training period (Fig. 6). Training induced a decrease (P < 0.05) in the level of eNOS monomers in the normotensive but not in the hypertensive subjects. Skeletal muscle eNOS uncoupling (the ratio between eNOS monomers and total eNOS) in both the normotensive (P < 0.05) and the hypertensive (P < 0.05) group was lower after training. This reduction in eNOS uncoupling was associated with a tendency towards an increased expression of total eNOS in both the normotensive (P= 0.067) and hypertensive (P= 0.052) subjects. When accounting for changes in total eNOS, training tended (P= 0.062) to promote dephosphorylation of eNOS at threonine residue 495 (eNOS-PThr495; phosphorylation here inhibits enzyme activity) in the normotensive group whereas no change was detected in the phosphorylation of eNOS at serine residue 1177 (eNOS-PSer1177; phosphorylation here increases enzyme activity) in either group. There was an increase (P < 0.05) in the protein level of nNOS in the normotensive group after training. Representative blots are presented in Supplemental Fig. 2.
Figure 6. Protein expression of total eNOS, eNOS monomers, and the ratio between eNOS monomers and total eNOS (A), and protein expression of Thr495-phosphorylated eNOS, Ser1177-phosphorylated eNOS and nNOS (B).
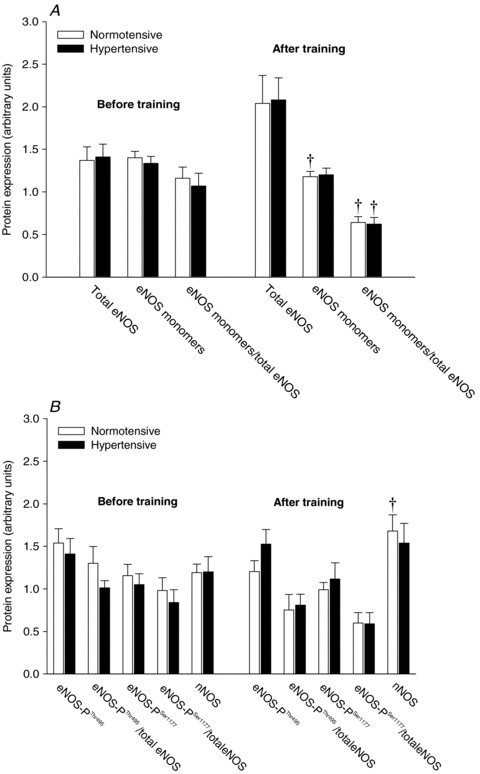
Protein expression and phosphorylation status in normotensive (n = 11) and hypertensive (n = 10) subjects before and after 8 weeks of aerobic high-intensity training. Values are means ± SEM. †Significantly different from before training.
Effect of sex and menopausal status
Careful examination of individual data for vascular responses, response to double blockade and NO synthase levels and properties provided no indication that either pre- or post-menopausal women differed from the males in this regard.
Discussion
In the current study, we show for the first time that blood flow to exercising skeletal muscle is lower in humans with essential hypertension and that this blunted exercise response is not associated with a reduced capacity of the NO and prostanoid systems to induce vasodilatation during muscle contraction or an attenuated vasodilator response to ACh. In the normotensive, but not the hypertensive, group, the period of training lowered leg blood flow during exercise at the same absolute workload, an effect which also was paralleled by a reduced dependency of the NO and prostanoid system in this subject group, as evidenced by a lower reduction in leg blood flow during infusion of l-NMMA and indomethacin after training. In contrast to the unaltered role of NO and prostanoids on the regulation of exercise hyperaemia at the local muscular level, the mechanism responsible for the training-induced lowering of systemic blood pressure appears to be associated with changes in the tonic effect of NO and/or prostanoids on vascular tone, as the effect of training was abolished when these systems were inhibited. Furthermore, despite indications of a lower resting arterial NO formation before training in the hypertensive group, skeletal muscle eNOS content, degree of uncoupling and phosphorylation status were similar between the two groups. However, in both groups, training induced a reduction in eNOS uncoupling.
Vascular physiology of essential hypertension
Regulation of blood flow to the exercising leg
The lower blood flow to contracting leg muscles in hypertensive subjects is an important novel finding that suggests that hypertension is associated with not only an elevation in systemic blood pressure, but also an inadequate blood flow to skeletal muscle which is essential for exercise capacity and which, with time and severity of the disease, could lead to tissue ischaemia. The mechanisms underlying the reduction in exercise induced blood flow are not completely clear. However, the function of both the NO and the prostanoid systems have been shown to be affected in the forearm vasculature of individuals with essential hypertension (Taddei et al. 1997), indicating that an inadequacy in the formation of NO and vasodilator prostanoids could potentially explain the lower hyperaemic response to exercise in these subjects. We examined this possibility in the present study by pharmacological inhibition of the synthesis of NO and prostanoids. The inhibition reduced leg blood flow during exercise to a similar extent in both groups, suggesting that the capacity to produce NO and vasodilator prostanoids during leg exercise is not affected in essential hypertension. In accordance with this finding, the hypertensive subjects showed a similar vascular relaxation to the mainly NO-dependent vasodilator ACh (Mortensen et al. 2009) and the two groups also had similar muscle protein levels and phosphorylation status of enzymes catalysing NO formation. The similar vasodilator response to ACh found in the current study is in accordance with a study on subjects with type 2 diabetes (Thaning et al. 2011), a disease state known to be associated with endothelial dysfunction, but in contrast to studies on forearm vasculature of hypertensive subjects showing reduced vascular relaxation (Taddei et al. 1997; Higashi et al. 1999). This discrepancy may reflect differences in the vasculature of the upper and lower extremities and/or the severity and hence effect of hypertension on endothelial function.
Combined, the above findings suggest that the lower blood flow during exercise is not associated with a reduced capacity to form NO or prostanoids. Alternatively, it is possible that the observed lower blood flow to contracting skeletal muscle was related to alterations in the blunting of sympathetic outflow normally observed in contracting muscles of healthy animals and humans (Rosenmeier et al. 2004), since this mechanism has been shown to be impaired in rat models of hypertension (Zhao et al. 2006) as well as in the forearm of human hypertensive subjects (Vongpatanasin et al. 2011).
Shear-stress induced NO formation
The likely stimulus for endothelial NO production has been identified as increased flow through the vessel lumen (Pohl et al. 1986). In animal models, this shear stress induced dilatation is reduced in hypertension due to impaired NO function (Koller, 2002). In accordance, a tendency towards lower resting femoral arterial plasma NOx concentration in the hypertensive subjects was observed before training, indicating that the shear stress induced formation of NO within the arterial system was impaired in these subjects. Interestingly, acute exercise at 20 W increased the femoral arterial NOx concentration in the hypertensive group to levels similar to that of the normotensive group, suggesting that a higher arterial shear stress was needed to stimulate NO production in the hypertensive subjects.
eNOS uncoupling
Uncoupling of eNOS has been suggested to be present in animal models of hypertension (Cosentino & Luscher, 1998; Kerr et al. 1999; Mollnau et al. 2002), as well as in essential hypertension (Higashi et al. 2002), and this alteration has been shown to increase superoxide formation and decrease vascular NO bioavailability (Mollnau et al. 2002). In the present study no difference in eNOS uncoupling was detected between the two groups. This discrepancy may be related to the vessel type investigated as the level of eNOS uncoupling was determined in the microvasculature of skeletal muscle, whereas earlier studies have used larger upstream vessels (Cosentino & Luscher, 1998; Kerr et al. 1999; Mollnau et al. 2002). Interestingly, in the study by Higashi and coworkers (2002), infusion of tetrahydrobiopterin, an essential cofactor for coupling of eNOS (Förstermann & Li, 2010), augmented endothelium-dependent vasodilatation in both normotensive and hypertensive individuals, suggesting that eNOS uncoupling was present in both groups. The lack of difference in eNOS coupling at the protein level between the two groups in the current study may, therefore, reflect that the extent of eNOS uncoupling at the microvascular level is related to age and/or inactivity and not to disease state.
Adaptive responses to exercise training in hypertensive individuals
Regulation of blood flow to the exercising leg
In the normotensive subjects, blood flow during exercise performed at the same absolute workload was ∼0.4 l min−1 lower after training, which is in congruence with findings on young healthy individuals (Kiens et al. 1993; Proctor et al. 2001; Krustrup et al. 2004). The lower blood flow to the trained leg during exercise in the normotensive group is thought to be due to training adaptations within the skeletal muscle that result in an optimized blood flow distribution and improved conditions for oxygen diffusion (Saltin et al. 1976; Kalliokoski et al. 2001; Proctor et al. 2001). In contrast, the ∼0.35 l min−1 lower blood flow to the working limb in the hypertensive subjects before training is likely to reflect a pathological reduction due to an imbalance between vasoconstriction and vasodilatation (Taddei et al. 1997b; Hansen et al. 2011), structural alterations of the blood vessels (Heerkens et al. 2007) and/or increased MSNA (Laterza et al. 2007). The ability of the hypertensive subjects to perform the same workload with less blood flow and oxygen delivery suggests that the reduction in blood flow was compensated for by an increased oxygen extraction, greater anaerobic energy production and/or better mechanical efficiency. We did not, however, detect any significant differences in leg oxygen extraction, oxygen uptake, or lactate release, indicating that more than one adaptation was responsible for the compensation. The unaltered leg blood flow after training in the hypertensive subjects may reflect that an improvement in vascular function occurred in parallel with some of the changes leading to the training induced lowering of blood flow.
In the normotensive subjects, the lower leg blood flow after training was paralleled by less reduction in exercise-induced blood flow with pharmacological inhibition of the NO and prostanoid system in the normotensive subjects. This novel observation suggests that a potential mechanism responsible for the lower leg blood flow after training was a reduced role of the NO and prostanoid systems for skeletal muscle blood flow regulation. Conversely, in the hypertensive subjects no difference in the effect of pharmacological inhibition was observed after training, suggesting an unaltered formation of NO and prostanoids during muscle contraction.
Blood pressure regulation in essential hypertension
From a meta-analysis including 30 hypertensive studies, it has been found that aerobic training reduces systolic and diastolic blood pressure by ∼7 and ∼5 mmHg, respectively (Cornelissen & Fagard, 2005). Consequently, the reductions of ∼9 and ∼12 mmHg found in the current study are well above the expected outcome and of high clinical relevance as a reduction of either 10 or 5 mmHg in systolic and diastolic blood pressure, respectively, will in the long term be associated with a 40% lower risk of stroke death and 30% lower risk of death from ischaemic heart disease or other vascular causes throughout middle age (Lewington et al. 2002).
An association between the training induced magnitude of blood pressure reduction and the gain in 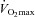 has previously been reported (Cornelissen & Fagard, 2005), and in congruence with this observation we did detect large reductions in blood pressure, which tended (P= 0.084) to correlate with the improvement in
has previously been reported (Cornelissen & Fagard, 2005), and in congruence with this observation we did detect large reductions in blood pressure, which tended (P= 0.084) to correlate with the improvement in 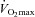 in the hypertensive group. These observations emphasize that high-intensity aerobic training, which is known for its large effect on improvements in
in the hypertensive group. These observations emphasize that high-intensity aerobic training, which is known for its large effect on improvements in 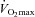 (Helgerud et al. 2007), is a powerful stimulus for reducing blood pressure. The mechanisms underlying this association are intriguing as central haemodynamic adaptations are likely to explain the improvement in
(Helgerud et al. 2007), is a powerful stimulus for reducing blood pressure. The mechanisms underlying this association are intriguing as central haemodynamic adaptations are likely to explain the improvement in 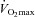 (Saltin et al. 1968), whereas the lowering of blood pressure is thought to be due to changes in peripheral vascular function (Taddei et al. 1997a), vascular remodelling (Heerkens et al. 2007) and/or a decrease in MSNA (Laterza et al. 2007).
(Saltin et al. 1968), whereas the lowering of blood pressure is thought to be due to changes in peripheral vascular function (Taddei et al. 1997a), vascular remodelling (Heerkens et al. 2007) and/or a decrease in MSNA (Laterza et al. 2007).
Role of NO and prostanoids in the regulation of blood pressure
A novel observation was that l-NMMA and indomethacin increased blood pressure after training in the hypertensive subjects. The unaltered blood pressure in normotensive subjects and hypertensive subjects before training is in agreement with previous studies (Mortensen et al. 2007, 2009) using a similar dose of these inhibitors in young healthy subjects. The blood pressure raising effect of combined inhibition after training is likely to be related to an increased formation and bioavailability of NO and possibly also improved balance between vasoconstrictor and vasodilator prostanoids after training. With regard to the latter possibility, the endothelium in hypertensive subjects has been suggested to produce cyclooxygenase-derived vasoconstrictor prostanoids as well as superoxide anions which reduce NO bioavailability (Taddei et al. 1997b, 1998).
The effect of l-NMMA and indomethacin on blood pressure could have contributed to activation of the arterial baroreflex and subsequent inhibition of central sympathetic outflow (Hansen et al. 1994), but the lack of change in heart rate makes this possibility unlikely. The increased perfusion pressure in the leg could be a confounding factor for the observed change in leg blood flow and vascular conductance. However, infusion of NG-nitro-l-arginine methyl ester and indomethacin has been shown to increase blood pressure in healthy subjects (Boushel et al. 2002), but despite this effect on central haemodynamics, exercise hyperaemia was reduced to a similar extent as in studies where no changes in blood pressure have been observed (Mortensen et al. 2007, 2009). In addition, Frandsen et al. (2001) reported no influence on leg blood flow during exercise with systemic NOS inhibition, despite an increase in blood pressure. The increased blood pressure during infusion of l-NMMA and indomethacin in trained hypertensive subjects is, therefore, unlikely to have altered the leg blood flow response to exercise to an extent that would have affected our conclusions.
Although it is well established that aerobic training lowers blood pressure in hypertensive subjects the underlying mechanisms remain unidentified. NO is important for whole body blood pressure regulation in humans (Joyner & Casey, 2009) and training has been shown to improve the function of eNOS, but in congruence with the findings from the current study, no correlation between blood pressure reduction and improved endothelial function was observed (Higashi et al. 1999). Despite the unaltered function of the NO and prostanoid system on local blood flow regulation during exercise, inhibition of these systems did fully abolish the training-induced reduction in resting and exercising blood pressure at the systemic level. This discrepancy between local and systemic effects suggests that vascular resistance is regulated upstream from the microvasculature, but more evidence is needed to confirm this proposition.
Shear-stress induced NO formation
Exercise is known to be associated with acute changes in endothelial shear stress (Tinken et al. 2010), and it has been shown that increases in intraluminal shear stress improve NO-mediated endothelium-dependent dilatation in animals (Woodman et al. 2005) and humans (Tinken et al. 2010). High-intensity exercise eliciting very high blood flows and hence shear stress may, therefore, be a potent exercise modality to reduce blood pressure in hypertensive subjects due to its effect on endothelial NO function, which is in accordance with the normalization of resting arterial NOx after training observed in the current study. Additional studies investigating the effect of different exercise intensities on shear stress induced alterations in endothelial NO function and blood pressure changes are warranted.
eNOS phosphorylation
Exercise training has been shown to increase phosphorylation of eNOS on serine residue 1177 (Hambrecht et al. 2003) and to decrease phosphorylation of eNOS on threonine residue 495 (Calvert et al. 2011), both leading to increased enzyme activity. In the current study no difference in phosphorylation status was detected between the groups with only a tendency towards a lower phosphorylation on threonine residue 495 in the normotensive subjects after training. What underlines this discrepancy is unclear, but it may reflect differences in the vessel (Hambrecht et al. 2003) and species (Calvert et al. 2011) investigated as well as disease state (Hambrecht et al. 2003; Calvert et al. 2011).
Exercise hyperaemia as an evaluation of vascular function
In the current study we demonstrate that although the vasodilator response to ACh was unaffected in the leg of hypertensive subjects, blood flow to contracting muscle was lower. This finding underscores that, whereas ACh infusion specifically tests the capacity of a few endothelium-dependent vasoactive systems, including NO synthesis, vasodilatation in response to exercise includes an integrated response involving sympathetic activity, and numerous endothelium- and non-endothelium-dependent vasoactive systems. Within the present conditions exercise hyperaemia appears to be a better indicator of vascular function than the vasodilator response to ACh.
Conclusion
The current study provides evidence for a lower blood flow to the exercising leg in hypertensive subjects that is not associated with a reduced capacity of the NO and prostanoid systems to induce vasodilatation or with altered ACh-induced response. In contrast to the unaffected function of these systems in the peripheral circulation, our data suggest that the observed reduction in systemic blood pressure is related to a training-induced change in the tonic effect of NO and/or prostanoids on vascular tone.
Acknowledgments
Assia A. Bada and Morten Overgaard are gratefully acknowledged for their excellent medical assistance. This work was supported by a grant from the Lundbeck foundation and the Danish Medical Research Council. M.N. was supported by a grant from the P. Carl Petersen foundation. S.P.M. was supported by a grant from the Danish Heart Foundation and the Danish Council for Independent Research – Medical Sciences.
Glossary
Abbreviations
- LBF
leg blood flow
- LVC
leg vascular conductance
- eNOS
endothelial nitric oxide synthase
- NOx
nitrite and nitrate
- l-NMMA
NG-monomethyl-l-arginine
Author contributions
The experiments were conducted at the Copenhagen Muscle Research Centre, Rigshospitalet, Denmark. The contributions of the authors were as follows: conception and design of the study: M.N., Y.H. and S.P.M; collection, analysis and interpretation of data: M.N., L.G.J., P.T., Y.H. and S.P.M.; drafting the article or revising it critically for important intellectual content: M.N., Y.H. and S.P.M. All authors approved the final version.
Supplementary material
Supplementary Table 1
Supplementary Table 2
Supplementary Table 3
Supplementary Table 4
Supplementary Figure 1
Supplementary Figure 2
References
- Andersen P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangsbo J, Gunnarson TP, Wendell J, Nybo L, Thomassen M. Reduced volume and increased training intensity elevate muscle Na+-K+ pump α-2-subunit expression as well as short- and long-term work capacity in humans. J Appl Physiol. 2009;107:1771–1780. doi: 10.1152/japplphysiol.00358.2009. [DOI] [PubMed] [Google Scholar]
- Boushel R, Langberg H, Gemmer C, Olesen J, Crameri R, Scheede C, Sander M, Kjaer M. Combined inhibition of nitric oxide and prostaglandins reduces human skeletal muscle blood flow during exercise. J Physiol. 2002;543:691–698. doi: 10.1113/jphysiol.2002.021477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvert JW, Condit ME, Aragón JP, Nicholson CK, Moody BF, Hood RL, Sindler AL, Gundewar S, Seals DR, Barouch LA, Lefer DJ. Exercise protects against myocardial ischemia-reperfusion injury via stimulation of β3-adrenergic receptors and increased nitric oxide signaling: role of nitrite and nitrosothiols. Circ Res. 2011;108:1448–1458. doi: 10.1161/CIRCRESAHA.111.241117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen VA, Fagard RH. Effects of endurance training on blood pressure, blood pressure-regulating mechanisms, and cardiovascular risk factors. Hypertension. 2005;46:667–675. doi: 10.1161/01.HYP.0000184225.05629.51. [DOI] [PubMed] [Google Scholar]
- Cosentino F, Luscher TF. Tetrahydrobiopterin and endothelial function. Eur Heart J. 1998;19:G3–G8. [PubMed] [Google Scholar]
- Förstermann U, Munzel T. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- Förstermann U, Li H. Therapeutic effect of enhancing endothelial nitric oxide synthase (eNOS) expression and preventing eNOS uncoupling. Br J Pharmacol. 2010;164:213–223. doi: 10.1111/j.1476-5381.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambrecht R, Adams V, Erbs S, Linke A, Kränkel N, Shu Y, Baither Y, Gielen S, Thiele H, Gummert JF, Mohr FW, Schuler G. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation. 2003;107:3152–3158. doi: 10.1161/01.CIR.0000074229.93804.5C. [DOI] [PubMed] [Google Scholar]
- Frandsen U, Bangsbo J, Sander M, Höfner L, Betak A, Saltin B, Hellsten Y. Exercise-induced hyperaemia and leg oxygen uptake are not altered during effective inhibition of nitric oxide synthase with NG-nitro-L-arginine methyl ester in humans. J Physiol. 2001;531:257–264. doi: 10.1111/j.1469-7793.2001.0257j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen J, Jacobsen TN, Victor RG. Is nitric oxide involved in the tonic inhibition of central sympathetic outflow in humans? Hypertension. 1994;24:439–444. doi: 10.1161/01.hyp.24.4.439. [DOI] [PubMed] [Google Scholar]
- Hansen AH, Nyberg M, Bangsbo J, Saltin B, Hellsten Y. Exercise alters the balance between vasoactive compounds in skeletal muscle of individuals with essential hypertension. Hypertension. 2011;58:943–949. doi: 10.1161/HYPERTENSIONAHA.111.176529. [DOI] [PubMed] [Google Scholar]
- Helgerud J, Høydal K, Wang E, Karlsen T, Berg P, Bjerkaas M, Simonsen T, Helgesen C, Hjorth N, Back R, Hoff J. Aerobic high-intensity intervals improve VO2max more than moderate training. Med Sci Sports Exerc. 2007;39:665–671. doi: 10.1249/mss.0b013e3180304570. [DOI] [PubMed] [Google Scholar]
- Heerkens EHJ, Izzard AS, Heagerty AM. Integrins, vascular remodeling, and hypertension. Hypertension. 2007;49:1–4. doi: 10.1161/01.HYP.0000252753.63224.3b. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Kurisu S, Yoshimizu A, Sasaki N, Matsuura H, Kajiyama G, Oshima G. Regular aerobic exercise augments endothelium-dependent vascular relaxation in normotensive as well as hypertensive subjects: role of endothelium-derived nitric oxide. Circulation. 1999;100:1194–1202. doi: 10.1161/01.cir.100.11.1194. [DOI] [PubMed] [Google Scholar]
- Higashi Y, Sasaki S, Nakagawa K, Fukuda Y, Matsuura H, Oshima T, Chayama K. Tetrahydrobiopterin enhances forearm vascular response to acetylcholine in both normotensive and hypertensive individuals. Am J Hypertens. 2002;15:326–332. doi: 10.1016/s0895-7061(01)02317-2. [DOI] [PubMed] [Google Scholar]
- Jansson PA, Veneman T, Nurjhan N, Gerich J. An improved method to calculate adipose tissue interstitial substrate recovery for microdialysis studies. Life Sci. 1994;54:1621–1624. doi: 10.1016/0024-3205(94)90034-5. [DOI] [PubMed] [Google Scholar]
- Joyner MJ, Casey DP. The catacholamines strike back – what NO does not. Circ J. 2009;73:1783–1792. doi: 10.1253/circj.cj-09-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalliokoski KK, Iokonen V, Takala TO, Sipilä H, Knuuti J, Nuutila P. Enhanced oxygen extraction and reduced flow heterogeneity in exercising muscle in endurance-trained men. Am J Physiol Endocrinol Metab. 2001;280:1015–1021. doi: 10.1152/ajpendo.2001.280.6.E1015. [DOI] [PubMed] [Google Scholar]
- Kiens B, Éssen-Gustavsson B, Christensen NJ, Saltin B. Skeletal muscle substrate utilization during submaximal exercise in man: effect of endurance training. J Physiol. 1993;469:459–478. doi: 10.1113/jphysiol.1993.sp019823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt P, Schmidt K, Lehner D, Glatter O, Bächinger HP, Mayer B. Structural analysis of porcine brain nitric oxide synthase reveals a role for tetrahydrobiopterin and L-arginine in the formation of an SDS-resistant dimer. EMBO J. 1995;14:3687–3695. doi: 10.1002/j.1460-2075.1995.tb00038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- Koller A. Signaling pathways of mechanotransduction in arteriolar endothelium and smooth muscle cells in hypertension. Microcirculation. 2002;9:277–294. doi: 10.1038/sj.mn.7800142. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Hellsten Y, Bangsbo J. Intense interval training enhances human skeletal muscle oxygen uptake in the initial phase of dynamic exercise at high but not at low intensities. J Physiol. 2004;559:335–345. doi: 10.1113/jphysiol.2004.062232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterza MC, de Matos LD, Trombetta IC, Braga AM, Roveda F, Alves MJ, Krieger EM, Negrãu CE, Rondon MU. Exercise training restores baroreflex sensitivity in never treated hypertensive patients. Hypertension. 2007;49:1298–1306. doi: 10.1161/HYPERTENSIONAHA.106.085548. [DOI] [PubMed] [Google Scholar]
- Lewington S, Clarke R, Qizbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- Mollnau H, Wendt M, Szöcs K, Lasséque B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kieschow AL, Tsilimingas N, Walter U, Förstermann U, Meinertz T, Griendling K, Münzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of the nitric oxide/cGMP signaling. Circ Res. 2002;90:58–65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, González-Alonso J, Damsgaard R, Hellsten Y, Saltin B. Inhibition of nitric oxide and prostaglandins, but not endothelial derived hyperpolarizing factors, reduces blood flow and aerobic energy turnover in the exercising human leg. J Physiol. 2007;581:853–861. doi: 10.1113/jphysiol.2006.127423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortensen SP, Nyberg M, Thaning P, Saltin B, Hellsten Y. Adenosine contributes to blood flow regulation in the exercising human leg by increasing prostaglandin and nitric oxide formation. Hypertension. 2009;53:993–999. doi: 10.1161/HYPERTENSIONAHA.109.130880. [DOI] [PubMed] [Google Scholar]
- Nyberg M, Mortensen SP, Saltin B, Hellsten Y, Bangsbo J. Low blood flow at onset of moderate-intensity exercise does not limit muscle oxygen uptake. Am J Physiol Regul Integr Comp Physiol. 2010;298:843–848. doi: 10.1152/ajpregu.00730.2009. [DOI] [PubMed] [Google Scholar]
- Pohl U, Holtz J, Busse R, Bassenge E. Crucial role of endothelium in the vasodilator response to increased flow in vivo. Hypertension. 1986;8:37–44. doi: 10.1161/01.hyp.8.1.37. [DOI] [PubMed] [Google Scholar]
- Proctor DN, Miller JD, Dietz NM, Minson CT, Joyner MJ. Reduced submaximal leg blood flow after high-intensity aerobic training. J Appl Physiol. 2001;91:2619–2627. doi: 10.1152/jappl.2001.91.6.2619. [DOI] [PubMed] [Google Scholar]
- Rosenmeier JB, Hansen J, Gonzáles-Alonso J. Circulating ATP-induced vasodilatation overrides sympathetic vasoconstrictor activity in human skeletal muscle. J Physiol. 2004;558:351–365. doi: 10.1113/jphysiol.2004.063107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38:1–78. [PubMed] [Google Scholar]
- Saltin B, Nazar K, Costill DL, Stein E, Jansson E, Essén B, Gollnick D. The nature of the training response; peripheral and central adaptations of one-legged exercise. Acta Physiol Scand. 1976;96:289–305. doi: 10.1111/j.1748-1716.1976.tb10200.x. [DOI] [PubMed] [Google Scholar]
- Saunders NR, Dinenno FA, Pyke KE, Rogers AM, Tschakovsky ME. Impact of combined NO and PG blockade on rapid vasodilation in a forearm mild-to-moderate exercise transition in humans. Am J Physiol Heart Circ Physiol. 2005;288:214–220. doi: 10.1152/ajpheart.00762.2004. [DOI] [PubMed] [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and calculating tissue concentration from dialysate samples. J Neurosci Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Thaning P, Bune LT, Zaar M, Saltin B, Rosenmeier JB. Functional sympatholysis during exercise in patients with type 2 diabetes with intact response to acetylcholine. Diabetes Care. 2011;34:1–6. doi: 10.2337/dc10-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Mattei P, Ghiadoni L, Fasolo CB, Sudano I, Salvetti A. Hypertension causes premature aging of endothelial function in humans. Hypertension. 1997a;29:736–743. doi: 10.1161/01.hyp.29.3.736. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Cyclooxygenase inhibition restores nitric oxide activity in essential hypertension. Hypertension. 1997b;29:274–279. doi: 10.1161/01.hyp.29.1.274. [DOI] [PubMed] [Google Scholar]
- Taddei S, Virdis A, Ghiadoni L, Magagna A, Salvetti A. Vitamin C improves endothelium-dependent vasodilation by restoring nitric oxide activity in essential hypertension. Circulation. 1998;97:2222–2229. doi: 10.1161/01.cir.97.22.2222. [DOI] [PubMed] [Google Scholar]
- Tinken TM, Thijssen DHJ, Hopkins N, Dawson EA, Cable NT, Green DJ. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55:312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- Vongpatanasin W, Wang Z, Arbique D, Arbique G, Adams-Huet B, Mitchell JH, Victor RG, Thomas GD. Functional sympatholysis is impaired in hypertensive humans. J Physiol. 2011;589:1209–1220. doi: 10.1113/jphysiol.2010.203026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman CR, Price EM, Laughlin MH. Shear stress induces eNOS mRNA expression and improves endothelium-dependent dilation in senescent soleus muscle feed arteries. J Appl Physiol. 2005;98:940–946. doi: 10.1152/japplphysiol.00408.2004. [DOI] [PubMed] [Google Scholar]
- Zhao W, Swanson SA, Ye J, Li X, Shelton JM, Zhang W, Thomas GD. Reactive oxygen species impair sympathetic vasoregulation in skeletal muscle in angiotensin II-dependent hypertension. Hypertension. 2006;48:637–643. doi: 10.1161/01.HYP.0000240347.51386.ea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


